Abstract
Progressive neurodegeneration of the optic nerve and the loss of retinal ganglion cells is a hallmark of glaucoma, the leading cause of irreversible blindness worldwide, with primary open-angle glaucoma (POAG) being the most frequent form of glaucoma in the Western world. While some genetic mutations have been identified for some glaucomas, those associated with POAG are limited and for most POAG patients, the etiology is still unclear. Unfortunately, treatment of this neurodegenerative disease and other retinal degenerative diseases is lacking. For POAG, most of the treatments focus on reducing aqueous humor formation, enhancing uveoscleral or conventional outflow, or lowering intraocular pressure through surgical means. These efforts, in some cases, do not always lead to a prevention of vision loss and therefore other strategies are needed to reduce or reverse the progressive neurodegeneration. In this review, we will highlight some of the ocular pharmacological approaches that are being tested to reduce neurodegeneration and provide some form of neuroprotection.
Keywords: : glaucoma, drug targets, neuroprotection
Receptor Activation or Blockade and Neuroprotection
G-protein-coupled receptors
Glaucoma is a leading cause of irreversible blindness worldwide1 and primary open-angle glaucoma (POAG) is a major contributor.2 One of the major classes of receptors that have resulted in a number of therapeutic agents developed to interact with these receptors is the G-protein-coupled receptors (GPCRs) (Fig. 1). There are more than 800 receptors that are considered GPCRs and this occupies roughly 4% of the human genome.3,4 Having such a wide spectrum of receptors makes the GPCRs a frequent target for the development of drugs by the pharmaceutical industry, including those developed for treating eye disease. In fact, nearly 50% of all drugs on the market target GPCRs5 and the most common of the drug classes for treating the disease of glaucoma are drugs that act through GPCRs. For POAG, most agents developed focused on regulating aqueous humor inflow and outflow. These agents include β-adrenergic receptor antagonists like timolol (a nonselective β1 and β2 receptor antagonist) and betaxalol (a selective β1 antagonist), which act primarily to decrease aqueous humor formation and lower intraocular pressure (IOP). The actions of these agents in reducing IOP and slowing the progression of glaucoma were appreciated in the Advanced Glaucoma Intervention Study,6 which found that lowering IOP was associated with reduced progression of visual field loss, thus supporting the protective role of lowering IOP.
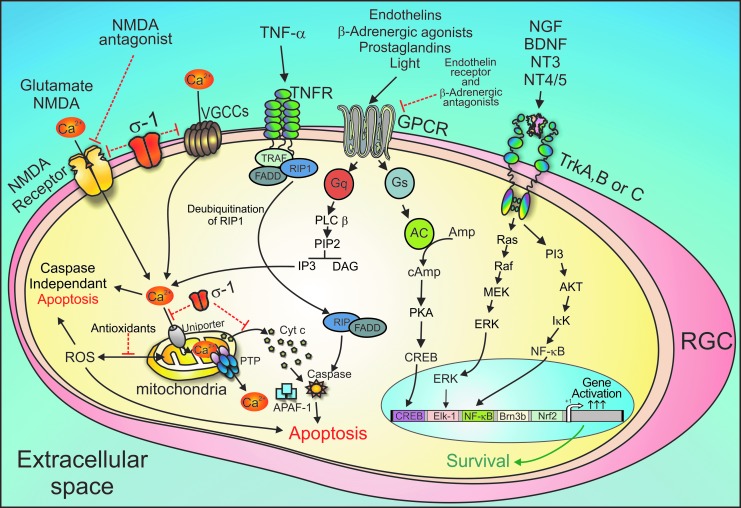
FIG. 1.
Potential targets for neuroprotection in glaucoma. There are multiple targets for neuroprotection based upon currently understood cellular mechanisms of cell death of RGCs. A number of agents target GPCRs. An important key second messenger contributing to cell death is intracellular calcium (Ca2+)i. Sigma receptors (σ-1) act by attenuating calcium uptake from ionotropic glutamate receptor (NMDA receptor). In addition, sigma receptor agonists could prevent formation of the permeability transition pore by sustaining the transmembrane potential of the inner mitochondrial membrane. This would prevent the release of cytochrome c from the mitochondria. Excessive ROS cause oxidative stress, which mediates RGC apoptosis through caspase-dependent and caspase-independent pathways. Application of antioxidative strategies might be an effective approach. Sigma receptors could also associate with voltage-dependent calcium channels and diminish calcium uptake from the extracellular milieu. Endothelin receptors antagonists could act on the Gq-coupled endothelin A receptor, blocking its ability to activate phospholipase C, and thereby prevent calcium release from the endoplasmic reticulum stores. Neurotrophins act through Trk receptors (which are receptor tyrosine kinases) and promote cell survival effects by activating the MAP kinase cascade, ultimately influencing gene expression in the nucleus through transcription factors such as Elk-1. TNF-α through its receptors can also trigger apoptotic signaling resulting in cell death. Targeting TNF-R is another protective target that would prevent cell death. The use of multiple agents could synergistically act to generate robust neuroprotection of RGCs. BNDF, brain-derived neurotrophic factor; GPCRs, G-protein-coupled receptors; NGF, nerve growth factor; NMDA, N-methyl-d-aspartate; RGCs, retinal ganglion cells; ROS, reactive oxygen species; TNF, tumor necrosis factor; VGCCs, voltage-gated calcium channels.
Prostaglandins (PGs) are another group of agents developed that target GPCRs and lower IOP by increasing outflow of aqueous humor predominantly through uveoscleral outflow mechanisms, including increasing the matrix metalloproteinase activity and remodeling of the extracellular matrix.7 There are a number of approved prostaglandin F (FP) receptor PG analogs used to lower IOP, including latanoprost, bimatoprost, travoprost, and trafluprost. It was found in the latanoprost for open-angle glaucoma study (UKGTS)8 that, lowering IOP showed significant preservation of the visual field and slowed the progression of the disease, supporting the notion that lowering IOP does provide some degree of protection against glaucoma progression. Interestingly, the EP2 receptor agonist, butaprost, has been shown to be neuroprotective in primary rat retinal cell cultures9 and that the EP2 agonist (N)-AW1-259-01 reduced IOP in mice, but not in EP2 knockout mice, suggesting a link between IOP reduction and neuroprotection. It is unclear if direct effects of PGs on the optic nerve head (ONH) also contribute to the neuroprotection properties of PGs. Currently, no EP2 agonists are approved for glaucoma treatment.
Consensus guidelines by the American Academy of Ophthalmology recommend that the β-adrenergic antagonists and PG analogs should be used as first-line medication therapy for individuals with elevated IOP. While for many patients, this treatment can slow progression of the disease, it is not always effective in significantly attenuating disease progression in glaucoma. Therefore, other agents with specific actions on the optic nerve and on the process of neurodegeneration can provide additional protection.
Other GPCRs that had shown promise were agents that affect the α-adrenergic receptor system. These receptors are the predominant effector sites for the sympathetic nervous system. While there are multiple subtypes of receptors, there has been a major interest in the α-2 receptor because of their location in the ciliary process and retina, including the presence on retinal ganglion cells (RGCs). Since the RGC is the major cell type that is affected in glaucoma, the α-2 receptor represented a potential drug target. Brimonidine, an α-2 agonist, was developed for lowering of IOP through reductions in aqueous humor formation; it was also proposed to have an additional benefit by acting on receptors on RGCs to provide neuroprotection,10 and one study found less visual field loss with brimonidine treatment than with timolol.11 However, while it is uncertain as to the mechanism responsible for this outcome, it could just be a result of lowering pressure than direct actions on RGCs. In another study, the Low-pressure Glaucoma Study Group,12 it was found that while timolol- and brimonidine-treated patients had similar IOP lowering effects, only brimonidine preserved visual function as measured by white on white perimetry, leading the investigators to propose that brimonidine had actions independent of IOP lowering that contributed to its neuroprotective role. While the mechanism for this effect is still unclear, it has been shown that brimonidine can upregulate brain-derived neurotrophic factor (BDNF) in RGCs.13 However, the exact mechanisms for the differences from its IOP effects remain uncertain.
Glutamate receptors
There are two major classes of glutamate receptors, those associated with ligand-gated ion channels (ionotropic) and those associated with second messenger systems (metabotropic). The N-methyl-d-aspartate (NMDA) receptor system (Fig. 1), which is part of the ionotropic class, has also been examined as a potential target for a neuroprotective strategy. Glutamate, while an essential neurotransmitter, has been implicated, when present in high concentrations, as contributing to the excitotoxicity of a number of neurodegenerative diseases. When glutamate is present in high concentrations, it results in the activation of ionotropic glutamate NMDA receptors. One of the promising developments associated with this excitotoxicity effect of activation of NMDA receptors was the finding that memantine, a selective noncompetitive antagonist of the NMDA receptor, which blocks the toxic effects of glutamate excitotoxicity, would be a new approach in the treatment of neurodegenerative diseases like glaucoma. Unfortunately, the phase 3 clinical trial for memantine treatment of glaucoma failed to demonstrate a significant effect on progression of the disease from the control group. This finding was a setback for developing potential neuroprotective drugs; however, memantine has shown clinical efficacy in patients with Alzheimer's disease and Parkinson's disease.14,15
Perhaps, a potential problem with the memantine glaucoma study stems from the uncertainty of how RGCs die in glaucoma. For instance, there are a number of different morphologically distinct RGC subtypes16,17 and some of these RGCs may be more susceptible to excitotoxity than others. For instance, Ullian et al.18 demonstrated that RGCs treated with 500 μM glutamate for 1 h did not result in RGC death, whereas hippocampal neurons treated with glutamate for 1 h resulted in 100% cell death. Recent data using neuronal and glial cell cultures suggest that memantine can also release neurotrophic factors from astroglia and provide anti-inflammatory actions, and prevent microglial activation.19 Thus, actions of memantine may include several mechanisms that bring about neuroprotection in neurodegenerative diseases like Alzheimer's or Parkinson's disease. Perhaps, further investigations into other related pathways for glaucoma progression are needed to identify better targets.
In addition to NMDA receptors, there are other receptors, like α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors that belong to the ionotropic glutamate receptors (iGluRs), which have been implicated in neurodegeneration. AMPA glutamate receptors are associated with the majority of fast excitatory neurotransmission in the central nervous system (CNS) and their dysregulation has been associated with several neurodegenerative disorders.20,21 There has been an increased interest in the role of AMPA receptors in glutamate-induced excitotoxicity in RGCs.22–27 The subunits of the AMPA receptor can be posttranscriptionally modified by alternative splicing, forming flip and flop isoforms.28 The flip isoform has a slower desensitization time and faster recovery time, and produces a larger current amplitude and steady state compared to the flop isoform.28 These differences in response rate and recovery play a role in excitotoxicity during disease conditions in which changes in the AMPA receptors may increase the susceptibility of neurons to excitotoxicity. Changes in AMPA receptor flip to flop ratio were seen in neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), ischemia, retinitis pigmentosa, and Parkinson's disease,29–32 and more recently with RGCs.27 Therefore, modification of these AMPA receptor isoform ratios may occur during glaucoma pathogenesis, which contribute to the progressive pathology that occurs in glaucoma. Additional studies are needed in which shifts in isoform ratios of AMPA receptor flip to flop can be observed in different RGC types and their susceptibility to noxious stimuli determined. Furthermore, it will be beneficial if we can measure these differences in animal models of RGC neurodegeneration.
Another agent that has been found to protect against NMDA-induced retinal neuronal death is the endogenous oligopeptide ligand to the GPCR for apelin (APJ receptor).33,34 In support of this action has been the observation that apelin deficiency in mice accelerates the progression of the neurodegenerative disease, ALS.35 This apelin peptide appears to act through G-protein inhibition of adenylate cyclase.36 How this functions in protecting neurons is not clear. Recently, it has been shown that apelin protects against NMDA-induced retinal neuronal death by activation of Akt and ERK1/2, while suppressing tumor necrosis factor-α (TNF-α).37 In addition, nonpeptide synthetic agents that interact with the APJ receptor have been developed, and these agents may show promise as potential drugs to test as neuroprotective agents on RGCs.
Sigma receptors
The Sigma-1 receptor (σ-1r) has also emerged as a potential new neuroprotective target that protects RGCs from noxious stimuli and prevents RGC loss that is associated with neurodegeneration.38 σ-1rs are a 26 kDa transmembrane protein found on the endoplasmic reticulum (ER) that translocate from the ER to interact with ionotropic channels located on the plasma membrane.39 This molecular signaling pathway enables the ubiquitously expressed σ-1r to associate and regulate many ligand-gated ion channels that are found on the plasma membrane of many different cell types throughout the body.39,40 In the retina, σ-1r has been identified with major expression seen in the RGC layer.41 The mechanism for σ-1r neuroprotection is still not clear; however, several observations suggest that the σ-1r can interact with l-type voltage-gated calcium channels to regulate neuronal intracellular calcium concentrations when the receptor translocates to the plasma membrane.42 Furthermore, when RGCs are exposed to ischemic conditions or subjected to excitotoxicity, activation of σ-receptors results in cell protection. This is enhanced when RGCs are treated with the σ-1r agonist (+)-pentazocine.38,43,44 σ-1r also appears to decrease cell death signal elements, thereby promoting cell viability as well as regulating intracellular calcium signaling.43,45
The σ-1r also regulates mitochondrial function by regulation of calcium-dependent and calcium-independent mechanisms. The signaling mechanisms by which the σ-1r modulates calcium entry into the mitochondria are being elucidated. Calcium overload in the mitochondria may lead to apoptosis by the generation of reactive oxygen species (ROS), triggering of the permeability transition pore, and release of cytochrome c with subsequent cell death. Activation of σ-1r protects cells and neurons from death by attenuating multiple components in the cell death pathway, including inducing changes in the mitochondrial membrane potential and preventing cytochrome c release43,46 (Fig. 1). Recent observations have suggested that the σ-1r decreases calcium currents in RGCs,42,45 inhibits glutamate-induced increase in intracellular calcium concentrations, prevents overexpression of the proapoptotic protein Bax and the activation of caspase-3, and protects against β-amyloid toxicity by inhibiting overexpression of Bax and TNF-related apoptosis inducing ligand and phosphorylation of c-Jun N-terminal kinase (JNK) in rat retina.43,47 In support of these cellular findings is the observation that σ-1r stimulation provides in vivo protection against retinal degeneration in a diabetic retinopathy animal model.48 It was further shown that there is a direct relationship between σ-1r stimulation and the neuroprotective effects of the ERK1/2 pathway in purified RGCs when they are subjected to oxygen and glucose deprivation.49 The σ-1r agonist (+) pentazocine prevented RGC death in an NMDA-induced murine model that was absent in σ-1r−/− (σ-1r knockout) mice.50 Collectively, these findings suggest that the activation of σ-1r may be a useful therapeutic target for RGC neuroprotection. More preclinical research needs to be performed to demonstrate the neuroprotective efficacy of σ-1r in glaucomatous animal models.
Endothelins
Another promising target for neuroprotection are the receptors belonging to the endothelin family of peptides, the endothelin A receptor (EDNRA or ETA) and the endothelin B receptor (EDNRB or ETB). Endothelins are potent 21-amino acid vasoconstrictor peptides that exist as three isoforms, endothelin-1 (ET-1), endothelin-2 (ET-2), and endothelin-3, each encoded by a separate gene, and are produced from the action of endothelin-converting enzyme from the 38 amino–amino acid precursor Big endothelin. ET-1 has gained recognition as playing an important role in neurodegeneration, particularly as it relates to glaucoma.51 While the endothelin pathway has been implicated in a wide variety of studies both in human and animal models of glaucoma, the exact mechanism responsible for these actions is not clear. Early studies have shown that ET-1 levels are elevated in the aqueous humor of POAG patients52,53 and in animal models of glaucoma.54–56 Recently it was shown that one of the early changes noted in an animal model of glaucoma was the persistent elevation of ET-257 and EDNRB receptors.58 Continuous peribulbar administration of ET-1 in primates produces optic neuropathy,59,60 and administration of ET-1 into rat eyes can promote loss of RGCs61 acting through EDNRB receptors.62–65 Since endothelins are potent vasoactive agents, the mechanisms responsible for ET actions could include a vascular component, particularly as reduced ONH blood flow and vascular dysregulation is thought to contribute to the progression of glaucoma. Chauhan et al.,66 however, demonstrated that chronic administration of low-dose ET-1 that results in loss of RGCs had no significant effect on blood flow of the optic nerve, supporting the notion that ETs have a direct action on RGCs. Wang et al.62 showed an increased immunohistochemical staining for EDNRB receptors in human glaucomatous optic nerves compared with age-matched controls, and Rogers et al.67 observed increases in EDNRB expression in glial cells in the optic nerve following optic nerve transection.
