Abstract
Rhodopsin is the classical light sensor. While rhodopsin is important for image formation in the eye, the requirements for opsins in non-image formation and in extra-ocular light sensation were revealed later. Most recent is the demonstration that an opsin in the fruit fly, Drosophila melanogaster, is expressed in pacemaker neurons in the brain and functions in circadian photoentrainment. After more than a century of analysis, the dogma has been that opsins are exclusive light sensors. Remarkably, through studies in Drosophila, light-independent roles for opsins in multiple senses are emerging. These include roles in temperature sensation and hearing. While these findings are uncovered in the fruit fly, there are hints that opsins have light-independent roles in a wide array of animals, including mammals. Thus, despite the decades of focus on opsins as light detectors, they represent an important new class of polymodal sensory receptors.
Keywords: rhodopsin, thermosensation, taste, hearing, circadian rhythm
INTRODUCTION
Discovery of opsins and the dogma that they function exclusively as light sensors
Rhodopsin is the founding member of the opsin family, and no G protein-coupled receptor (GPCR) has undergone greater scrutiny. This classical light sensor was discovered by Franz Boll in 1877 (Boll 1877a, Boll 1877b). Soon thereafter, Walter Kühne described that light absorption by rhodopsin causes pigment instability (Kühne 1878). However, it was not until 1936 when George Wald showed that rhodopsin is comprised of two components—a protein (opsin) and a light-responsive chromophore (retinal) (Wald 1936, Wald 1938, Wald 1968). 47 years later, the primary amino acid sequence of bovine opsin was deciphered, thereby revealing the first sequence of a GPCR (Hargrave et al 1983, Nathans & Hogness 1983, Ovchinnikov Iu et al 1983). This work led to the model that opsins have seven membrane-spanning α-helices (Figure 1), an idea later confirmed by structural studies (Baldwin et al 1997, Davies et al 1996, Herzyk & Hubbard 1998, Krebs et al 1998, Palczewski et al 2000, Schertler & Hargrave 1995, Shieh et al 1997, Unger et al 1997).
Figure 1. Cartoon of rhodopsin.
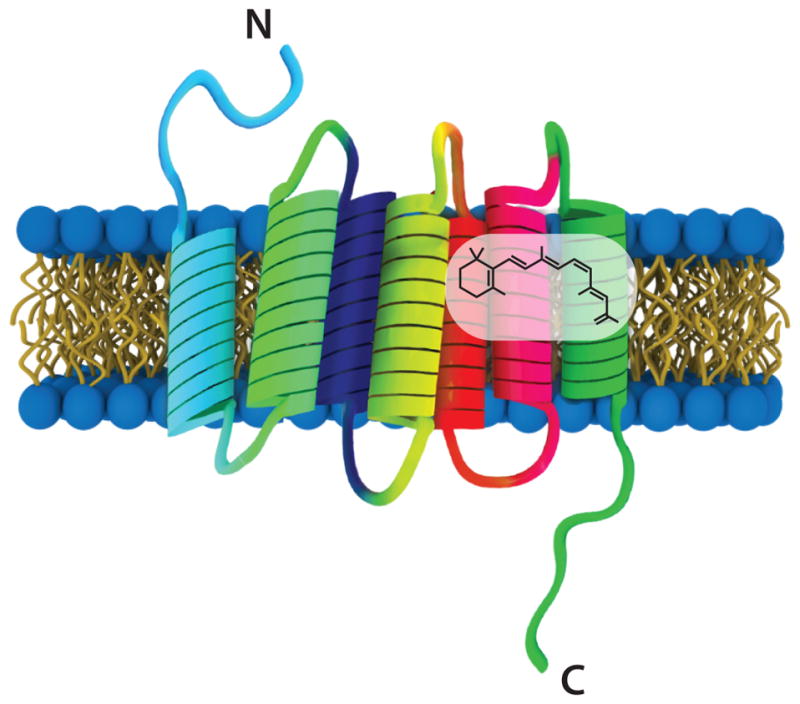
Rhodopsin is comprised of an opsin protein made up of seven transmembrane α-helices and a light-sensitive retinal chromophore. The chromophore in mammalian rods and cones is 11-cis-retinal and in Drosophila photoreceptor cells it is 3-hydroxy 11-cis-retinal.
A seminal advance was the demonstration that light-activated rhodopsin initiates a signal transduction cascade through the engagement of a trimeric G protein (Fung & Stryer 1980, Godchaux & Zimmerman 1979). In mammalian rods and cones, following light-induced conversion of 11-cis retinal to all-trans retinal, the G protein (transducin) releases GDP, binds GTP, and stimulates cyclic GMP (cGMP) phosphodiesterase (PDE), which leads to the closing of cGMP-gated cation channels (Figure 2a) (Yau & Hardie 2009). However, in mammalian intrinsically photosensitive retinal ganglion cells (ipRGCs) and in photoreceptor cells of Drosophila compound eyes, the effector for the G protein is a phospholipase C-β (PLC), which in turn causes the opening of TRP channels (Figure 2b) (Bloomquist et al 1988, Hardie & Minke 1992, Montell & Rubin 1989, Xue et al 2011).
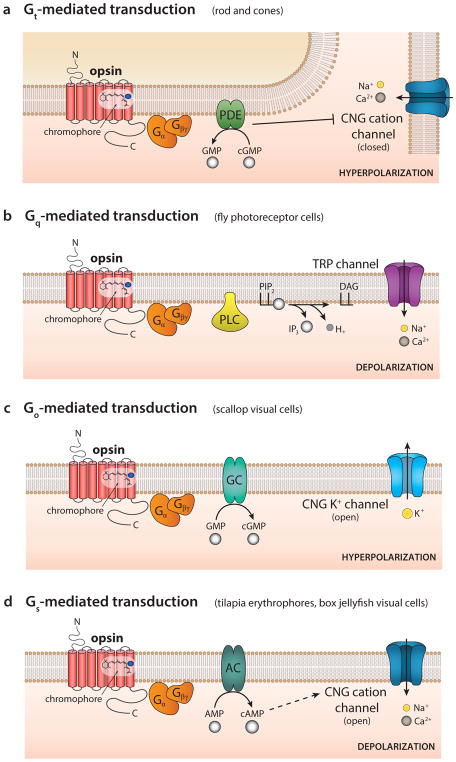
Figure 2. Multiple opsin-driven G protein-coupled signaling cascades.
(A) Gt-mediated transduction (e.g. mammalian rods and cones) couples an activated opsin to a G protein called transducin (Gt). Gt stimulates a phosphodiesterase (PDE), which causes a decline in cyclic GMP (cGMP) levels and subsequent closure of the cyclic nucleotide-gated (CNG) cation channels resulting in hyperpolarization.
(B) Gq-mediated transduction (e.g. Drosophila photoreceptor cells) couples an activated opsin to Gq, which stimulates phospholipase C (PLC), resulting in hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2). The subsequent openings of TRP channels result in depolarization. IP3, inositol triphosphate; DAG, diacylglycerol.
(C) Go-mediated transduction (e.g. scallop visual cells) links an activated opsin to Go, which activates guanylyl cyclase (GC). This leads to opening of CNG K+ channels and hyperpolarization. Go-mediated signaling cascades have also been observed in sea slug simple photoreceptors and in the lizard parietal eye. Several encephalopsins (pufferfish TMT and mosquito Opn3) are suggested to couple to Go and Gi, but the downstream components are less clear. Moreover, vertebrate neuropsins (Opn5) couple to Gi and likely inhibits adenylyl cyclase activity.
(D) Gs-mediated transduction (e.g. tilapia erythrophores and box jellyfish visual cells) links an activated opsin to Gs, which activates adenylyl cyclase (AC). The consequent increase in cAMP opens CNG cation channels resulting in depolarization.
The discoveries of these phototransduction cascades were major breakthroughs as they provided molecular explanations for two critical properties of photoreceptor cells. The first is signal amplification (Stryer 1986). This phenomenon permits light sensitivity that is so exquisite that animals can detect the smallest elemental particle of light—a photon. Absorption of a single photon by rhodopsin changes the activities of many cGMP-gated or TRP channels. Because rhodopsin in vertebrate rods or fly photoreceptor cells comprises 65–90% of the membrane protein in these cells (Heitzmann 1972, Papermaster & Dreyer 1974, Paulsen & Schwemer 1983), quantum efficiency is high. In response to dim light, roughly one out of every five photons is captured in rods (Alpern & Pugh 1974, Zwas & Alpern 1976), and nearly every photon is captured in flies (Song et al 2012). A second striking property of photoreceptor cells is adaptation to variations in light intensities over many orders of magnitude (Stryer 1986). This latter phenomenon is mediated largely by changes in Ca2+ levels as a consequence of light-dependent closing or opening of cGMP-gated and TRP channels, respectively (Fain et al 2001, Gu et al 2005).
Based on many decades of intense scrutiny, the dogma was that rhodopsin and related opsins function exclusively in light detection. In mammals, the long-held view was that photoreception is limited to the eye since enucleation of laboratory animals eliminates light entrainment (Nelson & Zucker 1981). However, there is evidence that mammals can detect light in skin (Haltaufderhyde et al 2015, Kim et al 2013, Toh et al 2016, Tsutsumi et al 2009, Wicks et al 2011), the brain (Sun et al 2016, Wade et al 1988), and in blood vessels (Sikka et al 2014). Non-mammalian vertebrates, such as fish and birds, have extra-ocular light sensory cells in multiple tissues, including the pineal (Bailey & Cassone 2004, Chaurasia et al 2005, Okano et al 1994), the hypothalamus (Fischer et al 2013, Halford et al 2009), and in dermal melanophores (Provencio et al 1998b). These sites express opsins, suggesting that they function in detecting environmental light but not in image formation (Fernandes et al 2012, Peirson et al 2009, Surbhi 2015, van Veen et al 1976, von Frisch 1911).
