Abstract
Schwann cells (SCs) are specialized glial cells that myelinate and protect axons in the peripheral nervous system (PNS). Although myelinating SCs are more commonly studied, the PNS also contains a variety of non-myelinating SCs, including but not limited to Remak SCs (RSCs), terminal SCs, enteric glia. While the field currently lacks many robust tools for interrogating the functions of non-myelinating SCs, recent evidence suggests that, like their myelinating counterparts, non-myelinating SCs are critical for proper PNS function. In this review, we focus specifically on RSCs and highlight recent advances in understanding regulators of RSC development, function, and participation in PNS regeneration.
INTRODUCTION
Schwann cells (SCs) have long been recognized for their essential function of myelinating the peripheral nervous system (PNS). During development, promyelinating SCs associate 1:1 with large caliber axons [1], and following key signaling events [2], spirally wrap and compact their membranes around axons to generate the mature myelin sheath (Figure 1). Peripheral nerves also contain non-myelinating SCs, which have been traditionally understudied relative to myelinating SCs. This is in part due to a relative lack of genetic and imaging tools for studying non-myelinating SCs compared to tools available for myelinating SCs. Here, we focus specifically on Remak SCs (RSCs), which ensheath multiple small-caliber axons in “Remak bundles” (Figure 1) [1]. Although RSCs do not generate myelin, these cells are essential for proper PNS development and function. Furthermore, RSCs play key roles in regeneration following peripheral nerve injury. In this review, we discuss recent advances in RSC biology, and highlight areas for future discovery.
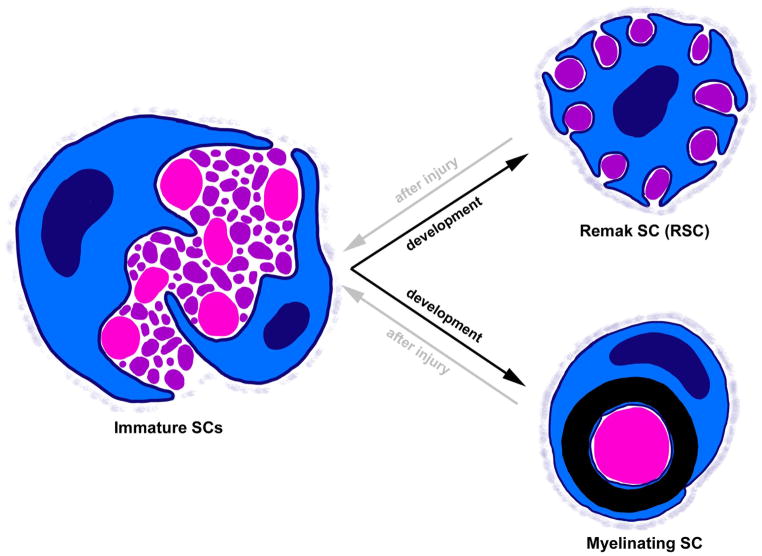
Figure 1. SC fate determination during development and after injury.
During development (black arrows), immature SCs sort axons by diameter in a process called radial sorting. SCs associated with large diameter axons (>1 μm, pink) differentiate into myelinating SCs, while small axons (purple) will be ensheathed by RSCs. After injury (gray arrows) both RSCs and myelinating SCs can dedifferentiate back to an immature state and then re-differentiate to aid in nerve regeneration. SCs at all stages secrete a basal lamina, which becomes denser as SCs mature.
RSC FATE DETERMINATION
During radial sorting, immature SCs extend cytoplasmic projections into bundles of axons [3]. Immature SCs then differentiate into either myelinating SCs or RSCs (Figure 1, black arrows). Unlike myelinating SCs, RSCs associate with small caliber axons (<1 μm diameter) to form Remak bundles. How the myelinating versus RSC fate is determined is not entirely understood, and it is clear that SC fate is quite plastic. Seminal studies demonstrated that when nerve segments containing mostly myelinating SCs are grafted into a largely unmyelinated nerve, these SCs do not myelinate. Conversely, RSCs are capable of generating myelin when sections of predominately unmyelinated nerve are grafted into a primarily myelinated nerve [4,5]. In addition to showing that SC fate determination is reversible (Figure 1, gray arrows), these studies also suggest that signals dictating SC fate derive at least in part from axons.
