Abstract
Sickle cell disease (SCD) is a genetic blood disorder that affects the shape and transportation of red blood cells (RBCs) in blood vessels, leading to various clinical complications. Many drugs that are available for treating the disease are insufficiently effective, toxic, or too expensive. Therefore, there is a pressing need for safe, effective, and inexpensive therapeutic agents from indigenous plants used in ethnomedicines. The potential of aqueous extracts of Cajanus cajan leaf and seed, Zanthoxylum zanthoxyloides leaf, and Carica papaya leaf in sickle cell disease management was investigated in vitro using freshly prepared 2% sodium metabisulfite for sickling induction. The results indicated that the percentage of sickled cells, which was initially 91.6% in the control, was reduced to 29.3%, 41.7%, 32.8%, 38.2%, 47.6%, in the presence of hydroxyurea, C. cajan seed, C. cajan leaf, Z. zanthoxyloides leaf, and C. papaya leaf extracts, respectively, where the rate of polymerization inhibition was 6.5, 5.9, 8.0, 6.6, and 6.0 (×10−2) accordingly. It was also found that the RBC resistance to hemolysis was increased in the presence of the tested agents as indicated by the reduction of the percentage of hemolyzed cells from 100% to 0%. The phytochemical screening results indicated the presence of important phytochemicals including tannins, saponins, alkaloids, flavonoids, and glycosides in all the plant extracts. Finally, gas chromatography–mass spectrometry analysis showed the presence of important secondary metabolites in the plants. These results suggest that the plant extracts have some potential to be used as alternative antisickling therapy to hydroxyurea in SCD management.
Keywords: sickle cell disease, antisickling, medicinal plants, secondary metabolites, drug discovery
Graphical abstract
INTRODUCTION
Sickle cell disease (SCD) is an inherited genetic disorder affecting red blood cells (RBCs). At the genome level, this is due to a mutation in hemoglobin beta gene (HBB) causing a single amino acid substitution of valine for glutamic acid at the sixth position in the beta-globin chain, resulting in hemoglobin S (HbS).1 Hemoglobin is the main component of the RBC with the primary function of oxygen transport.2,3 Hemoglobin A (HbA) is the most common adult form of hemoglobin, but numerous variants of hemoglobin have been described.4 Normal RBCs have a flexible biconcave disk-like shape that allows for unimpeded passage through microvasculature with an approximate 120 day life span.5 Under hypoxic condition, HbS polymerizes, resulting in rigid and distorted RBCs termed “sickle cells”, which cause impaired microcirculation, hemolysis, and reduced life span. Numerous clinical manifestations of sickle cell disease include pain, vaso-occlusive crisis, splenic sequestration, acute chest syndrome, aplastic anemia, hemolytic anemia, and stroke.6 The rigidity of a sickle cell renders it fragile and prone to osmotic lysis. A normal red blood cell is flexible and elastic, which enables it to move through narrow blood vessels. Thus, sickle cells are described as being rigid and distorted because their resistance to hemolysis is reduced. A rigid cell cannot expand, meaning that it is not flexible and therefore cannot easily move along the narrow human blood vessels. When the osmotic fragility decreases, the resistance increases and vice versa. Therefore, the reduction in osmotic fragility by antisickling agents is an advantage in that it increases the RBCs’ resistance to lysis. In other words, a rigid and distorted red blood cell with low elasticity can be fragile and may break with little stress.7–10
Recent attempts at producing a targeted therapy for sickle cell disease management have focused on the inhibition of HbS polymerization by binding to small molecules. The polymerization occurs due to interactions among the amino acids in the hypoxic states. In normal RBCs, hemoglobin exists as a tetrameric protein with two alpha- and two beta-chains. The interactions among the tetramers of hemoglobin molecule can lead to polymerization. For instance, in homozygous SS patients, both beta-chains contain valine at the sixth position. Val6 interacts hydrophobically with Phe85 and Leu88 of the other hemoglobin molecules (i.e., beta-2 of the first Hb molecule interact with beta-1 of the second Hb molecule). This interaction constitutes the basis for polymerization. The same beta-2 chain of the first Hb molecule also contains glutamic acid at position 121, which interacts with Gly16 of the beta-1 chain of a third Hb molecule. Meanwhile, between the first and the third Hb molecules, His20 of the first Hb molecule of alpha-2 chain interacts with Glu6 of beta-1 of the third Hb molecule. Another interaction is that beta-2 Val6 of the first Hb is interacting with Phe85 and Leu88 of beta-2 of the second Hb molecule. In addition, the Asp73 of Beta-2 of the first Hb interacts with Thr4 of beta-2 of the fourth Hb molecule. There is also an interaction between Glu121 of the first Hb molecule on beta-1 chain and proline of alpha-2 and His116 of beta-2 chain of the fifth Hb molecule. This interaction model is supported by earlier reports on the nature of polymerization of sickle cells.9,11 These complex molecular interactions among the hemoglobin tetramers and with neighboring hemoglobin molecules play a vital role in the polymerization of the HbS cells, which result in deformation or sickled shape.8,9 Therefore, we speculate that therapeutic agents that interfere with these interactions may have the potential to be useful in sickle cell therapy.
Several antisickling agents have been investigated and confirmed to possess ameliorative properties.12 For instance, hydroxyurea has been shown to decrease the number and severity of sickle cell crises by increasing fetal hemoglobin production significantly in patients with sickle cell anemia.13 In fact, there was no specific therapy available for sickle cell disease patients before the 1970s. However, subsequent studies have shown that patients with a higher concentration of fetal hemoglobin (HbF) in the red blood cell had less adverse clinical complications.14 In 1984, it was shown that hydroxyurea induced HbF in two adults with sickle cell anemia, while a subsequent report showed the efficacy and tolerability of the drug in the patients. The US Food and Drug Administration has approved the use of hydroxyurea since 1998 for the treatment of sickle cell patients with frequent painful crises. In 2007, the European Medicines Agency also authorized hydroxyurea for treatment of sickle cell disease. A review was later published by the Agency for Healthcare Research and Quality in 2008 on the use of hydroxyurea for sickle cell diseases. In addition, the National Institutes of Health Consensus Development Conference was held on the use of hydroxyurea for the treatment of sickle cell disease.13–15
Hydroxyurea achieved this function by activating the production of fetal hemoglobin to replace the hemoglobin S that causes sickle cell anemia. One of the mechanisms for the action is based on its ability to inhibit the reaction that leads to the production of deoxyribonucleotides by acting on the enzyme of ribonucleotide reductase. The production of deoxyribonucleotides requires tyrosyl group (which is a free radical). So, hydroxyurea captures these tyrosyl free radicals thereby preventing the production of deoxyribonucleotides. Another mechanism is that it increases nitric oxide levels. This brings about the activation of soluble guanylyl cyclase, which results in an increase in the cyclic GMP. It also activates gammaglobulin synthesis, which is required for the production of fetal hemoglobin (by removing the rapidly dividing cells that preferentially produce sickle hemoglobin).16 In addition, there are other actions of the agent on the membrane of human erythrocytes in vitro. The effects of hydroxyurea on sickled red blood cells and how to reverse the sickling state of the cells by acting on the membrane are important properties of hydroxyurea. Several reports are available online on the in vitro effects of hydroxyurea on the erythrocyte membrane deformability.17–25 These studies show that hydroxyurea acts on the erythrocyte membrane. In fact, hydroxyurea also acts on hematological parameters as a mechanism to reduce sickling.26–28 One of the purposes of this work is to compare the effect of antisickling plants with that of hydroxyurea in ameliorating or reverse the deformability of the erythrocyte membrane during sickling. Overall, although there are other agents tested and confirmed for the treatment of sickle cell disease, such as phenylalanine,29 vanillin,30 pyridyl derivatives,31 acetyl-3,5-dibromosalicylic acid,32 and 5-hydroxymethyl-2-furfural,33 these are not yet clinically accepted for the management of the disease. So, hydroxyurea still remains the most widely accepted therapy for sickle cell disease.
