Abstract
Background
The effectiveness of intraoral appliances (IOA), maxillary expansion (ME), and maxillomandibular advancement (MMA) in the treatment of children and adults with obstructive sleep apnea (OSA) has not yet been adequately assessed.
Methods
An umbrella review was performed based on established guidelines for evidence-based medicine. Data synthesis was performed only from randomized controlled trials with Paule-Mandel random-effects meta-analyses / meta-regressions using mean differences (MDs) and 95% confidence intervals (CIs) and was followed by the qualitative evaluation of the meta-evidence.
Results
29 systematic reviews were included, 7 of which provided quantitative data. IOA were effective in improving apnea hypopnea index (AHI) compared to both, placebo appliances (12 trials; 525 patients; MD = –11.70; 95% CI: [–15.38; –8.01]; p<0.001) and no treatment (1 trial; 24 patients; MD = –14.30; [–21.59; –7.01]; p<0.001). Only the former comparison was supported by robust meta-evidence. Effectiveness of IOA as measured by the Epworth Sleepiness Scale, on the other hand, was not supported by robust meta-evidence. No randomized or prospective controlled trials were found on the effectiveness of ME (conventional or surgically assisted) and MMA.
Conclusion
Intraoral appliances are effective in reducing AHI and their use is substantiated by robust evidence. There is no evidence from high-quality research to support treatment with ME (conventional or surgically assisted) or MMA in patients with OSA.
Obstructive sleep apnea (OSA) is a sleep-related disorder in which the repetitive narrowing or collapse of the upper airway leads to a partial decrease in airflow (hypopnea) or to complete airflow cessation (apnea) during sleep (1).
OSA is a common medical condition that affects 1% to 4% of children, with a higher prevalence in boys than in girls (2), as well as 2% to 5% of women and 3% to 7% of men (3).
Risk factors for OSA development include:
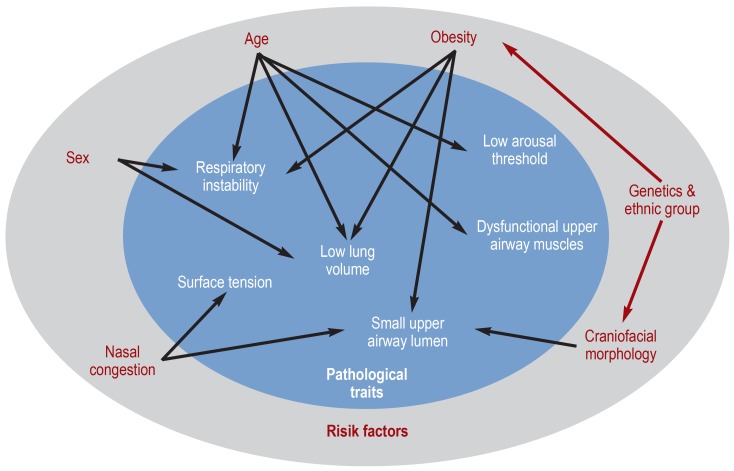
An overview of the pathophysiology of OSA and associated risk factors is given in Figure 1 (adapted with permission from Jordan et al. [14]).
Figure 1.
Diagram summarizing the pathophysiology of obstructive sleep apnea [adapted with permission from Jordan et al. (14)]
Since some well-established risk factors have a genetic background, OSA aggregates within families (15). However, susceptibility to OSA among family members is not fully explained by familial aggregation of other risk factors, such as obesity (16, 17).
The impact of OSA on patients is considerable. Lack of energy seems to be the most important complaint (18), although daytime sleepiness is also reported by 46–47% of OSA patients (18, 19).
Additionally, OSA sufferers have an elevated risk of motor vehicle crashes, although the actual number of accidents is still quite low (20). Furthermore, their cognitive performance is impaired in proportion to OSA severity (21, 22), while their perceived quality of life appears to resemble that of other chronic diseases (23).
Available symptomatic or causative treatments include (24, 25):
Lifestyle interventions—especially weight loss
Intraoral appliances (IOA)
Continuous positive airway pressure (CPAP)
Pharmacological agents
Surgery.
Additionally, in orthodontics, maxillary expansion has been thought to increase the upper airway dimensions and thus alleviate OSA symptoms (26). Overall, dental science is implicated in many OSA treatment modalities including IOA, rapid maxillary expansion (RME) in children, surgically assisted rapid maxillary expansion (SARME) in adults, and surgical maxillomandibular advancement (MMA) (24– 30).
Here, we aimed to comparatively investigate the effectiveness of OSA treatment modalities for children and adults that are of interest to dentists/orthodontists by conducting an umbrella review of systematic reviews. Furthermore, we intended to systematically assess the available scientific evidence on these interventions and to identify potential biases that could affect the study findings.
Methods
Protocol, registration, conduct, and reporting
The protocol for this study was made a priori and registered in PROSPERO (CRD42016045840). All post hoc changes are appropriately mentioned. The review was conducted according to the Cochrane Handbook (31) and reported according to the PRISMA statement (32). We also considered the guidelines provided by Aromataris et al. (33).
Eligibility criteria, study identification and selection
Three search queries were created and appropriately adjusted to each electronic database for a systematic search from database inception to August 14th, 2016 (etable 1). Firstly, systematic reviews were checked for eligibility according to the criteria for systematic reviews listed in eBox 1. Secondly, all primary studies of each included systematic review were extracted and assessed according to the eligibility criteria for primary studies (ebox 1). A detailed methodological description is provided in the eMethods.
eTable 1. Identification of studies: databases. dates. search strategies. and hits.
| Database and date searched | Search query | Hits per query | Hits per database |
| PubMed www.ncbi.nlm.nih.gov/pubmed/advanced. am 13.08.2016 |
((((appliance OR device OR splint*)) AND (mandib* OR “lower jaw”)) AND (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*)) Filters: Review | 132 | 245 |
| ((((maxilla* OR palat*)) AND expansion) AND (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*)) Filters: Review | 43 | ||
| ((((“maxillomandibular advancement” OR osteotomy OR BSSO OR “bilateral sagittal split osteotomy” OR “mandibular advancement” OR “Le Fort” OR “maxillary advancement”))) AND ((“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*))) Filters: Meta-Analysis; Systematic Reviews | 70 | ||
| Cochrane Database of Systematic Reviews http://onlinelibrary.wiley.com/cochranelibrary/search. am 13.08.2016 |
(appliance OR device or splint*) and (mandib* OR “lower jaw”) and (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA or apnea OR apnoea OR snor* OR breath* OR respir* OR ‧hypopnea OR sleep*) in Cochrane Reviews (Search all text) | 46 | 80 |
| (“maxillary expansion” OR “palatal expansion”) and (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*) in Cochrane Reviews (Search all text) | 6 | ||
| (“maxillomandibular advancement” OR osteotomy OR BSSO or “bilateral sagittal split osteotomy” OR “mandibular advancement” OR “Le Fort” or “maxillary advancement”) and (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA or apnea or apnoea or snor* OR breath* OR respir* OR hypopnea OR sleep*) in Cochrane Reviews (Search all text) | 28 | ||
| Virtual Health Library http://pesquisa.bvsalud.org/portal/advanced/?lang=en. am 13.08.2016 |
(tw:(appliance OR device OR splint*)) AND (tw:(mandib* OR “lower jaw”)) AND (tw:(“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*)) AND (tw:(“systematic review” OR “meta-analysis”)) in Title. abstract. subject | 16 | 57 |
| (tw:(“maxillary expansion” OR “palatal expansion”)) AND (tw:(“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypo‧pnea OR sleep*)) AND (tw:(“systematic review” OR “meta-analysis”)) in Title. abstract. subject | 9 | ||
| (tw:(“maxillomandibular advancement” OR osteotomy OR BSSO OR “bilateral sagittal split osteotomy” OR “mandibular advancement” OR “Le Fort” OR “maxillary advancement”)) AND (tw:(“obstruc‧tive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*)) AND (tw:(“systematic review” OR “meta-analysis”)) in Title. abstract. subject | 32 | ||
| Scopus www.scopus.com/search/. am 14.08.2016 |
(TITLE-ABS-KEY (“maxillomandibular advancement” OR osteotomy OR bsso OR “bilateral sagittal split osteotomy” OR “mandibular advancement” OR “Le Fort” OR “maxillary advancement”) AND TITLE-ABS-KEY (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR osa OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*) AND TITLE-ABS-KEY (“systematic review” OR “meta-analysis”)) | 61 | 124 |
| (TITLE-ABS-KEY (((((appliance OR device OR splint*)) AND (mandib* OR “lower jaw”)) AND (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR osa OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*))) AND TITLE-ABS-KEY (“systematic review” OR “meta-analysis”)) | 53 | ||
| (TITLE-ABS-KEY (((((maxilla* OR palat*)) AND expansion) AND (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR osa OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*))) AND TITLE-ABS-KEY (“systematic review” OR “meta-analysis”)) | 10 | ||
| Web of Science http://apps.webofknowledge.com/. am 13.08.2016 |
TOPIC: (appliance OR device OR splint*) AND TOPIC: (mandib* OR “lower jaw”) AND TOPIC: (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*) Refined by: DOCUMENT TYPES: (REVIEW) All Databases | 208 | 548 |
| TOPIC: (“maxillary expansion” OR “palatal expansion”) AND TOPIC: (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*) Refined by: DOCUMENT TYPES: (REVIEW) All Databases | 64 | ||
| TOPIC: (“maxillomandibular advancement” OR osteotomy OR BSSO OR “bilateral sagittal split osteotomy” OR “mandibular advancement” OR “Le Fort” OR “maxillary advancement”) AND TOPIC: (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*) Refined by: DOCUMENT TYPES: (REVIEW) All Databases | 276 | ||
| UMI Proquest http://search.proquest.com/advanced/reset?accountid=13478. am 13.08.2016 | (appliance OR device OR splint*) AND (mandib* OR “lower jaw”) AND (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*) in Dissertations & Theses / Anywhere | 0 | 0 |
| (“maxillary expansion” OR “palatal expansion”) AND (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR respir* OR hypopnea OR sleep*) in Dissertations & Theses / Anywhere | 0 | ||
| (“maxillomandibular advancement” OR osteotomy OR BSSO OR “bilateral sagittal split osteotomy” OR “mandibular advancement” OR “Le Fort” OR “maxillary advancement”) AND (“obstructive sleep apnoea” OR “obstructive sleep apnea” OR OSA OR apnea OR apnoea OR snor* OR breath* OR ‧respir* OR hypopnea OR sleep*) in Dissertations & Theses / Anywhere | 0 | ||
| Sum | 1054 |
eBOX 1. Eligibility criteria for selecting systematic reviews and primary studies.
