Summary
FliK–FlhB interaction switches export specificity of the bacterial flagellar protein export apparatus to stop hook protein export at an appropriate timing for hook length control. The hook structure is required for the productive FliK–FlhB interaction to flip the switch but it remains unknown how it works. Here, we characterize the role of FliK in the switching probability in the absence of the hook. When RflH/Flk was missing in the hook mutants, the switching occurred at a low probability. Overproduction of FliK significantly increased the switching probability although not at the wild-type level. An in-frame deletion of residues 129 through 159 of FliK weakened the interaction with the hook protein but not with the hook-capping protein, producing polyhooks with filaments attached. We suggest that temporary association of FliK with the inner surface of the hook during FliK secretion results in a pause in the secretion process to allow the C-terminal switch domain of FliK to be positioned and appropriately oriented near FlhB for catalysing the switch and that RflH/Flk interferes with premature switch by preventing access of cytoplasmic FliK to FlhB and even that of FliK during its secretion until hook length reaches 55 nm; only then FliKC passes the RflH/Flk block.
Introduction
Bacteria such as Salmonella enterica serovar Typhimurium and Escherichia coli swim in liquid environments by means of flagella, which are rotary nanomachines made of three parts: the basal body as a rotary motor; the hook as a universal joint; and the filament as a helical propeller (Fig. 1). Flagellar assembly begins with the basal body, followed by the hook and finally the filament (Macnab, 2003; Minamino and Namba, 2004; Minamino et al., 2008). Most of flagellar component proteins are translo-cated into the axial central channel of the growing structure by the flagellar type III protein export apparatus, which is believed to be located within the central pore of the basal body MS ring (Minamino and Macnab, 1999; 2000a). The component proteins of the flagellar protein export apparatus share substantial sequence similarities with those of the type III secretion system of pathogenic animal and plant bacteria, which direct inject virulence factors into eukaryotic host cells (Cornelis, 2006). One remarkable feature of both flagellar and virulence type III secretion systems is that the export apparatus switches export substrate specificity, thereby fairly well controlling the length of their substructures (Minamino and Pugsley, 2005).
Fig. 1.
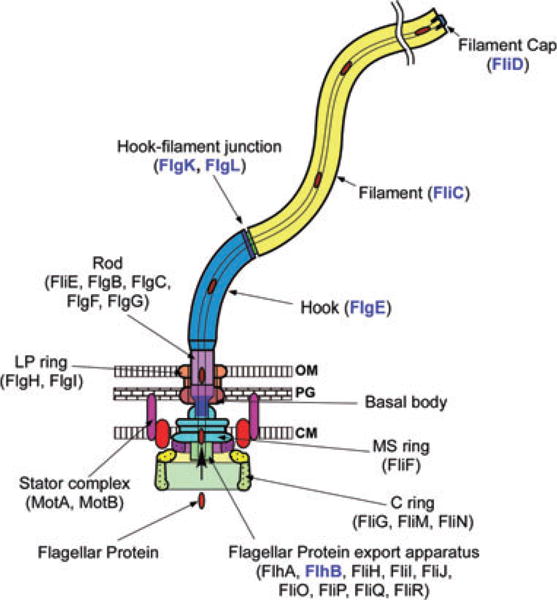
The bacterial flagellum can be roughly divided into three parts: the basal body, the hook and the filament. The different colours represent different protein components: FliF, MS ring protein; FliG, FliM and FliN, C ring component proteins; FlhA, FlhB, FliH, FliI, FliJ, FliO, FliP, FliQ and FliR, component proteins of the flagellar type III protein export apparatus; FliE, FlgB, FlgC, FlgF and FlgG, rod proteins; FlgH, Ling protein; FlgI, P ring protein; FlgE, hook protein; FlgK and FlgL, hook–filament junction proteins; FliC, filament protein (flagellin); FliD, filament-capping protein; MotA and MotB, the stator proteins. After the MS-C ring structure is assembled by FliF in the cytoplasmic membrane and FliG, FliM and FliN on the cytoplasmic surface of the FliF ring, flagellar component proteins are translocated into the central channel by the flagellar protein export apparatus, which is postulated to be located in a putative central pore of the MS ring. The rod assembly begins with FliE, FlgB, FlgC and FlgF followed by FlgG with the help of the rod cap made of the putative rod-capping protein FlgJ. Upon completion of the rod structure, the rod cap is presumably replaced by the hook cap, which is made of the hook-capping protein FlgD. After the rod is formed, FlgI and FlgH assemble around the rod in the peptidoglycan layer and outer membrane, respectively, to form the L–P ring complex. FlgE self-assembles into the hook at the tip of the distal rod with the aid of the hook cap. When the hook reaches its mature length, the ruler protein FliK interacts with FlhB, thereby allowing the export apparatus to switch its export specificity to substrates needed for the structure and assembly of the flagellar filament including the anti-sigma factor FlgM. The hook cap is replaced by FlgK, and then FlgL and FliD are bound at the distal end in this order. FliC then starts self-assembling into the long helical filament onto the hook–filament junction with the help of the filament cap made of FliD. FlgD, FlgJ, FliK and FlgM are not in the final structure of the flagellum. Location of RflH/Flk remains unknown but it is postulated to associate with the export apparatus. Component proteins relevant to this study are highlighted in blue.
During hook-basal body (HBB) assembly, the flagellar protein export apparatus is specific for substrates needed for the structure and assembly of the rod and hook (Minamino and Macnab, 1999; Minamino et al., 1999a; Hirano et al., 2003). The hook length control protein FliK is also secreted as a rod-hook substrate and is required, upon HBB completion, to facilitate the substrate specificity switch from the rod-hook class of secretion substrates to the filament class of substrates (Minamino et al., 1999b). At this point the four filament-type substrates that are expressed along with the HBB genes, FlgK, FlgL, FliD and FlgM, are exported (Minamino et al., 1999a). FlgK and FlgL form the hook–filament junction and FliD is the filament cap (Fig. 1). The FlgM protein is an anti-sigma factor (Ohnishi et al., 1992). Prior to HBB completion FlgM binds the flagellar-specific sigma factor, σ28, to prevent transcription of filament genes (fliC or fljB), motor force generator genes (motA and motB), and the 15 genes that comprise the chemosensory system (Ohnishi et al., 1992). Upon FlgM secretion σ28 is free to transcribe the genes that are only needed after HBB completion (Hughes et al., 1993; Kutsukake, 1994). In this way the flagellar transcriptional hierarchy is coupled to the assembly process.
An integral membrane component of the export apparatus, FlhB, plays an important role in substrate recognition (Minamino and Pugsley, 2005; Ferris and Minamino, 2006; Minamino et al., 2008). FlhB is a 384-amino-acid protein that is composed of four N-terminal transmembrane segments followed by a 170-amino-acid cytoplasmic domain (FlhBC) (Minamino et al., 1994). FlhBC undergoes an auto-cleavage event between N269 and P270 that is essential for the substrate specificity switch to occur (Minamino and Macnab, 2000b; Fraser et al., 2003; Ferris et al., 2005). Recently, molecular mechanism of autocleavage has been demonstrated by X-ray crystallography of the FlhB homologues (Zarivach et al., 2008). A second requirement for the switch to occur is the interaction between the C-terminal domain of FliK (FliKC) and FlhBC (Kutsukake et al., 1994; Williams et al., 1996; Hirano et al., 2005; Minamino et al., 2006). Thus, in strains that are either missing FliK or mutant in FlhB that are unable to undergo autocleavage (N269A), the specificity switch will not occur. These strains remain in the rod-hook secretion mode and produce polyhook structures, in which the hooks reach lengths of up to 1 μm (the length of the cell body) (Patterson-Delafield et al., 1973; Suzuki and Iino, 1981; Fraser et al., 2003). In wild-type cells, FliK catalyses the export specificity switch when the hook has reached its mature length of c. 55 nm (Hirano et al., 1994). One model to explain the FliK-dependent export specificity switch is that FliK is a molecular ruler (Minamino et al., 2004; Moriya et al., 2006). During FliK secretion, FliKC is positioned in the vicinity of FlhB when the hook reaches c. 55 nm in length to induce a conformational change in the cleaved FlhBC that results in the substrate specificity switch.