One of the fundamental signaling pathways in SC-axon interactions is the Neuregulin 1 type III (Nrg1-III)/ErbB2/3 receptor cascade. Nrg1-III is produced by neurons and either anchored to axonal membranes or released from axons by proteases such as BACE1. Nrg1-III interacts with ErbB2/ErbB3 receptor tyrosine kinase heterodimers on the SC membrane, which then activate a variety of downstream pathways such as Ras/MAPK and PI3K/Akt. Nrg1-III/ErbB2/3 signals are required at nearly every stage of SC development in both myelinating SCs and RSCs (reviewed in [6,7]). During myelinating SC development, Nrg1-III levels on the surface of an axon are thought to directly correlate with axonal diameter, and this promotes the differentiation of SCs associated with large axons into myelinating SCs and thus the generation of myelin [8,9] (reviewed in [10]). Nrg1-III signaling is also critical for RSC development as loss of Nrg1 specifically in sensory axons results in Remak bundles with many more axons, and dramatically reduced RSC ensheathment of axons [11]. Interestingly, however, Nrg1 does not appear to have the same effect on SC fate during remyelination after injury. Using an inducible Nrg1 mouse mutant in which Nrg1 is deleted ubiquitously using CAG-Cre™ in adult animals, Fricker and colleagues demonstrated that loss of Nrg1 delays, but does not prevent remyelination or proper RSC ensheathment after sciatic nerve injury [12]. This suggests that Nrg1 regulates the rate of remyelination but is not absolutely critical in SC fate choice in the context of nerve regeneration. Thus, either the requirements of Nrg1 are quite different between early SC development and during remyelination, or perhaps Nrg1 does not actually strictly control SC fate choice, but rather is important more for controlling the efficiency of myelination even during development. The authors go on to test this somewhat by using a conditional Nrg1 mutant in which Nrg1 is deleted in a subset of small caliber axons using Nav1.8-Cre. The authors had previously shown that in young (10 weeks) Nav1.8Cre/Nrg1fl/fl mutant nerves, Remak bundles were severely disrupted and on some axons there was very thin or no myelin [11]. Importantly, in the more recent study, the authors aged these Nav1.8Cre/Nrg1fl/fl mutant animals to 1 year, and saw that there is no compensation for the developmental loss of Nrg1 over time as the Remak bundles still contained many large diameter axons and thinner myelin [12]. Thus, the authors conclude that Nrg1 controls SC development and remyelination by distinct mechanisms. It is important to note however, that the inducible Nrg1 mutants result in loss of all Nrg1 isoforms in all cell types, which makes it difficult to directly compare to cell-type specific loss of specifically Nrg1-III during development. Further, this data does not firmly rule out the possibility that Nrg1 does not actually dictate SC fate choice but simply makes developmental myelination and RSC ensheathment more efficient. Future work should continue to focus on parsing out the mechanism by which Nrg1 controls (or not) SC fate, but regardless, it is clear that axonal-derived Nrg1 is an important signal in the development of peripheral nerves.
Although axonal cues are certainly critical, several studies suggest some genes controlling SC fate decision may act cell-autonomously within SCs [13–15]. For example, SCs that have de-differentiated after injury can cell-autonomously upregulate Nrg1 to spur re-differentiation and remyelination [16]. During development, the gamma-aminobutyric acid type B1 receptor (GABBR1) localizes primarily, albeit not exclusively, in RSCs rather than myelinating SCs [17]. Faroni et al. deleted Gabbr1 in embryonic SCs and observed increased numbers of small diameter axons, although the axons were properly ensheathed, which correlated with an increase in the number of Remak bundles relative to controls as well as a corresponding decrease in the number of larger diameter axons [15]. Myelin protein levels were significantly increased, and the myelin was slightly thicker in Gabbr1 mutant nerves, however these measurements did take into consideration axonal diameter. Thus it is unclear why myelin protein levels are increased. Nrg1-III expression was decreased in Gabbr1 mutants, which is probably related to the decrease in average axonal diameter. The authors also observed enhanced ErbB2 and ErbB3 receptor mRNA and protein levels, a likely compensatory mechanism in response to decreased Nrg1-III levels. One of the intracellular pathways that can be activated by ErbB2/3 signaling is the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk) cascade. Activation of the MAPK/ERK pathway in SCs has been reported to suppress the myelinating state [13,14]; therefore, the authors assessed the levels of phosphorylated Erk2 (pErk2) in Gabbr1 mutant nerves and found enhanced levels of pErk2. Thus, the authors concluded that GABBR1 functions cell-autonomously in SCs and potentially non-cell-autonomously in neurons to regulate SC differentiation. Given the observed elevation in myelin protein levels and myelin thickness, as well as the impact on axon size, future work should further dissect the requirement of GABBR1 in SCs and neurons and exactly how GABBR1 functions to control the differentiation fate of myelinating SCs versus RSCs.
