Abstract
Based upon observations in murine models, we have developed protocols to induce renal allograft tolerance by combined kidney and bone marrow transplantation (CKBMT) in non-human primates (NHP) and in humans. Induction of persistent mixed chimerism has proved to be extremely difficult in major histocompatibility complex (MHC)-mismatched primates, with detectable chimerism typically disappearing within 30–60 days. Nevertheless, in MHC mismatched NHP, long-term immunosuppression-free renal allograft survival has been achieved reproducibly, using a non-myeloablative conditioning approach that has also been successfully extended to human kidney transplant recipients. CKBMT has also been applied to the patients with end stage renal disease with hematologic malignancies. Renal allograft tolerance and long-term remission of myeloma have been achieved by transient mixed or persistent full chimerism. This review summarizes the current status of pre-clinical and clinical studies for renal and non-renal allograft tolerance induction by CKBMT. Improving the consistency of tolerance induction with less morbidity, extending this approach to deceased donor transplantation and inducing tolerance of non-renal transplants, are critical next steps for bringing this strategy to a wider range of clinical applications.
Keywords: Allograft tolerance, Chimerism, Hematopoietic stem cell transplantation, Donor bone marrow transplantation, Kidney transplantation
1. Introduction
Development of increasingly effective immunosuppressive medications has improved short-term results in organ transplantation dramatically [1]. Unfortunately, long-term administration of these medications results in significant morbidity and mortality, including nephrotoxicity, infections [2], neoplasm [3] and cardiovascular diseases [4]. These complications adversely affect eventual patient and graft survival [5]. Thus, although renal transplant recipients currently have a one-year survival rate of over 90% after deceased-donor transplantation, the subsequent relentless annual attrition rate of approximately 5% results in a 10-year survival of only 50%. About a half of the graft loss is due to death of the recipient despite continuing function of the graft. Most of the other failures are due to chronic rejection, which is not prevented even with potent immunosuppression. Therefore, induction of allograft tolerance, which would remove the requirement for life-long immunosuppression, is a major goal in the field of organ transplantation.
2. Preclinical studies in large animals
Based on mice studies by Sharabi and Sachs, in which tolerance of MHC fully mismatched skin grafts was achieved by induction of persistent mixed chimerism, we developed a nonmyeloablative conditioning regimen for induction of renal allograft tolerance in NHPs.
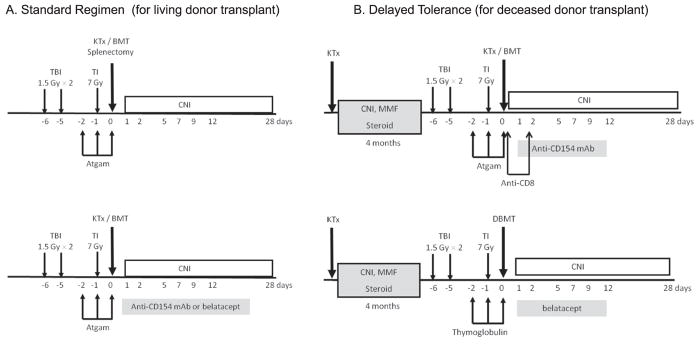
The initial conditioning regimen for NHPs consisted of TBI (1.5 Gy × 2), TI (7 Gy), and anti T-cell antibody [horse anti-thymocyte globulin (Atgam)], thus including all of the components in the protocol previously developed in mice studies. Interestingly, this regimen failed to induce chimerism in NHPs, likely due to the facts that 1) T cell depletion is much more effective in mice than it is in large animals; and 2) there are abundant memory T-cells in NHPs, which are not present in pathogen-controlled laboratory mice [6]. Consistent with this hypothesis, following the addition of splenectomy and an one-month course of cyclosporine (CyA) (Fig. 1A, upper panel) to the preparative regimen, NHP recipients of simultaneous kidney and donor bone marrow transplantation (SKBMT) developed transient multilineage chimerism, lasting for up to two months. Surprisingly, the renal allografts were not rejected, despite disappearance of detectable chimerism. The longest renal allograft survival in the initial NHP study reached > 13 years without further immunosuppression after discontinuation of CyA (Fig. 2, left panel). Moreover, a skin allograft from the kidney donor was accepted in donor-specific fashion (Fig. 2, right panel)[7]. With this protocol, recipients without splenectomy developed humoral rejection, even though chimerism was induced [8]. The protocol was subsequently modified by substituting a short course of anti-CD40L (anti-CD154mAb) for splenectomy (Fig. 1A, lower panel) [9]. This modification significantly promoted hematopoietic stem cell engraftment and improved the consistency of renal allograft tolerance induction. However, since anti-CD154 mAb remained clinically unavailable due to its thrombophilic complications [10], we then tested CTLA4-Ig (belatacept), in place of anti-CD154 mAb (Fig. 1A, lower panel). With the belatacept regimen, 80% of recipients developed chimerism, and 75% of these achieved long-term renal allograft survival without ongoing immunosuppression [11]. This study suggested that CD28/B7 blockade with belatacept may provide a clinically applicable alternative to anti-CD154 mAb for promoting chimerism and renal allograft tolerance.
