Abstract
Background
The standard treatment option for medication-refractory essential tremor is invasive neurosurgery. A new, noninvasive alternative is magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery. We aimed to determine the effectiveness, safety, and cost-effectiveness of MRgFUS neurosurgery for the treatment of moderate to severe, medication-refractory essential tremor in Ontario. We also spoke with people with essential tremor to gain an understanding of their experiences and thoughts regarding treatment options, including MRgFUS neurosurgery.
Methods
We performed a systematic review of the clinical literature published up to April 11, 2017, that examined MRgFUS neurosurgery alone or compared with other interventions for the treatment of moderate to severe, medication-refractory essential tremor. We assessed the risk of bias of each study and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic review of the economic literature and created Markov cohort models to assess the cost-effectiveness of MRgFUS neurosurgery compared with other treatment options, including no surgery. We also estimated the budget impact of publicly funding MRgFUS neurosurgery in Ontario for the next 5 years. To contextualize the potential value of MRgFUS neurosurgery as a treatment option for essential tremor, we spoke with people with essential tremor and their families.
Results
Nine studies met our inclusion criteria for the clinical evidence review. In noncomparative studies, MRgFUS neurosurgery was found to significantly improve tremor severity and quality of life and to significantly reduce functional disability (GRADE: very low). It was also found to be significantly more effective than a sham procedure (GRADE: high). We found no significant difference in improvements in tremor severity, functional disability, or quality of life between MRgFUS neurosurgery and deep brain stimulation (GRADE: very low). We found no significant difference in improvement in tremor severity compared with radiofrequency thalamotomy (GRADE: low). MRgFUS neurosurgery has a favourable safety profile.
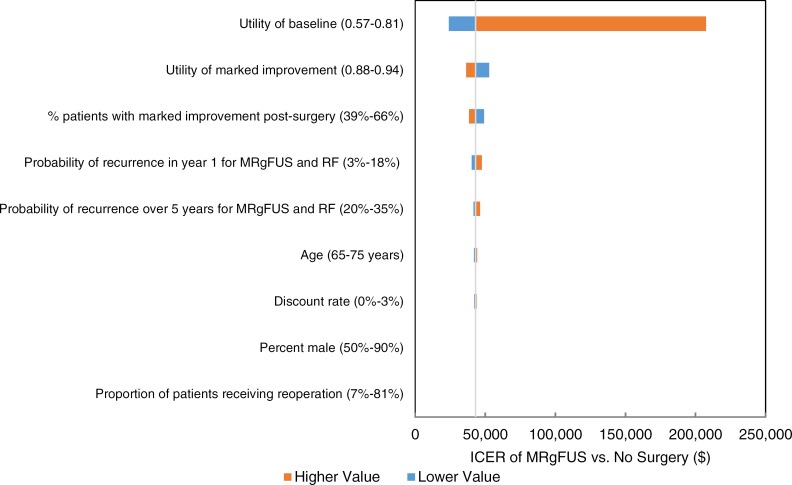
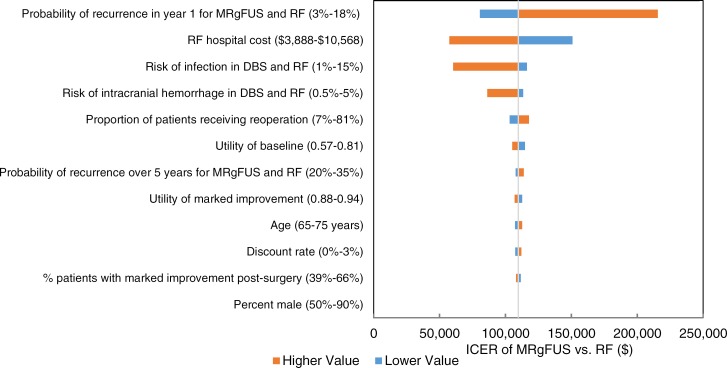
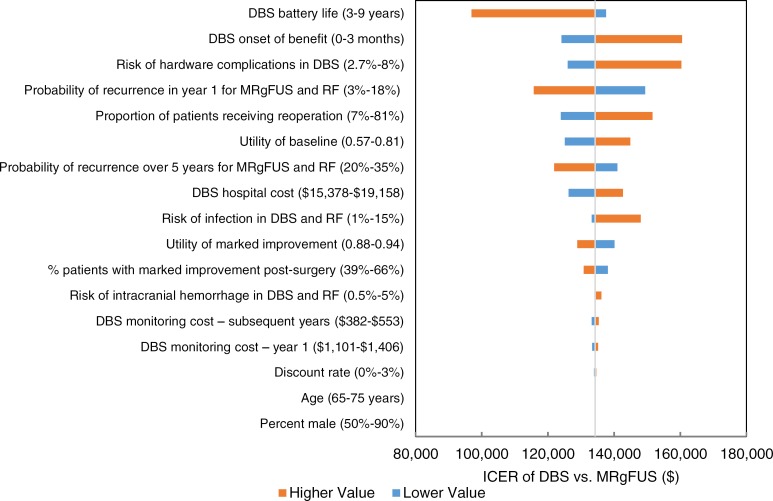
We estimated that MRgFUS neurosurgery has a mean cost of $23,507 and a mean quality-adjusted survival of 3.69 quality-adjusted life-years (QALYs). We also estimated that the mean costs and QALYs of radiofrequency thalamotomy and deep brain stimulation are $14,978 and 3.61 QALYs, and $57,535 and 3.94 QALYs, respectively. For people ineligible for invasive neurosurgery, we estimated the incremental cost-effectiveness ratio (ICER) of MRgFUS neurosurgery compared with no surgery as $43,075 per QALY gained. In people eligible for invasive neurosurgery, the ICER of MRgFUS neurosurgery compared with radiofrequency thalamotomy is $109,795 per QALY gained; when deep brain stimulation is compared with MRgFUS neurosurgery, the ICER is $134,259 per QALY gained. Of note however, radiofrequency thalamotomy is performed very infrequently in Ontario. We also estimated that the budget impact of publicly funding MRgFUS neurosurgery in Ontario at the current case load (i.e., 48 cases/year) would be about $1 million per year for the next 5 years.
People with essential tremor who had undergone MRgFUS neurosurgery reported positive experiences with the procedure. The tremor reduction they experienced improved their ability to perform activities of daily living and improved their quality of life.
Conclusions
MRgFUS neurosurgery is an effective and generally safe treatment option for moderate to severe, medication-refractory essential tremor. It provides a treatment option for people ineligible for invasive neurosurgery and offers a noninvasive option for all people considering neurosurgery.
For people ineligible for invasive neurosurgery, MRgFUS neurosurgery is cost-effective compared with no surgery. In people eligible for invasive neurosurgery, MRgFUS neurosurgery may be one of several reasonable options. Publicly funding MRgFUS neurosurgery for the treatment of moderate to severe, medication-refractory essential tremor in Ontario at the current case load would have a net budget impact of about $1 million per year for the next 5 years.
People with essential tremor who had undergone MRgFUS neurosurgery reported positive experiences. They liked that it was a noninvasive procedure and reported a substantial reduction in tremor that resulted in an improvement in their quality of life.
OBJECTIVE
This health technology assessment examines the effectiveness, safety, and cost-effectiveness of magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery for the treatment of moderate to severe, medication-refractory essential tremor. It also assesses the budget impact of publicly funding MRgFUS neurosurgery in Ontario and examines the experiences of people with essential tremor and their perspectives on treatment options, including MRgFUS neurosurgery.
BACKGROUND
Health Condition
Essential tremor is a benign, chronic, progressive condition of tremors (rhythmic, oscillatory, involuntary movements) that occur during rest or action, which is not attributed to another cause (e.g., Parkinson's disease).1 Typically, essential tremor manifests in one or more of the upper limbs (in about 95% of cases), the head, trunk, or voice.2 The condition is the most common movement disorder, estimated to affect 0.4% to 3.9% of the general adult population, with an increase in severity and prevalence with age.3 The average age of onset is 45 years; however, essential tremor can occur at any age, including adolescence and early adulthood.4 The condition tends to run in families.1
Diagnosis and Assessment
There are several types of tremors and tremor-like syndromes, and these are classified based on their primary characteristics: affected area(s), frequency of oscillation (measured in Hertz [Hz]), accompanying features, age at onset, and rapidity of onset.5 Essential tremor is distinguished by middle- to high-frequency tremors (i.e., 4–12 Hz) at rest, during movement (kinetic), and when maintaining the body in a position against gravity (postural; e.g., holding arms outstretched).2 The diagnostic workup may include brain imaging, tests such as electromyography (EMG; to determine the frequency of the tremor), and a complete medication history. A diagnosis of essential tremor is challenging, but is reached when the tremor characteristics fit the profile, there are no other neurological signs, and other types and causes of tremor have been ruled out.2
Tremor severity is evaluated by a person's assessment of the impact of essential tremor on their quality of life and via tools such as the Clinical Rating Scale for Tremors (CRST; an adaptation of the Fahn–Tolosa–Marín [FTM] Clinical Rating Scale for Tremor).6 These essential tremor–specific severity scales include three subscales—tremor; tasks of writing, drawing, and pouring; and activities of daily living—as well as global assessments by both patient and clinician.7
One component of the CRST is a spiral-drawing task, sometimes called the “Archimedes spiral.” This task is clinically useful, as people with essential tremor demonstrate a distinct tremor pattern and spiral diameter on this task.8,9 Repeated assessment can aid in evaluating tremor severity, tremor progression, and response to therapy. The CRST has been validated as sensitive to changes in essential tremor over time.7 Table 1 outlines the FTM scale and provides examples of the components of each subscale.7 (Note that the overall scoring of the CRST differs slightly from that of the FTM.)
Table 1:
Summary of the Fahn–Tolosa–Marín Clinical Rating Scale for Tremor
| Scale | Objective | Scoring (Examples of Meaning) |
|---|---|---|
| Part A | Quantifies tremor while holding posture, and with action/intention in nine body parts | 0: None 1: Amplitude < 0.5 cm (slight, may be intermittent) 2: Amplitude 0.5–1.0 cm (moderate, may be intermittent) 3: Amplitude 1–2 cm (marked) 4: Amplitude > 2 cm (severe) |
| Part B | Action tremor of the upper extremities during writing, drawing, and water pouring | 0: Normal 1: Mild (untidy, may cross lines, no spilling) 2: Moderate (consistent tremor but legible, crosses lines frequently, spills up to 10%) 3: Marked (illegible, great difficulty drawing, spills > 10–50%) 4: Severe (unable to keep pen on paper, complete drawing, or pour without spilling most of the water) |
| Part C | Functional disability, including speaking, eating, drinking, hygiene, dressing, working, and domestic tasks | 0: Normal 1: Mild (spills food rarely, moves more carefully when doing tasks than the average person) 2: Moderate (unable to use spoon, able but many errors in dressing, hygiene, work tasks) 3: Marked (uses two hands to eat or for hygiene, unable to do regular job, requires assistance with dressing) 4: Severe (some words difficult to understand, needs help to feed, unable to do any fine movements, requires assistance for even gross motor tasks, unable to work) |
| Total | Overall assessment of severity and disability (parts A, B, and C), as well as each of clinician's and patient's subjective assessments of severity |
Expressed as a percentage representing severity 1–24%: Mild disability 25–49%: Moderate disability 50–74%: Marked disability 75–100%: Severe disability |
Source: Fahn et al, 1988.6
Current Treatment Options
There is no cure for essential tremor; however, treatments can help patients manage the symptoms. Many people live with essential tremor untreated for an extended period of time, often decades, not seeking treatment until tremor severity progresses to a point at which it interferes with daily life. Severe essential tremor is disabling, limiting a person's ability to perform routine activities of daily living, such as eating, writing, walking, and self-care, thus considerably impacting quality of life, the ability to work or participate in social activities, and independence.1 Pharmacotherapy is the first-line treatment for essential tremor; the beta-blocker propranolol and the anticonvulsant primidone are most commonly trialed.1,10,11 However, as many as half of all people with essential tremor experience recurrent tremor or inadequate tremor control while taking medication, have contraindications to medication, or cannot tolerate the side effects of medication.11
Although the exact pathophysiology of essential tremor is not well understood,12 it is known to involve the cerebello-thalamo-cortical network.13 The area of the brain targeted during neurosurgery is typically the ventral intermediate nucleus of the thalamus or, less commonly, the cerebellothalamic tract.10
Two invasive neurosurgical treatment options are available for people with medication-refractory, disabling essential tremor: lesional surgery and deep brain stimulation. Lesional surgery involves creating a permanent injury to the part of the brain causing the tremor, whereas deep brain stimulation involves implanting permanent electrodes into the affected brain areas to inhibit neural activity.11 Although these surgical techniques can alleviate tremor in about 40% to 80% of people with essential tremor who undergo them, there are risks involved with each procedure that must be considered for each person.10
The principal lesional surgery for essential tremor is unilateral thalamotomy, in which neurons in the thalamus on one side of the brain are destroyed. This procedure has historically been performed via craniotomy (opening the skull). During the surgery, a probe is inserted to deliver radiofrequency electrical current to heat the target site and create a lesion, a method called ablation.10 The patient is kept awake so that the clinical team can assess the accuracy of the target location before creating a permanent lesion. Radiofrequency thalamotomy has been in practice since the mid-twentieth century and was the main surgical option for essential tremor prior to the introduction of other surgical techniques.10 As an invasive surgery, radiofrequency thalamotomy is inappropriate for people with surgical contraindications (e.g., unstable cardiac disease) and has inherent risks, including hemorrhage, infection, and seizure.10
Thalamotomy can also be performed noninvasively by focusing radiation beams through the skull via a technology called Gamma Knife. The effects of Gamma Knife thalamotomy, in terms of both tremor control and adverse effects, do not appear until weeks or months after the procedure. Importantly, people who undergo the procedure have variable responses to radiation, and there is a risk of progressive neurological deficits associated with the radiation.14,15 Documented procedure-related complications include mild numbness, hemiparesis (paralysis on one side of the body), dysphagia (difficulty swallowing), and death.14 Gamma Knife thalamotomy also relies entirely on anatomical imaging to identify the target area in the brain. Further, in contrast to all other surgeries for essential tremor, this procedure does not allow for testing to refine and confirm the correct target location prior to ablation.15 There may be a role for Gamma Knife thalamotomy in rare cases of essential tremor; for example, elderly people who cannot undergo an invasive procedure such as deep brain stimulation.14,15 However, a shortage of long-term data on effectiveness and safety led the American Academy of Neurology to conclude that the evidence is insufficient to make a recommendation regarding the use of Gamma Knife thalamotomy for the treatment of essential tremor.16
Deep brain stimulation is the current standard of care for the neurosurgical treatment of essential tremor, as it is considered reversible and adjustable. This procedure is an invasive, nondestructive surgery that can be applied unilaterally (to one side of the brain) or bilaterally (to both sides of the brain).17 This is different from lesional surgery, which is typically performed unilaterally. Deep brain stimulation involves craniotomy and the implantation of permanent electrodes into the brain that electrically stimulate the target area, inhibiting neural activity and thus alleviating the tremor.18 As with thalamotomy, the patient is kept awake so that the clinical team can assess the accuracy of the target location. In a subsequent surgery, a pulse generator (a device similar to a pacemaker) is surgically implanted below the clavicle to adjust the stimulation delivered by the implanted electrodes.17 A unique advantage of deep brain stimulation over lesional surgery is that tremor control and adverse effects can be titrated (adjusted) or reversed by adjusting the stimulation settings.18 The implanted pulse generator must be programmed by a clinician to optimize the stimulation settings, and the battery of the generator must be replaced at intervals of 2 to 5 years.17 In 2005, the Ontario Health Technology Advisory Committee recommended increased access to deep brain stimulation in Ontario for people with medication-refractory movement disorders, including essential tremor.19
Adverse effects of both radiofrequency thalamotomy and deep brain stimulation can arise from inaccurate target localization, acute swelling in response to surgery, or the size of the lesion created during radiofrequency thalamotomy.20 These can be transient or permanent and include ataxia (alterations in control of body movements), paresthesias (bodily sensations of burning, tingling, or pricking), dysarthria (slowed or slurred speech), hemorrhage (bleeding in the brain), or seizures.10 The risk of intracranial hemorrhage associated with invasive stereotactic neurosurgeries (including radiofrequency thalamotomy and deep brain stimulation) ranges between 1% and 4%.20,21
The few studies comparing radiofrequency thalamotomy with deep brain stimulation have found that both procedures are effective in controlling tremor in essential tremor, both in the short term20,21 and 5 years post-surgery.22 Despite their similar effectiveness in tremor control, radiofrequency thalamotomy may be associated with more complications and neurological adverse effects than deep brain stimulation20; the existing literature estimates a risk of permanent complications following radiofrequency thalamotomy of 13% to 38%.23,24 Although deep brain stimulation has been found to be effective in terms of tremor control,17,25 as many as half of all people with essential tremor who have undergone the procedure report diminished tremor control 5 years post-surgery,22 and improvements in activities of daily living and quality of life may not be sustained 1 to 7 years following surgery.22,26
A recent systematic review reported that both thalamotomy and deep brain stimulation may produce speech and language difficulties (most frequently hypophonia, dysarthria, and dysphasia), which are two to three times more likely to occur after bilateral than unilateral procedures.27 For people with essential tremor in particular, the risk of language adverse effects has been found to be higher with deep brain stimulation than with radiofrequency thalamotomy.27
The surgical options for a person with no contraindications to surgery are typically considered by weighing the benefits and risks of each procedure, along with the local availability of types of procedure. Deep brain stimulation is now the preferred surgical procedure for people with essential tremor in many jurisdictions, largely replacing radiofrequency thalamotomy.21 A further consideration is the willingness of the person to accept aspects of invasive neurosurgical procedures such as craniotomy, general anaesthetic, and permanent, implanted hardware.
Health Technology Under Review
Magnetic resonance-guided focused ultrasound (MRgFUS) is a noninvasive surgical technology with two components: magnetic resonance imaging (MRI) and high-intensity focused ultrasound. In MRgFUS neurosurgery, MRI provides detailed images of the brain in real time during the surgery, allowing for precision in identifying the target area, thus minimizing risk to surrounding tissue.28 The high-intensity focused ultrasound transducer contains 1,024 rays of ultrasound waves, which are emitted to a focal point through the intact skull.28 As the rays are focused and converge, they ablate the target brain tissue.28 Throughout the procedure, ongoing, real-time feedback of thermal data allows the clinical team to precisely adjust the location and temperature parameters.28 During the procedure, the patient is conscious, and the functional effects of the procedure are clinically assessed throughout. This allows the multidisciplinary clinical team to refine and confirm the correct target and appropriate number of sonications (repetitions of ultrasound beam application).28
With MRgFUS neurosurgery, tremor improvement is immediate, and because the procedure is noninvasive, recovery is quick, and surgical complications such as hemorrhage and infection are minimized.28 Because it is noninvasive, MRgFUS neurosurgery offers a treatment option for people who otherwise have none. This includes people ineligible for the currently available surgical procedures (e.g., elderly people for whom the risks of invasive surgery outweigh the potential benefits), people with surgical contraindications (e.g., those with unstable cardiac disease), and people who find invasive procedures or the associated risks unacceptable.
MRgFUS neurosurgery is inappropriate for people with contraindications to MRI and for the small proportion of people with a skull density that would prevent the therapeutic temperature from being reached.
Regulatory Information
Health Canada approved the use of InSightec's (Tirat Carmel, Israel) Exablate Neuro (also called Exablate 4000) MRgFUS system for the unilateral treatment of idiopathic medication-refractory essential tremor in May 2016 (licence 96969; personal communication, Health Canada, October 11, 2016).
Ontario Context
The Exablate Neuro MRgFUS system is currently available in just two centres in Canada, both in Ontario. At the time of writing, the total capacity to perform MRgFUS neurosurgery was estimated at four patients per month. Local experts advised that current capacity is insufficient to meet the demand of people with essential tremor for whom this surgery is appropriate, owing to limitations on the necessary MRI suite time, as well as financial considerations, as the procedure is presently financed only with research funds. At the time of writing, the Ontario Ministry of Health and Long-Term Care advised that MRgFUS neurosurgery for the treatment of essential tremor is not publicly funded in any province in Canada. However, the N124 billing code in the Ontario Schedule of Benefits: Physician Services Under the Health Insurance Act addresses functional stereotactic neurosurgery for physician remuneration of thalamotomy.29
CLINICAL EVIDENCE
Research Question
What are the effectiveness and safety of magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery for the treatment of moderate to severe, medication-refractory essential tremor?
Methods
We developed the research questions in consultation with people with essential tremor, clinical experts, and other health system stakeholders.
Clinical Literature Search
We performed a literature search on April 11, 2017, to retrieve studies published from inception to the search date. We used the Ovid interface to search the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, and National Health Service Economic Evaluation Database (NHSEED).
Medical librarians developed the search strategies using controlled vocabulary (i.e., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.30 We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the health technology assessment review.
We performed targeted grey literature searching of health technology assessment agency websites and clinical trial registries. See Appendix 1 for the literature search strategies, including all search terms.
Literature Screening
A single reviewer used DistillerSR management software to conduct an initial screening of titles and abstracts and obtained the full text of studies that appeared eligible for the review, according to the inclusion criteria. The author then examined the full-text articles and selected studies that were eligible for inclusion. We reported citation flow and primary reason for exclusion of full-text articles according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.31
Inclusion Criteria
English-language full-text publications
Studies published prior to April 11, 2017
-
Randomized controlled trials, systematic reviews, and nonrandomized studies of MRgFUS neurosurgery alone or MRgFUS neurosurgery compared with one or more of the following:
-
∘
Radiofrequency thalamotomy
-
∘
Deep brain stimulation (unilateral or bilateral)
-
∘
Gamma Knife thalamotomy
-
∘
Control intervention (e.g., sham)
-
∘
Exclusion Criteria
Animal and in vitro studies
Feasibility, clinico-radiologic, and technical (e.g., targeting accuracy) studies
Editorials, case reports, and commentaries
Conference abstracts and posters
Studies of people with untreated or mild essential tremor or with a primary presentation of voice, head, or trunk tremor or ataxia
Studies of people with surgical contraindications (e.g., advanced age, unstable cardiovascular or respiratory disease, inability to communicate) or who have other specific contraindications or clinical or physical features that preclude them from eligibility for receiving MRgFUS neurosurgery (e.g., contraindications to MRI scanning)
Studies of people with movement disorders other than essential tremor (e.g., Parkinson's disease, Wilson disease, enhanced physiologic tremor)
Studies including mixed–movement disorder populations that do not analyze data or provide data or results by diagnostic subgroup (i.e., essential tremor alone)
Studies on medication, superficial brain stimulation, experimental treatments, and surgery
Outcomes of Interest
-
Tremor severity and disability assessed by tools such as the following:
-
∘
Versions of the Fahn–Tolosa–Marín (FTM) Clinical Rating Scale for Tremor (e.g., the Clinical Rating Scale for Tremor [CRST])
-
∘
Essential Tremor Rating Assessment Scale (TETRAS)
-
∘
Columbia University Assessment of Disability in Essential Tremor (CADET)
-
∘
Quality of life (assessed using a validated tool; e.g., Quality of Life in Essential Tremor [QUEST])
Activities of daily living (assessed using a validated tool)
Durability of effect over time
Adverse effects (i.e., complications and side effects)
Adverse events
During scoping, we did not detect any potential health inequities related to the effect of MRgFUS neurosurgery for the treatment of essential tremor. We report any relevant equity issues with regard the effect of MRgFUS neurosurgery for the treatment of essential tremor across different populations as defined by the PROGRESS-Plus categories identified during the review process.32
Data Extraction
A single reviewer extracted relevant information on study context, methods, population, intervention, comparators, outcomes, results, and risk-of-bias items into a data form, based on the information available in the published articles.
Statistical Analysis
We report the results from each included study. We calculated measures of central tendency, confidence intervals, and proportions from the reported data, as needed. We did not perform a meta-analysis of the results, as we had planned, owing to heterogeneity in study designs, analyses, and outcome measurement across the studies.
Critical Appraisal of Evidence
We assessed risk of bias to evaluate the internal validity of individual studies using the Cochrane Risk of Bias tool for randomized controlled trials33 and the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS)34 for nonrandomized studies.
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.35 The quality reflects our certainty about the evidence. We assessed the body of evidence based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias.
We could not formally assess publication bias using funnel-plot methodology or statistical methods owing to an insufficient number of studies.
Expert Consultation
Beginning in February 2017, we solicited local expert feedback on the use of MRgFUS neurosurgery for the treatment of medication-refractory essential tremor. We consulted with experts in specialty areas including neurology, neurosurgery, medical physics, and health care administration. The role of the expert advisors was to provide important contextual information on essential tremor, the use of MRgFUS neurosurgery to treat essential tremor, the diffusion of the MRgFUS technology, and clinical issues related to the treatment of Ontarians with essential tremor. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Literature Search
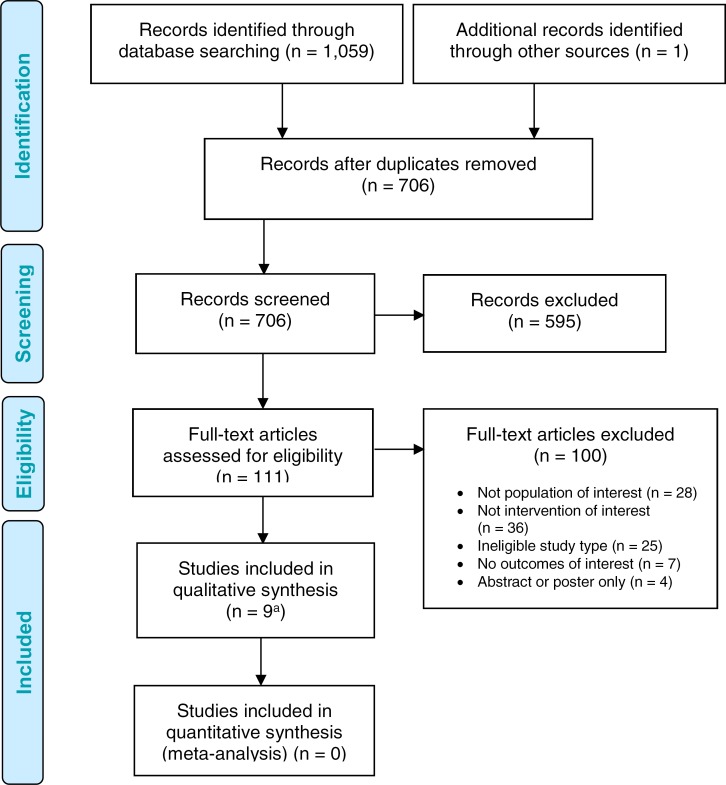
The literature search yielded 706 citations published from inception to April 11, 2017, after removing duplicates. Eight studies reported in ten articles36–45 (seven nonrandomized studies and one randomized control trial) met the inclusion criteria. We reviewed the reference lists of the included studies but identified no additional citations. We identified one further eligible study published since conducting our systematic literature search, for a total of nine included studies.
Figure 1 presents the PRISMA flow diagram. The characteristics of the included studies are presented in Table 2. Appendix 3 provides a selected list of studies excluded after full-text review that includes the primary reason for exclusion.
Figure 1: PRISMA Flow Diagram—Clinical Evidence Review.
Source: Adapted from Moher et al.31
aNine studies reported in 11 articles.
Table 2:
Characteristics of Included Studies
| Author, Year | Country | Study Design | Enrolment Time Frame | N | Intervention(s) | MRgFUS Target | Outcomes of Interest Reported (Time of Evaluation) |
|---|---|---|---|---|---|---|---|
| Kim et al, 201746 | Republic of Korea | Retrospective analysis (chart review) | 1995–2014 | 59 | MRgFUS DBS RF thalamotomy |
VIM | Degree of tremor improvement (1 month, 12 months) Recurrence Side effects/complications |
| Schreglmann et al, 201742 | Switzerland | Prospective, uncontrolled, single-centre, interventional study | NR | 6 | MRgFUS | CTT | Tremor severity (6 months) QUEST (6 months) Side effects/complications AE |
| Zaaroor et al, 201745 | Israel | Single cohort | Nov. 2013–Jan. 2016 | 18a | MRgFUS | VIM | Tremor severity (1 month, 6 months) QUEST (1 month, 6 months) Recurrence AE |
| Elias et al, 201638 | Canada, Japan, Republic of Korea, United States | Prospective, sham-controlled, multicentre RCT | Aug. 2013–Sep. 2014 | 76 | MRgFUS Sham MRgFUS |
VIM | Tremor severity (3 months) QUEST (3 months) Recurrence (12 months) Side effects/complications AE |
| Gallay et al, 201639 | Switzerland | Single cohort | NR | 21 | MRgFUS | CTT | Tremor severity (12 months) AE |
| Huss et al, 201640 | United States | Retrospective analysis (chart review) | Jan. 2004–Jul. 2013 | 85 | MRgFUS DBS |
VIM | Tremor severity (pre- vs. post-operative)b QUEST (pre- vs. post-operative)b Side effects/complications AE |
| Chang et al, 201536 | Republic of Korea | Single cohort | Mar. 2012–Nov. 2012 | 11 | MRgFUS | VIM | Tremor severity (6 months) AE |
| Elias et al, 201337,43,44 | United States | Open-label uncontrolled study (phase I) | Feb. 2011–Dec. 2011 | 15 | MRgFUS | VIM | Tremor severity (3 months, 12 months) QUEST (12 months) Side effects/complications AE |
| Lipsman et al, 201341 | Canada | Single cohort | May 2012–Jan. 2013 | 4 | MRgFUS | VIM | Tremor severity (1 month, 3 months) Side effects/complications AE |
Abbreviations: AE, adverse events; CTT, cerebellothalamic tract of the posterior thalamic area; DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; NR, not reported; QUEST, Quality of Life in Essential Tremor questionnaire; RCT, randomized controlled trial; RF, radiofrequency; VIM, ventral intermediate nucleus of the thalamus.
The study population included people with tremor conditions other than essential tremor (n = 12); some results were reported separately by condition.
For deep brain stimulation, the timing of postoperative measurement varied from 3 to 24 months (mean: 13.1 months) after the device was optimized. For MRgFUS thalamotomy, postoperative measurement occurred at 12 months, except for n = 1, who was assessed at 3 months (mean: 11.8 months).
Tremor Severity
All nine included studies36–42,45,46 reported on the impact of MRgFUS neurosurgery on tremor severity. Eight studies assessed severity with either the original FTM scale or its later version, the CRST. Both versions generate an overall score that reflects tremor severity, as well as three subscales that quantify the tremor (part A), action tremor of the upper extremity (part B; assesses tremor during movement via handwriting, drawing, and liquid-pouring tasks), and functional disability in activities of daily living (part C).6 For simplicity, we use CRST to refer to both versions of the scale throughout and report intra-study results for the version used in each study. The study by Kim and colleagues46 did not use CRST, but categorized treatment response according to extent of tremor abolition after surgery. All study participants across studies had moderate to severe, disabling essential tremor upon study enrolment, and of those who underwent MRgFUS neurosurgery, none had contraindications to the procedure. All studies used the Exablate Neuro ultrasound array paired with a compatible 3-Tesla MRI for MRgFUS procedures.
Comparative Studies
MRgFUS Thalamotomy Versus Sham
In the 2016 randomized controlled trial by Elias et al,38 76 people with essential tremor were randomized in a 3:1 ratio to receive MRgFUS ventral intermediate nucleus thalamotomy or a sham procedure. Upper extremity tremor severity (as measured by parts A and B of the CRST) was assessed via video 3 months postoperatively by a neurologist not involved in patient care or the trial and who was blinded to treatment allocation. The mean age of participants was 71 years (+/− 8.2 years), 75% were of Caucasian ethnicity with most others being Asian, 83% were right-handed, and 68% were male. There were no differences between the two groups in baseline demographic characteristics or tremor severity.38 The primary outcome of upper extremity tremor (Table 3) was analyzed for differences between groups at 3 months, and also for difference from baseline at 3 months and 12 months in the MRgFUS arm.
Table 3:
Intention-to-Treat Analysis of Upper Extremity Tremor Severity—MRgFUS Thalamotomy Versus Sham
| Time Point | Mean Score (SD) | Mean Improvement vs. Baseline | Mean Difference, Points (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| MRgFUS | Sham | MRgFUS | Sham | |||
| Baseline | 18.1 (4.8) | 16.0 (4.4) | – | – | – | NS |
| 3 months | 9.6 (5.1) | 15.8 (4.9) | 47% | 0.1% | −8.3 (−5.9 to −10.7) | < .001a |
| 12 months | 10.9 (4.5) | NAb | 40% | NAb | −7.2 (−6.1 to −8.3) | < .001c |
Abbreviations: CI, confident interval; MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable; NS, not significant; SD, standard deviation.
Note: Upper extremity tremor severity was assessed via parts A and B of the Clinical Rating Scale for Tremors (CRST), where higher scores indicate greater severity. The maximum possible score is 32.
P value is for the between-groups difference at 3 months follow up.
After the 3-month blinded period, sham participants could cross over to receive MRgFUS thalamotomy.
P value is for the change from baseline within the MRgFUS thalamotomy group.
Source: Elias et al, 2016.38
Three months after the procedure, people who had undergone MRgFUS thalamotomy experienced a 47% improvement in upper extremity tremor; this result was statistically significant compared with both their baseline severity (P < .001) and with the 0.1% improvement seen in the sham group over the same time frame (P < .001).38 This result translated to an absolute mean change in score at 3 months between the MRgFUS and sham groups of −8.3 points (95% confidence interval [CI]: 5.9–10.7, P < .001).
