Abstract
Children from lower-SES families exhibit smaller hippocampal volume than do their higher-SES peers. Few studies, however, have compared hippocampal developmental trajectories as a function of SES. Thus, it is unclear whether initial rank-order stability is preserved, or whether volumes diverge/converge over the course of adolescence. In a sample of 101 girls ages 10–24 years, we examined the longitudinal association between family income and parental education, proxies for SES, and changes in hippocampal volume. Hippocampal volume was obtained using MRI; using mixed modeling, we examined the effects of income and education on hippocampal volume across age. As expected, changes in volume were non-linear across development. Further, trajectories diverged in mid-adolescence, with lower-income girls exhibiting reductions in hippocampal volume. Maximal income-related differences were observed at 18 years, and trajectories converged thereafter. This interaction remained significant when accounting for maternal hippocampal volume, suggesting a unique contribution of environment over potential heritable differences. In contrast, the association between parental education and offspring hippocampal volume appeared to be stable across adolescence, with higher levels of parental education predicting consistently larger hippocampal volume. These findings constitute preliminary evidence that girls from lower-income homes exhibit unique trajectories of hippocampal growth, with differences most evident in late adolescence.
Keywords: Adolescence, Adversity, Environmental influences, Family factors, Socioeconomic status
1. Introduction
Growing up in a home with fewer economic and educational resources has been shown to be a risk factor for a range of negative life outcomes. Compared with children raised in higher socioeconomic status (SES) households, children from lower SES backgrounds are more likely to perform poorly in school, exhibit behavioral problems, and develop psychopathology (Duncan et al., 1994; Hackman et al., 2010; McLoyd, 1998). Researchers have proposed several mechanisms through which this risk may be conferred, including a lack of cognitively stimulating experiences (Johnson et al., 2016; Weisleder and Fernald, 2013), increased stress in the home (Evans et al., 2005; Evans and English, 2002), unequal access to educational and health resources (Coleman, 1968; Graham, 2008), and unfair treatment within these systems (Alexander and Entwisle, 1987; Marks et al., 2006; McLoyd, 1998).
While there is a long history of psychosocial and epidemiological research examining the causes and consequences of disparities in SES, there has been a recent impetus to examine the neural mechanisms through which risk factors may exert their adverse effects. Understanding the neural regions implicated in risk − and particularly the ages at which these effects have the greatest adverse impact on neural regions − may facilitate the development of interventions that utilize sensitive periods in children’s development (Gabrieli and Bunge, 2016; Lawson et al., 2017). Indeed, it is clear that different neural systems undergo significant transformation during distinct periods of development, which may lead people to be particularly sensitive to relevant forms of environmental input at different times. For example, in the context of SES, it is noteworthy that critical connections between regions supporting executive function are developing rapidly during adolescence (Murty et al., 2016; Ordaz et al., 2013).
In this context, researchers have recently demonstrated that the hippocampus, a brain region that is particularly sensitive to stressful effects of the environment (Frodl and O’Keane, 2013; Lupien et al., 2009), is smaller in children and adolescents from lower- than from higher-SES homes (Hanson et al., 2015, Hanson et al., 2011; Luby et al., 2013; Noble et al., 2012). Rao and colleagues found that in a sample of lower-SES children, parental nurturance at age 4, but not at later ages, was significantly associated with smaller hippocampal volume in early adolescence (Rao et al., 2010), suggesting a direct association between the early environment and hippocampal volume, although the effects were in the opposite direction than would be expected from other research. Further supporting an association between the environment and hippocampal development, Hanson and colleagues found that early life stress was significantly linked to smaller hippocampal volume, and that hippocampal volume partially mediated the relation between stress and behavioral problems (Hair et al., 2015). Recently, Noble and colleagues (2015) examined this association in a large, cross-sectional sample of children ages 3–20. They found a significant association between parental education and left hippocampal volume. Moreover, their analyses revealed that effects of education on the hippocampus was most pronounced for those children whose parents had the least formal education. Interestingly, there was no significant association between income and hippocampal volume in their sample, despite significant support for the link between these variables from other work (for a review, see Farah, 2017). Notably, consistent with prior studies (Mills and Tamnes, 2014), Noble and colleagues found that a quadratic model was the best fit for modeling hippocampal volume across this age range (Noble et al., 2015).
Importantly, the majority of research linking SES to hippocampal volume has thus far been cross-sectional, comparing hippocampal volume of low- and high-SES children at a single time point. This approach has critical limitations, including the difficulty of separating age-related differences from cohort effects or age-related measurement errors (Church et al., 2010). Longitudinal analyses can model between- and within-subject variation separately to describe growth processes more accurately. Indeed, a longitudinal approach is particularly useful in examining the relation between SES and hippocampal volume, given the documented inverted-U trajectory of hippocampal development (Gogtay et al., 2006; Mills and Tamnes, 2014). Not only does this protracted and nonlinear growth make it difficult to interpret the meaning of volumetric differences at a single time point during childhood, but the teenage years may be particularly significant, given that important connections between the hippocampus and prefrontal cortex that support cognitive control are developing rapidly during this time (Murty et al., 2016).
Despite evidence of the protracted development of the hippocampus, most studies have focused on the relation between SES and hippocampal volume in childhood. This limits our understanding of this association, given the likelihood that SES exerts different levels of influence on the hippocampus throughout development. For example, maternal sensitivity influences trajectories of hippocampal growth when children are preschool-aged, but not older, suggesting a sensitive period for maternal sensitivity (Luby et al., 2016). Similarly, socioeconomic factors may be differentially salient during specific developmental periods. For example, when children shift in the relative importance of their peer group compared to their family at 12–13 years of age (Claes, 1992), SES-related differences in cortisol levels have been found to disappear (Dowd et al., 2009; Lupien et al., 2001). Moreover, higher-SES children experience more stress during school transitions than do lower-SES children (Lupien et al., 2001). In fact, a recent study showed no association between childhood SES and hippocampal volume in adulthood (Lawson et al., 2017). Thus, lower-SES children might exhibit hippocampal recovery over late adolescence and early adulthood. On the other hand, however, there is evidence that childhood poverty influences hippocampal function and associated memory-related functioning in adulthood (Duval et al., 2017). While some longitudinal research has documented SES-related differences in trajectories of brain growth in infants (Hanson et al., 2013), we know little about SES-related differences in hippocampal growth through adolescence. In fact, it may be that the null results reported by Noble and colleagues (2015) is due to varying effects of income on hippocampal volume over this large age range. Elucidating whether these differences vary as a function of children’s age or remain stable over development has critical implications for the generation of timely and sensitive interventions to improve child outcomes.
Importantly, more recent studies have examined the effects of SES on hippocampal development longitudinally, with mixed results. Hair and colleagues (2015) found that children living below the federal poverty level had hippocampal gray matter that was on average 6–8% below developmental norms across the ages of 4–22. Moreover, these differences partially mediated income-related differences in scores on academic tests, suggesting that hippocampal volume is associated with academic outcomes. These authors calculated developmental norms for hippocampal gray matter by modeling its developmental trajectory in their sample, strategically accounting for its nonlinear development over adolescence. The results that they presented in the paper, however, were based on an average of comparisons across ages, and did not specifically examine whether the effects of income on hippocampal development varied systematically across these age ranges. In another study, Whittle et al. (2017) explicitly tested possible interactions of age and SES in a longitudinal sample of adolescents. Contrary to expectations, there was no main effect of SES or interaction of age and SES on hippocampal volume. However, the authors’ models only tested for linear effects, despite evidence of the nonlinear development of the hippocampus; a quadratic model may have yielded different results. Therefore, it remains an open question whether the association between SES and hippocampal volume varies as a function of age over adolescence.