Fig. 1.
Nonmyeloablative regimens for NHPs: A. Standard regimen for living donor transplant. The regimen consisted of TBI (1.5 Gy × 2) on Day −6, −5, TI (7 Gy) on Day −1, intravenous horse ATG (50 mg/kg/day) on Day −2, −1 and 0 and kidney and bone marrow transplantation, followed by a 1-month course of cyclosporine. The initial regimen (upper panel) included Splenectomy on Day 0. The modified regimen (lower panel) included treatment with anti-CD154 mAb (20 mg/kg/day on Day 0, 2, 4, 6, 8 and 10) or belatacept (20 mg/kg/day on Day 0 and 2, and 10 mg/kg/day on Day 5 and 15) in place of splenectomy. B. Delayed tolerance regimen for deceased donor transplant: All recipients initially underwent kidney transplantation alone with a conventional triple drug immunosuppressive regimen consisting of tacrolimus, mycophenolate mofetil and prednisone. The recipients then undergo conditioning and donor bone marrow transplantation 4 months later. The initial conditioning regimen consisted of TBI (1.5 Gy × 2) on Day −6, −5, TI (7 Gy) on Day −1, intravenous horse ATG (50 mg/kg/day) on Day −2, −1 and 0, followed by anti-CD8 mAb (5 mg/kg/day on Day 0 and 2). Frozen BBM is administered on Day 0, followed by anti-CD154 mAb (20 mg/kg/day on Day 0, 2, 5, 7, 9 and 12) and a one-month course of cyclosporine. In most recent regimen, that uses only clinically available reagents (lower panel), thymoglobulin (20 mg/kg/day on Day −2 and −1) and belatacept (20 mg/kg/day on Day 0 and 2, and 10 mg/kg/day on Day 5 and 12) are substituted for horse ATG and anti CD154 mAb, respectively.
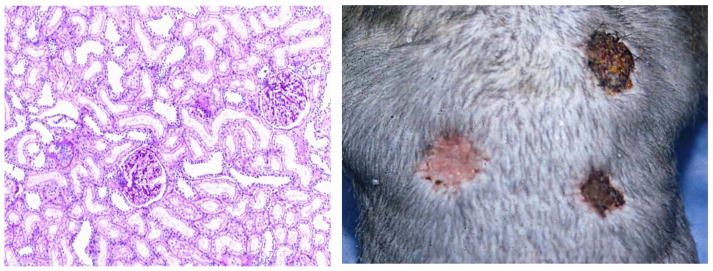
Fig. 2.
Full-thickness skins from a kidney donor and an allogeneic third-party were grafted on 337 days after kidney transplantation. Photograph (right panel) shows that acceptance of kidney donor skin surviving in perfect condition over 100 days was observed while third-party frozen and fresh skin grafts were rejected by day 10. Renal allograft biopsy was performed at 13 years after kidney transplantation. Histology (left panel) of the donor kidney graft showed no evidence of rejection, interstitial fibrosis and tubular atrophy.
3. Preclinical studies – the approach for deceased donor transplantation
The conditioning in our protocols described above requires recipient treatment beginning 6 days prior to the planned SKBMT, thereby limiting applicability to living donor transplant recipients. To apply this tolerance strategy to deceased donor transplantation, we have subsequently developed a “delayed tolerance” regimen, in which recipients initially undergo kidney transplantation (KTx) with conventional immunosuppression and several months later receive non-myeloablative conditioning and donor bone marrow transplantation (DBMT) from the original kidney donor. This “delayed tolerance” approach has the theoretical disadvantage that donor-specific memory T cells (TMEM) might be elicited prior to conditioning for tolerance induction even during administration of potent suppressive agents. Indeed, the original protocol, which was effective for SKBMT, failed to induce chimerism in the delayed tolerance protocol. Since rapid homeostatic recovery of CD8 TMEM was noted after DBMT in these recipients, we added anti-CD8 mAb to the regimen (Fig. 1B, upper panel). With this modification, 85% (11/13) of recipients successfully developed chimerism and 55% (6/11) of these recipients achieved long-term renal allograft survival without ongoing immunosuppression. However, 3/11 died due to either CMV infection or EBV related PTLD after prolonged CD8 TMEM depletion. As anti-CD8 mAb is not clinically available and was possibly too potent, we have sought alternative approaches to overcome the TMEM response in the delayed tolerance induction protocol. LFA3-Ig (alefacept) is a humanized chimeric fusion protein that is known to deplete selectively CD8+ effector memory T cells while sparing naïve CD8+ T cells [12] and that has been approved by the FDA for treatment of psoriasis. Conditioning with the protocol, in which alefacept was substituted for anti-CD8 mAb, successfully induced mixed chimerism in three consecutively treated recipients and all three achieved long-term renal allograft survival [13]. Unfortunately, however, alefacept production was discontinued by the manufacturer, forcing us to seek another alternative to overcome the TMEM response. Thymoglobulin has been shown to have more potent T cell depleting properties than Atgam [14] and is widely used as an induction therapy in clinical kidney transplantation. Therefore, Atgam and anti-CD154 mAb were replaced with Thymoglobulin and belatacept, respectively in our most recent “delayed tolerance” conditioning protocol. With this modified regimen (Fig. 1B, lower panel), which uses only clinically available reagents, all four recipients developed mixed chimerism and achieved long-term renal allograft survival (Hotta et al. manuscript submitted). This encouraging result suggests that induction of delayed renal allograft tolerance may now be possible using only clinically available reagents.
