Abstract
Spatial learning and memory are critically dependent on the integrity of hippocampal systems. Functional MRI and neuropathological studies show that hippocampal circuitry is prominently affected among HIV-seropositive individuals, but potential spatial learning and memory deficits have not been studied in detail in this population. We investigated the independent and interactive effects of sex and HIV serostatus on performance of a spatial learning and memory task in a sample of 181 individuals with a history of cocaine dependence. We found that men showed faster times to completion on immediate recall trials compared with women and that delayed recall was significantly poorer among HIV-infected compared with HIV-uninfected participants. Additionally, a sex × serostatus effect was found on the total number of completed learning trials. Specifically, HIV-infected men successfully completed more learning trials compared with HIV-infected women. Results are discussed in the context of recent reports of sex and HIV serostatus effects on episodic memory performance.
Keywords: Spatial memory, Cocaine, HIV, Cognitive impairment, Sex differences
Introduction
Deficits in episodic memory, the acquisition and retention of information for specific events or personal experiences, are common (approximately 40–60%) among HIV-infected individuals (Woods et al. 2009; Reger et al. 2002), significantly affect the capacity for everyday function (Heaton et al. 2004), and have been linked to a dysregulation of activity in prefrontal-striatal and hippocampal networks (Thompson et al. 2005; Castelo et al. 2006; Maki et al. 2009). For example, Moore et al. (2006) found that the extent of postmortem putamen and hippocampal pathology in HIV-infected individuals significantly and independently predicted the severity of lifetime neurocognitive impairment. In an fMRI study of 54 HIV-infected and 12 HIV-uninfected women with a verbal memory probe task, Maki and colleagues demonstrated that HIV-infected women showed significantly decreased hippocampal activation during encoding and increased hippocampal activation during recall. These hippocampal alterations were also associated with poorer performance on the Hopkins Verbal Learning Test (HVLT) (Maki et al. 2009).
Most published investigations of episodic memory among HIV-infected individuals have targeted verbal (e.g., Murji et al. 2003; Woods et al. 2005) or visual information processing (Keutmann et al. 2016; Morgan et al. 2009). Memory for spatial and navigational learning has been relatively understudied among persons living with HIV/AIDS, despite converging evidence from the animal literature of deleterious effects of HIV on spatial memory (Vigorito et al. 2007; Carey et al. 2012).
The Morris water maze (MWM) has been employed in numerous studies of spatial learning and memory in rodents and is highly sensitive to both hippocampal pathology (Vorhees and Williams 2006; Redish and Touretzky 1998) and HIV (Sanchez-Alavez et al. 2000; Vigorito et al. 2007; Lashomb et al. 2009). Raber and colleagues introduced the Memory Island (MI) task as an MWM analogue for use with human subjects (Rizk-Jackson et al. 2006). The task requires participants to traverse a virtual island to locate and recall four different objects. Task performance includes indices of spatial learning, immediate recall, and delayed recall. Thus, the MI task allows for a translational model of spatial learning and memory, which we employ in the current study.
In one such study using the MI task, Morales et al. (2012) found that HIV-infected women learned the task more slowly and showed significantly poorer delayed recall than HIV-uninfected women. Furthermore, these effects were most prominent among HIV-infected women with documented neurocognitive impairment. The Morales study is unique as it is the first published study to examine HIV serostatus effects on spatial and navigational learning within an all-female sample. To our knowledge, no studies have examined MI performance in HIV-infected versus HIV-uninfected men. An examination of the performance of HIV-infected men is of interest given that men typically outperform women on spatial learning and memory tasks among non-clinical samples (Maeda and Yoon 2013; Driscoll et al. 2005; Moffat et al. 1998).
Additionally, the Morales study participants were largely free of potentially confounding comorbid conditions including substance use, which may limit generalization of their results to a large subset of persons living with HIV. Notably, converging evidence suggests that crack cocaine use increases the risk of AIDS-related mortality and disease progression (Cook et al. 2008) and recent studies have reported an 11% prevalence of seroconversion among non-injection drug users (Kuo et al. 2011). A study of 1395 women enrolled in the Women’s Interagency HIV Study (WIHS) reported that verbal memory impairment was most evident among HIV-infected women who reported recent use of crack cocaine, compared with HIV-uninfected women (Meyer et al. 2013). Additionally, a recent study of visuospatial episodic memory among HIV-infected and HIV-uninfected men and women substance users reported that impaired performance of the Brief Visuospatial Memory Test-Revised (BVMT-R, see Benedict 1997) was most prominent among HIV-infected women with a history of cocaine dependence (Keutmann et al. 2016).
