Abstract
The Threshold Toxicological Concern (TTC) is based on the concept that in absence of experimental data reasonable assurance of safety can be given if exposure is sufficiently low.
Using the REACH database the low 5th percentile of the NO(A)EL distribution, for prenatal developmental toxicity (OECD guideline 414) was determined. For rats, (434 NO(A)ELs values) for maternal toxicity, this value was 10 mg/kg-bw/day. For developmental toxicity (469 NO(A)ELs): 13 mg/kg-bw/day. For rabbits, (100 NO(A)ELs), the value for maternal toxicity was 4 mg/kg-bw/day, for developmental toxicity, (112 NO(A)EL values): 10 mg/kg-bw/day. The maternal organism may thus be slightly more sensitive than the fetus. Combining REACH- (industrial chemicals) and published BASF-data (mostly agrochemicals), 537 unique compounds with NO(A)EL values for developmental toxicity in rats and 150 in rabbits were evaluated. The low 5th percentile NO(A)EL for developmental toxicity in rats was 10 mg/kg-bw/day and 9.5 mg/kg-bw/day for rabbits. Using an assessment factor of 100, a TTC value for developmental toxicity of 100 µg/kg-bw/day for rats and 95 µg/kg-bw/day for rabbits is calculated. These values could serve as guidance whether or not to perform an animal experiment, if exposure is sufficiently low. In emergency situations this value may be useful for a first tier risk assessment.
Keywords: TTC, Developmental toxicity, Rat, Rabbit, NOAEL
1. Introduction
The Threshold of Toxicological Concern (TTC) concept is a risk assessment tool that is based on the idea that reasonable assurance of safety can be given even in the absence of chemical-specific toxicity data if the exposure is negligible. An important prerequisite is a sufficiently low intake to define an exposure level below which no significant risk to human health can be expected (JECFA, 2006). The TTC refers to the development of generic human exposure threshold values for groups of chemicals below which no appreciable risk to human health is assumed (Barlow et al., 2001). The concept of a TTC evolved from the review (Munro, 1990) of the Threshold of Regulation as applied by the FDA for food contact chemicals and was refined (Munro et al., 1996, 1999), based on an extensive analysis of available chronic oral toxicity data of substances.
The approach was adopted by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) to evaluate flavoring substances (JECFA, 1993, 1995; 1999; Munro et al., 1999) and has been used already from 1997 for the safety evaluation of 1259 flavoring substances (Renwick, 2004). A TTC decision tree developed by Kroes et al. (2004) provides a systematic structured approach for the consistent application of the TTC approach to chemicals that are present in food at low concentrations.
The application of the TTC concept in the absence of chemical-specific data is a pragmatic approach that allows the safety evaluation of chemicals to which humans are exposed via food and the environment. Thus, the TTC can be considered to be a first-tier assessment. If exposure levels are below the TTC value then no immediate action is required. If exposure levels are above the TTC value then more information on the hazard potential is needed. Initially this may include structure-activity approaches, read-across to similar chemicals or in vitro assays. The strength of this approach is that unnecessary animal studies are not performed because it identifies those chemicals that need additional testing. JECFA considered extension of the TTC concept to other substances present in the diet in small amounts (e.g., processing aid residues, packaging materials, and contaminants) and recommended development of guidelines for the application of the approach in the risk assessment of such substances for which full toxicological datasets are not available or are unnecessary (JECFA, 2005). Regulatory TTC values have been defined for migrant substances from packaging material in food (Food and Drug Administration, 1995), flavoring substances in food (JECFA, 2003; Renwick, 2004), genotoxic impurities in pharmaceutical preparations (EMEA, 2003, 2004; Müller et al., 2006) and were proposed for cosmetic ingredients (Kroes et al., 2007). The approach is used by the European Food Safety Authority to evaluate flavoring substances (EFSA, 2004) and has been endorsed by the WHO International Program on Chemical Safety for the risk assessment of chemicals (IPCS, 1998) and by the EU Scientific Committee on Toxicology, Ecotoxicology and the Environment. The approach has also been suggested for application to aquatic environmental exposure (De Wolf et al., 2005), constituents of consumer products (Blackburn et al., 2005) and occupational exposure in drug manufacturing (Dolan et al., 2005). More recent and novel applications for the TTC concept include its use to derive tolerable concentrations for “non-relevant metabolites” formed from plant protection products (Melching-Kollmuβ et al., 2010).
Most of the reported work within the context of TTC development refers to chronic toxicity and carcinogenicity following oral exposure. Only a limited number of reports deal with end point-specific TTC values, i.e. TTC values for other toxicological end points than chronic toxicity and carcinogenicity. One area of particular interest is prenatal developmental toxicity. The reason for this is manifold; irreversible changes to the unborn are considered as the most grave in toxicology, theoretically such changes may be induced by a single or only a few exposures and under REACH extensive animal testing for reproduction toxicity is required. Therefore, a solid database to derive an acceptable TTC value for developmental toxicity could serve as a starting point in the assessment if (further) studies are needed from a regulatory perspective and could give risk managers a tool that could be used in emergency situations. Unfortunately, only few data evaluations relating to a TTC value for prenatal developmental toxicity in rats (OECD, 2001) have entered the scientific literature (Kroes et al., 2004; Bernauer et al., 2008; Laufersweiler et al., 2012). To increase the number of data available for TTC consideration for developmental toxicity we have published the NOAEL and LOAEL values of 93 different OECD 414 guideline studies in rats performed in our laboratories over the last two decades (van Ravenzwaay et al., 2011). Even less data are available with respect to the second species used for the determination of prenatal developmental toxicity, i.e. the rabbit. We have contributed to this database by publishing BASF’s database, which contains 48 compounds for which the NOAEL and LOAEL values for maternal and developmental toxicity based on the OECD 414 guideline. ECHA’s public database of REACH registration dossier offers a large toxicology dataset useful for assessing the outcome of toxicological studies for different end-points (Luechtefeld et al., 2016a). Here, we have collected the NO(A)EL and LO(A)EL values obtained for prenatal developmental toxicity studies in rats and rabbits. We have examined the studies for adherence to regulatory guidelines and used the data to establish TTC values for developmental toxicity for both species. Subsequently we have compared the obtained TTC values for these largely industrial chemicals with the TTC values obtained from our database, which consist mainly of active ingredients.
2. Materials and methods
2.1. Identification of developmental toxicity studies
The database for these analyses was created from ECHA dossier pages as described (Luechtefeld et al., 2016a). Automated extraction by linguistic search engines of data from ECHA online dossiers enables analysis of diverse chemical study data. Extracted REACH data were stored as a query-able collection of documents in a Mongo database (Chodorow, 2013; Godbillon, 2015). The public release of the REACH database is under discussion with ECHA; at this stage, it is available for collaborative analyses from the authors. Analyses for endpoints such as oral acute toxicity, eye irritation and skin sensitization have been published earlier (Luechtefeld et al., 2016b, 2016c, 2016d).
Briefly, data was downloaded from ECHA using HtmlUnit in an iterative manner in order not to hinder data flow, using an open source Java “Guiless browser” library (Bowler, 2002). Implementation of ECHA dossier download automation used the functional programming language SCALA (Odersky et al., 2004). A MongoDB database (https://www.mongodb.org/) was generated from REACH data (Chodorow, 2013). Extracted REACH data is stored as a query able collection of documents in this Mongo database. The database was generated by automated data extraction from ECHA dossier URLs via the SCALA driver ReactiveMongo (Godbillon, 2015).
Every document is identified by a unique set of three fields:
-
–
ECNumber: Substance identifier (“415-890-1”)
-
–
Type: Study description (e.g., “Exp Key Eye irritation”)
-
–
Num: disambiguates repeat studies (1, 2, 3, …)
The constructed database, downloaded December 17, 2014, contains 816,048 such documents with 9801 unique substances (identified by ECNumber) and 3609 unique study descriptions.
The prenatal developmental toxicity study data and NO(A)EL/LO(A)EL values extracted from the ECHA files were carried out according to OECD guideline 414. For some performed after 1997, also according to U.S. EPA Health Effects Test Guidelines OPPTS 870.3700. The studies evaluated, were performed in the period from 1971 to 2014 according to the REACH database. Studies carried out in rats and rabbits were evaluated for maternal- and developmental-toxicity. Only studies in which maternal and developmental NOAEL/LOAEL or NOEL/LOEL values were reported in units of “mg/kg bw/d” were recruited for the analysis. The most frequently used rat strains were Sprague-Dawley and Wistar and for rabbits the New Zealand White, Himalayan and Dutch strains. The numbers of selected studies reported NOAEL/LOAEL and NOEL/LOEL values of maternal and developmental toxicity are illustrated in Fig. 1 (rat) and in Fig. 2 (rabbit).
Fig. 1.
The numbers of selected studies reported NOAEL/LOAEL and NOEL/LOEL values of maternal and developmental toxicity in rats.
Fig. 2.
The numbers of selected studies reported NOAEL/LOAEL and NOEL/LOEL values of maternal and developmental toxicity in rabbits.
2.2. Statistical analysis
A total of 480 chemicals tested in rats (477 of which contained developmental toxicity data) and 112 (all with developmental toxicity data) in rabbits were finally obtained taking into account the criteria mentioned above and used for evaluation. In some cases several NOAEL/LOAEL values for maternal- and developmental toxicity were available for the same chemical due to multiple studies. In this situation, the lowest NOAEL and LOAEL values were used as the input parameter for the evaluation of the chemical in question, thus resulting in a lower LOAEL/NOAEL values than potentially correct. Therefore, the TTC for oral prenatal toxicity values are determined in our analysis represent a conservative evaluation. The cumulative distribution functions of the NOAEL/LOAEL values were calculated and are displayed. Additionally, the median values as well as the 5th, 10th, 90th and 95th percentiles were determined. Since some studies merely reported LOEL or NOEL values, the same analyses were performed to compare the results when LOEL and NOEL values were included in the distribution. In this paper, NO(A)EL/LO(A)EL denotes the combination of NOAEL/LOAEL and NOEL/LOEL. The influence of various routes of administration (ROA) on determining NOAEL/LOAEL values was also considered in our evaluations. In most studies (>90%) test chemicals were administered by gavage. Other ROAs were administration via the feed and drinking water.
