Abstract
Advancement in neurotechnologies for electrophysiology, neurochemical sensing, neuromodulation, and optogenetics are revolutionizing scientific understanding of the brain while enabling treatments, cures, and preventative measures for a variety of neurological disorders. The grand challenge in neural interface engineering is to seamlessly integrate the interface between neurobiology and engineered technology, to record from and modulate neurons over chronic timescales. However, the biological inflammatory response to implants, neural degeneration, and long-term material stability diminish the quality of interface overtime. Recent advances in functional materials have been aimed at engineering solutions for chronic neural interfaces. Yet, the development and deployment of neural interfaces designed from novel materials have introduced new challenges that have largely avoided being addressed. Many engineering efforts that solely focus on optimizing individual probe design parameters, such as softness or flexibility, downplay critical multi-dimensional interactions between different physical properties of the device that contribute to overall performance and biocompatibility. Moreover, the use of these new materials present substantial new difficulties that must be addressed before regulatory approval for use in human patients will be achievable. In this review, the interdependence of different electrode components are highlighted to demonstrate the current materials-based challenges facing the field of neural interface engineering.
Keywords: Bioelectronics, Electrodes, Microelectromechanical Systems, Sensors/Biosensors, Photonics
Graphical abstract
Neural interface engineering aims to apply advanced functional materials to seamlessly integrate neural technology with the nervous system in order to restore brain function in patients and uncover at least some of the brain’s mysteries. This review highlights the challenges and interdependence of material components for long-term functional performance, and compiles a “roadmap” to guide materials-based neural interface engineering.
1. Introduction
Implantable neural interfaces are important tools for capturing and modulating the sophisticated computations of the nervous system. This technology has seen an explosion in research, innovation and potential applications. In order to better understand plastic changes in neural networks, these interface components must maintain stability over the long time periods associated with memory formation and learning.[1] Clinical scientists have also applied knowledge gained from basic neuroscience studies to develop interfaces with the nervous system for therapeutic or assistive purposes in patients with injury and disease.[2–4] For example, electrical stimulation using implantable neural interfaces –or neuromodulation devices– have received FDA market approval for the treatment of a diverse set of conditions including epilepsy, depression, Parkinson’s Disease, sleep apnea, blindness, deafness, obesity, urinary and fecal incontinence, and hypertension. Patients have also used similar devices to detect and direct brain signals to control robotic limbs, bypassing their injured or degenerated spinal cord.[3–4] While these clinical successes highlight the potential of these technologies, concerns over large variability in therapeutic/assistive efficacy, long-term reliability, and health risks[5–8] prevent these devices from reaching their full potential. This article reviews the recent advances and continued materials based challenges of this rapidly expanding field.
1.1. Neural Signals
The success of an implanted neural interface is contingent upon the quality of the signals that the interface can detect from the nervous system, as well as the reliability and precision with which the interface can modulate the nervous system. Extra-cellular neural recordings can be used to isolate individual neuronal action potentials (also known as “single-units” or spikes), which represent the most basic code in neuron communication. Extra-cellular recordings that do not isolate individual action potentials are called multi-unit recordings which arise when two or more single-units from different nearby neurons are recorded simultaneously. Finally, local field potentials (LFP) are generated by the summation of neural activity from a large population of neurons within a spatial region. LFP is often comprised of low frequency oscillations (0.1 Hz – 120 Hz) that are less sensitive to spatial interference. For neuroprosthetics applications, single-unit and multi-unit recordings are often preferred over LFPs, due to the specificity of the information conveyed but all have demonstrated useful read-out information.
Although neuronal signal transduction is electrical in nature, it is propagated at each axon/dendrite terminal, called a synapse, by the release of neurotransmitters. Unlike action potentials and LFPs, neural chemical sensing electrodes are specific to certain electroactive neurotransmitters such as dopamine or adenosine. These biosensors rely on a technique called Fast-Scan Cyclic Voltammetry (FSCV) to measure the concentration of molecules based on their reduction potential. While these devices do not operate in the same temporal domain as traditional electrodes, their sensitivity to neurotransmitter-specific signaling is critical for assessing the activity from these types of neurons. Neurochemical recordings utilizing FSCV electrodes have been obtained in human patients by multiple groups,[9–10] and have been proposed as a fundamental human research tool as well as a critical sensing component for closed-loop neuromodulation systems.[11–12] When combined with traditional electrophysiological recording modalities, FSCV neurochemical measurements can be instrumental in understanding the complex multimodal therapeutic effects and limiting side effects from neuromodulation therapies to treat psychiatric disorders.
In addition to sensing electrical current or neurochemicals, electrode-based neural interfaces can also be used to introduce signal into the nervous system by injecting current into local tissue (0.1 Hz to >10,000 Hz). Electrode designs can vary dramatically depending upon the anatomical location and therapeutic application for stimulation. For example, simple bipolar or tripolar electrode configurations are typically used to stimulate the vagus nerve for epilepsy and depression, while indwelling microcortical arrays with hundreds of electrode sites are used to provide sensory feedback for a prosthetic.
Recent advances in genetics have expanded the neural interface toolset beyond electrode-based technologies. Optogenetics combines genetics with light-sensitive proteins to instill membrane channels or activity reporters in selective cell populations. Genetic targeting provides a level of control not possible with traditional electrode technology and is attained using several approaches. A common approach is to rely on specific promoters that regulate the expression of genes in cells targeted by the promoter. Major constraints for optogenetics are the requirement for genetic manipulation, the limited availability of promoters, and the potential variability of genetic transcription. Many of the biological challenges for optogenetics are due to poor control over the number of transgenes inserted into the genome, gene insertion loci, promoter enhancers/inhibitors/silencers activity based on insertion loci, and for virus optogenetics, trade-offs between diffusion radius and transduction rates.[13] In addition, due to biophysics and protein kinetics limitations, high sensitivity opsins will have slower channel kinetics while fast opsins will have lower sensitivity.[14] For the purpose of this work, the focus will be on the challenges in delivering and extracting light over the desired spatial resolution with implantable devices (section 2.15). However, it is critical to understand that optogenetic control will be an ongoing challenge for both biological engineering and materials engineering of neural interfaces.
In general, neural interfaces intended to stimulate nervous tissue or record neural activity from the nervous system face a similar material based challenge. The fundamental goal is to enable a higher number of input/output channels from the nervous system, while only creating a minimal disruption of tissue. Avoiding tissue damage is important both for the long-term patency of the interface as well as for minimizing safety risks to the patient. As will be explained in subsequent sections, this fundamental trade-off motivates a number of interdependent design choices with interactions that are often not considered.
1.2. Current Challenges
Current challenges with chronically implantable neural interfaces can largely be categorized into performance reliability and variability issues. Invasive surgical procedures to implant chronic neural interfaces carry inherent risks, such as brain infection, surgery related hemorrhaging, blood clots, edema, etc. As such, these interfaces would ideally be designed to minimize tissue trauma, remain both functional and biocompatible for the life-time of the patient to avoid corrective surgery or re-implantation, and be removable in the event of unforeseen complications. Across all neural implant classes, there is an average decline of the signal-to-noise ratio (SNR) in the communication between the nervous system and neural interfaces over time. The resulting decline in neural interface reliability is believed to be, in part, due to the inflammatory tissue response triggered by the surgical implantation procedure. This initial reaction can progress into a chronic immune response, ultimately turning into a foreign body response and even leading to migration of the tissue or implant.[7, 15] While the details of the biological challenges with chronic neural interfaces have been discussed elsewhere,[8] this reactive tissue response degrades the electrical characteristics of the interface by forming a high impedance encapsulation sheath in conjunction with neural degeneration. This response decreases the amplitude of the detected neural signals by increasing the minimum distance from the electrode to the nearest neuron firing action potentials, thereby increasing the applied current necessary for exciting nearby neurons, and limiting the precision of activation via electrical stimulation.[16–17] A number of acute and delayed stab wound studies show that the tissue can recover to some extent when the device is removed,[17–20] suggesting that certain physical properties of chronically implanted neural interfaces are responsible for this tissue reaction.[20]
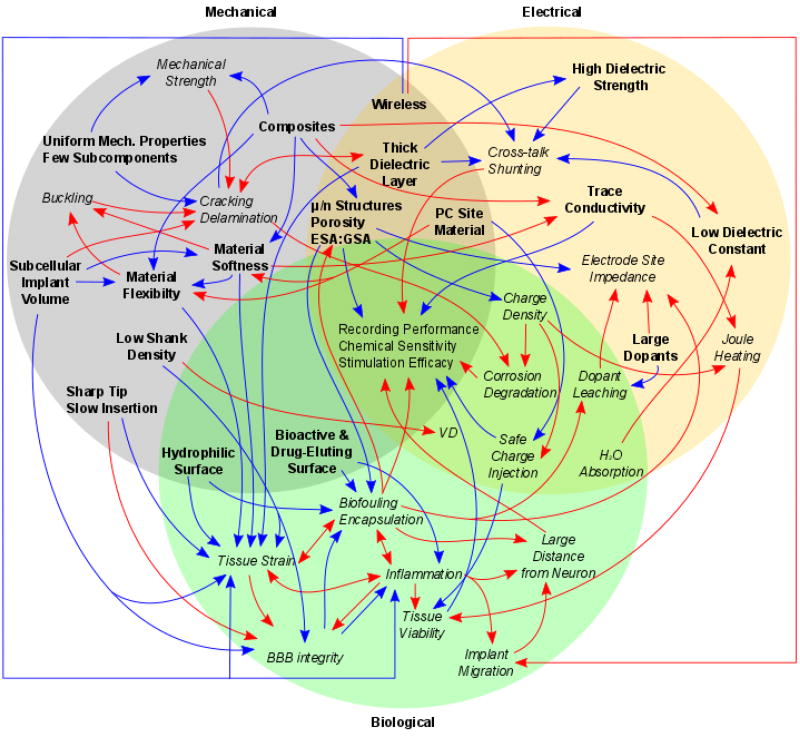
The focus on minimizing the foreign body response has increased the emphasis on studying advanced materials to overcome specific design parameters hypothesized to attenuate the inflammatory tissue response (see reviews[8, 21–28]). However, large histology and recording performance variability[8, 29–34] have made it difficult to disentangle contributions from the many physical properties of these devices to the myriad of observed multi-modal failure modes.[5, 8, 35–37] These complex failure modes produce histological outcomes that, in isolation, are poor predictors of actual performance outcomes as measured by brain-machine neural communication with high degrees of freedom and SNR.[34–35] Similarly, for stimulation electrode sites, small amounts of platinum particulates expelled over the course of stimulation, and the resulting foreign body response, have not been linked to any additional clinically adverse tissue reaction beyond those of a recording electrode.[38–41] Current literature often highlights the advances of individual material properties and therefore has a limited discussion of the broader obstacles limiting the advancement of the field. While excellent reviews can be found on advancing individual design properties[21–28, 42] or expanding upon the biological tissue reaction,[8] this review aims to highlight the interdependence of multiple design and material properties as well as to guide the success of balancing and titrating these properties. Through this multi-dimensional cost-benefit analysis, engineers can design functional neural interfaces capable of obtaining selective, high-fidelity, long-lasting readouts of brain activity. Material selection, the design and assembly of the materials, and strategies for surgical delivery of the implantable components will likely provide critical breakthroughs in achieving improved device performance.
