Abstract
The blood-testis barrier (BTB) is an important ultrastructure in the testis that supports meiosis and postmeiotic spermatid development since a delay in the establishment of a functional Sertoli cell barrier during postnatal development in rats or mice by 17–20 day postpartum (dpp) would lead to a delay of the first wave of meiosis. Furthermore, irreversible disruption of the BTB by toxicants also induces infertility in rodents. Herein, we summarize recent findings that BTB dynamics (i.e., disassembly, reassembly, and stabilization) are supported by the concerted efforts of the actin- and microtubule (MT)-based cytoskeletons. We focus on the role of two actin nucleation protein complexes, namely, the Arp2/3 (actin-related protein 2/3) complex and formin 1 (or the formin 1/spire 1 complex) known to induce actin nucleation, respectively, by conferring plasticity to actin cytoskeleton. We also focus on the MT plus (+)-end tracking protein (+TIP) EB1 (end-binding protein 1) which is known to confer MT stabilization. Furthermore, we discuss in particular how the interactions of these proteins modulate BTB dynamics during spermatogenesis. These findings also yield a novel hypothetical concept regarding the molecular mechanism that modulates BTB function.
Keywords: Testis, Sertoli cell, Blood-testis barrier, Spermatogenesis, Ectoplasmic specialization, Tight junction, Gap junction, Desmosome, Seminiferous epithelial cycle
1 Introduction
The blood-testis barrier (BTB) is constituted by multiple junction types at the base of the seminiferous epithelium between adjacent Sertoli cells near the basement membrane, which include the actin-based tight junction (TJ) and gap junction (GJ). These junctions, in turn, are supported by coexisting basal ectoplasmic specialization (ES, a testis-specific actin-rich adherens junction (AJ)) and the intermediate filament-based desmosome (Fig. 1a, b) [1–4]. Recent studies have shown that the actin- and microtubule (MT)-based cytoskeletons in the seminiferous epithelium are playing a crucial role to the homeostasis of the BTB in the mammalian testis, in particular the cytoskeletal elements at the basal ES [5–11]. Specifically, it was shown that besides serving as the attachment sites for the adhesion protein complexes at the BTB, these cytoskeletons also provide the track-like structures to support (1) intracellular trafficking of endocytic vesicles and other cargoes (e.g., phagosomes, endosomes) and (2) the paracellular transport of preleptotene spermatocytes connected in clones across the immunological barrier at stage VIII of the epithelial cycle in rodent testes [12–14]. This conclusion is supported based on studies by disrupting these track-like structures such as through a transient knockdown of nucleation protein formin 1 (Fig. 1c) by RNAi. It is now known that in the testis, formin 1, likely working in concert with other actin regulatory proteins, such as plastin 3 (an actin bundling protein) and the Arp2/3 complex (a branched actin nucleation protein complex which effectively converts an actin microfilament to a branched configuration), are crucial to support the generation of linear actin microfilaments across the Sertoli cells to confer the establishment of actin microfilaments at the basal ES [15–18] (Fig. 1c, d). This coordinated effort is necessary to support the timely transport of step 19 spermatids across the epithelium in stage VII–VIII tubules to prepare for their release at spermiation in stage VIII tubules [19, 20]. But they are also necessary to facilitate the timely remodeling of the BTB to support the transport of preleptotene spermatocytes across the immunological barrier at stage VIII of the epithelial cycle [15, 16]. Studies have shown that these two cellular events that take place at the opposite ends of the seminiferous epithelium are coordinated at stage VIII of the epithelial cycle [9, 21, 22]. On the other hand, studies have shown that formin 1 is capable of binding to microtubules (MT) [15], possibly through the presence of a MT-binding domain near the N-terminus of formin 1 as earlier reported [23]. In fact, it was recently shown that a loss of formin 1 in the testis in vivo by RNAi led to a gross disruption of MT-based cytoskeletal network across the entire seminiferous epithelium in virtually all stages of the tubules [15]. Additionally, studies based on the use of the adjudin model have also shown that a disruption of the MT-based tracks mediated by changes in the spatiotemporal expression of EB1 (end-binding protein 1, an MT plus (+)-end tracking protein (TIP)) (Fig. 1e) also perturbed the transport of step 19 spermatids across the epithelium in stage VII–VIII tubules [13]. Adjudin also induced disruptive changes in the actin-based cytoskeleton mediated by its effects on the actin regulatory proteins such as Arp2/3 complex (a branched actin polymerization protein) (Fig. 1d), Eps8 (an actin barbed end capping and bundling protein) [13, 17, 24], and palladin (an actin-bundling protein) [25]. In both animal models, namely, the adjudin and the formin 1 knockdown model, step 19 spermatids are persistently found in the seminiferous epithelium that are trapped near the base of the epithelium in stage IX–XII tubules until they are eliminated by the Sertoli cell through lysosomal degradation, sometimes through the appearance of multinucleated round and/or spermatocytes [13, 15, 16], as noted in other toxicant-induced aspermatogenesis models [26–29].
Fig. 1.