4. Preclinical studies – tolerance for non-renal organs/tissues
Extension of these approaches to non-renal organs/tissues is another important next goal. We have so far evaluated our mixed chimerism approach in heart, lung and islet allograft recipients.
Islet allografts: We first attempted to transplant islets from the original donor in the presence of already established renal allograft tolerance after SKBMT. Three years after SKBMT, the kidney recipient was made diabetic by streptozocin (STZ) injection and islets from the kidney donor were transplanted into the recipient’s portal vein and under the renal capsule. The STZ-induced diabetes was reversed following the islet transplantation, without any immunosuppression. The recipient again became insulin dependent 300 days after islet transplantation, but, at autopsy examination, viable transplanted islets were found in the liver and under the kidney capsule, without any evidence of rejection. This study indicated that the recipient, rendered tolerant to the renal allograft after induction of transient mixed chimerism, also appeared to be tolerant of the islet allograft. We subsequently performed studies to determine whether tolerance of islet allografts can be induced without the need for the primarily vascularized renal allograft in our mixed chimerism approach. However, no islet alone recipients achieve allograft tolerance despite successful induction of transient mixed chimerism comparable to that observed in renal recipients [15]. It therefore appears that induction of transient mixed chimerism is not sufficient to induce tolerance of isolated islet allografts and that either presence of the renal allograft or more robust chimerism may be required to achieve islet allograft tolerance.
Heart allografts: In collaboration with the Madsen group, we have also applied our approach to induce isolated heart allograft tolerance. However, similar to the results observed in islet transplants, no recipients achieved heart allograft tolerance despite induction of transient mixed chimerism [16]. Interestingly, consistent induction of heart allograft tolerance was achieved when the kidney allograft from the same donor was co-transplanted [17]. The removal of the renal allograft resulted in abrogation of allograft tolerance within a month, indicating the critical involvement of renal allograft in induction/maintenance of heart allograft tolerance (Madsen et al. manuscript in preparation). Although combined heart/kidney transplantation is a potential clinical indication which accounts for about 5% of all clinical heart transplantation, alternative approaches to induce isolated heart allograft tolerance without co-transplantation of the renal allograft is of much greater relevance and remains under investigation.
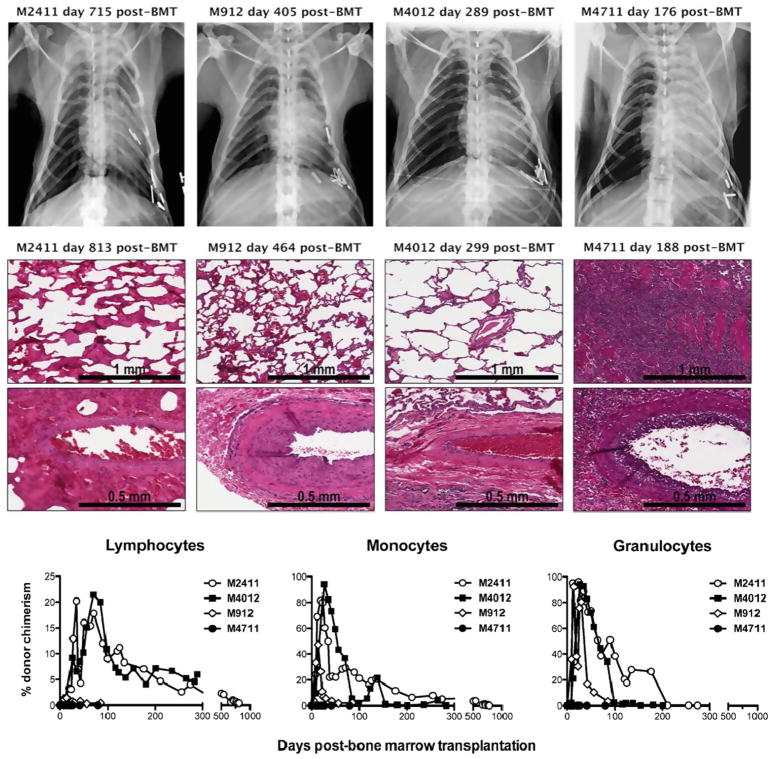
Lung allografts: In collaboration with the Allan group, our delayed tolerance approach has been tested in lung transplant recipients. By adding anti-IL6R mAb to the regimen with anti-CD8 and anti-CD154 mAbs, three of four lung transplant recipients achieved long-term lung allograft survival (> 813, > 464 and > 299 days) without immunosuppression (Fig. 3). Interestingly, two of three recipients achieved persistent mixed chimerism until euthanasia (Fig. 3), which has never been achieved in kidney transplant recipients. Addition of anti-IL6R mAb to the conditioning regimen in kidney transplants did not similarly induce persistent chimerism, suggesting that the persistent chimerism may have required lung allograft-specific mechanisms. For clinical application, the delayed protocol with Thymoglobulin and belatacept is currently being tested in lung-transplant recipients.
