Abstract
Patients with schizophrenia self-administer nicotine at rates higher than is self-administered for any other psychiatric illness. Although the reasons are unclear, one hypothesis suggests that nicotine is a form of ‘self-medication’ in order to restore normal levels of nicotinic signaling and target abnormalities in neuronal function associated with cognitive processes. This brief review discusses evidence from neurophysiological and neuroimaging studies in schizophrenia patients that nicotinic agonists may effectively target dysfunctional neuronal circuits in the illness. Evidence suggests that nicotine significantly modulates a number of these circuits, although relatively few studies have used modern neuroimaging techniques (e.g. functional magnetic resonance imaging (fMRI)) to examine the effects of nicotinic drugs on disease-related neurobiology. The neuronal effects of nicotine and other nicotinic agonists in schizophrenia remain a priority for psychiatry research.
Keywords: Schizophrenia, nicotine, fMRI, electrophysiology, neuroimaging
Introduction
According to a 2005 meta-analysis, patients with schizophrenia are over five times as likely to be cigarette smokers than the general population, with over 70% of male patients and 40% of female patients being smokers (de Leon and Diaz, 2005). Smoking prevalence is also higher in schizophrenia than any other psychiatric disease (George and Krystal, 2000). Furthermore, patients ingest more nicotine per cigarette (Olincy et al., 1997) and smoke more cigarettes per day (Olincy et al., 1997) than otherwise healthy smokers.
Researchers have yet to reach a consensus as to why schizophrenia patients smoke at higher rates than other populations. Among the hypotheses put forth are to improve cognition, relieve anxiety or withdrawal, relieve boredom, reluctance on the part of clinicians to encourage cessation for the aforementioned reasons, various psychosocial factors (e.g. socioeconomic disadvantage), and/or poor access to cessation support programs (Winterer, 2010; Ziedonis et al., 2008). Although these viewpoints are supported by patient questionnaires (Barr et al., 2008a; Herran et al., 2000), they are unlikely to fully explain the disproportionate percentage of schizophrenia patients who smoke relative to other populations. A hypothesis that has gained substantial traction in the previous two decades is that nicotinic receptor signaling is fundamentally decreased in the illness, and that patients are using the most readily available method for pharmacologically targeting this system in an attempt to restore signaling to appropriate levels. Indeed, in addition to acute effects of the drug, chronic nicotine is associated with increased nicotinic receptor expression in patients (Esterlis et al., 2014; Mexal et al., 2010). Smoking, therefore, may be a way for patients to ‘self-medicate’ a pharmacologic deficit.
It should be noted that the ‘self-medication’ hypothesis in the context of this review does not necessarily imply improvement of positive or negative symptomatology. Indeed, evidence for these effects in the illness is highly inconclusive (Aguilar et al., 2005; Kumari and Postma, 2005). Rather, by ‘self-medication’ we specifically refer to physiological and functional abnormalities in neuronal processes (including cognitive processes). Evidence for this hypothesis is discussed in the proceeding sections following an introduction to the pharmacology of nicotinic receptors.
Neuropharmacology of nicotinic receptors
Neuronal nicotinic receptors are ionotropic, ligand-gated channels that consist of heteromeric or homomeric arrangements of five α (α2–α10) and/or β (β2–β4) subunits (Iversen, 2009). The most ubiquitous arrangements in the central nervous system are the α4β2 and α7 (homomeric) subtypes (Iversen, 2009). Ligand-gated activation of a nicotinic receptor enables positively charged ions to diffuse across their concentration into the neuron, leading to depolarization. This depolarizing current is specifically carried by an influx of sodium through the α4β2 receptor subtype, and an influx of calcium through the α7 receptor subtype (Role, 1992). Furthermore, calcium influx through presynaptic α7 receptors activates second-messenger systems that can induce the release of neurotransmitters into the synaptic cleft (Wonnacott et al., 2006). The diversity of nicotinic receptors allows for considerable variation in affinity and consequently, differential targeting by pharmacologic compounds. For example, nicotine, the exogenous direct agonist found in tobacco products, binds with high (nM) affinity to the α4β2 receptor and 100 to 1000-fold lower affinity to the α7 receptor (Le Houezec, 1998).
Nicotinic receptor subtypes display a variety of regional, cell-specific, and subcellular localizations that convey functional specialization. Brain regions of relatively high expression include the cingulate cortex, hippocampus, insula, and subcortical areas, depending on receptor subtype (reviewed by Paterson and Nordberg (2000)). Nicotinic receptors are expressed in both excitatory cells and inhibitory interneurons, where they exert localized control over activity and influence oscillatory firing patterns and synchronization across neuronal populations over a range of frequencies. To varying degrees and depending on subtype, receptors may be situated on dendritic arbors, cell bodies, and/or pre/postsynaptic terminals. Nicotinic receptors are thus situated to influence neuronal input and output, and affect brain response on a global and local scale (Picciotto et al., 2012).
Nicotinic receptors in schizophrenia
Multiple lines of evidence suggest that nicotinic cholinergic signaling is fundamentally altered in schizophrenia. Single nucleotide polymorphisms (SNPs) in the 5’ upstream regulatory region of the α7 nicotinic receptor gene are associated with reduced expression of α7 receptors and increased risk for schizophrenia (Freedman et al., 2001; Gault et al., 2003; Leonard and Freedman, 2006; Sinkus et al., 2009; Stephens et al., 2009). Decreased postmortem α7 receptor expression has been observed in schizophrenia in the hippocampus, thalamus and cingulate cortex, among other areas (Freedman et al., 1995). As the α7 receptor is primarily expressed on interneurons but not pyramidal cells in the adult hippocampus, loss of these receptors is hypothesized to predominantly affect inhibitory neuronal function (Frazier et al., 1998).
The other prominent nicotinic receptor subtype, the α4β2 receptor, is also implicated in schizophrenia. Loss of α4β2 expression has been observed in the illness in a postmortem study (Breese et al., 2000) and in vivo (D’Souza et al., 2012; Esterlis et al., 2014). Genes that control the expression of α4 and β2 subunits (the CHRNA4 and CHRNB2 genes) are in linked in combination to schizophrenia (De Luca et al., 2006). A SNP in the CHRNA4 has also been linked to heavy smoking in the disease (Voineskos et al., 2007).
Although genetic factors account for a substantial (>50%) proportion of the risk for schizophrenia (Gottesman and Shields, 1967) and significantly influence receptor expression, environmental factors play an important role as well. For example, prenatal stress, whether brought about by maternal physical factors (e.g. infection, famine) or mental illness (e.g. depression) is associated with high risk for schizophrenia in offspring (Brown and Derkits, 2010; Fine et al., 2014; Susser et al., 1996). Related to this review, one potential effect of stress during pregnancy is that it causes sequestration of choline in the stressed mother, which prevents adequate levels of the acetylcholine precursor from reaching the fetal brain during development (Freedman and Ross, 2015). Although the effects of stress on nicotinic receptor levels in humans are unknown, restraint stress in pregnant mothers has been shown to reduce α7 receptor expression in the cortex and hippocampus in their newborn pups (Baier et al., 2015). The potential applications of dietary-based interventions in the treatment and/or prevention of schizophrenia are currently being investigated or considered (Freedman, 2014; Freedman and Ross, 2015; Kimhy et al., 2015).