-
1.
Criteria for the inclusion of systematic reviews (with or without meta-analysis)
Inclusion criteria
At least one database was systematically searched and
-
The study follows a systematic methodology (at least rudimentarily) using methods such as:
Methodological quality assessment of the included studies using any tool or
Conduct of a meta-analysis or
Duplicate study selection or data extraction by two independent reviewers.
Exclusion criteria
Systematic reviews of animal studies, narrative reviews, and clinical practice guidelines merely based on a literature search
-
2.
Criteria for selecting primary studies included in the systematic reviews
Inclusion criteria
Patients with an a priori diagnosis of OSA (AHI = 5 or RDI = 5 for adults and AHI = 1 for children) of any age or sex
IOA, RME, SARME, or MMA to alleviate / treat OSA
Direct comparisons between two or more interventions or between patients receiving the intervention and untreated / placebo matched controls
Randomized controlled trials or prospective non-randomized clinical trials of parallel or crossover design
Any clinical setting
Primary outcome: AHI measured with polysomnography before and after the intervention
Secondary outcomes measured before and after the intervention: RDI, oximetry indices, sleep efficiency, REM sleep latency, and ESS
Exclusion criteria
Studies reporting on patients suffering from conditions other than OSA
Non-clinical studies, retrospective clinical studies, case series (less than 10 patients), and case reports
OSA, obstructive sleep apnea; AHI, apnea hypopnea index; RDI, respiratory disturbance index, IOA, intraoral appliance; RME, rapid maxillary expansion; SARME, surgically assisted rapid maxillary expansion; MMA, maxillomandibular advancement; REM, rapid eye movement; ESS, Epworth Sleepiness Scale
Data extraction
Two authors (VK and SNP) independently extracted descriptive data from each eligible systematic review. For further data extraction of quantitative results from primary studies, we considered only such systematic reviews in which meta-analyses with comparison groups were performed. If an article presented separate meta-analyses on more than one eligible outcome, those outcomes were assessed separately. Data extraction was based on the results provided in the included systematic reviews; where discrepancies existed between reviews, we directly extracted data from their corresponding primary studies. For the predefined subgroup analyses, additional data were directly extracted from the primary studies. For further details, refer to the eMethods.
Assessment of pooled effects and heterogeneity
For meta-analyses of continuous outcomes, mean differences (MDs) of the treatment-induced increments were chosen as effect estimates, while risk ratios (RRs) were chosen for categorical outcomes, both with their 95% confidence intervals (CI). Based on clinical and statistical reasoning (e3), we estimated all pooled effects with a random-effects model using the Paule–Mandel estimator instead of the commonly used DerSimonian–Laird, due to the improved performance of the former (e4). All calculations were performed in STATA SE version 12 (StataCorp, College Station, TX, USA). Further details on the assessment of pooled effects and heterogeneity, assessment of small-study effects, and criteria for epidemiological associations are provided in the eMethods.
Results
Characteristics of included systematic reviews
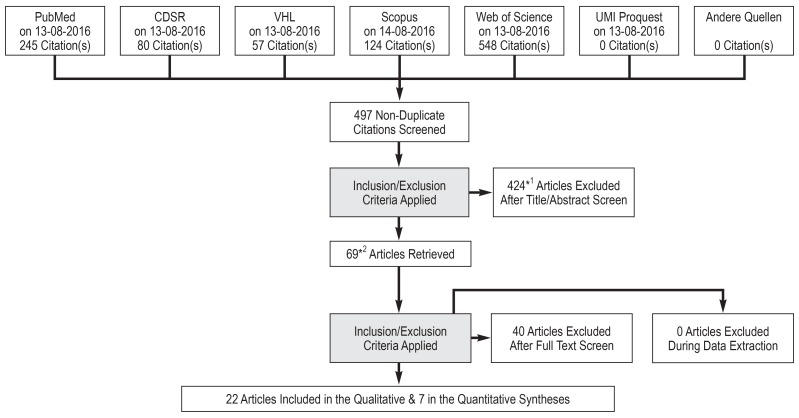
The electronic searches yielded a total of 497 hits (eFigure 1). After excluding inappropriate studies (eTable 2), a total of 29 systematic reviews remained for inclusion in our study. Table 1 provides an overview of the characteristics of the included systematic reviews and eBox 2 gives a detailed description.
eFigure.
Flow diagram for the selection of included studies
CDSR, Cochrane Database of Systematic Reviews; VHL, Virtual Health Library.
*1 Three abstracts could not be found; *2 One full text could not be found
Table 1. Characteristics of included systematic reviews.
| Nr. | Systematic review | Databases searched | Tx | Internal validity | Outcomes *1 | MA | Evidence quality | Conflict of Interest |
| 1 | Abdullatif (2016) (e11) | 9 | ME | NICE tool | AHI. Min satur | Yes | No | NR |
| 2 | Ahrens (2011) (e12) | 4 | IOA | AASM criteria | AHI. RDI | No | No | External. non-profit |
| 3 | Bartolucci (2016) (e13) | 6 | IOA | EPHPP tool | AHI | Yes | GRADE | None existing |
| 4 | Bratton (2015) (e14) | 2 | IOA | Cochrane RoB | ESS | Yes | No | External / internal non-profit |
| 5 | Bridgman and Dunn (2000) (e15) | 4 | MMA | Jadad scale | OD | No | No | External. non-profit |
| 6 | Caldas (2009) (e16) | 1 | IOA | Jadad scale | AHI. ESS. OD | No | No | NR |
| 7 | Camacho (2015) (e17) | 4 | MMA | NICE tool | AHI. RDI. ESS. Min satur |
Yes | No | External. non-profit |
| 8 | Caples (2010) (e18) *2 | 4 | MMA | NR | AHI | Yes | GRADE | External for one author. research support from ResMed and Ventus Medical |
| 9 | Carvalho (2007. 2016) (e19, e20) | 6 | IOA | Cochrane RoB | AHI | No | GRADE | External. non-profit |
| 10 | Health Quality Ontario (2009) (e21) | 6 | IOA | Custom (Goodman) |
AHI. ESS | Yes | GRADE | None existing |
| 11 | Hoekema (2004) (e22) | 4 | IOA | Custom (e41) | AHI. ESS | Yes | No | External. non-profit |
| 12 | Holty and Guilleminault (2010) (e23) | 1 | MMA | – | AHI. ESS. Min satur. SE |
Yes | No | None existing |
| 13 | Hsieh and Liao (2013) (e24) | 1 | MMA | Jadad scale | AHI | No | No | External. non-profit |
| 14 | Huynh (2016) (e25) | 4 | IOA. ME | ARRIVE (modif. for humans) |
AHI. OS | Yes | No | None existing |
| 15 | Knudsen (2015) (e26) | 2 | MMA | – | AHI. Min satur | Yes | No | None existing |
| 16 | Li (2013) (e27) | 3 | IOA | Cochrane RoB | AHI. ESS. Min satur | Yes | No | None existing |
| 17 | Lim (2004) (e28) | 2 | IOA | Jadad scale | AHI. Min satur | Yes | No | External. non-profit |
| 18 | Machado-Júnior (2016) (e29) | 1 | ME | – | AHI | Yes | No | None existing |
| 19 | Marcus (2012) (e30) | 6 | ME | AAN criteria | AHI | No | AAP criteria | None existing |
| 20 | Marklund (2012) (e31) | 2 | IOA | CEBM criteria | AHI. ESS. OS | No | CEBM criteria | None existing |
| 21 | Nazarali (2015) (e32) | 6 | IOA | Cochrane RoB | AHI. OD | No | No | NR |
| 22 | Okuno (2014) (e33) | 3 | IOA | Cochrane RoB (modif.) |
AHI. ESS | Yes | GRADE | None existing |
| 23 | Pirklbauer (2011) (e34) | 1 | MMA | CEBM criteria | AHI. ESS | No | No | NR |
| 24 | Ramar (2015) (e35) | 2 | IOA | Cochrane RoB (modif.) |
AHI. RDI. ESS. Min satur. SE |
Yes | GRADE | External for some authors. profit / non-profit |
| 25 | Serra-Torres (2016) (e36) | 3 | IOA | CONSORT | AHI. ESS. OS | No | No | None existing |
| 26 | Sharples (2016) (e37) | 3 & existing database |
IOA | Jadad scale | AHI. ESS | Yes | No | None existing |
| 27 | Sher (1996) (e38) | 1 | MMA | - | AHI. Min satur | No | No | NR |
| 28 | Zaghi (2016) (e39) | 4 | MMA | NR | AHI. RDI | Yes | No | None existing |
| 29 | Zhu (2015) (e40) | 5 | IOA | Cochrane RoB | AHI. ESS. Min satur. SE |
Yes | GRADE | External. non-profit |
AAN, American Academy of Neurology; AAP, American Academy of Pediatrics; AASM, American Academy Sleep Medicine; AHI, apnea hypopnea index; CEBM, Center for Evidence-Based
Medicine in Oxford; EPHPP, Effective Public Health Practice Project; ESS, Epworth Sleepiness Scale; Min Satur, mimimum oxygen saturation; MA, meta-analysis; ME, maxillary expansion;
MMA, maxillomandibular advancement; NR, not reported; IOA, intraoral appliances; OD, oxygen desaturation; OS, oxygen saturation; RDI, respiratory disturbance index; SE, sleep efficiency;
Tx, treatment.
*1 Only among those that were included in our study protocol
*2 Data extracted only on MMA
eBOX 2. Characteristics of the included systematic reviews.
Twenty eight reviews were published in scientific journals, while one was published as a Health Technology Assessment.
All reviews were published in English between 1996 and 2016 and each searched between one and nine literature databases.
Ten (34%) reviews included only RCTs, 7 (24%) included both RCTs and non-RCTs, and the remaining 12 (41%) only included non-RCTs.
The majority of them (16 reviews; 55%) assessed intraoral appliances, 9 (31%) assessed surgical maxillomandibular advancement, 3 (10%) assessed maxillary expansion, and one assessed more than one intervention.
