Abstract
Objective
Breast cancer simulation models must take changing mortality rates into account to evaluate the potential impact of cancer control interventions. We estimated mortality rates due to breast cancer and all other causes combined to determine their impact on overall mortality by year, age and birth cohort.
Methods
Based on mortality rates from publicly available datasets, an age-period-cohort model was used to estimate the proportion of deaths due to breast cancer for US women aged 0–119 with birth years 1900–2000. Breast cancer mortality was calculated as all-cause mortality multiplied by the proportion of deaths due to breast cancer; other-cause mortality was the difference between all-cause and breast cancer mortality.
Results
Breast cancer and other-cause mortality rates were higher for older ages and birth cohorts. The percent of deaths due to breast cancer increased across birth cohorts from 1900 to 1940 then decreased. Among 50-year-old women, in the 1920 birth cohort, 52/100,000 deaths (9.9%, 95% confidence interval 9.8–10.1%) were attributed to breast cancer with 476/100,000 due to other causes; in the 1960 birth cohort, 22/100,000 deaths (8.5%, 95% confidence interval 8.3–8.7%) were attributed to breast cancer with 242/100,000 due to other causes. The percent of all deaths due to breast cancer was highest (4.1–12.9%) for women in their 40s and 50s for all birth cohorts.
Conclusions
This study offers evidence that advances in breast cancer screening and treatment have reduced breast cancer mortality for women across the age spectrum, and provides estimates of age-, year- and birth cohort-specific competing mortality rates for simulation models. Other-cause mortality estimates are important in these models because the majority of women die from causes other than breast cancer.
Keywords: Breast Cancer, Mortality, Age-period-cohort Modeling, Simulation Modeling
INTRODUCTION
Breast cancer is a leading cause of death among women in the United States with over 40,000 women dying from breast cancer annually [1, 2]. Breast cancer mortality rates have steadily declined over the past 25 years [3]. Between 1990 and 2015, breast cancer mortality decreased approximately 39% [2, 4]. However, these declines have not been equal across all age groups, with the youngest and oldest women having a smaller decline in breast cancer mortality than other women [5, 6]. Previous studies have also identified differences in breast cancer mortality patterns by birth cohort, with those born between 1900 and 1950 having a higher proportion of deaths from breast cancer than women born after 1950 [7, 8].
Population-based simulation modeling of breast cancer within the Cancer Intervention and Simulation Modeling Network (CISNET) has been used to quantify relative contributions of screening and treatment to these observed patterns of declines in breast cancer mortality rates. The models have also been used to forecast the benefits, harms, and costs of alternative screening and treatment interventions on breast cancer death rates [9–13]. Such simulations require accurate estimates of trends in non-breast cancer (“other cause”) mortality over age, time periods and birth cohorts to account for competing risks of death [7, 8].
Since both all-cause and breast cancer-specific mortality rates have been changing over time, estimates of other-cause mortality rates (which are the simple difference of the two) require regular updates. Therefore, the overarching goal of this paper was to apply statistical methods to the latest available data to derive breast cancer and other-cause mortality by age and birth cohort through 2010. These data are intended for use in simulation modeling. In addition, these data can be used to illustrate the relative and absolute impact of breast cancer mortality on overall mortality for different birth cohorts.
METHODS
The objective of this analysis was to estimate rates of death due to breast cancer (breast cancer mortality) and deaths due to causes other than breast cancer (other-cause mortality) according to birth year and age. This study was done as part of the CISNET Breast Working Group using observed mortality trends through 2014; prior analyses were based on data available through 1999 [7]. Only publicly available anonymous data were used, so this study was determined to be exempt from human subjects review by the University of Wisconsin Health Sciences Institutional Review Board.
