Abstract
Since ancient times, opioids have been used clinically and abused recreationally. In the early stages (about 1,000 AD) of opium history, an Arab physician, Avicenna, administered opioids to control diarrhea and eye diseases.1 Opioids have very strong pain relieving properties and they also regulate numerous cellular responses. Opioid receptors are expressed throughout the body, including the nervous system, heart, lungs, liver, gastrointestinal tract, and retina.2–6 Delta opioid receptors (DORs) are a very attractive target from the perspective of both receptor function and their therapeutic potential. Due to a rapid progress in mouse mutagenesis and development of small molecules as DOR agonist, novel functions and roles of DORs have emerged in recent years. This review article focuses on the recent advances in the neuroprotective roles of DOR agonists in general and retina neuroprotection in particular. Rather than being exhaustive, this review highlights the selected studies of DOR function in neuroprotection. We also highlight our preclinical studies using rodent models to demonstrate the potentials of DOR agonists for retinal neuroprotection. Based on existing literature and our recently published data on the eye, DOR agonists possess therapeutic abilities that protect the retina and optic nerve injury against glaucoma and perhaps other retinopathies as well. This review also highlights the signaling events associated with DOR for neuroprotection in the eye. There is a need for translational research on DORs to recognize their potential for clinical application such as in glaucoma.
Keywords: : opioid receptors, glaucoma pharmacology, retinal neuroprotection
Opioid Receptors and Endogenous Ligands
It is difficult to find when and where the opium poppy was used first because description of drugs by ancient authors is often ambiguous. History shows the use of opium in the ancient Sumerian civilization, nearly 5,000 years ago when this drug was often used for recreational purposes. However, the drug's earliest use was primarily linked to religion and mysticism. The diverse biological effects of opioids, including cytoprotection, analgesia, neuroendocrine regulation, immunomodulation, behavioral modifications, and neuroprotection, are manifested through three classes of transmembrane G-protein-coupled opioid receptors (e.g., μ, κ, and δ), distributed throughout the body. In general, the opioid receptors, on binding of exogenous opioid drugs or endogenous opioid peptides, regulate a multitude of intracellular signaling pathways, including neural, endocrine, and immune responses,7–9 as well as energy conserving/protective responses such as hibernation.10 Synthetic opioids have been shown to mimic the cytoprotective effects of endogenous opioidergic ligands and the physiological effects are initiated by the activation of μ-, δ-, and κ-opioid receptors.11 Delta opioid receptor (DOR) genes have been cloned from rat12 and human13 tissues. Studies have shown the messenger RNA (mRNA) expression of DOR in the heart, lungs, adrenal glands, stomach, small and large intestine, kidney, spleen, brain, sex organs, and retina.3,6,14,15 Studies have also shown that endogenous levels of opioid peptides are increased under stress conditions,16,17 which counteract the detrimental pathways, including the production of tumor necrosis factor alpha (TNF-α),3,18 secretion of matrix metalloproteinases (MMPs),19 and activation of nuclear factor-κB (NF-κB) and p38 mitogen-activated protein (MAP) kinase.19 In contrast, reduced levels of endogenous opioids have been found in the patients suffering from depression and other stress conditions,20–23 which will reduce the activity of opioid receptors and their ability to fight against stressful conditions. Unlike other opioid receptors, DORs possess unique properties to provide beneficial effects such as antidepressant, antioxidant, and neuroprotective ability against cytotoxicity and hypoxic insult. As a result, numerous nonpeptidic DOR agonists have been developed over the last several years and it was assumed that such compounds will have antinociceptive properties like other opioids, without unwanted side effects such as respiratory depression, physical dependence, and abuse potential. Interestingly, nonpeptidic DOR agonists were found to possess less analgesic properties as measured in morphine-sensitive antinociceptive assays, and they also lacked the other side effects of opioids, including opioid-withdrawal systems.
In the eye, experimental and/or clinical evidence has suggested key roles of endogenous opioids and their receptors in the regulation of iris function, accommodative power, aqueous humor dynamics, corneal wound healing, retinal development, and retinal neuroprotection.3–5,24–28 For example, an endogenous peptide ligand for DOR, enkephalin, has been shown to be present in the rat retina.28 Although opioids have been demonstrated to alter a variety of ocular functions, the detailed mechanisms by which DOR activation elicits pharmacological actions and subsequently neuroprotection have not been clearly defined in the eye.
DORs: Antidepressant Properties
DOR agonists have been shown to provide promising results in preclinical models for depression. Depression, a mental disorder that affects more than 18 million Americans, is most often characterized by a sad or blunted mood. Selective serotonin reuptake inhibitors such as fluoxetine (Prozac) and sertraline (Zoloft) have been used to treat depression; however, the treatment has not been fully effective in the majority of cases. Therefore, researchers have been looking for alternative medications to treat depression. Limited studies performed in preclinical models found that DOR agonists are promising drugs to treat depression. For example, DOR null mice showed depression-like behavior.29 Exogenous administration of enkephalins provides antidepressant-like effects in the forced swim test.30 In addition, DOR peptides resulted in antidepressant-like effects in rodents models,23,31 and such effects were blocked by a selective DOR antagonist, naltrindole. Nonpeptide DOR agonist such as [(+)-4-[(α)-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-mthoxyphenyl)methyl]-N,N-diethylbenzamide], commonly known as SNC-80, produces antidepressant-like properties in the forced swim test models of rodents.32,33 Many other opioid ligands such as buprenorphine, oxycodone, and oxymorphone have also been suggested to have antidepressant actions in humans,21,22,34 but there are few clinical data in humans specifically linking the DOR activation with depression or antidepressant activity. Therefore, further studies are warranted to establish that DOR agonists can act as effective antidepressant drugs in humans.
