Abstract
Background
Heart Involvement is the most important prognostic determinant in AL amyloidosis patients. Echocardiography is a cornerstone for the diagnosis and provides important prognostic information.
Methods and Results
We studied 754 patients with AL amyloidosis who underwent echocardiographic assessment including a Doppler derived measurement of stroke-volume (SV) within 30 days of their diagnosis to explore the prognostic role of echocardiographic variables in the context of a well-established soluble cardiac biomarker staging system. Reproducibility of SV, MCF and LV-strain was assessed in a separate, yet comparable, study cohort of 150 patients from the Pavia Amyloidosis Center.
The echocardiographic measures most predictive for overall survival were SV-index (SVI)<33 mL/min, myocardial contraction fraction <34%, and cardiac-index <2.4 L/min/m2 with respective hazard ratios [HR] (95% confidence intervals) of 2.95 (2.37, 3.66), 2.36 (1.96, 2.85), and 2.32 (1.91, 2.80). For the subset who had left ventricular strain (LV-strain) performed, the prognostic cut-point was -14% [HR 2.70 (1.84, 3.96)]. Each parameter was independent of systolic blood pressure, Mayo staging system (NT-proBNP and troponin), and ejection fraction on multi-variable analysis. Simple predictive models for survival including biomarker staging along with SVI or LV-strain were generated.
Conclusions
SVI prognostic performance was similar to LV-strain in predicting survival in AL amyloidosis, independently of biomarker staging. Since SVI is routinely calculated and widely available, it could serve as the preferred echocardiographic measure to predict outcomes in AL amyloidosis patients.
Introduction
The systemic amyloidoses are disorders of protein conformation and metabolism that result in tissue deposition of insoluble fibrils, organ dysfunction, and death. In light chain amyloidosis (AL), fibrils are composed mainly by the N-terminus of a monoclonal immunoglobulin light chain1. AL is a systemic disease, and most patients have clinical involvement of more than one organ at diagnosis. Heart involvement is present in almost two-thirds of patients with this disease, representing the most common cause of death2, 3.
Cardiac amyloidosis typically causes a restrictive cardiomyopathy,4 in which the deposition of misfolded amyloid proteins in the myocardium, causes structural alterations whereas cardiotoxic light chains cause myocardial dysfunction5, 6, eventually leading to heart failure. Despite the progression of the disease, ejection fraction (EF) often remains within the “normal” or “preserved” range, and a reduced EF is a sign of late or end stage disease7, 8. More subtle echocardiographic parameters such as increased left ventricular wall thickness and decreased fractional shortening, which have been found to be independent predictors of cardiac mortality in AL amyloidosis7, 9, are often overlooked. Myocardial strain imaging10-14 and midwall fractional shortening are useful for diagnosis and prognostication of survival among patients with cardiac amyloidosis15-18, but are not routinely performed. Myocardial strain imaging is limited by technical challenges in acquisition and vendor specific variation in calculated results. The myocardial contraction fraction (MCF), which is defined as the ratio of stroke volume (SV) to myocardial volume (MV), was proposed by King et al. as a novel index of myocardial function,19 being a volumetric measure of myocardial shortening, which differentiates myocardial performance in patients with pathological versus physiologic hypertrophy. In a recent paper of 34 patients, MCF was superior to EF in predicting overall survival among AL amyloidosis patients20. N-terminal pro-natriuretic type B (NT-proBNP)21 and cardiac troponins (cTn)22-24 are currently the most important markers for the assessment of prognosis in AL amyloidosis and are combined in different staging systems25-27. Unfortunately, the general cardiologist often overlooks the prognostic implications of these variables and/or they are tests that are ordered only after an index of suspicion for AL amyloidosis has been raised. We, therefore set out to assess the potential prognostic value of widely accessible echocardiographic variables in a larger cohort of AL amyloidosis patients.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The study included 754 patients who underwent hematologic assessment, as well as complete echocardiographic evaluation at Mayo Clinic, Rochester, MN, USA, within 30 days of their AL amyloidosis diagnosis. Patients were diagnosed from January 1, 1996 through February 1, 2015. The diagnosis of AL amyloidosis was predicated on the presence of organ involvement as previously defined28 and a tissue biopsy specimen that stained positive with Congo red and exhibited green birefringence under polarized light. The diagnosis of AL amyloidosis was confirmed by tissue typing with immunohistochemistry, immunofluorescence, or laser-microdissection mass spectrometry according to the technique available at the time of diagnosis29, 30. The Mayo Foundation institutional review board approved the study, and all patients consented to have their medical records reviewed according to institutional review board practices.
