Abstract
Growing evidence points to synaptic pathology as a core component of the pathophysiology of schizophrenia (SZ). Significant reductions of dendritic spine density and altered expression of their structural and molecular components have been reported in several brain regions, suggesting a deficit of synaptic plasticity. Regulation of synaptic plasticity is a complex process, one that requires not only interactions between pre- and post-synaptic terminals, but also glial cells and the extracellular matrix (ECM). Together, these elements are referred to as the ‘tetrapartite synapse’, an emerging concept supported by accumulating evidence for a role of glial cells and the extracellular matrix in regulating structural and functional aspects of synaptic plasticity. In particular, chondroitin sulfate proteoglycans (CSPGs), one of the main components of the ECM, have been shown to be synthesized predominantly by glial cells, to form organized perisynaptic aggregates known as perineuronal nets (PNNs), and to modulate synaptic signaling and plasticity during postnatal development and adulthood. Notably, recent findings from our group and others have shown marked CSPG abnormalities in several brain regions of people with SZ. These abnormalities were found to affect specialized ECM structures, including PNNs, as well as glial cells expressing the corresponding CSPGs. The purpose of this review is to bring forth the hypothesis that synaptic pathology in SZ arises from a disruption of the interactions between elements of the tetrapartite synapse.
Keywords: Extracellular matrix, Perineuronal nets, Chondroitin sulfate proteoglycans, Astrocytes, NG2 cells, Microglia
1. Introduction
Growing evidence points to synaptic pathology across several brain disorders, including schizophrenia (SZ), bipolar disorder, major depression, autism spectrum disorder and Alzheimer’s disease. Research on the underlying mechanisms for this pathology has only very recently begun to make headway, and important questions arise with regard to the potential common denominators of synaptic pathology among these disorders, and their timeframe across lifespan. With regard to the latter, it is important to consider that synaptic remodeling occurs constantly throughout life. During postnatal development, excessive synaptic formation is followed by elimination of less active synapses, a process named synapse pruning [1, 2]. During adult life, synapses are highly dynamic, with constant, experience-driven synaptic growth and elimination. It is plausible to postulate that timeframe-, mechanism- and brain region- specificity underlying synaptic pathology across a spectrum of brain disorders may at least in part contribute to their diverse clinical and pathophysiological manifestations. Within this context, we suggest that the concept of ‘tetrapartite synapse’ may be a useful starting point for investigating synaptic pathology. We focus on schizophrenia as a notable example.
1.1. The tetrapartite synapse
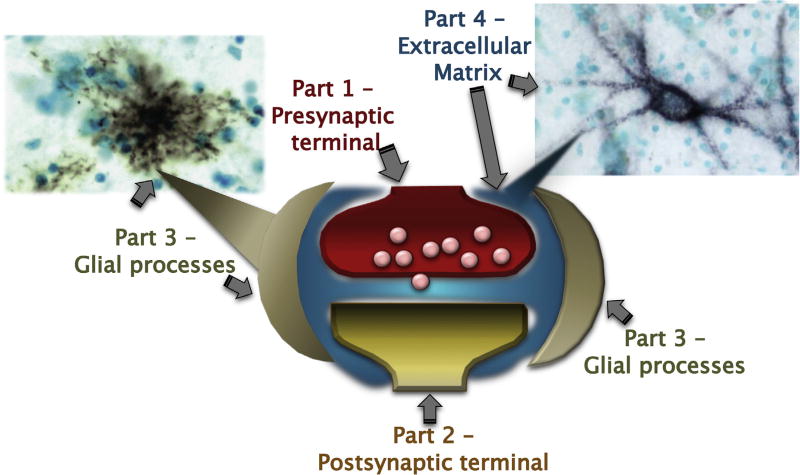
The chemical synapse has classically been considered as composed of two main elements, i.e. the presynaptic and postsynaptic elements. This concept evolved over the past two decades to include a third element, i.e. the astrocyte, as processes from these cells envelope the synapse and play a key role in regulating its functions [3,4]. The ensemble of pre- and postsynaptic elements and astrocytes has been proposed to form a functional complex referred to as the ‘tripartite synapse’ [5]. Growing evidence indicates that other populations of glial cells, including NG2 glia and microglia, also play critical roles in regulating synaptic functions and plasticity. Thus, we suggest that distinct populations of glial cells with specific functions may be considered together to represent the third element of tripartite synapse. Yet more recently, the extracellular matrix (ECM) has come to the forefront of neuroscience as an active component of neural functions and, in particular, synaptic regulation. On the basis of this evidence, Dityatev et al., proposed the elegant concept of the ‘tetrapartite synapse’, composed of pre- and post-synaptic elements, glial processes and ECM, and elegantly documented the interactions between these components [6–8] (Fig. 1). Here, we review evidence supporting the idea that synaptic functions and plasticity result from interactions between all elements of the tetrapartite synapse, and focus on evidence that these interactions are disrupted in SZ.
Fig. 1.
Diagramatic representation of the tetrapartite synapse. Elements composing it are the pre- and post-synaptic terminals, astrocytic processes surrounding them and perisynaptic extracellular matrix condensations interposed between these elements.
1.1.1. The tetrapartite synapse: pre- and post-synaptic elements
The brain possesses the extraordinary ability to continuously reshape itself throughout the entire lifespan. This property, defined as plasticity, is based on the highly dynamic properties of synaptic contacts, i.e. the ability to generate new synapses, eliminate them, and alter the electrophysiological, molecular and structural properties of existing ones in response to experience. The mechanisms underlying synaptic plasticity have been the object of intense work and exciting discoveries over the past few decades, focused initially on the interplay between the presynaptic and postsynaptic elements. For example, the discovery that trains of presynapticaction potentials induce a long-lasting increase in synaptic strength during long term potentiation (LTP) generated intense debate over whether the predominant underlying changes may be related to postsynaptic modification in α-amino-3-hydroxy-5-methyl-4-isoxazolepro-pionic acid receptors (AMPARs) or altered presynaptic transmitter release [9,10]. Ensuing work demonstrated that these mechanisms include a series of steps, from receptor phosphorylation, to protein synthesis and, eventually, structural changes including growth of new dendritic spines and increased size of pre-existing spines, mediated by changes dendritic spine molecular cytoskeleton, including long-lasting increases in F-actin content within spines and condensation of the post-synaptic density (PSD), a dense aggregate of scaffolding proteins implicated in structural maintenance and signal transduction [11–13]. Similarly, long-term depression (LTD) of synaptic strength results in spine shrinkage and elimination [14–17]. A review of current knowledge on the role of pre-and post-synaptic elements in plasticity is beyond the scope of this manuscript; arguably plastic modifications of these elements may be considered to be the final result of complex, experience-driven mechanisms, and the underlying substrate of learning and memory.
1.1.2. The tetrapartite synapse: role of glial cells
1.1.2.1. Astrocytes
Astrocytic processes envelop the pre- and postsynaptic elements and closely approach the synaptic cleft, thus representing a key component of the synapse [3,18] (Fig. 1). Their robust expression of the high-affinity glutamate transporters EAAT1-EAAT2 allows them to rapidly re-uptake excess of glutamate released from the presynaptic terminals, thus restricting excitatory transmission [19]. In turn, astrocytes actively contribute to synaptic transmission and plasticity. Although electrically silent, they respond to presynaptic activation with G-protein-mediated Ca2 signals, triggering the release of ‘gliotransmitters’, including glutamate, ATP, and GABA, which modulate local synaptic transmission [20–25]. Astrocyte-derived glutamate facilitates NMDA receptor activation on the postsynaptic sites, enhancing the probability of triggering LTP [26]. Moreover, stimuli inducing synaptic LTP rapidly induce structural remodeling of astrocytic processes enwrapping synapses, resulting in changes in their ability to modulate synaptic transmission [27]. Together, these considerations compellingly point to astrocytes as key active mediators of synaptic plasticity.
1.1.2.2. NG2 cells
In the early nineties, a novel population of cells was identified by their expression of the CSP GNG2, platelet-derived growth factor α receptors (PDGFαR), and O4 sulfatide (O4) [28–30]. These cells are abundant in the white and gray matter, and have been shown to represent the main source of mature oligodendrocytes in the mature brain, earning the name of oligodendrocyte precursor cells [31]. However, morphological features of NG2 cells closely resemble those of mature astrocytes, while their molecular signature is quite distinct [29,32,33]. Further studies showed that NG2 cells represent a distinct matureglial type with unique properties. Indeed, a growing body of evidence indicates that NG2 cell express voltage-gated ion channels and ligand-gated ionotropic neurotransmitter receptors, typically found in neurons but not in glial cells; this peculiar pattern of molecular expression endows them with a unique electrophysiological profile [34]. They are the only glial cell type capable of receiving synaptic GABAergic and glutamatergic contacts from neurons, and to respond to excitatory inputs with excitatory postsynaptic currents and activity-dependent modifications analogous to LTP at excitatory synapses, although with some notable differences [35–37]. Electron microscopy studies demonstrated that NG2 cell processes often encapsulate neuronal cell bodies and contact synapses [38]. Potential effects of NG2 cells on synaptic transmission are suggested by experiments showing that downregulation of NG2 expression results in altered subunit composition of AMPA receptors and marked reduction of NMDA-dependent LTP [39]. The full range of NG2 cell functions is only partially understood, and is likely to be age-, stage- and brain region-specific; however, plastic responses of these cells to excitatory neurotransmission and their intimate contacts with synaptic complexes strongly suggest that they play a significant role in the regulation of synaptic functions and plasticity [34,40–43].
