Summary
Air pollution is composed of particulate matter (PM) and gaseous pollutants, such as nitrogen dioxide and ozone. PM is classified according to size into coarse particles (PM10), fine particles (PM2.5) and ultrafine particles. We aim to provide an original review of the scientific evidence from epidemiological and experimental studies examining the cardiovascular effects of outdoor air pollution. Pooled epidemiological studies reported that a 10 μg/m3 increase in long-term exposure to PM2.5 was associated with an 11% increase in cardiovascular mortality. Increased cardiovascular mortality was also related to long-term and short-term exposure to nitrogen dioxide. Exposure to air pollution and road traffic was associated with an increased risk of arteriosclerosis, as shown by premature aortic and coronary calcification. Short-term increases in air pollution were associated with an increased risk of myocardial infarction, stroke and acute heart failure. The risk was increased even when pollutant concentrations were below European standards. Reinforcing the evidence from epidemiological studies, numerous experimental studies demonstrated that air pollution promotes a systemic vascular oxidative stress reaction. Radical oxygen species induce endothelial dysfunction, monocyte activation and some proatherogenic changes in lipoproteins, which initiate plaque formation. Furthermore, air pollution favours thrombus formation, because of an increase in coagulation factors and platelet activation. Experimental studies also indicate that some pollutants have more harmful cardiovascular effects, such as combustion-derived PM2.5 and ultrafine particles. Air pollution is a major contributor to cardiovascular diseases. Promotion of safer air quality appears to be a new challenge in cardiovascular disease prevention.
Keywords: Air pollution, Cardiovascular mortality, Particulate matter, Myocardial infarction, Oxidative stress
Background
Air pollution is a major public health issue, leading to millions of premature deaths worldwide. Cardiovascular diseases account for 60–80% of air pollution-related deaths [1]. In line with the campaign launched in 2015 by the European Society of Cardiology, which aims to “raise awareness of the detrimental effects that the environment can have on the heart”, we performed a review of publications reporting on the cardiovascular effects of air pollution. Although both indoor and outdoor air pollution exposure have a strong impact on cardiovascular diseases, we have focused our review on outdoor air pollution. We first introduce some basics on air pollution definition and sources, which are needed to present evidence arising from long-and short-term epidemiological studies. We then present some pathophysiological evidence, and propose a schematic overview of mechanistic pathways linking air pollution exposure to clinical events. We also present recent data on the enhancing effects of mitigation measures, and some future directions to overcome the limitations in environmental research.
Air pollution basics
General definition
Air pollution is composed of particulate matter (PM) and gaseous components. PM is classified as coarse particles (diameter < 10 μm, ≥ 2.5 μm), fine particles (diameter < 2.5 μm, ≥ 0.1 μm) and ultrafine particles (nanoparticles, diameter < 0.1 μm). PM has a different composition depending on its source. Carbonaceous particles are derived from combustion sources, such as traffic emission or residential heating, while inorganic particles are represented by, for example, desert dust and mineral dust from agriculture. Carbonaceous particles are carbon based, but carry on their surface an amount of organic chemicals, such as polycyclic aromatic hydrocarbons and reactive metals [2]. Gaseous pollutants are nitrogen oxides including nitrogen dioxide (NO2) and nitric oxide (NO), ozone, sulphur dioxide (SO2), volatile organic compounds and carbon monoxide (CO). Besides their own toxicity, SO2 and nitrogen oxides also contribute to particle formation through complex atmospheric photochemical reactions involving ammonia from agriculture. As they result from gaseous transformation, these particles are called secondary particles, and are essentially composed of inorganic compounds, such as ammonia, sulphates and nitrates. Ozone is a secondary gaseous pollutant, formed through a photochemical reaction involving sunlight and gaseous precursors such as nitrogen oxides or volatile organic compounds.
Outdoor air pollution sources
In Europe, agriculture is a major source of PM2.5. Nevertheless, particles from agriculture are mainly inorganic particles, which are usually considered to be less toxic than carbonaceous particles from combustion sources, such as road traffic. Assuming this difference in toxicity, road traffic and residential heating have the largest impact on outdoor air pollution-related mortality in Europe [1]. In North America, industry and power generation using fossil fuels are also important sources of PM. In Africa, natural sources, such as desert dust and biomass burning (natural or man-made fires), contribute largely to ambient air pollution concentrations. In Asia, residential heating and cooking are the main sources of particles in both outdoor and indoor emissions [1]. Besides the differences between countries and continents, strong differences exist between the main sources of pollutants within the same country, depending on local sources. In large cities, road traffic is a major contributor to global pollutant emissions, and is also the main source of NO2, arising mainly from diesel vehicles. In Paris, for example, road traffic accounts for 30% of PM emissions, as much as the residential sector, and nearly 60% of emissions of nitrogen oxides [3]. SO2 mainly arises from industrial emissions and maritime transport; however, its contribution to air pollution is decreasing over the years.