Echocardiography
Because the study covered a 19 years period, clinical echocardiography protocols varied, with more of an emphasis on two-dimensional (2D) measurements. Patients were evaluated with standard echocardiographic protocols used in the Mayo Clinic Echocardiography Laboratory at the time of diagnosis. Measurements of diastolic thickness of the inter-ventricular septum and LV posterior wall, end-systolic and end-diastolic LV diameters 2D techniques, LV-mass, EF, left atrial volume and Doppler evaluation of diastolic function and SV were carried out as previously described31, 32. MCF was calculated as left ventricular SV (obtained from a Doppler derived method) divided by left ventricular myocardial volume, which is defined as LV-mass divided by the mean density of myocardium (1.05 g/mL)19. Using a previously validated technique, LV end-diastolic and end-systolic volumes were calculated from two-dimensional echo guided M-mode echocardiographic dimensions as previously reported33-35. In addition, a subset of 238 patients had measurement of left ventricular global peak longitudinal strain (LV-strain) using speckle-tracking as previously described36. Sonographers with experience in obtaining 2D-STE performed the echocardiographic studies using a Vivid 7 or E9 GE Medical System with a 2.5- to 4.0-MHz transducer in accordance with the guidelines of the American Society of Echocardiography31. LV-strain measurements were performed during the echocardiographic examination with dedicated vendor-specific software (EchoPAC PC version 6.0, GE Healthcare Co).
Reproducibility of SV, MCF and LV-strain was assessed in a separate, yet comparable, study cohort from the Pavia Amyloidosis Center. Briefly, echo data from 150 consecutive patients with AL amyloidosis, were analyzed twice by the same observer, allowing a 2-week time interval between two blinded measurements. This allowed assessing the intra-observer reproducibility of the different measures. A second observer repeated the same measures independently, to assess inter-observer reproducibility. Reproducibility was assessed in terms of coefficients of variation (CV) and percentage difference (PD), which is defined as the absolute difference between two paired values divided by the average of the two values, and expressed as a percentage.
Biomarkers
In all patients, blood was collected at the time of diagnosis. Creatinine, glomerular filtration rate (GFR), alkaline phosphatase, albumin and 24-hour protein loss were measured at the time of the diagnosis in all the patients. Since 2004, measurement of NT-proBNP, cardiac troponin T and free light chain were added to the routine panel. The cardiac biomarkers NT-proBNP (cutoff 1800 ng/L) and troponin T (cutoff 0.025 mcg/L), difference between amyloidogenic and non-amyloidogenic free light chain (dFLC, cutoff 180 mg/L), were combined into the Mayo 2012 cardiac staging system26. Stage I patients have all markers below the cutoffs, stage II only one marker above the cutoff, stage III patients three markers above the cutoffs and stage IV all markers above the cutoffs.
Statistical analysis
Continuous variables were expressed as median values and interquartile range, and categorical variables as frequencies and percentages. Correlation among variables was summarized using Pearson's correlation coefficient. Survival was analyzed using the Kaplan-Meier method to estimate the distribution of survival as a function of the follow-up duration, while censoring those not known to be deceased at last known follow-up. The best cutoffs of all the variables were evaluated using the Contal and O'Quigley method. Using these cutoffs, sensitivity and specificity were calculated based on 2 years mortality. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were estimated using Cox proportional hazards. When analyzing continuous variables, HR are reported per 1 standard deviation in each variable to allow comparability between variables with different scales. In the subset of patients assessable for cardiac biomarkers, Harrell's survival c-Statistics were calculated to assess the discriminatory ability of the models and compare different models for predicting overall mortality37. Comparisons among c-Statistics were computed using the standard errors derived from 1000 boostrap samples. Survival curves were plotted according to the Kaplan-Meier method and differences in survival were tested using the log-rank test. All analyses were performed using SAS software V.9.4 and JMP 10.0 (Cary, North Carolina, USA). All p-values were two-sided and p≤0.05 were considered to be statistically significant.