1.1.2.3 Microglia
Microglial cells serve diverse roles during brain development and adulthood, from regulation of synaptic pruning and plasticity to removal of apoptotic cells and debris, and immune responses, representing primary sources of immune response factors such as cytokines (see 44). Notably, these seemingly unrelated functions may in fact share similar mechanisms, as growing evidence indicates that immune factors play important roles in the regulation of synaptic plasticity [45]. Microglial cells express an impressive variety of receptors, including not only immune receptors, such as pattern-recognition receptors that allow them to recognize pathogen-associated molecular patterns and tissue damage–associated molecular patterns and chemokine receptors, but also a surprisingly large number of receptors for neurotransmitters and neuropeptides [44]. These two latter categories include ionotropic and metabotropic glutamate receptors, and receptors for GABA, dopamine, catecholamines and acetylcholine, which mediate neural-glia communications. These receptors modulate the release of cytokines and guide microglial processes toward active synapses, where they regulate synaptic structural plasticity [44–46]. In particular, microglia represent a key player in the remodeling, particularly removal, of inactive synapses during brain development and adulthood. Microglia processes are highly dynamic, continually extending, retracting and interacting with synapses, thus as acting like sentinels to assess surrounding synapses and contribute to their remodeling when needed [20–23]. Among the immune-related molecules involved in these mechanisms are several complement factors, which act as signal to microglia to find and engulf pre and post-synaptic elements [47–49]. Complement signaling pathways also mediate microglia-induced long term depression (LTD), involved in brain circuitry optimization, and potentially in memory impairments and synaptic disruptions in neuroinflammation-related brain disorders [50]. Notably, the strongest genetic association observed in SZ involves variation in the Major Histocompatibility Complex locus, shown to arise predominantly from alleles of the complement component 4 (C4) genes [51,52]. Elegant work by Sekar et al. recently showed that these alleles do affect C4 expression and that this factor mediates synapse elimination during postnatal development [52]. In summary, microglial cells play a key role in shaping synaptic connectivity in an activity-dependent manner – the underlying mechanisms involve molecular factors with strong relevant to the pathophysiology of SZ.
1.1.3. The tetrapartite synapse: role of the extracellular matrix
The brain ECM is a complex molecular network that surrounds all cells, occupying approximately a 20% volume fraction of the adult brain [53]. It’s main components include hyaluronan, proteoglycans, glycoproteins and a variety of posttranslational remodeling proteases, such as matrix metalloproinases (MMPs), ‘a disintegrin and matrix metalloproteases’ (ADAMS), and ‘ADAMS with a thrombospondin domain’ (ADAMTS), which cleave ECM molecules, allowing for highly dynamic functional adaptations [54–58]. As discussed below, organized forms of ECM surround the synapse, fill the synaptic cleft and interact with cell surface receptors (Fig. 1). Converging evidence points to peri-synaptic ECM aggregates as a critical player contributing to synaptic signaling and plasticity.
1.1.3.1. ECM factors regulating synaptic plasticity
Chondroitin sulfate proteoglycans (CSPGs) have been described as the organizers of the ECM, of which they represent a main component [59–64]. These macromolecules consist of core proteins linked to varying numbers of chondroitin sulfate (CS) glycosaminoglycan (GAG) chains. The number and length of GAG chains, and particularly their sulfation patterns (e.g. CS-6, CS-4), are key factors in determining their functions, resulting in highly dynamic structural and functional diversity to these molecules [65–68]. While their functions in the developing and mature brain are highly diversified, mounting evidence indicates that CSPGs play a complex role in developmental and adult regulation of synaptic plasticity. For example, enzymatic CSPG removal in vitro mouse hippocampal slices causes a two-fold decrease in long-term potentiation (LTP) [69]. Overexpression of CS-6 sulfation in mice leads to failure to instate an adult form of restricted plasticity [66]. Altered expression of several CSPGs, such as PTPRZ1, neurocan and brevican, was found to be associated with synaptic remodeling LTP abnormalities and learning impairment [70–75]. Notably, several CSPGs have been shown to actively stabilize dendritic spines, while their removal by enzymatic digestion results in increased spine motility [76–79].
Several other ECM molecules have been found to be involved in the regulation of synaptic plasticity. For instance, genetic or pharmacological removal of the ECM component tenascin-C and thrombospondins 1 and 2 resulted in reduced calcium signaling and impaired LTP in rodents (Evers et al.; Dityatev et al.). Hyaluronan, considered to be the backbone of the ECM and enriched in PNNs and other forms of ECM perisynaptic aggregates, was found to regulate hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2+ channels [80]. Other ECM components have been shown to modulate chemical transmission by acting on glutamate NMDA and AMPA receptors and impacting adaptive synapse modifications. Particularly relevant to several brain disorders, including SZ, is the ECM glycoprotein Reelin. Reelin’s effects are mediated through its main lipoprotein receptors, apolipoprotein E receptor 2 and very-low-density lipoprotein receptor [81,82], as well as through the integrin family and the Src family kinases [82–85]. Reelin is secreted into the ECM, where it regulates the composition of NMDA receptors, controlling the predominance and/or phosphorylation of the NR2 NMDA receptor subunits, augments AMPA responses by increasing the number of AMPA receptors on the postsynaptic membrane, and robustly enhances LTP [85–87]. Reelin powerfully promotes spine remodeling, regulating spine size and stability, and number of synaptic contacts per spine [88–93]. Integrins, known to interact with Reelin and other ECM molecules, regulate AMPA receptor internalization, surface mobility of NMDA receptor subunits, and synaptic dwell time of glycine receptors and their scaffolding molecule gephyrin [94–97]. These mechanisms have been postulated to allow integrins to play complex roles in synaptic plasticity, including carrying out structural and functional changes that accompany LTP [97–99]. Secreted ECM proteases, such as MMPs, affect excitatory transmission and have extensively been investigated as mediators of synaptic plasticity [100–103]. During development, MMPs play a key role in spine formation and maturation [104–106]. In mature neurons, MMPs and their interactions with integrins, are required for spine volume changes induced by LTP and LTD [107,108]. MMP-9 is transiently released in response to enhanced neuronal activity and impacts both synaptic potentiation and dendritic spine enlargement in a dependent manner [108,109]. Notably, several MMPs have been shown to be represented in WFA-labeled PNNs [110], suggesting a role in regulating their functions. Semaphorins, key components of the ECM, have also been shown to regulate synaptogenesis (Pasterkamp & Giger 2009). For instance, semaphorin 3A, a key component of at least a subpopulation of PNNs, exerts a powerful effect on synapses, possibly through its plexin and neuropilin receptors [106,111–114].
1.1.3.2. ECM perisynaptic aggregates
1.1.3.2.1. Perineuronal nets
In addition to a loosely organized molecular lattice, the ECM forms organized, structured aggregates with distinct molecular composition. PNNs are arguably the most extensively investigated. They tightly surround synaptic contacts on distinct populations of neurons, including GABAergic interneuron populations and GABAergic projection neurons, such as those in the reticular nucleus of the thalamus, central nucleus of the amygdala and Purkinje cells in the cerebellum, as well as subpopulations of cortico-cortical pyramidal cells and spinal cord motor neurons [62,115–121]. PNNs represent key players in the regulation of synaptic connectivity and plasticity [111,122–129]. They mature late in postnatal development, in an activity-dependent manner [130–133]. Their maturation brings to a closure critical periods of development, inducing a profound shift from juvenile forms of plasticity to more restricted mature forms, consolidating successfully established synaptic connectivity and controlling formation of new synapses [76,124,129,134]. Enzymatic CSPG digestion dramatically disrupts PNN integrity, reverting local circuits and learning modalities, from visual perception to emotional learning, to a juvenile state [129,134]. The molecular composition of mature PNNs is thought to be species-, neuron- and brain region-specific, but to include CSPGs, hyaluronan and a variety of glycoproteins described above in relationship to synaptic plasticity regulation. Thus, functions such as modulation of glutamatergic transmission, LTP and LTD and synaptic motility and structural plasticity, demonstrated for these molecules, are inherent to PNNs. In addition, recent evidence shows that PNNs are in themselves dynamically regulated [123]. Fear learning and consolidation in response to pure tones was shown to induce marked PNN changes in the adult auditory cortex [123]. Expression CSPG mRNA and numbers of PNN were increased within hours following fear conditioning and returned to baseline 24 h later. CSPG enzymatic digestion in the auditory cortex impaired fear learning and consolidation, demonstrating that PNNs are necessary for fear learning [123]. Notably, in the amygdala, CSPG digestion also affected fear learning, reinstating juvenile forms of extinction-vulnerable conditioning [129].