Neuroprotection and Preclinical Studies in Nonocular System
In general, opioids provide neuroprotection against various forms of injuries (acute to chronic injury) in numerous organelles.4,5,35,36 Earlier studies have shown that the turtle brain has a higher density of DOR than rat's brain.37 Furthermore, turtle brain showed more resistance to hypoxic/ischemic injury compared with rat brain.38,39 These studies established an initial evidence showing a potential neuroprotective role of DOR. In addition, a growing body of evidence has demonstrated the neuroprotective effects of DOR agonists in cytotoxic, hypoxic, ischemic stress and neurodegeneration.4,36,40–45 For example, DOR activation has been shown to be involved in neuroprotection in Alzheimer's46 and Parkinson's disease,47 cardiac arrest, and stroke.48–50 Pretreatment with DOR agonist [D-Ala2, D-leu5] enkephalin attenuates hippocampal CA1 neuronal damage in response to asphyxial cardiac arrest in rats.51 DADLE ([6 D-Ala2, D-Leu5]-enkephalin) has been shown to be neuroprotective against ischemia/reperfusion injury following transient MCAO injury.48 DOR agonists have neuroprotective effects on dopamine transporters, dopamine neurons, and cortical neurons.52–54 In addition, DADLE has been shown to suppress P-53 mRNA expression, a marker for apoptosis characteristically associated with MCAO and stroke models.48–50 Another DOR agonist, SNC-80, decreases astrogliosis in the cingulate cortex in mice.55 DOR also plays key roles in maintaining K+ homeostasis in neonatal brain in response to ischemia.56 In another study, DADLE administration through the abdominal aorta provided dose-dependent protection of the spinal cord against ischemia/reperfusion-induced injury in rabbits.57 DOR agonist, BW373U86, which can cross the blood–brain barrier, significantly reduces neuronal loss in response to asphyxial cardiac arrest.58
The retina has a high metabolic activity and is highly sensitive to hypoxia.59 Intraocular pressure (IOP) impairs blood supply and leads to retinal ischemia.60 Studies have also shown that hypoxia decreases the expression pattern of DOR and activation of DOR counteracted ischemia-induced disruption of ionic homeostasis.40 DOR activation by DADLE reduced glutamate and hypoxia-induced injury effectively, and antagonist of DOR reversed the beneficial effects in neocortical neurons.41,45,54,61 In addition, hypoxic preconditioning provides neuroprotection, which is blocked by DOR antagonist.40,62 Moreover, SNC-80 promoted neuronal differentiation from neural stem cells obtained from embryonic C3H mouse forebrain.63
Neuroprotection and Preclinical Studies in the Eye
Limited data show that DOR agonists provide retinal neuroprotection in preclinical studies performed in animal models, but cellular/molecular mechanisms associated with such neuroprotection are not fully known. Pharmacological treatment of rabbits with morphine (a broad range of opioid agonists) provides retinal neuroprotection against acute ischemic injury.64 In our laboratory, we have shown the presence of functional opioid receptor subtypes in the retina, optic nerve, and optic nerve head (ONH) astrocytes.6,65 A strong immunostaining for DORs was seen in the nerve fiber layer and retinal ganglion cell (RGC) layer, whereas a diffuse staining was noticed in the inner plexiform layer.6 In addition, we have shown that activation of opioid receptors by a broad range of opioid agonists (e.g., morphine) provides retinal neuroprotection against ischemia/reperfusion and ocular hypertension-induced injury.6,65 In addition, we have shown that activation of DOR by a selective agonist, SNC-121, provides retinal neuroprotection in a chronic ocular hypertensive glaucoma rat model.3,66 IOP was raised in Brown Norway rats by injecting 2.0 M hypertonic saline into limbal veins, which is a well-established and routinely used procedure in our laboratory.3,18,66 We have seen a significant elevation in IOP as early as 7 days, posthypertonic saline injection and IOP remained significantly elevated up to 6 weeks postinjury. To determine the physiological changes in the retina, we measured pattern electroretinogram (pattern-ERG) in normal rats and ocular hypertensive rats at different time intervals. Pattern-ERG is known to be a reliable measure of RGC function. This technique has been utilized widely in rodents and humans to measure RGC function.3,18,67–69 Pattern-ERG amplitudes were significantly reduced (24%; P < 0.05) at week 6, postinjury in ocular hypertensive eyes.3 Furthermore, we determined the changes in the total number of RGCs in normal and ocular hypertensive eyes to establish a relationship between pattern-ERG and RGC numbers. RGCs were visualized by retrograde labeling with bilateral injections of Fluorogold into the superior colliculus as shown earlier in our laboratory.3,18,66 There was a clear loss of RGCs in the ocular hypertensive eye compared with the contralateral normal eye. The mean number (±standard error) of Fluorogold-positive RGCs was as follows: normal eyes, 1572 ± 6 81, and ocular hypertensive eyes, 1122 ± 6 38 (28.6% less than healthy eyes).3
To determine the effects of a selective DOR agonist, animals were treated intraperitoneally (i.p.) with SNC-121 (1 mg/kg) for 7 days, once daily. Interesting, we found that short-term (7 days) DOR agonist treatment provides a long-term RGC neuroprotection.3 More specifically, SNC-121 treatment preserved RGC function significantly as measured by pattern-ERG. We did not see any significant loss of pattern-ERG amplitudes in SNC-121-treated ocular hypertensive eyes.3 In addition, the loss of RGCs was reduced in SNC-121-treated ocular hypertensive animals. We found 28.6% less RGCs in ocular hypertensive eyes compared with contralateral normal eyes. We also found 36.6% greater RGCs in SNC-121-treated ocular hypertensive eyes compared with ocular hypertensive eyes.3 It is important to emphasize that SNC-121-induced retinal neuroprotection seen in our glaucoma model was IOP independent because IOP was not reduced in SNC-121-treated ocular hypertensive animals. We speculate that long-term neuroprotective response of opioids could be due to epigenetic changes in the retina and optic nerve. Such epigenetic changes will allow RGCs to maintain their functional integrity under conditions that normally lead to progressive neuronal loss. Overall, our recently published data provide evidence that DOR activation by the exogenous ligand, SNC-121, protects RGC function and integrity against glaucomatous injury.