RSC MATURATION
When mature, RSCs individually ensheathe axons with membrane such that each axon in a Remak bundle is separated from the surrounding axons (Figure 2, top left). Thus a hallmark of failed RSC maturation is “naked axons” that have not been completely ensheathed by RSC cytoplasm and are therefore directly contact other axons (Figure 2, top middle). Note that this is distinct from radial sorting defects in which large caliber axons (>1 μm) remain within Remak bundles (Figure 2, top right) (reviewed in [3]). Recently, McFerrin and colleagues found that increased expression of Neuropathy target esterase (Nte) correlates with the timing of SC maturation, and that Nte protein levels are highest in mature non-myelinating SCs. Further, Nte expression is induced after nerve injury [18]. Using Gfap:cre (Gfap is expressed in immature and Remak SCs), the authors observed incomplete ensheathment of unmyelinated axons and degeneration of these axons, without obvious defects in myelinating SCs [18]. These results suggest that Nte is dispensable in myelinating SCs, but may be involved in promoting RSC maturation.
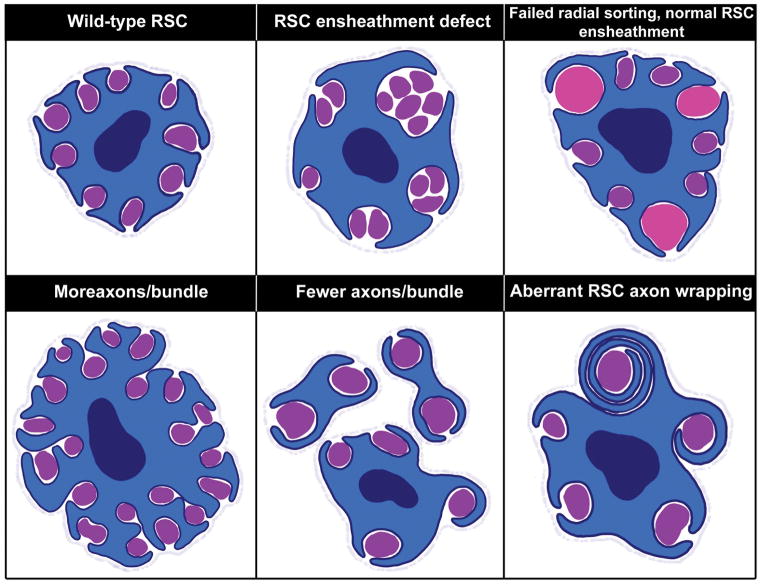
Figure 2. Morphological phenotypes observed when RSCs are disrupted.
Normally, a fully mature RSC will completely ensheathe every axon with cytoplasm to insulate it from other axons (top left). However, when RSCs are disrupted in different mutants, one of the most commonly observed defects is incomplete ensheathment of axons such that some axons remain in direct contact with other axons (top middle). This is distinct from radial sorting deficits in immature SCs where large diameter axons (pink) remain within bundles (top right), although the two phenotypes are often observed together. Another phenotype often observed with failed RSC ensheathment, is an increase in the number of axons per Remak bundle (bottom left). In contrast, some mutants display smaller Remak bundles (bottom middle). Finally, a few mutants have described phenotypes in which RSCs wrap multiple layers of membrane around axons (bottom right). Interestingly, the mutants displaying RSC wrapping have also displayed smaller Remak bundles.
In contrast to Nte, many molecules function in both myelinating SC and RSC biology. For example, PI3K/mTOR signaling is critical in SC development and myelination and has recently proven to be important in non-myelinating SCs as well [19–22]. SC-specific loss of mTOR complex I adaptor, Raptor, resulted in increased axon numbers per Remak bundle (Figure 2, bottom left) as well as more naked axons within bundles [21]. In contrast, elevated mTOR signaling in SCs resulted in fewer axons per Remak bundle (Figure 2, bottom middle) and the aberrant ability of RSCs to iteratively wrap axons with multiple layers of membrane (Figure 2, bottom right) [19,22]. Together, these studies suggest that RSCs, like myelinating SCs, require a precise balance of mTOR signaling for proper development and function.
