Abstract
Background and aims
Airflow limitation, i.e. reduced forced expiratory volume in one-second (FEV1) is associated with increased prevalence of atherosclerosis, however, causal mechanisms remain elusive. The objective of the study was to determine if the association between airflow obstruction and markers of atherosclerosis were mediated by systemic inflammation.
Methods
1154 subjects from the longitudinal AGES Reykjavik study were included. Population characteristics, systemic inflammation markers from blood (white blood cell counts (WBC) and level of C-reactive protein (CRP)) were compared between patients with and without airflow limitation defined by reduced FEV1 on spirometry. Atherosclerosis burden was quantified by measurements of coronary artery calcium, aortic arch and distal aortic calcification in addition to carotid intimal media thickness (CIMT).
Results
Subjects were split into four groups according to smoking status and if airflow limitation was present. There was a higher overall burden of atherosclerosis in ever-smokers compared to never-smokers, and in individuals with airflow obstruction compared to individuals without airflow obstruction. After adjusting for population characteristics, Framingham cardiovascular risk factors and markers of systemic inflammation (WBC and CRP), there was a significantly more aortic arch and distal aorta calcification and higher measurement of CIMT for individuals with airflow obstruction compared to individuals without airflow obstruction. After adjusting for population characteristics, Framingham cardiovascular risk factors and markers of systemic inflammation (WBC and CRP), there was a significantly more aortic arch and distal aorta calcification and higher measurement of CIMT for individuals with airflow obstruction compared to individuals without airflow obstruction.
Conclusions
Systemic inflammation (WBC and CRP) does not appear to mediate the association between airflow limitation and atherosclerosis. Only airflow limitation and not systemic inflammation (WBC and CRP) appears to be an independent predictor of atherosclerosis.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease characterized by expiratory airflow obstruction (reduced FEV1 (forced expiratory volume in 1 sec)) that is not reversible by bronchodilators. The cardinal symptoms are longstanding cough and shortness of breath, and common clinical findings are hyperinflation of the lungs, gas trapping and gas exchange abnormalities. COPD is associated with significant morbidity and mortality (1,2).
Cardiovascular disease is a common comorbidity in patients with COPD and is a frequent cause of mortality (3). Although there is some heterogeneity in the results across different studies, several studies indicate that reduced FEV1 is an important risk factor for cardiovascular mortality, independent of established cardiovascular risk factors such as cigarette smoking status, cholesterol, body mass index and systemic hypertension (3,4,5). It has been shown that for every 10% decrease in FEV1 all-cause mortality increases by 14%, cardiovascular mortality increases by 28% and the frequency of non-fatal coronary events increases by 20% (3).
Chronic obstructive pulmonary disease is also associated with a systemic inflammatory response that is more pronounced with advanced disease and during exacerbations (3,4,5). Patients with COPD have elevation of markers of systemic inflammation, such as C-reactive protein (CRP) that also increases with severity of obstruction and in exacerbations (4,5,6). However, It is still unclear whether these associations between markers of systemic inflammation and airflow obstruction are a consequence of shared risk factors such as cigarette smoking or whether airflow limitation is truly causal factor.
The causal mechanisms linking airflow obstruction with atherosclerosis remain for most part unknown (7). It has been hypothesized that systemic inflammation mediates the association between airflow obstruction and atherosclerosis.
Here, we assessed expiratory airflow, systemic inflammation (WBC and CRP), and carotid/coronary/aortic atherosclerosis in a well phenotyped longitudinal cohort study from a subset of participants from the Reykjavik AGES study. We hypothesized that increased systemic inflammation would mediate the association between airflow obstruction and atherosclerosis so after adjusting for WBC and CRP as markers of systemic inflammation, the association between those two would be lost.
