Abstract
Most kidney stones are composed of calcium oxalate, and minor changes in urine oxalate affect the stone risk. Obesity is a risk factor for kidney stones and a positive correlation of unknown etiology between increased body size and elevated urinary oxalate excretion has been reported. Here, we used obese ob/ob (ob) mice to elucidate the pathogenesis of obesity-associated hyperoxaluria. These ob mice have significant hyperoxaluria (3.3-fold) compared with control mice, which is not due to overeating as shown by pair-feeding studies. Dietary oxalate removal greatly ameliorated this hyperoxaluria, confirming that it is largely enteric in origin. Transporter SLC26A6 (A6) plays an essential role in active transcellular intestinal oxalate secretion, and ob mice have significantly reduced jejunal A6 mRNA (− 80%) and total protein (− 62%) expression. While net oxalate secretion was observed in control jejunal tissues mounted in Ussing chambers, net absorption was seen in ob tissues, due to significantly reduced secretion. We hypothesized that the obesity-associated increase in intestinal and systemic inflammation, as reflected by elevated proinflammatory cytokines, suppresses A6-mediated intestinal oxalate secretion and contributes to obesity-associated hyperoxaluria. Indeed, proinflammatory cytokines (elevated in ob mice) significantly decreased intestinal oxalate transport in vitro by reducing A6 mRNA and total protein expression. Proinflammatory cytokines also significantly reduced active mouse jejunal oxalate secretion, converting oxalate transport from net secretion in vehicle-treated tissues to net absorption in proinflammatory cytokines-treated tissues. Thus, reduced active intestinal oxalate secretion, likely secondary to local and systemic inflammation, contributes to the pathogenesis of obesity-associated hyperoxaluria. Hence, proinflammatory cytokines represent potential therapeutic targets.
Keywords: Obesity, intestinal oxalate secretion, hyperoxaluria, inflammation, SLC26A6
INTRODUCTION
Kidney stone disease (KSD) is the second most prevalent kidney disease in USA after hypertension, with a rising prevalence and complications including CKD and ESRD.1 It remains a major source of patient discomfort and disability, lost working days, and health-care expenditure (~$10 billion annually). Hyperoxaluria is a major risk factor for KS, and 70–80% of KS are composed of calcium oxalate (CO).2 Urinary oxalate is an important determinant of the level of supersaturation than calcium, and the risk for stone formation is affected by small increases in urine oxalate.3
Obesity is a leading preventable cause of death worldwide, with increasing prevalence in adults and children, and reaching an epidemic level in USA. Obesity is a critical causative factor in the development of metabolic syndrome (MS).4 Obesity and MS are associated with higher rates of KS in several studies.4–6 Although a greater proportion of KS in obese patients are uric acid stones, COKS are also common in these patients, as reported by Li et al who found that 53.1% of uric acid stone formers were obese compared with 42.7% COKS formers.7 Mild to moderate hyperoxaluria is frequently seen in obese stone formers and a positive correlation between increased body size and elevated urinary oxalate excretion has been reported in population based studies.8–10 Urinary oxalate is significantly higher in COKS formers with MS compared to those without MS (μmol/day: no MS = 353±141; MS = 454±206), and it significantly rises with increasing features of the MS (356±141 to 504±203; zero to four features).11 In addition, analysis of individuals with and without KS in the NHS1, NHSII, and HPFS, revealed that urinary oxalate excretion is directly related to BMI in women.12 Moreover, overweight children have significantly higher urine oxalate compared with normal weight children.13 A reduced man-woman ratio for KS from 1.7:1 in 1997 to 1.3:1 in 2002 was reported, with the greater increase of overweight and obese women compared with men a possible explanation for the changing trend.14
The mechanism(s) underlying the positive correlation between body size and urinary oxalate excretion and hence increased incidence of KSD, beyond dietary indiscretion, remain(s) unknown. With the rising prevalence of obesity and MS, it is expected that the incidence of KSD will continue to increase at a significant rate, leading to a substantial increase in care costs. Better understanding of the mechanisms underlying the obesity-associated hyperoxaluria (OAH) is crucial for identification of potential targets for therapeutic intervention. To this end, we initiated studies in the obese ob/ob (ob; an extensively used animal model of obesity in which the obesity is primarily due to leptin deficiency15) mice and observed that these mice have significant hyperoxaluria compared with their lean controls. We find reduced active transcellular intestinal oxalate secretion, which is likely mediated by proinflammatory cytokines (PCs), contributes to OAH. We also find that several PCs significantly reduce SLC26A6 (A6)-mediated oxalate transport, both in vitro and in vivo. For the convenience of oxalate measurements, oxalate uptake and not efflux (secretion), has been determined in human colonic Caco2-BBE (C2) cells since A6 operates in both directions.16
RESULTS
We used the ob mice to elucidate the molecular mechanisms underlying the positive correlation between body size and urinary oxalate excretion.8, 9 To establish the ob mice as a useful model for the OAH, we assessed whether the ob mice have increased urinary oxalate excretion compared to their lean controls. Shown in Figure 1A, ob mice have significantly higher (3.3-fold) urine oxalate (adjusted for creatinine = Cr) compared to controls using urine samples collected directly from the bladders at the time of euthanasia or collected over a 1 h period as described in Methods. Importantly, we also observed significantly higher (3.2-fold) urine oxalate in the ob mice compared to controls using 24 h urine samples collected by placing the mice in metabolic cages (Figure 1B). These results show that the ob mice have similar levels of hyperoxaluria when urinary oxalate is adjusted for Cr, which corrects for any variations in urinary water excretion, or expressed as an excretion rate over 24 h. Collectively, these results indicate that the ob mice have significant hyperoxaluria, and therefore establish them as a useful model to elucidate the mechanisms of OAH.
Fig. 1.