The authors also analyzed change in tremor severity from baseline within each group. In the MRgFUS group, upper extremity tremor severity scores were significantly reduced (i.e., tremor improved) at 1 month, 3, months, 6 months, and 12 months following treatment (P < .001).38 There was no change from baseline scores in the sham group.38
Total CRST scores were analyzed similarly post hoc; Table 4 presents these results. The mean total CRST tremor score (based on scores from parts A, B, and C) for participants at baseline was 49.5, out of a possible 152, with no difference between groups.38
Table 4:
Post-hoc Analysis of Overall Tremor Severity—MRgFUS Thalamotomy Versus Sham
| Time Point | Mean Score (SD) | Mean Improvement vs. Baseline | Mean Difference, Points (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| MRgFUS | Sham | MRgFUS | Sham | |||
| Baseline | 50.1 (14.0) | 44.1 (12.7) | – | – | – | NS |
| 3 months | 29.6 (13.0) | 43.1 (13.1) | 41% | 2% | NR | < .001a |
| 12 months | 32.4 (14.5) | NAb | 35% | NAb | NR | NA |
Abbreviations: CI, confident interval; MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable; NS, not significant; SD, standard deviation.
Note: Overall tremor severity was assessed via parts A, B, and C of the Clinical Rating Scale for Tremors (CRST), where higher scores indicate greater tremor severity. The maximum possible score is 152.
P value is for the between-groups difference at 3 months follow up.
After the 3-month blinded period, sham participants could cross over to receive MRgFUS thalamotomy. Only the within-group differences from baseline for the MRgFUS thalamotomy group was reported for the 12-month time point.
Source: Elias et al, 2016.38
At 3 months post-procedure, those in the MRgFUS group experienced a statistically significant 41% improvement in total tremor, compared with 2% in the sham group (P < .001; Figure 2).38 In the MRgFUS group, 27 patients (48%) experienced an improvement in tremor severity of more than 50%, 24 (43%) experienced a change of between 10% and 50%, and 5 (9%) experienced an improvement of less than 10%.38 The tremor improvement seen at 3 months in the MRgFUS group was sustained at 1 year (35%). No P values for change from baseline were reported.
Figure 2: CRST Scores at 3 Months Post-surgery—MRgFUS Thalamotomy Versus Sham (Post-hoc Analysis).
Source: Elias et al, 2016.38
Unblinded Cohort of Randomized Controlled Trial
In the 2016 randomized controlled trial by Elias et al,38 participants allocated to the sham arm were permitted to cross over to receive MRgFUS thalamotomy after 3 months and were then followed, with their outcomes analyzed in the same blinded manner as the MRgFUS group. Of those in the sham group, 19 of 20 participants crossed over, and two participants from the MRgFUS group in whom the procedure was incomplete (therapeutic temperature was not reached in the initial procedure) underwent MRgFUS thalamotomy.38 Three months postoperatively, upper extremity tremor improved significantly by 55% compared with baseline (16.5 +/− 4.2 to 7.4 +/−3.9, P < .001).38 At 6 months, there was a statistically significant 52% improvement in upper extremity tremor compared with baseline (8.0 +/− 3.9, P < .001).38 Participants' overall tremor severity also significantly improved between baseline and 1 month, 3 months, 6 months, and 12 months (P < .001 for all).38
MRgFUS Thalamotomy Versus Deep Brain Stimulation
Huss et al40 conducted a retrospective analysis of people with essential tremor treated with either MRgFUS thalamotomy (n = 15) or deep brain stimulation (unilateral or bilateral, n = 70) at one U.S. centre. This was done by reviewing the charts of patients with essential tremor who had been treated with these neurosurgeries over a period of approximately a decade. The authors compared patients' functional and quality-of-life outcomes both within and between groups. The authors analyzed outcomes for patients treated by one neurosurgeon between January 2004 and July 2013 who had completed both pre- and postoperative CRST and QUEST assessments. The groups were two-thirds male, with mean ages ranging from 63.5 to 71.7 years.40
At baseline, patients who had received MRgFUS thalamotomy had significantly lower overall CRST scores and tremor scores than patients who had received bilateral deep brain stimulation (P < .05 for both).40 No differences were seen in either task performance or dominant-hand subscores between groups. Importantly, the authors found some evidence that patients with marked tremor in the midline of the body may have been more likely to undergo bilateral deep brain stimulation than MRgFUS thalamotomy, as they scored significantly higher on axial tremor scores than patients in the MRgFUS group (P < .05).40
Regardless of treatment, patients with essential tremor experienced significant improvement postoperatively in total CRST score, tremor score, functional tasks, and disability compared with baseline (P < .05).40 Improvements in total CRST score were 79.5% for those who underwent bilateral deep brain stimulation, 62.8% for those who underwent unilateral deep brain stimulation, and 55.7% for those who underwent MRgFUS thalamotomy.40 The upper-extremity tremor of all patients improved significantly after treatment, regardless of surgery type (P < .05).40 However, no significant change in axial tremor was seen in the postoperative observation of patients who received MRgFUS thalamotomy (14.8%, P = .52), in contrast to the significant improvement seen with both unilateral and bilateral deep brain stimulation (P < .05 for all).40 Bilateral deep brain stimulation yielded lower scores on part B of the CRST (tremor during tasks) than MRgFUS thalamotomy (P < .05), indicating greater improvement.40
Kim et al46 also conducted a retrospective analysis of people with medication-refractory essential tremor who had undergone unilateral MRgFUS thalamotomy (n = 23), unilateral deep brain stimulation (n = 19), or unilateral radiofrequency thalamotomy (n = 17) at their medical centre in Seoul, Korea, over about 20 years. The study groups had median ages of between 63 and 66 years (interquartile ranges varied from 26 to 73 years). The MRgFUS thalamotomy group was 87% male, the deep brain stimulation group was 68% male, and the radiofrequency thalamotomy group was 53% male.46
Tremor improvement was measured on a different disease-specific scale than the CRST, as the CRST was unavailable for the radiofrequency thalamotomy group. The authors categorized symptoms as absent (100% tremor abolition), occasional (> 90% abolition), partial improvement (> 50% abolition), or showing no improvement (< 50% improvement).46 The results were then dichotomized as either success (absent or occasional tremor) or failure (partial or no improvement). It is important to note that this high threshold for success (> 90% tremor abatement) would have led to a conservative estimate of effectiveness. The authors analyzed their data using a generalized estimating equation model to compare outcomes between the three groups.
Tremor severity improved significantly from baseline in all groups at both 1 month and 12 months postoperatively (P < .001 and P < .0001, respectively).46 The model found no significant differences between the three groups in either treatment success (P = .54 at 1 month; P = .62 at 12 months) or rate of complete remission (i.e., 100% abolition) at 1 month (P = .14) or 12 months of follow-up (P = .62).46 The proportion of treatment success for MRgFUS thalamotomy compared with deep brain stimulation, and for MRgFUS thalamotomy compared with radiofrequency thalamotomy, respectively, are described separately in the paragraphs that follow. We calculated 95% confidence intervals for the proportions of success and remission rates for each group.
MRgFUS thalamotomy resulted in an improvement of more than 90% in 91.3% of patients at the 1 month follow-up (95% CI: 79.8–100%), and in 78.3% of patients (95% CI: 66.8–89.8%) at the 12-month follow-up.46 Among patients who had undergone deep brain stimulation, 89.5% experienced treatment success (i.e., > 90% improvement) at 1 month (95% CI: 75.7–100%), and 84.2% experienced treatment success at 12 months (95% CI: 67.8–100%).
At 1 month, 43.5% (95% CI: 23.2–63.8%) of patients who had undergone MRgFUS thalamotomy experienced complete tremor abolition, compared with 31.6% (95% CI: 10.7–52.5%) of those who had undergone deep brain stimulation. At 12 months, 34.8% (95% CI: 15.3–54.2%) of patients who had undergone MRgFUS thalamotomy experienced complete tremor abolition, compared with 47.4% (95% CI: 24.9–69.9%) of patients who had undergone deep brain stimulation.
MRgFUS Thalamotomy Versus Radiofrequency Thalamotomy
In the study by Kim and colleagues,46 the authors compared MRgFUS thalamotomy with radiofrequency thalamotomy and deep brain stimulation in terms of tremor control and complications.46 The generalized estimating equation model found no significant differences between the three groups with regard to treatment success or remission at any time point.46 Using the categorization of either success (complete or > 90% tremor abolishment) or failure (< 90% tremor improvement) described above, MRgFUS thalamotomy was successful in 91.3% of patients at 1 month follow-up (95% CI: 79.8–100%) and in 78.3% (95% CI: 66.8–89.8%) of patients at 12 months follow-up. Radiofrequency thalamotomy was successful in 100% (95% CI: 78.3–100%) of patients at 1 month and in 70.6% (95% CI: 48.9–92.3%) of patients at 12 months.
At 1 month, 43.5% (95% CI: 23.2–63.8%) of patients who had undergone MRgFUS thalamotomy and 76.5% (95% CI: 56.3–95.7%) of patients who had undergone radiofrequency thalamotomy experienced complete tremor abolition.46 At 12 months, 34.8% (95% CI: 15.3–54.2%) of patients in the MRgFUS group and 29.4% (95% CI: 25.7–51.1%) of patients in the radiofrequency group experienced complete tremor abolishment.
Noncomparative Cohort Studies
Six studies36,37,39,41,42,45 examined the impact of MRgFUS neurosurgery (ventral intermediate nucleus thalamotomy or cerebellothalamic tractotomy) in cohorts of people with essential tremor. Table 5 summarizes the characteristics of these cohorts.
Table 5:
Participant Characteristics in Cohort Studies of MRgFUS Neurosurgery
| Author, Year | N | Mean Age, Years (SD) | Age Range, Years | % Male |
|---|---|---|---|---|
| Schreglmann et al, 201742 | 6 | 70.7 (8.5) | 58–82 | 33 |
| Zaaroor et al, 201745 | 18a | 73.1 (6.2) | 64–87 | 67 |
| Gallay et al, 201639 | 21 | 69.1 (9.2) | NR | 71 |
| Chang et al, 201536 | 11 | 64.8 (7.71) | 53–78 | 82 |
| Elias et al, 201337,43,44 | 15 | 66.6 (8) | 53–79 | 67 |
| Lipsman et al, 201341 | 4 | 70.8 (NR) | 58–77 | 100 |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NR, not reported; SD, standard deviation.
There were 30 study participants, of whom 18 had essential tremor and 12 had either Parkinson's disease or tremor-dominant Parkinson's disease. The characteristics described reflect means, ranges, and proportions for the subset of participants with essential tremor.45
Sample sizes of participants with essential tremor ranged from 4 to 21, with mean ages in the mid- to late 60s. The authors analyzed total or subscale CRST scores after MRgFUS treatment to determine the effect of treatment on tremor severity and disability. Follow-up timing ranged from 1 month to 1 year. Table 6 presents the findings of these studies.
Table 6:
Improvement in Tremor Severity Observed in Cohort Studies of MRgFUS Neurosurgery
| Author, Year | N | CRST Scale(s) Assessed | Baseline Score, Mean (SD) | Follow-Up | Post-treatment Score, Mean (SD) | Improvement | P Value |
|---|---|---|---|---|---|---|---|
| Ventral Intermediate Nucleus Thalamotomy | |||||||
| Zaaroor et al, 201745 | 18a | Total | 40.7 (11.6) | 1 mo 6 mo |
9.3 (7.1) 8.2 (5.0) |
77% 80% |
< .001 < .001 |
| Chang et al, 201536 | 11b | Part A Part B Part C |
5.1 13.0 13.5 |
6 mo 6 mo 6 mo |
1.4 2.6 2.8 |
73% 80% 79% |
NR NR NR |
| Elias et al, 201337,43,44 | 15 | Part A Total |
20.4 (5.2) 54.9 (14.4) |
3 mo 12 mo 12 mo |
4.3 (3.5) 5.2 (4.8) 24.3 (14.8) |
79% 75% 56% |
NR .001 .001 |
| Lipsman et al, 201341 | 4 | Part A Part B Part C Total Part A Part B Part C Total |
21.5 (11.2) 28.5 (6.5) 20.8 (4.5) 70.8 (19.7) |
1 mo 1 mo 1 mo 1 mo 3 mo 3 mo 3 mo 3 mo |
7.3 (2.2) 16 (7.6) 8 (4.2) 31.3 (15.0) 7.8 (4.6) 17.3 (6.6) 10.3 (3.3) 35.3 (11.0) |
66% 45.5% 62% 56% 65% 39.6% 50% 50% |
NR NR NR NR NR NR NR NR |
| Cerebellothalamic Tractotomy | |||||||
| Schreglmann et al, 201742 | 6 | Total CRST Unilateral (treated side) Speaking and working Drawing and pouring |
43.8 (9.8) 14.3 (4.9) 13.8 (3.4) 8.7 (2.7) |
6 mo 6 mo 6 mo 6 mo |
19.8 (6.8) 2.5 (2.6) 2.5 (0.8) 2.3 (2.3) |
−24 points (95% CI: −18.1 to −29.9) −11.8 points (95% CI: −8.4 to −15.2) −11.3 (95% CI: −8.8 to −13.9) −6.3 (95% CI: −3.8 to −8.9) |
< .001 < .001 < .001 < .001 |
| Gallay et al, 201639 | 21c | Total | 57.6 (13.2) | 12 mo | 25.8 (17.6) | 55% | NR |
| Hand function, targeted hand only | 12.4 (1.3) | 3 mo 12 mo |
NR NR |
74% 78% |
NR NR |
||
| Subgroup 1 (Severe) | 7 | Hand function, targeted hand only | 15.3 (1.3) |
3 mo 12 mo |
NR NR |
41% 40% |
NR NR |
| Subgroup 2 | 14 | Hand function, targeted hand only | 11.0 (3.3) |
3 mo 12 mo |
NR NR |
92% 90% |
NR NR |
Abbreviations: CI, confident interval; MRgFUS, magnetic resonance-guided focused ultrasound; NR, not reported; SD, standard deviation.
Note: Essential tremor severity was assessed via the Clinical Rating Scale for Tremor (CRST).
There were 30 study participants, of whom 18 had essential tremor and 12 had either Parkinson's disease or tremor-dominant Parkinson's disease. The characteristics described reflect means, ranges, and proportions for the subset of participants with essential tremor.45
There were outcome data for 8 of 11 participants at follow-up.
There were outcome data for 10 of 21 participants at the 12-month follow-up.
In all six studies, tremor improved markedly following treatment compared with baseline.36,37,39,41,42,45 Three studies reported that the improvements were statistically significant (P < .001 for all),37,42,45 whereas the others did not report P values for the comparison.36,39,41 Across studies, total CRST scores improved by 50% to 77%; tremor subscale (part A) scores improved by 66% to 83%; task subscale (part B) scores improved by more than 40%; and functional disability subscale (part C) scores improved by 50% or more.36,37,39,41,42,45
In the study of MRgFUS cerebellothalamic tractotomy by Gallay et al,39 participants self-reported that they perceived a 77% improvement in tremor at 1 year post-procedure. Two participants underwent staged bilateral cerebellothalamic tractotomy, involving two procedures performed 1 year apart. One year after the second surgery, these participants reported an improvement of 75% to 88% in their dominant hand and an improvement of 56% to 78% in their nondominant hand.39
Gallay et al39 found that their sample differed in terms of baseline severity measured on the CRST and therefore conducted separate subgroup analyses of participants whose essential tremor was more severe (n = 7) versus moderate (n = 21). Those in the moderate group experienced greater improvement following surgery than did those in the severe group at 3 months (92% vs. 41%), and these within-group improvements persisted to 1 year.39 However, no statistics were reported for the subgroup comparisons.
Table 7 provides the GRADE assessment of our certainty in this body of evidence.
Table 7:
GRADE Evidence Profile for Tremor Severity After MRgFUS Neurosurgery
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Compared With Sham | |||||||
| 1 (RCT)38 | No serious limitations | No serious limitationsa | No serious limitations | No serious limitationsb | Undetecteda | NA | ⊕⊕⊕⊕ High |
| Compared With Deep Brain Stimulation | |||||||
| 2 (Observational)40,46 | Serious limitations (−1)c | No serious limitations | No serious limitations | Serious limitations (−1)d | Undetecteda | NA | ⊕ Very Low |
| Compared With Radiofrequency Thalamotomy | |||||||
| 1 (Observational)46 | Serious limitations (−1)c | No serious limitationsa | No serious limitations | No serious limitations | Undetecteda | NA | ⊕ Very Low |
| MRgFUS Only | |||||||
| 6 (Observational)36,37,39,41,42,45 | No serious limitationse | No serious limitationsf | No serious limitations | No serious limitationsg | Undetectedh | NA | ⊕⊕ Low |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable; RCT, randomized controlled trial.
Cannot definitively assess presence or absence because the evidence is derived from a single study.
Study adequately powered, confidence interval for absolute difference in severity scores relatively narrow, mean difference and upper and lower bounds are clinically meaningful, as is relative improvement.
Risk of bias was highest owing to retrospective data collection methods. The full risk-of-bias assessment is presented in Appendix 2, Table A2.
Considerable imbalance in group sizes for comparisons, optimal information size criteria not met, and no measures of variance or confidence intervals provided; therefore, uncertainty remains in the precision of estimates.
Risk of bias overall judged to be low or unclear for most studies; one study (Zaaroor et al45) judged to be at high risk of bias resulting from participant selection. The full risk-of-bias assessment is presented in Appendix 2, Table A2.
Variability in exact magnitude of effect, but of questionable importance as all estimates indicate clinically meaningful benefit.
Some studies did not meet optimal information size criterion, yet found both statistically significant and clinically meaningful results that are on the same side of the clinical decision threshold.
Inadequate information reported from studies to formally assess using funnel plot or statistical tests; however, the studies vary in terms of sample size and are generally small.
Functional Disability in Activities of Daily Living
Three studies provided a separate analysis of disability in people with essential tremor receiving MRgFUS thalamotomy.37,38,40 As a secondary outcome, functional disability in activities of daily living (assessed via part C of the CRST) was analyzed before and after either MRgFUS thalamotomy or a sham procedure in the 2016 randomized controlled trial by Elias et al.38 At baseline, the greatest degree of disability was observed in drinking and writing.38 Table 8 presents the results from this study.
Table 8:
Reduction in Functional Disability—MRgFUS Thalamotomy Versus Sham
| Time Point | Mean Score (SD) | Mean Improvement vs. Baseline | P Value | ||
|---|---|---|---|---|---|
| MRgFUS | Sham | MRgFUS | Sham | ||
| Baseline | 16.5 (4.6) | 16.0 (4.3) | – | – | NS |
| 3 months | 6.2 (5.6) | 15.6 (4.6) | 62% | 3% | < .001a |
| 12 months | 6.3 (6.2) | NAb | 62% | NAb | NA |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable; NS, not significant; SD, standard deviation.
Note: Functional disability was assessed via part C of the Clinical Rating Scale for Tremors (CRST), where higher scores indicate greater severity. The maximum possible score is 32.
P value is for the between-groups difference at 3 months follow up.
After the 3-month blinded period, sham participants could cross over to receive MRgFUS thalamotomy. Only the within-group difference from baseline for the MRgFUS thalamotomy group was reported for the 12-month time point.
Source: Elias et al, 2016.38
A significant reduction was seen in functional disability in the MRgFUS group compared with sham (P < .001).38 By 12 months follow-up, participants who had received MRgFUS thalamotomy experienced improvement in every activity to the level of either normal (score: 0) or mild disability (score: 1), with the exception of writing (mean score: 1.21 +/− 1.14).38
In their retrospective study, Huss et al40 analyzed reduction in disability (assessed via part C of the CRST) in people with essential tremor who had undergone unilateral or bilateral deep brain stimulation versus MRgFUS thalamotomy, as well as within each group compared with baseline. Although a statistically significant postoperative improvement (P < .05) was seen in all groups, no significant differences in improvement were found between those who had undergone bilateral deep brain stimulation versus MRgFUS thalamotomy (P = .59), or between those who had undergone unilateral versus bilateral deep brain stimulation (P = .42).40
In their 2013 cohort study, Elias et al37 found a statistically significant improvement in disability from baseline to 12 months following MRgFUS thalamotomy (18.2 +/− 4.1 vs. 2.8 +/− 3.4, P < .001). This result corresponds to an 85% improvement postoperatively.37
Table 9 summarizes the disability outcome results for the studies by Huss et al40 and Elias et al.37,43,44 Table 10 provides the GRADE assessment of the body of evidence from these studies.
Table 9:
Reduction in Functional Disability Observed in Nonrandomized Studies of MRgFUS Thalamotomy
| Author, Year (Study Design) | N | Intervention | Baseline Score, Mean (SD) | Follow-Up | Post-treatment Score, Mean (SD) | Improvement | P Value, Change From Baseline |
|---|---|---|---|---|---|---|---|
| Huss et al, 201540 (Retrospective analysis) |
15 13 57 |
MRgFUS Unilateral DBS Bilateral DBS |
18.2 18.9 19.9 |
NR | 2.8 3.2 2.3 |
85.4% 88.4% 83.1% |
< .05a < .05a < .05a |
| Elias et al, 201337,43,44 (Cohort) | 15 | MRgFUS | 18.2 (4.1) | 12 mo | 2.8 (3.4) | 85% | < .001 |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; NR, not reported; SD, standard deviation.
Note: Disability was assessed via part C of the Clinical Rating Scale for Tremors (CRST), where higher scores indicate greater severity. The maximum possible score is 32.
P values presented reflect change from baseline. In the study by Huss et al,40 the between-groups comparisons for degree of reduction in disability were not significant for the comparison of bilateral deep brain stimulation with either unilateral deep brain stimulation (P = .42) or MRgFUS thalamotomy (P = .59).
Table 10:
GRADE Evidence Profile for Functional Disability After MRgFUS Thalamotomy
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Compared With Sham | |||||||
| 1 (RCT)38 | No serious limitations | No serious limitationsa | No serious limitations | No serious limitationsb | Undetecteda | NA | ⊕⊕⊕⊕ High |
| Compared With Deep Brain Stimulation | |||||||
| 1 (Observational)40 | Serious limitations (−1)c | No serious limitationsa | No serious limitations | Serious limitations (−1)d | Undetecteda | NA | ⊕ Very Low |
| MRgFUS Only | |||||||
| 1 (Observational)37 | No serious limitationse | No serious limitationsa | No serious limitations | No serious limitationsf | Undetecteda | NA | ⊕⊕ Low |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable; RCT, randomized controlled trial.
Cannot definitively assess presence or absence because evidence is derived from a single study.
Study adequately powered; absolute change and relative improvement are both clinically meaningful and statistically significant.
Risk of bias was highest owing to retrospective data collection methods. The full risk-of-bias assessment is presented in Appendix 2, Table A2.
Considerable imbalance in group sizes for comparisons, optimal information size criteria not met, no measures of variance or confidence intervals provided; therefore, uncertainty remains in precision of estimates.
Risk of bias judged to be low for all considerations except participant selection, which was unclear. The full risk-of-bias assessment is presented in Appendix 2, Table A2.
Confidence interval for absolute difference in severity scores relatively narrow, mean difference and upper and lower bounds are clinically meaningful, as is relative improvement.
Tremor Recurrence
Two studies reported on the recurrence of tremor in people treated with MRgFUS thalamotomy separately from the stability of tremor improvement over time.45,46 In the study by Zaaroor et al,45 two patients (11%) experienced some tremor recurrence within the 6 months post-surgery, although tremor severity was less disabling than at baseline. In one case, tremor recurred after 3 weeks but was less debilitating than at baseline. In the other case, tremor recurred after 3 months but appeared only during writing, whereas at baseline the tremor precluded all activities.45
The retrospective study by Kim et al46 reported tremor recurrence, defined as return to baseline tremor severity, in each of three groups: people who had undergone MRgFUS thalamotomy, deep brain stimulation, or radiofrequency thalamotomy. Twelve months after surgery, one participant who had undergone MRgFUS thalamotomy (4.3%), one who had undergone deep brain stimulation (5.2%), and three who had undergone radiofrequency thalamotomy (17.6%) experienced tremor recurrence.46
Table 11 provides the GRADE assessment of the body of evidence for the outcome of tremor recurrence.
Table 11:
GRADE Evidence Profile for Tremor Recurrence After MRgFUS Neurosurgery
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| MRgFUS Only | |||||||
| 2 (Observational)45,46 | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitationsb | Undetected | NA | ⊕ Very Low |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable.
Judged to be at high risk of selection bias owing to participants being offered a choice between two surgeries and choosing MRgFUS neurosurgery in one study,45 and retrospective data collection in the other.46 The full risk-of-bias assessment is presented in Appendix 2, Table A2.
Quality of Life
Participants' self-reported quality of life was measured with the Quality of Life in Essential Tremor (QUEST) questionnaire in five studies.37,38,40,42,45 The QUEST questionnaire captures several aspects of quality of life, including communication, work and finances, hobbies and leisure, physical activities of daily living, and psychosocial well-being.47 Higher scores reflect poorer quality of life.
In their 2016 randomized controlled trial, Elias et al38 compared differences in QUEST scores at 3 months after the procedure between the MRgFUS thalamotomy and sham groups. Within the MRgFUS group, the authors also assessed change in quality of life between baseline and 12 months following surgery. Table 12 summarizes these results.
Table 12:
Impact on Quality of Life—MRgFUS Thalamotomy Versus Sham
| Time Point | Mean Score (SD) | Mean Improvement vs. Baseline | P Value | ||
|---|---|---|---|---|---|
| MRgFUS | Sham | MRgFUS | Sham | ||
| Baseline | 42.6 (18.3) | 42.8 (19.5) | – | – | NS |
| 3 months | 23.1 (16.9) | 41.4 (19.4) | 46% | 3% | < .001a |
| 12 months | 21.7 (17.2) | NAb | 49% | NAb | NA |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable; NS, not significant; SD, standard deviation.
Note: Quality of life assessed via the Quality of Life in Essential Tremor (QUEST) questionnaire.
P value is for the between-groups difference at 3 months follow up.
After the 3-month blinded period, sham participants could cross over to receive MRgFUS thalamotomy. Only the within-group difference for the MRgFUS thalamotomy group was reported for the 12-month time point.
Source: Elias et al, 2016.38
The authors found that quality of life improved significantly more in the MRgFUS group than in the sham group (46% vs. 3%, P < .001).38 For participants treated with MRgFUS thalamotomy, the improvement was sustained at 12-months post-procedure. The largest change from baseline was observed in psychosocial well-being.38 Figure 3 illustrates the mean difference in QUEST scores at 3 month follow-up between the MRgFUS and sham groups.
Figure 3: Mean Difference in QUEST Scores at 3 Months—MRgFUS Thalamotomy Versus Sham.
Source: Elias et al, 2016.38
In their retrospective study, Huss et al40 compared QUEST scores only for participants who had undergone MRgFUS neurosurgery or bilateral deep brain stimulation; QUEST scores were not available for participants who had undergone unilateral deep brain stimulation. After surgery, both the MRgFUS and bilateral deep brain stimulation groups experienced significant improvements in overall quality of life compared with preoperative assessments: MRgFUS participants improved by 68.0%, and bilateral deep brain stimulation participants improved by 72.0% (P < .05 for both). However, participants who had undergone MRgFUS thalamotomy did not experience a significant change from baseline in the communication subdomain. No significant between-groups differences were observed postoperatively in any of the QUEST subdomains.40
Caution must be exercised when interpreting the between-groups comparisons, as there were significant between-groups differences at baseline in age and tremor severity. Participants who underwent bilateral deep brain stimulation were older and had more severe tremor than participants in the other groups. Bilateral deep brain stimulation recipients also experienced significantly worse preoperative total quality of life than those who received MRgFUS thalamotomy (QUEST scores of 52.1 vs. 37.5, P = .009).40 Further, participants who underwent bilateral deep brain stimulation experienced significantly higher dysfunction in the communication and psychosocial subdomains of QUEST preoperatively than patients who underwent MRgFUS (P <.05 for both).40
Two cohort studies of MRgFUS ventral intermediate nucleus thalamotomy37,45 and one of cerebellothalamic tractotomy42 found statistically significant and clinically meaningful improvements in quality of life at various time points after MRgFUS neurosurgery.
As shown in Table 13, quality of life improved significantly after MRgFUS neurosurgery in all studies (P < .05 for all).37,42,45 The significant improvement was observed at 1 month, 6 months, and 12 months post-procedure.37,42,45
Table 13:
Improvement in Quality of Life Observed in Cohort Studies of MRgFUS Neurosurgery
| Author, Year | N | Target | Baseline, Mean Score (SD) | Follow-Up | Post-treatment, Mean Score (SD) | Improvement | P Value |
|---|---|---|---|---|---|---|---|
| Zaaroor et al, 201745 | 18a | VIM | 44.8 (12.9) | 1 mo 6 mo |
13.1 (12.3) 12.3 (7.2) |
71% 73% |
< .001 < .001 |
| Schreglmann et al, 201742 | 6 | CTT | 50.5 (19.4) | 6 mo | 24.8 (11.4) | 52% | .046 |
| Elias et al, 201337,43,44 | 15 | VIM | 37 (NR) | 12 mo | 12 (NR) | 68% | .001 |
Abbreviations: CTT, cerebellothalamic tract; MRgFUS, magnetic resonance-guided focused ultrasound; NR, not reported; SD, standard deviation; VIM, ventral intermediate nucleus of the thalamus.
Note: Quality of life was assessed via the Quality of Life in Essential Tremor (QUEST) questionnaire.
There were 30 study participants, of whom 18 had essential tremor and 12 had either Parkinson's disease or tremor-dominant Parkinson's disease. The characteristics described reflect means, ranges, and proportions for the subset of participants with essential tremor.45
Table 14 provides the GRADE assessment of the body of evidence for quality of life after MRgFUS neurosurgery.
Table 14:
GRADE Evidence Profile for Quality of Life After MRgFUS Neurosurgery
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Compared With Sham | |||||||
| 1 (RCT)38 | No serious limitations | No serious limitationsa | No serious limitations | No serious limitationsb | Undetecteda | NA | ⊕⊕⊕⊕ High |
| Compared With Deep Brain Stimulation | |||||||
| 1 (Observational)40 | Serious limitations (−1)c | No serious limitationsa | No serious limitations | Serious limitations (−1)d | Undetecteda | NA | ⊕ Very Low |
| MRgFUS Only | |||||||
| 3 (Observational)37,42,45 | No serious limitationse | No serious limitationsa | No serious limitations | No serious limitationsf | Undetecteda | NA | ⊕⊕ Low |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable; RCT, randomized controlled trial.
Cannot definitively assess presence or absence because the evidence is derived from a single study.
Absolute change and relative improvement are both clinically meaningful and statistically significant.
Risk of bias was highest owing to retrospective data collection methods. The full risk-of-bias assessment is presented in Appendix 2, Table A2.
Considerable imbalance in group sizes for comparisons, optimal information size criteria not met, no measures of variance or confidence intervals provided; therefore, uncertainty remains in the precision of estimates.
Risk of bias judged to be low for all considerations except participant selection, which was unclear. The full risk-of-bias assessment is presented in Appendix 2, Table A2.
Statistical power an issue in one study; however, a clinically meaningful and statistically significant, large effect was found.
Adverse Effects
MRgFUS Neurosurgery
As reported in four studies, the most frequent sensations and events experienced during the MRgFUS procedure were vestibular symptoms such as dizziness, vertigo, nausea, and vomiting.36,37,41,42 In these studies, all symptoms resolved once the sonications were stopped. The frequency and nature of these procedure-related adverse effects were reported as follows:
Chang et al36: Five of 11 participants experienced vestibular symptoms during sonications (e.g., dizziness, nausea, vomiting). No statically significant difference was observed in the duration of the procedure between participants who did and did not experience symptoms (P = .361)
Elias et al37: Vestibular symptoms experienced during sonications among the 15 participants included one episode of syncope, nine occurrences of head pain, and four instances of a warm or flushed feeling
Lipsman et al41: One of four participants experienced paresthesia during sonications, which resolved once the sonications were stopped
Schreglmann et al42: Four of six participants who underwent cerebellothalamic tractotomy experienced reversible vestibular symptoms, but only during the final, highest-power sonications
MRgFUS neurosurgery requires the attachment of a metal stereotactic head frame to keep the patient's head absolutely still and to facilitate precise localization of the target area for ablation. In their 2013 study, Elias et al37 reported a few minor adverse effects associated with use of the frame, including the pins used to secure the frame. Among the 15 participants, there were four (27%) headaches lasting more than 24 hours; four (27%) instances of scalp numbness; one pin-site laceration (7%); one instance of swelling around the eyes; and two scalp burns from pin-site MRI heating (13%).37 All events resolved without intervention.37
The study by Zaaroor et al45 included participants with essential tremor and other movement disorders. The authors reported overall adverse effects for the study population but did not provide separate findings for the subset of participants with essential tremor (n = 18). The authors reported occurrences of postoperative gait ataxia (n = 3; resolved within 1–3 months) and hand ataxia (n = 2; resolved within 1–4 weeks). All cases were transient and resolved gradually.45 The relative frequency of these events among participants with essential tremor is unclear.