Despite its wide acceptance, hydroxyurea is moderately toxic especially when administered long term.21 In search of inexpensive but effective and readily available drugs, several investigations have been conducted on indigenous plant materials. Among the commonly used plants in Nigeria and other African nations for the management of many ailments including sickle cell disease are the leaves of Terminalia catapa34,35 and Carica papaya.36 Others include Cajanus cajan seeds,37 unripe fruit of Carica papaya, leaves of Parquetina nigrescens, leaves of Citrus sinensis, leaves of Persia Americana, and leaves of Zanthoxyllum zanthoxyloides.38
These naturally occurring sources contain phytochemicals or secondary metabolites that may have beneficial properties.39 Potentially, medicinal plants could be used alongside pharmaceutical drugs for management of sickle cell disease. Because of the high number of sickle cell patients worldwide, especially in Nigeria, Africa, the high cost of pharmaceutical products, and the limited efficacy of the available drugs, there is a pressing need for the development of new drugs that are inexpensive but effective and readily available in rural communities as well as the world at large, for the management of sickle cell disease. Investigation of the antisickling properties of substances derived from indigenous plants is an attractive line of research. To this end, we investigated and compared the ability of the aqueous extracts of several widely used ethnomedicinal plants, including Cajanus cajan leaf, Cajanus cajan seeds, Zanthoxylum zanthoxyloides leaf, and Carica papaya leaf, to reverse the sickling of HbS-containing RBCs, inhibit the HbS polymerization, and increase the RBC resistance to hemolysis using hydroxyurea as a reference. Although the antisickling properties of some of the plants have been previously reported,36,38,40 there is a need to systematically examine their efficacy in control with known and well-established agents. In addition to the phytochemical screening of the plants, identification of bioactive components in the plants is also an essential step toward further investigation and development of new efficient antisickling drugs.
MATERIALS AND METHODS
Human Blood Samples
Human blood samples were obtained from residual clinical samples submitted to the clinical chemistry laboratory of the University of Michigan Health System for hemoglobin electrophoresis. Samples from patients who had been transfused in the prior three months, had received an allogenic bone marrow transplant, or were taking antisickling drugs were excluded. The phenotype of each sample was confirmed by electrophoretic analysis. Freshly collected blood samples were used throughout, and only one type of human blood sample was used at a time for each experiment. The research was approved by the University of Michigan Medical School Institutional Review Board (IRB).
Plant and Chemical Material Collection
The plant materials (Cajanus cajan leaf and seed, Zanthoxylum zanthoxyloides leaf, and Carica papaya leaf) were collected in Ilorin, Kwara State, Nigeria, West Africa, and authenticated in the Department of Plant Biology, Faculty of Life Sciences, University of Ilorin, Nigeria. The voucher numbers were deposited in the herbarium of the department. Hydroxyurea, sodium metabisulfite, and NaCl were products of Sigma-Aldrich and were of analytical grade.
Extraction of Plant Samples
After being air-dried in the laboratory and ground into powder using a clean electric grinder, 100 g of each plant sample was extracted in 1 L of distilled water for 48 h, filtered, and dried using a LAB-KIT freeze-dryer machine. The percentage yields were 9.2%, 7.6%, 4.2%, and 8.3% (w/w) for C. cajan leaf, C. cajan seed, Z. zanthoxyloides leaf, and C. papaya leaf, respectively. The resulting extracts were stored in the freezer at −20 °C. A working solution of 1% (w/v) of each of the plant extracts was made with distilled water and stored at −20 °C until used.
Phytochemical Screening of Plant Extracts
The method previously described41 was used in the determination of the presence of alkaloids in the extracts. One milliliter of 1% (v/v) HCl was added to 3 mL of 10 mg/mL plant extract and heated for 20 min. The resulting solution was cooled and filtered. To 1 mL of the filtrate, 2 drops of Mayer’s reagent was added. A creamy precipitate observed indicated the presence of alkaloids. Two drops of Wagner’s reagent was also added to a fresh 1 mL of the extract. A reddish brown precipitate indicated the presence of alkaloid in the extract.41 For tannins, dried sample (0.5 g) was dissolved in 20 mL of distilled water in a test tube and then filtered. A few drops of 0.1% ferric chloride were added. Appearance of brownish green or a blue-black color indicated the presence of tannins.42 In the test for saponins, two (2 g) of the plant extract was dissolved and boiled in 20 mL of distilled water in a water bath for 10 min, cooled, and filtered. Ten milliliters (10 mL) of the filtrate was taken and 5 mL of distilled water added and mixed thoroughly by shaking. A stable persistent froth indicated the presence of saponins.41 Presence of flavonoids in the extracts was tested by adding 2 mL of 1% (v/v) aluminum solution to 3 mL of the aqueous extract. A yellow color observed indicated the presence of flavonoid.41 The Keller–Killani test was used to test the presence of glycosides. Acetic acid (2 mL) containing one drop of ferric chloride solution was added to 5 mL of the aqueous plant extract. Then 1 mL of concentrated sulfuric acid was gently added. A brown ring observed at the interface indicated a deoxysugar characteristic of cardenolides.42
Gas Chromatography–Mass Spectrometry
After the confirmation of the presence of some phytochemicals in the plant extracts, they were analyzed by gas chromatography–mass spectrometry (GC–MS) (Shimadzu QP-2010-S) to identify secondary metabolites. This method employs electron impact ionization (ionizing potential 70 eV) and a capillary column (Supelco SLB-5 ms, 30 m × 0.25 mm × 0.25 μm film thickness). The ion source temperature was set to 200 °C. The inert gas helium (UHP grade, from Cryogenic Gases) was used as a carrier, with a linear velocity of 35.9 cm/s. The injector temperature was 200 °C with a splitless injection conducted. The oven temperature was held at 60 °C for 3 min, then heated to 325 °C at 40 deg/min, and then held at 325 °C for 10 min. The transfer line interface temperature was 250 °C. The mass spectrometer was scanned from m/z 35 to m/z 400 at every 0.5 s, with a solvent cut time of 3.0 min. The data were processed with Shimadzu’s GCMS Solution software V4.3 by comparing the compounds with the database at the National Institute of Standards and Technology. For each compound, the name, molecular weight, molecular structure, and percent peak area were determined.
Sickling Reversal Test
The test of the ability of the plants to reverse the sickling state of the RBCs was performed by a previously described procedure.43 The blood sample was washed twice in five volumes of phosphate buffered saline (1 mL of blood in 5 mL of PBS) with pH 7.4 by centrifugation at 1200g. Into a clean Eppendorf tube, 100 μL of the washed red blood cells and 100 μL of freshly prepared 2% sodium metabisulfite were added and incubated for 2 h at 37 °C. Then 100 μL of antisickling agent (1% w/v) was added and incubated for another 2 h at 37 °C. Ten microliters (10 μL) of the incubated cells was taken and transferred to a hemocytometer, and the cells were viewed and counted using an Olympus CK2 microscope at 40× magnification. A control test was performed by replacing 100 μL of drug/extract with 100 μL of PBS. Replacing drug/extract with PBS was performed to make the concentration of metabisulfite in total 300 μL final volume the same as in the test experiment, so that its effect on the red blood cells is not affected and the number of red blood cells in 100 μL of blood remains the same. The cells were classified as normal or sickled by observing their shapes. Biconcave or disk-like shapes were taken to be normal while the elongated, star-like, or wrinkled shapes were considered sickled. The percentage sickled cells was calculated using the following formula: percent sickling (%) = (number of sickled cells divided by the total number of counted cells) × 100. The experiment was repeated five times.
Polymerization Inhibition Test
The polymerization inhibition test was carried out following the method of Nwaoguikpe et al.44 This procedure involves the measurement of the turbidity of the polymerizing solution of RBCs at a wavelength of 700 nm at 26 °C. Freshly prepared 2% sodium metabisulfite (0.88 mL) was transferred into a cuvette followed by 0.1 mL of PBS and 0.02 mL of SS blood. Absorbance was read at 700 nm immediately and at every 2 min for 30 min. This serves as control test. For the inhibition test, 0.1 mL of phosphate buffered saline (PBS) was replaced by 0.1 mL of hydroxyurea/plant extracts. The rate of polymerization in percentage was calculated using the following formula: rate of polymerization (Rp) = [(final absorbance − initial absorbance)/30] × 100. The experiment was repeated five times as well.
Osmotic Fragility Test
The osmotic fragility test was carried out following a modification of the previously described method.43 The modifications include incubation at 37 °C instead of at room temperature and for 4 h instead of 24 h. Varying concentrations of normal saline were prepared (0–0.9% NaCl), followed by the addition of 0.05 mL of SS blood prewashed in PBS (pH 7.4) and then incubated at 37 °C for 4 h. The tube with 0.9% NaCl served as blank. For the test samples, 0.1 mL of solutions of the 1% hydroxyurea and 1% each of plant extracts were incubated separately with varying concentrations of NaCl as described for the control. After incubation, the mixture was centrifuged at 3000 rpm for 5 min. The supernatant was removed with its absorbance measured at 540 nm. Percent hemolysis was calculated as absorbance of the supernatant in all tubes divided by the absorbance of the supernatant in the tube with zero concentration of NaCl times 100. Results were presented graphically as percent hemolysis plotted against the concentration of NaCl.