1.1.3.2.2. CS-6/Glia clusters
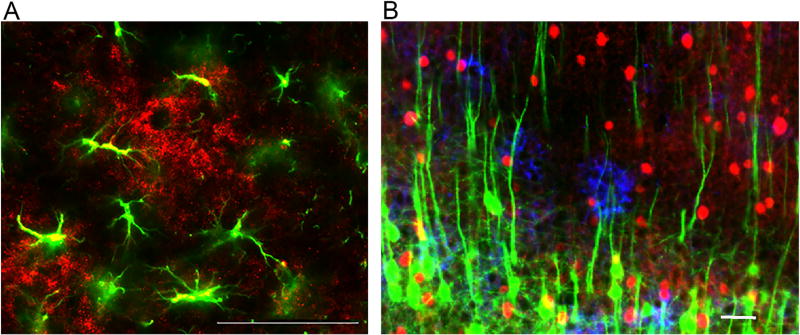
Contrary to what initially thought, PNNs are not unique as forms of organized ECM in the brain. Among other forms, CS-6/Glia clusters, or DAndelion-like Clock Structure, may be equally relevant to synaptic plasticity [135,136]. CS-6/Glia clusters are detectable in human and rodent brain using antibodies raised against the CS-6 sulfation patterns (Fig. 2). Their morphology may vary across brain regions, but in general they present as round rosettes of diffuse immunolabeling, with an overall diameter of 100–200 µm, often organized in short, dense segments. Several dendrites, and occasionally some neuronal and glial cell bodies are embedded in these clusters, while several glial cells surround them (Chelini et al; unpublished observations) [135,137]. In the mouse brain, CS-6/Glia clusters were found to develop in late postnatal development, suggesting a role in regulation of synaptic connectivity similar to that shown for PNNs [135,137–141]. Increases of CS-6 clusters in response to ketamine treatment suggest that these structures may be responsive to changes of glutamatergic transmission [142]. Although still preliminary, information on CS-6/Glia clusters is consistent with their involvement in synaptic functions, potentially representing segregated microenvironments regulated by predominant expression of CS-6 sulfation. The functional effects of these sulfation patterns are currently poorly understood, but evidence suggests a role for CS-6 sulfation patterns in facilitating plasticity. A switch from CS-6 to CS-4 sulfation patterns during postnatal development, and persistent cortical plasticity induced by CS-6 upregulation, suggest that the former may be more permissive, facilitating plasticity, while CS-4 may represent the mature, less permissive, CSPG form [66] (Fig. 3).
Fig. 2.
CS-6/Glia clusters in the healthy human amygdala, immunolabeled with CS-6 antibody CS56. Scale bar 100 µm.
Fig. 3.
(A) Rodent CS-6 cluster (red; immunolabeled with CS56) surrounded by astrocytes (green; immunoreactive for glial fibrillary acidic protein (GFAP). (B) Immunolabeled CS-6/Glia clusters (blue) in the mouse hippocampus. These clusters are crossed by several dendrites arising from projection neurons (green, immunolabeled for Thy1) and are often surrounded by interneurons expressing parvalbumin (red). Scale bar 100 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
1.2. Synaptic pathology in SZ
Growing evidence overwhelmingly points to synaptic abnormalities as core component of the pathology of SZ. Significant reductions of dendritic spines have been reported in several cortical areas, including prefrontal and auditory cortical areas and the hippocampus [143–147]. Anomalous dendritic spine morphology, expression of PSD proteins, including PSD95 and Homer-1, and associated glutamate signaling pathway proteins have also been reported [148,149]. Altered expression of molecules involved in the actin cytoskeleton strongly suggests that dendritic spines in SZ may reflect structural deficits [150–152]. A recent study shows dramatic changes affecting genes involved in synaptic functions in the amygdala [153], notably one of the main regions shown to have significant ECM abnormalities in this disorder. Decreases of dendritic spines in SZ have been proposed to represent the consequence of overpruning during adolescence [154,155].This hypothesis predicts a loss of large, mature spines in this disorder. Contrary to this expectation, a recent report shows that, at least in the primary auditory cortex, loss of dendritic spines in SZ may predominantly reflect decreases of smaller spines, and potentially be related to the SZ risk gene CACNB4 [154]. These findings are prompting novel hypotheses on the potential causes of synaptic pathology in SZ, and its links to genetic vulnerabilities. Importantly, GWAS data and de novo CNV analyses strongly support the involvement of genes involved in synaptic plasticity, and specifically, encoding for elements of the postsynaptic density, as risk factors for SZ [51,156]. Among these are alleles of the complement component 4 (C4) genes which, as mentioned above, were recently shown to play a key role in synapse elimination during postnatal development [51,52]. Additional genetic loci associated with genetic vulnerability to SZ also include genes encoding for ECM molecules and particularly ECM remodeling proteases [51]. Together, these studies support the possibility that risk genes affecting synaptic functions and ECM may interact with each other and with secondary or environmental factors to affect elements of the tetrapartite synapse, ultimately resulting in disruption of synaptic functions and plasticity.
2. Methods
Methods briefly described here refer to investigations on postmortem human tissue carried out by our group.
2.1. Human subjects
Tissue blocks containing the regions of interest (e.g. the amygdala) from cohorts of normal control and SZ donors (n = 12–25/group) were used for histochemical, immunocytochemical and qRT-PCR studies. All tissue blocks were obtained from the Harvard Brain Tissue Resource Center (HBTRC), McLean Hospital, Belmont, MA, USA. Diagnoses of SZ and BD were made by two psychiatrists on the basis of retrospective review of medical records, extensive questionnaires concerning social and medical history provided by family members and neuropathological report. Cohorts did not include subjects with evidence for gross and/or macroscopic brain changes, or clinical history, consistent with cerebrovascular accident or other neurological disorders. Subjects with Braak stages III or higher [157] (modified Bielschowsky stain) were not included.
2.2. Tissue processing and data collection
Tissue blocks were dissected from fresh brains, lightly fixed and cryoprotected (for immunohystochemistry (IHC) only), or quickly frozen in liquid nitrogen vapor (for Western blotting and qRT-PCR), then sectioned using a freezing microtome or a cryostat. Specificity of primary antibodies for IHC was tested by immunoblotting and pre-absorption with the corresponding antigen. WFA, and a variety of antibodies raised against CSPG protein cores (e.g. aggrecan), or CSPG CS-6 sulfation patterns (CS56, 3B3) were used to label PNNs and CS-6 clusters. Procedures for protein and mRNA detection and data collection were carried out as reported previously [e.g. 136,158].
2.3. Statistical analysis
Differences between groups relative to the main outcome measures in each of the regions examined were assessed for statistical significance using an ANCOVA stepwise linear regression process. Effect sizes were calculated according Hedges’ g. A logarithmic transformation was uniformly applied to all original values because the data were not normally distributed. Age, gender, postmortem time interval, inflammation (classified as positive or negative for inflammatory condition at time of death), hemisphere, cause of death, brain weight, exposure to alcohol, nicotine, electroconvulsive therapy, and lifetime, as well as final six months’, exposure to antipsychotic drugs, exposure to selective serotonin reuptake inhibitors classified as positive or negative for exposure, and lithium treatment were tested systematically for their effects on the main outcome measures, and included in the model if they significantly improved the model goodness of-fit (see also 136,158]
3. Results
3.1. ECM pathology in SZ
Human postmortem studies from our group consistently show marked decreases of PNNs in people with SZ [136,138,158–160]. Our first findings showed significant reduction of PNNs labeled with the lectin Wisteria floribunda agglutinin (WFA) in the amygdala and entorhinal cortex of subjects with SZ [158]. Similar decreases of WFA-labeled PNNs were also detected in the prefrontal cortex and hippocampus, but not in the visual cortex, consistent with specific involvement of brain regions impacted in SZ [138,159]. Notably, numbers of neurons predominantly associated with WFA-labeled PNNs, i.e. interneurons expressing parvalbumin [161–164], were not decreased in these regions [165–169]. This latter finding supports the idea that PNN decreases do not depend on a corresponding reduction of the neurons they envelope, while at the same time suggest that they may contribute to functional abnormalities affecting these neurons in SZ [169,170]. WFA labels a distal N-acetylgalactosamine on the CS chains of a group of CSPGs. Thus, decreases of WFA-labeled PNNs suggest CSPG involvement in SZ. To test the hypothesis that PNN decreases in SZ may not be restricted to WFA-positive PNNs, we focused on aggrecan, one of the main CSPGs in the brain and a major component of a population of PNNs, and on CS-6 sulfation patterns. Our results showed marked decreases of PNNs containing aggrecan and CS-6 sulfation in the amygdala of people with SZ [136]. Decreases of aggrecan-positive and WFA-positive PNNs were detected exclusively in the lateral nucleus of the amygdala; however, only one-third of WFA-positive PNNs also expressed aggrecan, raising the possibility that neuronal populations affected by WFA- and aggrecan-positive PNN loss only partially overlap. Decreases of CS-6 immunoreactive PNNs were much broader, impacting several amygdala nuclei, including the lateral, basal, accessory basal, cortical, and medial nuclei. Together, these findings show that PNN abnormalities in the amygdala of people with SZ include several distinct PNN phenotypes encompassing multiple neuronal populations. Such heterogeneity in PNN phenotypes is consistent with previous findings in several other brain regions [171–173].