Tables 15 and 16 summarize the adverse effects reported in the cohort studies, and in the intervention arm and unblinded cohort of the 2016 randomized controlled trial of ventral intermediate nucleus thalamotomy for the treatment of essential tremor by Elias et al.38
Table 15:
Adverse Effects Reported in Studies of MRgFUS Ventral Intermediate Nucleus Thalamotomy
| Author, Year (n) | Time Point | Paresthesias or Numbness, n (%) | Gait or Motor Disturbance, n (%) | Speech Problems, n (%) | Headache, n (%) | Vestibular Issues, n (%) | Other, n (%) |
|---|---|---|---|---|---|---|---|
| Elias et al, 201638,a | Totalb | Face and hand, 6 (11) | Ataxia, 11 (20) | Dysarthria, 1 (2) | > 1 day, 8 (14) | Disequilibrium sensation, 5 (9) | Contralateral weakness, 2 (4) |
| Randomized to MRgFUS (n = 56) | Face, lips, and tongue, 8 (14) Hand and fingers, 6 (11) Leg, 1 (2) |
Subjective unsteadiness, 9 (16) Dysmetria, 7 (12) |
Dysphagia, 1 (2) | Tinnitus, 3 (5) | Fatigue, 3 (5) Taste disturbance, 3 (5) |
||
| 12 mo | Face and hand, 8 (14) Face, lips, and tongue, 5 (9) Hand and fingers, 2 (4) Leg, 0 |
Ataxia, 2 (4) Subjective unsteadiness, 3 (5) Dysmetria, 2 (4) |
Dysarthria, 0 Dysphagia, 0 |
— | Disequilibrium sensation, 1 (2) Tinnitus, 0 |
Contralateral weakness, 1 (2) Fatigue, 0 Taste disturbance, 2 (4) |
|
| Elias et al, 201638,b Unblinded crossover cohort (n = 21) |
Totalc | Face, lips, and tongue, 6 (29) Hand and fingers, 5 (24) |
Ataxia, 3 (14) Subjective unsteadiness, 5 (24) Dysmetria, 3 (14) |
Dysarthria, 3 (14) | Any, 7 (33) | Disequilibrium sensation, 3 (14) | Contralateral weakness, 3 (14) Fatigue, 4 (19) Taste disturbance, 2 (10) |
| 12 mo | Face, lips, and tongue, 5 (24) Hand and fingers, 1 (5) |
Ataxia, 0 Subjective unsteadiness, 1 (5) Dysmetria, 2 (10) |
Dysarthria, 2 (10) | Any, 0 | Disequilibrium sensation, 1 (5) | Contralateral weakness, 1 (5) Fatigue, 0 Taste disturbance, 2 (10) |
|
| Chang et al, 201536 (n = 11) | 1 mo | – | – | – | – | Mild balance problem, 1 (9) | – |
| Elias et al, 201337 (n = 15) | Postoperative | Lip or tongue, 9 (60) Finger, 5 (33) |
Ataxia, 4 (27) Subjective unsteadiness, 5 (33) Dysmetria, 1 (7) |
Slurred speech (dysarthria), 1 (7) | – | – | Weak grip, 1 (7) |
| 12 mo | Lip or tongue, 2 (13) Finger, 1 (7) |
Ataxia, 0 Subjective unsteadiness, 0 Dysmetria 0 |
Slurred speech (dysarthria), 0 | > 1 day, 0 | – | Weak grip, 0 | |
| Lipsman et al, 201341 (n = 4) | 3 mo | Thumb and index finger, 1 (20) | – | – | – | – | – |
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
Note: The adverse effects described include all those as reported in the published articles.
The events reported are those occurring in the MRgFUS arm of the randomized controlled trial. Events at 12 months persist.
The events reported are those occurring in the unblinded cohort of crossover participants from the sham arm. Events at 12 months persist.
The total reflects the sum of all events occurring any time during the 3-month randomized study period.
Table 16:
Adverse Effects Reported in Studies of MRgFUS Cerebellothalamic Tractotomy
| Author, Year (n) | Time Point | Paresthesias or numbness, n (%) | Gait or Motor Disturbance, n (%) | Speech Problems, n (%) | Headache, n (%) | Vestibular Issues, n (%) | Other, n (%) |
|---|---|---|---|---|---|---|---|
| Schreglmann et al, 201742 (n = 6) | Postoperativea | – | Gait instability, 1 (17) Clumsiness of treated hand, 1 (17) |
– | – | Tendency to veer to treated side, 1 (17) | – |
| 3 mo | – | Gait instability, 0 Clumsiness of treated hand, 0 |
– | – | Tendency to veer to treated side, 0 | – | |
| Gallay et al, 201639 (n = 21) | Postoperativea | – | Worsening of pre-existing gait instability, 5 (24) | – | – | – | – |
| Last follow-upa,b | – | Worsening of pre-existing gait instability, 1 (5); 0.5/4 points worse than baseline) | – | – | – | – |
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
Note: The adverse effects described include all those as reported in the published articles.
The exact time point was not reported.
The last follow-up ranged from 3 months to 1 year after surgery.
Among the studies of MRgFUS ventral intermediate nucleus thalamotomy, the most common adverse effects were paresthesias or numbness in the face or fingers and, more rarely, gait or motor disturbances. About half of participants in these studies experienced one or more adverse effects immediately following the MRgFUS procedure. Most adverse effects occurred shortly after the procedure were transient, related to postoperative edema (swelling near the lesion site following surgery), resolving by follow-up in most cases. Across the studies' 107 participants, persistent adverse effects included paresthesias in 1% to 14%, gait or motor disturbance in up to 7%, balance problems or disequilibrium in less than 1%, contralateral weakness in less than 1%, and taste disturbance in up to 2%.36–38,41
Two studies reported data on the adverse effects of MRgFUS cerebellothalamic tractotomy, the most frequent of which was gait instability (either new or an exacerbation of pre-existing instability).39,42 With the exception of one participant in the study by Gallay et al,39 who had persistent mild gait instability (i.e., a worsening of 0.5 points out of a possible 4 from baseline), all adverse effects completely resolved by follow-up, as shown in Table 16.
MRgFUS Thalamotomy Versus Sham
In the 2016 randomized controlled trial by Elias et al,38 six participants who underwent MRgFUS thalamotomy (11%) and 8 who underwent a sham procedure (40%) experienced no adverse effects. Table 17 summarizes the occurrence of adverse effects over the 3-month randomized study period for each group.
Table 17:
Adverse Effects Reported Over 3 Months in a Randomized Controlled Trial of MRgFUS Thalamotomy Versus Sham
| Adverse Effect | MRgFUS (N = 56), Totala n (%) | Sham (N = 20), Totala n (%) |
|---|---|---|
| Procedural Events | ||
| Head discomfort | 17 (30) | 0 |
| Stereotactic frame pin-site edema, pain, or bruising | 17 (30) | 7 (35) |
| Vertigo | 12 (21) | 0 |
| Nausea | 11 (20) | 2 (10) |
| Back pain | 5 (9) | 1 (5) |
| Scalp tingling | 4 (7) | 1 (5) |
| Anxiety | 3 (5) | 2 (10) |
| Vomiting | 2 (4) | 0 |
| Paresthesias or Numbness | ||
| Face, lips, and tongue | 8 (14) | 0 |
| Face and hand | 6 (11) | 0 |
| Hand and fingers | 6 (11) | 1 (5) |
| Leg | 1 (2) | 0 |
| Gait or Motor Disturbance | ||
| Ataxia | 11 (20) | 0 |
| Subjective unsteadiness | 9 (16) | 1 (5) |
| Dysmetria | 7 (12) | 0 |
| Speech Problems | ||
| Dysarthria | 1 (2) | 0 |
| Dysphagia | 1 (2) | 0 |
| Vestibular Symptoms | ||
| Disequilibrium sensation | 5 (9) | 0 |
| Tinnitus | 3 (5) | 0 |
| Other Effects | ||
| Headache lasting more than 1 day | 8 (14) | 4 (20) |
| Fatigue | 3 (5) | 1 (5) |
| Taste disturbance | 3 (5) | 0 |
| Contralateral weakness | 2 (4) | 0 |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; N, number of participants in the group; n, number of events reported.
The total reflects the sum of all events occurring any time during the 3-month randomized study period.
Source: Elias et al, 2016.38
MRgFUS Thalamotomy Versus Deep Brain Stimulation
Huss et al40 retrospectively compared MRgFUS thalamotomy with bilateral and unilateral deep brain stimulation. Table 18 presents the transient adverse effects (occurring within 0–3 months of the procedure) and long-term adverse effects (occurring at 12 months following the procedure) reported for each of the three procedures.
Table 18:
Adverse Effects Reported in a Study Comparing MRgFUS Thalamotomy With Unilateral and Bilateral Deep Brain Stimulation
| Adverse Effect | MRgFUS (N = 15), n transient (n at 12 mo) | Bilateral DBS (N = 57), n transient (n at 12 mo) | Unilateral DBS (N = 13), n transient (n at 12 mo) |
|---|---|---|---|
| Procedural Events | |||
| Vertigo | 11 (0) | 0 (0) | 0 (0) |
| Headache | 9 (0) | 0 (0) | 0 (0) |
| Nausea or vomiting | 8 (0) | 0 (0) | 0 (0) |
| Warm or flushed feeling | 4 (0) | 0 (0) | 0 (0) |
| Stereotactic frame pin-site burn | 2 (0) | 0 (0) | 0 (0) |
| Side Effects | |||
| Paresthesia (any) | 14 (3) | 2 (1) | 1 (2) |
| Gait instability | 5 (0) | 10 (0) | 11 (0) |
| Dysarthria | 1 (0) | 10 (6) | 1 (0) |
| Weakness | 1 (0) | 4 (1) | 1 (0) |
| Dysphagia | 0 (0) | 2 (0) | 0 (0) |
| Mental status change | 0 (0) | 3 (3) | 1 (0) |
| Complications | |||
| DBS hardware infection | NA | 0 (1) | 0 (0) |
| DBS lead erosion | NA | 1 (2) | 0 (0) |
| Hemorrhage | 0 (0) | 2 (0) | 0 (0) |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; N, number of participants in the group; n, number of events reported.
Source: Adapted from Huss et al, 2015.40
Huss et al40 did not statistically quantify the frequency of the side effects and complications associated with MRgFUS neurosurgery or deep brain stimulation. As depicted in Table 18, the most common transient adverse effects of MRgFUS thalamotomy were paresthesias and gait instability; however, at 1 year post-procedure, the majority (nearly 85%) of these occurrences had resolved.40 There were no serious complications associated with MRgFUS thalamotomy in the study. The most common adverse effects associated with deep brain stimulation were gait instability and dysarthria, of which about half of the latter persisted at 12 months post-surgery.40 Mental state changes were not seen in any patients who had undergone MRgFUS, but did occur among patients who had undergone deep brain stimulation. All instances of mental status change persisted in the bilateral deep brain stimulation group at 12 months.40 In the bilateral deep brain stimulation group, hardware complications occurred in four instances over 12 months, and two intracranial hemorrhages occurred. Among patients who had undergone unilateral deep brain stimulation, two paresthesias developed over 12 months.40
The authors reported that the incidence and resolution of the adverse effects associated with MRgFUS thalamotomy and deep brain stimulation that occurred in the study were consistent with previously reported adverse event profiles for these procedures.40 However, they did not conduct a statistical analysis.
MRgFUS Thalamotomy Versus Deep Brain Stimulation or Radiofrequency Thalamotomy
In a retrospective study, Kim et al46 analyzed the rates of adverse effects associated with unilateral MRgFUS thalamotomy, deep brain stimulation, and radiofrequency thalamotomy by reviewing the charts of patients with essential tremor who had undergone these surgeries. For deep brain stimulation, the authors considered complications to include both side effects that were modifiable by adjusting the stimulation settings and those that were non-modifiable. Of the 59 patients in the study, 31 (52.5%) did not experience any postoperative or persistent adverse effects.46
Ten participants in the radiofrequency thalamotomy group (58.5%), one in the deep brain stimulation group (5.3%), and one in the MRgFUS thalamotomy group (4.3%) experienced adverse effects within 1 month following surgery. At 12 months, the numbers of participants experiencing persistent adverse effects were as follows: two (11.7%) who had undergone radiofrequency thalamotomy, four (21.1%) who had undergone deep brain stimulation (including both modifiable and non-modifiable complications), and one (4.3%) who had undergone MRgFUS thalamotomy.46
The authors compared occurrence rates of adverse effects between the three groups at 1 and 12 months post-procedure. A generalized estimating equation model found a statistically significant difference in complication rates between the groups at each time point (P = .01).46 The authors counted more than two adverse effects in a given patient as one. Table 19 lists the frequency of adverse effects associated with each surgery.
Table 19:
Adverse Effects Reported in a Study Comparing MRgFUS Thalamotomy, Deep Brain Stimulation, and Radiofrequency Thalamotomy
| Follow-Up | MRgFUS (N = 23), na (%) | Deep Brain Stimulation (N = 19), na (%) | Radiofrequency Thalamotomy (N = 17), na (%) |
|---|---|---|---|
| 1 month | Mild facial paresis, 1 (4.3) Balance problems, 1 (4.3) Loss of taste, 1 (4.3) |
Mild facial paresis, 1 (5.3) | Mild facial paresis, 3 (17.6) Intracerebral hemorrhage, 2 (11.8) Loss of taste, 1 (5.9) Cognitive deterioration, 1 (5.9) Mild dysarthria, 5 (29.4) Hypesthesia, 1 (5.9) |
| 12 months | Very mild facial paresis, 1 (4.3) | Balance problems,b 3 (15.8) Forearm muscle twitch, 1 (5.3) |
Mild facial paresis, 1 (5.9) Mild dysarthria, 1 (5.9) |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; N, number of patients in the group; n, number of events reported.
Represents the number of events experienced; more than one adverse effect may have been experienced by an individual patient. More than two complications in a single patient were counted as one.
Considered to be a modifiable side effect, which was resolved by adjusting the stimulation settings.
Source: Adapted from Kim et al 2017.46
At the 1 month follow-up, the complication rates were 13% (n = 3) in the MRgFUS thalamotomy group, 5.3% (n = 1) in the deep brain stimulation group, and 58.8% (n = 10) in the radiofrequency thalamotomy group. At 12 months, the complication rates were 4.4% (n = 1) for MRgFUS thalamotomy, 21.1% (n = 4) for deep brain stimulation, and 11.8% (n = 2) for radiofrequency thalamotomy.46 Post-hoc analyses determined that the complication rate was significantly lower in the deep brain stimulation group than in the radiofrequency thalamotomy group (P < .001). At 12 months, the lowest complication rate of all three surgeries occurred in the MRgFUS thalamotomy group; this rate was statistically significantly lower than for both deep brain stimulation (P < .01) and radiofrequency thalamotomy (P < .01).46 When only the non-modifiable complications associated with deep brain stimulation were included in the analysis, there was no longer a statistically significant difference between the complication rates for MRgFUS thalamotomy and deep brain stimulation.46
Table 20 provides the GRADE assessment of the complication rate analysis from the study by Kim et al.46
Table 20:
GRADE Evidence Profile for the Comparison of MRgFUS Thalamotomy, Deep Brain Stimulation, and Radiofrequency Thalamotomy
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Treatment-Related Complication Rate | |||||||
| 1 (Observational)46 | Serious limitations (−1)a | No serious limitationsb | No serious limitations | No serious limitations | Undetectedb | NA | ⊕ Very Low |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; NA, not applicable.
Risk of bias was highest owing to retrospective data collection methods. The full risk-of-bias assessment is presented in Appendix 2, Table A2.
Cannot definitively assess presence or absence because the evidence is derived from a single study.
Serious Adverse Events
A small number of serious adverse events were reported in the included studies. In the 2013 pilot study by Elias et al,37 one participant experienced dysesthesia of the index finger (abnormal sensation that can manifest as pain or discomfort), which began within 3 months of undergoing MRgFUS ventral intermediate nucleus thalamotomy and persisted to 12 months. In the study by Lipsman et al,41 one participant developed lower-leg deep vein thrombosis 1 week after undergoing MRgFUS ventral intermediate nucleus thalamotomy, which may have been related to the long duration of the procedure (i.e., 5–6 hours). In response, this person was treated with anticoagulant therapy for 3 months. In the study of MRgFUS cerebellothalamic tractotomy by Schreglmann et al,42 one participant experienced a fall at home 4 weeks after the procedure that required hospitalization; they then made a full recovery. However, the authors later discovered that this participant had also experienced an unexplained fall 6 months prior to surgery. The article reporting on the 2016 randomized controlled trial by Elias et al38 provides a full list of adverse events determined to be unrelated to the study interventions.
Discussion
MRgFUS neurosurgery significantly reduces tremor and functional disability, improves quality of life, and has a favorable safety profile for people with medication-refractory, disabling essential tremor. We identified some evidence that people with predominant unilateral upper extremity tremor may experience the most benefit from MRgFUS neurosurgery. However, the evidence showed that those with some bilateral or axial tremor also experienced clinically meaningful improvements in tremor severity, quality of life, and functional independence following MRgFUS neurosurgery. The included studies were conducted in many international settings, including Ontario, so the findings can be generalized to local practice.
In Ontario, people with moderate to severe, medication-refractory essential tremor may pursue one of three neurosurgical options as the next line treatment following medication: radiofrequency thalamotomy, deep brain stimulation, or Gamma Knife thalamotomy. Although Gamma Knife thalamotomy is a noninvasive procedure, it is recommended only for very select patients, owing to its considerable unpredictability, risk of complications, and modest therapeutic effect. Therefore, people with medication-refractory, moderate to severe essential tremor who are unable to undergo an invasive surgery owing to contraindications or who find the risks involved unacceptable currently have no effective treatment options. MRgFUS neurosurgery, a noninvasive procedure, would provide an option for these people. In particular, the elderly, who are disproportionately affected by moderate to severe essential tremor, as well as people with multiple comorbidities, currently experience inequitable access to effective treatment that could be mitigated with access to MRgFUS neurosurgery.
Further, people with essential tremor who have no contraindications to invasive neurosurgery may choose not to undergo such a procedure for many reasons, including finding craniotomy, general anaesthetic, or implanted hardware unacceptable. With radiofrequency thalamotomy infrequently being performed anymore, deep brain stimulation has become the favoured option and the standard of care in many jurisdictions, as it is an effective and titratable treatment for essential tremor. However, people may be unable to comply with the intensive follow-up regime of adjustments to the stimulation settings, which can render this option unfeasible for some, particularly in terms of geography, mobility, and social support. We did not assess each surgical option separately because the question of which is the optimal neurosurgery for essential tremor was beyond the scope of this review.
Our findings are consistent with previous research findings that MRgFUS neurosurgery improves tremor, function, and quality of life and has a generally satisfactory safety profile.10,48,49 While the ideal outcome of MRgFUS neurosurgery would be to achieve total remission in tremor, there is no precise magnitude of improvement that is broadly considered a success. However, a tremor reduction of around 50% often enables people to perform key tasks that they were unable to perform before, such as drinking or writing, even if with some difficulty. With this degree of improvement, people can typically return to living more independently, engaging in social activities, and possibly working. The most common persistent adverse effect of MRgFUS neurosurgery, paresthesia, tends to resolve sufficiently that it does not interfere with people's daily lives. The loss of some treatment effect over time in some people can be expected because essential tremor is a progressive condition, and tremor is likely to recur with any therapy.12
There are some limitations to our review. First, although each study we reviewed found a clinically meaningful magnitude of effect in patient-important outcomes following MRgFUS neurosurgery, the sample sizes of these studies were small. Unfortunately, owing to methodological heterogeneity between studies, we could not meta-analyze these data. A challenge in assessing this body of literature is the variability in the reporting of outcome data and analyses. In the studies we reviewed, the measures of variance were inconsistently reported, the use of statistical comparisons varied, and it was unclear in many instances whether the study design fell under a cohort study or case series. We therefore took an inclusive approach to be comprehensive in our review.
Second, a pervasive issue in this field of research is the issue of unblinded outcome assessment. This potential methodological problem has particularly been quantified in studies of Gamma Knife thalamotomy whereby open-label trials have tended to find marked improvement, whereas blinded trials have found little or modest effect.50 However, estimates tend to be more consistent between blinded and unblinded evaluations of outcomes of radiofrequency thalamotomy.51 While it is unclear to what extent this issue affected our review, three studies explicitly blinded outcome assessors, and results were consistent irrespective of blinding across the studies.
Third, comparative data against other surgeries were scant, and we identified only one randomized controlled trial demonstrating efficacy compared with sham. This is a widespread limitation in this field; no studies have been published comparing deep brain stimulation with sham procedures since its introduction decades ago.12
Fourth, the duration of follow-up among the studies was relatively shorty, varying from 1 to 12 months. Such limited follow-up precludes characterization of the long-term durability or adverse effects of the procedure. Future studies with longer follow-up are needed to provide insight into the longevity of therapeutic and adverse effects. This is an active area of research, and MRgFUS neurosurgery continues to be studied in essential tremor and other tremor conditions,52 as well as in other patient populations, such as people with chronic neuropathic pain.53
Ongoing Studies
Two forthcoming studies registered on ClinicalTrials.gov may be relevant to this topic. One is an open-label, continued-access protocol to assess primarily intraoperative adverse events and also effectiveness via the CRST up to 12 months after MRgFUS thalamotomy in people with essential tremor (NCT02289560; last updated March 28, 2017; study completion December 2017). The second is an international registry to assess the effectiveness of MRgFUS neurosurgery in people with a movement disorder or neuropathic pain at 5 years following treatment (NCT03100474; last updated April 3, 2017; study completion 2024).
Conclusions
MRgFUS neurosurgery is an effective treatment for moderate to severe, medication-refractory essential tremor and has an acceptable safety profile. Specifically:
In noncomparative studies, MRgFUS neurosurgery has been found to significantly improve tremor severity and quality of life and to significantly reduce functional disability in daily activities (low certainty in the evidence); MRgFUS neurosurgery has been found to be significantly more effective than a sham procedure (high certainty in the evidence)
There are no significant differences in tremor severity or quality-of-life improvement or in functional disability reduction conferred by MRgFUS neurosurgery compared with deep brain stimulation (very low certainty in the evidence)
There is no significant difference in tremor severity improvement conferred by MRgFUS neurosurgery compared with radiofrequency thalamotomy (very low certainty in the evidence)
MRgFUS neurosurgery is associated with few complications and adverse effects according to noncomparative studies and when compared with radiofrequency thalamotomy and deep brain stimulation (very low certainty in this evidence); the vast majority of adverse effects are transient and resolve either entirely or to a point at which there is minimal interference with people's lives
ECONOMIC EVIDENCE
Research Question
What is the cost-effectiveness of magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery compared with standard care for the treatment of moderate to severe, medication-refractory essential tremor?
Methods
Economic Literature Search
We performed an economic literature search on April 11, 2017, for studies published from inception to the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the health technology assessment.
We performed a targeted grey literature search of health technology assessment agency websites, clinical trial registries, and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Evidence, Literature Search, above, for further details on the methods used. See Appendix 1 for the literature search strategies, including all search terms.
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using the DistillerSR management software and then obtained the full text of studies that appeared eligible for the review, according to the inclusion criteria. The author then examined the full-text articles and selected studies eligible for inclusion. We also examined reference lists for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
Studies in people with moderate to severe, medication-refractory essential tremor
Studies reporting on MRgFUS neurosurgery compared with no surgery or with other surgical procedures for essential tremor
Cost–utility, cost-effectiveness, cost–benefit, cost–consequence, or cost analyses
Exclusion Criteria
Narrative reviews, letters/editorials, case reports, commentaries, abstracts, posters, or unpublished studies
Outcomes of Interest
Incremental cost-effectiveness ratio
Incremental costs
Incremental effectiveness
Data Extraction
We extracted relevant data on the following:
Source (i.e., name, location, year)
Population and comparator
Interventions
Outcomes (i.e., health outcomes, costs, incremental cost-effectiveness ratio)
We contacted study authors to provide clarification as needed (Ravikumar et al,54 Martinez-Martin et al55).
Study Applicability and Methodological Quality
We identified one study54 and determined its usefulness for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom. The original checklist is used to inform the development of clinical guidelines by NICE.56 We modified the wording of the questions to remove references to guidelines and to make it Ontario specific. We also separated the checklist into two sections. The first section assesses the applicability of studies to the research question as directly applicable, partially applicable, or not applicable. For studies deemed directly applicable or partially applicable to the research question, we assess their methodological quality using the second section of the checklist. Based on this assessment, we consider each study to have minor limitations, potentially serious limitations, or very serious limitations (Appendix 4, Tables A4 and A5).
Results
Literature Search
The literature search yielded 31 citations published from inception to April 11, 2017, after removing duplicates. We excluded 30 articles based on information in the title and abstract. We then obtained the full text of the one potentially relevant article for further assessment, and this study met the inclusion criteria.54 We also hand-searched the reference list of the included study but did not find any additional eligible studies. Figure 4 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 4: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al.31
Review of the Included Economic Study
Table 21 provides a summary of the included study. In this study, Ravikumar et al54 conducted a cost-effectiveness analysis and systematic review of 32 studies that examined treatment effectiveness and treatment-related complications. The outcomes of interest were total costs, total quality-adjusted life years (QALYs), and incremental cost per QALY. The authors used a decision tree model to perform a cost–utility analysis of MRgFUS thalamotomy compared with deep brain stimulation and stereotactic radiosurgery (e.g., Gamma Knife) in the United States. Deep brain stimulation was modeled as unstaged (consisting of one operation) or staged (consisting of two operations). The model population was people with medication-refractory essential tremor. The authors defined treatment effectiveness as percent improvement in functional disability, assessed via part C of the Fahn–Tolosa–Marín (FTM) Clinical Rating Scale for Tremor or the Clinical Rating Scale for Tremors (CRST). The model used a U.S. societal perspective. Time horizon was not reported but appears to be less than 1 year based on the results reported.
Table 21:
Results of Economic Literature Review—Summary
| Author, Year, Location | Study Design and Perspective | Population | Interventions | Results | ||
|---|---|---|---|---|---|---|
| Health Outcome, QALYs (SD) | Costs, 2017 USD (SD) | Cost-Effectiveness | ||||
| Ravikumar et al, 2017, United States54 |
|
|
|
|
|
|
Abbreviations: DBS, deep brain stimulation; ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; QALY, quality-adjusted life-year; SD, standard deviation (from probabilistic analysis); SRS, stereotactic radiosurgery.
The results showed that MRgFUS thalamotomy was more effective and less expensive than both unstaged and staged deep brain stimulation.54 Compared with stereotactic radiosurgery, the least costly procedure, MRgFUS thalamotomy was associated with an incremental cost of $580 and an incremental QALY of 0.078, resulting in an incremental cost-effectiveness ratio (ICER) of $7,436 per QALY gained.54 The ICER of unstaged deep brain stimulation versus stereotactic radiosurgery was estimated to be $483,500 per QALY gained, because deep brain stimulation is more expensive (by $7,893) and only slightly more effective (an additional 0.018 QALYs gained).54 Two-way sensitivity analyses showed that the ICER of MRgFUS thalamotomy versus stereotactic radiosurgery was sensitive to assumptions regarding the relative cost and effectiveness of MRgFUS thalamotomy compared with stereotactic radiosurgery.54
Applicability and Methodological Quality of the Included Study
Appendix 4 provides the results of the methodology checklist for economic evaluations applied to the included study.54 We considered this study only partially applicable to our research question because it was conducted from a U.S. perspective and did not include all treatments available in Ontario (i.e., it did not assess radiofrequency thalamotomy). Although the authors used valid methods to conduct their cost–utility analysis, we considered the study to have minor limitations. In particular, it did not include long-term clinical outcomes such as tremor recurrence. Further, the authors did not specify the time horizon of the model, and they did not clearly report the methods used to derive comparative treatment effectiveness and utility values.
Discussion
Ravikumar et al54 estimated the effectiveness of MRgFUS thalamotomy, deep brain stimulation (unstaged and staged), and stereotactic radiosurgery for the treatment of medication-refractory essential tremor based on a systematic review and meta-analysis of relevant clinical studies. However, the authors did not explicitly report the results of their meta-analysis. Aside from one phase III randomized controlled trial of MRgFUS thalamotomy,38 all studies reviewed by the authors included evidence based on uncontrolled observational studies, which may have introduced biases into the cost-effectiveness results.
Another limitation of this study is that the authors did not clearly state the method used to derive utilities. The authors suggested that utilities of essential tremor could be derived from percent change in functional disability using a mapping algorithm. However, they did not report or reference data supporting the mapping algorithm, nor did they report the baseline (pre-surgery) utility value.
In addition, since the study was conducted from a U.S. perspective and costs were based on U.S. Medicare reimbursement rates, we deemed its findings not generalizable to the Ontario setting.
Conclusions
We identified one economic study suggesting that MRgFUS thalamotomy may be cost-effective compared with deep brain stimulation and stereotactic radiosurgery for the treatment of medication-refractory essential tremor. However, this study has some methodological limitations and is not applicable to the Ontario setting.
PRIMARY ECONOMIC EVALUATION
Although the published economic evaluation that we identified in the literature review addressed the interventions of interest, it did not take a Canadian or Ontario-specific perspective. Owing to this and the methodological limitations described, we conducted a primary economic evaluation using Ontario-specific costs and clinical care pathways.
Through consultation with clinical experts, we determined a priori that there are two subgroups within our target population of people with moderate to severe, medication-refractory essential tremor: (1) those who are ineligible for invasive neurosurgeries owing to contraindications or preference; and (2) those who are eligible for invasive neurosurgeries (see the Clinical Evidence section for more details on this subgroup). Therefore, we formulated the following two research questions.
Research Questions
-
1.
What is the cost-effectiveness of MRgFUS neurosurgery compared with standard treatment (i.e., no surgery) for people with moderate to severe, medication-refractory essential tremor, who are ineligible for invasive neurosurgery, within the context of the Ontario Ministry of Health and Long-Term Care?
-
2.
What is the cost-effectiveness of MRgFUS neurosurgery compared with standard treatment (i.e., radiofrequency thalamotomy or deep brain stimulation) for people with moderate to severe, medication-refractory essential tremor, who are eligible for invasive neurosurgery, within the context of the Ontario Ministry of Health and Long-Term Care?
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards Statement.57
Type of Analysis
We conducted a cost–utility analysis to determine the costs and health outcomes (i.e., QALYs) associated with each treatment strategy. We chose this type of analysis because utility inputs are available and a generic outcome measure such as QALYs allows decision-makers to make comparisons across different conditions and interventions. The outcomes reported are total costs and total QALYs for each treatment and incremental cost per QALY gained compared with the next most effective strategy.
Target Population
The population evaluated in the model was people with moderate to severe, medication-refractory, disabling essential tremor; we then divided this population into two subgroups: those ineligible for invasive neurosurgery and those eligible for invasive neurosurgery. We based patient characteristics on the 2016 phase III randomized controlled trial of MRgFUS thalamotomy by Elias et al.38 We validated these characteristics via clinical experts (in-person communication, May 25, 2017; telephone communication, May 30 and 31, 2017) as representative of the Canadian population. At the start of the model, the average age of the population was 71 years, and the majority were male (68%).
Perspective
For the reference case, we conducted the analyses from the perspective of the Ontario Ministry of Health and Long-Term Care.
Since treatment for essential tremor may improve people's motor control, possibly allowing them to return to work, we also conducted scenario analyses incorporating people's out-of-pocket and lost productivity costs from a societal perspective.
Intervention and Comparators
Thalamotomy and deep brain stimulation are both effective treatments for moderate to severe, medication-refractory essential tremor.10,58 Thalamotomy works by creating a permanent injury to the part of the brain causing the tremor, whereas deep brain stimulation involves using an implanted electrode or electrodes to block the brain activity causing the tremor. Thalamotomy can be performed invasively (e.g., via radiofrequency) or noninvasively (e.g., via MRgFUS or Gamma Knife). Thalamotomy is typically performed unilaterally, whereas deep brain stimulation can be performed unilaterally or bilaterally. The treatments have different adverse effect profiles.17 Radiofrequency thalamotomy and deep brain stimulation involve craniotomy and therefore involve the risks of open surgical procedures, such as infection and intracranial hemorrhage. MRgFUS and Gamma Knife thalamotomy are noninvasive but involve making a permanent lesion in the brain.59 Deep brain stimulation is the current surgical standard of care because it is considered reversible and adjustable. However, the implanted device requires long-term management, including repeat programming and periodic battery replacement. Deep brain stimulation is also associated with hardware-related complications such as lead fracture and migration and device malfunction.17,60 Owing to these limitations, there is an unmet need for less invasive surgical options for the treatment of essential tremor.61
For research question 1, we compared MRgFUS thalamotomy with no surgery based on the 2016 phase III randomized controlled trial comparing MRgFUS thalamotomy with a sham procedure by Elias et al.38 For people ineligible for invasive neurosurgery, Gamma Knife thalamotomy is a surgical option. However, there is insufficient evidence regarding the effectiveness and safety of this procedure.16 Further, the clinical experts we consulted do not view Gamma Knife as an effective treatment option owing to its uncertain effectiveness and high risk of adverse effects. Therefore, we excluded Gamma Knife as a comparator.
For research question 2, we compared MRgFUS neurosurgery with radiofrequency thalamotomy and deep brain stimulation in people eligible for invasive neurosurgery.
On July 11, 2017, we performed a targeted literature search in MEDLINE and the Cochrane Database of Systematic Reviews for systematic reviews on the effectiveness and safety of radiofrequency thalamotomy and deep brain stimulation published from inception to the search date. See Appendix 1 for the literature search strategies, including all search terms. Based on the clinical literature16,20,40 and expert judgment (in-person communication, May 25 and June 19, 2017; telephone communication, May 30 and 31, 2017), we assumed that MRgFUS neurosurgery, radiofrequency thalamotomy, and deep brain stimulation are equally effective for tremor relief but have different adverse event profiles.
Table 22 summarizes the two reference case analyses.
