Abstract
Pleural injury and associated air leaks are a major influence on patient morbidity and healthcare costs after lung surgery. Pectin, a plant-derived heteropolysaccharide, has recently demonstrated potential as an adhesive binding to the glycocalyx of visceral mesothelium. Since bioadhesion is a process likely involving the interpenetration of the pectin-based polymer with the glycocalyx, we predicted that the pectin-based polymer may also be an effective sealant for pleural injury. To explore the potential role of an equal (weight%) mixture of high-methoxyl pectin and carboxymethylcellulose as a pleural sealant, we compared the yield strength of the pectin-based polymer to commonly available surgical products. The pectin-based polymer demonstrated significantly greater adhesion to the lung pleura than the comparison products (p < 0.001). In a 25 g needle-induced lung injury model, pleural injury resulted in an air leak and a loss of airway pressures. After application of the pectin-based polymer, there was a restoration of airway pressure and no measurable air leak. Despite the application of large sheets (50 mm2) of the pectin-based polymer, multifrequency lung impedance studies demonstrated no significant increase in tissue damping (G) or hysteresivity (η)(p > 0.05). In 7-day survival experiments, the application of the pectin-based polymer after pleural injury was associated with no observable toxicity, 100% survival (N = 5), and restored lung function. We conclude that this pectin-based polymer is a strong and nontoxic bioadhesive with the potential for clinical application in the treatment of pleural injuries.
Keywords: : pectin, mesothelium, adhesion, pleura, pneumothorax
Introduction
Pulmonary air leaks are commonly the result of pleural injury caused by trauma or surgical manipulation.1 Because of the pressure gradient that exists between the peripheral lung and the pleural space, there is a net movement of air into the pleural space. The accumulating intrapleural air (pneumothorax) results in lower lung volumes; if left untreated, a growing pneumothorax will eventually lead to impaired ventilation and inefficient gas exchange. To avoid these complications, pleural injuries are treated with tube thoracostomies that function to evacuate the accumulated air. Because the tubes remain in the pleural space until the lung is healed, air leaks can result in prolonged hospitalization2 and significant healthcare costs.3,4
To address this problem, previous approaches have used bioadhesive sealants and chemical crosslinkers to seal pleural air leaks. Bioadhesive sealants based on fibrin (Evicel; Ethicon, Somerville, NJ)5 or albumin and polyethylene glycol (Progel, Bard-Davol, Warwick, RI)6 have the advantage of being fast-setting, biocompatible, and biodegradable. Because of the dependence upon antigenic proteins, however, their use has been associated with allergic reactions.7 In addition, poor adhesivity to the pleural surface has limited their effectiveness.8–10 Chemical crosslinking of tissue surfaces has been attempted using albumin-glutaraldehyde adhesive (BioGlue, CryoLife, Kennesaw, GA)11–13 or cyanoacrylate derivatives (Histoacryl, Braun, Woburn, MA).14–16 These chemicals are fast-setting with effective adhesive strength, but their use has been limited by the risk of local toxicity and long-term carcinogenicity.17
In previous work, we have studied the bioadhesion of pectin-based polymers to the mesothelial surface glycocalyx of the lung, liver, bowel, and heart.18 Pectins are structural heteropolysaccharides that comprise 30% of the primary cell walls of plants.19 Pectins are not typical pressure sensitive adhesives; they are not “tacky” to touch nor do they bind to most nonbiologic compounds. The selective and strong binding of pectins to the mesothelial glycocalyx18 is likely the result of a mechanism of interpenetration; that is, the adhesivity is a result of the entanglement of branched chain polysaccharides20 that involves chemical bonds and weak chemical interactions.21,22 The binding strength of the pectin-based adhesive to the pleural mesothelium suggested a potential role for the pectin mixture as a sealant for pleural air leaks.
In this report, we studied the relative binding strength of pectin-based polymers to the mouse pleura. We tested the pectin-based adhesive for its effectiveness in limiting air leak, influence on pulmonary mechanics, and effect on pleural healing.
Materials and Methods
Animals
Male mice, 8–10-week-old wild-type C57BL/6 (Jackson Laboratory, Bar Harbor, ME) were anesthetized before euthanasia.23 The care of the animals was consistent with guidelines of the American Association for Accreditation of Laboratory Animal Care (Bethesda, MD) and approved by the Brigham and Women's Hospital Institutional Animal Care and Use Committee.
Anesthesia and intubation
The animals were anesthetized with an intraperitoneal injection of Ketamine 100 mg/kg (Fort Dodge Animal Health, Fort Dodge, IO) and Xylazine 10 mg/kg (Phoenix Scientific, Inc., St. Joseph, MO). Before mechanical ventilation, the glottis was directly visualized and intubated with a standard 18 gauge Angiocatheter to facilitate forced oscillation measurements (BD Insyte, Sandy, UT). After intubation, the animal was transferred to a FlexiVent rodent ventilator (Scireq, Montreal, Canada) as previously described.23
Pectins
The proportion of galacturonic acid residues in the methyl ester form determined the degree of methoxylation. High-methoxyl pectins (HMP) were defined as those pectins with a degree of methoxylation greater than 50%. Previously, we defined the optimal concentraton of carboxymethylcellulose (CMC).18 The HMP and CMC used in this study were obtained from a commercial source (Cargill, Minneapolis, MN).