In the current study, we administered the Memory Island task (Rizk-Jackson et al. 2006) to 67 HIV-infected and 114 HIV-uninfected men and women with a history of cocaine dependence. The purpose of this study was to investigate spatial learning and memory performance among HIV-infected individuals compared with HIV-uninfected individuals and among women compared with men. We hypothesized that (1) HIV-infected individuals would perform more poorly on the MI task compared with HIV-uninfected individuals and that (2) women would perform the task significantly more poorly compared with men.
Method
Participants
Participants included 67 HIV-infected and 114 enzyme-linked immunosorbent assay (ELISA)-verified HIV-uninfected cocaine-dependent individuals enrolled in a larger NIH-funded study on the effects of sex and HIV serostatus on neurocognition. Participants were recruited from infectious disease clinics at Rush University Medical Center (RUMC), University of Illinois at Chicago (UIC), Ruth M. Rothstein CORE Center at Stroger (formerly Cook County) Hospital, community agencies, and by word of mouth. Potential participants with AIDS-defining or other CNS illness or injury, closed head injury with loss of consciousness greater than 30 min, seizure disorder, schizophrenia, untreated bipolar disorder, or current antipsychotic medication were not eligible for the larger study. Participants were 18–60 years old and fluent in English and had completed 8 or more years of education. All participants in the current study met DSM-IV criteria for lifetime cocaine dependence. At the time of testing, the majority of participants met criteria for early (24%) or sustained (68%) remission. Median number of days since last use was 60 for alcohol (range 4–11,688), 227 for cocaine (range 7–8034), and 730 for heroin 730 (range 7–10,227). Participants dually diagnosed with cocaine and opioid dependence were excluded from the study.1 The sample was predominantly African American (86%), reflecting the demographic characteristics of cocaine-dependent individuals (CDIs) receiving medical and substance use care through UIC, CORE, Rush, and local substance use treatment programs. The study was approved by institutional review boards at Rush, the CORE Center, and UIC.
Procedure
Tests administered were part of a larger NIH-funded study protocol administered over two 120–150-min visits to RUMC’s Department of Outpatient Psychiatry. Testing was conducted by bachelor’s level research assistants under the supervision of a board-certified clinical neuropsychologist (EMM). Written informed consent was obtained upon arrival at the first study visit. To detect recent substance use (within 2–3 days prior to testing), all participants underwent a rapid urine toxicology screen at the start of each visit, which tested for ten illicit and prescription drugs, including opioids, cocaine, cannabis, benzodiazepines, and amphetamines. Participants also completed a breathalyzer test to ensure abstinence from alcohol at the time of testing. If a potential participant tested positive for alcohol or other drugs, the visit was terminated, the participant received no payment, and the visit was rescheduled.2 Participants were informed of these contingencies prior to the testing visit.
Measures
Clinical and personality measures
Participants completed the Wechsler Test of Adult Reading (WTAR; Wechsler 2001) to estimate premorbid verbal IQ and as an index of educational quality (Manly et al. 2011). Participants also completed a series of paper and pencil measures of conditions comorbid with substance use disorders with potentially confounding effects on neurocognitive test performance. These included the Mood Disorders modules of the Structured Clinical Interview for DSM-IV (SCID; First et al. 1995), PTSD Checklist—Civilian Version (Blake et al. 1995), Wender Utah Rating Scale of symptoms of attention deficit disorder (Stein et al. 1995), and Levenson Self-Report Psychopathy Scale, a measure of antisocial behavior for use with non-incarcerated individuals (Levenson et al. 1995). HIV-infected participants’ antiretroviral regimens were rated with the CNS Penetrance Effectiveness (CPE) scale (Smurzynski et al. 2011; Letendre et al. 2008), which ranks each antiretroviral compound in the participant’s current regimen according to its capacity to cross the blood-brain barrier. Total CPE score is obtained by summing the individual ranks.
Substance use
Participants were administered the SCID Substance Use Disorders module using the NetSCID (TeleSage, http://www.telesage.com/products/netscid.html), a web-based computer-assisted version of the SCID (First et al. 1995). Interviewers administered the Addictions Severity Index (ASI; McLellan et al. 1980). Finally, participants completed the Kreek-McHugh-Schluger-Kellogg scale (KMSK; Kellogg et al. 2003), a questionnaire employed as a proxy for lifetime severity of alcohol, cocaine, and opioid use derived from participant’s self-reported amount of money spent, frequency of use, and duration of the period of maximal use.