A major surprise is the discovery that opsins have sensory roles that are light independent. These insights, which emerged from studies in the fruit fly, Drosophila melanogaster, reveal roles for opsins in thermosensation (Shen et al 2011, Sokabe et al 2016) and hearing (Senthilan et al 2012) (Table 1). There are hints that vertebrate opsins also have light-independent roles. However, such roles for mammalian opsins are largely unexplored. In fact, when a mammalian opsin is detected in a cell type that is not associated with light sensation, the cell is assumed to be a photosensor. An important concept of this review is that opsins have multiple light-independent roles and are evolutionarily conserved polymodal sensors. While light-independent roles have been discovered in Drosophila, we hope this review will motivate similar studies in vertebrates, including mammals.
Table 1. Non-classical functions of Drosophila opsins.
A list of fly opsins and their expression in classical photoreceptors in the adult compound eyes and ocelli, along with their non-classical functions outside of the eye.
| Flyopsin | Classical photoreceptors | Non-classical function |
|---|---|---|
| Rh1 | R1-R6 (outer) | thermosensation |
| Rh2 | ocelli | - |
| Rh3 | R7 (inner) | - |
| Rh4 | R7 (inner) | |
| Rh5 | R8 (inner) | thermosensation, hearing |
| Rh6 | R8 (inner) | thermosensation, hearing |
| Rh7 | - | circadian photoentrainment in central pacemaker neurons |
An overview of opsin classification
The original classification of opsins is based on the photoreceptor cell type that houses the photopigment: ciliary or rhabdomeric (c-opsins and r-opsins) (Eakin 1965, Yau & Hardie 2009). Ciliary photoreceptors, which include human rods and cones, have stacked and flattened membranous discs. Rods are specialized for low light conditions and express rhodopsin, whereas human cones express three color pigments: OPN1-SW, OPN1-MW, and OPN1-LW. Rhabdomeric photoreceptors have densely packed membranous microvilli projections, which are present in the compound eye of flies and many other invertebrates. These visual photoreceptor cells express r-opsins that are distantly related to c-opsins. The c-opsins and r-opsins couple to distinct signaling cascades that result in hyperpolarization and depolarization of photoreceptor cells, respectively (Figure 2a and b). Another important difference is that following light-activation of c-opsins in rods and cones, the chromophore dissociates, whereupon the all-trans retinal is regenerated to 11-cis retinal in the neighboring retinal pigment epithelium (RPE) (Travis et al 2007, Wright et al 2015). However, the all-trans retinal does not typically dissociate from r-opsins, such as those in the fly eye (Montell 2012). Rather, it is converted back to 11-cis retinal by absorption of a second photon of light.
The early notion was that c-opsins were restricted to vertebrates, while r-opsins were exclusive to invertebrates. However, this is an oversimplification. For example, mammalian melanopsin (Opn4) is highly related to r-opsins, such as those in the fly eye (Provencio et al 1998b), and two opsins in the mosquito, Anopheles gambiae, are c-opsin-like (Arendt et al 2004). The marine ragworm, Platynereis dumerilii, expresses an r-opsin in its eyes and a c-opsin in the brain (Arendt et al 2004). In addition, other opsins classified as c-opsins based on sequence relationships initiate signaling cascades distinct from the phototransduction cascade observed in rods and cones (green opsin in tilapia, encephalopsin, parapinopsin, VA-opsin, parietopsin, neuropsin, pteropsin) (Figure 2c and d, Tables 2–3).
Table 2. Classification and expression of selected vertebrate opsins.
The opsins belong to three main opsin classes (c-opsin, r-opsin, RGR/Go-opsins). The opsin names that are based on the human isoforms are indicated (*). An X denotes opsins expressed in humans, rodents, teleosts, and birds. The ocular and extra-ocular cell types or tissues expressing a given opsin are listed, along with the animal in parenthesis: B, bird; H, human; R, rodent; T, teleost. Additional abbreviations: HC, horizontal cells; ipRGC, intrinsically photosensitive retinal ganglion cells; LW, long-wave; MW, medium-wave; OS, outer segment; RGR, retinal G protein-coupled receptor opsin; RPE, retinal pigment epithelium; RRH, retinal pigment epithelium-derived rhodopsin homolog; SW, short-wave; TMT, teleost multiple tissue; VA, vertebrate ancient; VAL, vertebrate ancient long.
| Group | G protein | Name | Other name (s) | Animal | Expression | ||||
|---|---|---|---|---|---|---|---|---|---|
| Human | Rodent | Teleost | Bird | Ocular | Extra-ocular | ||||
| c-opsin (ciliary) | Gt | rhodopsin* | × | × | × | × | rods (H) | skin (H,R), pineal (R), sperm (H,R) | |
| Gt | OPN1-SW* | blue opsin | × | × | × | × | cones (H) | skin (H,R), pineal (R), sperm (H,R) | |
| Gt Gs |
OPN1-MW* | green opsin | × | × | × | × | cones (H) | skin (H), sperm (H,R) erythrophore (T) |
|
| Gt | OPN1-LW* | red opsin | × | × | × | cones (H) | skin (H) | ||
| Gi/Go | OPN3* | encephalopsin panopsin TMT |
× | × | × | × | retina (H,R), HC (B), interneurons (T) | brain (R,T), spinal cord, skin, testis (R), sperm (H,R), pineal, heart (T), placenta (H), kidney, liver (H,T) | |
| Gt/Ggust | OPNP | pinopsin pineal opsin |
× | retina OS (gecko) | pineal (B, gecko, lizard) | ||||
| Gi | parapinopsin | × | parapineal, pineal, testis (T) | ||||||
| Gi | VA-opsin | VAL-opsin | × | × | HC (T), iris (T) | brain (T,B), ovary (T) | |||
| Go | parietopsin | × | parietal eye (lizard) | ||||||
| r-opsin (rhabdomeric) | Gq | OPN4* | melanopsin | × | × | × | × | ipRGCs (H,R), cones (H), HC (T,B), RPE (frog), iris (frog), cornea (frog) | brain (H,T,B), trigeminal ganglia (H,R), skin (R, frog), blood vessels (R), pineal (B), sperm (H,R) |
| RGR/Go -opsins | RGR opsin* | × | × | × | × | RPE, Müller cells (bovine) | brain (T,B), pineal (B), liver (T) | ||
| peropsin* | RRH | × | × | × | × | RPE (H), ganglion cells (B) | brain (B), skin (H), pineal (B) | ||
| Gi | OPN5* | neuropsin Gpr136, PGR12, TMEM13 | × | × | × | × | retina (H,R) | brain (H,R,B), spinal cord (H,R), skin (R), sperm (H,R), testis (H,R), outer ears (R), adrenal gland (B) | |
Table 3. Classification and expression of selected invertebrate opsins.
nvertebrate c-opsin, r-opsin, RGR/Go-opsins, and cnidops. The opsin names based on Drosophila are indicated (*). Expression of opsins in flies, mosquitoes, and squid are indicated with an X. The ocular and extra-ocular expression of the opsins is indicated followed by the animal or animal group: F, fly; M, mosquito; Oc, Onychophora (velvet worm); S, squid. Additional abbreviations: IS, inner segment; OS, outer segment; PNS, peripheral nervous system.
| Group | G protein | Name | Other name (s) | Animal Expression | ||||
|---|---|---|---|---|---|---|---|---|
| Fly | Mosquito | Squid | Ocular | Extra-ocular | ||||
| c-opsin (ciliary) | c-opsin | GPROP12 (M) | × | eye (Oc) | brain (spider, Oc), pedicellariae (urchin) | |||
| Gi/Go | pteropsin | brain (honey bee) | ||||||
| Platynereis c-opsin |
brain (ragworm) | |||||||
| r-opsin (rhabdomeric) | Gq | Rh1* | × | × | R1–R6 (F) | PNS (F) | ||
| Gq | Rh2* | × | ocelli (F) | testis (F) | ||||
| Gq | Rh3* | × | × | R7 (F) | ||||
| Gq | Rh4* | × | R7 (F) | |||||
| Gq | Rh5* | × | × | R8 (F) | Johnston’s organ, brain, PNS (F) | |||
| Gq | Rh6* | × | × | R8 (F) | Johnston’s organ, brain, PNS (F) | |||
| Rh7* | × | × | brain (F) | |||||
| r-opsin | onychopsin (Oc) | retina OS (S), eye (Oc) | brain (butterfly, Oc), nerve cord, tail (crayfish), skin (S, octopus), tube feet, pedicellariae (urchin), light organ et al. (S), genitalia (butterfly) | |||||
| melanopsin | neural tube (amphioxus) | |||||||
| arthropsin | brain (spider, Oc), ventral nerve cord (Oc) | |||||||
| RGR/Go-opsins | retinochrome | × | retina IS, OS (S) | parolfactory vesicles et al. (S) | ||||
| RGR opsin | Ci-opsin3 (tunicate) | ocelli (tunicate) | ||||||
| peropsin | non-visual cells (spider) | brain (spider, horseshoe crab), abdomen (krill) | ||||||
| Go | Go-opsin | retina (scallop) | tube feet, pedicellariae (urchin) | |||||
| cnidops | Gq/Gs | lens eye (jellyfish) | neuropil (jellyfish), battery complex (hydra) | |||||
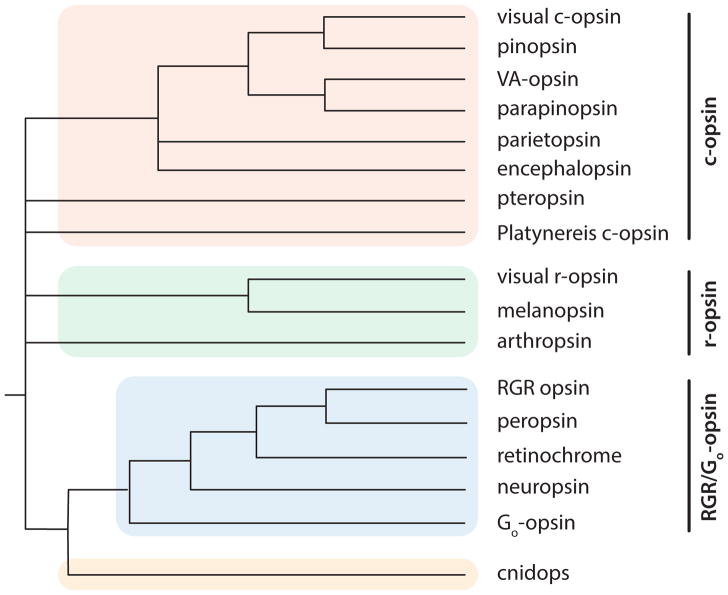
There is a third class of opsins distinct from c-opsins and r-opsins. This group called RGR/Go-opsins (also referred to as Group 4 opsins) includes retinal G protein-coupled receptor opsins (RGR), peropsin, and neuropsin (Opn5) (Figure 3, Tables 2–4). These opsins function as photoisomerases or couple to different signaling cascades (Figure 2c, Tables 2–3). For simplicity, we use three opsin groups in this review. However, analyses that include opsins from cnidarians, ctenophores, placozoans, and a large diversity of bilateria assign opsins to a greater number of groups (Feuda et al 2012, Feuda et al 2014, Porter et al 2012, Ramirez et al 2016) (Table 4).