Materials and methods
Subject Population
Subjects were identified among subjects in the Age Gene/Environment Susceptibility (AGES)-Reykjavik study (8). The AGES-Reykjavik study is a longitudinal epidemiologic study with primary focus on the process of aging. It originates from the Reykjavik study (RS), a large longitudinal population based study primarily focusing on cardiovascular disease. In 2002, 11,549 previously examined RS cohort members were still alive and invited for participation in the AGES-Reykjavik study. Recruitment took place from 2002 to 2006 with a total sample size of 5764 individuals. The mean age was 76.1 (SD 5.7) years with a range from 66 to 96 years. They were all Caucasians. The individuals answered detailed questionaire and had an extensive laboratory measurements, physiological measurements (including spirometry) and imaging performed. Details of collected variables are provided elsewhere (8). The AGES-Reykjavik study was approved by the Icelandic National Bioethics Committee (VSN: 00-063), the Icelandic Data Protection Authority and the Institutional Review Board of the Intramural Program of the National Institute for Aging. Written, informed consent was obtained from all participants.
Identification of smokers
Smoking status was assessed by questionnaire. To be considered an ever smoker, the individuals either responded positively to smoking a cumulative number of 20 packs of cigarettes (more than 400 cigarettes over their lifetime) over their lifetime, or 1 cigarette/day for a whole year (more than 365 cigarettes over their lifetime). In the total population of 5764 individuals there were 12% current smokers, 43% previous smokers and 45% never smokers.
Spirometry
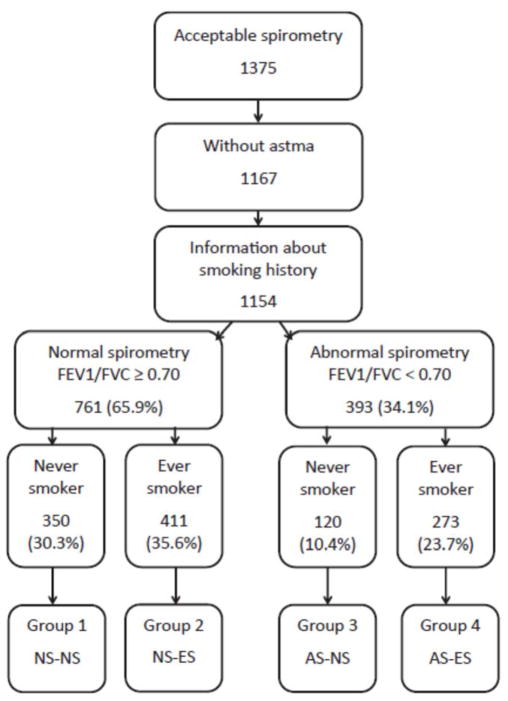
Spirometry was performed with a Vitalograph Gold Standard Plus (Vitalograph Ltd., Buckingham, UK). The procedure was explained in details before starting and was done in a sitting position in a standardized manner. Three attempts were made for each individual. Inclusion criteria for acceptable spirometry was completion of at least two spirometry attempts, no more than 300 ml difference between the attempts and ability to exhale for at least six seconds. No bronchodilator was given, so airflow limitation was defined as FEV1/FVC ratio less than 0.7. Details of included individuals are in Figure 1.
Fig. 1. Classiffication or individuals based on smoking and spirometry results.
Flowchart of inclusion of individuals into the study after applying exclusion criteria, and their categorization into four groups based on spirometry (normal spirometry(NS) or abnormal spirometry (AS)) and smoking status (never smoker (NS) or ever smoker ES)). The final four groups used in analysis are Group 1: NS-NS. Normal spirometry and never-smokers: Group 2: NS-ES. Normal spirometry and ever-smokers: Group 3: AS-NS: Normal spirometry and never-smokers and Group 4: AS-ES: Abnormal spirometry and never-smokers.
Cardiovascular risk factors
The presence of Framingham cardiac risk factors were evaluated in all individuals. These include: diabetes, hypertension, systolic blood pressure, cholesterol, HDL cholesterol and body mass index. Diabetes was defined as a history of diabetes, use of glucose-modifying medication, or fasting blood glucose of more than 7 mmol/L. Blood pressure was assessed from the mean value of two measurements using a large-cuff mercury sphygmomanometer. Hypertension was defined as measured systolic blood pressure of more than 140 mm Hg, diastolic blood pressure of more than 90 mm Hg, self-reported doctor’s diagnosis of hypertension or usage of antihypertensive medications. High-density lipoprotein (HDL) cholesterol, and glucose were measured in fasting blood samples using reagents from RocheDiagnostics (Mannheim, Germany) on a Hitachi 912 analyzer (Hitachi Ltd., Tokyo, Japan) according to the manufacturer’s instructions. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation. Body mass index (BMI) (weight(kg)/height (m)2) was calculated from measured height (cm) and weight (kg).