Almost all of the reviews (27 reviews; 93%) reported on the primary outcome AHI, 14 (48%) reported on ESS, and 15 (52%) reported on oxygen saturation indices.
From the included systematic reviews, 12 (41%) had no conflicts of interest, 9 (31%) declared non-profit support, 2 (7%) involved company support, and 6 (21%) did not declare any status.
At the time this umbrella review was conducted, 4 included reviews had not been cited, while the rest gathered in total 2980 citations in Google Scholar (median=21; range=1–1016)
AHI, Apnoe-Hypopnoe-Index; ESS, Epworth Sleepiness Scale
Risk of bias and methodological adequacy
Table 1 reports on the assessment of risk of bias in the included systematic reviews and eTable 3 provides results for their methodological adequacy. A detailed description is provided in eBox 3.
eTable 3. Methodological quality of included systematic reviews.
| Citation | 1. Was an ‘a priori’ design provided? |
2. Was there duplicate study selection and data extraction by two independent reviewers? |
3. Was a comprehensive and systematic literature search performed? |
4. Were unpublished study data as well as grey literature appropriately considered? |
5. Was a list of studies (included and excluded) provided? |
6. Were the characteristics (patient characteristics. interventions outcomes) of the included studies_provided? |
7. Was the scientific quality of the included studies assessed and documented? |
8. Was the scientific quality of the included studies used appropriately in formulating conclusions? |
9. Were the methods appropriate that were used to combine the findings of studies? |
10. Was the likelihood of publication bias assessed? |
11. Was the conflict of interest included? |
| Abdullatif (2016) (e11) |
No | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No |
| Ahrens (2011) (e12) |
No | Yes | Yes | No | No | No *2 | No | No | NA | No | No |
| Bartolucci (2016) (e13) |
No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No |
| Bratton (2015) (e14) |
Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | No |
| Bridgman (2000) (e15) |
No | Yes | Yes | Yes | No | No | Yes | Yes | NA | No | No |
| Caldas (2009) (e16) |
No | Yes | No | No | No | Yes | Yes | No | NA | No | No |
| Camacho (2015) (e17) |
No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Caples (2010) (e18) |
Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | No | No |
| Carvalho (2007. 2016) (e19, e20) |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | No |
| Health Quality Ontario (2009) (e21) |
No | No | Yes | No | No | Yes | Yes | Yes | Yes | No | No |
| Hoekema (2004) (e22) |
No | No | Yes | Yes | No | Yes | Yes | Yes | No | No | No |
| Holty (2010) (e23) |
No | Yes | No | No | No | Yes | No | No | No | No | No |
| Hsieh and Liao (2013) (e24) |
No | Yes | No | No | No | Yes | Yes | Yes | NA | No | No |
| Huynh (2016) (e25) |
Yes | Yes | Yes | No | Yes | Yes | No *3 | Yes | No | No | No |
| Knudsen (2015) (e26) |
No | No | Yes | No | No | No | No | No | Yes | Yes | No |
| Li (2013) (e27) | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | No |
| Lim (2004) (e28) |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No |
| Machado-Júnior (2016) (e29) |
No | No | No | No | No | Yes | No | No | No | No | No |
| Marcus (2012) (e30) |
No | No | Yes | No | No | No | Yes | Yes | n.a. | No | No |
| Marklund (2012) (e31) |
No | No | Yes | No | No | Yes | Yes | Yes | n.a. | No | No |
| Nazarali (2015) (e32) |
No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Okuno (2014) (e33) |
No | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | No |
| Pirklbauer (2011) (e34) |
No | No | No | No | No | Yes | Yes | No | n.a. | No | No |
| Ramar (2015) (e35) |
No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | No |
| Serra-Torres (2016) (e36) |
No | No | Yes | No | No | Yes | Yes | No | n.a. | No | No |
| Sharples (2016) (e37) |
No | Yes | Yes | No | No | No | Yes | No | Yes | Yes | No |
| Sher (1996) (e38) *1 |
Yes | Yes | No | No | No | No | No | No | n.a. | No | No |
| Zaghi (2016) (e39) |
No | Yes | Yes | No | No | No | Yes | No | Yes | No *4 | No |
| Zhu (2015) (e40) |
No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No |
NA. not applicable.
*1 Data extracted only on MMA
*2 Dead link
*3 Inappropriate tool
*4 Funnel-like plot provided. but no statistical confirmation
eBOX 3. Risk of bias and methodological adequacy of the included systematic reviews.
Out of the 29 included systematic reviews, 23 (79%) assessed the risk of bias in the included primary studies; 7 (24%) of these used the Cochrane Collaboration’s risk of bias tool.
The quality of evidence (strength of recommendations) from the performed meta-analyses was assessed with the GRADE approach in a mere 7 out of 29 reviews (24%) and with other approaches in another 2 reviews (7%). The strength of recommendations of the included reviews ranged from very low to high.
The AMSTAR scores for the included reviews ranged from 1 to 9 out of 11 possible points, with a mean score of 5 (excluding non-applicable ratings) and a standard deviation of 2, with no review scoring full points.
The main shortcomings were a lack of a priori design (in 22 [76%] of the reviews), incomplete reporting of included / excluded studies (in 24 [83%] of the reviews), absence of grey literature searches (in 21 [72%] of the reviews), and missing statements for possible conflicts of interest (in all of the reviews).
Pooled effect sizes
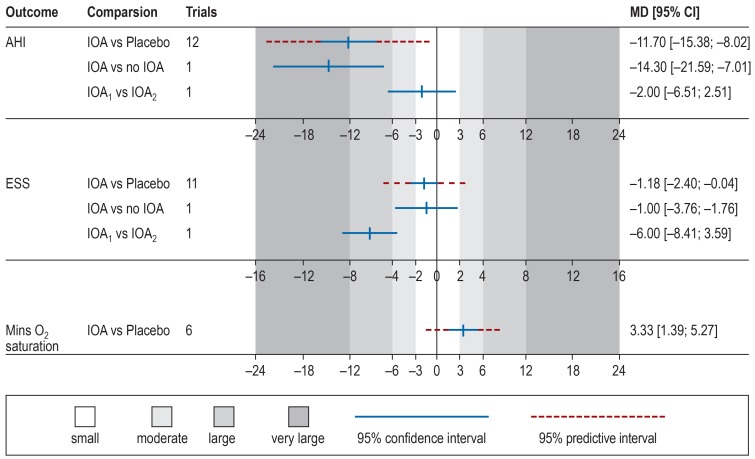
Of the included systematic reviews, 18 (62%) performed meta-analyses of primary studies of any kind. After applying the eligibility criteria to their corresponding primary studies (including primary studies with comparison groups and excluding inappropriate study designs), data from 7 systematic reviews based on 20 primary studies (ebox 4) were extracted. After removing duplicate primary trials and pooling studies on the same comparison identified from different systematic reviews, 8 meta-analyses of cumulative evidence could be conducted for the primary outcome apnea hypopnea index (AHI) and the secondary outcomes Epworth Sleepiness Scale (ESS) and minimum oxygen saturation (MOS). These meta-analyses pertained to comparisons of IOA with placebo appliances, no treatment, or different appliances (custom IOA based on impressions, pre-fabricated IOA, or tongue suction IOA) in the treatment of OSA in adults (table 2). No comparisons were available for RME, SARME, or MMA.
eBOX 4. List of included primary studies.
-
1
Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Oral appliance therapy versus nasal continuous positive airway pressure in obstructive sleep apnea: a randomized, placebo-controlled trial. Respiration. 2011; 81(5): 411–9.
-
2
Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, Pierce RJ. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004 Sep 15; 170(6): 656–64.
-
3
Blanco J, Zamarrón C, Abeleira Pazos MT, Lamela C, Suarez Quintanilla D. Prospective evaluation of an oral appliance in the treatment of obstructive sleep apnea syndrome. Sleep Breath. 2005 Mar;9(1): 20–5.
-
4
Bloch KE, Iseli A, Zhang JN, Xie X, Kaplan V, Stoeckli PW, Russi EW. A randomized, controlled crossover trial of two oral appliances for sleep apnea treatment. Am J Respir Crit Care Med. 2000 Jul; 162(1): 246–51.
-
5
Dal-Fabbro C, Garbuio S, D‘Almeida V, Cintra FD, Tufik S, Bittencourt L. Mandibular advancement device and CPAP upon cardiovascular parameters in OSA. Sleep Breath. 2014 Dec; 18(4): 749–59.
-
6
de Britto Teixeira, Andressa Otranto, Luciana Baptista Pereira Abi-Ramia, and Marco Antonio de Oliveira Almeida. „Treatment of obstructive sleep apnea with oral appliances.“ Progress in orthodontics 14.1 (2013): 10.
-
7
Dort L, Brant R. A randomized, controlled, crossover study of a noncustomized tongue retaining device for sleep disordered breathing. Sleep Breath. 2008 Nov;12 (4): 369–73.
-
8
Durán-Cantolla J, Crovetto-Martínez R, Alkhraisat MH, Crovetto M, Municio A, Kutz R, Aizpuru F, Miranda E, Anitua E. Efficacy of mandibular advancement device in the treatment of obstructive sleep apnea syndrome: A randomized controlled crossover clinical trial. Med Oral Patol Oral Cir Bucal. 2015 Sep 1; 20(5): e605–15.
-
9
Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002 Sep 1; 166(5): 743–8. / Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004 Aug 1;27(5): 934–41. / Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. Clin Sleep Med. 2005 Oct 15; 1(4): 374–80.
-
10
Hans MG, Nelson S, Luks VG, Lorkovich P, Baek SJ. Comparison of two dental devices for treatment of obstructive sleep apnea syndrome (OSAS). Am J Orthod Dentofacial Orthop. 1997 May; 111(5): 562–70.
-
11
Johnston CD, Gleadhill IC, Cinnamond MJ, Gabbey J, Burden DJ. Mandibular advancement appliances and obstructive sleep apnoea: a randomized clinical trial. Eur J Orthod. 2002 Jun; 24(3): 251–62.
-
12
Lam B, Sam K, Mok WY, Cheung MT, Fong DY, Lam JC, Lam DC, Yam LY, Ip MS. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007 Apr; 62(4): 354–9.
