Abstract
Purpose of review
Bone is constantly being remodeled throughout adult life through constant anabolic and catabolic actions that maintain tissue homeostasis. A number of hormones, cytokines growth factors and the proximity of various cells to bone surfaces influence this process. Inflammatory changes at the bone microenvironment result in alterations leading to both excessive bone loss and bone formation. Detailed understanding of the physiological and pathological mechanisms that dictate these changes will allow us to harness inflammatory signals in bone regeneration.
Recent Findings
Recent reports have suggested that inflammatory signals are able to stimulate transcription factors that regulate osteoblast differentiation from their precursors.
Summary
In this review we summarized current understanding of the roles of inflammation in bone resorption and bone formation, which give rise to different pathologies and discuss the huge potential of harnessing these inflammatory signals to achieve bone regeneration.
Keywords: Inflammation, osteoclasts, bone loss, osteoblasts bone formation
1. Introduction
Tissue regeneration is a biological process appreciated since the Bronze Age, from the ancient Greek myth of Prometheus and his punishment for deceiving the gods and protecting mankind. In the Greek myth, an eagle fed from his liver each day, but the liver regenerated overnight. Although, Prometheus is a mythical creature, liver regeneration in humans is a scientific fact. Indeed epimorphic regeneration occurs in certain animals, such as salamanders and frogs, which are able to regenerate limbs, tails, jaws, eye lenses. In mammals, deer can regenerate their antlers and mouse their ears. These unusual regenerative properties of certain tissues are a logical adaptation of organisms that are likely to be injured. Specifically, the liver is the main detoxifying organ of the body and is likely to be injured by ingested toxins. Similarly, the skeleton is likely to be fractured which necessitates the ability of fractured bone to regenerate and undergo repair. Apart from fracture healing which is most commonly observed, bone regeneration has also been observed in humans after amputations, especially in children.
Clinical Evidence
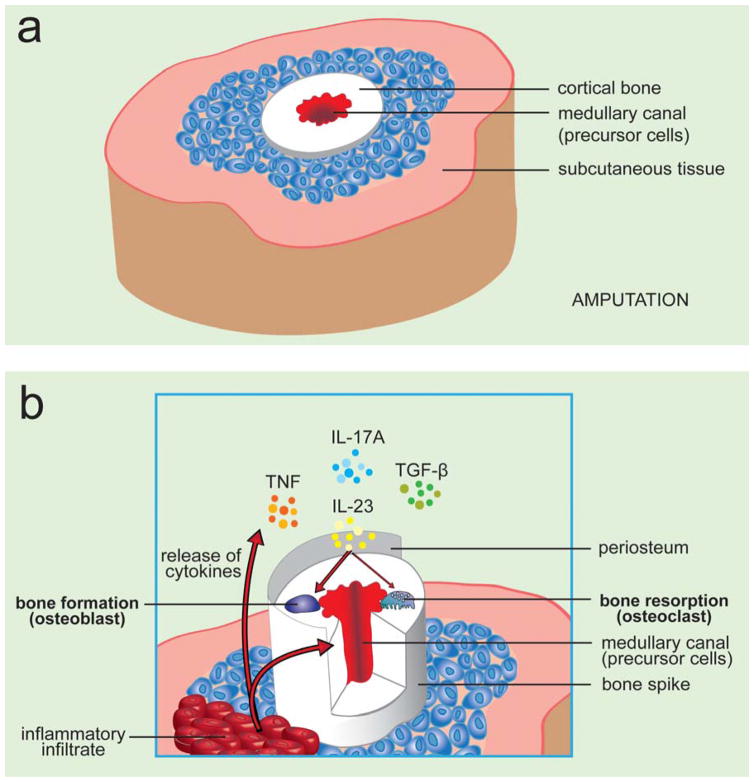
The ability of bone to regenerate after congenital transdiaphyseal amputation has been recognized since the 1940s and includes numerous case reports of digital phalanx regeneration and a more striking skeletal condition unique to children, known as Juvenile Amputee Overgrowth (JAO). This unique pathological condition is characterized by the growth of a skeletal spike that, in extreme cases, perforates the soft tissue envelope[1]. JAO is certainly no myth and has a prevalence of up to 35 percent in pediatric amputees and its cellular and molecular mechanisms underlying this phenomenon remain undetermined. A wide range of surgical procedures have been proposed for the management of JAO. Occlusion of the medullary canal appears to significantly retard the progression of JAO and is the current