Nicotine targets P50 gating deficits in schizophrenia
Schizophrenia, with its tripartite symptomatology (positive, negative, and cognitive symptoms), is an extraordinarily complex disease. It is not surprising, therefore, that no single gene has been discovered that can account for all of its symptoms on a pharmacological, cellular, circuit, or neuronal systems level. In the search for genetic influences, therefore, researchers have turned towards discovering endophenotypes, or stable phenotypes with clear genetic influences. A primary aim of this research is to identify associations between genes that may influence expression of proteins that can in turn disrupt simple neuronal circuits associated with electrophysiological abnormalities in the illness.
A prominent example of this approach is the development of P50 gating as a nicotinic receptor-associated endophenotype for schizophrenia. The study of P50 gating impairment in schizophrenia has its origins from work in the 1960s by McGhie and Chapman (1961) as well as Venables (1964), who published extensive patient case reports of perceptual abnormalities. Many of these reports described a ‘hypervigilant’ state in which patients were unable to ignore persistent distracting noises in the environment. As a result, patients found it hard to concentrate on any one stimulus in a noisy environment, such as the voice of a single person in a bustling crowd. Hypervigilance was later hypothesized to play a role in the emergence of positive symptoms. For example, increased salience of the sounds of squealing tires may cause the noises to be reinterpreted as a screaming baby (Freedman et al., 1991).
The hypervigilant state found in schizophrenia led Adler et al. (1982) to postulate that patients may show a deficit in the ability of the brain to physiologically decrease, or ‘gate’, its response to repeated stimuli. This brain response is postulated to play a major role in the ability of healthy subjects to subconsciously ignore irrelevant, incessant stimuli in the environment such as a clock ticking (Freedman et al., 1991). Based on electroencephalographic (EEG) methods developed in the 1960s for studying repetitive auditory stimuli (Davis et al., 1966), Adler et al. (1982) observed reduced capacity in schizophrenia to diminish early (50 ms post-stimulus, or P50) responses to the second of a pair of identical, closely-spaced (~0.5 s) clicks. This phenomenon has since been replicated in many laboratories, is predictive of cognitive function in several domains, including attention (Cullum et al., 1993; Potter et al., 2006; Smith et al., 2010; Smucny et al., 2013) and is one of the most frequently investigated electrophysiological endophenotypes in schizophrenia.
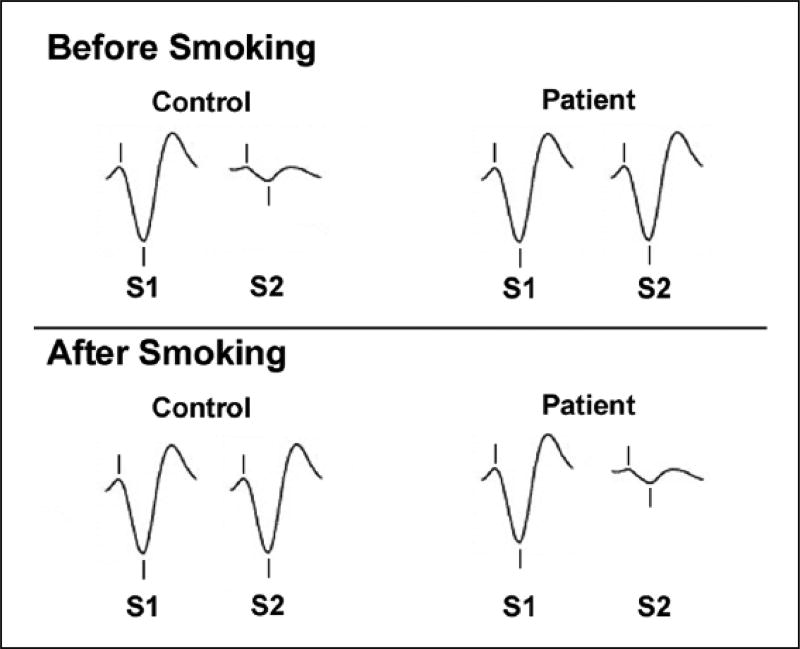
After the initial discovery in schizophrenia, Adler et al. (1992) postulated that gating deficits had a cholinergic basis, based on findings in rats in which lesions to the septal nucleus (a cholinergic input to the hippocampus) disrupted auditory gating (Vinogravoda, 1975). In support of this hypothesis, Adler et al. (1993) found that cigarette smoking improves P50 gating in patients (Figure 1). In another study, Adler et al. (1992) observed that nicotine improved gating in unaffected first-degree relatives, suggesting that normalization could occur independent of diagnosis and possibly have a genetic component. Nicotinic agonists more specific for receptor subtypes (e.g. the α7 partial agonist 3-2,4-dimethoxybenzylidene anabaseine (DMXB-A and the α4β2 agonist varenicline) have also been shown to improve P50 gating in patients (Hong et al., 2011b; Olincy et al., 2006). Interestingly, in the Olincy et al. (2006) trial the low dose of DMXB-A (75 mg followed by a half-dose of 37.5 mg) had more robust effects on gating than the high dose (150 mg followed by a half-dose of 75 mg), possibly due to receptor desensitization. A genetic linkage between nicotinic receptors and P50 gating in schizophrenia was later found in the α7 receptor gene (Freedman et al., 1997), and α7 promoter variants predict P50 gating deficits in healthy subjects (Leonard et al., 2002). Smoking, therefore, may be a method of ‘self-medicating’ an electrophysiological abnormality associated with an intrinsic signaling deficit caused by the loss of nicotinic receptors associated with polymorphisms in the α7 gene.
Figure 1.
Graphical summary illustration of the effects of nicotine (cigarette smoking) on P50 gating in schizophrenia. S1: event-related potential after presentation of the first auditory stimulus. S2: event-related potential after presentation of the second auditory stimulus. Gating is measured by the ratio of S2/S1 amplitude. Hash marks indicate the P50 waveform. Before smoking, patients show worse P50 gating relative to controls. After smoking, P50 gating is restored to control levels in patients. Smoking impaired P50 gating in healthy subjects (Adler et al., 1993).
A potentially important factor in regards to drug effects on the P50 and other physiological endpoints is the effect of baseline performance. Work by Knott and colleagues has demonstrated that nicotine as well as the dietary α7 agonist choline have differential effects on P50 gating in healthy subjects depending on baseline levels of P50 suppression (Knott et al., 2013, 2014b). Specifically, the researchers found that these compounds improve gating in subjects that show poor gating at baseline, but have no effect on moderate ‘gaters’ and deleterious effects on subjects with high baseline suppression. Researchers may thus wish to take baseline P50 levels into consideration when analyzing the effects of nicotinic and other drugs on P50 suppression in patient populations.
Later waveforms: The mismatch negativity and P300
Researchers have also examined the effects of nicotine on event-related potentials that occur after the P50 wave in schizophrenia. These potentials represent complex stimulus processing and may be related to more advanced cognitive processes (e.g. ‘top-down’ processes).