Markers of systemic inflammation
Total white blood cell count (WBC) was measured in fasting whole blood samples using an automated cell counter, Coulter HmX AL Hematology Analyzer (Beckman Coulter, High Wycombe, England, UK). Level of C-reactive protein (CRP) was measured in plasma from fasting blood samples using reagents from Roche Diagnostics (Mannheim, Germany) on a Hitachi 912 analyzer (Hitachi Ltd., Tokyo, Japan) according to the manufacturer’s instructions. Both within- and between-assay quality control procedures were used and the coefficient of variation of the method was 1.3% to 3.4%, respectively, through the preriod of data collection. All participants in this study had detectable CRP levels.
Evaluation of atherosclerosis
Carotid Intima-Media Thickness (CIMT)
The CIMT, measured in millimeters (mm) were measured using high resolution ultrasonography (US) on all study subjects. The ultrasound protocol of the CIMT is described in more detail elsewhere (9,10). In brief, images of the right and left carotid artery (CCA), bifurcation, and internal carotid artery (ICA) were acquired with an Acuson Sequoia C256 with a two-dimensional 8 MHz linear array transducer. Images of the intima-meida thickness were acquired from a prediefined 10 mm segment (extending from 10 mm to 20 mm proximal to the tip of the flow divider) at defined interrogation angles using the Meijers Arc. Standard images were obtained from 4 angles at each side. The mean IMT of the near and far walls was determined from a single image at each interrogation angle for both the right and left CCA. The average of all these IMT values comprised the CCA-IMT outcome parameter. All IMT measurements were carried out using the Artery Measurement System (AMSII) software (v1.141)
Coronary artery and aorta calcification
Calcium was quantified by Agatston scores of coronary arteries, proximal aortic arch and distal aorta using computerized tomography (CT) images of the chest (10,11,12) Cerebral infarction: The scoring of cerebral infarcts has been described in detail elsewhere (13,14). In brief, infarcts were scored by trained radiographers and defined as defects of the brain parenchyma with a signal intensity isointense to cerebrospinal fluid on all MR images (FLAIR, PD/T2-weighted images), associated hyperintensity on T2-weighted and FLAIR images with a minimal diameter of 4 mm, except for infarcts in the cerebellum and brain stem which had no size criteria and did not require associated hyperintensity.
Statistical Analysis
Stata 13.1 was used to perform the statistical analyses. Baseline characteristics for continuous variables were presented as mean values and standard deviations (SDs) or medians with 25th and 75th centiles and numbers and percentages were used for categorical variables. Logtrans formed outcomes for CRP were used. The overall comparison given in Table 1 is done with ANOVA except for the artery calcification variables where the Kruskal-Wallis test was used. We performed our analyses in three steps, one model for each step with outcome variable as given in Table 1. In the first step all the analyses were adjusted for age, airflow obstruction, smoking status (including interaction with airflow obstruction) and sex. In the second step, all analyses were adjusted for age, airflow obstruction, smoking status (including interaction with airflow obstruction), sex, as well as Framingham cardiac risk factors. These include: diabetes, hypertension, systolic blood pressure, cholesterol, HDL cholesterol and body mass index. In the third step markers of systemic inflammation were added. They were CRP levels and WBC count. Relationship between the two variables was measured by the Pearson correlation coefficient (r). Quantile regression was used for the outcome variables coronary calcium and aortic and distal calcium. Generalized linear model was used for CIMTmean and logit model was used for brain infarcts. Post estimation of adjusted differences was evaluated by the margins command in Stata and for covariates fixed at their means, see Table 2. Significance was defined as p<0.05.
Table 1.