Statistical Analysis
The data obtained were expressed as means ± standard error of mean (SEM) of five determinations. XLSTAT_2015 and SPSS V16.0 were used for data analysis. The statistical significance of differences was calculated using analysis of variance. Values of p < 0.05 were considered significantly different.
RESULTS AND DISCUSION
Phytochemical Screening of the Plants
Research has shown the importance of ethnomedicinal plants in the treatment of various ailments including sickle cell disease.34,35,37,38,40 To investigate the chemical components of the plants that are responsible for their therapeutic potentials, there is a need to screen the plants for the presence of phytochemicals. The presence of important phytochemicals in some plant extracts has been previously investigated. For instance, the phytochemical screening of C. cajan leaf and seed, Z. zanthoxyloides leaf, and C. papaya leaf has been reported by several investigators.45–47,57 To confirm the presence of the reported phytochemicals, we first carried out a phytochemical screening of the aqueous plant extracts of C. cajan leaf and seed, Z. zanthoxyloides leaf, and C. papaya leaf using the procedure described in Materials and Methods.
The results of this screening demonstrated the presence of tannins, saponins, alkaloids, flavonoids, and glycosides in all the plant extracts. These phytochemicals are bioactive components that possess various therapeutic properties useful in medicine. For instance, tannins are group of phenolic compounds that can bind and precipitate protein, a property that could be utilized in receptor-targeted drug design.44 Saponins are group of polycyclic aglycons (steroids or triterpenes) with attached monosaccharides, polysaccharides, or oligosaccharide side chains. Saponins have foaming characteristic and are used in the management of cancer, immune system stimulation in patients with low immune system, and blood cholesterol levels.48 Uses of alkaloids include antiarrhythmic, anticholinergic, antiprotozoal agent, analgesic, stimulant, inhibitors of acetylcholinesterase, remedy for gout, cough medicine, antihypertensive, vasodilating, and as aphrodisiac.49 In the same line, flavonoids are 15-carbon compounds with two phenyl rings and a heterocyclic ring usually referred to as rings A, B, and C. The use of glycosides and other phytochemicals in some plants as antisickling agents has also been reported.50,51 Thus, the results of this present work are comparable with earlier reports by Adesina and others where C. cajan leaf,47 Z. zanthoxyloides,34,57 and C. papaya leaf45 have been screened for the presence of phytochemicals such as alkaloids, tannins, saponins, flavonoids, and glycosides. Each phytochemical has a variety of uses in ethnomedicines. Their presence in these plant extracts probably explains the reason that the plants possess the antisickling properties.
GC–MS Analysis of Bioactive Components of Plant Extracts
The observation in the presence of different phytochemicals such as alkaloids, tannins, flavonoids, etc. in C. cajan leaf, C. cajan seed, Z. zanthoxyloides leaf, and C. papaya is consistent with the report in several previous studies.45–47,57 There is however a need to examine the identification of the bioactive chemicals, which will help us understand the type of phytochemicals that are responsible for therapeutic potential of the plants.
We employed gas chromatography–mass spectrometry to characterize each of the plant extracts. The effectiveness of this method has been previously shown in identifying bioactive components in plant extracts.39,52–55 The results of our tests are listed in the Tables 1–4, where 28, 29, 30, and 29 secondary metabolites were identified from C. cajan leaf, C. cajan seed, Z. zanthoxyloides leaf, and C. papaya aqueous leaf extracts, respectively. Despite the effectiveness of GC–MS analysis in identifying possible secondary metabolites in plant extracts, it cannot be used for classifying the constituents into functional or structural groups, since GC–MS is designed to identify the compounds by comparing the candidates with known molecules in the database/library. Classification of the identified constituents is therefore left for further research and investigations.
Table 1.
GC–MS Analysis of Secondary Metabolites Present in Aqueous Extract of C. cajan Leaf
| peaks | retention time (min) | compound name | molecular weight | molecular formula | peak area % |
|---|---|---|---|---|---|
| 1 | 5.7 | 1-dodecene | 168 | C12H24 | 3.880 |
| 2 | 7.6 | 2-tridecene | 182 | C13H26 | 6.68 |
| 3 | 7.8 | γ-elemene | 204 | C15H24 | 2.64 |
| 4 | 8.1 | bisabolene | 204 | C15H24 | 3.82 |
| 5 | 8.0 | 2,4-di-tert-butylphenol | 206 | C14H22O | 7.13 |
| 6 | 8.1 | 1,11-hexadecadiyne | 218 | C16H26 | 4.62 |
| 7 | 8.2 | nerolidol | 222 | C15H26O | 1.87 |
| 8 | 8.2 | 1-hexadecene (cetene) | 224 | C16H32 | 6.12 |
| 9 | 8.0 | 8-methyl-6-nonenoic acid | 170 | C10H18O2 | 2.24 |
| 10 | 8.5 | 2,3-epoxydecahydronaphthalene | 152 | C10H16O | 2.11 |
| 11 | 8.6 | caryophyllene oxide | 220 | C15H24O | 2.64 |
| 12 | 8.7 | 2-isopropyl-5-methylhexyl acetate | 200 | C12H24O2 | 1.41 |
| 13 | 8.8 | 3,7,11-trimethyl-1-dodecanol | 228 | C15H32O | 2.23 |
| 14 | 9.0 | 1-octadecyne | 250 | C18H34 | 2.96 |
| 15 | 9.3 | 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid; fenozan | 292 | C18H28O3 | 2.17 |
| 16 | 9.7 | 2-undecenoic acid (undec-2-enoic acid) | 184 | C11H20O2 | 1.89 |
| 17 | 9.8 | N-(2-oxo-5-hexenyl)acetamide | 155 | C8H13NO2 | 1.51 |
| 18 | 10.3 | M-diphenylmethanecarboxylic acid methyl ester | 226 | C15H14O2 | 3.73 |
| 29 | 10.6 | chalcone, 2′,6′-dihydroxy-4′-methoxy | 270 | C16H14O4 | 8.20 |
| 20 | 10.8 | cyclotrisiloxane, hexamethyl- (dimethylsiloxane cyclic trimer) | 222 | C6H18O3Si3 | 1.75 |
| 21 | 11.1 | trans-3-methoxy-4-propoxy-β-methyl-β-nitrostyrene | 251 | C13H17NO4 | 4.14 |
| 22 | 11.4 | 3H-cycloocta[c]pyran-3-one, 5,6,7,8,9,10-hexahydro-4-phenyl-1- (trifluoromethyl)- | 322 | C18H17F3O2 | 7.50 |
| 23 | 11.8 | methylidene]-2-pyridinecarbohydrazonamide | 238 | C14H14N4 | 1.71 |
| 24 | 13.5 | hexasiloxane | 430 | C12H38O5Si6 | 1.49 |
| 25 | 13.6 | 1,2-bis(trimethylsilyl)benzene | 222 | C12H22Si2 | 1.47 |
| 26 | 14.8 | urs-12-ene | 410 | C30H50 | 4.25 |
| 27 | 15.0 | 2-nitro-4-trimethylsilyl-benzaldehyde | 223 | C10H13NO3Si | 1.89 |
| 28 | 15.2 | lupeol (fagaresterol) | 426 | C30H50O | 4.57 |
Table 4.