The N200, or ‘mismatch negativity’ (MMN), is a negative potential that occurs 200 ms post-stimulus. It is elicited during ‘oddball’ tasks in which a stream of identical stimuli is interrupted by a stimulus of a different duration, amplitude, or frequency. It is now well-established that the MMN is decreased in schizophrenia, with a large effect size (Umbricht and Krljes, 2005). Although not well-studied, the effects of nicotine on the MMN in schizophrenia show mixed results. One study observed reversal of MMN deficits after nicotine in patients during duration but not frequency MMN (Dulude et al., 2010). Other studies, however, have reported no effect of nicotine on MMN amplitude in schizophrenia (Fisher et al., 2012; Inami et al., 2007). Possible reasons for the discrepancy include differences in the probability of the variant occurring (5% in the Dulude et al. (2010) and Inami et al. (2007) studies and 17% in the Fisher et al. (2012) study), subject smoking status (nonsmokers in the Inami et al. (2007) study and either a mix of smokers/nonsmokers or all smokers in the other studies), the dose of nicotine administered (2 mg higher in the Dulude et al. (2010) study versus the Fisher et al. (2012) study), and route of administration (patch in the Inami et al. (2007) paper and gum in the other studies). Another possibility is that nicotinic effects were influenced by differences baseline MMN; nicotine increases MMN amplitude in healthy subjects with low baseline MMN but has the opposite effect in healthy subjects with high baseline MMN (Knott et al., 2014a). In line with these findings, a similar pattern of baseline dependence was observed in healthy subjects given an acute dose of choline (Knott et al., 2015). All three patient studies also had relatively low sample sizes (n = 10–12) and the effects of nicotine on MMN in schizophrenia are therefore inconclusive.
As its name implies, the P300 is a positive deflection that occurs 300 ms post-stimulus. Like the MMN, the P300 is most commonly elicited during oddball tasks, although its amplitude is also dependent on attention level and the subject’s level of engagement with the trial. Higher levels of engagement (driven, for example, by stimulus unpredictability) produce higher P300 amplitudes. Meta-analyses have demonstrated that the P300 is reduced in schizophrenia (Bramon et al., 2004; Jeon and Polich, 2003). To our knowledge, only one studied has examined the effects of nicotine on the P300. In a small sample size (n = 14 patients), Mobascher et al. (2012) reported no effect of the drug on P300 amplitude, although it did increase brain response (measuring by functional neuroimaging) correlated with the waveform.
Nicotinic agonists modulate brain networks in schizophrenia
The continued development of noninvasive, whole-brain neuroimaging techniques such as functional magnetic resonance imaging (fMRI) has enabled researchers to examine the neuronal effects of nicotine in schizophrenia with unprecedented specificity and complexity. The fMRI is the only noninvasive human neuroimaging technique that allows millimeter-level resolution and whole-brain coverage of neuronal processes, and therefore is extremely useful in providing evidence of target engagement and testing causal hypotheses when used to study treatment effects (Borsook et al., 2006). The following sections briefly review evidence from fMRI studies that suggest that nicotine and nicotinic agonists may target many aspects of brain dysfunction in schizophrenia, including low-level sensory processes, intrinsic network connectivity, and finally higher-level cognitive functions.
It is important to note that a synthesis of the present state of clinical trials involving nicotinic receptor targeting compounds in schizophrenia is beyond the scope of this review. For this information, the reader is encouraged to consult Beinat et al. (2015) and Rowe et al. (2015).
Effects of nicotine on oculomotor networks in schizophrenia
Tregellas and colleagues (2002, 2004, 2005, 2010) have examined neuronal response during smooth pursuit eye movements (SPEMs) in a series of studies that were among the first to use fMRI to characterize a functional neuronal abnormality in schizophrenia and its modulation by nicotinic agonists.
In order to follow important moving objects (such as prey in the wild), mammalian carnivores have developed two oculomotor processes: one to rapidly saccade to stimuli of interest, and another to slowly track stimuli as they move across the visual field. The latter process, known as SPEMs, was first shown to be abnormal in schizophrenia by Diefendorf and Dodge (1908) and is now one of the most consistently reported abnormalities of the disease (Levy et al., 1993, 2010). SPEM performance is quantified by the relative ability of the retina to follow a moving target without shifting its focus to another location, and may be a measure of inhibitory dysfunction. Interestingly, a meta-analysis by O’Driscoll and Callahan (2008) found that increased intrusive anticipatory saccade rate is the specific feature of SPEMs with the largest effect size in schizophrenia (d = 1.31).
Although eye-tracking deficits had been reported in schizophrenia for almost a century, the neuronal circuitry involved in the deficit was not known until relatively recently. Tregellas et al. (2004) found that schizophrenia patients showed regionalized hyperactivity in the hippocampus, fusiform gyrus, thalamus, and parietal eye fields and hypoactivity in the frontal eye fields, cingulate gyrus, and occipital gyrus. Subsequent studies have largely replicated these findings (Hong et al., 2005; Tu et al., 2006).
Based on previous findings demonstrating that abnormal intrusive saccades are decreased in schizophrenia patients after cigarette smoking (Olincy et al., 1998), the neuronal effects of acute nicotine administration (4–6 mg gum) in schizophrenia during SPEMs have also been examined (Tregellas et al., 2005). Increased activity after nicotine (versus placebo) was observed in the cingulate gyrus, occipital gyrus, and precuneus. Decreased activity was observed in the hippocampus and parietal eye fields. Collectively, these results suggest that nicotine effectively reversed many of the abnormalities observed in schizophrenia in the initial study comparing patients to control subjects. A later study found that 150 mg twice daily (b.i.d.) DMXB-A also reduced hippocampal hyperactivity in patients during the SPEM task (Tregellas et al., 2010). A lower dose of drug (75 mg b.i.d.) was ineffective.
Effects of nicotine on intrinsic networks: The default network
The fMRI studies have traditionally focused on ‘task-related’ activity (i.e. how local brain areas are recruited during sensory stimulation and cognitive functions). These studies observed that a network of brain areas was consistently deactivated, reflecting suppression during the more cognitively demanding ‘task’ condition compared to the baseline or low-load condition (Greicius et al., 2003). Due to its tendency to be down-modulated during many tasks, and therefore be active as a ‘default’, the network was coined the Default Mode Network (DMN).
The primary hubs of the DMN are (1) the precuneus/posterior cingulate cortex (PCC), (2) the medial prefrontal cortex (mPFC), and (3) the bilateral inferior parietal lobule (IPL). The bilateral hippocampus/medial temporal lobe is considered an accessory hub of the network due its being less functionally connected to the other three DMN hubs, on average, relative to the primary hubs (Buckner et al., 2008). The DMN is readily and reproducibly detectable regardless of the analytic technique used, and irrespective of the cognitive state of the individual, be it during an effortful task, rest, or even during sleep (Whitfield-Gabrieli and Ford, 2012). The ease of which this network is extracted across states is particularly advantageous for patient populations that may be unable to perform complex cognitive operations (Smucny and Tregellas, 2013). The network is particularly active during actions that are self-referential, such as reflecting on the past, planning for the future, or monitoring internal state (Buckner et al., 2008). Abnormally high DMN activity during cognitively challenging tasks is associated with poor performance, likely due to competing resource allocation towards task-irrelevant thoughts (Gordon et al., 2012).