Another example of a shared molecule between myelinating SCs and RSCs is the G protein-coupled receptor Gpr126/Adgrg6, which controls radial sorting, myelination, remyelination after injury, and long-term SC-axon interactions via several binding partners [23–29]. Gpr126 may also be required in RSCs, as Gpr126 deletion from embryonic SCs resulted in naked small caliber axons in adults [25]. One Gpr126 binding partner, Laminin-211, has been repeatedly implicated in both myelinating SC and RSC biology [27,30] (reviewed in [2]). Additionally, the scaffold protein AHNAK1 was recently shown to bind the laminin receptor β-dystroglycan to facilitate communication between the SC basal lamina and the actin cytoskeleton to control SC motility and RSC ensheathment [31]. Together with studies of the Nrg1-III/ErbB and PI3K/mTOR signaling pathways, the effects of Gpr126 signaling clearly illustrate that, currently, most of the molecules known to impact RSCs also have strong roles in myelinating SCs.
METABOLIC SUPPORT OF AXONS BY RSCs
Significant evidence has been mounting supporting the hypothesis that SCs metabolically support axons to maintain axonal integrity [32]. As a result, an area of recent interest has been SC mitochondria, as mitochondrial dysfunction has emerged as a major mechanism behind several peripheral neuropathies [33]. To understand the roles of SC mitochondria, Viader and colleagues deleted the mitochondrial transcription factor A (Tfam) in embryonic SCs [34]. Interestingly, although SCs survive, loss of Tfam caused preferential degeneration of unmyelinated axons and subsequent loss of myelinated axons. Importantly, neuronal cell bodies had not upregulated markers of apoptosis, suggesting that axonal degeneration in mutants was not secondary to neuronal death. Thus, the authors concluded that SC-specific mitochondrial dysfunction likely contributes to peripheral neuropathies by disrupting axoglial interactions critical for axonal maintenance [34].
Similarly, multiple groups have independently shown that the serine/threonine kinase LKB1 regulates SC maintenance of axons through its roles in SC polarity [35] and mitochondrial metabolism [36,37]. When Lkb1 is deleted in embryonic SCs, myelination initiation is delayed, nerves are hypomyelinated, RSC ensheathment is impaired, and animals display behavioral symptoms of neuropathy. Like other differentiating cells, SCs undergo a shift from glycolytic to mitochondrial oxidative metabolism myelination, and that LKB1 regulates this shift by elevating citrate synthase activity [36]. The fact that oxidative metabolism generates more ATP than glycolysis and Lkb1 mutants display reduced lipid synthesis implies that the metabolic switch during SC differentiation is required for the increased energy production necessary to generate myelin lipids. Finally, in addition to the developmental defects already described, Beirowski et al. found that SC-specific Lkb1 mutants display progressive axon degeneration [37]. To determine if this was due to the developmental deficits, the authors deleted Lkb1 specifically in adult SCs. These animals also showed severe axon degeneration leading to peripheral neuropathy, suggesting that LKB1 function in SCs is critical for long-term axon maintenance. This finding agrees with observations by Pooya et al., that while SCs can survive via primarily glycolysis, loss of mitochondrial function impairs SC ability to redifferentiate after injury [36]. Interestingly, axon degeneration caused by Lkb1 loss preferentially affected unmyelinated sensory axons [38] reminiscent of what was described in Tfam mutants [34]. In their Lkb1 mutant model, Beirowski and colleagues found similar SC mitochondrial dysfunction as described by Pooya et al. in addition to other metabolic changes including energy depletion and increased lactate levels. Although axon degeneration in Lkb1 mutants appeared largely independent of AMPK and mTOR, the authors observed elevated AMPK signaling in Lkb1 mutant nerves and concluded that AMPK-related kinases also associated with LKB1 metabolic functions are likely contributing to SC metabolic support of axons [37].
Collectively, the studies of Tfam and LKB1 in SC mitochondrial function suggest that SC metabolism is critical for axonal integrity. Since both studies observed preferential loss of unmyelinated axons upon disrupted SC metabolism, this leads to the interesting speculation that perhaps unmyelinated axons are particularly dependent on glial support. Not mutually exclusive with this hypothesis is the possibility that RSCs are particularly sensitive to metabolic changes relative to myelinating SCs, and that this sensitivity leads to faster axoglial disruptions upon loss of metabolic regulation.