Spatial learning and memory
Participants completed the MI task (Rizk-Jackson et al. 2006), a computerized spatial learning measure.3 MI requires participants to traverse a virtual island using a joystick to find a series of four different objects located in four different quadrants. The task consists of four visible target trials (1–4), four hidden target trials (5–8), and a single delayed recall trial 15 min after the last hidden target trial.
During each visible target trial (1–4), the object is marked with a spatial cue (i.e., a tall flag) to assist in navigating the island. Participants are instructed to move the cursor from a fixed geographic starting point to the object. Upon completion of each trial, participants returned to the central fixed starting point, but their spatial position was reoriented 90°. Following four visible target practice trials, participants completed four hidden target trials. During the hidden target trials (5–8), if a participant did not locate a hidden object within 120 s, an arrow cue appeared directing the participant to the target object. The computer recorded time required to locate the target item for each trial. Performance on the visible and hidden trials is indexed by (1) the mean times to completion for each trial block and (2) the total number of “successful” trials (i.e., the participant locates the object within 120 s) for each trial block.
Fifteen minutes following the last hidden target trial, a single delayed recall trial was administered. The delayed recall trial required participants to locate a specific target object within a 30-s time limit without cued assistance. Delayed recall was indexed by the percentage of time participants spent in the quadrant that contained the target. All dependent variables were selected based on the published MI literature (e.g., Morales et al. 2012; Rizk-Jackson et al. 2006).
Results
Statistical analyses
Demographic, substance use, and comorbidity data were compared using factorial analyses of variance (ANOVA) for parametric data and chi-square tests for categorical data. Mean scores for times to completion for visible (practice) and hidden (immediate recall) trials were log transformed to handle the skewness in the distributions as these were time variables. A series of generalized linear models were used to examine the separate and interactive associations between HIV serostatus and sex on the Memory Island task. In addition, mean hidden scores were co-varied in the analysis of delayed recall performance to characterize amount of information retained over the delay period more precisely. All follow-up tests were computed using the appropriate error term from the primary mixed factor analysis. Greenhouse-Geisser corrected p values were used to control for family-wise error. Significance was set at p < 0.05.
Tables 1 and 2 show demographic, comorbid characteristics and substance dependence grouped by sex and HIV serostatus. Groups were generally comparable on measures of demographic characteristics and comorbid psychiatric disorders, except for significantly higher mean PTSD-C scores among women compared with men (F(1, 177) = 4.37, p = 0.04) and a non-significant trend (p = 0.06) toward a higher percentage of African Americans among men compared with women. Subsequent MI analyses therefore controlled for PTSD and race. There were no significant group differences in alcohol and drug use severity or lifetime severity of cocaine, opioid, or alcohol use, (all ps > 0.08) except for a non-significant trend toward higher prevalence of lifetime cannabis use disorder among men compared with women (p = 0.06) (see Table 2). A higher percentage of HIV-infected women had undetectable viral loads compared with HIV-infected men (p = 0.04); however, there were no significant sex differences on other HIV disease characteristics, including percentage of participants prescribed combination antiretroviral therapy (cART), as well as current and nadir CD4 count (all ps > 0.07) (see Table 1).
Table 1.
Demographic, HIV disease severity, and comorbidity characteristics for all participants
| Group
|
p value | ||||||
|---|---|---|---|---|---|---|---|
| Men
|
Women
|
|
|||||
| HIV− (n = 35) | HIV+ (n = 41) | HIV− (n = 79) | HIV+ (n = 26) | Sex | HIV status | Sex × HIV status | |
| Demographics | |||||||
| Age, M (SD) | 51.49 (4.70) | 49.83 (5.53) | 48.75 (8.39) | 49.00 (8.28) | 0.13 | 0.55 | 0.41 |
| WTAR, M (SD) | 90.06 (11.90) | 89.23 (10.97) | 90.51 (10.50) | 87.62 (11.06) | 0.75 | 0.30 | 0.56 |
| African American, n (%) | 34 (97.1) | 36 (87.8) | 65 (82.3) | 22 (84.6) | 0.06 | 0.96 | 0.16 |
| HIV disease | |||||||
| CPE scores (n = 63) | – | 8.45 (1.93) | – | 7.61 (1.44) | 0.07 | – | – |
| Current CD4 | – | 539.88 (297.91) | – | 560.69 (349.05) | 0.80 | – | – |
| Nadir CD4 (n = 61) | – | 205.64 (189.89) | – | 202.32 (184.62) | 0.95 | – | – |
| Undetectable VL, n (%) | – | 30 (73.2) | – | 24 (92.3) | 0.04 | – | – |
| HAART, n (%) (n = 66) | – | 39 (95.1) | – | 23 (88.5) | 0.61 | – | – |
| Comorbidities | |||||||
| HCV+, n (%) | 2 (5.7) | 10 (24.4) | 10 (12.7) | 3 (11.5) | 0.55 | 0.08 | 0.11 |
| MDD, n (%) | 10 (28.6) | 15 (36.6) | 25 (31.6) | 10 (38.6) | 0.95 | 0.36 | 0.92 |
| PCLC | 36.29 (13.72) | 34.15 (13.71) | 39.94 (14.58) | 40.04 (14.21) | 0.04 | 0.66 | 0.63 |
| WURS | 25.89 (19.65) | 28.56 (19.74) | 31.08 (23.00) | 33.54 (20.94) | 0.14 | 0.46 | 0.97 |
| SRPS | 50.06 (10.77) | 50.78 (10.20) | 48.48 (9.99) | 49.58 (9.65) | 0.39 | 0.57 | 0.91 |
All values represent mean scores unless otherwise indicated
WTAR Wechsler Test of Adult Reading, CPE CNS Penetration Effectiveness, CD4 T-cell count, VL viral load (HIV RNA), HAART highly active antiretroviral therapy, HCV hepatitis C virus, MDD major depressive disorder, PCLC PTSD Check List—Civilian version, WURS Wender Utah Rating Scale, SRPS Self-Report Psychopathy Scale
Table 2.