Soon after the discovery of the DMN, abnormalities in its function were observed in schizophrenia. Patients inappropriately recruit the DMN, as evidenced by hyperactivity of the network during an auditory oddball task (Garrity et al., 2007), working memory tasks (Meyer-Lindenberg et al., 2005; Pomarol-Clotet et al., 2008; Whitfield-Gabrieli et al., 2009), and language (semantic priming) tasks (Jeong and Kubicki, 2010). Patients are similarly impaired in their ability to deactivate the DMN as task difficulty is increased (Meyer-Lindenberg et al., 2005; Pomarol-Clotet et al., 2008; Whitfield-Gabrieli et al., 2009). DMN abnormalities extend to task-free (resting) states, during which DMN hyperactivity and hyperconnectivity have been frequently observed (Jafri et al., 2008; Liu et al., 2012; Skudlarski et al., 2010). DMN dysfunction is correlated with the severity of positive symptoms (Garrity et al., 2007), negative symptoms (Bluhm et al., 2007), and impaired social cognition (Holt et al., 2011). DMN hyperactivity and hyperconnectivity have been reported in unaffected first-degree relatives of schizophrenia patients, albeit to a lesser extent (Liu et al., 2012; Whitfield-Gabrieli et al., 2009). These findings suggest that DMN pathology has both state-dependent and independent characteristics.
Given that DMN pathology (e.g. hyperactivity) may predict symptoms of schizophrenia as well as cognitive function, nicotinic targeting of the DMN may represent a neurobiological form of ‘self-medication’. To test the hypothesis that a nicotinic agonist could target DMN pathology, Tregellas and colleagues (2011) examined DMN activity in 16 patients after chronic (1 month) treatment with the nicotinic agonist DMXB-A. The DMN was extracted by independent component analysis of resting state imaging data. The investigators found that relative to placebo, 75 and 150 mg b.i.d., DMXB-A reduced DMN activity in its PCC, bilateral IPL, and mPFC hubs. Decreased PCC activity was correlated with improved symptomatology. Interestingly, the investigators also found that a SNP located on the CHRNA7 locus (the α7 gene) predicted response to DMXB-A. If variation in this SNP influences receptor expression, this finding suggests that patients with lower levels of α7 expression may be less responsive to effects of the drug. Overall, these results suggest that α7 receptor activation may normalize DMN hyperactivity in schizophrenia and have clinical benefit, with the caveat that genetic factors may influence responsiveness to α7 agonists.
To our knowledge, no other studies have examined the effects of nicotinic agonists on DMN activity in schizophrenia. Effects of nicotine on DMN function, however, have been reported in healthy subjects. Tanabe et al. (2011) found that 7 mg of acute nicotine reduced activity of the DMN in nonsmoking participants during the resting state. Hahn et al. (2007) observed that nicotine deactivated the DMN during a visuospatial attention task in minimally deprived smokers, and that this effect predicted improved performance on the task. In line with these findings, Beaver et al. (2011) later reported that nicotine reduced DMN activation in deprived smokers during a rapid visual processing task. Future research may examine if these effects persist in schizophrenia.
Effects of nicotine on intrinsic networks: The salience network
The brain is constantly inundated by information from external cues and internal stores. In order to process what is important, ignore what is irrelevant, and update its internal model of the external world – in other words, process saliency – it must have a way of shifting focus from self-monitoring to monitoring external information (and vice versa) as needed. In this manner, a stimulus that is pleasurable, threatening, or important to a task is given precedence. This process is the primary task of a functionally and structurally interconnected set of cortical and subcortical areas called the Salience Network (SN).
The SN is anchored by the anterior cingulate cortex (ACC) and bilateral insula. These hubs in turn are connected to limbic structures as well as to the PCC and dorsolateral prefrontal cortex (DLPFC). Inputs into the insula provide sensory and emotional information (Craig, 2009). Inputs into the anterior cingulate from the DLPFC provide information regarding goals, expectations, and internal representations (Menon, 2011). As a result, the SN effectively integrates external and internal information so that a course of action (or inaction) can be chosen. The integrative nature of SN processing may be why the network is hypothesized to be important for distinguishing ‘self’ from ‘nonself’ as well as establishing a strong sense of identity, purpose, and self-worth (Palaniyappan and Liddle, 2012; Wylie and Tregellas, 2010).
The functions of the SN suggest that pathology of the network may be tied to symptom etiology in schizophrenia. Hallucinations, for example, occur when patients have difficulty distinguishing external stimuli from internal thoughts. Given that separating self from nonself is an important function of the SN, dysfunction of the network may thus contribute to this positive symptom. Loss of affect and motivation may arise in part due to pathology of the network; accordingly, loss of GM volume in the insula is associated with severity of negative symptoms (Koutsouleris et al., 2008). Cognitive symptoms may be partially explained by a relative inability of the SN to modulate activity in sensory areas according to perceptual expectations (Chambon et al., 2011). Patients may thus become overly reliant on sensory evidence to interpret their surroundings. As a result, the salience of external stimuli is inappropriately enhanced, leading to perceptual abnormalities (Palaniyappan et al., 2012).
Structural and functional neuroimaging evidence suggests that the SN is indeed dysfunctional in schizophrenia. Loss of gray matter volume in the insula and ACC are among the most striking and consistently replicated structural brain abnormalities in the illness (reviewed by Wylie and Tregellas (2010)). Gray matter loss of the insula and ACC is present at the first episode of psychosis, but also may be progressive in chronic schizophrenia (Chan et al., 2011; Ellison-Wright et al., 2008). Insula and ACC structural deficits have been linked to reality distortion, suggesting that SN dysfunction is associated with abnormal stimulus processing and positive symptoms (Palaniyappan et al., 2011). Functionally, abnormalities in SN activation across a variety of paradigms have been observed in schizophrenia, including working memory (Repovs and Barch, 2012), social and affective processing (Mitchell et al., 2004; Phillips et al., 1999; Seiferth et al., 2009), and error processing (Laurens et al., 2003; Polli et al., 2008). Network analyses have found a loss of connectivity between nodes of the SN and other brain areas both at rest (Moran et al., 2012; Tu et al., 2012) and during tasks (Gradin et al., 2012; Tu et al., 2010; White et al., 2010) in patients. This loss of connectivity has been proposed to underlie the relative inability of patients to adjust DMN activity according to task demands, resulting in performance deficits (Menon, 2011).
Like the DMN, immunohistochemical evidence suggests that the SN would be responsive to nicotinic modulation. The insula and ACC receive cholinergic input from the basal forebrain (Selden et al., 1998), and show enriched nicotinic receptor expression (Breese et al., 1997; Paterson and Nordberg, 2000). Reduced α7 receptor expression and increased α4β2 receptor expression has been observed in the ACC in patient postmortem brain (Marutle et al., 2001). In addition, nicotine administration increases basal cerebral blood flow in the ACC and insula (Stein et al., 1998).