Table 22:
Reference Case Analysis Summary
| Analysis | Patient Population | Intervention | Comparator(s) | Clinical Effectiveness Determination |
|---|---|---|---|---|
| Research question 1 | People ineligible for invasive neurosurgery | MRgFUS neurosurgery | No surgery | Based on direct comparison only (1 RCT)38 |
| Research question 2 | People eligible for invasive neurosurgery | MRgFUS neurosurgery | Radiofrequency thalamotomy Deep brain stimulation | Based on indirect comparison20,40 and clinical expert opinion: assumes all surgeries are equally effective for tremor improvement but have different adverse event profiles |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; RCT, randomized controlled trial.
Time Horizon and Discounting
In the reference case analyses, we used a 5-year time horizon to capture the long-term outcomes of the disease (e.g., tremor recurrence, reoperation, long-term complications). Although essential tremor is a chronic illness, we did not use a lifetime horizon because MRgFUS is a new technology and long-term clinical data are not yet available. We also explored different time horizons in scenario analyses (e.g., 1 year, 10 years).
We discounted both costs and outcomes at a rate of 1.5% per year, as per the Canadian Agency for Drugs and Technologies in Health guidelines for economic evaluations.62 Rates of 0% and 3% were considered in sensitivity analyses.
Model Structure
We developed a Markov model to estimate the long-term clinical and economic outcomes of MRgFUS neurosurgery versus standard treatment for people with moderate to severe, medication-refractory essential tremor. We used a cycle length of 1 year, as patients are usually monitored annually for disease progression. We applied a half-cycle correction on all health state transitions. Figure 5 illustrates the model structure. For research question 1, we compared MRgFUS neurosurgery with no surgery. For research question 2, we compared MRgFUS neurosurgery with radiofrequency thalamotomy and with deep brain stimulation; patients in the comparator arms followed the same clinical pathway and model structure as those in the MRgFUS arm.
Figure 5: Cost–Utility Model.
The clinical pathway and Markov health states are as follows:
-
All patients enter the model with disabling tremor
-
∘
Patients in the “no-surgery” arm continue to live with disabling tremor and may die from natural causes in each model cycle
-
∘
Patients in the “surgery” arm (i.e., those undergoing MRgFUS neurosurgery, radiofrequency thalamotomy, or deep brain stimulation) undergo surgery right away and enter the “improved tremor, post–primary surgery” health state
-
∘
Patients in the “improved tremor, post–primary surgery” health state may have one of two surgical outcomes: Some may experience marked tremor improvement (from 50% to 100% improvement compared with baseline), whereas others may experience mild to moderate improvement (from 10% up to 50% improvement). This categorization is based on the work of Fahn et al6
In the first year post-surgery, some patients in the “improved tremor, post–primary surgery” health state may experience short-term adverse effects (i.e., side effects) or complications (e.g., temporary paresthesia that resolves within a few months). In addition, a certain proportion of patients in the “improved tremor, post–primary surgery” health state may experience long-term, permanent adverse effects (persisting for more than 1 year after surgery)
-
Patients in the “improved tremor, post–primary surgery” health state may progress and enter one of two tremor recurrence states:
-
∘
“Tremor recurrence, no reoperation”: Patients in this health state again experience disabling tremor but do not undergo a further surgery. According to clinical experts, the majority of patients do not undergo reoperation when tremor recurs (in-person communication, May 25, 2017; telephone communication, May 30 and 31, 2017)
-
∘
“Tremor recurrence, reoperation”: Patients in this health state experience tremor recurrence and undergo a second surgery (the same type as the previous one) right away. Based on clinical expert opinion, patients who undergo a second surgery are usually treated with the procedure they received the first time. Patient quality of life differs based on the surgical outcome: marked improvement or mild to moderate improvement
-
∘
All patients in the “tremor recurrence, reoperation” health state enter the “improved tremor, post-reoperation” health state after 1 year if they do not die
At any point during the model time frame, a patient may die from natural causes. Since essential tremor does not affect life expectancy,38,63 we used age-specific natural mortality We developed the model using Microsoft Excel 2013 (Microsoft Corporation, Redmond, MA) and then replicated the analysis using TreeAge Pro 2017 (TreeAge Software, Williamstown, MA) for validation.
Main Assumptions
To simplify our analysis, we made the following assumptions:
Since essential tremor is a slowly progressive disease, patients in the “no surgery” arm have stable disease and do not receive any surgery throughout the model time frame
The onset of treatment benefit (i.e., tremor relief) differs based on surgery type.49 The benefit of MRgFUS neurosurgery and radiofrequency thalamotomy appears right away. The benefit of deep brain stimulation appears about 1 month following the procedure, as it takes time to program and optimize the device settings (in-person communication, May 25, 2017; telephone communication, May 30 and 31, 2017). Patients continue to experience treatment benefit until tremor recurrence
Owing to the limited data available on tremor recurrence after surgery, we assume that each patient can have only one recurrence during the model time horizon
Owing to the lack of clinical evidence on the effectiveness of reoperation, we assume that reoperation will result in the same outcomes as the primary surgery (some with marked improvement, some with mild to moderate improvement)
There is no disutility associated with reoperation
Patients who undergo reoperation receive the same surgical procedure as their first procedure
Patient quality of life returns to baseline (i.e., that associated with disabling tremor) when tremor recurs
Patients have the same mortality rate as that of the general population38,63
Clinical Parameters
We populated the model with a number of clinical parameters:
Proportion of patients with marked or mild to moderate improvement following each surgery
Probability of recurrence
Probability of reoperation
Probability of death
Probability of experiencing adverse effects or complications
Natural History and Treatment Effect
Improvement Post-surgery
In the 2016 randomized controlled trial by Elias et al,38 3 months after MRgFUS thalamotomy, 27 patients (53%) experienced marked improvement (50–100% improvement), 24 (47%) experienced mild to moderate improvement (10–50% improvement), and 5 (8.9%) experienced no improvement or a worsening of their tremor (less than 10% improvement). These 5 patients were considered to have experienced tremor recurrence (initial improvement but loss of treatment effect by 3 months post-surgery). Based on the literature64 and clinical expert opinion (in-person communication, May 23, 2017), tremor relief after thalamotomy that persists for 3 months is nearly always permanent. Therefore, we determined the probability of recurrence to be 8.9% in year 1. Among patients who did not experience tremor recurrence, we considered the proportion of those experiencing marked improvement to be 53%, and the proportion experiencing mild to moderate improvement to be 47%.
For research question 2, we assumed that MRgFUS neurosurgery, radiofrequency thalamotomy, and deep brain stimulation are equally effective, based on the results of a targeted literature review as well as clinical expert opinion. A case–control study of people with essential tremor by Pahwa et al20 found that radiofrequency thalamotomy and deep brain stimulation are equally effective for tremor relief but that radiofrequency thalamotomy is associated with a higher rate of neurological adverse effects. Another retrospective study, by Huss et al,40 also showed that unilateral MRgFUS thalamotomy and deep brain stimulation are equally effective. The 2011 American Academy of Neurology guidelines16 state that there is insufficient evidence to support or refute the superiority of deep brain stimulation or thalamotomy for the treatment of essential tremor. In addition, a randomized controlled trial by Schuurman et al21,22 showed that deep brain stimulation and radiofrequency thalamotomy are equally effective in people with medication-refractory, severe tremor owing to Parkinson's disease, multiple sclerosis, or essential tremor.
Very few studies of deep brain stimulation and radiofrequency thalamotomy report the proportion of patients with marked versus mild to moderate improvement. The most commonly reported outcome is percent change in tremor score from baseline. In the 2016 study by Elias et al38 of MRgFUS thalamotomy, the mean score for hand tremor improved by 47% at 3 months. One systematic review identified three studies that reported percent improvement from baseline following deep brain stimulation.17 In studies in which outcome assessors were not blinded or not reported to be blinded, percent improvement ranged from 65% to 76%.65,66 However, a study by Hariz et al,67 in which outcome assessors were blinded, reported a percent improvement of 47%, which is similar to that seen with MRgFUS thalamotomy.17 For radiofrequency thalamotomy, a systematic review by the American Academy of Neurology59 found that open-label trials (n = 181) have reported an 80% to 90% tremor reduction following thalamotomy in people with essential tremor. This is also consistent with the findings of the 2016 study of MRgFUS thalamotomy by Elias et al.38
Since there are have been no head-to-head clinical trials comparing MRgFUS neurosurgery with deep brain stimulation or radiofrequency thalamotomy, we assumed that each type of surgery results in the same proportions of patients with marked improvement (53%) and mild to moderate improvement (47%). Clinical experts validated this assumption (email communication, July 13, 2017).
Tremor Recurrence
After surgery, some people may initially experience satisfactory tremor relief but experience tremor recurrence within a few months. This could be the result of suboptimal lesion location (for thalamotomy) or lead placement (for deep brain stimulation) owing either to inaccuracy in the stereotactic procedure or to inter-individual anatomic variability in the target.68–71 Some people may experience tremor recurrence after several years, potentially as a result of disease progression.68,69,72 Recurrence is not well reported in the literature, and its definition varies from study to study, especially with regard to deep brain stimulation. Based on crude estimates in the literature73 and clinical expert option (in-person communication, May 23, May 25, and June 19, 2017; telephone communication, May 30 and 31, 2017), the rate of recurrence 5 years following radiofrequency thalamotomy is about 20% to 30%. Since MRgFUS neurosurgery and radiofrequency thalamotomy are both lesional surgeries, we assumed that the recurrence rate would be the same for both procedures in the reference case (confirmed by clinical experts). Therefore, we calculated the annual probability of recurrence in years 2 to 5 to be about 4.7%, assuming the 5-year recurrence rate to be 25% and the year 1 recurrence rate to be 8.9%.
However, since MRgFUS neurosurgery is a new technology and little is known about its long-term efficacy, clinical experts suggested that a larger number of people may experience tremor recurrence over a longer follow-up time, possibly 50% over 5 years (email communication, July 13, 2017). We considered this possibility in our sensitivity analysis.
According to the literature64 and clinical expert opinion (email communication, July 13, 2017), tremor recurrence following deep brain stimulation can nearly always be controlled by adjusting the stimulation level of the device (reprogramming) and therefore does not require reoperation. As a result, we assumed the probability of tremor recurrence following deep brain stimulation to be 0%.
Reoperation After Tremor Recurrence
Owing to resource limitations (e.g., wait list) and/or patient preferences, not all people whose tremor recurs following surgery will undergo reoperation. Following Elias et al (2016),38 we estimated that 40% of people who undergo either MRgFUS neurosurgery or radiofrequency thalamotomy may undergo a second surgery once tremor recurs.
As a result of a lack of clinical evidence on the effectiveness of reoperation, we assumed the second operation would result in the same possibility of outcomes as the primary surgery (53% with marked improvement and 47% with mild to moderate improvement).
Table 23 presents the clinical effectiveness parameters used in the economic model.
Table 23:
Clinical Effectiveness Parameters Used in the Economic Model
| Parameter | Value | Assumption | Source |
|---|---|---|---|
| Improvement Post-surgery (DBS, MRgFUS, RF) | |||
| Percentage of patients with marked improvement | 53% | DBS, MRgFUS, and RF are equally effective | Elias et al, 201638; expert opinion |
| Percentage of patients with mild to moderate improvement | 47% | ||
| Probability of Recurrencea (MRgFUS, RF) | |||
| Year 1 | 8.9% | MRgFUS and RF have the same recurrence rate; calculation based on a 5-year recurrence rate of 25% | Elias et al, 201638; expert opinion |
| Years 2–5 | 4.7% | Hirai et al, 198373; expert opinion | |
| Probability of Recurrencea (DBS) | |||
| Years 1–5 | 0% | Tremor recurrence following DBS can be controlled by adjusting the stimulation level of the device (reprogramming) and does not require reoperation | Tasker et al, 199864; expert opinion |
| Probability of Reoperation After Recurrence (MRgFUS or RF) | |||
| Years 1–5 | 40% | The probability of reoperation is the same for MRgFUS and RF | Elias et al, 201638 |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; RF, radiofrequency thalamotomy.
Recurrence refers to diminished tremor control to the point of requiring reoperation.
Mortality
The literature38,63 and clinical experts generally suggest that essential tremor does not affect life expectancy (in-person communication, May 25 and June 19, 2017; telephone communication, May 30 and 31, 2017). Therefore, we assumed people with essential tremor have the same mortality rate as the general population.38,63 We obtained the annual probability of all-cause natural mortality from Statistics Canada life tables for 2009 to 2011.74,75
Based on the literature38,63 and clinical expert opinion (in-person communication, May 25 and June 19, 2017; telephone communication, May 30 and 31, 2017), the overall mortality resulting from any surgical complication is very small. A small proportion of people may experience intracranial hemorrhage following open surgery (e.g., radiofrequency thalamotomy or deep brain stimulation). However, according to the literature,20 most intracranial hemorrhage events are asymptomatic, and those that are symptomatic usually resolve over time and do not lead to death. Therefore, we did not consider any surgery-related mortality risk.
Treatment-Related Adverse Effects and Complications
In our analysis, we considered the cost and quality-of-life impact of key adverse effects and complications associated with treatment (Table 24). We included those that are frequent (occurring in ≥ 5% of people), severe, expensive to treat, or have a large impact on a person's health-related quality of life. We excluded those with a negligible impact on health effects or resources from the analysis. We considered adverse effects persisting for more than 1 year after surgery to be long term.
Table 24:
Treatment-Related Adverse Effects and Complications Included in the Economic Model
| Adverse Effect or Complication | Proportion of Patients | Source | Average Duration | Source |
|---|---|---|---|---|
| MRgFUS Neurosurgery | ||||
| 1 year post-surgery | ||||
| Gait disturbance | 27% | Elias et al, 201638 | 2.8 months | Elias et al, 201638 |
| Paresthesia or numbness | 24% | Elias et al, 201638 | 4.3 months | Elias et al, 201638 |
| Headache | 14% | Elias et al, 201638 | 1.8 months | Elias et al, 201638 |
| Speech problema | 4% | Elias et al, 201638 | 6 months | Elias et al, 201638 |
| Permanent | ||||
| Paresthesia or numbness | 14% | Elias et al, 201638 | Permanent | Elias et al, 201638 |
| Gait disturbance | 9% | Elias et al, 201638 | Permanent | Elias et al, 201638 |
| Radiofrequency Thalamotomy | ||||
| 1 year post-surgery | ||||
| Gait disturbance | 27% | Assume same as MRgFUS | 2.8 months | Assume same as MRgFUS |
| Paresthesia or numbness | 24% | Assume same as MRgFUS | 4.3 months | Assume same as MRgFUS |
| Infection | 2% | Expert opinion | 1 month | Bjerknes et al, 201480; expert opinion |
| Intracranial hemorrhage | 1% | Expert opinion | 1 month | Pahwa et al, 200120 |
| Permanent | ||||
| Paresthesia or numbness | 14% | Assume same as MRgFUS | Permanent | Assume same as MRgFUS |
| Gait disturbance | 9% | Assume same as MRgFUS | Permanent | Assume same as MRgFUS |
| Speech problema | 4.5% | Alomar et al, 201727 | Permanent | Zesiewicz et al, 201376 |
| Deep Brain Stimulation | ||||
| 1 year post-surgery, procedure related | ||||
| Infection | 2% | Fishman et al, 201761 | 1 month | Bjerknes et al, 201480; expert opinion |
| Intracranial hemorrhage | 1% | Fishman et al, 201761 | 1 month | Pahwa et al, 200120 |
| Hardware related | ||||
| Device malfunction | 2% | Fishman et al, 201761 | 1 month | Assume same as infection |
| Lead fracture | 2% | Fishman et al, 201761 | 1 month | Assume same as infection |
| Stimulation relatedb | ||||
| Paresthesia, tingling | 19.5% | Flora et al, 201017 | Temporary; resolvable by reprogramming | Flora et al, 201017; Pahwa et al, 200120 |
| Speech problema | 15.2%c | Alomar et al, 201727 | Temporary; resolvable by reprogramming | Flora et al, 201017; Pahwa et al, 200120 |
| Disequilibrium, gait, incoordination, motor disturbance | 8.1% | Flora et al, 201017 | Temporary; resolvable by reprogramming | Flora et al, 201017; Pahwa et al, 200120 |
| Headache | 7.2% | Flora et al, 201017 | Temporary; resolvable by reprogramming | Flora et al, 201017; Pahwa et al, 200120 |
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
The category of “speech problem” includes adverse effects such as hypophonia, dysarthria, aphasia/dysphasia, and dysphagia.
For deep brain stimulation–related adverse effects, we considered only the cost implications and not the quality-of-life impact because these events are short-lived and resolve after reprogramming.
Weighted average: 15.2% = 12.3% × 90% (unilateral deep brain stimulation) + 41.4% × 10% (bilateral deep brain stimulation).
For MRgFUS neurosurgery, we obtained the proportion of people experiencing adverse effects and complications from the 2016 study of MRgFUS thalamotomy by Elias et al.38 Most adverse effects associated with MRgFUS thalamotomy were categorized as mild or moderate.38 The most common adverse effects were gait disturbance (36%) and paresthesia or numbness (38%), which persisted in 9% and 14% of people at 1 year, respectively.38 Transient speech problems (dysarthria and dysphagia) were reported in 4% of people. Headache was reported in 14% of people.38
For radiofrequency thalamotomy and deep brain stimulation, we obtained rates of adverse effects and complications (i.e., adverse events) from a targeted search of published systematic reviews. There are some limitations with the available evidence. The majority of studies included in these systematic reviews are noncomparative case series and case reports. It is also difficult to summarize adverse event rates from these studies, as there are large variations in study design and reporting (e.g., definition of adverse events, whether an event is temporary or permanent, duration of follow-up, and consequences of the events). We thus obtained adverse event rates from published systematic reviews when possible; when not possible, we obtained this information from selected studies based on the following criteria: larger sample sizes, long-term follow-up, reporting of average follow-up time, and reporting of adverse event consequences. We validated these adverse event rates with clinical experts.
Possible adverse effects following radiofrequency thalamotomy include paresthesia, dysarthria, gait disorder, and ataxia.76 These adverse effects usually resolve over time, although speech difficulties may persist in some people.76 For simplicity, we assumed that radiofrequency thalamotomy and MRgFUS neurosurgery have similar short- and long-term neurological adverse effects (i.e., gait disturbance, paresthesia or numbness), since both are lesional surgeries. Because radiofrequency thalamotomy is an invasive procedure, it is associated with surgical complications such as infection (2%) and intracranial hemorrhage (1%); these rates are based on expert opinion (telephone communication, May 31, 2017). We obtained the incidence of speech disorders (4.5%) in people with essential tremor undergoing thalamotomy from a systematic review and meta-analysis.27
The adverse effects and complications of deep brain stimulation are often classified into three categories: stimulation related, procedure related, and hardware related.60 Common stimulation-related adverse effects include paresthesia, dysarthria, and headache.17 These are usually mild and short-lived and may resolve after reprogramming the device.17,20 Therefore, we did not consider the negative quality-of-life impact caused by stimulation-related adverse effects; we captured only the cost impact as part of the reprogramming visits (see Ongoing Costs section). Similar to radiofrequency thalamotomy, deep brain stimulation is associated with procedure-related complications such as intracranial hemorrhage (0.5%–2.0%) and infection (1–3%).61,77,78 There are also deep brain stimulation–specific hardware-related complications, such as lead migration and fracture (1–3%), and device malfunction requiring surgical correction (1–3%).61,79
The rates of procedure- and hardware-related complications vary widely among studies and centers for several reasons80,81:
Varying definitions of adverse effects and complications
Varying follow-up times
Differences in patient selection or number of patients included
Changes in hardware over time
Variation in local practice (e.g., surgeon experience, surgical technique, use of prophylactic antibiotics)
Because of this variability, we tested a range of procedure- and hardware-related complication rates in sensitivity analyses to assess the impact of these parameters on the cost-effectiveness results.
Health State Utility Parameters
We obtained health state utility values (Table 25) from a targeted literature search of MEDLINE performed on April 11, 2017, of studies published from inception to the search date. We based the search on the clinical search strategy and applied a methodological filter82 to limit retrieval to health state utility values. See Appendix 1 for the literature search strategies, including all search terms.
Table 25:
Health State Utility Parameters Used in the Economic Model
| Mean Utility | Standard Deviation | Sample Size | Source | |
|---|---|---|---|---|
| Health State | ||||
| Alive with disabling tremor (baseline) | 0.69 | 0.30 | 24 | Herceg et al, 201283 |
| Improved tremor, post-surgery (marked improvement) | 0.91 | 0.08 | 24 | Herceg et al, 201283 |
| Improved tremor, post-surgery (mild to moderate improvement) | 0.80 | – | – | Assumption: calculated as the average of baseline and marked improvement post-surgery |
| Tremor recurrence, no reoperation | 0.69 | 0.30 | 24 | Assumption: quality of life returns to baseline |
| Tremor recurrence, reoperation (marked improvement) | 0.91 | – | – | Assumption: same as no recurrence, marked improvement |
| Tremor recurrence, reoperation (mild to moderate improvement) | 0.80 | – | – | Assumption: same as no recurrence, mild to moderate improvement |
| Adverse Effect or Complication | ||||
| Gait disturbance | 0.82 | – | – | Yardley et al, 201284 |
| Paresthesia or numbness | 0.81 | – | – | Thoma et al, 200685 |
| Speech disorder | 0.54 | – | – | Tengs et al, 200086 |
| Headache | 0.77 | – | – | Tengs et al, 200086 |
| Infection | 0.64 | – | – | Lee et al, 201087; Gheorghe et al, 201588 |
| Intracranial hemorrhage | 0.60 | – | – | Lenert et al, 199789 |
| Lead fracture or device malfunction | 0.66 | – | – | Gada et al, 201290 |
We found only one study, Herceg et al,83 that measured utility using the European Quality of Life—5 Dimensions (EQ-5D) instrument in people with disabling essential tremor. Although essential tremor is a common movement disorder, studies that report utility values for this condition are sparse. Most studies we found employed either disease-specific questionnaires (e.g., QUEST or a modified version of the Parkinson's Disease Questionnaire) or non-preference–based instruments (e.g., Nottingham Health Profile, Sickness Impact Profile). For example, the 2016 phase III randomized controlled trial of MRgFUS thalamotomy by Elias et al38 assessed quality of life before and after MRgFUS thalamotomy using the QUEST questionnaire. However, these non-preference–based quality-of-life data do not provide a single summary score for a given health state and thus cannot be used directly for economic modelling. We were also unable to identify any mapping algorithm that could convert these various quality-of-life measures (e.g., QUEST) into utility values. Appendix 5 provides a summary of the utility search results.
We decided to use utility values from Herceg et al83 because the patient population and health states measured are similar to our model population and health states. Based on this study, we assumed that people with disabling essential tremor have a utility (quality of life) of 0.69, and that people with marked improvement after surgery have a utility of 0.91. For those with mild to moderate improvement after surgery, we assumed a utility of 0.80: half-way between 0.69 and 0.91 ([0.69 + 0.91] ÷ 2 = 0.80). Following tremor recurrence, we assumed that a person's utility would return to baseline. The utility following reoperation would be 0.91 for marked improvement and 0.80 for mild to moderate improvement. We also included a decrease in quality of life owing to treatment-related adverse effects or complications. Table 25 summarizes the utility values we extracted from the literature.
Cost Parameters
We included the following costs in our model:
Diagnostic and imaging tests
Professional fees (e.g., neurologist, neurosurgeon, MRI technician, nurse, surgical assistant, anesthesiologist)
Procedure (including deep brain stimulation device)
Long-term monitoring
Medications for essential tremor
Managing adverse effects and complications
We obtained cost inputs from standard Ontario sources and the published literature. The fees for professional visits, procedures, and consultations were obtained from the Ontario Schedule of Benefits for Physician Services.91 Hospitalization costs were obtained from the Ontario Case Costing database of the Ministry of Health and Long-Term Care.92 Diagnostic and laboratory fees were obtained from the Ontario Schedule of Benefits for Laboratory Services.93 Drug costs were obtained from the Ontario Drug Benefit Formulary.94 Costs related to the maintenance and operation of the equipment used in MRgFUS neurosurgery were obtained from clinical experts and the manufacturer (in-person communication, May 25 and June 19, 2017; telephone communication, May 30 and 31, 2017).
We report all costs in 2017 Canadian dollars. Where 2017 costs were unavailable, we used the health care component of the Statistics Canada Consumer Price Index (CPI) to adjust costs to 2017 Canadian dollars (2017 CPI = 125.7; 2015 CPI = 123.1; 2016 CPI = 124.7).30 We describe our costing methods in detail below.
No Surgery
According to clinical experts (in-person communication, May 23 and 25, 2017), patients who do not undergo surgery usually receive both propranolol and primidone (at a cost of $269 per year; Table 26). We obtained average daily doses from the literature76,95,96 and unit costs for the drugs from the Ontario Drug Benefit Formulary.94 Patients are also typically monitored annually by a neurologist for disease progression ($176 for a neurology consultation91).
Table 26:
Essential Tremor Medication Costs
| Drug | Dose Range, mg/day | Average Dose, mg/day | Unit Cost, $/mg | Daily Cost, $ | Annual Cost, $ |
|---|---|---|---|---|---|
| Propranolol | 60–320 | 185 | 0.0031 | 0.57 | 207 |
| Primidone | 50–1,000 | 482 | 0.0004 | 0.17 | 62 |
| Average cost per year | 269 |
Surgery
Table 27 summarizes the cost inputs for all surgical procedures. The total mean costs per procedure are $19,786 for MRgFUS neurosurgery, $37,377 for deep brain stimulation, and $11,774 for radiofrequency thalamotomy.
Table 27:
Average Surgical Procedure Costs
| Resource Item | MRgFUS Neurosurgery, $ | Deep Brain Stimulation, $ | Radiofrequency Thalamotomy, $ | Data Source and Comments |
|---|---|---|---|---|
| Pre-procedure | ||||
| Neurology consultation | 176 | 176 | 176 | Schedule of Benefits (A185)91 |
| Neurosurgery consultation | 121 | 121 | 121 | Schedule of Benefits (A045)91 |
| MRI scan | ||||
| Professional fee | 73 | 73 | 73 | Schedule of Benefits (X421)91 |
| Procedure cost | 972 | 972 | 972 | OCC 2015 (CCI code 3.AN.40), ambulatory92 |
| CT scan | ||||
| Professional fee | 65 | NA | NA | Schedule of Benefits (X401)91 |
| Procedure cost | 513 | NA | NA | OCC 2015 (CCI code 3.AN.20), ambulatory92 |
| Total pre-procedure costs | 1,921 | 1,343 | 1,343 | |
| Peri-procedure | ||||
| Inpatient procedure cost | 14,095a | 17,221b | 6,822c | MRgFUS thalamotomy (clinical expert, email communication, June 21, 2017); deep brain stimulation (OCC 201592); radiofrequency thalamotomy (OCC 201692) |
| Device cost | NA | 14,355 | NA | Medtronic 2011; 90% unilateral DBS based on expert opinion (email communication, July 13, 2017) |
| Physician fees | 1,551 | 1,551 | 1,551 | Schedule of Benefits (N124)91 |
| Surgical assistant | 445 | 590 | 373 | Schedule of Benefits (N124)91 (see Appendix 6, Table A8) |
| Anesthesiologist | 585 | 766 | 495 | Schedule of Benefits (N124)91 (see Appendix 6, Table A9) |
| Total peri-procedure costs | 16,677 | 34,483 | 9,242 | |
| Post-procedure | ||||
| MRI scan | ||||
| Professional fee | 73 | 73 | 73 | Schedule of Benefits (X421)91 |
| Procedure cost | 972 | 972 | 972 | OCC 2015 (CCI code 3.AN.40.), ambulatory92 |
| Neurology, repeat consultation | 85 | 85 | 85 | Schedule of Benefits (A186)91 |
| Neurosurgeon, repeat consultation | 58 | 58 | 58 | Schedule of Benefits (A046)91 |
| Initial DBS programming | NA | 363 | NA | Schedule of Benefits (G547, G549)91; 1.8 visits for initial programming based on Ondo et al, 2005,97 and Picillo et al, 201698 |
| Total post-procedure costs | 1,189 | 1,551 | 1,189 | |
| Total cost per procedure | 19,786 | 37,377 | 11,774 | |
Abbreviations: CCI, Canadian Classification of Health Interventions; CT, computerized tomography; MRgFUS, magnetic resonance-guided focused ultrasound; MRI, magnetic resonance imaging; NA, not applicable; OCC, Ontario Case Costing database.
Note: All costs are presented in 2017 Canadian dollars.
This is the operational cost only; capital costs (i.e., those related to equipment, installation, and maintenance) are excluded in the reference case analysis.
CCI procedure code 1AE53SEJA (implantation, thalamus and basal ganglia, of electrodes [e.g., recording, stimulating] using burr hole approach) for essential tremor (G250); inflated from 2015 to 2017.
CCI procedure code 1AE59SEAW (destruction, thalamus and basal ganglia, using burr hole approach and radiofrequency probe) for all diagnoses; inflated from 2016 to 2017.
Pre-procedure Costs
Before each surgery, we assumed that patients would receive two consultations: one each by a neurologist and a neurosurgeon. Further, we assumed patients would undergo an MRI scan to identify the target location. For MRgFUS neurosurgery, an additional CT scan is needed to measure the thickness of the skull. Therefore, we estimated the total mean preoperative costs to be $1,921 for MRgFUS neurosurgery, $1,343 for deep brain stimulation, and $1,343 for radiofrequency thalamotomy (see Table 27).
Peri-procedure Costs
MRgFUS Neurosurgery
We estimated the inpatient procedure cost of MRgFUS neurosurgery through consultation with clinical experts (email communication, June 21, 2017) and an MRgFUS device manufacturer (in-person communication, June 19, 2017) (Table 28). We excluded the capital and fixed costs (i.e., equipment, installation, and maintenance costs) of the MRgFUS system in the reference case analyses since the equipment is already in place (either donated to or purchased by the hospital) at the two centres currently providing MRgFUS neurosurgery in Ontario. Since MRgFUS neurosurgery is a highly specialized procedure, we assumed that if it were to be publicly funded, it would most likely take place in these two centres. In this case, the Ministry of Health and Long-Term Care would be providing funding only for operational costs.
Table 28:
Inpatient Procedure Costs—MRgFUS Neurosurgery
| Resource Item | Cost, $ |
|---|---|
| Insightec system and equipment | 1,950,000 |
| Installation | 180,000 |
| Capital/Fixed Costs | |
| Equipment depreciation per year | 336,000 |
| Annual service contract | 125,000 |
| Full-time coordinator (−100,000 if 100 cases per year) | 0 |
| Total fixed cost per year | 461,000 |
| Annual case load (current level) | 24 |
| Average fixed cost per case | 19,208 |
| Operational/Variable Costs | |
| Procedure costs, including labour and materials (e.g., medical imaging) | 7,147 |
| Other diagnostic procedures | 404 |
| Post-procedure recovery costs (mainly nursing) | 4,822 |
| Other support services (e.g., pharmacy, nutrition, respiratory therapy) | 1,722 |
| Total variable cost per case, used for reference case analysis | 14,095 |
| Full cost per case (fixed + variable), used for scenario analysis | 33,303 |
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound. Note: All costs are presented in 2017 Canadian dollars.
We estimated the operational (variable) cost to be about $14,095 per case; this cost includes the procedure costs related to medical imaging, other diagnostic procedures, post-procedure recovery (mainly nursing), and other support services (e.g., pharmacy, nutrition, respiratory therapy). In a scenario analysis, we reduced the operational cost to $10,095 per case, considering the possibility that hospitals may send patients home on the same day as the surgery. In this case, some post-procedure recovery costs can be avoided, and the variable cost can be reduced by $4,000 per case.
We considered the full cost of MRgFUS neurosurgery (including both fixed and variable costs) in another scenario analysis. We estimated the total fixed cost per year to be $461,000; this cost consists of the equipment cost, annual service contract, and salary of a full-time coordinator (employed only once there are 100 cases per year). We then divided this amount by an annual case load of 24 (the current level) to derive an average fixed cost of $19,208 per case. Therefore, if the annual case load remains 24 patients per year, the full cost per MRgFUS procedure would be $33,303 ($19,208 + $14,095).
We did not include the cost of training medical personnel to use the MRgFUS technology since this training is provided by the manufacturer at no extra cost. We did include the professional fees for a neurosurgeon ($1,551), surgical assistant ($445), and anaesthesiologist ($585) (see Table 27).
Deep Brain Stimulation
According to the Ontario Case Costing database, the hospital cost of deep brain stimulation is $16,865 per procedure (standard deviation [SD]: $2,980, in 2015 CAD), with an average length of stay of 1.4 days. A unilateral deep brain stimulation device costs about $13,649, and a bilateral device costs about $20,709 (Appendix 6, Table A7). According to clinical expert opinion (email communication, July 13, 2017), about 90% of patients who undergo deep brain stimulation have a unilateral procedure; thus, we estimated the average cost of deep brain stimulation to be $14,355. In the reference case analysis, we assumed that Medtronic's Activa SC (single-channel) and PC (double-channel) systems are the most commonly used deep brain stimulation devices. The battery of the implantable pulse generator generally lasts 5 to 7 years, at which point it must be replaced at an additional cost.99 We also included the professional fees for a neurosurgeon ($1,551), surgical assistant ($590), and anaesthesiologist ($766) (see Table 27).
Radiofrequency Thalamotomy
According to the Ontario Case Costing database, the hospital cost of radiofrequency thalamotomy is $6,768 per procedure (SD: $5,099, in 2016 CAD), with an average length of stay of 1.4 days. We also included the professional fees for neurosurgeon ($1,551), surgical assistant ($373), and anaesthesiologist ($495) (see Table 27).
Post-procedure Costs
MRgFUS Neurosurgery and Radiofrequency Thalamotomy
We assumed that after MRgFUS neurosurgery or radiofrequency thalamotomy, a patient would receive one repeat consultation with a neurosurgeon ($58) and one from a neurologist ($85). We also assumed one follow-up MRI scan to check the location of the lesion.
