Abstract
Determining neurobiological factors that contribute to individual variance in drug addiction vulnerability allows for identification of at-risk populations, use of preventative measures and personalized medicine in the treatment of substance use disorders. Rodents that exhibit high locomotor activity when exploring an inescapable novel environment (high-responder; HR) are more susceptible to the reinforcing effects of many abused compounds, including nicotine, as compared to animals that exhibit low locomotor activity (low-responder; LR). Given that nicotinic acetylcholine receptor (nAChR) modulation of reward-related dopamine signaling at accumbal dopamine terminals is critical for the acquisition of drug self-administration, we hypothesized that nAChR modulation of dopamine release would be predicted by an animal’s novelty response. Using voltammetry in the nucleus accumbens core of rats, we found that nicotine produced opposite effects in HR and LR animals on stimulation frequencies that model phasic dopamine release, whereby release magnitude was either augmented or attenuated, respectively. Further, nicotine suppressed stimulation frequencies that model tonic release in LR animals, but had no effect in HR animals. The differential effects of nicotine were likely due to desensitization of nAChRs, since the nAChR antagonists mecamylamine (non-selective, 2 µM), dihydro-beta-erythroidine (β2-selective, 500 nM), and α-conotoxin MII (α6-selective,100 nM) produced effects similar to nicotine. Moreover, dihydro-beta-erythroidine failed to show differential effects in HR and LR rats when applied after α-conotoxin MII, suggesting a critical role of α6β2 compared non α6-containing nAChRs in the differential effects observed in these phenotypes. These results delineate a potential mechanism for individual variability in behavioral sensitivity to nicotine.
Keywords: Nicotine, Voltammetry, Striatum, Phasic, Tonic
1.0 Introduction
Approximately twenty percent of individuals who have used drugs recreationally ultimately develop a substance use disorder (SAMHSA,2008). Therefore, the biological underpinnings of individual differences in the propensity to develop a substance use disorder have been an area of much interest and research. In preclinical rodent models, drug abuse vulnerability can be predicted by an animal’s locomotor responsiveness to an inescapable novel environment. Indeed, animals with higher response to the novel environment (high-responder; HR) acquire drug self-administration more rapidly and at lower doses than their low-responder (LR) counterparts for many drugs of abuse, including psychostimulants such as cocaine and nicotine (Suto et al.,2001; Ferris et al.,2013a; Piazza et al.,1989). Thus, the HR/LR model is a powerful tool for determining antecedent neurochemical characteristics that contribute to drug abuse vulnerability.
Dopamine cell firing in the ventral tegmental area (VTA) switches between tonic (single-spikes at 0.5–10 Hz with majority at 4–5 Hz) and phasic (2 to 5 spikes at ≥ 20 Hz) patterns to encode information concerning salient stimuli and the discrete and contextual cues that predict them (Waelti et al.,2001:Tolber et al.,2005:Marinelli and McCutcheon,2014). As a result, dopamine signaling in the nucleus accumbens (NAc) is critical in guiding organisms towards advantageous outcomes, and is necessary for acquisition of responding for both natural and drug reinforcers (Woolverton et al.,1989). Dopamine release in the NAc is heavily modulated by nicotinic acetylcholine receptors (nAChR) located in both the VTA and directly on dopamine terminals in the NAc. nAChRs in the VTA are essential for nicotine reinforcement and nicotineinduced dopamine release in the NAc (Corrigall et al., 1994; Maskos et al, 2005). Recent evidence suggests a critical role of α6 containing nAChR in the VTA in modulate dopamine release elicited by electrical stimulation of the VTA (Wickham et al., 2013).
In the NAc, dopamine release is modulated by striatal cholinergic interneurons that signal through nAChRs. These interneurons exhibit decreased firing rates and corresponding decreases in acetylcholine release in a synchronous manner with dopamine neuron firing during salient environmental events (Morris et al.,2004), but have also been shown to mediate an increase in acetylcholine overflow in the NAc core during acquisition of drug reinforcement (Crespo et al.,2006). Cholinergic interneurons in the striatum can elicit dopamine release via α4β2* nAChRs located on dopamine terminals in a manner that is independent of VTA dopamine neuron firing (Threlfell et al., 2012; Cachope et al., 2012). Moreover, desensitization or pharmacological blockade of nAChRs in the NAc attenuates dopaminergic output at lower frequency electrical stimulations that model tonic firing, while either increasing, or leaving unaffected, dopamine release at higher stimulation frequencies that model phasic firing (Rice and Cragg,2004). Indeed, nAChRs in the NAc are poised to dynamically modulate the range of dopaminergic influence on accumbal efferents (Zhang and Sulzer,2004:Rice and Cragg,2004). nAChR blockade in the NAc prevents acquisition of drug self-administration (Exley and Cragg,2008a:Crespo et al.,2006;2008) but does not block nicotine self-administration once animals have been well trained (Corrigall et al., 1994). Therefore, while VTA nAChRs are critical for nicotine reinforcement throughout all phases of nicotine self-administration, the interplay between cholinergic and dopaminergic signaling via nAChRs in the NAc core is critical for reward learning.
Given that nAChRs are integrally involved in modulating learning and reward-related dopamine neurotransmission, and that HR and LR animals vary greatly in reward learning and acquisition of drug self-administration, we hypothesized that nAChR modulation of dopamine signaling in the NAc would be predicted by the HR/LR phenotype. To address these questions we used ex vivo fast-scan cyclic voltammetry (FSCV) in the NAc core to measure dopamine release across a range of stimulation parameters of animals previously screened for their locomotor response to an inescapable novel environment. We then used various pharmacological manipulations to examine nAChR-modulation of dopamine release in these phenotypes.
2.0 Methods and Materials
2.1 Animals
Male Sprague-Dawley rats (375–400 g, Harlan Laboratories, Frederick, Maryland), maintained on a 12:12 hour reverse light/dark cycle (3:00 am lights off; 3:00 pm lights on) with food and water ad libitum. All animals were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine.
2.2 Locomotor Assessment
Animals were allowed seven days to acclimate to the housing environment and light cycle prior to the start of experiments. All locomotor testing occurred during the dark/active cycle (9:00AM). We avoided the light/inactive portion of the cycle to prevent sleep from contributing to variability (or lack thereof) in locomotor activity. Animals were first transferred to the locomotor testing room (lights off) and allowed to habituate within their home cages for one hour. Animals were then placed in activity monitors (Med Associates, St. Albans, Vermont) and their horizontal activity was monitored for 90 minutes. The activity chambers were acrylic boxes measuring 43 × 43 × 30 cm and contained two infrared beam arrays. Horizontal activity was measured by beam breaks, which were recorded by a computer.
