Summary
Background
Although exercise is a vital component of the therapy prescribed to individuals with cystic fibrosis (CF), it is not a priority due to a finite amount of treatment time and the view that exercise is not as beneficial as pharmacological treatments by many individuals with CF. We sought to compare the therapeutic benefits of exercise and their prescribed bronchodilator albuterol.
Methods
CF (n = 14) and healthy (n = 16) subjects completed three visits, a baseline screening with VO2 max test and two treatment visits. On the two treatment visits, subjects completed spirometry and diffusing capacity of the lungs for nitric oxide (DLNO) maneuvers either at baseline, 60, and 110 min post-albuterol administration, or at baseline and the midway point of three separate 15 min exercise bouts at low, moderate and vigorous intensity (25, 50 and 65% of the maximum workload, respectively).
Results
With moderate exercise the increase in DLNO was double (39 ± 8 vs 15 ±6% change) and the level of bronchodilation similar (23% change) when compared to 110 min post-albuterol in individuals with CF. During exercise FVC became reduced (−309 ± 66 mL with moderate exercise) and the increase in FEV1 was attenuated (103 ± 39 vs 236 ± 58 mL, exercise vs. albuterol) when compared with the response to albuterol in individuals with CF. Epinephrine (EPI) release increased 39, 72 and 144% change with low, moderate and vigorous intensity exercise respectively for individuals with CF, but this increase was blunted when compared to healthy subjects.
Conclusion
Our results suggest that moderate intensity exercise is the optimal intensity for individuals with CF, as low intensity exercise increases EPI less than 50% and vigorous intensity exercise is over taxing, such that airflow can be restricted. Although the duration of the beneficial effect is uncertain, exercise can promote greater improvements in gas diffusion and comparable bronchodilation when compared to albuterol.
Keywords: Albuterol, Bronchodilation, Submaximal exercise, Cycle ergometer, Catecholamines, Diffusion capacity of the lungs for nitric oxide
Background
Research investigating the effects of exercise as therapy in cystic fibrosis (CF) has demonstrated that exercise can increase aerobic capacity (VO2peak) and exercise tolerance [1], facilitate sputum clearance [2,3], and that individuals with CF with better aerobic fitness have better survival [4]. Although exercise is a vital component of the therapy regimen prescribed to individuals with CF, Myers et al. demonstrated that adherence is poor, finding only 24% of adults with CF are completing their exercise program [5]. Reasons individuals with CF may not make exercise a priority are the belief that exercise is not as beneficial as pharmacological treatments, and not feeling they have the time and/or energy to engage in physical activity [6–9].
In the CF lung the abnormal or absent cystic fibrosis transmembrane conductance regulator (CFTR) chloride (Cl−) channel results in reduced or absent Cl− secretion and hyperabsorption of sodium (Na+) due to the loss of the CFTR-mediated inhibition of epithelial sodium channels (ENaC), with water being reabsorbed as it follows the osmotic gradient, or salt. This improper ion transport, causes a drastic depletion of the airway surface liquid (ASL) and compression of the cilia that impairs mucociliary clearance leaving thick, stagnant mucus to obstruct the distal airways and submucosal glands and becomes a stagnant breeding ground for infection. The therapeutic goals of the treatment of CF are to prevent the annual decline in pulmonary function and to improve ion regulation in the lung. With habitual exercise demonstrating the ability to attenuate the expected 2–3% annual decline in pulmonary function, exercise as a treatment achieves one of these goals [10]. Additionally, exercise can activate two pathways that could potentially improve ion regulation and thus hydration of the lung and subsequently facilitate improvements in mucus clearance. Exercise activates both the adrenergic pathway, through endogenous release of catecholamines, and the purinergic pathway, through sheer stress on the airway epithelia as a result of increases in ventilation. Stimulation of the β-2 adrenergic receptor will result in activation of ENaC and CFTR [11,12], stimulate an increase in ciliary beating [11,13–15], and promote bronchodilation [16–20]. Activation of the purinergic pathway will activate the CFTR-independent calcium-activated chloride channels and inhibit ENaC [21,22]. Recent work has demonstrated that exercise can inhibit ENaC function in individuals with CF [23,24], which suggests that exercise may have beneficial effects on ion regulation in CF, but further investigation is needed. Therefore, exercise through activation of the adrenergic and purinergic pathways can provide a mechanism for Cl− secretion that is CFTR-independent and inhibit hyperabsorption of Na+ helping to ameliorate the two ion regulation deficiencies plaguing individuals with CF, and facilitate improvements in mucus clearance directly through stimulation of ciliary beating and as an airway clearance technique and secondary to improved hydration of the lungs. These improvements in ion regulation along with mediating bronchodilation collectively have the potential to improve airflow and provide better ventilation and perfusion matching to improve gas distribution, diffusion, and spirometry during and post an exercise bout.
Exogenous stimulation of the adrenergic pathway by a bronchodilator, β2-adrenergic agonist, is commonly found in the therapy regimen for individuals with CF, prescribed in over 80% of individuals with CF, primarily to treat the symptoms of wheezing and breathlessness and preemptively combat airway hyperreactivity that can result as a consequence of other medications [25,26]. A goal of this study was to compare the systemic effects of submaximal exercise, at varying intensities, to that of the short-acting β2-adrenergic agonist, albuterol, which is a standard medication for individuals with CF taken 3–4 times daily for its bronchodilatory benefits.
Through this study we first, wanted to determine the intensity of exercise necessary to beneficially activate the adrenergic and purinergic pathways, but not limit pulmonary function in individuals with CF. Second, we sought to compare the therapeutic benefits between exercise and a prescribed pharmacologic bronchodilator, albuterol. We evaluated changes in airflow and ability to mediate bronchodilation through basic spirometry and the potential improvements in mucus hydration, facilitation of mucus clearance and better gas distribution through assessment of the diffusion capacity of the lungs for nitric oxide (DLNO), a measure of alveolar-capillary membrane conductance. Healthy control subjects also completed the study to provide a measure of the normal response to these treatments and allow for a comparison to the response observed in our CF population. We hypothesized that vigorous intensity exercise (60–65% maximum workload) would allow for significant activation of the adrenergic and purinergic pathways, to mediate bronchodilation and improve DLNO, but were uncertain if subjects with CF would be able to maintain this intensity. We expected minimal effect with low intensity exercise (25% maximum workload), but that moderate intensity exercise (50% maximal workload) would likely be a manageable intensity and could provide sufficient adrenergic and purinergic stimulation to alter pulmonary function and diffusion capacity of the lungs.
Methods
Study design
The subjects completed three visits in a crossover design with measurements made pre and post treatment: albuterol or submaximal exercise. First, subjects completed a screening visit to determine his/her baseline pulmonary function and maximal exercise capacity. The two subsequent visits were randomized; on one visit the subjects received nebulized albuterol and on the alternative visit subjects performed three separate fifteen minute sub-maximal exercise bouts at low, moderate and vigorous intensity.
Subjects
Fourteen individuals with CF with mild to moderate lung disease (FEV1 > 50% predicted), clinically diagnosed with a positive sweat test (>60 mmol/L Cl−), and who had not participated in another clinical trial within the past 30 days, or experienced a pulmonary exacerbation within two weeks were recruited for the present study. All individuals with CF abstained from taking any long acting β-agonist 12 h prior to any study visit and did not take albuterol the morning of the visits. Individuals with CF continued any other medications or treatments as normal. Additionally, sixteen healthy individuals matched for age, gender, height and weight were also recruited. Participants with CF were recruited through the University of Arizona Cystic Fibrosis Center. Control subjects were recruited through advertising posted around the University of Arizona and by word of mouth. Due to the limited number of female individuals with CF meeting the study’s inclusion criteria and willing to participate, the CF and healthy populations were primarily male in this study. The protocol was reviewed and approved by the University of Arizona Institutional Review Board. All participants provided written informed consent prior to the study, and all aspects of the study were performed according to the Declaration of Helsinki.