The idea that FliK could act as a molecular ruler is an extension of the elegant work on the FliK functional homologue, YscP, in the control of needle length in the virulence-associated type III secretion system of Yersinia enterocolitica (Journet et al., 2003; Agrain et al., 2005; Wagner et al., 2009). FliK and YscP are conserved in the structure of the C-terminal domain that catalyses the substrate specificity switch, known as the T3S4 domain (Agrain et al., 2005; Pallen et al., 2005). Loss-of-function mutations in yscP result in uncontrolled needle length (Journet et al., 2003). It has been demonstrated that the length of YscP is directly proportional to the length of the needle: amino acid insertions and deletions in YscP resulted in a concomitant increase or decrease in final needle length by c. 0.2 nm per amino acid inserted or deleted. A similar, follow-up study in FliK also demonstrated that hook length increased or decreased by c. 0.2 nm per amino acid inserted or deleted (Shibata et al., 2007).
Only 5–10 molecules of FliK per flagellum are secreted during HBB assembly (Muramoto et al., 1998; Minamino et al., 1999b). If FliK secretion is prevented, the export apparatus cannot efficiently switch export specificity at the normal hook length of 55 nm. This suggests that proper FliK secretion is required for the efficient switching process (Minamino et al., 1999b; 2004; Hirano et al., 2005). However, it is not understood how FliK secretion could allow a productive interaction between FliKC and FlhBC when proper hook length is achieved. For example, FliK is constantly secreted in strains with mutations in the hook-capping protein gene flgD or the hook protein gene flgE (Minamino et al., 1999b). However, the flagellar export apparatus never switches its substrate specificity even though FliKC is continuously passing by FlhBC during the export process in the flgD or flgE mutant strains (Minamino et al., 1999b). Thus, secretion of FliK alone is not sufficient for the specificity switch to occur. We presume that a mechanism is in place that allows for a productive interaction between FliKC and FlhBC when hook length is c. 55 nm.
In this article we investigate one mechanism that explains how FliKC and FlhBC could interact with each other to catalyse the substrate specificity switch at a hook length of c. 55 nm. Since FliK continuously passes FlhBC without a productive interaction to catalyse the switch, one model predicts that there is a pause in FliK secretion as the N-terminal domain of FliK (FliKN) reaches the hook cap and initiates its exit from the flagellar structure (Moriya et al., 2006). When hook reaches c. 55 nm, FliKC is in the vicinity of FlhB to interact and catalyse the switch. At shorter hook lengths FliKC does not interact with FlhBC and once the N-terminus of FliK exits the structure the speed of further FliK secretion does not permit a productive interaction of FliKC with FlhBC as it passes by. Such a pause could be mediated by an interaction between FliKN and the hook-capping protein FlgD. Evidence in support of this comes from the finding that FliKN binds to the hook-capping protein FlgD with high affinity and FlgE with low affinity in vitro (Moriya et al., 2006). These interactions could allow for the export specificity switch at the time of hook completion and control hook length by FliK during its translocation through the central channel of the growing flagellum. Although such mechanism seems plausible, it remains to be further supported by the presence of the interactions of FliKN with the hook cap and the hook during FliK secretion in vivo.
In this study, we analysed the interaction of FliKN with the hook in vivo. We show that the presence of the hook and hook cap allows FliKC to efficiently communicate with FlhBC. We also show that an in-frame deletion of 31 residues in FliKN weakens the FliKN–FlgE interaction but not the FliKN–FlgD interaction.
Results
Effect of RflH/Flk on the substrate specificity switch in the absence of the hook
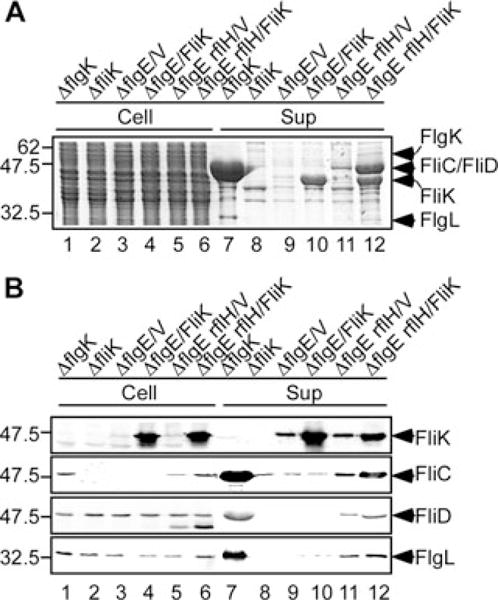
It has been shown that RflH/Flk inhibits the premature secretion of filament-type substrates prior to HBB completion (Kutsukake, 1997; Aldridge et al., 2006). Recently, it has been proposed that RflH/Flk will interact with FliKC during the process of FliK secretion to prevent FliKC from interacting with FlhBC before the hook reaches its terminal length (Hirano et al., 2009). To test this, culture supernatants were prepared from the flgD and flgE mutants with or without RflH/Flk and were analysed by both CBB staining and immunoblotting (Fig. 2). Since a fliK mutant cannot switch specificity and hence remains in the rod/hook-type specificity state (Kutsukake et al., 1994), we used wild type and fliK mutant as a positive and a negative control respectively. Very strong FlgK, FlgL, FliC and FliD bands were detected in the culture supernatant of wild-type cells (Fig. 2B, right panel lane 1). In contrast, only very faint or no bands were seen in the culture supernatant of the fliK, flgD or flgE mutants (lanes 3, 5 and 7) although higher amounts of FliK molecules were secreted compared with wild-type cells (lanes 5 and 7). Thus, the substrate specificity switch does not occur in the absence of either FliK or the hook. In addition, FlgM (not shown) is retained in the cytoplasm and σ28-dependent transcription of the fliC genes is inhibited, resulting in both the absence of FliC in the cytoplasm and the secretion from the cell of the fliK, flgD and flgE mutants (lanes 3, 5 and 7).
Fig. 2.

Effect of RflH/Flk on the secretion of filament-type proteins in the absence of the hook structure. (A) Coomassie-stained SDS-PAGE gel and (B) immunoblotting using polyclonal anti-FliK, anti-FliC, anti-FliD, anti-FlgK and anti-FlgL antibodies, of whole-cell proteins (Cell) and culture supernatants (Sup). Lane 1, SJW1103 (WT); lane 2, SJW1103RflH (ΔrflH∷tetRA); lane 3, TH8426 (ΔfliK); lane 4, TH8426RflH (ΔfliK, ΔrflH∷tetRA); lane 5, SJW156 (ΔflgD); lane 6, SJW156RflH (ΔflgD, ΔrflH∷tetRA); lane 7, NME001(ΔflgE); lane 8, NME001RflH (ΔflgE, ΔrflH∷tetRA); lane 9, MMEK001 (ΔflgE, ΔfliK); lane 10, MMEK001RflH (ΔflgE, ΔfliK, ΔrflH∷tetRA). The positions of FliK, FliC, FliD, FlgK and FlgL are indicated by arrows. Molecular mass markers (kDa) are shown on the left.
The levels of filament-type substrates, FliC, FliD, FlgK and FlgL, secreted by the rflH/flk mutant was at the wild-type levels (Fig. 2B, right panel lanes 1 and 2). This is in agreement with a previous report (Kutsukake, 1997). Interestingly, when RflH/Flk was removed from the flgD and flgE mutants, these filament-type proteins were secreted into the culture supernatant although at very low levels (lanes 6 and 8). However, they were not secreted from the fliK rflH/flk and flgE fliK rflH/flk mutant (lanes 4 and 10); the enhancement of the switch by RflH/Flk did not occur as far as FliK was absent. These results suggest that RflH/Flk acts as a negative regulator to interfere with the productive FliK–FlhB interaction to switch the specificity before the hook reaches its mature length of c. 55 nm as proposed before (Hirano et al., 2009).
