Abstract
The blood-testis barrier is a unique ultrastructure in the mammalian testis, located near the basement membrane of the seminiferous tubule that segregates the seminiferous epithelium into the basal and the adluminal (apical) compartment. Besides restricting paracellular and transcellular passage of biomolecules (e.g., paracrine factors, hormones), water, electrolytes, and other substances including toxicants and/or drugs to enter the adluminal compartment of the epithelium, the BTB is an important ultrastructure that supports spermatogenesis. As such, a sensitive and reliable assay to monitor its integrity in vivo is helpful for studying testis biology. This assay is based on the ability of an intact BTB to exclude the diffusion of a small molecule such as sulfo-NHS-LC-biotin (C20H29N4NaO9S2, Mr. 556.59, a water-soluble and membrane-impermeable biotinylation reagent) from the basal to the apical compartment of the seminiferous epithelium. Herein, we summarize the detailed procedures on performing the assay and to obtain semiquantitative data to assess the extent of BTB damage when compared to positive controls, such as treatment of rats with cadmium chloride (CdCl2) which is known to compromise the BTB integrity.
Keywords: Testis, Blood-testis barrier, Spermatogenesis, Sertoli cells, Tight junction, Ectoplasmic specialization, Actin filaments, Assay
1 Introduction
When small electron-dense substances are loaded into the blood vessel, the blood-testis barrier (BTB) is known to exclude their diffusion across the barrier from entering the seminiferous tubules for more than a century (for reviews, see [1, 2]). For instance, when peroxidase was injected into the testicular interstitium, the majority of the enzyme was accumulated in the lamina propria of the seminiferous tubules, in the intercellular spaces of the Leydig cells and in the lymphatic capillaries, and virtually no peroxidase was detected in the adluminal (apical) compartment of the seminiferous tubules [3]. The BTB is almost exclusively contributed by Sertoli cells which are the epithelial cells of the seminiferous epithelium even though peritubular myoid cells in rodents (but not primates) are known to confer part of the barrier function (for a review, see [1]). Studies have shown that the BTB is constituted by coexisting actin-based tight junction (TJ), basal ectoplasmic specialization (basal ES, a testis-specific atypical adherens junction) and gap junction, as well as intermediate filament-based desmosome [2, 4–8]. Under physiological conditions, the BTB is under precise regulation by different signaling molecules and signal pathways [9, 10], so that it remains tightly “sealed” at different stages of the seminiferous epithelial cycle including stage VIII when preleptotene spermatocytes connected in clones via intercellular bridges are to be transported across the immunological barrier [11–13]. Nonetheless, the BTB can be compromised during virus infection such as Zika [14] and HIV [15] such that these viruses can use the testis as a safe haven. In fact, the testis serves as one of the viral reservoirs, preventing HIV-1 eradication even though the serum viral load is virtually undetectable [16, 17]. Additionally, environmental toxicants (e.g., cadmium, bisphenol A, PFOS) are also known to induce BTB disruption [18, 19]. Studies have shown that scrotal heat stress [20], electromagnetic pulse irradiation [21], and reduced intratesticular testosterone level [22, 23] also play a role in perturbing the Sertoli cell BTB function in vivo. More importantly, BTB disruption is often associated with impaired spermatogenesis in particular germ cell exfoliation and hence subfertility and/or infertility [24, 25]. Collectively, these findings illustrate that a simple, reliable, and noninvasive assay that monitors BTB integrity in vivo would be of great help to investigators in the field.
An earlier version of the in vivo BTB integrity assay requires the use of a small fluorescence probe such as FITC (Mr 389.39) alone or inulin-FITC (Mr ~5 kDa) which was administered into the rats via the jugular vein under anesthesia using ketamine HCl/xylazine [26, 27]. This assay is based on the ability of an intact BTB to block the diffusion of either FITC or inulin-FITC conjugate from entering the apical compartment from the interstitial space so that the fluorescence tag was limited to the basal compartment near the basement membrane in the tunica propria. The extent of the BTB disruption is thus quantified by comparing the distance traveled by FITC or inulin-FITC versus the radius of the seminiferous tubule. However, this is an invasive procedure that requires an administration of either FITC or inulin-FITC via the jugular vein. The procedure is time-consuming and requires researchers to receive relevant recovery surgical training. Moreover, since FITC or inulin-FITC is released into the whole body through circulation following administration, the amount of FITC or inulin- FITC that eventually accumulates around the seminiferous tubules in the testis is limited, making the green fluorescence tracking by fluorescence microscopy less optimal and requiring a lengthy exposure time of ~10–15 s to capture optimal images. Herein, we describe an easy to perform and highly reproducible procedure of the BTB integrity assay based on two earlier reports with minor modifications [23, 28]. Instead of using FITC or inulin-FITC, a membrane-impermeable protein biotinylation reagent sulfo-NHS-LC-biotin is used based on the similar idea that biotin can be retained by an intact BTB. This assay is easy to manage since the sulfo-NHS-LC-biotin is aqueous soluble and a small aliquot of the biotin reagent can be loaded onto the testis under the tunica albuginea through the scrotum using a 28-gauge needle. Furthermore, since the sulfo-NHS-LC-biotin is loaded directly into the testis locally instead of through the systemic circulation, relatively small amount of the reagent is needed. Thereafter, cross sections of testes are obtained using frozen testes in a cryostat, and fluorescence signal is considerably enhanced by incubating cross sections with fluorophore-conjugated streptavidin.