Fig. 3.
Two recipients (M2411 and M4012) developed persistent mixed and achieved lung-allograft tolerance. The third recipient (M912) developed only transient chimerism up to day 75 but achieved long-term lung-allograft survival without immunosuppression. One recipient that failed to develop chimerism rejected lung allograft on day 176.
5. Mechanism of tolerance through transient mixed chimerism
The tolerance of skin allografts achieved in mice via persistent mixed hematopoietic chimerism has been shown to be primarily due to persistent elimination of anti-donor T cell repertoires in the thymus (thymic deletion) [18]. Since, tolerance of kidney allografts in NHPs has been induced by only transient mixed chimerism, we hypothesized that peripheral regulatory mechanisms rather than thymic deletion must be involved, at least after disappearance of the chimerism. The critical importance of regulatory mechanisms, rather than deletional mechanism, was supported by a recent study which showed that previously established renal allograft tolerance can be abrogated by daily administration of IL-2 [19].
Our studies have also shown that donor specific expansion of regulatory T-cells (Tregs) appears to be a major mechanism of maintaining peripheral tolerance in our kidney transplant recipients [20]. In this study, tolerant recipients consistently lost donor-specific CD8+ T-cell responses, while maintaining a proliferative CD4+ T-cell response to donor antigen. The majority of the proliferating CD4+ T cells in tolerant recipients appeared to be Tregs and the expansion of Tregs against donor antigens was greater than that observed against third-party antigens. Finally, the expanded Tregs appeared to be induced Tregs (iTregs) that were converted from non-Tregs by stimulating donor-antigen.
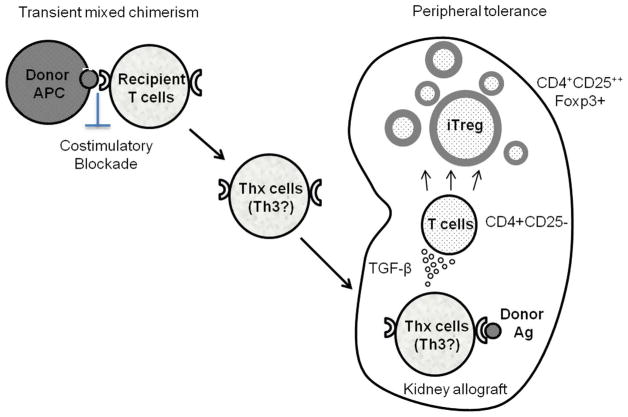
In another study, inhibition of Treg expansion by transforming growth factor-β(TGF-β) blockade was observed in tolerant recipients, resulting in restoration of anti-donor CD8+ T cell responses. Since some T cell subsets, such as Th3, have been shown to produce large amounts of TGF-β [21], we hypothesize that specific T cell subsets (Thx) that promote conversion of Tregs from non-Tregs are induced during the transient chimerism under costimulatory blockade. Upon encountering donor MHC class II antigens expressed on renal allograft endothelium [22,23], these T cells may be activated and produce cytokines that convert non-Tregs to Tregs, resulting in local enrichment of Tregs in the allograft (Fig. 4). Such enrichment of Tregs in the renal allograft has been observed in our previous studies [17,24] as well as our most recent study that showed intra-graft FOXP3 RNA expression to be significantly higher in tolerant recipients than in recipients that developed rejection (M. Matsunami et al. manuscript submitted). These locally enriched Tregs in the renal allograft may be protecting the allograft from rejection.
Fig. 4.
A hypothesis of renal allograft tolerance via transient mixed chimerism: Chimeric donor cells provide significant donor-antigen presentation under co-stimulatory blockade. This may result in generation of donor-specific memory T-cells that can produce cytokines (such as Th3 that produce TGF-β) upon stimulation by donor antigens. Upon encountering donor antigens in the graft, these T cells locally produce cytokines that favor conversion of no Tregs cells to Tregs in the renal allograft.
6. Clinical studies
At MGH, we have conducted clinical trials for induction of renal allograft tolerance for two different patient populations; 1) Patients with end-stage renal disease (ESRD) without malignancy and 2) patients with ESRD associated with hematologic malignancies.