2.3 Ex Vivo Voltammetry
FSCV was used to characterize presynaptic dopamine release in the NAc core. Animals were sacrificed within one week, but no earlier than 24 hours, after locomotor assessment. Animals were briefly anesthetized with isoflurane before decapitation was performed in a ventilated area free of any blood or tissue from previous animals. A vibrating tissue slicer was used to prepare 400 µm thick coronal brain sections containing the NAc core as previously described (Siciliano et al.,2014). We selected the NAc core given our interest in understanding individual differences in a brain region that is critical for conditioned learning and acquisition of drug self-administration. The tissue was immersed in oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), L-ascorbic acid (0.4) and pH was adjusted to 7.4. Once sliced, the tissue was transferred to the testing chambers containing bath aCSF (32°C), which flowed at 1 ml/min. A carbon fiber microelectrode (100–200 µM length, 7 µM diameter) and bipolar stimulating electrode were placed into the core of the NAc. Dopamine release was evoked by a single electrical pulse (750 µA, 2 msec, monophasic) applied to the tissue every 5 minutes. Extracellular dopamine was recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4V vs Ag/AgCl, 400 V/s). Once the extracellular dopamine response was stable (3 collections within 10% variability), 5 pulse stimulations were applied to the slice with varying burst frequencies (5, 10, 20 or 100 Hz) in order to encompass the physiological range of dopamine neuron firing. After assessing the dopaminergic response to single pulse and multiple pulse stimulations across a range of frequencies, various compounds targeting nAChRs (nicotine, 500 nM; Mecamylamine [MEC] 2 µM; dihydro-beta-erythroidine [DhβE] 500 nM; α- conotoxin MII [H9A; L15A] [α-Ctx] 100 nM (McIntosh et al., 2004)) were bath applied and dopamine response to single pulse stimulation was allowed to equilibrate to the drug (3 collections within 10% variability). We targeted α6-containing nAChRs given their dominant role in mediating the effect of nicotine on dopamine release in the NAc (Exley et al.,2008b). Separate slices were used in order to test each drug independently, and the same frequency-response curves assessed under drug-free conditions were reassessed following drug application in each slice. In a separate set of experiments, to test the independent contributions of α6 and non-α6 containing nAChRs, we repeated experiments described above and modified the procedure to add combinations of DhβE and α –Ctx in a cumulative fashion, starting with application and equilibration of α-Ctx following by DhβE. The difference in dopamine signaling across all frequencies between α-Ctx followed by α-Ctx + DhβE isolates the contribution of (non-α6)α4β2-containing nAChRs. Notably, although α-Ctx can have off-target effects at α3 subunits, α-Ctx binding in NAc is α3 independent, confirming selectivity in this region (Whiteaker et al., 2002; Champtiaux et al., 2002).
2.4 Data Analysis
For all analysis of FSCV data Demon Voltammetry and Analysis software was used (Yorgason et al.,2011). Recording electrodes were calibrated by recording responses (in electrical current; nA) to a known concentration of dopamine (3 µM) using a flow-injection system. This was used to convert electrical current to dopamine concentration. Michaelis–Menten modeling kinetics were used to determine maximal rate of dopamine uptake (Ferris et al.,2013b).
2.5 Statistics
Bivariate regression (correlation) was the primary analysis used to assess the relationship between locomotor response to novelty and nAChR modulation of dopamine release. We performed a tertiary split of locomotor data (comparing top and bottom third of animals based on their locomotor data) in order to provide informative graphical representations of the effects of nAChR compounds on dopamine release. These groups were subject to a repeated measures two-way analysis of variance (ANOVA) with burst frequency as the within-subjects factor and group as the between-subjects factor. Differences between groups were tested using a Bonferroni post-hoc test.
3.0 Results
3.1 HR and LR animals do not differ in dopamine signaling
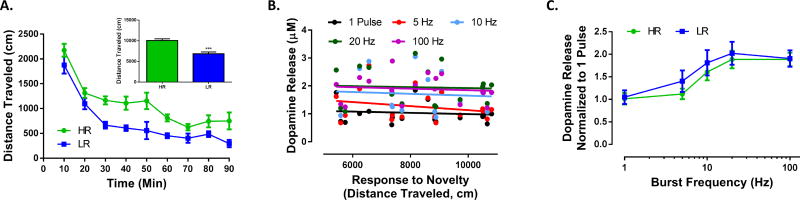
As expected, splitting animals into HR and LR groups revealed greater total distance traveled in HR animals (Figure 1 inset; t9=5.298, p < 0.0005). We first sought to determine if locomotor activity predicted accumbal dopamine signaling across multiple frequencies. To examine the frequency dependence of dopamine signaling, dopamine was elicited by 5 pulse stimulations across the physiological range of dopamine neuron firing. Consistent with previous results (Ferris et al.,2013a) response to novelty did not predict dopamine release magnitude in response to single pulse stimulations (Figure 1B) (r = −0.10, p = 0.68). Further, response to novelty did not predict dopamine release magnitude for any of the frequencies tested (Figure 1B; 5 Hz: r = −0.23, p = 0.35; 10 Hz: r = −0.08, p = 0.74; 20 Hz: r = −0.05, p = 0.85; 100 Hz: r = − 0.10, p = 0.69). A comparison of HR and LR phenotypes revealed that while both groups exhibited frequency-dependent changes in dopamine release, tonic and phasic dopamine signaling did not differ between the groups (Figure 1C; phenotype (F(1, 16) = 0.3199, p = 0.5795), frequency (F(4, 64) = 29.19, p < 0.0001)). Consistent with our previous finding (Ferris et al.,2013a), response to novelty did not predict maximal rate of dopamine uptake (Vmax) (r = − 0.24, p = 0.41), and comparison of HR and LR animals showed no difference in uptake rate (HR Vmax = 2.07 µM/S−1 vs LR Vmax = 2.45 µM/S−1, p > 0.05) (data not shown).
Figure 1. HR and LR animals do not differ in dopamine signaling.
(A) Locomotor activity over a 90 minute session in a novel environment. Data represented are from the upper (HR, n = 5) and lower (LR, n = 6) thirds of total distance traveled. Sum of distance traveled for each group is displayed in the inset. (B) Response to novelty does not predict dopamine release across a range of tonic and phasic stimulation frequencies. (C) HR and LR phenotypes do not differ in tonic or phasic dopamine release. N (number of rats) = 18.