DORs: ONH Astrocytes
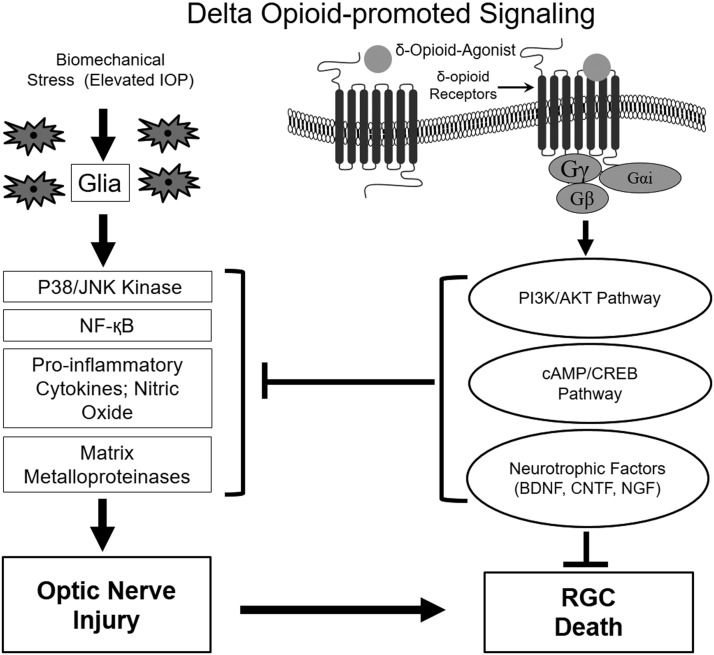
ONH astrocytes interact and provide cellular support to the axons of RGCs by regulating the ionic balance of the region70,71 and provide neurotrophic support to RGCs.72 In healthy eyes, astrocytes in the lamina cribrosa of the ONH are generally exposed to a hydrostatic pressure gradient between the intraocular compartment and the retrolaminar tissue pressure. This hydrostatic pressure gradient normally fluctuates, due to the ocular pulse and the diurnal changes in IOP. In pathological conditions such as glaucoma, there is an elevated IOP and spikes of IOP also occur, which deform lamina cribrosa more aggressively. As a result, biomechanical stress can activate/reactivate glial cells that subsequently lead to the initiation of changes in glial cell physiology and biochemistry, alteration in matrix remodeling, and release of potential toxins (TNF-α, transforming growth factor β1, nitric oxide (NO), and synthesis of MMPs); all contributing to the characteristic glaucomatous optic neuropathy (Fig. 1).66,72–74 The increased production of MMPs and cytokines in the glaucomatous ONH has been shown to play key roles in the tissue remodeling of the optic nerve.75 A direct pathological role for MMPs in glaucoma is not clear, MMPs have been shown to play detrimental roles in destabilization of the ONH by excessive remodeling, thereby predisposing RGCs to damage.75,76 In our laboratory, we have shown that DOR activation by the exogenous ligand, SNC-121, attenuated the TNF-α-induced MMP-2 production from ONH astrocytes. The mechanistic data also provided evidence that SNC-121 blocked the sustained activation of p38 MAP kinase and NF-κB, which subsequently reduced the TNF-α-induced MMP-2 secretion from ONH astrocytes.19
FIG. 1.
Signaling schematic showing the pathways potentially involved during optic nerve injury and RGC death in response to ocular hypertension, and neuroprotective pathways involved in response to delta opioid receptor activation. CREB, cAMP response element binding protein; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; IOP, intraocular pressure; NF-κB, nuclear factor-κB; NGF, nerve growth factor; RGC, retinal ganglion cell.
DORs: Mechanisms of Action
Opioid receptors regulate a variety of signaling pathways on endogenous and exogenous opioid receptor activation. These pathways include protein kinase A, MAP kinase, protein kinase C (PKC), PI3K/Akt, cAMP response element binding protein, and neurotrophic factor production (Fig. 1).4,5,77,78 The mechanisms involved in DOR agonist-induced antidepressant effects and neuroprotection are largely unknown. However, there are some speculations and limited data supporting the idea that DOR activation can increase the production of neurotransmitters (e.g., dopamine, serotonin, and norepinephrine), which will subsequently regulate the downstream signaling pathways to provide antidepressant-like effects.79–81 In addition, studies have shown that acute administration of DOR agonists rapidly increases the expression of BDNF (brain-derived neurotrophic factor) mRNA,23,82 and such neurotrophins may play a crucial role in the antidepressant-like effects, growth, and maintenance of the neurons.83
DOR agonists provide a variety of beneficial effects, which are often inhibited by DOR antagonists. A selective list of DOR agonists and antagonists is provided in Table 1. Opioids have been shown to regulate water and electrolyte homeostasis in the kidneys of animals and humans.84,85 Subsequently, it has been suggested that opioid receptors should have the capacity to regulate fluid formation/transfer in the eye by direct and indirect actions on the regulation of IOP, which can subsequently provide retinal neuroprotection. Studies have shown that a DOR agonist, DPDPE, reduced IOP bilaterally by suppressing aqueous flow in New Zealand rabbits.86 In contrast, we have shown that systemic application of DOR agonist SNC-121 (1 mg/kg; i.p.) for 7 days once a day had no effects on IOP.3,66 Studies have also shown that a selective serotonin receptor agonist efficaciously lowered IOP in the monkey model of ocular hypertension.87 Numerous pathways have been shown to play key roles in neuroprotection in nonocular systems. For example, the role of ERK (extracellular signal-related kinase) and PKC has been shown in DOR-mediated neuroprotection and neurogenesis.43,63,88 Activation of DOR by DADLE but not MOR and KOR reduces glutamate-induced injury to the neurons.45 DOR agonists also block lipopolysaccharide-induced p38 MAP kinase activation and expression pattern of TNF-α and MIP-2 in macrophages.89 DOR agonists also possessed free radical scavenger properties,53 and regulate MAP kinase activity.88 Activation of DOR reduces oxidative stress in the brain exposed to ischemia/reperfusion and also attenuated caspase activity.44 DADLE provides neuroprotection against oxygen glucose deprivation-induced neuronal injury via inhibition of p38 MAP kinase phosphorylation and activation.88
Table 1.
List of Selective Actions of Delta Opioid Receptor Agonists and Antagonists in Nonocular and Ocular Tissues/Cells
| Agonist/antagonist | Function in nonocular tissues | Function in ocular tissues | |
|---|---|---|---|
| DADLE (enkephalin) | Agonist | Activates PI3K/Akt and provides neuroprotection against cerebral ischemia,111 reduces infarct size,112 inhibits glutamate toxicity to neurons,41,45 inhibits brain ischemia,42 inhibits oxidative injury in ischemic brain,44 neuroprotective against stroke,48 increases dopamine secretion from neurons,52 antioxidative stress,44,88 involved in hibernation and neuroprotection,113 antidepressant,30,31,82 attenuates neuronal damage to asphyxia cardiac arrest,51 provides neuroprotection in spinal cord against ischemia,57 and free radical scavenger.53 | Increases the cell viability of ARPE-19 cells114 and lowers IOP and melatonin in iris ciliary body.115 |
| SNC-80 | Agonist | Neural differentiation,63 antidepressant,33 and decreases astrogliosis.55 | Increases inositol phosphate production.116 |
| SNC-121 | Agonist | Reduces myocardial infarct size in response of ischemia,117 SNC-121 induces postconditioning and protects myocardium against ischemia/reperfusion injury,118 provides cardioprotection against ischemia reperfusion.119 | Provides RGC neuroprotection in glaucoma model,3,66 inhibits NO production in the glaucomatous retina,66 inhibits proinflammatory cytokine production in glaucoma model,3 inhibits TNF-α-induced proinflammatory production from ONH astrocytes,19 inhibits p38 MAP kinase activation in glaucoma model and isolated ONH astrocytes,3,19 and inhibits MMP production from astrocytes.19 |
| ALD5747 | Agonist | Regulates inflammatory pain.120 | |
| UFP-512 | Agonist | Neuroprotective against Parkinson's disease47 and maintains K+ homeostasis in neonatal brain in response to ischemia.56 | |
| DPDPE | Agonist | Lowers IOP bilaterally by reducing aqueous flow86,121 and suppressed both basal and isoproterenol-stimulated cAMP accumulation in iris ciliary body.86 | |
| Naltrindole | Antagonist | Inhibits cardioprotection112 and inhibits hypoxic preconditioning-induced neuroprotection.43,45,122 | Inhibits SNC-80-induced inositol phosphate production,116 inhibits SNC-121-induced RGC neuroprotection,3 inhibits SNC-121-induced attenuation in NO production,66 and inhibits hypoxic preconditioning-induced retinal neuroprotection and ERK1/2 activation.122 |
DADLE, [6 D-Ala2, D-Leu5]-enkephalin; ERK, extracellular signal-related kinase; IOP, intraocular pressure; MAP, mitogen-activated protein; MMP, matrix metalloproteinase; NO, nitric oxide; ONH, optic nerve head; RGC, retinal ganglion cell; TNF-α, tumor necrosis factor alpha.