Results from these postmortem studies also show complex interactions between PNN decreases and glial cells abnormalities. In the amygdala and entorhinal cortex, WFA-positive PNN decreases were accompanied by a robust increase of WFA-positive astrocytes affecting all amygdala nuclei and entorhinal cortex subregions tested, in contrast to the more restricted PNN changes. We speculate that impaired CSPG secretion in glial cells may be causally linked to WFA-positive PNN decreases. In contrast to results with WFA, aggrecan-positive PNN decreases were accompanied by marked reductions of glial cells expressing this CSPG [136], suggesting decreased aggrecan supply from glial cells. Together, these findings are consistent with the hypothesis that interactions between glial cells and ECM may be disrupted in SZ. This hypothesis is also supported by our findings that CS-6/Glia clusters, labeled with two different CS-6 antibodies (CS56 and 3B3), are markedly decreased in the amygdala of SZ [136]. As mentioned above, CS-6/Glia clusters putatively represent a novel form of structured ECM, postulated to affect dendritic spines. Their decreases may represent a clear example of disruption of the interactions between elements of the tetrapartite synapse, i.e. glial cells, ECM and pre- and post-synaptic elements. Additional evidence for the involvement of the ECM in SZ comes from findings in the olfactory epithelium, a peripheral sensory organ where neurogenesis and axon growth occur throughout adult life [174–176]. ECM components, and CSPGs in particular, are suspected to play a key role in these functions. Postmortem findings from our group show that cytoplasmic CSPG expression is altered in olfactory receptor neurons, potentially contributing to a disruption of olfactory functions observed in people with SZ [177–185].
4. Discussion
In summary, our findings show consistent ECM abnormalities in people with SZ, including reductions of PNNs, altered CSPG expression in glial cells and reduced numbers of CS-6/Glia clusters. These abnormalities are large in magnitude, robust to confounding factors and shared by several brain regions involved in SZ {[136] Pantazopoulos #1181; [138] Mauney #7280; [158] Pantazopoulos #17815}. Yet, they are restricted in their distribution to specific cortical layers and amygdala nuclei, suggesting specialized mechanisms affecting distinct neuronal populations.
The causative mechanisms underlying ECM abnormalities are not yet well understood. However, support for the contribution of genetic factors can be found in a growing number of studies. A common variation of NCAN, encoding neurocan, a brain-specific CSPG highly represented in the brain, has been reported and replicated in several studies, including a large GWAS with more than 36000 SZ cases [51,186,187]. This latter study also discovered SZ risk factors from several other ECM molecules, including MMP-16 [51]. Several additional studies reported the involvement of other MMPs, of which MMP-9 may be the most notable. For instance, polymorphisms of MMP-9 have been shown to be associated with SZ, although some negative findings have also been reported, and plasma levels of MMP-9 were found to be altered in this disorder [109,188–190]. Together, these findings led to suggestions that MMPs may represent a novel therapeutic target for SZ [190,191]. Notably, dose-dependent MMP-9 activity is crucially modulated by mir-132, a microRNA identified as pivotal for synaptic plasticity and found to be decreased in blood and brain tissue of people with SZ [192–195]. Together, these findings support the hypothesis of aberrant CSPGs processing during activity dependent plasticity, potentially linking CSPGs abnormalities to synaptic pathology in SZ.
4.1. The tetrapartite synapse: role in synaptic pathology in SZ
Converging evidence reviewed here and elsewhere compellingly support the idea that all elements of the tetrapartite synapse may be altered in SZ. Briefly, several molecular factors contributing to presynaptic functions, including synaptic vesicle trafficking, have been found to be disrupted in SZ [196–199]. Similarly, altered expression of PSD proteins, such as PSD95 and Homer-1, and glutamate signaling pathway, as well as proteins molecules involved in the actin cytoskeleton has been reported in people with this disorder [148–152]. Emerging evidence for glial involvement in SZ indicates that all glial populations shown to affect synaptic functions, i.e. astrocytes, NG2 cells and microglia, contribute to the pathophysiology of this disorder [200–209]. Finally, ECM abnormalities in SZ have been reviewed above, including evidence for altered CSPG expression in glial cells [see also 121,210].
It is plausible to postulate that abnormalities affecting distinct elements of the tetrapartite synapse may be causally related, and/or interact with each and result in synaptic dysfunction. Although the underlying mechanisms are not yet understood, current data raises a number of possible hypotheses. For instance, altered CSPG expression in glial cells and decreased PNNs in the same subjects suggests that decreased availability of PNN molecular components may contribute to PNN abnormalities. ECM molecules bind to neuronal surface receptors (e.g. integrins), which in turn link the postsynaptic density to the actin cytoskeleton on one side, and to the ECM and pre-synaptic terminal of the other side. Through this arrangement, cell adhesion molecules (CAMs)-mediate ECM and PSD signaling may impact the dendritic spine actin network, and thus the spine shape [211–217]. This possibility is supported by evidence that several ECM molecules, including CSPGs and Reelin, known to modulate spine formation, size and stability through ECM receptors, are also implicated in the pathology of SZ [76–79, 88–92, 218]. The potential contribution of decreased Reelin expression to dendritic spine decreases in SZ has long been postulated [93,219]. As reviewed above, ECM proteases such as MMPs, secreted into the ECM by astrocytes and microglia in addition to neurons, have been shown to robustly affect dendritic spine stability [106].
5. Conclusions
We reviewed evidence that each element of the tetrapartite synapse plays a role in synaptic plasticity and is involved in SZ. Several glial populations, including astrocytes, NG2 cells and microglia have been shown to regulate synaptic functions and plasticity. Each of these glial cell populations has been shown to have complex, intimate relationships with the ECM. Several ECM molecules have been shown to have powerful effects on synaptic plasticity, and to be represented in ECM organized perisynaptic structures such as PNNs. Genetic and human postmortem evidence supports the involvement of the ECM in SZ, including loss of PNNs and CS-6/Glia clusters. Synaptic pathology, including loss of dendritic spines and molecular factors involved in spine structural stability and enriched in the PSD, is well established in SZ. We put forth the hypothesis that this pathology results from a disruption of interactions between elements of the tetrapartite synapse. Given the clinical, genetic and pathological heterogeneity of SZ, it is possible that synaptic pathology in specific brain regions may represent a point of convergence, potentially caused by a number of distinct molecular mechanisms in different individuals.
Acknowledgments
The authors thank the Harvard Brain Tissue Resource Center, funded through NIH—NeuroBiobank HHSN-271-2013-00030C (The National Institute of Mental Health (NIMH), National Institute of Neurological Diseases and Stroke (NINDS) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and brain donors and their families for the tissue samples used in these studies.
Funding sources
This work was supported by the National Institutes of Health: R01 MH086522,R01 MH104488, R01 MH105608.