Deep Brain Stimulation
We assumed that after deep brain stimulation, a patient would receive one repeat consultation with a neurologist and one from a neurosurgeon (to check the patient's condition and incisions),99 as well as an average of 1.8 visits for initial device programming.97 This assumption is based on the North American Survey of Placement and Adjustment Strategies for Deep Brain Stimulation97 and the Toronto Western Hospital algorithm for deep brain stimulation programming.98 Initial device programming is performed in clinic at least 3 weeks after surgery to lessen the impact of the dynamic impedance changes caused by tissue reaction to the electrode placement. After the first programming visit, some patients may come back for a further 1 to 2 appointments to optimize stimulation settings (to maximize tremor control while minimizing stimulation-induced adverse effects). Most patients complete programming in less than 2 months. Clinicians may set a given parameter range and instruct patients how to slowly tune the stimulation at home with the help of a remote control. Therefore, we estimated the total expected postoperative physician reimbursement to be $506 ($58 + $85 + $363).91 We also assumed one follow-up MRI scan to check the location of the lesion.
Ongoing Costs
Medication Costs
After surgery, some people may stop taking medication, whereas some may need to continue taking medication to manage residual tremor. According to clinical experts (email communication, July 13, 2017), 50% of people who experience marked improvement after surgery typically reduce the dose of their tremor medication by half (post-surgery cost: $202 per year); people who experience mild to moderate improvement following surgery tend to remain on the same dose as before surgery (at a cost of $269 per year) (see Table 26).
Monitoring Costs
According to clinical experts, people who undergo MRgFUS neurosurgery or radiofrequency thalamotomy are monitored annually by a neurologist for disease progression ($176 per year).91
People who undergo deep brain stimulation typically have more frequent visits to adjust the stimulation level and/or to check the battery status of the device every 6 to 12 months. The number of visits is estimated to be 6.2 in the first year following surgery and 2.3 in subsequent years.97 The cost of a reprogramming session is $201 (Schedule of Benefits, codes G547 and G549, assuming a rate of 90% unilateral procedures).91 Therefore, we estimated the monitoring cost of deep brain stimulation to be $1,249 per patient in the first year and $463 per patient in subsequent years.
Deep Brain Stimulation Device Battery Replacement Costs
For people who have undergone deep brain stimulation, we assumed that reoperation to replace the device battery is needed every 5 years. This procedure is performed on an outpatient basis with local anaesthesia. We estimated the cost of the battery replacement surgery to be $11,329 (includes the operating cost of $2,434 [2009 CAD]100 and the device cost of $8,689, assuming a rate of 90% unilateral procedures).
Costs of Reoperation After Tremor Recurrence
For MRgFUS neurosurgery and radiofrequency thalamotomy, the cost of reoperation is the same as the cost of the initial procedure.
Costs of Managing Adverse Effects and Complications
We based the treatment costs for adverse effects and complications on the published literature and clinical expert opinion (see Table 24 and Treatment-Related Adverse Effects and Complications section). The resources used were multiplied by the unit costs to obtain the costs of managing each adverse effect and complication (Table 29).
Table 29:
Average Costs of Managing Adverse Effects and Complications
| Resource Item | Total Cost, $ | Resources Used (Source) |
|---|---|---|
| Short-term adverse effects and complications | ||
| Gait disturbance | 108.95 | One repeat neurologist visit (assumption); 25% of patients prescribed physical therapy (Elias et al, 201638) |
| Paresthesia or numbness | 84.95 | One repeat neurologist visit (assumption) |
| Speech disorder | 84.95 | One repeat neurologist visit (assumption) |
| Headache | 84.95 | One repeat neurologist visit; over-the-counter pain medication (assumption) |
| Intracranial hemorrhage | 7,634.74 | Half of cases symptomatic (assumption; cost of symptomatic intracranial hemorrhage obtained from OCC) |
| Infection following deep brain stimulation | 22,461.04 | 60% require reoperation; 40% treated as outpatients (Fenoy et al, 2012101; Fenoy et al, 201478) |
| Infection following radiofrequency thalamotomy | 5,591.82 | 60% treated as inpatients, 40% treated as outpatients (expert opinion) |
| Permanent adverse effects | ||
| Gait disturbance | 0 | No treatment required (expert opinion); 20% of patients require a walker (Elias et al, 201638)a |
| Paresthesia or numbness | 0 | No treatment required (expert opinion) |
| Speech disorder | 0 | Speech therapy for some patients (expert opinion)a |
| Deep brain stimulation–hardware-related complications | ||
| Lead fracture or migration | 25,111.88 | Reoperation to replace the lead (Fenoy et al, 201478) |
| Device malfunction | 37,377.05 | Reoperation to replace the device (Fenoy et al, 201478) |
We did not include the costs of assistive devices and speech therapy, as they are usually not covered by public payers.
For deep brain stimulation, to avoid double-counting, we considered the costs of managing stimulation-related adverse effects already captured under the reprogramming visit costs (see Ongoing Costs section).
For intracranial hemorrhage, the literature reports that about half of cases are symptomatic and all symptoms usually resolve within a month.20,102 No treatment is needed for asymptomatic intracranial hemorrhage. Patients with symptomatic hemorrhage may be hospitalized. We obtained the cost of hospitalization for intracranial hemorrhage from the Ontario Case Costing database ($15,148 per case [2016 CAD]).92
The literature and clinical experts report that for infection following deep brain stimulation, 60% of patients must have all device components removed and replaced, whereas 40% can be treated as outpatients with oral antibiotics (cephalexin 500 mg orally every 6 hours) for 1 month.78,92,94,101 For infection following radiofrequency thalamotomy, we assumed that 60% of patients would be hospitalized and 40% would be treated with oral antibiotics on an outpatient basis.92,94
Based on the literature and clinical expert opinion, all deep brain stimulation–hardware-related complications require reoperation.78 For device malfunction, the entire deep brain stimulation device must be removed and reimplanted. For lead fracture or migration, a reoperation is needed to replace the lead.
Societal Cost
In a scenario analysis, we used a societal perspective and included the following types of costs:
Out-of-pocket cost (i.e., parking at hospital)
Cost of travel to receive treatment
Cost of people's time spent travelling and receiving treatment
Cost of lost productivity
For out-of-pocket costs, we considered the cost of parking at the hospital. We estimated the number of days patients spent at the hospital and applied a maximum daily parking rate of $26.92 Based on clinical expert opinion (in-person communication, May 23 and 25, 2017; telephone communication, May 30 and 31, 2017), patients undergoing MRgFUS neurosurgery, radiofrequency thalamotomy, and deep brain stimulation stay at the hospital for about 2 days, 3 to 4 days, and 4 to 5 days, respectively.
We estimated the cost of travel to receive treatment by multiplying the average cost per trip by the number of round-trips each year (for physician consultation and annual monitoring, and to the hospital for surgery). We estimated the average cost per round-trip to be about $10.80.103 We obtained the number of programming visits for deep brain stimulation from the literature.97
We based people's time spent travelling and receiving treatment on both the literature104 and expert opinion (in-person communication, May 23 and 25, 2017; telephone communication, May 30 and 31, 2017). According to a Canadian study by Hunka et al,104 in the first year following deep brain stimulation surgery, the mean total time spent programming the stimulator and assessing a person with essential tremor is about 22.3 hours. To value the time of people undergoing a procedure (the majority of whom are not in the work force), we used the Ontario minimum wage of $11.60 per hour plus a 30% employee benefit.105
Last, we considered the cost of lost productivity for people undergoing neurosurgery for essential tremor. The average age of the model population is 71 years, and 7% of people aged 70 years and older are still in the work force according to Statistics Canada.106 Therefore, we assumed that 7% of the model population are unable to work because of their disease and used the median income in Ontario ($33,840) to estimate the cost of lost productivity.91 We did not consider the lost productivity cost of caregivers since, according to clinical expert opinion (in-person communication, May 23 and 25, 2017; telephone communication, May 30 and 31, 2017), the majority of people with essential tremor do not require caregiver support.
Table 30 presents the societal costs included in the economic model.
Table 30:
Societal Costs per Person by Treatment Strategy
| No Surgery | MRgFUS Neurosurgery | Radiofrequency Thalamotomy | Deep Brain Stimulation | Source | |
|---|---|---|---|---|---|
| Out-of-Pocket Cost (parking) | |||||
| Year 1, days spent in hospital | – | 2 | 3.5 | 4.5 | Sunnybrook hospital, 201792; expert opinion |
| Year 1, cost | – | $52.00 | $91.00 | $117.00 | |
| Cost of Travel to Receive Treatment | |||||
| Year 1, round-trips | 1 | 5 | 5 | 13 | Ondo et al, 200597; expert opinion |
| Subsequent years, round-trips | 1 | 1 | 1 | 2.3 | |
| Year 1, cost | $10.80 | $54.00 | $54.00 | $140.40 | |
| Subsequent years, cost | $10.80 | $10.80 | $10.80 | $24.84 | |
| Time Spent Travelling and Receiving Treatment | |||||
| Year 1, hours | 2 | 56 | 92 | 146.3 | Hunka et al, 2005104; expert opinion |
| Subsequent years, hours | 2 | 2 | 2 | 4.6 | |
| Year 1, cost | $30.16 | $844.48 | $1,387.36 | $2,206.20 | |
| Subsequent years, cost | $30.16 | $30.16 | $30.16 | $69.37 | |
| Lost Productivity | |||||
| Cost per year | $2,369.00 | – | – | – | Statistics Canada, 201691 |
Analysis
For the reference case analyses (research questions 1 and 2), we performed both deterministic and probabilistic analyses. We calculated the deterministic results using the best point estimates for each parameter. We conducted the probabilistic analysis by running 5,000 Monte Carlo simulations to capture parameter uncertainty. When possible, we specified distributions around input parameters using the mean and standard deviation. Costs were characterized by gamma distributions, and probabilities and utilities were characterized by beta distributions. If not specified, other parameters were assumed fixed. See Appendix 7, Table A10, for a list of the model variables and their corresponding distributions.
We determined the costs and quality-adjusted life-years (QALYs) for each treatment and calculated the incremental cost-effectiveness ratio (ICER) of MRgFUS neurosurgery compared with other treatments. We also assessed the probability of each treatment being cost-effective over a range of thresholds.
We conducted deterministic sensitivity analyses (e.g., changing from the mean to the upper and lower limits of the 95% confidence interval of the variables) to assess the impact of key variables on the ICER. We also conducted several scenario analyses related to methodological and structural uncertainty. These are summarized in Table 31.
Table 31:
Scenario Analyses
| Parameter | Parameter/Assumption Used in Reference Case | Parameter/Assumption Used in Scenario Analysis |
|---|---|---|
| Cost perspective | Public payer (Ontario Ministry of Health and Long-Term Care) | Societal |
| Time horizon | 5 years | 1 year; 10 years |
| Capital cost of MRgFUS neurosurgery | Excluded (inpatient procedure cost of MRgFUS neurosurgery: $14,095 per case) | Included (inpatient procedure cost of MRgFUS neurosurgery increased to $33,033 per case) |
| Post-surgery recovery cost of MRgFUS neurosurgery | Included (inpatient procedure cost of MRgFUS neurosurgery: $14,095 per case) | Reduced (inpatient procedure cost of MRgFUS neurosurgery reduced to $10,095 per case) |
| MRI scan cost | $972 per scan (based on OCC data92) | Reduced by one-third to $324 per scan (based on expert opinion) |
| Probability of recurrence over 5 years for MRgFUS neurosurgery | 25% (based on Hirai et al, 198373 and expert opinion) | 50% (based on expert opinion) |
| Utility of mild to moderate improvement, post-surgery | 0.80 (based on Herceg et al, 201283) | 0.71 (based on Herceg et al, 201283) |
| Probability of recurrence in year 1 for MRgFUS neurosurgery and radiofrequency thalamotomy | 8.9% (based on Elias et al, 201638) | 11% (based on Zaaroor et al, 201745) |
| Surgical effectiveness of MRgFUS neurosurgery, radiofrequency thalamotomy, and deep brain stimulation | Same for all surgeries: 53% with marked improvement; 47% with mild to moderate improvement (based on Elias et al, 201638) | MRgFUS neurosurgery: 91.3% with marked improvement (absence of tremor or occasional tremor); 8.7% with mild to moderate improvement (partial tremor) Radiofrequency thalamotomy: 100%; 0% Deep brain stimulation: 89.5%; 10.5% (Based on Kim et al, 201746) |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; MRI, magnetic resonance imaging; OCC, Ontario Case Costing database.
We validated our economic evaluation by verifying the Microsoft Excel model and its equations, communicating with clinical experts to ensure the model had face validity, and cross-validating the results with previously published economic evaluations addressing similar decision problems. We also replicated the analysis using a different modelling program (TreeAge Pro). The two models produced the same results.
Generalizability
The findings of this economic analysis cannot be generalized to all people with essential tremor. They may, however, be used to guide decision-making about the specific patient populations addressed in the studies evaluated by Health Quality Ontario.
Expert Consultation
Throughout the development of this model, we consulted with clinical and methodological experts with expertise in neurology, neurosurgery, radiology, and medical imaging, as well as with other specialists, advisors, and clinicians with relevant experience. The role of the expert advisors was to review the structure and inputs of the economic model to confirm that the information we used reasonably reflected the clinical setting. However, the statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
Results
Research Question 1: MRgFUS Neurosurgery Versus No Surgery in People With Essential Tremor Ineligible for Invasive Neurosurgery
Reference Case Deterministic Analysis
Table 32 presents the results of the deterministic reference case analysis for the comparison of MRgFUS neurosurgery versus no surgery. Over a 5-year time horizon, MRgFUS neurosurgery has a total mean cost of $23,507 and a total mean of 3.69 QALYs. Compared with no surgery, MRgFUS neurosurgery is associated with an incremental cost of $21,448 and an incremental QALY of 0.50. The ICER of MRgFUS neurosurgery compared with no surgery is $43,075 per QALY gained.
Table 32:
Deterministic Reference Case Analysis Results—Cost-Effectiveness of MRgFUS Neurosurgery Versus No Surgery
| MRgFUS Neurosurgery | No Surgery | |
|---|---|---|
| Cost of primary surgery | $19,786 | – |
| Cost of monitoring | $814 | $814 |
| Cost of medications | $1,095 | $1,245 |
| Cost of reoperation | $1,741 | – |
| Cost of managing adverse events | $71 | – |
| Total costs | $23,507 | $2,060 |
| Life-years | 4.63 | 4.63 |
| QALYs | 3.69 | 3.19 |
| Incremental cost | $21,448 | |
| Incremental life-years | 0.00 | |
| Incremental QALYs | 0.50 | |
| ICER (cost/QALY) | $43,075 | |
Abbreviations: ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; QALY, quality-adjusted life-year.
Reference Case Probabilistic Analysis
For the comparison of MRgFUS neurosurgery versus no surgery, the results of the probabilistic reference case analysis were similar to those of the deterministic analysis (Appendix 8, Table A11). Over a 5-year time horizon, MRgFUS neurosurgery has a total mean cost of $23,497 (95% CI: $22,044–$25,287) and a total mean of 3.70 QALYs (95% CI: 3.48–3.89). Compared with no surgery, MRgFUS neurosurgery is associated with an incremental cost of $21,438 (95% CI: $19,985–$23,227) and an incremental quality-adjusted survival of 0.47 QALYs (95% CI: 0.10–0.90). The ICER of MRgFUS neurosurgery compared with no surgery is $45,817 per QALY gained.
The cost-effectiveness acceptability curve presented in Figure 6 shows the probability of MRgFUS neurosurgery being cost-effective compared with no surgery across a range of willingness-to-pay thresholds. At common willingness-to-pay thresholds of $50,000 per QALY and $100,000 per QALY, MRgFUS neurosurgery is 56.0% and 89.5% likely to be cost-effective, respectively.
Figure 6: Cost-Effectiveness Acceptability Curve—MRgFUS Neurosurgery Versusu No Surgery.
Deterministic Sensitivity Analyses
Figure 7 presents a tornado diagram illustrating the results of the deterministic sensitivity analyses. The ICER of MRgFUS neurosurgery compared with no surgery was most sensitive to assumptions regarding baseline utility. When assuming a utility of 0.81 (the upper limit of the 95% confidence interval for baseline utility) for people with disabling tremor, the incremental cost of MRgFUS neurosurgery versus no surgery remains the same, while the incremental effectiveness decreases to 0.10 QALYs, resulting in an ICER of more than $200,000 per QALY. Our results remained robust when other parameters were varied.
Figure 7: Tornado Diagram—Influence of Key Parameters on the Incremental Cost-Effectiveness Ratio of MRgFUS Neurosurgery Versus No Surgery.
Abbreviations: ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; RF, radiofrequency
Scenario Analysis
Table 33 presents our scenario analysis results. These results were sensitive to the following model parameters or assumptions: time horizon, inclusion of the capital cost of MRgFUS neurosurgery, utility of people experiencing mild to moderate improvement after surgery, and cost perspective. When the time horizon was shortened to 1 year, the ICER increased to $241,520 per QALY. When we used a 10-year time horizon, the ICER was reduced by 40% compared to the reference case ($25,980/QALY). When the capital cost of MRgFUS neurosurgery was included, the ICER almost doubled compared to the reference case ($85,047/QALY). When we assumed that people would experience only a modest improvement in quality of life following mild to moderate improvement post-surgery (utility of 0.71 vs. 0.80), the ICER became $65,637 per QALY. When incorporating people's out-of-pocket and lost productivity costs into the reference case analysis, the cost difference between MRgFUS neurosurgery and no surgery was reduced from $21,448 to $11,423, resulting in an ICER of $22,943 per QALY (a reduction of 47% compared to the reference case).
Table 33:
Deterministic Scenario Analysis Results—MRgFUS Neurosurgery Versus No Surgery
| MRgFUS Neurosurgery vs. No Surgery | |||
|---|---|---|---|
| Incremental Cost | Incremental QALYs | ICER | |
| Reference case | $21,448 | 0.50 | $43,075 |
| Societal perspective | $11,423 | 0.50 | $22,943 |
| Time horizon | |||
| 1 year | $20,171 | 0.08 | $241,520 |
| 10 years | $22,345 | 0.86 | $25,980 |
| Including capital cost of MRgFUS | $42,346 | 0.50 | $85,047 |
| Reducing post-surgery recovery cost of MRgFUS | $17,096 | 0.50 | $34,334 |
| Reducing MRI scan cost for all surgeries | $20,037 | 0.50 | $40,243 |
| Utility of mild to moderate improvement post-surgery (0.71, based on Herceg et al, 201283) | $21,448 | 0.33 | $65,637 |
| Probability of recurrence in year 1 for MRgFUS and RF (11%, based on Zaaroor et al, 201745) | $21,478 | 0.49 | $43,544 |
| Probability of recurrence over 5 years for MRgFUS (50%, based on expert opinion) | $23,129 | 0.45 | $51,965 |
| Surgical effectiveness (based on Kim et al, 201746) | $21,339 | 0.67 | $31,924 |
Abbreviations: ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; QALY, quality-adjusted life-year; RF, radiofrequency.
Research Question 2: MRgFUS Neurosurgery Versus Radiofrequency Thalamotomy or Deep Brain Stimulation in People With Essential Tremor Eligible for Invasive Neurosurgery
Reference Case Deterministic Analysis
Tables 34 and 35 present the results of the deterministic reference case analysis for the comparison of MRgFUS neurosurgery versus radiofrequency thalamotomy or deep brain stimulation. Over a 5-year time horizon, the total mean costs of MRgFUS neurosurgery, radiofrequency thalamotomy, and deep brain stimulation are $23,507, $14,978, and $57,535, with a total mean of 3.69, 3.61, and 3.94 QALYs, respectively. Radiofrequency thalamotomy is the least costly and least effective procedure, and deep brain stimulation is the most costly and most effective. The ICER of MRgFUS neurosurgery compared with radiofrequency thalamotomy is $109,795 per QALY gained. The ICER of deep brain stimulation compared with MRgFUS neurosurgery is $134,259 per QALY gained.
Table 34:
Deterministic Reference Case Analysis Results—Cost-Effectiveness of MRgFUS Neurosurgery, Radiofrequency Thalamotomy, and Deep Brain Stimulation
| MRgFUS Neurosurgery | Radiofrequency Thalamotomy | Deep Brain Stimulation | |
|---|---|---|---|
| Cost of primary surgery | $19,786 | $11,774 | $37,377 |
| Cost of monitoring | $814 | $814 | $3,307 |
| Cost of medications | $1,095 | $1,095 | $1,081 |
| Cost of reoperation | $1,741 | $1,036 | – |
| Cost of managing adverse events | $71 | $259 | $6,307 |
| Cost of battery replacement | – | – | $9,463 |
| Total cost | $23,507 | $14,978 | $57,535 |
| Life-years | 4.63 | 4.63 | 4.63 |
| QALYs | 3.69 | 3.61 | 3.94 |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; QALY, quality-adjusted life-year.
Table 35:
Deterministic Reference Case Analysis Results—Incremental Cost-Effectiveness Ratios for MRgFUS Neurosurgery Versus Radiofrequency Thalamotomy or Deep Brain Stimulation
| Intervention | Total Mean Cost | Total Mean QALYs | Incremental Cost | Incremental QALYs | Sequential ICER |
|---|---|---|---|---|---|
| Radiofrequency thalamotomy | $14,978 | 3.61 | – | – | – |
| MRgFUS neurosurgery | $23,507 | 3.69 | $8,530a | 0.08a | $109,795a |
| Deep brain stimulation | $57,535 | 3.94 | $34,028b | 0.25b | $134,259b |
Abbreviations: ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; QALY, quality-adjusted life-year.
Compared with radiofrequency thalamotomy.
Compared with MRgFUS neurosurgery.
Reference Case Probabilistic Analysis
For the comparison of MRgFUS neurosurgery versus radiofrequency thalamotomy or deep brain stimulation, the results of the probabilistic reference case analysis are similar to those of the deterministic analysis (Appendix 8, Tables A11 and A12). Over a 5-year time horizon, the total mean costs of MRgFUS neurosurgery, radiofrequency thalamotomy, and deep brain stimulation are $23,497 (95% CI: $22,044–$25,287), $14,972 (95% CI: $14,096–$16,051), and $57,523 (95% CI: $55,507–$59,772), with a total mean of 3.70 (95% CI: 3.48–3.89), 3.63 (95% CI: 3.42–3.82), and 3.96 (95% CI: 3.75–4.14) QALYs, respectively. Radiofrequency thalamotomy is the least costly and least effective, and deep brain stimulation is the most costly and most effective. The ICER of MRgFUS neurosurgery compared with radiofrequency thalamotomy is $119,607 per QALY gained, and the ICER of deep brain stimulation compared with MRgFUS neurosurgery is $130,850 per QALY gained.
Deterministic Sensitivity Analyses
Figures 8 and 9 present tornado diagrams illustrating the results of the deterministic sensitivity analyses. The ICER of MRgFUS neurosurgery compared with radiofrequency thalamotomy was most sensitive to assumptions regarding the probability of recurrence in the first year following surgery, the hospital cost of radiofrequency thalamotomy, and the risk of infection related to the radiofrequency thalamotomy procedure (see Figure 8). When the upper limit of the 95% confidence interval for recurrence in year 1 was used (18%), the ICER of MRgFUS neurosurgery versus radiofrequency thalamotomy became $215,586 per QALY. When assuming a 15% risk of infection for people undergoing radiofrequency thalamotomy, MRgFUS neurosurgery became more cost-effective with an ICER of $60,307 per QALY.
Figure 8: Tornado Diagram—Influence of Key Parameters on the Incremental Cost-Effectiveness Ratio of MRgFUS Neurosurgery Versus Radiofrequency Thalamotomy.
Abbreviations: DBS, deep brain stimulation; ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; RF, radiofrequency.
Figure 9: Tornado Diagram—Influence of Key Parameters on the Incremental Cost-Effectiveness Ratio of Deep Brain Stimulation Versus MRgFUS Neurosurgery.
Abbreviations: DBS, deep brain stimulation; ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; RF, radiofrequency.
The ICER of deep brain stimulation versus MRgFUS neurosurgery was sensitive to several assumptions related to deep brain stimulation, such as battery life, onset of benefit, risk of hardware complications, and risk of infection (see Figure 9). When assuming the battery of the deep brain stimulation device can last longer (8–10 years if the device is turned off at night), the ICER of deep brain stimulation versus MRgFUS neurosurgery became smaller ($96,924/QALY). When assuming a 3-month delay in the onset of treatment benefit with deep brain stimulation, the ICER of deep brain stimulation vs. MRgFUS neurosurgery increased to $160,560 per QALY. When the risk of hardware complications was doubled (from 4% to 8%), the ICER of deep brain stimulation versus MRgFUS neurosurgery increased to $160,390 per QALY.
Scenario Analysis
Table 36 presents our scenario analysis results. These results were sensitive to time horizon and the inclusion of the capital cost of MRgFUS neurosurgery. When the time horizon was shortened to 1 year, the ICER of MRgFUS neurosurgery versus radiofrequency thalamotomy increased to $546,289 per QALY, and the ICER of deep brain stimulation versus MRgFUS neurosurgery increased to $297,776 per QALY. When we used a 10-year time horizon, the ICER of MRgFUS neurosurgery versus radiofrequency thalamotomy decreased to $63,579 per QALY, and the ICER of deep brain stimulation versus MRgFUS neurosurgery decreased to $90,486 per QALY. When we included the capital cost of MRgFUS neurosurgery, the ICER of MRgFUS neurosurgery versus radiofrequency thalamotomy increased to $378,806 per QALY, and the ICER of deep brain stimulation versus MRgFUS neurosurgery was reduced to $51,803 per QALY. The results did not change much when we applied a societal perspective, since these surgeries are considered to improve people's productivity similarly. When assuming that MRgFUS neurosurgery will have a much higher probability of recurrence over 5 years (50% compared to 25% in the reference case analysis), MRgFUS neurosurgery became less cost-effective compared with radiofrequency thalamotomy, with an ICER of $410,779 per QALY.
Table 36:
Deterministic Scenario Analysis Results—MRgFUS Neurosurgery vs. Radiofrequency Thalamotomy or Deep Brain Stimulation
| MRgFUS vs. RF | DBS vs. MRgFUS | |||||
|---|---|---|---|---|---|---|
| Incremental Cost | Incremental QALYs | ICER | Incremental Cost | Incremental QALYs | ICER | |
| Reference case | $8,530 | 0.08 | $109,795 | $34,028 | 0.25 | $134,259 |
| Societal perspective | $7,948 | 0.08 | $102,305 | $35,815 | 0.25 | $141,310 |
| Time horizon | ||||||
| 1 year | $7,980 | 0.01 | $546,289 | $19,996 | 0.07 | $297,776 |
| 10 years | $8,925 | 0.14 | $63,579 | $45,855 | 0.51 | $90,486 |
| Including capital cost of MRgFUS | $29,428 | 0.08 | $378,806 | $13,129 | 0.25 | $51,803 |
| Reducing post-surgery recovery cost of MRgFUS | $4,178 | 0.08 | $53,775 | $38,380 | 0.25 | $151,430 |
| Reducing MRI scan cost for all surgeries | $8,530 | 0.08 | $109,795 | $33,886 | 0.25 | $133,702 |
| Utility of mild to moderate improvement post-surgery (0.71, based on Herceg et al, 201283) | $8,530 | 0.07 | $115,288 | $34,028 | 0.23 | $145,485 |
| Probability of recurrence in year 1 for MRgFUS and RF (11%, based on Zaaroor et al, 201745) | $8,541 | 0.08 | $110,090 | $33,998 | 0.26 | $131,715 |
| Probability of recurrence over 5 years for MRgFUS (50%, based on expert opinion) | $10,211 | 0.02 | $410,779 | $32,346 | 0.31 | $105,612 |
| Surgical effectiveness (based on Kim et al, 201746) | $8,554 | 0.04 | $196,484 | $34,023 | 0.26 | $128,867 |
Abbreviations: DBS, deep brain stimulation; ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; RF, radiofrequency; QALY, quality-adjusted life year.
Discussion
Our reference case analysis showed that in people with essential tremor ineligible for invasive neurosurgery, MRgFUS neurosurgery is cost-effective compared with no surgery (ICER: $43,075) at commonly used willingness-to-pay thresholds between $50,000 and $100,000 per QALY. Our deterministic sensitivity analyses showed that the results are most sensitive to the baseline utility of people with essential tremor (0.69 ± 0.30 [mean ± SD], based on Herceg et al, 2012,83 using the EQ-5D instrument). When we used the upper limit of the 95% confidence interval for baseline utility, the ICER of MRgFUS neurosurgery versus no surgery increased to more than $200,000 per QALY. For people who are not as severely affected by the disease, MRgFUS neurosurgery may not be a cost-effective treatment option compared with no surgery. However, essential tremor typically does have a negative impact on people's quality of life. Several studies have found that people with essential tremor have a much poorer quality of life compared with peers without the disease.83,107–109 A large Spanish study,55 which also used EQ-5D to assess quality of life, reported a utility of 0.73 ± 0.30 (mean ± SD) in people with essential tremor, which is similar to that of people with other chronic illnesses. We did not use this study for our reference case analysis since the majority of the participants in the study had mild to moderate disease and were not considered to have medication-refractory disease.
We excluded the capital cost of MRgFUS neurosurgery in the reference case analysis since the two centres currently providing this surgery in Ontario already have the equipment installed, meaning the Ministry of Health and Long-Term Care would be responsible only for the operational costs if MRgFUS neurosurgery becomes publicly funded. Based on expert input, for a variety of reasons (e.g., specialized skill and high capital costs), MRgFUS neurosurgery is probably most feasible to be implemented at a few large centres. If we consider the full cost of MRgFUS neurosurgery (both capital and operational costs, resulting in $33,303 per case), the ICER almost doubles compared with the reference case ($85,047/QALY). In another scenario, we lowered the operational cost of MRgFUS neurosurgery by $4,000 per case, based on expert opinion that hospitals may convert the procedure into a same-day service model. In this case, the ICER of MRgFUS neurosurgery compared with no surgery is reduced to $34,334 per QALY. However, the likelihood of this reduction occurring will depend on the health condition of people undergoing the procedure and will thus be determined on a case-by-case basis.
Our analysis also showed that in people eligible for invasive neurosurgery, radiofrequency thalamotomy is the least expensive but also least effective treatment option. This is because radiofrequency thalamotomy, as an open surgical procedure, is associated with a risk of intracranial hemorrhage and infection. It is also associated with a higher rate of long-term speech disorders than either MRgFUS neurosurgery or deep brain stimulation. Compared with radiofrequency thalamotomy, MRgFUS neurosurgery is associated with an ICER of $109,795 per QALY. We found that deep brain stimulation is more effective than radiofrequency thalamotomy and MRgFUS neurosurgery, but it is also more costly. The ICER of deep brain stimulation compared with radiofrequency thalamotomy is $128,520 per QALY, and the ICER of deep brain stimulation compared with MRgFUS neurosurgery is $134,259 per QALY.
Deep brain stimulation is currently the surgical standard for moderate to severe, medication-refractory essential tremor. Although no trials have demonstrated the superiority of deep brain stimulation over radiofrequency thalamotomy, deep brain stimulation is the preferred surgical treatment because it is considered reversible and modifiable. In this analysis, we made two assumptions favouring deep brain stimulation: (1) the procedure does not result in tremor recurrence requiring reoperation; and (2) any stimulation-related adverse effects are short-lived and resolvable by reprogramming, thus having no negative impact on the patient's quality of life. However, clinical studies have shown that, in rare cases, people who undergo deep brain stimulation may experience tremor recurrence requiring reoperation.
Both MRgFUS neurosurgery and radiofrequency thalamotomy are good treatment options for people with essential tremor who would have difficulty meeting the follow-up requirements for deep brain stimulation (i.e., for device programming) and for those unable or unwilling to accept the risks associated with an implantable device.76 MRgFUS neurosurgery has advantages over both radiofrequency thalamotomy and deep brain stimulation because it is a noninvasive procedure. Thus, it provides a treatment option for people with medication-refractory essential tremor who are ineligible for an invasive procedure. However, MRgFUS neurosurgery is a relatively new technology for which the long-term effectiveness is currently unknown. Therefore, there is some uncertainty as to whether results achieved in the short term will persist. Clinical experts have reported seeing stability in effect up to 2 years following the procedure, and that treatment effects seen at 1 month tend to be stable at 1 year (in-person communication, May 23 and 25, 2017; telephone communication, May 30 and 31, 2017). Although it remains to be demonstrated, some researchers have suggested that outcomes such as durability of effect and adverse effects owing to suboptimal lesion location may improve over time as centres gain experience with the MRgFUS neurosurgery procedure.
Our primary economic evaluation has several strengths. It is the first economic analysis estimating the economic value of MRgFUS neurosurgery for the treatment of essential tremor in Canada. We assessed the cost-effectiveness of MRgFUS neurosurgery compared with standard treatment in two distinct patient populations: those who are ineligible for invasive neurosurgery and those who are. Our analysis comparing MRgFUS neurosurgery with no surgery in people ineligible for invasive neurosurgery was based on a randomized, double-blinded, placebo-controlled trial,38 a study design considered to provide high-quality evidence. For our analysis comparing MRgFUS neurosurgery with radiofrequency thalamotomy or deep brain stimulation in people eligible for invasive neurosurgery, we assumed that all surgeries are similarly effective but have different safety profiles, based on the literature38 and clinical expert opinion (in-person communication, May 23 and 25, 2017; telephone communication, May 30 and 31, 2017). Our analysis incorporated important long-term clinical outcomes, such as tremor recurrence, reoperation, and permanent or ongoing adverse effects and complications, compared with the study by Ravikumar et al,54 which did not include these outcomes.