Effect of overproduction of FliK on the switch in the absence of the hook
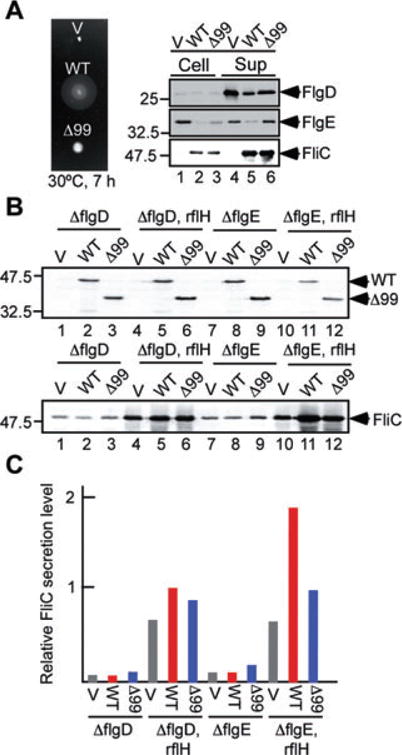
FliK secretion is dependent on its N-terminal type III secretion signal and FliK secretion is essential in order to couple hook completion at c. 55 nm to the substrate specificity switch (Minamino et al., 1999b). A deletion of amino acids 2 through 99 of FliK (FliKΔ99) resulted in loss of secretion (Hirano et al., 2005). Interestingly, when FliKΔ99 was overexpressed it catalyses the substrate specificity switch, suggesting that overexpressed FliKΔ99 interacts with FlhBC to flip the switch without secretion. Therefore, to test for the ability of overexpressed FliK to flip the switch in the absence of the hook protein (FlgE), we analysed the secretion levels of filament-type substrates, FliC, FliD and FlgL. We used a flgK mutant as a positive control because the flgK mutant forms hooks of normal length and hence switches its specificity to the filament-type class properly but does not form the filament, permitting assay of the secretion of FlgL, FliC and FliD without complication by filament assembly, which reduces the secretion level of FliC (Minamino et al., 1999a). In agreement with a previous report (Homma et al., 1984), very strong FlgL, FliC and FliD bands were detected in the culture supernatant of the flgK mutant (Fig. 3, lane 7). The overproduction of FliK in a flgE null mutant and a flgE rflH(flk) double null mutant considerably increased both the cellular and secretion levels of FliK by about 1000-fold (lanes 4, 6, 10 and 12). When RflH/Flk was present, overproduction of FliK did not affect the secretion levels of FlgL, FliD and FliC (lanes 9 and 10), indicating that the specificity switch did not occur. However, when RflH/Flk was missing, FlgL, FliD and FliC were secreted (lane 11), and their secretion levels were significantly enhanced by overproduction of FliK (lane 12), although the amounts were still four or five times less than those of the flgK mutant (lane 7). These results suggest that overproduction of FliK must have increased the probability of the FliK–FlhB interaction to switch the specificity in the flgE rflH(flk) double null mutant.
Fig. 3.
Multicopy effects of FliK on export specificity switch in the absence of the hook protein FlgE. (A) Coomassie-stained SDS-PAGE gel and (B) immunoblotting using polyclonal anti-FliK, anti-FliC, anti-FliD and anti-FlgL antibodies, of whole-cell proteins (Cell) and culture supernatants (Sup). Lanes 1 and 7, SJW2177 (ΔflgK); lanes 2 and 8, TH8426 (ΔfliK); lanes 3 and 9, NME001(ΔflgE) carrying pTrc99A (V); lanes 4 and 10, NME001(ΔflgE) carrying pKM002 (FliK); lanes 5 and 11, NME001RflH (ΔflgE, ΔrflH∷tetRA) carrying pTrc99A (V); lanes 6 and 12, NME001RflH (ΔflgE, ΔrflH∷tetRA) carrying pKM002 (FliK). The positions of FliK, FliC, FliD and FlgL are indicated by arrows. Molecular mass markers (kDa) are shown on the left.
Multicopy effects of FliKΔ99 on export specificity switch
The first 40 amino acid residues of FliK are required for its secretion during HBB assembly (Minamino et al., 1999b). It has been shown that FliKΔ99, an export-deficient FliK variant, catalyses the substrate specificity switch when overexpressed (Hirano et al., 2005), raising the possibility that overexpressed FliKΔ99 can catalyse the switch even in the absence of the hook. To test this, we first analysed the ability of FliKΔ99 to flip the substrate specificity switch in the presence of FlgD and FlgE. In agreement with a previous study (Hirano et al., 2005), FliKΔ99 complemented the fliK null mutant to some degree although not at the wild-type levels (Fig. 4A, left panel). The poor motility was a consequence of an elongated hook structure (data not shown), indicating that hook length control is lost. Consistently, higher amounts of FlgD and FlgE were detected in the culture supernatants compared with full-length FliK (Fig. 4A, right panel lane 6), indicating that a deletion of residues 2–99 leads the secretion mode specific for rod/hook-type substrates to continue for a longer period. In agreement with data shown by Hirano et al. (2005), FliC was detected at the wild-type FliK level. These results indicate that FliKΔ99 can switch export specificity but its switching efficiency is much less than wild-type FliK in the presence of the hook structure.
Fig. 4.
Switching probability of FliK and FliKΔ99 in the absence of the hook structure.
A. Swarming motility (left panel) and flagellar protein export (right panel) of TH8426 (ΔfliK) transformed with pTrc99A (V), pKM002 (WT), pNM201 (Δ99). For swarming motility assays, soft agar plates were incubated at 30°C for 7 h. Secretion assays of FlgD, FlgE and FliC were performed by immunoblotting.
B. Multicopy effects of FliK and FliKΔ99 on FliC secretion. Immunoblotting, using polyclonal anti-FliK (upper panel) and anti-FliC antibodies (lower panel), of whole-cell proteins from SJW156 (ΔflgD), SJW156RflH (ΔflgD, ΔrflH∷tetRA), NME001 (ΔflgE) and NME001RflH (ΔflgE, ΔrflH∷tetRA) transformed with pTrc99A (V), pKM002 (WT), pNM201 (Δ99).
C. Relative secretion level of FliC measured on the data presented in lower panel of (B). Grey bar, V; red bar, WT; blue bar, Δ99. These data are the average of three independent experiments. The experimental errors are within 10%.
To test the ability of FliKΔ99 to flip the substrate specificity switch in the absence of the hook, we analysed the secretion level of FliC by overproducing FliK or FliKΔ99 in the flgE mutant (Fig. 4B, lower panel, and Fig. 4C). When overproduced, their cellular levels were almost the same regardless of the mutant background used here (Fig. 4B, upper panel). Overproduction of FliK in the flgE mutant has no effect on the probability of the specificity switch (Fig. 4B, lower panel lanes 7 and 8), but overproduction of FliKΔ99 increased it about twice (lane 9), indicating that the hook is required for secreted FliK to switch and that FliKΔ99 can catalyse the switch in the absence of the hook but not so efficiently. When RflH/Flk was further removed from the flgE mutant, however, the level of FliC secretion was several fold higher than that of the flgE mutant at a normal level of FliK (lane 10), almost 20 times higher by overproduction of FliK (lane 11), and several fold higher by overproduction of FliKΔ99 (lane 12), indicating that RflH/Flk interferes with the interaction of FliKΔ99 with FlhB as well as that of FliK with FlhB as shown in Figs 2 and 3. Interestingly, the secretion level of FliC became threefold further higher by FliK overexpres-sion but only 1.5-fold higher by FliKΔ99 overexpression compared with the vector control, which produces FliK at wild-type level (lanes 10–12 and Fig. 4C). This suggests that the switching probability of FliKΔ99 is less than that of wild-type FliK in the flgE mutant.
We also tested how overproduction of FliK and FliK Δ99 affects the probability of export specificity switch in the flgD rflH/flk double null mutant background as compared with the flgE rflH/flk background. The increase in the secretion level of FliC by FliK overproduction was only one and half times in the flgD rflH/flk double null mutant, which is much smaller than the three times increase observed in the flgE rflH/flk background (Fig. 4B, lower panel lanes 4 and 5, lanes 10 and 11, and Fig. 4C) and little difference was observed by FliKΔ99 overproduction (Fig. 2B, lanes 4 and 6, lanes 10 and 12, and Fig. 4C). As the flgE mutant has the FlgD cap at the tip of the rod while the flgD mutant does not (Ohnishi et al., 1994), the higher level of increase in FliC secretion from the flgE rflH/flk double mutant by overproduction of FliK seems to be due to the presence of the FlgD cap. This suggests that the interaction of FliK with FlgD increases the probability of the substrate specificity switch.