Indoor air pollution
In 2010, air pollution led to 7 million premature deaths worldwide, with similar contributions made by indoor and outdoor air pollution [1]. However, indoor air pollution is more heterogeneous, with wide variations in pollutants and sources between countries. Worldwide, second-hand smoke is a major source of indoor air pollution. In Asia, cooking and heating with solid fuels are the main sources of indoor air pollution, whereas in Europe, several sources contribute to indoor pollution, such as volatile organic compounds from organic solvents, household products and PM from cooking and wood burning. Nevertheless, a recent European study demonstrated that 60% of the global burden of indoor air pollution-related disease comes from outdoor PM2.5 penetrating inside via air exchange [4]. Consequently, decreasing the burden of disease from indoor air pollution requires measures that affect indoor air pollution sources, ventilation and filtration of outdoor air [4].
Epidemiological evidence
Long-term exposure studies have investigated the cardiovascular effects of air pollution after annual variations in pollutant concentration, whereas short-term exposure studies have focused mainly on daily or hourly variations in pollutant concentrations. Most of the studies extrapolated the level of individual exposure to air pollution, taking into account background pollutant concentrations or road traffic exposure at the residential address.
Air pollution and cardiovascular mortality
Long-term exposure
A 2013 meta-analysis found that an average increase of 11% in cardiovascular mortality was associated with a 10 μg/m3 increase in annual PM2.5 concentration [5]. The strongest associations were observed for coronary artery disease-associated mortality [6], and were persistent even after adjustment for cardiovascular factors and socioeconomic status. However, PM2.5 adverse effects were higher for individuals with the lowest education in some studies, probably related to low antioxidant intake because of low fruit intake. Fine and ultrafine particles had the most important impact on cardiovascular mortality, compared with coarse particles. Furthermore, the composition of PM is an important issue to consider, with some findings showing higher cardiovascular toxicity of carbonaceous particles from combustion-derived sources, such as road traffic, fossil fuels and wood burning [7]. Combustion sources are also the leading source of NO2. A meta-analysis of cardiovascular effects of long-term exposure to NO2 reported a 13% increase in cardiovascular mortality after a 10 μg/m3 increase in annual NO2 concentrations [8]. The effect of ozone appears to be less pronounced, with some long-term exposure studies showing a small increase in cardiopulmonary causes of death, but this was only observed during the warm season, and not in a yearly-based analysis. This may be explained by the fact that, unlike NO2, ozone pollution typically occurs during warm and sunny days, because its formation requires photochemical reactions involving sunlight. Long-term exposure to SO2 has been strongly associated with respiratory mortality, although its impact on cardiovascular mortality remains unclear [9].
Independent of air pollutant background concentration, long-term exposure to road traffic was strongly linked to cardiovascular mortality [10]. In a large-scale prospective study in women, living within 50 m of a major roadway increased the risk of sudden cardiac death by 38% compared with living ≥ 500 m away, and this effect remained, even after adjusting for potential confounders and cardiovascular risk factors [11].
Short-term exposure
In a recent meta-analysis, a daily increment of 10 μg/m3 in PM2.5 exposure was associated with an increase in cardiovascular mortality of 0.84% [12]. A study examining short-term variations in NO2 reported an increase in cardiovascular mortality of 0.4–0.88% for a 10 μg/m3 daily increase in NO2 [13]. Analysis of the effects of short-term variations in ozone on cardiovascular mortality remains controversial [14].
Air pollution and coronary artery disease
Long-term exposure
Several studies reported a strong association between long-term exposure to air pollution and acute myocardial infarction. In a large-scale prospective European study, annual increases of 10 μg/m3 in PM10 and 5 μg/m3 in PM2.5 were associated with increased risks of myocardial infarction of 12% and 13%, respectively [15]. Above all, these positive associations were observed despite air pollutant concentrations being below the current European policy recommended thresholds. Several studies indicated that long-term exposure to PM2.5 promotes premature arteriosclerosis. In a prospective cohort study, exposure to PM2.5 and traffic-related air pollution were significantly associated with an increased coronary artery calcium score [16]. A similar observation was made with road traffic exposure, with respective increased risks of a high coronary artery calcium score of 63% and 34% for people living within 50 m and 50–100 m from a major road, compared with people living 200 m away [17].
Short-term exposure
A short-term increase in air pollution has been shown to be capable of triggering acute coronary syndromes [18], and the strongest associations were observed with concurrent-day or last 2 days’ mean air pollutant exposure [19]. In a meta-analysis reviewing triggers for non-fatal myocardial infarction, exposure to air pollution and road traffic were the most important factors at the population level, considering that a large number of people are exposed [20]. Regarding more specifically the risk of ST-segment elevation myocardial infarction, a recent study demonstrated that each 10 μg/m3 increase in PM2.5 during the 24 hours preceding the event was associated with an increased risk of 2.8%, whereas a similar increase in NO2 was associated with an increased risk of 5.1%. The risk related to PM appeared to be greater among the elderly, while younger patients appeared to be more susceptible to NO2 exposure [21]. A short-term increase in ozone was also associated with acute coronary events in middle-aged adults with no pre-existing cardiovascular diseases [22].