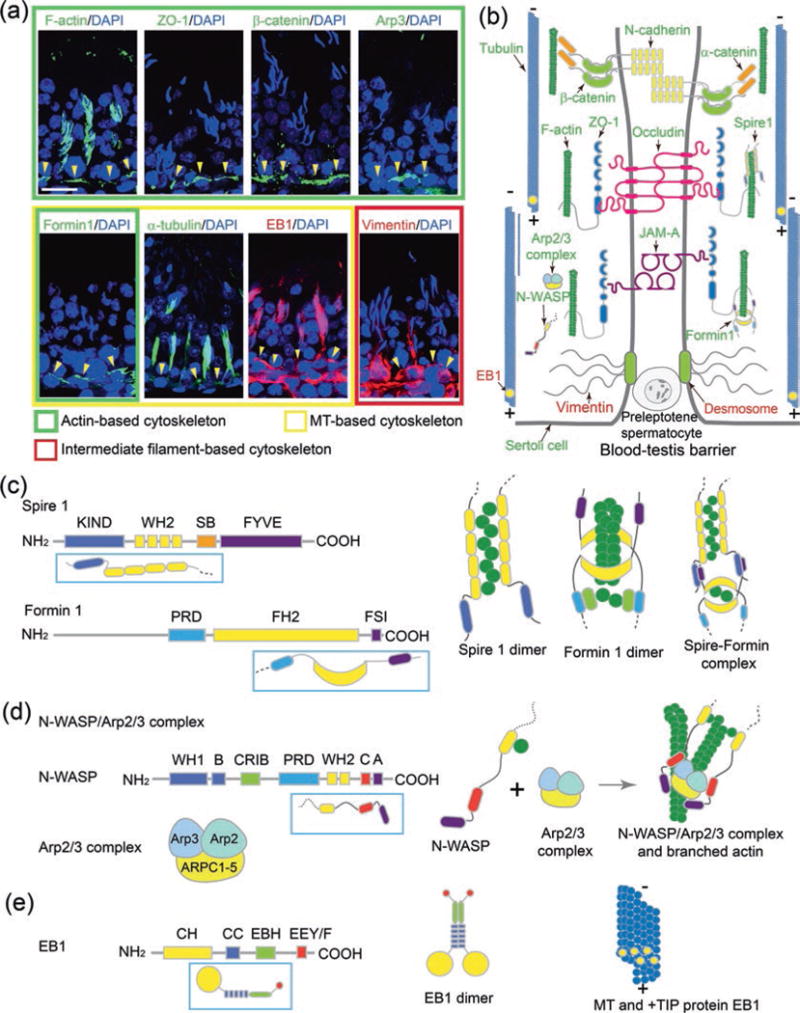
Illustrations on the relative localization of F-actin-, MT-, and vimentin-based cytoskeletons in the testis and selected regulatory and structural proteins plus their functional domains and organization. (a) The relative localization of F-actin network and the basal ES/BTB proteins ZO-1 and ß-catenin and nucleation proteins Arp3 and formin 1 in cross sections of adult rat testes vs. the MT (α-tubulin)- and intermediate filament (vimentin)-based cytoskeletons and EB1 (a +TIP protein) using corresponding specific antibodies was performed by immunofluorescence analysis as earlier described [15, 17, 18, 81]. The relative location of the BTB is annotated by the yellow arrowheads. It is noted that F-actin, MT, and intermediate filaments also form some track-like structures that lay perpendicular to the basement membrane to support germ cell and organelle transport. Cell nuclei were visualized by DAPI staining. Scale bar, 80 μm, which applies to all other micrographs. (b) This is a schematic drawing illustrating the intact BTB of a stage VII to early stage VIII tubule and the association of different regulatory and nucleation proteins with the corresponding F-actin- and MT-based cytoskeletons. Also shown are the structural adhesion proteins of the BTB. (c) Functional domains of nucleation proteins spire 1 and formin 1 and the simplified models of the two proteins (boxed in rectangles). (d) Functional domains of barbed end nucleation protein complex N-WASP/Arp2/3 and the simplified model of N-WASP (boxed in rectangle). (e) Functional domains of MT regulatory protein EB1, a +TIP protein known to stabilize MTs, and the simplified model of EB1. For (c–e), the illustrations on the right panels illustrate the models by which the corresponding proteins function in tissues including the seminiferous epithelium in the rat testis. Abbreviations: +TIP plus (+)-end microtubule tracking protein, A acidic domain, Arp2/3 complex actin-related protein 2 and 3 complex, B basic domain, C central region, CC coiled-coil domain, CH calponin homology, CID α-catenin interacting domain, CRIB Cdc42/Rac-interactive binding, DAD diaphanous autoregulatory domain, DD dimerization domain, DID diaphanous inhibitory domain, EB1 end-binding protein 1 (a +TIP protein), EBH end-binding protein homology, EEY/F C-terminal EEY/F motif, EF hand calcium-binding motif, FH formin homology, FSI formin-spire interaction motif, FYVE Fab1/YOTB/Vac1/EEA1 zinc-binding domain, GBD GTPase-binding domain, KIND kinase noncatalytic C-lobe domain, MTB microtubule-binding domain, PRD proline-rich domain, N-WASP neuronal Wiskott-Aldrich syndrome protein, SB spire box, WH1 domain WASP homology domain 1, WH2 domain WASP homology domain 2, ZO-1 zonula occludens 1
On the other hand, recent studies have shown that BTB dynamics in the rat testis are supported and regulated by local functional axis involving biologically active fragments released from the apical ES (at the Sertoli-spermatid interface in the adluminal compartment from step 8–19 spermatids) such as the F5 peptide from the laminin chains [30–32], and also peptides from the collagen α3 (IV) chain (e.g., non-collagenous 1, NC1, domain peptide) [33] and laminin α2 chain (e.g., 80 kDa fragment) [34, 35]. These findings are also supported by studies using toxicant-induced Sertoli cell injury animal models, such as the use of phthalates [36, 37]. More important, these biologically active fragments released from the constituent components of the apical ES (e.g., laminin γ3 chain) and the basement membrane (e.g., collagen α3 (IV) chain, laminin α2 chain) through the plausible action of MMP 2 (matrix metalloprotease 2) [38] and MMP 9 [39] at the corresponding site are shown to exert their regulatory effects through their action on the actin- and/or MT-based cytoskeletons [31, 32, 34, 35]. Collectively, these findings illustrate that the actin- and MT-based cytoskeletons are working in concert to maintain the homeostasis of the BTB dynamics to support spermatogenesis.