Polymer thickness
The thickness of the pectin-based polymer was measured three times on different preparations with an Ames engineering micrometer (Ames, Waltham, MA) gently applied parallel to the sheet surface. Data represented the mean of replicate measurements.
Pleural injury
The standard pleural injury was created with a 25 g needle (Becton Dickinson) with a diameter of 0.51 mm, inserted 1–2 mm into the mouse lung. For comparison, the surface area injury, scaled to the average human lung surface area, would be 2.1 cm2.
Adhesion strength testing
The bioadhesion test was similar to a previously described method.18 In brief, the pectin-based adhesive and the comparison surgical products provided a rigid interface upon which the pleural adherend was applied. The forces were applied with a 6-0 Prolene suture (Ethicon) passed through the tissue within 2 mm of the adhesive interface. The test was performed with a controlled rate of uniaxial load applied to a comparable cross-sectional area. Adhesion strength was tested at a 90° load application.
Pulmonary mechanics
Before all measurements, the pressure transducers and ventilator tubing of the FlexiVent (SciReq) were calibrated as described.24,25 After general anesthesia and intubation, the mice were transferred to the FlexiVent system (SciReq) for pulmonary mechanics studies. The animals were hyperventilated at a rate of 200/min and allowed to acclimate to the ventilator for 2 min before standardization of the volume history with three consecutive positive pressure recruitment maneuvers (“TLC” by SciReq). After recruitment, two maneuvers were performed. First, dynamically determined pressure volume loops (PV-P by SciReq) were created starting from a positive end expiratory pressure (PEEP) of 3 cm H2O by an 8-s steady inflation ramp to 30 cm H2O with an 8-s passive deflation. Second, mechanical ventilation was briefly stopped and the animal passively exhaled to functional residual capacity; an 8-s multifrequency (0.5–19.5 Hz) oscillatory signal (Prime-8 by SciReq) was delivered. Mechanical ventilation was resumed on completion of the Prime-8 maneuver. The pulmonary mechanics were performed before and after lung injury as well as following the application of the pectin sealant.
Input impedance model
Respiratory system impedance (Zrs) includes the components representing the lung (ZL) and chest wall (Zw).26 The constant phase model27 was fit to the input impedance to derive Newtonian resistance (Rn), tissue damping (G), tissue elastance (H), and hysteresivity (η) as described below:
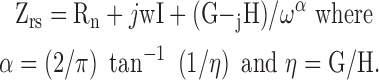 |
For each animal, a minimum of three measurements were obtained. A minimum coherence of 0.89 was ensured for quality control.
Scanning electron microscopy
The specimen was fixed by immersion in 2.5% buffered glutaraldehyde. After coating with 20–25 A gold in argon atmosphere, the mesothelial layer was imaged using a Philips XL30 ESEM scanning electron microscope (Philips, Eindhoven, Netherlands) at 15 Kev and 21 μA. Stereopair images were obtained using a tilt angle difference of 6° on a eucentric sample holder using standardized computerized control.
Surgical products
The surgical products compared to the pectin-based adhesive sealant were obtained from commercial sources and stored at controlled room temperature. Surgicel Original, oxidized regenerated cellulose, was obtained from Ethicon (Neuchatel, Switzerland). Seprafilm Adhesion Barrier, chemically modified sodium hyaluronate/carboxymethylcellulose absorbable adhesion barrier, was obtained from Genzyme Biosurgery (Framingham, MA). TachoSil fibrin sealant patch was obtained from Baxter Healthcare (Deerfield, IL). Surgifoam absorbable gelatin sponge was obtained from Ethicon (Somerville, NJ). The Prolene Surgical Mesh was obtained from Ethicon (Somerville, NJ). Vetbond Surgical Adhesive was obtained from 3 M (St. Paul, MN).