2.0 Material Considerations for Engineering
The simplest electrode design consists of a conductive component to transfer electrical charge, insulation to spatially constrain neuronal signals, and a connector that provides external interfacing with signal processing devices. However, there are numerous intrinsic material properties (conductivity, flexibility, material strength, and chemical stability) as well as extrinsic design parameters (geometry, density, insertion, and packaging) that must be considered to achieve long-term biocompatibility and functionality. These individual intrinsic and extrinsic properties are interdependent on one another and optimizing one can negatively impact overall performance or lead to loss of functionality. The goal of this review is to introduce a number of the key material and design properties as well as their complex interdependence on one another. Understanding these engineering challenges are critical for the development of devices with long-term functionality.
2.1. Electrical Property/Electrode Traces
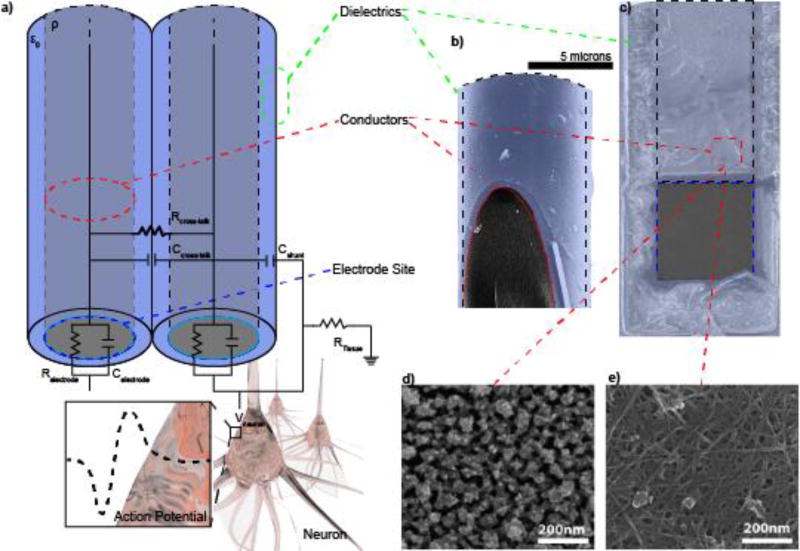
One of the most important components of any electrode technology is the conductor, which forms the electrical connection, also known as electrical trace, that carries current (Figure 1a). Every electrode must include a conductive element which transfers detected signal from the tissue to an external electrical device, such as a headcap for cortical recording electrodes, or transmits current to electrode site to a percutaneous connector or transducer. The conductance (G) is the material property that describes the ease with which charges can move within it:
| (1) |
| (2) |
where R is the resistance of the conductor, A is the cross-sectional area of the conductor, l is the length, σ is the electrical conductivity in siemens per meter (S·m−1), and ρ is the electrical resistivity or specific electrical resistance (Ω·m).
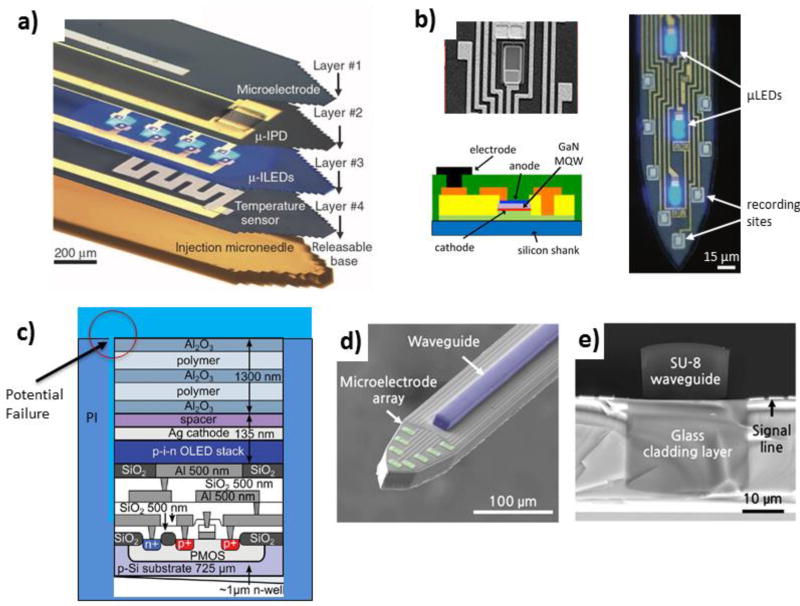
Figure 1. Basic Electrode Anatomy.
a) Randles circuit representation of the electrode, which includes the electrode trace (red dash), insulation (green dash), and electrode site (blue dash: Relectrode and Celectrode) where neural potentials (Vneuron) are recorded from. Note: Post-electrode equipment is not shown. b) Cross-section of a Microthread Electrode[251] showing the carbon fiber core and Parylene insulation. c) Layer-by-Layer (LbL) assembled composite electrodes using Au nanoparticles (d) or carbon nanotubes (e). Reprinted with permission from[569] Copyright 2012, American Chemical Society.
As shown in Eq. 1 and Eq. 2, the dimensions of the material can play a crucial role in determining electrical properties. With the emergence of nanoscale and angstrom diameter wires, it is important to consider how the size of the electrical trace can constrain the maximum signal or stimulation current that can safely be passed through the device. The minimum cross-sectional area (A) is limited by the heating power (P, Joule heating, also known as ohmic or resistive heating), especially for electrical stimulation and chemical sensing electrodes;
| (3) |
where I is electrical current. At some finite A the electric current will produce enough heat that it might burn/oxidize or melt the conductor, reducing its conductivity. Given all of these considerations, specific materials and the method of deposition have unique advantages and disadvantages that should be considered when choosing an electrical trace conductor for particular applications.
Metals, particularly noble metals, are perhaps the oldest and most common electrical conductors used for neural electrodes.[43–44] Their high electrical conductivity originates from the overlapping valence and conductance bands of metal atoms, allowing for relatively free movement of electrons through the solid ‘electron sea’ lattice.[45] Selection of metal conductors requires careful consideration of conductivity, mechanical stability for handling, chemical stability (namely, corrosion resistance), and biocompatibility. In general, heavy transition metals such as gold (Z=79), platinum (Z=78), and iridium (Z=77), as well as tungsten (Z=74) or tantalum (Z=73; for short-term applications) display a number of these desirable properties. The noble metals and alloys can generally remain relatively chemically inert over chronic timescales that would affect other conductor classes.
Advances in microfabrication and photolithography techniques enable well controlled and reproducible batch fabrication, which has led to the use of semi-conductors and polycrystalline materials which are more compatible with these microfabrication processes. Semi-conductors are materials with small band-gaps (between the valence band and conductance band) that can be made conductive by adding electron acceptors (p-dopants) or electron donors (n-dopants) impurities. Polycrystalline materials are composed of small crystalline regions separated by grain boundaries.[46] These grain sizes dramatically impact the conductivity, making them more conductive than their amorphous material counterparts.[47–49] Polycrystalline silicon (Polysilicon) is a highly pure, polycrystalline form of silicon that is easily fabricated into microelectrodes and is compatible with complementary metal-oxide-semiconductor (CMOS)[50] and MEMS[51] technology. Additionally, the conductive properties of polysilicon can be modulated by varying processing conditions or dopant densities.[49] For these reasons, polysilicon is a popular material used in microfabricated silicon arrays. While silicon technology can be more brittle than metal, it offers a greater design space than traditional microwires.
Unlike inorganic semi-conductors, conductive polymers (CPs) are organic polymers that conduct current.[52–57] CPs are composed of monomeric compounds linked in chains of alternating single and double bonds and doped with a stabilizing counter-ion.[58] Conjugated polymers have narrower band gaps, allowing electrons to move easily between the conducting band and valence band. Dopants increase the conductivity of polymers by either removing electrons from the valence band (p-doping) or adding electrons to the conduction band (n-doping). The most common biocompatible conductive polymers used for neural interfaces are Polypyrrole (PPy),[59] poly(ethylene dioxythiophene) (PEDOT)[59] and polyaniline (PAni),[60] with PEDOT being the most electrochemically stable.[28] Most conductive polymers, however, do not contain intrinsic charge carriers and must be doped with a counter ion to reduce electrical resistance and heating, and improve conductance. Therefore, they require counter ions that are partially oxidizing (p-doping) electron acceptor (such as LiClO4, I2, or AsF3) or alternatively partially reducing (n-doping) electron donors such as Na or K. While CPs may be either chemically or electrochemically synthesized,[61–62] careful consideration of the counter-ion’s charge or polarity (zwitterions), and concentration is necessary for electrochemical deposition.[28] Conductive polymers generally have lower conductivity than most metals, but other advantageous properties make them popular for coating electrode sites (see section 2.4).[63] However, recent efforts are aimed at developing conductive polymer composite wires with improved mechanical properties.[64–66] Currently, considerable challenges remain due the brittle deposition of CPs which complicate the fabricattion of electrodes comprised entirely of CPs.
Carbon-based materials (graphene, carbon fiber, carbon nanotubes) are also being developed as the conductive components of electrodes. Some carbon allotropes are highly conductive due to the carbon-carbon pi bonds. Carbon-based materials also have useful mechanical properties such as high tensile strength and modulus. Single carbon fibers (CF) are implemented similarly to metal microwires[67–71] (Figure 1b). Graphene, a hexagonal lattice monolayer of carbon,[72] has exceptional flexibility, surface area, and conductivity.[73] Graphene and graphene oxide (GO) have been implemented as bulk conductors,[73] coatings,[74] and dopants.[75–76] The flat structure of graphene makes it ideal for patterning planar implants.[77–79] Recently developed high aspect ratio all-carbon crumpled graphene transistors demonstrate high sensitivity and improved spatial resolution, with no observed increase in source–drain resistance at up to 56% of compressive strain.[80] In contrast, carbon nanotubes (CNTs) are cylindrically-wrapped graphene sheets. Single-walled carbon nanotubes (SWCNTs) are comprised of a single layer and multi-walled carbon nanotubes (MWCNTs) have multiple layers. These CNTs are emerging as novel substrates for a variety of neural engineering applications due to their high mechanical strength and electrical properties.[81–83] CNTs also can have a range of electrical properties based on the orientation in which the graphene is wrapped in a tube. This property is represented by a pair of indices (n,m), and it modulates the CNTs’ conductive properties: from metallic, to quasi-metallic, or semiconductive[84–85] (see review[28]). Due to challenges of fabricating long CNTs, they are generally employed for the electrode site materials (see section 2.4),[83] however, there has been some effort in developing and patterning CNT containing composite conductors, such as through layer-by-layer deposition methods for neural interfaces (Figure 1c–e).[28, 86–89] For applications where stretchability rather than flexibility is required, CNT-doped copolymer matrices can achieve up to 130% strain.[90] These composite conductors are engineered by bringing conductive particles close enough for electrons to jump from one particle to another known as percolation networks. The small spaces between each particle can absorb more strain before breakage, improving the mechanical properties of the device. However, the tradeoff is that these small spaces lead to increased resistivity compared to solid metals.