Data Sources
Breast cancer (ICD-10 C50) and all-cause female deaths by single year of age 0–99 for calendar year of death (period) 1999–2014 were obtained from the Detailed Mortality file on CDC WONDER [1]. (Data for single years of age >99 are not available from CDC WONDER.) Breast cancer (ICD-9 174 for 1979–1998 and ICD-8 174 for 1968–1978) and all-cause female deaths by age group (<1, 1–4, 5–9, 10–14, 15–19, 20–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, 85+) for calendar year of death (period) 1968–1998 were obtained from the Compressed Mortality files on CDC WONDER [1]. We found no evidence of differences associated with the transition between ICD codes (p=0.57 by score test). The logistic regression models (described below) use data from calendar years 1968–2014. Estimates for prior (and future) calendar years assume that the definition of breast cancer in prior years is consistent with the definition of breast cancer in 1968–2014.
Female all-cause mortality cohort life tables by single year of age from 0–119 for years of birth (cohorts) 1900–2000 were obtained from the Berkeley Mortality Database [14].
Analysis
We used an age-period-cohort (APC) model [15] to estimate the proportion of deaths due to breast cancer for all ages (0–119) and birth years (1900–2000) of interest from the Detailed and Compressed Mortality Data with an over-dispersed (quasibinomial) generalized additive logistic regression model [16]. The APC modeling approach was chosen since it provided estimates for the proportion of deaths due to breast cancer for all single years of age, years of birth, and calendar years; 95% confidence intervals (CI) around the proportions were calculated using the standard approach (+/− 1.96 x standard error) on the logit scale. Age, period (calendar year of death) and cohort (calendar year of birth) were entered into the logistic regression model as additive thin-plate regression splines [17]. The APC model cannot be uniquely parameterized due to the linear dependence of age, period and cohort (age = period-cohort), which prevents the estimation of linear trends for all three factors (age, period and cohort). Following the identification strategy of Carstensen [15], linear terms were included in the age and cohort effects, while the period effect was constrained to have 0 slope on average. The thin-plate splines were constrained to be linear for age >99 years and periods before 1968 and after 2014. The smoothing parameters for the thin plate regression splines were selected to optimize the goodness-of-fit of the regression by generalized cross-validation, which penalized possible overfitting [18]. Data for single years of age were only available for calendar years 1999–2014; for earlier years, age groups were converted to the corresponding central age of the five-year group for analysis (0, 2.5, 7, 12, 17, 22, 29.5, 39.5, 49.5, 59.5, 69.5, 79.5 and 92).
Breast cancer mortality was calculated as all-cause mortality from the Berkeley Mortality Database multiplied by the proportion of deaths due to breast cancer. Other-cause mortality was the difference between all-cause mortality and breast cancer mortality.
Estimates are provided for birth years 1900 through 1990 since 1990 is the last decade with observed breast cancer deaths by 2014; extrapolations through birth year 2000 are available in an online interactive resource (https://resources.cisnet.cancer.gov/projects/#bcr/bcmort). Analysis was conducted using the mgcv [16, 17] and ggplot2 [19] packages in R v3.3.1 [20].
RESULTS
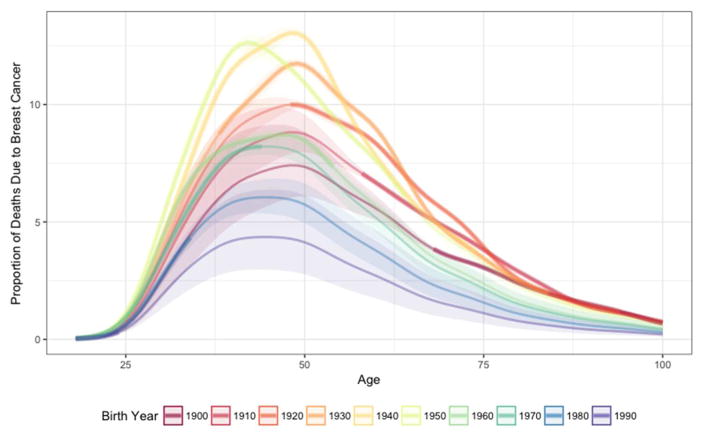
As a fraction of all deaths, breast cancer mortality increased from the 1900 to the 1940 birth cohort, and was estimated to have since declined through 1990 to a level lower than observed in 1900 (Figure 1); the percent of deviance explained by the model is 99.7%. The percent of all deaths attributable to breast cancer in 50-year-olds increased from 7.3% (95% CI 6.1–8.8%) in the 1900 birth cohort to peak at 12.9% (95% CI 12.7–13.1%) for the 1940 birth cohort. We estimate that 4.1% (95% CI 2.8–6.1%) of deaths in the 1990 birth cohort among 50-year-olds will be due to breast cancer.