2 Materials
2.1 Animal Surgical Procedures
Ketamine hydrochloride (800 mg)/xylazine hydrochloride (120 mg) mixture in 10-ml double distilled water (note: xylazine is an analgesic and a muscle relaxant).
EZ-Link™ Sulfo-NHS-LC-Biotin.
Cadmium chloride.
Insulin syringe (28 gauge).
2.2 Sample Preparation and Image Requisition
Standard cryostat operating at −22 °C.
Paraformaldehyde.
Streptavidin, Alexa Fluor® 555 conjugate (red fluorescence) (streptavidin conjugated with other types of fluorophore is also suitable based on specific experimental needs).
Standard wide-field microscope or confocal microscope.
3 Methods
3.1 Pre-surgery Setup
Treat a group of adult Sprague-Dawley male rats (or other rodents based on specific experimental needs) (n = 3, ~270–300 gm b.w.) with CdCl2 (3 mg/kg b.w., i.p.) which is known to induce irreversible BTB disruption [29, 30] about 2 to 3 days before surgery. This group of rats serves as a positive control.
On the day of surgery, each rat (~270–300 gm b.w.) receives 400 μl mixture of ketamine hydrochloride with xylazine hydrochloride so that the final concentration of ketamine hydrochloride is 32 mg per rat (i.e., 80 mg/ml) and the xylazine hydrochloride is 4.8 mg per rat (i.e., 12 mg/ml).
Clean area for surgery with Betadine, to be followed by 70% ethanol, and cover the area with clean tissue towel. Turn on a heat lamp to maintain proper temperature of the surgical area. Use sterile scissors and forceps for all procedures.
Maintain a clean rest area (with animal beddings) with a heat lamp so that anesthetized rats can maintain its body temperature.
Freshly prepare a stock of 10 mg/ml EZ-Link™ sulfo-NHSLC-biotin in PBS (10 mM sodium phosphate, 0.15 M NaCl) containing 1 mM CaCl2.
3.2 Surgery
Weigh rats and record.
Inject ketamine/xylazine anesthetic mixture (~400 μl per rat at ~300 gm b.w.) intramuscularly onto his right thigh using the 28-gauge insulin needle. It usually takes ~1–2 min before rats are under anesthesia. Turn on heat lamp to maintain normal body temperature of the anesthetized rats. Keep rats in a clean rest area (with animal beddings).
Allow injected rats to rest for 5–10 min. Observe the loss of eye reflex response and maintenance of a steady breathing pattern to ensure rats are under complete anesthesia. Move rats to the surgery area, with their head/eye area covered with a moist tissue paper to avoid eye injury.
Ensure both testes have descended to the scrotum. Rinse scrotal area with 70% ethanol.
Expose testes by making a small incision of approximately 1 cm in the middle of the scrotum.
Load 100 μl of EZ-Link™ sulfo-NHS-LC-biotin (at 10 mg/ml, biotin reagent dissolved in PBS containing 1 mM CaCl2) stock gently under the tunica albuginea using a 28-gauge insulin syringe.
After biotin reagent administration, move rats to a clean rest area.
Allow rats to rest for 30 min. These rats remain under anesthesia for at least ~40–50 min.
3.3 Sample Preparation
After 30 min, euthanize rats by CO2 asphyxiation using slow displacement of chamber air with compressed carbon dioxide from a CO2 tank at 20–30% per min.
Following euthanasia, remove testes from the scrotum using a pair of sharp scissors, immediately rinse each testis in ~20-ml ice-cold PBS in a Petri dish to remove any blood contamination (only handle the testis with a pair of forceps, avoiding touching the organ with hands).
Snap-freeze each testis in liquid nitrogen and store them in labeled specimen bags at −80 °C.