-
33
Maguire J, Steele JG, Gibson GJ, Wilson JA, Steen N, McCracken GI. Randomised cross-over study of oral appliances for snoring. Clin Otolaryngol. 2010 Jun; 35(3): 204–9.
-
14
Marklund M, Carlberg B, Forsgren L, Olsson T, Stenlund H, Franklin KA. Oral Appliance Therapy in Patients With Daytime Sleepiness and Snoring or Mild to Moderate Sleep Apnea: A Randomized Clinical Trial. JAMA Intern Med. 2015 Aug; 175(8): 1278–85.
-
15
Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001 May; 163(6): 1457–61.
-
16
16 Petri N, Svanholt P, Solow B, Wildschiødtz G, Winkel P. Mandibular advancement appliance for obstructive sleep apnoea: results of a randomised placebo controlled trial using parallel group design. J Sleep Res. 2008 Jun; 17(2): 221–9.
-
17
Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. 2002 Sep 15; 166(6): 860–4.
-
18
Quinnell TG, Bennett M, Jordan J, Clutterbuck-James AL, Davies MG, Smith IE, Oscroft N, Pittman MA, Cameron M, Chadwick R, Morrell MJ, Glover MJ, Fox-Rushby JA, Sharples LD. A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO). Thorax. 2014 Oct; 69(10): 938–45. / Sharples L, Glover M, Clutterbuck-James A, Bennett M, Jordan J, Chadwick R, Pittman M, East C, Cameron M, Davies M, Oscroft N, Smith I, Morrell M, Fox-Rushby J, Quinnell T. Clinical effectiveness and cost-effectiveness results from the randomised controlled Trial of Oral Mandibular Advancement Devices for Obstructive sleep apnoea-hypopnoea (TOMADO) and long-term economic analysis of oral devices and continuous positive airway pressure. Health Technol Assess. 2014 Oct; 18(67): 1–296.
-
19
Rose E, Staats R, Virchow C, Jonas IE. A comparative study of two mandibular advancement appliances for the treatment of obstructive sleep apnoea. Eur J Orthod. 2002 Apr; 24(2): 191–8.
-
20
Villa MP, Bernkopf E, Pagani J, Broia V, Montesano M, Ronchetti R. Randomized controlled study of an oral jaw-positioning appliance for the treatment of obstructive sleep apnea in children with malocclusion. Am J Respir Crit Care Med. 2002 Jan 1; 165(1): 123–7.
Table 2. Results of available comparisons regarding the primary and secondary outcomes in adult patients with obstructive sleep apnea.
| Outcome | Trials | Patients | Effect (95% CI) | p value | Sign. | Heterogeneity | Largest trial | Egger’s test | ||||
| τ2 | I2 | Comment | Consistent | Effect (95% CI) | Sign. | |||||||
| Intraoral appliance versus placebo appliance | ||||||||||||
| A | AHIcon*1 | 12 | 525 | MD: −11.7 [−15.38; −8.01] | <0.001 | ** | 20.2 | 93.6 % | High | Yes | −9.3 [−12.00; –6.60] | NS |
| B | ESS*2 | 11 | 475 | MD: −1.18 [−2.38; 0.03] | 0.055 | 2.1 | 60.6 % | Moderate | No | −2. 01 [−2.70; −1.32] | NS | |
| C | Min satur*3 | 6 | 286 | MD: 3.33 [1.38; 5. 28] | 0.007 | * | 2.2 | 96.8 % | High | Yes | 1.90 [0.51; 3.29] | – |
| Intraoral appliance versus no appliance | ||||||||||||
| D | AHIcon | 1 | 24 | MD: –14.30 [−21.59; −7.01] | <0.001 | ** | – | – | – | – | Same | – |
| E | AHIbin | 1 | 23 | RR: 0.37 [0.15; 0.90] | 0.029 | * | – | – | – | – | Same | – |
| F | ESS | 1 | 23 | MD: –1.00 [–3.77; 1.77] | 0.479 | – | – | – | – | Same | – | |
| Intraoral appliance1 versus intraoral appliance2 | ||||||||||||
| G | AHI | 1 | 23 | MD: –2.00 [–6.51; 2.51] | 0.385 | – | – | – | – | Same | – | |
| Intraoral appliance1 versus intraoral appliance2 | ||||||||||||
| H | ESS | 1 | 67 | MD: – 6.00 [−8.41; −3.59] | <0.001 | ** | – | – | – | – | Same | – |
AHI. apnea hypopnea index; con. continuous; bin. binary; CI. confidence interval; ESS. Epworth Sleepiness Scale; MD. mean difference; Min Satur. mimimum oxygen saturation;
Sign.. statistically significant at 5%.
intraoral appliance1 intraoral appliance with 4 mm opening; intraoral appliance2. intraoral appliance with 14 mm opening
*1 95% Predictive Intervals; Estimate: –22.55.–0.85; Consistent: Yes
*2 95% Predictive Intervals; Estimate: –4.76.2.40; Consistent: No
*3 95% Predictive Intervals; Estimate: –1.62.8.28; Consistent: No
As far as the comparison of IOA versus placebo appliances is concerned (Table 2; Figure 2), a considerable improvement was evident with IOA in apnea hypopnea index scores (MD: –11.7; 95% CI: [–15.38; –8.01]; p<0.001), small, marginally non-significant effects were noticed on Epworth Sleepiness Scale scores (MD: –1.18 [–2.38; 0.03]; p = 0.055), and moderate effects on minimum oxygen saturation (MD: 3.33 [1.38; 5.28]; p = 0.007). High heterogeneity (I2 >75%) was found in the meta-analyses of AHI and MOS. However, this posed a threat to the results only for the latter meta-analysis. For the former meta-analysis, the 95% prediction interval that incorporates existing heterogeneity was consistent to the left side of the forest plot. Additionally, compared to no treatment, IOA were found effective in improving AHI (MD: –14.30 [–21.59; –7.01]; p <0.001 as continuous and RR: 0.37 [0.15; 0.90]; p = 0.029 as binary outcome), but not in improving ESS (MD: –1.00 [–3.77; 1.77]; p = 0.479). Finally, as far as comparisons between different appliance designs are concerned, increased vertical opening (14 mm instead of 4 mm) did not influence AHI (MD: –2.00 [–6.51; 2.51]; p = 0.385) and significantly hampered improvement in ESS (MD: –6.00 [–8.41; –3.59]; p <0.001). However, this was based on a single trial and additional evidence is needed before any robust conclusions can be drawn.
Associations meeting the epidemiological criteria
From the cumulative evidence on the performance of IOA, only the large improvement in AHI compared to placebo appliances was supported by robust evidence, i.e. sample sizes were adequate, heterogeneity was not an issue, the random-effects predictive intervals were consistent in favour of the intervention, and no signs of reporting bias were found (table 3).
Table 3. Results on epidemiological criteria regarding the primary and secondary outcomes.
| Outcome | p<0.001 | Adequate sample size |
Heterogeneity not a problem |
PrI consistent | Egger’s test NS | Criteria met | |
| Intraoral appliance versus placebo appliance | |||||||
| A | AHIcon | Yes | Yes | Yes | Yes | Yes | Yes |
| B | ESS | No | No | No | No | Yes | No |
| C | Min satur | No | No | Yes | No | – | No |
| Intraoral appliance versus no appliance | |||||||
| D | AHIcon | Yes | No | – | – | – | No |
| E | AHIbin | No | No | – | – | – | No |
| F | ESS | No | No | – | – | – | No |
| Intraoral appliance1 versus intraoral appliance2 | |||||||
| G | AHI | No | No | – | – | – | No |
| H | ESS | Yes | No | – | – | – | No |
PrI, predictive intervals; NS, not significant; AHI, apnea hypopnea index; Min Satur, minimum oxygen saturation; ESS, Epworth Sleepiness Scale; con, continuous;
bin, binary; intraoral appliance1, intraoral appliance with 4 mm opening; intraoral appliance2, intraoral appliance with 14 mm opening
Discussion
This umbrella review of systematic reviews summarizes the existing evidence from randomized trials on the effectiveness of IOA, RME, SARME, and MMA in the treatment of OSA. A total of 29 systematic reviews were included in the qualitative synthesis, while eligible trials from 7 of these also contributed to the quantitative synthesis. Comparisons were available only for IOA, since for RME, SARME, and MMA no high-quality evidence was identified.
Considerable evidence indicated that IOA are effective in treating OSA in adults and improving AHI compared to both placebo appliances and no treatment. The former comparison was the only one that met all the criteria for strong epidemiological associations (e42) indicating strength of evidence. By mechanically holding the mandible in a forward position, IOA not only affect the anteroposterior dimension but also provoke an increase in the lateral diameter of the velopharynx (e43). In growing children with malocclusion, functional appliances for stimulating mandibular growth may alleviate OSA symptoms (e44, e45), but evidence is scarce and thus more research is needed. In regard to the secondary outcomes, IOA might have a positive effect on ESS and MOS in adults (table 2). However, the epidemiological strength of the associations (e42, e49) was poor, mostly due to the limited number of contributing studies.