Results
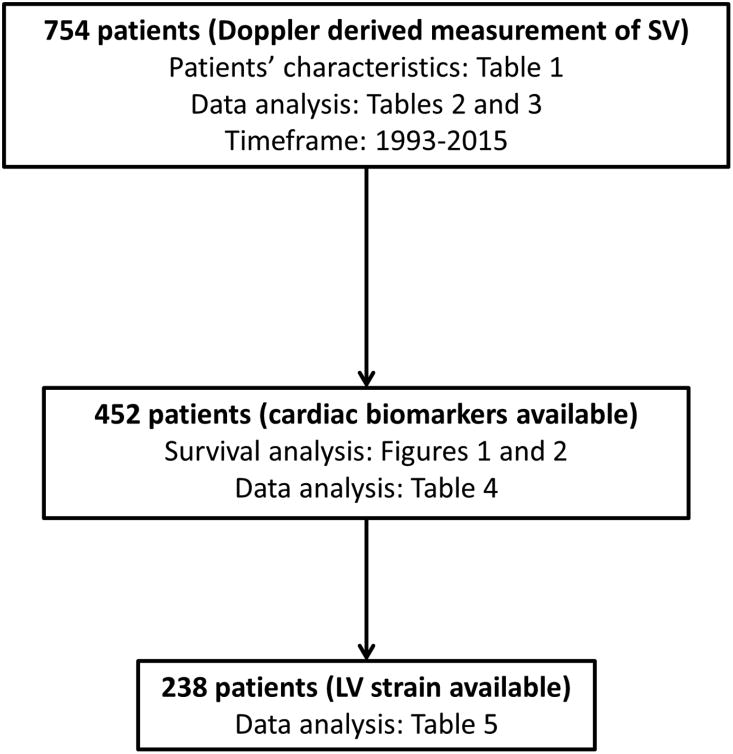
The study population was composed of 754 patients with a biopsy proven AL amyloidosis diagnosis.A total of 495 (66%) of patients had a positive biopsy of the abdominal fat aspirate and 80 (10%) had a positive endomyocardial biopsy; 465 (61%) had amyloid deposits in the bone marrow biopsy. In Figure 1 we defined how the patient population was divided according to the time of diagnosis. Patient characteristics are shown in Supplemetal Table 1. Median age was 64 years, and 65% were male. A total of 591 (78%) patients were considered to have cardiac amyloidosis based on diagnostic criteria (IVS>12 mm in the absence of other cardiac causes and an elevated NT-proBNP (>332 ng/L)28, 38. During a median follow-up of living patients of 40.8 months [interquartile range (IQR): 19-72 months], 480 (63%) have died. Standard two-dimensional echocardiographic parameters, SVI, LV-strain, and MCF are illustrated in Supplemental Table 2 along with their impacts on the risk of death expressed by HR. In particular, an SVI below the cutoff of 33 mL/m2 was able to significantly predict overall survival [HR:2.95 (CI: 2.37, 3.66), P<0.001]. In addition, MCF <34% significantly predicted survival [HR:2.36 (1.96, 2.85)]. The measures of MCF, SVI and CI were associated with a reduced survival (Figure 2, Panel A-C). These cutoffs were able to discriminate the overall survival if the study population was divided into different cohorts according to the time of diagnosis, that had a different outcome as previously described by our group39. In particular, in Supplemental Figure 1 we reported the outcome of patients in the cohort of 2005-2009 (Panel A/B) and the cohort 2010-2015 (Panel C/D) for both SVI and MCF. It is also shown the effect of circulating cardiac biomarkers, assessed by the prognostic impact of the cardiac biomarker staging system (Supplemental Figure 2). Sensitivity and specificity data of the different cutoffs are reported in Supplemental Table 3.
Figure 1.
Consort diagram of the study population.
Figure 2.
- OS according to MCF.
- OS according to SVI.
- C. OS according to CI.
In the 754 patients evaluable for MCF, SVI and CI, incremental multi-variable modeling was performed to assess the association of echocardiographic variables with risk of death, excluding LV-strain due to the limited number of patients with available strain measurements. Since MCF, SVI, and CI all contain SV as part of their calculation, separate models were generated for each of them (Table 1). The three variables (MCF, SVI, CI) were independent predictors of survival along with EF, systolic blood pressure (SBP) and type of first-line therapy (autologous stem cell transplant, ASCT). Other echocardiographic parameters, such as IVS and PW, and the presence of pericardial effusion were not independent of MCF, but IVS remained an independent predictor in the models with SVI and CI (Table 1).
Table 1.
Multi-variable proportional hazards model for risk of death using echocardiographic parameters only (n=754).