6.1. Renal allograft tolerance for ESRD patients without malignancy
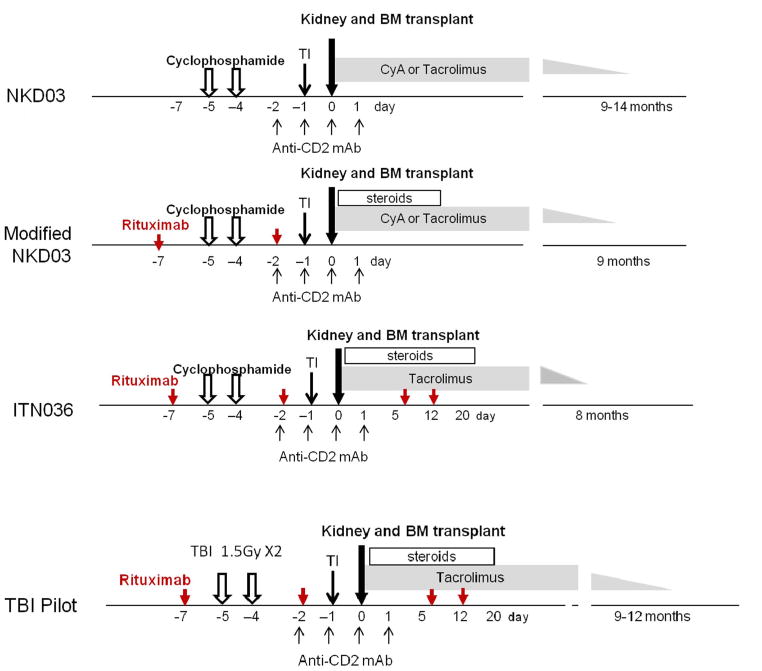
In application of the mixed chimerism approach to achieve renal allograft tolerance for regular ESRD patients without malignancy, any risk of GVHD cannot be justified. In our approach, induction of renal allograft tolerance has been achieved by induction of only transient mixed chimerism, and therefore, the risk of GVHD is very low. Indeed, none of more than 100 NHP recipients and 13 human recipients who received our conditioning regimen and CKBMT have developed GVHD. The initial conditioning regimen (NKD03, Fig. 5) for HLA-mismatched kidney transplantation consisted of cyclophosphamide (60 mg/kg) administered intravenously (i.v.) on Days −5 and −4; humanized anti-CD2 mAb (MEDI 507) on Days −2, −1, 0 and +1; and TI (700 cGy) on Day −1. On Day 0, kidney transplantation was performed, followed by i.v. infusion of fresh, unprocessed donor bone marrow (DBM; 2–3 × 108 cells/kg). CyA was administered postoperatively and then slowly tapered after 6 months and completely discontinued by 9–14 months. This regimen was modified, after the occurrence of acute humoral rejection in the third patient, to include administration of two doses of rituximab (375 mg/m2/dose) on days −7 and −2. To mitigate acute kidney injury (AKI), presumably caused by cytokine syndrome associated with the loss of chimerism, a short course of post-transplant prednisone was also added (mod NKD03). Since delayed humoral responses were still observed in recipients treated with the mod NKD03, the regimen was further modified in its next iteration (ITN036) by adding two more doses of rituximab on days +5 and +12 and substituting tacrolimus for CyA. Tacrolimus was slowly tapered over several months and completely discontinued by 8 months, after confirming absence of rejection by protocol biopsy at 6 months. Together these three protocols were evaluated in 10 HLA-mismatched kidney transplant recipients (Table 1). Transient mixed chimerism for up to 3 weeks was induced in all recipients, without any evidence of graft versus host disease. Immunosuppression was successfully discontinued in 7 of the 10 recipients for more than 4 years. Four have remained off immunosuppression and remain well, with normal kidney function, after more than 7–14 years. In the other three, immunosuppression was resumed after 5, 7 and 8 years (i.e. with immunosuppression-free graft survivals of 4, 6 and 7 years), due either to recurrence of the original kidney disease or development of chronic rejection [25].
Fig. 5.
The initial conditioning regimen (NKD03) consisted of cyclophosphamide (60 mg/kg) on Days −5 and −4; humanized anti-CD2 mAb (MEDI 507) (0.6 mg/kg/dose) on Days −2, −1, 0 and 0.1; cyclosporine A (CyA) (5 mg/kg) i.v. on Day −1 and thymic irradiation (7 Gy) on Day −1. On Day 0, kidney transplantation was followed by i.v. infusion of unprocessed donor bone marrow (DBM; 2–3 × 108 mononuclear cells/kg). Oral CyA (Neoral) was administered postoperatively at 8–12 mg/kg/day with target trough blood levels of 250–350 ng/mL, then tapered and discontinued over several months. The protocol was modified after treatment of the third subject, with the addition of rituximab, 375 mg/m2/dose on Days −7 and −2 (red arrows); and prednisone, 2 mg/kg/dose starting on the day of transplantation and tapering to withdrawal over the next 10 post-transplant days (mod NKD03). Since subjects treated with this mod NKD03 still developed donor-specific antibodies (DSAs) after discontinuation of immunosuppression, the regimen was further modified to add two more doses of rituximab (375 mg/m2/dose) on Days 5 and 12 (red arrows), plus a more prolonged course of prednisone until Day 20, and tacrolimus in place of CyA (ITN036). Tacrolimus was slowly tapered over several months and completely discontinued at 8 months after confirming no rejection by a 6-month protocol biopsy. To overcome acute kidney injury (AKI) observed in 9/10 recipients of cyclophosphamide-based regimen, a pilot study with a modified regimen, in which TBI substituted cyclophosphamide, was tested in two patients (TBI Pilot)(For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Table 1.