According reported NOAEL and LOAEL values of the maternal toxicity and developmental toxicity, chemical can be divided into different categories of the developmental toxicity. To obtain an estimate how frequently selective developmental toxicity is observed we tried to estimate this by selecting studies according to the following criteria:
- Selective development toxicity:
- the LOAEL value of maternal toxicity > the LOAEL value of developmental toxicity;
- the NOAEL value of maternal toxicity ≥ the LOAEL value of developmental toxicity;
- the NOAEL value of maternal toxicity > the NOAEL value of developmental toxicity, if the LOAEL value of developmental toxicity was not reported.
Using these criteria selective development toxicity means that a chemical has developmental toxic effect at doses lower than its maternal toxic effect. Developmental toxicity was only taken into account for dose level ≤1000 mg/kg bw/d as specified as limit dose in the OECD guidelines.
All calculations and visualizations were performed with the statistical software R version 3.2.0 (R Development Core Team, 2013).
3. Results
3.1. Calculation of a TTC value for oral prenatal developmental toxicity for rats
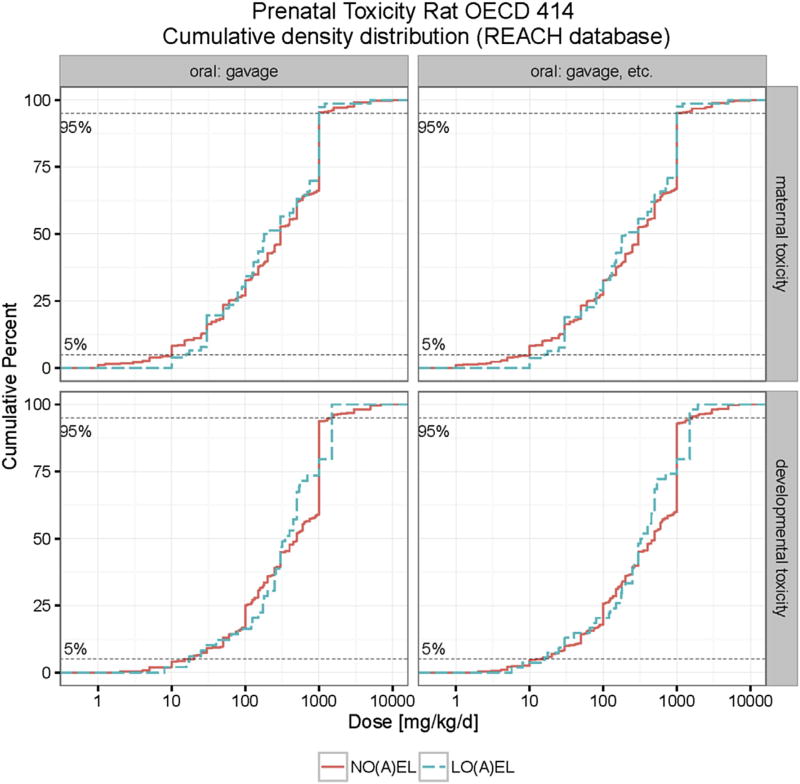
A data set of 480 chemicals was collected for the evaluation of maternal and developmental NOAELs and LOAELs in rats. In studies by gavage, the median NOAEL for maternal toxicity is 300 mg/kg bw/d with a median LOAEL of 200 mg/kg bw/d. The median NOAEL and LOAEL values for developmental toxicity are higher, i.e. 500 mg/kg bw/d and 310 mg/kg bw/d, respectively. At the 5th percentile the maternal NOAEL (10 mg/kg bw/d) is slightly lower than the developmental NOAEL (17.8 mg/kg bw/d). At the 10th percentile the maternal NOAEL (15 mg/kg bw/d) is clearly much lower than the developmental NOAEL (50 mg/kg bw/d)., The 5th and 10th percentiles maternal LOAELs are slightly higher than the developmental LOAELs, i.e. 22 mg/kg bw/d and 30 mg/kg bw/d for maternal toxicity and 16.5 mg/kg bw/d and 28.5 mg/kg bw/d for developmental toxicity (see Table 1).
Table 1.
Median and percentiles of NOAELs and LOAELs for maternal and developmental toxicity studies (OECD 414) in rats (measured in mg/kg/day).
| N | 5th | 10th | Median | 90th | 95th | |
|---|---|---|---|---|---|---|
| Oral: gavage | ||||||
| Maternal NOAEL | 360 | 10 | 15 | 300 | 1000 | 1200 |
| Maternal LOAEL | 72 | 22 | 30 | 200 | 1000 | 1000 |
| Developmental NOAEL | 390 | 17.5 | 50 | 500 | 1000 | 1550 |
| Developmental LOAEL | 47 | 16.525 | 28.5 | 310 | 1500 | 1500 |
| Oral: gavage, drinking water, feed and unspecified | ||||||
| Maternal NOAEL | 380 | 10 | 16 | 300 | 1000 | 1350 |
| Maternal LOAEL | 75 | 23.125 | 30 | 250 | 1000 | 1000 |
| Developmental NOAEL | 415 | 11.525 | 32.5 | 500 | 1000 | 1600 |
| Developmental LOAEL | 51 | 12.4 | 25.5 | 310 | 1500 | 1500 |
In a second evaluation, we evaluated studies including all routes of administration (gavage, drinking water, feed and unspecified); the corresponding distributions of maternal and developmental NOAELs and LOAELs and the numbers of their involved studies are also shown in Table 1. Only a slight difference was found between the gavage only group and the group in which all routes of administration were evaluated. The 5th percentile developmental NOAEL (11.5 mg/kg bw/d) and LOAEL (12.4 mg/kg bw/d) based on all routes of administration are only marginally different in the overall evaluation compared to the gavage only group. As verified in Fig. S1 (Appendix), the effect of route of administration on the cumulative distribution of maternal and developmental toxicity can be neglected in rats.
Since some studies merely reported maternal and developmental NOELs and LOELs, we used the lower value from NOEL/LOEL and NOAEL/NOAEL as the end point for the evaluation of maternal and developmental toxicity. As shown in Fig. S2 (Appendix), the cumulative distributions of NOAEL and LOAEL values do virtually not change when LOEL and NOEL values are included in analysis. Table 2 and Fig. 3 illustrate percentiles and cumulative distributions of NO(A)EL and LO(A)EL values including NOELs and LOELs for maternal and developmental toxicity.
Table 2.
Median and percentiles of NO(A)ELs and LO(A)ELs for maternal and developmental toxicity studies (OECD 414) in rats (measured in mg/kg/day).
| N | 5th | 10th | Median | 90th | 95th | |
|---|---|---|---|---|---|---|
| Oral: gavage | ||||||
| Maternal NO(A)EL | 413 | 10 | 15 | 300 | 1000 | 1000 |
| Maternal LO(A)EL | 76 | 14.8 | 30 | 180 | 1000 | 1000 |
| Developmental NO(A)EL | 443 | 15.75 | 50 | 500 | 1000 | 1477.5 |
| Developmental LO(A)EL | 49 | 16.675 | 29.5 | 335 | 1500 | 1500 |
| Oral: gavage, drinking water, feed and unspecified | ||||||
| Maternal NO(A)EL | 434 | 10 | 15 | 300 | 1000 | 1000 |
| Maternal LO(A)EL | 79 | 15.7 | 30 | 190 | 1000 | 1000 |
| Developmental NO(A)EL | 469 | 13.35 | 30 | 500 | 1000 | 1600 |
| Developmental LO(A)EL | 54 | 13.6 | 27 | 320 | 1500 | 1500 |
Fig. 3.
Cumulative distribution of maternal and developmental toxicity (NO(A)EL and LO(A)EL) in rats. The developmental toxicity LO(A)EL (NO(A)EL) values range from 5.7 (2) to 1920 (7000) mg/kg bw/d. The maternal toxicity LO(A)EL (NO(A)EL) values range from 10 (1) to 5000 (10000) mg/kg bw/d.
The numbers of various rat strains used in the two databases are given in Table 3. The most common rat strains used for the investigation of maternal and developmental toxicity are Wistar and Sprague-Dawley.
Table 3.
The number of rat strains distributed in maternal and developmental toxicity studies.
| Wistar | Sprague- Dawley |
Fischer 344 |
Others | in total |
|
|---|---|---|---|---|---|
| Maternal NOAEL | 171 | 251 | 4 | 8 | 434 |
| Maternal LOAEL | 15 | 63 | 0 | 1 | 79 |
| Developmental NOAEL | 182 | 274 | 4 | 9 | 469 |
| Developmental LOAEL | 11 | 42 | 0 | 1 | 54 |
As TTC values are normally derived from the 5th percentile of the evaluated studies (with a sufficiently large data base), in rats the relevant NOAEL values for maternal toxicity is 10 mg/kg bw/d (based on 434 values) and for developmental toxicity is 11.5 mg/kg bw/d (based on 469 values).