To summarize, ideal conductors should achieve exceptional conductivity, long-term functional durability, electrochemical stability, low cross-sectional area, and the ability to withstand stress and strain forces. As devices become smaller and vary in their geometries, materials that can be handled easily during microfabrication are preferred since their conductive properties can be altered with processing. Metals wires are traditional materials with good conductivity and the ability to be assembled in large arrays, but have limited stretchability. Some other conductors trade compatibility with microfabrication techniques and increased design space for assembly into arrays with increased brittleness. Composite materials generally trade decreased conductivity with increased stretchability and/or durability. Often, the decreased conductivity needs to be compensated by increasing the cross-sectional surface area. As such, for developing functional implants, it is critically important to balance all of these material properties as well as those in the following sections.
2.2. Insulation Materials
The other critical component of chronically implantable microelectrode technology is the dielectric layer, or insulation layer. Some commonly empoyed insulating materials include varnish, epoxy, parylene, glass, Teflon, polyimide, fused silica, silicon oxide, silicon nitride, or silicone elastomers.[28, 91] Dielectric materials generally have a large bandgap between the conductive and valence band, which blocks electrical conduction at low voltages. While the functional component of any electrode is the conductive element, insulating dielectric materials are equally critical to preventing parasitic capacitance from allowing the signal to leak into the tissue or into adjacent conductors. The insulating material property is defined primarily by its resistivity (ρ) and the dielectric constant (εr) or the relative static permittivity. For simplicity one may consider the effect of a dielectric material in a parallel plate capacitor (C):
| (4) |
where ε0 is the electrical constant (ε0 ≈ 8.854×10−12 F·m−1), d is the thickness of the insulation layer, and A is the cross-sectional area of the insulation layer along the electrical trace. Insulating materials are characterized as having a relatively low εr, that is, the ratio of the capacitance of a dielectric material to that of a vacuum which tends to 1. The shunt capacitance (Cshunt, Figure 1a) is the capacitance across the insulation layer from the center of the electrode conductor to the conductive electrolyte bath conductor, which results in an inversely frequency dependent impedance across the electrode, behaving as a low pass RC filter with frequency fc:[92–94]
| (5) |
| (6) |
where ω is the angular frequency of the waveform (rad/s). With respect to equations 3 and 4, materials with high dielectric constants or low dielectric thickness lead to high capacitance, coupling of electrical signal to adjacent traces, and attenuating the recorded signal (Vout-shunt). At very thin dielectric thicknesses, the insulation becomes less effective due to electron tunneling.
For high density arrays, current density accumulation in a wire or electrode can cause a change in current density in adjacent wires through capacitive charging with the two wires acting as the capacitor (Ccross-talk: aka, cross-talk capacitance, capacitive coupling or mutual capacitance, Figure 1a). In multi-channel electrode arrays, cross talk is caused by capacitive coupling of one circuit (the “aggressor”) to another (the “victim”):
| (7) |
where ΔVvictim is the potential change induced by the “aggressor” circuit onto the “victim” circuit, ΔVaggressor is the potential change in the “aggressor circuit”, Cgrd is the total capacitance relative to ground, Cadj describes the capacitor formed between the circuits, Ragressor and Rvictim are the resistive loads of the coupled circuit on the conductors respectively, Cgrd−a and Cgrd−v are the capacitances between the aggressor and ground and the victim to ground respectively. Equivalent circuit models developed for electrodes implanted into tissue show that crosstalk increases the effective amplifier impedance (comprised of the head-stage impedance and shunt capacitance) above the effective electrode impedance (comprised of the resistance of the electrolyte solution as well as resistance and capacitance of the electrode) with increasing signal frequency.[93–94] As a result, crosstalk can attenuate recorded signals, cause false signal in adjacent electrodes during recording, or result in unwanted accumulation of charge density between adjacent electrodes during stimulation.[95–96] This effect is especially prominent in small multi-electrode arrays in which resolution is important, as signal in one wire can affect signals in nearby wires. Inert materials or materials with low dielectric constants, such as ceramics or polyimide, reduce crosstalk compared to other poor insulating materials such as silicon, a more conductive substrate.[97] While the source of cross-talk is traditionally in the interconnects and packaging, it becomes an increasingly important issue as engineers attempt to design higher density arrays.[98]
A number of polymer insulating materials have become popular due to the ability to uniformly and reproducibly coat complex micro-scale shapes, lower elastic modulus, attain chronic stability in physiological environments, and achieve compatibility with other microfabrication techniques for selectively controlled de-insulation of active sites.[99] These include parylene and polyimide variants where the side chains or R groups alter the material’s dielectric, mechanical, and sometimes even bioactive properties.[99–101] Careful selection or combination of functional R-groups can also be used to functionalize bioactive surfaces (see section 2.14). Polydimethylsiloxane (PDMS) is a another insulator for neural tissue interfaces due to its biocompatibility.[102] However, effective PDMS-based dielectrics for implants require substantial thickness due to its high water absorption rate, which increases its dielectric constant and shunt capacitance, and decreases the cutoff frequency. Other polymers with very high water absorption cannot be used as insulation materials for chronic implants. For example, many epoxies are unsuitable for use as insulators for chronic electrodes because it absorbs water, negatively impacting dielectric properties as well as allowing for corrosion conditions in devices utilizing dissimilar metal connections within the insulation, especially under stimulation conditions.[103–104]
Another property that requires consideration, especially for electrical stimulation, is the material’s dielectric strength. Dielectric strength, in contrast to the dielectric constant, decreases with surface defects (pin-hole defects; Figure 2a), increases with temperature, increases with signal frequency, and can decrease with water absorption. Careful consideration of the dielectric property, water absorption, and insulation thickness are important for chronic neural interfaces. At high voltages (on the order of 106 V/cm, the limit increases with decreasing relative permittivity and thickness) dielectric materials ‘break-down’ and become conductive.[105] Exceeding these voltages will usually also lead to other failure modes such as gross degradation or melting.[106] However, for chronic electrical stimulation, lower DC voltages that are either deliberately induced to power active components on the electrode, or naturally arise during stimulation can exacerbate failure of the insulation, especially at points of defects. For example, the increase in time to failure for an Al2O3 and parylene bilayer is 13 times faster when a 5V bias is applied.[107] Also, thin dielectric films risk the possibility of forming a simple low-pass RC circuit. An RC low-pass filter allows frequencies from 0 Hz (DC) to its cutoff frequency to pass while attenuating higher frequencies, which can contain critical single-unit information. In this case, a negative capacitance amplifier can help correct for the various capacitive impedances introduced by the conductor/insulator capacitors.[92, 108]
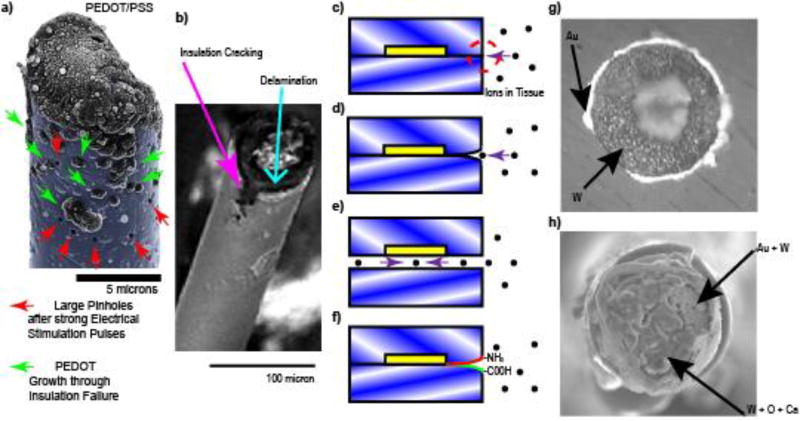
Figure 2. Insulation Failures.
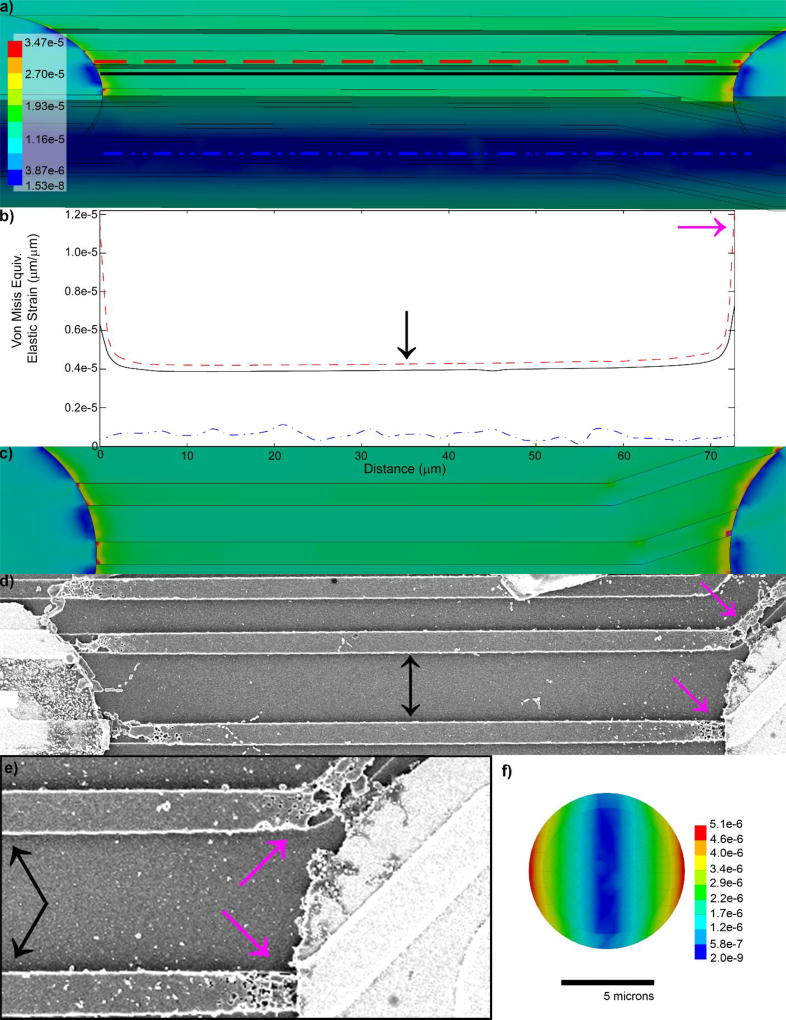
a) Strong stimulation currents lead to insulation failure that starts as pin hole defects (Red Arrow) in the insulation (cyan). Complete Insulation failure (green arrow) can be identified over regions of PEDOT/PSS electrochemical deposition through the insulation (cyan). b) Many dielectric polymers such as parylene are susceptible to cracking (magenta arrow) and delamination (cyan arrow). © IOP Publishing. Reproduced with Permission from[210]. All rights Reserved. c) Planar arrays made from crystalline polymers form triple junctions (red dashed circle) when implanted. The two crystalline layers cannot be perfectly aligned due to entropy (blue/white angles). d) Ions and water molecules in the tissue thermodynamically degrade the interface of the two crystalline layers forming a crack. e) Cracks thermodynamically propagate until insulation fails. Note: this is not an issue if the triple junction crack propagation failure rate is slower than the lifetime of the patient. f) Self-healing smart polymers with self-annealing functional groups and re-seal delamination. g) Insulation adhesion layers (eg. Au) are sometimes used to reduce insulation delamination failures. h) In the tissue, the wire (W), exposed adhesion layers (Au), and the tissue environment creates a galvanic cell, accelerating corrosion. Reprinted with permission from[209] Copyright 2011, Elsevier
Dielectrics are essential in ensuring electrical current travels unimpeded through the conductor preventing any loss in electrical quality through shunting or attenuation. As a result, an ideal insulator would be thin and have a low dielectric constant, high dielectric strength, achieve virtually no capacitance, have minimal potential for water absorption, and are simultaneously thick enough to occlude fluid ingress. These optimal properties would allow small electrical traces to be packed close together, while limiting parasitic capacitance and cross-talk, leading to limited signal loss or attenuation. In addition, the material must be flexible (low Young’s modulus) and remain durable under chronic cycling. Since a wide range of materials fit into the insulator class, other mechanical and adhesion properties must also be considered for applicability to specific electrode designs and functionality requirements.