treatment of choice with the best-reported results occurring with the use of autogenous bone graft[2,3]. Therefore, current thinking has focused on the progenitor cells and cytokines such as TNF, IL-23, IL-17 and TGFβ that are present within the medullary canal and their interplay under inflammatory conditions with inflamed tissue (Figure 1). The notion that circulatory cells are able to contribute to bone formation is not observed only in JAO. Interestingly, recent reports suggest that cells with osteogenic potential can be found in a variety of tissues and circulating cells of hematopoietic origin can serve as osteogenic precursors at remote sites of tissue inflammation [4]. This study shows that CD45+ hematopoietic-derived circulating osteogenic precursor (COP) cells with osteogenic potential migrate to inflammatory sites where they increase heterotopic bone formation in patients with fibrodysplasia ossificans progressiva (FOP). FOP is an extremely rare autosomal-dominant genetic disorder of connective tissue defined by progressive, disabling, heterotopic skeletogenesis in predictable anatomic patterns[5]. Histopathologic analysis of FOP lesions have also revealed an extended inflammatory infiltrate consisting of monocytes and lymphocytes that are commonly associated with pathology and more importantly accompany spontaneous and trauma-induced exacerbations or flare-ups of FOP[6].
Figure 1. Pathophysiology of juvenile amputation overgrowth.
Schematic shows the a) physiological and b) pathological bone remodeling as it occurs in JAO. Inflammatory infiltrate present after tissue injury (amputation) is responsible for the release of pro-inflammatory cytokines IL-23 IL-17, TNF and TGF-β that come into contact with stromal and hematopoietic precursor cells. These molecular changes allow the transcriptional activation of bone cells to promote bone formation causing an abnormal increase in bone growth forming a bone spike, which in extreme cases the terminal skeletal spike may perforate the soft tissue envelope.
2. Inflammation in bone physiology and pathology
Increased inflammation in arthritis or after amputation and trauma has always been linked with bone loss via osteoclast activation, and many laboratories have studied this phenomenon extensively in autoimmune diseases but the role of inflammation in bone formation is less clear and not well defined. Approximately 2 million people have limb amputations in the United States due to disease or injury with more than 185,000 new amputations every year. The ability to promote epimorphic regeneration, or the regrowth of a biologically based digit or limb, would radically change the prognosis for amputees. However, the significance and impact of undertsanding molecular pathways that regulate physiological and pathological bone remodelling is far greater and more far-reaching than the amputee populations. In fact, the ability to harness inflammatory signals to regenerate bone may enable the understanding of tissue repair mechanisms that can be applied to a large number of tissues with fundamental benefits.