Figure 1.
Proportion of deaths due to breast cancer in women by age according to decade of birth, 1900 to 1990, United States. Thick lines show estimates based on observed data; thin lines based on extrapolated estimates and the shaded regions show corresponding 95% confidence intervals.
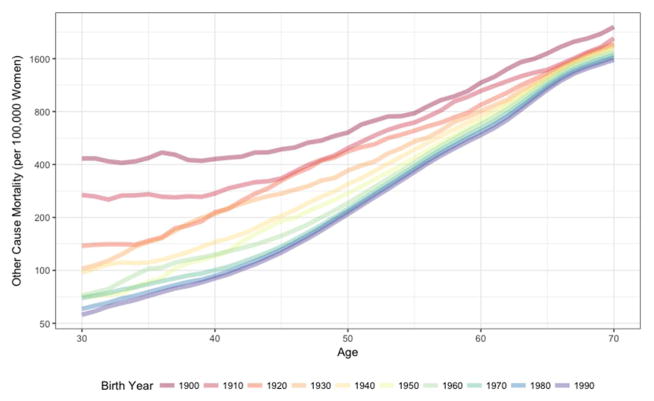
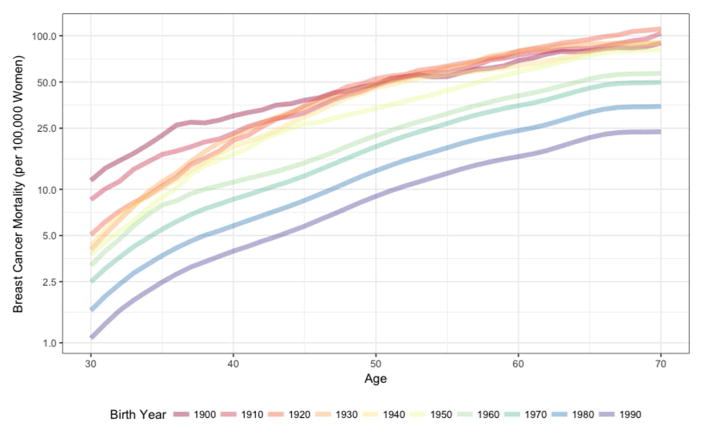
As expected, other-cause mortality rates generally increased with age (Figure 2A). For example, in the 1940 birth cohort, other-cause mortality increased from 97 per 100,000 30-year-old women to 1,874 per 100,000 70-year-old women (Table). Other-cause mortality rates have steadily fallen across birth cohorts across all years 1900–1990. For example, we estimate that among 50-year-olds, other-cause mortality decreased from 608 per 100,000 women born in 1900 to 309 per 100,000 women born in 1940 (a 49% decrease) to 209 per 100,000 women born in 1990 (an additional 32% decrease). Breast cancer mortality rates also generally increased with age (Figure 2B); breast cancer mortality rates increased from 4 per 100,000 for 30-year-olds to 90 per 100,000 70-year-old women born in 1940 (Table).
Figure 2.
Mortality rates per 100,000 women for (A) other-cause mortality and (B) breast cancer mortality by age according to decade of birth, 1900 to 1990, United States.
Table.