Results
Pectin binding to the visceral pleura
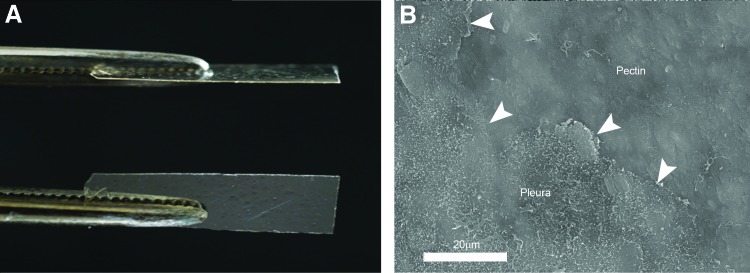
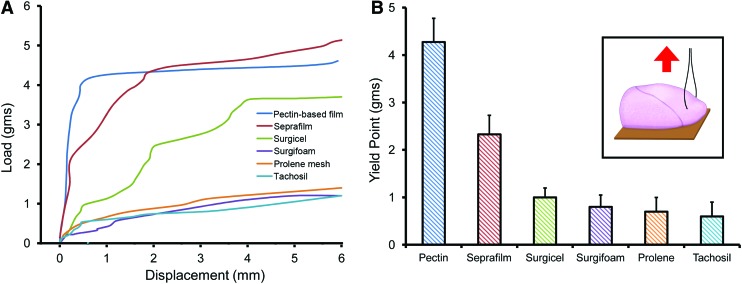
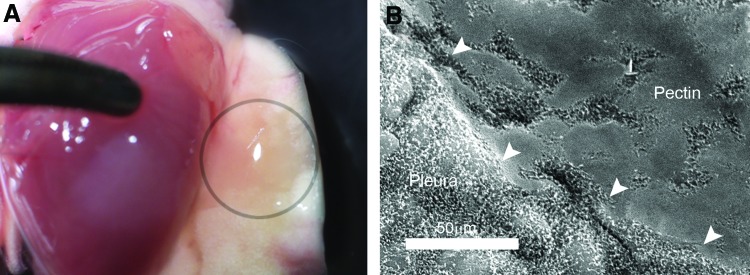
Previous work has demonstrated the adhesion of a 50–50 (wt%) mixture of HMP and CMC to the lung pleura.18 The HMP and CMC mixture was prepared as a viscous liquid, poured into the desired mold, and allowed to dry overnight. In most experiments, the pectin-based mixture was prepared as a translucent 50 μm thick sheet (48.3 ± 13 μm; Fig. 1A). With warming, the pectin-based sheet readily conformed to the pleural surface and demonstrated near-maximal adhesion within a 2–3 min development time. Scanning electron microscopy (SEM) images of the postapplication pectin-based polymer created the visual impression that the sheet had “melted” into the mesothelial surface (Fig. 1B). The relative strength of the pectin-based adhesion was demonstrated in comparison to commonly available commercial surgical products used for a variety of surgical applications (Fig. 2). The pectin-based polymer demonstrated significantly greater adhesive strength and greater yield strength than the comparison products (p < 0.001; Fig. 2).
FIG. 1.
Preparation and application of the pectin-based polymer. Based on previous work, a 50–50 (wt%) mixture of HMP and CMC was used.18 (A) The HMP and CMC mixture was prepared as a viscous liquid, poured into the desired mold, and allowed to dry overnight into a translucent 50 μm thick sheet (48.3 ± 13 μm). (B) The pectin-based polymer was applied to the lung surface with 1–2 min of gentle pressure. Subsequent SEM demonstrated intimate integration of the polymer with the existing pleura. Arrows highlight the edge of the pectin polymer covering the mesothelial microvilli. CMC, carboxymethylcellulose; HMP, high methoxyl pectin; SEM, scanning electron microscopy. Color images available online at www.liebertpub.com/tea
FIG. 2.
Load/displacement measurements. The adhesion of lung pleura to different surgical products was measured by a load/displacement assay (inset).18 Ninety degree loads were applied at a controlled rate to a suture passed through the tissue near the adhesive interface. Displacement was a measure of the separation at the adherend-substratum interface. (A) Representative load/displacement curves for the pectin-based polymer and five commercially available surgical products are shown. The curves were highly reproducible at displacement distances less than 3 mm; greater variability was noted between 3 and 6 mm (representative estimates shown). (B) Replicates of the initial separation load (yield point) demonstrated significantly greater adhesion of the pectin-based polymer than the surgical products (p < 0.001; N = 5). Color images available online at www.liebertpub.com/tea
Pectin adhesion after pleural injury
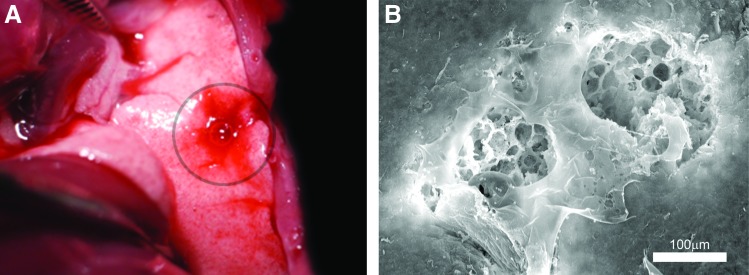
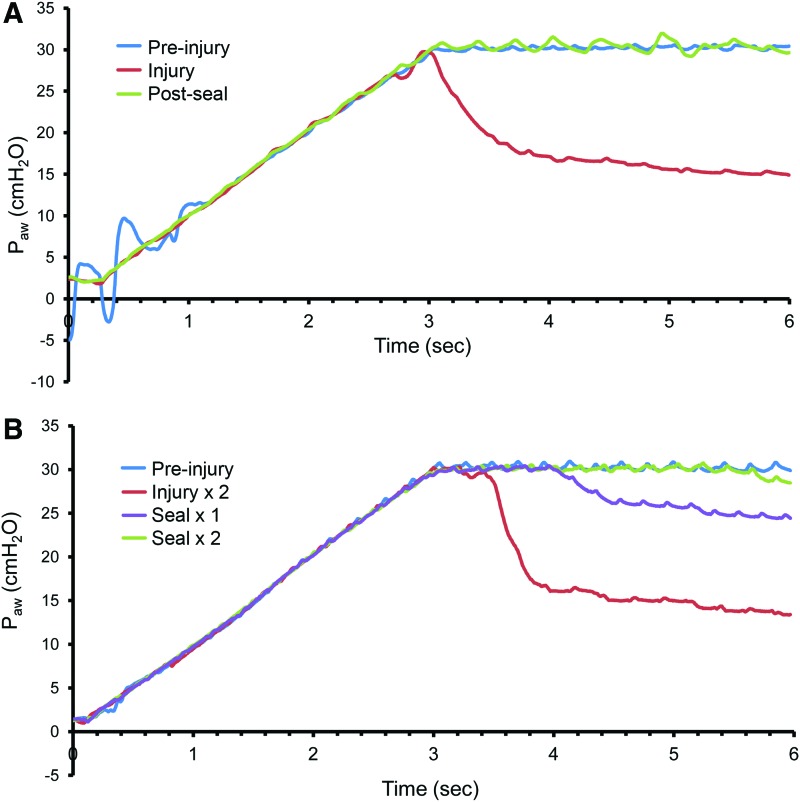
During general anesthesia and mechanical ventilation, a reproducible pleural injury was created with a 25 g needle inserted 1–2 mm into the visceral pleura. Leaking air and traces of blood were immediately observed after injury (Fig. 3A). SEM demonstrated a reproducible pleural injury with a consistent absence of a submesothelial matrix (Fig. 3B). To assess the potential function of the pectin-based polymer as an “air tight” sealant, the polymer was applied to the pleural wound. After 3–5 min, a lung recruitment maneuver