Accordingly, Moran et al. (2012) investigated the effects of nicotine administration on resting state brain activity in smoking patients with schizophrenia and healthy smokers. Overall, Moran and coworkers (2012) found (1) decreased connectivity within the SN in patients, and (2) increased connectivity between the SN and parietal cortex and SN and occipital cortex after nicotine administration. No effects of nicotine on connectivity within the SN were reported, however. These results suggest that nicotine affects connectivity between the SN and other networks, but not within the SN itself in schizophrenia.
Our laboratory has recently expanded upon this work by examining effects of acute nicotine on resting state SN connectivity and topology in nonsmoking schizophrenia patients and healthy controls (Smucny et al., 2017). The SN was extracted by independent component analysis, and peaks from the extracted network used as seeds in a functional connectivity analysis. Using a 7-mg patch, we observed a significant treatment (nicotine versus placebo) × diagnosis (control versus patient) interaction on connectivity between the anterior cingulate and lateral prefrontal/superior parietal cortices. The effect was driven by relative hypoconnectivity in patients (versus controls) during placebo administration and reversal of this effect in patients after nicotine. The opposite interaction effect was also observed on connectivity between the left insula and the middle cingulate cortex. Finally, we found that nicotine affected SN topology. Specifically, a treatment × diagnosis interaction was observed on between-ness centrality (a measure of global information flow through a region) of the anterior cingulate, driven by decreased centrality in patients during placebo and reversal of the effect after nicotine. Taken together, these results suggest that nicotine effectively reverses abnormalities in intrinsic SN connectivity and functional organization in nonsmoking patients with schizophrenia.
Neuroimaging the cognitive effects of nicotine in schizophrenia
The previous section primarily focused on fMRI studies that either examined the effects of nicotine on neuronal function in schizophrenia during a simple, low-level task (smooth pursuit) or at rest. Previous fMRI studies have also found effects of nicotine during cognitive tasks in schizophrenia, although the field remains poorly developed and findings are limited.
Working memory
Working memory (WM), the process by which information is held in short-term storage for immediate use and manipulation, is one of the most frequently examined cognitive deficits in schizophrenia. Indeed, several WM tasks were recently nominated by the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) consortium as potential neuroimaging biomarkers (biological indicators of disease state) due to their ability to probe the most deleterious aspects of WM in schizophrenia (goal maintenance and interference control) (Barch et al., 2012). Given that WM deficits are predictive of everyday functioning in schizophrenia (Evans et al., 2003; Shamsi et al., 2011) there is great interest in developing interventions that can target this core cognitive deficit.
Previous work suggests that nicotinic agonists improve WM in schizophrenia patients (D’Souza and Markou, 2012; Freedman et al., 2008; Mackowick et al., 2014; Radek et al., 2010; Sacco et al., 2005) and in animal models of schizophrenia (Mackowick et al., 2014; Rushforth et al., 2011). Conversely, nicotine deprivation in patient smokers impairs WM performance (George et al., 2002; Ghiasi et al., 2013; Sacco et al., 2005), and nicotinic receptor blockade impairs WM in rats (Pocivavsek et al., 2006). Nonetheless, surprisingly little work has examined the neuronal effects of nicotinic agents in WM tasks in schizophrenia. An early study by Jacobsen et al. (2004) reported enhanced performance in abstinent smoking patients given nicotine relative to placebo as well as increased recruitment of the anterior cingulate and thalamus during an n-back task. Nicotine also increased thalamic connectivity in patients to a greater extent than healthy smokers. The neuronal effects of α7 receptor agonists during WM in schizophrenia are unknown.
Attention
Deficits in attention in schizophrenia have been reported and replicated for many years across a variety of tasks (Cornblatt and Keilp, 1994; Hahn et al., 2012; Keefe and Harvey, 2012; Laurent et al., 1999; Mayer et al., 2015; Smith and Cornblatt, 2005; Suwa et al., 2004; Verleger et al., 2013). Both sustained attention (the ability to maintain focus on a task over time) and selective attention (the ability to focus on task-relevant stimuli among competing stimuli) are affected. Due to its ability to predict functional outcomes in schizophrenia, attention has been recently recognized by CNTRICS as an important area for future imaging biomarker development (Luck et al., 2012).
Nicotinic agonists have also been shown to improve performance on attention tasks in schizophrenia. A study by Harris et al. (2004) found that nicotine improved scores on the attention index of the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB) in nonsmoking patients. Performance enhancement after nicotine was also observed in nonsmoking patients during the continuous performance task (CPT) in a later study (Barr et al., 2008b). Acute nicotine has also been shown to improve performance during the CPT in smoking patients that abstain from smoking for 10–24 h (Sacco et al., 2005; Smith et al., 2006). Nicotinic effects on attention in schizophrenia may be α7 receptor-dependent, as the α7 receptor partial agonist and α4/β2 antagonist DMXB-A improved attention scores on the MCCB during the first arm of a double crossover phase II trial in nonsmoking patients given either 75 or 150 mg b.i.d. of drug (Freedman et al., 2008).
Functional neuroimaging studies have begun to elucidate the neuronal mechanisms that underlie nicotine’s effects on attention in schizophrenia. An fMRI study by Hong et al. (2011a) showed that in deprived patient smokers, nicotine improved accuracy and reaction time during a visual sustained attention task as well as increased activity in the thalamus, anterior cingulate, frontal cortex, parietal cortex, and precuneus. No task condition (i.e. task difficulty) × drug (nicotine versus placebo), diagnosis (control versus patient) × drug, or condition × drug × diagnosis interactions were observed, however, suggesting drug effects were nonspecific to the task or diagnostic group.
A more recent study by our lab has examined the effects of acute nicotine on neuronal response during a selective attention task (Smucny et al., 2016a). Briefly, the task required subjects to respond (button press) to auditory target stimuli (numbers) while ignoring distracting environmental noise. The task was designed to simulate noisy situations known to be bothersome to schizophrenia patients (e.g. loud parties) (McGhie and Chapman, 1961). Using a crossover, placebo-controlled design, a significant diagnosis (control versus patient) × drug (placebo versus nicotine) interaction was observed in the ventral parietal cortex (VPC) and hippocampus. The effect was driven by relative hyperactivity in patients under placebo administration and reversal of this difference after nicotine. Based on previous findings in the VPC (Shulman et al., 2007) and hippocampus (Tregellas et al., 2007, 2009) these results were interpreted as evidence for nicotinic targeting of neuronal abnormalities associated with sensory flooding and distractibility in the illness.
Related to this last point, the VPC and inferior frontal gyrus make up a functional brain network called the Ventral Attention Network (VAN). The VAN is the principle network associated with ‘bottom-up’ (stimulus-driven) attention processing, is modulated by shifts in attention, and may be a functional index of distractibility (Shulman et al., 2002, 2007). To examine the effects of nicotine on VAN connectivity in schizophrenia patients and controls, our group analyzed connectivity during the selective attention task described above (Smucny et al., 2016b). Using psychophysiological interaction-based modeling, we found that nicotine restored functional connectivity of the VAN during the task in schizophrenia. Interestingly, nicotine was unable to target abnormal VAN connectivity during the resting state in patients, suggesting the drug’s effects may be task-specific. Taken together with the findings on nicotinic effects on VPC response, these results suggest that nicotine effectively targets abnormalities in neuronal functional associated with selective attention in schizophrenia.