Female all-cause, breast cancer and other-cause mortality rates per 100,000 in the United States from 1900 to 1990 birth cohorts by decade of age.
| Birth Year | Mortality Rate (per 100,000 Women) | % Deaths Due to Breast Cancer | |||||
|---|---|---|---|---|---|---|---|
| Age | All Cause | Breast Cancer | Other Causes | Estimate | 95% CI | ||
| 1900 | 20 | 535 | 0 | 535 | 0.1% | 0.0% | 0.1% |
| 30 | 444 | 11 | 433 | 2.6% | 1.7% | 3.8% | |
| 40 | 460 | 30 | 430 | 6.5% | 4.9% | 8.7% | |
| 50 | 656 | 48 | 608 | 7.3% | 6.1% | 8.8% | |
| 60 | 1237 | 69 | 1168 | 5.6% | 5.1% | 6.1% | |
| 70 | 2513 | 90 | 2423 | 3.6% | 3.5% | 3.6% | |
| 80 | 5620 | 134 | 5486 | 2.4% | 2.3% | 2.4% | |
|
| |||||||
| 1910 | 20 | 341 | 0 | 341 | 0.1% | 0.0% | 0.1% |
| 30 | 277 | 9 | 268 | 3.2% | 2.3% | 4.1% | |
| 40 | 297 | 23 | 274 | 7.8% | 6.5% | 9.4% | |
| 50 | 543 | 47 | 496 | 8.7% | 8.0% | 9.6% | |
| 60 | 1123 | 75 | 1048 | 6.7% | 6.5% | 6.8% | |
| 70 | 2194 | 104 | 2090 | 4.7% | 4.6% | 4.8% | |
| 80 | 5072 | 145 | 4927 | 2.9% | 2.8% | 2.9% | |
|
| |||||||
| 1920 | 20 | 190 | 0 | 190 | 0.1% | 0.1% | 0.1% |
| 30 | 143 | 5 | 138 | 3.5% | 2.9% | 4.3% | |
| 40 | 235 | 21 | 214 | 8.8% | 8.1% | 9.7% | |
| 50 | 528 | 52 | 476 | 9.9% | 9.8% | 10.1% | |
| 60 | 954 | 79 | 875 | 8.3% | 8.2% | 8.5% | |
| 70 | 2054 | 111 | 1943 | 5.4% | 5.3% | 5.5% | |
| 80 | 4835 | 123 | 4712 | 2.5% | 2.5% | 2.6% | |
|
| |||||||
| 1930 | 20 | 92 | 0 | 92 | 0.1% | 0.1% | 0.1% |
| 30 | 106 | 4 | 102 | 3.8% | 3.4% | 4.2% | |
| 40 | 231 | 22 | 209 | 9.5% | 9.3% | 9.7% | |
| 50 | 418 | 49 | 369 | 11.7% | 11.7% | 11.9% | |
| 60 | 884 | 79 | 805 | 9.0% | 9.0% | 9.1% | |
| 70 | 1991 | 90 | 1901 | 4.5% | 4.5% | 4.6% | |
| 80 | 4616 | 114 | 4502 | 2.5% | 2.5% | 2.5% | |
|
| |||||||
| 1940 | 20 | 63 | 0 | 63 | 0.1% | 0.1% | 0.1% |
| 30 | 101 | 4 | 97 | 4.2% | 4.1% | 4.4% | |
| 40 | 164 | 19 | 145 | 11.5% | 11.3% | 11.8% | |
| 50 | 355 | 46 | 309 | 12.9% | 12.7% | 13.1% | |
| 60 | 817 | 64 | 753 | 7.8% | 7.7% | 7.9% | |
| 70 | 1964 | 90 | 1874 | 4.6% | 4.5% | 4.6% | |
| 80 | 4389 | 106 | 4283 | 2.4% | 2.3% | 2.6% | |
|
| |||||||
| 1950 | 20 | 71 | 0 | 71 | 0.1% | 0.1% | 0.1% |