was performed (3-s ramp to 30 cm H2O pressure). After a single pleural injury, there was a reproducible loss of pressure at 30 cm H2O and a new plateau pressure at 15 cm H2O (Fig. 4A). After the pectin-based polymer was applied to the injury and a repeat recruitment maneuver was performed, there was a consistent restoration of the normal plateau pressures (Fig. 4A). To test the effectiveness of the pectin-based sealant in more complex injuries, a “through-and-through” injury of the lung was created with a 25 g needle. During subsequent recruitment maneuvers, there was a loss of plateau pressures similar to the single injury. After sequential applications of the pectin-based sealant, there was a progressive restoration of the baseline plateau pressures (Fig. 4B).
FIG. 3.
Model of pleural injury. (A) A pleural injury in the left lung was produced with a 25 g needle inserted 1–2 mm into the lung parenchyma. Blood and a bubbling air leak were immediately noted. (B) Scanning electron microscopy of a double puncture of the lung demonstrated the associated pleural injury. Note the absence of a subpleural extracellular matrix to support wound healing. Color images available online at www.liebertpub.com/tea
FIG. 4.
Representative respiratory system mechanics after pleural injury. After standardizing volume history, a recruitment maneuver was performed consisting of a 3-s ramp to a 30 cm H2O airway pressure followed by a 3-s plateau. (A) After a single 25 g needle-induced pleural injury, the recruitment maneuver demonstrated a plateau pressure of ∼15 cm H2O. After sealing the injury with the pectin-based polymer, the repeat recruitment maneuver restored the 30 cm H2O airway pressures. (B) After a through-and-through injury of the lung, the pressures similarly dropped to less than half of the 30 cm H2O plateau pressures (Injury × 2). Sealing one of the injuries with the pectin-based polymer increased plateau pressures (Seal × 1). Also sealing the second injury (Seal × 2) resulted in restoration of baseline airway pressures. Note the two phases of the pressure curve: the initial exponential decline in airway pressures followed by a quasi-plateau phase. Summary data of a single pleural injury are shown in Figure 7. Color images available online at www.liebertpub.com/tea
Effect of pectin sealant on lung mechanics
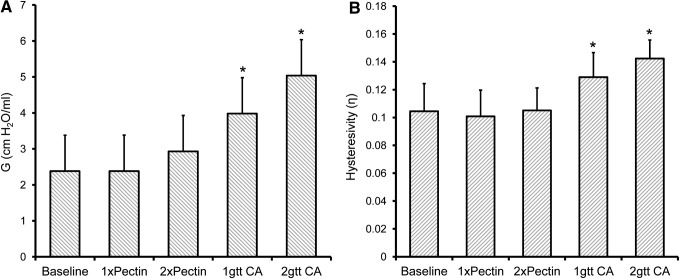
A potential limitation of any pleural sealant is lung restriction; that is, the effect of the sealant on limiting lung expansion. To separate the peripheral and central mechanical effects of the sealant, two oversized polymers (25 and 50 mm2) were applied to the lung surface and compared with no sealant (baseline) and two cyanoacrylate (Vetbond) controls. After application of the sealant, multifrequency lung impedance data were fitted to the constant-phase model.27 As expected, the application of the sealant had no effect on central airway resistance (no sealant Rn = 0.475 ± 0.017 cm H2O.s/mL; pectin-based sealant 50 mm2 Rn = 0.484 ± 0.026 cm H2O.s/mL). In contrast, measures of tissue damping demonstrated a slight effect with the larger pectin-based sealant (p > 0.05) and a significant effect with both cyanoacrylate (Vetbond) conditions (p < 0.01) (Fig. 5A). Hysteresivity (η), reflecting the relationship between energy dissipation and energy storage, demonstrated no significant change after the application of the pectin-based polymer (Fig. 5B).