| MCF<33.6% model | SVI<33 mL/m2model | CI<2.44 model | ||||
|---|---|---|---|---|---|---|
| Variable | HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P |
| MCF, SVI, or CI | 1.72(1.39, 2.13) | <0.001 | 1.90(1.47, 2.46) | <0.001 | 1.50(1.20, 1.87) | <0.001 |
| EF<55% | 1.73(1.40, 2.14) | <0.001 | 1.60(1.24, 2.06) | <0.001 | 1.73(1.39, 2.16) | <0.001 |
| SBP<95 mmHg | 1.44(1.16, 1.81) | 0.001 | 1.47(1.13, 1.90) | 0.004 | 1.42(1.14, 1.78) | 0.002 |
| IVS>12 mm | Not-included | 1.44(1.07, 1.93) | 0.02 | 1.48(1.17, 1.86) | 0.001 | |
| ASCT | 0.57(0.43, 0.76) | <0.001 | 0.50(0.35, 0.72) | <0.001 | 0.56(0.42, 0.74) | <0.001 |
Additional analysis was performed using the subset of 452 patients with available circulating cardiac biomarkers. This subgroup could be stratified with the use of the Mayo 2012 cardiac biomarker staging that identified four stages with significant different overall survival (Supplemental Figure 2). A significant worsening of the echocardiographic parameters according to the different biomarkers staging was noted (Supplemental Table 4). In particular, while the median EF in stage IV resulted still>50%, MCF, SVI and LV-strain were significantly reduced compared to the median value. MCF, SVI and CI each retained prognostic value upon addition of cardiac biomarkers staging (Mayo 2012 stage III and IV) to the models shown in Table 2. Results were similar when staging was analyzed across 4 levels rather than combined, though MCF was only of borderline significance (Supplemental Table 5). Other useful echocardiographic variables that resulted significant predictor of survival at univariate analysis were tested in a stepwise multivariate approach. In particular, IVS, PW, deceleration time and relative wall thickness were added to the analyses but none of these variables were prognostic in the models with SVI in addition to cardiac biomarker staging. To identify the model most predictive of survival we compared the three systems containing the prognostic variables (SVI, MCF and CI) using the survival c-Statistic. A baseline model of EF, SBP and biomarker staging had a c-Statistic 0.70 (95%CI 0.67, 0.74). The addition SVI—but not MCF or CI—added prognostic power to the basal model (Table 2) with respective c-Statistics of 0.72 (0.68, 0.75), P=0.010. To further validate these analyses, a second method of measuring SV, the Simpson method incorporating 2D data, was used to calculate MCF and SVI; MCF was no longer independently prognostic, but SVI was (Supplemental Table 6).
Table 2.
Multi-variable proportional hazards model for risk of death incorporating circulating cardiac biomarkers (n=452).
| MCF<33.6% model | SVI<33 mL/m2 model | CI<2.44 model | ||||
|---|---|---|---|---|---|---|
| Variable | HR(95%CI) | P | HR(95%CI) | P | HR(95%CI) | P |
| MCF, SVI, or CI | 1.54(1.11, 2.14) | 0.009 | 1.92(1.42, 2.60) | <0.001 | 1.48(1.10, 1.99) | 0.01 |
| EF<55% | 1.59(1.20, 2.10) | 0.001 | 1.41(1.05, 1.88) | 0.02 | 1.58(1.19, 2.10) | 0.002 |
| SBP<95 mmHg | 1.51(1.13, 2.02) | 0.006 | 1.40(1.04, 1.88) | 0.03 | 1.50(1.11, 2.01) | 0.007 |
| Stage III&IV | 1.83(1.30, 2.58) | <0.001 | 1.89(1.36, 2.63) | <0.001 | 2.07(1.50, 2.86) | <0.001 |
| ASCT | 0.62(0.40, 0.95) | 0.03 | 0.61(0.40, 0.94) | 0.02 | 0.65(0.42, 0.99) | 0.05 |
| c-Statistic | 0.70(0.67, 0.74) | 0.72(0.68, 0.75) | 0.70(0.67, 0.74) | |||
In order to evaluate the contribution of LV-strain, the 238 patients who had baseline LV-global peak systolic strain assessment were included. The optimal cutoff of LV-strain predicting mortality at 2 years was greater than or equal to -14% (sensitivity:86.2%; specificity:57.1%) and was able to sharply discriminate the overall survival of patients (Supplemental Figure 3, A). Of note, MCF was highly correlated with LV-strain (r:-0.85, P<0.001, Supplemental Figure 3, B). The correlation between LV-strain and SVI was less strong (r:-0.70, P<0.001, not shown). When LV-strain was added to EF, SBP and cardiac biomarker stages III and IV, LV-strain was an independent risk factor (Table 3, model A); however, the addition of SVI to model A drove LV-strain from the model (Table 3, models B and C). This analysis led us to investigate a simplified model containing circulating biomarker stage and each of the most powerful cardiac measures in the group of patients evaluable for cardiac biomarkers (Supplemental Table 7). The addition of SVI to biomarker staging system had the higher c-Statistic as compared to biomarkers alone. In the subset of patients with advanced cardiac stages (stage III and IV and cardiac stage IIIa and IIIb, according to Wechalekar et al.27) SVI was able to identify a subgroup of patients with a significant better overall survival (Figure 3).
Table 3.