GC–MS Analysis of Secondary Metabolites Present in Aqueous Extract of C. papaya Leaf
| sample no. | retention time (min) | compound name | molecular weight | molecular formula | peak area % |
|---|---|---|---|---|---|
| 1 | 5.7 | 2,4-dimethyl-2-nitropentane | 145 | C7H15NO2 | 2.12 |
| 2 | 6.8 | 1-nonanol | 144 | C9H20O | 4.80 |
| 3 | 7.6 | cis-3-tetradecene | 196 | C14H28 | 9.19 |
| 4 | 7.8 | 3-methylbutanal | 86 | C5H10O | 2.56 |
| 5 | 8.0 | 3,5-di-tert-butylphenol | 206 | C14H22O | 7.12 |
| 6 | 8.1 | 2-(tert-butylperoxy)-2-ethylbutyl butyrate | 260 | C14H28O4 | 1.79 |
| 7 | 8.2 | 1-tetradecene | 196 | C14H28 | 8.65 |
| 8 | 8.8 | 3-octadecene | 252 | C18H36 | 3.34 |
| 9 | 9.0 | 1-octadecyne | 250 | C18H34 | 7.16 |
| 10 | 9.1 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 296 | C20H40O | 7.06 |
| 11 | 9.2 | cyclopentaneundecanoic acid, methyl ester | 268 | C17H32O2 | 3.55 |
| 12 | 9.3 | methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate | 292 | C18H28O3 | 3.88 |
| 13 | 9.4 | propan-2-yl decanoate | 214 | C13H26O2 | 2.14 |
| 14 | 9.7 | heptadecanoic acid (15-ethyl-, methyl ester) | 312 | C20H40O2 | 2.34 |
| 15 | 10.4 | 3,5-bis(trimethylsilyl)-2,4,6-cycloheptatrien-1-one | 250 | C13H22OSi2 | 2.49 |
| 16 | 10.6 | ethyl 2-(3-methoxy-4-trimethylsilyloxyphenyl)acetate | 282 | C14H22O4Si | 1.50 |
| 17 | 11.1 | 1-trimethylsilyl-4-(1-methyl-1-silacyclobutyl)benzene | 234 | C13H22Si2 | 2.47 |
| 18 | 11.4 | decamethylpentasiloxane | 356 | C10H32O4Si5 | 2.45 |
| 29 | 11.6 | undec-10-enyl 6-bromohexanoate | 346 | C17H31BrO2 | 1.60 |
| 20 | 11.8 | tricyclo[3.2.1.1(3,6)]nonane-5-methanol (3-bromo-2,2-ethylenedioxy-) | 288 | C12H17BrO3 | 2.06 |
| 21 | 13.0 | 1,3,5-cycloheptatriene (7,7-dimethyl-2,4-bis(trimethylsilyl)-) | 264 | C15H28Si2 | 1.38 |
| 22 | 14.3 | mandelic acid (ethyl 2-phenyl-2-trimethylsilyloxyacetate) | 252 | C13H20O3Si | 1.47 |
| 23 | 14.8 | 1,2,3,5-tetramethyl-4,6-dinitrobezene (dinitroisodurene) | 224 | C10H12N2O4 | 1.88 |
| 24 | 15.0 | diethyl bis(trimethylsilyl) orthosilicate | 296 | C10H28O4Si3 | 2.53 |
| 25 | 15.2 | 4-(4-chlorophenyl)-1-methyl-3,6-dihydro-2H-pyridine | 207 | C12H14ClN | 3.44 |
| 26 | 15.4 | pentamethylcyclopentadienyl-(N,N,N′-trimethyl)-o-phenylenediamine-N′-o-nickel | 342 | C19H28N2Ni | 2.40 |
| 27 | 16.4 | (p-trimethylsilyloxyphenyl)-1-trimethylsilyloxypropane | 296 | C15H28O2Si2 | 1.34 |
| 28 | 18.5 | 2,2,4,4,6,6-hexamethyl-1,3,5,2,4,6-trioxatrisilinane | 222 | C6H18O3Si3 | 2.30 |
| 29 | 18.6 | silicic acid (diethyl bis(trimethylsilyl) ester) | 296 | C10H28O4Si3 | 2.14 |
Among the secondary metabolites identified, urs-12-ene and lupeol in C. cajan leaf have been used as anticancer agent and anti-inflammatory agents, respectively. Similarly, hexestrol in C. cajan seed is a derivative that has been used in detection of estradiol receptor sites; bromazepam and nickel detected in Z. zanthoxyloides and C. papaya leaf extracts act on neuro-transmitters to reduce anxiety and are used as hepatoprotective agent. While the direct benefits of these components for antisickling remain to be elucidated, the examination of the presence of specific compounds presented here should help for future studies in characterizing the therapeutic properties of these plants.56
Reduction of Sickle Cells by Hydroxyurea and Plants Extracts
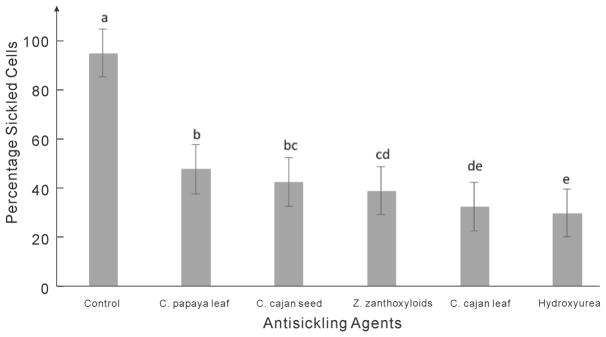
Sickle cell disease affects the shape and flexibility of RBCs in such a way that it prevents their smooth movement through small human blood vessels. Normal red blood cells are biconcave and flexible, a property that enables them to move freely and smoothly through narrow blood vessels. It also enables them to live longer to about 120 days. One of the motives for antisickling drug design is to have a drug that can prevent or reverse the sickle shape phenotype of the RBCs. Here we investigated the potentials of C. cajan leaf and seed, Z. zanthoxyloides leaf, and C. papaya leaf in reversing the sickling of human RBCs, with data presented in Figures 1 and 2. First, we compared the effects of the respective plant extracts to hydroxyurea in treating sickle cells. The results indicated a high potential of the plant extracts in reversing the sickling of RBCs. When viewed under microscope with 40× magnification, the sickle cells were found to have elongated or spike-like shapes while normal RBCs appeared biconcave or disk-like (Figure 2).
Figure 1.
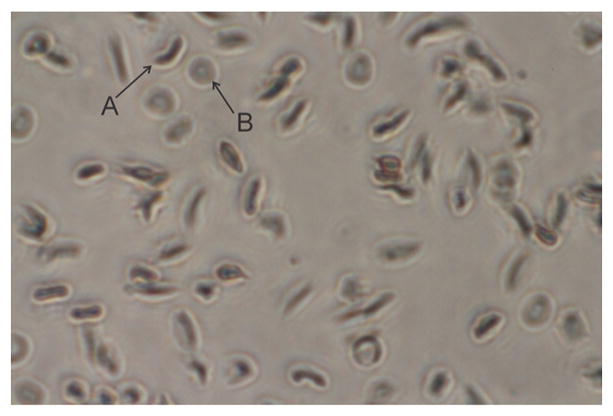
Structural shape of sickled (A) and normal (B) red blood cells when viewed under microscope at 40× magnification.
Figure 2.
Inhibition of sickling of RBCs by aqueous extracts of studied plants and hydroxyurea. Letters a, b, c, d, and e are used to differentiate the level of significance between the percentage values, obtained by the IBM SPSS Statistics package. Here, two distinct letters (e.g., a and b) indicate significant difference (p-value <0.05) and two letters of mixture (e.g., b and bc) indicate nonsignificant difference (p-value >0.05).
To quantitatively compare their antisickling properties, we have carried out an experiment to test the ability to reverse the sickling of RBCs for each of the antisickling agents. Figure 2 compares the percentage of sickled cells in different antisickling agents. The number of sickled cells in the control (RBCs in PBS) was about 91.6%, while in the presence of hydroxyurea, the percentage of sickled cells was reduced to about 30%. The reduction was largest for hydroxyurea, followed by extracts of C. cajan leaf, Z. zanthoxyloides leaf, C. cajan seed, and C. papaya, which have the reduction rate ranging from 32 to 47%. These data were obtained from the average of five repeated experiments for each antisickling agent and the control. The mean and SEM of the original RBC numbers and the reduction rates are also listed in Table 5.
Table 5.
Mean and Standard Error of Mean in Five Repeated Experiments on the Reversal of Sickling of RBCs by Aqueous Extracts of Studied Plants and Hydroxyurea
| sample no. | antisickling agents | sickled RBCs | normal RBCs | total RBCs Counted | percent sickled RBCs |
|---|---|---|---|---|---|
| 1 | control | 2500.67 ± 0.02 | 229.33 ± 0.61 | 2730.00 ± 0.98 | 91.60 ± 0.71 |
| 2 | C. papaya leaf | 1048.33 ± 1.21 | 1156.00 ± 0.22 | 2204.33 ± 0.15 | 47.56 ± 0.31 |
| 3 | C. cajan seed | 1004.00 ± 2.21 | 1406.00 ± 3.89 | 2410.00 ± 2.10 | 41.66 ± 7.45 |
| 4 | Z. zanthoxyloides leaf | 848.33 ± 0.09 | 1371.33 ± 2.12 | 2413.67 ± 0.28 | 38.22 ± 0.87 |
| 5 | C. cajan leaf | 791.33 ± 0.88 | 1622.33 ± 3.11 | 2413.67 ± 0.55 | 32.79 ± 0.55 |
| 6 | hydroxyurea | 535.33 ± 0.49 | 1293.33 ± 0.89 | 1828.67 ± 0.92 | 29.28 ± 0.68 |
While all plant extracts tested have shown some level of reductions in the number of sickled cells compared to the control, the slightly higher potential observed in hydroxyurea as compared to the plant extracts may be attributed to its purity and synthetic nature. Being a synthetic drug, there is little or no interference of other components with its action. Hydroxyurea is the most widely acceptable and used drug for the treatment of sickle cell disease. It increases the fetal hemoglobin production by increasing nitric oxide production. This occurs through a series of other reactions leading to reduction of HbS concentration. Plant extracts, on the other hand, are mixtures of different bioactive chemicals that may be antagonists of one another. Thus, if the bioactive compounds in each plant extracts are isolated and purified, their potential and effectiveness may be further enhanced.