Partial defect in hook length control by FliKΔ(129–159) is overcome by its overexpression
Insertion of peptide fragments into the N-terminal region of FliK (FliKN) results in increase in hook length in proportion to the size of inserted fragments, suggesting that FliKN acts as a molecular ruler to control hook length (Shibata et al., 2007). In contrast, in-frame deletions in only three small regions within FliKN shorten hook length; most of deletions within FliKN cause loss of function, producing polyhooks (Shibata et al., 2007), suggesting that FliKN plays an important role in export specificity switch. To understand the role of FliKN in the switching mechanism, we characterized an in-frame deletion variant of FliK, FliKΔ(129–159), missing amino acids 129–159, and the effect of the mutation on hook length and cell motility because it is known to produces longer hooks with filament attached (Fig. 5). FliKΔ(129–159) did not sustain wild-type motility (Fig. 5A, left panel) although it was stable and efficiently secreted (Fig. 5B). In agreement with previous data (Williams et al., 1996; Shibata et al., 2007), the length distribution of hooks produced by this in-frame deletion mutant was significantly broader than that of the wild type with a mean value of 80 44 nm, but with a peak length still at 55 nm, the same value as that of the wild type (Fig. 6A). This is consistent with the data showing that slightly higher amounts of FlgD and FlgE were detected in the culture supernatants compared with the wild-type levels (Fig. 5B, lanes 5 and 6). As the in-frame deletion did not decrease the secretion level of FliC (Fig. 5B, lane 6), we conclude that the in-frame deletion makes the average rod/hook-type export mode continue for a longer period, producing many longer hooks, but the export specificity eventually switches to form the filaments.
Fig. 5.
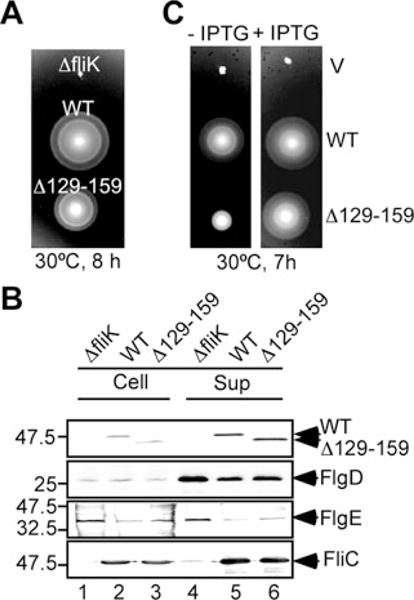
Characterization of the fliKΔ(129–159) mutant.
A. Swarming motility assay of TH8426 (ΔfliK), SJW1103 (WT) and MY2870 (Δ129–159) on soft tryptone agar plates. The plates were incubated at 30°C for 8 h.
B. Secretion assays of FlgD, FlgE and FliC by immunoblotting.
C. Swarming motility assay of TH8426 (ΔfliK) transformed with pTrc99A (V), pKM002 (WT) and pMMIK2807 (Δ129–159) on soft agar plates with or without 1 mM IPTG. The plates were incubated at 30°C for 7 h.
Fig. 6.
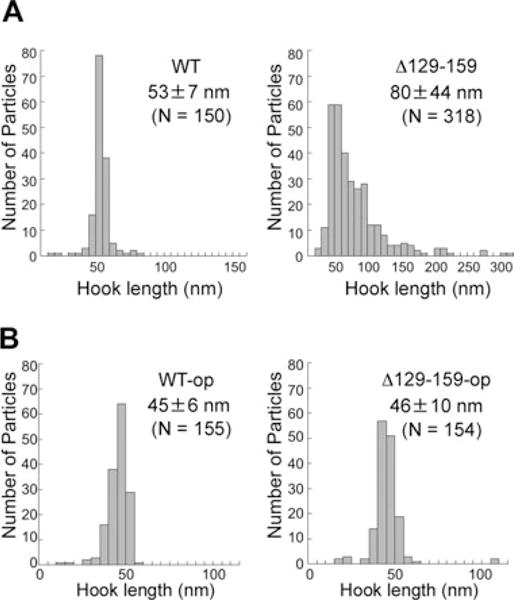
Effect of an in-frame deletion Δ(129–159) of FliK on hook length control.
A. Hook length distribution of SJW1103 (WT) on the left and MY2807 (Δ129–159) on the right.
B. Hook length distribution of MY2807 (Δ129–159) transformed with pKM002 (FliK) on the left (WT-op) and pMMIK2807 [FliKΔ(129–159)] on the right (Δ129–159-op). The cells were grown exponentially in the presence of 1 mM IPTG.
It has been shown that overproduction of FliKΔ(129–159) restores the mutant’s motility to wild-type levels (Minamino et al., 1999b). To confirm this, we compared the swarming motility of fliK null mutant cells overexpressing either FliK or FliKΔ(129–159) on soft tryptone agar plates (Fig. 5C). In the presence of 1 mM IPTG, both the cellular and secretion levels of wild-type FliK and FliKΔ(129–159) increased (data not shown). As shown previously by Minamino et al. (1999), under inducing conditions, the motility improved to near wild-type levels (Fig. 5C), indicating that an excess of FliKΔ(129–159) will restore wild-type motility.
To see if the improved motility is a consequence of hook length shortening, we measured the hook length of fliK null mutant cells that overproduce either FliK or FliKΔ(129–159) by electron microscopy (Fig. 6B) and compared them with those of the wild type and fliKΔ(129–159) mutant (Fig. 6A). Because overproduction of FliK causes a slight shortening of hook length (Muramoto et al., 1998), we used cells overproducing FliK as a control. Overproduction of FliKΔ(129–159) resulted in shortening of the hook length to a mean value of 46 ± 10 nm, which is essentially the same as that of the cells overproducing FliK (45 ± 6 nm), suggesting that overproduction of FliKΔ(129–159) recovers its efficiency to measure hook length and to switch export specificity to the wild-type FliK level. These results suggest that FliKΔ(129–159) is somewhat defective in the switching capability, possibly due to a weaker interaction between FliKN and the hook, resulting in a greater number of polyhook structures and this defect is overcome by overexpression.
Interaction of FliKΔ(129–159) with FlgD and FlgE
The data presented in Fig. 3 demonstrate a role of FlgD and FliK secretion in the substrate specificity switch. We have previously shown that FliKN interacts strongly with FlgD and less so with FlgE (Moriya et al., 2006). As the secretion of an excess amount of FliKΔ(129–159) is necessary to achieve the wild-type ruler function, we tested if the partial defect in the switch of the FliKΔ(129–159) mutant might be due to a defect in interaction with FlgD or FlgE by affinity blotting (Fig. 7A). Since it has been shown that FlgD and FlgE binds to FliK(1–147) but not to FliK(265–405), we used FliK(265–405), the filament-capping protein FliD and a non-flagellar protein GST as negative controls. The in-frame deletion did not affect the interaction with FlgD (middle panel lane 5). In contrast, FlgE bound to full-length FliK (right panel lane 2) and FliK(1–147) (right panel lane 3), more weakly to FliKΔ(129–159) (right panel lane 5), and not at all to FliK(265–405) (right panel lane 4), FliD (data not shown) or GST (data not shown).
Fig. 7.

Interactions of FliKΔ(129–159) with FlgD and FlgE.
A. Coomassie-stained SDS-PAGE gel (left panel) of whole-cell lysates from BL21(DE3)pLysS carrying pET19b (V), pKM003 encoding His-FliK (WT), pBGIKN encoding His-FliK(1–147) (FliKN), pBGIKN encoding His-FliK(265–405) (FliKC) or pMMIK2807ET encoding His-FliKΔ(129–159) (Δ129–159). Affinity blots of the same samples as in the left panel using purified His-FlgD (middle panel) and His-FlgE (right panel) as probes and detection of the probe with polyclonal anti-FlgD and anti-FlgE antibodies respectively. Exposure times for the middle and right panels were 8 and 60 s respectively. The positions of the target proteins are indicated by arrowheads.
B and C. Pull-down assays by Ni-NTA affinity chromatography. The mixture of the soluble fractions (L) from cells overexpressing His-FliK or His-FliKΔ(129–159) with those from cells overexpressing FlgD (B) or FlgE (C) was loaded onto a Ni+-NTA agarose column. After washing with buffers containing 25 mM imidazole, proteins were eluted with buffers containing 50, 100, 250 or 500 mM imidazole. The eluted fractions were analysed by CBB staining (upper panel) and immnunoblotting with polyclonal FlgD (B) and FlgE (C) antibodies (lower panel). The positions of molecular mass markers (kDa) are shown on the left.