The role of astrocytes in the ET response was supported from studies demonstrating that ET-1 stimulation involves ONH astrocyte activation and proliferation, both implicated in the progressive neurodegeneration in glaucoma.61 It was also found that there was an early increase in EDNRB messenger RNA (mRNA) expression in the retina that was sustained for 8 weeks in a rodent elevated IOP model of glaucoma.58 Could an increase in EDNRB receptors be an early biomarker for glaucoma? Krishnamoorthy et al.63 showed that EDNRB expression increased in RGCs after intravitreal injection of ET-1 and this was accompanied by apoptosis of RGCs, and ET-1-mediated apoptosis was attenuated in EDNRB-deficient rats.63 Recent studies from Simon John's laboratory showed that blocking endothelin receptors, using bosentan or macitentan (antagonists for both EDNRA and EDNRB receptors), is neuroprotective in a mouse ocular hypertension model of glaucoma.68 Whether these observations are translatable into clinical efficacy awaits further evidence and clinical trials with endothelin antagonists. A clinical trial involving the EDNRA/EDNRB receptor antagonist, bosentan, is being assessed for efficacy in treating nonarteritic anterior ischemic optic neuropathy (NAION). However, no intermediate results are available yet for another 2 years. In another study, bosentan was also administered to healthy volunteers and glaucoma patients. It was found that significant increases in ocular blood flow occurred in both groups after 8 days of treatment.69 Unfortunately, this short-term treatment regime did not provide for any collection of data concerning visual fields or progression of the disease. Long-term studies are needed with bosentan to determine if this will be an efficacious treatment for glaucoma. These studies suggest that the endothelin system appears to play a pivotal role in the neurodegeneration process and ET antagonists might be a potential treatment as a neuroprotective agent in glaucoma.
Tumor necrosis factor-α
Another molecule that has emerged as a key contributor to glaucoma progression is the cytokine TNF-α70 and its superfamily of receptors. TNF-α interacts with two receptors TNF-R1 (p55) and TNF-R2 (p75) that are expressed on neurons, astrocytes, and microglia, with TNF-R1 containing the death domain that is involved in cell apoptosis71 (Fig. 1). Yang et al.58 showed an increase in TNF-R1A receptor expression in an ocular hypertension model of glaucoma in rodents and an increased immunohistochemistry intensity for TNF-α and its receptor was observed in glaucomatous eyes compared to controls.72 Furthermore, TNF-α was predominantly positive in the glial cells, while TNF-R1 receptors were found mainly on RGCs. Nakazawa et al.73 found increased concentrations of TNF-α in the ocular hypertensive mouse model retina, which was followed by microglial activation and delayed loss of RGCs. It was also reported that deleting genes encoding for TNF-α or its TNF-R2 receptor resulted in an attenuation of RGC death following elevation of the IOP.73 This supported their conclusion that TNF-α acts through the TNF-R2 receptor to produce neurodegeneration in glaucoma.
TNF-α also promotes ET-1 release from nonpigmented ciliary epithelial cells74 as well as from ONH astrocytes,75 and from retinal pigmented epithelial cells,76 suggesting that some of TNF-α's effects could be due to the release of endothelin. Increasingly, it has been proposed that a neuroinflammation component may play an important role in the progression of glaucoma.77 In animal models, intravitreal injection of TNF-α mimics glaucomatous damage in control mice73 and an inhibitor of TNF-α prevents RGC loss in a rat model of glaucoma.78 An important protein associated with the TNF-α pathway is optineurin. The protein optineurin has various cell functions that include nuclear factor-κB (NF-κB) regulation, autophagy, membrane trafficking, and exocytosis, and a role in reorganization of actin and microtubules.79 Optineurin interacts with E3-14.7k protein that inhibits TNF-α-induced apoptosis.80 Mutations in optineurin are responsible for a significant portion of low-pressure glaucoma/POAG families.79 Overexpression of optineurin inhibits the TNF-α increase in the NF-κB activity.81 Such actions prevent TNF-α-induced cell death. Activation of NF-κB is associated with chronic neuroinflammatory conditions and the ability of optineurin in preventing NF-κB activation suggests that alterations in the optineurin activity might be an important target for controlling neuroinflammation associated with glaucoma. The role of TNF-α in neuroinflammation and RGC death is also known and may involve astrocytes and microglia.82 What contribution TNF-α plays in the progression of neuroinflammation seen in glaucoma remains to be established.
Potential Targets of Neuroprotection in Glaucoma-Oxidative Stress and Antioxidants
It is widely known that oxidative stress is involved in the etiology of glaucoma.83–89 The correlation between oxidative stress and glaucoma was also confirmed from a variety of human subject studies and animal experiments.86,90 In this study, we summarize the major contributing factors of oxidative stress, role of ROS, oxidative stress-induced apoptosis in glaucoma, and the potential therapeutic approaches to alleviate oxidative stress in glaucoma.
Understanding the mechanisms of ROS-induced oxidative stress is very crucial for designing strategies in prevention of damage caused by ROS. Based on the involvement of ROS in the etiology of glaucoma, there are several strategies to control the balance of ROS and antioxidants. They include the following: control endogenous antioxidants and antioxidant enzymes; administration of antioxidants; control of ROS production; and regulating the expression of antioxidative factors or genes.
Antioxidants
Administration of antioxidants is a direct approach to neutralize ROS in cells to decrease the possible damages caused by oxidative reactions. Clinical studies and animal experiments have investigated the effects of antioxidants in heart attack, neurodegeneration, cancer, and eye diseases, including age-related macular degeneration, cataract, and glaucoma. There are a variety of antioxidants being applied in animal studies and clinical studies, including vitamin C, vitamin E, lutein, CoQ10, and flavonoids, Ginkgo biloba, Lycium barbarum, α-lipoic acid, and ghrelin. In this study, we review and summarize some studies in glaucoma using antioxidants in animal models91–103 (Table 1) and human patients104–110 (Table 2).
Table 1.
Application of Antioxidants in Animal Models of Glaucoma
| Antioxidants | Administration | Effects in animal models | Reference |
|---|---|---|---|
| Vitamin B3 | Oral | Prevented RGC death and axon loss, and protected mitochondrial intact in DBA/2J mice with elevated IOP. | Williams et al.91 |
| CoQ10 | Oral | Promoted RGC survival and attenuated axon loss in DBA/2J mice and C57BL/6 mice, and rats with retinal ischemia. Normalized the expression of genes and proteins associated with oxidative stress and apoptosis. | Lee et al.,92,93 Nucci et al.,94 Russo et al.95 |
| Ghrelin | Intraperitoneal injection | Less cell apoptosis in IOP-elevated rats treated with ghrelin. | Can et al.96 |
| Lycium barbarum | Oral | Attenuated RGC loss and decreased retina damages in rats with optic nerve transection and mice with elevated IOP. | Li et al.,97 Mi et al.98 |
| α-Lipoic acid | Oral | Reduction of RGC death and decreased oxidative genes and proteins in DBA/2J mice. | Inman et al.,99 Razali et al.100 |
| Resveratrol and flavonoids | Eye drop/oral/Intraperitoneal injection | Lowered steroid-induced IOP, reduced oxidative stress-mediated damages, attenuate RGC loss in rats and mice in IOP-elevated and optic nerve crush models. | Pirhan et al.,101 Lindsey et al.,102 Razali et al.103 |
IOP, intraocular pressure; RGC, retinal ganglion cell.
Table 2.
Application of Antioxidants in Human Subjects of Glaucoma
| Antioxidants | Administration and patient number | Effects in human subjects | Start and end time | Reference |
|---|---|---|---|---|
| CoQ10+Vitamin E | Eye drop | Increased integrity of the visual pathways by visual-evoked potential and RGC's visual function by the pattern electroretinogram in OAG patients. | January 2010 to April 2011 | Parisi et al.104 |
| 43 OAG patients (22 treated patients, 21 control) | ||||
| GBE | Oral | Beneficial to the ocular blood flow. | January 2003 to December 2004 | Park et al.105 |
| 30 NTG patients (15 GBE, 15 placebo) | ||||
| 27 NTG patients (14 GBE treatment followed with placebo, 13 placebo followed with GBE) | Improved visual field test and contrast sensitivity in both groups treated with GBE. | September to December 1999 | Quaranta et al.106 | |
| 35 NTG patients (17 treated, 18 placebo treated)103 | No protective or beneficial effects detected for visual field test and contrast sensitivity. | June 2010 to December 2011 | Guo et al.107 | |
| ICAPS R® (Alcon) | Oral antioxidant supplements. | No significant effects identified in visual field test, peripapillary retinal nerve fiber layer, and macular ganglion cell complex. | June 2007 to December 2011 | Garcia-Medina et al.108 |
| 2-year follow-up for patients | ||||
| OFTAN MACULA® (Laboratorios Esteve) | 117 POAG patients: | |||
| 26 with ICAPS R | ||||
| 28 with OFTAN MACULA | ||||
| 63 control | ||||
| Resveratrol and flavonoids | Oral | A comprehensive summary of flavonoids in human patients: | The information was extracted publication from 1980 to present. | Milea and Aung,109 Patel et al.110 |
| 214 patients from 6 studies | Restore visual field of human subjects, but no effect on lowering IOP. |
GBE, Ginkgo biloba extract; NTG, normal-tension glaucoma; OAG, open-angle glaucoma; POAG, primary open-angle glaucoma.
Vitamin B3
Vitamins C and E have been given as food supplements in the treatment of glaucoma83,111; however, the effects of vitamins C/E in glaucoma patients are still ambiguous. Recently, Simon John's group showed that intake of vitamin B3 prevented RGC death, axon loss, mitochondrial damage, and malfunction of axonal transport in DBA/2J mice with elevated IOP, although vitamin B3 administration did not lower IOP.91 Nicotinamide/nicotinic acid mononucleotide adenylyltransferase 1 (NMNAT1) is a key enzyme that converts vitamin B3 to nicotinamide adenine dinucleotide. The latter plays an important role in redox reactions. Accordingly, adeno-associated virus type 2 (AAV2)-mediated overexpression of NMNAT1 also protected eyes from glaucomatous neuron degeneration in DBA/2J mice.91 The improvement in RGC survival and anterograde transport was found in mice with NMNAT1 gene therapy. RNA-Seq-detected gene expression profiles were also carried out to confirm the protective effects of B3 intake and NMNAT1 gene therapy.91 This study suggests that antioxidant effects of B3 may play a crucial role in neuroprotection in glaucoma.
Flavanoids
G. biloba contains flavonoids and is one of herbs commonly used in Chinese traditional medicine. G. biloba extract (GBE) was considered an antioxidant in defense of oxidative stress in glaucoma90 and is used to alleviate oxidative damage in glaucoma patients and animal models. GBE administration attenuated the RGC loss in IOP-elevated rats orally supplied EGb 761 (a type of GBE),112 restored hydrogen peroxide-mediated mitochondrial membrane potential loss,90 and altered remodeling of extracellular matrix at trabecular meshwork from dexamethasone-induced IOP elevation in rabbits.113 GBE application was also beneficial to blood flow, visual field test, and contrast sensitivity in normal tension patients105,106,114; however, Guo et al.107 were unable to detect any effect of GBE on mean deficit or contrast sensitivity in their group of normal-tension glaucoma patients. These differences in outcomes appear to be related to patient selection criteria, including confounding effects of local and systemic therapies. Additional clinical studies are needed to determine the usefulness of GBE for low-tension glaucoma.107
Epigallocatechin-3-gallate (EGCG), the ester of catechin belonging to the family of flavonoids and a type of polyphenol, is found in green tea. It is approved to be a powerful antioxidant115,116 as an ROS scavenger, as well as an anti-inflammation117,118 and anticancer reagent.119–122 Administration of EGCG significantly promoted the survival of RGCs123,124 and increased the higher protein concentrations of neurofilament triplet l of the optic nerve in glaucomatous models in rats and mice.123 It was suggested that EGCG has neuroprotective effects to prevent RGC death and axon loss. EGCG treatment also attenuated upregulation of neuronal nitric oxide synthase (nNOS) and Bax proteins caused by optic nerve axotomy in rats and induced an increase in phosphorylated ERK1/2 and phosphorylated-Akt.125 Other proapoptotic genes and proteins like caspases 3 and 8 were downregulated by EGCG application in an ischemia/reperfusion (I/R) rat model.126 EGCG exerted its neuroprotective effect not only on prevention of RGC death and axon loss but also on deactivation of astrocytes and Müller cells in glaucoma.127
Antioxidant enzymes
Protective mechanisms against oxidative stress are tightly linked with the activities of antioxidant enzymes, including superoxide dismutases (SODs), and catalases. Mutations of these enzymes alter the enzymatic reactions; thus the balance between ROS production and clearance is altered leading to conditions of oxidative stress.
Myocilin mutation at Y437H in mouse is considered a glaucoma model that results in elevated IOP. A heterozygous deletion of SOD2 in mice (Tg-MYOC(Y437H+/+)/SOD+/−) produced a higher IOP and a greater loss of RGC in retina compared to mice carrying either Tg-MYOC(Y437H+/+) or Tg-SOD2+/−.128 In addition, some mutations of these antioxidant enzymes are associated with glaucoma in human patients. For example, a single nucleotide polymorphism (SNP; rs4880; c.47T>C) in the manganese superoxide dismutase gene (Mn-SOD) was detected in POAG patients who had slightly higher IOP than controls.129 Although other mutations in SOD were also detected in glaucoma patients130,131; there was no confirmed direct association between the mutations of SOD and onset of glaucoma in humans. Several different studies confirm the alterations of SOD enzyme activities that occur in glaucoma patients. An increase of SOD, glutathione peroxidase (GPx), and catalase in the red blood cells was identified in 30 POAG patients compared to the control group (25 cataract patients).132 Furthermore, a significant increase of SOD and GPx was also detected from aqueous humor of 30 POAG and 30 primary angle closure glaucoma patients compared to the age-matched cataract patients. This increased enzyme activity was associated with decreased levels of vitamins C and E. Taken together, modulation of antioxidant enzyme activity plays a key role in prevention and protection of damage caused by glaucoma.
Changes or control of antioxidant enzyme activity directly regulates the concentration of ROS in cells through adjustments of production and neutralization of ROS. Overexpression of SOD2 has been shown to be an effective approach to alleviate the damage caused by ROS in glaucoma animal models. In a laser-induced IOP elevation in rat, intravitreal injection of AAV-encoding SOD2 protected RGC death and decreased malondialdehyde, which is a product of lipid peroxidation and a marker of oxidative stress.133 In addition, upregulation of SOD2 or catalase in an ischemia/perfusion mouse/rat model decreased levels of superoxide, 8-hydroxy-2′-deoxyguanosine, and nitrotyrosine, and correspondingly had less cell apoptosis.134,135
Regulating expression of antioxidative factors or genes
Oxidative stress is thought to be a contributing factor in cell death in various neurodegenerative diseases, including glaucoma.86,90 One of the genes activated by oxidative stress and ROS is the transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2). In addition, Kelch-like ECH-associated protein 1 (Keap1), NF-κB, hypoxia-inducible factor 1, mitogen-activated protein kinases (MAPK), and phosphoinositide 3-kinase/Akt136 are also tightly controlled by gene regulation in pathological conditions. There are several well-identified pathways involved in maintaining the balance between ROS production and clearance. For example, NF-κB is a crucial transcription factor regulating Mn-SOD, ferritin heavy chain.137 Activating transcription factor 1 (ATF-1) is an upstream regulator for GPx1138 and PPARγ coactivator 1α (PGC-1α) plays a key role in regulating GPx1 and SOD2.139 Nrf2 is a transcription factor belonging to a basic leucine zipper family. Nrf2 is responsible for oxidative stress, translocates into the nucleus, and binds to antioxidant response elements (ARE). In response to these oxidative stimuli, it regulates many antioxidative genes, including thioredoxin reductase 1, heme oxygenase-1, and glutathione S-transferase,140 following binding to ARE and triggers the downstream gene expression.