The osteoclast and the osteoblast are the two main cell types that regulate bone remodeling within the bone. They work in tandem, exhibiting opposing functions; to remove and replace bone, respectively. Under physiological conditions, osteoblasts secrete macrophage colony stimulating factor (M-CSF) which signals through the tyrosine kinase receptor Csf1r, which is ubiquitously expressed early during myeloid lineage commitment, and its expression is maintained on nearly all mononuclear phagocytic cells [7]. The differentiation of osteoclast precursors is regulated by the receptor activator of nuclear factor κ B ligand (RANKL) [8]. RANKL-mediated osteoclast differentiation depends on the RANK receptor, which is upregulated by M-CSF in early stage osteoclast precursors [9]. M-CSF activity generates a specific myeloid subset that expresses both Csf1r and Rank. This Csf1r+/Rank+ myeloid precursor is destined to become a terminally-differentiated osteoclast on bone surfaces upon stimulation with MCSF and RANKL. Osteoblasts control the availability of MCSF and RANKL to osteoclast precursors and also regulate the production and secretion of osteoprotegerin (OPG). OPG is a soluble decoy receptor for RANKL; thus, the main determinants of osteoclastogenesis are the relative concentrations of MCSF, RANKL and OPG which regulate a network of gene transcriptions as previously reviewed [10]. The coupling between the osteoblast and the osteoclast at the bone surface, balance bone formation and resorption and maintain bone homeostasis by removing mature bone tissue, following fractures or micro-fractures, through bone resorption and replacing it with new bone tissue by a process called ossification. The amount of resorption and ossification is therefore tightly linked to the number and activity of osteoclasts and osteoblasts and their regulation makes it a necessary event for bone homeostasis and fracture repair [11]. Under inflammatory conditions such as autoimmune diseases, these pathways seem to be disturbed.
Inflammatory Bone Remodeling
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that exhibits various clinical manifestations including synovial inflammation and bone loss. Immune cells such as Th17 cells, B cells, macrophages, neutrophils, mast cells and fibroblast-like synoviocytes are critical for inducing and maintaining synovial inflammation in RA pathology [12,13]. This chronic inflammation leads to secretion of a plethora of pro-inflammatory cytokines and RANKL, which are primarily responsible for the activation of osteoclasts and the subsequent bone destruction.
Transcriptional regulation of bone formation
RANKL regulates a number of transcription factors including NFkB, which is critical in osteoclast differentiation [10,14]. The IKK kinase complex, comprised of two kinases (IKKα and IKKβ) and a regulatory subunit, NEMO/IKKγ is the core element of the NF-κB cascade. This can be activated by RANKL and TNF in both physiological and pathological (inflammatory) conditions to regulate osteoclastogenesis [15]. Interestingly, the same complex seems to have dramatic effects in the regulation of bone formation in osteoblasts [16]. The roles of NFkB transcriptional regulation are diverse and expand in many different cell types that regulate numerous cellular processes. Their functions in bone remodeling include physiological (RANKL) and pathological (TNF) induced bone loss via osteoclastogenesis and bone formation via the osteoblasts, which have been reviewed elsewhere [17].
Although RANKL is the most potent osteoclastogenic signal in the aforementioned Csf1r+/Rank+ myeloid populations, osteoclasts have also been generated under inflammatory conditions in RANK−/− mice [17,18,19,20]. In these alternative pathways of osteoclastogenesis that is independent of RANKL, it is clearly evident that a few pro-inflammatory cytokines including TNF [21,22] and IL-23 [23] regulate the activation of calcium signaling and nuclear factor of activated T cells cytoplasmic 1 (NFATc1). NFATc1−/− cells are unable to generate osteoclasts, despite normal development into the monocyte/macrophage lineage, highlighting the specific needs of osteoclastogenesis [24]. NFATc1 is a transcription factor activated by calcium signaling, as Ca2+ activates calcineurin, which in turn dephosphorylates multiple phosphoserines on NFAT, leading to its nuclear translocation and activation. NFATc1 is responsible for the regulation of genes related to osteoclast function as well as numerous genes non-essential to osteoclast function [25,26]; therefore the significance of this pathway may extend beyond our current understanding. This is particularly important as other research groups, paradoxically, have observed a role of NFATc1 in bone formation. Although NFATc1 in osteoclasts induces bone loss in osteoblasts, NFATc1 induces bone formation as mice expressing a constitutively nuclear NFATc1 variant (NFATc1nuc) develop high bone mass [27]. Surprisingly, NFATc1nuc mice have massive osteoblast overgrowth and enhanced osteoblast proliferation [27]. Although Runx2/Cbfa1 is considered the major transcription factor to trigger activation of osteoblast specific genes [28], at least in one report, NFATc1 regulates bone mass via the osteoblast. The emerging importance of NFATc1 in bone remodeling is due to the fact that Runx2/Cbfa1 is expressed restrictively in osteoblasts whereas NFATc1 is expressed in both osteoblasts and osteoclasts. More importantly, if indeed that is the case, it seems that NFATc1 transcription factor can regulate bone formation and bone loss in both cell types. This is of particular importance as pro-inflammatory cytokines such as IL-23 and TNF can induce NFATc1 [22,23].