Comparison between four groups according to smoking status and spirometry
| d | Normal spirometry and never smoker | Normal spirometry and ever smoker | Abnormal spirometry and never smoker | Abnormal spirometry and ever smoker | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | mean | sd | n | mean | sd | n | mean | sd | n | mean | sd | Total | p | |
| Age | 350 | 76.0 | 5.5 | 411 | 74.9 | 5.5 | 120 | 79.4 | 5.4 | 273 | 76.6 | 5.6 | 1154 | <0.0001 |
| BMIa | 350 | 27.0 | 4.4 | 411 | 27.6 | 3.9 | 120 | 25.3 | 3.7 | 273 | 25.7 | 4.1 | 1154 | <0.0001 |
| FVCb L | 350 | 2.99 | 0.78 | 411 | 3.29 | 0.80 | 120 | 2.84 | 0.83 | 273 | 2.92 | 0.85 | 1154 | <0.0001 |
| FEV1c L | 350 | 2.28 | 0.62 | 411 | 2.50 | 0.64 | 120 | 1.75 | 0.57 | 273 | 1.81 | 0.57 | 1154 | <0.0001 |
| FEV1/FVC Ratio | 350 | 0.76 | 0.04 | 411 | 0.76 | 0.04 | 120 | 0.62 | 0.09 | 273 | 0.62 | 0.08 | 1154 | |
| Smoking | 350 | 411 | 0.16 | 120 | 273 | 0.30 | 1154 | |||||||
| Packyears | 350 | 367 | 21.34 | 120 | 256 | 28.9 | 1093 | |||||||
| Systolic BPd | 350 | 142 | 20 | 411 | 141 | 20 | 120 | 146 | 24 | 273 | 141 | 21 | 1154 | 0.099 |
| Diastolic BPd | 350 | 74 | 9 | 411 | 74 | 9 | 120 | 72 | 10 | 273 | 73 | 9 | 1154 | 0.063 |
| LDLe | 350 | 3.68 | 1.01 | 411 | 3.47 | 1.01 | 120 | 3.66 | 1.05 | 273 | 3.40 | 0.97 | 1154 | 0.001 |
| WBCf | 349 | 5.79 | 1.84 | 411 | 5.99 | 1.59 | 120 | 5.72 | 1.60 | 273 | 6.36 | 1.83 | 1153 | 0.0002 |
| centiles | centiles | centiles | centiles | |||||||||||
| median | 25th –75th | median | 25th –75th | median | 25th –75th | median | 25th –75th | |||||||
| CRPg mg/l | 350 | 1.6 | 1–1.3 | 411 | 2.1 | 1.1–4.3 | 119 | 1.7 | 1.0–2.8 | 273 | 2.0 | 1.1–4.3 | 1153 | 0.011 |
| Coronary calciumi | 346 | 171 | 14–622 | 410 | 430 | 69–1174 | 120 | 255 | 66–775 | 269 | 553 | 128–1288 | 1145 | <0.0001 |
| Aortic arch calcificationi | 347 | 1145 | 343–3023 | 410 | 1614 | 575–3621 | 120 | 2276 | 1076–4910 | 267 | 2481 | 1072–5547 | 1144 | <0.0001 |
| Distal aorta calcificationi | 346 | 98 | 5–720 | 410 | 157 | 16–876 | 120 | 562 | 62–1166 | 269 | 454 | 95–1397 | 1145 | <0.0001 |
| CIMT mm | 344 | 0.93 | 0.85–1.03 | 401 | 0.98 | 0.88–1.08 | 119 | 0.98 | 0.87–1.09 | 270 | 0.99 | 0.90–1.09 | 1134 | <0.0001 |
BMI: body mass index (kg/m2)
FVC: forced vital capacity in liters
FEV1: Forced expiratory volume in one second in liters
BP: blood pressure in mmHg
LDL: low density cholesterol in mmol/l
WBC: white blood cell count x10E9/L
CRP: C-reactive protein
CIMT: Carotid Intima media thickness
(Agatston units)
Table 2.