In animal models with glaucomatous damage, activation of Nrf2 alleviated RGC loss. Optic nerve crush induced RGC death and axon loss was prevented in wild-type mice treated with an Nrf2 activator, 1-(2-cyano-3-, 12-dioxooleana-1, 9 (11)-dien-28-oyl) imidazole (CDDO-Im), and CDDO-Im-activated neuroprotection was abolished in Nrf2 knockout mice.141 Furthermore, enhanced antioxidant and related enzymes were detected after CDDO-Im treatment.141 This study suggests that Nrf2 plays an important role in regulating the antioxidant system that prevents the damage caused by oxidative stress. In another study of glaucomatous damage induced by I/R injury in mice, it was found that the damage was more severe in Nrf2 knockout mice compared to that in wild-type mice.142 It was suggested that neurodegeneration seen in optic nerve crush and I/R appeared to be a result of the overproduction of ROS-mediated oxidative stress, leading to apoptotic signaling and cell death.135,143–145 Keap1 is an endogenous repressor protein of Nrf2, which acts by binding to Nrf2, and promotes Nrf2 ubiquitination. Ubiquitylated Nrf2 rapidly undergoes degradation by the proteasome.146 Using genetic pharmacological approaches to regulate the protein levels of Nrf2 or Keap1 could be a potential tool to protect cells against oxidative stress. As already mentioned, AAV2-mediated overexpression of NMNAT1 had neuroprotective effects resulting in an increased RGC survival and axon function in DBA/2J mice with an elevated IOP.91 Gene therapy may be a promising approach to regulate gene expression to attenuate oxidative stress that leads to RGC loss.
ROS-induced damage is involved in the etiology of glaucoma and targeting ROS and oxidative stress could be a beneficial therapeutic target. Although a number of reports have shown beneficial effects of antioxidative strategies in animal glaucomatous models and cell cultures, additional studies are needed to demonstrate that these effects would be useful approaches in clinical therapeutics.
Mitochondria and Neurodegeneration
RGCs and their nerve fibers of the optic nerves are specialized neurons that are tasked with the transmission of visual stimuli from the environment to the visual processing centers of the brain. There are increases in the amount of mitochondria in the unmyelinated nerve fibers that comprise the ONH. Their presence in the optic nerve may be necessary for the high energy demands of RGCs and transmission of nerve impulses. Neurons and other cells utilize mitochondria for several processes, including generation of energy in the form of ATP. In addition, it has become increasingly clear that mitochondria are controllers and contributors to the process of cell death involving apoptosis, and are tasked with regulating the cells' response to oxidative stress and other toxic stimuli.147 Mitochondria also contribute to the maintenance of calcium homeostasis.
Understanding the role of mitochondria in the retina, including RGCs, is relevant to human diseases as there are mitochondrial abnormalities in patients with retinal diseases, including POAG, Leber hereditary optic neuropathy (LHON), and dominant optic atrophy.148–153 Perturbations in mitochondrial dynamics as a result of mutations in nuclear or mitochondrial genes involved in regulating cell processes are linked to diseases such as LHON, dominant optic atrophy, and Parkinson's disease.148–151,154–156 Interestingly, mitochondrial dysfunction has also been implicated as a secondary causative factor in the development of glaucoma.157 Recently, Khawaja et al.,158 using gene set analyses of a large cohort of patients, identified mitochondrial enzyme pathways critical to the pathogenesis of POAG, particularly identifying lipid and carbohydrate pathways.
In addition, in POAG patients, mitochondrial DNA mutations and increased mitochondrial DNA content were identified, suggesting increased pathological changes in RGCs and the inability to provide increased response to oxidative stress.152,159 Furthermore, there was reduced mitochondrial respiration in a significant population of POAG patients.152,159 In lymphocytes from POAG patients, there were decreased ATP produced from mitochondrial complex 1 with no measurable changes in ATP production from complex 2.157 Taken together, these data suggest that the optic neurodegeneration observed in glaucoma may be associated with mitochondrial dysfunction.
Neurotrophins
Neurotrophins (NTs) are promising agents for neuroprotection as they act by promoting cell survival effects in neurons both in the CNS as well as the peripheral nervous system. During development, only those neurons that are able to innervate NT-releasing target tissues survive, while other neurons deprived of trophic support are eliminated by apoptosis. The ability of NTs to promote neuronal survival and facilitate synaptic pruning during development provides a strong rationale for the use of NTs for neuroprotection. Our focus will be mainly on the following members of the NT family, namely, nerve growth factor (NGF), BDNF, NT-3, ciliary neurotrophic factor (CNTF), and NT-4/5, and their potential for neuroprotection in glaucoma. NTs are not only synthesized in the brain but also locally produced in the retina. Surprisingly, NTs and Trk receptors are also expressed in the lamina cribrosa and ONH astrocytes,160 which may serve as additional sources of NTs in the microenvironment of the optic nerve axons. BDNF along with its TrkB receptor is taken up from the brain by RGC axon terminals and retrograde transported to the somas of RGCs. Upon binding of NT to their Trk receptor, there is a dimerization and cross-phosphorylation of the receptor subunits,161 which trigger signaling cascades leading, in general, to prosurvival cellular responses. One of the prototypical signaling cascades activated by NT signaling through Trk receptors is the Ras→Raf→MEK→ERK1/2 MAP kinase pathway162,163 (Fig. 1). Activation of Trk receptors by NTs also induces the PI3-K→Akt→mTOR pathways evoking prosurvival and/or progrowth cellular responses. These prosurvival signaling effects are consistent with the neuroprotective actions of NTs.
The multiple receptor types bound by NTs and their diverse signaling leading to different cell fates are some of the challenges to be considered when NTs are to be used as agents for neuroprotection. BDNF administration in the superior colliculus of neonatal hamster leads to a 13- to 15-fold reduction in pyknosis of RGCs, suggesting a key role for target-derived BDNF in promoting RGC survival.164 In adult retinas, a stable complex of NTs and their Trk receptor is formed, which is taken up by endocytosis and retrograde transported by the axon to the cell body.165 Retrograde and orthograde axonal transport can be blocked by elevated IOP.166–168 This decline in axonal transport is thought to be a main cause of neurotrophic deprivation in RGCs,169–171 which could lead to neurodegenerative changes seen in glaucoma. Survival of adult RGCs is maintained by transported as well as locally produced NTs.172–174 During glaucoma, due to degenerative changes in the RGCs and ONH, decreased local production of NTs in the retina as well as additional obstruction to retrograde transport contribute to disease progression. There was a decrease in immunostaining for endogenous BDNF and NT-4/5 from the superior retina following IOP elevation for 7 days, which coincided with the evidence of axonal degeneration in rats.175 However, NT deprivation by itself could not be the only explanation for RGC death during glaucoma. It was possible that in case of retrograde transport blockade, NTs expressed in situ could compensate for deficits in the brain-derived NTs. However, the relative contribution of brain and locally derived NTs toward neuronal survival, as well as various effects of NTs on somal and axonal compartments, are not completely understood.169,176,177
While NTs are promising neuroprotective agents that act by decreasing RGC loss during axonal injury, this strategy results only in temporary results.178–181 This could be due to a deficit in Trk receptors in injured RGCs.182 Conflicting effects on NT neuroprotection could be due to differential expression of Trk and p75 receptors. For instance, Coassin et al.183 demonstrated that during elevated IOP, retinal NGF expression was upregulated and it correlated with RGC death, possibly because the expression of the proapoptotic p75 was greatly increased relative to that of TrkB. Similarly, Rudzinski et al.184 found that during IOP elevation, there was a dysregulation of NT receptors and proposed a neuroprotective strategy of a combination of Trk receptor agonists and p75 receptor antagonists. It is clear that mere administration of NTs would not be sufficient as a neuroprotective strategy, suggesting that a concurrent regulation of receptor expression would be necessary for optimal neuroprotection. These findings underscore some of the difficulties involved in a neuroprotective approach using NT administration. NGF (200 μg/mL) administered as eye drops has been shown to be neuroprotective for RGCs through blocking apoptosis in rat eyes. The authors also tested NGF drops on three human glaucoma patients and evaluated changes in pattern electroretinography, visual field, and visual evoked potential assays. The NGF topical administration was found to improve inner retinal function; however, no placebo controls were included in this study.185 In another study, using Morrison's model of IOP elevation in rats, Colafrancesco et al.186 found an increase in NGF in the retina, followed by a drastic drop below normal levels leading to RGC apoptosis. The authors found a significant protection of RGCs in this model by topical treatment with NGF drops.
CNTF is another NT, which has neuroprotective properties as it prevents RGC loss in models of glaucoma and ischemic optic neuropathy.187,188 In addition to its neuroprotective role, CNTF can also stimulate axonal regeneration. In an optic tract injury model, CNTF was shown to participate with other factors in the formation of functional synapses and enhancement of visual function.189 Recently, a cell-based intraocular device was developed with the ability to secret CNTF. This encapsulated cell therapy-based device Renexus (NT-501) was implanted in patients with chronic retinal degenerative diseases, retinitis pigmentosa, and geographic atrophy. The patients tolerated this implant well and the pharmacokinetics indicated that the implants produced CNTF consistently over a 2-year period.190 This implant has yet to be tried in a glaucoma patient cohort. The technology indicates that encapsulated cell-based technology could be used to produce NTs for extended periods of time. Such a profile may be of benefit for studying effects of various NTs on the progression of glaucoma.
The use of AAV to target gene expression has also emerged as a safe and promising approach for the treatment of ocular disorders after the successful phase 1 clinical trial for Leber's Congenital Amaurosis using AAV-mediated gene delivery of the RPE65 gene.191,192 In a rat model of glaucoma, AAV-mediated BDNF expression in RGCs significantly increased RGC survival (up to 52%) after 4 weeks of IOP elevation.193 Cheng et al.194 used a combination of AAV vector-mediated TrkB expression and intraocular injections of BDNF to promote RGC survival following a similar IOP elevation. Another attractive approach is the transplantation of progenitor or stem cells for neurodegenerative conditions of the CNS, and in particular the retina. Some of the neuroprotective effects of transplanted cells occur by the production and secretion of NTs.195 Clearly, neuroprotection using NTs is an evolving field with additional refinements needed to enhance the efficacy and block some of the receptor cross talk, particularly the opposing effects of Trk and p75 receptors.
Nitric Oxide
Most of the current therapies of glaucoma are aimed at lowering IOP by either reducing aqueous humor formation or increasing outflow through the uveoscleral pathway.196 There is evidence that nitric oxide (NO) induction may mediate IOP-lowering effects predominantly through an increase in the aqueous outflow, through the conventional pathway of trabecular meshwork and Schlemm's canal. NO donors have been found to lower IOP in several different species, including rabbits, dogs, and monkeys.197–199 Intravitreal or intracameral administration with the NO donor 3-morpholinosydnonimine (SIN-1) or S-nitroacetylpenicillamine (SNAP) produced a drastic IOP-lowering effect in the rabbit eye.200 Acharya et al.201 reported the synthesis of a hybrid compound comprising SIN-1 and an SOD-mimetic group, which has the potential for prolonged NO release by quenching ROS. Topical administration of NO donors SNP and SNAP in rabbits were shown to increase NO and cyclic guanosine monophosphate (cGMP) in the aqueous humor and iris ciliary body.202 Several derivatives of PG analogs with NO donor moieties have been tested.
Mechanisms contributing to NO actions include cGMP-mediated changes in cytoskeletal elements such as inhibition of Rho kinase and myosin light chain kinase activities. In addition, NO could reduce calcium influx through l-type calcium channels by acting through potassium channels leading to membrane hyperpolarization.203 In the trabecular meshwork, Rho kinase inhibitors have been shown to inhibit MLC-2 phosphorylation and affect actomyosin organization, and cell contractility, thereby contributing to increased aqueous humor outflow.204
While there is abundant evidence for the ability of NO to increase aqueous humor outflow through the conventional outflow pathways of trabecular meshwork and Schlemm's canal, there are no clear data regarding the involvement of NO in neurodegeneration. Neufeld et al.205 demonstrated an increase in NOS-1, NOS-2, and NOS-3 as well as NOS enzymes in the ONH astrocytes of glaucoma patients. Subsequently, several studies from the same laboratory demonstrated an increase in NOS-2 expression in rats with an elevated IOP (by cauterization of their episcleral veins) and provided evidence for neuroprotective effects of NOS-2 inhibition using aminoguanidine.206,207 These studies suggested that high levels of NO released by upregulated NOS-2 contribute to damaging effects on RGCs and their cell death. However, no increase in NOS-2 was found using the Morrison's model of IOP elevation in rats; and treatment with the NOS-2 inhibitor (aminoguanidine) did not have any protective effects on neurodegeneration of the optic nerve in this model.208 The authors also found that NOS-2 immunoreactivity in ONHs and optic nerves from POAG patients was similar to those of control human eyes. Some of the discrepancies between these findings could be due to differences in the animal models of IOP elevation. In the congenital DBA/2J mouse model of glaucoma, neither deletion of NOS-2 nor treatment with aminoguanidine protected against optic neuropathy.209 On the other hand, Chen et al.210 showed an increase in nNOS (NOS-1) in Muller cells in the DBA/2J mice, and intraperitoneal administration of L-NAME protected against loss of Brn3a-positive RGCs. A recent study by Husain et al.211 reported increased immunostaining for NOS-2 in the ONHs of female rats with elevated IOP, which was attenuated by treatment with the δ-opioid receptor agonist. The precise involvement of NOS-2 in mediating neurodegeneration is still not completely clear, some of the differences in findings could be due to the transient nature of expression of the protein, which could occur in a narrow time window and vary depending upon the specific model of optic neuropathy.
The glaucoma drug nipradilol (a donor of NO and an antagonist of α-1 and β-1 and −2 adrenoreceptors) has been shown to have neuroprotective effects attributable to its NO release. Karim et al.212 demonstrated significant neuroprotection of RGCs in a rat optic nerve crush model following topical application of nipradilol. Watanabe et al.213 found nipradilol treatment produced a fourfold increase in the number of cat RGCs regenerating their axons into a transplanted peripheral nerve 4 and 6 weeks after axotomy. In another study in rabbits, Mizuno et al.214 found an increase in the number of surviving RGCs and IPL thickness following intravitreal administration of nipradilol after NMDA-induced retinal damage. The authors also demonstrated that topical application of nipradilol, but not timolol, significantly reduced ET-1-induced contraction of the retinal artery. On the other hand, Takahata et al.215 found that intravitreal administration of the NO-releasing compound N-ethyl-2-(1-ethyl-2-hydroxy-2-nitrosohydrazino)ethanamine (NOC12) produced a significant and dose-dependent decrease in cell density in the ganglion cell layer and a reduction in thickness of the inner plexiform layer. Clearly, the type of NO donor, the amount of NO released, and its duration of action could have disparate effects on neuronal survival in the retina. However, it is within the realm of possibilities to develop an NO donor that releases NO at an optimal dose with beneficial effects on both IOP lowering and neuroprotection.
Heat Shock Proteins
Heat shock proteins (HSPs) are proteins that were serendipitously found by Ritossa216 to be elevated following heat shock in drosophila. Subsequently, it was found that a variety of cellular and environmental stressors, including ultraviolet (UV) damage, low temperatures, wound healing, and tissue remodeling, also cause rapid induction of HSPs. The HSPs are important components of a cellular defense mechanism mediated by its various activities, including chaperone, antiapoptotic, and signal transduction. One classification of HSPs is according to their molecular size and distributes them into six families, including (1) small HSPs, (2) HSP40 (DNAJ), (3) HSP60 (HSPD), (4) HSP70 (HSPA), (5) HSP90 (HSPC), and (6) HSP110 (HSPH). We will focus mainly on HSP70 and crystallins since there is a considerable amount of literature on their role in glaucomatous optic neuropathy. Members of the HSP70 family are found principally in the cytosol as well as other organelles, including ER and mitochondria. HSP70 proteins comprise two main functional domains, namely, a C-terminal peptide binding domain and an N-terminal ATPase domain.217 HSP70 is able to sense the conformation of unfolded proteins, bind to them, and permit folding of denatured proteins. HSP90 interacts with various signaling molecules, steroid hormone receptors, and protein kinases. Several HSPs are coordinately induced in response to cellular stress, suggesting a shared mechanism of gene expression. The basis for this coordinated induction of gene expression is the presence of a heat shock element in all HSP gene promoters that is bound by several heat shock transcription factors (HSFs) including HSF1, HSF2, HSF3, HSF4, HSF5, HSFX, and HSFY. The predominant HSF found in vertebrates is HSF-1 that plays a pivotal role in regulation of several HSPs during conditions of cellular stress. The status of HSF-1 during glaucomatous insults has not been adequately explored and this may generate an additional avenue to upregulate HSPs as a means to promote neuroprotection.