FIG. 5.
Effect of sealants on peripheral lung mechanics. Lung impedance measurements were made using a forced oscillation technique (FlexiVent). The no sealant condition (Baseline) was compared to two sizes of the pectin-based polymer (1xPectin, 25 mm2; 2xPectin, 50 mm2) as well as two volumes of a cyanoacrylate (CA, VetBond) sealant (1gtt, 25 μL; 2gtt, 50 μL). (A) Measurement of tissue damping (G) demonstrated a slight increase in the 2xPectin condition (p > 0.05), but a significant increase in both cyanoacrylate conditions (asterisk, p < 0.01). (B) Hysteresivity, reflecting the relationship between energy dissipation and energy conservation (elastance), demonstrated no difference in the pectin-based polymer conditions, but a significant increase in the cyanoacrylate treatment groups (asterisk, p < 0.01). Triplicate measures per mouse; each data point represents N = 3 mice.
Long-term results of pleural adhesion
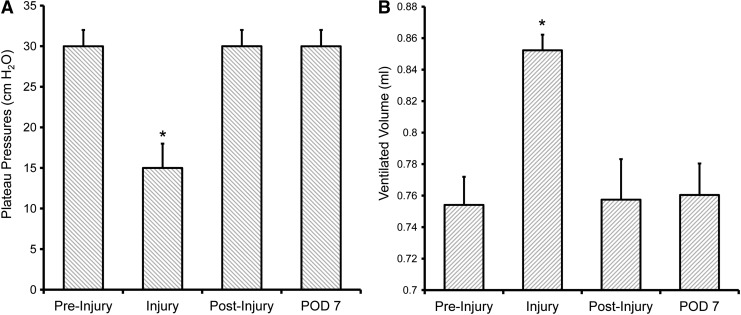
Since the pectin-based mixture is bioabsorbable, the effects of the pectin were studied during the 7-day timeframe typical for pleural healing.28 Although ethical restrictions precluded a no treatment control condition, experience with inadvertent lung injuries after murine surgery suggested that untreated air leaks are near-uniformly fatal. In this study, application of the pectin-based pleural sealant resulted in 100% survival (N = 5). Daily observations of control littermates demonstrated no difference in respiratory rate, respiratory effort, ruffled fur, nesting behavior, and general activity level. Similarly, there was no detectable change in body weight (preinjury 26.5 ± 2.4 g vs. 7 days postinjury + sealant 26.6 ± 1.8 g). Examination of the site of pleural injury 7 days later demonstrated a detectable residual sealant (Fig. 6A), but no pleural effusion and no pleural adhesions. SEM demonstrated some suggestion of resorption of the sealant, but residual polymer with an appearance consistent with its original application (Fig. 6B). Functional studies of the mice 7 days after their original injury demonstrated normal plateau pressures (Fig. 7A) and no detectable air leak (Fig. 7B).
FIG. 6.
Appearance of pectin-based polymer 7 days after application onto a pleural injury. (A) A left lung injury (25 g needle) was acutely sealed with the pectin-based polymer and the thoracotomy closed. After 7 days, the mice were euthanized. The lungs were flushed to facilitate identification of the polymer (circle). (B) SEM of the pleura demonstrated a similar appearance to the acute application with some discontinuous or “patchy” areas near the edges (arrows) of the applied pectin. Color images available online at www.liebertpub.com/tea
FIG. 7.
Respiratory system mechanics 7 days after application of the pectin-based polymer. (A) Acutely after injury with a 25 g needle, significantly decreased plateau pressures (asterisk, p < 0.001) were restored after application of the pectin-based polymer. The plateau pressures remained at baseline levels after 7 days. (B) Similarly, the significant increase in ventilated volume (“leak volume”) after pleural injury (asterisk, p < 0.001) was normalized after application of the pectin-based sealant. The ventilatory efficiency remained unchanged after 7 days. Triplicate measures per mouse; each data point represents, N = 5 mice.
Discussion
Air leaks after pleural injury can have a significant impact on patient care and healthcare costs.4 In this study, we tested the sealant properties of an equal mixture (wt%) of HMP and CMC, for the treatment of pleural injury. We identified four features of the pectin-based sealant. (1) The pectin-based polymer demonstrated a significantly stronger pleural bond than commonly available surgical products. (2) A 2–3-min development time was sufficient to seal air leaks under positive pressure (30 cm H2O). (3) Despite application of the polymer to 30% of the lung surface area, there was no significant compromise of lung expansion. (4) The sealant was partially reabsorbed, without detectable adhesions or pleural effusions, within 1 week after application. We conclude that the pectin-based polymer is a promising sealant for the treatment of pleural injury.