In the eye, detrimental roles of TNF-α and NF-κB have been shown during optic nerve degeneration.3,90 We have shown that TNF-α plays a detrimental role in the early stages of glaucoma development, and DOR agonist, SNC-121, counterbalances TNF-α production. Mechanistic data provide clues that TNF-α is produced mainly from glial cells during the early phase of glaucoma development, which was almost fully blocked by DOR agonist treatment.3 The proinflammatory cytokines such as TNF-α could be released from a variety of cells, including activated macrophages, astrocytes, microglia, Muller cells, and/or neuronal cells under stress/glaucomatous conditions. We reported that glial cells, but not the Muller cells, are the primary source for TNF-α production in glaucomatous conditions. In addition, we have shown that resident ONH astrocytes and microglia cells are the major sources of TNF-α production and the site of opioid agonist actions for TNF-α suppression during acute ischemic and ocular hypertension injury.3,19,65 These studies provided evidence that TNF-α-mediated signaling pathways could be a potential target for development of antiglaucoma therapies, and enhancement of opioidergic activity by exogenous means may be a vital neuroprotective strategy for glaucoma therapy.
We have also provided evidence that p38 MAP kinase plays a detrimental role in glaucoma pathology and it is one of the potential targets of DOR agonist during retinal neuroprotection.3 MAP kinases are serine/threonine kinases and play instrumental roles in the regulation of cell growth, differentiation, gene expression, and protein synthesis/secretion.91 Inhibition of p38 MAP kinase confers neuroprotection against excitotoxic insult92 and reduces acute ischemic injury in vivo.93 We have shown that TNF-α increases the activation of p38 MAP kinase in ONH astrocytes, which was further significantly inhibited by the DOR agonist, SNC-121.3,19 These findings provide clues that p38 MAP kinase plays a detrimental role during glaucoma development and DOR activation mitigates such injuries.3
To dissect out downstream targets of TNF-α and p38 MAPK pathways, we determined MMP secretion from ONH astrocytes. Based on our previous findings, we hypothesized that the production of TNF-α in response to glaucomatous injury is an early event within the optic nerve that leads to the activation of downstream signaling molecules (e.g., p38 MAPK and NF-κB), subsequently leading to excessive production of MMPs. Mechanistically, we have shown a significant upregulation of NF-κB expression by TNF-α treatment. We believed that NF-κB is a downstream target of p38 MAPK and both NF-κB and p38 MAPK are required for the production of MMPs. In the eye, a pivotal role for NF-κB has been suggested in TNF-α-induced optic neuropathy.90 Subsequently, we demonstrated that DOR activation by SNC-121 interferes in the dissociation mechanisms of IκB and NF-κB, which can regulate TNF-α-induced MMP production from ONH astrocytes. Both p38 MAPK activation and NF-κB upregulation were significantly inhibited in the presence of SNC-121, suggesting that both p38 MAPK and NF-κB were the downstream targets of SNC-121. The presence of MMP-1, MMP-2, MMP-3, MMP-9, MT1-MMP, and TIMPs 1–3 has been documented in human vitreous, interphotoreceptor matrix, RGCs, and astrocytes.76,94,95 Studies have also shown that reactive astrocytes express increased levels of MT1-MMP and MMP-1 in glaucomatous human astrocytes and a glaucomatous monkey model.76,94,95 Overall, the therapeutic approaches that primarily inhibit excessive astrocyte reactivity, proinflammatory cytokine activity, and downstream activation and/or expression of signaling targets (e.g., stress-activated protein kinase and p38 kinase) should impede deleterious changes in the optic nerve and RGCs in the eyes predisposed to glaucoma (Fig. 1).
NO is a pleiotropic mediator that regulates various physiological processes in numerous tissues. Studies have shown that morphine-induced NO can regulate IOP,96,97 and small quantities could be beneficial to maintain metabolites and blood circulation.98 However, excessive production of NO may cause direct tissue toxicity and contribute to neuronal degeneration.99 Generally, NO is produced by nitric oxide synthase (NOS); three isoforms of NOS have been cloned: constitutive (NOS-1 or nNOS), endothelial (NOS-3 or eNOS), and inducible (iNOS or NOS-2). Inducible nitric oxide synthase (iNOS) has been implicated in several neurodegenerative human diseases, including Parkinson's and stroke.100 Conflicting reports have been shown for the role of NO and NOS in glaucoma-induced retinal degeneration and RGC loss.101–104
Elevation in the iNOS has been reported in astrocytes and the ONH of primary open-angle glaucoma (POAG) patients102,105,106 A selective inhibitor of iNOS, aminoguanidine (AG), provides RGC neuroprotection in an extraocular vein cauterization glaucoma model.103 NO has been implicated in ischemia-induced retinal degeneration, which is mainly produced from iNOS.107,108 In addition, inhibition of iNOS resulted in RGC neuroprotection against ischemia109 and axotomy.110 In contrast, studies have shown that iNOS levels are not changed in POAG patients104 and iNOS does not play a detrimental role in glaucomatous injury in a chronically elevated IOP glaucoma model (i.e., a model of aqueous humor outflow obstruction). Moreover, iNOS expression was not correlated with the severity of optic nerve damage.104 Additional studies have shown that iNOS does not play a detrimental role in optic neuropathy in the DBA/2J glaucoma model.101 Considering such discrepancies in the literature, our laboratory has also confirmed the role of iNOS and NO in glaucoma pathology. Furthermore, we determined if DOR activation regulates NO production in the eye. Our data were in agreement with previously published reports showing that systemic application of AG treatment had no significant effect on IOP. In addition, we have shown that blocking NO either directly by AG or indirectly via activating DOR by SNC-121 provided RGC neuroprotection.66 We have shown that changes in iNOS expression are occurring at an early stage of glaucoma progression (day 7), while other studies103,104 have measured such changes in iNOS expression at 6–9 months, postglaucomatous injury. Overall, our findings confirmed that the enhanced levels of NO seen in ocular hypertensive eyes were due to upregulation of iNOS, which may be playing a crucial role in orchestrating RGC death during the pathogenesis of glaucoma. We have shown that DOR activation attenuates iNOS activity/expression that will lead to the attenuation of NO production and rescue RGCs from ocular hypertension injury.66
Conclusions
DOR pharmacology has gained a better understanding in the last two decades in nonocular systems. However, similar attention has not been given to the ocular systems. In recent years, our laboratory has demonstrated that DOR activation provides a significant amount of retinal neuroprotection in the glaucoma model. As a result of work done in nonocular and ocular systems, numerous novel and highly selective DOR agonists with potent in vivo activity have been shown to provide neuroprotection. Studies have provided substantial evidence that DOR agonists are highly beneficial to treat chronic pain and emotional disorders; however, the usefulness of DOR agonists for neuroprotection particularly in the eye requires additional work. Taken together, our findings suggest that short-term (7 days) opioid treatment results in long-term (42 days) neuroprotection, which brings up possibilities of epigenetic changes in the retina and optic nerve. However, such speculations required confirmation with additional experiments.