RSCs IN NERVE INJURY AND NOCICEPTION
Many of the axons in Remak bundles are sensory c-fibers, which transmit pain information to the brain. Accordingly, recent work suggests that RSC disruption results in neuropathic pain. As mentioned above, SC-specific loss of Gabbr1 resulted in increased numbers of unmyelinated c-fibers. This increase in Remak bundle density resulted in hypersensitivity to mechanical and thermal stimuli as well as altered locomotor coordination even in the absence of injury, although it is still somewhat unclear if this may be simply due to the changes in axon size distribution [15]. Additionally, loss of the LDL receptor related protein (LRP1), which is a potent regulator of SC migration and survival in vitro [38,39], resulted in slight hypomyelination and impaired RSC ensheathment in vivo, which then lead to mechanical allodynia in the absence of injury [40]. Further, after partial nerve ligation, Orita and colleagues observed dramatically accelerated axon degeneration and increased SC apoptosis, which severely impaired regeneration in Lrp1 mutant nerves. Thus, Lrp1 mutants displayed severe and sustained motor dysfunction and mechanical allodynia after injury, which the authors show is likely due to elevated phosphorylation of p38-MAPK in microglia in the dorsal spinal cord resulting in their activation [40]. Importantly, these data agree with previous work showing that a shed form of LRP1 injected into mouse sciatic nerves prior to injury prevented p38-MAPK activation and attenuated neuropathic pain in those animals [41]. Together, these data suggest that RSCs are critical in the development of neuropathic pain both with and without nerve injury.
CONCLUSIONS
Although less is known about non-myelinating SCs relative to myelinating SCs, recent evidence demonstrates that RSCs play critically important roles in peripheral nerve development, maintenance of normal PNS function, and regeneration after injury. Thus, future efforts should continue to parse out the molecular mechanisms regulating the development of RSCs and their potential utility in understanding PNS diseases and injury conditions.
However, a major hindrance to progress in understanding RSCs is the difficulty in imaging these cells without using electron microscopy in an animal model amenable to forward genetic screens. Genetic screens in zebrafish have led to the discovery and characterization of multiple regulators of myelinating SC development, including gpr126 [42]. Although a robust method to screen for defects in RSCs in zebrafish has not been described, this possibility should be explored further. Another solution that has been proposed recently would be to look more closely at wrapping glia in Drosophila melanogaster. In peripheral nerves, Drosophila wrapping glia ensheath and separate single or small bundles of axons with their membranes within the nerve, similar to the segregation of individual axons by RSCs [43,44]. Recent work suggests that several genes implicated in RSC development, including NRG1 [45] and Laminin [30,46], appear to perform analogous functions, and act through similar molecular mechanisms in Drosophila wrapping glia. Thus, one possible avenue for further exploration into basic RSC biology might be comparative analyses with wrapping glia in Drosophila.
Another significant impediment to studying RSCs is the inability to perform cell type specific knockouts and molecular studies due to the lack of highly specific markers that do not overlap with immature SCs. Without RSC-specific markers, the field cannot delete genes specifically in RSCs while leaving myelinating SCs intact. Similarly, an RSC-specific marker would allow individual separation of these cells from other SCs for deep molecular characterization. Importantly, this lack of tools is true not only for RSCs, but also for other types of non-myelinating PNS glia including terminal SCs, enteric glia, etc.
These tools would help the field address many outstanding questions such as: why does axon degeneration after metabolic challenge preferentially affect unmyelinated sensory fibers? Are RSCs more susceptible to metabolic changes and thus less capable of maintaining their axons after insult? Further, it remains unclear if RSCs and myelinating SCs are equivalent after injury. Although early nerve graft studies suggest these two fates are interchangeable, it is unknown whether RSC vs. myelinating SC repair responses are equivalent. Finally, a particularly fascinating question is why do RSCs never make myelin, and myelinating SCs never interact with more than one axon? Even in Pten and Akt mutants, where the RSCs aberrantly generate multiple membrane wraps around their axons, RSCs never proceed to myelinate axons. The importance of RSCs is highlighted by their essential role in proper regeneration after injury, and loss of axonal support by RSCs is sufficient to drive neuropathic pain even in the absence of injury. Therefore, efforts to discover therapeutic options for human neuropathy patients are dependent upon further advances in RSC biology.
HIGHLIGHTS.