Figure 3. Phylogenetic tree of selected opsin family members.
There are four main groups: c-opsin (red), r-opsin (green), RGR/Go-opsin (blue), and cnidops (yellow). The relationships shown are based on Ramirez et al. (2016), Hering & Mayer (2014), and Feuda et al (2012, 2014).
Table 4. Classes and total number of opsins in different species.
The “Other” opsin class includes cnidops and echinopsins. Opsin numbers are based on whole genome data unless indicated (*). Of note, many opsin sequences are not functionally verified and may be pseudogenes. The following species were used for the table: amphioxus (Branchiostoma floridae), comb jelly (Mnemiopsis leidyi), horseshoe crab (Liminus polyphemus), hydra (Hydra magnipalillata), mosquito (Aedes aegypti), octopus (Octopus bimaculoides), ragworm (Platynereis dumerilii), sea anemone (Nematostella vectensis), sea urchin (Strongylocentrotus purpuratus), scallop (Pectinidae), squid (Dorytheuthis pealeii), tunicate (Ciona intestinalis), water flea (Daphnia pulex).
| Animal | Opsin groups
|
Total | |||
|---|---|---|---|---|---|
| C-opsin | R-opsin | RGR/Go | Other | ||
| Mammal | |||||
|
| |||||
| human | 5 | 1 | 3 | - | 9 |
| mouse | 4 | 1 | 3 | - | 8 |
|
| |||||
| Non-mammalian vertebrate | |||||
|
| |||||
| zebrafish | 23 | 5 | 14 | - | 42 |
| chicken | 9 | 2 | 6 | - | 17 |
|
| |||||
| Arthropod | |||||
|
| |||||
| fruit fly | - | 7 | - | - | 7 |
| mosquito | 1 | 9 | - | - | 10 |
| horseshoe crab | 2 | 14 | 2 | - | 18 |
| water flea | 9 | 37 | - | - | 46 |
|
| |||||
| Mollusc | |||||
|
| |||||
| squid/scallop* | - | 1 | 1 | - | 2 |
| octopus | - | 2 | 2 | - | 4 |
|
| |||||
| Chordate | |||||
|
| |||||
| tunicate | 2 | - | 1 | - | 3 |
| amphioxus | 5 | 7 | 8 | - | 20 |
|
| |||||
| Echinoderm | |||||
|
| |||||
| sea urchin | 1 | 1 | 5 | 2 | 9 |
|
| |||||
| Annelid | |||||
|
| |||||
| ragworm | 1 | 5 | 5 | - | 11 |
|
| |||||
| Cnidarian | |||||
|
| |||||
| sea anemone | - | - | - | 31 | 31 |
| hydra | - | - | - | 63 | 63 |
|
| |||||
| Ctenophore | |||||
|
| |||||
| comb jelly | - | - | - | 3 | 3 |
NON-IMAGING FORMING FUNCTIONS OF OPSINS IN THE MAMMALIAN EYE
In the mammalian eye, light detection occurs in three types of neurons: rods, cones, and a small subset of retinal ganglion cells, which are intrinsically photosensitive (ipRGCs) (Berson et al 2002). While rods and cones facilitate image formation, direct light sensation by ipRGCs primarily drives non-image forming functions, although, it also promotes a rudimentary form of image formation (Ecker et al 2010, Hughes et al 2016, Schmidt et al 2011). The discovery of non-image forming functions in the eye traces back to observations that blind mice can shift their circadian rhythms according to an external light-dark cycle (Foster et al 1991, Freedman et al 1999, Provencio et al 1994). A few years later, ipRGCs were found to be photosensitive and mediate circadian photoentrainment (Berson et al 2002). In addition to these three classes of photoreceptor cells, at least two types of neuronal cells in the mammalian eye are also intrinsically photosensitive. These include sphincter muscle cells in the iris, which control pupillary constriction (Xue et al 2011), and RPE cells (Radu et al 2008).
The light responsiveness of ipRGCs and iris sphincter muscles are dependent on the photopigment melanopsin (Berson et al 2002, Hattar et al 2002, Lucas et al 2003, Xue et al 2011). This opsin, which was first described in Xenopus laevis dermal melanophores (Provencio et al 1998b), shares greater biophysical properties and amino acid homology to Drosophila rhodopsins (Provencio et al 1998a), and couples to a Gq-PLC-TRP signaling pathway highly reminiscent of fly phototransduction (Díaz et al 2016, Montell 2012, Xue et al 2011) (Figure 2b).
Melanopsin serves as the photopigment for an impressive range of non-image forming light responses. These include photoentrainment of circadian rhythms (Freedman et al 1999, Panda et al 2002), light-induced pupillary constriction (Lucas et al 2003, Xue et al 2011), suppression of pineal melatonin (Lucas et al 1999), light aversion (Johnson et al 2010, Semo et al 2010), control of sleep states (Altimus et al 2008, Hubbard et al 2013, Lupi et al 2008, Milosavljevic et al 2016, Pilorz et al 2016, Tsai et al 2009), and other functions (Atkinson et al 2013, Esquiva et al 2016, Rao et al 2013, Reifler et al 2015, Zhang et al 2008). The discoveries of melanopsin and its myriad of non-image and rudimentary image forming functions in the eye are covered in detail in other reviews (Do & Yau 2010, Hankins et al 2008, Hughes et al 2016, Lucas 2013, Matynia 2013, Schmidt et al 2011).
Mammalian retinal G protein-coupled receptor opsin (RGR) is another opsin with non-classical functions in the eye. It is expressed in the RPE and Müller glial cells, but has slightly greater sequence homology to an opsin in squid (retinochrome), which has photoisomerase activity, than to mammalian rhodopsin (Jiang et al 1993, Radu et al 2008). In RPE maintained in the dark, RGR is a negative regulator of two enzymes that catalyze reactions that contribute to the visual cycle (Radu et al 2008). The enzyme activities are restored in light, indicating that RGR is an important regulator of the visual cycle (Radu et al 2008).
EXTRA-OCULAR LIGHT-DEPENDENT FUNCTIONS OF OPSINS
Mouse Opn4 functions in the photorelaxation of blood vessels
Light sensation is not limited to the eye. A long-known phenomenon is the photorelaxation of blood vessels (Furchgott et al 1955), but the receptor mediating this response has been enigmatic. Melanopsin mRNAs are expressed in blood vessels of mice and light-induced vasorelaxation is essentially eliminated in Opn4 null mutants, indicating that melanopsin functions in extra-ocular light detection (Sikka et al 2014). Given that vascular function is under circadian control (Paschos & FitzGerald 2010), melanopsin in blood vessels may serve as the vascular sensor that drives the circadian control of blood flow and pressure in mammals (Sikka et al 2014).
Melanopsin in ipRGCs couples to a PLC-dependent pathway that resembles the phototransduction cascade in fly photoreceptors (Figure 2b). However, light-induced vasorelaxation is dependent on cGMP, PDE6, guanylyl cyclase (GC), and K+ channels (Sikka et al 2014). Moreover, membrane potentials of vascular smooth muscle cells hyperpolarize rather than depolarize in response to light (Sikka et al 2014). If melanopsin uses cGMP-dependent signaling in blood vessels, then melanopsin has the remarkable ability to couple two types of G protein signaling pathways to drive different physiological responses.
Interestingly, the photorelaxation signaling pathway resembles the phototransduction cascade observed in invertebrates, such as scallop (SCOP2) and sea slugs (Ip-1, Ip-2), which involves a Go-type G protein coupled to GC and the opening of K+ channels leading to hyperpolarization (Gotow & Nishi 2007, Kojima et al 1997) (Figure 2c). However, the G protein responsible for photorelaxation has not been identified and further biochemical studies are needed to establish whether or not melanopsin can initiate a Go signaling cascade. Moreover, it remains possible that other non-image forming opsins, including Opn3 and Opn5, may be involved in vascular responses.
Potential roles for opsins in skin
The earliest evidence of an opsin in skin is the discovery of melanopsin in Xenopus dermal melanophores (Provencio et al 1998b). Since then, almost all human opsins have been documented in skin (Haltaufderhyde et al 2015, Kojima et al 2011, Tsutsumi et al 2009). RNAi knockdown of rhodopsin in primary human epidermal melanocytes reduces Ca2+ increases in response to ultraviolet A (UVA) illumination (Wicks et al 2011). However, the spectral sensitivity of rhodopsin does not extend into the UVA range, raising the possibility that rhodopsin might heterodimerize with another light receptor to achieve this sensitivity (Wicks et al 2011). Unexpectedly, the UVA response might occur via a Gq-PLC signaling pathway (Bellono et al 2014, Wicks et al 2011). In keratinocytes, violet light downregulates expression of differentiation markers, and this effect is blunted upon siRNA-mediated suppression of rhodopsin (Kim et al 2013). An inhibitor of Gi signaling prevents the light-induced downregulation of differentiation markers (Kim et al 2013), raising the possibility that rhodopsin couples to Gt, Gq, and Gi cascades in rods, melanocytes, and keratinocytes, respectively. However, the link between rhodopsin and Gq/Gi signaling remains speculative.