Red Blood Cell Polymerization Inhibition Test
Considerable effort has been made to elucidate the nature of sickle cell disease in the past decades, and it has been well established that the genetic mutation in the globin chain is where the problem originated. One of the clinical manifestations of this genetic RBC disorder is polymerization of hemoglobin in the hypoxic condition.8,24,57–60 Therefore, inhibition or prevention of hemoglobin polymerization is one of the avenues of drug design against sickle cell disease.
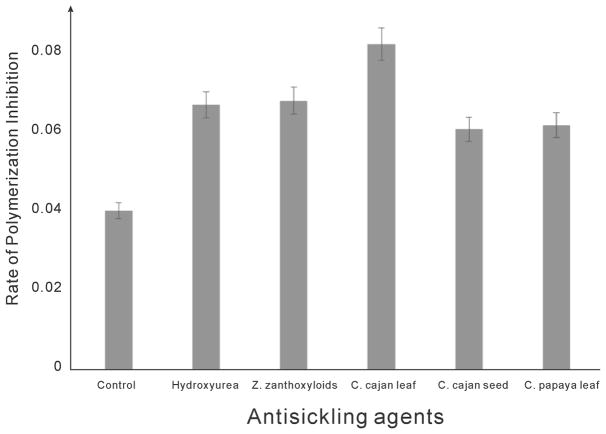
In Figure 3, we presented the effect of various plant extracts in preventing RBC polymerization. The results of these tests indicated that C. cajan leaf and seed, Z. zanthoxyloides leaf, and C. papaya leaf could all prevent RBC polymerization at some level. The initial absorbance of the polymerizing cells was measured at time zero (i.e., immediately after addition of sodium metabisulfite) and subtracted from the final absorbance taken at the end of 30 min. The resulting value divided by 30 gives the rate of polymerization inhibition. It is observed that C. cajan leaf possesses the fastest rate in hemoglobin polymerization inhibition, followed by hydroxyurea, Z. zanthoxyloides, C. papaya leaf, and C. cajan seed in descending order. Rate of polymerization inhibition in the control is the lowest because there are no antisickling agents to prevent the polymerization reactions. The characteristic trend observed here is due to the ability of the agents to interact with RBC membrane or probably with any of the amino acids that are involved in the polymerization reactions. It suggests that some of the bioactive components in the plant extracts were able to interact with hemoglobin molecules.
Figure 3.
RBC polymerization inhibition rates by aqueous extracts of studied plants and hydroxyurea.
We inferred that some of the bioactive components in the plant extracts, in addition to the interaction with RBCs membrane, were able to interact with two or more amino acid residues to bring about inhibition of the polymerization reactions. Thus, the medicinal plants may be effective antisickling agents as alternatives to the more expensive hydroxyurea, a synthetic drug.
Resistance of RBCs to Hypotonic Lysis
Osmotic fragility test is an effective approach to identify potential antisickling agents by attempting to increase the resistance of RBCs to hypotonic lysis. Hypotonic lysis occurs when water molecules move into the cells through osmosis against a solute concentration gradient. It has been reported that an increase in surface-to-volume ratio can increase the resistance of RBCs to hemolysis (i.e., decrease the osmotic fragility).61,62 Examples include cases in iron-defficient anemia, thalassemia, sickle cell anemia, and liver disease. On the other hand, a decrease in surface-to-volume ratio can decrease the resistance of RBCs to hemolysis (i.e., increase the osmotic fragility), with examples in the hemolytic anemias and hereditary spherocytosis.
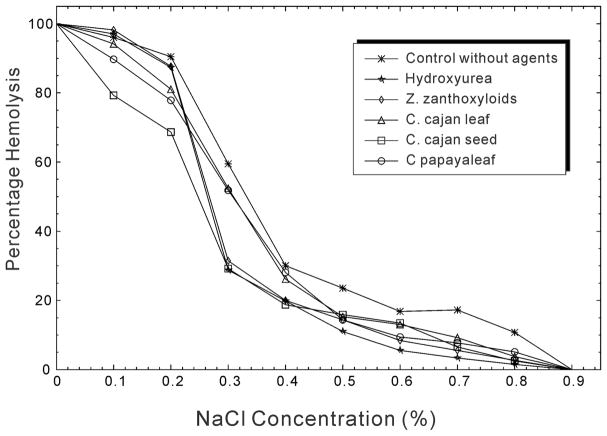
The results of this experiment are presented in Figure 4 and Table 6 to show the effects of hydroxyurea and the plant extracts on the resistance of RBCs to hemolysis. While the figure shows the graphical tendency of the changes of percentage hemolysis at different NaCl concentrations, the table lists the number of hemolyzed cells with the standard error and the statistical significance level across different agents. The osmotic fragility of red blood cell is usually fully observed between 0.45% and 0.35% NaCl concentration, representing the onset and the completion of the hemolysis during the increasing hypotonicity. Considering the NaCl concentration at 0.4% and 0.5%, when compared with the control across the row, there were significant differences (p-value <0.05) among the effects of different antisickling agents on the resistance of RBCs to hemolysis. This significance level is represented by the different superscript letters in Table 6. Although hydroxyurea has the lowest number of hemolyzed cells (0.139 ± 0.01) at 0.4%NaCl, most of the extracts are compared with the control (0.212 ± 0.00 hemolyzed cells). Overall, the extract with the least effects or with the highest number of hemolyzed cells (0.219 ± 0.00%) is C. papaya leaf, a trend of effect observed at almost every concentration of NaCl.
Figure 4.
Osmotic fragility results of the human RBCs by different antisickling agents.
Table 6.
Effect of Hydroxyurea and Plant Extracts on the Resistance of RBCs to Hemolysisa
| % NaCl | control | hydroxyurea | Z. zanthoxyloides leaf | C. cajan leaf | C. cajan seed | C. papaya leaf |
|---|---|---|---|---|---|---|
| 0 | 0.708 ± 0.01 a | 0.699 ± 0.02 b | 0.701 ± 0.00 b | 0.764 ± 0.08 c | 0.757 ± 0.05 d | 0.779 ± 0.09 e |
| 0.1 | 0.680 ± 0.00 a | 0.678 ± 0.01 b | 0.688 ± 0.01 c | 0.719 ± 0.00 d | 0.600 ± 0.01 e | 0.699 ± 0.01 f |
| 0.2 | 0.640 ± 0.00 a | 0.610 ± 0.01 b | 0.615 ± 0.02 c | 0.619 ± 0.02 d | 0.520 ± 0.08 e | 0.607 ± 0.05 f |
| 0.3 | 0.421 ± 0.03 a | 0.201 ± 0.00 b | 0.221 ± 0.00 c | 0.400 ± 0.00 d | 0.221 ± 0.00 c | 0.404 ± 0.00 e |
| 0.4 | 0.212 ± 0.00 a | 0.139 ± 0.01 b | 0.140 ± 0.04 b | 0.200 ± 0.06 c | 0.142 ± 0.00 d | 0.219 ± 0.00 e |
| 0.5 | 0.167 ± 0.00 a | 0.077 ± 0.00 b | 0.101 ± 0.02 c | 0.117 ± 0.01 d | 0.120 ± 0.03 e | 0.112 ± 0.06 f |
| 0.6 | 0.119 ± 0.00 a | 0.039 ± 0.01 b | 0.059 ± 0.00 c | 0.100 ± 0.00 d | 0.102 ± 0.04 e | 0.073 ± 0.01 f |
| 0.7 | 0.122 ± 0.00 a | 0.024 ± 0.00 b | 0.039 ± 0.07 c | 0.070 ± 0.00 d | 0.049 ± 0.03 e | 0.060 ± 0.02 f |
| 0.8 | 0.076 ± 0.01 a | 0.011 ± 0.00 b | 0.019 ± 0.05 c | 0.029 ± 0.08 d | 0.019 ± 0.00 c | 0.040 ± 0.00 e |
| 0.9 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
The spaced letters a–f indicate the level of significance of the difference between different agents and the control, which was obtained by SPSS Statistics, i.e., two distinct letters (e.g., a and b) indicate significant difference (p-value <0.05) and two letters of the same identity indicate nonsignificant difference (p-value >0.05).