To confirm this, we carried out pull-down assays by Ni-NTA affinity chromatography. FlgD was co-purified with both His-FliK and His-FliKΔ(129–159) (Fig. 5B). In agreement with Fig. 5A, FlgE was co-purified with His-FliK but not with His-FliKΔ(129–159) (Fig. 5C). These results strongly suggest that the interaction with FlgE is considerably weakened by the FliKΔ(129–159) deletion.
FliK interacts with assembled FlgD and FlgE
Thus far, our results suggest that interactions of FliK with both FlgD and FlgE play a role in the ability of FliK to efficiently catalyse the substrate specificity switch. The results of the FliK–FlgD and FliK–FlgE affinity assay presented in the previous section are consistent with at least two mechanisms by which FliKN interacts with FlgD and FlgE to facilitate export specificity switch. First, FlgD and FlgE proteins could interact with FliK in the initial process of its export in the cytoplasmic side of the basal body to target FliK to the export apparatus. All of the filament-type substrates have secretion chaperones that facilitate their secretion (Minamino and Namba, 2004), but no such chaperone has been identified for the rod/hook-type substrates. It is possible that FlgD and FlgE act as secretion chaperones for FliK through a direct interaction in the cytoplasm. Alternatively, it is FlgD assembled into the hook cap and FlgE assembled into the hook that interact with FliKN to allow the FliK secretion process to pause and to facilitate the interaction between FliKC and FlhBC to switch export specificity when the hook reaches a length of c. 55 nm.
To distinguish between these two possibilities, the effect of secretion-competent, assembly-defective alleles of flgD and flgE on the ability of FliK to catalyse export specificity switch was examined. Mutants defective in HBB assembly fail to switch export substrate specificity, resulting in the accumulation of the anti-σ28 factor FlgM in the cytoplasm and inhibition of σ28-dependent class 3 flagellar promoter transcription (Hughes et al., 1993; Kutsukake, 1994). However, strains defective in L- or P-ring assembly switches export specificity prematurely in the absence of RflH/Flk (Karlinsey et al., 1997). We used a ΔflgH-flgI deletion mutant strain defective in LP-ring formation to examine the effect of mutations in flgD and flgE on the ability to switch export specificity (Table 2). We used a fliC–lac operon fusion as a reporter for flagellar class 3 transcription. As shown in Table 1, loss of RflH/Flk in a ΔflgH-flgI double null mutant resulted in more than 20-fold increase in σ28-dependent class 3 transcription. This is because a loss of RflH/Flk allows export specificity switch to occur in the LP-ring mutant strain, resulting in the secretion of FlgM into the periplasm where it is degraded (Aldridge et al., 2006), which is coupled to increased σ28-dependent class 3 transcription (Kutsukake, 1997).
Table 2.
Strains and plasmids used in this study.
| Strains and plasmids | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| BL21(DE3)pLysS | Overproduction of proteins | Novagen |
| Salmonella | ||
| SJW1103 | Wild type for motility and chemotaxis | Yamaguchi et al. (1984) |
| SJW1103RflH | ΔrflH∷tetRA | This study |
| SJW156 | flgD | Ohnishi et al. (1994) |
| SJW156RflH | flgD ΔrflH∷tetRA | This study |
| SJW2177 | flgK | Homma et al. (1984) |
| MMEK001 | ΔflgE ΔfliK | This study |
| MMEK001RflH | ΔfigE ΔfliK ΔrflH∷tetRA | This study |
| MY2807 | Δfl/K(129–159) | Williams et al. (1996) |
| NME001 | ΔflgE | This study |
| NME001RflH | ΔflgE ΔrflH∷tetRA | This study |
| TH8426 | ΔfliK6140(6–405) | Minamino et al. (2006) |
| TH8426RflH | ΔfliK6140 ΔrflH∷tetRA | Minamino et al. (2006) |
| TH14136 | ΔflgHI958 fliC5050∷MudJ | This study |
| TH14137 | ΔflgHI958 fliC5050∷MudJ flk-5212∷Tn 10dTc | This study |
| TH14140 | fliC5050∷MudJ | This study |
| TH14141 | ΔflgHI958 ΔflgJ7677 fliC5050∷MudJ | This study |
| TH14142 | flgD157(S+A−) ΔflgHI958 fliC5050∷MudJ | This study |
| TH14144 | ΔflgD6541 ΔflgHI958 fliC5050∷MudJ | This study |
| TH14145 | flgE1353(S+A−) ΔflgHI958 fliC5050∷MudJ | This study |
| TH14146 | ΔflgE7616 ΔflgHI958 fliC5050∷MudJ | This study |
| TH14147 | ΔflgDE7639 ΔflgHI958 fliC5050∷MudJ | This study |
| TH14148 | flk-5212∷Tn 10dTc fliC5050∷MudJ | This study |
| TH14149 | ΔflgHI958 ΔflgJ7677 flk-5212∷Tn 10dTc fliC5050∷MudJ | This study |
| TH14150 | flgD157(S+A−) ΔflgHI958 flk-5212∷Tn 10dTc fliC5050∷MudJ | This study |
| TH14152 | ΔflgD6541 ΔflgHI958 flk-5212∷Tn10dTcfliC5050∷MudJ | This study |
| TH14153 | flgE1353(S+A−) ΔflgHI958 flk-5212∷Tn10dTcfliC5050∷MudJ | This study |
| TH14154 | ΔflgE7616 ΔflgHI958 flk-5212∷Tn10dTcfliC5050∷MudJ | This study |
| TH14155 | ΔflgDE7639 ΔflgHI958 flk-5212∷Tn10dTcfliC5050∷MudJ | This study |
| TH14211 | flgD157(S+A−) fliC5050∷MudJ | This study |
| TH14212 | ΔflgD6541 fliC5050∷MudJ | This study |
| TH14213 | flgE1353(S+A−) fliC5050∷MudJ | This study |
| TH14214 | ΔflgE7616 fliC5050∷MudJ | This study |
| TH14215 | ΔflgDE7639 fliC5050∷MudJ | This study |
| TH14216 | ΔflgJ7598∷tetRΔ fliC5050∷MudJ | This study |
| TH14218 | flgD157(S+A−) flk-5206∷Tn10dCm fliC5050∷MudJ | This study |
| TH14219 | ΔflgD6541 flk-5206∷Tn10dCm fliC5050∷MudJ | This study |
| TH14220 | flgE1353(S+A−) flk-5206∷Tn 10dCm fliC5050∷MudJ | This study |
| TH14221 | ΔflgE7616 flk-5206∷Tn10dCm fliC5050∷MudJ | This study |
| TH14221 | ΔflgDE7639 flk-5206∷Tn10dCm fliC5050∷MudJ | This study |
| TH14223 | ΔflgJ7598∷tetRΔ flk-5206∷Tn 10dCm fliC5050∷MudJ | This study |
| TH16207 | ΔfliK(129-159) fliC5050∷MudJ | This study |
| TH16208 | ΔflgHI958 Δfl/K(129-159) fliC5050∷MudJ | This study |
| TH16209 | ΔfliK(129-159) flk-5212∷Tn 10dTc fliC5050∷MudJ | This study |
| TH16210 | ΔflgHI958 ΔfliK(129-159) flk-5212∷Tn 10dTc fliC5050∷MudJ | This study |
| Plasmids | ||
| pET19b | Expression vector | Novagen |
| pGEX-6p-1 | Expression vector, GST | GE Healthcare |
| pBGIKN | pET19b/His-FliK(1-147) | Minamino et al. (2004) |
| pBGIKC | pET19b/His-FliK(265-405) | Minamino et al. (2004) |
| pKM002 | pTrc99Δ/FliK | Muramoto et al. (1998) |
| pKM003 | pET19b/His-FliK | Minamino et al. (1999) |
| pMM711 | pET19b/His-FlgD | Minamino and Macnab (2000a) |
| pMM1601 | pET19b/His-FlgE | Minamino and Macnab (2000a) |
| pMMIK2807 | pTrc99Δ/FliKΔ(129-159) | Minamino et al. (1999) |
| pMMIK2807ET | pET19b/His-FliKΔ(129-159) | This study |
| pNM001 | pTrc99Δ/FlgE | Moriya et al. (2006) |
| pNM201 | pTrc99Δ/FliKΔ(1-99) | This study |
| pRcD001 | pTrc99Δ/FlgD | Hirano et al. (2003) |
Table 1.