| 30 | 75 | 4 | 71 | 5.0% | 4.8% | 5.2% | |
| 40 | 137 | 17 | 120 | 12.3% | 12.1% | 12.6% | |
| 50 | 308 | 34 | 274 | 10.9% | 10.7% | 11.1% | |
| 60 | 764 | 58 | 706 | 7.6% | 7.5% | 7.7% | |
| 70 | 1895 | 81 | 1814 | 4.3% | 4.0% | 4.5% | |
| 80 | 4156 | 94 | 4062 | 2.3% | 2.0% | 2.6% | |
|
| |||||||
| 1960 | 20 | 60 | 0 | 60 | 0.1% | 0.1% | 0.1% |
| 30 | 75 | 3 | 72 | 4.3% | 4.1% | 4.4% | |
| 40 | 134 | 11 | 123 | 8.3% | 8.1% | 8.5% | |
| 50 | 264 | 22 | 242 | 8.5% | 8.3% | 8.7% | |
| 60 | 720 | 41 | 679 | 5.6% | 5.3% | 6.0% | |
| 70 | 1811 | 57 | 1754 | 3.1% | 2.8% | 3.6% | |
| 80 | 3944 | 65 | 3879 | 1.6% | 1.4% | 2.0% | |
|
| |||||||
| 1970 | 20 | 50 | 0 | 50 | 0.1% | 0.1% | 0.1% |
| 30 | 72 | 2 | 70 | 3.5% | 3.3% | 3.6% | |
| 40 | 109 | 9 | 100 | 7.9% | 7.8% | 8.2% | |
| 50 | 243 | 19 | 224 | 7.8% | 7.6% | 8.3% | |
| 60 | 678 | 35 | 643 | 5.2% | 4.6% | 5.9% | |
| 70 | 1734 | 50 | 1684 | 2.9% | 2.4% | 3.5% | |
| 80 | 3753 | 56 | 3697 | 1.5% | 1.2% | 1.9% | |
|
| |||||||
| 1980 | 20 | 45 | 0 | 45 | 0.1% | 0.1% | 0.1% |
| 30 | 62 | 2 | 60 | 2.6% | 2.4% | 2.9% | |
| 40 | 99 | 6 | 93 | 5.8% | 5.3% | 6.5% | |
| 50 | 230 | 13 | 217 | 5.7% | 5.0% | 6.6% | |
| 60 | 639 | 24 | 615 | 3.8% | 3.1% | 4.6% | |
| 70 | 1664 | 35 | 1629 | 2.1% | 1.6% | 2.7% | |
| 80 | 3578 | 39 | 3539 | 1.1% | 0.8% | 1.5% | |
|
| |||||||
| 1990 | 20 | 40 | 0 | 40 | 0.1% | 0.0% | 0.1% |
| 30 | 57 | 1 | 56 | 1.9% | 1.3% | 2.7% | |
| 40 | 94 | 4 | 90 | 4.2% | 2.9% | 6.1% | |
| 50 | 218 | 9 | 209 | 4.1% | 2.8% | 6.1% | |
| 60 | 605 | 16 | 589 | 2.7% | 1.7% | 4.2% | |
| 70 | 1599 | 24 | 1575 | 1.5% | 0.9% | 2.4% | |
| 80 | 3420 | 26 | 3394 | 0.8% | 0.4% | 1.3% | |
Note: Shaded cells are based on observed data. Cells without shading are projections. Abbreviation: CI, confidence interval.
Breast cancer mortality rates decreased for most but not all birth cohorts since 1900, with rates among 50-year-old women in early birth cohorts virtually unchanged (48 deaths per 100,000 women in the 1900 birth cohort and 46 deaths per 100,000 women in 1940) before declining to 22 deaths per 100,000 women in the 1960 birth cohort and an estimated 9 deaths per 100,000 women in the 1990 birth cohort (Table).