Substance use characteristics
| Group
|
p value | ||||||
|---|---|---|---|---|---|---|---|
| Men
|
Women
|
|
|||||
| HIV− (n = 35) | HIV+ (n = 41) | HIV− (n = 79) | HIV+ (n = 26) | Sex | HIV status | Sex × HIV status | |
| KMSK scores, median, IQR | |||||||
| Alcohol | 13 (3) | 12 (3) | 12 (2) | 12 (2) | 0.95 | 0.83 | 0.99 |
| Cocaine | 16 (3) | 16 (2) | 16 (2) | 16 (2) | 0.99 | 0.69 | 0.75 |
| Heroin | 1 (2) | 0 (2) | 2 (4) | 0.5 (2) | 0.40 | 0.09 | 0.08 |
| Tobacco | 11 (3) | 11 (2) | 11 (2) | 11 (3) | 0.91 | 0.48 | 0.36 |
| DSM-IV lifetime dependence, n (%) | |||||||
| Alcohol | 23 (65.7) | 30 (73.2) | 50 (63.3) | 18 (69.2) | 0.48 | 0.29 | 0.90 |
| Cannabis | 14 (40) | 18 (43.9) | 25 (31.6) | 5 (19.2) | 0.06 | 0.99 | 0.25 |
KMSK Kreek-McHugh-Schluger-Kellogg scale, IQR interquartile range, DSM Diagnostic and Statistical Manual of Mental Disorders
Memory Island
A total of 97% of participants completed at least one successful “visible” trial (i.e., successfully located at least one of the four objects) and 71% of all participants successfully located at least one of four hidden objects. Table 3 shows the results of the separate and interactive associations between sex and HIV serostatus on the Memory Island visible, hidden, and delayed recall conditions.
Table 3.
Memory Island outcome measures
| Group
|
p valuea | η2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men
|
Women
|
|
||||||||
| HIV− (n = 35) | HIV+ (n = 41) | HIV− (n = 79) | HIV+ (n = 26) | Sex | HIV status | Sex × HIV status | Sex | HIV status | Sex × HIV status | |
| Visible trials (M, SD) | ||||||||||
| Mean times to completiona,b | 101.96 (37.02) | 89.45 (38.80) | 116.29 (77.01) | 97.05 (49.45) | 0.45 | 0.97 | 0.06 | 0.00 | 0.00 | 0.02 |
| Total successful | 2.91 (0.85) | 3.29 (1.03) | 3.16 (0.94) | 2.73 (1.37) | 0.41 | 0.78 | 0.02 | 0.00 | 0.00 | 0.03 |
| Hidden trials (M, SD) | ||||||||||
| Mean times to completion | 166.17 (60.27) | 157.31 (83.23) | 184.72 (84.92) | 189.35 (75.90) | 0.01 | 0.63 | 0.27 | 0.04 | 0.00 | 0.01 |
| Total successful | 1.43 (1.33) | 1.95 (1.47) | 1.41 (1.31) | 1.38 (1.20) | 0.08 | 0.26 | 0.25 | 0.02 | 0.01 | 0.01 |
| Delayed recall (%) | 41.04 | 36.35 | 49.18 | 30.46 | 0.23 | 0.01 | 0.51 | 0.01 | 0.04 | 0.00 |
All models control for race and PCLC. Delayed recall also controlled for hidden trials
For clarity of presentation, raw means and standard deviations are included; however, the analyses were log transformed
Measured in seconds
Visible trials (practice)
Results from the visible (practice) trials (1–4) were analyzed as a validity check to verify participants’ understanding of task requirements and ability to use the joystick. There were no significant main effects of sex or HIV serostatus for either times to completion or number of successful trials. There was no significant sex × HIV serostatus interaction on times to completion. There was a significant sex × HIV serostatus interaction for the total number of successful visible trials (F(1,175) = 5.39, p = 0.02). Analysis of simple main effects indicated that HIV-infected men successfully completed more visible trials compared with HIV-infected women (p = 0.04, d = 0.45). Among HIV-uninfected individuals, there were no sex differences for the total number of successful trials (p = 0.25) (Fig. 1a).