Protocol
Visit 1
Subjects arrived in a two-hour fasted state. Subjects were then outfitted with a 12-lead electrocardiogram (ECG) (Marquette electronics, Milwaukee, WI) to allow for continuous monitoring of heart rhythms and heart rate (HR), and performed a basic spirometry test. A maximal exercise test was performed on a cycle ergometer (Corvial Lode B.V., The Netherlands) which progressed in a manner appropriate for the subject’s body size and his/her reported type, speed, and intensity of exercise training. Information about habitual activity was evaluated from mode, frequency and duration physical activity questions included in the medical history questionnaire that subjects completed following consenting. Subjects exercised at an initial workload ranging between 20 and 40 W (mean initial workload was: healthy, 32 ± 7 W; CF, 25 ± 8 W) with the workload increasing by this initial workload (wattage) every two minutes until exhaustion following exercise testing standards and guidelines by the American Heart Association [27]. Exhaustion was determined to have been reached when two of the following three were observed: an inability to maintain a pedal rate of 60–80 revolutions per minute, a respiratory exchange ratio (RER) greater than or equal to 1.15, or a rating of perceived exertion (RPE) 18 or greater out of 20 [28]. Peripheral oxygen saturation (SpO2) was continuously monitored by pulse oximetry using a finger sensor (Nellcor N-600 Pulse Oximeter, Bolder, CO), and blood pressure was assessed by cuff auscultation midway through each stage of exercise by the same technician. Mean arterial pressure (MAP) was calculated using the equation: MAP = DBP + 1/3(SBP-DBP), where DBP is diastolic blood pressure and SBP is systolic blood pressure. Accurate SpO2 values were ensured by instructing the subject to maintain a relaxed grip on the handle bars and confirming there were no discrepancies in HR between the pulse oximeter and ECG. Following two minutes of recovery pedaling, subjects were dismissed once their heart rate and blood pressure had returned to baseline.
Oxygen uptake (VO2), carbon dioxide production (VCO2), respiratory rate (RR), tidal volume (VT), and minute ventilation (VE) were continuously monitored and averaged over the last 30 s of the stage for reported values. For flow and gas exchange analysis, a Medical Graphics CPX/D (St. Paul, MN) metabolic cart was interfaced with a Perkin Elmer MGA-1100 mass spectrometer (Wesley, MA). Predicted VO2PEAK was calculated according to the equation by Hansen et al. [29].
For the treatment visits, subjects were randomized to one of the following four sequences, which randomized both the intervention and exercise intensity, A: visit 2 albuterol and visit 3 submaximal exercise low-moderate-vigorous; B: visit 2 albuterol and visit 3 submaximal exercise vigorous-moderate-low; C: visit 2 submaximal exercise low-moderate-vigorous and visit 3 albuterol; D: visit 2 submaximal exercise vigorous-moderate-low and visit 3 albuterol.
Albuterol visit
Subjects arrived in a fasted state and were equipped with a 12-lead ECG. Baseline assessment of diffusion capacity of the lungs for nitric oxide (DLNO), cardiac output (Q) and maximal expiratory flow maneuver (MEFV) were performed. Subjects were then administered nebulized albuterol (2.5 mg diluted in 3 mL 0.9% saline) using a Power Neb2 nebulizer (Drive Medical, Port Washington, NY). During the nebulization subjects sat quietly, breathing while wearing a nose piece until all the solution had been nebulized (usually in ten to twelve minutes). Additionally, subjects were asked to take a deep breath every two minutes to assist with dispersion of the nebulized particles into the lower airways. DLNO, Q, and MEFV measurements were repeated at 60 min and 110 min post albuterol nebulization. Subjects were then dismissed.
Submaximal exercise visit
Once the ECG electrodes were placed on the fasted subjects, a venous catheter was placed to allow blood samples to be collected throughout the visit. A baseline sample was taken 20 min later. Catecholamines were measured on the exercise visit using HPLC by ARUP laboratories (Salt Lake City, UT). Previous research has demonstrated that twenty minutes of seated rest between six minute bouts of moderate intensity exercise was sufficient for full recovery, seen as a return to baseline of HR and oxygen consumption between bouts (Spencer, 2010). As such, we used a 40 min recovery period following each exercise bout. Even with this recovery, we randomized the order of the exercise intensities, either starting with the highest and finishing with the lowest workload or starting with the lowest and ending with the highest workload. The protocol for the submaximal exercise bouts was the same for all intensities. Prior to commencement of each bout, triplicate DLNO, Q, and MEFV measurements were performed. Next, for the first five minutes of exercise, the workload increased incrementally every minute until the desired low (25% maximal workload, HR 55% max), moderate (50% max, HR 75% max), or vigorous (60–65% max, HR 90% max) workload/exercise intensity was reached. At this time, subjects continued to pedal at this workload for the remaining ten minutes of the exercise bout. Similar to the maximal exercise test, VO2, VCO2, VT, VE, SpO2 and HR were continuously monitored and blood pressure was measured every three minutes. Additionally, DLNO, Q, and MEFV maneuvers were performed at the ten minute mark, and a blood sample was collected twelve minutes into exercise. The exercise protocol of fifteen minutes of submaximal exercise on a cycle ergometer was chosen as this duration would be similar to the administration time used with albuterol nebulization and would be sufficient to mediate an appropriate catecholamine response to induce bronchodilation [20]. The cycle ergometer was chosen as the mode of exercise to provide the most stable torso for the measurement of DLNO and Q. This exercise duration was shorter than the length used in most aerobic exercise intervention studies previous, but was best suited for the aims and measurements of this study [10,30]. After fifteen minutes, the workload was dropped to the initial workload for two minutes of recovery pedaling. This was followed with the 40 min recovery period. Once all three exercise bouts were completed subjects were dismissed.
Experimental procedures
Measurement of diffusion capacity of the lungs for nitric oxide and assessment of cardiac output
Measurement of the DLNO and Q were performed simultaneously, in triplicate during rest/pre-albuterol and recovery/post-albuterol and once during exercise, as the subjects were in an upright seated position using the rebreathing technique described previously [31–34]. Briefly, DLNO and Q were assessed by a rebreathe technique using a 5-L anesthesia bag containing 40 PPM NO (diluted immediately before the test in the bag from an 800 PPM gas mixture), 0.7% acetylene, 9% helium and 35% O2 at a respiratory rate of 32 breaths/minute. Gas concentration was analyzed using the mass spectrometer and NO analyzer (Sievers Instruments, Boulder, CO), which was integrated with custom analysis software for the assessment of DLNO and Q. The NO analyzer and mass spectrometer were calibrated with zero and first inspiratory breath bag concentration calibration points. A pneumo-tachograph was connected to a pneumatic switching valve, which allowed for rapid switching from room air to the test gas mixture. The volume of gas placed in the bag was a standardized volume of 1575 mL at rest and was dictated by the subjects tidal volume during exercise, with an extra 500 ml added to ensure the bag did not collapse during inhalation, but also did not cause an unnecessary excess of gas in the bag during the maneuver. At the end of a normal expiration (end-expiratory lung volume), the subjects were switched into the rebreathe bag and instructed to nearly empty the bag with each breath for 8–10 consecutive breaths. Alveolar volume (VA) was also concurrently assessed using helium during the rebreathing technique. Accurate bag volumes were assured using a timed switching circuit, which given a constant rate of flow from the tank produced the desired volume. The switching circuit and tank were calibrated prior to each test to ensure accurate volumes. Following each maneuver, the rebreathe bag was emptied with a suction device and refilled immediately prior to the next maneuver. At the start of each maneuver, there was no residual gas in the dead space of the apparatus, nor from the exhaled air from the subjects, as determined through gas sampling with the mass spectrometer.