DISCUSSION
Our results show that mortality, breast cancer mortality and other-cause mortality rates have decreased for women of all ages in recent birth cohorts. Overall, age-adjusted breast cancer mortality rates have been decreasing since 1990 [3]; between 1990 and 2015, breast cancer mortality rates dropped by 39% [4]. Early detection efforts with mammography screening and improvements in breast cancer therapy have both contributed to declines in breast cancer mortality rates [21]. Breast cancer deaths in the most recent birth cohort (1990) contribute less to overall mortality than in the 1940 birth cohort—when breast cancer had its greatest impact; breast cancer also contributes less to overall mortality in the most recent birth cohort than in the 1900 birth cohort, the first cohort for whom we estimated death rates.
The reduction in mortality seen for breast cancer over time has also been observed for other cancers in women affected by improvements in early detection and treatment; for example, colorectal cancer mortality rates declined by 44% in women between 1990 and 2015 [4].
Our study extends previous work by Rosenberg et al (Appendix Table) [7], who used similar life table methods applied to earlier versions of the Berkeley Mortality Database and breast cancer mortality data provided by the National Center for Health Statistics to obtain other-cause mortality rates. We estimate that, among 50-year-old women born in 1940, 12.9% (95% CI 12.7–13.1%) of deaths were attributable to breast cancer, while Rosenberg [7] estimated that 13.4% of deaths were attributable to breast cancer, resulting in estimated other-cause mortality of 309 per 100,000 women from our model versus 307 per 100,000 women from Rosenberg. Rosenberg’s model estimated that breast cancer’s relative contribution to overall mortality peaked at 15% in 43-year-olds born in 1947, whereas we estimated a similar peak of 14% in 46-year-olds born in 1945 [7]. Larger differences are evident for younger women in earlier periods. For example, for 30-year-olds, we estimate 11 breast cancer deaths per 100,000 women for the 1900 birth cohort, 9 per 100,000 women for the 1910 birth cohort and 5 per 100,000 women for the 1920 birth cohort, while Rosenberg estimates 4 breast cancer deaths per 100,000 women for all three cohorts, essentially using the estimated breast cancer mortality for the 1920 cohort for all prior cohorts. Similarly, we assumed that period effects in future years followed a linear trend, so that our estimates for breast cancer mortality after the year 1999 are larger than estimates provided by Rosenberg. To the extent that period effects on the percent of deaths attributable to breast cancer after the year 2014 deviates from a linear pattern on the logit scale, our estimates will be in error.
Appendix Table.
Female all-cause, breast cancer and other-cause mortality rates per 100,000 in the United States for the 1910, 1920, 1930 and 1940 birth cohorts by decade of age (Source: Rosenberg, 2006)
| Birth Year | Mortality Rate (per 100,000 Women) | % Deaths Due to Breast Cancer | |||
|---|---|---|---|---|---|
| Age | All Cause | Breast Cancer | Other Causes | ||
| 1910 | 30 | 277 | 4 | 273 | 1.6% |
| 40 | 297 | 23 | 275 | 7.6% | |
| 50 | 543 | 54 | 490 | 9.9% | |
| 60 | 1123 | 77 | 1046 | 6.9% | |
| 70 | 2194 | 101 | 2093 | 4.6% | |
|
| |||||
| 1920 | 30 | 143 | 4 | 139 | 3.0% |
| 40 | 235 | 22 | 213 | 9.2% | |
| 50 | 528 | 54 | 474 | 10.3% | |
| 60 | 954 | 81 | 873 | 8.5% | |
| 70 | 2054 | 112 | 1942 | 5.5% | |
|
| |||||
| 1930 | 30 | 106 | 4 | 102 | 4.0% |
| 40 | 231 | 22 | 210 | 9.3% | |
| 50 | 418 | 50 | 368 | 11.9% | |
| 60 | 884 | 80 | 804 | 9.0% | |
| 70 | 1991 | 91 | 1900 | 4.6% | |
|
| |||||
| 1940 | 30 | 101 | 5 | 96 | 4.6% |
| 40 | 164 | 19 | 145 | 11.8% | |
| 50 | 355 | 48 | 307 | 13.4% | |
| 60 | 817 | 61 | 756 | 7.5% | |
| 70 | 1964 | NA | NA | NA | |
Abbreviation: NA, not available
Vilaprinya [8] calculated other-cause mortality for Catalonia, Spain using a life table approach. Results were similar to our findings, showing that the impact of breast cancer on all-cause mortality was greatest for women born around 1950 in the 40–49 age group, and that the proportion of deaths due to breast cancer has declined in recent birth cohorts. Other investigators have used time series analysis to forecast future breast cancer rates for the United States [5]; since source data are essentially identical for all researchers—arising from national death statistics—study findings are concordant. Another study used survival analysis to calculate other-cause mortality restricted to adult (ages ≥50) cancer patients to provide insight into non–cancer-related health issues among cancer patients and their risk of dying from other causes [22]. Their cumulative survival probability estimates are specifically designed for research investigating the impact of various treatment approaches in adults diagnosed with cancer (among whom the risk of death from cancer is relatively high), and are not directly comparable to our mortality rates that based on women at risk of breast cancer (among whom the risk of death from cancer is relatively low).