Fig. 1.
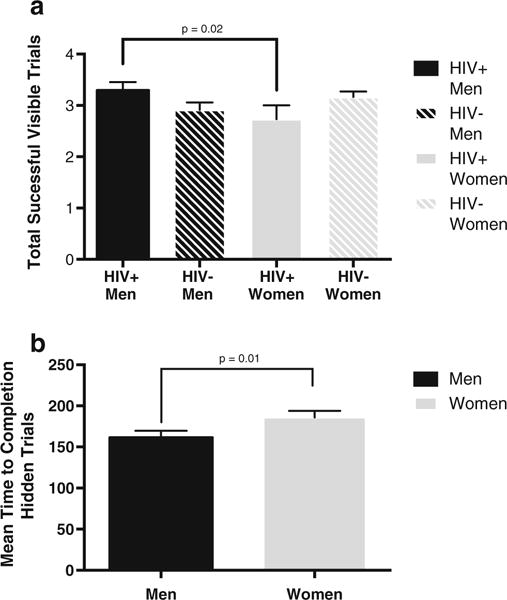
a Total number of successful visible trials as a function of sex × HIV serostatus. The x-axis represents number of completed visible trials. Solid bars indicate HIV-infected individuals (M = 41, F = 26). Striped bars indicate HIV-uninfected individuals (M = 35, F = 79). Unidirectional brackets indicate one SEM. b Mean time to completion for hidden trials as a function of sex. The x-axis represents mean time to completion. Black bars indicate males (N = 76). Gray bars indicate females (N = 105). Uni-directional brackets indicate one SEM. Higher scores indicate poorer performance
Hidden trials (immediate recall)
Results from analyses of the hidden trials showed a significant main effect for sex, with faster times to completion among the men compared with women (F(1175) = 7.34, p = 0.01, d = 0.36) (Fig. 1b). No significant main effect for HIV serostatus or sex × HIV interaction were found on either mean time to completion or total successful hidden trials (ps > 0.05).
Delayed recall
Delayed recall performance is indexed by the percentage of time spent within the correct quadrant within a 30-s time limit. We found a significant main effect for HIV serostatus, with poorer recall among HIV-infected compared with HIV-uninfected individuals (F(1, 174) = 6.63, p = 0.01, d = 0.35) (Fig. 2). There was no significant main effect for sex (p = 0.23) or sex × HIV serostatus interaction (p = 0.51).
Fig. 2.
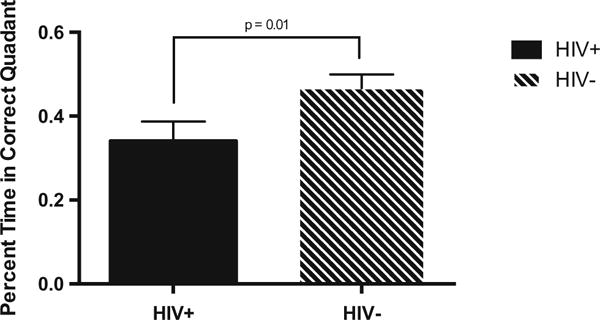
Percent time spent in the correct quadrant on the delayed recall trial as a function of HIV serostatus. The x-axis represents percent time spent in the correct quadrant. Solid bars indicate HIV-infected individuals (N = 67). Striped bars indicate HIV-uninfected individuals (N = 114). Unidirectional brackets indicate one SEM
HIV disease characteristics
Independent-sample t tests were conducted to investigate effects of HIV disease severity on MI outcomes. There were no significant differences between participants with undetectable (HIV RNA < 40) and those with detectable viral loads on any Memory Island outcome (ps > 0.06). There was a significant difference in delayed recall between HIV-infected individuals with a current AIDS-defining CD4 count (< 200) (M = 0.20, SD = 0.25) and those without (M = 0.47, SD = 0.39) (t(65) = 2.01, p = 0.05). This is most likely an incidental finding, as only nine (13%) of the 67 HIV-infected participants had a current immunologic AIDS diagnosis.