Air pollution and other cardiovascular outcomes
Arrhythmia and sudden death
Associations between air pollution and arrhythmia are still a matter of debate. However, in several studies, daily changes in PM2.5 or NO2 concentrations were associated with an increased risk of hospital admissions for arrhythmia [23]. Several studies reported associations between short- and long-term exposure to particulate air pollution and reduced heart rate variability, which is considered to be a marker of autonomic nervous system imbalance, and a risk factor for cardiovascular mortality [24]. Studies in patients with no cardiovascular diseases also reported some association between long-term exposure to PM and QT prolongation [25]. Associations between air pollution and malign arrhythmia were also reported by studies on patients with implantable defibrillators [26], and short-term variations in PM2.5 and ozone have been associated with out- of-hospital cardiac arrest [27].
Heart failure
A meta-analysis concluded that there was a positive association between short-term increases in PM and gaseous components (NO2, SO2, CO) and an increased risk of hospitalization or death from congestive heart failure, with the strongest associations on the day of exposure, and more persistent effects for PM2.5 [28].
Stroke
Data from the Global Burden of Diseases 2013, collected in 188 countries between 1990 and 2013, demonstrated that air pollution contributed to 29% of the burden of stroke [29]. Several studies found positive associations between long- and short-term exposure to air pollution and stroke incidence and mortality [30,31]. A prospective European study found a 19% increased risk of stroke associated with a 5 μg/m3 increase in annual PM2.5, with the strongest associations found among those who had never smoked. As previously described for myocardial infarction, the risk was still increased at pollution levels below current European air quality standards [32]. Exposure to road traffic also increased the risk of stroke, with strong associations with ischaemic stroke observed in subjects living within 75 m of a main road [33]. Hourly or daily changes in pollutant concentrations were also related to an increased risk of stroke and stroke mortality, with a strong association between ultrafine particles and stroke mortality [34]. Short-term increases in gaseous compounds, such as NO2, SO2 and CO, also increased the risk of stroke. Regarding ozone, some studies reported an increased risk of stroke after a short-term increase in ozone [35], while some investigations did not find any association [36].
Hypertension
Several studies have recently demonstrated that the risk of hypertension is increased by exposure to air pollution – most especially, traffic-related pollutants [37,38].
Association between air pollutants and biomarkers of oxidative stress and inflammation
Long-term exposure to PM2.5, especially exposure to the polycyclic aromatic hydrocarbons found at the surface of traffic-related PM, was associated with an increased level of 8-hydroxy-2′-deoxyguanosine, a stable and reliable biomarker of oxidative DNA damage, in exposed individuals such as street policemen, bus drivers and garage workers [39]. Similarly, the presence of etheno-DNA adducts were twice as high in diesel engine workers compared with the non-diesel-exposed workers [40]. Different studies reported an association between air pollution exposure and increased plasma concentrations of oxidized low-density lipoprotein, homocysteine, inflammatory markers (interleukin-1, interleukin-6, tumour necrosis factor-α, C-reactive protein) and fibrinogen [41]. More recently, increased concentrations of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 have been associated with air pollution exposure [42]. Exposure to air pollution may facilitate the expression of inflammatory genes, as indicated in studies that reported upregulation of antioxidant genes and a decrease in “global” DNA methylation [42].
Effects of mitigation manoeuvers
Tokyo introduced a diesel emission control ordinance in 2003 to improve air quality. This regulation resulted in a 44% decrease in PM from traffic between 2003 and 2012. During the same period, mortality rates in Tokyo were compared with those in Osaka, which introduced such a regulation later (in 2009). After adjustment for mortality rates in Osaka, global cardiovascular mortality in Tokyo decreased by 11%, driven by a 10% decrease in ischaemic heart disease mortality [43]. The effectiveness of strategies to eliminate or reduce particle exposure, such as particle traps or face-masks, remains under investigation. At first glance, the efficiency of a facemask as individual protection against particle exposure appears limited, given that ultrafine particles, which constitute the major part of traffic-related PM, are not stopped by facemasks. However, some studies on patients with coronary heart disease living in high-polluted areas reported that high-efficiency facemasks reduced the acute cardiovascular effects related to air pollution [44].