3.2. Calculation of a TTC value for oral prenatal developmental toxicity for rabbits
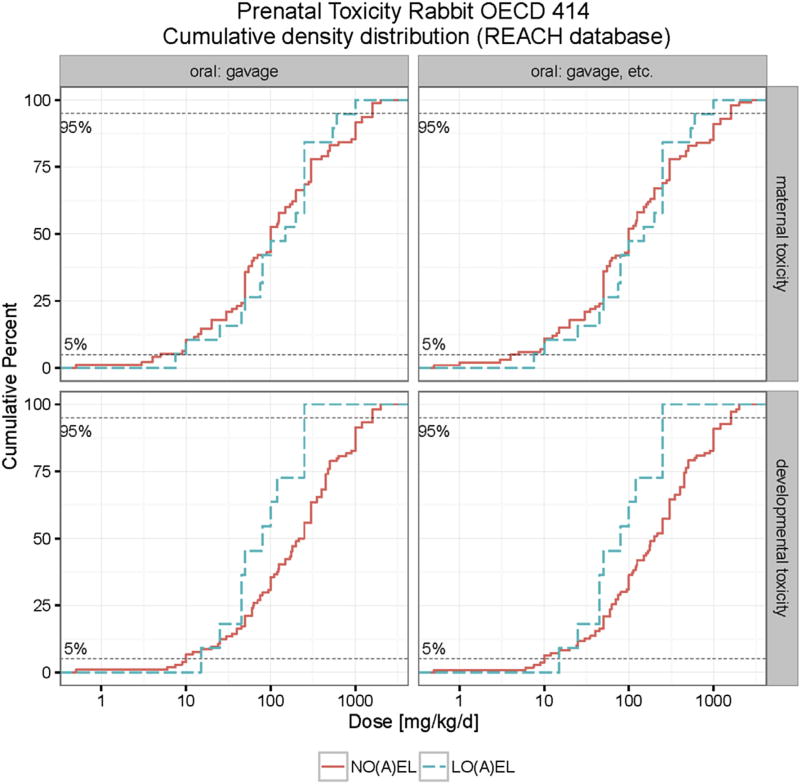
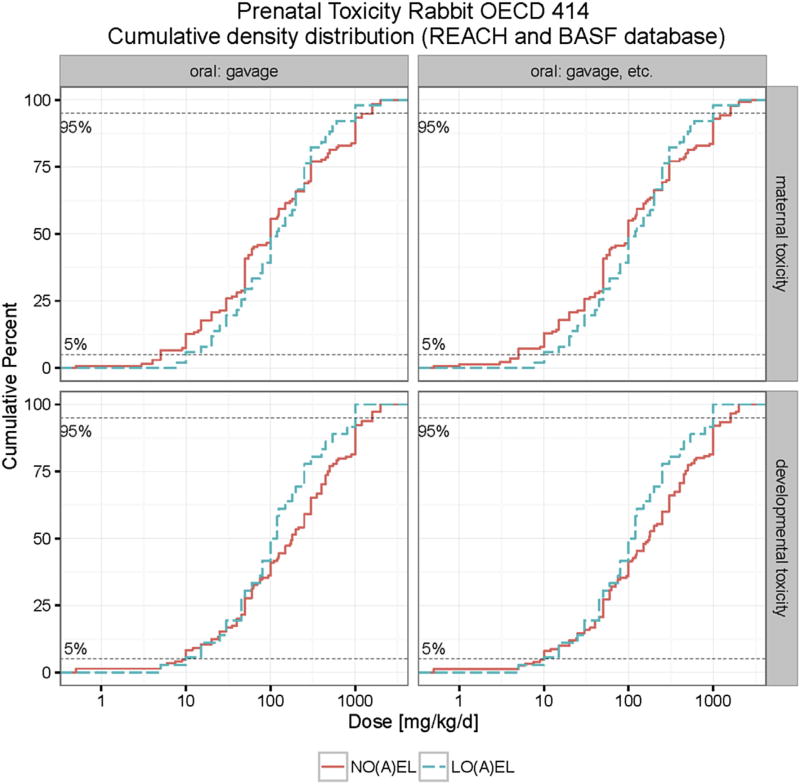
A data set of 112 chemicals was collected for the evaluation of maternal and developmental NOAELs and LOAELs in rabbits. In studies performed by gavage, the median NOAEL and LOAEL for maternal toxicity are close, i.e. 100 mg/kg bw/d for NOAEL and 125 mg/kg bw/d for LOAEL. For developmental toxicity the median NOAEL value (250 mg/kg bw/d) is much higher, whereas the median LOAEL value (50 mg/kg bw/d) is lower. Nevertheless, at the 5th and 10th percentiles the NOAEL or LOAEL for maternal and developmental toxicity do not differ greatly (see Table 4). The 5th percentile values for maternal NOAEL and LOAEL is 5.2 mg/kg bw/d and 7.5 mg/kg bw/d, respectively; the 5th percentile value for developmental NOAEL and LOAEL is 10 mg/kg bw/d and 15 mg/kg bw/d, respectively. Table 4 also displays the percentiles of maternal and developmental NOAELs and LOAELs in the group of all routes of administration to compare the percentiles calculated in the gavage group. At 5th and 10th percentiles the NOAEL and LOAEL for maternal and developmental toxicity by all routes of administration were almost the same as those purely by gavage, since other routes of administration were chosen only in a small number of studies. At the 5th percentile the maternal NOAEL (4.3 mg/kg bw/d) and based on all routes (N = 86) of administration is slightly lower than that (5.2 mg/kg bw/d) in the gavage group (N = 81), whereas, at 5th percentile the developmental NOAEL (10 mg/kg bw/d) in the group of all routes of administration (N = 93) is identical to that in the gavage group (N = 87, 10 mg/kgbw/d). As verified in Fig. S3 (Appendix), the overall effect of route of administration on the cumulative distribution of maternal and developmental toxicity can also be neglected in rabbits.
Table 4.
Median and percentiles of NOAELs and LOAELs for maternal and developmental toxicity studies (OECD 414) in rabbits (measured in mg/kg/day).
| N | 5th | 10th | Median | 90th | 95th | |
|---|---|---|---|---|---|---|
| Oral: gavag | ||||||
| Maternal NOAEL | 81 | 5.2 | 12.65 | 100 | 1000 | 1600 |
| Maternal LOAEL | 17 | 7.5 | 19.75 | 125 | 337 | 549 |
| Developmental NOAEL | 87 | 10 | 22.8 | 250 | 1060 | 1600 |
| Developmental LOAEL | 10 | 15 | 15 | 50 | 250 | 250 |
| Oral: gavage, drinking water, feed and unspecifie | ||||||
| Maternal NOAEL | 86 | 4.3 | 11,5 | 100 | 1080 | 1600 |
| Maternal LOAEL | 17 | 7.5 | 19.75 | 125 | 337 | 549 |
| Developmental NOAEL | 93 | 10 | 24.3 | 232.5 | 1140 | 1600 |
| Developmental LOAEL | 10 | 15 | 15 | 50 | 250 | 250 |
As shown in Fig. S4 (Appendix), the cumulative distributions of NOAEL and LOAEL values do not change significantly when LOEL and NOEL values are included in analysis. Table 5 and Fig. 4 illustrate percentiles and cumulative distributions of NO(A)EL and LO(A)EL values for maternal and developmental toxicity. In rabbits the relevant (5th percentile) NOAEL value for maternal is toxicity 4 mg/kg bw/d (N = 100) and for developmental toxicity is 10 mg/kg bw/d (N = 112).
Table 5.
Median and percentiles of NO(A)ELs and LO(A)ELs for maternal and developmental toxicity studies (OECD 414) in rabbits (measured in mg/kg/day).
| N | 5th | 10th | Median | 90th | 95th | |
|---|---|---|---|---|---|---|
| Oral: gavage | ||||||
| Maternal NO(A)EL | 95 | 4.75 | 10 | 100 | 1000 | 1600 |
| Maternal LO(A)EL | 19 | 7.5 | 9.75 | 125 | 546 | 620 |
| Developmental NO(A)EL | 104 | 10 | 21,6 | 215 | 1000 | 1600 |
| Developmental LO(A)EL | 11 | 15 | 16 | 65 | 250 | 250 |
| Oral: gavage, drinking water, feed and unspecified | ||||||
| Maternal NO(A)EL | 100 | 4 | 10 | 100 | 1000 | 1600 |
| Maternal LO(A)EL | 19 | 7.5 | 9.75 | 125 | 546 | 620 |
| Developmental NO(A)EL | 110 | 10 | 24 | 200 | 1000 | 1600 |
| Developmental LO(A)EL | 11 | 15 | 16 | 65 | 250 | 250 |
Fig. 4.
Cumulative distribution of maternal and developmental toxicity (NO(A)EL and LO(A)EL) in rabbits. The developmental toxicity LO(A)EL (NO(A)EL) values range from 15 (0.5) to 250 (2000) mg/kg bw/d. The maternal toxicity LO(A)EL (NO(A)EL) values range from 7.5 (0.5) to 1000 (2765) mg/kg bw/d.
3.3. Comparison of oral prenatal developmental toxicity for rats and rabbits
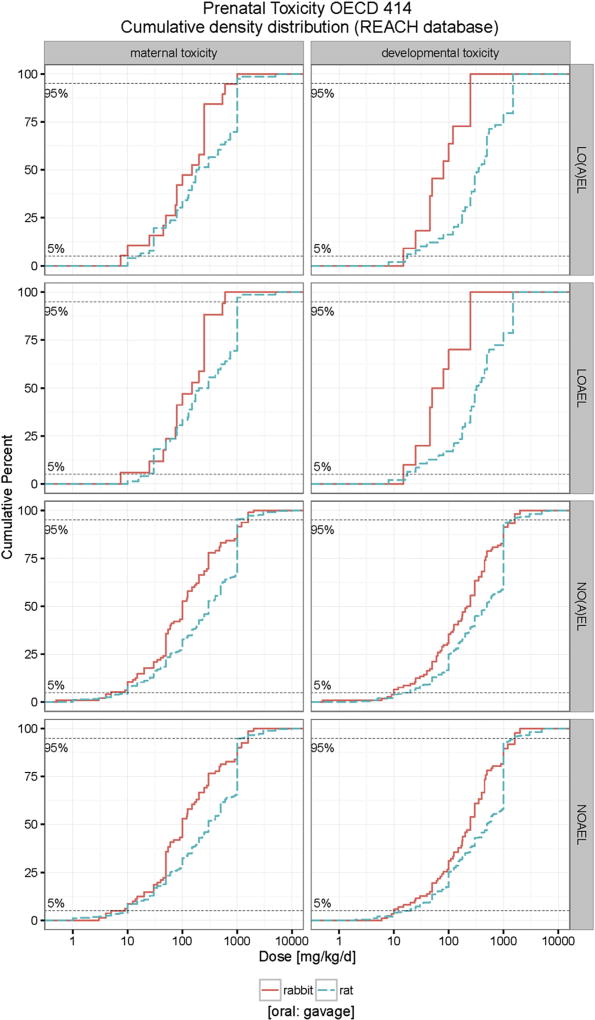
From REACH database it is evident that more studies were performed rats than with rabbits. As shown in Fig. 5, the cumulative distribution curves of NOAEL and LOAEL values for gavage studies in rat for maternal and developmental toxicity are right shifted (i.e. have higher values) compared to those for rabbits. The corresponding cumulative distribution curves of NOAEL and LOAEL values maternal and developmental toxicity for rats and rabbits for all routes of administration are displayed in Fig. S5, giving the same results. Comparing Table 1 (Table 2) with Table 4 (Table 5), the 5% percentile NOAEL value for developmental toxicity in both species are quite similar (11.5 mg/kg bw/d for rats and 10 mg/kg bw/d for rabbits), however the 5% percentile NOAEL value for maternal toxicity in rats (10 mg/kg bw/d) is clearly higher than that for rabbits (4 mg/kg bw/d).
Fig. 5.