Abbreviations
- ADAMS
a disintegrin and matrix metalloproteases
- ADAMTS
ADAMS with a thrombospondin domain
- AMPAR
α amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- ATP
adenosine trisphosphate
- C4
complement component 4
- CS
chondroitin sulfate
- CSPG
Chondroitin sulfate proteoglycan
- EAAT1
excitatory amino acid transporter 1
- EAAT2
excitatory amino acid transporter 2
- ECM
extracellular matrix
- GABA
γ-Aminobutyric acid
- GAG
glycosaminoglycan chains
- GWAS
genome wide association study
- IHC
immunocytochemistry
- LTD
long term depression
- LTP
long term potentiation
- MMP
matrix metalloproinase
- NG2
neural/glial antigen 2
- NMDA
N-methyl-d-aspartate
- O4
O4 sulfatide
- PDGFαR
platelet-derived growth factor α receptors
- PNNs
perineuronal nets
- PSD
postsynaptic density
- SZ
schizophrenia
- WFA
Wisteria floribunda agglutinin
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 2.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19(16):6897–906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006;4(11):e343. doi: 10.1371/journal.pbio.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 6.Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21(2):353–9. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dityatev A, Frischknecht R, Seidenbecher CI. Extracellular matrix and synaptic functions. Results Probl Cell Differ. 2006;43:69–97. doi: 10.1007/400_025. [DOI] [PubMed] [Google Scholar]
- 8.Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull. 2018;136:101–8. doi: 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Nicoll RA, Kauer JA, Malenka RC. The current excitement on long-term potentiation. Neuron. 1988;1:97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- 10.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Banker G, Churchill L, Cotman CW. Proteins of the postsynaptic density. J Cell Biol. 1974;63(2 Pt 1):456–65. doi: 10.1083/jcb.63.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granger AJ, Nicoll RA. Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on. Philos Trans R Soc Lond B Biol Sci. 2014;369(1633):20130136. doi: 10.1098/rstb.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy MB. The postsynaptic density. Curr Opin Neurobiol. 1993;3(5):732–7. doi: 10.1016/0959-4388(93)90145-o. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7(10):1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 15.Wiegert JS, Oertner TG. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc Natl Acad Sci U S A. 2013;110(47):E4510–9. doi: 10.1073/pnas.1315926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44(5):759–67. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44(5):749–57. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Witcher MR, Kirov SA, Harris KM. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 2007;55(1):13–23. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- 19.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 20.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–55. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6(6):983–92. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- 22.Parpura V, Zorec R. Gliotransmission: exocytotic release from astrocytes. Brain Res Rev. 2009 doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81(4):728–39. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada K, Kamiya T, Tsuboi T. Gliotransmitter release from astrocytes: functional, developmental, and pathological implications in the brain. Front Neurosci. 2015;9:499. doi: 10.3389/fnins.2015.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera A, Vanzulli I, Butt AM. A central role for ATP signalling in glial interactions in the CNS. Curr Drug Targets. 2016;17(16):1829–33. doi: 10.2174/1389450117666160711154529. [DOI] [PubMed] [Google Scholar]
- 26.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317(5841):1083–6. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci. 2014;34(38):12738–44. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996;43(3):315–30. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47(5):455–70. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7(9):2737–44. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61(5):471–9. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Levine JM, Card JP. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci. 1987;7(9):2711–20. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207(6):695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson VA, Zhang Y, Bergles DE. Electrophysiological properties of NG2(+) cells: matching physiological studies with gene expression profiles. Brain Res. 2016;1638(Pt B):138–60. doi: 10.1016/j.brainres.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, et al. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312(5779):1533–7. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- 36.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405(6783):187–91. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 37.Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004;47(3):290–8. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- 38.Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31(6–7):551–65. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- 39.Sakry D, Neitz A, Singh J, Frischknecht R, Marongiu D, Biname F, et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol. 2014;12(11):e1001993. doi: 10.1371/journal.pbio.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eugenin-von Bernhardi J, Dimou L. More than progenitor cells. Adv Exp Med Biol. 2016;949:27–45. doi: 10.1007/978-3-319-40764-7_2. [DOI] [PubMed] [Google Scholar]
- 41.Sakry D, Karram K, Trotter J. Synapses between NG2 glia and neurons. J Anat. 2011;219(1):2–7. doi: 10.1111/j.1469-7580.2011.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63(8):1429–51. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill RA, Nishiyama A. NG2 cells (polydendrocytes): listeners to the neural network with diverse properties. Glia. 2014;62(8):1195–210. doi: 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–68. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36(10):605–13. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30(10):527–35. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Bialas AR, Presumey J, Das A, van der Poel CE, Lapchak PH, Mesin L, et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature. 2017;546(7659):539–43. doi: 10.1038/nature22821. [DOI] [PubMed] [Google Scholar]
- 48.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 49.Schafer DP, Lehrman EK, Stevens B. The quad-partite synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai X, Fan L, Shao H, Lu X, Zhang W, Li J, et al. Reelin induces branching of neurons and radial glial cells during corticogenesis. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu216. [DOI] [PubMed] [Google Scholar]
- 51.Ripke S. Schizophrenia working group of the psychiatric genomics C: biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530(7589):177–83. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88(4):1277–340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci. 2010;30(46):15337–57. doi: 10.1523/JNEUROSCI.3467-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, et al. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100(1–2):103–17. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 56.Abdolmaleky HM, Thiagalingam S, Wilcox M. Genetics and epigenetics in major psychiatric disorders: dilemmas, achievements, applications, and future scope. Am J Pharmacogenom. 2005;5(3):149–60. doi: 10.2165/00129785-200505030-00002. [DOI] [PubMed] [Google Scholar]
- 57.Medina-Flores R, Wang G, Bissel SJ, Murphey-Corb M, Wiley CA. Destruction of extracellular matrix proteoglycans is pervasive in simian retroviral neuroinfection. Neurobiol Dis. 2004;16(3):604–16. doi: 10.1016/j.nbd.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 58.Hobohm C, Gunther A, Grosche J, Rossner S, Schneider D, Bruckner G. Decomposition and long-lasting downregulation of extracellular matrix in perineuronal nets induced by focal cerebral ischemia in rats. J Neurosci Res. 2005;80(4):539–48. doi: 10.1002/jnr.20459. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57(2):276–89. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giamanco KA, Matthews RT. Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience. 2012;218:367–84. doi: 10.1016/j.neuroscience.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281(26):17789–800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 62.Gati G, Lendvai D. [The dress makes the neuron – different forms of the extracellular matrix in the central nervous system of vertebrates] Orv Hetil. 2013;154(27):1067–73. doi: 10.1556/OH.2013.29646. quiz 78–9. [DOI] [PubMed] [Google Scholar]
- 63.Jager C, Lendvai D, Seeger G, Bruckner G, Matthews RT, Arendt T, et al. Perineuronal and perisynaptic extracellular matrix in the human spinal cord. Neuroscience. 2013;238:168–84. doi: 10.1016/j.neuroscience.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 64.Lendvai D, Morawski M, Negyessy L, Gati G, Jager C, Baksa G, et al. Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer’s disease. Acta Neuropathol. 2013;125(2):215–29. doi: 10.1007/s00401-012-1042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karus M, Samtleben S, Busse C, Tsai T, Dietzel ID, Faissner A, et al. Normal Sulphation levels regulate spinal cord neural precursor cell proliferation and differentiation. Neural Dev. 2012;7(1):20. doi: 10.1186/1749-8104-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci. 2012;15(3):414–22. S1–2. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- 67.Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121(Pt 18):3083–91. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maeda N. Structural variation of chondroitin sulfate and its roles in the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10(1):22–31. doi: 10.2174/187152410790780136. [DOI] [PubMed] [Google Scholar]