The effect of flgD and flgE alleles on flagellar class 3 (fliC–lac) transcription in the presence and absence of the LP-rings and RflH/Flk.
| Strain set | Rod/hook mutation | Relative β-galactosidase activity
|
|||
|---|---|---|---|---|---|
|
rflH/flk+
|
rflH/flk−
|
||||
| flgH+I+ | ΔflgHI | flgH+I+ | ΔflgHI | ||
| 1. | None | 100 | 2 | 100 | 43 |
| 2. | flgD(S+A−) | 4 | 2 | 18 | 9 |
| 3. | ΔflgD | 3 | 2 | 21 | 11 |
| 4. | flgE(S+A−) | 3 | 1 | 9 | 11 |
| 5. | ΔflgE | 4 | 2 | 9 | 18 |
| 6. | ΔflgDE | 4 | 2 | 26 | 15 |
| 7. | ΔflgJ | 3 | 2 | 11 | 5 |
| 8. | ΔfliKa | 79 | 2 | 74 | 18 |
This ΔfliK allele is the in-frame deletion of amino acids 129 through 159 from strain MY2807 (Williams et al., 1996).
The S+A− designations for strains 2 and 4 represent secretion-proficient, assembly-deficient mutants in flgD and flgE respectively. The β-galactosidase activities were assayed twice in triplicate. The β-galactosidase activities of the wild-type strains were set to 100 and the average for the two independent sets of triplicate assays were normalized to the wild-type as described (Maloy, 1990).
In this experiment, the expression level of a fliC–lac fusion is the measure of the degree that export specificity switch occurs in the absence of RflH/Flk. Transcription of fliC–lac is an indirect measure of FlgM secretion and therefore is a good measure of the degree to which export specificity switch has occurred. Loss of either flgD or flgE lowered the efficiency of export specificity switch in the ΔflgH-flgI rflH/flk mutant background, suggesting that FliK has an increased access to FlhB only when both FlgD and FlgE are present in the ΔflgH-flgI mutant. Essentially the same results were obtained for deletion mutants in flgD or flgE that were isolated as mutants producing stable, secretion-proficient, but assembly-deficient FlgD or FlgE, demonstrating that only assembled FlgD and FlgE facilitate the FliK-mediated switch.
The genetic experiment above strongly supports the model that only assembled FlgD and FlgE are involved in the switch via the interaction with FliK. Our binding studies demonstrate a defect in the interaction of FliKΔ(129–159) with FlgE. From this one can predict that the FliKΔ(129–159) deletion mutant should give the same phenotype in this assay system as a flgE mutant. In the presence of the LP-ring the FliKΔ(129–159) showed a reduced β-gal activity relative to wild type (Table 1), which is consistent with its hook length distribution phenotype and motility defect, both a result of reduced switching capability. In the absence of the LP-rings, the FliKΔ(129–159) showed the exact same β-gal activities in the presence and absence of RflH/Flk as the ΔflgE allele (Table 1). This is strong genetic evidence that FliKΔ(129–159) is defective in the interaction with FlgE in vivo.
Discussion
Almost all flagellar proteins that are exported by the flagellar type III protein export apparatus have been grouped into two classes, the rod/hook-type class and the filament-type class (Minamino and Macnab, 1999; Minamino et al., 1999a; Hirano et al., 2003). Substrate specificity of the flagellar protein export apparatus is switched from rod/hook-type to filament-type by autocleavage of FlhBC and an interaction of FlhBC with FliKC that occurs upon completion of hook assembly (Kutsukake et al., 1994; Williams et al., 1996; Minamino and Macnab, 2000b; Fraser et al., 2003; Ferris et al., 2005). The productive FliKC–FlhBC interaction to flip the switch is required for hook completion (Minamino et al., 2004; 2006; Moriya et al., 2006). However, little is known about the role of the hook in the export specificity-switching mechanism. Here, we have analysed multicopy effects of FliK on export specificity switch in the hook mutants with or without RflH/Flk, which also acts as a negative regulator to prevent premature secretion of filament-type proteins prior to completion of hook assembly (Kutsukake, 1997; Aldridge et al., 2006), and characterized the effect of an in-frame deletion mutation, fliKΔ(129–159), that is defective in an interaction with FlgE. We have obtained evidence that RflH/Flk interferes with premature interaction between FliK and FlhB and that the association of FliKN with the hook is required for normal switching to occur.
RflH/Flk inhibits the interaction of FliKC with FlhBC
RflH/Flk has been reported to inhibit the export of filament-type substrates prior to completion of HBB assembly in a manner that is independent of the FliK–FlhB switch mechanism (Kutsukake, 1997). Recently, it has been proposed that RflH/Flk prevents FliKC from interacting with FlhB until hook assembly is completed (Hirano et al., 2009). Here, we showed that, even in the flgD and flgE mutant backgrounds, a deletion of RflH/Flk allows export specificity switch to occur with some degree although not at wild-type level (Fig. 2). Overex-pression of full-length FliK and FliKΔ99 considerably increased the switching efficiency (Figs 3 and 4). However, the export specificity switch never occurs when RflH/Flk is present (Figs 3 and 4). These results suggest that RflH/Flk specifically prevents FliKC from interacting with FlhBC unless FliKC is restrained in an appropriate position for a certain period of time by FliKN temporarily tethered within the central channel of the complete hook as proposed before (Hirano et al., 2009). Although it may look redundant, the double-lock mechanism by RflH/Flk must be playing an important role in suppressing premature switching at an inappropriate stage of flagellar morphogenesis.
Association of FliKN with the hook cap is required for the efficient switching process
FliK consists of at least two domains, FliKN and FliKC (Minamino et al., 2004). FliKN is responsible not only for FliK secretion during hook assembly (Minamino et al., 1999b) but also for its molecular ruler function (Minamino et al., 2004; 2006; Moriya et al., 2006; Shibata et al., 2007). Residues 301 through 350 and the last five residues in FliKC are directly involved in the interaction with FlhBC to switch export specificity of the flagellar export apparatus (Minamino et al., 2006). Interestingly, FliKΔ99, which is not secreted into the culture medium, can flip the specificity switch with considerable efficiency, but only when it is overproduced (Hirano et al., 2005; Fig. 4A). Even then, it still produces hooks longer than the wild-type length with filaments attached, indicating that the timing of export specificity switch is significantly delayed (Hirano et al., 2005; Fig. 4A). In agreement with this, higher amounts of FlgD and FlgE were detected in the culture supernatant compared with the wild-type levels (Fig. 4A, right panel). Overproduction of FliKΔ99 is required probably because its access to FlhBC is limited when uncoupled to its secretion. In contrast, the cellular level of wild-type FliK is 40–80 molecules per cell, which corresponds to 5–10 molecules per flagellum (Muramoto et al., 1998). Since such a low level of FliK production is sufficient for the proper switch of export specificity, FliKN must be playing an important role in the efficient recruitment of FliKC to an appropriate place near FlhBC to facilitate the FliKC–FlhBC interaction, presumably in part by directing FliK to the flagellar secretion apparatus for export.
FliKN binds strongly to FlgD and relatively weakly to FlgE in vitro, suggesting physical communications of FliKN with the growing hook structure for facilitating the FliKC–FlhBC interaction and controlling hook length (Moriya et al., 2006). We found in this study that, even in the absence of the hook, overproduction of FliK in the absence of RflH/FlK allowed filament-type substrates such as FlgL, FliC and FliD to be secreted with considerable efficiency (Fig. 3). The cellular and secretion levels of FliK, however, were c. 1000-fold higher than those of the flgK mutant, which forms hooks of normal length and hence switches export specificity properly. These results strongly suggest that the hook structure is required for the efficient recruitment of FliKC to an appropriate position and orientation for its interaction with FlhBC to switch export specificity. This is also strongly supported by our finding that loss of either flgD or flgE lowered the switching efficiency in the ΔflgH-flgI rflH/flk mutant (Table 1).
Multicopy effects of FliK were more prominent in the presence of the hook cap than in its absence (Fig. 4). Even in the absence of the hook, the switching efficiency as monitored by the level of FliC secretion was twofold higher by FliK than by FliKΔ99 in the presence of the hook cap, but no significant difference was observed between the two when the hook cap was missing (Fig. 4). Since the hook cap is composed of FlgD (Ohnishi et al., 1994), to which FliKN binds strongly, these observations suggest that a temporary binding of FliKN to the hook cap at the tip of the growing hook is important for the efficient switching process.