The diffusion of the lungs for nitric oxide is theoretically based solely on membrane conductance as nitric oxide is scavenged 280 times faster by hemoglobin than CO, meaning its uptake into the blood is nearly instantaneous. For this reason, DLNO is considered a relatively direct measure of alveolar-capillary membrane conductance (DMNO), as the diffusion resistance of the blood is trivial [34–38]. In addition, more recent work has demonstrated that DLNO is closely related to anatomical abnormalities determined using computed tomography in patients with CF [39].
The rate of disappearance of acetylene from the exhaled gas mixture was used to assess Q. Acetylene does not bind to hemoglobin so the rate of disappearance of acetylene is limited primarily by the rate at which a new volume of blood is transported through the lungs. Since the output from the right and left sides of the heart are equal in a normal heart, the measure of the disappearance of acetylene provides a measure of cardiac output [40]. The rate of disappearance of acetylene with each breath was calculated from the slope of the exponential disappearance of acetylene with respect to the inert gas helium using custom software [32]. The accuracy and reliability of Q assessed by noninvasive inert gas rebreathing (IGR) techniques in patients with chronic lung disease has been demonstrated most recently by Saur et al. involving the Innocor system which uses the same rebreathe technique just different gases: soluble gas nitrous oxide and the insoluble gas sulfur hexafluoride (SF6) vs. the soluble gas acetylene and the insoluble gas helium used in this study [41], and that done by Pierce et al. in patients with chronic airway obstruction [42]. The direct comparison of IGR to cardiac magnetic resonance imaging found good agreement between both methods, and that there was no significant impact on bias between the two methods influenced by pulmonary function (FEV1/FVC, VC, DLCO/VA). As such the Q values reported for our subjects with CF should be fairly true with minimal compromise to the accuracy of the acetylene rebreathe technique in these subjects who demonstrate reduced DLCO and some pulmonary obstruction.
Pulmonary function testing
On the first visit, spirometry was performed according to American Thoracic Society (ATS) guidelines using the Medical Graphics CPXD (Minneapolis, MN) to determine: forced vital capacity (FVC), forced expiratory volume in one second of the FVC (FEV1) and forced expiratory flow at 25–75% of the FVC (FEF25–75). For each subject, the FVC maneuver was performed a minimum of three times such that three repeatable values were obtained (measured values of FVC and FEV1 within 150 ml of each other). Subjects performed a maximal expiratory flow volume (MEFV) maneuver according to ATS guidelines at each time point following diffusion capacity of the lungs measurements during the albuterol and submaximal exercise visits [43]. Predicted values for all pulmonary function measures were based on predicted equations from NHANES III [44].
Statistical analysis
The SPSS statistical software package (v.19; SPSS, Inc., Chicago, IL) was used for all statistical analyses. After confirming equality of variance with a Levene’s Test, paired samples t-tests were performed between the moderate exercise and 60-min post albuterol pulmonary function measures values for healthy and CF groups with a Bonferroni correction of 0.025 to determine statistical significance. Also baseline and treatment differences (60-min post albuterol or moderate exercise) between healthy and CF groups were assessed using two-sided independent samples t-tests, and a Bonferroni correction of 0.025 to determine statistical significance. To analyze whether exercise can provide comparable changes to albuterol in pulmonary function, we compared the percent change from baseline between the moderate intensity exercise and 60-min post albuterol for both the CF and healthy groups, using a Bonferroni correction of 0.025 to determine statistical significance. All values presented are mean ± SD unless otherwise stated.
Results
The overall goal of this project was to compare the effects of exercise as a therapeutic modality to standard pharmacologic therapy, albuterol, in CF using changes in lung function as an outcome variable. To do this our first aim was to determine the ideal intensity of exercise for individuals with CF based on the changes in catecholamines, pulmonary function and diffusion capacity of the lungs. Second, we determined if exercise at this intensity could provide comparable improvements pulmonary function for individuals with CF as their standard medication albuterol.
There were no differences in gender, age, height, weight, body mass index, or body surface area between the 14 individuals with CF and 16 healthy individuals (Table 1). Baseline pulmonary function (FEV1, FEV1/FVC and FEF25–75) was significantly lower for individuals with CF (Table 1). Both groups were active with similar activity levels and only two subjects from each group reported being sedentary. The types of activities were similar including for healthy subjects running (8), biking (5), weight lifting (5), rock climbing (2), swimming/dance/basketball/hiking (1 participant each) and for CF subjects included weight lifting (6), running (4), walking (4), biking (3), swimming (2), hiking/ baseball/basketball/soccer/baseball/dance (1 participant each). However, the maximal exercise testing demonstrated that there were some exercise limitations for subjects with CF. The absolute workload, percentage of predicted maximum workload, minute ventilation (VE), tidal volume (VT) and aerobic capacity (VO2PEAK) were lower in the individuals with CF when compared to their healthy counterparts (Table 2). Although the absolute intensity of exercise based on workload may have been different between CF and healthy participants, there was no difference in relative intensity, assessed by RPE.
Table 1.
Subject demographics and baseline pulmonary function.
| Healthy | CF | |
|---|---|---|
| n | 16 | 14 |
| Gender male (%) | 10 (63) | 12 (86) |
| Age (years) | 24 ± 6 | 22 ± 8 |
| Height (cm) | 168 ± 8 | 166 ± 17 |
| Weight (kg) | 70 ± 12 | 62 ±12 |
| BMI (kg/m2) | 24 ± 3 | 22 ±2 |
| BSA (m2) | 1.8 ± 0.2 | 1.7 ± 0.3 |
| VO2PEAK (% predicted) | 106 ± 23 | 78 ± 18* |
| Exercise (hr/wk) | 5.5 ± 4.5 | 6.8 ± 4.5 |
| Pulmonary function | ||
| FVC (L) | 4.6 ± 0.9 | 4.0 ± 1.2 |
| FVC (% predicted) | 100 ± 9 | 96 ± 30 |
| FEV1 (L) | 3.8 ± 0.9 | 2.9 ± 1.1* |
| FEV1 (% predicted) | 99 ± 12 | 83 ± 28 |
| FEV1/FVC (%) | 84 ± 4 | 73 ± 10* |
| FEF25–75 (L/sec) | 4.0 ± 1.2 | 2.4 ± 1.4* |
| FEF25–75 (% predicted) | 96 ± 26 | 60 ± 30* |
BMI = body mass index; BSA = body surface area; VO2peak= maximal oxygen consumption. FVC = forced vital capacity; FEV1 = forced expiratory volume after one second of FVC; FEF25–75 = forced expiratory flow at 25–75% of FVC. Data presented are mean ± SD
p < 0.0025 vs. healthy.
Table 2.
Response to maximal exercise.
| Healthy | CF | |
|---|---|---|
| FVC (L) | 4.6 ± 0.9 | 4.0 ± 1.2 |
| FVC (% predicted) | 100 ± 9 | 96 ± 30 |
| FEV1 (L) | 3.8 ± 0.9 | 2.9 ± 1.1* |
| FEV1 (% predicted) | 99 ± 12 | 83 ± 28 |
| FEV1/FVC (%) | 84 ± 4 | 73 ± 10* |
| FEF25–75 (L/sec) | 4.0 ± 1.2 | 2.4 ± 1.4* |
| FEF25–75 (% predicted) | 96 ± 26 | 60 ± 30* |
CF = cystic fibrosis; HR = heart rate; VO2 = oxygen consumption; VCO2 = carbon dioxide production; RER = respiratory exchange ratio; RPE = rating of perceived exertion; RR = respiratory rate; VT = tidal volume; VE = minute ventilation; SpO2 = peripheral oxygen saturation. Data are present as mean ± SD.
p < 0.025 vs. healthy.