Current study limitations and future directions
In most of the epidemiological studies available, individual exposure assessment uses either a mean pollutant outdoor concentration recorded at a regional level or an extrapolated concentration at the individual address estimated from a concentration recorded at the nearest fixed monitoring station. Such an approach does not take into account time spent inside, and can generate some exposure misclassification. A better assessment of air pollutant exposure is required through the development of an individual exposure monitoring system or, as recently performed, the use of real-time modelling of air pollutant concentration [21]. Another study limitation is related to the underestimation of the contribution of fine and ultrafine particles in ambient air particle measurements. Indeed, actual standards for particle measurements only take into account the particulate mass, which is ideal for the measurements of coarse particles, such as PM10, but not for ultrafine particles, which have a negligible mass [45]. Furthermore, ultrafine particles account for more than 90% of particles emitted by road traffic. Thus, there is an urgent need for studies assessing population exposure, by taking into account the number of particles – and not only their mass – using, for example, particle counters, which can detect each particle whatever its size.
In addition to exposure assessment, other methodological issues include the procedures for case ascertainment (hospital- or population-based, exhaustiveness, misclassification of time of event onset results), the study design (case crossover, time series study) and the outcome evaluated (incidence, hospital admission, mortality, etc.). Moreover, studies are limited for the evaluation of the interaction between pollutants because of their high statistical correlation. Estimation of the joint effects of exposure to PM, gas and noise is challenging, and indicates a need for new multipollutant modelling.
Pathophysiological evidence
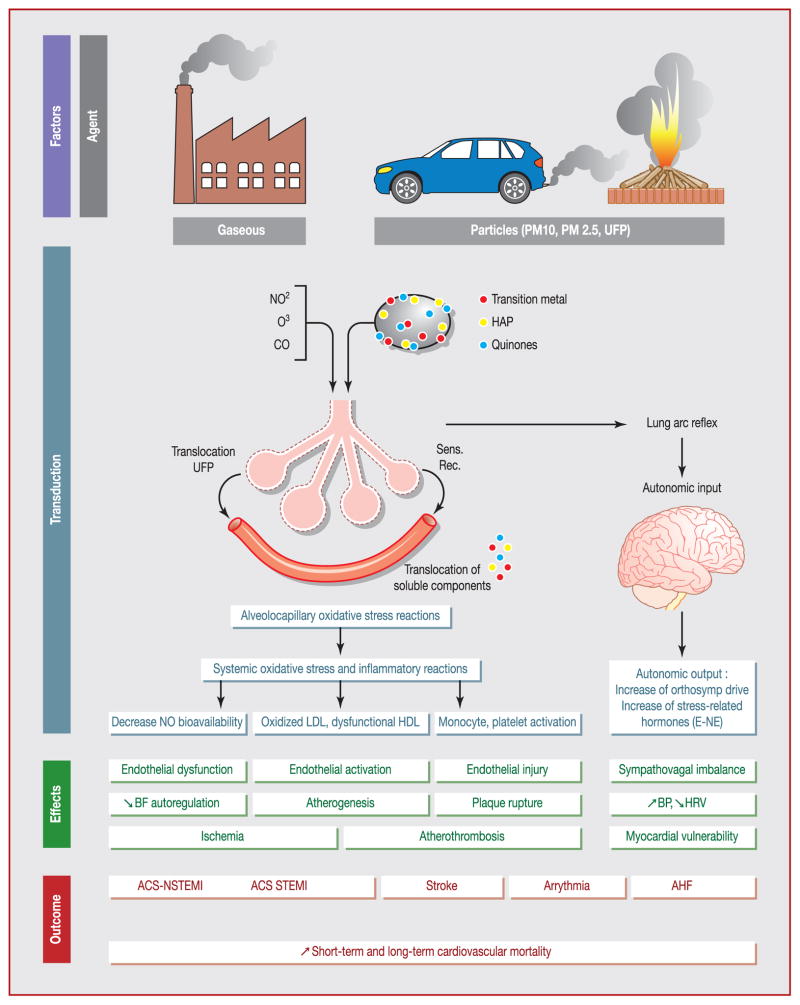
Environmental research on air pollution integrates results of numerous animal and human interventional studies. In humans, such studies consist mostly of randomized double-blind crossover studies performed in dedicated chambers, allowing full standardization of air pollutant exposure. In these exposure chambers, healthy volunteers, but also patients with stable coronary artery disease are usually exposed to controlled amounts of dilute diesel exhaust or filtered air, and perform exercise on a bicycle ergometer. The duration of exposure is generally limited to 1 or 2 hours, and the achieved concentrations of PM are close to those observed during a severe air pollution episode. Because of the previously described limitations of epidemiological studies, these studies reinforce the evidence of air pollution-mediated cardiovascular toxicity. Furthermore, these experimental designs describe the pathophysiological pathways involved in air pollution-related cardiovascular disease (Fig. 1).
Figure 1.