Comparison of cumulative distribution of maternal and developmental toxicity by gavage in rats and rabbits. The developmental toxicity LO(A)EL (NO(A)EL) values range from 15 (0.5) to 250 (2000) mg/kg bw/d in rabbits and range from 8 (2) to 1500 (7000) mg/kg bw/d in rats. The maternal toxicity LO(A)EL (NO(A)EL) values range from 7.5 (0.5) to 1000 (2000) mg/kg bw/d in rabbits and range from 10 (1) to 5000 (10000) mg/kg bw/d in rats.
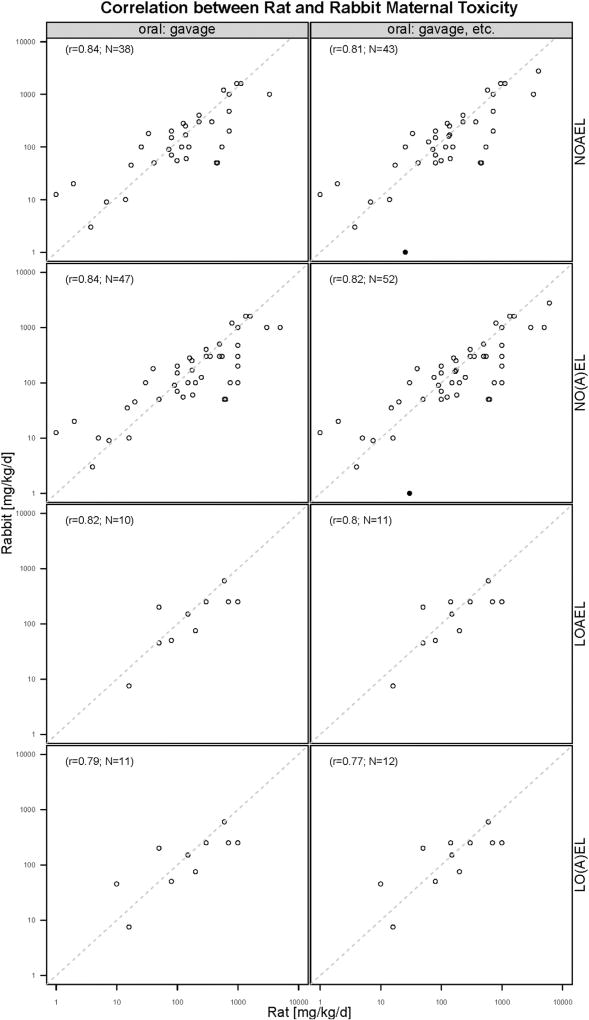
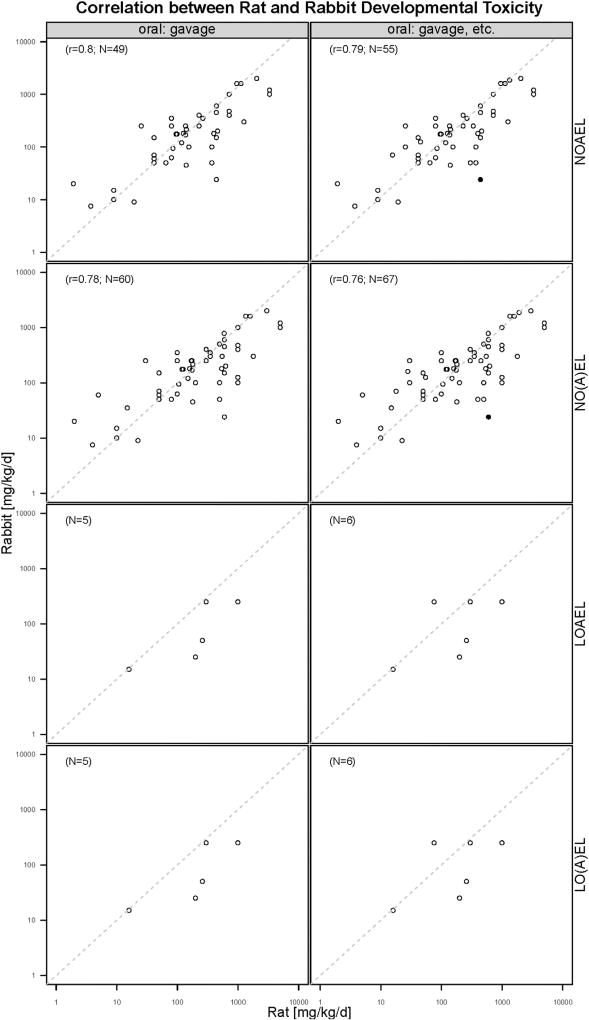
The dataset containing the information about maternal and developmental toxicity evaluated in both rats and rabbits for the same chemical was merged to investigate the correlation between rat and rabbit toxicity. As shown in Fig. 6, Pearson correlation coefficients between rat and rabbit maternal toxicity range from 0.77 to 0.84, indicating a strong positive correlation. In addition, in most cases NOAEL and LOAEL values for rabbit maternal toxicity are lower than those for rat, especially obviously for LOAEL values, although it should be noted that only for a few chemicals maternal LOAEL values were determined for both species. Notably, the NOAEL value for rabbit maternal toxicity for ammonium perchlorate is 1 mg/kg bw/d and much lower than that for rat (30 mg/kg bw/d). This case is indicated as an outlier (filled dots) in Fig. 6. Pearson correlation coefficients between rat and rabbit developmental toxicity are illustrated in Fig. 7, ranging from 0.59 to 0.81. Only six chemicals, tested in both rats and rabbits, were available with LOAEL values for developmental toxicity, therefore the sample size is too small to evaluate their correlations. Similar as the maternal toxicity, in most cases NOAEL and LOAEL values for rabbit developmental toxicity are lower than those for rat. Notably, the NOAEL value for rabbit developmental toxicity of the chemical butanone oxime is 24 mg/kg bw/d and much lower than that for rat (600 mg/kg bw/d), this outlier is displayed as filled dots in Fig. 7.
Fig. 6.
Pearson correlation coefficient between rat and rabbit maternal toxicity.
Fig. 7.
Pearson correlation coefficient between rat and rabbit developmental toxicity.
3.4. Selective developmental toxicity for rats and rabbits
According to the category of selective developmental toxicity defined in the previous section we have estimated the prevalence of compounds causing selective developmental toxicity. There are 34 chemicals (7.1%) in rats and 5 chemicals (4.6%) in rabbits show selective developmental toxicity. Only one chemical (N,N-dime-thylacetamide) has selective developmental toxicity observed in both species.
4. Discussion
The major goal of this paper is to provide further data to calculate a general TTC value for prenatal developmental toxicity after oral administration. Such a value can potentially be used to reduce animal testing for industrial chemicals (e.g. within the context of the REACH legislation), for low exposure situations such as for impurities or environmental contaminants as well as in emergency situations for risk assessors and risk managers. The availability of a TTC value should, however, not be considered as a replacement for the testing of compounds, such as active ingredients (e.g. pesticides or drugs) for which there are clear data requirements.
In the current evaluation, we were able to take into account 434 NO(A)EL values for maternal toxicity and 469 NO(A)EL values for developmental toxicity in rats from the REACH database. The calculated TTC values, based on the lowest 5th percentile of the NO(A)EL distributions were very similar if gavage only was considered, or if all routes of oral administration were compared. To increase the number of chemicals available for inclusion and to enlarge the data base, we recommend using the data from “gavage” and “all routes of oral administration” in the calculation of a TTC value for maternal and developmental toxicity in rats. The fact that the results from both groups were similar was somewhat surprising because in gavage studies the entire dose is delivered within a few seconds, whereas in feeding or drinking water studies the dose is distributed over 24 h. Specifically, for compounds causing toxicity based on peak plasma levels (e.g. neurotoxicants), administration by gavage normally produces more pronounced toxicity than when the same dose is given via food or drinking water over 24 h. The lowest 5th percentile of the NO(A)EL distribution curve for maternal toxicity in rats was 10 mg/kg bw/d, whereas that for developmental toxicity was 13.35 mg/kg bw/d. In our earlier evaluations (van Ravenzwaay et al., 2011), dealing with rat prenatal developmental toxicity studies, we calculated the 5th percentile of the NOAEL values for maternal toxicity to be 4 mg/kg bw/d (based on 92 studies) and that for developmental toxicity to be 4 mg/kg bw/d (based on 111 studies). Although the differences between the two evaluations are rather small (approximately a factor of 3) they may still be of importance. In the present investigation we have used the results of studies submitted under REACH regulation, which therefore pertain almost exclusively only to a particular cohort of potential toxicants (i.e. industrial chemicals). The earlier evaluation however, was performed using in-house data which were predominantly derived from agrochemical active ingredients. Although the present sample size for the evaluation of chemicals was clearly much larger (about 4-fold) than that from the active ingredients, it is conceivable that compounds purposely designed to elicit a biological response would have higher toxicity and thus a lower NO(A)EL than general industrial chemicals, which, rather by chance, elicit a toxic response.
In the present evaluation we used 100 NO(A)EL values for maternal toxicity and 112 NO(A)EL values for developmental toxicity in rabbit. As observed in the rat data, the calculated TTC values, based on the lowest 5th percentile of the NO(A)EL distributions, were very similar if gavage only data was included, or all routes of oral administration were compared, even though the number of studies for other oral routes was rather small. Again, we propose using values obtained from the combined routes of exposure groups to calculate a TTC value for maternal and developmental toxicity in rabbits. The lowest 5th percentile of the NO(A)EL distribution curve for maternal toxicity in rabbits was 4 mg/kg bw/d, while that for developmental toxicity was 10 mg/kg bw/d.
The earlier evaluation of our database for prenatal developmental toxicity studies in rabbits following oral exposure (OECD guideline 414) demonstrated a NOAEL for maternal and developmental toxicity in 48 cases (van Ravenzwaay et al., 2012). The 5th percentile for the NOAEL value for maternal effects as well as for developmental effects was 5 mg/kg bw/d. Nearly all (44 out of 48 cases) of the developmental toxicity studies performed in our laboratories used Himalayan Rabbits (Crl:Chbb:HM). The data set for New Zealand White Rabbits (Crl:KBL[NZW]) was comprised of 4 studies.
Comparing the values from the present evaluation of the REACH database with the earlier evaluation of the BASF database, it can be seen that the 5th percentile for the NO(A)EL for maternal toxicity is quite similar in both cases: 4 and 5 mg/kg bw respectively (see Table 6). However, for the 5th percentile of the NO(A)EL for developmental toxicity a more apparent discrepancy can be observed: the values being 5 and 10 mg/kg bw/d respectively. Again, it should be reiterated that the BASF database consisted predominantly of active ingredients, whereas the REACH database is made up of industrial chemicals.