Cut 7 μm-thick frozen cross sections of the testes in a cryostat at −22 °C.
Fix frozen cross sections of the testes at room temperature (RT) in 4% PFA (paraformaldehyde) (in PBS, 10 mM sodium phosphate, 0.15 M NaCl, pH 7.4 at 22 °C) (w/v) for 10 min.
Wash cross sections with PBS, three times at RT.
Stain cross sections with Alexa Fluor 555-streptavidin at a dilution of 1:250 for 30 min at RT.
Wash cross sections with PBS for three times at RT.
Stain cell nuclei in cross sections with 4′,6-diamidino-2-phenylin-dole (DAPI).
3.4 Image Requisition and Data Analysis
Acquire images using a standard fluorescence microscope or confocal microscope. Typical images are shown in Fig. 1, illustrating the intact BTB in normal rat testes which blocks the diffusion of biotin across the barrier to enter the adluminal compartment. However, the BTB which has been disrupted by cadmium treatment fails to block the diffusion of biotin across the immunological barrier.
- To semi-quantify the extent of BTB damage, measure the distance traveled by biotin in the tubule (DBiotin) and the radius of the same tubule (DRadius). For an oval-shaped tubule, the radius is the average of the shortest and the longest distance of the tubule. The extent of the BTB damage (E) can be expressed in percentage (n = 3 rats, a total of ~150–300 randomly selected tubules) as:
At least 50–100 tubules are randomly selected and quantified from a total of n = 3 rats with a total of 150–300 tubules.
Calculate the E for each experimental group, and compare each group using appropriate statistical method.
Fig. 1.
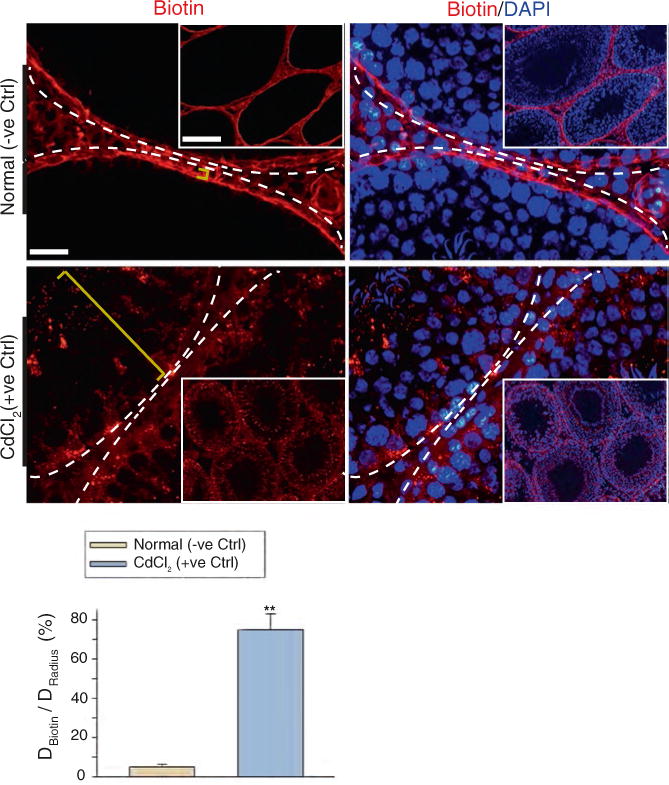
A study to assess the BTB integrity based on an in vivo functional assay. A membrane-impermeable protein biotinylation reagent sulfo-NHS-LC-biotin was loaded under the tunica albuginea of the rat testis under anesthesia using ketamine HCl/xylazine. The sulfo-NHS-LC-biotin rapidly diffused across the entire testis. An intact BTB blocked the passage of biotin into the apical compartment of the seminiferous epithelium. Thus, the biotin (red fluorescence) was detected near the basement membrane of the seminiferous tubules and the interstitial space as noted in the normal (−ve Ctrl) testis group. However, in the positive control group wherein rats were treated with CdCl2 at 3 mg/kg b.w. by i.p. (for 3 days) which was earlier shown to induce irreversible disruption of the BTB [29, 31], biotin diffused into the apical compartment of the seminiferous epithelium. The yellow bracket indicates the distance traveled by biotin. Basement membrane was annotated by dashed white lines. Image in inset is the lower magnified view of the testis cross section. Scale bar, 30 μm; 220 μm in insets. The bar graph below summarizes the semi-quantified data regarding the extent of BTB damage (E) shown in the representative micrographs above between normal (−ve Ctrl) group and CdCl2-treated (+ve Ctrl) group. DBiotin, Distance traveled by biotin in the tubule; DRadius, the radius of the same tubule. **, p < 0.01 by student’s t-test
Acknowledgments
This work was supported by grants from the National Institutes of Health, NICHD R01 HD056034 to C.Y.C., and U54 HD029990 Project 5 to C.Y.C.; Hong Kong Research Grants Council (RGC)/National Natural Science Foundation of China Joint Research Scheme (N_HKU 717/12) to W.M.L., Hong Kong RGC Grant GRF17100816 to W.Y.L. and GRF 771513 to W.M.L., Hong Kong University Seed Funding to W.Y.L. and W.M.L.; NSFC Grant 81730042 to R.S.G. and NSFC Grant 31371176 to X.X., and Zhejiang Province Department of Science Technology Funding 2016F10010 to X.X.; H.C. was supported in part by The S.Y. Law Memorial Fellowship and The F. Lau Memorial Fellowship.