Interaction of FliKN with the hook protein FlgE is required for the proper switch of export specificity
It has been shown that most fliK deletions cause loss of function (Shibata et al., 2007), suggesting that FliKN has an additional role for its ruler function. Here, we characterized an in-frame deletion of residues 129–159 in FliKN [FliKΔ(129–159)]. This deletion caused extended hooks with filaments attached (Figs 5 and 6). Thus, FliKΔ(129–159) allows the rod/hook-type export mode to continue for a longer period of time than wild-type cells (Figs 5 and 6), suggesting that this deletion variant is somehow deficient in catalysing the export specificity switch. Overproduction of FliKΔ(129–159) improved motility to wild-type levels (Fig. 5C). The improved motility resulted from shortening of the hook length nearly to the wild-type length distribution (Fig. 6B). Since an excess level of secretion of FliKΔ(129–159) is necessary for the wild-type function, association of FliK with the growing hook is probably the limiting step. Affinity blotting and pull-down assays revealed that the deletion significantly reduced the binding affinity of FliKN for FlgE but not for FlgD (Fig. 7). Taken all together, we suggest that the interaction of FliK with FlgE contributes significantly to the stable attachment of FliKN with the inner surface of the growing hook structure to allow FliKC to trigger the switch of export specificity properly. Identification of the FlgE-binding regions of FliKN as well as analysis of the functional role of FliKN in binding to FlgD is in progress.
Possible molecular ruler model
Shibata et al. (2007) have proposed that FliK regulates hook length as an internal ruler because secreted FliK was not detected in culture supernatant from any of the three different fliK in-frame deletion mutants that produced shorter hooks of relatively well-controlled length. They also showed that all the other deletions in the presumed ruler region not interfering with FliK secretion resulted in polyhooks of uncontrolled length, instead of shorter hooks as predicted if FliKN functions as a ruler to actually measure hook length. However, most deletions in FliKN result in polyhooks (Shibata et al., 2007) possibly because those deletions reduce the affinity of FliK to FlgE just as we observed for FliKΔ(129–159) and hence impair the ruler function of FliK. Also, the number of FliK molecules required to achieve the ruler function for export specificity switching is very small, and detection of such extremely small amounts of FliK secreted into culture supernatant could be tricky. It should be noted that the three FliK deletion variants that produced shorter hooks and were not detected in culture supernatant were not detected in the cytoplasm either (Shibata et al., 2007). Therefore, the conclusion by Shibata et al. (2007) that FliK is the internal ruler and that its secretion is not required for export specificity switch is not conclusive. In contrast, we found that the interaction of FliKN with the hook and the hook cap is important for the efficient switching process, suggesting that FliK measures hook length inside the hook during the process of FliK secretion.
Based on available information, we propose a model that explains how export specificity switch by FliK and FlhB occurs at the appropriate timing during hook assembly (Fig. 8). During hook assembly, interactions of FliKN with the hook and the hook cap temporarily tether FliKN inside the hook for a certain period of time to allow FliKC to interact with FlhBC and catalyse a conformational change in FlhBC that results in export specificity switch. The FliKC–FlhBC interaction does not occur when hook length is shorter than its mature length of 55 nm. RflH/Flk presumably prevents FliKC from interacting with FlhBC unless the position and the orientation of FliKC are appropriately restrained for a certain period of time by FliKN temporarily tethered inside the hook of mature length. The FliKC–FlhBC interaction for switch does not occur until hook length becomes sufficient for the FliKC domain to pass the RflH/Flk block. Only at the hook length of 55 nm, the interaction between the N-terminus of FliK and the hook cap as well as that between multiple regions of FliKN and parts of FlgE exposed in the inner surface of the hook allows a stretched conformation of FliKN to remain in the secretion channel for a sufficient period of time to facilitate the specific binding between FliKC and FlhBC, which in turn triggers export specificity switch to occur.
Fig. 8.

Model for hook length control and export specificity switch. Exported hook protein molecules self-assemble at the tip of the growing hook with the help of the hook cap. FliK is occasionally exported during hook assembly, which involves sequential folding and binding of FlgE subunits at the tip underneath the hook cap. RflH (Flk) presumably prevents premature interaction of FliKC with FlhBC during rod/hook assembly and hence the switch does not occur. When the hook length is around 55 nm, an unfolded and stretched conformation of FliKN within the central channel temporarily anchors its N-terminus to the hook cap and multiple regions to the inner surface of the hook so that the stretched conformation of FliKN and part of FliKC can measure the length of the rod and the hook together (90 nm), letting FliKC pass the RflH/Flk block to be positioned and oriented near FlhBC to allow for the FliKC–FlhBC binding to occur. OM, outer membrane; PG, peptidoglycan layer; CM, cytoplasmic membrane.
Experimental procedures
Bacterial strains, plasmids and media
Bacterial strains and plasmids used in this study are listed in Table 2. L-broth (LB) and soft tryptone agar plates were prepared as described (Minamino and Macnab, 1999). Ampicillin was added at a final concentration of 100 μg ml−1.
Preparation of whole cell and culture supernatant fractions and immunoblotting
Whole cells and culture supernatant fractions were prepared as described before (Minamino and Macnab, 1999). Immunoblotting with polyclonal anti-FlgD, anti-FlgE, anti-FlgK, anti-FlgL, anti-FliC, anti-FliD and anti-FliK antibodies was carried out as described (Minamino and Macnab, 1999).
Preparation of HBB structures
Preparation of the HBB particles and measurement of hook length was performed as described (Moriya et al., 2006).
Affinity blotting
The N-terminally His-tagged FlgD and FlgE proteins were purified from the soluble fractions of BL21 (DE3) pLysS cells transformed with the appropriate plasmids and purified by affinity chromatography with a nickel-nitrilacetic acid (Ni-NTA) agarose column as described (Minamino and Macnab, 2000a). Affinity blotting was carried out as described before (Minamino and Macnab, 2000a).
Pull-down assays by Ni-NTA affinity chromatography
For co-purification of His-FliK and His-FliKΔ(129–159) with FlgD or FlgE, the soluble fractions prepared from BL21(DE3) pLysS expressing His-FliK or His-FliKΔ(129–159) were mixed with those from the BL21(DE3) pLysS cells transformed with pRCD001(FlgD) or pNM001 (FlgE) and then the mixtures were loaded onto a Ni-NTA column. After washing with binding buffer (20 mM Tris-HCl, 500 mM NaCl) containing 25 mM imidasol, bound proteins were eluted with binding buffer containing imidazole by a stepwise increase in the imidazole concentration of 50, 100, 250 and 500 mM.
Acknowledgments
We acknowledge M. Kihara and A. Blocker for critical reading of the manuscript and helpful comments. N.M. was a research fellow of the Japan Society for the Promotion of Science. This work has been supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan to T.M., N.M. and K.N.