The pectin-based adhesive polymer demonstrated several additional features characteristics of an ideal sealant.20,29,30 The pectin-based sealant appeared to be safe and nontoxic. Although the focus of our study was the 7-day time frame of pleural healing, no toxicity was observed in mice treated with the pleural pectin as long as 14 days. Constituted as a 50 μm thick film, the pectin-based sealant was easily prepared and readily applied to the injured pleura. The pectin-based film required only 2–3 min of gentle pressure to seal the air leak. SEM suggested that the sealant persisted throughout the typical 7-day healing process without evidence of pulmonary edema or pleural effusion.
Pectin is one of many naturally occurring polysaccharides used in tissue engineering applications. Other polysaccharide biopolymers include alginate,31 agarose,32 cellulose,33 and chitin.34 Pectin is a particularly intriguing polysaccharide because of its structural features35 as well as its potential anti-inflammatory activity.36,37 Pectin is a structural heteropolysaccharide that comprises ∼30% of the primary cell walls of plants.19 Pectin consists predominantly of partially esterified linear chains of (1,4)-α-d-galacturonic acid residues.38,39 When exposed to calcium, pectin forms egg box-like structures that facilitate the immobilization of substances within the gel structure.40 The bioadhesivity of pectin, combined with the ability to trap drugs or growth factors within the gel structure, has led to considerable interest in using pectin to target drug delivery41 as well as facilitate wound healing.42 Although little is known about mesothelial growth factors, an intriguing potential application is the incorporation of wound healing adjuvants into the pectin-based polymer.
The dehydrated pectin-based polymer was relatively inflexible; the polymer was not tacky to touch nor adhesive to nonpolysaccharide surfaces. With the warming and gentle hydration associated with pleural application, however, the pectin-based polymer acquired its distinctive properties. The polymer became soft, flexible, and strongly adherent to the pleural surface. The application of a densely adherent polymer to the pleural surface raised the possibility of functional restriction43; that is, the polymer might limit lung expansion and diminish parenchymal compliance. In this study, we applied the pectin-based polymer to larger surface areas than would be anticipated in clinical use—more than 30% of the lung surface—and studied the consequences using a forced oscillation technique.44 The multifrequency lung impedance data were fitted to the constant-phase model to discriminate central versus peripheral effects.27 As expected, the presence or absence of the polymer had no effect on central airway resistance. Unexpectedly, there was also no significant difference in tissue damping (G) and hysteresivity (η) before and after the application of the polymer. A potential explanation for this observation is that pectin—depending upon ion concentration, solvent conditions, and degree of crosslinking—can demonstrate significant elasticity.45 Also, greater viscoelastic properties of interacting polysaccharides (including pectins) have been observed with increased crosslinking and enhanced polysaccharide structure.46,47 Based on these observations, it is possible that the pectin film mirrors the endogenous glycocalyx in accommodating the tidal changes in pleural surface area.
The key to the function of the pectin-based sealant is the mesothelial glycocalyx, a polysaccharide coating of the mesothelium of the lung, liver, heart, bowel, and tunica vaginalis. Although early electron microscopy studies had suggested an extracellular polysaccharide-rich coating of many eukaryotic48 and prokaryotic cells,49 Bennett coined the word glycocalyx (Greek, “sweet husk”) to describe the polysaccharide coating of cells.50 In subsequent electron microscopy studies of the mesothelium, a variety of cytochemical techniques demonstrated an extracellular polysaccharide layer, composed of negatively charged sialomucins,51 ∼25–50 nm thick.52,53 The glycocalyx coats the mesothelial cell surface between microvilli. The glycocalyx, at least in endothelial cells, is anchored to the cell membrane and cytoskeleton via a variety of transmembrane proteoglycans and glycoproteins.54,55 Although the function of the mesothelial glycocalyx is unknown, the glycocalyx may function to limit fluid loss56 and minimize frictional forces.57 In these experiments, the mesothelial glycocalyx also functions as the target substrate for the pectin-based sealant.
The potential clinical application of the pectin-based sealant depends upon the homology of the murine and human glycocalyx. Although some species differences in lectin binding have been observed,58 there are many structural and chemical similarities between human and murine mesothelium.53 Elucidating these similarities will be an objective of future work.
Acknowledgments
This study was supported, in part, by NIH Grants HL94567, HL007734, HL134229, CA009535, and ES000002.
Disclosure Statement
No competing financial interests exist.