3.2 Response to novelty predicts nicotine effects on dopamine signals and locomotor response to systemic nicotine administration
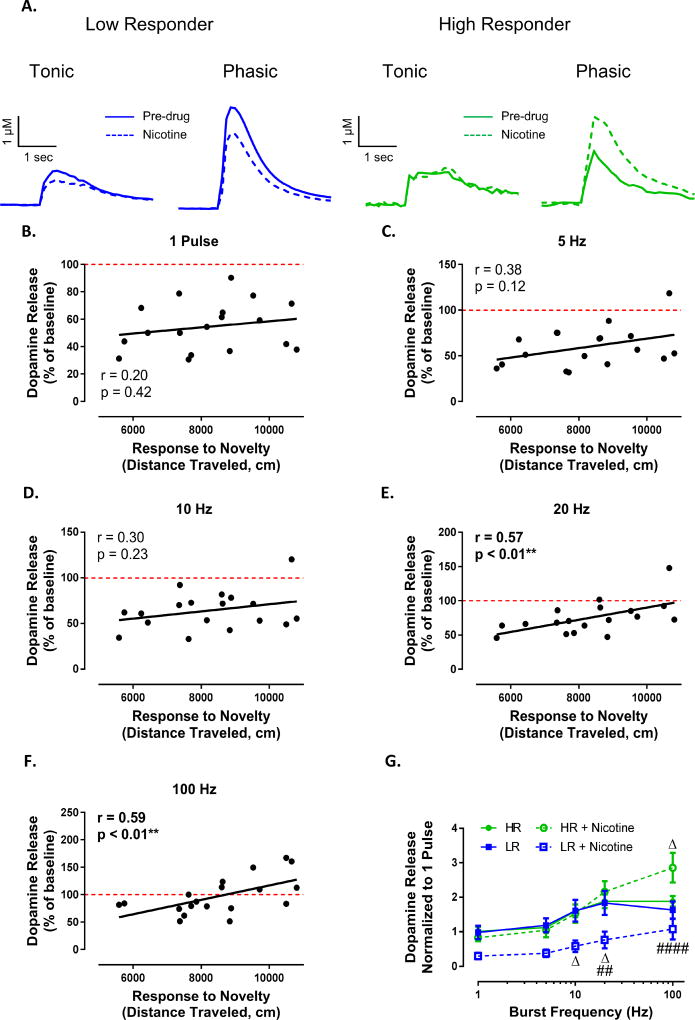
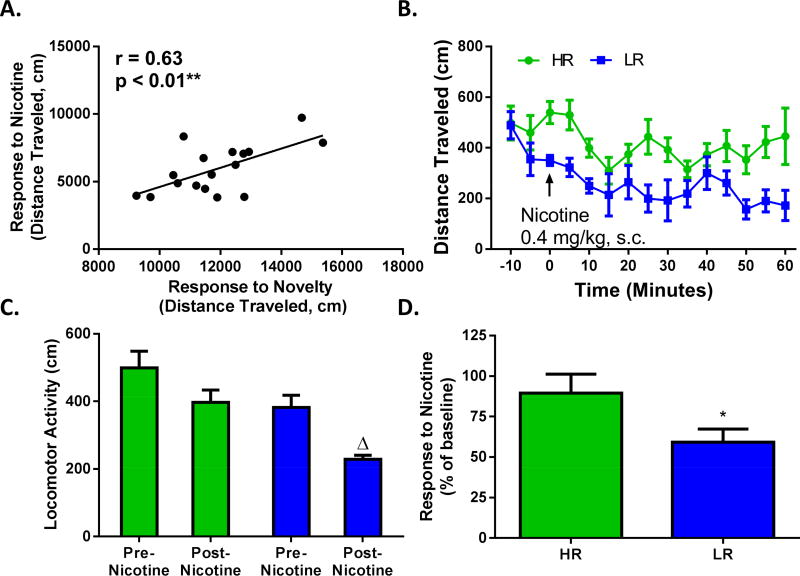
To determine the relationship between response to novelty and nAChR modulation of dopamine signaling, frequency-response curves were reassessed following bath application of nicotine (500 nM) (Figure 2A). We found that there was no relationship between response to novelty and the effects of nicotine on dopamine release elicited by single pulse and low frequency stimulations (Figure 2B,C,D; 1 pulse: r = 0.20, p = 0.42; 5 Hz: r = 0.38, p = 0.12; 10 Hz: r = 0.30, p = 0.23). However, for higher frequency stimulations we found that response to novelty positively predicted dopamine release magnitude (Figure 2E,F; 20 Hz: r = 0.57, p < 0.01; 100 Hz: r = 0.59, p < 0.01). Splitting the data into HR and LR groups in Figure 2G revealed that the dopamine release was affected with differential directionality between the two phenotypes. Nicotine facilitated the amplitude of phasic dopamine signaling without affecting tonic stimulations in HR animals while tonic and phasic signaling was suppressed in LR animals (phenotype (F(3, 24) = 3.788, p = 0.0235), frequency (F(4, 96) = 55.04, p < 0.0001), interaction (F(12, 96) = 4.444, p < 0.0001)). To explore whether response to novelty can predict behavioral outcome measures in response to nicotine, we assessed locomotor response to an acute, systemic injection of nicotine (0.4 mg/kg, s.c.) immediately following assessment of each animal’s response to a novel environment in a separate set of animals (Figure 3). Total locomotor activity elicited by response to novelty significantly predicted locomotor response following a single systemic injection of nicotine (r = 0.63, p < 0.01; Figure 3A). As expected, the acute injection of nicotine significantly decreased locomotor activity in all animals (F(1, 10) = 14.93, p < 0.01), but did so to a greater extend in LR animals compared to HR animals (F(1, 10) = 9.4, p < 0.05) (Figure 3B). The difference between HR and LR animals response to nicotine is apparent when averaging locomotor activity (cm) that occurs within each of the 5 minutes bins across the session, and comparing pre- vs. post-nicotine in Figure 3C. Indeed, only LR animals show a significant nicotine-induced decrease in locomotor activity compared to their own baseline (p < 0.05) in Figure 3C, and when the effect of nicotine is normalized to each groups respective baseline in Figure 3D (t10 = 1.95, p < 0.05).
Figure 2. Nicotine facilitates dopamine release in HR animals and suppresses dopamine release in LR animals.
(A) Representative traces showing the effects of nicotine on tonic (5 Hz) and phasic (100 Hz) stimulations in LR (left) and HR (right) animals. Following bath application of 500 nM nicotine, correlation analysis shows no relationship between response to novelty and dopamine release magnitude elicited by single pulse stimulations (B), 5 pulse 5 Hz stimulations (C) or 5 pulse 10 Hz stimulations (D). For phasic stimulations of 20 Hz (E) and 100 Hz (F) response to novelty positively predicted the effects of nicotine on dopamine release. (G) Tertiary split of the data into HR and LR revealed that nicotine had differential effects on tonic and phasic dopamine release between the two phenotypes whereby phasic stimulations were amplified in HR animals and both tonic and phasic stimulations were attenuated in LR animals. ##, p < 0.01 LR + Nicotine vs HR+Nicotine; #### p < 0.0001 LR + Nicotine vs HR + Nicotine; Δ, p < 0.05 vs pre-drug condition of respective group. N (number of rats) = 18.