Table 6.
The 5% NO(A)EL maternal and developmental toxicity for rats and rabbits.
| 5th % NO(A)EL maternal toxicity (mg/kg bw/d) |
5th % NO(A)EL developmental toxicity (mg/kg bw/d) |
||
|---|---|---|---|
| rat | BASF DB | 4 | 4 |
| REACH DB | 10 | 13.35 | |
| BASF + REACH DB | 7.625 | 10 | |
|
| |||
| rabbit | BASF DB | 5 | 5 |
| REACH DB | 4 | 10 | |
| BASF + REACH DB | 5 | 9.5 | |
For TTC calculations for systemic toxicity (including carcinogenicity) it is quite common to assign Cramer classes to the individual compounds and perform TTC calculations for each class individually (Cramer et al., 1978; Tluczkiewicz et al., 2011). To our understanding this has not yet been done for developmental toxicity.
Evaluations of the use of Cramer classes in TTC value calculation have indicated that there is a good distinction between classes 1 (simple chemical structures and efficient modes of metabolism, suggesting low order oral toxicity) and 3 (chemical structures that permit no strong initial presumption of safety or may even suggest significant toxicity or have reactive functional groups). There are, however, problems in defining class 2 (substances which possess structures that are less innocuous than class I substances but do not contain structural features suggestive of toxicity like those substances in class III) compounds (Escher et al., 2010). Based on the work of Munro et al. (1996) the EFSA Scientific Committee (2011) suggests “[…] that consideration should be given to treating substances that would be classified in Cramer Class II under the Cramer decision tree as if they were Cramer Class III substances.”
The EFSA Scientific Committee (2011) concluded that “[…] NOELs for reproductive and developmental toxicity for substances classified as such under EU legislation, the TTC values for Cramer Classes I and III are considered sufficiently protective for adverse effects on reproduction or development”, the use of Cramer classes will necessitate a significant expansion of the database for the refinement of a general TTC for prenatal developmental toxicity, which should take into account both rat and rabbit data.
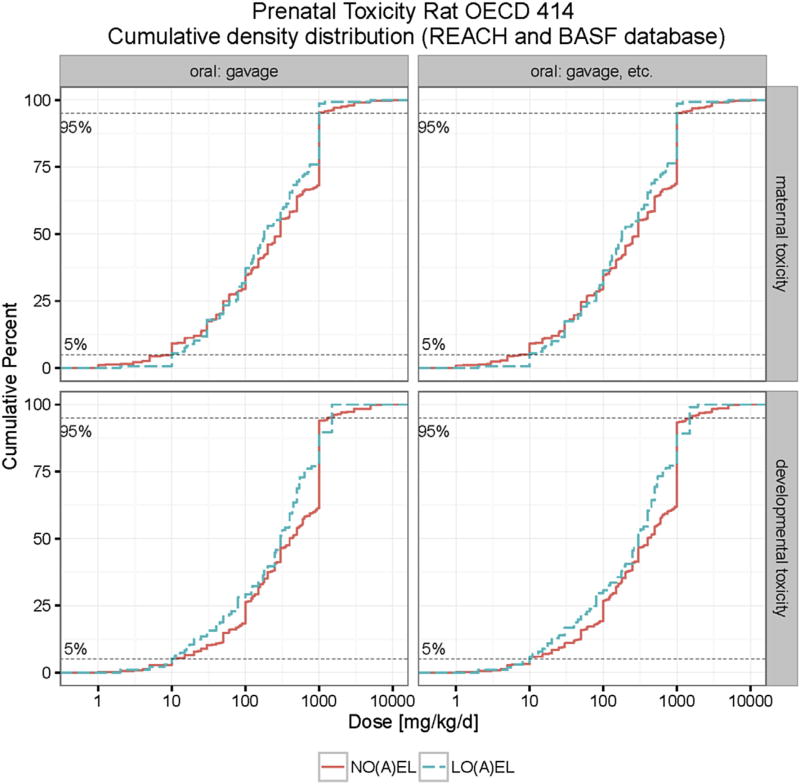
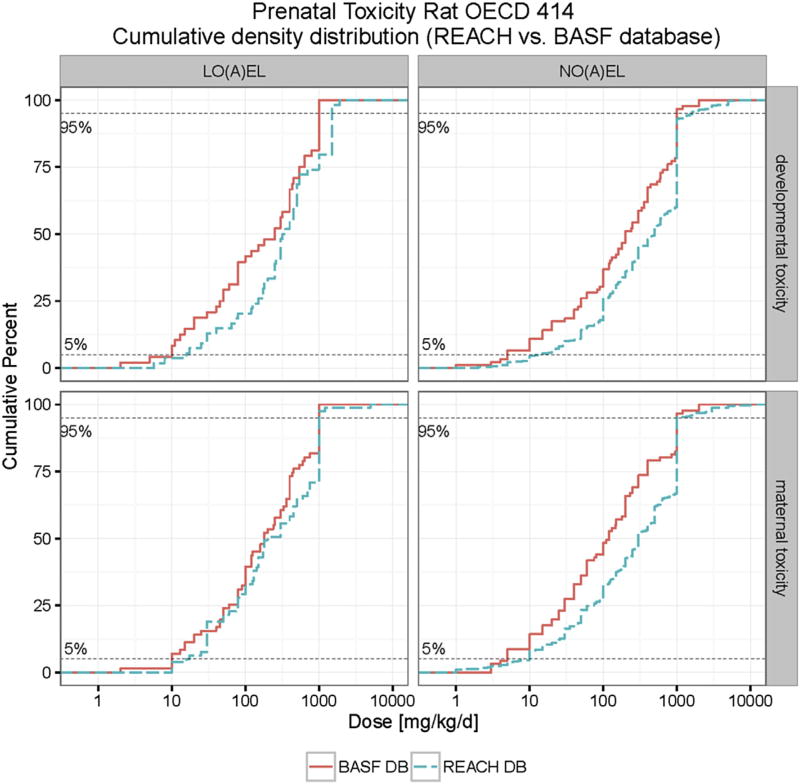
In the present paper we provide a significant expansion of the developmental toxicity database for rats, and in doing so, a significant advance in to fulfilling the EFSA requirements for this species. Combining the REACH and BASF database for rat developmental toxicity studies, and excluding double entries (24 in total), we obtain a total of 537 chemicals with appropriate NO(A)EL values for developmental toxicity. In cases where the BASF and REACH databases did not provide the same reference values (i.e. NO(A)EL values for developmental toxicity) always the lower value was used for calculation purposes. The lowest 5th percentile for the NO(A)EL for developmental toxicity in rats was calculated to be 10 mg/kg bd/d. The lowest 5th percentile for the NO(A)EL for maternal toxicity was calculated to be 7.6 mg/kg bw/d), see Figs. 8 and 9, Table 7.
Fig. 8.
Cumulative distribution of maternal and developmental toxicity (NO(A)EL and LO(A)EL) in rats combined BASF and REACH databases. The developmental toxicity LO(A)EL (NO(A)EL) values range from 2 (1) to 1920 (7000) mg/kg bw/d. The maternal toxicity LO(A)EL (NO(A)EL) values range from 2 (1) to 5000 (10000) mg/kg bw/d.
Fig. 9.
Comparison of cumulative distribution of maternal and developmental toxicity (NO(A)EL and LO(A)EL) in rats between BASF and REACH database. The developmental toxicity LO(A)EL (NO(A)EL) values range from 2 (1) to 1000 (2000) mg/kg bw/d for BASF database and range from 5.7 (2) to 1920 (7000) mg/kg bw/d for REACH database. The maternal toxicity LO(A)EL (NO(A)EL) values range from 2 (3) to 1000 (2000) mg/kg bw/d for BASF database and range from 10 (1) to 5000 (10000) mg/kg bw/d for REACH database.
Table 7.
Median and percentiles of NO(A)ELs and LO(A)ELs for maternal and developmental toxicity studies (OECD 414) in rats (measured in mg/kg/day) combined BASF and REACH datasets.
| N | 5th | 10th | Median | 90th | 95th | |
|---|---|---|---|---|---|---|
| Oral: gavage | ||||||
| Maternal NO(A)EL | 480 | 10 | 15 | 300 | 1000 | 1000 |
| Maternal LO(A)EL | 145 | 10 | 20 | 180 | 1000 | 1000 |
| Developmental NO(A)EL | 511 | 10 | 30 | 400 | 1000 | 1322.5 |
| Developmental LO(A)EL | 97 | 10 | 17.05 | 300 | 1150 | 1500 |
| Oral: gavage, drinking water, feed and unspecified | ||||||
| Maternal NO(A)EL | 501 | 7.625 | 15 | 300 | 1000 | 1000 |
| Maternal LO(A)EL | 148 | 10 | 20 | 180 | 1000 | 1000 |
| Developmental NO(A)EL | 537 | 10 | 30 | 400 | 1000 | 1515 |
| Developmental LO(A)EL | 102 | 10 | 16.3 | 300 | 1400 | 1500 |
Given the size of the database, and the representation of chemicals and agrochemical active ingredients a standard assessment factor of 100 is proposed to calculate a TTC value for developmental toxicity. As the use of Cramer classes has not been well established for developmental toxicity, we consider it more prudent to work with the entire database. As the difference in the 5th percentile values between the REACH database (containing mostly industrial chemicals) and the BASF database (containing mostly agrochemical active ingredients) is not very large (approximately factor of 3) this may also be an indication that differentiating in Cramer classes may not be appropriate for developmental toxicity.
Based on the assumptions we calculate a TTC value for developmental toxicity in rats of 10 / 100 = 0.1 mg/kg bw/d.