Due to the lower maximal workload, the absolute workloads at the low, moderate and vigorous intensities for the individuals with CF were lower than the healthy subjects, but the relative workloads, watts as a percentage of their maximum, VO2 as a percentage of maximum, HR as a percentage of maximum and RPE were similar between CF and healthy subjects at each intensity suggesting we were successful in matching relative intensities between healthy and CF subjects (Tables 3 and 4). At rest, HR was elevated in individuals with CF compared healthy subjects, but the increase in response to exercise (percent change from baseline) was blunted in individuals with CF resulting in higher HR for the healthy individuals by the end of the moderate and vigorous workloads (Table 3). There was no significant difference in baseline HR at the start of each exercise bout suggesting the recovery period was sufficient for the subjects (Table 3, RM-ANOVA p > 0.05). Although there was no difference in Q at rest or low intensity exercise between groups, Q was lower in individuals with CF moderate and vigorous intensities. There were no differences in vascular parameters (systolic blood pressure (SBP), diastolic blood pressure (DBP) or mean arterial pressure (MAP)) at rest or in response to exercise. Epinephrine levels were higher in individuals with CF at baseline, but the change in response to exercise was lower in individuals with CF compared to healthy individuals across all exercise intensities (Table 3).
Table 3.
Cardiovascular parameters and catecholamines in response to submaximal exercise.
| Baseline | Low | Percent change | Baseline | Moderate | Percent change | Baseline | Vigorous | Percent change | |
|---|---|---|---|---|---|---|---|---|---|
| Workload (Watts) | |||||||||
| Healthy | – | 51 ± 16 | – | – | 97 ± 29 | – | – | 123 ± 38 | – |
| CF | – | 30 ± 12* | – | – | 61 ± 26* | – | – | 80 ± 36* | – |
| Watts/maxWatts (%) | |||||||||
| Healthy | – | 28 ± 8 | – | – | 53 ± 18 | – | – | 64 ± 30 | – |
| CF | – | 25 ± 0.2 | – | – | 47 ± 5 | – | – | 62 ± 8 | – |
| HR (bpm) | |||||||||
| Healthy | 74 ± 11 | 113 ± 18 | 54 ± 16 | 76 ± 11 | 145 ± 17 | 93 ± 24 | 80 ± 12 | 168 ± 16 | 110 ± 30 |
| CF | 83 ± 14* | 110 ± 13 | 34 ± 9* | 86 ± 15* | 132 ± 16† | 55 ± 18* | 84 ± 13 | 148 ± 18* | 78 ± 28* |
| Q (L/min) | |||||||||
| Healthy | 2.7 ± 1.2 | 6.3 ± 4.7 | 241 ± 124 | 2.7 ± 1.2 | 10.7 ± 2.6 | 354 ± 227 | 2.7 ± 1.2 | 12.1 ± 2.8 | 433 ± 298 |
| CF | 2.7 ± 1.6 | 5.9 ± 2.0 | 223 ±318 | 2.7 ± 1.6 | 7.3 ± 2.6* | 347 ± 611 | 2.7 ± 1.6 | 8.8 ± 3.8* | 375 ±512 |
| SpO2 (%) | |||||||||
| Healthy | 98 ± 2 | 97 ± 2 | −0.1 ± 1.4 | 97 ± 2 | 97 ±2 | −1.2 ± 1.6 | 98 ± 2 | 96 ±2 | −2.2 ± 1.8 |
| CF | 97 ± 2 | 96 ± 2 | −0.9 ± 1.7 | 96 ± 2 | 95 ±2 | −1.1 ± 1.4 | 97 ± 1 | 95 ± 3 | −2.5 ± 3.5 |
| EPI (pg/dL) | |||||||||
| Healthy | 33.0 ± 19.2 | 49.1 ± 29.3 | 74 ± 84 | 33.0 ± 19.2 | 83.9 ± 47.6 | 190 ± 135 | 33.0 ± 19.2 | 289.9 ± 270.7 | 928 ± 742 |
| CF | 53.1 ± 23.3* | 66.3 ± 28.6 | 39 ± 43 | 53.1 ± 23.3* | 77.4 ± 28.9 | 72 ± 76* | 53.1 ± 23.3* | 113 ± 54 | 144 ± 113* |
| NE (pg/dL) | |||||||||
| Healthy | 273.4 ± 92.4 | 516.3 ± 124.6 | 91 ± 53 | 273.4 ± 92.4 | 779 ±115 | 215.9 ± 111.8 | 273.4 ± 92.4 | 1621 ± 695 | 555 ±417 |
| CF | 258.3 ± 89.6 | 428.7 ± 127.7 | 75 ± 46 | 258.3 ± 89.6 | 662 ± 235 | 175.9 ± 108.4 | 258.3 ± 89.6 | 1093 ± 569 | 350 ±212 |
CF = cystic fibrosis; HR = heart rate; Q = cardiac output; SpO2 = peripheral oxygen saturation EPI = epinephrine; NE = norepinephrine. Baseline column is the baseline at the start of each workload, except catecholamines where baseline is from start of visit. Exercise values are from the last 2 min of each exercise bout. The percent change has been calculated from the respective baseline. Data are present as mean ± SD.
p < 0.025 vs. healthy;
p < 0.05.
Table 4.
Ventilatory response to submaximal exercise.
| Baseline | Low | Baseline | Moderate | Baseline | Vigorous | |
|---|---|---|---|---|---|---|
| VO2 (mL/kg/min) | ||||||
| Healthy | 5.8 ± 1.4 | 17.3 ± 4.5 | 6.1 ± 1.0 | 25.9 ± 5.4 | 6.6 ± 1.0 | 31.1 ± 6.2 |
| CF | 6.6 ± 2.2 | 15.1 ± 3.8 | 7.2 ± 2.2 | 21.0 ± 5.7* | 6.4 ± 2.2 | 25.3 ± 8.1 |
| VO2 % of Max | ||||||
| Healthy | 15 ± 4 | 45 ± 11 | 15± 5 | 68 ± 12 | 17 ± 7 | 82 ± 13 |
| CF | 22 ± 7* | 50 ± 12 | 24 ± 9* | 68 ± 15 | 21 ± 7* | 81 ± 19 |
| VO2 (mL/min) | ||||||
| Healthy | 404 ± 114 | 1158 ± 264 | 424 ± 93 | 1788 ± 367 | 466 ±110 | 2172 ± 463 |
| CF | 409 ± 131 | 938 ± 230* | 449 ± 141 | 1323 ± 439* | 399 ± 129 | 1598 ± 614* |
| VCO2 (mL/min) | ||||||
| Healthy | 340 ± 64 | 1043 ± 233 | 380 ± 92 | 1713 ± 369 | 377 ± 81 | 2172 ± 481 |
| CF | 342 ± 93 | 866 ± 219* | 371 ±112 | 1286 ± 431* | 341 ± 103 | 1634 ± 688* |
| RER | ||||||
| Healthy | 0.85 ± 0.21 | 0.86 ± 0.07 | 0.86 ± 0.24 | 0.96 ± 0.05 | 0.82 ± 0.18 | 1.00 ± 0.05 |
| CF | 0.83 ± 0.14 | 0.92 ± 0.06 | 0.81 ± 0.15 | 0.98 ± 0.05* | 0.79 ± 0.25 | 0.99 ± 0.07 |
| RPE | ||||||
| Healthy | 6 | 10 ± 2 | 6 | 14 ±2 | 6 | 16 ± 1 |
| CF | 6 | 12 ± 3 | 6 | 14 ± 3 | 6 | 16 ±2 |
| RR (breaths/min) | ||||||
| Healthy | 17 ± 5 | 24 ± 5 | 18±7 | 28 ±6 | 18± 5 | 33 ±6 |
| CF | 20 ± 4* | 30 ± 9* | 22 ± 4 | 34 ± 8† | 21 ± 4 | 38 ± 8 |
| Vt BTPS (mL) | ||||||
| Healthy | 925 ± 328 | 1363 ±318 | 966 ± 270 | 1860 ±513 | 909 ± 236 | 2061 ± 511 |
| CF | 774 ± 162 | 1108 ± 272* | 757 ± 141* | 1363 ± 351* | 745 ± 178* | 1531 ± 462* |
| VE BTPS (L/min) | ||||||
| Healthy | 13.7 ± 2.9 | 30.9 ± 7.4 | 15.3 ± 4.2 | 50.0 ±11.1 | 14.8 ± 2.7 | 65.8 ± 15.1 |
| CF | 14.8 ± 2.8 | 30.7 ± 4.2 | 15.7 ± 3.7 | 43.7 ± 9.3 | 14.7 ± 3.1 | 54.8 ± 14.7 |
CF = cystic fibrosis; VO2 = oxygen consumption; VCO2 = carbon dioxide production; RER = respiratory exchange ratio; RPE = rating of perceived exertion; RR = respiratory rate; VT = tidal volume; VE = minute ventilation. Exercise values are from the last 30 s of each exercise bout. Data are present as mean ± SD.
p < 0.025 vs. healthy;
p < 0.05.