The summary of clinical experience.
| # | Protocol | Transient chimerism | Engraftment syndrome | Outcome | I.S. free graft survival |
|---|---|---|---|---|---|
| 1 | NKD03 | + | − | No rejection | > 13 years |
| 2 | NKD03 | + | + | MPGN recurrence | 7 years |
| 3 | NKD03 | + | + | AHR on day 10 (Pre-sensitized) | – |
| 4 | Mod NKD03 | + | + | Chronic rejection after 5 years | 4 years |
| 5 | Mod NKD03 | + | + | Chronic rejection after 7 years | 6 years |
| 6 | ITN036 | + | + | No rejection | > 7.2 years |
| 7 | ITN036 | + | + | No rejection | > 7 years |
| 8 | ITN036 | + | + | Lost graft 6 month due to TMA | – |
| 9 | ITN036 | + | + | No rejection | > 6.8 years |
| 10 | ITN036 | + | + | Acute rejection | 2 months |
| 11 | TBI/aCD2 | + | − | No rejection | > 2.5 years |
| 12 | TBI/aCD2 | − | − | Stable under I.S. | Under I.S. |
I.S.:immunosuppression, TBI: Total Body Irradiation.
Acute kidney injury (AKI) was observed in all but the first of these initial 10 study subjects, between 10 and 20 days after SKBMT. The AKI was associated with the loss of chimerism as well as with the recovery of host T cells. Biopsies taken at the time of AKI showed capillary endothelial injury with some CD8+ and CD68+ cells in peritubular and glomerular capillaries [26]. Among these nine recipients that developed AKI, three recovered without additional treatment. Renal allograft function normalized in four recipients in conjunction with anti-rejection therapy, including thymoglobulin, plasma exchange and steroid therapy. In two patients (subjects #3 and #8) the renal allografts failed to recover. Subject #3 was known to have high PRA, although donor-specific antibody (DSA) was negative by ELISA before transplant. This subject developed irreversible humoral rejection on day 10. Subsequent assessment of pre-transplant serum, by the more sensitive Luminex technique, revealed significant DSA (> 6000 MFI), which had been missed by ELISA and was presumably an indication of the presensitization which led to acute humoral rejection. The second failure (subject #8) developed AKI which was misdiagnosed as rejection and treated with higher levels of tacrolimus, leading to thrombotic microangiopathy (TMA), likely due to tacrolimus-induced toxicity. Kidney function remained compromised thereafter and at 6 months post-transplant the subject returned to dialysis.
Since AKI had not been observed in NHPs which received TBI–based conditioning regimen, a pilot study, in which cyclophosphamide was replaced with low-dose TBI, was recently conducted in two recipients (Fig. 5, TBI pilot). Both recipients did well without development of AKI and one of them has been off immunosuppression for more than 2 years (manuscript in preparation).
We compared postoperative complications and Quality of Life (QOL) of five tolerant recipients (tolerant group) with thirty-one comparable live donor kidney recipients on conventional immunosuppression (conventional group) (Table 2) [27]. Patients in the tolerant group required significantly less treatment after transplant for hypertension and no medications for diabetes (P < 0.01). In addition, there was no dyslipidemia nor malignancy in the tolerant group. In contrast, diabetes, dyslipidemia and malignancy were observed in 12.5%, 40.6%, and 11.8% of the conventional group, respectively. Tolerant patients experienced better overall health (P < 0.01) and scored higher on kidney transplant-targeted scales and healthy survey scales than patients in the conventional group according to the KDQOL SF-36 (P < 0.05). Tolerant patients were less likely to experience depression, dyspnea, excessive appetite/thirst, flatulence, hearing loss, itching, joint pain, lack of energy, muscle cramps, and lack of libido than conventional patients according to the MTSOSD-59R (P < 0.05) [27]. These observations emphasize the induction of tolerance induction for maintenance of overall QOL.
Table 2.