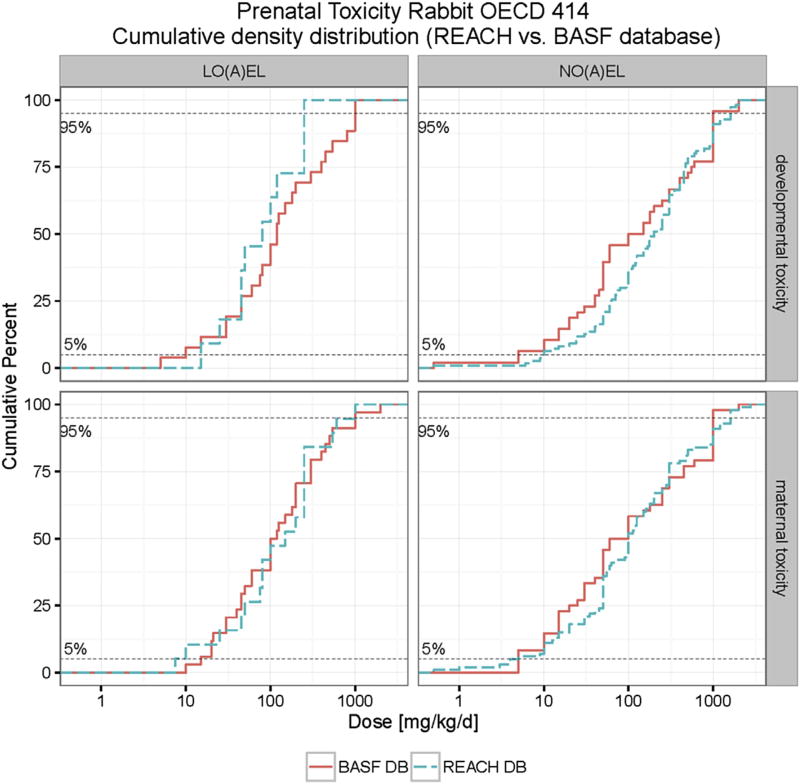
For rabbits the analysis of the REACH database provided 112 values for developmental toxicity. The BASF database consisted of 48 cases. Excluding double entries we now have a total of 150 (8 chemicals contained in both databases) values. In cases where the BASF and REACH databases did not provide the same reference values (i.e. NO(A)EL values for developmental toxicity) always the lower value was used for calculation purposes. The combined evaluation of both databases results in a lowest 5th percentile value of 9.5 mg/kg bw/d, see Figs. 10 and 11, Table 8.
Fig. 10.
Cumulative distribution of maternal and developmental toxicity (NO(A)EL and LO(A)EL) in rabbits combined BASF and REACH databases. The developmental toxicity LO(A) EL (NO(A)EL) values range from 5 (0.5) to 1000 (2000) mg/kg bw/d. The maternal toxicity LO(A)EL (NO(A)EL) values range from 7.5 (0.5) to 2000 (2765) mg/kg bw/d.
Fig. 11.
Comparison of cumulative distribution of maternal and developmental toxicity (NO(A)EL and LO(A)EL) in rabbits between BASF and REACH database. The developmental toxicity LO(A)EL (NO(A)EL) values range from 5 (0.5) to 1000 (2000) mg/kg bw/d for BASF database and range from 15 (0.5) to 250 (2000) mg/kg bw/d for REACH database. The maternal toxicity LO(A)EL (NO(A)EL) values range from 10 (5) to 2000 (2000) mg/kg bw/d for BASF database and range from 7.5 (0.5) to 1000 (2765) mg/kg bw/d for REACH database.
Table 8.
Median and percentiles of NO(A)ELs and LO(A)ELs for maternal and developmental toxicity studies (OECD 414) in rabbits (measured in mg/kg/day) combined BASF and REACH datasets.
| N | 5th | 10th | Median | 90th | 95th | |
|---|---|---|---|---|---|---|
| Oral: gavage | ||||||
| Maternal NO(A)EL | 135 | 5 | 10 | 100 | 1000 | 1300 |
| Maternal LO(A)EL | 51 | 10 | 20 | 110 | 536 | 1000 |
| Developmental NO(A)EL | 144 | 9.2 | 15 | 180 | 1000 | 1600 |
| Developmental LO(A)EL | 36 | 9 | 15 | 100 | 644 | 1000 |
| Oral: gavage, drinking water, feed and unspecified | ||||||
| Maternal NO(A)EL | 140 | 5 | 10 | 100 | 1000 | 1600 |
| Maternal LO(A)EL | 51 | 10 | 20 | 110 | 536 | 1000 |
| Developmental NO(A)EL | 150 | 9.5 | 15 | 175 | 1000 | 1600 |
| Developmental LO(A)EL | 36 | 9 | 15 | 100 | 644 | 1000 |
As for rats, the REACH database consists mainly of industrial chemicals, while the BASF database is almost exclusively populated with data from agrochemicals. Here, in contrast to the rat values, there is absolutely no difference in the 5th percentile values of the REACH, BASF and combined data sets. This strengthens our earlier preliminary, conclusion that for developmental toxicity Cramer class evaluation may not be appropriate.
Based on the same assumptions as for the rat, the TTC value for developmental toxicity in rabbits is 9.5/100 = 95 µg kg bw/d.
The present evaluation has some important limitations. Details with respect to the nature of the developmental toxicity observed cannot be obtained from the database. We assumed that not all the observed developmental toxicity was related to maternal toxicity, and that consequently a good number of potential modes of action for developmental toxicity were covered. As the reference values used to calculated TTCs in our previous publications (van Ravenzwaay et al., 2011, 2012) were rather similar, but obtained from pesticides, which includes at least some classes of chemistry (e.g. triazoles) with modes of action known to cause selective developmental toxicity, it would seem that at least for pesticides and industrial chemicals there is sufficient data to validate the correctness of the presented values, without a particular risk that certain modes of action with a very high potency would not be covered. The database, however does not contain pharmaceutical active ingredients and therefore cannot be used for such compounds until proven that the TTC reference values are similar.
Of particular interest are those compounds which are found to have NO(A)EL values below the 5th percentile distribution. In rats we found 19 compounds and in rabbits seven compounds below the 5th percentile, i. e lower than 10 mg/kg bw/day. For rats the lowest values range from 1 mg/kg bw (lowest overall value in this species) to approximately 8 mg/kg bw/day/day) In rabbits the range is from 0.5 mg/kg bw/day (overall lowest value) to 9 mg/kg bw/day. Thus, the lowest NOAEL value for both species and all tests (N = 647 studies) is 0.5 mg/kg bw/day. In this group of compounds with low NO(A)EL values there were nine agrochemical active ingredients and 17 industrial chemicals. With respect to the LOAEL values, the lowest value obtained in rats is 2 mg/kg bw and in rabbits 5 mg/kg bw. Consequently, the overall lowest dose which caused developmental toxicity was 2 mg/kg bw/day (in rats), obtained for Vitamin A acid. Moreover, we evaluated how many of these “high potency” chemicals had selective developmental toxicity. In total we found three compounds; two in rats (one of which had no maternal toxicity data and was assumed to have a selective effect) and one in rabbits.
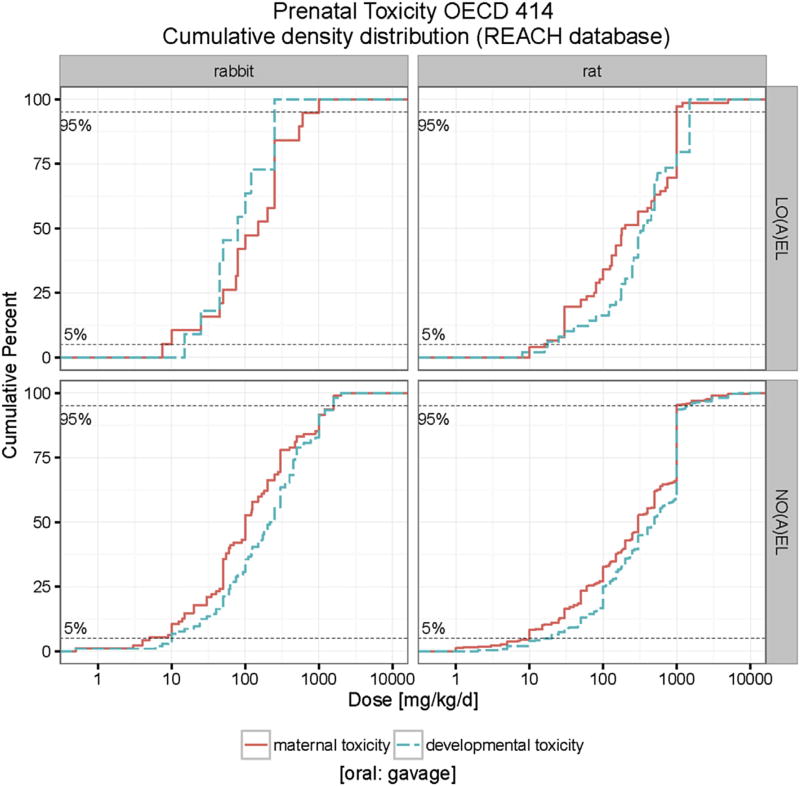
The database also provides an opportunity to compare, on a large scale, the relative sensitivities of the maternal organism and its unborn offspring. Fig. 12 displays the cumulative distribution curves of maternal and developmental toxicity NO(A)EL and LO(A) EL values for gavage studies in rats and rabbits. The curves of NO(A) EL and LO(A)EL values for maternal toxicity are generally shifted to the left compared to those for developmental toxicity, except for the higher end of the LO(A)EL curve for rabbits. The differences, however are rather marginal. The data would suggest the maternal organism might be slightly more susceptible than the fetus to the toxic effects of the administered chemicals. It should be noted here however, that the investigations to determine maternal toxicity are relatively crude and incomparable to the detailed investigations performed in the fetus, and that therefore, the difference in sensitivity may, in reality, be greater.
Fig. 12.
Comparison of the cumulative distribution curves of maternal and developmental toxicity NO(A)EL and LO(A)EL values for gavage studies in rats and rabbits. The developmental toxicity LO(A)EL and NO(A)EL values (in parenthesis) range from 15 (0.5) to 250 (2000) mg/kg bw/d in rabbits and range from 8 (2) to 1500 (7000) mg/kg bw/d in rats. The maternal toxicity LO(A)EL and NO(A)EL values range from 7.5 (0.5) to 1000 (2000) mg/kg bw/d in rabbits and range from 10 (1) to 5000 (10000) mg/kg bw/d in rats.