Urinary oxalate levels in the ob/ob (ob) mice and their lean controls. A: Urine samples were collected either directly from the bladders of the ob mice and their controls at the time of euthanasia or collected over a 1 h period as described in Methods. The ob mice have significantly higher (μmol Oxalate/mg creatinine) urinary oxalate levels (* P < 9.9E−20 for ob compared with Controls, by unpaired t-test, n = 47–49). B: The ob mice and their controls were placed individually in metabolic cages and 24 h urine samples were collected. The ob mice have significantly higher (μmol/24 h) urinary oxalate levels (* P < 0.0002 for ob compared with Controls, by unpaired t-test, n = 7–8).
db/db (db) mice are deficient in leptin receptor and are another commonly used animal model of obesity.17 We similarly assessed urinary oxalate levels in the db mice and their controls to ensure that the observed hyperoxaluria is not specific to the ob mice. Interestingly, the db mice have significantly higher (2.3-fold) urine oxalate compared to their lean controls (Figure 2), indicating the hyperoxaluria observed in the ob mice is not specific to these mice.
Fig. 2.
Urinary oxalate levels in the db/db (db) mice and their lean controls. Urine samples were collected directly from the bladders of the db mice and their controls at the time of euthanasia. The db mice have significantly higher (μmol Oxalate/mg creatinine) urinary oxalate levels (* P < 0.03 for db compared with Controls, by unpaired t-test, n = 3).
ob and db mice are hyperphagic, and therefore it is highly possible that the observed hyperoxaluria is largely coming from diet. To control for this possibility, ob mice and their controls were housed individually (one mouse/cage) and their daily food consumption was assessed over a period of 4 days, during which they have free access to food. The ob mice consumed significantly higher (1.8-fold) food per day compared to controls (Figure 3A). To rule out the possibility that the observed hyperoxaluria is due to overeating, the ob mice and their controls were pair-fed (4 g/mouse/day) for up to 4 days, with the pair-fed mice in the two groups consuming all the food. Importantly, the ob mice were still found to have significantly higher (2.6-fold) urine oxalate compared with controls (Figure 3B). Taken together, these results strongly support the hypothesis that the observed hyperoxaluria is largely due to specific defect(s) in the ob mice and it is clearly not solely due to overeating.
Fig. 3.
Effect of pair-feeding on urinary oxalate levels in the ob/ob (ob) mice and their lean controls. A: Average daily food consumption by the ob mice and their controls. The ob mice and their controls were housed individually and their daily food consumption was assessed over a period of 4 days, during which they have free access to food. The ob mice consumed significantly higher food per day (*P < 0.006 for ob compared with Controls, by unpaired t-test, n = 4–5). B: Urinary oxalate levels in pair-fed (4g/mouse/day) ob mice and their controls. Urine samples were collected either directly from the bladders at the time of euthanasia or collected over a 1 h period as described in Methods. The ob mice have significantly higher (μmol Oxalate/mg creatinine) urinary oxalate levels (* P < 0.007 for ob compared with Controls, by unpaired t-test, n = 4).
Anion exchanger SLC26A6 (A6) is apically expressed in enterocytes and plays an essential role in small intestinal active transcellular oxalate secretion, thereby preventing hyperoxaluria and related KS (A6 null mice develop hyperoxalemia, hyperoxaluria, and KS).18, 19 SLC26A2 (A2) is also apically expressed in enterocytes and it transports oxalate when expressed in heterologous systems;20–22 however, its role in intestinal oxalate transport is unknown. In addition, SLC26A3 (A3) is also apically expressed in enterocytes and plays a critical role in mouse intestinal active transcellular oxalate absorption.23 Active transcellular intestinal oxalate secretion requires oxalate influx into the enterocyte from the blood side, where SLC26A1 (A1) is likely involved, and then its efflux from the luminal side by A6 (± other transporters, potentially including A2). Therefore, we evaluated whether there is altered mRNA expression of one or more of these transporters in intestinal tissues from the ob mice using qPCR. Interestingly, Jejunal A6 mRNA expression is significantly reduced (80%) in ob mice compared with controls, without a change in A1, A2, and A3 mRNA expression levels (Figure 4). These results indicate that the observed decrease in A6 mRNA is specific and is not due to global changes in mRNA expression in the ob mice.
Fig. 4.
SLC26A1 (A1), SLC26A2 (A2), SLC26A3 (A3), and SLC26A6 (A6) mRNA expression in the ob/ob (ob) mice and their lean controls. Total RNA was isolated from the ob mice and their controls for real-time PCR analysis. Values are means ± SD of 4–5 independent experiments each of which was done in duplicate or triplicate. Relative A1, A2, A3, and A6 mRNA expression levels were expressed as a percentage of Control normalized to GAPDH. The ob mice have significantly reduced A6 mRNA expression level (* P < 0.002 for ob compared with Controls, by unpaired t-test).
To evaluate whether the observed reduction in A6 mRNA expression is translated into decreased A6 protein expression, A6 total protein expression was assessed by immunoblotting. Indeed, the ob mice have significantly reduced (62%) jejunal A6 total protein expression compared with controls (Figure 5A & B). Due to lack of good antibodies, we did not assess A1, 2, & 3 total protein expression. Of note is that we did not observe a significant change in the expression of the apical membrane protein villin (data not shown).
Fig. 5.
SLC26A6 (A6) total protein expression in the ob/ob (ob) mice and their lean controls. A: A representative Western blot analysis of total A6 protein expression. A6 protein expression was evaluated in jejunal mucosal scrapings (15 μg protein/lane) isolated from the ob mice and their controls (CON). The lower part of the same blot was probed with an anti-β-actin (Actin) antibody to normalize loading of protein in each lane (lower panel). B: Densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means ± SD for 4 independent experiments (blots shown in Supplementary Fig. S1) of relative total A6 abundance to β-actin and are presented as a percentage of the Control value. The ob mice have significantly reduced A6 protein expression (* P < 0.004 for ob compared with Controls, by unpaired t-test).