Piri et al.218 described the expression of HSP70 and crystallins in the retina and changes in gene expression following various animal models of glaucoma. It is evident from many of these studies that HSPs play a beneficial role in promoting survival of retinal neurons by a multitude of mechanisms, some of which are already described. Interest in this area of research was stimulated by reports of elevation of antibodies to HSP27, crystallin αA, as well as crystallin αB in patients with normal-tension glaucoma, as well as POAG.219 In addition, the authors demonstrated that incubation of human retinas with antibodies to αA-crystallin, αB-crystallin, and HSP27 produced apoptotic cell death particularly in the RGC layer.219 Immunization of rats with HSP27 as well as HSP60 resulted in a significant loss of RGCs and damage to optic nerve axons.220,221 These observations are indicative of a neuroprotective role of these HSPs in RGCs.
The sHSP family contains a signature sequence of 80–100 amino acids, which is called the α-crystallin domain. Crystallins are members of the sHSP family that were originally identified in the lens fiber cells of the eye and shown to be responsible for maintaining the transparency of the lens. Subsequently, crystallins were found in several tissues, including the eye, and have been shown to protect against cellular stress and apoptosis. There are three main families of crystallins namely, α, β, and γ. Fischer et al.222 demonstrated that lens injury generated neuroprotective and neuroregenerative effects on RGC axons. These dramatic neuroprotective effects following lens injury involved the orchestration of several events, including activation and infiltration of macrophages into the eye, and the activation of various glial components, including microglia, retinal astrocytes, and Müller cells.223–226 Apart from neuroinflammation, lens-derived factors also contribute to axon regrowth of RGCs in culture.226–228 A recent publication by Bohm et al.229 demonstrated that neural progenitor cells overexpressing βB2 crystallin produced an increase in the number of neurofilament 200-positive axons from the injured site following optic nerve crush in Sprague Dawley rats.
The mRNA and protein expression of αA- and αB-crystallins are downregulated by IOP elevation following trabecular photocoagulation230; however the mRNA levels return back to control levels following 5 weeks of IOP elevation.230 Ahmed et al.231 found a decrease in mRNA expression of αA, αB, and βB2 in the retinas of Brown Norway rats subjected to the Morrison's method of IOP elevation (by administration of hypertonic saline through episcleral veins). The initial downregulation of crystallin expression could be one factor that predisposes RGCs to neurodegeneration. Data in support of this possibility come from the work of Munemasa et al.232 who demonstrated an increased RGC survival following optic nerve transection in retinas treated with αA-crystallin as well as αB-crystallin expression vectors. In another study, rats intravenously administered 50, 500 and 5,000 mg/kg of α-crystallin had an increased RGC survival that was associated with reduced microglial activation through downregulation of TNF-α and NOS-2.233,234 It is, however, unclear if this protective effect is mediated by the full-length protein or a peptide derived from proteolysis of α-crystallin HSPs, particularly HSP70 and crystallins, could act through diverse cellular mechanisms to promote neuroprotective effects. HSPs appear to be excellent candidates for robust neuroprotection due to their chaperone activities and prosurvival effects that occur under a variety of stress conditions. Several challenges remain to be addressed for HSPs, including stability, transport across the blood retinal barrier, and targeted delivery of HSPs to the RGCs.
Transcription Factors
Many diseases and syndromes are linked to mutations in the gene regulatory regions or that result in dysregulation in expression of transcription factors. The mutations are associated with various afflictions, including cancer, neurological disorders, autoimmunity, developmental syndromes, cardiovascular disease, and diabetes, among others. Specific to neurological disorders, loss of function mutations of transcription factors is connected to devastating phenotypes resulting from compromised neuronal functions. For example, loss of function mutation of early growth response protein 2 leads to the development of hypomyelinating neuropathy and axonal Charcot–Marie–Tooth disease,235 homeobox protein engrailed-2 is connected to autism,236 forkhead box protein G1 to Rett syndrome,237 forkhead box protein P2 to speech language disorder,238 and Pit-Oct-Unc (POU) class 3 homeobox 4 (Brn4) to nonsyndromic mixed deafness.239 In this study, we will provide information regarding the involvement of transcription factors in glaucoma pathogenesis, some of which emerged from the understanding of gene regulation in various neurodegenerative diseases. Retrograde axonal injury activates various transcription factors in the cell body, which results in changes to the cell's transcriptome.240,241 Recent studies have highlighted the link between disease-associated variants in regulatory DNA sequences and glaucoma.
Sine oculis homeobox homolog 6 transcription factor
Sine oculis homeobox homolog 6 (Six6) is a member of the homeodomain transcription factor family and is required for normal eye development.242 Recent genome-wide association studies have found that the Six1–Six6 and cyclin-dependent kinase inhibitor 2A, isoform INK4a (P16INK4A) loci, correlate with risk genes associated with POAG.243–245 Recently, Six6-rs33912345 (His141Asn) risk variant was found to be strongly associated with POAG.246–248 Highly upregulated expression levels of Six6 in conjunction with its target P16INK4A (as an indicator of cell senescence and aging) were detected in a mouse model 5 days following acute IOP elevation to 90 mmHg.249 Lack of either Six6 or P16INK4A protected RGCs against apoptosis in this acute IOP model.249 IOP elevation caused an increase in the levels of P16INK4A through upregulation of Six6 His variant and its binding to the p16INK4A promoter causing irreversible cell cycle arrest.249 These data could help explain the molecular link between genetic susceptibility and other factors leading to the pathogenesis of glaucoma, in particular in the context of IOP elevation.
JNK/AP-1 pathway
Two transcription factor proteins, proto-oncogenes Jun (c-Jun) and Fos (c-Fos), are the main components of the ubiquitous transcription factor, activator protein-1 (AP1), which is a key regulator of RGC death and regeneration.250 JNK belongs to the MAPK family and is associated with RGC death after axonal injury by activation of the c-Jun-dependent pathway.251 Multiple AP1 family members were induced by retrograde axonal injury and differed in their expression profile between Jun knockout retinas compared to wild-type retinas.252 Stressful conditions, such as growth factor withdrawal or UV radiation, also lead to binding of JNK to the N-terminal region of c-Jun and cause phosphorylation of c-Jun. In glaucomatous retinas (both following IOP elevation and optic nerve transection), phosphorylated JNK (p-JNK) and phosphorylated c-Jun were elevated in RGCs 2 and 7 days after laser-induced IOP elevation in rats.253 Analysis of glaucomatous human donor eyes revealed increased levels of p-JNK in nonglial retinal cells, including RGCs.254 Related to the activation of the JNK pathway is the upstream serine/threonine protein kinase, dual leucine zipper kinase (DLK), which activates the JNK pathway and initiates steps leading to apoptosis.251 If one either inhibits DLK or institutes a conditional knockdown of DLK, this prevents cells from undergoing cell death from neural insults.255 Similarly, an increase in DLK expression occurs following axonal injury and appears to be an early indicator of activation of the cell death pathway. Moreover, a small molecule inhibitor (tozasertib) binds to DLK and promotes RGC survival in an in vivo rodent optic nerve transection model.256 These studies point to DLK as an important component in the RGC death pathway that is linked to JNK activation. Recent work by Fernandes et al., suggests that blocking DLK signaling will not prevent axonal degeneration following axonal injury.255
Specific inhibition of JNK, using blocking cell permeable d-JNKI1 peptide, protected RGCs from degeneration in optic nerve crush model in mice.257 Inhibition of JNK activity with d-JNKI1 and Tacrolimus (FK506) in Morrison's model of glaucoma in rats preserved optic nerve axons specifically by inhibiting phosphorylation of 14-3-3 protein and preventing further translocation of proapoptotic BCL2-associated agonist of cell death (Bad) protein to mitochondria.258 Another JNK inhibitor, SP600125, has also been shown to prevent RGC death following a suture-pulley compression method of IOP elevation in rats.259 Furthermore, application of an SP600125 was found to significantly protect RGCs against ocular hypertension-induced cell death.260 SP600125-mediated JNK inhibition was also found to be profoundly neuroprotective in mouse retinal I/R injury model in mice.261 Other experiments have shown that deficiency in JNK2 and JNK3 isoforms, activated in retrograde-injured axons, resulted in robust neuroprotection by preventing phosphorylation of Jun and blocking downregulation of transcription factor Brn3b in controlled optic nerve crush in mice.251 Blocking N-terminal phosphorylation of c-Jun provides protection against apoptosis of RGCs in optic nerve transection model in mice.262 The interventions to block JNK signaling and AP-1 transcription factor complex activation bring up possibilities for suitable neuroprotective strategies for glaucoma. On the other hand, a lack of protection was also reported in experimental IOP elevation in bead/viscoelastic injection model in JNK3 homozygous knockout mice with an increased RGC loss compared to wild-type mice.263 Authors speculate that the removal of JNK3 during development caused compensatory upregulation of other pathways, including JNK1 and JNK2, causing RGC death.
Brn3b transcription factor
Brn3a (Pou4f1), Brn3b (Pou4f2), and Brn3c (Pou4f3) belong to the POU family of transcription factors. In the mouse retina, Brn3 proteins are localized almost exclusively in the ganglion cells.264 In Brn3b-deficient mice, during development, RGCs are specified and migrate to the ganglion cell layer. However, they are unable to develop axons and more than 70% of RGCs undergo apoptosis.265 Moreover, Brn3b plays a key role in axon genesis and axon path-finding during RGC development.264,266,267 Brn3a- and Brn3c-deficient mice do not display any retinal abnormalities, indicative of the key role of Brn3b in development of RGCs.268 Expression of Brn3b drastically decreases in the retinas in rodent models of glaucoma.251,269–271 Recombinant AAV2-mediated overexpression of Brn3b (rAAV-2-Brn3b) was found to significantly enhance survival of RGCs, maintain the integrity of optic nerve axons, and restore visual functions following Morrison's method of IOP elevation in Brown Norway rats.168 A reduction in Brn3b levels decreases expression of neuroprotective target genes, including Brn3b itself, Bcl-2, GAP-43, neurofilament l, abLIM, and KLF-6,269,272,273 thereby predisposing RGCs to axonal degeneration and cell death. AAV-mediated upregulation of transcription factor Brn3b (rAAV-2-Brn3b) increased the expression of Brn3b, Bcl-2, neurofilament-l, abLIM, and GAP-43 and enhanced the phosphorylation of Akt, thereby activating intrinsic neuroprotective mechanisms in glaucomatous RGCs.274,168 However, a study by Huang et al. showed that a conditional double knockout of Brn3a and Brn3b does not delay RGC loss following acute glaucoma model, an optic nerve crush in mice.275 This possibly could be due to other redundant protective mechanisms involving Brn3c, since it has been shown that Brn3b/Brn3c demonstrated a worse phenotype than Brn3b knockout mice, with almost complete loss of RGCs.268
ATF transcription factors
ATFs are the ATF/cyclic AMP-responsive elements and are often described as adaptive response genes, whose activity is usually regulated by stressful stimuli. ATFs interact with a number of other transcription factors, including c-Jun, to form complexes repressing or activating various target genes. It was reported that the level of phosphorylated ATF-2 (p-ATF2), the substrate of P38, was significantly elevated in the translimbal photocoagulation laser glaucoma model in rats.276 In a subsequent study, the same group demonstrated the detrimental role of p-ATF2 in primary and secondary degeneration following partial transection model of the optic nerve in rats.277 In the optic nerve crush model in rats, RGCs had a robust long-term upregulation of another member of ATF family, ATF3, along with a decreased expression of ATF2. These data suggested the prosurvival role of ATF3 during the initial phase of optic nerve injury in rats.278 ATF3 gene expression was also found to be elevated in RGCs of rats following other models of glaucoma, such as experimental IOP elevation and optic nerve transection in rats.58
Postmitotic-differentiated RGCs undergo neurodegeneration through an alteration of their transcriptional and translational regulatory mechanisms. The dissection of the regulatory networks and signaling pathways engaged by transcription factors will facilitate development of novel therapeutic strategies to protect RGCs from degeneration in glaucoma. There are several technologies available for correcting the transcription factor-imbalanced expression or repairing its mutated variant. One strategy is using RNAi fused to transcription factors for regulating target gene expression.279 Another commonly used option is antisense and RNA interference as seen with an example of a clinical trial being successful with the SMN1 gene in spinal muscular atrophy.280 Virally derived expression of transcription factors that were found to be downregulated in the disease process can be used to protect RGCs is another potential approach translatable to the clinic.168 The approach with the highest potential is the use of synthetic nucleases to allow genome editing, especially RNA-guided Clustered Regularly Interspaced Short Palindromic Repeat systems (CRISPR/Cas9). This approach allows for the selective knockout or/and repair of a defective gene with the possibility to repair patient pluripotent cells.281 CRISPR/Cas9 system may be ideal for correcting mutated transcription factors involved in glaucoma phenotypes seen during development, for example, optineurin, Six6, tumor protein 63, and GLI-similar 3.
Conclusion
The challenges in neuroprotection for glaucoma are many, including the development of adequate animal models equivalent to human disease. The above review regarding potential targets for neuroprotection were not all inclusive, but represent those areas that have received increased attention. While animal models of neurodegeneration have been developed, only a few approaches have been able to be translated into clinical trials in humans. Some of these have shown potential, while others have not. As we develop new methods and advances in monitoring and imaging technologies as well as genetic approaches to identifying key pathways to disease, new processes will be unveiled. The development of Rho kinase agents is an example of new approaches to lower IOP that may have an impact on the progression of glaucoma. Other targets, such as affecting transcription factor expression, can also have the potential if such approaches are cell or tissue specific. Such studies will provide additional understanding into the many steps and mechanisms that lead to neurodegeneration in glaucoma with the hope that future targets will be identified, which will provide optimum opportunities for therapeutic intervention and treatment.