References
- Agrain C, Callebaut I, Journet L, Sorg I, Paroz C, Mota LJ, Cornelis GR. Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol Microbiol. 2005;56:54–67. doi: 10.1111/j.1365-2958.2005.04534.x. [DOI] [PubMed] [Google Scholar]
- Aldridge P, Karlinsey JE, Becker E, Chevance FF, Hughes KT. Flk prevents premature secretion of the anti sigma factor FlgM into the preiplasm. Mol Microbiol. 2006;60:630–642. doi: 10.1111/j.1365-2958.2006.05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Ferris HU, Minamino T. Flipping the switch: bringing order to flagellar assembly. Trends Microbiol. 2006;14:519–526. doi: 10.1016/j.tim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Ferris HU, Furukawa Y, Minamino T, Kroetz MB, Kihara M, Namba K, Macnab RM. FlhB regulates ordered export of flagellar components via auto-cleavage mechanism. J Biol Chem. 2005;280:41236–41242. doi: 10.1074/jbc.M509438200. [DOI] [PubMed] [Google Scholar]
- Fraser GM, Hirano T, Ferris HU, Devgan LL, Kihara M, Macnab RM. Substrate specificity of type III flagellar protein export in Salmonella is controlled by subdomain interactions in FlhB. Mol Microbiol. 2003;48:1043–1057. doi: 10.1046/j.1365-2958.2003.03487.x. [DOI] [PubMed] [Google Scholar]
- Hirano T, Yamaguchi S, Oosawa K, Aizawa SI. Roles of FliK and FlhB in determination of flagellar hook length in Salmonella typhimurium. J Bacteriol. 1994;176:5439–5449. doi: 10.1128/jb.176.17.5439-5449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Minamino T, Namba K, Macnab RM. Substrate specificity class and the recognition signal for Salmonella type III flagellar export. J Bacteriol. 2003;185:2485–2492. doi: 10.1128/JB.185.8.2485-2492.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Shibata S, Ohnishi K, Tani T, Aizawa SI. N-terminal signal region of FliK is dispensable for length control of the flagellar hook. Mol Microbiol. 2005;56:346–360. doi: 10.1111/j.1365-2958.2005.04615.x. [DOI] [PubMed] [Google Scholar]
- Hirano T, Mizuno S, Aizawa SI, Hughes KT. Mutations in Flk, FlgG, FlhA, and FlhE that affect the flagellar type III secretion specificity switch in Salmonella enterica. J Bacteriol. 2009;181:3938–3949. doi: 10.1128/JB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M, Fujita H, Yamaguchi S, Iino T. Excretion of unassembled flagellin by Salmonella typhimurium mutants deficient in hook-associated proteins. J Bacteriol. 1984;159:1056–1059. doi: 10.1128/jb.159.3.1056-1059.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KT, Gillen KL, Semon MJ, Karlinsey JE. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- Journet L, Agrain C, Broz P, Cornelis GR. The needle length of bacterial injectisomes is determined by a molecular ruler. Science. 2003;302:1757–1760. doi: 10.1126/science.1091422. [DOI] [PubMed] [Google Scholar]
- Karlinsey JE, Pease AJ, Winkler ME, Bailey JL, Hughes KT. The flk gene of Salmonella typhimurium couples flagellar P- and L-ring assembly to flagellar morphogenesis. J Bacteriol. 1997;179:2389–2400. doi: 10.1128/jb.179.7.2389-2400.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake K. Excretion of the anti-sigma factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet. 1994;243:605–612. doi: 10.1007/BF00279569. [DOI] [PubMed] [Google Scholar]
- Kutsukake K. Hook-length control of the export-switching machinery involves a double-locked gate in Salmonella typhimurium flagellar morphogenesis. J Bacteriol. 1997;179:1268–1273. doi: 10.1128/jb.179.4.1268-1273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake K, Minamino T, Yokoseki T. Isolation and characterization of FliK-independent flagellation mutants from Salmonella typhimurium. J Bacteriol. 1994;176:7625–7629. doi: 10.1128/jb.176.24.7625-7629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM. How bacteria assemble flagella. Annu Rev Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- Maloy SR. Experimental Techniques in Bacterial Genetics. Boston, MA: Jones and Bartlett; 1990. [Google Scholar]
- Minamino T, Macnab RM. Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol. 1999;181:1388–1394. doi: 10.1128/jb.181.5.1388-1394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Macnab RM. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol Microbiol. 2000a;35:1052–1064. doi: 10.1046/j.1365-2958.2000.01771.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Macnab RM. Domain structure of Salmonella FlhB, a flagellar export component responsible for substrate specificity switching. J Bacteriol. 2000b;182:4906–4919. doi: 10.1128/jb.182.17.4906-4914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Namba K. Self-assembly and type III protein export of the bacterial flagellum. J Mol Microbiol Biotechnol. 2004;7:5–17. doi: 10.1159/000077865. [DOI] [PubMed] [Google Scholar]
- Minamino T, Pugsley AP. Measure for measure in the control of type III secretion hook and needle length. Mol Microbiol. 2005;56:303–308. doi: 10.1111/j.1365-2958.2005.04611.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Iino T, Kutsukake K. Molecular characterization of the Salmonella typhimurium flhB operon and its protein products. J Bacteriol. 1994;176:7630–7637. doi: 10.1128/jb.176.24.7630-7637.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Doi H, Kutsukake K. Substrate specificity switching of the flagellum-specific export apparatus during flagellar morphogenesis in Salmonella typhimurium. Biosci Biotechnol Biochem. 1999a;63:1301–1303. doi: 10.1271/bbb.63.1301. [DOI] [PubMed] [Google Scholar]
- Minamino T, González-Pedrajo B, Yamaguchi K, Aizawa SI, Macnab RM. FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol Microbiol. 1999b;34:295–304. doi: 10.1046/j.1365-2958.1999.01597.x. [DOI] [PubMed] [Google Scholar]
- Minamino T, Saijo-Hamano Y, Furukawa Y, González-Pedrajo B, Macnab RM, Namba K. Domain organization and function of Salmonella FliK, a flagellar hook-length control protein. J Mol Biol. 2004;341:491–502. doi: 10.1016/j.jmb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Minamino T, Ferris HU, Morioya N, Kihara M, Namba K. Two parts of the T3S4 domain of the hook-length control protein FliK are essential for the substrate specificity switching of the flagellar type III export apparatus. J Mol Biol. 2006;362:1148–1158. doi: 10.1016/j.jmb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Minamino T, Imada K, Namba K. Mechanisms of type III protein export for bacterial flagellar assembly. Mol Biosyst. 2008;4:1105–1115. doi: 10.1039/b808065h. [DOI] [PubMed] [Google Scholar]
- Moriya N, Minamino T, Hughes KT, Macnab RM, Namba K. The type III flagellar export specificity switch is dependent on FliK ruler and a molecular clock. J Mol Biol. 2006;359:466–477. doi: 10.1016/j.jmb.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Muramoto K, Makishima S, Aizawa SI, Macnab RM. Effect of cellular level of FliK on flagellar hook and filament assembly in Salmonella typhimurium. J Mol Biol. 1998;277:871–882. doi: 10.1006/jmbi.1998.1659. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Kutsukake K, Suzuki H, Iino T. A novel transcriptional regulation in the flagellar regulon of Salmonella typhimurium: an anti-sigma factor inhibits the activity of the flagellum-specific sigma factor, sigma F. J Bacteriol. 1992;176:2272–2281. doi: 10.1111/j.1365-2958.1992.tb01771.x. [DOI] [PubMed] [Google Scholar]
- Ohnishi K, Ohto Y, Aizawa SI, Macnab RM, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen MJ, Penn CW, Chaudhuri RR. Bacterial flagellar diversity in the post-genomic era. Trends Microbiol. 2005;14:143–149. doi: 10.1016/j.tim.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Patterson-Delafield J, Martinez RJ, Stocker BAD, Yamaguchi S. A new fla gene in Salmonella typhimurium – flaR – and its mutant phenotype-superhooks. Arch Microbiol. 1973;90:107–120. doi: 10.1007/BF00414513. [DOI] [PubMed] [Google Scholar]
- Shibata S, Takahashi N, Chevance FFV, Karlinsey JE, Hughes KT, Aizawa SI. FliK regulates flagellar hook length as an internal ruler. Mol Microbiol. 2007;64:1404–1415. doi: 10.1111/j.1365-2958.2007.05750.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Iino T. Role of the flaR gene in flagellar hook formation in Salmonella spp. J Bacteriol. 1981;148:973–979. doi: 10.1128/jb.148.3.973-979.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Sorg I, Degiacomi M, Journet L, Dal Peraro M, Cornelis GR. The helical content of the YscP molecular ruler determines the length of the Yersinia injectisome. Mol Microbiol. 2009;71:692–701. doi: 10.1111/j.1365-2958.2008.06556.x. [DOI] [PubMed] [Google Scholar]
- Williams AW, Yamaguchi S, Togashi F, Aizawa SI, Kawagishi I, Macnab RM. Mutations in fliK and flhB affecting flagellar hook and filament assembly in Salmonella typhimurium. J Bacteriol. 1996;178:2960–2970. doi: 10.1128/jb.178.10.2960-2970.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Fujita H, Sugata K, Taira T, Iino T. Genetic analysis of H2, the structural gene for phase-2 flagellin in Salmonella. J Gen Microbiol. 1984;130:255–265. doi: 10.1099/00221287-130-2-255. [DOI] [PubMed] [Google Scholar]
- Zarivach R, Deng W, Vuckovic M, Felise HB, Nguyen HV, Miller SI, et al. Structural analysis of the essential self-cleaving type III secretion proteins EscU and SpaS. Nature. 2008;453:124–127. doi: 10.1038/nature06832. [DOI] [PubMed] [Google Scholar]