- 69.Bukalo O, Schachner M, Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience. 2001;104(2):359–69. doi: 10.1016/s0306-4522(01)00082-3. [DOI] [PubMed] [Google Scholar]
- 70.Niisato K, Fujikawa A, Komai S, Shintani T, Watanabe E, Sakaguchi G, et al. Age-dependent enhancement of hippocampal long-term potentiation and impairment of spatial learning through the Rho-associated kinase pathway in protein tyrosine phosphatase receptor type Z-deficient mice. J Neurosci. 2005;25(5):1081–8. doi: 10.1523/JNEUROSCI.2565.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawachi H, Tamura H, Watakabe I, Shintani T, Maeda N, Noda M. Protein tyrosine phosphatase zeta/RPTPbeta interacts with PSD-95/SAP90 family. Brain Res Mol Brain Res. 1999;72(1):47–54. doi: 10.1016/s0169-328x(99)00204-1. [DOI] [PubMed] [Google Scholar]
- 72.Snyder SE, Li J, Schauwecker PE, McNeill TH, Salton SR. Comparison of RPTP zeta/beta, phosphacan, and trkB mRNA expression in the developing and adult rat nervous system and induction of RPTP zeta/beta and phosphacan mRNA following brain injury. Brain Res Mol Brain Res. 1996;40(1):79–96. doi: 10.1016/0169-328x(96)00039-3. [DOI] [PubMed] [Google Scholar]
- 73.Nishiwaki T, Maeda N, Noda M. Characterization and developmental regulation of proteoglycan-type protein tyrosine phosphatase zeta/ RPTPbeta isoforms. J Biochem. 1998;123(3):458–67. doi: 10.1093/oxfordjournals.jbchem.a021959. [DOI] [PubMed] [Google Scholar]
- 74.Shintani T, Watanabe E, Maeda N, Noda M. Neurons as well as astrocytes express proteoglycan-type protein tyrosine phosphatase zeta/RPTPbeta: analysis of mice in which the PTPzeta/RPTPbeta gene was replaced with the LacZ gene. Neurosci Lett. 1998;247(2–3):135–8. doi: 10.1016/s0304-3940(98)00295-x. [DOI] [PubMed] [Google Scholar]
- 75.Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H, et al. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22(21):7417–27. doi: 10.1128/MCB.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103(22):8517–22. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Majewska A, Sur M. Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proc Natl Acad Sci U S A. 2003;100(26):16024–9. doi: 10.1073/pnas.2636949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Vivo L, Landi S, Panniello M, Baroncelli L, Chierzi S, Mariotti L, et al. Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat Commun. 2013;4:1484. doi: 10.1038/ncomms2491. [DOI] [PubMed] [Google Scholar]
- 79.Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O. Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci. 2012;32(50):18009–17. doi: 10.1523/JNEUROSCI.2406-12.2012. 17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kochlamazashvili G, Henneberger C, Bukalo O, Dvoretskova E, Senkov O, Lievens PM, et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron. 2010;67(1):116–28. doi: 10.1016/j.neuron.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24(2):471–9. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 82.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7(11):850–9. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 83.Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, et al. Reelin binds alpha 3 beta 1 integrin and inhibits neuronal migration. Neuron. 2000;27(1):33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25(36):8209–16. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26(50):12943–55. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One. 2009;4(5):e5505. doi: 10.1371/journal.pone.0005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hellwig S, Hack I, Kowalski J, Brunne B, Jarowyj J, Unger A, et al. Role for Reelin in neurotransmitter release. J Neurosci. 2011;31(7):2352–60. doi: 10.1523/JNEUROSCI.3984-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, et al. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci. 2010;30(13):4636–49. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pribiag H, Peng H, Shah WA, Stellwagen D, Carbonetto S. Dystroglycan mediates homeostatic synaptic plasticity at GABAergic synapses. Proc Natl Acad Sci U S A. 2014;111(18):6810–5. doi: 10.1073/pnas.1321774111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci. 2010;30(43):14560–72. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, et al. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J Neurosci. 2006;26(7):2041–52. doi: 10.1523/JNEUROSCI.4566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trotter JH, Klein M, Jinwal UK, Abisambra JF, Dickey CA, Tharkur J, et al. ApoER2 function in the establishment and maintenance of retinal synaptic connectivity. J Neurosci. 2011;31(40):14413–23. doi: 10.1523/JNEUROSCI.3135-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eastwood SL, Harrison PJ. Cellular basis of reduced cortical reelin expression in schizophrenia. Am J Psychiatry. 2006;163(3):540–2. doi: 10.1176/appi.ajp.163.3.540. [DOI] [PubMed] [Google Scholar]
- 94.Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11(11):735–46. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- 95.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66(3):337–51. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charrier C, Machado P, Tweedie-Cullen RY, Rutishauser D, Mansuy IM, Triller A. A crosstalk between beta 1 and beta 3 integrins controls glycine receptor and gephyrin trafficking at synapses. Nat Neurosci. 2010;13(11):1388–95. doi: 10.1038/nn.2645. [DOI] [PubMed] [Google Scholar]
- 97.McGeachie AB, Cingolani LA, Goda Y. Stabilising influence: integrins in regulation of synaptic plasticity. Neurosci Res. 2011;70(1):24–9. doi: 10.1016/j.neures.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Babayan AH, Kramar EA, Barrett RM, Jafari M, Haettig J, Chen LY, et al. Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation. J Neurosci. 2012;32(37):12854–61. doi: 10.1523/JNEUROSCI.2024-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chan CS, Chen H, Bradley A, Dragatsis I, Rosenmund C, Davis RL. alpha8-integrins are required for hippocampal long-term potentiation but not for hippocampal-dependent learning. Genes Brain Behav. 2010;9(4):402–10. doi: 10.1111/j.1601-183X.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13(11):743–57. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iryna M, Ethell DWE. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;9999(9999) doi: 10.1002/jnr.21273. NA. [DOI] [PubMed] [Google Scholar]
- 102.Smith AC, Scofield MD, Kalivas PW. The tetrapartite synapse: extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res. 2015 doi: 10.1016/j.brainres.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verslegers M, Lemmens K, Van Hove I, Moons L. Matrix metalloproteinase-2 and — 9 as promising benefactors in development, plasticity and repair of the nervous system. Prog Neurobiol. 2013;105:60–78. doi: 10.1016/j.pneurobio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 104.Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K, et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J Cell Sci. 2011;124(Pt 19):3369–80. doi: 10.1242/jcs.090852. [DOI] [PubMed] [Google Scholar]
- 105.Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem. 2006;97(1):44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Levy AD, Omar MH, Koleske AJ. Extracellular matrix control of dendritic spine and synapse structure and plasticity in adulthood. Front Neuroanat. 2014;8:116. doi: 10.3389/fnana.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26(7):1923–34. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A. 2008;105(49):19520–5. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lepeta K, Matrix Kaczmarek L. Metalloproteinase-9 as a novel player in synaptic plasticity and schizophrenia. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rossier J, Bernard A, Cabungcal JH, Perrenoud Q, Savoye A, Gallopin T, et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts 8, Adamts 15 and Neprilysin. Mol Psychiatry. 2015;20(2):154–61. doi: 10.1038/mp.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fawcett JW. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog Brain Res. 2015;218:213–26. doi: 10.1016/bs.pbr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 112.Uesaka N, Uchigashima M, Mikuni T, Nakazawa T, Nakao H, Hirai H, et al. Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science. 2014;344(6187):1020–3. doi: 10.1126/science.1252514. [DOI] [PubMed] [Google Scholar]
- 113.Carulli D, Foscarin S, Faralli A, Pajaj E, Rossi F. Modulation of semaphorin 3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol Cell Neurosci. 2013;57:10–22. doi: 10.1016/j.mcn.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 114.Dick G, Tan CL, Alves JN, Ehlert EM, Miller GM, Hsieh-Wilson LC, et al. Semaphorin 3A binds to the perineuronal nets via chondroitin sulfate type E motifs in rodent brains. J Biol Chem. 2013;288(38):27384–95. doi: 10.1074/jbc.M111.310029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wegner F, Hartig W, Bringmann A, Grosche J, Wohlfarth K, Zuschratter W, et al. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABA(A) receptor alpha1 subunit form a unique entity in rat cerebral cortex. Exp Neurol. 2003;184(2):705–14. doi: 10.1016/S0014-4886(03)00313-3. [DOI] [PubMed] [Google Scholar]
- 116.Ajmo JM, Eakin AK, Hamel MG, Gottschall PE. Discordant localization of WFA reactivity and brevican/ADAMTS-derived fragment in rodent brain. BMC Neurosci. 2008;9:14. doi: 10.1186/1471-2202-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, et al. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8(3):183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 118.Hartig W, Bruckner G, Brauer K, Schmidt C, Bigl V. Allocation of perineuronal nets and parvalbumin-, calbindin- D28k- and glutamic acid decarboxylase-immunoreactivity in the amygdala of the rhesus monkey. Brain Res. 1995;698(1–2):265–9. doi: 10.1016/0006-8993(95)01016-o. [DOI] [PubMed] [Google Scholar]