Figure 3. Response to novelty predicts behavioral sensitivity to acute administration of nicotine.
(A) Locomotor activity in a novel environment (cm) correlates with total locomotion following a subsequent nicotine injection (0.4 mg/kg, s.c.). (B) Tertiary split of locomotor response to novelty showing the effect of nicotine on locomotor activity (cm) across time (5 minute bins) between HR and LR animals. (C) Average locomotor counts (cm) in 5 minute bins comparing pre- vs. post-nicotine injection in HR (green) and LR (blue) animals shows that LR animals exhibit a greater reduction in locomotor activity post-injection compared to HR animals. (D) Locomotor response to nicotine normalized to baseline (post-nicotine/pre-nicotine) for HR and LR animals shows that nicotine decreases locomotion significantly more in LR animals compared to HR animals. N (number of rats) = 17. Δ, p <0.05 vs predrug; *, p < 0.05 vs HR.
3.3 nAChR blockade differentially modulates dopamine signaling between HR and LR animals
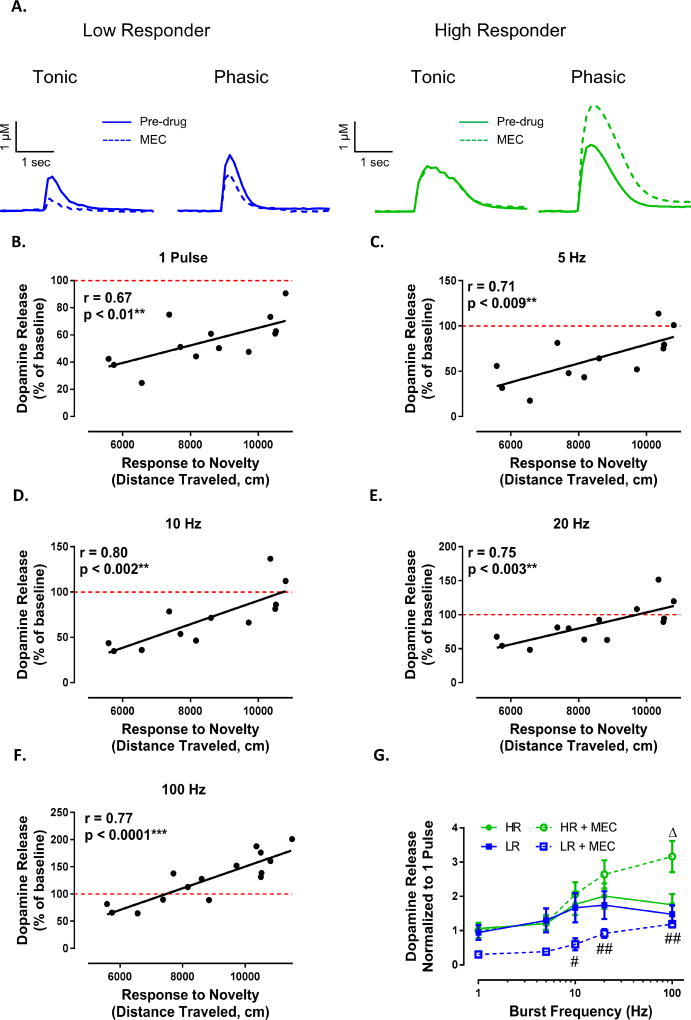
Having found that nicotine differentially modulated tonic and phasic dopamine release between HR and LR animals, we next determined if these effects could possibly be due to nicotine-induced desensitization of nAChR, rather than nicotine’s actions as a nAChR agonist. Thus, we performed an identical experiment with the non-selective, non-competitive nAChR antagonist MEC (Figure 4A). We found that following bath application of MEC (2 µM), response to novelty positively predicted dopamine release at both tonic and phasic frequencies (Figure 4B,C,D,E,F; 1 pulse: r = 0.67, p < 0.01; 5Hz: 0.71, p < 0.009; 10 Hz: r = 0.80, p < 0.002; 20 Hz: r = 0.75, p < 0.003; 100 Hz: r = 0.77, p < 0.0001). In agreement with the effects of nicotine, MEC differentially affected tonic and phasic dopamine signaling between HR and LR animals whereby release elicited by high frequency stimulations was increased in HR animals and decreased in LR animals (Figure 4G; phenotype (F(3, 14) = 2.579, p = 0.0951), frequency (F(4, 56) = 48.44, p < 0.0001), interaction (F(12, 56) = 6.161, p < 0.0001)).
Figure 4. Differential effect of nicotine on dopamine release between HR and LR animals is due to nAChR blockade.
(A) Representative traces demonstrating differential effect of MEC (non-selective nAChR antagonist) on tonic (5 Hz) and phasic (100 Hz) dopamine release between HR (right) and LR (left) animals. (B–F) Following bath application of MEC (2 µM) to brain slices, we found that response to novelty predicted the effects of MEC on dopamine release for both tonic and phasic stimulations. (G) MEC augmented phasic dopamine release in HR animals, while attenuating dopamine release across all frequencies in LR animals. # p < 0.05 LR + MEC vs HR + MEC; ##, p < 0.01 LR + MEC vs HR+MEC; Δ, p < 0.05 vs pre-drug condition of respective group. N (number of rats) = 13.