In summary, compared with the control experiment in which no agent was added, both hydroxyurea and the ethnomedicinal plant extracts reduced the osmotic fragility. When the osmotic fragility is reduced, the resistance to hemolysis is increased. It is shown that although hydroxyurea has the highest effect on the reduction of osmotic fragility of RBCs, the values obtained in the presence of the tested ethnomedicinal plant extracts also indicated considerable efficacy in the reduction of osmotic fragility. The curves for the tested agents were shifted to the left of the control (Figure 4). The slopes of the curves for the agents are higher than that of the control, suggesting a higher resistance with agents than the control. Generally, the resistance of RBCs to hemolysis was significantly increased in the presence of hydroxyurea and the plant extracts as compared to the control.
The plant extracts may therefore be used as alternatives to the synthetic drug hydroxyurea. The ability of all these plant extracts to increase resistance to hemolysis as presented in this work is consistent with several earlier reports that showed the efficacy of medicinal plants on the reduction of osmotic fragility of red blood cells.36,43,63–67 Since the plant extracts and the hydroxyurea increased the resistance of RBCs to hypotonic lysis, it can be inferred that they act on the membrane of the RBCs to prevent inward water movement, which suggests possible direct interactions in the agents and the RBC membrane.
CONCLUSION
The presence of some important phytochemicals and secondary metabolites was examined in three ethnomedicinal plant extracts, including C. cajan leaf and seed, Z. zanthoxyloides leaf, and C. papaya leaf. The medicinal plant extracts were able to reduce the percentage of sickled cells, the rate of hemoglobin polymerization, and the osmotic fragility of human sickled RBCs. Further data analyses suggest that the ability of these natural plant extracts to exhibit these properties is probably due to the presence of the identified bioactive compounds. Thus, C. cajan leaf, C. cajan seed, Z. zanthoxyloides leaf, and C. papaya leaf extracts may be used as alternative agents to hydroxyurea or a precursor in ameliorating the sickling in human HbS containing RBCs. Various bioactive components in the plant extracts may be isolated and developed to drugs. Further research on identifying the bioactive components from the ethnomedicinal plants and to experimentally examine their individual potential for controlling the sickle cell disease is in progress.
Table 2.
GC–MS Analysis of Secondary Metabolites Present in Aqueous Extract of C. cajan Seed
| peaks | retention time (min) | compound name | molecular weight | molecular formula | peak area % |
|---|---|---|---|---|---|
| 1 | 5.7 | 6-methyl-1-octene | 126 | C9H18 | 1.55 |
| 2 | 6.3 | 2,2-dimethylbutane | 86 | C6H14 | 3.63 |
| 3 | 6.8 | 1-dodecene | 168 | C12H24 | 5.43 |
| 4 | 7.1 | 1,3-bis(1,1-dimethylethyl)-benzene | 190 | C14H22 | 1.96 |
| 5 | 7.6 | n-tridecan-1-ol | 200 | C13H28O | 8.53 |
| 6 | 8.0 | 2,4-di-tert-butylphenol | 206 | C14H22O | 7.52 |
| 7 | 8.2 | n-tridecan-1-ol | 200 | C13H28O | 8.61 |
| 8 | 8.8 | 1-octadecene | 252 | C18H36 | 2.79 |
| 9 | 9.0 | 1-octadecyne | 250 | C18H34 | 4.90 |
| 10 | 9.0 | 2-tridecyne | 180 | C13H24 | 2.00 |
| 12 | 9.2 | undecanoic acid | 200 | C12H24O2 | 1.69 |
| 13 | 9.3 | methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate | 292 | C18H28O3 | 4.58 |
| 14 | 9.4 | 2-methylhexadecan-1-ol | 256 | C17H36O | 2.01 |
| 15 | 9.9 | N-acetyl-DL-methionine | 191 | C7H13NO3S | 1.71 |
| 16 | 10.8 | 1-deoxy-2,5-O-methylenehexitol | 178 | C7H14O5 | 2.42 |
| 17 | 11.8 | trimethylsilyl 3-methyl-4-[(trimethylsilyl)oxy]benzoate | 296 | C14H24O3Si2 | 5.24 |
| 18 | 12.3 | hexestrol | 414 | C24H38O2Si2 | 2.11 |
| 19 | 13.4 | 3,3-diethoxy-1,1,1,5,5,5-hexamethyltrisiloxane | 296 | C10H28O4Si3 | 1.54 |
| 20 | 13.6 | butyl(2-isopropyl-5-methylphenoxy)dimethylsilane | 264 | C16H28OSi | 2.18 |
| 21 | 13.8 | 1,2-bis(trimethylsilyl)benzene | 222 | C12H22Si2 | 2.00 |
| 22 | 14.9 | 5-hydroxypentyl-1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione | 266 | C12H18N4O3 | 1.50 |
| 23 | 16.0 | trimethylsilyl 3-methyl-4-[(trimethylsilyl)oxy]benzoate | 296 | C14H24O3Si2 | 2.25 |
| 24 | 16.8 | (3,3-dimethyl-4-methylidene-2-trimethylsilylcyclopenten-1-yl)methoxy-trimethylsilane | 282 | C15H30OSi2 | 1.62 |
| 25 | 17.1 | 1,1,3,3,5,5,7,7-octamethyltetrasiloxane | 282 | C8H26O3Si4 | 1.72 |
| 26 | 17.3 | 7,7,9,9,11,11-hexamethyl-3,6,8,10,12,15-hexaoxa-7,9,11-trisilaheptadecane | 384 | C14H36O6Si3 | 4.10 |
| 27 | 17.4 | decamethyltetrasiloxane | 310 | C10H30O3Si4 | 3.42 |
| 28 | 18.1 | 1,1,3,3,5,5,7,7,9,9-decamethylpentasiloxane | 356 | C10H32O4Si5 | 3.35 |
| 29 | 18.8 | hexamethylcyclotrisiloxane | 222 | C6H18O3Si3 | 2.56 |
Table 3.
GC–MS Analysis of Secondary Metabolites Present in Aqueous Extract of Z. zanthoxyloides Leaf
| peaks | retention time (min) | compound name | molecular weight | molecular formula | peak area % |
|---|---|---|---|---|---|
| 1 | 5.6 | glycerin (1,2,3-propanetriol or glycerol) | 92 | C3H8O3 | 14.95 |
| 2 | 6.4 | hexahydro-5-methyl-1,3-diphenyl-1,3,5-triazine-2-thione | 283 | C16H17N3S | 2.58 |
| 3 | 6.8 | 1-dodecene | 168 | C12H24 | 3.25 |
| 4 | 7.6 | 1-undecene | 154 | C11H22 | 6.17 |
| 5 | 7.8 | 4-nitrophenyl-beta-D-glucopyranoside | 301 | C12H15NO8 | 1.45 |
| 6 | 8.0 | 2,4-bis(1,1-dimethylethyl)-phenol | 206 | C14H22O | 5.28 |
| 7 | 8.2 | 1-hexadecene | 224 | C16H32 | 5.39 |
| 8 | 8.4 | caryophyllene oxide | 220 | C15H24O | 3.19 |
| 9 | 8.7 | 9-(2-cyclohexylethyl)-heptadecane | 350 | C25H50 | 1.21 |
| 10 | 8.8 | 1-tetradecene | 196 | C14H28 | 2.21 |
| 11 | 9.3 | 3,5-bis(1,1-dimethylethyl)-4-hydroxybenezene propanoic acid | 292 | C18H28O3 | 3.92 |
| 12 | 9.7 | 1,2-epoxydodecane | 184 | C12H24O | 4.19 |
| 13 | 9.9 | 1,1,3,3,5,5-hexamethyltrisiloxane | 208 | C6H20O2Si3 | 2.45 |
| 14 | 11.4 | thymol-TMS (trimethyl-5-methyl-2-(1-methylethylphenoxysilane) | 222 | C13H22OSi | 1.46 |
| 15 | 11.7 | 4-nitrocinnamic acid | 193 | C9H7NO4 | 1.36 |
| 16 | 11.8 | bromazepam | 315 | C14H10BrN3O | 11.36 |
| 17 | 12.6 | 3-ethyl-4,4-dimethyl-2-(2-methylpropenyl)cyclohex-2-enone | 206 | C14H22O | 1.26 |
| 18 | 13.2 | butyl(2-isopropyl-5-methylphenoxy)dimethylsilane | 264 | C16H28OSi | 1.46 |
| 19 | 13.4 | ethyl tris(trimethylsilyl) silicate | 340 | C11H32O4Si4 | 2.30 |
| 20 | 14.1 | 1,3-diphenyl-3-(trimethylsilyl)propan-1-one | 282 | C18H22OSi | 2.43 |
| 21 | 14.3 | hexamethylcyclotrisiloxane | 222 | C6H18O3Si3 | 1.64 |
| 22 | 14.5 | 1,1,1,5,5,5-hexamethyl-3-(trimethylsilyl)trisiloxane | 280 | C9H28O2Si4 | 2.09 |
| 23 | 14.8 | 2-(3,4-bis[(trimethylsilyl)oxy]phenyl)-N,N-dimethyl-2-[(trimethylsilyl)oxy]ethanamine | 413 | C19H39NO3Si3 | 1.72 |
| 24 | 15.0 | 1-(3-methylbutyl)-1H-pyrazole-4-boronic acid, pinacol ester | 264 | C14H25BN2O2 | 2.13 |
| 25 | 15.3 | 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyloctasiloxane | 578 | C16H50O7Si | 2.54 |