Finally, SES-related differences in children may represent, in part, heritable characteristics acquired from their parents. Indeed, twin studies show that 40% of variance in hippocampal volume is due to genetic influences (Sullivan et al., 2001). Further, hippocampal volumes of mothers and daughters are strongly correlated, significantly more so than for father–daughter, mother–son, or father–son pairings (Yamagata et al., 2016), suggesting matrilineal patterns of transmission for this region. It is important, therefore, to control for the influence of matrilineal transmission in characterizing SES-related differences in hippocampal volume.
We address these issues by examining longitudinally the effects of family income and parental education, two distinct indicators of SES (Braveman et al., 2013), on trajectories of hippocampal volume in 10- to 24-year-old females. We examined these variables separately because evidence suggests that they are differentially associated with early experiences and subsequent outcomes (Duncan and Magnuson, 2012). Many participants provided multiple time points of data, allowing us to investigate changes in hippocampal volume over development. Our first aim was to examine whether trajectories of hippocampal development vary as a function of family income and parental education. We tested two potential hypotheses. One possibility is that trajectories of hippocampal volume would diverge as a function of family income and parental education across adolescence, with children from less wealthy or educated households exhibiting a steady reduction in hippocampal volume compared to their higher-SES peers. This finding would be consistent with research demonstrating the compounding effects of factors associated with low SES (Hart and Risley, 1995), and with animal models showing a lack of synapse production following early exposure to stress (Andersen and Teicher, 2004). Another possibility is that trajectories would converge over adolescence, mirroring findings of SES-related differences in cortisol in childhood but not in adulthood (Dowd et al., 2009; Lupien et al., 2001).
In addition, because a subset of the girls’ biological mothers were also scanned, we were uniquely positioned to assess the matrilineal familial transmission of hippocampal volume. Therefore, our second aim was to examine the association between two metrics of SES (i.e., family income and parental education) and offspring hippocampal volume, controlling for maternal hippocampal volume. We conceptualize this relation as a proxy for both inherited traits and the shared environment of mothers and daughters. We hypothesized that family income and parental education would be associated with hippocampal trajectories during adolescence, above and beyond the variance accounted for by maternal hippocampal volume.
Finally, because the sample from which our study was drawn was a longitudinal study of familial risk for major depressive disorder (MDD) (i.e., daughters of mothers with recurrent or no MDD history) and followed these girls prospectively to assess onset of MDD, we performed exploratory analyses to examine whether socioeconomic-related effects on hippocampus were independent of, or moderated by, risk status and longitudinal onset of MDD. Further, we tested the specificity of our results to the hippocampus by examining the association of family income and parental education on trajectories of another stress sensitive subcortical brain region (i.e., the amygdala).
2. Methods
2.1. Participants
Participants were recruited as part of a larger, longitudinal study at Stanford University designed to examine the intergenerational transmission of depression. Mother–daughter pairs were recruited through local community outreach, and all interested participants completed a telephone screening interview to establish initial eligibility criteria. Pairs were recruited based on a maternal history of either recurrent Major Depressive Disorder (MDD) or no past MDD. Potential dyads were excluded if daughters met criteria for any past or current Axis I disorder, had experienced severe head trauma, had been diagnosed with a learning disability, or were taking medications that would affect cerebral blood flow (Gotlib et al., 2008). Adolescent girls were assessed using the Kiddie Schedule for Affective Disorders (Kaufman et al., 1997) and participants were rescreened annually for the development of MDD (for more details see LeMoult et al., 2015). This larger study restricted recruitment to girls to reduce heterogeneity of the sample, given sex differences in MDD risk (Gotlib et al., 2014; LeMoult et al., 2015). This study was approved by the Institutional Review Board at Stanford University, and all participants gave informed consent if they were over age 18 years or assent if they were under 18 years.
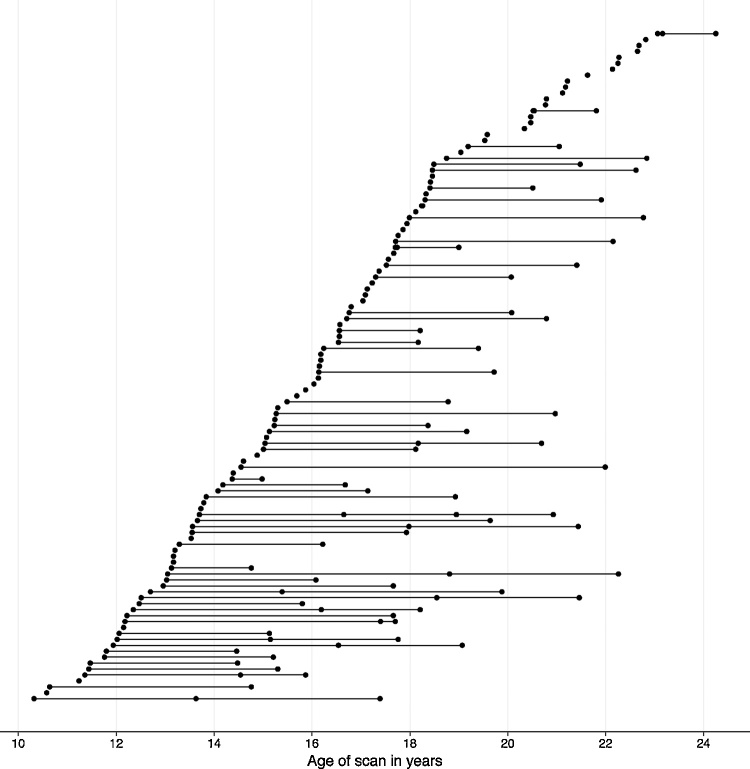
Daughters from this sample were included in the current study if they completed at least one scan with high-quality structural MRI data and had complete information on family income and parental education. 116 girls had 1–4 structural MRI scans, yielding a total of 194 scans. 16 scans from 15 girls were unusable due to poor scan quality (N = 14) or failed automated bilateral hippocampus segmentation (N = 2); thus, the final analyses focused on 101 girls (N = 178 scans) for whom estimates of unilateral hippocampal volume were usable for at least one time point. More specifically, girls completed baseline assessments between the ages of 9.12 and 15.44 years (M = 12.44 years, SD = 1.55) (at which time mothers reported family income and parental education), and completed subsequent scans between the ages of 10.32 and 24.25 years (M = 16.27 years, SD = 3.30; see Table 1). Thus, we were able to examine developmental changes from early adolescence through early adulthood. Participants contributed an average of 1.76 time points (SD = 0.81; see Supplemental Table 1); average time between first and last scans was 4.61 years (SD = 2.15; range: 0.61-9.93), and average time between baseline assessments and first scan was 2.38 years (SD = 2.57, range: 0.02–9.18). The distribution of scan ages is displayed in Fig. 1. A subset of mothers (N = 44) also completed scans at the time of their daughters’ first scan. In addition, 69 girls in our sample completed follow-up assessments through age 18 years or until the onset of an MDD episode; thus, for this subsample we could report the presence of absence of adolescent-onset MDD.
Table 1.