3.4 α6β2-containing nAChRs differentially modulate dopamine signaling between HR and LR animals
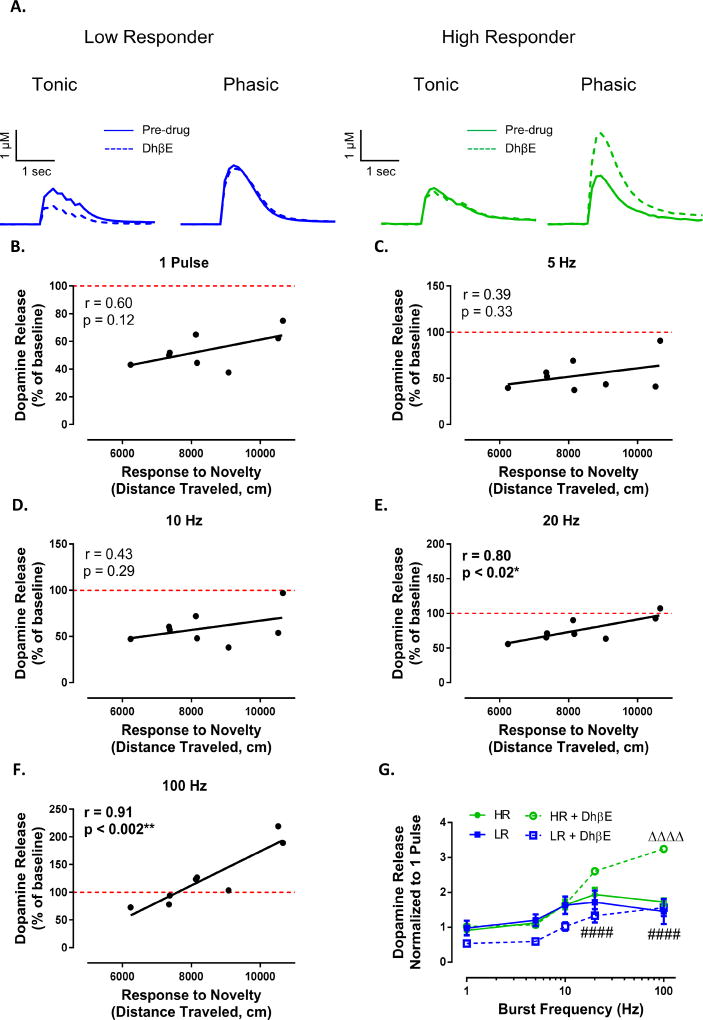
To further determine differences in nAChR modulation of tonic and phasic dopamine signaling between HR and LR animals we examined the effects of DhβE, an antagonist selective for β2 subunit containing nAChRs (Figure 5A). Following bath application of DhβE (500 nM), we found no relationship between response to novelty and dopamine release elicited by tonic stimulation frequencies (Figure 5B,C,D; 1 pulse : r = 0.60, p = 0.12; 5 Hz: r = 0.39, p = 0.33; 10Hz r = 0.43, p = 0.29). Similar to the effects of nicotine on dopamine release, we found a positive relationship between response to novelty and the effects of DhβE on phasic dopamine release (Figure 5E,F; 20 Hz: r = 0.80, p < 0.02; 100 Hz: r = 0.91, p < 0.002). Indeed, DhβE differentially effected phasic dopamine release between HR and LR animals whereby phasic signaling was amplified in HR animals and unaffected in LR animals (Figure 5G; phenotype (F(3, 12) = 5.678, p = 0.0117), frequency (F(4, 48) = 140.4, p < 0.0001), interaction (F(12, 48) = 17.26, p < 0.0001)).
Figure 5. Differential effect of nicotine on dopamine release between HR and LR animals is due to β2 subunit containing nAChR blockade.
(A) Representative traces demonstrating a differential effect of the selective β2 subunit containing nAChR antagonist DhβE on tonic (5 Hz) and phasic (100 Hz) dopamine release between LR (left) and HR (right) animals. Following bath application of DhβE (500 nM) we found that response to novelty did not correlate with dopamine release for single pulse (B), 5Hz (C) or 10 Hz (D) stimulations. However there was a positive relationship between response to novelty and dopamine release under phasic conditions of 20 Hz (E) 100 Hz (F). (G) DhβE augments phasic dopamine release in HR animals without effecting release in LR animals. #### p < 0.0001 LR+DhβE vs HR+DhβE; Δ Δ Δ Δ, p < 0.0001 vs pre-drug condition of respective group. N (number of rats) = 8.
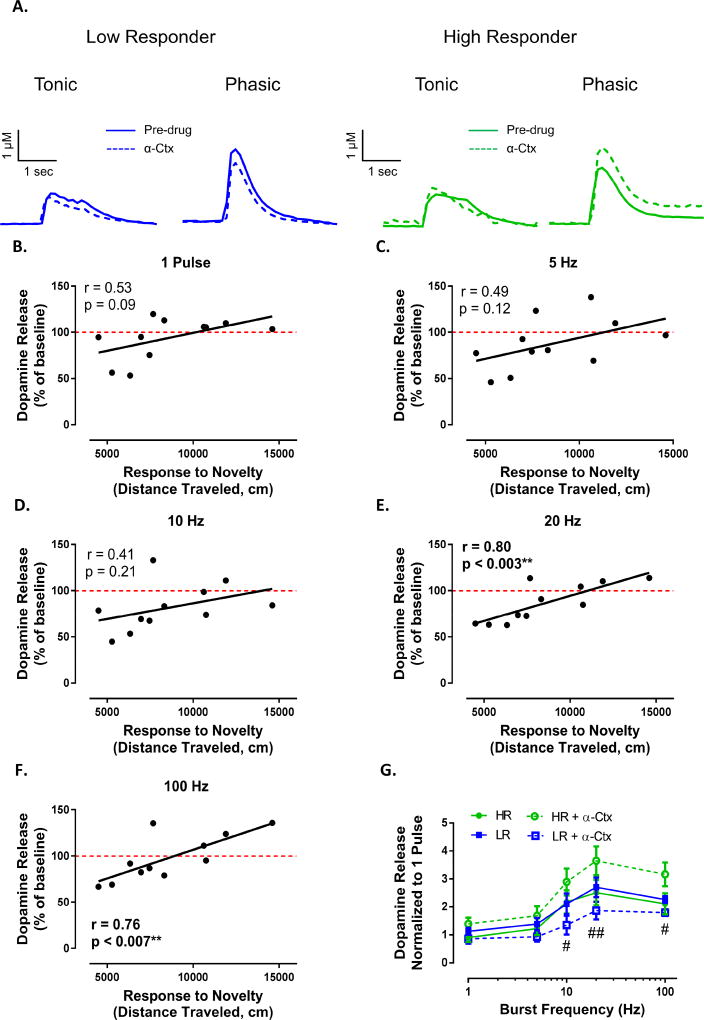
To further localize the differential effects of nicotine between HR and LR animals, we examined the effects of the α6 subunit containing nAChR selective toxin α-Ctx (Figure 6A). α6-containing nAChR subunits are the primary mediators of nicotine’s effect on dopamine release in the NAc (Exley et al.,2008b). Bath application of α-Ctx (100nM) revealed no relationship between the effects of α6 subunit containing nAChR blockade on dopamine release elicited by tonic stimulations (Figure 6B,C,D; 1 pulse: r = 0.53, p = 0.09; 5 Hz: r = 0.49, p = 0.12; 10 Hz: r = 0.41, p = 0.21). Similar to other drugs tested, there was a positive relationship between locomotor activity and α-Ctx-induced modulation of dopamine release elicited by phasic stimulations (Figure 6E,F; 20 Hz: r = 0.80, p < 0.003; 100 Hz: r = 0.76, p < 0.007). Tertiary split of the data revealed that α-Ctx augmented phasic dopamine signaling in HR animals while suppressing signaling in LR animals (Figure 6G; phenotype (F(3, 18) = 2.454, p = 0.0964), frequency (F(4, 72) = 80.77, p < 0.0001), interaction (F(12, 72) = 2.218, p = 0.0194).