As far as modifying factors on the effectiveness of IOA are concerned, subgroup analyses and meta-regressions indicated that baseline AHI levels were significantly associated with the observed AHI reduction (etable 4). This could mean that patients with more severe OSA symptoms are more likely to experience greater improvements in AHI. The same association between baseline severity and improvement of symptoms was also noticed for the effect of IOA on MOS (etable 5), but not ESS (etable 6). Although there were some indications pointing to small-study effects, these were not confirmed with the Egger’s test. Furthermore, this association could also be explained by “regression to the mean” and needs further confirmation. It is also important to note, that OSA baseline severity might directly influence treatment choices (30) leading to IOA being used more often in mild to moderate cases with non-compliance to CPAP, since CPAP appears to be more effective in complete resolution of OSA compared to IOA (28). Although IOA are not as effective as the first-line option, CPAP, in reducing AHI, they might be better preferred by patients (e47, e48), since CPAP is associated with more serious negative aspects, which influence adherence (e48, e49) and thus the effectiveness of treatment. Due to this fact, IOA could be used alternatively to CPAP in mild to moderate OSA and in severe OSA, if CPAP is not tolerated (30).
eTable 4. Overall results for the comparison of intraoral appliances vs placebo on the AHI.
| Data | Trials | Effect | 95% CI | p | 95% PrI | τ2 | I2 (%) | |
| Meta-analysis | MD | 12 | –11.69 | [–15.38; –8.01] | <0.001 | –22.55; –0.85 | 20.15 | 93.6 |
| Factor | Data | Trials | Effect | 95% CI | p | τ2 | I2 (%) | |
| Follow-up (months) | Coefficient | 11 | 0.25 | [–0.24; 0.75] | 0.273 | 21.23 | 94.7 | |
| Constant | –14.40 | [–21.20; –7.61] | 0.001 | |||||
| % of maximum protrusion |
Coefficient | 8 | 0.03 | [–0.42; 0.48] | 0.87 | 34.21 | 96.3 | |
| Constant | –14.18 | [–48.70; 20.34] | 0.354 | |||||
| Appliance type (thermoplastic vs impression-based) |
Coefficient | 13 | 6.25 | [–3.73; 16.24] | 0.196 | 17.12 | 89.4 | |
| Constant | –12.17 | [–15.68; 8.66] | <0.001 | |||||
| Appliance type (1- or 2-piece) | Coefficient | 9 | –0.09 | [–11.25; 11.08] | 0.986 | 15.79 | 87.9 | |
| Constant | –14.24 | [–24.81; –4.30] | 0.012 | |||||
| Baseline-BMI (kg/m2) | Coefficient | 12 | 1.94 | [–0.84; 4.72] | 0.152 | 17.42 | 88.7 | |
| Constant | –68.59 | [–150.39; 13.21] | 0.091 | |||||
| Baseline AHI (events/hour) |
Coefficient | 11 | –0.53 | [–0.72; –0.34] | <0.001 | 2.84 | 46.3 | |
| Constant | 1.17 | [–3.64; 5.99] | 0.595 | |||||
| Baseline age (years) | Coefficient | 12 | 0.44 | [–1.05; 1.93] | 0.524 | 21.46 | 91.3 | |
| Constant | –33.59 | [–107.42; 40.25] | 0.335 | |||||
| Ratio of male patients | Coefficient | 12 | –28.65 | [–78.34; 21.05] | 0.228 | 18.8 | 93.7 | |
| Constant | 10.39 | [–28.05; 48.82] | 0.561 | |||||
| Total sample | Coefficient | 12 | 0.14 | [0.04; 0.24] | 0.01 | 9.78 | 73.7 | |
| Constant | –19.13 | [–25.29; –12.98] | <0.001 | |||||
| Study design (crossover or parallel) |
Coefficient | 12 | –0.52 | [–8.85; 7.81] | 0.892 | 22.5 | 94.2 | |
| Constant | –11.38 | [–18.25; 4.51] | 0.004 | |||||
| Data type 1 | Coefficient | 12 | –1.20 | [–10.98; 8.58] | 0.79 | 22.39 | 94.2 | |
| Constant | –11.49 | [–15.81; –7.17] | <0.001 | |||||
| Data type 2 | Coefficient | 12 | 8.07 | [–1.72; 17.86] | 0.096 | 15.9 | 89.2 | |
| Constant | –12.77 | [–16.47; –9.07] | <0.001 | |||||
| Data type 1 | Coefficient | 12 | 0.22 | [–9.36; 9.79] | 0.274 | 18.15 | 90.2 | |
| Data type 2 | Coefficient | 8.12 | [–2.63; 18.88] | |||||
| Constant | –12.82 | [–17.25; –8.39 | <0.001 | |||||
| Data | Trials | Effect | 95% CI | p | ||||
| Reporting bias (Egger’s test) |
Coefficient | 12 | –1.24 | [–5.35; 2.87] | 0.516 |
CI. confidence interval; PrI. predictive interval; MD. mean difference; BMI. body mass index; Data type 1. origin of data used in the analysis 1 (increment calculated from parallel or cross-over trials); Data type 2. origin of data used in the analysis 2 (increment calculated from final values of cross-over trials)
eTable 5. Overall results for the comparison of intraoral appliances vs placebo on minimum oxygen saturation.
| Data | Trials | Effect | 95% CI | p | 95% PrI | τ2 | I2 (%) | |
| Meta-analysis | MD | 6 | 3.33 | [1.38; 5.28] | 0.007 | –1.62; 8.28 | 2.19 | 96.8 |
| Factor | Data | Trials | Effect | 95% CI | p | τ2 | I2 (%) | |
| Follow-up (months) | Coefficient | 5 | –0.37 | [–0.61; –0.14] | 0.015 | 0.26 | 45.6 | |
| Constant | 7.2 | [4.69; 9.70] | 0.003 | |||||
| % of maximum protrusion | Coefficient | NA | ||||||
| Constant | ||||||||
| Appliance type (1- or 2-piece) | Coefficient | 5 | 2.19 | [–13.13; 17.51] | 0.68 | 3.22 | 92 | |
| Constant | 1.5 | [–38.15; 57.79] | 0.77 | |||||
| Baseline BMI (kg/m2) | Coefficient | 6 | –0.22 | [–1.88; 1.43] | 0.725 | 2.7 | 92 | |
| Constant | 9.82 | [–38.15; 57.79] | 0.6 | |||||
| Baseline AHI (events/hour) |
NA | |||||||
| Baseline age (years) | Coefficient | 6 | –0.27 | [–1.14; 0.60] | 0.129 | 1.35 | 94.9 | |
| Constant | 16.42 | [–25.56; 58.39] | 0.2 | |||||
| Ratio of male patients | Coefficient | 6 | 22.38 | [–10.19; 54.96] | 0.129 | 1.35 | 94.9 | |
| Constant | –14.03 | [–39.43; 11.37] | 0.2 | |||||
| Total sample | Coefficient | 6 | –0.05 | [–0.11; 0.00] | 0.06 | 0.9 | 51.5 | |
| Constant | 6.48 | [2.91; 10.05] | 0.007 | |||||
| Study design (crossover or parallel) |
Coefficient | 6 | 1.82 | [–3.34; 6.97] | 0.383 | 2.21 | 97.4 | |
| Constant | 1.9 | [–2.67; 6.47] | 0.313 | |||||
| Data type 1 | Coefficient | NA | ||||||
| Constant | ||||||||
| Data type 2 | Coefficient | 6 | –1.87 | [–14.97; 11.22] | 0.712 | 2.69 | 97.4 | |
| Constant | 3.37 | [1.04; 5.70] | 0.016 | |||||
| Data type 1 | Coefficient | NA | ||||||
| Data type 2 | Coefficient | |||||||
| Constant | ||||||||
| Data | Trials | Effect | 95% CI | p | ||||
| Reporting bias (Egger’s test) |
Coefficient | NA |
CI. confidence interval; PrI. predictive interval; MD. mean difference; NA. not applicable; BMI. body mass index; AHI. apnea hypopnea index; Data type 1. origin of data used in the analysis 1 (increment calculated from parallel or cross-over trials); Data type 2. origin of data used in the analysis 2 (increment calculated from final values of cross-over trials).
eTable 6. Overall results for the comparison of intraoral appliances vs placebo on the Epworth Sleepiness Scale.
| Data | Trials | Effect | 95% CI | p | 95% PrI | τ2 | I2 (%) | |
| Meta-analysis | MD | 11 | –1.18 | [–2.38; 0.03] | 0.055 | –4.76; 2.40 | 2.12 | 60.6 |
| Factor | Data | Trials | Effect | 95% CI | p | τ2 | I2 (%) | |
| Follow-up (months) | Coefficient | 10 | 0.04 | [–0.21; 0.29] | 0.716 | 3.4 | 67.2 | |
| Constant | –1.56 | [–3.95; 0.83] | 0.17 | |||||
| % of maximum protrusion | Coefficient | 7 | –0.00 | [–0.13; 0.12] | 0.95 | 3.36 | 66.4 | |
| Constant | –1.48 | [–10.38; 7.42] | 0.686 | |||||
| Appliance type (thermoplastic or tongue suction versus impression-based) |
Coefficient (thermoplast) |
10 | –0.83 | [–4.38; 2.72] | 0.43 | 2.23 | 52.4 | |
| Coefficient (tongue suction) |
2.22 | [–2.34; 6.78] | ||||||
| Constant | –1.62 | [–3.31; 0.08] | 0.059 | |||||
| Appliance type (1- or 2-piece) | Coefficient | 8 | 1.57 | [–2.94; 6.07] | 0.428 | |||
| Constant | –1.87 | [–4.39; 0.65] | 0.12 | |||||
| Baseline BMI (kg/m2) | Coefficient | 11 | –0.21 | [–1.30; 0.88] | 0.671 | 2.6 | 56.5 | |
| Constant | 5.04 | [–27.15; 37.23] | 0.731 | |||||
| Baseline AHI (events/hour) |
Coefficient | 9 | –0.03 | [–0.16; 0.10] | 0.59 | 2.6 | 56.5 | |
| Constant | –0.50 | [–3.89; 2.88] | 0.735 | |||||
| Baseline age (years) | Coefficient | 11 | –0.28 | [–0.65; 0.09] | 0.119 | 1.29 | 40.9 | |
| Constant | 12.44 | [–5.41; 30.29] | 0.149 | |||||
| Ratio of male patients | Coefficient | 11 | –15.22 | [–33.47; 3.02] | 0.092 | 1.11 | 60.1 | |
| Constant | 10.78 | [–3.50; 25.05] | 0.122 | |||||
| Total sample | Coefficient | 11 | 0.01 | [–0.05; 0.06] | 0.839 | 2.7 | 58.3 | |
| Constant | –1.47 | [–4.57; 1.63] | 0.312 | |||||
| Study design (crossover or parallel) |
Coefficient | 11 | 1.87 | [–0.76; 4.50] | 0.141 | |||
| Constant | –2.63 | [–4.97; –0.28] | 0.032 | |||||
| Data type 1 | Coefficient | 11 | –1.87 | [–4.50; 0.76] | 0.141 | 1.42 | 60.3 | |
| Constant | –0.75 | [–1.93; 0.43] | 0.185 | |||||
| Data type 2 | Coefficient | 11 | –0.93 | [–4.88; 3.03] | 0.609 | 2.55 | 48.2 | |
| Constant | –1.08 | [–2.49; 0.32] | 0.115 | |||||
| Data type 1 | Coefficient | 11 | –2.12 | [–4.80; 0.55] | 0.197 | 1.27 | 41.6 | |
| Data type 2 | Coefficient | –1.52 | [–4.53; 1.49] | |||||
| Constant | –0.49 | [–1.76; 0.79] | 0.405 | |||||
| Data | Trials | Effect | 95% CI | p | ||||
| Reporting bias (Egger’s test) |
Coefficient | 11 | –0.26 | [–2.08; 1.56] | 0.752 |
CI, confidence interval; PrI, predictive interval; BMI, body mass index; AHI, apnea hypopnea index; Data type 1, origin of data used in the analysis 1 (increment calculated from parallel or crossover trials); Data type 2, origin of data used in the analysis 2 (increment calculated from final values of cross-over trials).