Acknowledgments
Supported, in part, by the National Institutes of Health (NIH)/National Eye Institute (NEI) grants EY019081 and EY027355, and BrightFocus Foundation, Clarksburg, Maryland.
Author Disclosure Statement
The authors have no financial or proprietary interest in any product mentioned in this article.
References
- 1.Norn S., Kruse P.R., and Kruse E. [History of opium poppy and morphine]. Dan. Medicinhist. Arbog. 33:171–184, 2005 [PubMed] [Google Scholar]
- 2.Hochberg U., Elgueta M.F., and Perez J. Interventional analgesic management of lung cancer pain. Front. Oncol. 7:17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdul Y., Akhter N., and Husain S. Delta-opioid agonist SNC-121 protects retinal ganglion cell function in a chronic ocular hypertensive rat model. Invest. Ophthalmol. Vis. Sci. 54:1816–1828, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husain S., Abdul Y., and Potter D.E. Non-analgesic effects of opioids: neuroprotection in the retina. Curr. Pharm. Des. 18:6101–6108, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Husain S., and Potter D.E. The opioidergic system: potential roles and therapeutic indications in the eye. J. Ocul. Pharmacol. Ther. 24:117–140, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Husain S., Potter D.E., and Crosson C.E. Opioid receptor-activation: retina protected from ischemic injury. Invest. Ophthalmol. Vis. Sci. 50:3853–3859, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Bodnar R.J., and Klein G.E. Endogenous opiates and behavior: 2003. Peptides. 25:2205–2256, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Malinowski K., Shock E.J., Rochelle P., Kearns C.F., Guirnalda P.D., and McKeever K.H. Plasma beta-endorphin, cortisol and immune responses to acute exercise are altered by age and exercise training in horses. Equine Vet. J. Suppl. 36:267–273, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Stanojevic S., Mitic K., Vujic V., Kovacevic-Jovanovic V., and Dimitrijevic M. Beta-endorphin differentially affects inflammation in two inbred rat strains. Eur. J. Pharmacol. 549:157–165, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cui Y., Lee T.F., and Wang L.C. State-dependent changes of brain endogenous opioids in mammalian hibernation. Brain Res. Bull. 40:129–133, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Tamura Y., Monden M., Shintani M., Kawai A., and Shiomi H. Neuroprotective effects of hibernation-regulating substances against low temperature-induced cell death in cultured hamster hippocampal neurons. Brain Res. 1108:107–116, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fukuda K., Kato S., Mori K., Nishi M., and Takeshima H. Primary structures and expression from cDNAs of rat opioid receptor delta- and mu-subtypes. FEBS Lett. 327:311–314, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Knapp R.J., Malatynska E., Fang L., et al. Identification of a human delta opioid receptor: cloning and expression. Life Sci. 54:PL463–PL469, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Wittert G., Hope P., and Pyle D. Tissue distribution of opioid receptor gene expression in the rat. Biochem. Biophys. Res. Commun. 218:877–881, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y., Hsu M.S., and Pintar J.E. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J. Neurosci. 18:2538–2549, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akil H., Watson S.J., Young E., Lewis M.E., Khachaturian H., and Walker J.M. Endogenous opioids: biology and function. Annu. Rev. Neurosci. 7:223–255, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Holaday J.W. Cardiovascular effects of endogenous opiate systems. Annu. Rev. Pharmacol. Toxicol. 23:541–594, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Husain S., Abdul Y., and Crosson C.E. Preservation of retina ganglion cell function by morphine in a chronic ocular-hypertensive rat model. Invest. Ophthalmol. Vis. Sci. 53:4289–4298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhter N., Nix M., Abdul Y., Singh S., and Husain S. Delta-opioid receptors attenuate TNF-alpha-induced MMP-2 secretion from human ONH astrocytes. Invest. Ophthalmol. Vis. Sci. 54:6605–6611, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarone S., Gambini O., Calabrese G., et al. Asymmetrical distribution of beta-endorphin in cerebral hemispheres of suicides: preliminary data. Psychiatry Res. 32:159–166, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Emrich H.M., Vogt P., and Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann. N. Y. Acad. Sci. 398:108–112, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Stoll A.L., and Rueter S. Treatment augmentation with opiates in severe and refractory major depression. Am. J. Psychiatry. 156:2017, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Torregrossa M.M., Jutkiewicz E.M., Mosberg H.I., Balboni G., Watson S.J., and Woods J.H. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 1069:172–181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell K.R., and Potter D.E. Dynorphin modulates ocular hydrodynamics and releases atrial natriuretic peptide via activation of kappa-Opioid receptors. Exp. Eye Res. 75:259–270, 2002 [PubMed] [Google Scholar]
- 25.Stiles J., Honda C.N., Krohne S.G., and Kazacos E.A. Effect of topical administration of 1% morphine sulfate solution on signs of pain and corneal wound healing in dogs. Am. J. Vet. Res. 64:813–818, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lehtosalo J., Uusitalo H., Mahrberg T., Panula P., and Palkama A. Nerve fibers showing immunoreactivities for proenkephalin A-derived peptides in the lacrimal glands of the guinea pig. Graefes Arch. Clin. Exp. Ophthalmol. 227:455–458, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Slaughter M.M., Mattler J.A., and Gottlieb D.I. Opiate binding sites in the chick, rabbit and goldfish retina. Brain Res. 339:39–47, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Howells R.D., Groth J., Hiller J.M., and Simon E.J. Opiate binding sites in the retina: properties and distribution. J. Pharmacol. Exp. Ther. 215:60–64, 1980 [PubMed] [Google Scholar]
- 29.Filliol D., Ghozland S., Chluba J., et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 25:195–200, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Kastin A.J., Scollan E.L., Ehrensing R.H., Schally A.V., and Coy D.H. Enkephalin and other peptides reduce passiveness. Pharmacol. Biochem. Behav. 9:515–519, 1978 [DOI] [PubMed] [Google Scholar]