Translational physiopathological mechanism between air pollution exposure and cardiovascular events. ACS: acute coronary syndrome; AHF: acute heart failure; BF: blood flow; BP: blood pressure; CO: carbon monoxide; E: epinephrine; PAH: polycyclic aromatic hydrocarbons; HDL: high-density lipoprotein; HRV: heart rate variability; LDL: low-density lipoprotein; NE: norepinephrine; NO: nitric oxide; NO2: nitrogen dioxide; NSTEMI: non-ST-segment elevation myocardial infarction; O3: ozone; PM: particulate matter; Sens. Rec.: sensitivity receptor; STEMI: ST-segment elevation myocardial infarction; UFP: ultrafine particle.
From oxidative stress to endothelial dysfunction
Numerous studies have confirmed that air pollution exposure is followed by an intense oxidative stress reaction initiated by PM entering the lung. However, this lung oxidative reaction is amplified through stimulation of different enzymatic pathways, and finally leads to a systemic vascular oxidative stress reaction. A recent study demonstrated an acquired oxidative capacity of serum from people exposed to diesel exhaust. After incubating endothelial cell cultures with serum from subjects who were exposed to air pollution voluntarily, superoxide anion production (reactive oxygen species [ROS]) was observed, following a dose-response pattern directly related to the amount of inhaled PM2.5 [46]. The major role of ROS production was also supported by in vitro studies, which reported that superoxide dismutase reverses the adverse vascular effect following diesel exhaust exposure [47]. Several studies demonstrated that the oxidative stress reaction was mostly related to the surface compounds coating diesel particles, such as transition metals, polycyclic aromatic hydrocarbons and quinones [47]. Although oxidative stress is mainly related to particles, gaseous compounds, such as NO2, are also involved in ROS generation as peroxynitrite.
One final common pathway of endothelial oxidative stress is the decrease in the availability of NO, a key regulator of vascular tone. Exposure to diesel exhaust, compared with filtered air, led to impaired endothelium-dependent mediated vasodilatation and decreased endothelial NO bioavailability [48]. Endothelial dysfunction is considered an early marker of atherosclerosis [2,49] and some early functional consequences can be observed on different vascular beds. When patients with stable coronary heart disease were exposed to diesel exhaust, a decrease in ischaemic threshold was observed, suggesting an adverse influence of air pollution on myocardial blood flow regulation [50]. Acute exposure to diesel exhaust is also followed by an increase in pulmonary vascular resistance and a decrease in pulmonary vessel distensibility at high cardiac output, which may participate in the influence of air pollution on an acute heart failure episode [51]. Acute arterial vasoconstriction was also observed in the systemic circulation in healthy adults exposed to diesel exhaust [52].
From endothelial dysfunction to atherogenesis
In addition to endothelial dysfunction, air pollution also induces oxidative stress, which is followed by alteration of circulating lipids. Air pollution favours oxidized low-density lipoprotein generation and the release of other highly oxidized phospholipids [49]. These proatherogenic molecules diffuse into the subendothelial space and further activate endothelial cells. Activation of endothelial cells following air pollution exposure is characterized by the release of proinflammatory adhesion molecules, such as vascular cell adhesion molecule-1 and monocyte chemotactic protein-1, which ensure monocyte recruitment and their differentiation into macrophages in the subendothelial space [2,49]. Furthermore, air pollution is associated with impairment of antiatherogenic molecules, such as high-density lipoprotein [53]. A reduced high-density lipoprotein antioxidant capacity was also found in an animal study, with ultra-fine particles having a larger effect compared with PM2.5 [49]. Consequently, air pollution exposure promotes vascular inflammation, lipid accumulation in foam cells [54] and plaque progression [55].
From atherogenesis to atherothrombosis
In healthy adults exposed to diesel exhaust, platelet activation and increased thrombus formation were reported [56]. Similar findings were also observed in patients with coronary artery disease exposed to diesel exhaust, as well as inhibition of endogenous fibrinolytic capacity, demonstrated by a reduction in acute tissue activator plasminogen release [50]. Air pollution exposure also induces some endothelial injuries, characterized by increased endothelial cell apoptosis [57], a decreased circulating level of endothelial progenitor cells [58] and tight junction protein degradation [49,59]. A rise in interleukin-6 observed after PM inhalation leads to increased fibrinogen, factor VIII and tissue factor release [2,52]. Furthermore, interleukin-6 and other proinflammatory molecules trigger platelet activation. With – at the same time – a disrupted endothelial cell barrier, an increase in coagulation factors, a reduction in fibrinolytic capacity and platelet activation, air pollution accumulates all the conditions needed to promote thrombus formation.