Our study contrasts with the approach of Wang and colleagues [23], who used Poisson regression and mortality data linked to the National Health and Nutrition Examination Survey (NHANES) to adjust standard life tables for relative risks of mortality due to age, race, smoking status and body mass index during 1970–2003. The estimates by Wang et al [23] were limited by the use of NHANES risk factor data collected at one time point to predict long term mortality. However, these estimates provide an approach for considering mortality according to demographic and lifestyle factors that influence both overall and breast cancer mortality differentially by age. Future research is needed to further refine the contribution of breast cancer to overall mortality for subgroups defined by age [5, 6, 8], race [2, 5, 24] as well as molecular subtype of breast cancer [6, 11] since these factors have important disparate impacts on risk of death before and after a breast cancer diagnosis.
While the results for mortality trends are consistent with prior research, there are some caveats that should be considered in evaluating our approach. As mentioned above, we made assumptions regarding extrapolations for age > 99 years and periods before 1968 and after 2014 to generate plausible estimates of other-cause mortality for simulation modeling. Estimates depended on the availability of accurate and complete mortality data. While historical national death data are available, annual all-cause mortality data were not available for single years of age, so that interpolations were necessary within 5-year age groups. CISNET simulation modelers may also make assumptions in applying these other-cause mortality estimates in their breast cancer investigations. For example, the simulation models may assume that survivors of breast cancer have the same other-cause mortality risk as women without breast cancer, or that women who obtain mammograms have similar other-cause mortality as women without a history of screening (e.g., ignoring a “healthy screener” effect). Recent analyses show that women with early stage breast cancer may have lower other-cause mortality compared to the general population [22], and that women who receive mammograms are also more likely to fill medication prescriptions and engage in physical activity and are less likely to smoke cigarettes [25, 26].
Overall, we found that the relative contribution of breast cancer to mortality from all causes has been decreasing for women of all ages in recent birth cohorts. These results provide evidence that the nation’s substantial investments in breast cancer screening and treatment have reduced breast cancer mortality for women across the age spectrum. Since the majority of women diagnosed with breast cancer die from causes other than breast cancer, our results should be useful as inputs for computer microsimulation and other studies seeking to identify optimal breast cancer prevention, detection, and treatment strategies to improve population health.
Acknowledgments
Financial support for this work was provided by grants U01 CA152958 and P30 CA014520 from the National Cancer Institute. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
The authors would like to thank Jeanne Mandelblatt and Karen Kuntz for their editorial suggestions as well as two anonymous referees for their comments regarding this manuscript, and David Vanness and Heide Jackson for project support.
Footnotes
Conflicts of Interest: None
Note An interactive resource for the figures is available at https://resources.cisnet.cancer.gov/projects/#bcr/bcmort
References
- 1.Centers for Disease Control and Prevention, U.S. Department of Health & Human Services. [Accessed December 1, 2016];WONDER Online Databases, Underlying Cause of Death. Accessed at http://wonder.cdc.gov.