Fish have specialized dermal light receptors for adjusting pigmentation. In tilapia erythrophores (red pigment cells), pigment migration depends on the wavelength of light. Green light results in pigment dispersion and red light induces pigment aggregation (Ban et al 2005). In erythrophores, a green opsin and a red opsin appear to be co-expressed, and they might couple to Gs and Gi proteins, respectively, to activate or inhibit adenylyl cyclase and regulate the opposing effects of pigment dispersion and aggregation (Figure 2d) (Ban et al 2005).
In cephalopod molluscs, such as squid, cuttlefish, and octopus, chromatophores mediate dynamic changes in skin color (Kingston et al 2015, Mäthger et al 2010, Ramirez & Oakley 2015). In octopus, the skins cells express an r-opsin and retinochrome (Ramirez & Oakley 2015). The r-opsin may also function in mechanosensation, raising the provocative suggestion that it serves dual functions in mechanoreception and light reception (Ramirez & Oakley 2015). Evidence that an opsin is required for mechanosensation is discussed later in this review.
Drosophila Rh7 functions in circadian pacemaker neurons in the central brain
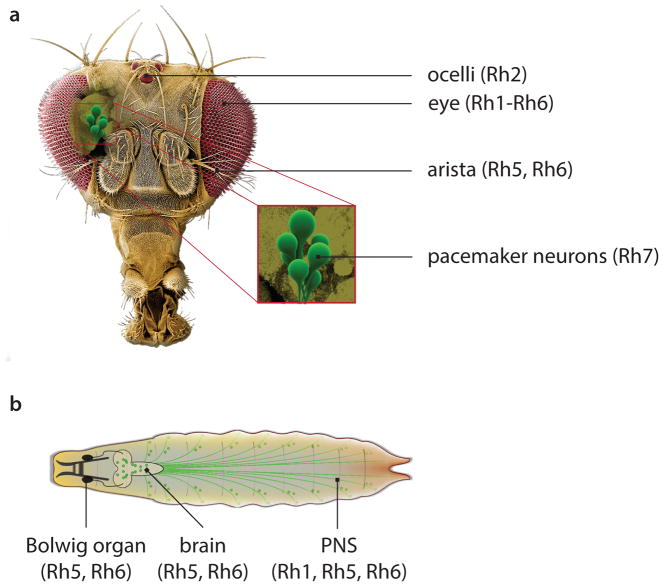
In addition to the six Drosophila rhodopsins that are expressed in the compound and simple eyes (ocelli), flies encode a seventh opsin (Rh7). Unexpectedly, this opsin is expressed in the central brain in a subset of circadian pacemaker neurons (Figure 4a) (Ni et al 2017). Despite this unusual cellular localization, Rh7 is a light sensor as it can substitute for Rh1 in the eyes and confers light sensitivity to tissue culture cells in the purple range. Of significance, loss of Rh7 greatly diminishes the sensitivity of pacemaker neurons to purple light. Thus, Rh7 functions as a light detector in the central brain (Ni et al 2017).
Figure 4. Opsins function in a diversity tissues in Drosophila.
(A) Tissues in the adult fly head requiring opsins.
(B) Larval tissues requiring opsins.
The observation that Rh7 is a photopigment in pacemaker neurons raises the possibility that it could function in circadian photoentrainment. Circadian rhythms are set most robustly by light/dark cycles and control an array of behaviors including sleep, movement, feeding, and mating (Allada & Chung 2010, Mohawk et al 2012, Peschel & Helfrich-Förster 2011). Opsins in the Drosophila compound eye, ocelli, and an eyelet between the retina and optic lobes contribute to photoentrainment (Helfrich-Förster et al 2001, Klarsfeld et al 2004, Mealey-Ferrara et al 2003, Rieger et al 2003, Saint-Charles et al 2016, Szular et al 2012). Notably, light sensation by the ~150 pacemaker neurons are of particular importance in setting circadian rhythms (Nitabach & Taghert 2008). These central brain neurons express another type of photoreceptor—a flavoprotein called cryptochrome (Cry), which helps establish circadian rhythms in response to light (Emery et al 2000b, Stanewsky et al 1998). The circadian clock can be entrained by dim light. However, Cry is not very light sensitive when expressed in vitro (Ozturk et al 2011) and in the absence of Cry, flies still photoentrain, indicating that there is another light detector in pacemaker neurons that contributes to setting circadian rhythms.
Rh7 is co-expressed in central pacemaker neurons with Cry, and with Pigment Dispersing Factor (PDF) (Ni et al 2017), which appears to be the primary neuropeptide controlling circadian behavior (Renn et al 1999). While loss of Rh7 or Cry alone results in relatively subtle impairments in photoentrainment, mutations eliminating both Rh7 and Cry cause a profound deficit. Nevertheless, loss of just Rh7 has strong effects under some conditions. In contrast to wild-type flies, which become arrhythmic upon exposure to constant light (Emery et al 2000a), rh7 mutant animals can retain rhythmicity, and this phenotype was particularly pronounced under dim light (Ni et al 2017).
The dual system for responding to light in circadian pacemaker neurons through Cry and Rh7 might provide independent mechanisms for setting circadian photoentrainment through bright and dim light, respectively. A rhodopsin-dependent mechanism could promote signal amplification, similar to a key feature of phototransduction in ocular photoreceptor cells. However, unlike the rhodopsins in the compound eye, which couple to PLC encoded by the norpA locus, Rh7 appears to engage the other Drosophila PLC-β (PLC21C). Because the phototransduction cascade in the Drosophila visual system culminates with opening of TRP channels (Hardie & Minke 1992, Montell & Rubin 1989), it is likely that one or more of the 13 fly TRP channels is activated downstream of Rh7. However, the TRP channels remain to be identified.
It is intriguing to speculate that Rh7 is the Drosophila functional equivalent of melanopsin. Both Rh7 and melanopsin couple to PLC-dependent signaling cascades, and are light receptors with important roles in circadian photoentrainment rather than in classic image formation. Given that extra-ocular opsins are also expressed in the mammalian brain (Blackshaw & Snyder 1999, Nissilä et al 2012), the question arises as to whether these opsins are sensors that detect red-shifted light penetrating the mammalian skull, or whether they have light-independent roles.
Opsins in photosensation in the vertebrate brain and central nervous system
Lampreys and teleosts, such as zebrafish, are endowed with extra-ocular light sensitivity in the pineal, and some fish also have photoreceptor cells within the deep brain, such as the hypothalamus (von Frisch 1911). These tissues express multiple opsins, suggesting that they function in photoreception in the brain (Davies et al 2015, Fischer et al 2013, Hang et al 2016a, Kingston & Cronin 2016, Kojima et al 2008, Koyanagi et al 2004).
At least two light-dependent behaviors are under control of opsins during zebrafish development. During embryogenesis, coiling behavior is promoted by spontaneous activity of the spinal central pattern generator (Friedmann et al 2015). Surprisingly, this behavior is suppressed by light, which inhibits the central pattern generator, and the key receptor is the opsin, VALopA (Friedmann et al 2015). At a later developmental stage, larvae sense light through deep brain photoreceptors in the preoptic area, which contributes to a light seeking behavior that is triggered by loss of illumination (Fernandes et al 2012). Based on its spatial distribution and overexpression studies, Opn4 might be the receptor underlying the “dark photokinesis.”
In birds, extra-ocular light sensation influences seasonal reproductive behavior and occurs through several brain regions, including the pineal gland, septal regions of the telencephalon, and the medio-basal hypothalamus (García-Fernández et al 2015, Surbhi 2015). The medio-basal hypothalamus is the essential brain region that regulates seasonal behavior (Benoit 1935a, Benoit 1935b, García-Fernández et al 2015, Oliver et al 1977, Yokoyama et al 1978). The hypothalamus expresses a VA opsin, which is the proposed light receptor (García-Fernández et al 2015).
Multiple opsins are expressed in the mammalian brain (Opn3, Opn4, and Opn5) (Blackshaw & Snyder 1999, Nissilä et al 2012) and spinal cord (Opn5) (Tarttelin et al 2003). The brains of some neonatal mammals may sense light (Johnson et al 2010, Routtenberg et al 1978, Viggiani et al 1970). However, it is unclear whether the adult mammalian brain is capable of detecting illumination. While there is evidence in favor of light modulation of cells deep in the brains of adult mammals, including humans (Flyktman et al 2015, Sun et al 2016, Vandewalle et al 2009, Wade et al 1988), this could be an indirect effect of light stimulation of cells outside the brain. Thus, it is intriguing to speculate that opsins in the mammalian brain have light-independent roles that remain to be revealed. In support of this provocative idea, light-independent roles for opsins have been discovered in Drosophila, and are described below.
LIGHT-INDEPENDENT ROLES FOR OPSINS
Drosophila opsins and temperature discrimination in the comfortable range
Animals have a strong preference to seek out their ideal temperature within the thermal landscape. This behavior is particularly notable in poikilothermic organisms such as fruit flies, in which their body temperatures equilibrate with the environment. Drosophila larvae are capable of distinguishing temperature differences within a fraction of a degree (Klein et al 2015). The comfortable range for 3rd instar larvae is 18–24°C, although mid- and late-3rd instar larvae prefer 18°C over higher temperatures, such as 19–24°C (Kwon et al 2008, Shen et al 2011, Sokabe et al 2016).