Multivariable analysis of cardiovascular outcomes using three models for different adjustments
| Adjusted difference between normal and abnormal spirometry | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Model 1a | Model 2b | Model 3c | ||||||||||
| N | Adjusted difference | 95% CI | p | Adjusted difference | 95% CI | p | Adjusted difference | 95% CI | p | ||||
| Coronary calcium | 1142 | 69.9 | −23.9 | 163.6 | =0.144 | 84.2 | −15.7 | 184.2 | =0.099 | 97.0 | −0.982 | 195.0 | =0.052 |
| Aortic arch calcification | 1141 | 510.7 | 167.4 | 853.9 | <0.004 | 504.2 | 148.2 | 860.3 | <0.006 | 481.9 | 112.1 | 851.8 | <0.011 |
| Distal aorta calcification | 1142 | 202.8 | 102.0 | 303.6 | <0.0001 | 158.3 | 52.2 | 264.5 | <0.004 | 146.4 | 37.8 | 255.1 | =0.008 |
| CIMTmean | 1131 | 0.017 | 0.000 | 0.034 | =0.054 | 0.019 | 0.002 | 0.036 | =0.027 | 0.019 | 0.002 | 0.036 | =0.029 |
| Brain Infarcts+ | 1013 | 0.013 | −0.051 | 0.076 | =0.694 | 0.017 | −0.048 | 0.083 | =0.600 | 0.016 | −0.050 | 0.081 | =0.636 |
| Adjusted difference between normal and abnormal spirometry for eversmokers | |||||||||||||
| Coronary Calcium | 1142 | 122.5 | 8.2 | 236.9 | =0.036 | 87.1 | −34.4 | 208.5 | =0.160 | 105.4 | −13.9 | 224.7 | =0.083 |
| Aortic arch calcification | 1141 | 706.5 | 287.5 | 1125.6 | <0.001 | 575.5 | 142.6 | 1008.4 | <0.009 | 540.9 | 90.3 | 991.5 | <0.019 |
| Distal aorta calcification | 1142 | 203.5 | 80.6 | 326.5 | <0.001 | 138.0 | 9.1 | 267.0 | =0.036 | 117.6 | −14.7 | 249.9 | =0.081 |
| CIMTmean | 1131 | 0.013 | −0.008 | 0.034 | =0.219 | 0.017 | −0.004 | 0.038 | =0.105 | 0.017 | −0.004 | 0.037 | =0.118 |
| Brain Infarctsd | 1013 | 0.018 | −0.060 | 0.096 | =0.657 | 0.024 | −0.056 | 0.104 | =0.557 | 0.020 | −0.059 | 0.100 | =0.616 |
Model 1 is adjusted for age, sex and contains main effects and interaction between smoking history and airflow obstruction
Model 2 is model 1 extended by adjusting for the Framingham risk factors.
Model 3 is model 2 extended by adjusting for CRP and WBC.
absolute difference between proportions
Results
There were a total of 1154 individuals included in the analysis. Median age was 76 years and 47% were males. Smoking was more common in males. Average BMI was 26.7 kg/m2 (SD 4.2). Subjects were divided into four groups according to smoking status and if airflow limitation was present or not. Comparisons between the four groups are shown in Table 1. Most of the subjects were ever smokers with normal spirometry and the group of neversmokers with abnormal spirometry had fewest individuals. There was no difference in blood pressure between the groups. WBC counts and CRP levels were higher in ever smokers than never smokers. Markers of atherosclerosis of coronaries/aorta/carotid arteries were highest in the smokers with airflow obstruction, especially compared with non-smokers.
Airflow obstruction and atherosclerosis
Table 1 describes the measurements of atherosclerosis burden between the four groups. Individuals with airflow obstruction had significantly higher burden of atherosclerosis, indicated by either coronary calcium (171 Agatston units (log) vs 255 Agatston units (log) for never-smokers, 430 Agatston units (log) vs. 553 Agatston units (log) for ever-smokers), aortic arch calcium (1145 vs 2276 for never-smokers, 1614 Agatston units (log) vs. 2481 Agatston units (log) for ever-smokers) distal aorta calcium (98 Agatston units (log) vs 562 Agatston units (log) for never-smokers, 157 Agatston units (log) vs. 454 Agatston units (log) for eversmokers) and CIMT (0.93 mm vs 0.98 mm for never-smokers, 0.98 mm vs. 0.99 mm for eversmokers). In general we found that neither CRP levels nor WBC counts showed significant association with any of the calcium variables. However, WBC counts was found to be associated with aortic arch calcium score (p=0.038) if CRP levels were not in the model. Correlation between log(CRP) and WBC was r=0.25 (p<0.0001).