Multi-variable proportional hazards models for risk of death incorporating LV-strain and SVI (n=238).
| Model A | Model B | Model C | ||||
|---|---|---|---|---|---|---|
| Variable | HR(95%CI) | P | HR(95% CI) | P | HR(95% CI) | P |
| LV-Strain≥-14% | 1.95(1.04, 3.66) | 0.04 | 1.75(0.93, 3.33) | 0.08 | Not-included | |
| EF<55% | 1.26(0.81, 1.97) | 0.31 | 1.02(0.64, 1.64) | 0.92 | 1.16(0.73, 1.84) | 0.54 |
| SBP†<95 mmHg | 1.60(1.01, 2.52) | 0.04 | 1.38(0.86, 2.20) | 0.18 | 1.36(0.85, 2.18) | 0.20 |
| Stage III&IV | 2.69(1.44, 5.01) | 0.002 | 2.51(1.34, 4.70) | 0.004 | 3.11(1.73, 5.59) | <0.001 |
| SVI<33 mL/m2 | Not-included | 2.00(1.24, 3.23) | 0.004 | 2.14(1.32, 3.47) | 0.002 | |
| ASCT | 0.34(0.12, 0.96) | 0.04 | 0.37(0.13, 1.05) | 0.06 | 0.36(0.13, 1.02) | 0.05 |
| c-statistic | 0.75(0.70, 0.80) | 0.76(0.72, 0.81) | 0.75(0.70, 0.80) | |||
Figure 3.
- OS according to SVI in Mayo 2012 stage III.
- OS according to SVI in Mayo 2012 stage IV.
- OS according to SVI in stage IIIa.
- OS according to SVI in stage IIIb.
The intra-observer and inter-observer coefficients of variations were, respectively, 0.4% and 0.7% for LV end-diastolic diameter, 3.0% and 3.4% for septal wall thickness, 2.9% and 3.2% for posterior wall LV-thickness. As to LV-strain data, intra-observer and inter-observer percentage difference were 5%±4% [r=0.98; 95%CI limits of agreements (LOA), 2.38% to 0.99%] and 6%±4% (r=0.96; 95%CI LOA, 3.02% to 1.39%), respectively. MCF and SV showed slightly worse reproducibility. Intra-observer and inter-observer percentage difference were 8%±5% (r=0.86; 95%CI LOA, 5.22% to 2.99%) and 9%±6% (r=0.82; 95%CI LOA, 5.99% to 3.23%) for MCF and 8%±5% (r=0.84; 95%CI LOA, 5.44% to 2.89%) and 10% ± 6% (r=0.78; 95%CI LOA, 6.66% to 3.43%) for SV.
Discussion
Understanding outcomes in patients with AL is essential given the heterogeneity of disease. Echocardiography was one of the first and most important diagnostic and prognostic tools for cardiac assessment among patients with AL40. In terms of prognosis, soluble cardiac biomarkers have largely supplanted echocardiography. We set out to assess how measures like MCF41, LV-strain42, SVI, and CI held up against other simple prognostic measures like troponin T, NT-proBNP26 and systolic blood pressure27. Using one of the largest cohorts of subjects with AL amyloidosis with echocardiographic assessment and soluble cardiac biomarkers, we found that MCF correlated quite well with LV-strain and that it was prognostic for overall survival even in the context of cardiac biomarkers. The prognostic impact of these variables was also independent of the type of therapy. The echocardiographic variables reported in our manuscript are similar to those previously reported by others15, 16, 20. In addition, SVI was able to identify a subset of patients with a better prognosis, even in the context of patients with severe cardiac involvement according to the cardiac biomarker staging systems. Therefore, we observed that SVI performed as well as MCF and LV-strain in survival models leading us to believe that this simple measure—SVI—is the favored echocardiographic parameter to predict outcomes among patients with AL amyloidosis. Interestingly, a similar cutoff of SVI (35mL/m2) has been found to be a useful prognostic marker in patients with aortic stenosis with preserved EF43. An advantage of SVI is that is routinely calculated as part of a standard echocardiographic study, requires little time, does not require additional calculations or analysis, is not limited by the challenges in determining left ventricular mass as is required for MCF, or those of LV-strain (2D-image-quality, sonographer expertise, and vendor and software variations). We did show, however, that LV-strain when using the same vendor and software data was slightly more reproducible than MCF and SV (that are measured by manual tracing and hence more operator-dependent).