Figure 6. Differential effect of nicotine on dopamine release between HR and LR animals is due to α6β2 subunit containing nAChR blockade.
(A) Representative traces demonstrating opposing actions of the α6 subunit containing nAChR antagonist α-Ctx on tonic and phasic dopamine signaling between LR (left) and HR (right) animals. (B–D) Response to novelty did not predict the effects of α-Ctx (100 nM) on dopamine release elicited by a single pulse, 5 Hz or 10 Hz stimulations. However we found that response to novelty positively predicted the effects of α6 subunit containing nAChR blockade on dopamine release elicited by 20 Hz (E) and 100 Hz (F) stimulations. (G) α-Ctx augments phasic dopamine release in HR animals and while suppressing release in LR animals. # p < 0.05 LR + α-Ctx vs HR + α-Ctx; ##, p < 0.01LR + α-Ctx vs HR+ α-Ctx. N (number of rats) = 11.
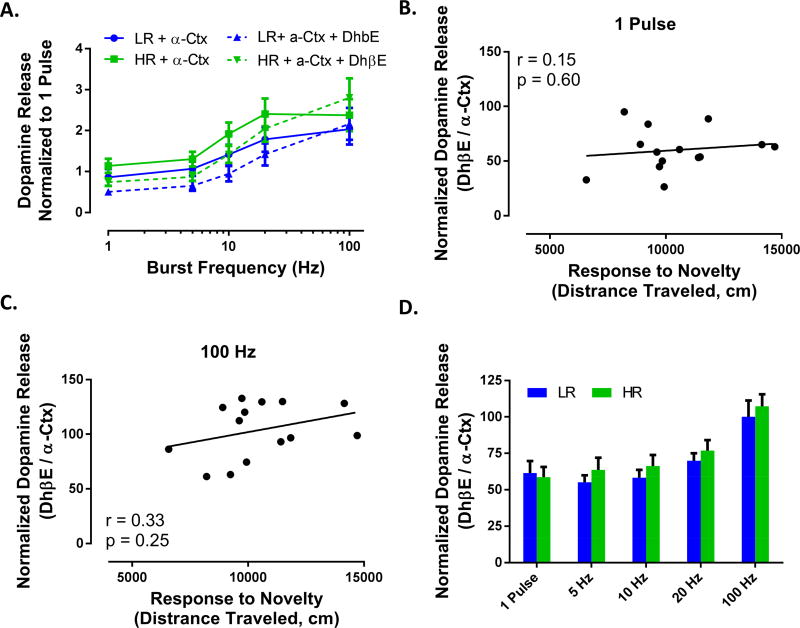
There is variation (albeit nonsignificant) in the extent of modulation of dopamine release for both low and high stimulations between DhβE and α-Ctx (e.g., Figure 5F vs 6F). Therefore, we bath applied α-Ctx first followed by DhβE in order to assess and confirm the relative contribution of both α6 and non-α6 subunits to individual differences in dopamine release. When DhβE is applied to slices after α-Ctx is applied and equilibrated, dopamine release magnitude is further modulated, but equally so in HR and LR animals (Figure 7). DhβE when applied in the presence of α-Ctx causes an equal, non-significant trend toward reduction dopamine release to low frequencies with restoration of release at high frequencies in both HR and LR animals (Figure 7A–D). Additionally, the relationship between locomotor activity and relative shifts in dopamine release magnitude that is observed for DHβE without α-Ctx (Figure 5) is no longer present with DhβE is applied after α-Ctx (Figure 7B and 7C). Therefore, non-α6 nAChRs may modulate dopamine release above and beyond α6-containing nAChRs to some degree, but only the α6-containing nAChRs are responsible for differential effects observed in HR and LR animals. To ensure that these effects were not due to off-target drug action or extended duration of the experiment, in a separate slice, we ran a control experiment in which these same drugs were applied in the opposite order (DhβE followed by α-Ctx). As expected, there was no additional effect of α-Ctx in this case (data not shown).
Figure 7. Non-α6 subunit containing nAChR blockade alters dopamine release, but does not differ between HR and LR phenotypes.
(A) Dopamine release magnitude across burst frequencies after bath application of α-Ctx (solid lines). After α-Ctx effects stabilized, DhβE was added to the slice (dashed lines); any additional effects of DhβE after α-Ctx-induced α6 subunit containing nAChR blockade are presumed to be due to non-α6β2 subunit containing nAChRs. Following bath application of DhβE we found that response to novelty did not correlate with dopamine release for tonic (B) or phasic (C) stimulations. (D) DhβE modulates dopamine release even after α6 subunit containing nAChR are blocked, however, the effect does not differ between HR and LR animals. N (number of rats) = 6.
4.0 Discussion
We show that response to novelty can predict nAChR modulation of dopamine signaling in the ventral striatum. Specifically, dopamine release magnitude following multiple pulse stimulations ≥ 20 Hz was positively correlated with locomotor response to novelty, but only while in the presence of nicotine, MEC, DhβE, or α-Ctx. Blockade of nAChR amplifies phasic dopamine release in HR animals and inhibits phasic dopamine release in LR animals. Further, we demonstrated that these opposing effects are likely attributed to α6β2-subunit containing nAChRs. The involvement of α6β2 is consistent with reports demonstrating that α6 subunits dominate dopamine release dynamics in the ventromedial striatum (Exley et al.,2008b). Moreover, response to novelty also predicted the degree of locomotor depression caused by acute administration of nicotine. These results demonstrate that nAChR modulation of dopamine release varies substantially across individuals and provides a potential mechanism for differential behavioral sensitivity to nicotine as well as augmented drug abuse vulnerability and general learning ability in HR animals (Suto et al.,2001:Piazza et al.,1989:Matzel et al.,2006).