Demographic characteristics of final sample.
| Mean (SD) | Minimum | Maximum | |
|---|---|---|---|
| Daughter age at baseline | 9.12 (1.55) | 9.12 | 15.44 |
| Daughter age of first scan | 14.82 (3.09) | 10.32 | 22.82 |
| Mother age at scan | 45.04 (5.43) | 27.96 | 55.19 |
| Time between first and last scans (years) | 4.61 (2.15) | 0.61 | 9.93 |
| Household income (in thousands) | 83.36 (24.66) | 17.50 | 100 |
| $10,000–25,000 | 4% | ||
| $25,000–50,000 | 11% | ||
| $50,000–75,000 | 11% | ||
| $75,000–100,000 | 20% | ||
| Greater than $100,000 | 55% | ||
| Maternal, paternal education (years) | 15.83 (2.16), 15.50 (2.51) | 12, 11 | 20, 20 |
| Less than high school/GED | 0%, 5% | ||
| High school diploma/GED | 7%, 11% | ||
| Some college | 15%, 17% | ||
| 2-year college degree | 7%, 1% | ||
| 4-year college degree | 44%, 36% | ||
| Master’s degree | 21%, 24% | ||
| Doctorate | 7%, 6% | ||
| Mother race | |||
| Caucasian | 78% | ||
| African-American | 4% | ||
| Latino | 7% | ||
| Asian-American | 8% | ||
| Other/Mixed | 3% | ||
| Daughter race | |||
| Caucasian | 64% | ||
| African-American | 3% | ||
| Latino | 2% | ||
| Asian-American | 3% | ||
| Other/Mixed | 28% | ||
| Mothers with a history of MDD | 50% | ||
| Daughters experiencing an episode of MDD | 32%* | ||
*This percentage is of girls who were followed through age 18 or until the onset of an episode of MDD (N = 69).
Fig. 1.
Distribution of participant ages for scans with usable data. Each scan is represented by a circle; scans from the same participant are connected with a line.
2.2. Baseline assessments
At the first laboratory visit, mothers reported family income on a scale from 0 (less than $10,000) to 5 (greater than $100,000), as shown in Table 1. Consistent with prior published reports (Hair et al., 2015; Hanson et al., 2011), income midpoints for each income category were calculated and used in subsequent analyses. Mothers also reported the highest level of education achieved for both parents on a scale from 0 (no GED/no high school diploma) to 6 (Doctorate); average years of parental education were estimated based on the mean of these selections. We examined family income and parental education as two separate components of SES (Braveman et al., 2013; Noble et al., 2012), based on evidence that these variables exert distinct influences on the environment and outcomes (Duncan and Magnuson, 2012). Race of mother and father were indicated as Caucasian, African-American, Latina/o, Asian-American, Native American, or Other/Multiracial. If the same race was reported for the child’s mother and father, that same assignment was made for the daughter; if different races were reported, the daughter was assigned “Other/Multiracial.”
2.3. MRI data acquisition
At the beginning of the study, neuroimaging data were acquired on a 1.5T GE Signa Excite MR scanner (scanner 1). Six years later, the 1.5T MRI system was decommissioned and we used a 3T GE MR750 Discovery MRI system (scanner 2). Baseline and follow-up scans on scanner 2 were collected with different head coils. Scan parameters and scans collected on each scanner and head coil are presented in the Supplemental Material. In all but three cases, mothers were scanned on the same scanner as their daughters’ first scan. An analysis of variance (ANOVA) yielded no significant differences in family income as a function of scanner (dummy coded scanner 1, scanner 2, or both; income: F(2,98) = 0.13, p = 0.880), though there was a marginal difference in average years of parental education (F(2,97) = 3.03, p = 0.053). Post hoc Fisher’s least significant differences (LSD) tests revealed that the 32 girls who were scanned with scanner 2 only had more highly educated parents than those who were scanned only at scanner 1 (p = 0.021) and marginally more than those who were scanned at both facilities (p = 0.067).
2.4. Hippocampus segmentation
Automated segmentation of subcortical volumes from the T1-weighted images was obtained using the Freesurfer software suite (v5.3; http://surfer.nmr.mgh.harvard.edu/) (Fischl et al., 2002). This approach has been shown to be robust to anatomic variability and to have accuracy comparable to manual labeling techniques (Fischl et al., 2002; Fischl and Dale, 2000) and acceptable scan-rescan reliability (Jovicich et al., 2009). We used the cross-sectional Freesurfer stream, as this more general image processing procedure ensured that analyses were not influenced by changing whole brain volume and ICV across development (Mills et al., 2016; Reuter, 2016). All hippocampi and amygdalae segmentations were visually inspected for major errors and poorly segmented bilateral hippocampi and amygdalae were excluded.
2.5. Data analysis
To examine the effect of family income and parental education on trajectories of hippocampal volume, we used linear mixed modeling (also referred to as hierarchical linear modeling or multilevel modeling). This approach accounts for the non-independence of repeated measures within individuals and handles data from participants with differing numbers of time points and intervals between time points. We used restricted maximum likelihood estimation in Mixed Models in SPSS (version 23, IBM Corporation), specifying an autoregressive heterogeneous covariance matrix with time point as a repeated measure. Degrees of freedom were calculated using the Satterthwaite method (Satterthwaite, 1946) which can be fractional.
Previous research has shown that trajectories of hippocampal development are best described with a quadratic model (Dima et al., 2015; Gogtay et al., 2006); therefore, we entered both linear and quadratic measures of age in years (i.e., age and age-squared) as time-varying covariates, specifying a model with random slope and intercept terms to allow for differences in intercepts and slopes for each individual and examining left and right hippocampi separately. We used total intracranial volume (ICV) and scanner (dummy coded scanner 1, scanner 2 8-channel head coil, scanner 2 32-channel head coil) as covariates and tested the effects of family income and parental education on hippocampal volume by adding income/education and interaction terms for age and income/education. Age, income, and education values were mean-centered in all analyses.
To examine the relation of mother and daughter hippocampal volume, we used hierarchical linear regression to predict daughter hippocampal volume from mother hippocampal volume, using daughter hippocampal volume at her first visit – the time closest to when mothers were scanned. We entered daughter age and ICV in step 1, and mother hippocampal volume, age, and ICV in step 2. Then, to examine the effects of family income and parental education on daughter hippocampal trajectories controlling for mother hippocampal size, we created residuals of mother hippocampal volume, regressing out age and ICV. We repeated analyses using the mixed models described above, with mother hippocampal volume residuals (controlling for maternal age and ICV) and interaction terms for child age and mother hippocampal residuals.
As exploratory analyses, we examined whether hippocampal trajectories and their associations with family income and parental education varied as a function of familial risk or depression onset. We first examined whether mothers with past MDD and daughters who developed MDD differed from the rest of the sample in terms of family income and parental education. When tests for equal variances revealed significant differences between groups, we present statistics for equal variances not assumed. We then conducted models with income and education predicting bilateral hippocampal volume, including familial risk status and their interactions with age. Next, we tested for possible moderation by risk status on effects of both income and education. In addition, for the girls for whom data about MDD status were available, we examined whether hippocampal trajectories predicted the onset of MDD. For each time point of usable data, we characterized whether the girls had experienced an episode of MDD prior to the scan. We then repeated analyses for income and education with the addition of MDD onset and age interactions, and tested whether MDD onset moderated the association of income and education on hippocampal volume.
3. Results
Demographic characteristics of the participants are presented in Table 1. Seventy-two percent of mothers and 66% of fathers completed a Bachelor’s degree or higher, compared to local averages around 50% (U.S. Census Bureau). In addition, 55% of families in the sample earned above $100,000 per year. For comparison, the average household income in the area between 2011 and 2015 was $80,000–$100,000 (San Francisco County: $81,294, San Mateo County: $96,623, Santa Clara County: $96,310; U.S. Census Bureau). Thus, the majority of participants in our sample were highly educated and financially well-off.