Although these were relatively healthy individuals with CF, their baseline oxygen consumption was a greater percentage of their maximum aerobic capacity, suggesting a higher metabolic rate in individuals with CF (Table 4). Similar to what we observed during maximal exercise; healthy subjects demonstrated greater oxygen consumption than their CF counterparts at the moderate and vigorous submaximal exercise intensities, but the VO2 as a percentage of their maximum from visit 1 was similar between groups. Ventilatory response to meet the increase in oxygen demand was different between the two groups. Tidal volume was higher at all three intensities in healthy subjects when compared to individuals with CF, in contrast the elevation in respiratory rate (RR) tended to be higher in individuals with CF than in healthy subjects at the low and moderate intensities. There was no difference in SpO2 at rest or during submaximal exercise.
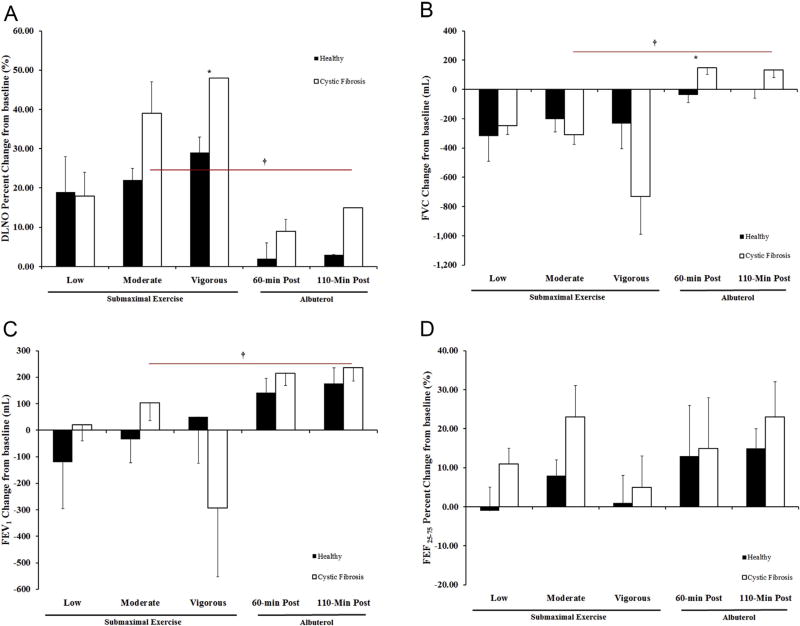
The same baseline impairments in pulmonary function (FEV1 and FEF25–75), observed on the first visit in individuals with CF, were also present on the submaximal exercise visit and remained through submaximal exercise (Table 5). Although the pulmonary function changes in response to exercise are quite variable between individuals, our results do provide some information about ideal exercise intensity for individuals with CF. Unlike the healthy subjects where the increase in DLNO with exercise was similar with each submaximal workload, the increase in DLNO was greater with each increase in intensity for individuals with CF (Fig. 1 Panel A). Also the degree of change in DLNO from baseline was greater for CF compared to healthy subjects especially at the vigorous workload. This higher intensity exercise resulted in decline in pulmonary function (FVC and FEV1, Fig. 1 Panel B and C). Moderate intensity exercise provided the greatest bronchodilation (FEF25–75, Fig. 1 Panel D) and an increase in DLNO with minimal to zero decline in FEV1 and FVC respectively, suggesting this was the ideal exercise intensity for individuals with CF.
Table 5.
Respiratory response to submaximal exercise and albuterol.
| Exercise
|
Albuterol
|
||||||
|---|---|---|---|---|---|---|---|
| Baseline | Low | Moderate | Vigorous | Baseline | 60-min Post | 110-Min post | |
| FVC (L) | |||||||
| Healthy | 4.6 ± 0.8 | 3.8 ± 0.2 | 3.8 ± 1.1 | 4.1 ± 1.1 | 4.8 ± 0.9 | 4.7 ± 0.7 | 4.8 ± 0.7 |
| CF | 3.9 ± 1.3 | 3.6 ± 1.3 | 3.6 ± 1.3 | 3.2 ± 1.0 | 4.0 ± 1.3 | 4.2 ± 1.2 | 4.1 ± 1.3 |
| FVC (% predicted) | |||||||
| Healthy | 98 ± 17 | 82 ± 11 | 81 ± 25 | 86 ± 28 | 104 ± 18 | 100 ± 16 | 101 ± 16 |
| CF | 90 ± 26 | 82 ± 24 | 81 ± 24 | 67 ± 24 | 91 ± 26 | 95 ± 26 | 94 ± 26 |
| FEV1 (L) | |||||||
| Healthy | 3.9 ± 0.8 | 3.4 ± 0.2 | 3.5 ± 1.2 | 3.8 ± 1.2 | 3.9 ± 0.9 | 4.1 ± 0.9 | 4.1 ± 0.9 |
| CF | 3.0 ± 1.2* | 2.9 ± 1.2* | 3.0 ± 1.2* | 2.7 ± 1.0 | 3.0 ± 1.2 | 3.2 ± 1.3 | 3.2 ± 1.3 |
| FEV1 (% predicted) | |||||||
| Healthy | 112 ± 19 | 102 ± 9 | 95 ±28 | 104 ± 27 | 113 ± 20 | 116 ± 20 | 117 ± 21 |
| CF | 86 ± 27* | 80 ± 25* | 82 ± 25* | 71 ± 23* | 83 ± 28* | 90 ± 30 | 91 ± 30 |
| FEF25–75 (L/sec) | |||||||
| Healthy | 4.2 ± 0.9 | 3.7 ± 0.1 | 4.2 ± 2.1 | 4.2 ± 2.0 | 3.9 ± 1.3 | 4.6 ± 1.6 | 4.6 ± 1.5 |
| CF | 2.7 ± 1.5* | 2.7 ± 1.4* | 3.1 ± 1.5* | 2.7 ± 1.4 | 2.4 ± 1.4* | 2.9 ± 1.9 | 2.9 ± 1.6 |
| FEF25–75 (%predicted) | |||||||
| Healthy | 116 ± 21 | 105 ± 17 | 105 ± 47 | 108 ± 44 | 105 ± 33 | 123 ±41 | 125 ± 38 |
| CF | 70 ± 36* | 66 ± 29* | 74 ± 32* | 64 ± 30 | 62 ± 33* | 73 ± 43* | 74 ± 38* |
| DLNO (mL/min/mmHg) | |||||||
| Healthy | 92.1 ± 17.5 | 103.1 ± 22.9 | 111.8 ± 19.2 | 119.8 ± 28.8 | 80.9 ± 15.5 | 81.4 ± 13.4 | 82 ± 14 |
| CF | 62.7 ± 25.1* | 70.3 ± 28.5* | 83.1 ± 28.9* | 90.7 ± 35.6 | 64.0 ± 25.6 | 68.8 ± 26.3 | 71 ± 25 |
| VA (mL) | |||||||
| Healthy | 2916 ± 429 | 3469 ± 648 | 2916 ± 429 | 3423 ± 821 | 2692 ± 706 | 2872 ± 569 | 2894 ± 662 |
| CF | 2372 ± 726 | 2344 ± 755* | 2372 ± 726* | 2390 ± 772* | 2411 ± 596 | 2397 ± 696 | 2431 ± 746 |
| DLNO/VA | |||||||
| Healthy | 0.03 ± 0.0 | 0.07 ± 0.08 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.0 | 0.03 ± 0.0 |
| CF | 0.03 ± 0.01 | 0.03 ± 0.01* | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
FVC = forced vital capacity; FEV1 = forced expiratory volume after one second of FVC; FEF25–75 = forced expiratory flow at 25–75% of FVC. DLNO = diffusion capacity of the lungs for nitric oxide; VA = alveolar volume; DLNO/VA = DLNO corrected for VA. Data presented are mean ± SEM
p < 0.0025 vs. healthy.