| 26 | 15.4 | 1,1,3,3,5,5,7,7,9,9-decamethylpentasiloxane | 356 | C10H32O4Si5 | 2.19 |
| 27 | 15.7 | butyl-dimethyl-(5-methyl-2-propan-2-ylphenoxy)silane | 264 | C16H28OS | 1.68 |
| 28 | 16.1 | 4,4,6a,6b,8a,11,11,14b-octamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picen-3-one | 424 | C30H48O | 5.45 |
| 29 | 18.0 | 2-(3-trimethylsilyloxyphenyl) trimethylsilyloxyethane | 282 | C14H26O2Si2 | 1.36 |
| 30 | 18.8 | 1-methylethyl)phenoxy]-silane | 222 | C13H22OSi | 1.31 |
Acknowledgments
The project is supported in part by the US National Institutes of Health R01 grants (GM083107 and GM116960) to Y.Z. and the Nigeria Tertiary Education Trust Fund (TETFUND) to I.O.N.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957;180(4581):326–8. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- 2.Ahrens T, Rutherford K, Basham KAR. Essentials of oxygenation: implication for clinical practice. Jones & Bartlett Learning; 1993. [Google Scholar]
- 3.Voet D, Voet JG, Pratt CW. Fundamentals of Biochemistry. John Wiley & Sons, Inc; 1999. [Google Scholar]
- 4.Basset P, Braconnier F, Rosa J. An update on electrophoretic and chromatographic methods in the diagnosis of hemoglobinopathies. J Chromatogr, Biomed Appl. 1982;227(2):267–304. doi: 10.1016/s0378-4347(00)80384-9. [DOI] [PubMed] [Google Scholar]
- 5.Geller AK, O’Connor MK. The sickle cell crisis: a dilemma in pain relief. Mayo Clin Proc. 2008;83(3):320–323. doi: 10.4065/83.3.320. [DOI] [PubMed] [Google Scholar]
- 6.Iyamu EW, Turner EA, Asakura T. In vitro effects of NIPRISAN (Nix-0699): a naturally occurring, potent antisickling agent. Br J Haematol. 2002;118(1):337–43. doi: 10.1046/j.1365-2141.2002.03593.x. [DOI] [PubMed] [Google Scholar]
- 7.Allison A. Properties of sickle-cell haemoglobin. Biochem J. 1957;65(2):212. doi: 10.1042/bj0650212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton WA, Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987;70(5):1245–1266. [PubMed] [Google Scholar]
- 9.Ferrone FA, Ivanova M, Jasuja R. Heterogeneous nucleation and crowding in sickle hemoglobin: An analytic approach. Biophys J. 2002;82(1):399–406. doi: 10.1016/S0006-3495(02)75404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngolet LO, Moyen Engoba M, Kocko I, Elira Dokekias A, Mombouli JV, Moyen GM. Sickle-Cell Disease Healthcare Cost in Africa: Experience of the Congo. Anemia. 2016;2016:2046535. doi: 10.1155/2016/2046535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrone FA, Hofrichter J, Eaton WA. Kinetics of sickle hemoglobin polymerization: II. A double nucleation mechanism. J Mol Biol. 1985;183(4):611–631. doi: 10.1016/0022-2836(85)90175-5. [DOI] [PubMed] [Google Scholar]
- 12.Sahu M, Singh V, Yadav S, Harris K. Plant extracts with antisickling propensities: a feasible succour towards sickle cell disease management-a mini review. J Phytol. 2012;4(3):24–29. [Google Scholar]
- 13.Cokic VP, Smith RD, Beleslin-Cokic BB, Njoroge JM, Miller JL, Gladwin MT, Schechter AN. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest. 2003;111(2):231–9. doi: 10.1172/JCI16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124(26):3850–3857. doi: 10.1182/blood-2014-08-435768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halsey C, Roberts IA. The role of hydroxyurea in sickle cell disease. Br J Haematol. 2003;120(2):177–186. doi: 10.1046/j.1365-2141.2003.03849.x. [DOI] [PubMed] [Google Scholar]
- 16.Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood. 2008;111(3):1117–1123. doi: 10.1182/blood-2007-05-088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Athanassiou G, Moutzouri A, Kourakli A, Zoumbos N. Effect of hydroxyurea on the deformability of the red blood cell membrane in patients with sickle cell anemia. Clin Hemorheol Microcirc. 2006;35(1, 2):291–295. [PubMed] [Google Scholar]
- 18.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 19.Covas DT, de Lucena Angulo I, Palma PVB, Zago MA. Effects of hydroxyurea on the membrane of erythrocytes and platelets in sickle cell anemia. Haematologica. 2004;89(3):273–280. [PubMed] [Google Scholar]
- 20.Huang Z, Louderback JG, King SB, Ballas SK, Kim-Shapiro DB. In vitro exposure to hydroxyurea reduces sickle red blood cell deformability. Am J Hematol. 2001;67(3):151–156. doi: 10.1002/ajh.1098. [DOI] [PubMed] [Google Scholar]
- 21.Oyewole O, Malomo S, Adebayo J. Comparative studies on antisickling properties of thiocyanate, tellurite and hydroxyurea. Pak J Med Sci. 2008;24(1):18. [Google Scholar]
- 22.Adragna N, Fonseca P, Lauf PK. Hydroxyurea affects cell morphology, cation transport, and red blood cell adhesion in cultured vascular endothelial cells. Blood. 1994;83(2):553–560. [PubMed] [Google Scholar]
- 23.Charache S. Mechanism of action of hydroxyurea in the management of sickle cell anemia in adults. Semin Hematol. 1997:15–21. [PubMed] [Google Scholar]
- 24.Eaton WA, Hofrichter J. The biophysics of sickle cell hydroxyurea therapy. Science. 1995;268(5214):1142. doi: 10.1126/science.7539154. [DOI] [PubMed] [Google Scholar]
- 25.Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N Engl J Med. 2008;358(13):1362–1369. doi: 10.1056/NEJMct0708272. [DOI] [PubMed] [Google Scholar]
- 26.Ballas SK, Dover GJ, Charache S. Effect of hydroxyurea on the rheological properties of sickle erythrocytes in vivo. Am J Hematol. 1989;32(2):104–111. doi: 10.1002/ajh.2830320206. [DOI] [PubMed] [Google Scholar]
- 27.Haynes J, Obiako B, Hester RB, Baliga BS, Stevens T. Hydroxyurea attenuates activated neutrophil-mediated sickle erythrocyte membrane phosphatidylserine exposure and adhesion to pulmonary vascular endothelium. Am J Physiol: Heart Circ Physiol. 2008;294(1):H379–H385. doi: 10.1152/ajpheart.01068.2007. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers GP, Dover GJ, Noguchi CT, Schechter AN, Nienhuis AW. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N Engl J Med. 1990;322(15):1037–1045. doi: 10.1056/NEJM199004123221504. [DOI] [PubMed] [Google Scholar]
- 29.Ekeke G, Shode F. Phenylalanine is the predominant antisickling agent in Cajanus cajan seed extract. Planta Med. 1990;56(1):41–43. doi: 10.1055/s-2006-960880. [DOI] [PubMed] [Google Scholar]
- 30.Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP, Orringer EP. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991;77(6):1334–1341. [PubMed] [Google Scholar]
- 31.Nnamani IN, Joshi GS, Danso-Danquah R, Abdulmalik O, Asakura T, Abraham DJ, Safo MK. Pyridyl derivatives of benzaldehyde as potential antisickling agents. Chem Biodiversity. 2008;5(9):1762–1769. doi: 10.1002/cbdv.200890165. [DOI] [PubMed] [Google Scholar]
- 32.Walder JA, Zaugg RH, Iwaoka RS, Watkin WG, Klotz IM. Alternative aspirins as antisickling agents: acetyl-3, 5-dibromosalicylic acid. Proc Natl Acad Sci U S A. 1977;74(12):5499–5503. doi: 10.1073/pnas.74.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells†,‡. Br J Haematol. 2005;128(4):552–561. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- 34.Moody JO, Ojo OO, Omotade OO, Adeyemo AA, Olumese PE, Ogundipe OO. Anti-sickling potential of a Nigerian herbal formula (ajawaron HF) and the major plant component (Cissus populnea L. CPK) Phytother Res. 2003;17(10):1173–6. doi: 10.1002/ptr.1323. [DOI] [PubMed] [Google Scholar]