Acknowledgments
This article was partially supported by the Department of Defence (W81XH-10-2-0003) to T.Y., the NEI grant (1R01EY019952) to R.R.K. and a grant from 2016 Fight for Sight Grant-in-Aid to D.S.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Quigley H.A. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 80:389–393, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudnicka A.R., Mt-Isa S., Owen C.G., Cook D.G., and Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest. Ophthalmol. Vis. Sci. 47:4254–4261, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Katritch V., Cherezov V., and Stevens R.C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53:531–556, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredriksson R., Lagerstrom M.C., Lundin L.G., and Schioth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63:1256–1272, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Xu H.E., and Xiao R.P. A new era for GPCR research: structures, biology and drug discovery. Acta. Pharmacol. Sin. 33:289–290, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 130:429–440, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Toris C.B., Gabelt B.T., and Kaufman P.L. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv. Ophthalmol. 53(Suppl 1):S107–S120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garway-Heath D.F., Crabb D.P., Bunce C., Lascaratos G., Amalfitano F., Anand N., Azuara-Blanco A., Bourne R.R., Broadway D.C., Cunliffe I.A., Diamond J.P., Fraser S.G., Ho T.A., Martin K.R., McNaught A.I., Negi A., Patel K., Russell R.A., Shah A., Spry P.G., Suzuki K., White E.T., Wormald R.P., Xing W., and Zeyen T.G. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 385:1295–1304, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Andrade da Costa B.L., Kang K.D., Rittenhouse K.D., and Osborne N.N. The localization of PGE2 receptor subtypes in rat retinal cultures and the neuroprotective effect of the EP2 agonist butaprost. Neurochem. Int. 55:199–207, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Lambert W.S., Ruiz L., Crish S.D., Wheeler L.A., and Calkins D.J. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol. Neurodegener. 6:4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sena D.F., and Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev. 1:Cd006539, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krupin T., Liebmann J.M., Greenfield D.S., Ritch R., and Gardiner S. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am. J. Ophthalmol. 151:671–681, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Gao H., Qiao X., Cantor L.B., and WuDunn D. Up-regulation of brain-derived neurotrophic factor expression by brimonidine in rat retinal ganglion cells. Arch. Ophthalmol. 120:797–803, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Reisberg B., Doody R., Stoffler A., Schmitt F., Ferris S., and Mobius H.J. Memantine in moderate-to-severe Alzheimer's disease. N. Engl. J. Med. 348:1333–1341, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Merello M., Nouzeilles M.I., Cammarota A., and Leiguarda R. Effect of memantine (NMDA antagonist) on Parkinson's disease: a double-blind crossover randomized study. Clin. Neuropharmacol. 22:273–276, 1999 [PubMed] [Google Scholar]
- 16.Sun D., Lye-Barthel M., Masland R.H., and Jakobs T.C. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J. Comp. Neurol. 516:1–19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badea T.C., and Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J. Comp. Neurol. 480:331–351, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ullian E.M., Barkis W.B., Chen S., Diamond J.S., and Barres B.A. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol. Cell Neurosci. 26:544–557, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Wu H.M., Tzeng N.S., Qian L., Wei S.J., Hu X., Chen S.H., Rawls S.M., Flood P., Hong J.S., and Lu R.B. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology. 34:2344–2357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka K., Nogawa S., Nagata E., Ito D., Suzuki S., Dembo T., Kosakai A., and Fukuuchi Y. Persistent CREB phosphorylation with protection of hippocampal CA1 pyramidal neurons following temporary occlusion of the middle cerebral artery in the rat. Exp. Neurol. 161:462–471, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kwak S., and Weiss J.H. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr. Opin. Neurobiol. 16:281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Lebrun-Julien F., Duplan L., Pernet V., Osswald I., Sapieha P., Bourgeois P., Dickson K., Bowie D., Barker P.A., and Di Polo A. Excitotoxic death of retinal neurons in vivo occurs via a non-cell-autonomous mechanism. J. Neurosci. 29:5536–5545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otori Y., Wei J.Y., and Barnstable C.J. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 39:972–981, 1998 [PubMed] [Google Scholar]
- 24.Sivakumar V., Foulds W.S., Luu C.D., Ling E.A., and Kaur C. Hypoxia-induced retinal ganglion cell damage through activation of AMPA receptors and the neuroprotective effects of DNQX. Exp. Eye Res. 109:83–97, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Wang A.L., Carroll R.C., and Nawy S. Down-regulation of the RNA editing enzyme ADAR2 contributes to RGC death in a mouse model of glaucoma. PLoS One. 9:e91288, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park Y.H., Mueller B.H., 2nd, McGrady N.R., Ma H.Y., and Yorio T. AMPA receptor desensitization is the determinant of AMPA receptor mediated excitotoxicity in purified retinal ganglion cells. Exp. Eye Res. 132:136–150, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Park Y.H., Broyles H.V., He S., McGrady N.R., Li L., and Yorio T. Involvement of AMPA receptor and its flip and flop isoforms in retinal ganglion cell death following oxygen/glucose deprivation. Invest. Ophthalmol. Vis. Sci. 57:508–526, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Sommer B., Keinanen K., Verdoorn T.A., Wisden W., Burnashev N., Herb A., Kohler M., Takagi T., Sakmann B., and Seeburg P.H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 249:1580–1585, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Tomiyama M., Rodriguez-Puertas R., Cortes R., Pazos A., Palacios J.M., and Mengod G. Flip and flop splice variants of AMPA receptor subunits in the spinal cord of amyotrophic lateral sclerosis. Synapse. 45:245–249, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Hatip-Al-Khatib I., Iwasaki K., Egashira N., Ishibashi D., Mishima K., and Fujiwara M. Comparison of single- and repeated-ischemia-induced changes in expression of flip and flop splice variants of AMPA receptor subtypes GluR1 and GluR2 in the rats hippocampus CA1 subregion. J. Pharmacol. Sci. 103:83–91, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Namekata K., Okumura A., Harada C., Nakamura K., Yoshida H., and Harada T. Effect of photoreceptor degeneration on RNA splicing and expression of AMPA receptors. Mol. Vis. 12:1586–1593, 2006 [PubMed] [Google Scholar]
- 32.Kobylecki C., Crossman A.R., and Ravenscroft P. Alternative splicing of AMPA receptor subunits in the 6-OHDA-lesioned rat model of Parkinson's disease and L-DOPA-induced dyskinesia. Exp. Neurol. 247:476–484, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Khaksari M., Aboutaleb N., Nasirinezhad F., Vakili A., and Madjd Z. Apelin-13 protects the brain against ischemic reperfusion injury and cerebral edema in a transient model of focal cerebral ischemia. J. Mol. Neurosci. 48:201–208, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Yang Y., Zhang X., Cui H., Zhang C., Zhu C., and Li L. Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3K/Akt and ERK1/2 signaling pathways. Neurosci. Lett. 568:44–49, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto K., Murakami Y., Sawada S., Ushikubo H., Mori A., Nakahara T., and Ishii K. Apelin-36 is protective against N-methyl-d-aspartic-acid-induced retinal ganglion cell death in the mice. Eur. J. Pharmacol. 791:213–220, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Habata Y., Fujii R., Hosoya M., Fukusumi S., Kawamata Y., Hinuma S., Kitada C., Nishizawa N., Murosaki S., Kurokawa T., Onda H., Tatemoto K., and Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta. 1452:25–35, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Ishimaru Y., Sumino A., Kajioka D., Shibagaki F., Yamamuro A., Yoshioka Y., and Maeda S. Apelin protects against NMDA-induced retinal neuronal death via an APJ receptor by activating Akt and ERK1/2, and suppressing TNF-alpha expression in mice. J. Pharmacol. Sci. 133:34–41, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Dun Y., Thangaraju M., Prasad P., Ganapathy V., and Smith S.B. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest. Ophthalmol. Vis. Sci. 48:4785–4794, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi T., and Su T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 131:596–610, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Zhang H., and Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J. Neurophysiol. 87:2867–2879, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Mavlyutov T.A., Epstein M., and Guo L.W. Subcellular localization of the sigma-1 receptor in retinal neurons - an electron microscopy study. Sci. Rep. 5:10689, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchedre K.T., Huang R.Q., Dibas A., Krishnamoorthy R.R., Dillon G.H., and Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Invest. Ophthalmol. Vis. Sci. 49:4993–5002, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Tchedre K.T., and Yorio T. sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest. Ophthalmol. Vis. Sci. 49:2577–2588, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Katnik C., Guerrero W.R., Pennypacker K.R., Herrera Y., and Cuevas J. Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. J. Pharmacol. Exp. Ther. 319:1355–1365, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Mueller B.H., 2nd, Park Y., Daudt D.R., 3rd, Ma H.Y., Akopova I., Stankowska D.L., Clark A.F., and Yorio T. Sigma-1 receptor stimulation attenuates calcium influx through activated L-type voltage gated calcium channels in purified retinal ganglion cells. Exp. Eye Res. 107:21–31, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Ellis D.Z., Li L., Park Y., He S., Mueller B., and Yorio T. Sigma-1 receptor regulates mitochondrial function in glucose- and oxygen-deprived retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 58:2755–2764, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Cantarella G., Bucolo C., Di Benedetto G., Pezzino S., Lempereur L., Calvagna R., Clementi S., Pavone P., Fiore L., and Bernardini R. Protective effects of the sigma agonist Pre-084 in the rat retina. Br. J. Ophthalmol. 91:1382–1384, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith S.B., Duplantier J., Dun Y., Mysona B., Roon P., Martin P.M., and Ganapathy V. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest. Ophthalmol. Vis. Sci. 49:4154–4161, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller B.H., 2nd, Park Y., Ma H.Y., Dibas A., Ellis D.Z., Clark A.F., and Yorio T. Sigma-1 receptor stimulation protects retinal ganglion cells from ischemia-like insult through the activation of extracellular-signal-regulated kinases 1/2. Exp. Eye Res. 128:156–169, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Zhao J., Mysona B.A., Qureshi A., Kim L., Fields T., Gonsalvez G.B., Smith S.B., and Bollinger K.E. (+)-Pentazocine reduces NMDA-induced murine retinal ganglion cell death through a sigmaR1-dependent mechanism. Invest. Ophthalmol. Vis. Sci. 57:453–461, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yorio T., Krishnamoorthy R., and Prasanna G. Endothelin: is it a contributor to glaucoma pathophysiology? J. Glaucoma. 11:259–270, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Noske W., Hensen J., and Wiederholt M. Endothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataract. Graefe's Arch. Clin. Exp. Ophthalmol. 235:551–552, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Tezel G., Kass M.A., Kolker A.E., Becker B., and Wax M.B. Plasma and aqueous humor endothelin levels in primary open-angle glaucoma. J. Glaucoma. 6:83–89, 1997 [PubMed] [Google Scholar]
- 54.Kallberg M.E., Brooks D.E., Garcia-Sanchez G.A., Komaromy A.M., Szabo N.J., and Tian L. Endothelin 1 levels in the aqueous humor of dogs with glaucoma. J. Glaucoma. 11:105–109, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Thanos S., and Naskar R. Correlation between retinal ganglion cell death and chronically developing inherited glaucoma in a new rat mutant. Exp. Eye Res. 79:119–129, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Prasanna G., Hulet C., Desai D., Krishnamoorthy R.R., Narayan S., Brun A.M., Suburo A.M., and Yorio T. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol. Res. 51:41–50, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Howell G.R., Macalinao D.G., Sousa G.L., Walden M., Soto I., Kneeland S.C., Barbay J.M., King B.L., Marchant J.K., Hibbs M., Stevens B., Barres B.A., Clark A.F., Libby R.T., and John S.W. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Investig. 121:1429–1444, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Z., Quigley H.A., Pease M.E., Yang Y., Qian J., Valenta D., and Zack D.J. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest. Ophthalmol. Vis. Sci. 48:5539–5548, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Orgul S., Cioffi G.A., Bacon D.R., and Van Buskirk E.M. An endothelin-1-induced model of chronic optic nerve ischemia in rhesus monkeys. J. Glaucoma. 5:135–138, 1996 [PubMed] [Google Scholar]
- 60.Cioffi G.A., and Sullivan P. The effect of chronic ischemia on the primate optic nerve. Eur. J. Ophthalmol. 9(Suppl 1):S34–S36, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Lau J., Dang M., Hockmann K., and Ball A.K. Effects of acute delivery of endothelin-1 on retinal ganglion cell loss in the rat. Exp. Eye Res. 82:132–145, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Wang L., Fortune B., Cull G., Dong J., and Cioffi G.A. Endothelin B receptor in human glaucoma and experimentally induced optic nerve damage. Arch. Ophthalmol. 124:717–724, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Krishnamoorthy R.R., Rao V.R., Dauphin R., Prasanna G., Johnson C., and Yorio T. Role of the ETB receptor in retinal ganglion cell death in glaucoma. Can. J. Physiol. Pharmacol. 86:380–393, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Minton A.Z., Phatak N.R., Stankowska D.L., He S., Ma H.Y., Mueller B.H., Jiang M., Luedtke R., Yang S., Brownlee C., and Krishnamoorthy R.R. Endothelin B receptors contribute to retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 7:e43199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He S., Park Y.H., Yorio T., and Krishnamoorthy R.R. Endothelin-mediated changes in gene expression in isolated purified rat retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 56:6144–6161, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chauhan B.C., LeVatte T.L., Jollimore C.A., Yu P.K., Reitsamer H.A., Kelly M.E., Yu D.Y., Tremblay F., and Archibald M.L. Model of endothelin-1-induced chronic optic neuropathy in rat. Invest. Ophthalmol. Vis. Sci. 45:144–152, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Rogers S.D., Demaster E., Catton M., Ghilardi J.R., Levin L.A., Maggio J.E., and Mantyh P.W. Expression of endothelin-B receptors by glia in vivo is increased after CNS injury in rats, rabbits, and humans. Exp. Neurol. 145:180–195, 1997 [DOI] [PubMed] [Google Scholar]
- 68.Howell G.R., MacNicoll K.H., Braine C.E., Soto I., Macalinao D.G., Sousa G.L., and John S.W. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol. Dis. 71:44–52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Resch H., Karl K., Weigert G., Wolzt M., Hommer A., Schmetterer L., and Garhofer G. Effect of dual endothelin receptor blockade on ocular blood flow in patients with glaucoma and healthy subjects. Invest. Ophthalmol. Vis. Sci. 50:358–363, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog. Brain Res. 173:409–421, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park K.M., and Bowers W.J. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell. Signal. 22:977–983, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tezel G., Li L.Y., Patil R.V., and Wax M.B. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 42:1787–1794, 2001 [PubMed] [Google Scholar]