In multivariable analysis for all individuals after adjusting for age, sex and smoking history, airflow obstruction was associated with a higher burden of atherosclerosis as measured by the difference in both aortic arch (511, 95% CI 167–854 Agatston units (log)), distal aorta (203, 95% CI 102–304 Agatston units (log)) calcification and CIMT (0.017,95% CI 0.0–0.034), This relationship was further strengthened after additional adjustment for Framingham risk factors and markers of inflammation (Table 2, model 2 and 3).
Discussion
In this study we confirmed the previously known association between reduced airflow and atherosclerosis in a large prospectively collected cohort. Furthermore, we showed that association holds even after correcting the association for markers of systemic inflammation (WBC and CRP). Thus, it seems unlikely that markers of systemic inflammation (WBC and CRP) mediates the association between airflow limitation and atherosclerosis. Rather, both airflow limitation and markers of systemic inflammation (WBC and CRP) appear to be independent predictors of atherosclerosis.
Although cigarette smoking is widely accepted as the most important risk factor for COPD, it is now recognized that never-smokers may account for between one fourth and one third of all COPD patients (15). Several studies have demonstrated that risk factors for airflow limitation differ in smokers and never-smokers (15,16). In addition studies have shown that in older individuals definition of airflow obstruction as a ratio of FEV1/FVC lower that 0.7 may overestimate the incidence of airflow obstruction compared with using lower limits of normal in elderly subjects (17). Thus it is important to study the observation in both ever- and never-smokers. We compared subjects both according to airflow limitation and smoking status by splitting them up into four groups as others have done previously (18). We found that the relationship between airflow obstruction and atherosclerosis after correcting for markers of systemic inflammation (WBC and CRP) is similar in both never- and ever-smokers.
Our findings that both coronary calcium, aortic arch calcification, distal aorta calcification and CIMT are worse in patients with airflow obstruction, both in ever-smokers and never-smokers, can be compared to several previous studies. In the National Health and Nutrition Examination Survey (NHANES) database the estimated self-reported cardiovascular disease prevalence was 20.0% and 7.4% in COPD and non-COPD groups, respectively (19). In multivariable analysis, COPD was an independent risk factor for self-reported cardiovascular disease. A study from Japan found that mean CIMT was greater in smokers with airflow limitation compared to either control smokers without airflow limitation or control never-smokers (20). Multivariable analyses showed significant associations between both thickened CIMT and decreased percent FEV1 independent of age, pack-years of smoking, body mass index, peripheral mean arterial pressure, heart rate, glucose, and low density lipoprotein cholesterol. Another study from Japan categorized 234 patients with coronary artery disease into four groups: neversmokers with normal pulmonary function (group A), never-smokers with airflow limitation (group B), ever-smokers with normal pulmonary function (group C), and ever-smokers with airflow limitation (group D)(18). They found the prevalence of airflow limitation was 23.1%. The prevalence of carotid atherosclerosis was 28.2, 29.4, 41.3, and 45.9%, respectively, in the four groups (group D vs. group A, p = 0.035). Even after multivariable adjusting for confounding factors, eversmokers with airflow limitation were independently associated with carotid atherosclerosis (odds ratio 2.89, 95% confidence interval, 1.19–7.00, p=0.019). The Mesa study also found that FEV1 was associated with the extent of distal aortic calcification (0.76; 95%CI 0.60–0.97, p=0.02) but not proximal aortic calcification (21). A study by Chae et al found that the amount of vascular calcification was associated with the extent of emphysema on computed tomography and airflow limitation (22). This is in contrast with a recent study from China on elderly smokers with airflow limitation that found no evidence that the association of pulmonary function with CIMT varied by smoking status (23).