Multiple TRP channels in vertebrates and invertebrates are direct thermosensors (Fowler & Montell 2013, Julius 2013), and thermotaxis of Drosophila larvae towards 18°C is dependent on TRPA1 (Kwon et al 2008). However, the dependence on this channel is indirect since none of the TRPA1 isoforms are directed activated until the temperatures exceed 27°C (Kang et al 2012, Luo et al 2016, Viswanath et al 2003, Zhong et al 2012). Rather, the gating of TRPA1 in mid-3rd instar larvae depends on a signaling cascade that includes Gq and PLC (NORPA) (Kwon et al 2008). Remarkably, the cascade is initiated by an opsin (Rh1) (Shen et al 2011). The requirement for Rh1 is light independent, since thermotaxis assays are performed in the dark and light does not interfere with this behavior (Shen et al 2011).
How is it possible that illumination does not disrupt the thermotaxis behavior, since rhodopsin is a photosensor? Because the chromophore is the light-sensitive subunit of rhodopsin, a potential solution to this question is that the opsin alone, and not the chromophore, is required for thermosensation. However, the chromophore is essential for thermotaxis (Shen et al 2011). This most likely reflects a requirement for the chromophore for trafficking, since it has dual roles as a light sensor and as a molecular chaperone in photoreceptor cells in the compound eye (Ozaki et al 1993). In the absence of the chromophore, the opsins do not exit the endoplasmic reticulum.
The low expression level of Rh1 in thermosensory neurons is the most plausible explanation for the lack of interference of light on Rh1-dependent thermotaxis. In classical photoreceptor cells in the visual system, rhodopsins are expressed at high levels for efficient photon capture. However, Rh1 is expressed at extremely low levels in the thermosensory neurons in the larval peripheral nervous system (Shen et al 2011) (Figure 4b). As a consequence, photon capture in these cells is very inefficient.
Another surprising finding is that there is a switch in requirements for opsins in late-3rd instar larvae, when the preference for 18°C becomes even more pronounced (Sokabe et al 2016). At this stage, Rh1 is no longer required. Rather, two other opsins, Rh5 and Rh6, are essential in trpA1-expressing neurons in the brain and in the peripheral nervous system (Figure 4b) for selecting 18°C over slightly higher temperatures in the comfortable range. As with Rh1, the requirements for Rh5 and Rh6 are light-independent, and depend on a signaling cascade that includes Gq, PLC (NORPA), and TRPA1 (Sokabe et al 2016).
Two notable features of phototransduction cascades are signal amplification and adaptation (Stryer 1986). Opsin-dependent signaling cascades may be employed in allowing larvae to choose their preferred temperature (18°C) over slightly higher temperatures for the same two reasons. Amplification may allow the larvae to detect very small differences in a shallow gradient. If the animals cannot find 18°C in their environment, they can adapt to slightly higher temperatures, which are still positive for growth and survival.
Multiple questions remain. It is not clear why there is a switch from dependence on Rh1 to Rh5/Rh6 between the mid- and late-3rd instar larval periods. Whether adult flies use opsins to sense small temperature differences in the comfortable range is not known. A crucial question discussed later in this review is whether mammals use opsins to detect small deviations in innocuous temperatures over shallow gradients.
The major unresolved question concerns the mechanism through which opsins serve as temperature sensors. Rhodopsin and color visual pigments in classical photoreceptor cells have high thermal stability (Barlow 1988). This is important to limit noise in photoreceptor cells, which would otherwise reduce sensitivity to dim light (Barlow 1988). Nevertheless, there is a very low level of electrical activity in the dark that results from thermal activity of rhodopsin (Hardie et al 2002, Yau et al 1979).
Dark noise is a two-step process. The first step is the deprotonation of the 11-cis Schiff-base linkage and the second step involves the thermally-induced isomerization of 11-cis to all-trans retinal (Barlow et al 1993). The deprotonation decreases the energy barrier required for the cis to trans isomerization from ~45 to 27–30 kcal/mol (Barlow et al 1993). This latter number is in line with the activation energy for thermal noise. Thus, thermal activation (dark noise) may not occur on random rhodopsins, but on those molecules that are deprotonated. If the pH in the vicinity of rhodopsin is increased slightly, then deprotonation of rhodopsin occurs. Thus, we propose that in neurons that function in thermosensation, the local pH near the rhodopsins is elevated, which results in the deprotonation of rhodopsin, thereby greatly decreasing the energy barrier for thermal activation.
Auditory role for Drosophila opsins
Another surprise is that opsins are expressed and function in the Drosophila auditory system (Senthilan et al 2012). The fly ear is located in the antenna where sound is received by vibrations of arista—a feathery organ extending out from the 3rd antennal segment (Figure 4a). The movement of the arista and the associated 3rd antennal segment then causes activation of two or three stretch-activated chordotonal neurons housed in dozens of separate sensory units (scolopidia) in the Johnston’s organ on the 2nd antennal segment (Ishikawa & Kamikouchi 2016). In flies, hearing is important for courtship. Males produce a courtship song by vibrating their wings in a particular pattern that is attractive to females of the same species (Albert & Göpfert 2015, Ishikawa & Kamikouchi 2016).
The identification of opsins as receptors important in Drosophila hearing was not expected. Rather, this insight emerged from an unbiased screen for genes expressed in the Johnston’s organ (Senthilan et al 2012). The approach took advantage of a mutation in atonal, which eliminates the Johnston’s organ. Therefore, genes expressed in wild-type but not atonal mutant 2nd antennal segments were likely to be enriched in the Johnston’s organ. Among the 274 genes that surfaced in this screen, many function in phototransduction in the compound eye. These include Arrestin2, G-protein subunits, PLC (NORPA), TRP, TRPL, and others. Amazingly, four out of the seven opsins showed up as well: Rh3, Rh4, Rh5 and Rh6.
Mutation of either rh5 or rh6 cause pronounced deficits in sound-evoked electrical responses of the Johnston’s organ and greatly decrease mechanical amplification. Flies there are doubly mutant for rh5 and rh6 display similarly severe deficits in mechanical amplification as by the single mutants. However, louder sounds were required to evoke compound action potentials from the antennal nerve of the double mutant versus the single mutants, indicating that the two opsins have some non-redundant roles. These roles are light-independent. However, reminiscent of thermosensation, the chromophore is required for the auditory response, presumably to facilitate trafficking of opsins out of the endoplasmic reticulum.
The key question is whether Rh5 and Rh6 function in mechanotransduction. Consistent with this possibility, both opsins are expressed in Johnston’s organ neurons (Senthilan et al 2012). Moreover, they are required for gating mechanotransduction channels, which include multiple TRP channels. An open question is whether Rh5 and Rh6 are themselves mechanically gated. Do the opsins in the Johnston’s organ neurons engage a Gq/PLC signaling cascade? Stimulation of PIP2 generates DAG, IP, and H+. Because DAG is smaller than PIP2, this could cause a conformation change in the plasma membrane, and contribute to mechanical gating of the TRP channels, similar to the proposed mechanism for mechanical gating of TRP and TRPL in photoreceptor cells (Hardie & Franze 2012). However, a caveat is that mutation of the norpA PLC, which is essential for phototransduction in the eye, does not appear to function in the auditory response (Senthilan et al 2012). It is possible that Rh5 and Rh6 couple to PLC21C in Johnston’s organ neurons, reminiscent of the engagement of Rh7 with PLC21C in pacemaker neurons (Ni et al 2017). Because Rh3 and Rh4 are also expressed in the Johnston’s organ, an additional question is whether these opsins also play roles in the auditory response.
Potential light-independent roles for opsins in vertebrates
Several mammalian opsins are expressed in the brain (Opn3, Opn4, and Opn5) (Blackshaw & Snyder 1999, Nissilä et al 2012) and spinal cord (Opn5) (Tarttelin et al 2003) (Table 2). While there is evidence in favor of light modulation of cells deep in the brains of mammals, including humans (Flyktman et al 2015, Sun et al 2016, Vandewalle et al 2009, Wade et al 1988), it is not known whether light is sensed directly by the mammalian brain. Moreover, it is unclear whether the opsins in the mammalian brain are expressed at sufficiently high levels for effective photon capture. Thus, opsins in the mammalian brain are candidates for having light-independent roles.
One recent study reported a knockout of the first “deep brain” opsin (valop or val opsin) in a non-mammalian vertebrate (Hang et al 2016b). The only phenotypes apparent in the mutant zebrafish are impairments in chorion formation and hatching of embryos. This raises the intriguing possibility that VAL opsin may have a light-independent role in reproduction.
There is potential that an opsin might function in the immune system. OPN3 is implicated as an asthma susceptibility gene, and OPN3 mRNA is detected in T-cells, B-cells, and bronchiolar epithelial cells (White et al 2008). RNAi-mediated knockdown of OPN3 in a T-cell line, Jurkat cells, inhibited secretion of interleukin-2 (IL-2) following activation by the T-cell receptor (CD3/CD28). If OPN3 indeed functions in promoting IL-2 secretion, a key unresolved question is whether the opsin is activated by light, a chemical agonist, or mechanical force.
An exciting possibility is that mammalian opsins might function in sperm thermotaxis, reminiscent of the function of opsins in larval thermotaxis. After reaching the storage site near the beginning of the Fallopian tube, sperm must traverse several centimeters to reach the egg. One long-range cue is rheotaxis—the ability to move in response to fluid flow (Miki & Clapham 2013). Chemotaxis is unlikely to be a long-range cue since peristaltic movements within the oviduct would disrupt chemical gradients. As the sperm approaches the egg, chemotaxis come into play (Eisenbach & Giojalas 2006), and this may be mediated by odorant receptors in sperm (Fukuda et al 2004, Spehr et al 2003).
Thermotaxis is also a potential navigation mechanism since in mammals, such as pigs and rabbits, there is a 1° to 2°C temperature difference between the sites of sperm storage and fertilization (David et al 1972). Both rabbit and human sperm are capable of thermotaxis in vitro (Bahat et al 2003). However, only about 7–17% and 3–5% exhibit thermotaxis, respectively (Bahat et al 2003). This low percentage may reflect a requirement for capacitation for thermotaxis, since the fraction of capacitated sperm in the preparations is low.