2.3. Adhesion of the Insulator
During microelectrode production, the insulator is typically either deposited uniformly over the entire conductive material, as in the case of fabricated microelectrodes, or hand-made using backfill in the case of neuromodulatory devices. Active electrode sites are formed by removing insulation in a controlled fashion or by controlling the wire length that protrudes from the insulation sleeve. Exposure methods for microwires, such as mechanical impact, cutting with a razor, pyrolysis (flame), electric arc, plasma arc, and laser can lead to large variability in site size, impedance, and recording quality.[109–113] These methods can also create collateral damage to the surrounding insulation layer, such as cracking and delamination (Figure 2b). Cracking and delamination lead to electrode failure as the geometric area of the exposed conductor becomes too large.[114] This averages the recorded potentials across a larger electrode surface area and attenuates the neural signal (see section 2.4). This underscores the importance of carefully considering the biocompatibility of the underlying materials. For example, many semi-conductors (i.e. gallium or arsenide) are toxic in vivo.[115] While adhesion layer coatings such as Au and Ti have been employed to limit delamination, this can lead to rapid corrosion of the electrode site material (Figure 2g–h; see section 2.5).[116]
A much more controlled approach for exposing the electrode site or depositing the same electrode site is through lithographic techniques, such as through the use of photoresist masks.[117] However, unlike microwires that have conformal coatings of insulation, planar microelectrodes are generally created by sandwiching conductors between two insulation materials. With silicon technology, low pressure chemical vapor deposition of silicon oxide is used to conformally insulate the polycrystalline silicon traces,[118] and provides one of the highest quality SiO2 films available. However, thin films of silicon oxide have a tendency to bow from compressive internal stresses.[119] For this reason, an additional silicon nitride dielectric layer or silicon nitride followed by another silicon oxide dielectric layer are used to balance the bowing and provide additional biostability compared to a single SiO2 layer.[120–121] When two crystalline materials are used to sandwich electrical traces and then are implanted into tissue, a triple junction forms (Figure 2c) often leading to delamination (Figure 2d–e). The thermodynamics of crack propagation at triple-junction sites between crystalline materials has been well studied, especially in electrolytic solutions such as the cerebral spinal fluid.[35, 122–124] One approach to reducing delamination is through thermal annealing, a method that utilizes heat and pressure to improve the seal between two polymer layers.[125] However, this has been shown to impact device performance and sensitivity by influencing bulk polymer properties.[125–126] Polymers that can withstand temperatures near their melting points for longer periods of time such as amorphous (non-crystalline) polymers may be another alternative to forming better thermally-annealed insulators for avoiding triple-junctions. Another approach to prevent crack propagation is through the use of ‘smart’ self-healing materials that can respond to incidences of damage.[127] For example, polymers can be functionalized with reactive chemical species,[128–140] two polymer layers with appropriate functional groups (-CO-NH2 and -COOH) have been shown to self-anneal into imine bonds in vitro that chemically seal two layers together over time without the need for thermal annealing (Figure 2f).[141] In the event of material degradation or delamination, the exposed functional groups may reform covalently through thermodynamic reactions. However, further investigations are required to determine the self-healing potential of these functionalized polymers in vivo. Unlike planer probes that involve polymer-polymer delamination failure, designs such as microwires and bed of needle arrays benefit from being coated with a single conformal layer of insulation. However, these devices still suffer from delamination and cracking at the conductor-polymer interface near their recording sites.[36, 114, 142] Careful material selection and deposition methods are critical for long-term functional performance.
2.4. Electrode size & materials
Electrode site materials and site sizes are important aspects that impact the sensitivity and performance capabilities of neural microelectrodes. Electrode site designs need to carefully consider the electric field strength to selectively attenuate signals resulting from more distant neurons:
| (8) |
| (9) |
where q is the magnitude of the action potential, r is the distance from the source, d is the thickness of the cell membrane, ε0 is the permittivity constant of the tissue, and θ, the angle between the current source and the electrode, is equal to 0. In reality, extracellular action potential characterization depends on the distance between the electrode and the cell, and the radius and dendritic density of the cell.[143] However, in general, empirical measurements indicate that the voltage drop-off is between that of Vmonopole and Vdipole.[144–147] This voltage can be expressed simply by:
| (10) |
where σe is the conductivity of the extracellular space, n is the number of current point sources which the electrode is exposed to, and x is 1 < x < 2 dependending on the size, geometry and type of neuron. Small contact sites (<<1000 µm2) have the advantage of greater selectivity, and can potentially distinguish between multiple single-units with higher signal amplitudes. This is because only a small number of neurons will be within close proximity to small recording sites and detected voltage of distant neurons decay rapidly with r (Figure 3a). However, small electrode sites are impaired by increased impedances and thermal noise from tissue.[145, 148] On the other hand, lowering the impedance by increasing the recording site surface area leads to attenuation of the signal amplitude.[145, 149] (Figure 3a–b) Large sites can be modeled as small sites in parallel with increasing r with distant parallel elements detecting very low V. Voltage elements in parallel are calculated as the average of all parallel elements. As a result, the fall-off of the electric field generated by a nearby neuron is steep; larger electrodes tend to average strong signal from the nearby neuron with weak signals that are more distant, effectively decreasing the signal recorded. Therefore, it is important to select electrode site materials that lower impedance without increasing the geometric site size. For single-unit recordings, the ideal electrode has the lowest possible surface area and impedance.
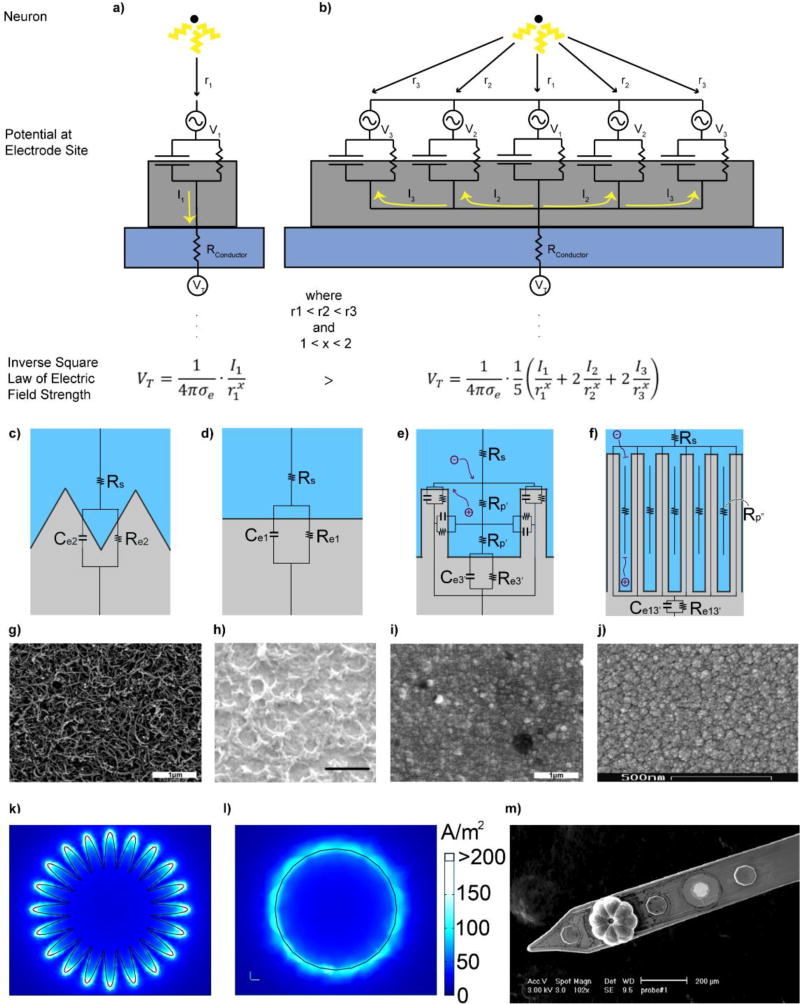
Figure 3. Electrode site size and material properties influence recording and stimulating performance.