There were several limitations to our analysis. There is currently no clinical evidence directly comparing MRgFUS neurosurgery with radiofrequency thalamotomy and deep brain stimulation. In the absence of such evidence, we relied on unadjusted comparisons and clinical expert judgment. Owing to a lack of data on the natural history of essential tremor, we were unable to model disease progression by severity. Other than tremor recurrence after the primary surgery, we assumed that in both the “surgery” and “no-surgery” arms of the model, a patient's condition is stable, since essential tremor is a slowly progressive disorder. Also, we were unable to model quality of life by disease severity, since very few studies have reported utility values for essential tremor. Also, we were unable to use quality-of-life data collected by the randomized controlled trial of MRgFUS thalamotomy38 because there is no mapping algorithm for converting QUEST scores into utility values.
Conclusions
In the subset of people with moderate to severe, medication-refractory essential tremor ineligible for invasive neurosurgery, MRgFUS neurosurgery is a cost-effective treatment option compared with no surgery. Further, as a noninvasive surgical procedure, MRgFUS neurosurgery is a good treatment option for people unable to undergo surgery owing to comorbidities and for those who find the risks of invasive surgery unacceptable. In the subset of people with moderate to severe, medication-refractory essential tremor who are eligible for invasive neurosurgery, MRgFUS neurosurgery is more effective but also more costly than radiofrequency thalamotomy. Compared with the current standard of care, deep brain stimulation, MRgFUS neurosurgery is less expensive but less effective.
BUDGET IMPACT ANALYSIS
Research Question
What is the 5-year budget impact of publicly funding MRgFUS neurosurgery for the treatment of moderate to severe, medication-refractory essential tremor, within the context of the Ontario Ministry of Health and Long-Term Care?
Methods
Target Population
The target population is people with moderate to severe, medication-refractory essential tremor. We estimated the size of the target population based on the published literature3,16,110,111 and clinical expert inputs (in-person communication, May 25, 2017; telephone communication, May 30 and 31, 2017). The condition is estimated to affect 4.6% of people 65 years of age and older3; in Ontario, this is about 109,311 people. Of these, an average of 6% seek medical care, and 30% have moderate to severe disease.110 According to the literature16,111 and clinical expert opinion (in-person communication, May 25, 2017; telephone communication, May 30 and 31, 2017), of those with no contraindications to drug therapy (99%), 50% will experience a suboptimal response to drugs and/or be unable to tolerate the adverse effects. According to experts, about 85% of these people, around 828 individuals, are eligible for surgery (in-person communication, May 25, 2017; telephone communication, May 30 and 31, 2017). According to the literature16,111 and clinical experts (in-person communication, May 25, 2017; telephone communication, May 30 and 31, 2017), for those with contraindications to drugs (1%), it is estimated that 50%, about 10 individuals, are eligible for surgery (less than in the case of those with no contraindications to drugs, since this subpopulation is considered likely to be more fragile and to have comorbidities negatively affecting their surgical risk). Thus, we estimate a total of 838 people with moderate to severe, medication-refractory essential tremor to be eligible for surgery. Table 37 and Figure 10 provide further details on these calculations.
Table 37:
Epidemiological Inputs Used to Derive Target Population
| Parameter | Value | Source |
|---|---|---|
| Ontario population aged ≥ 65 years (in 2018) | 2,376,327 | Ontario Ministry of Finance112 |
| Prevalence of essential tremor among those aged ≥ 65 years | 4.60% | Louis and Ferreira, 20103 |
| % seeking medical care | 6% (0.5–11%) | Louis et al, 1998110 |
| % with moderate to severe disease | 30% | Expert opinion |
| % with no contraindications to drug therapy | 99% | Expert opinion |
| % medication refractory or intolerant | 50% | Koller et al, 1994111; Zesiewicz et al, 201116; expert opinion |
| % eligible for surgery (without drug contraindications) | 85% | Expert opinion; assumption |
| % eligible for surgery (with drug contraindications) | 50% | Expert opinion |
Figure 10: Target Population Estimation.
Our estimate is consistent with the number of referrals for MRgFUS neurosurgery received by clinical experts (about 300 referrals from neurologists) (telephone communication, May 18, 2017; in-person communication, May 25, 2017).
Current Treatment Pattern
Although many people with essential tremor may be eligible for and interested in pursuing surgery, the number of those who actually receive surgery may be much lower owing to factors such as limited resources, referral patterns, limited awareness of treatment options, and aversion to surgery. We obtained the number of thalamotomy and deep brain stimulation procedures performed in Ontario over the past 5 years from the IntelliHealth administrative database (IntelliHealth Ontario, Inpatient Discharges 2012–2016, May 18, 2017) (Table 38). We identified the procedures using the Canadian Classification of Health Intervention codes (1AE) with a diagnosis of essential tremor (ICD-10 code G250). From 2012 to 2016, an average of 14 patients underwent a neurosurgical procedure for essential tremor each year (deep brain stimulation: 60%; Gamma Knife thalamotomy: 30%; radiofrequency thalamotomy: 10%).
Table 38:
Number of Deep Brain Stimulation and Thalamotomy Procedures Performed in Ontario, 2012 to 2016
| Procedure | CCI Code | Number of Procedures Performed | % | |||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | Average | |||
| Deep brain stimulation | 1AE53SEJA; 1AE53SZJA | 14 | 5 | 12 | 5 | 6 | 8.4 | 60% |
| Gamma Knife thalamotomy | 1AE27JX | 0 | 2 | 8 | 6 | 5 | 4.2 | 30% |
| Radiofrequency thalamotomy | 1AE59SEAW; 1AE59SZAW | 1 | 2 | 1 | 3 | 0 | 1.4 | 10% |
| Total | 15 | 9 | 21 | 14 | 11 | 14 | 100% | |
Abbreviations: CCI, Canadian Classification of Health Intervention.
Source: IntelliHealth Ontario, Inpatient Discharges 2012–2016, May 18, 2017.
From clinical experts, we also know that two centres in Ontario currently treat about two patients per month each with MRgFUS neurosurgery, resulting in 48 MRgFUS neurosurgery procedures per year (telephone communication, May 18, 2017; in-person communication, May 30, 2017). Since this procedure is not currently publicly funded, these patients are not reflected in the administrative databases.
Therefore, the total number of neurosurgical procedures currently performed for the treatment of essential tremor in Ontario per year is 62 (48 with MRgFUS neurosurgery; 14 with deep brain stimulation, Gamma Knife thalamotomy, or radiofrequency thalamotomy).
Uptake of MRgFUS Neurosurgery
The budget impact of publicly funding MRgFUS neurosurgery depends on how it is implemented. We assumed that MRgFUS neurosurgery may be implemented in several possible scenarios (Table 39). In the reference case, we assumed that the Ministry of Health and Long-Term Care would fund MRgFUS neurosurgery at the current level of 48 cases per year and, for simplicity, that MRgFUS neurosurgery would not replace other surgical procedures. In scenario analyses, we assumed that the Ministry of Health and Long-Term Care would fund a certain number of MRgFUS neurosurgery procedures each year, ranging from 60 to 144 cases. This increase in cases results from the fact that MRgFUS neurosurgery is a noninvasive procedure, and we therefore expected that demand for this procedure would be high were it to be publicly funded. With greater public awareness of MRgFUS neurosurgery, neurologists may refer more patients for the procedure. In the last scenario, we assumed that MRgFUS neurosurgery would replace both deep brain stimulation and radiofrequency thalamotomy once it is publicly funded. We did not include Gamma Knife thalamotomy since we did not include it as a comparator in the primary economic evaluation.
Table 39:
Number of Neurosurgical Procedures Performed in the Current and New Scenarios
| Scenario | Number of Publicly Funded MRgFUS Neurosurgery Procedures per Year | Treatment Replaced by MRgFUS Neurosurgery (n) |
|---|---|---|
| Current Scenario | ||
| Reference Case | 0 | – |
| New Scenario | ||
| Reference Case | 48 | No surgery |
| Scenario 1 | 60 | No surgery |
| Scenario 2 | 72 | No surgery |
| Scenario 3 | 96 | No surgery |
| Scenario 4 | 144 | No surgery |
| Scenario 5 | 62 | No surgery (52.2) Deep brain stimulation (8.4) Radiofrequency thalamotomy (1.4) |
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
Resources and Costs
We included both treatment- and disease-related costs in the budget impact analysis. We obtained the relevant costs by running the cost-utility analysis over 5 years without discounting. Table 40 presents the annual per-patient costs in years 1 to 5 for each treatment strategy.
Table 40:
Annual Per-Patient Cost for Each Treatment Strategy
| Year | No Surgery, $ | MRgFUS Neurosurgery, $ | Radiofrequency Thalamotomy, $ | Deep Brain Stimulation, $ |
|---|---|---|---|---|
| Year 1 | 437 | 20,964 | 12,844 | 40,582 |
| Year 2 | 427 | 731 | 599 | 1,868 |
| Year 3 | 417 | 700 | 576 | 1,824 |
| Year 4 | 406 | 668 | 554 | 1,777 |
| Year 5 | 394 | 637 | 531 | 11,769 |
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
Note: All costs presented in 2017 Canadian dollars.
Analysis
We estimated the net budget impact of MRgFUS neurosurgery as the cost difference between two scenarios: the current scenario (no public funding) and the new scenario (with public funding). We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. In sensitivity analyses, we explored the variability in budget impact resulting from changing key input parameters and model assumptions (see Table 39).
Results
Table 41 presents the results of the reference case analysis. In the current scenario, in which MRgFUS neurosurgery is not publicly funded, the total cost per year ranges from $20,957 in year 1 to $99,941 in year 5 for people receiving no surgery. In the new scenario, in which MRgFUS neurosurgery is publicly funded, the total cost per year ranges from $1,006,266 in year 1 to $1,137,586 in year 5 for people receiving MRgFUS neurosurgery. Table 42 presents the resulting 5-year net budget impact. When considering the cost of the primary surgery only, the net budget impact is $949,739 per year (i.e., the cost of funding 48 MRgFUS neurosurgery procedures per year), for a total of $4,748,697 over 5 years.
Table 41:
Reference Case Analysis Results
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | 5-Year Total | |
|---|---|---|---|---|---|---|
| Current Scenario: No Surgerya | ||||||
| Total cost, $ | 20,957 | 41,466 | 61,492 | 80,997 | 99,941 | 304,852 |
| Cost of primary surgery only, $ | – | – | – | – | – | – |
| New Scenario: MRgFUS Neurosurgerya | ||||||
| Total cost, $ | 1,006,266 | 1,041,364 | 1,074,947 | 1,107,021 | 1,137,586 | 5,367,185 |
| Cost of primary surgery only, $ | 949,739 | 949,739 | 949,739 | 949,739 | 949,739 | 4,748,697 |
| Net Budget Impacta | ||||||
| Total cost, $ | 985,309 | 999,898 | 1,013,456 | 1,026,025 | 1,037,645 | 5,062,332 |
| Cost of primary surgery only, $ | 949,739 | 949,739 | 949,739 | 949,739 | 949,739 | 4,748,697 |
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
Note: All costs presented in 2017 Canadian dollars.
48 patients per year.
Table 42:
Five-Year Net Budget Impact of Publicly Funding MRgFUS
| Year | Net Budget Impact, $ |
|---|---|
| Year 1 | 985,309 |
| Year 2 | 999,898 |
| Year 3 | 1,013,456 |
| Year 4 | 1,026,025 |
| Year 5 | 1,037,645 |
| Total | 5,062,332 |
Abbreviation: MRgFUS, magnetic resonance-guided focused ultrasound.
Table 43 presents the results of the scenario analyses. In scenarios 1 to 4, in which we assumed MRgFUS neurosurgery would only replace no surgery, the net budget impact increased proportionally as the number of MRgFUS procedures funded increased. In scenario 5, in which we assumed MRgFUS neurosurgery would replace no surgery, deep brain stimulation, and radiofrequency thalamotomy, the net budget impact was reduced to $831,882 in year 1 and $763,063 in year 5.
Table 43:
Scenario Analysis Results—Net Budget Impact
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | 5-Year Total | |
|---|---|---|---|---|---|---|
| Scenario 1: 60 patients per year (MRgFUS neurosurgery replacing no surgery) | ||||||
| Total cost, $ | 1,231,637 | 1,249,872 | 1,266,819 | 1,282,531 | 1,297,057 | 6,327,915 |
| Cost of primary surgery only, $ 1,187,174 | 1,187,174 | 1,187,174 | 1,187,174 | 1,187,174 | 1,187,174 | 5,935,871 |
| Scenario 2: 72 patients per year (MRgFUS neurosurgery replacing no surgery) | ||||||
| Total cost, $ | 1,477,964 | 1,499,846 | 1,520,183 | 1,539,037 | 1,556,468 | 7,593,498 |
| Cost of primary surgery only, $ 1,424,609 | 1,424,609 | 1,424,609 | 1,424,609 | 1,424,609 | 1,424,609 | 7,123,045 |
| Scenario 3: 96 patients per year (MRgFUS neurosurgery replacing no surgery) | ||||||
| Total cost, $ | 1,970,618 | 1,999,795 | 2,026,911 | 2,052,049 | 2,075,290 | 10,124,665 |
| Cost of primary surgery only, $ 1,899,479 | 1,899,479 | 1,899,479 | 1,899,479 | 1,899,479 | 1,899,479 | 9,497,394 |
| Scenario 4: 144 patients per year (MRgFUS neurosurgery replacing no surgery) | ||||||
| Total cost, $ | 2,955,928 | 2,999,693 | 3,040,367 | 3,078,074 | 3,112,936 | 15,186,997 |
| Cost of primary surgery only, $ 2,849,218 | 2,849,218 | 2,849,218 | 2,849,218 | 2,849,218 | 2,849,218 | 14,246,091 |
| Scenario 5: 62 patients per year (MRgFUS neurosurgery replacing no surgery, DBS, and RF) | ||||||
| Total cost, $ | 831,882 | 837,104 | 841,387 | 844,805 | 763,063 | 4,118,240 |
| Cost of primary surgery only, $ 813,194 | 813,194 | 813,194 | 813,194 | 813,194 | 813,194 | 4,065,971 |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; RF, radiofrequency.
Note: All costs presented in 2017 Canadian dollars.
Discussion
Our analysis shows that publicly funding MRgFUS neurosurgery at the current level of 48 cases per year would lead to a total 5-year budget impact of $5 million, about $1 million each year. If considering only the cost of the primary surgery, the budget impact would be $0.95 million each year.
Our budget impact analysis has several strengths. We estimated both the potential demand (number of patients potentially eligible for surgery) and supply (current volume of surgeries performed) for essential tremor neurosurgeries. We estimated the number of patients potentially eligible for surgery based on published epidemiological data and clinical expert opinion, and we also obtained the actual volume of neurosurgeries performed in Ontario from local administrative databases. These data suggest that, as with other surgeries, there may be a gap between supply and demand for essential tremor neurosurgeries. Further, although deep brain stimulation and radiofrequency thalamotomy are currently publicly funded, very few people actually undergo either surgery. This may indicate an unmet need for noninvasive surgical procedures for the treatment essential tremor.
A limitation of our budget impact analysis is that we estimated the peri-procedural costs of deep brain stimulation and radiofrequency thalamotomy using the Ontario Case Costing database. This database has data from only a few facilities in the province; thus, the costs may not be representative of all facilities performing these procedures in Ontario.
Conclusions
We estimate the budget impact of publicly funding MRgFUS neurosurgery for the treatment of moderate to severe, medication-refractory essential tremor at the current level to be about $1 million each year over the next 5 years.
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying values, needs, impacts, and preferences of those with lived experience of essential tremor. The treatment focus was magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery versus standard care.
Background
Public and patient engagement explores the lived experience of a person with a health condition. It includes the impact that the condition and its treatment have on the patient and the patient's family and other caregivers, and on the patient's personal environment. Public and patient engagement increases awareness and builds appreciation for the needs, priorities, and preferences of people at the centre of a treatment program.
Lived experience is a unique source of evidence about the personal impact of a health condition and how that condition is managed. It includes what it is like to navigate the health care system and how technologies may or may not make a difference in people's lives. Information shared from lived experience can also identify gaps or limitations in published research (e.g., sometimes typical outcome measures do not reflect what is important to those with lived experience).113–115 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of technologies and treatments.
Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored in the published literature, we contact and speak directly with people who live with a given health condition, including those who may have experience with the intervention we are exploring.
Essential tremor has a substantial impact on the quality of life of people with the condition and their families. To better understand its impact on quality of life, we spoke with people with essential tremor and their families.
Several people we heard from had received treatment for their essential tremor through MRgFUS neurosurgery. Understanding and appreciating the day-to-day experience of living with essential tremor helps us contextualize the potential value of this treatment from the perspective of those with lived experience.
Methods
Engagement Plan
Engagement as a concept captures a range of efforts used to involve the public and patients in various domains and stages of decision-making for a health technology assessment.116 Rowe and Frewer outline three types of engagement: communication, consultation, and participation.117 Communication constitutes a one-way transfer of information from the sponsor to the individual, whereas participation involves the sponsor and individual collaborating through real-time dialogue. Consultation, however, refers to the sponsor seeking out information about the experience of the public, patients, and caregivers affected by the technology or intervention in question.118
The engagement plan for this health technology assessment focused on consultation. Within this typology, our design focused on interviews to elicit the lived experience of people with essential tremor and their families, including their experiences with MRgFUS neurosurgery.
We selected a qualitative interview as an appropriate methodology because this approach allows us to explore the central themes in participants' lived experience. Our main task in interviewing is to understand what participants tell us.119 Interviews are particularly useful for getting the story behind a participant's experiences, which was the objective of this portion of the health technology assessment. The sensitive nature of exploring quality of life for this topic is another factor supporting the use of interviews for this project.
Recruitment of Participants
We used an approach called purposive sampling120–122 to actively recruit people with lived experience. We contacted people with essential tremor and their families through a variety of patient support organizations, health clinics, and foundations.
Inclusion Criteria
We sought to speak with people with essential tremor and their families. People with essential tremor were not required to have had direct experience with MRgFUS neurosurgery.
We sought broad geographic, cultural, and socioeconomic representations to elicit possible equity issues in accessing various treatment options for essential tremor.
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
We conducted interviews with 14 people, including people with essential tremor and family members. While a small number of people with essential tremor had direct experience with MRgFUS neurosurgery, all participants were familiar with the treatment option and were able to speak to the values and preferences that would inform their decision-making with regard to pursuing this treatment option. Most of our participants live in the Greater Toronto Area, and a few live outside this area.
Interview Approach
At the beginning of the interview, we explained the mandate of Health Quality Ontario, the role of the Ontario Health Technology Advisory Committee, and the purpose of this health technology assessment. We outlined the risks of participation, and, through a letter of information, we explained how participants' personal health information would be protected (Appendix 9). We obtained verbal consent from participants before starting each interview (Appendix 10). We recorded and transcribed the interviews.
The interview consisted of a series of open-ended questions and lasted 20 to 40 minutes. We based our questions on a list of questions developed by Health Technology Assessment International's Interest Group on Patient and Citizen Involvement in HTA to elicit lived experiences specific to the impact of a health technology or intervention.12
Interview questions focused on the impact of essential tremor on the quality of life of those living with the condition and their families, experiences with treatment options for essential tremor, and the perceived benefits and people's expectations of MRgFUS neurosurgery. Appendix 11 provides our interview guide.
Data Extraction and Analysis
To capture themes and compare elements of lived experience among participants, we selected a modified version of a grounded theory methodology to analyze interview transcripts. The inductive nature of grounded theory follows an iterative process of eliciting, documenting, and analyzing responses while simultaneously collecting and analyzing data using a constant comparative approach.13,14 We coded transcripts and compared themes using NVivo (QSR International, Doncaster, Victoria, Australia). This qualitative software program helped us identify and interpret meaningful patterns in the interview data.
Results
Lived Experience of Essential Tremor
People with essential tremor with whom we spoke were diagnosed at various ages, from the teen years into people's 50s and 60s. These participants described a range of disease severity with a corresponding impact on quality of life. The degree to which people with essential tremor and their families made adjustments to their activities of daily living increased with the progression of the disease and the spread of the tremor. Typically, people with the condition reported first noticing tremor in their dominant hand. In a number of people with whom we spoke, the tremor then progressed to their other hand, head, lips, or feet.
All participants with the condition had been living with their tremor for a number of years and had developed various methods of compensating for the impact of the disease. One of the most common impacts mentioned was on people's ability to use their hands to perform tasks involving fine motor skills, such as eating and holding containers without spilling the contents:
“I'm able to function in the day-to-day environment. I'm able to function at home. I can cut grass. I do things at home. I bring my dinner plate over. I just use two hands. Right now, I had a cup of hot water. I don't fill it to the top. I fill it half-way up. This way, I know that I won't spill it.”
“In the meantime, I can never be a waitress, and I can never be a brain surgeon, and I can't carry hot coffee or soup, and I can't carry much without spilling, but I've worked with it. So that's my story.”
“It was okay for a while because, you know what? You learn. When you have an affliction like this all your life, you learn to compensate for it.”
People with more severe tremor reported challenges with balance and an increased negative impact on their daily life. These people reported decreased participation in physical and social activities as a result of their tremor:
“I'm noticing I'm a little more…I'm all right most of the time, but sometimes when I move or something, I'm going somewhere and, like, even a familiar location like my home, all of a sudden, my balance just seems to get off for a second.”
“We have a brand-new grandson…and I can't feed him because my hands are shaking so much that I can't make…I want to get the food in the mouth without it going on his face.”
In addition, several people who had developed essential tremor at a younger age found that it had affected their employment. Depending on the severity of the tremor, people reported that their employment options were restricted or that they had required modifications to perform their duties:
“I wanted to go into nursing at one point in time, and I was told absolutely not.”
“My hands are my living.”
“If I wasn't careful in how I approached things or covered things up, I would probably have lost my job.”
Social situations were reported as particularly burdensome for those with essential tremor. People reported feeling embarrassed or frustrated in situations in which others would notice or ask about the tremor. A number of people reported feeling discriminated against, either in social situations or in the workplace:
“I remember what all people with tension tremor have to learn, which is either you better explain it clearly to everybody early, or you better hide it: one or the other. Because often they'll assume the wrong thing, which is an unfortunate form of bigotry, but it's reality, so you deal with it.”
“There was a problem with having to go use a computer and have coworkers make comments.”
“When you're having a fine dining [experience] or an important executive meeting, other people would notice it. And they would…you could see the look in their eyes that [said] they would wonder, of course, ‘Has this guy got the heebie-jeebies from alcoholism?” or just, ‘What happened?”'
People reported experiencing a variety of emotions related to their disease. Most commonly, people reported frustration and fear. Frustration was often a result of the consistent impact of tremor on people's daily activities and a sense of loss of control. A number of people also spoke of worrying about and fearing disease progression and what the future might hold:
“Yeah, the frustration. Oh, I've picked stuff up, and I start throwing things around the kitchen.”
“Sometimes while I'm thinking about the fact my hands are shaking…well, it angers me a little, or I'm disappointed or something.”
“I guess I don't like to look down the road.”
“It takes me longer than everybody else, but you know, you have to, you have to make the decision that this is what life is, and actually, the way I look at it is, if God wanted me to have a handicap, this is the one to have.”
Treatments for Essential Tremor
People with essential tremor typically reported taking medications to help control their tremor, although most felt these medications were only moderately effective. A number of people told us that they could not take medications for tremor owing to contraindications, and others reported unacceptable side effects. In addition, people told us that stressful or emotional situations could cause an exacerbation of tremor that could not be controlled by medication:
“[When I was] about 28 years old, the doctor put me on beta blockers, [but when] I was about 55, 60…I started to get side effects from beta blockers, and that's when I started looking into [MRgFUS].”
“But I didn't really feel that it [medication] did anything. I didn't bother taking [medication] anymore. So I haven't really…I've not had anything since about 2000.”
“Nowadays, it's even worse because [the doctor] is experimenting with different combinations to try to make the tremor stop, and it's getting a little worse actually.”
Some people reported self-medicating with alcohol to try to dull their tremor, and many of those who did not use alcohol in this way reported knowing or hearing of others who did:
“But if I had say, like, [with] three beers, it's fairly controllable. Like, it's still there, but…not too bad. I can sort of eat with a fork, that kind of thing. Usually if somebody invites me over for supper, I usually have two beers before I go or something like that, and they usually give me one there, and then I can sort of get through it, if you know what I mean.”
“The only good thing about it, that I saw online, that one of the possible [ways to] help is having a glass of vodka, and it does work. In other words, if we go out and I'm not driving, I'll have a vodka and tonic, and you know, that's two, two and a half hours I'm fine. I don't shake, no nothing.”
Disease progression and the lack of effective treatment with medication frustrated many people with essential tremor with whom we spoke and led them to seek out alternative treatments, such as surgery. To varying degrees, everyone we interviewed was familiar with surgical techniques to treat essential tremor, including MRgFUS neurosurgery. People often discussed weighing various factors, such as their degree of disability and their confidence in a particular procedure, when deciding whether to pursue a surgical treatment option. Most people expressed hesitation with regard to invasive surgical procedures, fearing the risks involved:
“I did go to one neurologist…about 10 years ago, and he suggested the surgery, deep brain stimulation…. I don't think I would do that. I can sort of get by. I think that would be a little…kind of pushing it.”
“So I chose not to fool around with that. And with the surgery, I just didn't want to have holes dug in my head.”
“I'm afraid of that kind of surgery. I don't want anybody [going] into my head. I don't want the possible, even if it's a little possibility, [negative] consequence of it…I never liked pain, right?…I don't think I would ever have it.”
MRgFUS Neurosurgery
Almost all people with essential tremor interviewed were familiar with the procedure of MRgFUS neurosurgery, even if they had not undergone the procedure. Recent media coverage of the procedure, along with information gained from social and patient groups, has raised the profile of this surgical option, and many people reported seeking out information about it. People reported speaking extensively with their health care providers about the procedure and conducting their own research online. The noninvasive aspect of the surgery is viewed positively compared with invasive surgical procedures:
“Surgically speaking, there was [deep brain stimulation], but [my tremor] was not serious enough at that time to warrant an operation like that. And then along comes MRgFUS, and, like I said, the rest was history. It had so many advantages, but the big one is, the huge one is the no cutting.”
“I wanted to proceed ASAP [based] on the recommendation of my neurologist and also with some readings that I did.”
For a number of people with essential tremor, making the decision to pursue surgery was difficult, despite receiving positive information about MRgFUS neurosurgery. Individual factors such as health, age, and the feelings of family members influenced people's decision-making, and some were uncertain whether they would ever undergo a surgical procedure. Given the length of wait times for surgical procedures, others expressed interest in being placed on the wait list and taking a wait-and-see approach:
“I thought if it got real bad, my daughters and my wife might say something to me, but I'm 86 and in fairly good health, and I move, and so I'm not so sure that I would consider something like that at that age unless it made me where I couldn't do anything.”
“In 20 years, if I'm still around, I'm going to have a heck of a time [with] everything, I'm guessing, from eating, and I would never be able to drink tea from a cup. But so I thought, well, why wait until that point before I start thinking about what I can maybe do to alleviate some of the symptoms? And that's why I talked to [a neurologist] in the first place. And I thought I'd like to do it now and have the next 20 years not get any worse.”
“You know, if the waiting list is a couple of years, then I can talk to my [doctor] and say, ‘Put me on that list,’ now.”
People who had undergone MRgFUS neurosurgery reported a generally positive experience. People felt that they had been well prepared for what the procedure involved and were aware of the results they could expect. All of these people praised the professionalism and skill of their surgical teams:
“I knew what was going to come out the other end. There couldn't be anything but an improvement in this, [what] I'll call it my 6-inch tremor.”
“And I met the guy who controls the machine for the intensity of the ultrasound. [He was a] very nice fellow, and he explained steps as we [went].”
Those who underwent MRgFUS neurosurgery did express frustration at the length of the wait time to receive the procedure. Many had lived with essential tremor for a number of years and were eager to resolve the issue:
“At the time, I felt some impatience because I had lived with it for so long, from 40 to 73 or so. So I had lived with it so long. And not knowing when death is going to come from some other reason, I was anxious to…I was very keen to progress forward in any way that I could help happen.”
“I think it could be very tough for people [who] have to wait two, three, four, five years for this. And I can only see the waiting lists increase and increase, because the team of people and the training they have to have to do everything so, so precisely.”
All people who had undergone the procedure reported positive results. Despite knowing what to expect, people still reported surprise at the immediate positive results. Being able to use their dominant hand again without tremor made it easier to perform daily tasks. Those who underwent the procedure considered themselves very lucky and reported being supportive of others undergoing this procedure:
“Having the treatment was, I thought, much of a miracle. Just having the ability to use one hand…that was a miraculous happening.”
“They pulled me out as normal, slid me out [of the MRI scanner] on the [sled]. And they said, “Okay, let's do the scroll [the spiral-drawing task] first.” And, I kid you not, as soon as I did that, you could have heard me…more than a block away with the word ‘w-o-w.’ ‘Wow'…because it was such an improvement. It was everything at that moment.”
One person who had undergone MRgFUS neurosurgery reported experiencing tremor recurrence over time, but to a lesser degree than before the procedure. Others mentioned slight frustration that only one hand could be treated, not both. However, no other side effects were reported by those who had undergone the procedure:
“A million times better than it was, but it could still be better; there's been a bit of a reverse.”
Discussion
Although only a small number of people with essential tremor have undergone MRgFUS neurosurgery in Ontario, we were able to speak with a number of these people, as well as others with essential tremor who have considered a variety of surgical treatments.
The majority of people with essential tremor with whom we spoke had mild to moderate symptoms, which they reported could have a large impact on their ability to perform daily tasks but did not require the use of additional supports or caregiver assistance. However, we did not interview any people with severe essential tremor who may require such supports or assistance. All people with the condition whom we interviewed had lived with their tremor for a number of years and could speak to the progression of the disease, symptom management, different treatment options, and potential surgical options.
Those interviewed were supportive of MRgFUS neurosurgery and its positive treatment effect. However, despite this support, people acknowledged that, although it is a noninvasive procedure, it is still a surgical procedure, and the benefits may not outweigh the perceived risks. People reported considering many personal factors and other variables when deciding whether to pursue a surgical procedure, including MRgFUS neurosurgery.
People who had undergone MRgFUS neurosurgery spoke positively of their experience of both the procedure and the results they obtained. The most beneficial effect reported was a reduction or elimination of tremor in the dominant hand, which people reported as having a substantial beneficial effect on their ability to perform daily tasks and on their quality of life. However, some frustration was expressed at the long wait times for the procedure and the treatment effect being limited to the dominant hand.
Conclusions
People with essential tremor reported positive experiences with the procedure and results experienced with MRgFUS neurosurgery. The reduction or elimination of tremor in the dominant hand increased people's ability to perform daily tasks and improved their quality of life.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
MRgFUS neurosurgery is an effective treatment for moderate to severe, medication-refractory essential tremor and has an acceptable safety profile. Important findings are as follows:
In noncomparative studies, MRgFUS neurosurgery has been found to significantly improve tremor severity and quality of life and to significantly reduce functional disability in daily activities (low certainty in the evidence); MRgFUS neurosurgery has been found to be significantly more effective than a sham procedure (high certainty in the evidence)
There are no significant differences in tremor severity or quality-of-life improvement or in functional disability reduction conferred by MRgFUS neurosurgery compared with deep brain stimulation (very low certainty in the evidence)
There is no significant difference in tremor severity improvement conferred by MRgFUS neurosurgery compared with radiofrequency thalamotomy (very low certainty in the evidence)
MRgFUS neurosurgery is associated with few complications and adverse effects, the vast majority of which are transient and resolve either entirely or to a point at which there is minimal interference with people's lives
Important findings of our economic analysis are as follows:
As a noninvasive surgical procedure, MRgFUS neurosurgery is a good treatment option for people unable to undergo surgery owing to comorbidities and is also one of several reasonable surgical options
In the subset of people with moderate to severe, medication-refractory essential tremor ineligible for invasive neurosurgery, the ICER of MRgFUS neurosurgery compared with no surgery is $43,075 per QALY gained
The province would need to spend about $1 million a year over the next 5 years to publicly fund MRgFUS neurosurgery for the treatment of moderate to severe, medication-refractory essential tremor at the current level of 48 cases per year
Our engagement with people with lived experience of essential tremor found the following:
As tremor severity increased, people reported a decrease in their ability to perform activities of daily living and in their quality of life
Many reported being unable to take medication owing to contraindications or unacceptable side effects
Most expressed hesitation regarding invasive neurosurgical procedures
The noninvasive aspect of MRgFUS neurosurgery was viewed favourably
People who had undergone MRgFUS neurosurgery reported positive experiences, both with the procedure itself and with the results
Acknowledgments
The medical editor was Kara Stahl; others involved in the production of this report were Paul Kolodziej, Kellee Kaulback, Ana Laing, Claude Soulodre, Sarah McDowell, Andrée Mitchell, Vivian Ng, Anil Thota, Nancy Sikich, and Irfan Dhalla.