- 2.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: Apr, 2016. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 4.Byers T, Wender RC, Jemal A, Baskies AM, Ward EE, Brawley OW. The American Cancer Society challenge goal to reduce US cancer mortality by 50% between 1990 and 2015: Results and reflections. CA Cancer J Clin. 2016;66(5):359–69. doi: 10.3322/caac.21348. [DOI] [PubMed] [Google Scholar]
- 5.Yasmeen F, Hyndman RJ, Erbas B. Forecasting age-related changes in breast cancer mortality among white and black US women: a functional data approach. Cancer epidemiology. 2010;34(5):542–9. doi: 10.1016/j.canep.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25(13):1683–90. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg MA. The impact of mammography and adjuvant therapy on US breast cancer mortality (1975–2000): collective results from the Cancer Intervention and Surveillance Modeling Network. Competing risks to breast cancer mortality. J Natl Cancer Inst Monogr. 2006;36:15–9. doi: 10.1093/jncimonographs/lgj004. [DOI] [PubMed] [Google Scholar]
- 8.Vilaprinyo E, Gispert R, Martinez-Alonso M, Carles M, Pla R, Espinas JA, et al. Competing risks to breast cancer mortality in Catalonia. BMC Cancer. 2008;8:331. doi: 10.1186/1471-2407-8-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandelblatt J, van Ravesteyn N, Schechter C, Chang Y, Huang AT, Near AM, et al. Which strategies reduce breast cancer mortality most? Collaborative modeling of optimal screening, treatment, and obesity prevention. Cancer. 2013;119(14):2541–8. doi: 10.1002/cncr.28087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, et al. Collaborative Modeling of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Ann Intern Med. 2016;164(4):215–25. doi: 10.7326/M15-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju289. pii: dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprague BL, Stout NK, Schechter C, van Ravesteyn NT, Cevik M, Alagoz O, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med. 2015;162(3):157–66. doi: 10.7326/M14-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stout NK, Lee SJ, Schechter CB, Kerlikowske K, Alagoz O, Berry D, et al. Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography. J Natl Cancer Inst. 2014;106(6):dju092. doi: 10.1093/jnci/dju092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkely Mortality Database. Data for the United States. 2016 Dec 1; ( http://u.demog.berkeley.edu/~bmd/states.html )
- 15.Carstensen B. Age-Period-Cohort models for the Lexis Diagram. Stat Med. 2007;26:3018–45. doi: 10.1002/sim.2764. [DOI] [PubMed] [Google Scholar]
- 16.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J Royal Stat Soc (B) 2011;73(1):3–36. [Google Scholar]
- 17.Wood SN. Thin-plate regression splines. J Royal Stat Soc (B) 2003;65(1):95–114. [Google Scholar]
- 18.Craven P, Wahba G. Smoothing noisy data with spline functions: estimating the correct degree of smoothing by the method of generalized cross-validation. Numer Math. 1979;31:377–403. [Google Scholar]
- 19.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. http://www.R-project.org/ [Google Scholar]
- 21.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 22.Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol. 2013;178(3):339–49. doi: 10.1093/aje/kws580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YC, Graubard BI, Rosenberg MA, Kuntz KM, Zauber AG, Kahle L, et al. Derivation of background mortality by smoking and obesity in cancer simulation models. Med Decis Making. 2013;33(2):176–97. doi: 10.1177/0272989X12458725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy AM, Yang J, Armstrong K. Increasing disparities in breast cancer mortality from 1979 to 2010 for US black women aged 20 to 49 years. Am J Public Health. 2015;105(Suppl 3):S446–8. doi: 10.2105/AJPH.2014.302297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166(3):348–54. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 26.Lagerlund M, Drake I, Wirfalt E, Sontrop JM, Zackrisson S. Health-related lifestyle factors and mammography screening attendance in a Swedish cohort study. Eur J Cancer Prev. 2015;24(1):44–50. doi: 10.1097/CEJ.0000000000000025. [DOI] [PubMed] [Google Scholar]