Herein, we briefly review these recent data to provide a molecular model regarding the regulation of BTB dynamics by the local area regulatory networks besides the hormonal hypothalamic-pituitary-testicular axis [40–44] which have been reviewed by other investigators.
2 Actin and Microtubule (MT) Networks and BTB Homeostasis
Blood-tissue barriers such as the blood-brain barrier (BBB) and the blood-retinal barrier (BRB) are created by tight junction (TJ) barrier between adjacent endothelial cells of the microvessels and supported by pericytes in the brain and the eye, respectively [45–48]. The BTB, however, is contributed almost exclusively by the coexisting TJ and basal ES between adjacent Sertoli cells near the base of the seminiferous tubule in the mammalian testis, whereas endothelial cells of the microvessels in the interstitium contribute to relatively little barrier function [1, 11, 49]. In rodent testes, but not in primates, the peritubular myoid cells in the tunica propria also play a role in conferring the barrier function at the BTB [1, 50]. As such, the Sertoli cells in the testis, in particular in primates and humans, are the major players of the BTB function. As noted in Fig. 1a, b, the Sertoli cell is supported by the extensive networks of the actin- and MT-based cytoskeletons across the cell cytosol, which together with the intermediate filament-based desmosome [4, 51] constitute the BTB such as shown in the rat testis (Fig. 1a, b). This unusual extensive networks of F-actin and MTs that contribute to the strength of the BTB, making it one of the tightest blood-tissue barriers in the mammalian body, are not static ultrastructures. Instead, these actin microfilaments and MTs continuously remodel, undergoing rapid disassembly and reassembly to support the transport of preleptotene spermatocytes across the immunological barrier (Fig. 2). Recent studies have shown that several actin-binding and regulatory proteins as well as MT-binding and regulatory proteins are crucial to modulate the dynamic conversion of actin microfilaments or MTs between their bundled, unbundled/branched, and/or truncated/defragmented state. These changes thus confer plasticity to these cytoskeletons, supporting their continuous remodeling during the epithelial cycle of spermatogenesis when preleptotene spermatocytes transformed from type B spermatogonia are being transported across the immunological barrier. Below is a critical review of these findings.
Fig. 2.
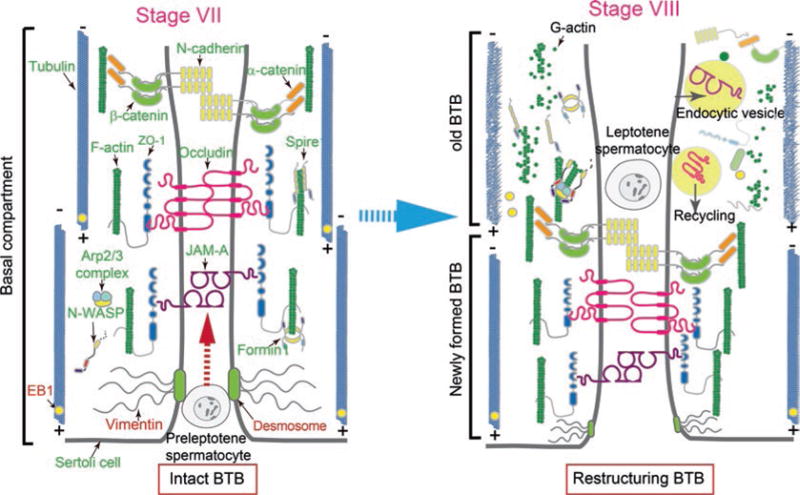
A schematic drawing that illustrates the dynamic BTB restructuring is mediated by changes in the spatial expression of actin- and MT-binding/regulatory and nucleation proteins. The left panel depicts a schematic stage VII (or early stage VIII) tubule with an intact BTB supported by proper organized F-actin and MT networks. At stage VIII (right panel), the N-WASP/Arp2/3 complex is activated, causing actin microfilaments to assume a branched configuration, no longer capable of supporting the actin filaments to assume a bundled configuration. EB1 is also moving away from MTs, failing to stabilize the MT network and causing a disruption of the “old” BTB to facilitate the transport of preleptotene spermatocytes across the immunological barrier with a concomitant assembly of a “new” BTB behind the preleptotene spermatocyte. See text for details
3 Actin-Based Cytoskeleton
Actin in Sertoli cells, similar to other mammalian cells, is organized as either filamentous actin (F-actin) or globular actin (G-actin). F-actin is a polymer and an intrinsic polarized structure formed by G-actin subunits assembled from the fast-growing end (also called the barbed end or plus (+) end) through the action of nucleation proteins vs. the slow-growing end known as the pointed end or minus (−) end [10, 52, 53]. In order for actin to undergo continuous remodeling, actin microfilaments undergo rapid changes including polymerization (or nucleation), depolymerization, bundling, severing (or cleavage), capping, and branching via the action of different actin-binding and regulatory proteins. Herein we discuss some recent findings based on studies of the following selected actin-binding/regulatory proteins in the testis and how these proteins work alone or in concert with others to modulate the organization of actin microfilaments, most notably at the ES to regulate (1) spermatid adhesion and transport and (2) BTB dynamics to facilitate the transport of preleptotene spermatocytes across the immunological barrier. Other regulatory proteins such as actin-bundling proteins plastin 3, Eps8, ezrin, and palladin and regulatory protein kinases (e.g., FAK, c-Src, c-Yes) pertinent to the regulation of spermatogenesis have recently been reviewed [14, 19], and thus, they are not being discussed herein to avoid redundancy. Instead, we focus on a group of actin nucleation proteins; however, these actin nucleation proteins are grouped into two types: one confers branched actin nucleation (e.g., the Arp2/3 complex), whereas the other type confers linear actin nucleation (e.g., formin 1 and Spire 1). Their concerted effects thus generate either a branched actin network or actin microfilaments that can be bundled by actin cross-linking/bundling proteins to facilitate ES degeneration or integrity to support ES breakdown or integrity in the seminiferous epithelium during the epithelial cycle. These changes in turn support germ cell transport across the epithelium.