Study limitations and future directions
Long-term exposure effects were only investigated in animals, especially rats, and confirmed an increase in arteriosclerosis [60]. All these experimental studies provide robust information regarding the acute effects of air pollution exposure and, above all, they provide strong evidence for diesel toxicity. While diesel exhaust has been widely studied, specific exposure to gasoline exhaust has not been tested in exposition chambers. Cars with gasoline engines produce, albeit to a lesser extent, fine and ultrafine particles, and their composition and health effects remain to be investigated, and cannot be extrapolated easily from diesel exhaust toxicity. Traffic emissions also include non-exhaust emissions, such as metals from brake wear particles, which could also lead to oxidative stress reactions, but their specific effects have not been studied extensively [61]. Compared with the large number of studies establishing some atherogenic effects of air pollution, an understanding of how acute air pollution exposure may trigger coronary plaque rupture is needed from further studies. At least, a human experimental model of pollutant exposure can be used to study the interaction between air pollutants and other pollutants, such as noise exposure.
Effects of mitigation manoeuvres
Exposure to diesel exhaust with commonly used particulate filters for diesel cars has not been investigated in interventional studies involving humans. One study reported a decreased adverse vascular reaction with filtered diesel exhaust, but the filter used in this study was highly efficient, and is not applicable to control vehicle emission at this stage [47]. However, a study performed on human bronchial epithelial cells exposed to diesel exhaust with a diesel particulate filter showed persistent oxidative stress reactions and a tumorigenic effect, probably caused by gaseous exhaust and the smallest nanoparticles not stopped by the filter [62]. Several studies reported that the intake of antioxidants and anti-inflammatory nutrients, such as vitamins B, C and E and omega-3 polyunsaturated fatty acids, could reduce air pollution oxidative effects [63]. For example, higher intake of vitamin B6, B12 and folate reduced the effect of air pollution on heart rate variability. Decreased levels of markers of lipid and protein oxidative damage were also associated with vitamin C and E supplementation [63]. However, even if intake of essential micronutrients could counteract the oxidative and inflammatory effects of air pollution, a clinical benefit of these nutritional strategies remains to be proved in the particular setting of air pollution.
Conclusions
Epidemiological studies have reported that exposure to air pollution increases long- and short-term cardiovascular mortality through an increase in myocardial infarction, stroke and heart failure events. Although the cardiovascular risk increases with the level and duration of exposure, all the studies concluded that there is no safe threshold below which there is no effect. Interventional controlled studies provide some explanations of the mechanisms involved. Air pollution – particularly diesel exhaust – is followed by a dramatic increase in ROS generation, which impairs nitric oxide-mediated vasodilatation and promotes vascular inflammation. Acute functional consequences of air pollution exposure have been clearly demonstrated in myocardial and pulmonary blood flow regulation, but also in coagulation function. Combustion-derived particles have strong adverse effects attributable to their small sizes (ultrafine particles) and to the polycyclic aromatic hydrocarbons and metals that they carry on their surface. While ultrafine particles have a major cardiovascular effect, their concentrations in ambient air are strongly underestimated by actual standards and measurements. Furthermore, in urban areas, traffic is also the leading source of NO2, which has been recently associated with the risk of ST-segment elevation myocardial infarction. As recently endorsed by European Society of Cardiology, the available scientific evidence supports the plan that efforts to reduce exposure to air pollution should urgently be intensified and supported by appropriate and effective legislation.
Acknowledgments
Sources of funding
Research reported in this publication was supported by the Office Of The Director, National Institutes Of Health, under Award Number DP5OD021412. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank Thierry Reeb, Jacques Reis and Professor Maurice Giroud for their valuable contributions.
Abbreviations
- CO
carbon monoxide
- NO
nitric oxide
- NO2
nitrogen dioxide
- PM
particulate matter
- ROS
reactive oxygen species
- SO2
sulphur dioxide
Footnotes
Disclosure of interest
Y.B. Honoraria for participating in advisory boards or speaking at symposia for the companies AstraZeneca France, Daiichi-Sankyo, BMS-Pfizer, Covidiem and MSD France.
T.B. Member of Strasbourg Respire, a non-profit, nongovernmental organization.
The other authors declare that they have no competing interest.
References
- 1.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–71. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 2.Miller MR, Shaw CA, Langrish JP. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012;8:577–602. doi: 10.2217/fca.12.43. [DOI] [PubMed] [Google Scholar]
- 3.Air Parif. Inventaire des émissions en Île de France : année de référence – élément synthétiques. 2012 Available at: https://www.airparif.asso.fr/pdf/publications/inventaire-emissions-idf-2012-150121.pdf.