The notion that inflammation is required for bone regeneration is supported by various observations, most importantly the impaired fracture healing in TNF deficient mice [29]. The ability of inflammatory signals to induce bone formation is also corroborated by the findings that deregulated bone formation occurs at erosion sites in inflammatory arthritis although its not clear whether this is a coupling effect where increased bone loss is leading to increased bone formation or whether it’s a directly inflammation induced bone formation.
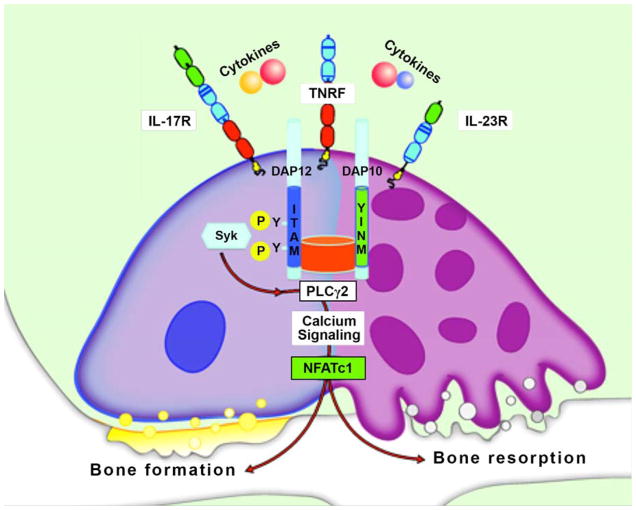
Paradoxical observations
The induction of bone overgrowth in JAO is indeed puzzling and reconciling the paradox of increased bone formation under inflammatory conditions is challenging. The significant overlaps in transcriptional regulation between osteoclasts, osteoblasts and T cells, by common transcription factors, NFkB and NFATc1 can only partly explain the clinical observations in JAO. This is because although osteoblasts and osteoclasts express TNF and IL-23 receptors the presence of immunoreceptors such as DAP12 and MDL-1 is critical for NFATc1 stimulation. Indeed a complex interplay between adaptors and immunoreceptors including DAP10, DAP12 and MDL-1 can relay activation signals that regulate osteoclastogenesis and bone mineral density [30] (Figure 2). This is of immense importance since those immunoreceptors are only expressed in hematopoietic cells and not mesenchymal cells. The assumption that circulating cells are of hematopoietic origin, can be manipulated to both bone resorbing and bone forming cells is supported by some evidence [4]. However, before we can explain a paradox with yet another paradox, more experiments in circulating cells need to be performed and a thorough investigation to dissect which cell type responds to inflammation-induced NFkB/NFATc1 activation is much needed. Whether bone formation on hematopoietic precursor cells is possible or not, the certainty is that the molecular mechanisms of inflammatory bone regenerations elude us. There are a lot of questions remain unanswered and the mechanisms of inflammatory bone gain need to be investigated, as since the observations by Winslow et al., the activation patterns of NFATc1 in osteoblast precursors remain unknown.
Figure 2. Cellular and molecular mechanisms of immune bone loss and bone formation.
Schematic representation of pro-inflammatory cytokine signaling showing activation of NFATc1 through calcium-dependent pathways. Although NFATc1 regulation of genes responsible for both bone loss and bone formation has been reported, certain immunoreceptors are restrictively expressed in myeloid cells. The possibility of cells of hematopoietic origin serving as osteogenic precursors at remote sites of tissue inflammation has also been proposed.