Perspectives and future directions
The overall pattern of results presented in this review suggest that high rates of smoking in schizophrenia may be explained by a combination of factors, including intrinsic deficits in nicotinic signaling, targeting by nicotine of functionally abnormal circuits, and improved cognition. It is not surprising, therefore, that a number of clinical trials using investigational compounds that target the nicotinic receptor have been completed or are currently being conducted in schizophrenia (Freedman et al., 2008; Lieberman et al., 2013; Preskorn et al., 2014; Shim et al., 2012; Umbricht et al., 2014; Winterer et al., 2013). The results of these trials on cognitive endpoints thus far have been mixed, perhaps because the neuronal effects of nicotinic agents in schizophrenia (particularly during cognitive tasks) remain poorly understood. With the aid of neurophysiological and neuroimaging techniques, the ability of these and other investigational compounds to target neuronal dysfunction in schizophrenia may become a priority for psychiatry research in the near future.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the VA Biomedical Laboratory and Clinical Science Research and Development Service, Washington, DC (grant to Dr Tregellas), the Brain and Behavior Research Foundation, New York, NY (grant to Dr Tregellas), National Institutes of Health (NIH), Bethesda, MD grant MH-086383-01 (grant to Dr Tregellas), and NIH fellowship 1F31-MH102879-01A1 (grant to Mr Smucny).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adler LE, Hoffer LD, Wiser A, et al. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Griffith J, et al. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, et al. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Aguilar MC, Gurpegui M, Diaz FJ, et al. Nicotine dependence and symptoms in schizophrenia: Naturalistic study of complex interactions. Br J Psychiatry. 2005;186:215–221. doi: 10.1192/bjp.186.3.215. [DOI] [PubMed] [Google Scholar]
- Baier CJ, Pallares ME, Adrover E, et al. Prenatal restraint stress decreases the expression of alpha-7 nicotinic receptor in the brain of adult rat offspring. Stress. 2015;18:435–445. doi: 10.3109/10253890.2015.1022148. [DOI] [PubMed] [Google Scholar]
- Barch DM, Moore H, Nee DE, et al. CNTRICS imaging biomarkers selection: Working memory. Schizophr Bull. 2012;38:43–52. doi: 10.1093/schbul/sbr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Procyshyn RM, Hui P, et al. Self-reported motivation to smoke in schizophrenia is related to antipsychotic drug treatment. Schizophr Res. 2008a;100:252–260. doi: 10.1016/j.schres.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008b;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Long CJ, Cole DM, et al. The effects of nicotine replacement on cognitive brain activity during smoking withdrawal studied with simultaneous fMRI/EEG. Neuropsychopharmacology. 2011;36:1792–1800. doi: 10.1038/npp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinat C, Banister SD, Herrera M, et al. The therapeutic potential of alpha7 nicotinic acetylcholine receptor (alpha7 nAChR) agonists for the treatment of the cognitive deficits associated with schizophrenia. CNS Drugs. 2015;29:529–542. doi: 10.1007/s40263-015-0260-0. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: Anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5:411–424. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, et al. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Breese CR, Adams C, Logel J, et al. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387:385–398. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chambon V, Pacherie E, Barbalat G, et al. Mentalizing under influence: Abnormal dependence on prior expectations in patients with schizophrenia. Brain. 2011;134:3728–3741. doi: 10.1093/brain/awr306. [DOI] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, et al. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: An activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel-now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, et al. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res. 1993;10:131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Esterlis I, Carbuto M, et al. Lower ss2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry. 2012;169:326–334. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62:1564–1573. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H, Mast T, Yoshie N, et al. The slow response of the human cortex to auditory stimuli: Recovery process. Electroencephalogr Clin Neurophysiol. 1966;21:105–113. doi: 10.1016/0013-4694(66)90118-0. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- De Luca V, Voineskos S, Wong G, et al. Genetic interaction between alpha4 and beta2 subunits of high affinity nicotinic receptor: Analysis in schizophrenia. Exp Brain Res. 2006;174:292–296. doi: 10.1007/s00221-006-0458-y. [DOI] [PubMed] [Google Scholar]
- Diefendorf AR, Dodge R. An experimental study of the ocular reactions of the insane from 840 photographic records. Brain. 1908;31:451–489. [Google Scholar]
- Dulude L, Labelle A, Knott VJ. Acute nicotine alteration of sensory memory impairment in smokers with schizophrenia. J Clin Psychopharmacol. 2010;30:541–548. doi: 10.1097/JCP.0b013e3181f0c9c6. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, et al. The anatomy of first-episode and chronic schizophrenia: An anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Ranganathan M, Bois F, et al. In vivo evidence for beta2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biol Psychiatry. 2014;76:495–502. doi: 10.1016/j.biopsych.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Heaton RK, Paulsen JS, et al. The relationship of neuropsychological abilities to specific domains of functional capacity in older schizophrenia patients. Biol Psychiatry. 2003;53:422–430. doi: 10.1016/s0006-3223(02)01476-2. [DOI] [PubMed] [Google Scholar]
- Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: Implications for neuropsychiatric disorders. Mol Psychiatry. 2014;19:641–651. doi: 10.1038/mp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DJ, Grant B, Smith DM, et al. Nicotine and the hallucinating brain: Effects on mismatch negativity (MMN) in schizophrenia. Psychiatry Res. 2012;196:181–187. doi: 10.1016/j.psychres.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, et al. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med. 2014;65:245–261. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, et al. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Gault JM, et al. Linkage disequilibrium for schizophrenia at the chromosome 15q13–14 locus of the alpha7-nicotinic acetylcholine receptor subunit gene (CHRNA7) Am J Med Genet. 2001;105:20–22. [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Ross RG. Prenatal choline and the development of schizophrenia. Shanghai Arch Psychiatry. 2015;27:90–102. doi: 10.11919/j.issn.1002-0829.215006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, et al. Elementary neuronal dysfunctions in schizophrenia. Schizophr Res. 1991;4:233–243. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, et al. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gault J, Hopkins J, Berger R, et al. Comparison of polymorphisms in the alpha7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:39–49. doi: 10.1002/ajmg.b.20061. [DOI] [PubMed] [Google Scholar]
- George TP, Krystal JH. Comorbidity of psychiatric and substance abuse disorders. Curr Opin Psychiatry. 2000;13:327–331. [Google Scholar]