- 4.Asikainen A, Carrer P, Kephalopoulos S, Fernandes Ede O, Wargocki P, Hanninen O. Reducing burden of disease from residential indoor air exposures in Europe (HEALTHVENT project) Environ Health. 2016;15(Suppl 1):35. doi: 10.1186/s12940-016-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoek G, Krishnan RM, Beelen R, et al. Long-term air pollution exposure and cardiorespiratory mortality: a review. Environ Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–58. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 7.Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six US cities. Environ Health Perspect. 2000;108:941–7. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faustini A, Rapp R, Forastiere F. Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur Respir J. 2014;44:744–53. doi: 10.1183/09031936.00114713. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson RW, Butland BK, Dimitroulopoulou C, et al. Long-term exposure to ambient ozone and mortality: a quantitative systematic review and meta-analysis of evidence from cohort studies. BMJ Open. 2016;6:e009493. doi: 10.1136/bmjopen-2015-009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet. 2002;360:1203–9. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- 11.Hart JE, Chiuve SE, Laden F, Albert CM. Roadway proximity and risk of sudden cardiac death in women. Circulation. 2014;130:1474–82. doi: 10.1161/CIRCULATIONAHA.114.011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69:660–5. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills IC, Atkinson RW, Kang S, Walton H, Anderson HR. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open. 2015;5:e006946. doi: 10.1136/bmjopen-2014-006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerrett M, Burnett RT, Pope CA, 3rd, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–95. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412. doi: 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman JD, Adar SD, Barr RG, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388:696–704. doi: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann B, Moebus S, Mohlenkamp S, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–96. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 18.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–5. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 19.Pope CA, 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–8. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 20.Nawrot TS, Perez L, Kunzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 2011;377:732–40. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- 21.Argacha JF, Collart P, Wauters A, et al. Air pollution and ST-elevation myocardial infarction: a case-crossover study of the Belgian STEMI registry 2009–2013. Int J Cardiol. 2016;223:300–5. doi: 10.1016/j.ijcard.2016.07.191. [DOI] [PubMed] [Google Scholar]
- 22.Ruidavets JB, Cournot M, Cassadou S, Giroux M, Meybeck M, Ferrieres J. Ozone air pollution is associated with acute myocardial infarction. Circulation. 2005;111:563–9. doi: 10.1161/01.CIR.0000154546.32135.6E. [DOI] [PubMed] [Google Scholar]
- 23.Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart. 2014;100:1093–8. doi: 10.1136/heartjnl-2013-304963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mordukhovich I, Coull B, Kloog I, Koutrakis P, Vokonas P, Schwartz J. Exposure to sub-chronic and long-term particulate air pollution and heart rate variability in an elderly cohort: the Normative Aging Study. Environ Health. 2015;14:87. doi: 10.1186/s12940-015-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mordukhovich I, Kloog I, Coull B, Koutrakis P, Vokonas P, Schwartz J. Association between particulate air pollution and QT interval duration in an elderly cohort. Epidemiology. 2016;27:284–90. doi: 10.1097/EDE.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ljungman PL, Berglind N, Holmgren C, et al. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J. 2008;29:2894–901. doi: 10.1093/eurheartj/ehn463. [DOI] [PubMed] [Google Scholar]
- 27.Raza A, Bellander T, Bero-Bedada G, et al. Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm. Eur Heart J. 2014;35:861–8. doi: 10.1093/eurheartj/eht489. [DOI] [PubMed] [Google Scholar]
- 28.Shah AS, Langrish JP, Nair H, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382:1039–48. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–24. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 30.Ljungman PL, Mittleman MA. Ambient air pollution and stroke. Stroke. 2014;45:3734–41. doi: 10.1161/STROKEAHA.114.003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin HH, Fann N, Burnett RT, Cohen A, Hubbell BJ. Outdoor fine particles and nonfatal strokes: systematic review and meta-analysis. Epidemiology. 2014;25:835–42. doi: 10.1097/EDE.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafoggia M, Cesaroni G, Peters A, et al. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect. 2014;122:919–25. doi: 10.1289/ehp.1307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yitshak Sade M, Novack V, Ifergane G, Horev A, Kloog I. Air pollution and ischemic stroke among young adults. Stroke. 2015;46:3348–53. doi: 10.1161/STROKEAHA.115.010992. [DOI] [PubMed] [Google Scholar]
- 34.Kettunen J, Lanki T, Tiittanen P, et al. Associations of fine and ultrafine particulate air pollution with stroke mortality in an area of low air pollution levels. Stroke. 2007;38:918–22. doi: 10.1161/01.STR.0000257999.49706.3b. [DOI] [PubMed] [Google Scholar]
- 35.Henrotin JB, Zeller M, Lorgis L, Cottin Y, Giroud M, Bejot Y. Evidence of the role of short-term exposure to ozone on ischaemic cerebral and cardiac events: the Dijon Vascular Project (DIVA) Heart. 2010;96:1990–6. doi: 10.1136/hrt.2010.200337. [DOI] [PubMed] [Google Scholar]