Figure 1.
Change in pulmonary function in response to submaximal exercise and albuterol. Panel A: Diffusion Capacity of the Lungs for Nitric Oxide (DLNO) Panel B: Forced Vital Capacity (FVC) Panel C: Forced Expiratory Volume in one second of the FVC (FEV1) Panel D: Forced Expiratory Flow at 25–75% of FVC (FEF25–75). The black bars represent healthy subjects and the white bars represent CF subjects. The error bars represent the standard error of the mean. * = p < 0.025 healthy vs. CF and † = p < 0.025 moderate intensity vs. 60-minutes post albuterol.
Albuterol administration did not result in any significant cardiovascular changes (HR, Q, SBP, DBP, MAP and SpO2) for both CF and healthy subjects. We did not collect cate-cholamines on the albuterol visit in this study as our previous study of albuterol administration in these populations found minimal change. In this unpublished work (Wheatley, 2011), we found that there was only a modest increase in norepinephrine (NE) and a decline in epinephrine (EPI) from baseline to 60-min post (NE = 23 ± 46 vs. 17 ± 64%; EPI = −35 ± 22 vs. −4 ± 48%, healthy vs. CF respectively). This highlights the purely exogenous β2-adrenergic receptor activation with albuterol, compared to the endogenous activation during exercise which is mediated by catecholamines. Regardless of the mechanism of stimulation, both treatments, 60-min post albuterol and moderate intensity exercise, resulted in similar levels of bronchodilation for CF subjects (Fig. 1 Panel D). In individuals with CF, albuterol administration did not diminish FVC or FEV1 as was observed with exercise (Fig. 1B and C), but exercise provided a greater increase in membrane conductance (DLNO) than albuterol, where with moderate exercise the increase in DLNO was double that of albuterol at 110-min post (39 ± 8 vs 15 ±6% change, Fig. 1A). In healthy subjects the only significant difference between albuterol and moderate exercise was the greater increase in DLNO with exercise likely being driven by the higher Q with exercise. Bronchodilation also tended to be greater with albuterol than with exercise in healthy subjects.
Discussion
In this study we aimed to demonstrate that exercise acts therapeutically for individuals with CF by comparing the effects of albuterol to those of submaximal exercise in both individuals with CF and in healthy control subjects. Most of the individuals with CF were taking a short-acting β-agonist three to four times a day. The primary reasons for albuterol use in CF are to improve pulmonary function and reduce symptoms of wheezing and breathlessness and as a preemptive measure to handle bronchospasms, through mediating bronchodilation, that are commonly caused by other medications a patient may be taking. Our study found that exercise mediated similar levels of bronchodilation to that resulting from albuterol administration (Fig. 1D). This is a key finding, as for exercise to be a comparable treatment and potentially be used as a replacement for one of the daily albuterol treatments, so to not increase therapy burden, it must provide the comparable levels of bron-chodilation. The specific therapy burden for albuterol can be relatively short if using a metered dose inhaler which takes seconds, but if administered using a nebulizer takes 10–15 min to complete. In our CF population nebulization remains a primary method of delivery, being used prior to other nebulized medications such as Pulmozyme, and can be occurring simultaneously with chest physiotherapy using the vibratory vest. In this study we had subjects perform submaximal exercise at three intensities (low, moderate and vigorous) to determine the optimal intensity for facilitating bronchodilation and improvements in DLNO, a measure of alveolar-capillary membrane conductance, in individuals with CF subjects. We found moderate intensity exercise provided the greatest bronchodilation at a level comparable to that mediated by albuterol administration, with minimal to no decline in FEV1 and FVC. Low intensity exercise did not mediate a large increase in EPI release and consequently lower levels of bronchodilation, and vigorous intensity, although producing the greatest catecholamine release, appeared to be working individuals with CF too hard, resulting in airflow restriction. Collectively, this suggests moderate exercise (50% maximal workload or 75% of maximum HR) is the optimal exercise intensity for individuals with CF.
Another sign of exercise’s therapeutic benefit was its ability to increase DLNO, and that there was a dose or intensity effect, with the greatest increase in DLNO occurring during vigorous exercise. That being said, moderate exercise was sufficient to increase DLNO to double (39 ± 8 vs 15 ± 6% change) what was seen with albuterol at 110 min post. Although the combined assessment of DLCO and DLNO provides important information about gas diffusion by allowing for the partitioning both components contributing to the DLCO measured: the resistance of gas to move across the alveolar-capillary membrane or alveolar-capillary membrane conductance (DM) and the resistance of the blood to the gas binding to hemoglobin, measurement of DLNO independently provides a direct measure of DM. Due to the high reaction rate of NO with hemoglobin, the influence of the blood resistance or blood flow is minimal, as such DLNO is limited primarily by the transfer of gas across the membrane which is dependent on surface area and diffusion distance [36,37,45]. Additionally, Dressel et al., demonstrated that quantification of membrane diffusion using DLNO was better correlated with CF-specific CT scores than DLCO, concluding that DLNO was a suitable measure to quantify destruction and structural alterations (bronchiectasis, fibrosis, mucus plugging, peribronchial thickening, etc) in CF lung function [39]. As such, we utilized DLNO as a means to assess the potential improvements in mucus hydration, facilitation of mucus clearance and better gas distribution, which we hypothesize exercise would be better able to mediate than albuterol alone due to its ability to activate the purinergic pathway to potentially provide improved ion regulation and the increased motion of the thoracic cavity, in addition to the β2–mediated increases in ciliary beating common to both albuterol and exercise. The increases in DLNO in the individuals with CF we observed suggest improvements in membrane conductance, which in CF could be due to 1) improved hydration of the mucus and improved mucus clearance to reduce the diffusion distance; 2) improved airflow to better facilitate ventilation, gas distribution and increases in alveolar volume which subsequently increase available surface area for diffusion; 3) increased perfusion through an increase in cardiac output recruiting and distending pulmonary capillaries, again improving ventilation-perfusion matching, which is likely the primary reason for the increases in healthy subjects with exercise. The increases in gas transfer achieved with moderate exercise further support the vital benefits of exercise in the therapy regimen for individuals with CF, as any improvement in lung diffusion is vital to adequate oxygen delivery.