3.1. Age-related changes in bilateral hippocampus by income
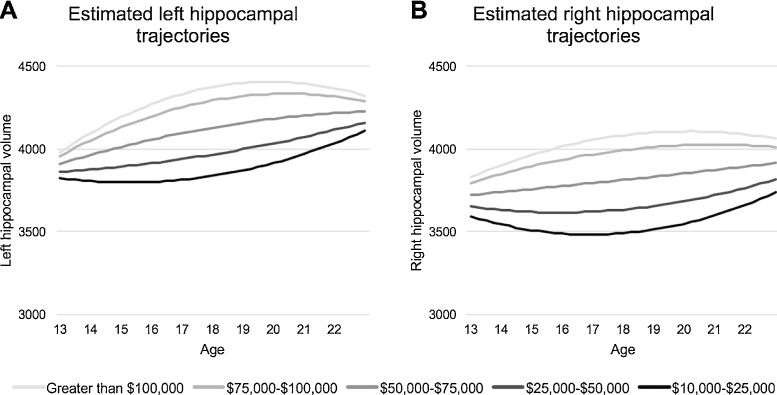
First, we tested the effect of family income as a predictor of bilateral hippocampal volume trajectories over development, controlling for total ICV and scanner (Table 2). In the left hippocampus, this analysis yielded a significant main effect of income (F(1,124.88) = 19.93, p < .001) and a significant interaction of income and the quadratic age term (F(1,103.08) = 7.93, p = 0.006). Similarly, the analysis of the right hippocampus yielded a significant main effect of income (F(1,135.08) = 23.56, p < .001) and a significant interaction of income and quadratic age (F(1,113.70) = 4.94, p = 0.028). As can be seen in Fig. 2, this model shows a curvilinear association of age on hippocampal volume. Lower income was related to a slight reduction followed by an increase over adolescence; based on plotting slope estimates from the model parameters, the largest effects of income were observed at ages 18.20 and 18.34 years in left and right hippocampus, respectively, and diminished into early adulthood. Given that longitudinal data were available for a subsample of participants, we repeated analyses on the 56 participants for whom we had multiple time points of usable data. These patterns persisted in both left (income: F(1,51.43) = 16.98, p < .001; income by age2: F(1,70.01) = 11.97, p = 0.001) and right hippocampi (income: F(1,59.77) = 13.02, p = 0.001; income by age2: F(1,71.65) = 3.44, p = 0.068).
Table 2.
Mixed model results for income left and right hippocampal volume over development.
| Left |
Right |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | 95% CI | t-statistic | p-value | Estimate | 95% CI | t-statistic | p-value |
| Intercept | 2424.39 | 1843.31, 3005.46 | 8.30 | <0.001 | 2194.87 | 1608.35, 2781.39 | 7.40 | <0.001 |
| Estimated ICV | 0.001 | 0.001, 0.002 | 5.64 | <0.001 | 0.001 | 0.001, 0.002 | 6.35 | <0.001 |
| Scanner (0) | −129.95 | −282.23, 22.34 | −1.69 | 0.094 | −423.76 | −589.23, −258.28 | −5.06 | <0.001 |
| Scanner (1) | 406.12 | 194.69, 617.54 | 3.81 | <0.001 | 345.38 | 123.95, 566.81 | 3.09 | 0.003 |
| Scanner (2) | 0 | 0 | – | – | 0 | 0 | – | – |
| Age in years | 43.37 | 20.38, 66.36 | 3.73 | <0.001 | 26.24 | 1.54, 50.93 | 2.10 | 0.037 |
| Age2 | −5.88 | −9.23, −2.53 | −3.51 | 0.001 | −2.79 | −6.49, 0.91 | −1.50 | 0.137 |
| Income | 6.32 | 3.52, 9.12 | 4.47 | <0.001 | 6.95 | 4.12, 9.78 | 4.85 | <0.001 |
| Age × Income | 0.37 | −0.05, 0.79 | 1.76 | 0.082 | 0.37 | −0.08, 0.83 | 1.61 | 0.109 |
| Age2 × Income | −0.17 | −0.28, −0.05 | −2.82 | 0.006 | −0.15 | −0.28, −0.02 | −2.22 | 0.028 |
ICV = intracranial volume; CI = confidence interval.
Fig. 2.
Estimated trajectories of left (A) and right (B) hippocampal volume for girls from varying household income levels at mean estimated intracranial volume (ICV). On average, the difference between the highest- and lowest-income groups is greatest at 18.20 years in the left hippocampus 18.34 years in the right hippocampus.
Because our sample was skewed toward the highest income bin, it is possible that the lack of specificity in this bin affected the accuracy of the model. To address this possibility, we broke the sample into low- or high-income groups based on a cut-off of $75 K. This value was chosen to best represent 200% of the California Poverty Level in surrounding counties from 2011 to 2013 (San Francisco: $73,252; San Mateo: $69,230; Santa Clara: $72,952; Public Policy Institute of California), and to fall below the average income during this time for all three counties (San Francisco: $81,294, San Mateo: $96,623, Santa Clara: $96,310). Classifying families at and below 200% of the poverty level as lower-income is consistent with other published reports (Hair et al., 2015; Hanson et al., 2013). We therefore repeated analyses, replacing our continuous income variable with this binary split. In the left hippocampus, these analyses confirmed a main effect of income and an income and quadratic age interaction (income: F(1,70.78) = 23.09, p < .001; income by age2: F(1,93.74) = 8.49, p = 0.004). In the right hippocampus, there was a main effect of income (F(1,123.24) = 21.55, p < .001) and a possible trend for the income and quadratic age interaction (F(1,118.07) = 2.79, p = 0.097).
3.2. Age-related changes in bilateral hippocampus by parental education
Next, we tested the effects of parental education level on offspring hippocampal volume trajectories over development. The same model and analysis described above yielded a significant main effect of parental education on left hippocampal volume (F(1,151.77) = 14.47, p < .001), but no significant interaction of age and parental education (linear: F(1,130.72) = 0.68, p = 0.413; quadratic: F(1,106.04) = 2.61, p = 0.109). For the right hippocampus, there was a significant main effect of parental education F(1,161.94) = 6.95, p = 0.009) but no significant interaction of age and education (linear: F(1,140.21) = 0.51, p = 0.477; quadratic: F(1,109.17) = 0.25, p = 0.621).
Because an ANOVA indicated that girls who were scanned on scanner 2 had marginally higher levels of parental education than did the rest of the sample, we reran analyses excluding these 32 girls. Paralleling our initial findings, in the left hippocampus there was a significant main effect of parental education (F(1,87.65 = 13.81, p < .001), but no significant interaction of parental education and age terms (linear: F(1,84.10) = 1.35, p = 0.248; quadratic: F(1,73.06) = 3.62, p = 0.061). In the right hippocampus there was a main effect of education (F(1,91.93 = 5.37, p=0.023) but no significant effect of age and parental education and age terms (linear: F(1,80.90) = 1.52, p = 0.221; quadratic: F(1,89.97) = 0.00, p = 0.949).
3.3. Association of mother and daughter hippocampal volume
Maternal hippocampal volume was moderately and positively associated with daughter’s hippocampal volume, controlling for mother’s and daughter’s age and ICV, in both left (β = 0.51, t = 2.98, p = 0.006, ΔR2 = 0.32) and right (β = 0.44, t = 3.02, p = 0.005, ΔR2 = 0.30) hippocampi. We also examined age-related changes in hippocampal volume related to family income and parental education, using maternal hippocampal residuals (controlling for maternal age and ICV) and age interactions as covariates. Results from the model with family income are presented in Table 3. In the model with income predicting left hippocampus, the main effect of income remained significant (F(1,38.48) = 15.86, p < .001), as did the income and quadratic age interaction term (F(1,38.79) = 8.32, p = 0.006). In the right hippocampus, there was a significant main effect of income (F(1,34.07) = 15.98, p < .001) and a trend for the interaction of income and quadratic age (F(1,37.62) = 3.60, p = 0.065). Similarly, the models with education yielded a significant main effect of education (F(1,43.95) = 22.47, p < .001) and a significant education and quadratic age interaction (F(1,34.68) = 13.27, p = 0.001) in the left hippocampus, and a significant main effect of education (F(1,50.96) = 4.81, p = 0.033) and possible trend for the income and quadratic age term (F(1,36.91) = 3.13, p = 0.085) in the right hippocampus. A similar pattern was observed when education analyses were repeated excluding the participants scanned only at scanner 2 in both left (education: F(1,27.80) = 18.96, p < .001; education by age2: F(1,19.18) = 16.19, p = 0.001) and right (education: F(1,35.28) = 4.93, p = 0.033; education by age2: F(1,29.28) = 8.18, p = 0.008) hippocampus, suggesting that differences in education by scanner did not significantly affect our findings.