- 119.Pantazopoulos H, Lange N, Hassinger L, Berretta S. Subpopulations of neurons expressing parvalbumin in the human amygdala. J Comp Neurol. 2006;496(5):706–22. doi: 10.1002/cne.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seeger G, Brauer K, Hartig W, Bruckner G. Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience. 1994;58(2):371–88. doi: 10.1016/0306-4522(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 121.Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015 doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JC, et al. Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci. 2016;36(45):11459–68. doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Banerjee SB, Gutzeit VA, Baman J, Aoued HS, Doshi NK, Liu RC, et al. Perineuronal nets in the adult sensory cortex are necessary for fear learning. Neuron. 2017;95(1):169–79. doi: 10.1016/j.neuron.2017.06.007. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bernard C, Prochiantz A. Otx2-PNN interaction to regulate cortical plasticity. Neural Plast. 2016;2016:7931693. doi: 10.1155/2016/7931693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frischknecht R, Chang KJ, Rasband MN, Seidenbecher CI. Neural ECM molecules in axonal and synaptic homeostatic plasticity. Prog Brain Res. 2014;214:81–100. doi: 10.1016/B978-0-444-63486-3.00004-9. [DOI] [PubMed] [Google Scholar]
- 126.Romberg C, Yang S, Melani R, Andrews MR, Horner AE, Spillantini MG, et al. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci. 2013;33(16):7057–65. doi: 10.1523/JNEUROSCI.6267-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349(1):147–60. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- 128.Maeda N, Fukazawa N, Ishii M. Chondroitin sulfate proteoglycans in neural development and plasticity. Front Biosci. 2010;15:626–44. doi: 10.2741/3637. [DOI] [PubMed] [Google Scholar]
- 129.Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325(5945):1258–61. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 130.Koppe G, Bruckner G, Brauer K, Hartig W, Bigl V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res. 1997;288(1):33–41. doi: 10.1007/s004410050790. [DOI] [PubMed] [Google Scholar]
- 131.Ye Q, Miao QL. Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 2013;32(6):352–63. doi: 10.1016/j.matbio.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 132.Mix A, Hoppenrath K, Funke K. Reduction in cortical parvalbumin expression due to intermittent theta-burst stimulation correlates with maturation of the perineuronal nets in young rats. Dev Neurobiol. 2015;75(1):1–11. doi: 10.1002/dneu.22205. [DOI] [PubMed] [Google Scholar]
- 133.Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sanchez-Aguilera A, Mantoan L, et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein Brevican. Neuron. 2017;95(3):639–55. doi: 10.1016/j.neuron.2017.06.028. e10. [DOI] [PubMed] [Google Scholar]
- 134.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–51. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 135.Hayashi N, Tatsumi K, Okuda H, Yoshikawa M, Ishizaka S, Miyata S, et al. DACS, novel matrix structure composed of chondroitin sulfate proteoglycan in the brain. Biochem Biophys Res Commun. 2007;364(2):410–5. doi: 10.1016/j.bbrc.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 136.Pantazopoulos H, Markota M, Jaquet F, Ghosh D, Wallin A, Santos A, et al. Aggrecan, chondroitin-6-sulfate abnormalities in schizophrenia, bipolar disorder: a postmortem study on the amygdala. Transl. Psychiatry. 2015;5:e496. doi: 10.1038/tp.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Horii-Hayashi N, Tatsumi K, Matsusue Y, Okuda H, Okuda A, Hayashi M, et al. Chondroitin sulfate demarcates astrocytic territories in the mammalian cerebral cortex. Neurosci Lett. 2010;483(1):67–72. doi: 10.1016/j.neulet.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 138.Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, et al. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry. 2013;74(6):427–35. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yamada J, Jinno S. Spatio-temporal differences in perineuronal net expression in the mouse hippocampus, with reference to parvalbumin. Neuroscience. 2013;253:368–79. doi: 10.1016/j.neuroscience.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 140.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30(45):14964–71. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 142.Matuszko G, Curreli S, Kaushik R, Becker A, Dityatev A. Extracellular matrix alterations in the ketamine model of schizophrenia. Neuroscience. 2017;350:13–22. doi: 10.1016/j.neuroscience.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 143.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 144.Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71(12):1323–31. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34(2):374–89. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am J Psychiatry. 2004;161(10):1848–55. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- 147.Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57(1):47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- 148.de Bartolomeis A, Latte G, Tomasetti C, Iasevoli F. Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: relevance to schizophrenia and other behavioral disorders pathophysiology, and implications for novel therapeutic approaches. Mol Neurobiol. 2014;49(1):484–511. doi: 10.1007/s12035-013-8534-3. [DOI] [PubMed] [Google Scholar]
- 149.MacDonald ML, Ding Y, Newman J, Hemby S, Penzes P, Lewis DA, et al. Altered glutamate protein co-expression network topology linked to spine loss in the auditory cortex of schizophrenia. Biol Psychiatry. 2015;77(11):959–68. doi: 10.1016/j.biopsych.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Datta D, Arion D, Corradi JP, Lewis DA. Altered expression of CDC42 signaling pathway components incortical layer 3 pyramidal cells in schizophrenia. Biol Psychiatry. 2015;78(11):775–85. doi: 10.1016/j.biopsych.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shelton MA, Newman JT, Gu H, Sampson AR, Fish KN, MacDonald ML, et al. Loss of microtubule-associated protein 2 immunoreactivity linked to dendritic spine loss in schizophrenia. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Konopaske GT, Subburaju S, Coyle JT, Benes FM. Altered prefrontal cortical MARCKS and PPP1R9A mRNA expression in schizophrenia and bipolar disorder. Schizophr Res. 2015;164(1–3):100–8. doi: 10.1016/j.schres.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Beach PA, Huck JT, Zhu DC, Bozoki AC. Altered behavioral and autonomic pain responses in Alzheimer’s disease are associated with dysfunctional affective, self-reflective and salience network resting-state connectivity. Front Aging Neurosci. 2017;9:297. doi: 10.3389/fnagi.2017.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.MacDonald ML, Alhassan J, Newman JT, Richard M, Gu H, Kelly RM, et al. Selective loss of smaller spines in schizophrenia. Am J Psychiatry. 2017;174(6):586–94. doi: 10.1176/appi.ajp.2017.16070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 156.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–84. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 Suppl):S85–8. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 158.Pantazopoulos H, Woo T-UW, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67(2):155–66. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pantazopolous H, Sawyer C, Heckers S, Berretta S, Markota M. Chondroitin sulfate proteoglycan abnormalities in the hippocampus of subjects with schizophrenia. Neuropsychopharmacology. 2014;39:S298–9. [Google Scholar]
- 160.Pantazopoulos H, Boyer-Boiteau A, Holbrook EH, Jang W, Hahn CG, Arnold SE, et al. Proteoglycan abnormalities in olfactory epithelium tissue from subjects diagnosed with schizophrenia. Schizophr Res. 2013;150(2–3):366–72. doi: 10.1016/j.schres.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pantazopoulos H, Lange N, Hassinger L, Berretta S. Subpopulations of neurons expressing parvalbumin in the human amygdala. J Comp Neurol. 2006;496(5):706–22. doi: 10.1002/cne.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hartig W, Brauer K, Bigl V, Bruckner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res. 1994;635(1–2):307–11. doi: 10.1016/0006-8993(94)91452-4. [DOI] [PubMed] [Google Scholar]
- 163.Brauer K, Hartig W, Bigl V, Bruckner G. Distribution of parvalbumin-containing neurons and lectin-binding perineuronal nets in the rat basal forebrain. Brain Res. 1993;631(1):167–70. doi: 10.1016/0006-8993(93)91205-7. [DOI] [PubMed] [Google Scholar]
- 164.Hartig W, Brauer K, Bruckner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3(10):869–72. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 165.Pantazopoulos H, Lange N, Baldessarini RJ, Berretta S. Parvalbumin neurons in the entorhinal cortex of subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;61(5):640–52. doi: 10.1016/j.biopsych.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, et al. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry. 2011;68(4):340–50. doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang AY, Lohmann KM, Yang CK, Zimmerman EI, Pantazopoulos H, Herring N, et al. Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol. 2011;122(5):615–26. doi: 10.1007/s00401-011-0881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chung DW, Fish KN, Lewis DA. Pathological basis for deficient excitatory drive to cortical parvalbumin interneurons in schizophrenia. Am J Psychiatry. 2016;173(11):1131–9. doi: 10.1176/appi.ajp.2016.16010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77(12):1031–40. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38(5):950–7. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22(17):7536–47. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Racz E, Gaal B, Matesz C. Heterogeneous expression of extracellular matrix molecules in the red nucleus of the rat. Neuroscience. 2016;322:1–17. doi: 10.1016/j.neuroscience.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 173.Yamada J, Jinno S. Molecular heterogeneity of aggrecan-based perineuronal nets around five subclasses of parvalbumin-expressing neurons in the mouse hippocampus. J Comp Neurol. 2017;525(5):1234–49. doi: 10.1002/cne.24132. [DOI] [PubMed] [Google Scholar]