- 36.Yorifuji T, Suzuki E, Kashima S. Cardiovascular emergency hospital visits and hourly changes in air pollution. Stroke. 2014;45:1264–8. doi: 10.1161/STROKEAHA.114.005227. [DOI] [PubMed] [Google Scholar]
- 37.Cai Y, Zhang B, Ke W, et al. Associations of short-term and long-term exposure to ambient air pollutants with hypertension: a systematic review and meta-analysis. Hypertension. 2016;68:62–70. doi: 10.1161/HYPERTENSIONAHA.116.07218. [DOI] [PubMed] [Google Scholar]
- 38.Fuks KB, Weinmayr G, Foraster M, et al. Arterial blood pressure and long-term exposure to traffic-related air pollution: an analysis in the European Study of Cohorts for Air Pollution Effects (ESCAPE) Environ Health Perspect. 2014;122:896–905. doi: 10.1289/ehp.1307725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagryantseva Y, Novotna B, Rossner P, Jr, et al. Oxidative damage to biological macromolecules in Prague bus drivers and garagemen: impact of air pollution and genetic polymorphisms. Toxicol Lett. 2010;199:60–8. doi: 10.1016/j.toxlet.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Shen M, Bin P, Li H, et al. Increased levels of etheno-DNA adducts and genotoxicity biomarkers of long-term exposure to pure diesel engine exhaust. Sci Total Environ. 2016;543:267–73. doi: 10.1016/j.scitotenv.2015.10.165. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs L, Emmerechts J, Hoylaerts MF, et al. Traffic air pollution and oxidized LDL. PLoS One. 2011;6:e16200. doi: 10.1371/journal.pone.0016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bind MA, Baccarelli A, Zanobetti A, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–40. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yorifuji T, Kashima S, Doi H. Fine-particulate air pollution from diesel emission control and mortality rates in Tokyo: a quasi-experimental study. Epidemiology. 2016;27:769–78. doi: 10.1097/EDE.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 44.Langrish JP, Li X, Wang S, et al. Reducing personal exposure to particulate air pollution improves cardiovascular health in patients with coronary heart disease. Environ Health Perspect. 2012;120:367–72. doi: 10.1289/ehp.1103898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kittelson DB. Engines and nanoparticles: a review. J Aerosol Sci. 1998;29:575–88. [Google Scholar]
- 46.Wauters A, Dreyfuss C, Pochet S, et al. Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension. 2013;62:352–8. doi: 10.1161/HYPERTENSIONAHA.111.00991. [DOI] [PubMed] [Google Scholar]
- 47.Mills NL, Miller MR, Lucking AJ, et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 2011;32:2660–71. doi: 10.1093/eurheartj/ehr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mills NL, Tornqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–6. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 49.Araujo JA. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual Atmos Health. 2010;4:79–93. doi: 10.1007/s11869-010-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mills NL, Tornqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–82. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 51.Wauters A, Vicenzi M, De Becker B, et al. At high cardiac output, diesel exhaust exposure increases pulmonary vascular resistance and decreases distensibility of pulmonary resistive vessels. Am J Physiol Heart Circ Physiol. 2015;309:H2137–44. doi: 10.1152/ajpheart.00149.2015. [DOI] [PubMed] [Google Scholar]
- 52.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 53.Ramanathan G, Yin F, Speck M, et al. Effects of urban fine particulate matter and ozone on HDL functionality. Part Fibre Toxicol. 2016;13:26. doi: 10.1186/s12989-016-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y, Long J, Ji Y, et al. Foam cell formation by particulate matter (PM) exposure: a review. Inhal Toxicol. 2016;28:583–90. doi: 10.1080/08958378.2016.1236157. [DOI] [PubMed] [Google Scholar]
- 55.Li R, Navab M, Pakbin P, et al. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res. 2013;54:1608–15. doi: 10.1194/jlr.M035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucking AJ, Lundback M, Mills NL, et al. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008;29:3043–51. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- 57.Pope CA, 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119:1204–14. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Toole TE, Hellmann J, Wheat L, et al. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res. 2010;107:200–3. doi: 10.1161/CIRCRESAHA.110.222679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T, Wang L, Moreno-Vinasco L, et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol. 2012;9:35. doi: 10.1186/1743-8977-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Q, Wang A, Jin X, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–10. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 61.Grigoratos T, Martini G. Brake wear particle emissions: a review. Environ Sci Pollut Res Int. 2015;22:2491–504. doi: 10.1007/s11356-014-3696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hawley B, L’Orange C, Olsen DB, Marchese AJ, Volckens J. Oxidative stress and aromatic hydrocarbon response of human bronchial epithelial cells exposed to petro- or biodiesel exhaust treated with a diesel particulate filter. Toxicol Sci. 2014;141:505–14. doi: 10.1093/toxsci/kfu147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peter S, Holguin F, Wood LG, et al. Nutritional solutions to reduce risks of negative health impacts of air pollution. Nutrients. 2015;7:10398–416. doi: 10.3390/nu7125539. [DOI] [PMC free article] [PubMed] [Google Scholar]