- 73.Nakazawa T., Nakazawa C., Matsubara A., Noda K., Hisatomi T., She H., Michaud N., Hafezi-Moghadam A., Miller J.W., and Benowitz L.I. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci. 26:12633–12641, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prasanna G., Dibas A., Tao W., White K., and Yorio T. Regulation of endothelin-1 in human non-pigmented ciliary epithelial cells by tumor necrosis factor-alpha. Exp. Eye Res. 66:9–18, 1998 [DOI] [PubMed] [Google Scholar]
- 75.Prasanna G., Krishnamoorthy R., Clark A.F., Wordinger R.J., and Yorio T. Human optic nerve head astrocytes as a target for endothelin-1. Invest. Ophthalmol. Vis. Sci. 43:2704–2713, 2002 [PubMed] [Google Scholar]
- 76.Narayan S., Prasanna G., Krishnamoorthy R.R., Zhang X., and Yorio T. Endothelin-1 synthesis and secretion in human retinal pigment epithelial cells (ARPE-19): differential regulation by cholinergics and TNF-alpha. Invest. Ophthalmol. Vis. Sci. 44:4885–4894, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Williams P.A., Marsh-Armstrong N., and Howell G.R. Neuroinflammation in glaucoma: a new opportunity. Exp. Eye Res. 157:20–27, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roh M., Zhang Y., Murakami Y., Thanos A., Lee S.C., Vavvas D.G., Benowitz L.I., and Miller J.W. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 7:e40065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minegishi Y., Nakayama M., Iejima D., Kawase K., and Iwata T. Significance of optineurin mutations in glaucoma and other diseases. Prog. Retin. Eye Res. 55:149–181, 2016 [DOI] [PubMed] [Google Scholar]
- 80.Sarfarazi M., and Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol. Clin. North Am. 16:529–541, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Zhu G., Wu C.-J., Zhao Y., and Ashwell J.D. Optineurin negatively regulates TNFα-induced NF-κB activation by competing with NEMO for ubiquitinated RIP. Curr. Biol. 17:1438–1443, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Munch A.E., Chung W.S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., and Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541:481–487, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pinazo-Durán M.D., Zanón-Moreno V., García-Medina J.J., and Gallego-Pinazo R. Evaluation of presumptive biomarkers of oxidative stress, immune response and apoptosis in primary open-angle glaucoma. Curr. Opin. Pharmacol. 13:98–107, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Saccà S.C., and Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog. Brain Res. 173:385–407, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Zanon-Moreno V., Marco-Ventura P., Lleo-Perez A., Pons-Vazquez S., Garcia-Medina J.J., Vinuesa-Silva I., Moreno-Nadal M.A., and Pinazo-Duran M.D. Oxidative stress in primary open-angle glaucoma. J. Glaucoma. 17:263–268, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog. Retin. Eye Res. 25:490–513, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benoist d'Azy C., Pereira B., Chiambaretta F., and Dutheil F. Oxidative and anti-oxidative stress markers in chronic glaucoma: a systematic review and meta-analysis. PLoS One. 11:e0166915, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuenca N., Fernández-Sánchez L., Campello L., Maneu V., De la Villa P., Lax P., and Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 43:17–75, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Erdurmus M., Yagci R., Atis O., Karadag R., Akbas A., and Hepsen I.F. Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Curr. Eye Res. 36:713–718, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Cybulska-Heinrich A.K., Mozaffarieh M., and Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Mol. Vis. 18:390–402, 2012 [PMC free article] [PubMed] [Google Scholar]
- 91.Williams P.A., Harder J.M., Foxworth N.E., Cochran K.E., Philip V.M., Porciatti V., Smithies O., and John S.W. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 355:756–760, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee D., Shim M.S., Kim K.Y., Noh Y.H., Kim H., Kim S.Y., Weinreb R.N., and Ju W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest. Ophthalmol. Vis. Sci. 55:993–1005, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee D., Kim K.-Y., Shim M.S., Kim S.Y., Ellisman M.H., Weinreb R.N., Ju W.-K. Coenzyme Q10 ameliorates oxidative stress and prevents mitochondrial alteration in ischemic retinal injury. Apoptosis. 19:603–614, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nucci C., Tartaglione R., Cerulli A., Mancino R., Spano A., Cavaliere F., Rombola L., Bagetta G., Corasaniti M.T., and Morrone L.A. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int. Rev. Neurobiol. 82:397–406, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Russo R., Cavaliere F., Rombola L., Gliozzi M., Cerulli A., Nucci C., Fazzi E., Bagetta G., Corasaniti M.T., and Morrone L.A. Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection. Prog. Brain Res. 173:575–582, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Can N., Catak O., Turgut B., Demir T., Ilhan N., Kuloglu T., and Ozercan I.H. Neuroprotective and antioxidant effects of ghrelin in an experimental glaucoma model. Drug Des. Devel. Ther. 9:2819–2829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H., Liang Y., Chiu K., Yuan Q., Lin B., Chang R.C., and So K.F. Lycium barbarum (wolfberry) reduces secondary degeneration and oxidative stress, and inhibits JNK pathway in retina after partial optic nerve transection. PLoS One. 8:e68881, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mi X.S., Feng Q., Lo A.C., Chang R.C., Lin B., Chung S.K., and So K.F. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS One. 7:e45469, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inman D.M., Lambert W.S., Calkins D.J., and Horner P.J. Alpha-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS One. 8:e65389, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Razali N., Agarwal R., Agarwal P., Kumar S., Tripathy M., Vasudevan S., Crowston J.G., and Ismail N.M. Role of adenosine receptors in resveratrol-induced intraocular pressure lowering in rats with steroid-induced ocular hypertension. Clin. Exp. Ophthalmol. 43:54–66, 2015 [DOI] [PubMed] [Google Scholar]
- 101.Pirhan D., Yuksel N., Emre E., Cengiz A., and Kursat Yildiz D. Riluzole- and resveratrol-induced delay of retinal ganglion cell death in an experimental model of glaucoma. Curr. Eye Res. 41:59–69, 2016 [DOI] [PubMed] [Google Scholar]
- 102.Lindsey J.D., Duong-Polk K.X., Hammond D., Leung C.K., and Weinreb R.N. Protection of injured retinal ganglion cell dendrites and unfolded protein response resolution after long-term dietary resveratrol. Neurobiol. Aging. 36:1969–1981, 2015 [DOI] [PubMed] [Google Scholar]
- 103.Razali N., Agarwal R., Agarwal P., Tripathy M., Kapitonova M.Y., Kutty M.K., Smirnov A., Khalid Z., and Ismail N.M. Topical trans-resveratrol ameliorates steroid-induced anterior and posterior segment changes in rats. Exp. Eye Res. 143:9–16, 2016 [DOI] [PubMed] [Google Scholar]
- 104.Parisi V., Centofanti M., Gandolfi S., Marangoni D., Rossetti L., Tanga L., Tardini M., Traina S., Ungaro N., Vetrugno M., and Falsini B. Effects of coenzyme Q10 in conjunction with vitamin E on retinal-evoked and cortical-evoked responses in patients with open-angle glaucoma. J. Glaucoma. 23:391–404, 2014 [DOI] [PubMed] [Google Scholar]
- 105.Park J.W., Kwon H.J., Chung W.S., Kim C.Y., and Seong G.J. Short-term effects of Ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J. Ophthalmol. 25:323–328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quaranta L., Bettelli S., Uva M.G., Semeraro F., Turano R., and Gandolfo E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 110:359–362; discussion 362–354, 2003 [DOI] [PubMed] [Google Scholar]
- 107.Guo X., Kong X., Huang R., Jin L., Ding X., He M., Liu X., Patel M.C., and Congdon N.G. Effect of Ginkgo biloba on visual field and contrast sensitivity in Chinese patients with normal tension glaucoma: a randomized, crossover clinical trial. Invest. Ophthalmol. Vis. Sci. 55:110–116, 2014 [DOI] [PubMed] [Google Scholar]
- 108.Garcia-Medina J.J., Garcia-Medina M., Garrido-Fernandez P., Galvan-Espinosa J., Garcia-Maturana C., Zanon-Moreno V., and Pinazo-Duran M.D. A two-year follow-up of oral antioxidant supplementation in primary open-angle glaucoma: an open-label, randomized, controlled trial. Acta. Ophthalmol. 93:546–554, 2015 [DOI] [PubMed] [Google Scholar]
- 109.Milea D., and Aung T. Flavonoids and glaucoma: revisiting therapies from the past. Graefe's Arch. Clin. Exp. Ophthalmol. 253:1839–1840, 2015 [DOI] [PubMed] [Google Scholar]
- 110.Patel S., Mathan J.J., Vaghefi E., and Braakhuis A.J. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefe's Arch. Clin. Exp. Ophthalmol. 253:1841–1850, 2015 [DOI] [PubMed] [Google Scholar]
- 111.Wang S.Y., Singh K., and Lin S.C. Glaucoma and vitamins A, C, and E supplement intake and serum levels in a population-based sample of the United States. Eye. 27:487–494, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirooka K., Tokuda M., Miyamoto O., Itano T., Baba T., and Shiraga F. The Ginkgo biloba extract (EGb 761) provides a neuroprotective effect on retinal ganglion cells in a rat model of chronic glaucoma. Curr. Eye Res. 28:153–157, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Jia L.Y., Sun L., Fan D.S., Lam D.S., Pang C.P., and Yam G.H. Effect of topical Ginkgo biloba extract on steroid-induced changes in the trabecular meshwork and intraocular pressure. Arch. Ophthalmol. 126:1700–1706, 2008 [DOI] [PubMed] [Google Scholar]
- 114.Chung H.S., Harris A., Kristinsson J.K., Ciulla T.A., Kagemann C., and Ritch R. Ginkgo biloba extract increases ocular blood flow velocity. J. Ocul. Pharmacol. Ther. 15:233–240, 1999 [DOI] [PubMed] [Google Scholar]
- 115.Tak E., Park G.C., Kim S.H., Jun D.Y., Lee J., Hwang S., Song G.W., and Lee S.G. Epigallocatechin-3-gallate protects against hepatic ischaemia-reperfusion injury by reducing oxidative stress and apoptotic cell death. J. Int. Med. Res. 44:1248–1262, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peluso I., and Serafini M. Antioxidants from black and green tea: from dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 174:1195–1208, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shan D., Fang Y., Ye Y., and Liu J. EGCG reducing the susceptibility to cholesterol gallstone formation through the regulation of inflammation. Biomed. Pharmacother. 62:677–683, 2008 [DOI] [PubMed] [Google Scholar]
- 118.Li M., Liu J.T., Pang X.M., Han C.J., and Mao J.J. Epigallocatechin-3-gallate inhibits angiotensin II and interleukin-6-induced C-reactive protein production in macrophages. Pharm. Rep. 64:912–918, 2012 [DOI] [PubMed] [Google Scholar]
- 119.Chung F.-L., Xu Y., Jin C.-L., and Wang M. Tea as antioxidant in prevention of lung cancer. In: Ohigashi H., Osawa T., Terao J., et al., eds. Food Factors for Cancer Prevention. Tokyo, Japan: Springer; 1997; p. 130–133 [Google Scholar]
- 120.Stoner G.D., and Mukhtar H. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. Suppl. 22:169–180, 1995 [DOI] [PubMed] [Google Scholar]
- 121.Yao Y.F., Liu X., Li W.J., Shi Z.W., Yan Y.X., Wang L.F., Chen M., and Xie M.Y. (-)-Epigallocatechin-3-gallate alleviates doxorubicin-induced cardiotoxicity in sarcoma 180 tumor-bearing mice. Life Sci. 180:151–159, 2016 [DOI] [PubMed] [Google Scholar]
- 122.Suganuma M., Takahashi A., Watanabe T., Iida K., Matsuzaki T., Yoshikawa H.Y., and Fujiki H. Biophysical approach to mechanisms of cancer prevention and treatment with green tea catechins. Molecules. 21:E1566, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xie J., Jiang L., Zhang T., Jin Y., Yang D., and Chen F. Neuroprotective effects of epigallocatechin-3-gallate (EGCG) in optic nerve crush model in rats. Neurosci. Lett. 479:26–30, 2010 [DOI] [PubMed] [Google Scholar]
- 124.Shen C., Chen L., Jiang L., and Lai T.Y. Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neurosci. Lett. 600:132–136, 2015 [DOI] [PubMed] [Google Scholar]
- 125.Peng P.H., Chiou L.F., Chao H.M., Lin S., Chen C.F., Liu J.H., and Ko M.L. Effects of epigallocatechin-3-gallate on rat retinal ganglion cells after optic nerve axotomy. Exp. Eye Res. 90:528–534, 2010 [DOI] [PubMed] [Google Scholar]
- 126.Zhang B., Safa R., Rusciano D., and Osborne N.N. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 1159:40–53, 2007 [DOI] [PubMed] [Google Scholar]
- 127.Peng P.H., Ko M.L., and Chen C.F. Epigallocatechin-3-gallate reduces retinal ischemia/reperfusion injury by attenuating neuronal nitric oxide synthase expression and activity. Exp. Eye Res. 86:637–646, 2008 [DOI] [PubMed] [Google Scholar]
- 128.Joe M.K., Nakaya N., Abu-Asab M., and Tomarev S.I. Mutated myocilin and heterozygous SOD2 deficiency act synergistically in a mouse model of open-angle glaucoma. Hum. Mol. Genet. 24:3322–3334, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abu-Amero K.K., Kondkar A.A., Mousa A., Osman E.A., and Al-Obeidan S.A. Association of Mn-SOD mutation (c.47T > C) with various POAG clinical indices. Ophthalmic. Genet. 35:85–90, 2014 [DOI] [PubMed] [Google Scholar]
- 130.Zhou Y., Shuai P., Li X., Liu X., Wang J., Yang Y., Hao F., Lin H., Zhang D., and Gong B. Association of SOD2 polymorphisms with primary open angle glaucoma in a Chinese population. Ophthalmic. Genet. 36:43–49, 2015 [DOI] [PubMed] [Google Scholar]
- 131.Celojevic D., Nilsson S., Kalaboukhova L., Tasa G., Juronen E., Sjolander A., Zetterberg H., and Zetterberg M. Genetic variation of superoxide dismutases in patients with primary open-angle glaucoma. Ophthalmic. Genet. 35:79–84, 2014 [DOI] [PubMed] [Google Scholar]
- 132.Rokicki W., Zalejska-Fiolka J., Pojda-Wilczek D., Kabiesz A., and Majewski W. Oxidative stress in the red blood cells of patients with primary open-angle glaucoma. Clin. Hemorheol. Microcirc. 62:369–378, 2016 [DOI] [PubMed] [Google Scholar]
- 133.Jiang W., Tang L., Zeng J., and Chen B. Adeno-associated virus mediated SOD gene therapy protects the retinal ganglion cells from chronic intraocular pressure elevation induced injury via attenuating oxidative stress and improving mitochondrial dysfunction in a rat model. Am. J. Transl. Res. 8:799–810, 2016 [PMC free article] [PubMed] [Google Scholar]
- 134.Chen B., Caballero S., Seo S., Grant M.B., and Lewin A.S. Delivery of antioxidant enzyme genes to protect against ischemia/reperfusion-induced injury to retinal microvasculature. Invest. Ophthalmol. Vis. Sci. 50:5587–5595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Y., Tang L., and Chen B. Effects of antioxidant gene therapy on retinal neurons and oxidative stress in a model of retinal ischemia/reperfusion. Free Radic. Biol. Med. 52:909–915, 2012 [DOI] [PubMed] [Google Scholar]
- 136.Schieber M., and Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24:R453–R462, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bubici C., Papa S., Pham C.G., Zazzeroni F., and Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol. Histopathol. 21:69–80, 2006 [DOI] [PubMed] [Google Scholar]
- 138.D'Autreaux B., and Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8:813–824, 2007 [DOI] [PubMed] [Google Scholar]
- 139.St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jager S., Handschin C., Zheng K., Lin J., Yang W., Simon D.K., Bachoo R., and Spiegelman B.M. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 127:397–408, 2006 [DOI] [PubMed] [Google Scholar]
- 140.Kensler T.W., Wakabayashi N., and Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47:89–116, 2007 [DOI] [PubMed] [Google Scholar]
- 141.Himori N., Yamamoto K., Maruyama K., Ryu M., Taguchi K., Yamamoto M., and Nakazawa T. Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. J. Neurochem. 127:669–680, 2013 [DOI] [PubMed] [Google Scholar]
- 142.Xu Z., Cho H., Hartsock M.J., Mitchell K.L., Gong J., Wu L., Wei Y., Wang S., Thimmulappa R.K., Sporn M.B., Biswal S., Welsbie D.S., and Duh E.J. Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion. J. Neurochem. 133:233–241, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCord J.M. Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 312:159–163, 1985 [DOI] [PubMed] [Google Scholar]
- 144.Zweier J.L., Flaherty J.T., and Weisfeldt M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. U. S. A. 84:1404–1407, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yokota H., Narayanan S.P., Zhang W., Liu H., Rojas M., Xu Z., Lemtalsi T., Nagaoka T., Yoshida A., Brooks S.E., Caldwell R.W., and Caldwell R.B. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest. Ophthalmol. Vis. Sci. 52:8123–8131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.-L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 1:45–49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tait S.W., and Green D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11:621–632, 2010 [DOI] [PubMed] [Google Scholar]