Remak Schwann cells ensheath multiple small caliber axons
Remak Schwann cells are critical for peripheral nerve development, maintenance, and regeneration
Genetic regulators of myelinating Schwann cells can also control Remak Schwann cell ensheathment
Both Remak Schwann cells and myelinating Schwann cells metabolically support axons
Remak Schwann cell disruptions result in neuropathic pain even in the absence of nerve injury
Acknowledgments
The authors are indebted to Megan Corty (OHSU) for helpful discussions and critical feedback on the manuscript. This work was supported by NIH/NINDS to BLH (F31 NS094004). We apologize for any citations omitted due to space limitations.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the period of review, have been highlighted as:
* recommended reading
** of particularly special interest
- 1.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 2.Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia. 2015;63:1376–1393. doi: 10.1002/glia.22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feltri ML, Poitelon Y, Previtali SC. How Schwann Cells Sort Axons: New Concepts. Neuroscientist. 2016;22:252–265. doi: 10.1177/1073858415572361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *4.Aguayo AJ, Charron L, Bray GM. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976;5:565–573. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- *5.Aguayo AJ, Epps J, Charron L, Bray GM. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976;104:1–20. doi: 10.1016/0006-8993(76)90643-0. [DOI] [PubMed] [Google Scholar]
- 6.Lemke G. Neuregulin-1 and myelination. Sci STKE. 2006;2006:pe11. doi: 10.1126/stke.3252006pe11. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56:1491–1497. doi: 10.1002/glia.20753. [DOI] [PubMed] [Google Scholar]
- 8.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 9.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 11.Fricker FR, Zhu N, Tsantoulas C, Abrahamsen B, Nassar MA, Thakur M, Garratt AN, Birchmeier C, McMahon SB, Wood JN, et al. Sensory axon-derived neuregulin-1 is required for axoglial signaling and normal sensory function but not for long-term axon maintenance. J Neurosci. 2009;29:7667–7678. doi: 10.1523/JNEUROSCI.6053-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Fricker FR, Antunes-Martins A, Galino J, Parasmothy R, La Russa F, Perkins J, Goldberg R, Brelstaff J, Zhu N, McMahon SB, et al. Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination. Brain. 2013;136:2279–2297. doi: 10.1093/brain/awt148. Fricker et al. used an inducible Nrg1 knockout to analyze the role of Nrg1 in remyelination after sciatic nerve injury. Interestingly, they found that loss of Nrg1, while critical for proper myelination during development appears largely dispensible during remyelination. After injury, remyelination was significantly delayed, but did still eventually occur in inducible Nrg1 mutants, suggesting that Nrg1 controls the rate of remyelination but is not required for SC fate choice during recovery. The authors then analyze conditional Nrg1 mutants in which Nrg1 has been deleted in sensory axons during early development using the Nav1.8-Cre transgene. Previously they had shown that loss of Nrg1 in sensory axons resulted in defective RSC ensheathment and hypomyelination (see reference 11), and when they assessed these nerves at 1 year, they saw that there had been no compensation for these phenotypes, which they suggest means that Nrg1 controls developmental myelination and remyelination in a distinct manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- **15.Faroni A, Castelnovo LF, Procacci P, Caffino L, Fumagalli F, Melfi S, Gambarotta G, Bettler B, Wrabetz L, Magnaghi V. Deletion of GABA-B receptor in Schwann cells regulates remak bundles and small nociceptive C-fibers. Glia. 2014;62:548–565. doi: 10.1002/glia.22625. In this study, conditional ablation of GABBR1 in SCs results in increased numbers of unmyelinated axons, particularly nociceptive c-fibers, and Remak bundles, with a corresponding decrease in the number of large fibers. Myelinating SCs were relatively unaffected in these mutants, suggesting that GABBR1 may play a role in Remak SC fate determination. Further, SC-specific GABBR1 mutant animals displayed neuropathic pain even in the absence of nerve injury. This supports other work suggesting that Remak SCs play critical roles in maintaining proper PNS function and in the development of neuropathic pain. [DOI] [PubMed] [Google Scholar]