Since A6 plays a crucial role in small intestinal oxalate secretion,18, 19 it is possible that the reduced jejunal A6 mRNA and total protein expression could lead to decreased active intestinal oxalate secretion and therefore potentially contribute to the observed hyperoxaluria. To test this possibility, jejunal tissues were isolated from the ob mice and their controls and mounted in Ussing chambers. Of note is that the jejunum was chosen because jejunal wall inflammation was reported in the db mice24, and which we confirmed in our ob mice (Fig. 8B), as well as the fact that A6 is highly expressed in mouse jejunum (at an equivalent level to the duodenum).25 Interestingly, while a net oxalate secretory flux (−14±11) was observed in control tissues, a large net oxalate absorptive flux (16±8) was seen in the ob tissues (Figure 6). This was mainly due to a significant reduction (31%) in jejunal oxalate secretion (JSM). In addition, we observed a non-significant increase (19%) in the absorptive flux (JMS), which also contributed to the reversal of oxalate transport from net secretion in control tissues to net absorption in ob tissues. Collectively, these findings indicate that reduced active transcellular intestinal oxalate secretion and potentially increased transcellular intestinal oxalate absorption contributes to the hyperoxaluria observed in the ob mice, and hence to the pathogenesis of OAH.
Fig. 8.
Plasma and jejunal cytokines levels in the ob/ob (ob) mice and their lean controls. A: Plasma TNF-α (TNF), IFN-γ(IFN), and IL-6 levels were measured as described in the Methods. Values are means ± SD of 10–19 independent measurements each of which was done in duplicate. The ob mice have significantly higher plasma TNF, IFN, and IL-6 levels (* P < 0.002, 3.2E−06, and 1.4E−09 for ob compared with Controls, with regards to TNF, IFN, IL-6, respectively, by unpaired t-test). B: Jejunal total RNA was isolated from the ob mice and their controls for real-time PCR analysis. Values are means ± SD of 7–11 independent experiments each of which was done in triplicate. Relative TNF, IFN, and IL-6 mRNA expression levels were expressed as a percentage of Control normalized to GAPDH. The ob mice have significantly higher jejunal wall TNF and IFN mRNA expression levels (* P < 0.002 and 0.008 for ob compared with Controls, with regards to TNF and IFN, respectively, by unpaired t-test).
Fig. 6.
Unidirectional [mucosa to serosa = JMS (absorptive flux), and serosa to mucosa = JSM (secretory flux)] and net (Jnet) transepithelial oxalate fluxes across jejunal tissues (n = 19 tissue pairs) isolated from the ob/ob (ob) mice and their lean controls and mounted ex vivo in modified Ussing chambers. While a net baseline oxalate secretory flux is observed in control tissues, a large net baseline oxalate absorptive flux is seen in ob tissues, which is mainly due to a significant reduction in JSM (* P < 0.007 and < 1.6E−10 for ob mice compared with Controls, with regards to JSM and Jnet, respectively, by unpaired t-test).
Reduced active intestinal oxalate secretion contributes to the pathogenesis of OAH by increasing the amount of net oxalate absorbed by the intestine, and indicates that the OAH is mainly enteric in origin. To support the hypothesis that the OAH is enteric in origin, the ob mice and their controls were placed on an oxalate-free diet to evaluate the effect of dietary oxalate removal on the observed hyperoxaluria. Shown in Figure 7, an oxalate-free diet greatly ameliorated the observed hyperoxaluria, confirming that it is largely enteric in origin.
Fig. 7.
Effect of dietary oxalate removal on urinary oxalate levels in the ob/ob (ob) mice and their lean controls. Urinary oxalate levels were assessed as described in Methods in ob and control mice consuming oxalate-containing diet (Regular Diet) or an oxalate-free diet. The oxalate-free diet significantly reduced urinary oxalate levels in the ob mice (* P < 8.2E−12 for the ob mice on the oxalate-free diet compared with the regular diet, by unpaired t-test, n = 7–10).
Although the oxalate-free diet greatly ameliorated the observed hyperoxaluria, urine oxalate still remains significantly higher (~1.3-fold) in the ob mice compared to urine oxalate in the control mice on regular diet, suggesting that a small component of the observed hyperoxaluria might be due to increased endogenous hepatic oxalate synthesis. To evaluate whether increased hepatic oxalate synthesis contributes to the observed hyperoxaluria, urinary glycolate (an indicator of hepatic oxalate synthesis26) levels in the ob mice and their controls were measured. There is no significant difference in the glycolate levels in the ob and their controls (μg/mg Cr: Control = 86.5±8; ob = 82.8±6.9; n = 6), suggesting that the ob mice have no evidence of enhanced hepatic oxalate synthesis through a glycolate-dependant pathway.
Obesity is characterized by chronic systemic inflammation27 and both ob and db mice have higher plasma levels of several PCs (e.g. TNF-α [TNF], IFN-γ[IFN], IL-1β, & IL-6).28 Obesity is also characterized by increased small intestinal inflammation.24, 29 To evaluate whether our ob mice have increased systemic and/or small intestinal wall inflammation under our housing conditions, plasma and jejunal TNF, IFN, and IL-6 levels were measured by ELISA and qpcr, respectively. The ob mice were found to have significantly higher plasma TNF, IFN, & IL-6 (Fig. 8A) and jejunal TNF & IFN (Fig. 8B) levels, confirming systemic and jejunal wall inflammation in these mice. The ob mice also have a non-significant increase in jejunal wall IL-6 levels.
A6-mediated intestinal oxalate secretion plays a critical role in limiting net intestinal absorption of ingested oxalate, thereby preventing hyperoxaluria and KS.18, 19 It is possible that the obesity milieu is associated with factors that could cause defective A6 regulation, leading to reduced active intestinal oxalate secretion. We hypothesized that the high circulating and intestinal PCs levels observed in obesity, which inhibit several intestinal transporters,30, 31 suppress A6-mediated intestinal oxalate secretion and thus contribute to OAH. To test this hypothesis, we examined the effects of several PCs on apical oxalate uptake (≥49% of which is mediated by A632, 33) by human intestinal Caco2-BBE (C2) cells (human epithelial colorectal adenocarcinoma cells). C2 cells were treated basolaterally with TNF (25 ng/ml × 48 h), IFN, IL-6, or IL-β (50 ng/ml × 48 h), before assessing 14C-oxalate uptake. TNF, IFN, and IL-6 significantly inhibited (30, 33, and 32%, respectively) apical 14C-oxalate uptake by C2 cells, while IL-1β had no effect (which reflects the specific inhibitory effects of TNF, IFN, and IL-6) (Figure 9). On the other hand, the PCs IL-2 and IL-8 (50 ng/ml × 48 h), which are elevated in obese human subjects,34 had no effect (data not shown). The PCs concentrations were selected based on previous work30, 35, 36 and on our findings in pilot studies (where several concentrations were tested) that the described doses gave the best inhibition. Taken together, these results show that active DIDS-sensitive (100 μM DIDS inhibits it by 91% as we previously reported37) apical 14C-oxalate uptake by C2 cells, is suppressed by several PCs.