Table 3.
Mixed model results for income left and right hippocampal volume over development controlling for mother left and right hippocampal residuals, respectively.
| Left |
Right |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | 95% CI | t-statistic | p-value | Estimate | 95% CI | t-statistic | p-value |
| Intercept | 2514.10 | 1337.91, 3690.29 | 4.42 | <0.001 | 1701.81 | 650.08, 2753.54 | 3.31 | 0.002 |
| Estimated ICV | 0.001 | 0.000, 0.002 | 2.91 | 0.008 | 0.002 | 0.001, 0.002 | 4.69 | <0.001 |
| Scanner (0) | −162.03 | −405.73, 81.67 | −1.34 | 0.188 | −540.37 | −824.41, −256.32 | −3.81 | <0.001 |
| Scanner (1) | 225.53 | −49.73, 500.80 | 1.64 | 0.106 | 183.23 | −116.67, 483.13 | 1.22 | 0.226 |
| Scanner (2) | 0 | 0 | – | – | 0 | 0 | – | – |
| Age in years | 39.05 | 2.22, 75.89 | 2.13 | 0.038 | 18.05 | −20.79, 56.89 | 0.93 | 0.356 |
| Age2 | −2.65 | −9.48, 4.17 | −0.79 | 0.436 | 2.65 | −3.65, 8.95 | 0.85 | 0.402 |
| Income | 9.15 | 4.50, 13.79 | 3.98 | <0.001 | 8.60 | 4.23, 12.96 | 4.00 | <0.001 |
| Age × Income | 0.83 | 0.30, 1.38 | 3.24 | 0.004 | 0.55 | −0.09, 1.18 | 1.76 | 0.088 |
| Age2 × Income | −0.23 | −0.40, −0.07 | −2.88 | 0.006 | −0.17 | −0.36, 0.01 | −1.90 | 0.065 |
| Mother hippocampus | 0.17 | −0.17, 0.50 | 1.01 | 0.318 | −0.17 | −0.54, 0.19 | −0.96 | 0.343 |
| Age × Mother hippocampus | −0.01 | −0.08, 0.06 | −0.30 | 0.768 | −0.01 | −0.07, 0.06 | −0.24 | 0.813 |
| Age2 × Mother hippocampus | 0.001 | −0.02, 0.02 | 0.14 | 0.890 | 0.01 | −0.01, 0.03 | 1.19 | 0.242 |
ICV = intracranial volume; CI = confidence interval.
3.4. Associations with familial risk for depression and with the development of MDD
An independent-sample t-test revealed that, on average, participants with a maternal history of depression had lower levels of family income than did participants with no maternal history of MDD (maternal MDD: M = $76,150, SD = $29,410; no maternal MDD: M = $90,440, SD = $16,310; t(76.23) = 3.01, p = 0.004); the groups did not differ in parental education (maternal MDD: M = 15.57, SD = 2.30; no maternal MDD: M = 15.80, SD = 1.78; t(98) = 0.56, p = 0.577). In the model with income, there was a significant main effect of income, a significant interaction of income and quadratic age, and a significant interaction of familial risk and quadratic age (income: F(1,118.97) = 22.05, p < .001; income by age2: F(1,110.00) = 9.80, p = 0.002; maternal MDD by age2: F(1,64.93) = 4.31, p = 0.042). Similarly, in the model with education, there was a significant main effect of parental education, a significant interaction of education and quadratic age, and a significant interaction of familial risk and quadratic age (education: F(1,139.37) = 14.90, p < .001; education by age2: F(1,44.64) = 5.43, p = 0.024; maternal MDD by age2: F(1,26.13) = 8.55, p = 0.007). Plotting this interaction revealed that daughters of depressed mothers showed a more linear increase in hippocampal volume with age, compared to a peak and later reduction observed among those without familial risk for MDD (Supplemental Fig. 1). Next, we tested for possible moderation by risk status in both cases. Risk status did not moderate the effects of either income or education on hippocampal trajectories (ps > 0.10).
Next, we examined whether hippocampal trajectories predicted the onset of MDD in the subset of girls for whom these data were available. Participants who had experienced an episode of MDD did not differ from those who did not in terms of family income (MDD onset: M = $78,590, SD = $30,440; no MDD onset: M = $84,930, SD = $21,230; t(54.29) = 0.988, p=0.327) or parental education (MDD onset: M = 15.48, SD = 2.18; no MDD onset: M = 15.54, SD = 1.99; t(79) = 0.12, p=0.908). In the model with income, MDD onset did not independently predict hippocampal volume (ps > 0.05), although previous patterns of results we reported above held with these variables accounted for, yielding a significant main effect of income (F(1,120.61) = 18.89, p < .001) and interaction with income and quadratic age (F(1,115.52) = 4.11, p = 0.045). Next, we tested whether MDD onset moderated the association of income on hippocampal volume. There was a significant interaction of MDD onset and income (F(1,65.84) = 4.32, p=0.042). Re-running the models separately for girls pre- versus post-MDD onset revealed that income significantly predicted hippocampal volume prior to the onset of MDD (income: F(1,45.62) = 7.10, p=0.011; income by age2: F(1,47.31) = 0.99, p = 0.325), but not after the onset of MDD (income: F(1,10.16) = 1.75, p = 0.215; income by age2: F(1,14.44) = 0.07, p = 0.796), suggesting that income-related effects on hippocampal volume disappeared after the onset of depression (Supplemental Fig. 2). In the models with education, there was a significant main effect of MDD onset (F(1,99.29) = 3.24, p = 0.025) and a significant interaction of MDD onset with quadratic age (F(1,92.43) = 4.25, p = 0.007), in addition to a main effect of education F(1,143.13) = 16.73, p < .001) and interaction of education with quadratic age F(1,92.24) = 4.77, p = 0.032). Plotting this interaction revealed that before MDD onset, hippocampal trajectories showed the typical increase and slight reduction in hippocampal volume; however, after MDD onset, hippocampal volume was relatively unchanging across adolescence (Supplemental Fig. 3). We note that average age of MDD onset was 16.59 (SD = 2.57) years, well before the age that hippocampal volume peaked in the rest of the sample. MDD onset did not moderate the association of education with hippocampal volume (ps > 0.10). Thus, while income appeared to influence hippocampal trajectories only prior to the onset of MDD, parental education and MDD onset exerted unique influences on hippocampal development.
3.5. Specificity of results
Finally, we tested the specificity of our results by conducting analyses predicting left and right amygdala volume. In the models with income, there was a significant main effect family income on left amygdala (F(1,117.10) = 4.39, p = 0.038) but no significant interactions with age (ps > 0.10); there were no significant effects of income or age interactions on right amygdala volume (ps > 0.10). Similarly, in the models with education, there were no significant main effects of parental education or interactions with age on left or right amygdala volume (ps > 0.05). Thus, the effects of family income and parental education on hippocampal volume were not also observed in the amygdala.