IL-23/IL17 in bone regeneration
Non-conventional γδ TCR+ cells are a small (<5% in human peripheral blood) subset of T cells that straddles between the adaptive and innate immune responses and exhibit a high percentage of IL-23R+ cells (~38%), compared to other innate cells (4–6%) [31,32]. Studies on fracture healing have shown differences in the biomechanical properties of fracture repair, upregulation of cell adhesion molecules in osteoblasts and secretion of inflammatory cytokines at the regenerative site in the absence of γδ TCR+ cells, suggesting that a more central role of γδ T cells in fracture healing [33]. Other recent studies have specifically shown that Vγ6+ γδ T cells are the major source of IL-17A in bone regeneration and the action of IL-17 is responsible for enhanced bone formation in a drill-hole injury animal model [34]. The contribution of IL-23 in the differentiation and expansion of IL-23R+ T cell subsets that produce IL-17 to enhance bone formation is also corroborated by other groups. Other groups have also shown that pro-inflammatory cytokines IL-17 and TNF have the ability to induce gene expression of genes Wnt5a, BMP2, and RUNX2, associated and required for osteoblast differentiation in isolated synoviocytes of arthritic patients [35].
3. Conclusions
The role of inflammation has been closely associated with bone loss and osteoclastogenesis, however, it becomes all the more clearer that inflammation has as strong a role in bone formation. The same pro-inflammatory cytokines that have been studied in the context of bone loss in animal models of arthritis are now being studied in bone fracture/repair models. The dual capacities of many transcription factors including NFkB and NFATc1 that regulate these processes, suggest that adaptor or transducer molecules may fine-tune these regulatory elements that need to be identified. Moreover, the specific cell subtypes that give rise to terminally-differentiated osteoclasts and osteoblasts need to be better defined [36]. Once we have overcome these obstacles, the exciting prospects of bone regeneration will be realized as a therapeutic approach. For now, although bone regeneration may not be a myth, it certainly merits further investigation.
Key points.
Inflammation regulates bone loss in pathological conditions (Rheumatoid arthritis)
Inflammation regulates bone formation in physiological conditions (fracture repair)
Inflammation may recruit different precursor cells and pathways than those observed in physiological bone remodeling.
Acknowledgments
We would like to thank Thanh Nguyen for her assistance with the illustration in the figures.
Financial support and sponsorship
This work was partly supported by NIH/NIAMS-R01AR062173 and the National Psoriasis Foundation Translational Research grant to IEA.
Footnotes
Conflicts of interest
None
References
- 1.O’Neal ML, Bahner R, Ganey TM, Ogden JA. Osseous overgrowth after amputation in adolescents and children. J Pediatr Orthop. 1996;16:78–84. doi: 10.1097/00004694-199601000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Davids JR, Meyer LC, Blackhurst DW. Operative treatment of bone overgrowth in children who have an acquired or congenital amputation. J Bone Joint Surg Am. 1995;77:1490–1497. doi: 10.2106/00004623-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Benevenia J, Makley JT, Leeson MC, Benevenia K. Primary epiphyseal transplants and bone overgrowth in childhood amputations. J Pediatr Orthop. 1992;12:746–750. doi: 10.1097/01241398-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Suda RK, Billings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, Porter DL, Shore EM, Pignolo RJ. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27:2209–2219. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan FS, Tabas JA, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J Bone Joint Surg Am. 1993;75:220–230. doi: 10.2106/00004623-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, Kurokawa T, Suda T. Macrophage Colony-Stimulating Factor Is Indispensable for Both Proliferation and Differentiation of Osteoclast Progenitors. Journal of Clinical Investigation. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 9.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and Differentiation of Osteoclast Precursor Cells by the Sequential Expression of c-Fms and Receptor Activator of Nuclear Factor {kappa}B (RANK) Receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 11.Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 2008;118:421–428. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 13.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes & development. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Amer Y, Tondravi MM. NF-kappaB and bone: the breaking point. Nat Med. 1997;3:1189–1190. doi: 10.1038/nm1197-1189. [DOI] [PubMed] [Google Scholar]