References
- 1.Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 2.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki A, Fawcett DW. Impermeability of sertoli cell junctions to prolonged exposure to peroxidase. Andrologia. 1975;7:63–76. doi: 10.1111/j.1439-0272.1975.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 4.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 5.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- 7.Kaur G, Thompson LA, Dufour JM. Sertoli cells–immunological sentinels of spermatogenesis. Semin Cell Dev Biol. 2014;30:36–44. doi: 10.1016/j.semcdb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanton PG. Regulation of the blood-testis barrier. Semin Cell Dev Biol. 2016;59:166–173. doi: 10.1016/j.semcdb.2016.06.018. https://doi.org/10.1016/j.semcdb.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Tang EI, Cheng CY. Regulation of blood-testis barrier by actin binding proteins and protein kinases. Reproduction. 2016;151(3):R29–R41. doi: 10.1530/REP-15-0463. https://doi.org/10.1530/REP-15-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lie PPY, Cheng CY, Mruk DD. Signalling pathways regulating the blood-testis barrier. Int J Biochem Cell Biol. 2013;45:621–625. doi: 10.1016/j.biocel.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology. 2014;29(4):286–298. doi: 10.1152/physiol.00001.2014. https://doi.org/10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Cheng CY. Planar cell polarity (PCP) proteins and spermatogenesis. Semin Cell Dev Biol. 2016;59:99–109. doi: 10.1016/j.semcdb.2016.04.010. https://doi.org/10.1016/j.semcdb.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith BE, Braun RE. Germ cell migration across Sertoli cell tight junctions. Science. 2012;338:798–802. doi: 10.1126/science.1219969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govero J, et al. Zika virus infection damages the testes in mice. Nature. 2016;540:438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenabian MA, et al. Immune tolerance properties of the testicular tissue as a viral sanctuary site in ART-treated HIV-infected adults. AIDS. 2016;30(18):2777–2786. doi: 10.1097/QAD.0000000000001282. https://doi.org/10.1097/QAD.0000000000001282. [DOI] [PubMed] [Google Scholar]
- 16.Darcis G, Coombs RW, Van Lint C. Exploring the anatomical HIV reservoirs: role of the testicular tissue. AIDS. 2016;30(18):2891–2893. doi: 10.1097/QAD.0000000000001281. https://doi.org/10.1097/QAD.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37:377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238(3):240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan HT, Mruk DD, Wong CKC, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr407–an in vitro study. Endocrinology. 2014;155:249–262. doi: 10.1210/en.2013-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai H, et al. Scrotal heat stress causes a transient alteration in tight junctions and induction of TGF-beta expression. Int J Androl. 2011;34:352–362. doi: 10.1111/j.1365-2605.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 21.Hou WG, Zhao J, Li Z, Li W, Li T, Xiong LZ, Zhang YQ. Effects of electromagnetic pulse irradiation on the mouse blood-testicle barrier. Urology. 2012;80(1):225 e221–225 e226. doi: 10.1016/j.urology.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. https://doi.org/10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao X, et al. N-wasp is required for structural integrity of the blood-testis barrier. PLoS Genet. 2014;10:e1004447. doi: 10.1371/journal.pgen.1004447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mruk DD, Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 2015;36:564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li MW, et al. Tumor necrosis factor {alpha} reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar O, Mathur PP, Cheng CY, Mruk DD. Interleukin 1 alpha (IL1A). is a novel regulator of the blood-testis barrier in the rat. Biol Reprod. 2008;78:445–454. doi: 10.1095/biolreprod.107.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuse M, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 30.Lui WY, Lee WM, Cheng CY. Transforming growth factor-b3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 31.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-b3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]