4. Discussion
The goal of this study was to examine whether trajectories of girls’ hippocampal development vary as a function of family income and parental education, two indicators of SES. We found a main effect of family income on hippocampal volume that was qualified by an interaction between income and girls’ age. Girls from lower-income families showed a slight reduction in volume during the teenage years, followed by an increase through early adulthood. In contrast, hippocampal volume in the highest-income girls peaked around 19 years, with later reductions. Differences between the low- and high-income groups were most pronounced around 18 years of age and converged thereafter. We also found a main effect of parental education on right hippocampal volume: higher education levels predicted larger hippocampal volume across development. Furthermore, the effects of income and parental education persisted even after controlling for the effects of mother hippocampal volume. Thus, income and education-related differences cannot be fully explained by maternal transmission of brain structure or by the shared environment of mothers and daughters, suggesting other environmental contributions to hippocampal volume.
In addition to the primary goals of the study, we also explored whether these trajectories were related to or moderated by mothers’ and daughters’ MDD history and onset, respectively. Maternal history of MDD was associated with daughters’ hippocampal trajectories independent of family income and parental education. Specifically, there was a significant interaction of familial risk and age on hippocampal volume, over and above the effects of the socioeconomic variables, which remained significant predictors. Plotting estimated model parameters suggested that at average levels of income and parental education, girls with a maternal history of MDD had more linear increases in hippocampal volume. Upon visual inspection, this pattern was similar to that observed for the lower-income girls, with potential recovery of hippocampal volume in late adolescence. For girls at familial risk of MDD, however, hippocampal volume appeared to start out relatively high in early adolescence, in contrast to the lower starting point for lower-income girls.
In addition, we examined whether hippocampal trajectories were related to MDD onset in daughters. MDD onset moderated the effects of income on hippocampal volume, suggesting that income predicts hippocampal trajectories prior to MDD onset, but not after the development of MDD. Specifically, prior to MDD onset, higher income predicted increases in hippocampal volume similar to the pattern observed in the whole sample, but after the experience of at least one episode of MDD, income was no longer a significant predictor of hippocampal volume. Hippocampal volume appeared to remain relatively steady or decline post-MDD onset. Interestingly, hippocampal volume appeared to be relatively constant after the onset of MDD, a pattern distinct from that observed in either the higher- or the lower-income girls. In contrast, in the models with education, both MDD onset and education independently predicted hippocampal volume.
Finally, these findings were specific to the hippocampus; though there was a significant main effect of income in the left amygdala, there was no evidence of significant interactions of income or education with age on amygdala volume. While this is in contrast to a recent study which showed the effects of SES on amygdala volume varied as a function of age (Merz et al., 2017), this discrepancy may be explained by the fact that our study focused on an older age range. Notably, others have also found a main effect of income in only the left amygdala (Luby et al., 2013).
This study is the first to show that the relation between family income and hippocampal volume varies as a function of children’s age. Specifically, differences in hippocampal volume between girls from higher- and lower-income families peaked in the teenage years and converged in early adulthood. This result extends prior findings that children and early adolescents from lower-SES homes exhibit reduced hippocampal volume (Hanson et al., 2015, Hanson et al., 2011; Luby et al., 2013; Noble et al., 2012; Ursache et al., 2017), and suggests that differences related to family income converge in adulthood after a peak divergence in late adolescence. This is consistent with recent findings that income is related to hippocampal development in childhood but not adulthood (Yu et al., 2017) and with reports that current financial hardship but not childhood SES is related to adult hippocampal volume (Butterworth et al., 2012). Moreover, effects of family income were evident even after covarying for maternal hippocampal volume, a proxy for both inherited and shared environmental contributions.
Although income-related trajectories of hippocampal volume appear to converge in early adulthood, the divergence in the trajectories observed during adolescence may be particularly important. Adolescence is a sensitive period of development; during this time the brain is generating critical connections that support executive function (Murty et al., 2016; Ordaz et al., 2013). It will be important in future research to examine more explicitly and systematically the implications of this divergence, and of the subsequent convergence, for adolescent functioning. It may be that this represents a period of particular vulnerability for children from less affluent homes, making it an ideal time to intervene. If this is the case, interventions during this period could be targeted toward alleviating stressors − or offering increased support in terms of access to educational resources − during this time. In fact, there is evidence that high self-esteem can buffer negative effects of lower-SES on hippocampal volume (Wang et al., 2016). Clearly, more work is needed to determine the most ideal targets for such interventions.
On the other hand, the observed convergence suggests that the neurobiological consequences of SES on the hippocampus are not necessarily long-lasting. Indeed, while lower childhood SES may lead to increased forms of biological stress in earlier adolescence, children may show recovery over late adolescence. This possibility is supported by findings that lower-SES is linked with higher cortisol in childhood but not in adulthood (Lupien et al., 2001). In this case, the authors show that lower-SES parents actually exhibit reduced stress related to transitions than higher-SES parents; thus, these children may be protected from some forms of stress which become more apparent in adolescence. This is an important direction for future research to take.
In contrast to the effects of family income, differences in hippocampal volume related to parental education appeared to be constant throughout development. Other studies have similarly shown differential effects of income and education on neural development. For example, Noble et al. found that income, but not parental education, predicted SES-related differences in hippocampal volume in 5- to 17-year-olds, and suggested that whereas parental education is a stronger predictor of caregiving behaviors, income is related more strongly to a lack of material and educational resources (Brito and Noble, 2014; Noble et al., 2012). Other researchers have posited that years of education may not be the best indicator of SES, given that people with the same levels of education vary considerably in the quality of education they received, depending on their relative societal status (Braveman et al., 2013).
In interpreting results related to family income and parental education, it is important to consider the context in which this study was conducted. For example, in the California Bay Area, intense academic pressure has led to a spike in psychopathology and even suicide among teenagers in more affluent areas (Garcia-Williams et al., 2016). Thus, lower-SES children, by virtue of their school and/or neighborhood characteristics, may not be exposed to this particular stressful environment, serving as a protective factor for the lower-SES population in this study. Supporting this possibility, a recent study found that high school demographics are a better predictor of teens’ engagement in risky behavior than is their own family income (Coley et al., 2017). In fact, the influence of socioeconomic factors on child outcomes is likely to be highly variable depending on the geographic context in which differences are being examined (i.e., urban versus rural neighborhoods), the structure of systems within those contexts, and the extent to which upward social comparisons are readily available (Duncan et al., 1994; Lipina, 2017; Marks et al., 2006; Reuman, 1989). All of these factors could systematically influence children’s exposures to different types of deviations from the “typical” environment, leading to differences in outcomes (Humphreys and Zeanah, 2015; Sheridan and McLaughlin, 2016).
Another important consideration is that both family income and parental education in our study were high relative to US population norms (U.S. Census Bureau). Indeed, our findings may not represent effects driven by more extreme poverty, but rather a gradient spanning from lower- to higher-SES. This is an important distinction, as it may be that associations with the hippocampus are driven more by the benefits conferred in particularly well-resourced environments than by stressors associated with poverty (Amso and Lynn, 2017). Future studies are needed to examine whether adolescents at more extreme ends of the lower-SES distribution show a similar pattern of recovery in hippocampal volume over adolescence.
It may be important that findings were observed in the hippocampus but not in the amygdala. Evidence from the animal literature suggests that these two regions are sensitive to different forms of stress. Specifically, whereas immobilization stress appears to affect hippocampal neurons and increase amygdalar cell branching, chronic unpredictable stress affects neurons in the amygdala but not in the hippocampus (Vyas et al., 2002). Indeed, there is reason to believe that effects related to lower SES (outside of cases of extreme poverty) may be driven by less access to cognitively stimulating experiences in these contexts to a greater extent than by stress as it is traditionally conceptualized (Amso and Lynn, 2017). Consistent with this possibility, the pattern of hippocampal trajectories we observed for girls at familial risk for onset of MDD was distinct from those observed in either the higher- or the lower-SES group, as were trajectories after the onset of MDD. This lends support to the formulation that these variables, while generally correlated, operate through distinct mechanisms to confer risk for MDD (Amso and Lynn, 2017).