A small contact site surface area (a) compared to a larger contact site surface area (b) records larger voltage amplitudes (VT) from a single point source (a neuron) provided by the inverse square law of electric field strength. Note: I2 and I3 are negative. c–f) Charge transfer during electrical stimulation with varying geometric surface areas at the electrode interface. Increasing surface area from (c) to (d) increases the capacitance (Ce2 > Ce1) and halves the resistance (Re1 > Re2). Creating a pore (e) introduces a resistance (Rp’) with a cross-sectional area of A and length l. Increasing pore length from (e) to (f) begins to impact ion movement within the electrode-tissue interface and attenuates electrode performance. g–j) SEM images of electrode site materials: PEDOT/CNT, scale bar 1 µm (g), PEDOT/GO, scale bar 1 µm (h), PEDOT/PSS, scale bar 1 µm (i), and IrOx, scale bar 500 nm (j). Reproduced with permisison from[238],[75], and[570] Copyright, 2016, 2013, and 2010 from IEEE, the Royal Society of Chemistry, and Elsevier, respectively. k,l) Distribution of charge density representing the edge effect for a planar high-surface area electrode (k) and circular electrode (l). Adapted with permission from[183]. Copyright 2009, Frontiers Media SA. m) Recording site electrochemically deposited with polypyrrole (PPy). The octagonal recording site geometry allowed the polymer to grow into eight teardrop segments with an absence of coating from the center. (c–f,m) Reprinted with permisison from[28] Copyright © 2014, Springer Science+Business Media New York
Stimulation electrodes require additional material considerations. The electrode site material and stimulation amplitude on stimulating electrodes determine whether the electrode is capacitive (non-faradaic), involving electrostatic or electrolytic interactions between the electrode-electrolyte interface, or faradaic, which involves redox reactions between the electrode and surrounding medium.[92, 150] Non-faradic electrodes stimulate neurons through the diffusion of ions by charging and discharging the capacitive layer that forms at the electrode surface, which is considerably safer than faradic charge injection methods since the electrode material is chemically stable. Non-noble metals, such as titanium, tantalum, and stainless steel metals inject charge mainly through this double layer capacitance.[151] However, there are charge transfer limitations compared to faradaic electrode materials.[152–153] Irreversible faradic interactions occur when oxidized ions diffuse away from the electrode before they can be reduced and redeposited onto the electrode site, which is called electrode dissolution and can have harmful effects on the surrounding tissue.[154] This is particularly true with metals that generate low molecular weight ions during stimulation. Noble metals such as platinum (Pt), iridium (Ir), palladium (Pd), and rhodium (Rh) are used during neural stimulation due to their corrosion resistance.[155–156] While platinum and iridium have limitations on charge injection capacity,[157–158] platinum-iridium alloys improve mechanical and electrochemical properties, making them suitable as implantable interfaces. Another concern during tissue stimulation is the formation of hydrogen and oxygen gas through the electrolysis of water. The potential range that occurs before the reduction of water (H2 formation) or oxidation of water (O2 formation) is defined as the water window. Platinum electrodes have reported charge injection limits between 50–150 µC/cm2 using biphasic 0.2 ms pulses, safely within limits of the water window.[159] However, platinum dissolution has been shown to occur at as little as 20 µC/cm2 so alternative parameters during stimulation must be carefully considered.[160]
On the other hand, pseudocapacitive electrodes are made from electrode materials that transfer charge through faradaic modes, but some of these metal ions can be reversed back onto the electrode before the ion diffuses away by rapidly applying the opposite charge. These electrode materials are sometimes considered ideal stimulating electrode materials because their method of charge injection involves reversible redox reactions that avoid the harmful dissolution of metal ions but have high charge transfer capabilities. For example, iridium oxide (IrO2) electrodes are used for their mixed conductivity and reversible Ir3+/Ir4+ redox reactions that permit charge injection capacities of about 0.5–8 mC/cm2.[161–162] Sputtered iridium oxide films (SIROF), electrodeposited iridium oxide films (EIROF), and activated iridium oxide films (AIROF) generate the same iridium oxide as an electrode material, but with widely different surface morphologies.[163–165] Other common materials for pseudocapacitors include transition metal oxides, nitrides, and sulfides such as manganese (MnO2), iron (Fe3O4), titanium nitride (TiN), and titanium sulfide (TiS2).[166–172]
While many safe stimulation electrodes exist, they are limited in their ability to spatially and selectively activate small populations of neurons.[173] Ideally, a stimulation electrode will have a very small surface area such that it minimizes activation of nearby axons and neurons to a locally confined area.[28, 174–176] However, attempting to increase specificity by lowering the geometric area results in higher charge densities (Q/A) that can damage tissue.[177–179] To address tissue safety issues, most stimulating electrodes are designed to have large surface areas. One way to safely alter the electrical properties of both recording and stimulating electrodes is to increase the electrochemical surface area (ESA) relative to the geometric surface area (GSA) (Figure 3b–d). The ESA of a probe involves introducing micro- and nanoscaled features that alter the roughness and porosity of the surface of the electrode, improving charge transfer with the surrounding electrolyte solution. Likewise, increasing ESA lowers impedance,[180] so charges of equivalent amplitude can be injected with lower electrode potentials, decreasing the risk of tissue damage. However, there is an upper limit to increasing the ESA, since at some point ions diffusion through the pore become negatively impacted (Figure 3e). For a sinusoidal AC waveform, the penetration depth λ into the pore of a rough surface can be modeled as:
| (11) |
where r is the pore radius (cm), κ is the electrolyte conductivity (1/Ω cm), cd is the surface capacitance (F/cm2), and ω is the angular frequency of the waveform (rad/s). For a given frequency ω, there will be a maximum penetration depth λ at which increasing the surface area beyond this distance will not influence charge transfer, due to resistance of ionic current as a result of increasing pore length(Figure 3e).[181] Likewise in chronic implants, resistance of ion diffusion across the coating can be exacerbated in vivo as reactive tissue and cells block pores across the surface of the electrode, likely overriding any beneficial enhancements over time from increasing the electrochemical surface area.[182] Lastly, it may be worth considering how the geometry of the electrode site leads to an “edge effect”, where charges are more concentrated on the edges of an electrode during stimulation due to repelling like charges,[183–184] leading to preferential corrosion at the edges of the electrode site (Figure 3k–j).[185] Therefore, even when relatively safe stimulation parameters are followed, irreversible redox reaction may still occur at the edges which may lead to corrosion of the electrode, damage of the tissue, as well as facilitate the delamination of the electrode sites from around its edges.
One method of increasing the ESA:GSA ratio to reduce impedance is with CNT, graphene, CP coatings, or other nanostructured surfaces (Figure 3f–j).[28, 81–83, 117, 186] While these materials generally have lower impedances per electrochemical surface area compared to many metals, they are easier to deposit with high ESA. For stimulation, CPs require more careful consideration of the counter ion.[187–188] Among the CPs, PEDOT is considered to be the most stable electrically conducting polymer coating that has demonstrated decreased impedance, similar charging injecting capabilities to iridium oxide electrodes, biocompatibility with tissue, and long-term mechanical stability in vivo.[28, 189–190] Doped CPs have the potential for high storage capacities. PEDOT doped with paratoluene sulfonate (pTS) can produce charge storage capacities of about 130 mC/cm2.[191] The specific dopant and deposition parameters can dramatically alter the surface morphology of the electrode thus influencing charge capacities. For example, poly(styrene sulfonate) (PSS)-doped PEDOT electrodes display a reduced storage capacity of about 100 mC/cm2.[191] These polymers can also be chemically modified to form a plethora of derivatives, each with unique electrochemical and biological properties(see section 2.14).[192]
In contrast to stimulating electrodes or electrodes to record electrophysiological signals, electrodes used to measure electroactive neurochemical concentrations using fast-scan cyclic voltammetry (FSCV) are critically dependent on the interaction between the electrode surface and adsorbed electroactive neurochemicals of interest.[193] FSCV measurements typically consists of two steps: 1) a small DC holding potential is applied to the electrode to promote adsorption of neurochemicals of interest to the electrode surface while repelling confounding interferents and 2) the application of an approximately 10 ms triangular voltage pulse that sweeps from −0.4V to 1.5V back to −0.4Vs (the cathodic/anodic limits and sweep rate may vary based off of the neurochemical of interest and electrode material). The triangle voltage pulse causes electroactive neurochemicals adsorbed to the surface to oxidize and reduce at specific potentials creating brief peaks in faradaic current that can be used to identify specific neurochemicals and changes in their concentration. Consequently, materials used for FSCV materials must 1) enable small electrode designs that minimize biofouling/scarring that would prevent the adsorption of the neurochemical to the surface, 2) minimize the number of other electrochemical reactions that occur at the surface that could confound measurement of the neurochemical of interest, 3) have minimal drift in open circuit potential that would generate variability in the true voltage applied during the FSCV pulse, and 4) be able to both adsorb the neurochemical of interest while allowing the neurochemical to desorb once oxidized/reduced so that changes in local concentrations of the neurochemical can be monitored over time.[194] The ultimate goal is for the FSCV electrode to have both high sensitivity (detect low concentrations of a neurochemical) and high selectivity (able to differentiate the neurochemical of interest from interferents).
The traditional electrode material for FSCV recordings are small carbon fiber electrodes, which can also be cycled at high levels to deliberately cause dissolution of carbon at the surface. This removes biofouling and uncovers new unoccupied sites for adsorption to enhance signal in a chronic environment.[195] Numerous alternatives to traditional carbon have been studied including noble metals like gold, platinum/iridium, iridium oxide, conductive polymers, boron-doped diamond, graphene, and pyrolyzed photoresist.[196–204] Boron-doped diamond is a promising material for chemical sensing due to its good conductivity, chemical stability, and biocompatibility, but it has a low double-layer capacitance that is not ideal for electrical stimulation.[205–206] Some of these newer materials like boron-doped diamond have yet to demonstrate sensitivity comparable to carbon fibers. For other new materials the inherent drift in electrochemical reactions at the surface of the material over time ultimately limit sensitivity, or the inability of the neurochemicals of interest to desorb after oxidation/reduction once adsorbed dramatically decrease sensitivity over time. FSCV waveforms and subsequent analysis to maximize sensitivity and selectivity utilizing a small carbon fiber electrode was optimized over the course of decades, while the same degree of optimization for other material candidates has yet to be performed. Of the recently explored new materials, pyrolyzed photoresist, which behaves the most like traditional carbon but is more amenable to modern multi-electrode fabrication processes, is arguably the most promising.