- 174.Cascella NG, Takaki M, Lin S, Sawa A. Neurodevelopmental involvement in schizophrenia: the olfactory epithelium as an alternative model for research. J Neurochem. 2007;102(3):587–94. doi: 10.1111/j.1471-4159.2007.04628.x. [DOI] [PubMed] [Google Scholar]
- 175.Graziadei PP, Monti Graziadei GA. Neurogenesis, neuron regeneration in the olfactory system of mammals III. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria in rat. J Neurocytol. 1980;9(2):145–62. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- 176.Monti Graziadei GA, Karlan MS, Bernstein JJ, Graziadei PP. Reinnervation of the olfactory bulb after section of the olfactory nerve in monkey (Saimiri sciureus) Brain Res. 1980;189(2):343–54. doi: 10.1016/0006-8993(80)90095-5. [DOI] [PubMed] [Google Scholar]
- 177.Turetsky BI, Moberg PJ. An odor-specific threshold deficit implicates abnormal intracellular cyclic AMP signaling in schizophrenia. Am J Psychiatry. 2009;166(2):226–33. doi: 10.1176/appi.ajp.2008.07071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brewer WJ, Pantelis C, Anderson V, Velakoulis D, Singh B, Copolov DL, et al. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158(1):107–15. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- 179.Coleman E, Goetz RR, Leitman D, Yale S, Stanford A, Gorman JM, et al. Odor identification impairments in schizophrenia: relationship with demographic measures, clinical variables, and diagnostic subtypes. CNS Spectr. 2002;7(1):43–8. doi: 10.1017/s1092852900022252. [DOI] [PubMed] [Google Scholar]
- 180.Good KP, Whitehorn D, Rui Q, Milliken H, Kopala LC. Olfactory identification deficits in first-episode psychosis may predict patients at risk for persistent negative and disorganized or cognitive symptoms. Am J Psychiatry. 2006;163(5):932–3. doi: 10.1176/ajp.2006.163.5.932. [DOI] [PubMed] [Google Scholar]
- 181.Kohler CG, Moberg PJ, Gur RE, O’Connor MJ, Sperling MR, Doty RL. Olfactory dysfunction in schizophrenia and temporal lobe epilepsy. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(2):83–8. [PubMed] [Google Scholar]
- 182.Kopala LC, Clark C, Hurwitz T. Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res. 1993;8(3):245–50. doi: 10.1016/0920-9964(93)90022-b. [DOI] [PubMed] [Google Scholar]
- 183.Moberg PJ, Arnold SE, Doty RL, Gur RE, Balderston CC, Roalf DR, et al. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28(8):1444–61. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- 184.Seidman LJ, Talbot NL, Kalinowski AG, McCarley RW, Faraone SV, Kremen WS, et al. Neuropsychological probes of fronto-limbic system dysfunction in schizophrenia: olfactory identification and Wisconsin Card Sorting performance. Schizophr Res. 1991;6(1):55–65. doi: 10.1016/0920-9964(91)90021-i. [DOI] [PubMed] [Google Scholar]
- 185.Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci Biobehav Rev. 2008;32(7):1315–25. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 186.Muhleisen TW, Mattheisen M, Strohmaier J, Degenhardt F, Priebe L, Schultz CC, et al. Association between schizophrenia and common variation in neurocan (NCAN), a genetic risk factor for bipolar disorder. Schizophr Res. 2012;138(1):69–73. doi: 10.1016/j.schres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 187.Cichon S, Muhleisen TW, Degenhardt FA, Mattheisen M, Miro X, Strohmaier J, et al. Genome-wide association study identifies genetic variation in neurocan as a susceptibility factor for bipolar disorder. Am J Hum Genet. 2011;88(3):372–81. doi: 10.1016/j.ajhg.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yamamori H, Hashimoto R, Ishima T, Kishi F, Yasuda Y, Ohi K, et al. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci Lett. 2013;556:37–41. doi: 10.1016/j.neulet.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 189.Bienkowski P, Samochowiec J, Pelka-Wysiecka J, Grzywacz A, Skibinska M, Jasiewicz A, et al. Functional polymorphism of matrix metalloproteinase-9 (MMP9) gene is not associated with schizophrenia and with its deficit subtype. Pharmacol Rep. 2015;67(3):442–5. doi: 10.1016/j.pharep.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 190.Vafadari B, Salamian A, Kaczmarek L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem. 2016;139(Suppl. 2):91–114. doi: 10.1111/jnc.13415. [DOI] [PubMed] [Google Scholar]
- 191.Chopra K, Baveja A, Kuhad A. MMPs: a novel drug target for schizophrenia. Expert Opin Ther Targets. 2015;19(1):77–85. doi: 10.1517/14728222.2014.957672. [DOI] [PubMed] [Google Scholar]
- 192.Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci U S A. 2012;109(8):3125–30. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jasinska M, Milek J, Cymerman IA, Leski S, Kaczmarek L, Dziembowska M. miR-132 regulates dendritic spine structure by direct targeting of matrix metalloproteinase 9 mRNA. Mol Neurobiol. 2016;53(7):4701–12. doi: 10.1007/s12035-015-9383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mazziotti R, Baroncelli L, Ceglia N, Chelini G, Sala GD, Magnan C, et al. Mir-132/212 is required for maturation of binocular matching of orientation preference and depth perception. Nat Commun. 2017;8:15488. doi: 10.1038/ncomms15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tognini P, Pizzorusso T. MicroRNA212/132 family: molecular transducer of neuronal function and plasticity. Int J Biochem Cell Biol. 2012;44(1):6–10. doi: 10.1016/j.biocel.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 196.Egbujo CN, Sinclair D, Hahn CG. Dysregulations of synaptic vesicle trafficking in schizophrenia. Curr Psychiatry Rep. 2016;18(8):77. doi: 10.1007/s11920-016-0710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, et al. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8(9):797–810. doi: 10.1038/sj.mp.4001319. [DOI] [PubMed] [Google Scholar]
- 198.Siegert S, Seo J, Kwon EJ, Rudenko A, Cho S, Wang W, et al. The schizophrenia risk gene product miR-137 alters presynaptic plasticity. Nat Neurosci. 2015;18(7):1008–16. doi: 10.1038/nn.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Landen M, Davidsson P, Gottfries CG, Grenfeldt B, Stridsberg M, Blennow K. Reduction of the small synaptic vesicle protein synaptophysin but not the large dense core chromogranins in the left thalamus of subjects with schizophrenia. Biol Psychiatry. 1999;46(12):1698–702. doi: 10.1016/s0006-3223(99)00160-2. [DOI] [PubMed] [Google Scholar]
- 200.Catts VS, Wong J, Fillman SG, Fung SJ, Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: association with neuroinflammation. Aust N Z J Psychiatry. 2014;48(8):722–34. doi: 10.1177/0004867414531078. [DOI] [PubMed] [Google Scholar]
- 201.Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology. 2011;36(6):1171–7. doi: 10.1038/npp.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mauney SA, Pietersen CY, Sonntag KC, Woo TW. Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophr Res. 2015;169(1–3):374–80. doi: 10.1016/j.schres.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115–21. doi: 10.1016/j.pnpbp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 204.Trepanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Weickert CS, Weickert TW. What’s hot in schizophrenia research? Psychiatr Clin North Am. 2016;39(2):343–51. doi: 10.1016/j.psc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 206.Williams AJ, Umemori H. The best-laid plans go oft awry: synaptogenic growth factor signaling in neuropsychiatric disease. Front Synaptic Neurosci. 2014;6:4. doi: 10.3389/fnsyn.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McCullumsmith RE, O’Donovan SM, Drummond JB, Benesh FS, Simmons M, Roberts R, et al. Shaping plasticity: alterations in glutamate transporter localization as a pathophysiological mechanism in severe mental illness. Mol Psychiatry. 2016;21(6):723. doi: 10.1038/mp.2016.79. [DOI] [PubMed] [Google Scholar]
- 208.Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 209.Duncan LE, Holmans PA, Lee PH, O’Dushlaine CT, Kirby AW, Smoller JW, et al. Pathway analyses implicate glial cells in schizophrenia. PLoS One. 2014;9(2): e89441. doi: 10.1371/journal.pone.0089441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pantazopoulos H, Berretta S. In sickness and in health: perineuronal nets and synaptic plasticity in psychiatric disorders. Neural Plast. 2016;2016:9847696. doi: 10.1155/2016/9847696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Huntley GW, Gil O, Bozdagi O. The cadherin family of cell adhesion molecules: multiple roles in synaptic plasticity. Neuroscientist. 2002;8(3):221–33. doi: 10.1177/1073858402008003008. [DOI] [PubMed] [Google Scholar]
- 212.Washbourne P, Dityatev A, Scheiffele P, Biederer T, Weiner JA, Christopherson KS, et al. Cell adhesion molecules in synapse formation. J Neurosci. 2004;24(42):9244–9. doi: 10.1523/JNEUROSCI.3339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–78. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Benson DL, Huntley GW. Synapse adhesion: a dynamic equilibrium conferring stability and flexibility. Curr Opin Neurobiol. 2012;22(3):397–404. doi: 10.1016/j.conb.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cheadle L, Biederer T. The novel synaptogenic protein Farp1 links postsynaptic cytoskeletal dynamics and transsynaptic organization. J Cell Biol. 2012;199(6):985–1001. doi: 10.1083/jcb.201205041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sloniowski S, Ethell IM. Looking forward to EphB signaling in synapses. Semin Cell Dev Biol. 2012;23(1):75–82. doi: 10.1016/j.semcdb.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14(8):536–50. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47(4):567–79. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 219.Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic spine hypoplasticity and downregulation of reelin and gabaergic tone in schizophrenia vulnerability. Neurobiol Dis. 2001;8(5):723–42. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]