References
- 1.Pompili C., and Miserocchi G. Air leak after lung resection: pathophysiology and patients' implications. J Thorac Dis 8, S46, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunelli A., Xiume F., Al Refai M., Salati M., Marasco R., and Sabbatini A. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications—a case-matched analysis. Chest 130, 1150, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Varela G., Jimenez M.F., Novoa N., and Aranda J.L. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardio-Thorac Surg 27, 329, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Liang S.Y., Ivanovic J., Gilbert S., Maziak D.E., Shamji F.M., Sundaresan R.S., et al. Quantifying the incidence and impact of postoperative prolonged alveolar air leak after pulmonary resection. J Thorac Cardiovasc Surg 145, 948, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Pedersen T.B., Honge J.L., Pilegaard H.K., and Hasenkam J.M. Comparative study of lung sealants in a porcine ex vivo model. Ann Thorac Surg 94, 234, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Fuller C. Reduction of intraoperative air leaks with Progel in pulmonary resection: a comprehensive review. J Cardiothorac Surg 8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan M. Fibrin glue. Blood Rev 5, 240, 1991 [DOI] [PubMed] [Google Scholar]
- 8.McCarthy P.M., Trastek V.F., Bell D.G., Buttermann G.R., Piehler J.M., Payne W.S., et al. The effectiveness of fibrin glue sealant for reducing experimental pulmonary air leak. Ann Thorac Surg 45, 203, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Fleisher A.G., Evans K.G., Nelems B., and Finley R.J. Effect of routine fibrin glue use on the duration of air leaks after lobectomy. Ann Thorac Surg 49, 133, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Kawai N., Suzuki S., Naito H., Kushibe K., Tojo T., Ikada Y., et al. Sealing effect of cross-linked gelatin glue in the rat lung air leak model. Ann Thorac Surg 102, 282, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Ennker J., Ennker I.C., Schoon D., Schoon H.A., Dorge S., Meissler M., et al. The impact of gelatin-resorcinol glue on aortic tissue—a histomorphologic evaluation. J Vasc Surg 20, 34, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Hewitt C.W., Marra S.W., Kann B.R., Tran H.S., Puc M.M., Chrzanowski F.A., et al. BioGlue surgical adhesive for thoracic aortic repair during coagulopathy: efficacy and histopathology. Ann Thorac Surg 71, 1609, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Chao H.H., and Torchiana D.F. BioGlue: alburnin/glutaraldehyde sealant in cardiac surgery. J Cardiac Surg 18, 500, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Kaplan M., Bozkurt S., Kut M.S., Kullu S., and Demirtas M.M. Histopathological effects of ethyl 2-cyanoacrylate tissue adhesive following surgical application: an experimental study. Eur J Cardiothorac Surg 25, 167, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Toriumi D.M., Raslan W.F., Friedman M., and Tardy M.E. Histotoxicity of cyanoacrylate tissue adhesives. A comparative study. Arch Otolaryngol Head Neck Surg 116, 546, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Petrella F., Borri A., Brambilla D., Calanca G., Vezzani N., Colantoni A., et al. Efficacy and safety of Innoseal for air leak after pulmonary resection: a case-control study. J Surg Res 206, 22, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Benigni R., Passerini L., and Rodomonte A. Structure-activity relationships for the mutagenicity and carcinogenicity of simple and alpha-beta unsaturated aldehydes. Environ Mol Mutagen 42, 136, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Servais A.B., Kienzle A., Valenzuela C.D., Ysasi A.B., Wagner W., Tsuda A., et al. Structural heteropolysaccharide adhesion to the glycocalyx of visceral mesothelium Tissue Eng Part A. DOI: 10.1089/ten.TEA.2017.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheller H.V., Jensen J.K., Sorensen S.O., Harholt J., and Geshi N. Biosynthesis of pectin. Physiol Plant 129, 283, 2007 [Google Scholar]
- 20.Mehdizadeh M., and Yang J. Design strategies and applications of tissue bioadhesives. Macromol Biosci 13, 271, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sriamornsak P., Wattanakorn N., Nunthanid J., and Puttipipatkhachorn S. Mucoadhesion of pectin as evidence by wettability and chain interpenetration. Carbohydr Polym 74, 458, 2008 [Google Scholar]
- 22.Boateng J., Okeke O., and Khan S. Polysaccharide based formulations for mucosal drug delivery: a review. Curr Pharm Des 21, 4798, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Gibney B., Lee G.S., Houdek J., Lin M., Chamoto K., Konerding M.A., et al. Dynamic determination of oxygenation and lung compliance in murine pneumonectomy. Exp Lung Res 37, 301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner W., Bennett R.D., Ackermann M., Ysasi A.B., Belle J.M., Valenzuela C.D., et al. Elastin cables define the axial connective tissue system in the murine lung. Anat Rec 298, 1960, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibney B., Houdek J., Lee G.S., Ackermann M., Lin M., Simpson D.C., et al. Mechanostructural adaptations preceding post-pneumonectomy lung growth. Exp Lung Res 38, 396, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hantos Z., Adamicza A., Govaerts E., and Daroczy B. Mechanical impedances of lungs and chest-wall in the cat. J Appl Physiol 73, 427, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Hantos Z., Daroczy B., Suki B., Nagy S., and Fredberg J.J. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72, 168, 1992 [DOI] [PubMed] [Google Scholar]