- 16.Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16:48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- 17.Procacci P, Ballabio M, Castelnovo LF, Mantovani C, Magnaghi V. GABA-B receptors in the PNS have a role in Schwann cells differentiation? Front Cell Neurosci. 2012;6:68. doi: 10.3389/fncel.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McFerrin J, Patton BL, Sunderhaus ER, Kretzschmar D. NTE/PNPLA6 is expressed in mature Schwann cells and is required for glial ensheathment of Remak fibers. Glia. 2017;65:804–816. doi: 10.1002/glia.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siefke C, et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherman DL, Krols M, Wu LM, Grove M, Nave KA, Gangloff YG, Brophy PJ. Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J Neurosci. 2012;32:1817–1825. doi: 10.1523/JNEUROSCI.4814-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Norrmen C, Figlia G, Lebrun-Julien F, Pereira JA, Trotzmuller M, Kofeler HC, Rantanen V, Wessig C, van Deijk AL, Smit AB, et al. mTORC1 controls PNS myelination along the mTORC1-RXRgamma-SREBP-lipid biosynthesis axis in Schwann cells. Cell Rep. 2014;9:646–660. doi: 10.1016/j.celrep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- **22.Domenech-Estevez E, Baloui H, Meng X, Zhang Y, Deinhardt K, Dupree JL, Einheber S, Chrast R, Salzer JL. Akt Regulates Axon Wrapping and Myelin Sheath Thickness in the PNS. J Neurosci. 2016;36:4506–4521. doi: 10.1523/JNEUROSCI.3521-15.2016. Previous work had shown that constitutively active Akt did not affect peripheral myelination. However, here the authors elevated Akt activity specifically in SCs and found that the PNS in fact responds to this manipulation in a manner similar to constitutively active Akt in the CNS. Increased Akt activity results in enhanced mTOR signaling, leading to hypermyelination and several Remak bundle defects including fewer axons per bundle and Remak SCs wrapping multiple layers of membrane around axons. These phenotypes resemble those observed in Pten mutants, which also display elevated mTOR signaling. Together with work from Sherman et al. and Norrmen et al. these studies show that SCs require a precise balance in the PI3K/mTOR axis to achieve proper development and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Monk KR, Oshima K, Jors S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. 2011;138:2673–2680. doi: 10.1242/dev.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33:17976–17985. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paavola KJ, Sidik H, Zuchero JB, Eckart M, Talbot WS. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci Signal. 2014;7:ra76. doi: 10.1126/scisignal.2005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, Mogha A, Ghidinelli M, Feltri ML, Schoneberg T, Piao X, et al. The Adhesion GPCR GPR126 Has Distinct, Domain-Dependent Functions in Schwann Cell Development Mediated by Interaction with Laminin-211. Neuron. 2015;85:755–769. doi: 10.1016/j.neuron.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogha A, Harty BL, Carlin D, Joseph J, Sanchez NE, Suter U, Piao X, Cavalli V, Monk KR. Gpr126/Adgrg6 Has Schwann Cell Autonomous and Nonautonomous Functions in Peripheral Nerve Injury and Repair. J Neurosci. 2016;36:12351–12367. doi: 10.1523/JNEUROSCI.3854-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuffer A, Lakkaraju AK, Mogha A, Petersen SC, Airich K, Doucerain C, Marpakwar R, Bakirci P, Senatore A, Monnard A, et al. The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6. Nature. 2016;536:464–468. doi: 10.1038/nature19312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu WM, Yu H, Chen ZL, Strickland S. Disruption of laminin in the peripheral nervous system impedes nonmyelinating Schwann cell development and impairs nociceptive sensory function. Glia. 2009;57:850–859. doi: 10.1002/glia.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Boxberg Y, Soares S, Fereol S, Fodil R, Bartolami S, Taxi J, Tricaud N, Nothias F. Giant scaffolding protein AHNAK1 interacts with beta-dystroglycan and controls motility and mechanical properties of Schwann cells. Glia. 2014;62:1392–1406. doi: 10.1002/glia.22685. [DOI] [PubMed] [Google Scholar]
- 32.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 33.Niemann A, Berger P, Suter U. Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:217–242. doi: 10.1385/nmm:8:1-2:217. [DOI] [PubMed] [Google Scholar]