Finally, we should note the highly plastic nature of the brain and its ability for recovery. Despite growing evidence linking reduced hippocampal volume with early stress, behavioral problems, and psychopathology (Gould et al., 2000; Hanson et al., 2015; Luby et al., 2013), it is not clear whether the trajectory of hippocampal development or the hippocampal volume attained in adulthood is more important. In fact, it is possible that the protracted peak of hippocampal volume observed among the lower-income girls in this study was an adaptive response to their environment (Ellis et al., 2017; Ellwood-Lowe et al., 2016). It is worth noting that in our subsample, MDD onset was independent of hippocampal trajectories, suggesting that trajectories associated with lower household income and parental education may not confer risk for adolescent-onset MDD.
4.1. Limitations
We should note several limitations of this study. First, the larger study from which we drew our sample was not designed to explore SES; thus, the range of income and education groups recruited is relatively restricted, the girls have higher rates of familial risk for MDD, and the sample is limited to females. In this context, we measured family income and parental education variables only in childhood, prior to the first scan; thus, we cannot say whether income and education levels were stable across the testing period, or whether they varied across adolescence. Even so, we note that other studies have similarly examined childhood SES and later neural development, and there is evidence that childhood SES has predictive power (e.g., Duval et al., 2017; Hair et al., 2015). While these factors limit our ability to generalize these findings to males and to more extreme ends of the wealth distribution, the restriction of our sample allowed us to avoid important confounds introduced by sex. Indeed, given sex differences in hippocampal trajectories (Gogtay et al., 2006) in addition to biological differences in HPA-axis reactivity and sensitivity to environmental factors (Oldehinkel and Bouma, 2011), it is likely that such an association between SES and hippocampal volume would not be as evident as strongly in males, or would follow a different trajectory. Future work is needed to examine this possibility.
Second, although we attempted to rule out the potential heritability of hippocampal volume by controlling for maternal hippocampal volume, this does not exclude the possibility that differences in hippocampal volume are driven by genetic or health factors. Maternal hippocampal volume appears to be a unique predictor of daughter hippocampal volume, a finding that is likely due to a complex interaction of genetic, prenatal, and postnatal factors (Yamagata et al., 2016). Moreover, genetic factors outside of this relation may contribute to both SES and hippocampal development; in the absence of experimental manipulations such as randomized-control trials, these effects are difficult to disentangle fully. Third, because we used different scanners to collect data, differences in scanner may influence our findings. While we addressed this by covarying for scanner in analyses, it is still possible that differences in head positioning across scanners added additional variance to hippocampal estimates that is unaccounted for in our analyses. In addition, in our study we used the cross-sectional Freesurfer processing stream; other researchers with similar designs have used Freesurfer’s longitudinal stream. It is not clear how this difference would affect comparability of hippocampal estimates across studies. Currently it is uncertain whether it is appropriate to use the longitudinal stream in studies of development (Reuter, 2016); it is important that future studies be conducted explicitly testing the longitudinal stream in these contexts.
Finally, our study did not examine environmental variables that may have contributed to disparities related to family income or parental education. Future research will be needed to investigate whether SES-related differences in hippocampal trajectories are driven by risk factors such as more extreme forms of stress in lower-SES families, or by benefits such as increased access to material and educational resources in higher-SES environments. In addition, researchers should more directly examine the potential role of stress hormones in the association between SES and hippocampal development.
5. Conclusion and future directions
This is the first study to examine age-related differences in the effect of family income and parental education on trajectories of hippocampal volume in girls through early adulthood, allowing us to characterize SES-related differences in hippocampal growth longitudinally. Further, this is the first study to also control for maternal hippocampal volume. We found that girls from low- and high-income families differ in trajectories of hippocampal volume, differences that are most pronounced in late adolescence and converge in early adulthood. In contrast, the effects of parental education on hippocampal volume appeared constant across adolescence: higher education was related to larger hippocampal volume. Income and education-related differences in trajectories persisted even after accounting for maternal hippocampal size. These findings highlight the need to elucidate links between the environment and hippocampal volume, particularly during adolescence, and raise the possibility that parental education and family income exert distinct influences on hippocampal volume. Although income-related differences in hippocampal volume appear to converge in early adulthood, the significant disparity observed during adolescence – a sensitive period for development – may represent a time during which interventions could be particularly beneficial.
Conflict of interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2017.12.005.
Appendix A. Supplementary data
The followingis Supplementary data to this article:
References
- Alexander K.L., Entwisle D.R. School performance, status relations, and the structure of sentiment: bringing the teacher back In. Am. Sociol. Rev. 1987;52:665–682. [Google Scholar]
- Amso D., Lynn A. Distinctive mechanisms of adversity and socioeconomic inequality in child development: a review and recommendations for evidence-based policy. Policy Insights Behav. Brain Sci. 2017 doi: 10.1177/2372732217721933. (237273221772193.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Braveman P., Cubbin C., Egerter S., Chideya S., Marchi K.S., Metzler M., Posner S. Vol. 294. 2013. pp. 2879–2888. (Socioeconomic Status in Health Research). [DOI] [PubMed] [Google Scholar]
- Brito N., Noble K. Socioeconomic status and structural brain development. Front. Neurosci. 2014;8:1–12. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth P., Cherbuin N., Sachdev P., Anstey K.J. The association between financial hardship and amygdala and hippocampal volumes: results from the PATH through life project. Soc. Cogn. Affect. Neurosci. 2012;7:548–556. doi: 10.1093/scan/nsr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J.A., Petersen S.E., Schlaggar B.L. The task B problem and other considerations in developmental functional neuroimaging. Hum. Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes M.E. Friendship and personal adjustment during adolescence. J. Adolesc. 1992;15:39–55. doi: 10.1016/0140-1971(92)90064-c. [DOI] [PubMed] [Google Scholar]
- Coleman J.S. Equality of educational opportunity (COLEMAN) study (EEOS) Equity Excell. Educ. 1968;6:19–28. [Google Scholar]
- Coley R.L., Sims J., Dearing E., Spielvogel B. Locating economic risks for adolescent mental and behavioral health: poverty and af fluence in families, neighborhoods and schools. Child Dev. 2017;0:1–10. doi: 10.1111/cdev.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D., Papachristou E., Turner J., Glahn D.C., Hibar D.P., Van Erp T.G.M., Medland S.E., Thompson P.M. Subcortical brain volumes across the lifespan based on 10, 722 people aged 2–92. Organization for Human Brian Mapping Annual Meeting (OHBM); Honolulu, Hawaii, USA; 2015. [Google Scholar]
- Dowd J.B., Simanek A.M., Aiello A.E. Socio-economic status, cortisol and allostatic load: a review of the literature. Int. J. Epidemiol. 2009;38:1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G.J., Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3:377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Duncan G.J., Brooks-Gunn J., Klebanov P.K. Economic deprivation and early childhood development. Child Dev. 1994;65:296–318. [PubMed] [Google Scholar]
- Duval E.R., Garfinkel S.N., Swain J.E., Evans G.W., Blackburn E.K., Angstadt M., Sripada C.S., Liberzon I. Childhood poverty is associated with altered hippocampal function and visuospatial memory in adulthood. Dev. Cogn. Neurosci. 2017;23:39–44. doi: 10.1016/j.dcn.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.J., Bianchi J., Griskevicius V., Frankenhuis W.E. Beyond risk and protective factors: an adaptation-Based approach to resilience. Perspect. Psychol. Sci. 2017;12:561–587. doi: 10.1177/1745691617693054. [DOI] [PubMed] [Google Scholar]