Human sperm may be extremely temperature sensitive as they are proposed to respond to an extraordinarily small difference (<0.0006°C) between the front and back of the cell (Bahat et al 2012). Such sensitivity would be comparable to the slime mold, Dictyostelium discoideum, which senses and responds to a gradient as shallow as 0.04°C/cm, which equates to 0.0004°C along the length of one amoeba (Poff & Skokut 1977).
Notably, mammalian sperm thermotaxis has been attributed to an opsin-dependent signaling cascade (Pérez-Cerezales et al 2015). Six opsins mRNAs are expressed in human sperm, with OPN3 and OPN5 at the highest levels, while rhodopsin, OPN1-SW, OPN1-MW, and OPN4 are detected at lower levels. The same six opsin mRNAs are expressed in mouse testis, although rhodopsin is the most abundant (Pérez-Cerezales et al 2015).
Genetic experiments using knockout mice suggest that rhodopsin contributes to thermotaxis. Wild-type sperm (1–8%) accumulate in the warmer zone of a gradient, and the overall fraction of rhodopsin mutant sperm that undergo thermotaxis declines ~70% (Pérez-Cerezales et al 2015). This reduction does not appear to be due to effects of the rhodopsin mutation or an impairment in capacitation. The residual thermotaxis exhibited by the mutant sperm could be due to the function of other opsins.
A question is whether the opsin(s) that may contribute to sperm thermotaxis engage the same signaling cascades that is employed in photoreceptor cells (Figure 2a). Transducin (Gt) is expressed in mouse and human sperm, and PDE inhibitors reduce thermotaxis (Pérez-Cerezales et al 2015). However, in a separate study by the same group, an inhibitor of PLC (U73122) reduces thermotaxis of human sperm (Bahat & Eisenbach 2010), an observation which is consistent with the idea that a Gq-coupled opsin (OPN4) might also promote thermotaxis. The findings on sperm thermotaxis (Pérez-Cerezales et al 2015), in combination with work on rheotaxis (Miki & Clapham 2013), suggest that two mechanisms (thermotaxis and rheotaxis) facilitate long-range navigation in the female genital tract. However, because temperature affects fluid flow, it is important to exclude that this force impacts on the observed thermotaxis of sperm.
CONCLUDING REMARKS
Archetypal role for opsins
The observations that opsins are not just photodetectors, but have light-independent roles in thermosensation and hearing raise the question of their primordial function. We posit it was not phototransduction. Both temperature sensation and touch are primitive senses that most likely pre-date light sensation, and in principle, they only require one component—the opsin. However, the opsins that function in fly thermosensation and audition require the chromophore, which was likely co-opted to serve as a molecular chaperone. Thus, the light-independent roles for opsins may have arisen prior to light sensation.
Unifying roles for opsins and future perspective
Two central functions of rhodopsin-dependent signaling are signal amplification and adaptation (Stryer 1986). These two key roles may also be employed in other sensory contexts. An amplification cascade would allow low levels of a stimulus to be detected. However, if the stimulus cannot be avoided but is present at low concentrations that are not dangerous, then the animal adapts.
The repertoire of senses that depend on opsins is likely to be greater than currently recognized. Do opsins have roles in chemosensation, including taste and smell? Are there additional mechanosensory roles for opsins, such as light touch, fine coordination (proprioception), or food texture sensation? Nevertheless, what it is already clear is that opsins are not just for light sensation. They represent a broad class of polymodal sensory receptor, which until recently has eluded discovery despite many decades of intense scrutiny. We hope this review will motivate studies to unravel the light-independent sensory roles for opsins in a wide variety of animals, including mammals.
Acknowledgments
The work in the CM laboratory is supported by the NIAID, NIDCD and the NEI. NL is supported in part by a Ruth L. Kirschstein Predoctoral Fellowship from the NEI.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- Albert JT, Göpfert MC. Hearing in Drosophila. Curr Opin Neurobiol. 2015;34c:79–85. doi: 10.1016/j.conb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–24. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern M, Pugh EN., Jr The density and photosensitivity of human rhodopsin in the living retina. J Physiol. 1974;237:341–70. doi: 10.1113/jphysiol.1974.sp010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimus CM, Güler AD, Villa KL, McNeill DS, Legates TA, Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–71. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- Atkinson CL, Feng J, Zhang DQ. Functional integrity and modification of retinal dopaminergic neurons in the rd 1 mutant mouse: roles of melanopsin and GABA. J Neurophysiol. 2013;109:1589–99. doi: 10.1152/jn.00786.2012. [DOI] [PubMed] [Google Scholar]
- Bahat A, Caplan SR, Eisenbach M. Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PloS one. 2012;7:e41915. doi: 10.1371/journal.pone.0041915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahat A, Eisenbach M. Human sperm thermotaxis is mediated by phospholipase C and inositol trisphosphate receptor Ca2+ channel. Biol Reprod. 2010;82:606–16. doi: 10.1095/biolreprod.109.080127. [DOI] [PubMed] [Google Scholar]
- Bahat A, Tur-Kaspa I, Gakamsky A, Giojalas LC, Breitbart H, Eisenbach M. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat Med. 2003;9:149–50. doi: 10.1038/nm0203-149. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Cassone VM. Opsin photoisomerases in the chick retina and pineal gland: characterization, localization, and circadian regulation. Invest Ophthalmol Vis Sci. 2004;45:769–75. doi: 10.1167/iovs.03-1125. [DOI] [PubMed] [Google Scholar]
- Baldwin JM, Schertler GF, Unger VM. An alpha-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J Mol Biol. 1997;272:144–64. doi: 10.1006/jmbi.1997.1240. [DOI] [PubMed] [Google Scholar]
- Ban E, Kasai A, Sato M, Yokozeki A, Hisatomi O, Oshima N. The signaling pathway in photoresponses that may be mediated by visual pigments in erythrophores of Nile tilapia. Pigment Cell Res. 2005;18:360–9. doi: 10.1111/j.1600-0749.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Barlow HB. The thermal limit to seeing. Nature. 1988;334:296–97. doi: 10.1038/334296a0. [DOI] [PubMed] [Google Scholar]
- Barlow RB, Birge RR, Kaplan E, Tallent JR. On the molecular origin of photoreceptor noise. Nature. 1993;366:64–6. doi: 10.1038/366064a0. [DOI] [PubMed] [Google Scholar]
- Bellono NW, Najera JA, Oancea E. UV light activates a Gαq/11-coupled phototransduction pathway in human melanocytes. J Gen Physiol. 2014;143:203–14. doi: 10.1085/jgp.201311094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J. Le rôle des yeux dans l’action stimulante de la lumière sur le developpement testiculaire chez le canard. CR Soc Biol. 1935a;118:669–71. [Google Scholar]
- Benoit J. Stimulation par la lumière artificielle du développement testiculaire chez des canards aveuglés par section du nerf optique. CR Soc Biol. 1935b;120:133–36. [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Snyder SH. Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19:3681–90. doi: 10.1523/JNEUROSCI.19-10-03681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–33. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Boll F. On the anatomy and physiology of the retina. Vision Res. 1877a;17:1249–65. doi: 10.1016/0042-6989(77)90112-2. [DOI] [PubMed] [Google Scholar]
- Boll F. Zur anatomie und physiologie der retina. Arch Anat Physiol. 1877b:1252.
- Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, et al. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem. 2005;92:158–70. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- David A, Vilensky A, Nathan H. Temperature changes in the different parts of the rabbit’s oviduct. Int J Gynaecol Obstet. 1972;10:52–56. [Google Scholar]
- Davies A, Schertler GF, Gowen BE, Saibil HR. Projection structure of an invertebrate rhodopsin. J Struct Biol. 1996;117:36–44. doi: 10.1006/jsbi.1996.0067. [DOI] [PubMed] [Google Scholar]
- Davies WI, Tamai TK, Zheng L, Fu JK, Rihel J, et al. An extended family of novel vertebrate photopigments is widely expressed and displays a diversity of function. Genome Res. 2015;25:1666–79. doi: 10.1101/gr.189886.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz NM, Morera LP, Guido ME. Melanopsin and the non-visual photochemistry in the inner retina of vertebrates. Photochem Photobiol. 2016;92:29–44. doi: 10.1111/php.12545. [DOI] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–81. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin RM. Evolution of photoreceptors. Cold Spring Harb Symp Quant Biol. 1965;30:363–70. doi: 10.1101/sqb.1965.030.01.036. [DOI] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach M, Giojalas LC. Sperm guidance in mammals - an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–85. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Hall JC, Rosbash M. A unique circadian-rhythm photoreceptor. Nature. 2000a;404:456–7. doi: 10.1038/35006558. [DOI] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Helfrich-Forster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000b;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Esquiva G, Avivi A, Hannibal J. Non-image forming light detection by melanopsin, rhodopsin, and long-middlewave (L/W) cone opsin in the subterranean blind mole rat, Spalax Ehrenbergi: immunohistochemical characterization, distribution, and connectivity. Front Neuroanat. 2016;10:61. doi: 10.3389/fnana.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–51. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Fernandes AM, Fero K, Arrenberg AB, Bergeron SA, Driever W, Burgess HA. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr Biol. 2012;22:2042–7. doi: 10.1016/j.cub.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuda R, Hamilton SC, McInerney JO, Pisani D. Metazoan opsin evolution reveals a simple route to animal vision. Proc Natl Acad Sci USA. 2012;109:18868–72. doi: 10.1073/pnas.1204609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuda R, Rota-Stabelli O, Oakley TH, Pisani D. The comb jelly opsins and the origins of animal phototransduction. Genome Biol Evol. 2014;6:1964–71. doi: 10.1093/gbe/evu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RM, Fontinha BM, Kirchmaier S, Steger J, Bloch S, et al. Co-expression of VAL- and TMT-opsins uncovers ancient photosensory interneurons and motorneurons in the vertebrate brain. PLoS Biol. 2013;11:e1001585. doi: 10.1371/journal.pbio.1001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flyktman A, Mänttäri S, Nissilä J, Timonen M, Saarela S. Transcranial light affects plasma monoamine levels and expression of brain encephalopsin in the mouse. J Exp Biol. 2015;218:1521–6. doi: 10.1242/jeb.111864. [DOI] [PubMed] [Google Scholar]
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol, A. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci. 2013;92:394–403. doi: 10.1016/j.lfs.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–4. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Friedmann D, Hoagland A, Berlin S, Isacoff EY. A spinal opsin controls early neural activity and drives a behavioral light response. Curr Biol. 2015;25:69–74. doi: 10.1016/j.cub.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda N, Yomogida K, Okabe M, Touhara K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J Cell Sci. 2004;117:5835–45. doi: 10.1242/jcs.01507. [DOI] [PubMed] [Google Scholar]
- Fung BK, Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci USA. 1980;77:2500–4. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Sleator WJ, Mccaman MW, Elchlepp J. Relaxation of arterial strips by light and the influence of drugs on this photodynamic effect. J Pharmacol Exp Ther. 1955;113:22. [Google Scholar]
- García-Fernández JM, Cernuda-Cernuda R, Davies WI, Rodgers J, Turton M, et al. The hypothalamic photoreceptors regulating seasonal reproduction in birds: A prime role for VA opsin. Front Neuroendocrinol. 2015;37:13–28. doi: 10.1016/j.yfrne.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Godchaux W, 3rd, Zimmerman WF. Membrane-dependent guanine nucleotide binding and GTPase activities of soluble protein from bovine rod cell outer segments. J Biol Chem. 1979;254:7874–84. [PubMed] [Google Scholar]
- Gotow T, Nishi T. Involvement of a Go-type G-protein coupled to guanylate cyclase in the phototransduction cGMP cascade of molluscan simple photoreceptors. Brain Res. 2007;1144:42–51. doi: 10.1016/j.brainres.2007.01.068. [DOI] [PubMed] [Google Scholar]
- Gu Y, Oberwinkler J, Postma M, Hardie RC. Mechanisms of light adaptation in Drosophila photoreceptors. Curr Biol. 2005;15:1228–34. doi: 10.1016/j.cub.2005.05.058. [DOI] [PubMed] [Google Scholar]
- Halford S, Pires SS, Turton M, Zheng L, González-Menéndez I, et al. VA opsin-based photoreceptors in the hypothalamus of birds. Curr Biol. 2009;19:1396–402. doi: 10.1016/j.cub.2009.06.066. [DOI] [PubMed] [Google Scholar]
- Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol. 2015;91:117–23. doi: 10.1111/php.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang CY, Kitahashi T, Parhar IS. Neuronal organization of deep brain opsin photoreceptors in adult teleosts. Front Neuroanat. 2016a;10:48. doi: 10.3389/fnana.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang CY, Moriya S, Ogawa S, Parhar IS. Deep brain photoreceptor (val-opsin) gene knockout using CRISPR/Cas affects chorion formation and embryonic hatching in the zebrafish. PloS one. 2016b;11:e0165535. doi: 10.1371/journal.pone.0165535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science. 2012;338:260–3. doi: 10.1126/science.1222376. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Martin F, Cochrane GW, Juusola M, Georgiev P, Raghu P. Molecular basis of amplification in Drosophila phototransduction: roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron. 2002;36:689–701. doi: 10.1016/s0896-6273(02)01048-6. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–51. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hargrave PA, McDowell JH, Curtis DR, Wang JK, Juszczak E, et al. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9:235–44. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann H. Rhodopsin is the predominant protein of rod outer segment membranes. Nat New Biol. 1972;235:114. doi: 10.1038/newbio235114a0. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–61. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Herzyk P, Hubbard RE. Combined biophysical and biochemical information confirms arrangement of transmembrane helices visible from the three-dimensional map of frog rhodopsin. J Mol Biol. 1998;281:741–54. doi: 10.1006/jmbi.1998.1981. [DOI] [PubMed] [Google Scholar]
- Hubbard J, Ruppert E, Gropp CM, Bourgin P. Non-circadian direct effects of light on sleep and alertness: Lessons from transgenic mouse models. Sleep Med Rev. 2013;17:445–52. doi: 10.1016/j.smrv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Hughes S, Jagannath A, Rodgers J, Hankins MW, Peirson SN, Foster RG. Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye. 2016;30:247–54. doi: 10.1038/eye.2015.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Kamikouchi A. Auditory system of fruit flies. Hear Res. 2016;338:1–8. doi: 10.1016/j.heares.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Jiang M, Pandey S, Fong HK. An opsin homologue in the retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34:3669–78. [PubMed] [Google Scholar]
- Johnson J, Wu V, Donovan M, Majumdar S, Rentería RC, et al. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci USA. 2010;107:17374–8. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–84. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Son ED, Jung JY, Choi H, Lee TR, Shin DW. Violet light down-regulates the expression of specific differentiation markers through Rhodopsin in normal human epidermal keratinocytes. PloS one. 2013;8:e73678. doi: 10.1371/journal.pone.0073678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AC, Cronin TW. Diverse distributions of extraocular opsins in crustaceans, cephalopods, and fish. Integr Comp Biol. 2016 doi: 10.1093/icb/icw022. [DOI] [PubMed] [Google Scholar]
- Kingston AC, Kuzirian AM, Hanlon RT, Cronin TW. Visual phototransduction components in cephalopod chromatophores suggest dermal photoreception. J Exp Biol. 2015;218:1596–602. doi: 10.1242/jeb.117945. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A, Malpel S, Michard-Vanhée C, Picot M, Chélot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–77. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Afonso B, Vonner AJ, Hernandez-Nunez L, Berck M, et al. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc Natl Acad Sci USA. 2015;112:E220–9. doi: 10.1073/pnas.1416212112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima D, Mori S, Torii M, Wada A, Morishita R, Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PloS one. 2011;6:e26388. doi: 10.1371/journal.pone.0026388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima D, Terakita A, Ishikawa T, Tsukahara Y, Maeda A, Shichida Y. A novel Go-mediated phototransduction cascade in scallop visual cells. J Biol Chem. 1997;272:22979–82. doi: 10.1074/jbc.272.37.22979. [DOI] [PubMed] [Google Scholar]
- Kojima D, Torii M, Fukada Y, Dowling JE. Differential expression of duplicated VAL-opsin genes in the developing zebrafish. J Neurochem. 2008;104:1364–71. doi: 10.1111/j.1471-4159.2007.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Kawano E, Kinugawa Y, Oishi T, Shichida Y, et al. Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci USA. 2004;101:6687–91. doi: 10.1073/pnas.0400819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs A, Villa C, Edwards PC, Schertler GF. Characterisation of an improved two-dimensional p22121 crystal from bovine rhodopsin. J Mol Biol. 1998;282:991–1003. doi: 10.1006/jmbi.1998.2070. [DOI] [PubMed] [Google Scholar]
- Kühne W. On the stable colours of the retina. J Physiol. 1878;1:109–2125. doi: 10.1113/jphysiol.1878.sp000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 2008;11:871–73. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- Lucas RJ. Mammalian inner retinal photoreception. Curr Biol. 2013;23:R125–33. doi: 10.1016/j.cub.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–7. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–7. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Luo J, Shen WL, Montell C. TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat Neurosci. 2017;20:34–41. doi: 10.1038/nn.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–73. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- Mäthger LM, Roberts SB, Hanlon RT. Evidence for distributed light sensing in the skin of cuttlefish, Sepia officinalis. Biol Lett. 2010;6:600–3. doi: 10.1098/rsbl.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matynia A. Blurring the boundaries of vision: novel functions of intrinsically photosensitive retinal ganglion cells. J Exp Neurosci. 2013;7:43–50. doi: 10.4137/JEN.S11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealey-Ferrara ML, Montalvo AG, Hall JC. Effects of combining a cryptochrome mutation with other visual-system variants on entrainment of locomotor and adult-emergence rhythms in Drosophila. J Neurogenet. 2003;17:171–221. [PubMed] [Google Scholar]