- George TP, Vessicchio JC, Termine A, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26:75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Ghiasi F, Farhang S, Farnam A, et al. The short term effect of nicotine abstinence on visuospatial working memory in smoking patients with schizophrenia. Nord J Psychiatry. 2013;67:104–108. doi: 10.3109/08039488.2012.687765. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Stollstorff M, Vaidya CJ. Using spatial multiple regression to identify intrinsic connectivity networks involved in working memory performance. Hum Brain Mapp. 2012;33:1536–1552. doi: 10.1002/hbm.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Shields J. A polygenic theory of schizophrenia. Proc Natl Acad Sci USA. 1967;58:199–205. doi: 10.1073/pnas.58.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Waiter G, O’Connor A, et al. Salience network-mid-brain dysconnectivity and blunted reward signals in schizophrenia. Psychiatry Res. 2012;211(2):104–111. doi: 10.1016/j.pscychresns.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, et al. Kraepelin and Bleuler had it right: People with schizophrenia have deficits sustaining attention over time. J Abnorm Psychol. 2012;121:641–648. doi: 10.1037/a0028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, et al. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Herran A, de Santiago A, Sandoya M, et al. Determinants of smoking behaviour in outpatients with schizophrenia. Schizophr Res. 2000;41:373–381. doi: 10.1016/s0920-9964(99)00082-1. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Cassidy BS, Andrews-Hanna JR, et al. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011;69:415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Schroeder M, Ross TJ, et al. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr Bull. 2011a;37:416–425. doi: 10.1093/schbul/sbp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Tagamets M, Avila M, et al. Specific motion processing pathway deficit during eye tracking in schizophrenia: A performance-matched functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Hong LE, Thaker GK, McMahon RP, et al. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 2011b;68:1195–1206. doi: 10.1001/archgenpsychiatry.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inami R, Kirino E, Inoue R, et al. Nicotine effects on mismatch negativity in nonsmoking schizophrenic patients. Neuropsychobiology. 2007;56:64–72. doi: 10.1159/000111536. [DOI] [PubMed] [Google Scholar]
- Iversen LL. Introduction to Neuropsychopharmacology. New York: Oxford University Press; 2009. [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, et al. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, et al. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Jeong B, Kubicki M. Reduced task-related suppression during semantic repetition priming in schizophrenia. Psychiatry Res. 2010;181:114–20. doi: 10.1016/j.pscychresns.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;213:11–37. doi: 10.1007/978-3-642-25758-2_2. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Bartels MN, et al. The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with schizophrenia: A single-blind, randomized clinical trial. Schizophr Bull. 2015;41:859–868. doi: 10.1093/schbul/sbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V, de la Salle S, Smith D, et al. Baseline dependency of nicotine’s sensory gating actions: Similarities and differences in low, medium and high P50 suppressors. J Psychopharmacol. 2013;27:790–800. doi: 10.1177/0269881113490449. [DOI] [PubMed] [Google Scholar]
- Knott V, Impey D, Philippe T, et al. Modulation of auditory deviance detection by acute nicotine is baseline and deviant dependent in healthy nonsmokers: A mismatch negativity study. Hum Psychopharmacol. 2014a;29:446–458. doi: 10.1002/hup.2418. [DOI] [PubMed] [Google Scholar]
- Knott V, Smith D, de la Salle S, et al. CDP-choline: Effects of the procholine supplement on sensory gating and executive function in healthy volunteers stratified for low, medium and high P50 suppression. J Psychopharmacol. 2014b;28:1095–1108. doi: 10.1177/0269881114553254. [DOI] [PubMed] [Google Scholar]
- Knott V, Impey D, Choueiry J, et al. An acute dose, randomized trial of the effects of CDP-Choline on Mismatch Negativity (MMN) in healthy volunteers stratified by deviance detection level. Neuropsychiatr Electrophysiol. 2015;1:1. [Google Scholar]
- Koutsouleris N, Gaser C, Jager M, et al. Structural correlates of psychopathological symptom dimensions in schizophrenia: A voxel-based morphometric study. Neuroimage. 2008;39:1600–1612. doi: 10.1016/j.neuroimage.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: The self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Ngan ET, Bates AT, et al. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Laurent A, Saoud M, Bougerol T, et al. Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res. 1999;89:147–159. doi: 10.1016/s0165-1781(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Le Houezec J. Nicotine: Abused substance and therapeutic agent. J Psychiatry Neurosci. 1998;23:95–108. [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Freedman R. Genetics of chromosome 15q13-q14 in schizophrenia. Biol Psychiatry. 2006;60:115–122. doi: 10.1016/j.biopsych.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, et al. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, et al. Eye tracking dysfunction and schizophrenia: A critical perspective. Schizophr Bull. 1993;19:461–536. doi: 10.1093/schbul/19.3.461. [DOI] [PubMed] [Google Scholar]
- Levy DL, Sereno AB, Gooding DC, et al. Eye tracking dysfunction in schizophrenia: Characterization and pathophysiology. Curr Top Behav Neurosci. 2010;4:311–347. doi: 10.1007/7854_2010_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38:968–975. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kaneko Y, Ouyang X, et al. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2012;38:285–294. doi: 10.1093/schbul/sbq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Ford JM, Sarter M, et al. CNTRICS final biomarker selection: Control of attention. Schizophr Bull. 2012;38:53–61. doi: 10.1093/schbul/sbr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowick KM, Barr MS, Wing VC, et al. Neurocognitive endophenotypes in schizophrenia: Modulation by nicotinic receptor systems. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:79–85. doi: 10.1016/j.pnpbp.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutle A, Zhang X, Court J, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22:115–126. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Hanlon FM, Teshiba TM, et al. An fMRI study of multimodal selective attention in schizophrenia. Br J Psychiatry. 2015;207:420–428. doi: 10.1192/bjp.bp.114.155499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mexal S, Berger R, Logel J, et al. Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. J Mol Neurosci. 2010;40:185–195. doi: 10.1007/s12031-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Mitchell RL, Elliott R, Barry M, et al. Neural response to emotional prosody in schizophrenia and in bipolar affective disorder. Br J Psychiatry. 2004;184:223–230. doi: 10.1192/bjp.184.3.223. [DOI] [PubMed] [Google Scholar]
- Mobascher A, Warbrick T, Brinkmeyer J, et al. Nicotine effects on anterior cingulate cortex in schizophrenia and healthy smokers as revealed by EEG-informed fMRI. Psychiatry Res. 2012;204:168–177. doi: 10.1016/j.pscychresns.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Moran LV, Sampath H, Stein EA, et al. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142:223–229. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll GA, Callahan BL. Smooth pursuit in schizophrenia: A meta-analytic review of research since 1993. Brain Cogn. 2008;68:359–370. doi: 10.1016/j.bandc.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Young DA, et al. Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology. 1998;18:175–185. doi: 10.1016/S0893-133X(97)00095-X. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, et al. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol Med. 2011;41:1701–1708. doi: 10.1017/S0033291710002205. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, White TP, Liddle PF. The concept of salience network dysfunction in schizophrenia: From neuroimaging observations to therapeutic opportunities. Curr Top Med Chem. 2012;12(21):2324–2338. doi: 10.2174/156802612805289881. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Icenogle L, Levin ED. Ventral hippocampal alpha7 and alpha4beta2 nicotinic receptor blockade and clozapine effects on memory in female rats. Psychopharmacology (Berl) 2006;188:597–604. doi: 10.1007/s00213-006-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarro S, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: Dysfunction of the default mode network? Psychol Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Potter D, Summerfelt A, Gold J, et al. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Gawryl M, Dgetluck N, et al. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: A proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract. 2014;20:12–24. doi: 10.1097/01.pra.0000442935.15833.c5. [DOI] [PubMed] [Google Scholar]