The disparities between HR and LR animals observed here are likely to have implications for both general and drug reinforcement learning because endogenous cholinergic signaling in the striatum is instrumental in modulating dopamine signals that underlie these behaviors (Cachope et al.,2012:Exley and Cragg,2008a).For example, burst firing and pauses in acetylcholine interneuron activity, which occur sequentially in response to salient environmental cues, can elicit dopamine release directly or modulate the magnitude of action-potential dependent dopamine release, respectively. The magnitude of these rapid dopamine signals influences both learning of associations between primary rewards and their predictors, as well as motivation/incentive salience induced by reward predictive cues (Beyene et al., 2010; Flagel et al.,2013). Indeed, nAChR signaling in the striatum is required for acquisition of drug self-administration as well as procedural learning for non-drug reinforcers, and it is thought that these effects are primarily mediated through interactions with dopaminergic signaling (Kitabatake et al.,2003:Exley and Cragg,2008:Crespo et al.,2006). Indeed, nAChRs located directly on dopamine terminals are in an ideal position to influence acquisition of drug self-administration and reinforcement learning. Previous work has shown that nAChRs in VTA and NAc appear to have modulatory actions on phasic dopamine signaling, as micro-infusions of MEC into the VTA attenuates (Wickham et al., 2013) while MEC into the NAc augments (Collins et al., 2016) NAc dopamine signaling in vivo. While previous literature has highlighted the importance of cholinergic signaling in both VTA and NAc to learning and reinforcement, here we show that accumbal nAChRs display wide individual variations in regard to modulation of axonal dopamine release.
We show here that signaling via nAChRs modulates the magnitude of phasic dopamine signals in an opposite manner in animals with differential response to novelty, which for over 30 years has served as a model for initiation of drug use (e.g., drug use prone vs resistant). Therefore, the current finding that nAChRs differentially modulate dopamine signals important for learning and motivation may provide a putative mechanism for the fact that HR animals not only acquire self-administration of many drugs of abuse faster than their LR counterparts (Ferris et al.,2013a;Piazza et al.,1989), but also have been shown to have better performance on general learning tasks (Matzel et al.,2006). Moreover, it suggests a possibility for how nicotine use, via facilitation of reward-related dopamine signals in a specific population, could subsequently facilitate dopamine signals necessary for developing associations between rewards and their predictors, ultimately leading to increased vulnerability to abuse other illicit substances (Picciotto et al.,2008). Dopamine signals in the NAc core are essential for learned associations between rewards and their predictors and this study as well as work from many others have highlighted the powerful modulatory role of local nAChRs on dopamine signaling.
To explore the possibility that HR and LR phenotypes may display differential behavioral sensitivity to nicotine, we examined the effects of acute injection of nicotine on locomotion. We found that LR animals displayed greater sensitivity to the locomotor attenuating effects of acutely administered nicotine. Dopamine driven changes in locomotor activity are thought to rely more on sustained alterations in tonic levels of extracellular dopamine (Giros et al., 1996; Jones et al., 1999; Rao et al., 2013), as opposed to subsecond phasic signals that are essential for learning. The augmented sensitivity to nicotine-induced depression of locomotor activity in LR rats is consistent with the increased sensitivity of LRs to nicotine-induced depression of in dopamine release elicited by tonic-like frequencies. The relationships between locomotor response to novelty, nicotine, and nAChR modulation of dopamine release is consistent with previous work showing genetic modulation of either β2- or α6-containing nAChRs can govern dopamine-induced locomotor response to novelty (Villegier et al., 2010; Cohen et al., 2012). It is unclear, however, the extent to which individual differences in response to novelty or accumbal nAChR modulation of phasic dopamine signals would predict differences in the reinforcing aspects of nicotine. While HR animals have been shown to acquire nicotine self-administration more rapidly, they also display increased motivation to obtain nicotine as measured by greater responding during a progressive ratio schedule of reinforcement, and LR animals exhibit equal responding on nicotine paired and inactive operanda suggesting relatively less reinforcing efficacy of nicotine in these animals (Suto et al.,2001). However, studies have shown that nAChRs in the NAc core possess little to no role in maintaining nicotine reinforcement in animals well-trained for nicotine self-administration (Corrigall et al., 1994; Maskos et al., 2005). Moreover, dopamine signaling in the NAc core, regardless of its modulation by nAChRs, has been shown to play less of a role in well-learned behavior as the locus of activity shifts from ventral striatum during goal directed behavior to more dorsal striatal regions during habitual and compulsive behavior (Everitt et al., 2005; Porrino et al., 2007; Willuhn et al., 2012). Consistent with the dichotomy in the role of accumbal nAChRs and dopamine in learning versus well-trained behavior is the fact that variability in the locomotor response to novelty can predict acquisition of many drugs of abuse (Ferris et al.,2013a;Piazza et al.,1989), but is less able to predict measures of maintenance of drug self-administration in well trained animals (Belin et al., 2011).
While dopamine signaling in HR and LR animals was differentially modulated by nicotine, we further elucidated that these effects were likely due to desensitization of nAChRs, since blockade of nAChRs with either MEC, DHβE, or α-Ctx produced nearly identical effects to nicotine. A single cigarette is sufficient to desensitize nAChRs in humans (Brody et al.,2006). Thus, these results provide a potential mechanism for increased subjective effects of nicotine in humans that are scored high in measures of sensation seeking (Perkins et al.,2000), particularly since this trait in humans is modeled in rodents by their locomotor response to novelty (Dellu et al.,1996).
In addition to the differential nAChR modulation of axonal dopamine release between HR and LR animals shown here, previous reports have demonstrated that HR animals also display a greater influence of nAChRs on excitatory synaptic inputs onto VTA dopamine neurons, as well as directly on the dopamine cell bodies (Fagen et al.,2007). Dopamine neurons in the VTA receive direct cholinergic inputs from mesopontine nuclei, which fire just prior to VTA neurons upon presentation of reward-predictive cues (Pan and Hyland, 2005) and, in turn, lead to phasic dopamine release in the NAc (Day et al.,2007). While our study is consistent with the Fagen et al. (2007) study, it is important to note that our approach of measuring dopamine release in NAc dopamine terminals using voltammetry is fundamentally different than measuring VTA cell firing. We hold firing constant in our studies since we apply exogenous electrical stimulation using the same number of pulses at the same frequency across all of our animals. Thus, we are studying two very different biological functions having controlled for (i.e., eliminated) variance that served as the primary observation for the Fagen et al. (2007) study. In other words, while we are studying the same phenotype, we are investigating a very different outcome measures (release vs firing) and have demonstrated individual differences in the ability of nAChRs to modulate dopamine release magnitude while holding firing frequency constant. Therefore, the current findings give novel insight into the neurobiological variations that underlie individual differences in learning and suggest a common mechanism in meditating susceptibility to abused compounds. Moreover, we postulate that HR animals display augmentation of reward-related dopamine signaling (Flagel et al.,2011) through differences in nAChR function in both the cell body (Fagen et al.,2007) and terminal regions (current study) of the mesolimbic dopamine system. Indeed, smoking cessation treatments that target nAChRs have been shown to engender wide individual variability in treatment outcomes (Russo et al.,2011). These data may inform personalization of pharmacotherapeutic interventions, perhaps based on measures of sensation seeking, and could also lead to novel therapeutic approaches to learning deficits in other neuropsychiatric disorders.