- 31.Tejedor-Real P., Mico J.A., Smadja C., Maldonado R., Roques B.P., and Gilbert-Rahola J. Involvement of delta-opioid receptors in the effects induced by endogenous enkephalins on learned helplessness model. Eur. J. Pharmacol. 354:1–7, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Broom D.C., Jutkiewicz E.M., Folk J.E., Traynor J.R., Rice K.C., and Woods J.H. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 26:744–755, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Jutkiewicz E.M., Rice K.C., Woods J.H., and Winsauer P.J. Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behav. Pharmacol. 14:509–516, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Bodkin J.A., Zornberg G.L., Lukas S.E., and Cole J.O. Buprenorphine treatment of refractory depression. J. Clin. Psychopharmacol. 15:49–57, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Barry U., and Zuo Z. Opioids: old drugs for potential new applications. Curr. Pharm. Des. 11:1343–1350, 2005 [DOI] [PubMed] [Google Scholar]
- 36.He X., Sandhu H.K., Yang Y., et al. Neuroprotection against hypoxia/ischemia: delta-opioid receptor-mediated cellular/molecular events. Cell. Mol. Life Sci. 70:2291–2303, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia Y., and Haddad G.G. Major difference in the expression of delta- and mu-opioid receptors between turtle and rat brain. J. Comp. Neurol. 436:202–210, 2001 [PubMed] [Google Scholar]
- 38.Sick T.J., Rosenthal M., LaManna J.C., and Lutz P.L. Brain potassium ion homeostasis, anoxia, and metabolic inhibition in turtles and rats. Am. J. Physiol. 243:R281–R288, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Xia Y., Jiang C., and Haddad G.G. Oxidative and glycolytic pathways in rat (newborn and adult) and turtle brain: role during anoxia. Am. J. Physiol. 262:R595–R603, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Chao D., and Xia Y. Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Prog. Neurobiol. 90:439–470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., Qian H., Zhao P., Hong S.S., and Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke. 37:1094–1099, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Charron C., Messier C., and Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta 1-opioid agonists in a rat model of global ischemia. Physiol. Behav. 93:502–511, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Ma M.C., Qian H., Ghassemi F., Zhao P., and Xia Y. Oxygen-sensitive {delta}-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J. Biol. Chem. 280:16208–16218, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yang Y., Xia X., Zhang Y., et al. delta-Opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol. 7:55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J., Haddad G.G., and Xia Y. delta-, but not mu- and kappa-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 885:143–153, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Thathiah A., and De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer's disease. Nat. Rev. Neurosci. 12:73–87, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Mabrouk O.S., Marti M., Salvadori S., and Morari M. The novel delta opioid receptor agonist UFP-512 dually modulates motor activity in hemiparkinsonian rats via control of the nigro-thalamic pathway. Neuroscience. 164:360–369, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Borlongan C.V., Hayashi T., Oeltgen P.R., Su T.P., and Wang Y. Hibernation-like state induced by an opioid peptide protects against experimental stroke. BMC Biol. 7:31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi T., Sakai K., Sasaki C., Itoyama Y., and Abe K. Loss of bag-1 immunoreactivity in rat brain after transient middle cerebral artery occlusion. Brain Res. 852:496–500, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Wang Y., Chang C.F., Morales M., et al. Bone morphogenetic protein-6 reduces ischemia-induced brain damage in rats. Stroke. 32:2170–2178, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Gao C.J., Li J.P., Wang W., et al. Effects of intracerebroventricular application of the delta opioid receptor agonist [D-Ala2, D-Leu5] enkephalin on neurological recovery following asphyxial cardiac arrest in rats. Neuroscience. 168:531–542, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Borlongan C.V., Su T.P., and Wang Y. Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport. 11:923–926, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Tsao L.I., Ladenheim B., Andrews A.M., Chiueh C.C., Cadet J.L., and Su T.P. Delta opioid peptide [D-Ala2,D-leu5]enkephalin blocks the long-term loss of dopamine transporters induced by multiple administrations of methamphetamine: involvement of opioid receptors and reactive oxygen species. J. Pharmacol. Exp. Ther. 287:322–331, 1998 [PubMed] [Google Scholar]
- 54.Zhang J., Gibney G.T., Zhao P., and Xia Y. Neuroprotective role of delta-opioid receptors in cortical neurons. Am. J. Physiol. Cell Physiol. 282:C1225–C1234, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Narita M., Kuzumaki N., Narita M., et al. Age-related emotionality is associated with cortical delta-opioid receptor dysfunction-dependent astrogliosis. Neuroscience. 137:1359–1367, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Chao D., Wang Q., Balboni G., Ding G., and Xia Y. Attenuating ischemic disruption of K+ homeostasis in the cortex of hypoxic-ischemic neonatal rats: DOR activation vs. acupuncture treatment. Mol. Neurobiol. 53:7213–7227, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Liu H., Chen B., Li S., and Yao J. Dose-dependent neuroprotection of delta-opioid peptide [D-Ala(2), D-Leu(5)] enkephalin on spinal cord ischemia-reperfusion injury by regional perfusion into the abdominal aorta in rabbits. J. Vasc. Surg. 63:1074–1081, 2016 [DOI] [PubMed] [Google Scholar]
- 58.Yang L., Zhao X., Sun M., et al. Delta opioid receptor agonist BW373U86 attenuates post-resuscitation brain injury in a rat model of asphyxial cardiac arrest. Resuscitation. 85:299–305, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Kaur C., Sivakumar V., and Foulds W.S. Early response of neurons and glial cells to hypoxia in the retina. Invest. Ophthalmol. Vis. Sci. 47:1126–1141, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Chauhan B.C., Pan J., Archibald M.L., LeVatte T.L., Kelly M.E., and Tremblay F. Effect of intraocular pressure on optic disc topography, electroretinography, and axonal loss in a chronic pressure-induced rat model of optic nerve damage. Invest. Ophthalmol. Vis. Sci. 43:2969–2976, 2002 [PubMed] [Google Scholar]