2.5. Chemical Stability of Materials
Developing chronically implantable devices require careful selection or development of materials and assemblies that outlast the lifetime of the subject. When the adhesion layer (e.g. Ti underneath Au or Ir) or the interface of the conductor and electrode site material becomes exposed to the tissue (due to dielectric cracking, delamination, electrode site dissolution,[207–208] or general preparation), the tissue fluid and the two separate metals form a short circuit galvanic cell that continuously corrodes the electrode material and degrades the electrical characteristics of the microelectrode. Gold-plated tungsten wires, for instance, have been shown to corrode readily in phosphate-buffered saline (Figure 2gh).[209–210] Not only does this negatively impact the electrical properties of the electrode, but it can also lead to toxic metal ion generation.[211] Silver electrodes, for example, have been shown to rapidly oxidize in the body, leading to large implant mass loss and rampant tissue inflammation.[212–214] Corrosion in tissue commonly occurs between two metals, metals of varying purity, or a heterogeneous alloy surface, where one metal dissolves and deposits onto the surface of the other metal through ion migration facilitated by the electrolyte solution.[215] This can be avoided through homogenously alloying multiple metal elements together. Corrosion resistant, higher atomic weight transition metals with good biocompatibility, such as platinum or iridium, are chosen as preferred electrode materials.[21] In addition to improving corrosion resistance, alloys combine the mechanical and electrochemical properties of two metals, allowing for tunable mechanical and electrical properties.[216–219] For example, pure platinum is too soft for use as a cortical penetrating microwire, so it is commonly implemented in an alloy with the stiffer metal iridium, thus resulting in a stiffer, lower impedance electrode.[218–219] Even without metal adhesion layers, some metals can be prone to delamination and corrosion in physiological environments. Platinum black,[220] iridium oxide,[221] and nanoporous materials[222] can all be used to increase the ESA of the probe. However, careful selection of material selection, site size, and deposition parameters are necessary as these coatings are often prone to delamination or electrode dissolution.[207–208, 223] For example, platinum black suffers from low mechanical stability and poor adhesion to the electrode substrate,[224] while iridium oxide films become more prone to degradation and instability during electrical stimulation as electrode site sizes becomes smaller and charge densities increase.[207]
Electrodeposition of CPs can also control corrosion. PEDOT is especially promising due to its stability in oxygen-rich, hydrated environments,[59] since oxidation further polymerizes the CP rather than corroding the metal.[28] Likewise, undoped PPy coatings have been shown to impart corrosion resistance on steel.[225] However, while CP coatings offer electrochemical benefits and a wide array of options for functionalization, they have difficulty maintaining lamination[226] and are brittle.[227] Therefore, when using CP coatings, it is also important to consider the underlying conductor material. Care must be taken to ensure that the CP adheres well to the electrode site material to prevent delamination. Adhesion can also be improved by appropriate dopant selection or by surface premodification such as roughening.[228] For example, addition of self-assembled alkylsilane monolayers improved PPy adhesion.[229] The size of the dopant molecule is an important factor to consider. For example, PEDOT doped with large polystyrene sulfonate (PSS: M.W. 100,000) had an increased tendency to delaminate due to smoother electrode morphology when compared to doping with smaller p-toluenesulfonate (pTS: M.W. 194.18) and perchlorate (ClO4).[230] However, small dopants can leach out of the CP matrix eventually leading to a decrease in conductivity and long-term instability in vivo.[231] On the other hand, large dopants such as PSS (M.W. 70,000)205 typically remain trapped within the CP matrix, and therefore do not contribute to a loss in conductivity.[232] Unsurprisingly, appropriate selection of the conductive polymers (monomers and/or oligomers) used for electrochemical deposition is also critically important for the success of the electrode. Although, PPy is commonly used, due to its ease of growth and low toxicity, PEDOT exhibits superior chemical and electrochemical stability. Coating thickness is also an important consideration, with thicker PEDOT coatings displaying lower impedance measurements on average compared to thinner coatings, but are more prone to delamination.[189] It has been suggested that rough-patterned surfaces offer better adhesion of polymers to the substrate than smooth surfaces.[228, 230, 233] Furthermore, the applied potentials required for electrochemical deposition can cause material dependent corrosion which can disturb the integrity of CP coating, where oxidation of the metal can block electron transfer resulting in a non-uniform, patchy coverage.[234]
The risk of corrosion can also vary depending on whether the electrode is used for stimulation or recording: excessive electrode polarization during stimulation has been shown to produce irreversible electrochemical reactions that can degrade metals (such as stainless steel[235]) which are biologically stable otherwise. This has led to the exploration of conductive polymers and CNT composite materials for recording and electrical stimulation electrode materials.[82, 236–241] CNT doping improves the charge injection capacity through several mechanisms, including increased coating surface area, CNT electrical conductivity, and the additional charge transfer mechanism of small cationic molecules entering the coating to serve as counter-ions against the trapped anionic CNTs when the CP is reduced. These attributes provide the coating with substantial non-faradaic (capacitive) and faradaic character in both oxidized and reduced states.[242] Mechanical stability tests following both acute and chronic stimulation, as well as chronic soaking, revealed PEDOT doped with multi-walled nanotubes (PEDOT/MWNT) and layer-by-layer CNT composites exhibited none of the delamination or cracking that are typical of PEDOT coatings or IrOx sites.[82, 236, 243] Interestingly, PEDOT doped with carboxylic acid functionalized CNT maintained recording quality despite increases in impedance over 3–8 weeks post implant.[244] However, careful consideration of size and concentration are necessary since ejected nanoparticles can cause frustrated phagocytosis, cell cycle arrest, apoptosis, and membrane lysis.[245–246]
Lastly, the biological response to the implant contributes to electrode failure in a variety of ways. Initially, biofouling, or protein adsorption on the electrode surface, leads to encapsulation, which increases the resistance and capacitance of the electrode, while reducing the electrochemical surface area and attenuating the high frequency content of the electrical stimulus.[232] This is particularly true for high ESA electrode materials where the protein and glial cells restrict ionic diffusion.[247] A study for cardiac pacing electrodes showed that the advantages for having a high ESA electrode were lost 3–8 weeks after implant unless anti-inflammatory steroids were released from the electrode site.[248] Biofouling has also been shown to be exacerbated by stimulating electrodes. For example, IrOx electrode sites used for chronic in vivo stimulation or CV testing demonstrated more biological adhesion compared to non-stimulating electrode sites or sites used only for electrochemical impedance spectroscopy.[221] Besides decreasing electrode performance, the tissue response also continuously facilitates the degradation of the insulation and electrode site materials. Cracked or delaminated insulation is further exacerbated by body heat and infiltrating immune cells that produce reactive oxygen species.[5, 249–250] Activated macrophages have been speculated to break down cracked parylene coatings.[99] Efforts towards reducing the biological factors’ contribution to material degradation and recording failure are aimed at reducing biofouling, and improving the stability of the underlying materials. For intracortical electrodes, biofouling can be minimized by modifying the electrode surface properties and minimizing damage to the blood-brain barrier (BBB) upon electrode insertion. Various hydrogel and polymer coatings can be applied to electrode surfaces to increase hydrophilicity or incorporate anti-biofouling molecules or drugs. Common anti-biofouling polymers used in surface functionalization include polyethylene glycol (PEG) and PEG methacrylate (PEGMA). Boron-doped-diamond electrodes have also been shown to exhibit reduced biofouling and more stable electrochemical properties than conventional TiN electrodes.[251–252] Combining anti-biofouling surface chemistries with image guidance technology to avoid tearing major blood vessels during insertion may minimize overall inflammation and glial encapsulation.[33] A combination of materials and design selection will help improve the long-term performance of implantable interfaces.
2.6. Size and Geometry: Surface Area and Diffusion
Initially, the size and geometry of implants were believed to play a small role in recording performance and tissue integration when comparing three microelectrode designs with cross-sectional areas ranging from 1,450 – 16,900 µm2.[253] However, in vitro and in vivo studies with polymer fibers show glial attachment significantly decreased when the fiber diameters were less than 12 to 5.9 µm, respectively.[254–255] In the brain, it was shown that 20, 50, and 150 µm2 cross-sectional areas significantly improved neural health and reduced glial encapsulation compared to 3,264 µm2 (Figure 4a–c).[256] Lastly, microelectrodes with 55.4 µm2 cross-sectional area demonstrated significant improvement in chronic single-unit recording performance when compared to traditional devices with a 1,875 µm2 cross-sectional footprint.[128] This has led to a number of hypotheses on how size and geometry may be designed to enhance chronic recording performance.[8, 244] For example, devices with holes or lattice structures might improve recording performance by allowing trophic factors to diffuse from one side of the probe to another, which can potentially improve the health of the tissue around the probe.[8, 244] In addition, tissue can regenerate through the probe holes, which both improve electrical and chemical signaling between the tissues on either side of the probe as well as to prevent probe migration by providing a soft anchor once the tissue heals after the initial insertion injury.[257] These, in turn, have been demonstrated to impact chronic neural recordings and the efficacy of stimulation therapies.[258] Another hypothesis is that these lattice structures reduce the surface area for biofouling,[128] as well as the diffusion barrier for pro-inflammatory cytokines, which cause an accumulation of these cytokines around the probe.[259] Similarly, ECoG arrays also benefit from a more open architecture due to tissue growth through the holes, which minimizes the amount of scarring formed between the electrode sites and the brain.[260–261] Reducing the overall cross-sectional area of the implant reduces the overall volume of the implant. For implantable probes, lower implant volumes reduces the tissue displacement volume that occurs during device implantation, which in turn, decreases the mechanical strain experienced by neighboring neurons (Figure 4de).[8, 34] This strain has been shown to inhibit activity of nearby neurons and cause additional inflammation that negatively impacts the nearby tissue health.[262–263] Finally, the location of the electrode sites on the implantable probe is thought to influence recording characteristics. For instance, electrode sites located at the tip of the probe are hypothesized to have a larger viewing radius (see section 2.7), or volume of tissue in which extracellular action potentials can be detected, compared to sites located along the shank.[117] This results from tissue located behind the implant, or on the opposite side of the shank where the electrode sites are located, is not directly exposed to those sites, but is more exposed to sites located on the tip.
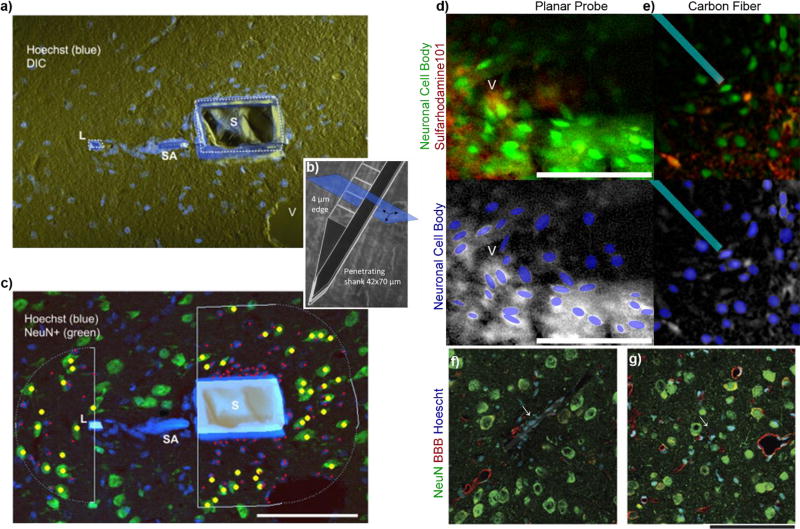
Figure 4. Implant size impacts acute and chronic tissue integration.
a) Tissue around the probe’s (b) thin polymer lattice structure showed significant reduction in encapsulating cells and improved neural density (c). (Adapted with permission from[256, 392]). d–e) Two photon imaging of tissue strain in vivo from a Michigan Electrode Array (d) and carbon fiber microthread electrode (e). Neurons are green, while recording sites and astrocytes are red. Cyan outline highlights microthread electrode. Neurons in panel c are much more compressed and oval/elliptical than neurons in panel d, indicating increased mechanical strain from the embedded electrode volume. Reprinted with permission from[34] Copyright 2014, Elsevier. f–g) Chronic histology shows carbon fiber microthreads with an 8-µm diameter reduced tissue reactivity and improved neuron density of microthread (g) compared to silicon electrodes. Reprinted from[128] Copyright 2012, with permission from Nature Publishing Group.