In this study we also assessed the cardiopulmonary response to the submaximal exercise, through measurement of Q, gas exchange and SpO2, to assess whether the impairments as we had previously observed in individuals with CF in response to maximal exercise when compared to healthy subjects [34] were still present during submaximal exercise, and to provide a gauge of the body’s response to the stimulus, as cardiac output should follow intensity. In this study, we found individuals with CF experience similar impairments in the responses to submaximal exercise as had been previously observed with maximal exercise. Specifically, lower absolute workload, Q, aerobic capacity (VO2), and DLNO than their healthy counterparts. Because VO2 and Q follow wattage, we also compared the responses after correcting for absolute wattage, finding that for a given wattage individuals with CF had a higher HR at all exercise intensities exercise (Low: VO2/W 0.37 ± 0.14 vs 0.56 ± 0.34; HR/W 2.49 ± 0.96 vs. 3.92 ± 1.95*; Q/W 0.16 ± 0.04 vs. 0.20 ± 0.08, Moderate: VO2/W 0.29 ± 0.09 vs 0.41 ± 0.24; HR/W 1.65 ± 0.60 vs. 2.57 ± 1.54*; Q/W 0.10 ±0.02 vs. 0.13 ± 0.05; vigorous: VO2/W 0.27± 0.08 vs 0.36 ± 0.18; HR/W 1.50 ± 0.56 vs. 2.21 ± 1.05*; Q/W 0.09 ± 0.02 vs. 0.12 ± 0.03 Healthy vs. CF respectively, *p < 0.05). This suggests that the reductions in absolute VO2 and Q are likely a consequence of deconditioning, muscle strength, and being unaccustomed to cycling, as absolute wattage is driving the differences, and individuals with CF are tending to have a higher a VO2 and Q per watt than their healthy counterparts. Although activity levels were similar between healthy and CF individuals, the differences in aerobic capacity and absolute workload we observed suggest the absolute intensity of exercise performed on a day to day basis may be lower for some of the CF subjects compared to their healthy counterparts. The relative intensities are likely fairly comparable between groups as we found no difference in watts, VO2 or HR as a percentage of maximum and RPE at any submaximal intensity between CF and healthy subjects. However, individuals with CF demonstrated a higher RER at the moderate and low workloads which taken together with the higher VO2 and Q per watt suggests, if anything the workload may have been a higher relative intensity for some of the individuals with CF. Not surprising due to the ventilatory constraints present in individuals with CF [46], there was also difference in how the two groups met the increase in oxygen demand. Healthy subjects had a higher VT than individuals with CF at all three intensities, whereas the individuals with CF tended to increase respiratory rate more than healthy subjects at the low and moderate intensities. Both methods were sufficient to meet the body’s oxygen demand, as SpO2 was not different between healthy and individuals with CF in this study, unlike we had previously observed during maximal exercise. This may be because the individuals with CF in this study were more fit as this group reached a higher percentage of their predicted VO2PEAK and participated in more vigorous activities when compared to our previous CF population [34].
Previous work has demonstrated there are autonomic nervous system abnormalities in individuals with CF (exaggerated cholinergic responses and reduced α- and β-adrenergic responses) [47]. In vitro evaluation of β2-adrenergic response has demonstrated that there is a reduced cAMP response to isoproterenol in leukocytes in CF and in vivo work has demonstrated that their cardiovascular system is less sensitive to β-agonists. Davis et al. demonstrated individuals with CF required higher doses of infused isoproterenol to increase pulse pressure by the same extent when compared to healthy individuals [48,49]. The higher basal EPI levels and apparent blunted EPI release we noted, along with the reduced β-adrenergic responsiveness previously reported, may explain the differences in baseline HR and change in HR in response to exercise.
Future directions
We determined the optimal exercise intensity was moderate, and exercise provided comparable and potentially additional beneficial effects as one of the current standard pharmacological therapies. The next step will be to determine the duration of the effects of a single bout of exercise and see if the exercise-induced bronchodilation, for example, lasts as long as albuterol-mediated broncho-dilation. Further, because we were asking our individuals with CF subjects to exercise at a vigorous intensity we restricted our subject recruitment to primarily mild individuals with CF, further research is therefore warranted to determine if these results can be applied to individuals with CF with more severe disease. Finally, the recruitment limitations left us with a primarily male CF population and to further validate these results a more balanced sex ratio needs to be recruited.
Conclusion
Our results suggest that moderate intensity exercise is the optimal dose for individuals with CF, as low intensity exercise does not mediate sufficient epinephrine release and vigorous intensity exercise restricted airflow. Although the duration of the beneficial effect is uncertain, exercise can promote greater improvements in gas diffusion and comparable bronchodilation to albuterol administration.
Acknowledgments
We are sincerely grateful to our subjects who were willing to give up their time and at times comfort to participate in this study and support research in cystic fibrosis. This work was performed at the University of Arizona and supported by the PEO Scholar Award, American College of Sports Medicine Doctoral Student Research Grant, Caldwell Health Sciences Research Fellowship and National Institute of Health (HL108962-01).
Abbreviations
- ASL
airway surface liquid
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cl−
chloride
- DLNO
diffusion capacity of the lung for nitric oxide
- ENaC
epithelial sodium channel
- FVC
forced vital capacity
- FEV1
forced expiratory volume in one second
- FEF25–75
forced expired flow at 25–75% of FVC
- MEFV
maximal expiratory flow volume
- Na+
sodium
- Q
cardiac output
- SpO2
peripheral oxygen saturation
- RR
respiratory rate
- VT
tidal volume
- VCO2
carbon dioxide production
- VO2
oxygen consumption
- VE
minute ventilation
- VA
alveolar volume
Footnotes
The authors have no conflict of interest to disclose.
Authors’ contributions
CMW, WJM, and EMS were responsible for the study concept and design, CMW was responsible for data analysis and interpretation, MAM was responsible for subject recruitment and all other authors assisted CMW with data collection. All authors were significant manuscript revisers and reviewers.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Orenstein DM, Franklin BA, Doershuk CF, et al. Exercise conditioning and cardiopulmonary fitness in cystic fibrosis. The effects of a three-month supervised running program. Chest. 1981;80(4):392–8. doi: 10.1378/chest.80.4.392. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin DR, Hill AL, Peckham DG, Knox AJ. Effect of addition of exercise to chest physiotherapy on sputum expectoration and lung function in adults with cystic fibrosis. Respir Med. 1994;88(1):49–53. doi: 10.1016/0954-6111(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 3.Dwyer TJ, Alison JA, McKeough ZJ, Daviskas E, Bye PT. Effects of exercise on respiratory flow and sputum properties in cystic fibrosis. Chest. 2011;139(4):870–7. doi: 10.1378/chest.10-1158. [DOI] [PubMed] [Google Scholar]
- 4.Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med. 1992;327(25):1785–8. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 5.Myers LB. An exploratory study investigating factors associated with adherence to chest physiotherapy and exercise in adults with cystic fibrosis. J Cyst Fibros. 2009;8(6):425–7. doi: 10.1016/j.jcf.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Gruber W, Orenstein DM, Braumann KM, Beneke R. Interval exercise trainingincystic fibrosis-effectsonexercise capacity in severely affected adults. J Cyst Fibros. 2014;13(1):86–91. doi: 10.1016/j.jcf.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Moola FJ, Faulkner GE, Schneiderman JE. “No time to play”: perceptions toward physical activity in youth with cystic fibrosis. Adapt Phys Act Q. 2012;29(1):44–62. doi: 10.1123/apaq.29.1.44. [DOI] [PubMed] [Google Scholar]