Fig. 9.
Effect of TNF-α, IFN-γ, IL-β, and IL-6 on apical 14C-oxalate uptake by Caco2-BBE (C2) cells. C2 cells were treated basolaterally with TNF-α (25 ng/ml × 48 h), IFN-γ, IL-β, or IL-6 (50 ng/ml × 48 h), or vehicle (Control) and then14C-oxalate uptake was measured as described in Methods. Values are means ± SD of 7 independent experiments each of which was done in triplicate and was normalized to the respective Control value. TNF-α, IFN-γ, and IL-6 significantly inhibited 14C-oxalate uptake (* P < 0.001 for TNF-α, IFN-γ, and IL-6 compared with Control or IL-β, by ANOVA).
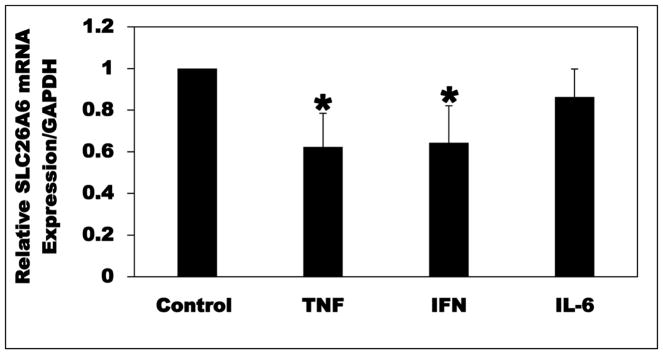
Since A6 mRNA and total protein expression levels are significantly reduced in the ob mice, we examined whether TNF, IFN, and/or IL-6 inhibit(s) apical 14C-oxalate transport by C2 cells by reducing A6 mRNA and/or total protein expression level(s). Using qPCR, TNF and IFN significantly reduced (38 and 36%, respectively) A6 mRNA expression, whereas IL-6 had no significant effect (Figure 10). These results show that TNF and IFN inhibit oxalate transport by C2 cells by reducing A6 mRNA expression.
Fig. 10.
Effect of TNF-α (TNF), IFN-γ(IFN), and IL-6 on SLC26A6 (A6) mRNA expression in Caco2-BBE (C2) cells. C2 cells were treated basolaterally with TNF (25 ng/ml × 48 h), IFN or IL-6 (50 ng/ml × 48 h), or vehicle (Control), and then total RNA was isolated for real-time PCR analysis. Values are means ± SD of 5–6 independent experiments each of which was done in triplicate. Relative A6 mRNA expression level was expressed as a percentage of Control normalized to GAPDH. TNF and IFN significantly reduced A6 mRNA expression level (* P < 0.05 for TNF and IFN compared with Control, by ANOVA).
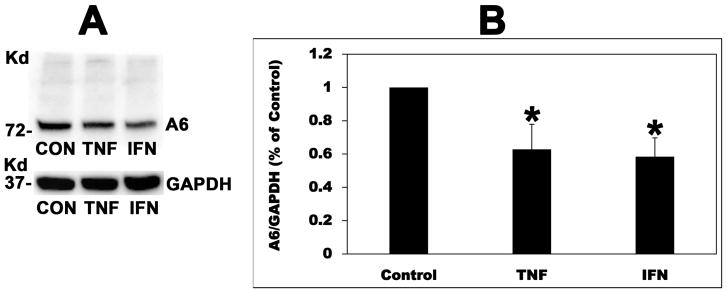
To confirm that the observed PC-induced reduction in A6 mRNA expression in C2 cells is translated into decreased A6 protein expression, A6 total protein expression was assessed by immunoblotting. TNF and IFN also significantly reduced A6 total protein expression in C2 cells (37 and 42%, respectively) (Figure 11). However, IL-6 had no significant effect on A6 total protein expression (data not shown). These results indicate that TNF and IFN decrease apical oxalate transport by C2 cells by reducing A6 total protein expression.
Fig. 11.
Effect of TNF-α (TNF) and IFN-γ(IFN) on SLC26A6 (A6) total protein expression in Caco2-BBE (C2) cells. A: A representative Western blot analysis of total A6 protein expression. A6 protein expression was evaluated in C2 cell lysate (10 μg protein/lane: CON, TNF, and IFN = C2 cells treated with vehicle, TNF, and IFN, respectively). The lower part of the same blot was probed with an anti-GAPDH antibody to normalize loading of protein in each lane (lower panel). B: Densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means ± SD for 4 independent experiments (blots shown in Supplementary Fig. S2) of relative total A6 abundance to GAPDH and are presented as a percentage of the Control value. TNF and IFN significantly reduced A6 protein expression (* P < 0.01 for TNF and IFN compared with Control, by ANOVA).
To ensure that PCs similarly regulate anion exchange-mediated active intestinal oxalate secretion in vivo as observed in vitro in C2 cells, age - and gender-matched BALB/c mice were given TNF [2 μg; IP × 135] or vehicle and then jejunal tissues were isolated and mounted in Ussing chambers 48 h later. Importantly, TNF caused significant inhibition (46%) of mouse jejunal active transcellular oxalate secretion, converting oxalate transport from net secretion in vehicle-treated tissues (−22±23) to net absorption in TNF-treated tissues (13±15) (Figure 12). TNF had no significant effect on the oxalate absorptive flux (Jms). These results confirm that PCs similarly inhibit active oxalate secretion in vivo as observed in vitro. Taken together, the above described findings clearly show that PCs can directly inhibit A6-mediated active intestinal oxalate secretion, and thus strongly support our hypothesis that PCs play an important role in the pathogenesis of OAH.