Similarly, our findings that markers of systemic inflammation are correlated with atherosclerosis are supported by a vast literature on the association between markers of systemic inflammation and atherosclerosis. An example is the recent study by Rein et al, showing that both peripheral vascular disease and coronary artery disease are associated with higher levels of CRP compared to controls (24). Secondarily, the study by Cushman and colleagues in which 3,971 men and women aged ≥65 years were followed for 10 years, found that patients with a CRP level >3.0 mg/L had a 1.45-fold increased risk of myocardial infarction or death due to coronary heart disease after adjustment for traditional CAD risk factors (25). There is also vast infomation available on the relationship between systemic inflammation and FEV1. In a recent study spirometry was measured in a population-based cohort at ages 32 and 38 years. CRP, fibrinogen, and WBC were measured at the same ages. There were no longitudinal associations between WBC and lung function. Higher CRP and fibrinogen at age 32 were associated with higher FEV1 and FEV1/FVC at age 38. The authors concluded that there was no evidence that systemic inflammation causes a decline in lung function (26). Metaanalysis has shown association between systemic inflammation and airway obstruction (5). Studies have also shown that markers of systemic inflammation are associated with rapid decline in FEV1 in individuals with COPD (27). As shown systemic inflammation has been linked to both atherosclerosis and airflow limitation. Possible pathways include complex interrelationships between chronic low-grade systemic inflammation and oxidative stress as well as shared risk factors such as age, cigarette smoking, and environmental pollutants.
The strength of the study is the well defined population of both males and females. They represent a sample of older individuals from the Reykjavik metropolitan area that have all lived within a 30 km radius and been exposed to rather similar amount of air pollution. The AGES-Reykjavik study has a very rigid data collection protocols. To our knowledge, none of the available studies has simultaneously available data on airflow obstruction, markers of systemic inflammation and atherosclerosis. We were in a unique position to contrast the three and test the association between airflow obstruction and atherosclerosis after correcting for the most common indicators of systemic inflammation and cardiovascular risk factors.
Our study has several limitations. One is the lack of perform postbronchodilator pulmonary function tests, so the study might overestimate the prevalence of subjects with non-reversible airflow limitation. This deficiency is partially mediated since we excluded the diagnosis of other respiratory diseases through self-reported diagnoses. Therefore it is most likely that these individuals had COPD. Another limitation is that we only use two markers of systemic inflammation, ie WBC and CRP. Most previous studies use one or two markers to represent systemic inflammation. It might be interesting to measure other markers of systemic inflammation. The third limitation is that there were only Caucasians in the study and results can possibly not directly be applied to other races. This was a study of elderly people and similarly results can therefore also not be applied to other age populations.
In summary our study shows that two markers of systemic inflammation (WBC and CRP) do not mediate the association between airflow limitation and atherosclerosis. Only airflow limitation and not markers of systemic inflammation (WBC and CRP) appear to be independent predictor of atherosclerosis.
Acknowledgments
Funding
This work was supported by the National Institute on Aging, US National Institutes of Health (grant N01-AG-12100), the National Institute on Aging Intramural Research Program, Hjartavernd (Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Gunnar Gudmundsson was funded by Landspitali Scientific Fund and by the Icelandic Research Fund, Project Grant 90414021 and 90414022
References
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3:2011–2030. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2:8–11. doi: 10.1513/pats.200404-032MS. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 5.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Translational research: the journal of laboratory and clinical medicine. 2013;162:237–251. doi: 10.1016/j.trsl.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment SusceptibilityReykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bots ML, Hofman A, Grobbee DE. Common carotid intima-media thickness and lower extremity arterial atherosclerosis: the Rotterdam Study. Arterioscler Thromb. 1994;14:1885–91. doi: 10.1161/01.atv.14.12.1885. [DOI] [PubMed] [Google Scholar]