The benefits of minimizing the volume and cross-sectional area of implants has motivated advances in microscale and nanoscale electrode technology. Wire shank devices with 1–10 µm diameters have been fabricated with silicon, gold, carbon fiber, and conductive polymer composite substrates with excellent electrical properties and minimal host-tissue response.[264–268] On the nano-scale, etching and chemical vapor deposition of silicon has enabled the creation of devices <150nm diameter for in vitro and in vivo applications and using tobacco mosaic virus as a biotemplate has enabled metal wires to be as thin as < 20nm in diameter.[269–275] However, these introduce new challenges,[28] including handling, packaging, resistive heating, and in vivo chronic implantation requirements.[168–169] In addition, decreasing the size of the implant corresponds with a smaller electrode site, which increases impedance. This can be addressed by increasing the ESA:GSA ratio(see section 2.4).[70–71, 186, 251]
Given that FSCV measurements require that neurochemicals of interest either directly adsorb to the electrode surface or diffuse into the vicinity directly adjacent to the electrode, there has been a fundamental push towards the use of FSCV electrodes with diameters of 10 microns or less.[276] The diameter of the carbon fiber electrode is important to decrease local damage to the synaptic end-terminals which are the neurochemical sources, as well as decrease biofouling and/or reactive gliosis which may create a barrier preventing the adsorption/diffusion of neurochemicals to the surface of the electrode.[276] Prior studies have demonstrated in-vitro that biofouling related to protein adsorption to the surface of the electrode can dramatically reduce sensitivity to dopamine and other neurochemicals of interest.[277] As carbon fibers are mechanically brittle, a stringent requirement of the use of carbon fiber FSCV electrodes with diameters 10 microns or less may be problematic in terms of handling and long-term implantation.[278]
There is no published work directly comparing different diameter sizes for FSCV electrodes and neurochemical recording quality in a chronic in-vivo experimental preparation. Nevertheless, difficulties have been observed in obtaining acute dopamine measurements when implanting a 50 micron diameter boron-doped diamond FSCV electrode, likely due to trauma to the adjacent tissue preventing dopamine release locally.[279] However, using a dopamine reuptake inhibitor to promote dopamine diffusion from intact tissue into traumatized tissue did enable dopamine detection with these larger devices.[279] These data support a substantial body of work demonstrating that 1) implantation of a 220 µm diameter dialysis probe can dramatically impact the release and reuptake of neurochemicals at least 220–250 µm from the microdialysis probe,[280–281] 2) tissue near probe tracks created by 280 µm diameter microdialysis probes exhibit ischemia and endothelial cell debris which is most prominent at 4 hours post implant,[282] 3) at 24 hours the microdialysis probe tracks are surrounded by hyperplasic and hypertrophic glial and glia processes,[282] and 4) 7 micron diameter carbon fiber probes produce a diffuse disruption of nanobead labeling, but no focal disruption of blood vessels, evidence of endothelial cell debris, or glial activation.[282] Across all interface modalities, there is increasing evidence that subcellular geometries improve long-term tissue integration.
2.7 Volumetric Density & Channel count
For recording applications, the demand for a greater number of channels has increased. More recording channels allows for a greater volume of tissue to be sampled, and further, multiple channels located closely together allows for the sampling of overlapping volumes of tissue.[147, 283–284] This makes triangulation of the signal across multiple electrodes possible. An important consideration for multi-shank devices is the impact of the overall footprint of the device on the tissue health. More specifically, the volumetric density that the device occupies in the brain over a given amount of space can impact performance. Even if the same volume is implanted into the brain, the maximum strain on the tissue can be reduced by distributing the implant volume over a larger tissue volume as demonstrated by finite element modeling (Figure 5). For example, a single shank tetrode made from four 50 μm diameter electrodes may generate a tissue response similar to a single 100 µm diameter wire, but would generate a very different tissue reaction when compared to four 50 µm diameter wires that are implanted with 1 mm spacing over a large region of the cortex. However, additional considerations may be required based on how the shank spacing impacts injury (tearing and compressing) to the underlying vasculature.
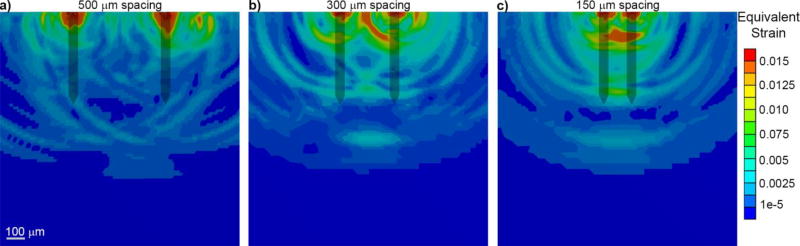
FIGURE 5. Probe insertion results in high strain between shanks of multi-shank devices, with more strain generated between densely packed shanks.
A finite element simulation of Michigan-style planar silicon probe insertion into a visco-elastic brain phantom is shown. The maximum strain value of each element following insertion is displayed. a) When the probe shanks are spread far apart (500 µm tip-to-tip spacing), there is little overlapping strain between shanks. b–c) As tip-to-tip spacing gets tighter, there is increased tissue strain between shanks (300 and 150 µm). Scale bar = 100 microns.
When considering configurations of multiple penetrating shanks, the most important factors are the density of the shanks and the configuration and footprint of the platform piece where the individual shanks come together. With a single shank, it is possible to avoid penetrating surface pial vessels and large penetrating blood vessels,[285] but with multiple, densely-packed shanks, there is a higher probability for vascular injury, microhemorrhage, and exacerbated scarring and cell death, which further increases with greater shank density.[286–289]. Despite this, there are few studies that define a “critical” shank spacing to avoid compounding damage. Both electrophysiological performance and gliosis are progressively worse for Michigan style devices and microwire devices at tip-to-tip spacings < 600 µm, while wire shanks that are 1 mm apart are indistinguishable from solitary electrodes.[290–292] For tapered devices, the base-to-base spacing of shanks should also be considered. The blood supply for the underlying cortex enters through the surface of the brain, and it is possible that the high-strain of narrow base-to-base separation could disrupt that blood flow.[293] Evaluating the effect of shank density on the hemodynamics of the underlying cortex should be examined in future studies.
Common types of multi-shank arrays (silicon planar Michigan arrays, silicon bed-of-needles Utah arrays, and metal microwire arrays) have been compared in 1–4 month-long rodent studies.[294–296] These studies show that metal wire shanks tend to outperform planar or bed-of-needles style silicon devices in chronic electrophysiology with higher SNRs and lower impedances, as well as with less long-term BBB dysfunction. In addition to different materials, these devices vary in shank density, shank shape, and platform area, complicating the interpretation of these results. Future studies should systematically vary these parameters to determine their significance for long-term probe viability.
2.8. Flexibility: Softness/Geometry
Once in the brain, a neural probe spends the rest of its life battling a wet, chemically dynamic, and mechanically tumultuous environment. This mechanical environment is dominated by “micromotions”, which are the movements of the brain in the cavity of the skull in response to respiration, pulsation, and everyday movement.[297] This effect is likely to be greater in larger primates than rodents due to larger and more intricate brains and interstitial space.[298] While the material composition of the probe will largely determine how it fares in the mechanical and chemical milieu of the brain, many groups have also shown that the flexibility of the probe can have an impact on how the host-tissue response and micromotion-related strain is generated by the probe.
Flexible implantable devices are believed to reduce the mechanical strain experienced by tissue due to motion especially in the brain when devices are anchored to the skull. The brain is normally floating inside a cerebrospinal fluid filled dural sac which in turn floats inside of the skull. In humans, the brain and skull are usually separated by about 2–4 mm while this number is closer to 100 µm in mice.[35] As such, large forceful movements or impacts to the skull can lead to substantial brain tissue displacement and mechanical strain to the tissue surrounding rigid implants. In addition, there are smaller micromotions and physiological motions in the brain due to respiration and heart-related blood flow pulsation.[299–300] These small movements can increase when craniotomies and duratomies are left open and unsealed.[301] Finite element modeling studies have shown that stiff or rigid devices that are anchored to the skull resist complying with these brain movements.[302–303] In turn, this can lead to a perpetual generation of mechanical strain and pro-inflammatory cytokines or even reinjure the tissue resulting in device failure.[5, 8, 34, 304] As such, there has been increased focus on developing implantable technologies that are much more compliant, which can be achieved in several ways:
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
where kc is compliance, l is the device length, A is the cross-sectional area, E is the elastic modulus (or Young’s modulus), w is the width of a planar devices, t is the thickness of a planar devices, do is the outer diameter of a cylindrical devices, di is the inner diameter of a cylindrical devices, and k is stiffness. The compliance of a device can be improved by employing materials with a low elastic modulus, reducing the cross-sectional area of the device, or increasing the length of the device. In order to reduce the rigidity of neural implants from silicon backbone substrates and large metal backbones (100’s GPa), engineers have turned to polymers and thin-film technology.[305–306] Polymers can have softer material properties (Young’s modulus), low thermal expansion, exceptional solvent tolerance, and good dielectric constants. However, polymers can also have high water absorption, poor solvent tolerance, and changing dielectric properties that increase when it has absorbed water or ions, which negatively impacts the chronic performance of implanted devices. Therefore, polymers selected for device design must satisfy these broad range of criteria.
Hydrogels are among the few materials that can be engineered to have similar mechanical properties to brain tissue due to their high water content.[307–309] Microglia have been shown to be responsive to the stiffness of a material, migrating from softer to stiffer surfaces.[310] However, hydrogels lack many of the other material properties necessary for functional performance, such as electrical, strength, and durability properties. Furthermore, the swelling of hydrogel materials can lead to additional neural cell death if the hydrophilicity, crosslink density, and weight of the polymer is not properly optimized.[311–312] This greatly limits the ability to construct functional devices from low elastic modulus hydrogels.
Reducing the cross-sectional area of the probe track has shown significant improvement in chronic neural recording performance, but as the dimensions decrease, other challenges emerge such as the mechanical handing requirement related to insertion and durability.[71] Similarly, overall increases in device length lead to new challenges. For specific anatomical targets, length can be increased by entering the tissue at a very distant region, though this substantially increases tissue injury. Alternatively, a meandering design, such as a sinusoid, could be employed to increase the length of the device without dramatically increasing the implantation related injury.[313–316] The placement of trifilar coils along the lead combined with strategic anchor points to prevent migration, such as with suture pads, may improve device performance, similarly to pacemakers.