Fig. 12.
Unidirectional [mucosa to serosa = JMS (absorptive flux), and serosa to mucosa = JSM (secretory flux)] and net (Jnet) transepithelial oxalate fluxes across jejunal tissues (n = 11 tissue pairs) isolated from vehicle - and TNF-α-treated BALB/c mice and mounted ex vivo in modified Ussing chambers. TNF-α caused significant inhibition of jejunal active transcellular oxalate secretion (JSM), converting oxalate transport from net secretion in vehicle-treated tissues to net absorption in TNF-α-treated tissues (* P < 0.011 and < 0.0008 for TNF-α-treated tissues compared with vehicle-treated tissues, with regards to JSM and Jnet, respectively, by unpaired t-test).
DISCUSSION
In the present study we used the ob mouse model of obesity to define the pathogenesis of OAH. The ob mice have significant hyperoxaluria compared with lean controls, which is not due to overeating as shown by pair-feeding experiments. Significant hyperoxaluria is also observed in another mouse model of obesity (db). Removal of dietary oxalate greatly ameliorated the hyperoxaluria observed in the ob mice, confirming that it is largely enteric in origin (i.e. dietary oxalate is the source of most of the excess oxalate in the urine of the ob mice). We also shown that the ob mice have remarkably reduced jejunal A6 mRNA and total protein expression. Assessment of unidirectional oxalate fluxes in jejunal tissues mounted in Ussing chambers showed net oxalate secretion in control tissues but net oxalate absorption in ob tissues, which is largely due to a significant reduction in active oxalate secretion. In addition, systemic and jejunal wall inflammation is demonstrated in the ob mice, and that several PCs significantly reduced A6-mediated oxalate transport, both in vitro and in vivo. Taken together, we have demonstrated that reduced active transcellular intestinal oxalate secretion, which is likely mediated by PCs, contributes to the pathogenesis of OAH.
Although dietary oxalate removal greatly ameliorated the observed hyperoxaluria and confirms that it is mainly enteric in origin, the finding that urine oxalate still remains significantly higher (~1.3-fold) in the ob mice compared to urine oxalate in the lean control mice on regular diet, suggests that a small component of the observed hyperoxaluria might be due to increased endogenous hepatic oxalate production. However, our finding that the ob and control mice have similar urine glycolate (a marker of hepatic oxalate synthesis26) levels does not support this notion. It should be noted that although urine glycolate is high in primary hyperoxaluria (PH) type 1, it is normal in PH types 2 & 3.38, 39 Therefore, the lack of an increase in urine glycolate does not totally rule out relative hepatic oxalate overproduction in the ob mice, and future studies will further explore this possibility and its contribution to the OAH.
We hypothesized that PCs are key mediators of the observed reduction in active transcellular intestinal oxalate secretion based on the following observations: 1. Obesity is characterized by chronic low-grade systemic inflammation in humans and animals,27, 40, 41 and both ob and db mice have high PCs circulating levels.28 2. In addition to chronic systemic inflammation, increased small intestinal inflammation is also observed in the db mice and obese human subjects with insulin resistance.24, 29 3. We demonstrated systemic and jejunal wall inflammation in our ob mice. 4. PCs are known to negatively regulate several intestinal transporters,30, 31 and we hypothesized that they inhibit A6-mediated active intestinal oxalate secretion. Indeed, we have shown that PCs can directly inhibit apical oxalate transport by C2 cells, through mechanisms including reduced A6 mRNA and total protein expression. The physiological relevance of the C2 findings is underscored by the fact that TNF significantly decreased (46%) mouse jejunal active oxalate secretion, converting oxalate transport from net secretion in vehicle-treated tissues to net absorption in TNF-treated tissues. Collectively, these findings strongly support our hypothesis that PCs play an important role in the pathogenesis of OAH by reducing active transcellular intestinal oxalate secretion. Of interest in this regard is that IFN was shown to reduce A6 mRNA expression, promoter activity, and transport function (assayed as DIDS-sensitive Cl/OH exchange activity) in C2 cells.42 In addition, TNF and IL-6, which are elevated in obese patients, contribute to the pathogenesis of obesity-associated insulin resistance.43 It will be of significant therapeutic interest to evaluate in future studies whether neutralizing antibodies against TNF, IFN, and IL-6 will normalize and/or ameliorate the hyperoxaluria observed in the ob mice. Future studies will also elucidate the mechanism(s) by which IL-6, which did not affect A6 mRNA and total protein expression, inhibits oxalate transport in C2 cells, which might involve modulation of the intrinsic activity of the preexisting A6 membrane transporters.
KSD remains a major public health problem in USA, with a rising prevalence and complications including CKD and ESRD.1 Importantly, the risk of stone formation begins to rise in both men and women even at urinary oxalate levels traditionally considered to be within the normal range (25–30 mg/day).3 Despite being a major risk factor for KSD, hyperoxaluria has no specific therapy. Lack of in-depth understanding of the pathophysiologic mechanisms leading to secondary hyperoxaluria, including OAH, is one reason behind delayed progress in new treatments for hyperoxaluria. An interesting and clinically relevant finding of this study is that reduced active transcellular intestinal oxalate secretion, which is likely mediated by the obesity-associated increased systemic and intestinal inflammation, plays an important role in the pathogenesis of OAH. Importantly, our findings should provide the basis for further mechanistic and interventional studies for the prevention and/or treatment of OAH, including evaluating the in vivo therapeutic potential of neutralizing antibodies against the involved PCs as described above.