Substance use
A series of Spearman’s rank-order correlations were conducted to investigate potential associations between severity of peak cocaine use and MI performance and potential differences in this association according to sex or HIV serostatus. No significant associations between MI outcomes and KMSK cocaine scores were observed (ps > 0.05).
Exploratory analyses
We conducted exploratory analyses of potential neurocognitive correlates of MI performance using the non-MI measures available for the study participants. These included measures of spatial working memory (n-back) (Hinkin et al. 2002), strategic memory (HVLT) (Benedict et al. 1998), index of semantic clustering (Woods et al. 2005, Meyer et al. 2014), and visuospatial episodic memory (BVMT-R Delayed Recall) (Benedict et al. 1996; Keutmann et al. 2016). We selected these measures based on known sensitivity to HIV effects and as validated measures of episodic memory or spatial processing. We conducted a forward multiple regression analysis with MI delayed recall as the dependent measure and the n-back, HVLT, and BVMT indices as predictor variables. Results showed that BVMT-R delayed recall and spatial n-back contributed significant and independent variance to MI delayed recall scores (β = .21, p = 0.008; β = 0.18, p = 0.04, respectively; multiple R = 0.31).
Discussion
The present study administered Memory Island, a spatial learning and memory task, to 67 HIV-infected and 114 HIV-uninfected men and women with a history of cocaine dependence per DSM-IV criteria. We elected to focus specifically on cocaine-dependent individuals because HIV seroprevalence remains elevated among non-injection drug users, and recent evidence that neurocognitive risk and HIV disease progression is increased among HIV+ women with a history of crack cocaine (Kuo et al. 2011; Cook et al. 2008; Meyer et al. 2013).
Participants were generally comparable on measures of demographic characteristics, substance use, and comorbid psychiatric disorders with potentially confounding effects on memory performance.
We had predicted that women would perform the MI task more poorly compared with men and that HIV-infected individuals would perform the task more poorly compared with HIV-uninfected individuals. Our results provide partial support for each hypothesis: the effects we observed were more selective than our initial predictions, which focused on more broadly descriptive MI scores. We found the predicted significant sex difference in MI performance, but only on the hidden (learning) trials component. Similarly, we observed significant differences in MI performance among HIV-infected compared with HIV-uninfected groups, but only on the delayed recall component.
Our finding of a sex difference in the amount of time required to locate the hidden objects is generally consistent with previous reports from non-clinical samples of a male advantage on the MI task (Piper et al. 2011; Rizk-Jackson et al. 2006). Our finding that both HIV-infected men and women showed poorer delayed recall compared with HIV-uninfected groups replicates and extends the previous report by Morales et al. (2012) by demonstrating that spatial episodic memory impairment among HIV-infected individuals is not sex specific and is detectable among substance users. Additionally, these results are generally consistent with previous reports of impairment in verbal and visual episodic memory among HIV-infected individuals (Maki et al. 2015; Keutmann et al. 2016; Heaton et al. 2011), indicating that episodic memory impairment associated with HIV disease is not modality specific.
Our results are not entirely consistent with previous studies of MI performance among non-clinical samples, as we observed a significant sex × HIV serostatus interaction rather than a main effect for sex for the number of successfully completed visible (practice) trials. Specifically, HIV-infected men significantly outperformed HIV-infected women, but performance among HIV-uninfected men and women did not differ. The significance of this finding is unclear. Compared with the non-clinical samples employed in most previous MI studies, our study population has considerably more complex medical and mental health histories and a much higher prevalence of potentially confounding comorbid disorders. This raises the question if increased variability might have masked more subtle within-group differences in MI performance on initial learning trials.
We previously reported that visuospatial memory performance was significantly poorer among HIV-infected women, but not HIV-infected men, compared with uninfected control groups (Keutmann et al. 2016). Our current finding of impaired spatial memory performance among both HIV-infected men and women indicates that sex differences in neurocognitive performance among individuals living with HIV are selective and that HIV-infected women’s neurocognitive performance is not universally impaired compared with HIV-infected men. Additional neurocognitive studies will be necessary to characterize how neuro AIDS manifests itself among men and women and its potential clinical significance.