We are grateful to Alfonso Fasano,a Suranga Fernando,b Kullervo Hynynen,c Anthony E. Lang,d Nir Lipsman,e Andres Lozano,f Michael Schwartz,g and Catherine Wangh for sharing their clinical and technical expertise during the development of this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of the consulted experts.
a Staff Neurologist and Co-director, Surgical Program, Morton and Gloria Shulman Movement Disorders Centre and Edmond J. Safra Program in Parkinson's Disease, Toronto Western Hospital–University Health Network; Associate Professor, Department of Medicine, Division of Neurology, University of Toronto Clinician Investigator, Krembil Research Institute
b Finance Director and Senior Finance Lead, Diagnostic Partnerships, Pharmacy, IPAC; Director, JDMI Research Office, University Health Network
c Director and Senior Scientist, Physical Sciences Platform, Sunnybrook Research Institute; Director, Centre for Research in Image-Guided Therapeutics, Canada Research Chair in Imaging Systems and Image-Guided Therapy (Tier 1); Professor, Department of Medical Biophysics and Institute of Biomaterial and Biomedical Engineering, University of Toronto
d Director, Morton and Gloria Shulman Movement Disorders Centre and Edmond J. Safra Program in Parkinson's Disease, Toronto Western Hospital–University Health Network, University of Toronto
e Neurosurgeon, Sunnybrook Health Sciences Centre; Scientist, Sunnybrook Research Institute; Assistant Professor, Department of Surgery, University of Toronto; Associate Member, Institute of Medical Science, University of Toronto
f Dan Family Chair of Neurosurgery, University of Toronto
g Professor of Surgery (Neurosurgery), University of Toronto
h Vice-President, Clinical Operations and Diagnostic Partnerships, University Health Network; Joint Department of Medical Imaging, University Health Network, Sinai Health System, Women's College Hospital; Laboratory Medicine Program, University Health Network; Department of Microbiology, Sinai Health System, University Health Network; Assistant Professor, Department of Medical Imaging, Faculty of Medicine, University of Toronto
ABBREVIATIONS
- CI
Confidence interval
- CRST
Clinical Rating Scale for Tremors (a version of the Fahn–Tolosa–Marín Clinical Rating Scale for Tremor; CRST is used throughout to refer to both versions of the scale)
- EQ-5D
European Quality of Life—5 Dimensions
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- FTM
Fahn–Tolosa–Marín Clinical Rating Scale for Tremor (note that CRST is used throughout to refer to both the CRST and FTM scales)
- ICER
Incremental cost-effectiveness ratio
- MRgFUS
Magnetic-resonance guided focused ultrasound
- MRI
Magnetic resonance imaging
- NICE
National Institute for Health and Care Excellence
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- QALY
Quality-adjusted life years
- QUEST
Quality of Life in Essential Tremor questionnaire
- SD
Standard deviation
GLOSSARY
- Blinded study
A study in which researchers, participants, or both are unaware of the treatment being administered.
- Cost-effective
Good value for money; the overall benefit of the technique or intervention justifies the cost.
- Cost–utility analysis
A type of analysis that estimates the value for money of an intervention by weighing the cost of the intervention against the improvements in length of life and quality of life. The result is expressed as a dollar amount per quality-adjusted life-year (QALY).
- Deterministic sensitivity analysis
A type of analysis that changes the variables to determine whether the final answer will change. The analysis is done by first setting values for each factor, and then substituting other possible values for one (in a one-way sensitivity analysis) or more (in a multi-way sensitivity analysis) factors to test how these changes affect the result.
- Discounting
A method that considers that costs and health benefits are worth more today than in the future.
- Health state utility
The strength of patient preferences for a given state of health using a scale on which 1 represents full health and 0 represents dead. (Negative scores, meaning worse than dead, are possible.) Health state utility is an important data input in cost–utility models, but is also among the most uncertain inputs.
- Incremental cost
The extra cost associated with using one test or treatment instead of another.
- Incremental cost-effectiveness ratio (ICER)
Determines a unit of benefit for an intervention by dividing the incremental cost by the effectiveness. The incremental cost is the difference between the cost of the treatment under study and an alternative treatment. The effectiveness is usually measured as additional years of life or as quality-adjusted life years (QALYs).
- Markov model
A type of modelling that measures the health state of a patient over the course of treatment. A patient may stay in one health state or move from one health state to another, depending on the effect of the treatment and the progression of the disease.
- Monte Carlo simulation
Determines the uncertainty in an economic model by running many trials of the model. In each trial, random numbers are assigned wherever values are uncertain to see how the model result changes.
- Open-label study
A study in which both researchers and participants are aware of the treatment being administered.
- Probabilistic sensitivity analysis (PSA)
A type of analysis where the value of one or more unknown factors is estimated through the use of a technique that determines the most likely value or range of values for that factor. For instance, the Monte Carlo simulation will run a scenario many times using randomly assigned numbers where the value of a particular factor is unknown. The simulation indicates which outcomes are most common, and therefore most probable.
- Quality-adjusted life-year (QALY)
A measurement that takes into account both the number of years gained by a patient from a procedure and the quality of those extra years (e.g., ability to function, freedom from pain). The QALY is commonly used as an outcome measure in cost–utility analyses.
- Randomized controlled trial
A type of study in which subjects are assigned randomly into different groups, with one group receiving the treatment under study and the other group(s) receiving a different treatment or a placebo (no treatment) in order to determine the effectiveness of one approach compared with the other(s).
- Reference case
A population or value used as a basis of comparison for the population under study. Where the population under study is said to deviate from a standard, this is the standard it deviates from.
- Sensitivity
The ability of a test to accurately identify persons with the condition tested for (how well it returns positive results in persons who have the condition).
- Sensitivity analysis
Every evaluation contains some degree of uncertainty. Study results can vary depending on the values taken by key parameters. Sensitivity analysis is a method that allows estimates for each parameter to be varied to show the impact on study results. There are various types of sensitivity analyses. Examples include deterministic, probabilistic, and scenario.
- Statistical significance
The outcome of an analysis is statistically significant if the assumption that there is no effect (the null hypothesis) is sufficiently unlikely to be true. Typically, the outcome is considered statistically significant if there is less than a 5% chance that the outcome would have occurred if the null hypothesis were true.
- Systematic review
A process to answer a research question by methodically identifying and assessing all available studies that evaluate the specified research question. The systematic review process is designed to be transparent and objective and is aimed at reducing bias in determining the answers to research questions.
- Time horizon
Costs and outcomes are examined within a chosen time frame. In an economic evaluation, this time frame is referred to as the time horizon.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: April 11, 2017
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <March 2017>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to April 4, 2017>, EBM Reviews -Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2017 Week 15>, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy
-
1
Essential Tremor/ (6490)
-
2
((essential or familial or hereditary or heredofamilial or heredo-familial or juvenile or presenile or senile or benign or idiopathic) adj2 tremor*).ti,ab,kf. (8213)
-
3
or/1–2 (9340)
-
4
exp Thalamus/su [Surgery] (2626)
-
5
((thalam* and (surg* or neurosurg* or ablat*)) or thalamotom*).ti,ab,kf. (9462)
-
6
Magnetic Resonance Imaging/ (693063)
-
7
Magnetic Resonance Imaging, Interventional/ (3110)
-
8
Ultrasonography, Interventional/ (20453)
-
9
Ultrasonic Therapy/ (17555)
-
10
High-Intensity Focused Ultrasound Ablation/ (3969)
-
11
((focus?ed ultrasound*1 or focus?ed ultrasonograph*) and (MRI or MR or MRI-guided or MR-guided or magnetic resonance* or unilateral or high-intensity)).ti,ab,kf. (7794)
-
12
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU* or TcMR?gFU*).ti,ab,kf. (1270)
-
13
(insightec* or exablate*).ti,ab,kf. (235)
-
14
or/4–13 (747080)
-
15
3 and 14 (1391)
-
16
exp Animals/ not Humans/ (16078021)
-
17
15 not 16 (1000)
-
18
Case Reports/ or Comment.pt. or Editorial.pt. or Letter.pt. or Congresses.pt. (4852163)
-
19
17 not 18 (880)
-
20
limit 19 to english language [Limit not valid in CDSR; records were retained] (780)
-
21
20 use ppez,coch,cctr,clhta,cleed (424)
-
22
essential tremor/ (6490)
-
23
((essential or familial or hereditary or heredofamilial or heredo-familial or juvenile or presenile or senile or benign or idiopathic) adj2 tremor*).tw,kw. (8320)
-
24
or/22–23 (9352)
-
25
thalamotomy/ (1375)
-
26
((thalam* and (surg* or neurosurg* or ablat*)) or thalamotom*).tw,kw,dv. (9745)
-
27
nuclear magnetic resonance imaging/ (652844)
-
28
interventional magnetic resonance imaging/ (3110)
-
29
interventional ultrasonography/ (20453)
-
30
ultrasound therapy/ (8099)
-
31
high intensity focused ultrasound/ (5690)
-
32
((focus?ed ultrasound*1 or focus?ed ultrasonograph*) and (MRI or MR or MRI-guided or MR-guided or magnetic resonance* or unilateral or high-intensity)).tw,kw,dv. (7933)
-
33
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU* or TcMR?gFU*).tw,kw,dv. (1329)
-
34
(insightec* or exablate*).tw,kw,dv. (354)
-
35
or/25–34 (697994)
-
36
24 and 35 (1588)
-
37
(exp animal/ or nonhuman/) not exp human/ (10171272)
-
38
36 not 37 (1573)
-
39
Case Report/ or Comment/ or Editorial/ or Letter/ or conference abstract.pt. (9188695)
-
40
38 not 39 (1029)
-
41
limit 40 to english language [Limit not valid in CDSR; records were retained] (927)
-
42
41 use emez (635)
-
43
21 or 42 (1059)
-
44
43 use ppez (401)
-
45
43 use emez (635)
-
46
43 use coch (0)
-
47
43 use cctr (21)
-
48
43 use clhta (2)
-
49
43 use cleed (0)
-
50
remove duplicates from 43 (721)
Economic Literature Search
Search date: April 11, 2017
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <March 2017>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to April 4, 2017>, EBM Reviews -Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2017 Week 15>, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy
-
1
Essential Tremor/ (6490)
-
2
((essential or familial or hereditary or heredofamilial or heredo-familial or juvenile or presenile or senile or benign or idiopathic) adj2 tremor*).ti,ab,kf. (8213)
-
3
or/1–2 (9340)
-
4
exp Thalamus/su [Surgery] (2626)
-
5
((thalam* and (surg* or neurosurg* or ablat*)) or thalamotom*).ti,ab,kf. (9462)
-
6
Magnetic Resonance Imaging/ (693063)
-
7
Magnetic Resonance Imaging, Interventional/ (3110)
-
8
Ultrasonography, Interventional/ (20453)
-
9
Ultrasonic Therapy/ (17555)
-
10
High-Intensity Focused Ultrasound Ablation/ (3969)
-
11
((focus?ed ultrasound*1 or focus?ed ultrasonograph*) and (MRI or MR or MRI-guided or MR-guided or magnetic resonance* or unilateral or high-intensity)).ti,ab,kf. (7794)
-
12
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU* or TcMR?gFU*).ti,ab,kf. (1270)
-
13
(insightec* or exablate*).ti,ab,kf. (235)
-
14
or/4–13 (747080)
-
15
3 and 14 (1391)
-
16
economics/ (254251)
-
17
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (794446)
-
18
economics.fs. (397845)
-
19
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).ti,ab,kf. (747961)
-
20
exp “costs and cost analysis”/ (544587)
-
21
(cost or costs or costing or costly).ti. (231064)
-
22
cost effective*.ti,ab,kf. (265682)
-
23
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (172651)
-
24
models, economic/ (174032)
-
25
markov chains/ or monte carlo method/ (70416)
-
26
(decision adj1 (tree* or analy* or model*)).ti,ab,kf. (34175)
-
27
(markov or markow or monte carlo).ti,ab,kf. (108821)
-
28
quality-adjusted life years/ (33723)
-
29
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (54963)
-
30
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).ti,ab,kf. (88737)
-
31
or/16–30 (2403893)
-
32
15 and 31 (25)
-
33
32 use ppez,coch,cctr,clhta (5)
-
34
15 use cleed (0)
-
35
33 or 34 (5)
-
36
limit 35 to english language [Limit not valid in CDSR; records were retained] (5)
-
37
essential tremor/ (6490)
-
38
((essential or familial or hereditary or heredofamilial or heredo-familial or juvenile or presenile or senile or benign or idiopathic) adj2 tremor*).tw,kw. (8320)
-
39
or/37–38 (9352)
-
40
thalamotomy/ (1375)
-
41
((thalam* and (surg* or neurosurg* or ablat*)) or thalamotom*).tw,kw,dv. (9745)
-
42
nuclear magnetic resonance imaging/ (652844)
-
43
interventional magnetic resonance imaging/ (3110)
-
44
interventional ultrasonography/ (20453)
-
45
ultrasound therapy/ (8099)
-
46
high intensity focused ultrasound/ (5690)
-
47
((focus?ed ultrasound*1 or focus?ed ultrasonograph*) and (MRI or MR or MRI-guided or MR-guided or magnetic resonance* or unilateral or high-intensity)).tw,kw,dv. (7933)
-
48
(MR?gFU* or MR?g-FU* or MR?gHIFU* or MR?-HIFU* or TcMR?gFU*).tw,kw,dv. (1329)
-
49
(insightec* or exablate*).tw,kw,dv. (354)
-
50
or/40–49 (697994)
-
51
39 and 50 (1588)
-
52
Economics/ (254251)
-
53
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (130520)
-
54
Economic Aspect/ or exp Economic Evaluation/ (435128)
-
55
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw. (771955)
-
56
exp “Cost”/ (544587)
-
57
(cost or costs or costing or costly).ti. (231064)
-
58
cost effective*.tw,kw. (276411)
-
59
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (173723)
-
60
Monte Carlo Method/ (57773)
-
61
(decision adj1 (tree* or analy* or model*)).tw,kw. (37865)
-
62
(markov or markow or monte carlo).tw,kw. (113775)
-
63
Quality-Adjusted Life Years/ (33723)
-
64
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (58721)
-
65
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw. (107774)
-
66
or/52–65 (1912130)
-
67
51 and 66 (34)
-
68
67 use emez (29)
-
69
limit 68 to english language [Limit not valid in CDSR; records were retained] (29)
-
70
36 or 69 (34)
-
71
70 use ppez (5)
-
72
70 use emez (29)
-
73
70 use coch (0)
-
74
70 use cctr (0)
-
75
70 use clhta (0)
-
76
70 use cleed (0)
-
77
remove duplicates from 70 (31)
Health State Utility Literature Search
Search date: April 11, 2017
Databases searched: All Ovid MEDLINE
Database: Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy
-
1
Essential Tremor/ (1597)
-
2
((essential or familial or hereditary or heredofamilial or heredo-familial or juvenile or presenile or senile or benign or idiopathic) adj2 tremor*).ti,ab,kf. (3287)
-
3
or/1–2 (3466)
-
4
Quality-Adjusted Life Years/ (9421)
-
5
(quality adjusted or adjusted life year*).tw. (12135)
-
6
(qaly* or qald* or qale* or qtime*).tw. (7843)
-
7
(illness state$1 or health state$1).tw. (5246)
-
8
(hui or hui1 or hui2 or hui3).tw. (1198)
-
9
(multiattribute* or multi attribute*).tw. (709)
-
10
(utility adj3 (score$1 or valu* or health* or cost* or measure* or disease* or mean or gain or gains or index*)).tw. (11110)
-
11
utilities.tw. (5641)
-
12
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eurqol5d or euro?qul or eur?qul5d or euro* quality of life or European qol).tw. (7574)
-
13
(euro* adj3 (5 d or 5d or 5 dimension* or 5dimension* or 5 domain* or 5domain*)).tw. (2564)
-
14
(sf36* or sf 36* or sf thirtysix or sf thirty six).tw. (18577)
-
15
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).tw. (1606)
-
16
((qol or hrqol or quality of life).ti. or *quality of life/) and ((qol or hrqol* or quality of life) adj2 (increas* or decreas* or improve* or declin* or reduc* or high* or low* or effect or effects of worse or score or scores or change$1 or impact$1 or impacted or deteriorate)).ab. (24011)
-
17
Cost-Benefit Analysis/ and (cost effectiveness ratio* and (perspective* or life expectanc*)).tw. (2537)
-
18
*quality of life/ and (quality of life or qol).ti. (43776)
-
19
quality of life/ and ((quality of life or qol) adj3 (improve* or chang*)).tw. (19022)
-
20
quality of life/ and ((quality of life or qol) adj (score$1 or measure$1)).tw. (9452)
-
21
quality of life/ and health-related quality of life.tw. (23854)
-
22
quality of life/ and ec.fs. (8622)
-
23
quality of life/ and (health adj3 status).tw. (7328)
-
24
(quality of life or qol).tw. and cost-benefit analysis/ (9635)
-
25
models, economic/ (8246)
-
26
or/4–25 (126359)
-
27
3 and 26 (33)
-
28
limit 27 to english language (32)
Targeted Literature Search
Search date: July 11, 2017
Databases searched: All Ovid MEDLINE, Cochrane Database of Systematic Reviews
Database: EBM Reviews – Cochrane Database of Systematic Reviews <2005 to July 6, 2017>, All Ovid MEDLINE(R) <1946 to Present>
Search Strategy
-
1
Essential Tremor/ (1642)
-
2
((essential or familial or hereditary or heredofamilial or heredo-familial or juvenile or presenile or senile or benign or idiopathic) adj2 tremor*).ti,ab,kf. (3355)
-
3
or/1–2 (3536)
-
4
Deep Brain Stimulation/ (6611)
-
5
(((brain or thalam* or subthalam*) adj3 (stimulat* or stimulus or stimuli)) or DBS).ti,ab,kf. (21617)
-
6
Electric Stimulation Therapy/ (19160)
-
7
Electric Stimulation/ (111699)
-
8
((electric* adj3 (stimulat* or stimulus or stimuli)) or electrotherap* or electro-therap* or electric therap* or electrostimulat* or electro-stimulat*).ti,ab,kf. (70115)
-
9
Thalamus/su [Surgery] (1396)
-
10
((thalam* and (surg* or neurosurg* or ablat*)) or thalamotom*).ti,ab,kf. (4113)
-
11
(radiofrequenc* or radio-frequenc*).ti,ab,kf. (36657)
-
12
Stereotaxic Techniques/ (14729)
-
13
(stereotactic or stereotaxic).ti,ab,kf. (25581)
-
14
or/4–13 (242728)
-
15
3 and 14 (932)
-
16
Meta Analysis.pt. (82825)
-
17
Meta-Analysis/ or Meta-Analysis as Topic/ or exp Technology Assessment, Biomedical/ (108105)
-
18
(((systematic* or methodologic*) adj3 (review* or overview*)) or pooled analysis or published studies or published literature or hand search* or handsearch* or medline or pubmed or embase or cochrane or cinahl or data synthes* or data extraction* or HTA or HTAs or (technolog* adj (assessment* or overview* or appraisal*))).ti,ab. (260255)
-
19
(meta analy* or metaanaly* or health technolog* assess*).mp. (157987)
-
20
or/16–19 (333350)
-
21
15 and 20 (29)
-
22
21 use ppez (29)
-
23
15 use coch (0)
-
24
22 or 23 (29)
-
25
limit 24 to english language [Limit not valid in CDSR; records were retained] (26)
Grey Literature
Search date: April 12–13, 2017
Websites searched: HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, clinicaltrails.gov, Tufts Cost Effectiveness Analysis Registry
Keywords used: tremor, tremors, tremblement, thalamus, thalamotomy, ultrasound, ultrasounds, ultrasonography, ultrasonographies, ultrason, ultrasonographie, MRgFU, MRg-FU, MRgHIFU, MR-HIFU, TcMRgFU, MRIgFU, MRIg-FU, MRIgHIFU, MRI-HIFU, TcMRIgFU, MRgFUS, MRg-FUS, MRgHIFUS, MR-HIFUS, TcMRgFUS, MRIgFUS, MRIg-FUS, MRIgHIFUS, MRI-HIFUS, TcMRIgFUS, insightec, exablate
Results: 0
Trial registry results not reported in PRISMA: 8
Appendix 2: Risk-of-Bias Assessment
Table A1:
Risk of Biasa Among Randomized Controlled Trials
| Author, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Elias et al, 201638 | Low | Low | Low | Low | Low | Lowb |
Possible risk of bias judgments: low, high, and unclear, based on the Cochrane Risk of Bias tool for randomized controlled trials.33
This study was supported by funds from InSightec, the Focused Ultrasound Foundation, and the Binational Industrial Research and Development Foundation, and some authors disclosed receipt of funding from InSightec. However, the statistical analysis was planned and conducted with the assistance of an outside biostatistics team according to the protocol approved by the U.S. Food and Drug Administration.
Table A2:
Risk of Biasa Among Nonrandomized Studies
| Author, Year | Selection of Participants | Confounding Variables | Measurement of Exposure | Blinding of Outcome Assessments | Incomplete Outcome Data | Selective Outcome Reporting |
|---|---|---|---|---|---|---|
| Kim et al, 201746 | Low | Low | Low | Unclear | Low | Low |
| Shreglmann et al, 201742 | Unclear | Low | Low | Low | Low | Low |
| Zaaroor et al, 201745 | Unclear | Low | Low | Unclear | Unclear | Low |
| Gallay et al, 201639 | Low | Low | Low | Unclear | High | Unclear |
| Chang et al, 201536,b | Unclear | Low | Low | Unclear | Low | Low |
| Huss et al, 201540 | High | Unclear | Low | Unclear | Low | Low |
| Elias et al, 201337,c | Unclear | Low | Low | Low | Low | Low |
| Lipsman et al, 201341,d | Unclear | Low | Low | High | Low | Low |
Possible risk of bias judgments: low, high, and unclear, based on the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS).
This study was supported by an InSightec research grant, and InSightec provided technical assistance for the procedures during the study.
This study was supported by funding from the Focused Ultrasound Surgery Foundation. InSightec provided technical assistance during procedures and financial contributions to the Focused Ultrasound Surgery Foundation, and their representatives were responsible for regulatory compliance throughout study. The University of Virginia collected and analyzed all data, and the authors vouch for accuracy and fidelity to protocol.
This study was supported by funding from the Focused Ultrasound Foundation. InSightec assisted with technical aspects of procedures and was responsible for regulatory compliance throughout study. The sponsor had no role in study design, data collection, analysis, interpretation, or writing the article, and the corresponding author had full access to the data and final decision on submission for publication.
Appendix 3: Selected Excluded Studies—Clinical Evidence Review
Table A3 provides a selection of studies that readers may have expected to see in the clinical evidence review of this health technology assessment. These studies were screened but did not meet the inclusion criteria.
Table A3:
Selected Excluded Studies
| Citation | Primary Reason for Exclusion |
|---|---|
| Bauer R, Martin E, Haegele-Link S, Kaegi G, von Specht M, Werner B. Noninvasive functional neurosurgery using transcranial MR imaging-guided focused ultrasound. Parkinsonism Relat Disord. 2014;20 Suppl 1:S197–9. | Study type (narrative review) |
| Dallapiazza R, McKisic MS, Shah B, Elias WJ. Neuromodulation for movement disorders. Neurosurg Clin N Am. 2014;25(1):47–58. | Intervention (deep brain stimulation) |
| Hariz M. Focused ultrasound thalamotomy improves essential tremor. Mov Disord. 2013;28(13):1803. | Study type (commentary) |
| Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32(1):E1. | Population (neuropathic pain) |
| Jolesz FA, McDannold NJ. Magnetic resonance-guided focused ultrasound: a new technology for clinical neurosciences. Neurol Clin. 2014;32(1):253–69. | Study type (narrative review) |
| Jung HH, Chang WS, Rachmilevitch I, Tlusty T, Zadicario E, Chang JW. Different magnetic resonance imaging patterns after transcranial magnetic resonance-guided focused ultrasound of the ventral intermediate nucleus of the thalamus and anterior limb of the internal capsule in patients with essential tremor or obsessive-compulsive disorder. J Neurosurg. 2015;122(1):162–8. | Outcome (MRI patterns) |
| Martin E, Werner B. Focused ultrasound surgery of the brain. Curr Radiol Rep. 2013;1(2):126–35. | Study type (narrative review) |
| Monteith S, Sheehan J, Medel R, Wintermark M, Eames M, Snell J, et al. Potential intracranial applications of magnetic resonance-guided focused ultrasound surgery. J Neurosurg. 2013;118(2):215–21. | Study type (narrative review) |
| Moser D, Zadicario E, Schiff G, Jeanmonod D. MR-guided focused ultrasound technique in functional neurosurgery: targeting accuracy. J Ther Ultrasound. 2013;1:3. | Outcome (targeting accuracy) |
| Ravikumar VK, Parker JJ, Hornbeck TS, Santini VE, Pauly KB, Wintermark M, et al. Cost-effectiveness of focused ultrasound, radiosurgery, and DBS for essential tremor. Mov Disord. 2017. | Study type (economic analysis) |
Abbreviations: DBS, deep brain stimulation; MRI, magnetic resonance imaging.
Appendix 4: Applicability and Methodological Quality of the Included Economic Study
Table A4:
Applicability of the Included Economic Study—Ravikumar et al, 201754
| Question | Response |
|---|---|
| Is the study population similar to the question? | Partially. The study evaluated people with medication-resistant essential tremor eligible for surgical treatment, but did not consider people unsuitable for surgical treatment. |
| Are the interventions similar to the question? | Partially. The study excluded radiofrequency thalamotomy in the main analysis; the estimated cost was included only in the appendix. |
| Is the health care system in which the study was conducted sufficiently similar to the current Ontario context? | No. U.S. societal perspective. |
| Was/were the perspective(s) clearly stated, and what were they? | Yes. U.S. societal perspective. |
| Are estimates of relative treatment effect from the best available sources? | Uncertain, since nearly all included reports are uncontrolled observational studies, and a detailed assessment of the quality of each study is not available. |
| Are all future costs and outcomes discounted? (If yes, at what rate?) | Unclear, as the publication did not report the model time horizon. |
| Is the value of health effects expressed in terms of quality-adjusted life-years? | Yes, although how utility was derived was not clearly reported. |
| Are costs and outcomes from other sectors fully and appropriately measured and valued? | No. The study appears to include only direct medical costs, although the authors stated they were using a societal perspective. |
| Overall judgment | Partially applicable |
Table A5:
Methodological Quality of Included Economic Study (Ravikumar et al 201754
| Question | Possible responses |
|---|---|
| Does the model structure adequately reflect the nature of the health condition under evaluation? | No. It did not include long-term clinical outcomes such as tremor recurrence. |
| Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? (e.g., if the rate of mortality differs between interventions, does the model take a lifetime horizon?) | No. The time horizon used is unclear; it appears to be short-term. |
| Are all important and relevant health outcomes included? | Partly. Improvement in functional disability was associated with a different treatment, which was not reported. |
| Are the estimates of relative treatment effects obtained from the best available sources? | Partly. Nearly all included reports are uncontrolled observational studies. |
| Do the estimates of relative treatment effect match the estimates contained in the clinical report? | Not applicable. We were unable to check since the estimates were not reported clearly. |
| Are all important and relevant (direct) costs included in the analysis? | No. The publication did not include long-term costs such as treatment for tremor recurrence and deep brain stimulation device battery replacement. |
| Are the estimates of resource use obtained from the best available sources? | Yes. |
| Are the unit costs of resources obtained from the best available resources? | Yes. |
| Is an appropriate incremental analysis presented, or can it be calculated from the reported data? | Yes. |
| Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Unclear. The sensitivity analysis results were not fully presented. |
| Is there a potential conflict of interest? | Unclear. |
| Overall assessment | Minor limitations |
Appendix 5: Health State Utility Search Results
Table A3 summarizes the results of our health state utility search.
We found one study that measured the health state utility of people with essential tremor before and after pharmacological treatment. Herceg et al83 included 29 people with essential tremor severe enough to produce disability. Nine of the 29 people had previously received propranolol or primidone as monotherapy or in combination without satisfactory outcome. In the study, people were treated with pramipexole for 16 weeks and evaluated every 4 weeks. At baseline, people had a mean score of 18.50 points on part A of the FTM scale (FTM-A) and a mean score of 0.69 on the EQ-5D. At 4 months, the FTM-A score had improved by 65% (6.60 points), and the EQ-5D score had increased to 0.91. We decided to use utility values from this study because the patient population was similar to our model population (as defined by having similar FTM-A and FTM total scores at baseline and after treatment).
One Spanish study measured the health state utility of people with essential tremor.55 The majority of people had mild to moderate tremor (42.4% and 43.2%, respectively); 13.6% had severe tremor. Also, most people had received no medication (32.2%) or just one medication (47.46%) for essential tremor. The mean EQ-5D score was 0.73 (SD: 0.30), and the mean QUEST summary index was 19.91. We did not use the value from this study because the patient population had less severe disease compared with our model population.
Three studies measured the health state utility of people with definite or probable essential tremor compared with healthy controls using the 36-Item Short-Form Survey (SF-36).107–109 We used a mapping algorithm from a study by Ara et al123 to convert the eight mean SF-36 dimension scores reported by these studies into a mean EQ-5D utility score. The utility value of people living with essential tremor was found to be between 0.7 and 0.8, compared with the utility value of healthy controls, which ranged from 0.84 to 0.90. We did not use the health state utility values from these studies since the patient population was not representative of our model population.
We also identified two studies reporting on quality-of-life improvement after deep brain stimulation, measured using a visual analog scale.26,67 In 2002, Hariz et al67 found that quality of life improved by 31.3 point between 6 and 26 months following the procedure. In 2008, Hariz et al26 found that quality of life improved by 34 points at 1 year, and by 18 points at 7 years. We did not use these data for the estimation of health state utility increments after MRgFUS neurosurgery as they were derived from a visual analog scale.
Last, we did not consider health state utility values of people with tremor resulting from other types of movement disorder (e.g., dystonia, multiple sclerosis, Parkinson's disease) as an approximation for those of people with essential tremor. This is because people with other types of movement disorder experience non-tremor–related symptoms and disabilities in addition to tremor, whereas tremor is the main symptom in essential tremor.
Table A6:
Summary of Results: Health State Utility Search
| Author, Year | Patient Population | Health State | Results |
|---|---|---|---|
| EQ-5D | |||
| Martinez-Martin et al, 201055 | Essential tremor, mild to moderate | Essential tremor, treatment unspecified | ET = 0.73 |
| Herceg et al, 201283 | Essential tremor, tremor severe enough to produce disability | Before and after drug treatment (pramipexole) | Baseline = 0.69 (FTM-A = 18.5; FTM total = 43.71) 4 months = 0.91 (FTM-A = 6.60; FTM total = 20.75) |
| VAS | |||
| Hariz et al, 200267 | Essential tremor, medication refractory | Before and after deep brain stimulation, 6–26 months | VAS improved by 31.3 points |
| Hariz et al, 200826 | Essential tremor, medication refractory | Before and after deep brain stimulation, 1 year, 7 years | VAS improved by 34 points at 1 year and by 18 points at 7 years |
| SF-36 | |||
| Lorenz et al, 2006107 | Essential tremor, definite or probable | Essential tremor, treatment unspecified | ET = 0.80; control = 0.90 |
| Nguyen et al, 2007108 | Essential tremor, community-dwelling, newly diagnosed, aged ≥ 65 years | Essential tremor, treatment unspecified | ET = 0.70; control = 0.84 |
| Musacchio et al, 2016109 | Essential tremor, definite or probable (FTM score at baseline = 36.8) | Essential tremor, treatment unspecified | ET = 0.76; control = 0.86 |
Abbreviations: EQ-5D, European Quality of Life—5 Dimensions; ET, essential tremor; FTM, Fahn–Tolosa–Marín Clinical Rating Scale for Tremor; SF-36, 36-Item Short-Form Survey; VAS, visual analog scale.
Appendix 6: Cost Inputs
Table A7:
Cost Components of the Deep Brain Stimulation System
| Item | Quantity | Unit Cost, $ (2011 CAD) | Unit Cost, $ (2017 CAD) |
|---|---|---|---|
| Activa SC (unilateral) | |||
| Implanted pulse generator | 1 | 7,995 | 8,375 |
| Deep brain electrode lead | 1 | 1,995 | 2,090 |
| Extension lead | 1 | 1,745 | 1,828 |
| Patient activator | 1 | 1,295 | 1,357 |
| Total cost | 13,030 | 13,649 | |
| Activa PC (bilateral) | |||
| Implanted pulse generator | 1 | 10,995 | 11,517 |
| Deep brain electrode lead | 2 | 1,995 | 2,090 |
| Extension lead | 2 | 1,745 | 1,828 |
| Patient activator | 1 | 1,295 | 1,357 |
| Total cost | 19,770 | 20,709 | |
Source: Medtronic of Canada Ltd., 2011.124
Table A8:
Surgical Assistant Service Costs
| Procedure | Source | |||
|---|---|---|---|---|
| MRgFUS | DBS | RF | ||
| Average surgery length, hours | 3.5 | 4.5 | 3 | Expert opinion |
| Number of basic units | 9 | 9 | 9 | Schedule of Benefits (N124)91 |
| Number of time units | 28 | 40 | 22 | Calculated based on average surgery length |
| Total number of units | 37 | 49 | 31 | |
| Total billing | $445 | $590 | $373 | |
Abbreviations: DBS, deep brain stimulation, MRgFUS, magnetic resonance-guided focused ultrasound; RF, radiofrequency.
Note: The amount payable for surgical assistant service is calculated by adding the number of basic and time units and multiplying the total by the surgical assistant unit fee (−12.04/unit). For surgical assistant, there are 9 base units and 1 unit per 15 minutes in the first hour, 2 units per 15 minutes after the first hour, and 3 units per 15 minutes after 2.5 hours.