Highlights.
Nicotine augments phasic dopamine signaling in animals with low response to novelty
Nicotine attenuates phasic dopamine signaling in animals with low response to novelty
Nicotine attenuates locomotor activity to a greater extend in animals with low response to novelty
Differential nicotine actions may be due to desensitization of α6β2 receptors
Acknowledgments
We would like to thank Lacey L. Sexton for her technical assistance. This work was funded by NIH grants K99 DA031791 (MJF), P50 DA006634 (SRJ, MJF), GM 103801, GM48677 (JMM), and F31 DA037710, T32 AA007565, F32 MH111216 (CAS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts to report.
References
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene M, Carelli RM, Wightman RM. Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation. Neuroscience. 2010;169:1682–1688. doi: 10.1016/j.neuroscience.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, Gracie CJ, Platt NJ, Cragg SJ. Gating of dopamine transmission by calcium and axonal N-, Q-, T-, and L-type voltage-gated calcium channels differs between striatal domains. Journal of Physiology. 2015;593:929–946. doi: 10.1113/jphysiol.2014.285890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63(8):907–15. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2(1):33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22(4):1208–17. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BN, Mackey ED, Grady SR, McKinney S, Patzlaff NE, Wageman CR, McIntosh JM, Marks MJ, Lester HA, Drenan RM. Nicotinic cholinergic mechanisms causing elevated dopamine release and abnormal locomotor behavior. Neuroscience. 2012;200:31–41. doi: 10.1016/j.neuroscience.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Aitken TJ, Greenfield VY, Ostlund SB, Wassum KM. Nucleus Accumbens Acetylcholine Receptors Modulate Dopamine and Motivation. Neuropsychopharmacology. 2016;41(12):2830–2838. doi: 10.1038/npp.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA, Sturm K, Saria A, Zernig G. Activation of muscarinic and nicotinic acetylcholine receptors in the nucleus accumbens core is necessary for the acquisition of drug reinforcement. J Neurosci. 2006;26(22):6004–10. doi: 10.1523/JNEUROSCI.4494-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JA, Stöckl P, Zorn K, Saria A, Zernig G. Nucleus accumbens core acetylcholine is preferentially activated during acquisition of drug- vs food-reinforced behavior. Neuropsychopharmacology. 2008;33(13):3213–20. doi: 10.1038/npp.2008.48. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;(8):1020–8. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- de Kloet SF, Mansvelder HD, De Vries TJ. Cholinergic modulation of dopamine pathways through nicotinic acetylcholine receptos. Biochemical Pharmacology. 2015 doi: 10.1016/j.bcp.2015.07.014. in press. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34(3):136–45. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008a;153(Suppl 1):S283–97. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, Mcintosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008b;33(9):2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Fagen ZM, Mitchum R, Vezina P, McGehee DS. Enhanced nicotinic receptor function and drug abuse vulnerability. J Neurosci. 2007;27(33):8771–8. doi: 10.1523/JNEUROSCI.2017-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Melchior JR, Roberts DC, España RA, Jones SR. Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur J Neurosci. 2013;38(4):2628–36. doi: 10.1111/ejn.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Yorgason JT, Jones SR. Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem Neurosci. 2013;4(5):693–703. doi: 10.1021/cn400026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;6(7328):53–7. doi: 10.1038/nature09588. 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Caron MG. Application of microdialysis and voltammetry to assess dopamine functions in genetically altered mice: correlation with locomotor activity. Psychopharmacology. 1999;147:30–32. doi: 10.1007/s002130051137. [DOI] [PubMed] [Google Scholar]
- Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci USA. 2003;100:7965–7970. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience. 2014;282C:176–197. doi: 10.1016/j.neuroscience.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Townsend DA, Grossman H, Han YR, Hale G, Zappulla M, Light K, Kolata S. Exploration in outbred mice covaries with general learning abilities irrespective of stress reactivity, emotionality, and physical attributes. Neurobiol Learn Mem. 2006;86(2):228–40. doi: 10.1016/j.nlm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65(4):944–52. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43(1):133–43. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–71. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25(19):4725–32. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Broge M, Grobe JE, Wilson A. Greater sensitivity to subjective effects of nicotine in nonsmokers high in sensation seeking. Exp Clin Psychopharmacol. 2000;8(4):462–71. doi: 10.1037//1064-1297.8.4.462. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Lemoal M, Simon H. Factors That Predict Individual Vulnerability to Amphetamine Self-Administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84(4):329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: A shifting target over the course of addiction. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Sorkin A, Zahnizer NR. Mice expressing markedly reduce striatial dopamine transporters exhibit increased locomotor activity, dopamine uptake turnover rate, and cocaine responsiveness. 2013;67:668–677. doi: 10.1002/syn.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7(6):583–4. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Russo P, Cesario A, Rutella S, Veronesi G, Spaggiari L, Galetta D, Margaritora S, Granone P, Greenberg DS. Impact of genetic variability in nicotinic acetylcholine receptors on nicotine addiction and smoking cessation treatment. Curr Med Chem. 2011;18(1):91–112. doi: 10.2174/092986711793979715. [DOI] [PubMed] [Google Scholar]
- Salamone A, Zappettini S, Grilli M, Olivero G, Agostinho P, Tome AR, Chen J, Pittaluga Am Cunha RA, Marchi M. Prolonged nicotine exposure down-regulates presynaptic NMDA receptors in dopaminergic terminals of the rat nucleus accumbens. Neuropharmacology. 2014;79:488–497. doi: 10.1016/j.neuropharm.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) National Survey on Drug Use and Health. 2008 [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Biphasic mechanisms of amphetamine action at the dopamine terminal. J Neurosci. 2014;34(16):5575–82. doi: 10.1523/JNEUROSCI.4050-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158(2):175–80. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75(1):58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412(6842):43–8. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaud AL, Collins AC, Marks MJ. Involvement of the alpha3 subunit in central nicotinic binding populations. J Neurosci. 2002;22(7):2522–9. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham R, Solecki W, Rathbun L, McIntosh JM, Addy NA. Ventral tegmental area α6β2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology (Berl) 2013;229(1):73–82. doi: 10.1007/s00213-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav. 1989;32(3):691–7. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202(2):158–64. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7(6):581–2. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]