Although Memory Island was designed as a human analogue to the Morris water maze probe of hippocampal systems in rats, our preliminary data suggest that the MI task engages a more complex network of cognitive and neural mechanisms. An exploratory analysis suggested that both prefrontal (as indexed by strategic verbal memory and spatial working memory) and hippocampal (visuospatial delayed recall) systems contributed independently to MI delayed recall performance. Further, given the complex task demands of MI, successful performance most likely engages additional non-memory cognitive mechanisms. In this regard, an early paper by Martin (1994) reported that compared with HIV-uninfected control participants, HIV-infected individuals showed deficits on a spatial navigational road map task, a measure of “egocentric” spatial processing critically dependent on integrity of prefrontal circuitry. It would be useful for follow-up spatial memory studies to include such navigational measures as comparison tasks to isolate cognitive components of MI performance more precisely.
More broadly, converging evidence suggests that HIV also exerts deleterious effects on non-conscious cognitive and neural memory processes, specifically in the domains of motor skills and probability learning, which are considered critically dependent on integrity of neostriatal-prefrontal systems (Fama et al. 2012; Martin et al. 2011). These studies emphasize that HIV disrupts activity of multiple neural systems and are consistent with previous reports that pathology of both the putamen and hippocampus independently contributes to severity of neurocognitive impairment among HIV-infected persons (Moore et al. 2006).
Our data are not without limitations. Our study sample was limited to cocaine-dependent men and women. Therefore, our findings may not be entirely generalizable to HIV-infected persons without a clinically significant drug history. Additionally, limiting our participants to those meeting lifetime DSM-IV criteria only for cocaine dependence was not feasible given that most SDIs misuse multiple substances. Thus, our findings do not reflect the effects of “pure” cocaine dependence.
Of note, addiction literature indicates that compared with men, women appear be more susceptible to “telescoping,” which is the rapid progression from initial use to dependence and from developing dependence to seeking treatment (Becker and Hu 2008; Greenfield et al. 2010). These findings indicate that greater neurocognitive risk among cocaine-dependent HIV-infected women may reflect additive or interactive effects of HIV with sex-specific vulnerability to stimulant effects. Follow-up studies with assays of sex-steroid hormones and multiple neuroimaging methods that include MR spectroscopy and arterial spin labeling will be critically important to distinguish these effects and identify neural mechanisms more precisely. Finally, studies of substance-dependent populations demand replication as well as detailed strategies for minimizing potentially confounding effects of psychiatric and medical disorders comorbid with substance dependence (see Martin-Thormeyer and Paul 2009).
Acknowledgments
This study was supported by HHS R01 DA12828 to Eileen Martin. We are grateful to Jacob Raber for generously providing the Memory Island task and Chrissy Franco, Stan Chen, Haley Sullins, and Leslie Ladd for data collection.
Footnotes
Data from 15 individuals on opioid substitution therapy were inadvertently included in the analyses. Follow-up sensitivity analyses were conducted removing those individuals on opioid substitution therapy to ensure that the present findings were not due to those select subjects, and since the findings did not change, those individuals were included in the final sample.
Participants who tested positive for cannabis were not excluded if testing was negative for all other substances. The presence of THC metabolites in the urine did not necessarily indicate cannabis use within 1–2 days prior to testing due to its much longer half-life.
Ten participants (5% of total sample) were unable or refused to complete the MI task due to difficulty in handling the joystick or understanding task instructions and were not included in the final total of 181 participants.
Authors’ contributions: All authors contributed scientifically to the manuscript and have read and approved the final version.
Compliance with ethical standards: Written informed consent was obtained upon arrival at the first study visit.