3.1 The Arp2/3 Complex
The Arp2/3 (actin-related protein 2/3) complex is an actin nucleation protein composed of Arp2, Arp3, and ARPC1-5 (actin-related protein 2/3 complex components 1–5). In short, the Arp2/3 is a protein complex composed of seven different proteins. Its intrinsic activity is to bind onto the barbed end of an existing actin microfilament, upon activation by N-WASP (neuronal Wiskott-Aldrich syndrome protein), and the N-WASP/Arp2/3 complex rapidly induces branched actin nucleation [54–59] (Fig. 1d). Thus, the Arp2/3 complex is effective in converting actin microfilaments from a bundled to a branched/unbundled configuration. The net result of these changes thus destabilizes the ES, facilitating internalization of adhesion protein complexes at the site via endocytosis. This thus promotes endocytic vesicle trafficking so that proteins at the ES, such as at the apical ES near the tubule lumen in a late stage VIII tubule getting ready for spermiation, can be endocytosed and recycled to assemble new apical ES to support newly differentiated step 8 spermatid. This notion is supported by the presence of an extensive network of proteins that are found at the apical ES near the concave (ventral) side of spermatid head, also known as the apical tubulobulbar complex (apical TBC) [60]. In fact, the apical ES is an ultrastructure that supports endocytic vesicle-mediated protein trafficking [61], which is being used to support the assembly of newly formed apical ES at the interface of step 8 spermatids and the Sertoli cell in stage VIII tubules. Similar events also take place at the BTB to support the transport of preleptotene spermatocytes across the immunological barrier in which adhesion proteins located above the preleptotene spermatocytes connected in clones via intercellular bridges are also rapidly endocytosed, creating an ultrastructure known as basal TBC [62], which is readily detected by electron microscopy [63]. As such, adhesion proteins (e.g., N-cadherin, occludin, JAM-1, ZO-1) can be rapidly endocytosed at the “old” BTB site located above preleptotene spermatocytes and recycled to the site behind the preleptotene spermatocytes for the assembly of a “new” BTB (Fig. 2). It is envisioned that the “new” BTB requires the rapid assembly of adhesion protein complexes at the site (e.g., N-cadherin/ß-catenin, occludin/ZO-1, JAM-1/ZO-1) through endocytosis and recycling, and this extensive series of protein trafficking events are supported by the actin network at the apical ES/apical TBC and basal ES/basal TBC. Furthermore, linear actin microfilaments through the action of actin nucleators formin 1 and possibly spire 1 are also required for the assembly of actin microfilament bundles at the “new” apical ES and “new” basal ES/BTB [15, 18].
3.2 Formin 1 and Spire 1
Formin 1 is a member of the morphoregulatory protein family called formins, and due to its intrinsic activity as an actin nucleator, it is capable of inducing actin polymerization from the fast-growing barbed end of an actin filament, forming a long stretch of linear actin microfilament [55, 64–66]. Thus, formins are essential actin nucleation proteins for cytoskeletal organization during development [64, 67]. A functional formin 1 molecule is a dimer in which two formin 1 polypeptide chains are recruited adjacent to one another via their coiled-coil (CC) domains and dimerization (DD) domains. These polypeptides in turn dimerize via their formin homology (FH) 2 domains at the barbed end of an actin microfilament [68, 69]. The dimerized formin 1 promotes addition of actin monomers from the barbed end using G-actin/profilin complexes so that long stretches of actin filaments can be rapidly formed [70]. Thus allows formation of actin microfilaments that can be assembled to actin filament bundles to support the ES through the action of actin bundling proteins such as palladin and Eps8 (epidermal growth factor receptor pathway substrate 8) in the testis. Additionally, formin 1 also possesses intrinsic actin-bundling activity via its FH2 domain [68, 71, 72] (Fig. 1c), capable of inducing actin microfilaments into bundled structures. It also possesses a prominent microtubule-binding (MTB) domain near its N-terminus [23, 69, 71], illustrating it may also be involved in MT organization. On the other hand, spire 1 is also an actin nucleator known to nucleate actin polymerization as a monomeric nucleator via a mechanism different from either formin 1 or the Arp2/3 complex [55, 73]. Spire 1 induces actin polymerization through its central four tandem WH2 domains including the linker-3 (between WH2 domains 3 and 4) [55] (Fig. 1c). Studies in Drosophila oocytes have shown that cappuccino (formin) and spire, however, form a functional complex to induce actin nucleation [74, 75] (Fig. 1c). Emerging evidence has shown that formins (e.g., formin 1) and spire proteins (e.g., spire 1) create a spire/formin actin nucleator complex in mammalian cells other than the oocyte to induce actin polymerization [76–78]. This is made possible due to the presence of a FSI (formin-spire interaction) motif near the C-terminus of formin 1 [76] (Fig. 1c). However, formation of this complex blocks the intrinsic nucleation and barbed end-binding activities of formins, but the spire nucleation activity is considerably enhanced [74, 75]. This is due to the assembly of a dimeric spire upon binding onto formins instead of a monomeric spire protein; thus, there are eight WH2 repeats in tandem to facilitate the recruitment of actin monomers to be polymerized to create long stretches of actin microfilaments across the cell (Fig. 1c) [76]. However, it remains to be investigated if spire/formin complex is a regulatory actin nucleation complex in the mammalian testis.