- 148.Wallace D.C., Singh G., Lott M.T., Hodge J.A., Schurr T.G., Lezza A.M., Elsas L.J., 2nd, and Nikoskelainen E.K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 242:1427–1430, 1988 [DOI] [PubMed] [Google Scholar]
- 149.Singh G., Lott M.T., and Wallace D.C. A mitochondrial DNA mutation as a cause of Leber's hereditary optic neuropathy. N. Engl. J. Med. 320:1300–1305, 1989 [DOI] [PubMed] [Google Scholar]
- 150.Delettre C., Lenaers G., Griffoin J.M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E., Astarie-Dequeker C., Lasquellec L., Arnaud B., Ducommun B., Kaplan J., and Hamel C.P. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat. Genet. 26:207–210, 2000 [DOI] [PubMed] [Google Scholar]
- 151.Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., Bhattacharya S.S., and Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 26:211–215, 2000 [DOI] [PubMed] [Google Scholar]
- 152.Abu-Amero K.K., Morales J., and Bosley T.M. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 47:2533–2541, 2006 [DOI] [PubMed] [Google Scholar]
- 153.Lopez Sanchez M.I., Crowston J.G., Mackey D.A., and Trounce I.A. Emerging mitochondrial therapeutic targets in optic neuropathies. Pharmacol. Ther. 165:132–152, 2016 [DOI] [PubMed] [Google Scholar]
- 154.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., and Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 392:605–608, 1998 [DOI] [PubMed] [Google Scholar]
- 155.Clark I.E., Dodson M.W., Jiang C., Cao J.H., Huh J.R., Seol J.H., Yoo S.J., Hay B.A., and Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 441:1162–1166, 2006 [DOI] [PubMed] [Google Scholar]
- 156.Park J., Lee S.B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J.M., and Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 441:1157–1161, 2006 [DOI] [PubMed] [Google Scholar]
- 157.Lee S., Sheck L., Crowston J.G., Van Bergen N.J., O'Neill E.C., O'Hare F., Kong Y.X., Chrysostomou V., Vincent A.L., and Trounce I.A. Impaired complex-I-linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts. Invest. Ophthalmol. Vis. Sci. 53:2431–2437, 2012 [DOI] [PubMed] [Google Scholar]
- 158.Khawaja A.P., Cooke Bailey J.N., Kang J.H., Allingham R.R., Hauser M.A., Brilliant M., Budenz D.L., Christen W.G., Fingert J., Gaasterland D., Gaasterland T., Kraft P., Lee R.K., Lichter P.R., Liu Y., Medeiros F., Moroi S.E., Richards J.E., Realini T., Ritch R., Schuman J.S., Scott W.K., Singh K., Sit A.J., Vollrath D., Wollstein G., Zack D.J., Zhang K., Pericak-Vance M., Weinreb R.N., Haines J.L., Pasquale L.R., and Wiggs J.L. Assessing the association of mitochondrial genetic variation with primary open-angle glaucoma using gene-set analyses. Invest. Ophthalmol. Vis. Sci. 57:5046–5052, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abu-Amero K.K., and Bosley T.M. Detection of mitochondrial respiratory dysfunction in circulating lymphocytes using resazurin. Arch. Pathol. Lab. Med. 129:1295–1298, 2005 [DOI] [PubMed] [Google Scholar]
- 160.Lambert W., Agarwal R., Howe W., Clark A.F., and Wordinger R.J. Neurotrophin and neurotrophin receptor expression by cells of the human lamina cribrosa. Invest. Ophthalmol. Vis. Sci. 42:2315–2323, 2001 [PubMed] [Google Scholar]
- 161.MacPhee I., and Barker P.A. Extended ceramide exposure activates the TrkA receptor by increasing receptor homodimer formation. J. Neurochem. 72:1423–1430, 1999 [DOI] [PubMed] [Google Scholar]
- 162.Marshall C.J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 80:179–185, 1995 [DOI] [PubMed] [Google Scholar]
- 163.Arevalo J.C., Yano H., Teng K.K., and Chao M.V. A unique pathway for sustained neurotrophin signaling through an ankyrin-rich membrane-spanning protein. EMBO J. 23:2358–2368, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma Y.T., Hsieh T., Forbes M.E., Johnson J.E., and Frost D.O. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J. Neurosci. 18:2097–2107, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ibanez C.F. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 17:519–528, 2007 [DOI] [PubMed] [Google Scholar]
- 166.Johansson J.O. Inhibition and recovery of retrograde axoplasmic transport in rat optic nerve during and after elevated IOP in vivo. Exp. Eye Res. 46:223–227, 1988 [DOI] [PubMed] [Google Scholar]
- 167.Crish S.D., Sappington R.M., Inman D.M., Horner P.J., and Calkins D.J. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 107:5196–5201, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stankowska D.L., Minton A.Z., Rutledge M.A., Mueller B.H., 2nd, Phatak N.R., He S., Ma H.Y., Forster M.J., Yorio T., and Krishnamoorthy R.R. Neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Invest. Ophthalmol. Vis. Sci. 56:893–907, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Quigley H.A., McKinnon S.J., Zack D.J., Pease M.E., Kerrigan-Baumrind L.A., Kerrigan D.F., and Mitchell R.S. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest. Ophthalmol. Vis. Sci. 41:3460–3466, 2000 [PubMed] [Google Scholar]
- 170.Pease M.E., McKinnon S.J., Quigley H.A., Kerrigan-Baumrind L.A., and Zack D.J. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 41:764–774, 2000 [PubMed] [Google Scholar]
- 171.Crish S.D., Dapper J.D., MacNamee S.E., Balaram P., Sidorova T.N., Lambert W.S., and Calkins D.J. Failure of axonal transport induces a spatially coincident increase in astrocyte BDNF prior to synapse loss in a central target. Neuroscience. 229:55–70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Raju T.R., Rao M.S., Nagaraja T.N., Meti B.L., and Schulz M. Retinal ganglion cell survival and neurite regeneration in vitro after cell death period are dependent upon target derived trophic factor and retinal glial factor(s). Brain Res. 664:247–251, 1994 [DOI] [PubMed] [Google Scholar]
- 173.Ugolini G., Cremisi F., and Maffei L. TrkA, TrkB and p75 mRNA expression is developmentally regulated in the rat retina. Brain Res. 704:121–124, 1995 [DOI] [PubMed] [Google Scholar]
- 174.Seki M., Fukuchi T., Tanaka T., Nawa H., Takei N., and Abe H. Quantitative analyses of mRNA and protein levels of neurotrophin-3 in the rat retina during postnatal development and aging. Jpn. J. Ophthalmol. 48:460–464, 2004 [DOI] [PubMed] [Google Scholar]
- 175.Johnson E.C., Deppmeier L.M., Wentzien S.K., Hsu I., and Morrison J.C. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest. Ophthalmol. Vis. Sci. 41:431–442, 2000 [PubMed] [Google Scholar]
- 176.Kimpinski K., Campenot R.B., and Mearow K. Effects of the neurotrophins nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF) on neurite growth from adult sensory neurons in compartmented cultures. J. Neurobiol. 33:395–410, 1997 [DOI] [PubMed] [Google Scholar]
- 177.Kuruvilla R., Ye H., and Ginty D.D. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 27:499–512, 2000 [DOI] [PubMed] [Google Scholar]
- 178.Clarke P.G., Posada A., Primi M.P., and Castagne V. Neuronal death in the central nervous system during development. Biomed. Pharmacother. 52:356–362, 1998 [DOI] [PubMed] [Google Scholar]
- 179.Di Polo A., Aigner L.J., Dunn R.J., Bray G.M., and Aguayo A.J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc. Natl. Acad. Sci. U. S. A. 95:3978–3983, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Isenmann S., Klocker N., Gravel C., and Bahr M. Short communication: protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. Eur. J. Neurosci. 10:2751–2756, 1998 [DOI] [PubMed] [Google Scholar]
- 181.Bahr M. Live or let die—retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 23:483–490, 2000 [DOI] [PubMed] [Google Scholar]
- 182.Chen H., and Weber A.J. Brain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult rats. Brain Res. 1011:99–106, 2004 [DOI] [PubMed] [Google Scholar]
- 183.Coassin M., Lambiase A., Sposato V., Micera A., Bonini S., and Aloe L. Retinal p75 and bax overexpression is associated with retinal ganglion cells apoptosis in a rat model of glaucoma. Graefe's Arch. Clin. Exp. Ophthalmol. 246:1743–1749, 2008 [DOI] [PubMed] [Google Scholar]
- 184.Rudzinski M., Wong T.P., and Saragovi H.U. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J. Neurobiol. 58:341–354, 2004 [DOI] [PubMed] [Google Scholar]
- 185.Lambiase A., Aloe L., Centofanti M., Parisi V., Bao S.N., Mantelli F., Colafrancesco V., Manni G.L., Bucci M.G., Bonini S., and Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucoma. Proc. Natl. Acad. Sci. U. S. A. 106:13469–13474, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Colafrancesco V., Parisi V., Sposato V., Rossi S., Russo M.A., Coassin M., Lambiase A., and Aloe L. Ocular application of nerve growth factor protects degenerating retinal ganglion cells in a rat model of glaucoma. J. Glaucoma. 20:100–108, 2011 [DOI] [PubMed] [Google Scholar]
- 187.Pease M.E., Zack D.J., Berlinicke C., Bloom K., Cone F., Wang Y., Klein R.L., Hauswirth W.W., and Quigley H.A. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 50:2194–2200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mathews M.K., Guo Y., Langenberg P., and Bernstein S.L. Ciliary neurotrophic factor (CNTF)-mediated ganglion cell survival in a rodent model of non-arteritic anterior ischaemic optic neuropathy (NAION). Br. J. Ophthalmol. 99:133–137, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kimura A., Namekata K., Guo X., Harada C., and Harada T. Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. Int. J. Mol. Sci. 17:E1584, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kauper K., McGovern C., Sherman S., Heatherton P., Rapoza R., Stabila P., Dean B., Lee A., Borges S., Bouchard B., and Tao W. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest. Ophthalmol. Vis. Sci. 53:7484–7491, 2012 [DOI] [PubMed] [Google Scholar]
- 191.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M., Rossi S., Lyubarsky A., Arruda V.R., Konkle B., Stone E., Sun J., Jacobs J., Dell'Osso L., Hertle R., Ma J.X., Redmond T.M., Zhu X., Hauck B., Zelenaia O., Shindler K.S., Maguire M.G., Wright J.F., Volpe N.J., McDonnell J.W., Auricchio A., High K.A., and Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358:2240–2248, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N., Petersen-Jones S., Bhattacharya S.S., Thrasher A.J., Fitzke F.W., Carter B.J., Rubin G.S., Moore A.T., and Ali R.R. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358:2231–2239, 2008 [DOI] [PubMed] [Google Scholar]
- 193.Martin K.R., Quigley H.A., Zack D.J., Levkovitch-Verbin H., Kielczewski J., Valenta D., Baumrind L., Pease M.E., Klein R.L., and Hauswirth W.W. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest. Ophthalmol. Vis. Sci. 44:4357–4365, 2003 [DOI] [PubMed] [Google Scholar]
- 194.Cheng L., Sapieha P., Kittlerova P., Hauswirth W.W., and Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J. Neurosci. 22:3977–3986, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bull N.D., Irvine K.A., Franklin R.J., and Martin K.R. Transplanted oligodendrocyte precursor cells reduce neurodegeneration in a model of glaucoma. Invest. Ophthalmol. Vis. Sci. 50:4244–4253, 2009 [DOI] [PubMed] [Google Scholar]
- 196.Schehlein E.M., Novack G.D., and Robin A.L. New classes of glaucoma medications. Curr. Opin. Ophthalmol. 28:161–168, 2017 [DOI] [PubMed] [Google Scholar]
- 197.Borghi V., Bastia E., Guzzetta M., Chiroli V., Toris C.B., Batugo M.R., Carreiro S.T., Chong W.K., Gale D.C., Kucera D.J., Jia L., Prasanna G., Ongini E., Krauss A.H., and Impagnatiello F. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J. Ocul. Pharmacol. Ther. 26:125–132, 2010 [DOI] [PubMed] [Google Scholar]
- 198.Impagnatiello F., Borghi V., Gale D.C., Batugo M., Guzzetta M., Brambilla S., Carreiro S.T., Chong W.K., Prasanna G., Chiroli V., Ongini E., and Krauss A.H. A dual acting compound with latanoprost amide and nitric oxide releasing properties, shows ocular hypotensive effects in rabbits and dogs. Exp. Eye Res. 93:243–249, 2011 [DOI] [PubMed] [Google Scholar]
- 199.Krauss A.H., Impagnatiello F., Toris C.B., Gale D.C., Prasanna G., Borghi V., Chiroli V., Chong W.K., Carreiro S.T., and Ongini E. Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2alpha agonist, in preclinical models. Exp. Eye Res. 93:250–255, 2011 [DOI] [PubMed] [Google Scholar]
- 200.Behar-Cohen F.F., Goureau O., D'Hermies F., and Courtois Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Invest. Ophthalmol. Vis. Sci. 37:1711–1715, 1996 [PubMed] [Google Scholar]
- 201.Acharya S., Rogers P., Krishnamoorthy R.R., Stankowska D.L., Dias H.V., and Yorio T. Design and synthesis of novel hybrid sydnonimine and prodrug useful for glaucomatous optic neuropathy. Bioorg. Med. Chem. Lett. 26:1490–1494, 2016 [DOI] [PubMed] [Google Scholar]
- 202.Carreiro S., Anderson S., Gukasyan H.J., Krauss A., and Prasanna G. Correlation of in vitro and in vivo kinetics of nitric oxide donors in ocular tissues. J. Ocul. Pharmacol. Ther. 25:105–112, 2009 [DOI] [PubMed] [Google Scholar]
- 203.Stumpff F., and Wiederholt M. Regulation of trabecular meshwork contractility. Ophthalmologica. 214:33–53, 2000 [DOI] [PubMed] [Google Scholar]
- 204.Prasanna G., Li B., Mogi M., and Rice D.S. Pharmacology of novel intraocular pressure-lowering targets that enhance conventional outflow facility: pitfalls, promises and what lies ahead? Eur. J. Pharmacol. 787:47–56, 2016 [DOI] [PubMed] [Google Scholar]
- 205.Neufeld A.H., Hernandez M.R., and Gonzalez M. Nitric oxide synthase in the human glaucomatous optic nerve head. Arch. Ophthalmol. 115:497–503, 1997 [DOI] [PubMed] [Google Scholar]
- 206.Shareef S., Sawada A., and Neufeld A.H. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest. Ophthalmol. Vis. Sci. 40:2884–2891, 1999 [PubMed] [Google Scholar]
- 207.Neufeld A.H., Sawada A., and Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc. Natl. Acad. Sci. U. S. A. 96:9944–9948, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pang I.H., Johnson E.C., Jia L., Cepurna W.O., Shepard A.R., Hellberg M.R., Clark A.F., and Morrison J.C. Evaluation of inducible nitric oxide synthase in glaucomatous optic neuropathy and pressure-induced optic nerve damage. Invest. Ophthalmol. Vis. Sci. 46:1313–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 209.Libby R.T., Howell G.R., Pang I.H., Savinova O.V., Mehalow A.K., Barter J.W., Smith R.S., Clark A.F., and John S.W. Inducible nitric oxide synthase, Nos2, does not mediate optic neuropathy and retinopathy in the DBA/2J glaucoma model. BMC Neurosci. 8:108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen C., Xu Y., Zhang J., Zhu J., Zhang J., Hu N., and Guan H. Altered expression of nNOS/NIDD in the retina of a glaucoma model of DBA/2J mice and the intervention by nNOS inhibition. J. Mol. Neurosci. 51:47–56, 2013 [DOI] [PubMed] [Google Scholar]
- 211.Husain S., Abdul Y., Singh S., Ahmad A., and Husain M. Regulation of nitric oxide production by delta-opioid receptors during glaucomatous injury. PLoS One. 9:e110397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Karim M.Z., Sawada A., Mizuno K., Kawakami H., Ishida K., and Yamamoto T. Neuroprotective effect of nipradilol [3,4-dihydro-8-(2-hydroxy-3-isopropylamino)-propoxy-3-nitroxy-2H-1-benzopyran] in a rat model of optic nerve degeneration. J. Glaucoma. 18:26–31, 2009 [DOI] [PubMed] [Google Scholar]
- 213.Watanabe M., Tokita Y., and Yata T. Axonal regeneration of cat retinal ganglion cells is promoted by nipradilol, an anti-glaucoma drug. Neuroscience. 140:517–528, 2006 [DOI] [PubMed] [Google Scholar]
- 214.Mizuno K., Koide T., Yoshimura M., and Araie M. Neuroprotective effect and intraocular penetration of nipradilol, a beta-blocker with nitric oxide donative action. Invest. Ophthalmol. Vis. Sci. 42:688–694, 2001 [PubMed] [Google Scholar]
- 215.Takahata K., Katsuki H., Kume T., Nakata D., Ito K., Muraoka S., Yoneda F., Kashii S., Honda Y., and Akaike A. Retinal neuronal death induced by intraocular administration of a nitric oxide donor and its rescue by neurotrophic factors in rats. Invest. Ophthalmol. Vis. Sci. 44:1760–1766, 2003 [DOI] [PubMed] [Google Scholar]
- 216.Ritossa F. New puffs induced by temperature shock, DNA and salicylate in salivary chromosomes of Drosophila melanogaster. Drosoph. Inf. Serv. 37:122–123, 1963 [Google Scholar]
- 217.Calderwood S.K., and Gong J. Heat shock proteins promote cancer: it's a protection racket. Trends Biochem. Sci. 41:311–323, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Piri N., Kwong J.M., Gu L., and Caprioli J. Heat shock proteins in the retina: focus on HSP70 and alpha crystallins in ganglion cell survival. Prog. Retin. Eye Res. 52:22–46, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tezel G., Seigel G.M., and Wax M.B. Autoantibodies to small heat shock proteins in glaucoma. Invest. Ophthalmol. Vis. Sci. 39:2277–2287, 1998 [PubMed] [Google Scholar]