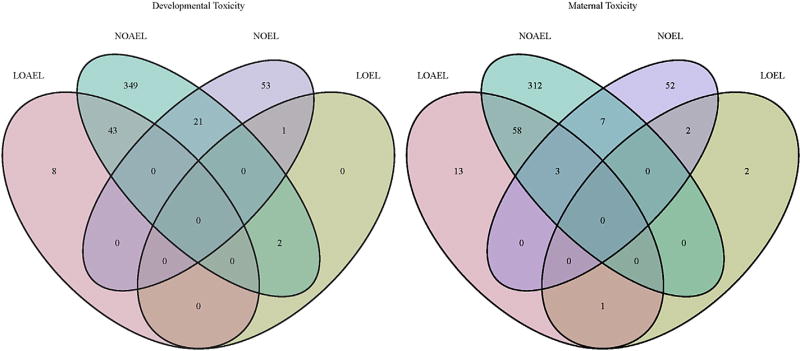
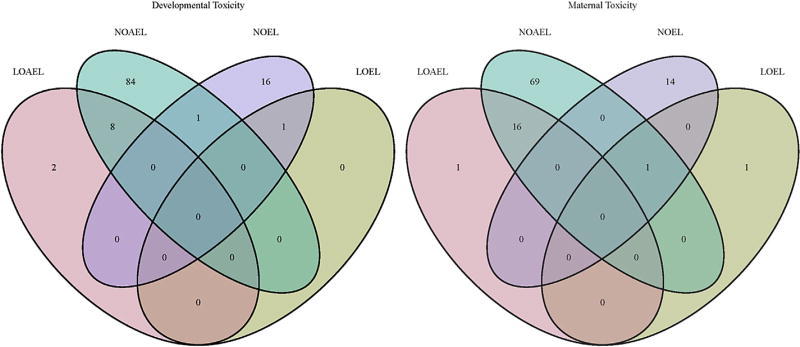
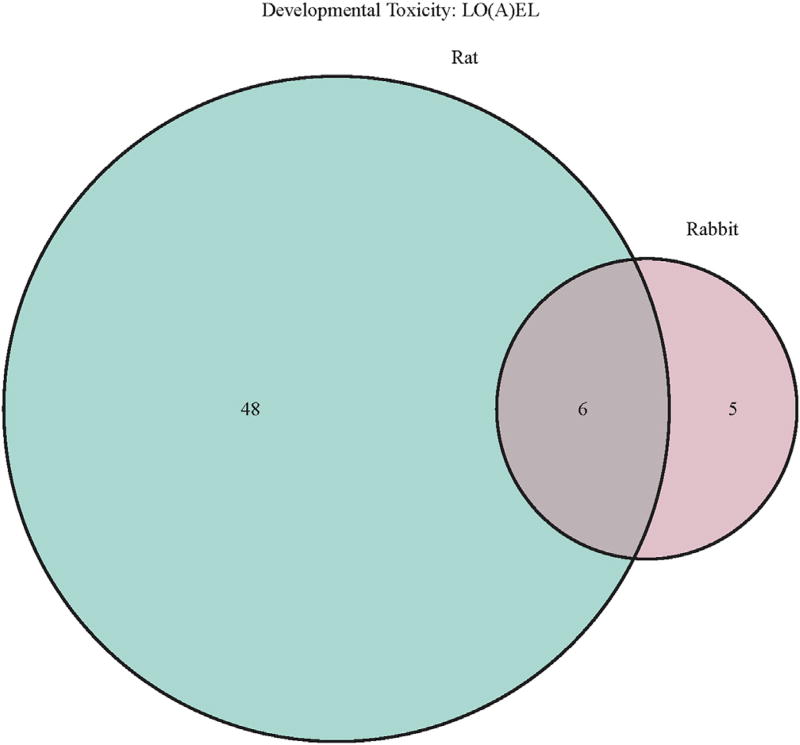
A further analysis of the database provides some insight of the unique aspects of species-specific developmental toxicity. We have evaluated how many chemicals had developmental toxicity (LOAEL) in only one species, i.e. how many chemicals had developmental toxicity LOAEL in rat but not rabbit and vice versa. As the number of rat studies is far higher than rabbit studies it is not surprising that the number of LOAEL values for developmental toxicity is much higher in rats. Nevertheless, and somewhat surprisingly, is that the prevalence of developmental toxicity in both species is virtually identical, i.e. ca. 12% in the rat studies and 11% in rabbit studies. The data also indicate that each species has its own particular sensitivity for developmental toxicity, as ca. 50% of the compounds (i.e. 5) with developmental toxicity observed in rabbits were not detected in rats. Fig. 13 illustrates the overall and uniqueness of with respect to developmental toxicity in rats and rabbits.
Fig. 13.
Venndiagram illustrates the number of LO(A)EL values for developmental toxicity in rats and rabbits.
We have proposed the use of a safety factor of 100 on the lowest 5th percentile of the NO(A)EL distribution. Most safety factors used for risk assessment purposes are somewhat arbitrary (unless derived with specific knowledge on the toxico-dynamics and -kinetics of the compound in question), in this case an SF = 100 is justifiable based on the size of the database, and the fact that two species have been taken into account. Moreover, it could be demonstrated that the calculated TTC value covers 100% of compounds for both NO(A)EL as well as LO(A)EL values.
The REACH legislation proposes to primarily use non-animal test systems and subsequently tailor-made testing approaches taking into account exposure and existing test results. However, there are currently no alternative methods for developmental toxicity available which have gained regulatory acceptance. Strategies to develop such alternatives have been proposed (Basketter et al., 2012; Leist et al., 2014), but will not lead to contributions before the REACH 2018 deadline, with the possible exception of read-across approaches (Patlewicz et al., 2014; Ball et al., 2016; Hartung, 2016). Thus, waiving (exemption from conducting individual toxicity tests) of studies based on negligible exposure is one of the few other options to reduce animal testing. It is, however, difficult to define what constitutes “no exposure” and the REACH Annexes VIII-XI use different terms to describe the conditions that allow waiving based on exposure considerations (“no relevant exposure”, “limited exposure”, “no exposure”, “no significant exposure” and “unlikely exposure”), but refrain from defining the level of exposure thought to be so small that no risk can result irrespective of the inherent toxicity of the chemical. Given that absence of relevant exposure has not been defined, the development of end point-specific TTC values could be used to derive these exposure values based on the likelihood of absence of adverse effects. An example of the use of an exposure based waiving concept is provided by Heath Canada, who, using the general TTC value obtained from the Munroe database identified 89 chemicals (out of approximately 1500) that are unlikely to pose a risk to human health based on exposure (Health Canada 2016) and for which animal testing was considered unnecessary. To request a waiver for not performing a developmental toxicity study considered necessary by the relevant authority, the margin of exposure should clearly be below the calculated TTC values and would also need to take the distribution of exposure into account.
However, in contrast to a waived animal test, no information on classification and labeling of a chemical, or on the potency of a specific effect could be provided by a TTC approach. One opportunity to fulfil such a requirement would be to take the chemical structure into account (Luechtefeld et al., 2016a; 2016b, 2016c). The study illustrates the enormous value of the REACH registration data set, which in December 2014 already included information pertaining to about 10,000 substances. It should be noted that in early 2016 already more than 15,000 substances had been registered, although these data are not yet publicly available. In view of the upcoming major registration effort in May 2018, there is an urgent need to enable analyses such as ours here to limit both animal testing and inform chemical safety evaluation.
Supplementary Material
Acknowledgments
Funding sources statement
The work was not funded by any sources.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.yrtph.2017.06.008.
Footnotes
Conflict of interest statement
Tom Luechtefeld is the cofounder of ToxTrack LLC developing computational toxicology tools. Thomas Hartung and Tom Luechtefeld are consulting Underwriter Laboratories (UL) in their efforts to develop computational tools and services for the safety assessments of chemicals and receive shares of revenue for their efforts. TTC approaches are not currently involved in these efforts. The other authors declare that there are no conflicts of interest.
If the end point specific TTC concept gains eventually regulatory acceptance, chemical industry, including BASF SE, employing Bennard van Ravenzwaay, may be able to reduce animal testing and reduce financial resources allocated to such tests.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.yrtph.2017.06.008.
References
- Ball N, Cronin MTD, Shen J, Adenuga MD, Blackburn K, Booth ED, Bouhifd M, Donley E, Egnash L, Freeman JJ, Hastings C, Juberg DR, Kleensang A, Kleinstreuer N, Kroese D, Lee AC, Luechtefeld T, Maertens A, Marty S, Naciff JM, Palmer J, Pamies D, Penman M, Richarz A-N, Russo DP, Stuard SB, Patlewicz G, van Ravenzwaay B, Wu S, Zhu H, Hartung T. Toward good read-across practice (GRAP) guidance. ALTEX. 2016;33:149–166. doi: 10.14573/altex.1601251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SM, Kozianowski G, Wurtzen G, Schlatter J. Threshold of toxico-logical concern for chemical substances present in the diet. Food Chem. Toxicol. 2001;39:893–905. doi: 10.1016/s0278-6915(01)00045-x. [DOI] [PubMed] [Google Scholar]
- Basketter DA, Clewell H, Kimber I, Rossi A, Blaauboer B, Burrier R, Daneshian M, Eskes C, Goldberg A, Hasiwa N, Hoffmann S, Jaworska J, Knudsen TB, Landsiedel R, Leist M, Locke P, Maxwell G, McKim J, McVey EA, Ouédraogo G, Patlewicz G, Pelkonen O, Roggen E, Rovida C, Ruhdel I, Schwarz M, Schepky A, Schoeters G, Skinner N, Trentz K, Turner M, Vanparys P, Yager J, Zurlo J, Hartung T. A roadmap for the development of alternative (non-animal) methods for systemic toxicity testing. ALTEX. 2012;29:3–89. doi: 10.14573/altex.2012.1.003. [DOI] [PubMed] [Google Scholar]
- Bernauer U, Heinemeyer G, Heinrich-Hirsch B, Ulbrich B, Gundert-Remy U. Exposure-triggered reproductive toxicity testing under the REACH legislation: a proposal to define significant/relevant exposure. Toxicol. Lett. 2008;176:68–76. doi: 10.1016/j.toxlet.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Blackburn K, Stickney JA, Carlson-Lynch HL, McGinnis PM, Chappell L, Felter SP. Application of the threshold of toxicological concern approach to ingredients in personal and household care products. Regul. Toxicol. Pharmacol. 2005;43:249–259. doi: 10.1016/j.yrtph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bowler M. HtmlUnit. 2002 http://sourceforge.net/p/htmlunit/mailman/message/5463073/
- Chodorow K. MongoDB: the Definitive Guide. O’Reilly Media, Inc; Sebastopol, CA: 2013. p. 432. [Google Scholar]
- Cramer GM, Ford RA, Hall RL. Estimation of toxic hazard — a decision tree approach. Food Cosmet. Toxicol. 1978;16:255–276. doi: 10.1016/s0015-6264(76)80522-6. [DOI] [PubMed] [Google Scholar]
- De Wolf W, Sieber-Sauer A, Lecloux A, Koch A, Holt M, Feytel T, Comber M, Boeije G. Mode of action and aquatic exposure thresholds of no concern. Environ. Toxic. Chem. 2005;24:479–485. doi: 10.1897/04-133r.1. [DOI] [PubMed] [Google Scholar]
- Dolan DG, Nauman BD, Sargent EV, Maier A, Dourson M. Application of the threshold of toxicological concern concept to pharmaceutica manufacturing operations. Regul. Toxicol. Pharmacol. 2005;43:1–9. doi: 10.1016/j.yrtph.2005.06.010. [DOI] [PubMed] [Google Scholar]
- EFSA. European Food Safety Authority. Scientific Panel on Food Additives, Flavorings, Processing Aids and Materials in Contact with Food. Opinion on Flavoring Group FGE.03 Acetals of branched- and straight-chain aliphatic saturated primary alcohols and branched- and straight-chain saturated aldehydes, and an orthoester of formic acid, from chemical groups 1 and 2. Opinion expressed on 7 October 2004. 2004 Available at: www.efsa.eu.int/science/afc/catindex_en.html.