- 10.Sturlaugsdottir R, Aspelund T, Bjornsdottir G, Sigurdsson S, Eiriksdottir G, Imai CM, Garcia M, Launer LJ, Harris TB, Gudnason V. Carotid atherosclerosis and cardiovascular health metrics in old subjects from the AGES-Reykjavik study. Atherosclerosis. 2015;242:65–70. doi: 10.1016/j.atherosclerosis.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 12.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 13.Saczynski J, Sigurdsson S, Jonsdottir M, Eiriksdottir G, Garcia M, Jonsson P, Kjartansson O, van Buchem M, Gudnason V, Launer L. Cerebral infarcts and cognitive performance: Importance of location and number of infarcts. Stroke. 2009;40:677–82. doi: 10.1161/STROKEAHA.108.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achten E, Brenteler M, de Leeuw F, de Groot J, Scheltens P, Heyboer R, Oudekerk M. Rating scale for age related brain changes. Imaging Decisions. 2002;4:10. [Google Scholar]
- 15.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, NizankowskaMogilnicka E, Studnicka M, Bateman E, Anto JM, Burney P, Mannino DM, Buist SA BOLD Collaborative Research Group. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–63. doi: 10.1378/chest.10-1253. Epub 2010 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng G, Sun B, Zhong N. Non-smoking-related chronic obstructive pulmonary disease: a neglected entity? Respirology. 2012;17:908–12. doi: 10.1111/j.14401843.2012.02152.x. [DOI] [PubMed] [Google Scholar]
- 17.Luoto JA, Elmståhl S, Wollmer P, Pihlsgård M. Incidence of airflow limitation in subjects 65–100 years of age. Eur Respir J. 2016;47:461–72. doi: 10.1183/13993003.006352015. Epub 2015 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto H1, Yokoyama A, Kitahara Y, Ishikawa N, Haruta Y, Yamane K, Hattori N, Hara H, Kohno N. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med. 2009;179:35–40. doi: 10.1164/rccm.200804-560OC. Epub 2008 Oct 17. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal S, Rokadia H, Senn T, Menon V. Burden of cardiovascular disease in chronic obstructive pulmonary disease. Am J Prev Med. 2014;47:105–14. doi: 10.1016/j.amepre.2014.03.014. Epub 2014 Jul 3. [DOI] [PubMed] [Google Scholar]
- 20.Hamrah MS, Suzuki S, Ishii H, Shibata Y, Tatami Y, Osugi N, Ota T, Kawamura Y, Tanaka A, Aso H, Takeshita K, Sakamoto J, Hasegawa Y, Murohara T. Impact of airflow limitation on carotid atherosclerosis in coronary artery disease patients. Respiration. 2015;89:322–8. doi: 10.1159/000375313. Epub 2015 Mar 17. [DOI] [PubMed] [Google Scholar]
- 21.McAllister DA, MacNee W, Duprez D, Hoffman EA, Vogel-Claussen J, Criqui MH, Budoff M, Jiang R, Bluemke DA, Barr RG. Pulmonary function is associated with distal aortic calcium, not proximal aortic distensibility. MESA lung study. COPD. 2011;8:71–8. doi: 10.3109/15412555.2011.558543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chae EJ1, Seo JB, Oh YM, Lee JS, Jung Y, Lee SD. Severity of systemic calcified atherosclerosis is associated with airflow limitation and emphysema. J Comput Assist Tomogr. 2013;37:743–9. doi: 10.1097/RCT.0b013e318299f9e7. [DOI] [PubMed] [Google Scholar]
- 23.Pan J, Xu L, Cai SX, Jiang CQ, Cheng KK, Zhao HJ, Zhang WS, Jin YL, Lin JM, Thomas GN, Lam TH. The association of pulmonary function with carotid atherosclerosis in older Chinese: Guangzhou Biobank Cohort Study-CVD Subcohort. Atherosclerosis. 2015;243:469–76. doi: 10.1016/j.atherosclerosis.2015.09.036. Epub 2015 Oct 3. [DOI] [PubMed] [Google Scholar]
- 24.Rein P, Saely CH, Silbernagel G, Vonbank A, Mathies R, Drexel H, Baumgartner I. Systemic inflammation is higher in peripheral artery disease than in stable coronary artery disease. Atherosclerosis. 2015;239:299–303. doi: 10.1016/j.atherosclerosis.2015.01.021. Epub 2015 Jan 28. [DOI] [PubMed] [Google Scholar]
- 25.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. Epub 2005 Jun 27. [DOI] [PubMed] [Google Scholar]
- 26.Hancox RJ, Gray AR, Sears MR, Poulton R. Systemic inflammation and lung function: A longitudinal analysis. Respir Med. 2016;111:54–9. doi: 10.1016/j.rmed.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–5. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]