- 220.Wax M.B., Tezel G., Yang J., Peng G., Patil R.V., Agarwal N., Sappington R.M., and Calkins D.J. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J. Neurosci. 28:12085–12096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Casola C., Schiwek J.E., Reinehr S., Kuehn S., Grus F.H., Kramer M., Dick H.B., and Joachim S.C. S100 alone has the same destructive effect on retinal ganglion cells as in combination with HSP 27 in an autoimmune glaucoma model. J. Mol. Neurosci. 56:228–236, 2015 [DOI] [PubMed] [Google Scholar]
- 222.Fischer D., Pavlidis M., and Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest. Ophthalmol. Vis. Sci. 41:3943–3954, 2000 [PubMed] [Google Scholar]
- 223.Yin Y., Henzl M.T., Lorber B., Nakazawa T., Thomas T.T., Jiang F., Langer R., and Benowitz L.I. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 9:843–852, 2006 [DOI] [PubMed] [Google Scholar]
- 224.Hauk T.G., Muller A., Lee J., Schwendener R., and Fischer D. Neuroprotective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp. Neurol. 209:469–482, 2008 [DOI] [PubMed] [Google Scholar]
- 225.Leon S., Yin Y., Nguyen J., Irwin N., and Benowitz L.I. Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci. 20:4615–4626, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lorber B., Berry M., and Logan A. Lens injury stimulates adult mouse retinal ganglion cell axon regeneration via both macrophage- and lens-derived factors. Eur. J. Neurosci. 21:2029–2034, 2005 [DOI] [PubMed] [Google Scholar]
- 227.Liedtke T., Naskar R., Eisenacher M., and Thanos S. Transformation of adult retina from the regenerative to the axonogenesis state activates specific genes in various subsets of neurons and glial cells. Glia. 55:189–201, 2007 [DOI] [PubMed] [Google Scholar]
- 228.Stupp T., Pavlidis M., Busse H., and Thanos S. Lens epithelium supports axonal regeneration of retinal ganglion cells in a coculture model in vitro. Exp. Eye Res. 81:530–538, 2005 [DOI] [PubMed] [Google Scholar]
- 229.Bohm M.R., Prokosch V., Bruckner M., Pfrommer S., Melkonyan H., and Thanos S. BetaB2-crystallin promotes axonal regeneration in the injured optic nerve in adult rats. Cell Transplant. 24:1829–1844, 2015 [DOI] [PubMed] [Google Scholar]
- 230.Piri N., Song M., Kwong J.M., and Caprioli J. Modulation of alpha and beta crystallin expression in rat retinas with ocular hypertension-induced ganglion cell degeneration. Brain Res. 1141:1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 231.Ahmed F., Brown K.M., Stephan D.A., Morrison J.C., Johnson E.C., and Tomarev S.I. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest. Ophthalmol. Vis. Sci. 45:1247–1258, 2004 [DOI] [PubMed] [Google Scholar]
- 232.Munemasa Y., Kwong J.M., Caprioli J., and Piri N. The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest. Ophthalmol. Vis. Sci. 50:3869–3875, 2009 [DOI] [PubMed] [Google Scholar]
- 233.Wu N., Wang Y.H., Zhao H.S., Liu D.N., Ying X., Yin Z.Q., and Wang Y. Alpha-crystallin downregulates the expression of TNF-alpha and iNOS by activated rat retinal microglia in vitro and in vivo. Ophthalmic. Res. 42:21–28, 2009 [DOI] [PubMed] [Google Scholar]
- 234.Wu N., Yu J., Chen S., Xu J., Ying X., Ye M., Li Y., and Wang Y. Alpha-crystallin protects RGC survival and inhibits microglial activation after optic nerve crush. Life Sci. 94:17–23, 2014 [DOI] [PubMed] [Google Scholar]
- 235.Sevilla T., Sivera R., Martinez-Rubio D., Lupo V., Chumillas M.J., Calpena E., Dopazo J., Vilchez J.J., Palau F., and Espinos C. The EGR2 gene is involved in axonal Charcot–Marie–Tooth disease. Eur. J. Neurol. 22:1548–1555, 2015 [DOI] [PubMed] [Google Scholar]
- 236.Provenzano G., Corradi Z., Monsorno K., Fedrizzi T., Ricceri L., Scattoni M.L., and Bozzi Y. Comparative gene expression analysis of two mouse models of autism: transcriptome profiling of the BTBR and EN2 (-/-) hippocampus. Front. Neurosci. 10:396, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Takahashi S., Matsumoto N., Okayama A., Suzuki N., Araki A., Okajima K., Tanaka H., and Miyamoto A. FOXG1 mutations in Japanese patients with the congenital variant of Rett syndrome. Clin. Genet. 82:569–573, 2012 [DOI] [PubMed] [Google Scholar]
- 238.Lopez-Gonzalez I., Palmeira A., Aso E., Carmona M., Fernandez L., and Ferrer I. FOXP2 expression in frontotemporal lobar degeneration-tau. J. Alzheimer's Dis. 54:471–475, 2016 [DOI] [PubMed] [Google Scholar]
- 239.Pollak A., Lechowicz U., Kedra A., Stawinski P., Rydzanicz M., Furmanek M., Brzozowska M., Mrowka M., Skarzynski H., Skarzynski P.H., Oldak M., and Ploski R. Novel and De Novo Mutations Extend Association of POU3F4 with distinct clinical and radiological phenotype of hearing loss. PLoS One. 11:e0166618, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hanz S., and Fainzilber M. Retrograde signaling in injured nerve—the axon reaction revisited. J. Neurochem. 99:13–19, 2006 [DOI] [PubMed] [Google Scholar]
- 241.Michaelevski I., Segal-Ruder Y., Rozenbaum M., Medzihradszky K.F., Shalem O., Coppola G., Horn-Saban S., Ben-Yaakov K., Dagan S.Y., Rishal I., Geschwind D.H., Pilpel Y., Burlingame A.L., and Fainzilber M. Signaling to transcription networks in the neuronal retrograde injury response. Sci. Signal. 3:ra53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Anderson A.M., Weasner B.M., Weasner B.P., and Kumar J.P. Dual transcriptional activities of SIX proteins define their roles in normal and ectopic eye development. Development. 139:991–1000, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Burdon K.P., Macgregor S., Hewitt A.W., Sharma S., Chidlow G., Mills R.A., Danoy P., Casson R., Viswanathan A.C., Liu J.Z., Landers J., Henders A.K., Wood J., Souzeau E., Crawford A., Leo P., Wang J.J., Rochtchina E., Nyholt D.R., Martin N.G., Montgomery G.W., Mitchell P., Brown M.A., Mackey D.A., and Craig J.E. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat. Genet. 43:574–578, 2011 [DOI] [PubMed] [Google Scholar]
- 244.Osman W., Low S.K., Takahashi A., Kubo M., and Nakamura Y. A genome-wide association study in the Japanese population confirms 9p21 and 14q23 as susceptibility loci for primary open angle glaucoma. Hum. Mol. Genet. 21:2836–2842, 2012 [DOI] [PubMed] [Google Scholar]
- 245.Wiggs J.L., Yaspan B.L., Hauser M.A., Kang J.H., Allingham R.R., Olson L.M., Abdrabou W., Fan B.J., Wang D.Y., Brodeur W., Budenz D.L., Caprioli J., Crenshaw A., Crooks K., Delbono E., Doheny K.F., Friedman D.S., Gaasterland D., Gaasterland T., Laurie C., Lee R.K., Lichter P.R., Loomis S., Liu Y., Medeiros F.A., McCarty C., Mirel D., Moroi S.E., Musch D.C., Realini A., Rozsa F.W., Schuman J.S., Scott K., Singh K., Stein J.D., Trager E.H., Vanveldhuisen P., Vollrath D., Wollstein G., Yoneyama S., Zhang K., Weinreb R.N., Ernst J., Kellis M., Masuda T., Zack D., Richards J.E., Pericak-Vance M., Pasquale L.R., and Haines J.L. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 8:e1002654, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sang J., Jia L., Zhao B., Wang H., Zhang N., and Wang N. Association of three single nucleotide polymorphisms at the SIX1–SIX6 locus with primary open angle glaucoma in the Chinese population. Sci. China Life Sci. 59:694–699, 2016 [DOI] [PubMed] [Google Scholar]
- 247.Carnes M.U., Liu Y.P., Allingham R.R., Whigham B.T., Havens S., Garrett M.E., Qiao C., Katsanis N., Wiggs J.L., Pasquale L.R., Ashley-Koch A., Oh E.C., and Hauser M.A. Discovery and functional annotation of SIX6 variants in primary open-angle glaucoma. PLoS Genet. 10:e1004372, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Iglesias A.I., Springelkamp H., van der Linde H., Severijnen L.A., Amin N., Oostra B., Kockx C.E., van den Hout M.C., van Ijcken W.F., Hofman A., Uitterlinden A.G., Verdijk R.M., Klaver C.C., Willemsen R., and van Duijn C.M. Exome sequencing and functional analyses suggest that SIX6 is a gene involved in an altered proliferation-differentiation balance early in life and optic nerve degeneration at old age. Hum. Mol. Genet. 23:1320–1332, 2014 [DOI] [PubMed] [Google Scholar]
- 249.Skowronska-Krawczyk D., Zhao L., Zhu J., Weinreb R.N., Cao G., Luo J., Flagg K., Patel S., Wen C., Krupa M., Luo H., Ouyang H., Lin D., Wang W., Li G., Xu Y., Li O., Chung C., Yeh E., Jafari M., Ai M., Zhong Z., Shi W., Zheng L., Krawczyk M., Chen D., Shi C., Zin C., Zhu J., Mellon P.L., Gao W., Abagyan R., Zhang L., Sun X., Zhong S., Zhuo Y., Rosenfeld M.G., Liu Y., and Zhang K. P16INK4a upregulation mediated by SIX6 defines retinal ganglion cell pathogenesis in glaucoma. Mol. Cell. 59:931–940, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Oshitari T., Dezawa M., Okada S., Takano M., Negishi H., Horie H., Sawada H., Tokuhisa T., and Adachi-Usami E. The role of c-fos in cell death and regeneration of retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 43:2442–2449, 2002 [PubMed] [Google Scholar]
- 251.Fernandes K.A., Harder J.M., Fornarola L.B., Freeman R.S., Clark A.F., Pang I.H., John S.W., and Libby R.T. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol. Dis. 46:393–401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fernandes K.A., Harder J.M., Kim J., and Libby R.T. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. Exp. Eye Res. 112:106–117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Levkovitch-Verbin H., Quigley H.A., Martin K.R., Harizman N., Valenta D.F., Pease M.E., and Melamed S. The transcription factor c-Jun is activated in retinal ganglion cells in experimental rat glaucoma. Exp. Eye Res. 80:663–670, 2005 [DOI] [PubMed] [Google Scholar]
- 254.Tezel G., Chauhan B.C., LeBlanc R.P., and Wax M.B. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest. Ophthalmol. Vis. Sci. 44:3025–3033, 2003 [DOI] [PubMed] [Google Scholar]
- 255.Fernandes K.A., Harder J.M., John S.W., Shrager P., and Libby R.T. DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol. Dis. 69:108–116, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Welsbie D.S., Yang Z., Ge Y., Mitchell K.L., Zhou X., Martin S.E., Berlinicke C.A., Hackler L., Fuller J., Fu J., Cao, L.-h., Han B., Auld D., Xue T., Hirai S., Germain L., Simard-Bisson C., Blouin R., Nguyen J.V., Davis C.H., Enke R.A., Boye S.L., Merbs S.L., Marsh-Armstrong N., Hauswirth W.W., DiAntonio A., Nickells R.W., Inglese J., Hanes J., Yau. K-W., Quigley H.A., and Zack D.J. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl. Acad. Sci. U. S. A. 110:4045–4050, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tezel G., Yang X., Yang J., and Wax M.B. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 996:202–212, 2004 [DOI] [PubMed] [Google Scholar]
- 258.Yang X., Luo C., Cai J., Pierce W.M., and Tezel G. Phosphorylation-dependent interaction with 14-3-3 in the regulation of bad trafficking in retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 49:2483–2494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liu H., Sun H., and Liu C. Interference of the apoptotic signaling pathway in RGC stress response by SP600125 in moderate ocular hypertensive rats. Chin. J. Physiol. 54:124–132, 2011 [PubMed] [Google Scholar]
- 260.Sun H., Wang Y., Pang I.H., Shen J., Tang X., Li Y., Liu C., and Li B. Protective effect of a JNK inhibitor against retinal ganglion cell loss induced by acute moderate ocular hypertension. Mol. Vis. 17:864–875, 2011 [PMC free article] [PubMed] [Google Scholar]
- 261.Kim B.J., Silverman S.M., Liu Y., Wordinger R.J., Pang I.H., and Clark A.F. In vitro and in vivo neuroprotective effects of cJun N-terminal kinase inhibitors on retinal ganglion cells. Mol. Neurodegener. 11:30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yoshida K., Behrens A., Le-Niculescu H., Wagner E.F., Harada T., Imaki J., Ohno S., and Karin M. Amino-terminal phosphorylation of c-Jun regulates apoptosis in the retinal ganglion cells by optic nerve transection. Invest. Ophthalmol. Vis. Sci. 43:1631–1635, 2002 [PubMed] [Google Scholar]
- 263.Quigley H.A., Cone F.E., Gelman S.E., Yang Z., Son J.L., Oglesby E.N., Pease M.E., and Zack D.J. Lack of neuroprotection against experimental glaucoma in c-Jun N-terminal kinase 3 knockout mice. Exp. Eye Res. 92:299–305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Xiang M., Zhou L., Macke J.P., Yoshioka T., Hendry S.H., Eddy R.L., Shows T.B., and Nathans J. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J. Neurosci. 15:4762–4785, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gan L., Xiang M., Zhou L., Wagner D.S., Klein W.H., and Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc. Natl. Acad. Sci. U. S. A. 93:3920–3925, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang S.W., Gan L., Martin S.E., and Klein W.H. Abnormal polarization and axon outgrowth in retinal ganglion cells lacking the POU-domain transcription factor Brn-3b. Mol. Cell Neurosci. 16:141–156, 2000 [DOI] [PubMed] [Google Scholar]
- 267.Erkman L., Yates P.A., McLaughlin T., McEvilly R.J., Whisenhunt T., O'Connell S.M., Krones A.I., Kirby M.A., Rapaport D.H., Bermingham J.R., O'Leary D.D., and Rosenfeld M.G. A POU domain transcription factor-dependent program regulates axon pathfinding in the vertebrate visual system. Neuron. 28:779–792, 2000 [DOI] [PubMed] [Google Scholar]
- 268.Wang S.W., Mu X., Bowers W.J., Kim D.-S., Plas D.J., Crair M.C., Federoff H.J., Gan L., and Klein W.H. Brn3b/Brn3c double knockout mice reveal an unsuspected role for Brn3c in retinal ganglion cell axon outgrowth. Development. 129:467–477, 2002 [DOI] [PubMed] [Google Scholar]
- 269.Buckingham B.P., Inman D.M., Lambert W., Oglesby E., Calkins D.J., Steele M.R., Vetter M.L., Marsh-Armstrong N., and Horner P.J. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J. Neurosci. 28:2735–2744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Soto I., Oglesby E., Buckingham B.P., Son J.L., Roberson E.D., Steele M.R., Inman D.M., Vetter M.L., Horner P.J., and Marsh-Armstrong N. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J. Neurosci. 28:548–561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Weishaupt J., Klöcker N., and Bähr M. Axotomy-induced early down-regulation of POU-IV class transcription factors Brn-3a and Brn-3b in retinal ganglion cells. J. Mol. Neurosci. 26:17–26, 2005 [DOI] [PubMed] [Google Scholar]
- 272.Pan L., Yang Z., Feng L., and Gan L. Functional equivalence of Brn3 POU-domain transcription factors in mouse retinal neurogenesis. Development. 132:703–712, 2005 [DOI] [PubMed] [Google Scholar]
- 273.Qiu F., Jiang H., and Xiang M. A comprehensive negative regulatory program controlled by Brn3b to ensure ganglion cell specification from multipotential retinal precursors. J. Neurosci. 28:3392–3403, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Phatak N.R., Stankowska D.L., and Krishnamoorthy R.R. Bcl-2, Bcl-xL, and p-AKT are involved in neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Mol. Vis. 22:1048–1061, 2016 [PMC free article] [PubMed] [Google Scholar]
- 275.Huang L., Hu F., Xie X., Harder J., Fernandes K., Zeng X.Y., Libby R., and Gan L. Pou4f1 and pou4f2 are dispensable for the long-term survival of adult retinal ganglion cells in mice. PLoS One. 9:e94173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Levkovitch-Verbin H., Harizman N., Dardik R., Nisgav Y., Vander S., and Melamed S. Regulation of cell death and survival pathways in experimental glaucoma. Exp. Eye Res. 85:250–258, 2007 [DOI] [PubMed] [Google Scholar]
- 277.Vander S., and Levkovitch-Verbin H. Regulation of cell death and survival pathways in secondary degeneration of the optic nerve—a long-term study. Curr. Eye Res. 37:740–748, 2012 [DOI] [PubMed] [Google Scholar]
- 278.Takeda M., Kato H., Takamiya A., Yoshida A., and Kiyama H. Injury-specific expression of activating transcription factor-3 in retinal ganglion cells and its colocalized expression with phosphorylated c-Jun. Invest. Ophthalmol. Vis. Sci. 41:2412–2421, 2000 [PubMed] [Google Scholar]
- 279.Garriga-Canut M., Agustín-Pavón C., Herrmann F., Sánchez A., Dierssen M., Fillat C., and Isalan M. Synthetic zinc finger repressors reduce mutant huntingtin expression in the brain of R6/2 mice. Proc. Natl. Acad. Sci. U. S. A. 109:E3136–E3145, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zanetta C., Nizzardo M., Simone C., Monguzzi E., Bresolin N., Comi G.P., and Corti S. Molecular therapeutic strategies for spinal muscular atrophies: current and future clinical trials. Clin. Ther. 36:128–140, 2014 [DOI] [PubMed] [Google Scholar]
- 281.Bassuk A.G., Zheng A., Li Y., Tsang S.H., and Mahajan V.B. Precision medicine: genetic repair of retinitis pigmentosa in patient-derived stem cells. Sci. Rep. 6:19969, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]