- 61.Feng Y., He X., Yang Y., Chen J., Yin K., and Xia Y. Effect of delta-opioid receptor over-expression on cortical expression of GABAA receptor alpha1-subunit in hypoxia. Chin. J. Physiol. 54:118–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson S.M., and Turner S.M. Protecting motor networks during perinatal ischemia: the case for delta-opioid receptors. Ann. N. Y. Acad. Sci. 1198:260–270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narita M., Kuzumaki N., Miyatake M., et al. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J. Neurochem. 97:1494–1505, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Riazi-Esfahani M., Kiumehr S., Asadi-Amoli F., Lashay A.R., and Dehpour A.R. Morphine pretreatment provides histologic protection against ischemia-reperfusion injury in rabbit retina. Retina. 28:511–517, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Husain S., Liou G.I., and Crosson C.E. Opioid receptor activation: suppression of ischemia/reperfusion-induced production of TNF-alpha in the retina. Invest. Ophthalmol. Vis. Sci. 52:2577–2583, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Husain S., Abdul Y., Singh S., Ahmad A., and Husain M. Regulation of nitric oxide production by delta-opioid receptors during glaucomatous injury. PLoS One. 9:e110397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holder G.E. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog. Retin. Eye Res. 20:531–561, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Porciatti V. The mouse pattern electroretinogram. Doc. Ophthalmol. 115:145–153, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Porciatti V., Saleh M., and Nagaraju M. The pattern electroretinogram as a tool to monitor progressive retinal ganglion cell dysfunction in the DBA/2J mouse model of glaucoma. Invest. Ophthalmol. Vis. Sci. 48:745–751, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson E.C., Deppmeier L.M., Wentzien S.K., Hsu I., and Morrison J.C. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest. Ophthalmol. Vis. Sci. 41:431–442, 2000 [PubMed] [Google Scholar]
- 71.Morgan J.E. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye. 14(Pt 3B):437–444, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Hernandez M.R. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog. Retin. Eye Res. 19:297–321, 2000 [DOI] [PubMed] [Google Scholar]
- 73.Gottschall P.E., and Yu X. Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J. Neurochem. 64:1513–1520, 1995 [DOI] [PubMed] [Google Scholar]
- 74.Tezel G., and Wax M.B. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J. Neurosci. 20:8693–8700, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan X., Tezel G., Wax M.B., and Edward D.P. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch. Ophthalmol. 118:666–673, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Agapova O.A., Kaufman P.L., Lucarelli M.J., Gabelt B.T., and Hernandez M.R. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 967:132–143, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Sharma S.K., Klee W.A., and Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc. Natl. Acad. Sci. U. S. A. 72:3092–3096, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Standifer K.M., and Pasternak G.W. G proteins and opioid receptor-mediated signalling. Cell. Signal. 9:237–248, 1997 [DOI] [PubMed] [Google Scholar]
- 79.Detke M.J., Rickels M., and Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 121:66–72, 1995 [DOI] [PubMed] [Google Scholar]
- 80.Hille C.J., Fox S.H., Maneuf Y.P., Crossman A.R., and Brotchie J.M. Antiparkinsonian action of a delta opioid agonist in rodent and primate models of Parkinson's disease. Exp. Neurol. 172:189–198, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Spina L., Longoni R., Mulas A., Chang K.J., and Di Chiara G. Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC 80: 1. Locomotion, rearing and stereotypies in intact rats. Behav. Pharmacol. 9:1–8, 1998 [PubMed] [Google Scholar]
- 82.Torregrossa M.M., Folk J.E., Rice K.C., Watson S.J., and Woods J.H. Chronic administration of the delta opioid receptor agonist (+)BW373U86 and antidepressants on behavior in the forced swim test and BDNF mRNA expression in rats. Psychopharmacology. 183:31–40, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Russo-Neustadt A.A., and Chen M.J. Brain-derived neurotrophic factor and antidepressant activity. Curr. Pharm. Des. 11:1495–1510, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Salas S.P., Roblero J., Ureta H., and Huidobro-Toro J.P. Diuretic effect of bremazocine, a kappa-opioid with central and peripheral sites of action. J. Pharmacol. Exp. Ther. 250:992–999, 1989 [PubMed] [Google Scholar]
- 85.Salas S.P., Roblero J.S., Lopez L.F., Tachibana S., and Huidobro-Toro J.P. [N-methyl-Tyr1,N-methyl-Arg7-D-Leu8]-dynorphin-A-(1–8)ethylamide, a stable dynorphin analog, produces diuresis by kappa-opiate receptor activation in the rat. J. Pharmacol. Exp. Ther. 262:979–986, 1992 [PubMed] [Google Scholar]
- 86.Wang D., and Potter D.E. Ocular action of an opioid peptide, DPDPE. J. Ocul. Pharmacol. Ther. 12:131–139, 1996 [DOI] [PubMed] [Google Scholar]
- 87.Sharif N.A., McLaughlin M.A., and Kelly C.R. AL-34662: a potent, selective, and efficacious ocular hypotensive serotonin-2 receptor agonist. J. Ocul. Pharmacol. Ther. 23:1–13, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Ke S., Dian-san S, Xiang-rui W. Delta opioid agonist [D-Ala2, D-Leu5] enkephalin (DADLE) reduced oxygen-glucose deprivation caused neuronal injury through the MAPK pathway. Brain Res. 1292:100–106, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Husted T.L., Govindaswami M., Oeltgen P.R., Rudich S.M., and Lentsch A.B. A delta2-opioid agonist inhibits p38 MAPK and suppresses activation of murine macrophages. J. Surg. Res. 128:45–49, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Kitaoka Y., Kwong J.M., Ross-Cisneros F.N., et al. TNF-alpha-induced optic nerve degeneration and nuclear factor-kappaB p65. Invest. Ophthalmol Vis. Sci. 47:1448–1457, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Marshall C.J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 4:82–89, 1994 [DOI] [PubMed] [Google Scholar]