Because the published data on MCF 41 was compelling, we speculated that SVI might be as useful and more readily available in a busy cardiology practice since MCF is defined as the ratio between SV and myocardial volume. The MCF evaluates shortening in relation to the degree of amyloid infiltration (as inferred from the myocardial chamber volume) and quantitates the effect of amyloid infiltration in the myocardium. Progressive amyloid deposition in the myocardium causes an increase in left ventricular myocardial volume concomitant with a decline in SV, which is indicated by declining MCF41. It is important to note that, in those echocardiographic laboratories in which the LV-strain is not yet (or readily) available, the measure of MCF appears to be a reasonable substitute for patients with AL amyloidosis, serving as the “poor man's LV-strain.” Similar to EF, the MCF is dependent on afterload19 but in contradistinction to EF in AL amyloid in which SV and EDV show parallel declines (and hence no change in EF), the MCF declines because increases in MV (due to amyloid deposition) are accompanied by SV reduction. Thus, in the setting of amyloid-induced LV mass increase, (that can be also viewed as amyloid-induced LV “hypertrophy”), MCF is reduced, analogous to mid-wall fractional shortening44. Although MCF does not require specific software or a specific training, since it is calculated from conventional echocardiographic measures, it is not automatically calculated by standard package software. In addition, the measurements that are needed for the calculation of LV-mass and myocardial volume are hampered by possible errors. To limit these, we focused only on 2D-derived data, excluding from the study all the subjects that had only M-mode based measurements of LV chamber size. However, the prognostic ability of MCF resulted limited, if the SV was calculated by only 2D measurement. Another important limitation of MCF is that the measurement of myocardial volume assumes a constant myocardial density (1.05g/ml) irrespective of the underlying pathology, which presumes that there is no difference between myocardial tissue and amyloid tissue density. Previous data have shown that myocardial density varies in several conditions such as cardiac infarction45. Moreover, a recent study with cardiac magnetic resonance documented a different degree of extracellular volume expansion in the main types of cardiac amyloidosis46. The use of SVI as a prognostic marker could overcome this assumption and the different limitations highlighted for MCF. LV-strain is a powerful tool, with the ability to promote early diagnosis by detection of subtle abnormalities in ventricular function and recognition of the characteristic pattern of apical sparing. However, unlike SVI, LV-strain requires specific vendor-dependent software, expertise, and time and is not available in all centers. In addition, the results are not standardized across ultrasound systems leading to a recommendation that serial studies be performed on the same machine. Recognizing the role of inter- and intra-observer variability in any measurement as a potential limitation, we integrated our data with an evaluation of reproducibility on a comparable patients' cohort. The reasonable reproducibility of Doppler-derived measurement of SV reinforce the role of our new proposed approach. Indeed, we documented a high prognostic role of this measurement in a real-world data setting, encompassing several years in a referral echocardiographic laboratory. In addition, a validation analysis confirmed our results by assessing the prognostic role of 2D-derived SVI. Unfortunately, a limitation of our study is that only few patients had cardiac magnetic resonance data and 3D echocardiographic data available. Therefore, no comparison with this method was possible. In addition, in this series we did not have a correlation with EKG data. Further studies in large series are warranted to assess the prognostic impact of SVI in the setting of cardiac biomarkers.
In conclusion, SVI is a simple, powerful, independent predictors of survival in patients with AL amyloidosis. In particular, the prognostic role of SVI was independent of EF, BP, the cardiac biomarkers staging, and LV-strain. SVI performed at least as well as LV-strain for predicting survival in AL, independent of biomarker staging. Since SVI is routinely calculated and widely available, it could serve as the preferred echocardiographic measure to predict outcomes in AL amyloidosis patients.
Supplementary Material
Acknowledgments
Sources of Funding: Dr. Maurer, NIH K24 AG036778 Midcareer Mentoring Award in Geriatric Cardiology.
Disclosures: Dr. Maurer or his institution receives funding for research and serving on advisory boards and DSMBs from Pfizer Inc., Alnylam Pharmaceuticals Inc., GSK Inc., ISIS Pharmaceuticals and Prothena Inc.; Dr. Merlini is advisory Board of Pfizer, Janssen, Takeda, Prothena Inc.;
Footnotes
all other Authors have nothing to disclose.