- 16.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyce BF, Yao Z, Xing L. Functions of nuclear factor kappaB in bone. Ann N Y Acad Sci. 2010;1192:367–375. doi: 10.1111/j.1749-6632.2009.05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, Yeh WC, Lee SK, Lorenzo JA, Choi Y. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. The Journal of experimental medicine. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. The Journal of experimental medicine. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.O’Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, Aliprantis AO, Charles JF. RANK-Independent Osteoclast Formation and Bone Erosion in Inflammatory Arthritis. Arthritis Rheumatol. 2016;68:2889–2900. doi: 10.1002/art.39837. This study describes the importance of RANKL-independent pathways in inflammatory arthtritis using RANK deficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukai T, Ishida S, Ishikawa R, Yoshitaka T, Kittaka M, Gallant R, Lin YL, Rottapel R, Brotto M, Reichenberger EJ, Ueki Y. SH3BP2 cherubism mutation potentiates TNF-alpha-induced osteoclastogenesis via NFATc1 and TNF-alpha-mediated inflammatory bone loss. J Bone Miner Res. 2014;29:2618–2635. doi: 10.1002/jbmr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarilina A, Xu K, Chen J, Ivashkiv LB. TNF activates calcium-nuclear factor of activated T cells (NFAT)c1 signaling pathways in human macrophages. Proc Natl Acad Sci U S A. 2011;108:1573–1578. doi: 10.1073/pnas.1010030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin HS, Sarin R, Dixit N, Wu J, Gershwin E, Bowman EP, Adamopoulos IE. Crosstalk among IL-23 and DNAX activating protein of 12 kDa-dependent pathways promotes osteoclastogenesis. J Immunol. 2015;194:316–324. doi: 10.4049/jimmunol.1401013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR, Glimcher LH. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118:3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charles JF, Hsu LY, Niemi EC, Weiss A, Aliprantis AO, Nakamura MC. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J Clin Invest. 2012;122:4592–4605. doi: 10.1172/JCI60920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winslow MM, Pan M, Starbuck M, Gallo EM, Deng L, Karsenty G, Crabtree GR. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell. 2006;10:771–782. doi: 10.1016/j.devcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 29.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 30.Inui M, Kikuchi Y, Aoki N, Endo S, Maeda T, Sugahara-Tobinai A, Fujimura S, Nakamura A, Kumanogoh A, Colonna M, Takai T. Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc Natl Acad Sci U S A. 2009;106:4816–4821. doi: 10.1073/pnas.0900463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul S, Shilpi, Lal G. Role of gamma-delta (gammadelta) T cells in autoimmunity. J Leukoc Biol. 2015;97:259–271. doi: 10.1189/jlb.3RU0914-443R. [DOI] [PubMed] [Google Scholar]
- 33.Colburn NT, Zaal KJ, Wang F, Tuan RS. A role for gamma/delta T cells in a mouse model of fracture healing. Arthritis Rheum. 2009;60:1694–1703. doi: 10.1002/art.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, Takayanagi H. IL-17-producing gammadelta T cells enhance bone regeneration. Nat Commun. 2016;7:10928. doi: 10.1038/ncomms10928. This study describes the role of Vγ6+ γδ T cells are the major source of IL-17A in bone regeneration and the action of IL-17 is responsible for enhanced bone formation in a drill-hole injury animal model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Osta B, Roux JP, Lavocat F, Pierre M, Ndongo-Thiam N, Boivin G, Miossec P. Differential Effects of IL-17A and TNF-alpha on Osteoblastic Differentiation of Isolated Synoviocytes and on Bone Explants from Arthritis Patients. Front Immunol. 2015;6:151. doi: 10.3389/fimmu.2015.00151. This study demonstrates a role of IL-17 in bone formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fierro FA, Nolta JA, Adamopoulos IE. Concise Review: Stem Cells in Osteoimmunology. Stem Cells. 2017;35:1461–1467. doi: 10.1002/stem.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]