Studies in the testis have shown that formin 1 is highly expressed at the basal ES at the BTB and also the apical ES, co-localizing with F-actin in adult rat testes [15, 18]. At the basal ES/BTB, the expression of formin 1 remains high in all stages of the epithelial cycle except at stage VIII when its expression is considerably diminished to an almost nondetectable level [15, 18], coinciding with the remodeling of the BTB at this stage to facilitate the transport of preleptotene spermatocytes across the immunological barrier [22]. At the apical ES, formin 1 expression is robust and prominent but limited mostly to stage VII tubules when elongated (step 19) spermatids firmly anchor onto the Sertoli cell in the epithelium [15, 18], requiring formin 1 to promote the generation of actin microfilaments to sustain apical ES function. In this context, it is of interest to note that at stage VII, extensive F-actin remodeling also takes place at the concave (ventral) side of spermatid heads known as the apical TBC – the site where formin 1 is highly expressed – to facilitate the endocytic vesicle-mediated trafficking events such as endocytosis and recycling [60, 61, 79, 80]. This thus requires an elevated expression of formin 1 to sustain actin microfilament turnover at the apical TBC. However, formin 1 expression at the apical ES diminished to an almost undetectable level in stage VIII tubules during apical ES degeneration to support the release of sperm at spermiation [15, 18]. In short, these findings suggest that the spatiotemporal expression of formin 1 at the apical and basal ES is to support (or promote) the integrity or its turnover (but not degeneration) of the actin microfilaments at the ES. Due to its intrinsic actin polymerization and bundling activity, a knockdown of formin 1 by RNAi in Sertoli cells cultured in vitro with an established functional TJ barrier has thus shown to perturb the Sertoli cell TJ permeability barrier function [18]. Formin 1 knockdown in Sertoli cell epithelium has also shown to downregulate the kinetics of actin polymerization and the overall Sertoli cell actin-bundling activity [18]. Thus, it is not unexpected that formin 1 knockdown in the testis in vivo considerably perturbed the transport of elongating/elongated spermatids across the seminiferous epithelium so that some fully developed elon gated spermatids (i.e., spermatozoa) fail to lineup at the luminal edge of the apical compartment for their release into the tubule lumen at spermiation [15, 18]. Instead, step 19 spermatids are routinely found embedded deep inside the epithelium; some are even close to the basement membrane in formin 1 silenced adult rat testes in stage IX–X tubules, and phagosomes also fail to be transported to the base of the epithelium in stage IX and X tubules for their eventual degradation by the Sertoli cell [15, 18]. This is likely due to defects in proper organization of F-actin network in the seminiferous epithelium in response to changes in stages of the epithelial cycle to support these cellular events. For instance, a knockdown of formin 1 considerably disrupts the track-like structures conferred by F-actin that lay across the epithelium such as in stage VIII tubules to support spermatid and phagosome transport [15].
4 MT-Based Cytoskeleton
The MT- and actin-based cytoskeletons are two distinctive cytoskeletal networks, supported by different binding and regulatory proteins in the testis [5, 8, 9, 20]. However, these two cytoskeletal networks are intimately associated structurally and functionally to support spermatogenesis [9, 19]. Interestingly, studies have shown that both cytoskeletal networks can be modulated by the same proteins. For instance, it has recently been shown that EB1 (end-binding protein 1, a +TIP (plus (+)-end tracking protein)) (Fig. 1e) modulates MT and F-actin organization since its knockdown by RNAi perturbs the organization of MTs and actin microfilaments in Sertoli cells [81]. On the other hand, formin 1, an actin nucleator which also possesses an MT-binding domain near its N-terminus [23] to confer its ability to bind to MTs [15], has also been shown to modulate both F-actin and MT organization in Sertoli cells [15, 18]. In fact, a knockdown of formin 1 in the testis in vivo was shown to induce extensive disorganization of MTs in the seminiferous epithelium in which the MT-conferred tracks were grossly disrupted, no longer stretched across the epithelium prominently as found in control testes transfected with nontargeting negative control siRNA duplexes besides perturbing F-actin organization [15]. Under electron microscopy, MTs lay adjacent to the actin microfilaments at the ES [9, 11], suggesting that these two cytoskeletons are likely working in concert to support the ES function. MTs, similar to F-actin, are also polymers, constituted by the α-and ß-tubulins that serve as the building blocks, and are polarized structures with the plus (+) and the minus (−) ends as the fast-growing and the slow-growing ends, respectively [5, 9]. A recent report has shown that germ cell transport across the seminiferous epithelium during the epithelial cycle requires the support of the actin- and MT-based cytoskeletons [13]. Based on the study using the adjudin model wherein rats were treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage, this male contraceptive drug was found to induce apical ES disruption, causing germ cell exfoliation across the entire seminiferous epithelium [13]. However, many step 19 spermatids were trapped deep inside the epithelium even though the apical ES that anchored these spermatids were degenerated. The inability of these spermatids to be empty into the tubule lumen was due to the absence of tracks conferred by MTs. As such, even though the apical ES function had been compromised following adjudin treatment, losing the ability to anchor spermatids onto the Sertoli cell in the epithelium, these step 19 spermatids were entrapped inside the epithelium because tracks were missing to support their transport to the adluminal edge for their release into the tubule lumen [13]. In short, there were no MT-conferred tracks that support the transport of these elongated spermatids to the tubule lumen [13], illustrating these two cytoskeletons are working in concert to support spermatid transport. This observation has also been reproduced in rats subjected to RNAi using testes transfected with formin 1-specific siRNA duplexes vs. nontargeting control siRNA duplexes in control testes [15]. For instance, many step 19 spermatids are found deeply embedded into the seminiferous epithelium in stage IX and X tubules when spermiation has already occurred [15]. A careful examination on the distribution and organization of MTs across the epithelium in these testes has noted that testes following formin 1 knockdown display gross disorganization of MTs so that the track-like structures are considerably diminished or virtually not existed [15]. This thus disables elongated spermatids from being transported to the adluminal edge of the apical compartment to be emptied into the tubule lumen at spermiation, as well as the transport of preleptotene spermatocytes across the immunological barrier. In short, the basal and apical ES rely heavily on the integrity of the actin- and MT-based cytoskeletons to support the transport of spermatids and other organelles (e.g., residual bodies and phagosomes), which are essential to maintain spermatogenesis.