The summary of QOL study.
| Characteristics | Tolerant group (n = 5) | Conventional group (n = 32) | P value |
|---|---|---|---|
| Complications –No. of events (%) | .33 | ||
| Malignancy | 0 | 4 (11.8) | .41 |
| Infection | 2 (40.0) | 14 (43.8) | .62 |
| Medications after transplant -% patients | .002 | ||
| Hypertension | 20 | 78.1 | .004 |
| Dyslipidemia | 0 | 40.6 | .07 |
| Diabetes requiring insulin | 0 | 12.5 | .40 |
| QOL measure | Tolerant group (n = 5) | Conventional group (n = 16) | |
| Kidney transplant-targeted scales | |||
| Burden of kidney transplantation | 98.75 ± 11.27 | 70.70 ± 30.50 | .04 |
| Sexual function | 100 | 70.83 ± 33.07 | .04 |
| 36-item health survey scales | |||
| Physical function | 98.89 ± 2.48 | 73.95 ± 31.6 | .04 |
| General health | 76.00 ± 6.52 | 51.87 ± 28.33 | .04 |
| Social function | 100 | 70.31 ± 32.87 | .03 |
| Energy/fatigue | 72.00 ± 5.70 | 52.50 ± 21.21 | .04 |
| Overall health | 90.00 ± 7.07 | 66.87 ± 23.30 | .01 |
| Symptom occurrence (mean % occurrence) | Tolerant group (n = 5) | Conventional group (n = 16) | |
| Depressed | 0 | 32.14 | .001 |
| Excessive appetite | 0 | 30.36 | .006 |
| Flatulence | 0 | 17.86 | .04 |
| Healing loss | 0 | 21.43 | .04 |
| Increased thirst | 6.25 | 35.71 | .04 |
| Itching | 0 | 17.86 | .04 |
| Joint pain | 0 | 23.21 | .02 |
| Lack of energy | 12.5 | 32.14 | .04 |
| Muscle cramps | 0 | 17.86 | .02 |
| Reduced libido | 0 | 28.57 | .04 |
| Shortness of breath | 0 | 17.86 | .02 |
6.2. Renal allograft tolerance for ESRD patients with hematologic malignancies
This patient population is unique as they are not eligible for kidney transplantation because of the malignancy and for bone marrow transplantation because of renal failure. In contrast to the ESRD patients without malignancy, induction of durable full or mixed chimerism is necessary to induce a graft versus tumor (GvT) effect. With durable chimerism, there is also a risk of GVHD. To achieve potent GvT responses and induce tolerance for the renal allograft, ten patients (median age: 50 years [range: 34–57 years]) with multiple myeloma and ESRD have undergone an HLA-matched CKBMT with lead follow-up time of more than 19 years. The initial preparative regimen for HLA matched transplants consisted of high-dose cyclophosphamide (60 mg/kg on days −6 and −5), equine antithymocyte globulin (Atgam 15–20 mg/kg on days −1, +1, +3 and +5) and pretransplant thymic irradiation (7Gy on day −1). Cyclosporine as the sole posttransplant immunosuppressive therapy was tapered and discontinued as early as day 73 post-transplant. All ten patients achieved mixed chimerism. Two patients developed acute GVHD and three chronic GVHD after their initial transplant (two patients underwent a second stem cell transplant from the same donor for progressive myeloma, which was complicated by chronic GVHD). Five of ten patients are alive, two with no evidence of myeloma from 7.2 to 19.2 years post-transplant. Three patients have normal or near-normal renal function without needing systemic immunosuppression. Two patients with normal renal function off immunosuppression were returned to immunosuppressive therapy because of chronic GVHD [28,29]. The patient survival of our approach with HLA-matched CKBMT for refractory myeloma has been 100% at 3 years, which is superior to the reported survival of myeloma patients with dialysis dependent ESRD without CKBMT (25% at 3 years) [30]. The regimen for HLA matched CKBMT has been revised to a TBI-based conditioning which has consisted of TBI 2-4Gy on day −1, Atgam (20 mg/kg on days −3, −1, +3 and +5) and post-transplant cyclosporine or tacrolimus. Two patients (one with myeloma and one with systemic AL amyloidosis and myelodysplastic syndrome) have been treated with this regimen. Both developed persistent full donor chimerism (one after a second transplant from the same donor for hematopoietic graft rejection) and are currently doing well without immunosuppression. This approach has recently been extended to HLA haplo-identical CKBMT using post-BMT cyclophosphamide for GVHD prophylaxis. The conditioning regimen consisted of pre-transplant conditioning with Thymoglobulin (replaced with fludarabine after the second patient), low-dose cyclophosphamide, and 2 Gy of TBI. GVHD prophylaxis was post-transplant high-dose cyclophosphamide (50 mg/kg/day on days +3 and +4) followed by tacrolimus and mycophenolate mofetil (MMF) starting on day +5 [31]. Five haplo-identical CKBMT have performed and two of them have been off immunosuppression [32].
7. Conclusions
Tolerance induction is now a clinical reality, at least for patients undergoing living donor KTx. Future efforts are being directed toward extension of the mixed chimerism approach to deceased-donor kidney transplantation and to transplantation of other organs and tissues. Further modifications of the conditioning regimen are being explored to develop the more robust mixed chimerism and reduced morbidity that will likely be required for extension to these wider clinical applications.