- 92.Legos J.J., McLaughlin B., Skaper S.D., et al. The selective p38 inhibitor SB-239063 protects primary neurons from mild to moderate excitotoxic injury. Eur. J. Pharmacol. 447:37–42, 2002 [DOI] [PubMed] [Google Scholar]
- 93.Legos J.J., Erhardt J.A., White R.F., et al. SB 239063, a novel p38 inhibitor, attenuates early neuronal injury following ischemia. Brain Res. 892:70–77, 2001 [DOI] [PubMed] [Google Scholar]
- 94.Brown D., Hamdi H., Bahri S., and Kenney M.C. Characterization of an endogenous metalloproteinase in human vitreous. Curr. Eye Res. 13:639–647, 1994 [DOI] [PubMed] [Google Scholar]
- 95.Plantner J.J., Jiang C., and Smine A. Increase in interphotoreceptor matrix gelatinase A (MMP-2) associated with age-related macular degeneration. Exp. Eye Res. 67:637–645, 1998 [DOI] [PubMed] [Google Scholar]
- 96.Dortch-Carnes J., and Russell K. Morphine-stimulated nitric oxide release in rabbit aqueous humor. Exp. Eye Res. 84:185–190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dortch-Carnes J., and Russell K.R. Morphine-induced reduction of intraocular pressure and pupil diameter: role of nitric oxide. Pharmacology. 77:17–24, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Sugiyama T., Oku H., Ikari S., and Ikeda T. Effect of nitric oxide synthase inhibitor on optic nerve head circulation in conscious rabbits. Invest. Ophthalmol. Vis. Sci. 41:1149–1152, 2000 [PubMed] [Google Scholar]
- 99.Dawson V.L., Dawson T.M., Bartley D.A., Uhl G.R., and Snyder S.H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J. Neurosci. 13:2651–2661, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dawson V.L., and Dawson T.M. Nitric oxide in neurodegeneration. Prog. Brain Res. 118:215–229, 1998 [DOI] [PubMed] [Google Scholar]
- 101.Libby R.T., Howell G.R., Pang I.H., et al. Inducible nitric oxide synthase, Nos2, does not mediate optic neuropathy and retinopathy in the DBA/2J glaucoma model. BMC Neurosc. 8:108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neufeld A.H., Hernandez M.R., and Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch. Ophthalmol. 115:497–503, 1997 [DOI] [PubMed] [Google Scholar]
- 103.Neufeld A.H., Sawada A., and Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc. Natl. Acad. Sci. U. S. A. 96:9944–9948, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pang I.H., Johnson E.C., Jia L., et al. Evaluation of inducible nitric oxide synthase in glaucomatous optic neuropathy and pressure-induced optic nerve damage. Invest. Ophthalmol. Vis. Sci. 46:1313–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 105.Shareef S., Sawada A., and Neufeld A.H. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest. Ophthalmol. Vis. Sci. 40:2884–2891, 1999 [PubMed] [Google Scholar]
- 106.Liu B., and Neufeld A.H. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia. 30:178–186, 2000 [DOI] [PubMed] [Google Scholar]
- 107.Neufeld A.H., Kawai S., Das S., et al. Loss of retinal ganglion cells following retinal ischemia: the role of inducible nitric oxide synthase. Exp. Eye Res. 75:521–528, 2002 [DOI] [PubMed] [Google Scholar]
- 108.Sennlaub F., Courtois Y., and Goureau O. Inducible nitric oxide synthase mediates retinal apoptosis in ischemic proliferative retinopathy. J. Neurosci. 22:3987–3993, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Geyer O., Almog J., Lupu-Meiri M., Lazar M., and Oron Y. Nitric oxide synthase inhibitors protect rat retina against ischemic injury. FEBS Lett. 374:399–402, 1995 [DOI] [PubMed] [Google Scholar]
- 110.Koeberle P.D., and Ball A.K. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp. Neurol. 158:366–381, 1999 [DOI] [PubMed] [Google Scholar]
- 111.Lv M.R., Li B., Wang M.G., et al. Activation of the PI3K-Akt pathway promotes neuroprotection of the delta-opioid receptor agonist against cerebral ischemia-reperfusion injury in rat models. Biomed. Pharmacother. 93:230–237, 2017 [DOI] [PubMed] [Google Scholar]
- 112.Ikeda Y., Miura T., Sakamoto J., et al. Activation of ERK and suppression of calcineurin are interacting mechanisms of cardioprotection afforded by delta-opioid receptor activation. Basic Res. Cardiol. 101:418–426, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Borlongan C.V., Wang Y., and Su T.P. Delta opioid peptide (D-Ala 2, D-Leu 5) enkephalin: linking hibernation and neuroprotection. Front. Biosci. 9:3392–3398, 2004 [DOI] [PubMed] [Google Scholar]
- 114.Pasovic L., Eidet J.R., Lyberg T., Messelt E.B., Aabel P., and Utheim T.P. Antioxidants improve the viability of stored adult retinal pigment epithelial-19 cultures. Ophthalmol. Ther. 3:49–61, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rohde B.H., Li B.H., and Chiou G.C. Effects of melatonin and haloperidol given via vortex vein on the intraocular pressure. Ophthalmic Res. 25:10–15, 1993 [DOI] [PubMed] [Google Scholar]
- 116.Dortch-Carnes J., and Potter D.E. Delta-opioid agonist-stimulated inositol phosphate formation in isolated, rabbit iris-ciliary bodies: role of G(i/o) proteins and Gbetagamma-subunits. Exp. Eye Res. 77:647–652, 2003 [DOI] [PubMed] [Google Scholar]
- 117.Tsutsumi Y.M., Kawaraguchi Y., Niesman I.R., Patel H.H., and Roth D.M. Opioid-induced preconditioning is dependent on caveolin-3 expression. Anesth. Analg. 111:1117–1121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsutsumi Y.M., Yokoyama T., Horikawa Y., Roth D.M., and Patel H.H. Reactive oxygen species trigger ischemic and pharmacological postconditioning: in vivo and in vitro characterization. Life Sci. 81:1223–1227, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patel H.H., Hsu A.K., and Gross G.J. COX-2 and iNOS in opioid-induced delayed cardioprotection in the intact rat. Life Sci. 75:129–140, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Le Bourdonnec B., Windh R.T., Leister L.K., et al. Spirocyclic delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl) benzamide (ADL5747). J. Med. Chem. 52:5685–5702, 2009 [DOI] [PubMed] [Google Scholar]
- 121.Jin J., Xu G.X., and Yuan Z.L. Influence of the hypothalamic arcuate nucleus on intraocular pressure and the role of opioid peptides. PLoS One. 9:e82315, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peng P.H., Huang H.S., Lee Y.J., Chen Y.S., and Ma M.C. Novel role for the delta-opioid receptor in hypoxic preconditioning in rat retinas. J. Neurochem. 108:741–754, 2009 [DOI] [PubMed] [Google Scholar]