- 8.White D, Stiller K, Haensel N. Adherence of adult cystic fibrosis patients with airway clearance and exercise regimens. J Cyst Fibros. 2007;6(3):163–70. doi: 10.1016/j.jcf.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Prasad SA, Cerny FJ. Factors that influence adherence to exercise and their effectiveness: application to cystic fibrosis. Pediatr Pulmonol. 2002;34(1):66–72. doi: 10.1002/ppul.10126. [DOI] [PubMed] [Google Scholar]
- 10.Schneiderman-Walker J, Pollock SL, Corey M, et al. A randomized controlled trial of a 3-year home exercise program in cystic fibrosis. J Pediatr. 2000;136(3):304–10. doi: 10.1067/mpd.2000.103408. [DOI] [PubMed] [Google Scholar]
- 11.Mutlu GM, Adir Y, Jameel M, et al. Interdependency of beta-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res. 2005;96(9):999–1005. doi: 10.1161/01.RES.0000164554.21993.AC. [DOI] [PubMed] [Google Scholar]
- 12.Mutlu GM, Koch WJ, Factor P. Alveolar epithelial beta 2-adrenergic receptors: their role in regulation of alveolar active sodium transport. Am J Respir Crit Care Med. 2004;170(12):1270–5. doi: 10.1164/rccm.200404-470CP. [DOI] [PubMed] [Google Scholar]
- 13.Davis PB, Silski CL, Perez A. cAMP does not regulate [Ca2+]i in human tracheal epithelial cells in primary culture. J Cell Sci. 1994;107(10):2899–907. doi: 10.1242/jcs.107.10.2899. [DOI] [PubMed] [Google Scholar]
- 14.Mutlu GM, Dumasius V, Burhop J, et al. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res. 2004;94(8):1091–100. doi: 10.1161/01.RES.0000125623.56442.20. [DOI] [PubMed] [Google Scholar]
- 15.Salathe M. Effects of beta-agonists on airway epithelial cells. J Allergy Clin Immunol. 2002;110(6 Suppl):S275–81. doi: 10.1067/mai.2002.129412. [DOI] [PubMed] [Google Scholar]
- 16.Gotshall RW. Airway response during exercise and hyperpnoea in non-asthmatic and asthmatic individuals. Sports Med. 2006;36(6):513–27. doi: 10.2165/00007256-200636060-00005. [DOI] [PubMed] [Google Scholar]
- 17.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- 18.Warren JB, Dalton N. A comparison of the bronchodilator and vasopressor effects of exercise levels of adrenaline in man. Clin Sci. 1983;64(5):475–9. doi: 10.1042/cs0640475. [DOI] [PubMed] [Google Scholar]
- 19.Johnson BD, Scanlon PD, Beck KC. Regulation of ventilatory capacity during exercise in asthmatics. J Appl Phys. 1995;79(3):892–901. doi: 10.1152/jappl.1995.79.3.892. [DOI] [PubMed] [Google Scholar]
- 20.Snyder EM, Beck KC, Dietz NM, Joyner MJ, Turner ST, Johnson BD. Influence of {beta}2-Adrenergic receptor genotype on airway function during exercise in healthy adults. Chest. 2006;129(3):762–70. doi: 10.1378/chest.129.3.762. [DOI] [PubMed] [Google Scholar]
- 21.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284(3):F419–32. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 22.O’Mullane LM, Cook DI, Dinudom A. Purinergic regulation of the epithelial Na+ channel. Clin Exp Pharmacol Physiol. 2009;36(10):1016–22. doi: 10.1111/j.1440-1681.2009.05256.x. [DOI] [PubMed] [Google Scholar]
- 23.Hebestreit A, Kersting U, Basler B, Jeschke R, Hebestreit H. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. Am J Respir Crit Care Med. 2001;164(3):443–6. doi: 10.1164/ajrccm.164.3.2007168. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt L, Wiebel M, Frese F, et al. Exercise reduces airway sodium ion reabsorption in cystic fibrosis but not in exercise asthma. Eur Respir J. 2011;37(2):342–8. doi: 10.1183/09031936.00197309. [DOI] [PubMed] [Google Scholar]
- 25.Halfhide C, Evans HJ, Couriel J. Inhaled bronchodilators for cystic fibrosis. Cochrane Database Syst Rev. 2005;(4):CD003428. doi: 10.1002/14651858.CD003428.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Colombo JL. Long-acting bronchodilators in cystic fibrosis. Curr Opin Pulm Med. 2003;9(6):504–8. doi: 10.1097/00063198-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1995;91(2):580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 28.Borg GAV. A category scale with ratio properties for inter-modal and interindividual comparisons. In: Geissler H, Pezold P, editors. Psychophysial judgement and the process of perception. Berlin: Veb Deutsche Verlag Wissen Schaften; 1982. pp. 25–34. [Google Scholar]
- 29.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 Pt 2):S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 30.Orenstein DM, Hovell MF, Mulvihill M, et al. Strength vs aerobic training in children with cystic fibrosis: a randomized controlled trial. Chest. 2004;126(4):1204–14. doi: 10.1378/chest.126.4.1204. [DOI] [PubMed] [Google Scholar]
- 31.Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Phys. 2006;101(6):1623–32. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- 32.Snyder EM, Johnson BD, Beck KC. An open-circuit method for determining lung diffusing capacity during exercise: comparison to rebreathe. J Appl Phys. 2005;99(5):1985–91. doi: 10.1152/japplphysiol.00348.2005. [DOI] [PubMed] [Google Scholar]
- 33.Wheatley CM, Baldi JC, Cassuto NA, Foxx-Lupo WT, Snyder EM. Glycemic control influences lung membrane diffusion and oxygen saturation in exercise-trained subjects with type 1 diabetes: alveolar-capillary membrane conductance in type 1 diabetes. Eur J Appl Physiol. 2011;111(3):567–78. doi: 10.1007/s00421-010-1663-8. [DOI] [PubMed] [Google Scholar]
- 34.Wheatley CM, Foxx-Lupo WT, Cassuto NA, et al. Impaired lung diffusing capacity for nitric oxide and alveolar-capillary membrane conductance results in oxygen desaturation during exercise in patients with cystic fibrosis. J Cyst Fibros. 2011;10(1):45–53. doi: 10.1016/j.jcf.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Phys. 1957;11(2):290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 36.Hsia CC. Recruitment of lung diffusing capacity: update of concept and application. Chest. 2002;122(5):1774–83. doi: 10.1378/chest.122.5.1774. [DOI] [PubMed] [Google Scholar]
- 37.Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120(6):1850–6. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 38.Hsia CC, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin. Am J Med. 2005;118(3):205–11. doi: 10.1016/j.amjmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Dressel H, Filser L, Fischer R, et al. Lung diffusing capacity for nitric oxide and carbon monoxide in relation to morphological changes as assessed by computed tomography in patients with cystic fibrosis. BMC Pulm Med. 2009;9:30. doi: 10.1186/1471-2466-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsia CC, Herazo LF, Ramanathan M, Johnson RL., Jr Cardiac output during exercise measured by acetylene rebreathing, thermodilution, and fick techniques. J Appl Phys. 1995;78(4):1612–6. doi: 10.1152/jappl.1995.78.4.1612. [DOI] [PubMed] [Google Scholar]
- 41.Saur J, Trinkmann F, Doesch C, et al. The impact of pulmonary disease on noninvasive measurement of cardiac output by the inert gas rebreathing method. Lung. 2010;188(5):433–40. doi: 10.1007/s00408-010-9257-0. [DOI] [PubMed] [Google Scholar]
- 42.Pierce RJ, McDonald CF, Thuys CA, Rochford PD, Barter CE. Measurement of effective pulmonary blood flow by soluble gas uptake in patients with chronic airflow obstruction. Thorax. 1987;42(8):604–14. doi: 10.1136/thx.42.8.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 44.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 45.Borland CD, Higenbottam TW. A simultaneous single breath measurement of pulmonary diffusing capacity with nitric oxide and carbon monoxide. Eur Respir J. 1989;2(1):56–63. [PubMed] [Google Scholar]
- 46.Regnis JA, Donnelly PM, Robinson M, Alison JA, Bye PT. Ventilatory mechanics at rest and during exercise in patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;154(5):1418–25. doi: 10.1164/ajrccm.154.5.8912758. [DOI] [PubMed] [Google Scholar]
- 47.Davis PB, Kaliner M. Autonomic nervous system abnormalities in cystic fibrosis. J Chronic Dis. 1983;36(3):269–78. doi: 10.1016/0021-9681(83)90062-0. [DOI] [PubMed] [Google Scholar]
- 48.Davis PB, Braunstein M, Jay C. Decreased adenosine 3′:5′-monophosphate response to isoproterenol in cystic fibrosis leukocytes. Pediatr Res. 1978;12(6):703–7. doi: 10.1203/00006450-197806000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Davis PB, Shelhamer JR, Kaliner M. Abnormal adrenergic and cholinergic sensitivity in cystic fibrosis. N Engl J Med. 1980;302(26):1453–6. doi: 10.1056/NEJM198006263022605. [DOI] [PubMed] [Google Scholar]