Although RME, SARME, and MMA in the treatment of OSA are not supported by robust evidence, the existing literature points to their possible effectiveness. In a randomized crossover trial comparing RME with adenotonsillectomy in children, AHI was reduced after RME in the first arm of the trial (e50). This reduction remained stable after 36 months (e51). In adults, SARME with mini-implants reduced AHI postoperatively (e52). Finally, although existing literature on MMA is mainly comprised by inappropriate study designs, MMA seems to be as effective as CPAP, with AHI pre- and postoperatively comparable to AHI pre- and post-CPAP (e53).
Strengths and limitations
The present umbrella review was based on robust methodology (e42, e49) that was set out a priori and registered in PROSPERO (e54).
Study selection and data extraction were performed on the level of the primary studies included in the identified systematic reviews. As a result, not only was the accuracy of extracted data ensured, but also only data from randomized trials were pooled (since no prospective non-randomized studies were found).
The Paule–Mandel estimator of random-effects model was used as it outperforms the DerSimonian–Laird variance estimator (e4).
The robustness of existing cumulative meta-evidence was judged on the basis of valid protocols of epidemiological strength (e42, e49).
On the other hand, several limitations should be considered in the interpretation of our findings:
We might have missed some individual studies, if these had not been identified and included in the original systematic reviews.
Most of the included systematic reviews had serious methodological inadequacies.
Funnel plot asymmetry was consistently investigated with Egger’s test for all meta-analyses, following the practice used in previous umbrella reviews (e42, e49, e55), although less than 10 trials were included in every case (e9).
Supplementary Material
eMETHODS
Eligibility criteria, study identification and selection
No limitations on publication date, language, and status were applied to our searches. Additional hand searches of the reference lists of eligible articles were undertaken. Finally, two electronic databases (Google Scholar and PROSPERO) were manually searched for additional systematic reviews and / or protocols.
Eligibility of systematic reviews was assessed on the basis of title, abstract, and full text. Authors were contacted whenever a full text could not be found. Study identification and selection were performed by one reviewer (VK) with a subsequent independent duplicate check by a second reviewer (SNP). Disagreements were resolved by discussion with a third party (TE).
For adults, OSA was defined as having an apnea-hypopnea index (AHI) of at least five episodes per hour of sleep or a respiratory disturbance index of at least five episodes per hour of sleep (34). For children, OSA was defined as having an AHI of at least one episode per hour of sleep (35, 36). Primary trials included in systematic reviews had to be randomized clinical trials or prospective non-randomized controlled trials from any type of clinical setting. We excluded studies with retrospective or historical-experimental control groups as they are associated with bias (37, 38).
Data extraction
For each included systematic review, we recorded whether or not their primary studies had been assessed for risk of bias and whether the quality of evidence had been assessed according to the GRADE approach (39). However, we did not perform these procedures ourselves, as this task was beyond the scope of this umbrella review of systematic reviews. The methodological quality of the included systematic reviews was further appraised using the AMSTAR tool (40).
From each eligible meta-analysis, the same two authors (VK and SNP) independently extracted information on first author, year of publication, outcome(s) examined, number of included primary studies, and reported data at the individual trial level. Eligible outcomes were extracted in either continuous or binary format. In order to deal with study overlaps across the included meta-analyses, the PubMed Unique Identifier (PMID) was used to characterize each trial included in the meta-analyses. All available trials were pooled according to PMID.
After checking for design suitability and lack or proper handling by the trialists of carry-over effects (31, e1), we decided to include and combine both parallel and crossover randomized trials, approximating a paired analysis for the latter. We extracted for each outcome the absolute increment of change through treatment as final minus baseline value. In case this was not reported in the original trials, we calculated this ourselves using appropriate methods for paired data (31), using a pre–post correlation of 0.5 from existing data (e2). For crossover trials, we additionally calculated the effect size by subtracting the increment of change from baseline of each trial arm using a similar paired approach (e1) and a correlation of 0.25, again, from existing data (e2).
Assessment of pooled effects and heterogeneity
A random-effects synthesis makes the assumption that individual studies are estimating different effects, which are assumed to have a normal distribution. For our umbrella review, we performed a random-effects meta-analysis to estimate the mean of this distribution of effects across different studies and the uncertainty about that mean (95% confidence interval [CI]). Cut-offs of one half, one, and two standard deviations of the response in the control group (taken from the largest included trials) were used to augment all forest plots with contours of effect magnitude.
We assessed heterogeneity between studies by calculating both the variance among effect sizes (tau2) and the I2 metric of inconsistency, which reflects the proportion of total variability in the results explained by heterogeneity and not by chance (e5). The I2 metric ranges between 0% and 100%, and is the ratio of variance between studies over the sum of variances within and between studies. We also assessed descriptively whether heterogeneity would influence the direction or only magnitude of effects (i.e. if pooled studies lay on both sides or on only one side of the forest plot, respectively) (e6). Finally, we calculated the 95% prediction interval (PrI) for the pooled random-effects estimates, which further accounts for heterogeneity between studies and indicates the uncertainty for the effect that would be expected in a new study with a similar design/setting examining that same association (e7).
Predefined mixed-effects subgroup analyses and random-effects meta-regressions were performed for the following factors: baseline patient characteristics (including mean age, ratio of male patients, mean AHI, mean BMI, and sample size), follow-up time, % of maximum protrusion in the construction of intraoral appliances (IOA), appliance category (custom IOA based on impressions, pre-fabricated IOA, tongue suction IOA), and appliance type (1- or 2-piece). Sensitivity analyses were performed to assess any systematic differences according to study design (parallel or crossover) and data calculations done for this umbrella review.
Assessment of small-study effects
We examined whether there were indications pointing to small-study effects—that is, if small studies tended to give higher estimates than large studies. Small-study effects can indicate publication bias or other reporting biases, but they may also reflect genuine heterogeneity, chance, or other reasons for differences between small and large studies (e8). We used the regression asymmetry test proposed by Egger et al. (e9) to investigate funnel plot asymmetry in meta-analyses of at least 10 studies (e8).
Epidemiological criteria for the robustness of associations
We further assessed which random-effects meta-analyses with statistically significant estimates fulfilled the following epidemiological criteria for robust associations:
All p-values were two-sided, while statistical significance was set at 5% for all tests, except for heterogeneity and Egger’s tests (10%). Although multiple p-values are reported in this umbrella review, the significance level was not adjusted, as we aimed to summarize the results of the included papers.
p<0.001, a threshold that has been suggested to reduce the number of false-positive findings (e10)
Associations based on adequate sample size (>500 patients)
Associations without large heterogeneity between studies (I2<75%) potentially affecting the direction of estimates
Their 95% PrI excluded the null value
No evidence of small-study effects (according to the Egger’s test).
The clinical perspective.
Intraoral appliances (IOA) for the treatment of obstructive sleep apnea (OSA) work mechanically, by holding and stabilizing the mandible in a forward position and thus increasing the upper airway dimensions. Custom IOA based on dental impressions are believed to be more effective than pre-fabricated ones and cooperation with a qualified dentist / orthodontist is desired. IOA are usually used in patients with mild to moderate OSA and those with severe OSA who cannot tolerate continuous positive airway pressure.
Rapid maxillary expansion to treat maxillary constriction can be performed in growing children by orthodontists. It opens the midpalatal suture and leads to a transverse expansion of the maxilla. Apart from the benefits of a balanced occlusion, this increases the nasopharyngeal airway and reduces nasal resistance facilitating nasal breathing. It is also believed to lead to anterior repositioning of the tongue.
Surgically assisted rapid maxillary expansion to treat maxillary constriction is performed in adults by maxillofacial surgeons and orthodontists cooperatively. In adults, due to the ossification of the midpalatal suture, maxillary expansion needs to be surgically assisted in order to obtain maximal skeletal changes, as it will otherwise lead to dentoalveolar compensation (tipping of teeth). Adults enjoy the same treatment benefits as children.
Maxillomandibular advancement (MMA) is performed in adults by maxillofacial surgeons in cooperation with orthodontists. It advances the maxillomandibular complex in the sagittal plane, thereby increasing the dimensions of the upper airway and the tone of the pharyngeal muscles. MMA is not a routine treatment in OSA and has to be anatomically indicated and carefully planned. Cephalometric analyses provide crucial help in identifying the anatomical site(s) of obstruction and in defining the limits of advancement to preserve facial aesthetics postoperatively.
Figure 2.
Forest plot providing an overview of included meta-analyses and their results
MD, mean difference; CI, confidence interval; AHI, apnea hypopnea index; IOA, intraoral appliance; IOA1 vs IOA2, intraoral appliance (4 mm opening) versus intraoral appliance (14 mm opening); ESS, Epworth Sleepiness Scale
Key messages.
According to the criteria for robust epidemiological associations, intraoral appliances (IOA) are effective in reducing apnea hypopnea index (AHI) in adult patients with obstructive sleep apnea (OSA) and this cannot be explained by placebo effects.
The epidemiological evidence for the effect of IOA on Epworth Sleepiness Scale scores and minimum oxygen saturation in adult OSA patients was poor.
There is no robust scientific evidence to support treatment of OSA patients with rapid maxillary expansion (RME), surgically assisted rapid maxillary expansion (SARME), or surgical maxillomandibular advancement (MMA).