Our findings could also have potential pathophysiologic relevance for other conditions characterized by chronic intestinal inflammation, including IBD and celiac disease.44, 45 IBD patients have a significantly increased risk of KS, often due to enteric hyperoxaluria.46, 47 This enteric hyperoxaluria has been largely attributed to fat malabsorption,48 where malabsorbed fat complexes with calcium and as a result oxalate becomes free and easily absorbed. Malabsorption occurs in crohn’s disease (CD) but not in ulcerative colitis (UC); however, hyperoxaluria is also seen in UC.49 Therefore, malabsorption might not be the only important risk factor predisposing to the IBD-associated hyperoxaluria, and it is possible that other mechanism(s) must be operative. In view of our findings that systemic and intestinal inflammation contribute to OAH, by likely mediating the observed reduction in active intestinal oxalate secretion, we hypothesize that the IBD-associated chronic intestinal inflammation also contribute to the enteric hyperoxaluria seen in IBD. Future studies will be directed at testing this hypothesis using IBD animal models. In addition, a case of subclinical celiac disease with significant hyperoxaluria leading to CKD was reported.50 Of remarkable interest is that this patient had significantly reduced small intestinal A6 apical expression compared with a controlled subject, which had likely contributed to reduced A6-mediated active small intestinal oxalate secretion and therefore to the reported hyperoxaluria.50 Moreover, diabetic patients [who also have increased systemic inflammation51] excrete 2 mg/day more urinary oxalate than those without diabetes.8 Furthermore, diabetic KS formers excrete significantly higher urine oxalate (>6 mg/day) than nondiabetic stone formers.52 Our findings could also potentially explain the association between DM and KSD with regards to increased urinary oxalate excretion.
The obesity-associated altered enteric microbiome might be another potential mediator of the reduced active intestinal oxalate secretion. Compared with lean individuals, an altered gut microbiota was reported in obese humans and animals.53, 54 LPS (endotoxin) is a structural part of the cell walls of gram-negative bacteria,55 and endotoxemia induces systemic inflammation through cytokine release.28, 56–58 Microbiota-mediated cytokine (e.g. TNF) secretion also contributes to intestinal mucosal inflammation.59 Significantly reduced gut colonization with oxalobacter formigenes (Of) is observed in obese subjects (16%) compared to 50–70% in the general population.60 In addition to degrading intraluminal dietary oxalate, Of also interacts with colonic epithelium by inducing distal colonic oxalate secretion, leading to reduced urinary excretion.61 Of colonization is associated with reduced stone risk,62–65 and the obesity-associated decreased Of colonization might potentially contribute to hyperoxaluria in obese subjects. However, it is unlikely contributing to the hyperoxaluria observed in the ob mice since laboratory mice are usually found not to be colonized with Of.66 Of interest in this regard is that we have recently shown that Of-derived bioactive factors remarkably stimulate oxalate transport by C2 cells (through mechanisms including increased intrinsic activity of A6 membrane transporters), as well as significantly reduced urinary oxalate excretion in hyperoxaluric mice.33 Besides Of, several other gut microbes also degrades intraluminal dietary oxalate, including lactobacillus Acidophillus, Bifidobacterium lactis, Eubacterium Lentum, Enterococcus faecalis, and Providencia Rettgeri.67 However, whether one or more of these bacteria is/are also capable of interacting with intestinal cells and modulate(s) intestinal oxalate transport as reported with Of61 remains unknown. By degrading dietary oxalate, these bacteria will reduce net intestinal oxalate absorption and lower urinary oxalate excretion. Based on these observations, it is possible that the altered gut microbiota also play an important role in the pathogenesis of OAH, by contributing to the observed reduced active intestinal oxalate secretion. Therefore, future studies will explore the role of gut microbiome in OAH, especially if neutralization and/or reduction of the associated inflammation is/are found to have no effect.
Our study has several limitations and they are summarized as follows: we concentrated on the jejunum for the reasons described above; however, we did not examine whether the ob mice also have reduced A6 mRNA/total protein expression and reduced active oxalate secretion in the duodenum and ileum. In addition, we did not determine whether the db mice have similar reduction in small intestinal A6 expression and active oxalate secretion. Moreover, due to lack of good antibodies, the potential roles of A1, 2, & 3 in the pathogenesis of the OAH could not be assessed at a protein level.
In summary, we have shown that obese mice have significant hyperoxaluria, which is largely enteric in origin, and that reduced active transcellular intestinal oxalate secretion contributes to the pathogenesis of OAH. The observed reduction in active intestinal oxalate secretion is likely mediated by the obesity-associated increased systemic and intestinal inflammation, and that PCs represent potential therapeutic targets for the prevention and/or treatment of OAH and related KSD.
METHODS
Animals
Mice were purchased and maintained as detailed in Supplementary Methods.
Urine collection for oxalate and glycolate measurements
Urine collection and oxalate & glycolate measurements were performed as detailed in Supplementary Methods. To evaluate the effects of dietary oxalate removal on the observed hyperoxaluria, the ob and control mice were placed on an oxalate-free diet, which has no detectable oxalate (<0.2 μmol/g), for 4 weeks.
Measurement of fluxes across jejunal tissues
Unidirectional oxalate fluxes (mucosa to serosa, JMS, and serosa to mucosa, JSM) were assessed in jejunal tissues mounted in Ussing chambers as we recently reported33 (see Supplementary Methods for details). Following a 15–30-min equilibration period, 14C-oxalate fluxes were measured for 75 min at 15-min intervals or for 150–180 min at 30 min intervals and the measurements were averaged.
Cell culture
C2 cells were grown and maintained as previously reported37, 68 and described in Supplementary Methods.
Radioactive flux studies
Apical 14C-oxalate flux studies in C2 cells were performed following our published methods,37, 68 and detailed in Supplementary Methods.
Real-time PCR
qPCR was performed as we recently reported33 and detailed in Supplementary Methods.
SDS/PAGE and Western Blotting
Total A6 protein expression is assessed by immunoblotting in jejunal and C2 cell lysates as previously reported33, 35 and detailed in Supplementary Methods.
Statistical Analysis
Experimental data are presented as means ± SD. Data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test, or by Student’s t test for unpaired samples when comparing two groups. P values < 0.05 were considered statistically significant
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01-DK101643 (HH) & P30DK42086 (the Digestive Disease Research Center of the University of Chicago), Harold Amos Medical Faculty Development Program Grant (RWJF grant) 65877 (HH), and American Society of Nephrology Gottschalk Research Scholar Grant (HH). We would like to thank Ming Cheng, Jan Stevens, Zhenguo Wang, Brad Hack, Mustafa Satti, and Rashda Norui for technical assistance.