References
- 1.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 2.Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, Falk RH. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM : monthly journal of the Association of Physicians. 1998;91:141–57. doi: 10.1093/qjmed/91.2.141. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 4.McCarthy RE, 3rd, Kasper EK. A review of the amyloidoses that infiltrate the heart. Clin Cardiol. 1998;21:547–52. doi: 10.1002/clc.4960210804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavatelli F, Imperlini E, Orru S, Rognoni P, Sarnataro D, Palladini G, Malpasso G, Soriano ME, Di Fonzo A, Valentini V, Gnecchi M, Perlini S, Salvatore F, Merlini G. Novel mitochondrial protein interactors of immunoglobulin light chains causing heart amyloidosis. FASEB J. 2015;29:4614–28. doi: 10.1096/fj.15-272179. [DOI] [PubMed] [Google Scholar]
- 6.Guan J, Mishra S, Qiu Y, Shi J, Trudeau K, Las G, Liesa M, Shirihai OS, Connors LH, Seldin DC, Falk RH, MacRae CA, Liao R. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol Med. 2014;6:1493–507. doi: 10.15252/emmm.201404190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristen AV, Perz JB, Schonland SO, Hegenbart U, Schnabel PA, Kristen JH, Goldschmidt H, Katus HA, Dengler TJ. Non-invasive predictors of survival in cardiac amyloidosis. European journal of heart failure. 2007;9:617–24. doi: 10.1016/j.ejheart.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Austin BA, Duffy B, Tan C, Rodriguez ER, Starling RC, Desai MY. Comparison of functional status, electrocardiographic, and echocardiographic parameters to mortality in endomyocardial-biopsy proven cardiac amyloidosis. The American journal of cardiology. 2009;103:1429–33. doi: 10.1016/j.amjcard.2009.01.361. [DOI] [PubMed] [Google Scholar]
- 9.Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, Salvi F, Ciliberti P, Pastorelli F, Biagini E, Coccolo F, Cooke RM, Bacchi-Reggiani L, Sangiorgi D, Ferlini A, Cavo M, Zamagni E, Fonte ML, Palladini G, Salinaro F, Musca F, Obici L, Branzi A, Perlini S. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–12. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 10.Gorcsan J, 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. Journal of the American College of Cardiology. 2011;58:1401–13. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 12.Bellavia D, Abraham TP, Pellikka PA, Al-Zahrani GB, Dispenzieri A, Oh JK, Bailey KR, Wood CM, Novo S, Miyazaki C, Miller FA., Jr Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J Am Soc Echocardiogr. 2007;20:1194–202. doi: 10.1016/j.echo.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Bellavia D, Pellikka PA, Abraham TP, Al-Zahrani GB, Dispenzieri A, Oh JK, Bailey KR, Wood CM, Lacy MQ, Miyazaki C, Miller FA., Jr Evidence of impaired left ventricular systolic function by Doppler myocardial imaging in patients with systemic amyloidosis and no evidence of cardiac involvement by standard two-dimensional and Doppler echocardiography. The American journal of cardiology. 2008;101:1039–45. doi: 10.1016/j.amjcard.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 14.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–52. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 15.Buss SJ, Emami M, Mereles D, Korosoglou G, Kristen AV, Voss A, Schellberg D, Zugck C, Galuschky C, Giannitsis E, Hegenbart U, Ho AD, Katus HA, Schonland SO, Hardt SE. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol. 2012;60:1067–76. doi: 10.1016/j.jacc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 16.Perlini S, Salinaro F, Musca F, Mussinelli R, Boldrini M, Raimondi A, Milani P, Foli A, Cappelli F, Perfetto F, Palladini G, Rapezzi C, Merlini G. Prognostic value of depressed midwall systolic function in cardiac light-chain amyloidosis. J Hypertens. 2014;32:1121–31. doi: 10.1097/HJH.0000000000000120. discussion 1131. [DOI] [PubMed] [Google Scholar]
- 17.Bellavia D, Pellikka PA, Al-Zahrani GB, Abraham TP, Dispenzieri A, Miyazaki C, Lacy M, Scott CG, Oh JK, Miller FA., Jr Independent predictors of survival in primary systemic (Al) amyloidosis, including cardiac biomarkers and left ventricular strain imaging: an observational cohort study. J Am Soc Echocardiogr. 2010;23:643–52. doi: 10.1016/j.echo.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging. 2010;3:333–42. doi: 10.1016/j.jcmg.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. Journal of the American College of Cardiology. 2002;40:325–9. doi: 10.1016/s0735-1097(02)01944-7. [DOI] [PubMed] [Google Scholar]
- 20.Tendler A, Helmke S, Teruya S, Alvarez J, Maurer MS. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid. 2015;22:61–6. doi: 10.3109/13506129.2014.994202. [DOI] [PubMed] [Google Scholar]
- 21.Palladini G, Campana C, Klersy C, Balduini A, Vadacca G, Perfetti V, Perlini S, Obici L, Ascari E, d'Eril G, Moratti R, Merlini G. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107:2440–5. doi: 10.1161/01.CIR.0000068314.02595.B2. [DOI] [PubMed] [Google Scholar]