5 Concluding Remarks and Future Perspectives
As briefly discussed above, the actin- and MT-based cytoskeletons are playing a crucial role to support BTB dynamics (i.e., disassembly, reassembly, and stabilization) to facilitate the paracellular transport of preleptotene spermatocytes across the Sertoli cell immunological barrier, such as at stage VIII of the epithelial cycle. The two actin nucleation protein complexes, namely, the Arp2/3 complex and formin 1 (or the spire/formin complex) that induce branched actin polymerization and linear actin filament polymerization, respectively, are working in concert with other actin regulatory proteins (e.g., actin-bundling proteins, cleavage and depolymerization proteins) to confer the plasticity of F-actin network in the Sertoli cell. On the other hand, MTs are also modulated by other MT-binding/regulatory proteins, such as EB1 which is known to induce MT stabilization [82–84]. However, formin 1, an actin nucleation protein, is also recently shown to modulate Sertoli cell MT organization, illustrating these two cytoskeletons are intimately engaged in the testis to support BTB dynamics during spermatogenesis. In fact, a recent report using the adjudin model has demonstrated the actin- and MT-based cytoskeletons are working closely together to support both germ cell adhesion and the transport of germ cells and other organelles (e.g., residual bodies, phagosomes, endocytic vesicles) across the seminiferous epithelium including the BTB during the epithelial cycle of spermatogenesis [13]. Future research shall explore the signaling molecules and/or pathways that are involved in modulating these actin- and MT-binding/regulatory proteins.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056034 to C.Y.C.; U54 HD029990 Project 5 to C.Y.C.); Hong Kong Research Grants Council (RGC)/National Natural Science Foundation of China Joint Research Scheme (N_HKU 717/12) to W.M.L., and Hong Kong University Seed Funding to W.M.L.; W.Q. was supported in part from The F. Lau Memorial Fellowship, The Noopolis Foundation, and The Economic Development Council.
References
- 1.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Stanton PG. Regulation of the blood-testis barrier. Semin Cell Dev Biol. 2016;59:166–173. doi: 10.1016/j.semcdb.2016.06.018. https://doi.org/10.1016/j.semcdb.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Mruk DD, Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 2015;36(5):564–591. doi: 10.1210/er.2014-1101. https://doi.org/10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell L, O’Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol. 2014;30:45–54. doi: 10.1016/j.semcdb.2014.01.003. https://doi.org/10.1016/j.semcdb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4(2):e979623. doi: 10.4161/21565562.2014.979623. https://doi.org/10.4161/21565562.2014.979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217:R13–R23. doi: 10.1530/JOE-12-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang EI, Mruk DD, Lee WM, Cheng CY. Cell-cell interactions, cell polarity, and the blood-testis barrier. In: Ebnet K, editor. Cell polarity 1. Springer International Publishing; Geneva: 2015. pp. 303–326. https://doi.org/10.1007/978-3-319-14463-4_13. [Google Scholar]
- 9.Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin Cell Dev Biol. 2016;59:35–45. doi: 10.1016/j.semcdb.2016.01.004. https://doi.org/10.1016/j.semcdb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 12.Wen Q, et al. Transport of germ cells across the seminiferous epithelium during spermatogenesis—the involvement of both actin-and microtubule-based cytoskeletons. Tissue Barriers. 2016;4(4):e1265042. doi: 10.1080/21688370.2016.1265042. https://doi.org/10.1080/21688370.2016.1265042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang EI, Lee WM, Cheng CY. Coordination of actin- and microtubule-based cytoskeletons supports transport of spermatids and residual bodies/phagosomes during spermatogenesis in the rat testis. Endocrinology. 2016;157(4):1644–1659. doi: 10.1210/en.2015-1962. https://doi.org/10.1210/en.2015-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, Wong CK, Cheng CY. Plastins regulate ectoplasmic specialization via its actin bundling activity on microfilaments in the rat testis. Asian J Androl. 2016;18:716–722. doi: 10.4103/1008-682X.166583. https://doi.org/10.4103/1008-682X.166583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Mruk DD, Tang EI, Lee WM, Wong CK, Cheng CY. Formin 1 regulates microtubule and F-actin organization to support spermatid transport during spermatogenesis in the rat testis. Endocrinology. 2016;157:2894–2908. doi: 10.1210/en.2016-1133. https://doi.org/10.1210/en.2016-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Lee WM, Cheng CY. Overexpression of plastin 3 in Sertoli cells disrupts actin microfilament bundle homeostasis and perturbs the tight junction barrier. Spermatogenesis. 2016;6(1):e1206353. doi: 10.1080/21565562.2016.1206353. https://doi.org/10.1080/21565562.2016.1206353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Mruk DD, Wong CKC, Han D, Lee WM, Cheng CY. Formin 1 regulates ectoplasmic specialization in the rat testis through its actin nucleation and bundling activity. Endocrinology. 2015;156:2969–2983. doi: 10.1210/en.2015-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N, Mruk DD, Cheng CY. Actin binding proteins in blood-testis barrier function. Curr Opin Endocrinol Diabetes Obes. 2015;22:238–247. doi: 10.1097/MED.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Tang EI, Cheng CY. Regulation of blood-testis barrier by actin binding pro teins and protein kinases. Reproduction. 2016;151(3):R29–R41. doi: 10.1530/REP-15-0463. https://doi.org/10.1530/REP-15-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 22.Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology. 2014;29(4):286–298. doi: 10.1152/physiol.00001.2014. https://doi.org/10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Leder P, Martin SS. Formin-1 protein associates with microtubules through a peptide domain encoded by exon-2. Exp Cell Res. 2006;312(7):1119–1126. doi: 10.1016/j.yexcr.2005.12.035. https://doi.org/10.1016/j.yexcr.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 24.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology. 2013;154:1907–1920. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KJ. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 2014;4:e979106. doi: 10.4161/21565562.2014.979106. https://doi.org/10.4161/21565562.2014.979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of reproductive disease and dysfunction. Semin Reprod Med. 2006;24:156–167. doi: 10.1055/s-2006-944423. [DOI] [PubMed] [Google Scholar]
- 28.Boekelheide K. Sertoli cell toxicants. In: Russell L, Griswold M, editors. The sertoli cell. Cache River Press; Clearwater: 1993. pp. 551–575. [Google Scholar]
- 29.Boekelheide K, et al. 2,5-Hexanedione-induced testicular injury. Annu Rev Pharmacol Toxciol. 2003;43:125–147. doi: 10.1146/annurev.pharmtox.43.100901.135930. [DOI] [PubMed] [Google Scholar]
- 30.Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs. 2012;3:1185. doi: 10.1038/ncomms2171. https://doi.org/10.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Mruk DD, Lui WY, Lee WM, Cheng CY. F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis. Oncotarget. 2016;7:64203–64220. doi: 10.18632/oncotarget.11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong EWP, Cheng CY. NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Spermatogenesis. 2013;3:e25465. doi: 10.4161/spmg.25465. https://doi.org/10.4161/spmg.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, Mruk D, Chen H, Lui WY, Lee WM, Cheng CY. Regulation of the blood-testis barrier by a local axis in the testis: role of laminin alpha2 in the basement membrane. FASEB J. 2017;31:584–597. doi: 10.1096/fj.201600870R. https://doi.org/10.1096/fj.201600870R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y, Chen H, Lui WY, Lee WM, Cheng CY. Basement membrane laminin α2 regulation of BTB dynamics via its effects on F-actin and microtubule (MT) cytoskeletons is mediated through mTORC1 signaling. Endocrinology. 2017;158:963–978. doi: 10.1210/en.2016-1630. https://doi.org/10.1210/en.2016-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod. 2010;82:516–527. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao PL, Lin YC, Richburg JH. TNFα-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 2009;80:581–589. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 39.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Mruk DD, Xiao X, Cheng CY. Human spermatogenesis and its regulation. In: Winters SJ, Huhtaniemi IT, editors. Male hypogonadism, contemporary endocrinology. Springer International Publishing AG; New York: 2017. pp. 1–24. https://doi.org/10.1007/978-3-319-53298-1_3. [Google Scholar]
- 41.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press; New York: 1994. pp. 1363–1434. [Google Scholar]
- 42.O’Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis. In: Neill JD, editor. Physiology of reproduction. 3rd. Elsevier; Amsterdam: 2006. pp. 1017–1069. [Google Scholar]
- 43.O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- 44.McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. Hormonal regula tion of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl. 2002;23:149–162. [PubMed] [Google Scholar]
- 45.Easton AS. Regulation of permeability across the blood-brain barrier. Adv Exp Med Biol. 2012;763:1–19. doi: 10.1007/978-1-4614-4711-5_1. [DOI] [PubMed] [Google Scholar]
- 46.Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- 47.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. https://doi.org/10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 48.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–1405. doi: 10.1038/nn.2946. https://doi.org/10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 51.Mruk DD, Cheng CY. Desmosomes in the testis. Moving into an unchartered territory. Spermatogenesis. 2011;1:47–51. doi: 10.4161/spmg.1.1.15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livne A, Geiger B. The inner workings of stress fibers—from contractile machinery to focal adhesions and back. J Cell Sci. 2016;129(7):1293–1304. doi: 10.1242/jcs.180927. https://doi.org/10.1242/jcs.180927. [DOI] [PubMed] [Google Scholar]
- 53.Spence EF, Soderling SH. Actin out: regulation of the synaptic cytoskeleton. J Biol Chem. 2015;290(48):28613–28622. doi: 10.1074/jbc.R115.655118. https://doi.org/10.1074/jbc.R115.655118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pizarro-Cerda J, Chorev DS, Geiger B, Cossart P. The diverse family of Arp2/3 complexes. Trends Cell Biol. 2017;27(2):93–100. doi: 10.1016/j.tcb.2016.08.001. https://doi.org/10.1016/j.tcb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominguez R. The WH2 domain and actin nucleation: necessary but insufficient. Trends Biochem Sci. 2016;41(6):478–490. doi: 10.1016/j.tibs.2016.03.004. https://doi.org/10.1016/j.tibs.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian X, Mruk DD, Cheng YH, Cheng CY. Actin cross-linking protein palladin and spermatogenesis. Spermatogenesis. 2013;3:e23473. doi: 10.4161/spmg.23473. https://doi.org/10.4161/spmg.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian X, Mruk DD, Cheng YH, Cheng CY. RAI14 (retinoic acid induced protein 14) is an F-actin regulator—lesson from the testis. Spermatogenesis. 2013;3:e24824. doi: 10.4161/spmg.24824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li SY, Mruk DD, Cheng CY. Focal adhesion kinase is a regulator of F-actin dynamics: new insights from studies in the testis. Spermatogenesis. 2013;3(3):e25385. doi: 10.4161/spmg.25385. https://doi.org/10.4161/spmg.25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol. 2013;303:319–355. doi: 10.1016/B978-0-12-407697-6.00008-8. [DOI] [PubMed] [Google Scholar]