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH. Brief visuospatial memory test—revised: professional manual 1997 [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: studies of normal performance, reliability, and validity. Psychol Assess. 1996;8:145–153. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test—Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo JM, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66:1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Golub ET, Schwartz RM, Howard AA, Ponath C, Plankey MW, Grey DD. Crack cocaine, disease progression, and mortality in a multi-center cohort of HIV-1 positive women. AIDS (London, England) 2008;22:1355. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Sassoon SA, Pfefferbaum A, Sullivan EV. Differential effect of alcoholism and HIV infection on visuomotor procedural learning and retention. Alcohol Clin Exp Res. 2012;36:1738–1747. doi: 10.1111/j.1530-0277.2012.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders. New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neuro Virol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Lam MN, Stefaniak M, Zolnikov B. Verbal and spatial working memory performance among HIV-infected adults. J Int Neuropsychol Soc. 2002;8:532–538. doi: 10.1017/s1355617702814278. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek–McHugh–Schluger–Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Keutmann MK, Gonzalez R, Maki PM, Rubin LH, Vassileva J, Martin EM. Sex differences in HIVeffects on visual memory among substance-dependent individuals. J Clin Exp Neuropsychol. 2016;15:1–3. doi: 10.1080/13803395.2016.1250869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo I, Greenberg AE, Magnus M, Phillips G, Rawls A, Peterson J, Hamilton F, West-Ojo T, Hader S. High prevalence of substance use among heterosexuals living in communities with high rates of AIDS and poverty in Washington, DC. Drug Alcohol Depend. 2011;117:139–144. doi: 10.1016/j.drugalcdep.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Lashomb AL, Vigorito M, Chang SL. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J NeuroVirol. 2009;15:14–24. doi: 10.1080/13550280802232996. [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. J Pers Soc Psychol. 1995;168:151. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Yoon SY. A meta-analysis on gender differences in mental rotation ability measured by the Purdue spatial visualization tests: visualization of rotations (PSVT: R) Educ Psychol Rev. 2013;25:69–94. [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women a preliminary study. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K. Cognitive function in women with HIV findings from the Women’s Interagency HIV Study. Neurology. 2015;84:231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Smith C, Crystal HA, Richardson J, Golub ET, Greenblatt R, Robison E, Martin EM, Young M. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV–women: the Women’s Interagency HIV Study (WIHS) Neurocognitive Substudy. J Clin Exp Neuropsychol. 2011;33:853–863. doi: 10.1080/13803395.2010.547662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. HIV, cognition and the basal ganglia. In: Grant I, Martin A, editors. Neuropsychology of HIV infection. Oxford University Press; New York: 1994. pp. 234–259. [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki P. HIV+ men and women show different performance patterns on procedural learning tasks. J Clin Exp Neuropsychol. 2011;33:112–120. doi: 10.1080/13803395.2010.493150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychol Rev. 2009;19:215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, Valcour V, Young MA, Crystal H, Anastos K, Aouizerat BE. HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr. 2013;63:67. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer VJ, Little DM, Fitzgerald DA, Sundermann EE, Rubin LH, Martin EM, Weber KM, Cohen MH, Maki PM. Crack cocaine use impairs anterior cingulate and prefrontal cortex function in women with HIV infection. J Neuro-Virol. 2014;20:352–361. doi: 10.1007/s13365-014-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “virtual” maze: sex differences and correlation with psychometric measures of spatial ability in humans. Evol Hum Behav. 1998;19:73–87. [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Morales D, Acevedo SF, Skolasky RL, Hechavarria R, Santiago S, De La Torre T, Maldonado E, Wojna V. Translational spatial task and its relationship to HIV-associated neurocognitive disorders and apolipoprotein E in HIV-seropositive women. J Neuro-Virol. 2012;18:488–502. doi: 10.1007/s13365-012-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Weber E, Dawson MS, Carey CL, Moran LM, Grant I. HIV-associated episodic memory impairment: evidence of a possible differential deficit in source memory for complex visual stimuli. J Neuropsychiatry Clin Neurosci. 2009;21:189–198. doi: 10.1176/appi.neuropsych.21.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murji S, Rourke SB, Donders J, Carter SL, Shore D, Rourke BP. Theoretically derived CVLT subtypes in HIV-1 infection: internal and external validation. J Int Neuropsychol Soc. 2003;9:1–6. doi: 10.1017/s1355617703910010. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Edwards KR, Curtiss AB, McGinnis GJ, Raber J. Age, sex, and handedness differentially contribute to neurospatial function on the Memory Island and Novel-Image Novel-Location tests. Physiol Behav. 2011;103:513–522. doi: 10.1016/j.physbeh.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. The role of the hippocampus in solving the Morris water maze. Neural Comput. 1998;10:73–111. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. JINS. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Rizk-Jackson AM, Acevedo SF, Inman D, Howieson D, Benice TS, Raber J. Effects of sex on object recognition and spatial navigation in humans. Behav Brain Res. 2006;173:181–190. doi: 10.1016/j.bbr.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Criado J, Gómez-Chavarín M, Jimenez-Anguiano A, Navarro L, Díaz-Ruiz O, Galicia O, Sanchez-Narvaez F, Murillo-Rodríguez E, Henriksen SJ, Elder JH. HIV-and FIV-derived gp120 alter spatial memory, LTP, and sleep in rats. Neurobiol Dis. 2000;7:384–394. doi: 10.1006/nbdi.2000.0302. [DOI] [PubMed] [Google Scholar]
- Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, Ellis R. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, Klein Z. Psychometric characteristics of the Wender Utah Rating Scale (WURS): reliability and factor structure for men and women. Psychopharmacol Bull. 1995;31:425–433. [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J NeuroImmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading: WTAR. Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Tröster AI, HIV Neurobehavioral Research Center (HNRC) Group Action (verb) fluency: test–retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc. 2005;11:408–415. [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