- Radek RJ, Kohlhaas KL, Rueter LE, et al. Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Curr Pharm Des. 2010;16:309–322. doi: 10.2174/138161210790170166. [DOI] [PubMed] [Google Scholar]
- Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW. Diversity in primary structure and function of neuronal nicotinic acetylcholine receptor channels. Curr Opin Neurobiol. 1992;2:254–262. doi: 10.1016/0959-4388(92)90112-x. [DOI] [PubMed] [Google Scholar]
- Rowe AR, Mercer L, Casetti V, et al. Dementia praecox redux: a systematic review of the nicotinic receptor as a target for cognitive symptoms of schizophrenia. J Psychopharmacol. 2015;29:197–211. doi: 10.1177/0269881114564096. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Steckler T, Shoaib M. Nicotine improves working memory span capacity in rats following sub-chronic ketamine exposure. Neuropsychopharmacology. 2011;36:2774–2781. doi: 10.1038/npp.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: Involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Seiferth NY, Pauly K, Kellermann T, et al. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology. 2009;34:477–487. doi: 10.1038/npp.2008.93. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, et al. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;21(Pt 12):2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Shamsi S, Lau A, Lencz T, et al. Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophr Res. 2011;126:257–264. doi: 10.1016/j.schres.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JC, Jung DU, Jung SS, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: A randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:660–668. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, et al. Right TPJ deactivation during visual search: Functional significance and support for a filter hypothesis. Cereb Cortex. 2007;17:2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- Shulman GL, d’Avossa G, Tansy AP, et al. Two attentional processes in the parietal lobe. Cereb Cortex. 2002;12:1124–1131. doi: 10.1093/cercor/12.11.1124. [DOI] [PubMed] [Google Scholar]
- Sinkus ML, Lee MJ, Gault J, et al. A 2-base pair deletion polymorphism in the partial duplication of the alpha7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 2009;1291:1–11. doi: 10.1016/j.brainres.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, et al. Brain connectivity is not only lower but different in schizophrenia: A combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Edgar JC, Huang M, et al. Cognitive abilities and 50-and 100-msec paired-click processes in schizophrenia. Am J Psychiatry. 2010;167:1264–1275. doi: 10.1176/appi.ajp.2010.09071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CW, Cornblatt BA. Attention deficits in the development of schizophrenia: Recent evidence from genetic high-risk and prodromal studies. Curr Psychos Ther Rep. 2005;3:152–156. [Google Scholar]
- Smith RC, Warner-Cohen J, Matute M, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Eichman LC, et al. Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophr Res. 2013;147:196–200. doi: 10.1016/j.schres.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Rojas DC, et al. Neuronal effects of nicotine during auditory selective attention in schizophrenia. Hum Brain Mapp. 2016a;37:410–421. doi: 10.1002/hbm.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Tregellas JR. Nicotine restores functional connectivity of the ventral attention network in schizophrenia. Neuropharmacology. 2016b;108:144–151. doi: 10.1016/j.neuropharm.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Tregellas J. Nicotinic modulation of intrinsic brain networks in schizophrenia. Biochem Pharmacol. 2013;86:1163–1172. doi: 10.1016/j.bcp.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Wylie KP, Kronberg E, et al. Nicotinic modulation of salience network connectivity and centrality in schizophrenia. J Psychiatr Res. 2017;89:85–96. doi: 10.1016/j.jpsychires.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, et al. Nicotine-induced limbic cortical activation in the human brain: A functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stephens SH, Logel J, Barton A, et al. Association of the 5’-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res. 2009;109:102–112. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Suwa H, Matsushima E, Ohta K, et al. Attention disorders in schizophrenia. Psychiatry Clin Neurosci. 2004;58:249–256. doi: 10.1111/j.1440-1819.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Nyberg E, Martin LF, et al. Nicotine effects on default mode network during resting state. Psychopharmacology (Berl) 2011;216:287–295. doi: 10.1007/s00213-011-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC, et al. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Ellis J, Shatti S, et al. Increased hippocampal, thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. Am J Psychiatry. 2009;166:354–360. doi: 10.1176/appi.ajp.2008.08030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Olincy A, Johnson L, et al. Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology. 2010;35:938–942. doi: 10.1038/npp.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe J, Rojas DC, et al. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry. 2011;69:7–11. doi: 10.1016/j.biopsych.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Martin LF, et al. fMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. Am J Psychiatry. 2005;162:391–393. doi: 10.1176/appi.ajp.162.2.391. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Miller DE, et al. Monitoring eye movements during fMRI tasks with echo planar images. Hum Brain Mapp. 2002;17:237–243. doi: 10.1002/hbm.10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Miller DE, et al. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: An fMRI study. Am J Psychiatry. 2004;161:315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- Tu P, Buckner RL, Zollei L, et al. Reduced functional connectivity in a right-hemisphere network for volitional ocular motor control in schizophrenia. Brain. 2010;133:625–637. doi: 10.1093/brain/awp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PC, Hsieh JC, Li CT, et al. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: A resting fMRI study. Neuroimage. 2012;59:238–247. doi: 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- Tu PC, Yang TH, Kuo WJ, et al. Neural correlates of antisaccade deficits in schizophrenia, an fMRI study. J Psychiatr Res. 2006;40:606–612. doi: 10.1016/j.jpsychires.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Keefe RS, Murray S, et al. A randomized, placebo-controlled study investigating the nicotinic alpha7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014;39:1568–1577. doi: 10.1038/npp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Krljes S. Mismatch negativity in schizophrenia: A meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Venables PH. Input Dysfunction in Schizophrenia. Prog Exp Pers Res. 1964;72:1–47. [PubMed] [Google Scholar]
- Verleger R, Talamo S, Simmer J, et al. Neurophysiological sensitivity to attentional overload in patients with psychotic disorders. Clin Neurophysiol. 2013;124:881–892. doi: 10.1016/j.clinph.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Vinogravoda OS. Functional Organization of the Limbic System in the Process of Registration of Information: Facts and Hypothesis. New York: Plenum Press; 1975. [Google Scholar]
- Voineskos S, De Luca V, Mensah A, et al. Association of alpha-4beta2 nicotinic receptor and heavy smoking in schizophrenia. J Psychiatry Neurosci. 2007;32:412–416. [PMC free article] [PubMed] [Google Scholar]
- White TP, Joseph V, Francis ST, et al. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123:105–115. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry. 2010;23:112–119. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]
- Winterer G, Gallinat J, Brinkmeyer J, et al. Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: A proof-of-mechanism study. Neuropharmacology. 2013;64:197–204. doi: 10.1016/j.neuropharm.2012.06.040. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Barik J, Dickinson J, et al. Nicotinic receptors modulate transmitter cross talk in the CNS: Nicotinic modulation of transmitters. J Mol Neurosci. 2006;30:137–140. doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10:1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]