- 61.Vogl AW, Du M, Wang XY, Young JS. Novel clathrin/actin-based endocytic machinery associated with junction turnover in the seminiferous epithelium. Semin Cell Dev Biol. 2014;30:55–64. doi: 10.1016/j.semcdb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood-testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat. 1979;155:259–279. doi: 10.1002/aja.1001550208. [DOI] [PubMed] [Google Scholar]
- 63.Xiao X, Mruk DD, Wong EWP, Lee WM, Han D, Wong CKC, Cheng CY. Differential effects of c-Src and c-Yes on the endocytic vesicle-mediated trafficking events at the Sertoli cell blood-testis barrier: an in vitro study. Am J Physiol Endocrinol Metab. 2014;307:E553–E562. doi: 10.1152/ajpendo.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeller R, et al. Formin defines a large family of morphorgulatory genes and functions in establishment of the polarising region. Cell Tissue Res. 1999;296:85–93. doi: 10.1007/s004410051269. [DOI] [PubMed] [Google Scholar]
- 65.Baarlink C, Brandt D, Grosse R. SnapShot: formins. Cell. 2010;142:172. doi: 10.1016/j.cell.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 66.Grikscheit K, Grosse R. Formins at the junction. Trends Biochem Sci. 2016;41(2):148–159. doi: 10.1016/j.tibs.2015.12.002. https://doi.org/10.1016/j.tibs.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Woychik RP, Maas RL, Zeller R, Vogt TF, Leder P. ‘Formins’: proteins deduced from the alternative transcripts of the limb deformity gene. Nature. 1990;346:850–853. doi: 10.1038/346850a0. [DOI] [PubMed] [Google Scholar]
- 68.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li N, Mruk DD, Tang EI, Wong CKC, Lee WM, Silvestrini B, Cheng CY. Formins: actin nucleators that regulate cytoskeletal dynamics during spermatogenesis. Spermatogenesis. 2015;5:e1066476. doi: 10.1080/21565562.2015.1066476. https://doi.org/10.1080/21565562.2015.1066476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 71.Breitsprecher D, Goode BL. Formins at a glance. J Cell Sci. 2013;126(Pt 1):1–7. doi: 10.1242/jcs.107250. https://doi.org/10.1242/jcs.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bohnert KA, Willet AH, Kovar DR, Gould KL. Formin-based control of the actin cytoskeleton during cytokinesis. Biochem Soc Trans. 2013;41(6):1750–1754. doi: 10.1042/BST20130208. https://doi.org/10.1042/BST20130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quinlan ME, Heuser JE, Kerkhoff E, Mullins RD. Drosophila spire is an actin nucleation factor. Nature. 2005;433:382–388. doi: 10.1038/nature03241. [DOI] [PubMed] [Google Scholar]
- 74.Vizcarra CL, et al. Structure and function of the interacting domains of Spre and Fmn-family formins. Proc Natl Acad Sci U S A. 2011;108:11884–11889. doi: 10.1073/pnas.1105703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quinlan ME, Hilgert S, Bedrossian A, Mullins RD, Kerkhoff E. Regulatory interactions between two actin nucleators, Spire and Cappuccino. J Cell Biol. 2007;179:117–128. doi: 10.1083/jcb.200706196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dietrich S, Weiβ S, Pleiser S, Kerkhoff E. Structural and functional insights into the Spir/formin actin nucleator complex. Biol Chem. 2013;394(12):1649–1660. doi: 10.1515/hsz-2013-0176. https://doi.org/10.1515/hsz-2013-0176. [DOI] [PubMed] [Google Scholar]
- 77.Carlier MF, Husson C, Renault L, Didry D. Control of actin assembly by the WH2 domains and their multifunctional tandem repeats in Spire and Cordon-Bleu. Int Rev Cell Mol Biol. 2011;290:55–85. doi: 10.1016/B978-0-12-386037-8.00005-3. https://doi.org/10.1016/B978-0-12-386037-8.00005-3. [DOI] [PubMed] [Google Scholar]
- 78.Carlier MF, Pernier J, Montaville P, Shekhar S, Kuhn S, Cytoskeleton D, Motility G. Control of polarized assembly of actin filaments in cell motility. Cell Mol Life Sci. 2015;72(16):3051–3067. doi: 10.1007/s00018-015-1914-2. https://doi.org/10.1007/s00018-015-1914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao X, Wong EWP, Lie PPY, Mruk DD, Wong CKC, Cheng CY. Cytokines, polarity proteins and endosomal protein trafficking and signaling—the Sertoli cell blood-testis barrier in vitro as a study model. Methods Enzymol. 2014;534:181–194. doi: 10.1016/B978-0-12-397926-1.00010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su WH, Mruk DD, Cheng CY. Regulation of actin dynamics and protein trafficking during spermatogenesis—insights into a complex process. Crit Rev Biochem Mol Biol. 2013;48:153–172. doi: 10.3109/10409238.2012.758084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis barrier in male rats—an in vitro study. Endocrinology. 2015;156:680–693. doi: 10.1210/en.2014-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16(12):711–726. doi: 10.1038/nrm4084. https://doi.org/10.1038/nrm4084. [DOI] [PubMed] [Google Scholar]
- 83.Nehlig A, Molina A, Rodrigues-Ferreira S, Honore S, Nahmias C. Regulation of end-binding protein EB1 in the control of microtubule dynamics. Cell Mol Life Sci. 2017;74(13):2381–2393. doi: 10.1007/s00018-017-2476-2. https://doi.org/10.1007/s00018-017-2476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bowne-Anderson H, Hibbel A, Howard J. Regulation of microtubule growth and catastrophe: unifying theory and experiment. Trends Cell Biol. 2015;25(12):769–779. doi: 10.1016/j.tcb.2015.08.009. https://doi.org/10.1016/j.tcb.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