Footnotes
Disclosures
John Asplin and Ignacio Granja are employed by LabCorp.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander RT, Hemmelgarn BR, Wiebe N, et al. Kidney stones and kidney function loss: a cohort study. Bmj. 2012;345:e5287. doi: 10.1136/bmj.e5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coe FL, Evan A, Worcester E. Kidney stone disease. The Journal of clinical investigation. 2005;115:2598–2608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney international. 2008;73:489–496. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 4.Gorbachinsky I, Akpinar H, Assimos DG. Metabolic syndrome and urologic diseases. Rev Urol. 2010;12:e157–180. [PMC free article] [PubMed] [Google Scholar]
- 5.Rendina D, Mossetti G, De Filippo G, et al. Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: role of gender, hypertension and abdominal obesity. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:900–906. doi: 10.1093/ndt/gfn548. [DOI] [PubMed] [Google Scholar]
- 6.West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Li WM, Chou YH, Li CC, et al. Association of body mass index and urine pH in patients with urolithiasis. Urological research. 2009;37:193–196. doi: 10.1007/s00240-009-0194-4. [DOI] [PubMed] [Google Scholar]
- 8.Taylor EN, Curhan GC. Determinants of 24-hour urinary oxalate excretion. Clinical journal of the American Society of Nephrology: CJASN. 2008;3:1453–1460. doi: 10.2215/CJN.01410308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemann J, Jr, Pleuss JA, Worcester EM, et al. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney international. 1996;49:200–208. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- 10.Kleinman JG. Bariatric surgery, hyperoxaluria, and nephrolithiasis: a plea for close postoperative management of risk factors. Kidney international. 2007;72:8–10. doi: 10.1038/sj.ki.5002284. [DOI] [PubMed] [Google Scholar]
- 11.Sakhaee K, Capolongo G, Maalouf NM, et al. Metabolic syndrome and the risk of calcium stones. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:3201–3209. doi: 10.1093/ndt/gfr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor EN, Curhan GC. Body size and 24-hour urine composition. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;48:905–915. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Sarica K, Eryildirim B, Yencilek F, et al. Role of overweight status on stone-forming risk factors in children: a prospective study. Urology. 2009;73:1003–1007. doi: 10.1016/j.urology.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Scales CD, Jr, Curtis LH, Norris RD, et al. Changing gender prevalence of stone disease. The Journal of urology. 2007;177:979–982. doi: 10.1016/j.juro.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice] ScientificWorldJournal. 2007;7:666–685. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Z, Grichtchenko II, Boron WF, et al. Specificity of anion exchange mediated by mouse Slc26a6. The Journal of biological chemistry. 2002;277:33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 17.Wua J, Wang HM, Li J, et al. The research applications of db/db mouse. Sheng li ke xue jin zhan [Progress in physiology] 2013;44:12–18. [PubMed] [Google Scholar]
- 18.Freel RW, Hatch M, Green M, et al. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. American journal of physiology Gastrointestinal and liver physiology. 2006;290:G719–728. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Asplin JR, Evan AP, et al. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nature genetics. 2006;38:474–478. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 20.Haila S, Hastbacka J, Bohling T, et al. SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2001;49:973–982. doi: 10.1177/002215540104900805. [DOI] [PubMed] [Google Scholar]
- 21.Satoh H, Susaki M, Shukunami C, et al. Functional analysis of diastrophic dysplasia sulfate transporter. Its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans The Journal of biological chemistry. 1998;273:12307–12315. doi: 10.1074/jbc.273.20.12307. [DOI] [PubMed] [Google Scholar]
- 22.Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Molecular aspects of medicine. 2013;34:494–515. doi: 10.1016/j.mam.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freel RW, Whittamore JM, Hatch M. Transcellular oxalate and Cl− absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. American journal of physiology Gastrointestinal and liver physiology. 2013;305:G520–527. doi: 10.1152/ajpgi.00167.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duparc T, Naslain D, Colom A, et al. Jejunum inflammation in obese and diabetic mice impairs enteric glucose detection and modifies nitric oxide release in the hypothalamus. Antioxidants & redox signaling. 2011;14:415–423. doi: 10.1089/ars.2010.3330. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Petrovic S, Mann E, et al. Identification of an apical Cl(−)/HCO3(−) exchanger in the small intestine. American journal of physiology Gastrointestinal and liver physiology. 2002;282:G573–579. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 26.Dawson PA, Russell CS, Lee S, et al. Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. The Journal of clinical investigation. 2010;120:706–712. doi: 10.1172/JCI31474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 28.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. American journal of physiology Gastrointestinal and liver physiology. 2007;292:G518–525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 29.Veilleux A, Grenier E, Marceau P, et al. Intestinal lipid handling: evidence and implication of insulin signaling abnormalities in human obese subjects. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:644–653. doi: 10.1161/ATVBAHA.113.302993. [DOI] [PubMed] [Google Scholar]
- 30.Rocha F, Musch MW, Lishanskiy L, et al. IFN-gamma downregulates expression of Na(+)/H(+) exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. American journal of physiology Cell physiology. 2001;280:C1224–1232. doi: 10.1152/ajpcell.2001.280.5.C1224. [DOI] [PubMed] [Google Scholar]
- 31.Musch MW, Clarke LL, Mamah D, et al. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. The Journal of clinical investigation. 2002;110:1739–1747. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freel RW, Morozumi M, Hatch M. Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. American journal of physiology Gastrointestinal and liver physiology. 2009;297:G918–929. doi: 10.1152/ajpgi.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arvans D, Jung YC, Antonopoulos D, et al. Oxalobacter formigenes-Derived Bioactive Factors Stimulate Oxalate Transport by Intestinal Epithelial Cells. Journal of the American Society of Nephrology: JASN. 2017;28:876–887. doi: 10.1681/ASN.2016020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosa JS, Heydari S, Oliver SR, et al. Inflammatory cytokine profiles during exercise in obese, diabetic, and healthy children. Journal of clinical research in pediatric endocrinology. 2011;3:115–121. doi: 10.4274/jcrpe.v3i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujiya M, Inaba Y, Musch MW, et al. Cytokine regulation of OCTN2 expression and activity in small and large intestine. Inflammatory bowel diseases. 2011;17:907–916. doi: 10.1002/ibd.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vavricka SR, Musch MW, Fujiya M, et al. Tumor necrosis factor-alpha and interferon-gamma increase PepT1 expression and activity in the human colon carcinoma cell line Caco-2/bbe and in mouse intestine. Pflugers Archiv: European journal of physiology. 2006;452:71–80. doi: 10.1007/s00424-005-0007-8. [DOI] [PubMed] [Google Scholar]