- EFSA. European Food Safety Authority. EFSA Scientific Committee. DRAFT Scientific Opinion on Exploring options for providing preliminary advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC) Endorsed for Public Consultation 2011. 2011 Available at: http://www.efsa.europa.eu/en/consultationsclosed/call/110712a.pdf.
- EMEA. The European Medical Control Agency. Committee for Medicinal Products for Human Use. Guideline on the limits of genotoxic impurities; London: 2003. Jun 23, 2003. http://eudra.org/emea.html. [Google Scholar]
- EMEA. European Medicines Evaluation Agency. Committee for Medicinal Products for Human Use (CHMP; London: 2004. Jun 23, Guideline on the limits of genotoxic impurities. CPMP/SWP/5199/02. 2004. http://www.emeaeu.int/pdfs/human/swp/519902en.pdf. [Google Scholar]
- EPA Health Effects Test Guidelines. OPPTS 870.3700: Prenatal Developmental Toxicity Study. 1998 Aug [Google Scholar]
- Escher SE, Tluczkiewicz I, Batke M, Bitsch A, Melber C, Kroese ED, Buist HE, Mangelsdorf I. Evaluation of inhalation TTC values with the data base RepDose. Regul. Toxicol. Pharmacol. 2010;58:259–274. doi: 10.1016/j.yrtph.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. US Food and Drug Administration, Department of Health and Human Services. 1995:174. Food Additives; Threshold of Regulation for Substances Used in Food-Contact Articles 21 CFR Parts 5, 25 170, 171. [Google Scholar]
- Godbillon S. ReactiveMongo. GitHub repository. 2015 https://github.com/ReactiveMongo.
- Hartung T. Making big sense from big data in toxicology by read-across. ALTEX. 2016;33:83–93. doi: 10.14573/altex.1603091. [DOI] [PubMed] [Google Scholar]
- Health Canada, Sience Approaóch Doucment. Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. 2016 http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=326E3E17-1.
- IPCS. International Program on Chemical Safety. World Health Organisation, WHO Food Additives Series 35; WHO, Geneva, Switzerland: 1998. [Google Scholar]
- JECFA. Forty-first Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report Series 837. World Health Organization; Geneva: 1993. Evaluation of Certain Food Additives and Contaminants. Safety Evaluation of Flavoring Agents. [PubMed] [Google Scholar]
- JECFA. Forty-fourth Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization; Geneva: 1995. Evaluation of Certain Food Additives and Contaminants. Safety evaluation of flavoring agents. WHO Technical Report Series 859; pp. 2–3. [Google Scholar]
- JECFA. Forty-ninth Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization, Geneva; 1999. Evaluation of Certain Food Additives and Contaminants. Procedure for the safety evaluation of flavoring agents. WHO Technical Report Series 884; pp. 3–6. [PubMed] [Google Scholar]
- JECFA. Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1956–2003) First through Sixty-first Meetings. 2003 http://jecfa.ilsi.org/
- JECFA. Joint FAO/WHO Expert Committee on Food Additives Sixty-fifth Meeting: Summary and Conclusions, June 7–16, 2005. Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO; Geneva, Switz: 2005. [JECFA/65/SC]. Available from: ftp://ftp.fao.org/es/esn/jecfa/jecfa65_summary.pdf. [Google Scholar]
- JECFA. Evaluation of Certain Food Additives and Contaminants. Sixty-fifth Report of the Joint FAO/WHO Expert Committee on Food Additives, June 7–16, 2005. Geneva. World Health Organization (WHO); Geneva, Switz: 2006. WHO Technical Report Series, No. 934. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_934.pdf. [Google Scholar]
- Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, van Schothorst F, Vos JG, Wurtzen G. Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem. Toxicol. 2004;42:65–83. doi: 10.1016/j.fct.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kroes R, Renwick AG, Feron V, Galli CL, Gibney M, Greim H, Guy RH, Lhuguenot JC, van de Sandt JJ. Applications of the threshold of toxicological concern (TTC) to the safety evaluation of cosmetic ingredients. Food Chem. Toxicol. 2007;45:2533–2562. doi: 10.1016/j.fct.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Laufersweiler MC, Gadagbui B, Baskerville-Abraham IM, Maier A, Willis A, Scialli AR, Carr GJ, Felter SP, Blackburn K, Daston G. Correlation of chemical structure with reproductive and developmental toxicity as it relates to the use of the threshold of toxicological concern. Regul. Toxicol. Pharmacol. 2012;62:160–182. doi: 10.1016/j.yrtph.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Leist M, Hasiwa N, Rovida C, Daneshian M, Basketter D, Kimber I, Clewell H, Gocht T, Goldberg A, Busquet F, Rossi A-M, Schwarz M, Stephens M, Taalman R, Knudsen TB, McKim J, Harris G, Pamies D, Hartung T. Consensus report on the future of animal-free systemic toxicity testing. ALTEX. 2014;31:341–356. doi: 10.14573/altex.1406091. [DOI] [PubMed] [Google Scholar]
- Luechtefeld T, Maertens A, Russo DP, Rovida C, Zhu H, Hartung T. Global analysis of publicly available safety data for 9,801 substances registered under REACH from 2008–2014. ALTEX. 2016a;33:95–109. doi: 10.14573/altex.1510052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luechtefeld T, Maertens A, Russo DP, Rovida C, Zhu H, Hartung T. Analysis of public oral toxicity data from REACH registrations 2008–2014. ALTEX. 2016b;33:111–122. doi: 10.14573/altex.1510054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luechtefeld T, Maertens A, Russo DP, Rovida C, Zhu H, Hartung T. Analysis of Draize eye irritation testing and its prediction by mining publicly available 2008–2014 REACH data. ALTEX. 2016c;33:123–134. doi: 10.14573/altex.1510053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luechtefeld T, Maertens A, Russo DP, Rovida C, Zhu H, Hartung T. Analysis of publically available skin sensitization data from REACH registrations 2008–2014. ALTEX. 2016d;33:135–148. doi: 10.14573/altex.1510055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melching-Kollmuβ S, Dekant W, Kalberlah F. Application of the “threshold of toxicological concern” to derive tolerable concentrations of “non relevant metabolites” formed from plant protection products in ground and drinking water. Regul. Toxicol. Pharmacol. 2010;56:126–134. doi: 10.1016/j.yrtph.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Müller L, Mauthe RJ, Riley CM, Andino MM, De Antonis D, Beels C, DeGeorge J, De Knaep AGM, Ellison D, Fagerland JA, Frank R, Fritschel B, Galloway S, Harpur E, Humfrey CDN, Jacks AS, Jagota N, Mackinnon J, Mohan G, Ness DK, ÓDonovan MR, Smith MD, Vudathala G, Yotti L. A rationale for determining, testing, and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity. Regul. Toxicol. Pharmacol. 2006;44:198–211. doi: 10.1016/j.yrtph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Munro IC. Safety assessment procedures for indirect food additives: an overview. Report of a Workshop. Regul. Toxicol. Pharmacol. 1990;12:2–12. doi: 10.1016/s0273-2300(05)80042-x. [DOI] [PubMed] [Google Scholar]
- Munro IC, Ford RA, Kennepohl E, Sprenger JG. Correlation of structural class with no-observed effect levels: a proposal for establishing a threshold of concern. Food Chem. Toxicol. 1996;34:829–867. doi: 10.1016/s0278-6915(96)00049-x. [DOI] [PubMed] [Google Scholar]
- Munro IC, Kennepohl E, Kroes R. A procedure for the safety evaluation of flavoring substances. Food Chem. Toxicol. 1999;37:207–232. doi: 10.1016/s0278-6915(98)00112-4. [DOI] [PubMed] [Google Scholar]
- OECD Guideline for Testing of Chemicals. Proposal for Updating Guideline 414: Prenatal Developmental Toxicity Study 2001 Jan 22; [Google Scholar]
- Patlewicz G, Ball N, Becker RA, Blackburn K, Booth E, Cronin M, Kroese D, Steup D, van Ravenzwaay B, Hartung T. Read-across approaches - misconceptions, promises and challenges ahead. ALTEX. 2014;31:387–396. doi: 10.14573/altex.1410071. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org. [Google Scholar]
- Renwick AG. Toxicology databases and the concept of thresholds of toxi-cological concern as used by the JECFA for the safety evaluation of flavoring agents. Toxicol. Lett. 2004;149:223–234. doi: 10.1016/j.toxlet.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Tluczkiewicz I, Buist HE, Martin MT, Mangelsdorf I, Escher SE. Improvement of the Cramer classification for oral exposure using the database TTC RepDose - A strategy description. Regul. Toxicol. Pharmacol. 2011;61:340–350. doi: 10.1016/j.yrtph.2011.09.005. [DOI] [PubMed] [Google Scholar]
- van Ravenzwaay B, Dammann M, Buesen M, Schneider S. The threshold of toxicological concern for prenatal developmental toxicity. Regul. Toxicol. Pharmacol. 2011;59:81–90. doi: 10.1016/j.yrtph.2010.09.009. [DOI] [PubMed] [Google Scholar]
- van Ravenzwaay B, Dammann M, Buesen R, Flick B, Schneider S. The threshold of toxicological concern for prenatal developmental toxicity in rabbits and a comparison of TTC values in rats. Regul. Toxicol. Pharmacol. 2012;64:1–8. doi: 10.1016/j.yrtph.2012.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.