- 35.Nwosu F, Dosumu O, Okocha J. The potential of Terminalia catappa (Almond) and Hyphaene thebaica (Dum palm) fruits as raw materials for livestock feed. Afr J Biotechnol. 2008;7(24):4576–4580. [Google Scholar]
- 36.Imaga N, Gbenle G, Okochi V, Akanbi S, Edeoghon S, Oigbochie V, Kehinde M, Bamiro S. Antisickling property of Carica papaya leaf extract. Afr J Biochem Res. 2009;3(4):102–106. [Google Scholar]
- 37.Ekeke G, Shode F. The reversion of sickled cells by Cajanus cajan. Planta Med. 1985;51(6):504–507. doi: 10.1055/s-2007-969576. [DOI] [PubMed] [Google Scholar]
- 38.Oduola T, Adeniyi F, Ogunyemi E, Bello I, Idowu T. Antisickling agent in an extract of unripe pawpaw (Carica papaya): is it real? Afr J Biotechnol. 2006;5(20):1947–1949. [Google Scholar]
- 39.Bewaji C, Olorunsogo O, Bababunmi E. Sickle-cell membrane-bound (Ca 2++ Mg 2+)-ATPase: Activation by 3, 4-dihydro-2, 2-dimethyl-2H-1-benzopyran-6-butyric acid, a novel anti-sickling agent. Cell Calcium. 1985;6(3):237–244. doi: 10.1016/0143-4160(85)90009-0. [DOI] [PubMed] [Google Scholar]
- 40.Imaga N, Gbenle G, Okochi V, Adenekan S, Edeoghon S, Kehinde M, Bamiro S, Ajiboye A, Obinna A. Antisickling and toxicological profiles of leaf and stem of Parquetina nigrescens L. J Med Plant Res. 2010;4:639–643. [Google Scholar]
- 41.Harborne JB. Phytochemical methods a guide to modern techniques of plant analysis. Springer Science & Business Media; 1998. [Google Scholar]
- 42.Odebiyi O, Sofowora E. Antimicrobial alkaloids from a Nigerian chewing stick (Fagara zanthoxyloides) Planta Med. 1979;36:204–207. doi: 10.1055/s-0028-1097271. [DOI] [PubMed] [Google Scholar]
- 43.Pauline N, Cabral BNP, Anatole PC, Jocelyne AMV, Bruno M, Jeanne NY. The in vitro antisickling and antioxidant effects of aqueous extracts Zanthoxyllum heitzii on sickle cell disorder. BMC Complementary Altern Med. 2013;13(1):162. doi: 10.1186/1472-6882-13-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nwaoguikpe RN, Ujowundu CO, Okwu GN. The Antisickling Potentials of Four Curcubits (T. Occidentalis, C. Maxima; C. Sativus and C. Lonatus) Scholars J Appl Med Sci. 2013;1(3):191–198. [Google Scholar]
- 45.Ayoola PB, Adeyeye A. Phytochemical and Nutrient Evaluation of Carica papaya (Pawpaw) Leaves. IJRRAS. 2010;5(3):325–328. [Google Scholar]
- 46.Banso A, Ngbede JE. Phytochemical screening and in vitro antifungal properties of Fagara zanthoxyloides. J Food Agric Environ. 2006;4(3/4):8. [Google Scholar]
- 47.Mohanty P, Chourasia N, Bhatt NK, Jaliwala Y. Preliminary Phytochemical Screening of Cajanus cajan Linn. Asian J Pharm Technol. 2011;1(2):49–52. [Google Scholar]
- 48.Marks D, Glyphis J, Leighton M. Measurement of protein in tannin-protein precipitates using ninhydrin. J Sci Food Agric. 1987;38(3):255–261. [Google Scholar]
- 49.Rajput ZI, Hu S-h, Xiao C-w, Arijo AG. Adjuvant effects of saponins on animal immune responses. J Zhejiang Univ, Sci, B. 2007;8(3):153–161. doi: 10.1631/jzus.2007.B0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dash BP, Archana Y, Satapathy N, Naik SK. Search for antisickling agents from plants. Pharmacogn Rev. 2013;7(13):53. doi: 10.4103/0973-7847.112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palcic MM, Heerze LD, Pierce M, Hindsgaul O. The use of hydrophobic synthetic glycosides as acceptors in glycosyltransferase assays. Glycoconjugate J. 1988;5(1):49–63. [Google Scholar]
- 52.Archana P, Sathishkumar N, Bharathi N. In Silico Docking Analysis of Curcumin–An Inhibitor for Obesity. Int J Pharma Bio Sci. 2010;1(4):224–235. [Google Scholar]
- 53.Mohan C, Dinakar S, Anand T, Elayaraja R, SathiyaPriya B. Phytochemical, GC-MS analysis and Antibacterial activity of a Medicinal Plant Acalypha indica. Int J PharmTech Res. 2012;4(3):1050–1054. [Google Scholar]
- 54.Ouattara B, Jansen O, Angenot L, Guissou I, Frédérich M, Fondu P, Tits M. Antisickling properties of divanilloylquinic acids isolated from Fagara zanthoxyloides Lam.(Rutaceae) Phytomedicine. 2009;16(2):125–129. doi: 10.1016/j.phymed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Sudeep H, Prasad KS. Computational studies on the antiobesity effect of polyphenols from pomegranate leaf. J Chem Pharm Res. 2014;6(9):278–281. [Google Scholar]
- 56.Doraiswamy H, Kathavarayan N, Sharma RC, Krishnamurthy V. Molecular Docking Analysis of Secondary Metabolites of Trigonella foenum graecum and Carica papaya with FTO: An Insilico Approach. Int J Pharm Sci Rev Res. 2014;27(1):105–110. [Google Scholar]
- 57.Eaton WA, Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 58.Nagel RL, Bookchin RM, Johnson J, Labie D, Wajcman H, Isaac-Sodeye WA, Honig GR, Schiliro G, Crookston JH, Matsutomo K. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A. 1979;76(2):670–672. doi: 10.1073/pnas.76.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noguchi CT, Rodgers GP, Schechter AN. Intracellular Polymerization. Ann N Y Acad Sci. 1989;565(1):75–82. doi: 10.1111/j.1749-6632.1989.tb24152.x. [DOI] [PubMed] [Google Scholar]
- 60.Stuart MJ, Setty BY. Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood. 1999;94(5):1555–1560. [PubMed] [Google Scholar]
- 61.Cooper RA, Jandl JH. Bile salts and cholesterol in the pathogenesis of target cells in obstructive jaundice. J Clin Invest. 1968;47(4):809. doi: 10.1172/JCI105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffman JF. The Use of Resealed Erythrocytes as Carriers and Bioreactors. Springer; 1992. On red blood cells, hemolysis and resealed ghosts; pp. 1–15. [DOI] [PubMed] [Google Scholar]
- 63.Adenkola A, Ayo J, Sackey A, Adelaiye A. Erythrocyte osmotic fragility of pigs administered ascorbic acid and transported by road for short-term duration during the harmattan season. Afr J Biotechnol. 2010;9(2):226–233. [Google Scholar]
- 64.Haut A, Tudhope G, Cartwright G, Wintrobe M. Studies on the osmotic fragility of incubated normal and abnormal erythrocytes. J Clin Invest. 1962;41(9):1766. doi: 10.1172/JCI104636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ibegbulem C, Eyong E, Essien E. Biochemical effects of drinking Terminalia catappa Linn. decoction in Wistar rats. Afr J Biochem Res. 2011;5(8):237–243. [Google Scholar]
- 66.Mpiana P, Ngbolua K, Mudogo V, Tshibangu D, Atibu E, Tshilanda D, Misengabu N. Anti Sickle Erythrocytes Haemolysis Properties and Inhibitory Effect of Anthocyanins Extracts of Trema orientalis (Ulmaceae) on the Aggregation of Human Deoxyhemoglobin S in vitro. Journal of Medical Sciences. 2011;11(3):129–137. [Google Scholar]
- 67.Naiho A, Okonkwor B, Okoukwu C. Anti-Sickling and Membrane Stabilizing Effects of Carica papaya Leaf Extract. British Journal of Medicine and Medical Research. 2015;6(5):484. [Google Scholar]