- 22.Kristen A, Giannitsis E, Lehrke S, Hegenbart U, Konstandin M, Lindenmaier D, Merkle C, Hardt S, Schnabel P, Röcken C, Schonland S, Ho A, Dengler T, Katus H. Assessment of disease severity and outcome in patients with systemic light-chain amyloidosis by the high-sensitivity troponin T assay. Blood. 2010;116:2455–61. doi: 10.1182/blood-2010-02-267708. [DOI] [PubMed] [Google Scholar]
- 23.Palladini G, Barassi A, Klersy C, Pacciolla R, Milani P, Sarais G, Perlini S, Albertini R, Russo P, Foli A, Bragotti LZ, Obici L, Moratti R, Melzi d'Eril GV, Merlini G. The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood. 2010;116:3426–30. doi: 10.1182/blood-2010-05-286567. [DOI] [PubMed] [Google Scholar]
- 24.Dispenzieri A, Kyle R, Gertz M, Therneau T, Miller W, Chandrasekaran K, McConnell J, Burritt M, Jaffe A. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003;361:1787–9. doi: 10.1016/S0140-6736(03)13396-X. [DOI] [PubMed] [Google Scholar]
- 25.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, Greipp PR, Witzig TE, Lust JA, Rajkumar SV, Fonseca R, Zeldenrust SR, McGregor CG, Jaffe AS. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–7. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–95. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, Foli A, Foard D, Milani P, Rannigan L, Hegenbart U, Hawkins PN, Merlini G, Palladini G. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121:3420–7. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 28.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79:319–28. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez de Larrea C, Verga L, Morbini P, Klersy C, Lavatelli F, Foli A, Obici L, Milani P, Capello GL, Paulli M, Palladini G, Merlini G. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125:2239–44. doi: 10.1182/blood-2014-11-609883. [DOI] [PubMed] [Google Scholar]
- 30.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–9. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 31.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA Doppler Quantification Task Force of the N and Standards Committee of the American Society of E. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 33.de Simone G, Devereux RB, Ganau A, Hahn RT, Saba PS, Mureddu GF, Roman MJ, Howard BV. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. The American journal of cardiology. 1996;78:801–7. doi: 10.1016/s0002-9149(96)00425-0. [DOI] [PubMed] [Google Scholar]
- 34.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. The American journal of cardiology. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 35.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 36.Fine NM, Crowson CS, Lin G, Oh JK, Villarraga HR, Gabriel SE. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann Rheum Dis. 2014;73:1833–9. doi: 10.1136/annrheumdis-2013-203314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Gertz M, Merlini G. Definition of organ involvement and response to treatment in AL amyloidosis: an updated consensus opinon. Amyloid. 2010;17(S1):48. Abstract CP-B. [Google Scholar]
- 39.Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, Grogan M, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV, Dispenzieri A. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129:2111–2119. doi: 10.1182/blood-2016-11-751628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–60. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 41.Tendler A, Helmke S, Teruya S, Alvarez J, Maurer MS. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2015;22:61–6. doi: 10.3109/13506129.2014.994202. [DOI] [PubMed] [Google Scholar]
- 42.Buss SJ, Emami M, Mereles D, Korosoglou G, Kristen AV, Voss A, Schellberg D, Zugck C, Galuschky C, Giannitsis E, Hegenbart U, Ho AD, Katus HA, Schonland SO, Hardt SE. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. Journal of the American College of Cardiology. 2012;60:1067–76. doi: 10.1016/j.jacc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 43.Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Survival by stroke volume index in patients with low-gradient normal EF severe aortic stenosis. Heart. 2015;101:23–9. doi: 10.1136/heartjnl-2014-306151. [DOI] [PubMed] [Google Scholar]
- 44.Aurigemma GP, Gaasch WH, McLaughlin M, McGinn R, Sweeney A, Meyer TE. Reduced left ventricular systolic pump performance and depressed myocardial contractile function in patients > 65 years of age with normal ejection fraction and a high relative wall thickness. Am J Cardiol. 1995;76:702–5. doi: 10.1016/s0002-9149(99)80201-x. [DOI] [PubMed] [Google Scholar]
- 45.Ford WR, Menon V, Bhambhani A, Liyanage R, Khan MI, Jugdutt BI. Changes in myocardial density during postinfarction healing: effect on estimation of in vivo left ventricular mass by echocardiographic imaging. Can J Physiol Pharmacol. 1997;75:1075–82. [PubMed] [Google Scholar]
- 46.Fontana M, Banypersad SM, Treibel TA, Abdel-Gadir A, Maestrini V, Lane T, Gilbertson JA, Hutt DF, Lachmann HJ, Whelan CJ, Wechalekar AD, Herrey AS, Gillmore JD, Hawkins PN, Moon JC. Differential Myocyte Responses in Patients with Cardiac Transthyretin Amyloidosis and Light-Chain Amyloidosis: A Cardiac MR Imaging Study. Radiology. 2015:141744. doi: 10.1148/radiol.2015141744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.