- **34.Viader A, Golden JP, Baloh RH, Schmidt RE, Hunter DA, Milbrandt J. Schwann cell mitochondrial metabolism supports long-term axonal survival and peripheral nerve function. J Neurosci. 2011;31:10128–10140. doi: 10.1523/JNEUROSCI.0884-11.2011. Evidence is mounting that SCs provide metabolic support to axons to maintain axonal integrity, and that SC mitochondrial metabolism is critical for this function. Here, the authors deleted mitochondrial transcription factor A (Tfam) in SCs and observed massive axonal degeneration, particularly of unmyelinated fibers. Importantly, these defects were not secondary to neuronal death as DRG neurons remained intact, nor were they the result of SC death as the SCs also survived this manipulation. Therefore, the authors concluded that SC mitochondrial dysfunction contributes to the onset of peripheral neuropathy by compromising axoglial interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen YA, Chen Y, Dao DQ, Mayoral SR, Wu L, Meijer D, Ullian EM, Chan JR, Lu QR. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. Nat Commun. 2014;5:4991. doi: 10.1038/ncomms5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Pooya S, Liu X, Kumar VB, Anderson J, Imai F, Zhang W, Ciraolo G, Ratner N, Setchell KD, Yoshida Y, et al. The tumour suppressor LKB1 regulates myelination through mitochondrial metabolism. Nat Commun. 2014;5:4993. doi: 10.1038/ncomms5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Beirowski B, Babetto E, Golden JP, Chen YJ, Yang K, Gross RW, Patti GJ, Milbrandt J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat Neurosci. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci. 2006;26:11197–11207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Yamauchi T, Gaultier A, Takimoto S, Campana WM, Gonias SL. Regulation of cytokine expression by Schwann cells in response to alpha2-macroglobulin binding to LRP1. J Neurosci Res. 2011;89:544–551. doi: 10.1002/jnr.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Orita S, Henry K, Mantuano E, Yamauchi K, De Corato A, Ishikawa T, Feltri ML, Wrabetz L, Gaultier A, Pollack M, et al. Schwann cell LRP1 regulates remak bundle ultrastructure and axonal interactions to prevent neuropathic pain. J Neurosci. 2013;33:5590–5602. doi: 10.1523/JNEUROSCI.3342-12.2013. Orita and colleagues used P0-cre to specifically delete the LDL receptor-related protein (LRP1) in SCs. This resulted in Remak SC ensheathment deficits and mild hypomyelination, leading to hypersensitivity to mechanical stimuli in the absence of injury. Upon partial nerve ligation, the authors observed severe axonal degeneration and SC apoptosis, which impaired regeneration and caused sustained motosensory dysfunction. Microglia were activated and p38-MAPK phosphorylation was enhanced in the spinal cords of Lrp1 mutants after injury, which the authors propose as the likely mechanism behind the neuropathic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Gaultier A, Arandjelovic S, Li X, Janes J, Dragojlovic N, Zhou GP, Dolkas J, Myers RR, Gonias SL, Campana WM. A shed form of LDL receptor-related protein-1 regulates peripheral nerve injury and neuropathic pain in rodents. J Clin Invest. 2008;118:161–172. doi: 10.1172/JCI32371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pogoda HM, Sternheim N, Lyons DA, Diamond B, Hawkins TA, Woods IG, Bhatt DH, Franzini-Armstrong C, Dominguez C, Arana N, et al. A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev Biol. 2006;298:118–131. doi: 10.1016/j.ydbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- *43.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schirmeier S, Klambt C. The Drosophila blood-brain barrier as interface between neurons and hemolymph. Mech Dev. 2015;138(Pt 1):50–55. doi: 10.1016/j.mod.2015.06.002. [DOI] [PubMed] [Google Scholar]
- **45.Matzat T, Sieglitz F, Kottmeier R, Babatz F, Engelen D, Klambt C. Axonal wrapping in the Drosophila PNS is controlled by glia-derived neuregulin homolog Vein. Development. 2015;142:1336–1345. doi: 10.1242/dev.116616. The homolog of mammalian Neuregulin in Drosophila melanogaster is Vein. In this manuscript, Matzat and colleagues delete Vein specifically in wrapping glia, which are analogous to mammalian RSCs. During mammalian PNS development, Nrg1-type III is axonally derived, but after injury SCs autonomously express Nrg1-type I to promote regeneration. This study shows that Vein is wrapping glial-derived and promotes membrane extension around axons via autocrine activation of EGF. This mechanism resembles reactivation of SCs after injury in the mammalian PNS. Additionally, Vein signaling in wrapping glia regulates multiple aspects of other glial types in the Drosophila nerve. [DOI] [PubMed] [Google Scholar]
- 46.Petley-Ragan LM, Ardiel EL, Rankin CH, Auld VJ. Accumulation of Laminin Monomers in Drosophila Glia Leads to Glial Endoplasmic Reticulum Stress and Disrupted Larval Locomotion. J Neurosci. 2016;36:1151–1164. doi: 10.1523/JNEUROSCI.1797-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]