Table A9:
Anaesthesia Service Costs
| Procedure | Source | |||
|---|---|---|---|---|
| MRgFUS | DBS | Radiofrequency | ||
| Average surgery length, hours | 3.5 | 4.5 | 3 | Expert opinion |
| Number of basic units | 11 | 11 | 11 | Schedule of Benefit (N124)91 |
| Number of time units | 28 | 40 | 22 | Calculated based on average surgery length |
| Total number of units | 39 | 51 | 33 | |
| Total billing | $585 | $766 | $495 | |
Abbreviations: DBS, deep brain stimulation, MRgFUS, magnetic resonance-guided focused ultrasound; RF, radiofrequency.
Note: The amount payable for anaesthesia service is calculated by adding the number of basic and time units and multiplying the total by the anaesthesiologist unit fee (−15.01/unit). For anaesthesia services, there are 11 base units and 1 unit per 15 minutes in the first hour, 2 units per 15 minutes after the first hour, and 3 units per 15 minutes after 2.5 hours.
Appendix 7: Probabilistic Analysis Model Variables and Distributions
Table A10:
Probabilistic Analysis Model Variables and Distributions
| Parameter | Distribution | Source | |||
|---|---|---|---|---|---|
| Improvement post-surgery (MRgFUS, RF, DBS) | Mean | Alpha | Beta | ||
| Proportion with marked improvement | Beta | 53% | 27 | 24 | Elias et al, 201638 |
| Proportion with mild to moderate improvement | Varies with the above | 47% | – | – | |
| Probability of recurrencea (MRgFUS, RF) | |||||
| Annual probability of recurrence after MRgFUS or RF thalamotomy in year 1 | Beta | 8.9% | 5 | 51 | Elias et al, 201638 |
| Annual probability of recurrence after MRgFUS or RF thalamotomy in years 2–5 | Varies with the above | 4.7% | – | – | Calculated |
| Probability of reoperation (MRgFUS, RF) | |||||
| Probability of reoperation after recurrence | Beta | 40% | 2 | 3 | Elias et al, 201638 |
| Costs | Mean | SD | Sample Size | ||
| DBS hospitalization cost | Gamma | $17,221 | $3,043 | 10 | OCC92 |
| RF hospitalization cost | Gamma | $6,822 | $5,140 | 9 | OCC92 |
| DBS monitoring cost, year 1 | Gamma | $1,249 | $468 | 36 | Ondo et al, 200597 |
| DBS monitoring cost, subsequent years | Gamma | $463 | $261 | 36 | Ondo et al, 200597 |
| Utilities | Mean | SD | Sample Size | ||
| Alive with disabling tremor (baseline) | Beta | 0.69 | 0.30 | 24 | Herceg et al, 201283 |
| No recurrence, marked improvement | Beta | 0.91 | 0.08 | 24 | |
Abbreviations: DBS, deep brain stimulation; MRgFUS, magnetic resonance-guided focused ultrasound; OCC, Ontario Case Costing database;
RF, radiofrequency.
Recurrence refers to diminished tremor control to the point of requiring reoperation.
Appendix 8: Additional Results Tables
Research Question 1: What is the cost-effectiveness of MRgFUS neurosurgery compared with standard treatment (i.e., no surgery) for people with moderate to severe, medication-refractory essential tremor, who are ineligible for invasive neurosurgery, within the context of the Ontario Ministry of Health and Long-Term Care?
Table A11:
Probabilistic Reference Case Analysis Results—MRgFUS Neurosurgery Versus No Surgery
| MRgFUS, Mean (95% CI) | No Surgery, Mean (95% CI) | |
|---|---|---|
| Cost of primary surgery | $19,786 | – |
| Cost of monitoring | $814 | $814 |
| Cost of medications | $1,096 | $1,245 |
| Cost of reoperation | $1,731 | – |
| Cost of managing adverse events | $71 | – |
| Total costs | $23,497 (−22,044–$25,287) | $2,060 |
| Life-years | 4.63 | 4.63 |
| QALYs | 3.70 (3.48–3.89) | 3.23 (2.65–3.74) |
| Incremental cost | $21,438 (−19,985–$23,227) | |
| Incremental life-years | 0.00 | |
| Incremental QALYs | 0.47 (0.10–0.90) | |
| ICER (cost/QALY) | $45,817 | |
Abbreviations: MRgFUS, magnetic resonance-guided focused ultrasound; QALY, quality-adjusted life-year.
Research Question 2: What is the cost-effectiveness of MRgFUS neurosurgery compared with standard treatment (i.e., radiofrequency thalamotomy or deep brain stimulation) for people with moderate to severe, medication-refractory essential tremor, who are eligible for invasive neurosurgery, within the context of the Ontario Ministry of Health and Long-Term Care?
Table A12:
Probabilistic Reference Case Analysis Results—Cost-Effectiveness of MRgFUS Neurosurgery, Radiofrequency Thalamotomy, and Deep Brain Stimulation
| MRgFUS, Mean (95% CI) | RF, Mean (95% CI) | DBS, Mean (95% CI) | |
|---|---|---|---|
| Cost of primary surgery | $19,786 | $11,774 | $37,366 |
| Cost of monitoring | $814 | $814 | $3,307 |
| Cost of medications | $1,096 | $1,096 | $1,081 |
| Cost of reoperation | $1,731 | $1,030 | – |
| Cost of managing adverse events | $71 | $259 | $6,306 |
| Cost of battery replacement | – | – | $9,463 |
| Total cost | $23,497 (−22,044–$25,287) | $14,972 (−14,096–$16,051) | $57,523 (−55,507–$59,772) |
| Life-years | 4.63 | 4.63 | 4.63 |
| QALYs | 3.70 (3.48–3.89) | 3.63 (3.42–3.82) | 3.96 (3.75–4.14) |
Abbreviations: AE, adverse events; DBS, deep brain stimulation; MRgFUS, magnetic resonance guided focused ultra sound; RF, radiofrequency.
Table A13:
Probabilistic Reference Case Analysis Results—Incremental Cost-Effectiveness Ratios for MRgFUS Neurosurgery Versus Radiofrequency Thalamotomy or Deep Brain Stimulation
| Intervention | Total Costs | Total QALYs | Incremental Costs | Incremental QALYs | Sequential ICER |
|---|---|---|---|---|---|
| Radiofrequency thalamotomy | $14,972 | 3.63 | – | – | – |
| MRgFUS neurosurgery | $23,497 | 3.70 | $8,525 | 0.07 | $119,607 |
| Deep brain stimulation | $57,523 | 3.96 | $34,026 | 0.26 | $130,850 |
Abbreviations: ICER, incremental cost-effectiveness ratio; MRgFUS, magnetic resonance-guided focused ultrasound; QALY, quality-adjusted life-year.
Appendix 9: Letter of Information
Appendix 10: Consent and Release Form
Appendix 11: Interview Guide
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario. The lead clinical epidemiologist was Alexis Schaink, the lead health economist was Chunmei Li, the secondary health economist was Olga Gajic-Veljanoski, the Patient, Caregiver, and Public Engagement analyst was David Wells, and the medical librarian was Caroline Higgins.
KEY MESSAGES
What Is This Health Technology Assessment About?
Essential tremor is the most common movement disorder and most often affects the dominant hand and arm. As the tremor gets worse, it can negatively impact quality of life. Medication is the first treatment, but it does not work for many people. The next treatment option is neurosurgery (brain surgery). Presently in Ontario, the neurosurgery options generally available are invasive procedures that involve craniotomy (opening the skull). Magnetic resonance-guided focused ultrasound (MRgFUS) neurosurgery is a new, noninvasive surgical technology for the treatment of essential tremor. Because MRgFUS neurosurgery is noninvasive, it does not have the same surgical risks as invasive procedures. Thus, it may be an option for people who cannot undergo invasive surgery and for people who find the risks of invasive surgery, such as craniotomy, general anaesthetic, and implanted hardware, unacceptable.
We reviewed the evidence to evaluate the effectiveness and safety of MRgFUS neurosurgery, assessed the cost-effectiveness of MRgFUS neurosurgery compared with other surgical treatments, and calculated the budget impact of publicly funding MRgFUS neurosurgery. We also interviewed people with essential tremor to learn about their experiences and to gain an understanding of their preferences and values with regard to treatment options for essential tremor, including MRgFUS neurosurgery.
What Did this Health Technology Assessment Find?
The evidence shows that MRgFUS neurosurgery is generally safe and effective at reducing tremor severity, improving quality of life, and helping people get back to their daily activities. MRgFUS neurosurgery offers a treatment option for people with essential tremor who otherwise have none if medication fails, and it offers a noninvasive option for people considering surgery who cannot safely have invasive surgery or who find the risks of invasive surgery unacceptable.
For people with essential tremor who cannot undergo invasive neurosurgery, MRgFUS neurosurgery represents good value for money compared with no surgery. For people who can undergo invasive neurosurgery, MRgFUS neurosurgery appears to be one of several reasonable options. Publicly funding MRgFUS neurosurgery for the treatment of moderate to severe, medication-refractory essential tremor in Ontario would result in additional spending of $1 million per year for the next 5 years.
People with essential tremor who had undergone MRgFUS neurosurgery reported positive experiences with the procedure and felt that it had improved their quality of life by substantially reducing their tremor.
Contributor Information
Health Quality Ontario:
Alexis Schaink, Chunmei Li, Olga Gajic-Veljanoski, David Wells, and Caroline Higgins
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Botzel K, Tronnier V, Gasser T. The differential diagnosis and treatment of tremor. Dtsch Arztebl Int. 2014;111(13):225–35; quiz 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Buijink AW, Contarino MF, Koelman JH, Speelman JD, van Rootselaar AF. How to tackle tremor - systematic review of the literature and diagnostic work-up. Front Neurol. 2012;3:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25(5):534–41. [DOI] [PubMed] [Google Scholar]
- (4).Elble RJ. Diagnostic criteria for essential tremor and differential diagnosis. Neurology. 2000;54(11 Suppl 4):S2–6. [PubMed] [Google Scholar]
- (5).Bain PG. Parkinsonism & related disorders. Tremor. Parkinsonism Relat Disord. 2007;13 Suppl 3:S369–74. [DOI] [PubMed] [Google Scholar]
- (6).Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovik J, Tolosa E, editors. Parkinson's disease and movement disorders. Baltimore: Urban & Schwarzenberg; 1988. p. 225–34. [Google Scholar]
- (7).Elble R, Bain P, Forjaz MJ, Haubenberger D, Testa C, Goetz CG, et al. Task force report: scales for screening and evaluating tremor: critique and recommendations. Mov Disord. 2013;28(13):1793–800. [DOI] [PubMed] [Google Scholar]
- (8).Elble RJ, Brilliant M, Leffler K, Higgins C. Quantification of essential tremor in writing and drawing. Mov Disord. 1996;11(1):70–8. [DOI] [PubMed] [Google Scholar]
- (9).Louis ED, Gillman A, Boschung S, Hess CW, Yu Q, Pullman SL. High width variability during spiral drawing: further evidence of cerebellar dysfunction in essential tremor. Cerebellum. 2012;11(4):872–9. [DOI] [PubMed] [Google Scholar]
- (10).Picillo M, Fasano A. Recent advances in essential tremor: surgical treatment. Parkinsonism Relat Disord. 2016;22 Suppl 1:S171–5. [DOI] [PubMed] [Google Scholar]
- (11).Zappia M, Albanese A, Bruno E, Colosimo C, Filippini G, Martinelli P, et al. Treatment of essential tremor: a systematic review of evidence and recommendations from the Italian Movement Disorders Association. J Neurol. 2013;260(3):714–40. [DOI] [PubMed] [Google Scholar]
- (12).Carranza MA, Snyder MR, Elble RJ, Boutzoukas AE, Zesiewicz TA. Methodological issues in clinical drug development for essential tremor. Tremor Other Hyperkinet Mov (N Y) 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Louis ED. Essential tremor. Handb Clin Neurol. 2011;100:433–48. [DOI] [PubMed] [Google Scholar]
- (14).Campbell AM, Glover J, Chiang VL, Gerrard J, Yu JB. Gamma knife stereotactic radiosurgical thalamotomy for intractable tremor: a systematic review of the literature. Radiother Oncol. 2015;114(3):296–301. [DOI] [PubMed] [Google Scholar]
- (15).Frighetto L, Bizzi J, Annes RD, Silva Rdos S, Oppitz P. Stereotactic radiosurgery for movement disorders. Surg Neurol Int. 2012;3(Suppl 1):S10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, Dewey RB, Jr., et al. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology. 2011;77(19):1752–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Flora ED, Perera CL, Cameron AL, Maddern GJ. Deep brain stimulation for essential tremor: a systematic review. Mov Disord. 2010;25(11):1550–9. [DOI] [PubMed] [Google Scholar]
- (18).Chopra A, Klassen BT, Stead M. Current clinical application of deep-brain stimulation for essential tremor. Neuropsychiatr Dis Treat. 2013;9:1859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ontario Health Technology Advisory Committee. OHTAC recommendation: deep brain stimulation in Parkinson's disease and other movement disorders [Internet]. Toronto (ON): Queen's Printer for Ontario; 2005. [cited 2017 Jun]. Available from: http://www.hqontario.ca/english/providers/program/ohtac/tech/recommend/rec_dbs_030205.pdf [Google Scholar]
- (20).Pahwa R, Lyons KE, Wilkinson SB, Troster AI, Overman J, Kieltyka J, et al. Comparison of thalamotomy to deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001;16(1):140–3. [DOI] [PubMed] [Google Scholar]
- (21).Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342(7):461–8. [DOI] [PubMed] [Google Scholar]
- (22).Schuurman PR, Bosch DA, Merkus MP, Speelman JD. Long-term follow-up of thalamic stimulation versus thalamotomy for tremor suppression. Mov Disord. 2008;23(8):1146–53. [DOI] [PubMed] [Google Scholar]
- (23).Goldman MS, Ahlskog JE, Kelly PJ. The symptomatic and functional outcome of stereotactic thalamotomy for medically intractable essential tremor. J Neurosurg. 1992;76(6):924–8. [DOI] [PubMed] [Google Scholar]
- (24).Nagaseki Y, Shibazaki T, Hirai T, Kawashima Y, Hirato M, Wada H, et al. Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg. 1986;65(3):296–302. [DOI] [PubMed] [Google Scholar]
- (25).Health Quality Ontario. Deep brain stimulation for Parkinson's disease and other movement disorders: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(2):1–56. [PMC free article] [PubMed] [Google Scholar]
- (26).Hariz GM, Blomstedt P, Koskinen LO. Long-term effect of deep brain stimulation for essential tremor on activities of daily living and health-related quality of life. Acta Neurol Scand. 2008;118(6):387–94. [DOI] [PubMed] [Google Scholar]
- (27).Alomar S, King NK, Tam J, Bari AA, Hamani C, Lozano AM. Speech and language adverse effects after thalamotomy and deep brain stimulation in patients with movement disorders: a meta-analysis. Mov Disord. 2017;32(1):53–63. [DOI] [PubMed] [Google Scholar]
- (28).Insightec. Insightec for neurosurgery [Internet]. Tirat Carmel (Israel): Insightec; 2017. [cited 2017 Mar 16]. Available from: http://www.insightec.com/clinical/neurosurgery [Google Scholar]
- (29).Ontario Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): The Ministry; 2015. [cited 2015 Jul 28]. Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/sob_master11062015.pdf [Google Scholar]
- (30).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (31).Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- (32).O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. [DOI] [PubMed] [Google Scholar]
- (33).Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–14. [DOI] [PubMed] [Google Scholar]
- (35).Schünemann H, Brożek J, Guyatt G, Oxman A, eds. GRADE handbook [Internet]. Hamilton (ON): GRADE Working Group; 2013. [cited 2017 Dec]. Available from: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html#h.hnedbo8gqjqk. [Google Scholar]
- (36).Chang WS, Jung HH, Kweon EJ, Zadicario E, Rachmilevitch I, Chang JW. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry. 2015;86(3):257–64. [DOI] [PubMed] [Google Scholar]
- (37).Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369(7):640–8. [DOI] [PubMed] [Google Scholar]
- (38).Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730–9. [DOI] [PubMed] [Google Scholar]
- (39).Gallay MN, Moser D, Rossi F, Pourtehrani P, Magara AE, Kowalski M, et al. Incisionless transcranial MR-guided focused ultrasound in essential tremor: cerebellothalamic tractotomy. J Ther Ultrasound. 2016;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Huss DS, Dallapiazza RF, Shah BB, Harrison MB, Diamond J, Elias WJ. Functional assessment and quality of life in essential tremor with bilateral or unilateral DBS and focused ultrasound thalamotomy. Mov Disord. 2015;30(14):1937–43. [DOI] [PubMed] [Google Scholar]
- (41).Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12(5):462–8. [DOI] [PubMed] [Google Scholar]
- (42).Schreglmann SR, Bauer R, Hagele-Link S, Bhatia KP, Natchev P, Wegener N, et al. Unilateral cerebellothalamic tract ablation in essential tremor by MRI-guided focused ultrasound. Neurology. 2017;88(14):1329–33. [DOI] [PubMed] [Google Scholar]
- (43).Wintermark M, Druzgal J, Huss DS, Khaled MA, Monteith S, Raghavan P, et al. Imaging findings in MR imaging-guided focused ultrasound treatment for patients with essential tremor. AJNR Am J Neuroradiol. 2014;35(5):891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Wintermark M, Huss DS, Shah BB, Tustison N, Druzgal TJ, Kassell N, et al. Thalamic connectivity in patients with essential tremor treated with MR imaging-guided focused ultrasound: in vivo fiber tracking by using diffusion-tensor MR imaging. Radiology. 2014;272(1):202–9. [DOI] [PubMed] [Google Scholar]
- (45).Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson's disease and essential tremor cases. J Neurosurg. 2017:1–9. [DOI] [PubMed] [Google Scholar]
- (46).Kim M, Jung NY, Park CK, Chang WS, Jung HH, Chang JW. Comparative evaluation of magnetic resonance-guided focused ultrasound surgery for essential tremor. Stereotact Funct Neurosurg. 2017;95(4):279–86. [DOI] [PubMed] [Google Scholar]
- (47).Troster AI, Pahwa R, Fields JA, Tanner CM, Lyons KE. Quality of life in essential tremor questionnaire (QUEST): development and initial validation. Parkinsonism Relat Disord. 2005;11(6):367–73. [DOI] [PubMed] [Google Scholar]
- (48).Fishman PS, Frenkel V. Treatment of movement disorders with focused ultrasound. J Cent Nerv Syst Dis. 2017;9:1179573517705670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Rohani M, Fasano A. Focused ultrasound for essential tremor: review of the evidence and discussion of current hurdles. Tremor Other Hyperkinet Mov (N Y). 2017;7:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Lim SY, Hodaie M, Fallis M, Poon YY, Mazzella F, Moro E. Gamma knife thalamotomy for disabling tremor: a blinded evaluation. Arch Neurol. 2010;67(5):584–8. [DOI] [PubMed] [Google Scholar]
- (51).Zirh A, Reich SG, Dougherty PM, Lenz FA. Stereotactic thalamotomy in the treatment of essential tremor of the upper extremity: reassessment including a blinded measure of outcome. J Neurol Neurosurg Psychiatry. 1999;66(6):772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Fasano A, Llinas M, Munhoz RP, Hlasny E, Kucharczyk W, Lozano AM. MRI-guided focused ultrasound thalamotomy in non-ET tremor syndromes. Neurology. 2017;89(8):771–5. [DOI] [PubMed] [Google Scholar]
- (53).Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32(1):E1. [DOI] [PubMed] [Google Scholar]
- (54).Ravikumar VK, Parker JJ, Hornbeck TS, Santini VE, Pauly KB, Wintermark M, et al. Cost-effectiveness of focused ultrasound, radiosurgery, and DBS for essential tremor. Mov Disord. 2017;03:03. [DOI] [PubMed] [Google Scholar]
- (55).Martinez-Martin P, Jimenez-Jimenez FJ, Carroza Garcia E, Alonso-Navarro H, Rubio L, Calleja P, et al. Most of the Quality of Life in Essential Tremor Questionnaire (QUEST) psychometric properties resulted in satisfactory values. J Clin Epidemiol. 2010;63(7):767–73. [DOI] [PubMed] [Google Scholar]
- (56).National Institute for Health and Care Excellence. The guidelines manual: appendix g: methodology checklist: economic evaluations [Internet]. London (UK): The Institute; 2012. [cited 2017 Nov]. Available from: http://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-appendices-bi-2549703709/chapter/appendix-g-methodology-checklist-economic-evaluations [Google Scholar]
- (57).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- (58).Fasano A, Deuschl G. Therapeutic advances in tremor. Mov Disord. 2015;30(11):1557–65. [DOI] [PubMed] [Google Scholar]
- (59).Zesiewicz TA, Elble R, Louis ED, Hauser RA, Sullivan KL, Dewey RB, Jr., et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64(12):2008–20. [DOI] [PubMed] [Google Scholar]
- (60).Chan DT, Zhu XL, Yeung JH, Mok VC, Wong E, Lau C, et al. Complications of deep brain stimulation: a collective review. Asian J Surg. 2009;32(4):258–63. [DOI] [PubMed] [Google Scholar]
- (61).Fishman PS. Thalamotomy for essential tremor: FDA approval brings brain treatment with FUS to the clinic. J Ther Ultrasound. 2017;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Paulden M, Galvanni V, Chakraborty S, Kudinga B, McCabe C. Discounting and the evaluation of health care programs [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016. [cited 2017 Mar]. Available from: http://www.cadth.ca/sites/default/files/pdf/CP0008_Economic_Evaluation_Guidelines_Discount_Rate_Report.pdf [Google Scholar]
- (63).Pal PK. Guidelines for management of essential tremor. Ann Indian Acad Neurol. 2011;14(Suppl 1):S25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Tasker RR. Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol. 1998;49(2):145–53; discussion 53–4. [DOI] [PubMed] [Google Scholar]
- (65).Lee JY, Kondziolka D. Thalamic deep brain stimulation for management of essential tremor. J Neurosurg. 2005;103(3):400–3. [DOI] [PubMed] [Google Scholar]
- (66).Murata J, Kitagawa M, Uesugi H, Saito H, Iwasaki Y, Kikuchi S, et al. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg. 2003;99(4):708–15. [DOI] [PubMed] [Google Scholar]
- (67).Hariz GM, Lindberg M, Bergenheim AT. Impact of thalamic deep brain stimulation on disability and health-related quality of life in patients with essential tremor. J Neurol Neurosurg Psychiatry. 2002;72(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Lozano AM, Levy R. Reoperation of deep brain stimulation in patients with essential tremor. World Neurosurg. 2012;78(5):442–4. [DOI] [PubMed] [Google Scholar]
- (69).Blomstedt P, Lindvall P, Linder J, Olivecrona M, Forsgren L, Hariz MI. Reoperation after failed deep brain stimulation for essential tremor. World Neurosurg. 2012;78(5):554.e1–5. [DOI] [PubMed] [Google Scholar]
- (70).Favilla CG, Ullman D, Wagle Shukla A, Foote KD, Jacobson CE, 4th, Okun MS. Worsening essential tremor following deep brain stimulation: disease progression versus tolerance. Brain. 2012;135(Pt 5):1455–62. [DOI] [PubMed] [Google Scholar]
- (71).Ghanouni P, Pauly KB, Elias WJ, Henderson J, Sheehan J, Monteith S, et al. Transcranial MRI-guided focused ultrasound: a review of the technologic and neurologic applications. AJR Am J Roentgenol. 2015;205(1):150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Nagy AM, Tolleson CM. Rescue procedures after suboptimal deep brain stimulation outcomes in common movement disorders. Brain Sci. 2016;6(4):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Hirai T, Miyazaki M, Nakajima H, Shibazaki T, Ohye C. The correlation between tremor characteristics and the predicted volume of effective lesions in stereotaxic nucleus ventralis intermedius thalamotomy. Brain. 1983;106 (Pt 4):1001–18. [DOI] [PubMed] [Google Scholar]
- (74).Statistics Canada. Table 7a: Complete life table, males, Ontario, 2009 to 2011 [Internet]. Ottawa (ON): Statistics Canada; 2015. [cited 2017 Jun 7]. Available from: http://www.statcan.gc.ca/pub/84-537-x/2013005/tbl/tbl7a-eng.htm [Google Scholar]
- (75).Statistics Canada. Table 7b: Complete life table, females, Ontario, 2009 to 2011 [Internet]. Ottawa (ON): Statistics Canada; 2015. [cited 2017 Jun 7]. Available from: http://www.statcan.gc.ca/pub/84-537-x/2013005/tbl/tbl7b-eng.htm [Google Scholar]
- (76).Zesiewicz TA, Shaw JD, Allison KG, Staffetti JS, Okun MS, Sullivan KL. Update on treatment of essential tremor. Curr Treat Options Neurol. 2013;15(4):410–23. [DOI] [PubMed] [Google Scholar]
- (77).Patel DM, Walker HC, Brooks R, Omar N, Ditty B, Guthrie BL. Adverse events associated with deep brain stimulation for movement disorders: analysis of 510 consecutive cases. Neurosurgery. 2015;11 Suppl 2:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Fenoy AJ, Simpson RK., Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120(1):132–9. [DOI] [PubMed] [Google Scholar]
- (79).Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery. 2008;62(2):360–6; discussion 6–7. [DOI] [PubMed] [Google Scholar]
- (80).Bjerknes S, Skogseid IM, Saehle T, Dietrichs E, Toft M. Surgical site infections after deep brain stimulation surgery: frequency, characteristics and management in a 10-year period. PLoS One. 2014;9(8):e105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Rezai AR, Kopell BH, Gross RE, Vitek JL, Sharan AD, Limousin P, et al. Deep brain stimulation for Parkinson's disease: surgical issues. Mov Disord. 2006;21 Suppl 14:S197–218. [DOI] [PubMed] [Google Scholar]
- (82).Glanville J, Arber M, Veale T, Garcia S. Sensitivity of a search filter designed to identify studies reporting health state utility values. Paper presented at: Mosaic, the 116th Annual Meeting of the Medical Library Association. 2016 May 13–18; Toronto, ON.
- (83).Herceg M, Nagy F, Pal E, Janszky J, Kesmarky I, Komoly S, et al. Pramipexole may be an effective treatment option in essential tremor. Clin Neuropharmacol. 2012;35(2):73–6. [DOI] [PubMed] [Google Scholar]
- (84).Yardley L, Barker F, Muller I, Turner D, Kirby S, Mullee M, et al. Clinical and cost effectiveness of booklet based vestibular rehabilitation for chronic dizziness in primary care: single blind, parallel group, pragmatic, randomised controlled trial. BMJ. 2012;344:e2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Thoma A, Wong VH, Sprague S, Duku E. A cost-utility analysis of open and endoscopic carpal tunnel release. Can J Plast Surg. 2006;14(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38(6):583–637. [DOI] [PubMed] [Google Scholar]
- (87).Lee BY, Wiringa AE, Bailey RR, Goyal V, Tsui B, Lewis GJ, et al. The economic effect of screening orthopedic surgery patients preoperatively for methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2010;31(11):1130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Gheorghe A, Moran G, Duffy H, Roberts T, Pinkney T, Calvert M. Health utility values associated with surgical site infection: a systematic review. Value Health. 2015;18(8):1126–37. [DOI] [PubMed] [Google Scholar]
- (89).Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Inform Assoc. 1997;4(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Gada H, Desai MY, Marwick TH. Cost-effectiveness of computed tomographic angiography before reoperative coronary artery bypass grafting: a decision-analytic model. Circ Cardiovasc Qual Outcomes. 2012;5(5):705–10. [DOI] [PubMed] [Google Scholar]
- (91).Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2017 Jun 7]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20151221.pdf [Google Scholar]
- (92).Ministry of Health and Long-Term Care. Health data branch web portal: Ontario case costing [Internet]. Toronto (ON): Queen's Printer for Ontario; c2017. [cited 2017 Jun 7]. Available from: http://hsim.health.gov.on.ca/HDBPortal/ [Google Scholar]
- (93).Ministry of Health and Long-Term Care. Schedule of benefits for laboratory services [Internet]. Toronto (ON): Queen's Printer for Ontario; 1999. [cited 2017 Jun 7]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_services_sched_01_19990401.pdf [Google Scholar]
- (94).Ministry of Health and Long-Term Care. Ontario drug benefit formulary/comparative drug index [Internet]. Toronto (ON): Queen's Printer for Ontario; c2017. [cited 2017 Jul 12]. Available from: http://www.formulary.health.gov.on.ca/formulary/ [Google Scholar]
- (95).Gironell A, Kulisevsky J. Diagnosis and management of essential tremor and dystonic tremor. Ther Adv Neurol Disord. 2009;2(4):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Schneider SA, Deuschl G. The treatment of tremor. Neurotherapeutics. 2014;11(1):128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Ondo WG, Bronte-Stewart H. The North American survey of placement and adjustment strategies for deep brain stimulation. Stereotact Funct Neurosurg. 2005;83(4):142–7. [DOI] [PubMed] [Google Scholar]
- (98).Picillo M, Lozano AM, Kou N, Munhoz RP, Fasano A. Programming deep brain stimulation for tremor and dystonia: the Toronto Western Hospital algorithms. Brain Stimul. 2016;9(3):438–52. [DOI] [PubMed] [Google Scholar]
- (99).Booth C. Deep brain stimulation: information for patients and families [Internet]. Toronto (ON): University Health Network; 2015. [cited 2017 May 27]. Available from: http://www.uhn.ca/PatientsFamilies/Health_Information/Health_Topics/Documents/Deep_Brain_Stimulation.pdf [Google Scholar]
- (100).Pan I, Dendukuri N. Subthalamic deep brain stimulation (DBS): clinical efficacy, safety and cost compared to medical therapy for the treatment of Parkinson's disease [Internet]. Montreal (QC): Technology Assessment Unit of the McGill University Health Centre; 2009. [cited 2017 May 27]. Available from: http://www.mcgill.ca/tau/files/tau/DBS_REPORT.pdf [Google Scholar]
- (101).Fenoy AJ, Simpson RK., Jr. Management of device-related wound complications in deep brain stimulation surgery. J Neurosurg. 2012;116(6):1324–32. [DOI] [PubMed] [Google Scholar]
- (102).Zrinzo L, Foltynie T, Limousin P, Hariz MI. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg. 2012;116(1):84–94. [DOI] [PubMed] [Google Scholar]
- (103).Government of Canada. Automobile allowance rates [Internet]. Ottawa (ON): Government of Canada; 2017. [cited 2017 Aug 3]. Available from: http://www.canada.ca/en/revenue-agency/services/tax/businesses/topics/payroll/benefits-allowances/automobile/automobile-motor-vehicle-allowances/automobile-allowance-rates.html [Google Scholar]
- (104).Hunka K, Suchowersky O, Wood S, Derwent L, Kiss ZH. Nursing time to program and assess deep brain stimulators in movement disorder patients. J Neurosci Nurs. 2005;37(4):204–10. [DOI] [PubMed] [Google Scholar]
- (105).Ministry of Labour. Minimum wage [Internet]. Toronto (ON): Queen's Printer for Ontario; 2017. [cited 2017 Aug 3]. Available from: http://www.labour.gov.on.ca/english/es/pubs/guide/minwage.php [Google Scholar]
- (106).Statistics Canada. CANSIM table 282-0002: Labour force survey estimates (LFS), by sex and detailed age group, annual (persons × 1,000) [Internet]. Ottawa (ON): Statistics Canada; 2017. [cited 2017 Aug 3]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=2820002&&pattern=&stByVal=1&p1=1&p2=-1&tabMode=dataTable&csid= [Google Scholar]
- (107).Lorenz D, Schwieger D, Moises H, Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord. 2006;21(8):1114–8. [DOI] [PubMed] [Google Scholar]
- (108).Nguyen HV, Ngian V, Cordato D, Shen Q, Chan DK. Quality of life in a random sample of community dwelling older patients with essential tremor. Acta Neurol Scand. 2007;116(5):289–92. [DOI] [PubMed] [Google Scholar]
- (109).Musacchio T, Purrer V, Papagianni A, Fleischer A, Mackenrodt D, Malsch C, et al. Non-motor symptoms of essential tremor are independent of tremor severity and have an impact on quality of life. Tremor Other Hyperkinet Mov (N Y). 2016;6:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? Estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13(1):5–10. [DOI] [PubMed] [Google Scholar]
- (111).Koller WC, Busenbark K, Miner K. The relationship of essential tremor to other movement disorders: report on 678 patients. Essential Tremor Study Group. Ann Neurol. 1994;35(6):717–23. [DOI] [PubMed] [Google Scholar]
- (112).Ministry of Finance. Ontario population projections update [Internet]. Toronto (ON): Queen's Printer for Ontario; 2017. [cited 2017 Oct]. Available from: http://www.fin.gov.on.ca/en/economy/demographics/projections/projections2016-2041.pdf [Google Scholar]
- (113).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (114).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (115).OHTAC Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2016 Jan]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (116).Tjornhoj-Thomsen T, Hansen HP. Knowledge in health technology assessment: who, what, how? Int J Technol Assess Health Care. 2011;27(4):324–9. [DOI] [PubMed] [Google Scholar]
- (117).Rowe G, Frewer LJ. A typology of public engagement mechanisms. Sci Technol Hum Val. 2005;30(2):251–90. [Google Scholar]
- (118).Brundisini F, Giacomini M, DeJean D, Vanstone M, Winsor S, Smith A. Chronic disease patients’ experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2013;13(15):1–33. [PMC free article] [PubMed] [Google Scholar]
- (119).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (120).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2016 Jan]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (121).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (122).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (123).Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health. 2008;11(7):1131–43. [DOI] [PubMed] [Google Scholar]
- (124).Medtronic of Canada Ltd. Neuromodulation price list [Internet]. Mississauga (ON): Medtronic of Canada Ltd.; 2011. [cited 2017 May 5]. Available from: http://www.thinnoxproductions.com/medtronic/Neuromodulation%20price%20list%20lowres.pdf [Google Scholar]