- Ellwood-Lowe M.E., Sacchet M.D., Gotlib I.H. The application of neuroimaging to social inequity and language disparity: a cautionary examination. Dev. Cogn. Neurosci. 2016;22:1–8. doi: 10.1016/j.dcn.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W., English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Gonnella C., Marcynyszyn L.A., Gentile L., Salpekar N. Chaos poverty and children’s socioemotional adjustment. Psychol. Sci. 2005;16:560–565. doi: 10.1111/j.0956-7976.2005.01575.x. [DOI] [PubMed] [Google Scholar]
- Farah M.J. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017;96:56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Van Der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Frodl T., O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D.E., Bunge S.A. The stamp of poverty. Sci. Am. Mind. 2016;28:54–61. [Google Scholar]
- Garcia-Williams A., O’Donnell J., Spies E., Azofeifa A., Vagi K. 2016. Undetermined Risk Factors for Suicide Among Youth, Ages 10–24 — Santa Clara County, CA, 2016. (Epi-2 Report) [Google Scholar]
- Gogtay N., Nugent T.F., Herman D.H., Ordonez A., Greenstein D., Hayashi K.M., Clasen L.S., Toga A.W., Giedd J.N., Rapoport J.L., Thompson P.M. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J., Minor K.L., Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J., Foland-Ross L.C. Understanding familial risk for depression. Perspect. Psychol. Sci. 2014;9:94–108. doi: 10.1177/1745691613513469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Tanapat P., Rydel T., Hastings N. Molecular and cellular hypotheses of antidepressant action: regulation of hippocampal neurogenesis in adulthood. Nat. Neurosci. 2000;3223:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Graham H. Unequal lives: health and socio-economic inequalities. Soc. Sci. 2008:587–588. [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair N.L., Hanson J.L., Wolfe B.L., Pollak S.D. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;53706:1–8. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Chandra A., Wolfe B.L., Pollak S.D. Association between income and the hippocampus. PLoS One. 2011;6:1–8. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D. Family poverty affects the rate of human infant brain growth. PLoS One. 2013:8. doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., Shirtcliff E.A., Pollak S.D., Davidson R.J. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B., Risley T.R. Paul H. Brookes Publishing; 1995. Meaningful Differences in the Everyday Experience of Young American Children. [Google Scholar]
- Humphreys K.L., Zeanah C.H. Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology. 2015;40:1–59. doi: 10.1038/npp.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.B., Riis J.L., Noble K.G. State of the art review: poverty and the developing brain. Pediatrics. 2016;137:1–17. doi: 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Han X., Salat D., Kouwe A., Quinn Van Der B., Pacheco J., Albert M., Killiany R., Blacker D., Rosas D., Makris N., Gollub R., Dale A. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lawson G.M., Camins J.S., Wisse L., Wu J., Duda J.T., Cook P.A., Gee J.C., Farah M.J. Childhood socioeconomic status and childhood maltreatment: distinct associations with brain structure. PLoS One. 2017;12:e0175690. doi: 10.1371/journal.pone.0175690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J., Ordaz S.J., Kircanski K., Singh M.K., Gotlib I.H. Predicting first onset of depression in young girls: interaction of diurnal cortisol and negative life events. J. Abnorm. Psychol. 2015;124:850–859. doi: 10.1037/abn0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina S.J. 2017. The Biological Side of Social Determinants: Neural Costs of Childhood Poverty. Prospects; pp. 1–16. [Google Scholar]
- Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C., Nishino T., Barch D. The effects of poverty on childhood brain development the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167:1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Belden A., Harms M.P., Tillman R., Barch D.M. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5742–5747. doi: 10.1073/pnas.1601443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., King S., Meaney M.J., McEwen B.S. Can poverty get under your skin?: basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marks G.N., Cresswell J., Ainley J. Explaining socioeconomic inequalities in student achievement: the role of home and school factors. Educ. Res. Eval. 2006;12:105–128. [Google Scholar]
- McLoyd V.C. Socioeconomic disadvantage and child development. Am. Psychol. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Merz E.C., Tottenham N., Noble K.G. Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. J. Clin. Child Adolesc. Psychol. 2017;4416:1–12. doi: 10.1080/15374416.2017.1326122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Tamnes C.K. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev. Cogn. Neurosci. 2014;9:172–190. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.-L., Herting M.M., Meuwese R., Blakemore S.-J., Crone E.A., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Tamnes C.K. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. doi: 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V.P., Calabro F., Luna B. The role of experience in adolescent cognitive development: integration of executive, memory, and mesolimbic systems. Neurosci. Biobehav. Rev. 2016;70:46–58. doi: 10.1016/j.neubiorev.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Kan E., Sowell E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Schork N.J., Murray S.S., Casey B.J., Chang L.D., Ernst T.M., Frazier J.A., Gruen J.R., Kennedy D.N., Van Zijl P., Mostofsky S., Kaufmann W.E., Kenet T., Dale A.M., Jernigan T.L., Sowell E.R. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18:773. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel A.J., Bouma E.M.C. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: a review of gender differences. Neurosci. Biobehav. Rev. 2011;35:1757–1770. doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 2013;33:18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H., Betancourt L., Giannetta J.M., Brodsky N.L., Korczykowski M., Avants B.B., Gee J.C., Wang J., Hurt H., Detre J.A., Farah M.J. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuman D.A. How social comparison mediates the relation between ability-grouping practices and students’ achievement expectancies in mathematics. J. Educ. Psychol. 1989;81:178–189. [Google Scholar]
- Reuter M. Vol. 13. 2016. (Re: Registration Errors in Longitudinal Analysis). (2016, January 13) [Freesurfer email list]. Retrieved from https://mail.nmr.mgh.harvard.edu/pipermail/freesurfer/2016-January/043178.html. [Google Scholar]
- Satterthwaite F. International biometric society. Biometrics Bull. 1946;2:110–114. [Google Scholar]
- Sheridan M.A., McLaughlin K.A. Neurobiological models of the impact of adversity on education. Curr. Opin. Behav. Sci. 2016;10:108–113. doi: 10.1016/j.cobeha.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E.V., Pfefferbaum A., Swan G.E., Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11:754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- Ursache A., Merz E.C., Melvin S., Meyer J., Noble K.G. Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology. 2017;78:142–150. doi: 10.1016/j.psyneuen.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Rao B.S.S., Chattarji S. Vol. 22. 2002. pp. 6810–6818. (Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang L., Kong X., Hong Y., Cheon B., Liu J. Pathway to neural resilience: self-esteem buffers against deleterious effects of poverty on the hippocampus. Hum. Brain Mapp. 2016;37:3757–3766. doi: 10.1002/hbm.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder A., Fernald A. Talking to children matters: early language experience strengthens processing and builds vocabulary. Psychol. Sci. 2013;24:2143–2152. doi: 10.1177/0956797613488145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J.G., Dennison M., Schwartz O., Pantelis C., Sheeber L., Byrne M.L., Allen N.B. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry. 2017;74:824. doi: 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata B., Murayama K., Black J.M., Hancock R., Mimura M., Yang T.T., Reiss A.L., Hoeft F. Female-specific intergenerational transmission patterns of the human corticolimbic circuitry. J. Neurosci. 2016;36:1254–1260. doi: 10.1523/JNEUROSCI.4974-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Daugherty A.M., Anderson D.M., Nishimura M., Brush D., Hardwick A., Lacey W., Raz S., Ofen N. 2017. Socioeconomic Status and Hippocampal Volume in Children and Young Adults I; pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.