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86:549–554. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobrowolska-Zarzycka M, Dunin-Wilczynska I, Szymanska J. Craniofacial structure in patients with obstructive sleep apnoea. Folia Morphol (Warsz) 2016;75:311–315. doi: 10.5603/FM.a2016.0003. [DOI] [PubMed] [Google Scholar]
- 5.Lee RWW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in caucasian and chinese patients with obstructive sleep apnea. Sleep. 2010;33:1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 9.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 10.Carskadon MA, Bearpark HM, Sharkey KM, et al. Effects of menopause and nasal occlusion on breathing during sleep. Am J Respir Crit Care Med. 1997;155:205–210. doi: 10.1164/ajrccm.155.1.9001313. [DOI] [PubMed] [Google Scholar]
- 11.McNicholas WT, Tarlo S, Cole P, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982;126:625–628. doi: 10.1164/arrd.1982.126.4.625. [DOI] [PubMed] [Google Scholar]
- 12.Kashyap R, Hock LM, Bowman TJ. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 2001;5:167–172. doi: 10.1007/s11325-001-0167-5. [DOI] [PubMed] [Google Scholar]
- 13.Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–2224. [PubMed] [Google Scholar]
- 14.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathur R, Douglas NJ. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med. 1995;122:174–178. doi: 10.7326/0003-4819-122-3-199502010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Carmelli D, Colrain IM, Swan GE, Bliwise DL. Genetic and environmental influences in sleep-disordered breathing in older male twins. Sleep. 2004;27:917–922. doi: 10.1093/sleep/27.5.917. [DOI] [PubMed] [Google Scholar]
- 17.Patel SR, Larkin EK, Redline S. Shared genetic basis for obstructive sleep apnea and adiposity measures. Int J Obes (Lond) 2008;32:795–800. doi: 10.1038/sj.ijo.0803803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–379. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- 19.Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28:472–477. doi: 10.1093/sleep/28.4.472. [DOI] [PubMed] [Google Scholar]
- 20.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–956. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 21.Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome. Arch Intern Med. 1992;152:538–541. [PubMed] [Google Scholar]
- 22.Naëgelé B, Thouvard V, Pépin JL, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18:43–52. [PubMed] [Google Scholar]
- 23.Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 24.Deacon NL, Jen R, Li Y, Malhotra A. Treatment of obstructive sleep apnea Prospects for personalized combined modality therapy. Ann Am Thorac Soc. 2016;13:101–108. doi: 10.1513/AnnalsATS.201508-537FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommer JU, Heiser C, Gahleitner C, et al. Tonsillectomy with uvulopalatopharyngoplasty in obstructive sleep apnea. Dtsch Arztebl Int. 2016;113:1–8. doi: 10.3238/arztebl.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichenberger M, Baumgartner S. The impact of rapid palatal expansion on children’s general health: a literature review. Eur J Paediatr Dent. 2014;15:67–71. [PubMed] [Google Scholar]
- 27.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280–2293. doi: 10.1001/jama.2015.16303. [DOI] [PubMed] [Google Scholar]
- 28.Iftikhar IH, Bittencourt L, Youngstedt SD, et al. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med. 2017;30:7–14. doi: 10.1016/j.sleep.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Hochban W, Brandenburg U, Peter JH. Surgical treatment of obstructive sleep apnea by maxillomandibular advancement. Sleep. 1994;17:624–629. doi: 10.1093/sleep/17.7.624. [DOI] [PubMed] [Google Scholar]
- 30.Deutsche Gesellschaft für Schlafforschung und Schlafmedizin (DGSM) S3-Leitlinie Nicht erholsamer Schlaf/Schlafstörungen - Kapitel „Schlafbezogene Atmungsstörungen“. Somnologie. 2017;20:97–180. [Google Scholar]
- 31.Higgins JPT, Green S, Cochrane Colloboration. Cochrane handbook for systematic reviews of interventions. www.handbook.cochrane.org/ (last accessed on 30 March. 2017) [Google Scholar]
- 32.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13:132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 34.Flemons WW, Buysse D, Redline S, et al. Sleep-related breathing disorders in adults. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 35.Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4:393–406. doi: 10.1016/j.jsmc.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–878. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 37.Papageorgiou SN, Xavier GM, Cobourne MT. Basic study design influences the results of orthodontic clinical investigations. J Clin Epidemiol. 2015;68:1512–1522. doi: 10.1016/j.jclinepi.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Papageorgiou SN, Koretsi V, Jäger A. Bias from historical control groups used in orthodontic research: a meta-epidemiological study. Eur J Orthod. 2017;39:98–105. doi: 10.1093/ejo/cjw035. [DOI] [PubMed] [Google Scholar]
- 39.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7 doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- E2.de Britto Teixeira AO, Abi-Ramia LBP, de Oliveira Almeida MA. Treatment of obstructive sleep apnea with oral appliances. Prog Orthod. 2013;14 doi: 10.1186/2196-1042-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Papageorgiou SN. Meta-analysis for orthodontists: part I—how to choose effect measure and statistical model. J Orthod. 2014;41:317–326. doi: 10.1179/1465313314Y.0000000111. [DOI] [PubMed] [Google Scholar]
- E4.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79. doi: 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- E6.Papageorgiou SN. Meta-analysis for orthodontists: part II—is all that glitters gold? J Orthod. 2014;41:327–336. doi: 10.1179/1465313314Y.0000000110. [DOI] [PubMed] [Google Scholar]
- E7.IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010247. e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4002. d4002. [DOI] [PubMed] [Google Scholar]
- E9.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Johnson VE. Revised standards for statistical evidence. Proc Natl Acad Sci U S A. 2013;110:19313–19317. doi: 10.1073/pnas.1313476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Abdullatif J, Certal V, Zaghi S, et al. Maxillary expansion and maxillomandibular expansion for adult OSA: a systematic review and meta-analysis. J Craniomaxillofac Surg. 2016;44:574–578. doi: 10.1016/j.jcms.2016.02.001. [DOI] [PubMed] [Google Scholar]
- E12.Ahrens A, McGrath C, Hägg U. A systematic review of the efficacy of oral appliance design in the management of obstructive sleep apnoea. Eur J Orthod. 2011;33:318–324. doi: 10.1093/ejo/cjq079. [DOI] [PubMed] [Google Scholar]
- E13.Bartolucci ML, Bortolotti F, Raffaelli E, D’Antò V, Michelotti A, Alessandri Bonetti G. The effectiveness of different mandibular advancement amounts in OSA patients: a systematic review and meta-regression analysis. Sleep Breath. 2016;20:911–919. doi: 10.1007/s11325-015-1307-7. [DOI] [PubMed] [Google Scholar]
- E14.Bratton DJ, Gaisl T, Schlatzer C, Kohler M. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869–878. doi: 10.1016/S2213-2600(15)00416-6. [DOI] [PubMed] [Google Scholar]
- E15.Bridgman S, Dunn K. Systematic review of the surgical treatment of obstructive sleep apnoea. J Clin Excell. 2000;1:223–226. [Google Scholar]
- E16.Caldas SGFR, Ribeiro AA, Santos-Pinto LAM dos, Martins LP, Matoso RM. Efetividade dos aparelhos intrabucais de avanço mandibular no tratamento do ronco e da síndrome da apneia e hipopneia obstrutiva do sono (SAHOS): revisão sistemática. Rev Dent Press Ortodon Ortop Facial. 2009;14:74–82. [Google Scholar]
- E17.Camacho M, Teixeira J, Abdullatif J, et al. Maxillomandibular advancement and tracheostomy for morbidly obese obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;152:619–630. doi: 10.1177/0194599814568284. [DOI] [PubMed] [Google Scholar]
- E18.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–1407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E19.Carvalho FR, Lentini-Oliveira D, Machado Ma. C, Prado GF, Prado LBF, Saconato H. Oral appliances and functional orthopaedic appliances for obstructive sleep apnoea in children. Cochrane Database Syst Rev. 2007;10 doi: 10.1002/14651858.CD005520.pub2. CD005520. [DOI] [PubMed] [Google Scholar]
- E20.Carvalho FR, Lentini-Oliveira DA, Prado LB, Prado GF, Carvalho LB. Oral appliances and functional orthopaedic appliances for obstructive sleep apnoea in children. Cochrane Database Syst Rev. 2016;10 doi: 10.1002/14651858.CD005520.pub3. CD005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Health Quality Ontario. Oral appliances for obstructive sleep apnea: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9:1–51. [PMC free article] [PubMed] [Google Scholar]
- E22.Hoekema A, Stegenga B, De Bont LGM. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: a systematic review. Crit Rev Oral Biol Med. 2004;15:137–155. doi: 10.1177/154411130401500303. [DOI] [PubMed] [Google Scholar]
- E23.Holty JEC, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010;14:287–297. doi: 10.1016/j.smrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- E24.Hsieh YJ, Liao YF. Effects of maxillomandibular advancement on the upper airway and surrounding structures in patients with obstructive sleep apnoea: a systematic review. Br J Oral Maxillofac Surg. 2013;51:834–840. doi: 10.1016/j.bjoms.2012.11.010. [DOI] [PubMed] [Google Scholar]
- E25.Huynh NT, Desplats E, Almeida FR. Orthodontics treatments for managing obstructive sleep apnea syndrome in children: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:84–94. doi: 10.1016/j.smrv.2015.02.002. [DOI] [PubMed] [Google Scholar]
- E26.Knudsen TB, Laulund AS, Ingerslev J, Homøe P, Pinholt EM. Improved apnea-hypopnea index and lowest oxygen saturation after maxillomandibular advancement with or without counterclockwise rotation in patients with obstructive sleep apnea: a meta-analysis. J Oral Maxillofac Surg. 2015;73:719–726. doi: 10.1016/j.joms.2014.08.006. [DOI] [PubMed] [Google Scholar]
- E27.Li W, Xiao L, Hu J. The comparison of CPAP and oral appliances in treatment of patients with OSA: a systematic review and meta-analysis. Respir Care. 2013;58:1184–1195. doi: 10.4187/respcare.02245. [DOI] [PubMed] [Google Scholar]
- E28.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2004;4 doi: 10.1002/14651858.CD004435.pub2. CD004435. [DOI] [PubMed] [Google Scholar]
- E29.Machado-Júnior AJ, Zancanella E, Crespo AN. Rapid maxillary expansion and obstructive sleep apnea: a review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2016;21:e465–e469. doi: 10.4317/medoral.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E30.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- E31.Marklund M, Verbraecken J, Randerath W. Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J. 2012;39:1241–1247. doi: 10.1183/09031936.00144711. [DOI] [PubMed] [Google Scholar]
- E32.Nazarali N, Altalibi M, Nazarali S, Major MP, Flores-Mir C, Major PW. Mandibular advancement appliances for the treatment of paediatric obstructive sleep apnea: a systematic review. Eur J Orthod. 2015;37:618–626. doi: 10.1093/ejo/cju101. [DOI] [PubMed] [Google Scholar]