- 28.DeCamp M.M., Blackstone E.H., Naunheim K.S., Krasna M.J., Wood D.E., Meli Y.M., et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 82, 197. [DOI] [PubMed] [Google Scholar]
- 29.Quinn JV. Tissue Adhesives in Clinical Medicine. 2nd ed. Lewiston, NY: BC Decker, 2005 [Google Scholar]
- 30.Smith A.M., and Callow J.A. Biological Adhesives. Berlin: Springer-Verlag, 2006 [Google Scholar]
- 31.Shachar M., Tsur-Gang O., Dvir T., Leor J., and Cohen S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater 7, 152, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Khanarian N.T., Haney N.M., Burga R.A., and Lu H.H. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials 33, 5247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pooyan P., Tannenbaum R., and Garmestani H. Mechanical behavior of a cellulose-reinforced scaffold in vascular tissue engineering. J Mech Behav Biomed Mater 7, 50, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Kumar PTS, Srinivasan S., Lakshmanan V-K, Tamura H., Nair S.V., and Jayakumar R. beta-Chitin hydrogel/nano hydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr Polym 85, 584, 2011 [Google Scholar]
- 35.Coimbra P., Ferreira P., de Sousa H.C., Batista P., Rodrigues M.A., Corriea I.J., et al. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int J Biol Macromol 48, 112, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Chen C.H., Sheu M.T., Chen T.F., Wang Y.C., Hou W.C., Liu D.Z., et al. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem Pharmacol 72, 1001, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Salman H., Bergman M., Djaldetti M., Orlin J., and Bessler H. Citrus pectin affects cytokine production by human peripheral blood mononuclear cells. Biomed Pharmacother 62, 579, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Monsoor M.A., Kalapathy U., and Proctor A. Determination of polygalacturonic acid content in pectin extracts by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem 74, 233, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Nunes C., Silva L., Fernandes A.P., Guine R.P.F., Domingues M.R.M., and Coimbra M.A. Occurrence of cellobiose residues directly linked to galacturonic acid in pectic polysaccharides. Carbohydr Polym 87, 620, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Munarin F., Guerreiro S.G., Grellier M.A., Tanzi M.C., Barbosa M.A., Petrini P., et al. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 12, 568, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Smistad G., Boyum S., Alund S.J., Samuelsen A.B.C., and Hiorth M. The potential of pectin as a stabilizer for liposomal drug delivery systems. Carbohydr Polym 90, 1337, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Munarin F., Tanzi M.C., and Petrini P. Advances in biomedical applications of pectin gels. Int J Biol Macromol 51, 681, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Loring S.H., Mentzer S.J., and Reilly J.J. Sources of graft restriction after single lung transplantation for emphysema. J Thorac Cardiovasc Surg 134, 204, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Pride N.B. Forced oscillation techniques for measuring mechanical-properties of the respiratory system. Thorax 47, 317, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abu-Lail N.I., and Camesano T.A. Polysaccharide properties probed with atomic force microscopy. J Microsc 212, 217, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Khondar D., Tester R.F., Hudson N., Karkalas J., and Morrow J. Rheological behaviour of uncross-linked and cross-linked gelatinised waxy maize starch with pectin gels. Food Hydrocoll 21, 1296, 2007 [Google Scholar]
- 47.Round A.N., MacDougall A.J., Ring S.G., and Morris V.J. Unexpected branching in pectin observed by atomic force microscopy. Carbohydr Res 303, 251, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Yamada E. The fine structure of the renal glomerulus of the mouse. J Biophys Biochem Cytol 1, 551, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suganuma A. Plasma membrane of staphylococcus aureus. J Biophys Biochem Cytol 10, 292, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett H.S. Morphological aspects of extracellular polysaccharides. J Histochem Cytochem 11, 14, 1963 [Google Scholar]
- 51.Wang N.S. Regional difference of pleural mesothelial cells in rabbits. Am Rev Respir Dis 110, 623, 1974 [DOI] [PubMed] [Google Scholar]
- 52.Schwarz W. Surface film on mesothelium of serous membranes of rat. Z Zellforsch Mikrosk Anat 147, 595, 1974 [DOI] [PubMed] [Google Scholar]
- 53.Ohtsuka A., Yamana S., and Murakami T. Localization of membrane-associated sialomucin on the free surface of mesothelial cells of the pleura, pericardium, and peritoneum. Histochem Cell Biol 107, 441, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Weinbaum S., Tarbell J.M., and Damiano E.R. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9, 121, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Tarbell J.M. Shear stress and the endothelial transport barrier. Cardiovasc Res 87, 320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.VanTeeffelen J.W., Brands J., Stroes E.S., and Vink H. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med 17, 101, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Bodega F., Sironi C., Porta C., Zocchi L., and Agostoni E. Pleural mesothelium lubrication after phospholipase treatment. Respir Physiol Neurobiol 194, 49, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Babal P., and Gardner W.A. Histochemical localization of sialylated glycoconjugates with Tritrichomonas mobilensis lectin (TLM). Histol Histopathol 11, 621, 1996 [PubMed] [Google Scholar]