- Miki K, Clapham DE. Rheotaxis guides mammalian sperm. Curr Biol. 2013;23:443–52. doi: 10.1016/j.cub.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic N, Cehajic-Kapetanovic J, Procyk CA, Lucas RJ. Chemogenetic activation of melanopsin retinal ganglion cells induces signatures of arousal and/or anxiety in mice. Curr Biol. 2016;26:2358–63. doi: 10.1016/j.cub.2016.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Drosophila visual transduction. Trends Neurosci. 2012;35:356–63. doi: 10.1016/j.tins.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–23. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Nathans J, Hogness DS. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983;34:807–14. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Zucker I. Absence of extraocular photoreception in diurnal and nocturnal rodents exposed to direct sunlight. Comp Biochem Physiol A Comp Physiol. 1981;69:145–48. [Google Scholar]
- Ni JD, Baik LS, Holmes TC, Montell C. A rhodopsin in central pacemaker neurons functions in circadian photoentrainment in Drosophila. Nature. 2017 doi: 10.1038/nature22325. (provisionally accepted pending minor revisions) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissilä J, Manttari S, Särkioja T, Tuominen H, Takala T, et al. Encephalopsin (OPN3) protein abundance in the adult mouse brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198:833–9. doi: 10.1007/s00359-012-0754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature. 1994;372:94–7. doi: 10.1038/372094a0. [DOI] [PubMed] [Google Scholar]
- Oliver J, Herbute S, Bayle JD. Testicular response to photostimulation by radioluminous implants in the deafferented hypothalamus of quail. J Physiol (Paris) 1977;73:685–91. [PubMed] [Google Scholar]
- Ovchinnikov Iu A, Abdulaev NG, Feigina M, Artamonov ID, Bogachuk AS. Visual rhodopsin. III Complete amino acid sequence and topography in a membrane. Bioorg Khim. 1983;9:1331–40. [PubMed] [Google Scholar]
- Ozaki K, Nagatani H, Ozaki M, Tokunaga F. Maturation of major Drosophila rhodopsin, ninaE, requires chromophore 3-hydroxyretinal. Neuron. 1993;10:1113–9. doi: 10.1016/0896-6273(93)90059-z. [DOI] [PubMed] [Google Scholar]
- Ozturk N, Selby CP, Annayev Y, Zhong D, Sancar A. Reaction mechanism of Drosophila cryptochrome. Proc Natl Acad Sci USA. 2011;108:516–21. doi: 10.1073/pnas.1017093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–6. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Dreyer WJ. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974;13:2438–44. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res. 2010;106:833–41. doi: 10.1161/CIRCRESAHA.109.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen R, Schwemer J. Biogenesis of blowfly photoreceptor membranes is regulated by 11-ci s-retinal. Eur J Biochem. 1983;137:609–14. doi: 10.1111/j.1432-1033.1983.tb07869.x. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Halford S, Foster RG. The evolution of irradiance detection: melanopsin and the non-visual opsins. Philos Trans R Soc Lond, B, Biol Sci. 2009;364:2849–65. doi: 10.1098/rstb.2009.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cerezales S, Boryshpolets S, Afanzar O, Brandis A, Nevo R, et al. Involvement of opsins in mammalian sperm thermotaxis. Sci Rep. 2015;5:16146. doi: 10.1038/srep16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N, Helfrich-Förster C. Setting the clock - by nature: Circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011;585:1435–42. doi: 10.1016/j.febslet.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Pilorz V, Tam SK, Hughes S, Pothecary CA, Jagannath A, et al. Melanopsin regulates both sleep-promoting and arousal-promoting responses to light. PLoS Biol. 2016;14:e1002482. doi: 10.1371/journal.pbio.1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poff KL, Skokut M. Thermotaxis by pseudoplasmodia of Dictyostelium discoideum. Proc Natl Acad Sci USA. 1977;74:2007–10. doi: 10.1073/pnas.74.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, et al. Shedding new light on opsin evolution. Proc Biol Sci. 2012;279:3–14. doi: 10.1098/rspb.2011.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Cooper HM, Foster RG. Retinal projections in mice with inherited retinal degeneration: implications for circadian photoentrainment. J Comp Neurol. 1998a;395:417–39. doi: 10.1002/(sici)1096-9861(19980615)395:4<417::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998b;95:340–5. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Wong S, Lederman AB, Argamaso SM, Foster RG. Visual and circadian responses to light in aged retinally degenerate mice. Vision Res. 1994;34:1799–806. doi: 10.1016/0042-6989(94)90304-2. [DOI] [PubMed] [Google Scholar]
- Radu RA, Hu J, Peng J, Bok D, Mata NL, Travis GH. Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells. J Biol Chem. 2008;283:19730–8. doi: 10.1074/jbc.M801288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MD, Oakley TH. Eye-independent, light-activated chromatophore expansion (LACE) and expression of phototransduction genes in the skin of Octopus bimaculoides. J Exp Biol. 2015;218:1513–20. doi: 10.1242/jeb.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MD, Pairett AN, Pankey MS, Serb JM, Speiser DI, et al. The last common ancestor of most bilaterian animals possessed at least 9 opsins. Genome Biol Evol. 2016 doi: 10.1093/gbe/evw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Chun C, Fan J, Kofron JM, Yang MB, et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–6. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Li BY, et al. All spiking, sustained ON displaced amacrine cells receive gap-junction input from melanopsin ganglion cells. Curr Biol. 2015;25:2763–73. doi: 10.1016/j.cub.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Rieger D, Stanewsky R, Helfrich-Forster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms. 2003;18:377–91. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Strop M, Jerdan J. Response of the infant rat to light prior to eyelid opening: mediation by the superior colliculus. Dev Psychobiol. 1978;11:469–78. doi: 10.1002/dev.420110510. [DOI] [PubMed] [Google Scholar]
- Saint-Charles A, Michard-Vanhée C, Alejevski F, Chélot E, Boivin A, Rouyer F. Four of the six Drosophila rhodopsin-expressing photoreceptors can mediate circadian entrainment in low light. J Comp Neurol. 2016 doi: 10.1002/cne.23994. [DOI] [PubMed] [Google Scholar]
- Schertler GF, Hargrave PA. Projection structure of frog rhodopsin in two crystal forms. Proc Natl Acad Sci USA. 1995;92:11578–82. doi: 10.1073/pnas.92.25.11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–80. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semo M, Gias C, Ahmado A, Sugano E, Allen AE, et al. Dissecting a role for melanopsin in behavioural light aversion reveals a response independent of conventional photoreception. PloS one. 2010;5:e15009. doi: 10.1371/journal.pone.0015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilan PR, Piepenbrock D, Ovezmyradov G, Nadrowski B, Bechstedt S, et al. Drosophila auditory organ genes and genetic hearing defects. Cell. 2012;150:1042–54. doi: 10.1016/j.cell.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–6. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- Shieh T, Han M, Sakmar TP, Smith SO. The steric trigger in rhodopsin activation. J Mol Biol. 1997;269:373–84. doi: 10.1006/jmbi.1997.1035. [DOI] [PubMed] [Google Scholar]
- Sikka G, Hussmann GP, Pandey D, Cao S, Hori D, et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc Natl Acad Sci USA. 2014 doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe T, Chen HS, Luo J, Montell C. A switch in thermal preference in Drosophila larvae depends on multiple rhodopsins. Cell Rep. 2016;17:336–44. doi: 10.1016/j.celrep.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Postma M, Billings SA, Coca D, Hardie RC, Juusola M. Stochastic, adaptive sampling of information by microvilli in fly photoreceptors. Curr Biol. 2012;22:1371–80. doi: 10.1016/j.cub.2012.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–8. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–92. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Sun L, Perakyla J, Kovalainen A, Ogawa KH, Karhunen PJ, Hartikainen KM. Human brain reacts to transcranial extraocular light. PloS one. 2016;11:e0149525. doi: 10.1371/journal.pone.0149525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surbhi VK. Avian photoreceptors and their role in the regulation of daily and seasonal physiology. Gen Comp Endocrinol. 2015;220:13–22. doi: 10.1016/j.ygcen.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Szular J, Sehadova H, Gentile C, Szabo G, Chou WH, et al. Rhodopsin 5- and Rhodopsin 6-mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-β signaling. J Biol Rhythms. 2012;27:25–36. doi: 10.1177/0748730411431673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–6. doi: 10.1016/s0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- Toh PP, Yap AM, Sriram G, Bigliardi-Qi M, Bigliardi P. Expression of peropsin in human skin is related to phototransduction of violet light in keratinocytes. Exp Dermatol. 2016 doi: 10.1111/exd.13226. [DOI] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, et al. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in opn4−/− mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Ikeyama K, Denda S, Nakanishi J, Fuziwara S, et al. Expressions of rod and cone photoreceptor-like proteins in human epidermis. Exp Dermatol. 2009;18:567–70. doi: 10.1111/j.1600-0625.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- Unger VM, Hargrave PA, Baldwin JM, Schertler GF. Arrangement of rhodopsin transmembrane α-helices. Nature. 1997;389:203–6. doi: 10.1038/38316. [DOI] [PubMed] [Google Scholar]
- van Veen T, Hartwig HG, Mueller K. Light-dependent motor activity and photonegative behavior in the eel (Anguilla anguilla L.): evidence for extraretinal and extrapineal photoreception. J Comp Physiol. 1976;111:209–19. [Google Scholar]
- Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–38. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Viggiani E, Ciesla M, Russo OL. The shielding power of the rat skull to visible light. Experientia. 1970;26:850–1. doi: 10.1007/BF02114217. [DOI] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–23. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- von Frisch K. Beiträge zur physiologie der pigmentzellen in der fischhaut. Pflügers Arch. 1911;138:319–87. [Google Scholar]
- Wade PD, Taylor J, Siekevitz P. Mammalian cerebral cortical tissue responds to low-intensity visible light. Proc Natl Acad Sci USA. 1988;85:9322–6. doi: 10.1073/pnas.85.23.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. Pigments of the retina: I. the bull frog. J Gen Physiol. 1936;19:781–95. doi: 10.1085/jgp.19.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. On rhodopsin in solution. J Gen Physiol. 1938;21:795–832. doi: 10.1085/jgp.21.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968;162:230–9. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- White JH, Chiano M, Wigglesworth M, Geske R, Riley J, et al. Identification of a novel asthma susceptibility gene on chromosome 1qter and its functional evaluation. Hum Mol Genet. 2008;17:1890–903. doi: 10.1093/hmg/ddn087. [DOI] [PubMed] [Google Scholar]
- Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol. 2011;21:1906–11. doi: 10.1016/j.cub.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Redmond TM, Nickerson JM. A history of the classical cisual cycle. Prog Mol Biol Transl Sci. 2015;134:433–48. doi: 10.1016/bs.pmbts.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–64. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, Matthews G, Baylor DA. Thermal activation of the visual transduction mechanism in retinal rods. Nature. 1979;279:806–7. doi: 10.1038/279806a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Oksche A, Darden TR, Farner DS. The sites of encephalic photoreception in phosoperiodic induction of the growth of the testes in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Cell Tissue Res. 1978;189:441–67. doi: 10.1007/BF00209132. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci USA. 2008;105:14181–6. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, et al. Thermosensory and non-thermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat sensor domains of a thermoTRP channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwas F, Alpern M. The density of human rhodopsin in the rods. Vision Res. 1976;16:121–7. doi: 10.1016/0042-6989(76)90088-2. [DOI] [PubMed] [Google Scholar]