Acknowledgments
The present work was supported in part by NIH/NIAID NO1 AI1541, the Immune Tolerance Network (ITN) and Grant 5U19AI102405, part of the NIH NHP Transplantation Tolerance Cooperative Study Group and sponsored by the National Institute of Allergy and Infectious Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- NHPs
non-human primates
- MHC
major histocompatibility complex
- mAb
monoclonal antibody
- TBI
total body irradiation
- TI
thymic irradiation
- ATG
anti-thymocyte globulin
- CyA
cyclosporine A
- SKBMT
simultaneous kidney and donor bone marrow transplantation
- DBMT
donor bone marrow transplantation
- Tregs
regulatory T-cells
- TGF-β
transforming growth factor-β
- HLA
human leukocyte antigen
- AKI
acute kidney injury
- DSA
donor-specific antibody
- QOL
quality of life
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 2.Dharnidharka VR, Caillard S, Agodoa LY, Abbott KC. Infection frequency and profile in different age groups of kidney transplant recipients. Transplantation. 2006;81:1662. doi: 10.1097/01.tp.0000226068.66819.37. [DOI] [PubMed] [Google Scholar]
- 3.London NJ, Farmery SM, Will EJ, Davison AM, Lodge JP. Risk of neoplasia in renal transplant patients. Lancet. 1995;346:403. doi: 10.1016/s0140-6736(95)92780-8. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2:807. doi: 10.1034/j.1600-6143.2002.20902.x. [DOI] [PubMed] [Google Scholar]
- 5.Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346:580. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 6.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256. [PubMed] [Google Scholar]
- 8.Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Ochiai T, Boskovic S, Nadazdin O, Oura T, Schoenfeld D, et al. Use of CTLA4Ig for induction of mixed chimerism and renal allograft tolerance in nonhuman primates. Am J Transplant. 2014;14:2704. doi: 10.1111/ajt.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in non-human primates. Nat Med. 2009;15:746. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Yamada Y, Tonsho M, Boskovic S, Nadazdin O, Schoenfeld D, et al. Alefacept promotes immunosuppression-free renal allograft survival in nonhuman primates via depletion of recipient memory T cells. Am J Transplant. 2013;13:3223. doi: 10.1111/ajt.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 15.Oura T, Ko DS, Boskovic S, O'Neil JJ, Chipashvili V, Koulmanda M, et al. Kidney versus islet allograft survival after induction of mixed chimerism with combined donor bone marrow transplantation. Cell Transplant. 2016;25:1331. doi: 10.3727/096368915X688966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Cosimi AB, Wee SL, Houser S, Andrews D, Sogawa H, et al. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation. 2002;73:1757. doi: 10.1097/00007890-200206150-00011. [DOI] [PubMed] [Google Scholar]
- 17.Tonsho MBG, Boskovic S, Nadazdin O, Smith N, Colvin R, Sachs D, Cosimi A, Kawai T, Madsen J. Successful tolerance induction of cardiac allografts in non-human primates through donor kidney co-transplantation. Am J Transplant. 2013;13(Suppl 5):181. [Google Scholar]
- 18.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087. [PubMed] [Google Scholar]
- 19.Yamada Y, Nadazdin O, Boskovic S, Lee S, Zorn E, Smith RN, et al. Repeated injections of IL-2 break renal allograft tolerance induced via mixed hematopoietic Chimerism in monkeys. Am J Transplant. 2015;15:3055. doi: 10.1111/ajt.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotta K, Aoyama A, Oura T, Yamada Y, Tonsho M, Huh KH, et al. Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI Insight. 2016;1 doi: 10.1172/jci.insight.86419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178:179. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 22.Muczynski KA, Ekle DM, Coder DM, Anderson SK. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. J Am Soc Nephrol. 2003;14:1336. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- 23.Shiao SL, Kirkiles-Smith NC, Shepherd BR, McNiff JM, Carr EJ, Pober JS. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. J Immunol. 2007;179:4397. doi: 10.4049/jimmunol.179.7.4397. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599. doi: 10.1111/ajt.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farris AB, Taheri D, Kawai T, Fazlollahi L, Wong W, Tolkoff-Rubin N, et al. Acute renal endothelial injury during marrow recovery in a cohort of combined kidney and bone marrow allografts. Am J Transplant. 2011;11:1464. doi: 10.1111/j.1600-6143.2011.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madariaga ML, Spencer PJ, Shanmugarajah K, Crisalli KA, Chang DC, Markmann JF, et al. Effect of tolerance versus chronic immunosuppression protocols on the quality of life of kidney transplant recipients. JCI Insight. 2016:1. doi: 10.1172/jci.insight.87019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68:480. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011;91:672. doi: 10.1097/TP.0b013e31820a3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy PT, Baldeo C, O'Kelly P, Sargant J, Thornton P, McCloy M, et al. Dialysis-dependent renal failure at diagnosis continues to be associated with very poor outcome in multiple myeloma. Br J Haematol. 2014;165:890. doi: 10.1111/bjh.12818. [DOI] [PubMed] [Google Scholar]
- 31.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YB, Kawai T, Spitzer TR. Combined bone marrow and kidney transplantation for the induction of specific tolerance. Adv Hematol. 2016;2016:6471901. doi: 10.1155/2016/6471901. [DOI] [PMC free article] [PubMed] [Google Scholar]