- E33.Okuno K, Sato K, Arisaka T, et al. The effect of oral appliances that advanced the mandible forward and limited mouth opening in patients with obstructive sleep apnea: a systematic review and meta-analysis of randomised controlled trials. J Oral Rehabil. 2014;41:542–554. doi: 10.1111/joor.12162. [DOI] [PubMed] [Google Scholar]
- E34.Pirklbauer K, Russmueller G, Stiebellehner L, et al. Maxilloman-dibular advancement for treatment of obstructive sleep apnea syndrome: a systematic review. J Oral Maxillofac Surg. 2011;69:e165–e176. doi: 10.1016/j.joms.2011.01.038. [DOI] [PubMed] [Google Scholar]
- E35.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11:773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E36.Serra-Torres S, Bellot-Arcís C, Montiel-Company JM, Marco-Algarra J, Almerich-Silla JM. Effectiveness of mandibular advancement appliances in treating obstructive sleep apnea syndrome: a systematic review. Laryngoscope. 2016;126:507–514. doi: 10.1002/lary.25505. [DOI] [PubMed] [Google Scholar]
- E37.Sharples LD, Clutterbuck-James AL, Glover MJ, et al. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev. 2016;27:108–124. doi: 10.1016/j.smrv.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- E39.Zaghi S, Holty JEC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2016;142:58–66. doi: 10.1001/jamaoto.2015.2678. [DOI] [PubMed] [Google Scholar]
- E40.Zhu Y, Long H, Jian F, et al. The effectiveness of oral appliances for obstructive sleep apnea syndrome: a meta-analysis. J Dent. 2015;43:1394–1402. doi: 10.1016/j.jdent.2015.10.008. [DOI] [PubMed] [Google Scholar]
- E41.Sindhu F, Carpenter L, Seers K. Development of a tool to rate the quality assessment of randomized controlled trials using a delphi technique. J Adv Nurs. 1997;25:1262–1268. doi: 10.1046/j.1365-2648.1997.19970251262.x. [DOI] [PubMed] [Google Scholar]
- E42.Belbasis L, Bellou V, Evangelou E, Ioannidis JPA, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14:263–273. doi: 10.1016/S1474-4422(14)70267-4. [DOI] [PubMed] [Google Scholar]
- E43.Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx. Thorax. 1999;54:972–977. doi: 10.1136/thx.54.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E44.Villa MP, Bernkopf E, Pagani J, Broia V, Montesano M, Ronchetti R. Randomized controlled study of an oral jaw-positioning appliance for the treatment of obstructive sleep apnea in children with malocclu-sion. Am J Respir Crit Care Med. 2002;165:123–127. doi: 10.1164/ajrccm.165.1.2011031. [DOI] [PubMed] [Google Scholar]
- E45.Cozza P, Gatto R, Ballanti F, Prete L. Management of obstructive sleep apnoea in children with modified monobloc appliances. Eur J Paediatr Den. 2004;5:24–29. [PubMed] [Google Scholar]
- E46.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350 doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- E47.Randerath WJ, Heise M, Hinz R, Ruehle KH. An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest. 2002;122:569–575. doi: 10.1378/chest.122.2.569. [DOI] [PubMed] [Google Scholar]
- E48.Tan YK, L’Estrange PR, Luo YM, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod. 2002;24:239–249. doi: 10.1093/ejo/24.3.239. [DOI] [PubMed] [Google Scholar]
- E49.Almeida FR, Henrich N, Marra C, et al. Patient preferences and experiences of CPAP and oral appliances for the treatment of obstructive sleep apnea: a qualitative analysis. Sleep Breath. 2013;17:659–666. doi: 10.1007/s11325-012-0739-6. [DOI] [PubMed] [Google Scholar]
- E50.Guilleminault C, Monteyrol PJ, Huynh NT, Pirelli P, Quo S, Li K. Adeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study. Sleep Breath. 2011;15:173–177. doi: 10.1007/s11325-010-0419-3. [DOI] [PubMed] [Google Scholar]
- E51.Villa MP, Rizzoli A, Miano S, Malagola C. Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up. Sleep Breath. 2011;15:179–184. doi: 10.1007/s11325-011-0505-1. [DOI] [PubMed] [Google Scholar]
- E52.Liu SY, Guilleminault C, Huon LK, Yoon A. Distraction Osteogenesis Maxillary Expansion (DOME) for adult obstructive sleep apnea patients with high arched palate. Otolaryngol Head Neck Surg. 2017;157:345–348. doi: 10.1177/0194599817707168. [DOI] [PubMed] [Google Scholar]
- E53.Vicini C, Dallan I, Campanini A, et al. Surgery vs ventilation in adult severe obstructive sleep apnea syndrome. Am J Otolaryngol. 2010;31:14–20. doi: 10.1016/j.amjoto.2008.09.002. [DOI] [PubMed] [Google Scholar]
- E54.Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1 doi: 10.1186/2046-4053-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E55.Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78:180–190. doi: 10.1055/s-0036-1592158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMETHODS
Eligibility criteria, study identification and selection
No limitations on publication date, language, and status were applied to our searches. Additional hand searches of the reference lists of eligible articles were undertaken. Finally, two electronic databases (Google Scholar and PROSPERO) were manually searched for additional systematic reviews and / or protocols.
Eligibility of systematic reviews was assessed on the basis of title, abstract, and full text. Authors were contacted whenever a full text could not be found. Study identification and selection were performed by one reviewer (VK) with a subsequent independent duplicate check by a second reviewer (SNP). Disagreements were resolved by discussion with a third party (TE).
For adults, OSA was defined as having an apnea-hypopnea index (AHI) of at least five episodes per hour of sleep or a respiratory disturbance index of at least five episodes per hour of sleep (34). For children, OSA was defined as having an AHI of at least one episode per hour of sleep (35, 36). Primary trials included in systematic reviews had to be randomized clinical trials or prospective non-randomized controlled trials from any type of clinical setting. We excluded studies with retrospective or historical-experimental control groups as they are associated with bias (37, 38).
Data extraction
For each included systematic review, we recorded whether or not their primary studies had been assessed for risk of bias and whether the quality of evidence had been assessed according to the GRADE approach (39). However, we did not perform these procedures ourselves, as this task was beyond the scope of this umbrella review of systematic reviews. The methodological quality of the included systematic reviews was further appraised using the AMSTAR tool (40).
From each eligible meta-analysis, the same two authors (VK and SNP) independently extracted information on first author, year of publication, outcome(s) examined, number of included primary studies, and reported data at the individual trial level. Eligible outcomes were extracted in either continuous or binary format. In order to deal with study overlaps across the included meta-analyses, the PubMed Unique Identifier (PMID) was used to characterize each trial included in the meta-analyses. All available trials were pooled according to PMID.
After checking for design suitability and lack or proper handling by the trialists of carry-over effects (31, e1), we decided to include and combine both parallel and crossover randomized trials, approximating a paired analysis for the latter. We extracted for each outcome the absolute increment of change through treatment as final minus baseline value. In case this was not reported in the original trials, we calculated this ourselves using appropriate methods for paired data (31), using a pre–post correlation of 0.5 from existing data (e2). For crossover trials, we additionally calculated the effect size by subtracting the increment of change from baseline of each trial arm using a similar paired approach (e1) and a correlation of 0.25, again, from existing data (e2).
Assessment of pooled effects and heterogeneity
A random-effects synthesis makes the assumption that individual studies are estimating different effects, which are assumed to have a normal distribution. For our umbrella review, we performed a random-effects meta-analysis to estimate the mean of this distribution of effects across different studies and the uncertainty about that mean (95% confidence interval [CI]). Cut-offs of one half, one, and two standard deviations of the response in the control group (taken from the largest included trials) were used to augment all forest plots with contours of effect magnitude.
We assessed heterogeneity between studies by calculating both the variance among effect sizes (tau2) and the I2 metric of inconsistency, which reflects the proportion of total variability in the results explained by heterogeneity and not by chance (e5). The I2 metric ranges between 0% and 100%, and is the ratio of variance between studies over the sum of variances within and between studies. We also assessed descriptively whether heterogeneity would influence the direction or only magnitude of effects (i.e. if pooled studies lay on both sides or on only one side of the forest plot, respectively) (e6). Finally, we calculated the 95% prediction interval (PrI) for the pooled random-effects estimates, which further accounts for heterogeneity between studies and indicates the uncertainty for the effect that would be expected in a new study with a similar design/setting examining that same association (e7).
Predefined mixed-effects subgroup analyses and random-effects meta-regressions were performed for the following factors: baseline patient characteristics (including mean age, ratio of male patients, mean AHI, mean BMI, and sample size), follow-up time, % of maximum protrusion in the construction of intraoral appliances (IOA), appliance category (custom IOA based on impressions, pre-fabricated IOA, tongue suction IOA), and appliance type (1- or 2-piece). Sensitivity analyses were performed to assess any systematic differences according to study design (parallel or crossover) and data calculations done for this umbrella review.
Assessment of small-study effects
We examined whether there were indications pointing to small-study effects—that is, if small studies tended to give higher estimates than large studies. Small-study effects can indicate publication bias or other reporting biases, but they may also reflect genuine heterogeneity, chance, or other reasons for differences between small and large studies (e8). We used the regression asymmetry test proposed by Egger et al. (e9) to investigate funnel plot asymmetry in meta-analyses of at least 10 studies (e8).
Epidemiological criteria for the robustness of associations
We further assessed which random-effects meta-analyses with statistically significant estimates fulfilled the following epidemiological criteria for robust associations:
All p-values were two-sided, while statistical significance was set at 5% for all tests, except for heterogeneity and Egger’s tests (10%). Although multiple p-values are reported in this umbrella review, the significance level was not adjusted, as we aimed to summarize the results of the included papers.
p<0.001, a threshold that has been suggested to reduce the number of false-positive findings (e10)
Associations based on adequate sample size (>500 patients)
Associations without large heterogeneity between studies (I2<75%) potentially affecting the direction of estimates
Their 95% PrI excluded the null value
No evidence of small-study effects (according to the Egger’s test).