- 37.Amin R, Sharma S, Ratakonda S, et al. Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-delta activation. American journal of physiology Cell physiology. 2013;305:C78–89. doi: 10.1152/ajpcell.00339.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobrowski AE, Langman CB. The primary hyperoxalurias. Seminars in nephrology. 2008;28:152–162. doi: 10.1016/j.semnephrol.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Hoppe B. An update on primary hyperoxaluria. Nature reviews Nephrology. 2012;8:467–475. doi: 10.1038/nrneph.2012.113. [DOI] [PubMed] [Google Scholar]
- 40.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 41.Trayhurn P. Adipose tissue in obesity--an inflammatory issue. Endocrinology. 2005;146:1003–1005. doi: 10.1210/en.2004-1597. [DOI] [PubMed] [Google Scholar]
- 42.Saksena S, Dwivedi A, Singla A, et al. Characterization of the 5′-flanking region and regulation of expression of human anion exchanger SLC26A6. Journal of cellular biochemistry. 2008;105:454–466. doi: 10.1002/jcb.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. European cytokine network. 2006;17:4–12. [PubMed] [Google Scholar]
- 44.Pascual V, Dieli-Crimi R, Lopez-Palacios N, et al. Inflammatory bowel disease and celiac disease: overlaps and differences. World journal of gastroenterology. 2014;20:4846–4856. doi: 10.3748/wjg.v20.i17.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du L, Kim JJ, Shen J, et al. Crosstalk between Inflammation and ROCK/MLCK Signaling Pathways in Gastrointestinal Disorders with Intestinal Hyperpermeability. Gastroenterology research and practice. 2016;2016:7374197. doi: 10.1155/2016/7374197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardi DS, Tremaine WJ, Sandborn WJ, et al. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93:504–514. doi: 10.1111/j.1572-0241.1998.156_b.x. [DOI] [PubMed] [Google Scholar]
- 47.Hueppelshaeuser R, von Unruh GE, Habbig S, et al. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn’s disease. Pediatric nephrology. 2012;27:1103–1109. doi: 10.1007/s00467-012-2126-8. [DOI] [PubMed] [Google Scholar]
- 48.Caspary WF, Tonissen J. Enteric hyperoxaluria. I. Intestinal oxalate absorption in gastrointestinal diseases (author’s transl) Klinische Wochenschrift. 1978;56:607–615. doi: 10.1007/BF01477009. [DOI] [PubMed] [Google Scholar]
- 49.Fukushima T, Ishiguro N, Matsuda Y, et al. Clinical and urinary characteristics of urolithiasis in ulcerative colitis. Am J Gastroenterol. 1982;77:238–242. [PubMed] [Google Scholar]
- 50.Capolongo G, Abul-Ezz S, Moe OW, et al. Subclinical celiac disease and crystal-induced kidney disease following kidney transplant. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;60:662–667. doi: 10.1053/j.ajkd.2012.02.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Kort S, Keszthelyi D, Masclee AA. Leaky gut and diabetes mellitus: what is the link? Obesity reviews: an official journal of the International Association for the Study of Obesity. 2011;12:449–458. doi: 10.1111/j.1467-789X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 52.Eisner BH, Porten SP, Bechis SK, et al. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. The Journal of urology. 2010;183:2244–2248. doi: 10.1016/j.juro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 55.Bayston KF, Cohen J. Bacterial endotoxin and current concepts in the diagnosis and treatment of endotoxaemia. Journal of medical microbiology. 1990;31:73–83. doi: 10.1099/00222615-31-2-73. [DOI] [PubMed] [Google Scholar]
- 56.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 57.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Deventer SJ, Buller HR, ten Cate JW, et al. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–2526. [PubMed] [Google Scholar]
- 59.Hausmann M. How bacteria-induced apoptosis of intestinal epithelial cells contributes to mucosal inflammation. International journal of inflammation. 2010;2010:574568. doi: 10.4061/2010/574568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duffey BG, Miyaoka R, Holmes R, et al. Oxalobacter colonization in the morbidly obese and correlation with urinary stone risk. Urology. 2011;78:531–534. doi: 10.1016/j.urology.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Hatch M, Cornelius J, Allison M, et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney international. 2006;69:691–698. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 62.Siener R, Bangen U, Sidhu H, et al. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney international. 2013;83:1144–1149. doi: 10.1038/ki.2013.104. [DOI] [PubMed] [Google Scholar]
- 63.Kaufman DW, Kelly JP, Curhan GC, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. Journal of the American Society of Nephrology: JASN. 2008;19:1197–1203. doi: 10.1681/ASN.2007101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Troxel SA, Sidhu H, Kaul P, et al. Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. Journal of endourology/Endourological Society. 2003;17:173–176. doi: 10.1089/089277903321618743. [DOI] [PubMed] [Google Scholar]
- 65.Mikami K, Akakura K, Takei K, et al. Association of absence of intestinal oxalate degrading bacteria with urinary calcium oxalate stone formation. International journal of urology: official journal of the Japanese Urological Association. 2003;10:293–296. doi: 10.1046/j.1442-2042.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 66.Hatch M, Gjymishka A, Salido EC, et al. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. American journal of physiology Gastrointestinal and liver physiology. 2011;300:G461–469. doi: 10.1152/ajpgi.00434.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller AW, Dearing D. The metabolic and ecological interactions of oxalate-degrading bacteria in the Mammalian gut. Pathogens. 2013;2:636–652. doi: 10.3390/pathogens2040636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hassan HA, Cheng M, Aronson PS. Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells. American journal of physiology Cell physiology. 2012;302:C46–58. doi: 10.1152/ajpcell.00075.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.