The last decade has seen intense excitement in the development of wearable and soft electronics[317–321] but rarely are these new materials used in vivo. Little is known about the performance of wearable systems for long-term in vivo use and the number of papers showing in vitro monitoring or actuation is quite limited. Another major challenge is that all of these devices currently require much larger substrate thickness and feature sizes relative to thin flexible arrays patterned with photolithographic approaches such as polyimide, parylene, or SU-8 substrates[125–126, 322–336]. Most applications currently favor thin substrates and small feature sizes because the tradeoffs for biomimetic elasticity at the expense of size are still unproven. In addition, as implantable probes become more flexible, motion related electromagnetic artifacts are likely to manifest within the probe. Motion artifacts are caused by the movement or bending of a current carrying conductor, resulting in a change in the induced electromagnetic field. Although these artifacts are typically observed in the external connector cable (see Section 2.12), increasing implant flexibility may increase the likelihood of motion artifact occurrences due to perturbances from physiological micromotion. These motion artifacts are further exacerbated by changes in the internal resistance of soft conductors or capacitance of the insulation due to mechanical strain, which alters the alignment of conductive molecules as well as the material‘s cross-sectional area. This, in turn, can dramatically impact the amplitude and waveform characteristics of the electrical signal. While improving the softness and flexibility of the device is hypothesized to improve chronic tissue interfaces, it can negatively impact conductivity, dielectric properties, impedances, handling requirements for insertion, material strength, packaging, and scaling.[64, 337] Balancing these properties with advanced composite materials or a blend of multiple materials has proven to be the critical challenge for developing functional implantable devices.[64, 66] Ideally these devices will be anchored to the region which they are intended to sample and deliver its signal reliably amid all the stresses and strains produced by normal brain motion.
2.9. Implantation Technology
Well controlled probe insertion techniques play a critical role in optimizing recording performance and minimizing injury during surgery.[338] This is particularly true for thin, flexible polymer probes that experience buckling when penetrating tough pia mater and, once successful, deflect from original site of implantation.[339–340] Insertion must generate enough strain to puncture the leptomeninges and brain tissue (as little as 0.05 shear strain or 1 mN penetration force), but without causing damage to the surrounding neural tissue, which occurs with as little as 0.1 strain at a 10/s strain-rate or 3% cyclic strain at 0.2 Hz.[340–345] The pia mater is relatively heterogeneous with differing regions of thickness and surface vasculature, so the location of insertion has implications on insertion success. Implanting through thicker pial blood vessels increases compression on the cortex before penetration occurs, while insertions through smaller vessels demonstrate reduced compression on the brain.[346]
In pursuing designs that minimize insertion injury in vivo and in vitro probe insertion experiments consistently show that rigid devices with sharper-tipped and lower cross-sectional area devices have lower penetration forces, less tissue dimpling, and less tissue strain during insertion.[342, 347–351] Considering a single penetrating shank, the major implicated parameters are tip shape, presence of edges, and cross-sectional area and profile. Devices with smaller profiles display reduced cortical insertion injury in post-mortem histological analysis <1 week post-implant.[352] However, sharper tips have other trade-offs such as leading to more vascular damage, especially at lower speeds in in vitro models.[342] In addition to device geometry, implant surface chemistry has been shown to play an important role in insertion. Notably, both hydrophobic (silane and Parylene C) or hydrophilic (acid functionalization) surface treatments were able to reduce penetration force (silane and acid treatment) or friction force during insertion (Parylene C).[348, 350]
There is less consensus about the effect of insertion speed on implantation damage. The brain is thought to be a hyper-viscoelastic material, which means the faster the tissue deforms, the higher the Young’s modulus (stiffer).[348, 351, 353–357] Thus, at higher insertion speeds it may require more stress to generate enough strain to puncture. In vivo puncture studies of single shank devices have corroborated this, showing more dimpling, higher penetration force, greater penetration damage, and ultimately more long-term gliosis at higher insertion speeds.[348, 351, 358–359] Interestingly, lower insertion speeds could lead to more vascular damage, which may be due to differing mechanical properties of brain and blood vessels. While strategies to map and avoid larger blood vessels with two-photon microscopy can minimize most of this vascular damage, this may be impossible for devices with multiple shanks.[285] For these multi-shank devices, high-speed insertion velocities > 8.3 m/s have demonstrated improved insertion success, reduced hemorrhaging, and improved gliosis.[286–287, 356]
So far insertion has been reviewed with respect to rigid, brittle substrates that can be machined into pointed shanks for facile penetration. However, stiff devices result in mechanical mismatch with the surrounding tissue (discussed previously in 2.8).[302, 360] In contrast, softer, more flexible substrates are more prone to buckling upon insertion.[340, 361] To address this, groups have proposed using delivery shuttles to deliver devices into the brain. Some simple designs use electrostatic interactions to adhere flexible probes to a silicon substrate.[362] Following implantation, moisture in the brain can disrupt the interactions between the silicon substrate and the flexible probe to allow for shuttle retrieval. This design can be scaled by using a “sewing machine” method, which uses an insertion needle that can mechanically attach and detach from a flexible probe for more rapid implantation.[363] However, these require additional design considerations for the coupling mechanism. Alternative approaches involve fully or partially coating a flexible device with a sacrificial, resorbable polymer.[364–370] In vivo studies have shown that insertion wounds around resorbable insertion shuttles can fully close with limited evidence of an inflammatory lesion at 4 weeks post-implant.[371] While this is a promising strategy, current fabrication methods are costly and labor intensive, and finished products are often very sensitive to moisture and handling.[314, 367] Material and design issues of scalability and ease-of-use must be addressed before these next generation techniques can be clinically realized.
2.10. Mechanical Strength
Materials must maintain robust mechanical strength despite implementation in smaller and more flexible devices, demanding careful material selection and geometry design. As devices become smaller and more flexible with material selection and design geometry, the strength of the material becomes an increasing problem. Mechanical strength varies with material, size, orientation and geometry. Therefore, mechanical properties are often quantified in different ways; yield strength, compressive strength, shear strength, tensile strength, fatigue strength, and impact strength. Decreasing cross-sectional area (A) leads to increased stress on the implant:
| (17) |
where the stress on the device (σstress) is a function of the force (F) applied on the device area (A). The stress on the implant causes strain (ε) or deformation of the material. While the stress-strain relationship is linear:
| (18) |
As the relationship become non-linear, elastic deformation of the implant becomes plastic, resulting in permanent deformation. Brittle materials such as varnish, glass, or even diamond undergo limited plastic deformation before failing.[372–373] In contrast, tough metals can undergo extensive plastic deformation before reaching failure, making them more resistant to mechanical stresses.[374] However, over time, small plastic deformations can cause failure through fatigue. Elastomers are a class of polymers that are capable of enduring large amounts of elastic deformations, and are the preferred choice for next generation devices.[22, 27]
While strong polymers such as aramid or polybenzoxazole (PBO) exist, their high resistivities, high dielectric properties, and sometimes poor chemical stabilities as thin films in physiological solutions make them difficult to implement as building blocks for neural interface technologies.[375–376] As such, a major challenge becomes balancing size with multiple mechanical, electrical, and chemical properties of both conductors and insulators. One approach to balance mechanical strength with conductivity is to incorporate carbon-based materials, such as graphene or carbon fibers into composite materials.[28, 128, 377] These composite conductors represent flexible alternatives to brittle thin-film metal traces.[28, 82, 87–88, 378–381] For example, Layer-by-Layer assembled of polyelectrolytes in particular have demonstrated some limited self-healing capacity.[382] The non-covalently bonded individual particles display some limited mobility within the composite material resulting in the material’s flexibility and capacity to heal defects. Alternatively, controlled addition of defects (adding tears or holes in strategic locations) in thin-film metal traces allow greater distribution of stress, allowing the device to undergo greater amounts of strain before failure.[383]
One material with exceptional mechanical strength and stiffness is silicon carbide (SiC). SiC is a wide band gap semiconductor with highly saturated electron drift velocity (leading to high electron mobility and good conductivity at high current frequencies) and a high thermal conductivity.[384] While SiC has more limitations in lithography and microfabrication compared to other forms of silicon, it can be grown readily and doped with p-type or n-type dopants.[384] It is also extremely resistant to physical wear and corrosion[384–385] and has improved biocompatibility compared to silicon[46] and surgical steel.[386] This has renewed interest in applying silicon carbide for chronic neural interfaces. The amorphous phase of SiC is used as an insulating coating, while the polycrystalline phase is conductive.[46] SiC has also been incorporated into nanocomposites with conductive polymers, augmenting the conductivity of SiC and the mechanical properties of the employed conductive polymer.[387] Additionally, the electrical conductivity of this composite can be ‘tuned’ by varying the particle loading.[387]
2.11 Mechanical mismatch of subcomponents and geometry
While the mechanical mismatch between the implant and tissue have been well characterized,[302–303] mechanical mismatch of implant subcomponents have recently been emphasized as critical points of failure.[35] For example, iridium and bulk silicon have an elastic modulus of 528 GPa and 200 GPa respectively.[35] As a result of this mismatch, mechanical strain occurs, especially on the lower modulus material during implant movement, such as from physiological motion. Finite element modeling revealed that these strains are further focused on protruding geometries, leading to material failure at these mechanically mismatched interfaces as demonstrated by in vivo experiments (Figure 6).[35] These finite element models also demonstrated that geometry and placement of these subcomponents impact the durability and mechanical strain experienced. Specifically, the center of the substrates experience the least amount of strain, while outer surfaces, especially on protrusion and edges, experience the greatest amount of strain. In turn, these impact the integrity of the electrical trace and insulation material. Furthermore, these models emphasized that with simple and uniform geometries, these strain profiles are more uniformly distributed and shared more evenly across the entire device rather than along sharp edges.[35] In some situations, simple and uniform geometries may have improved strength and durability compared to complex lithographic designs (Figure 6f).[35]
Figure 6. Mechanical failure from non-homogenous components and geometries.
a) A finite element model (FEM) of mechanical strain on a planar silicon array with iridium electrode sites under a 1 micron micromotion in tissue. b) A strain profile along the protruding electrical trace (red dashed line), substrate surface (black solid line), and at the center of the substrate (blue dash dot line) as indicated in (a). c) Strain profile of (a) from the top view. Lowest strain (black) and highest strain (purple) regions are indicated with arrows. (d) SEM of a planar silicon array explanted 189 days post implant in mice V1 cortex. e) 2× zoom in of (d). f) FEM showing an order of magnitude less, evenly distributed strain profiles along simple elegant geometries. Adapted from[35]. Copyright 2015, with permission from Elsevier.
2.12 Tethering & Packaging
The penetrating shaft of an intracortical electrode brings electrode sites close to neurons of interest, but in many applications, the neural signal must be transferred to an internal pulse generator or an external system. To accomplish this, the shanks and electrode traces of the device are joined at a platform at the surface of the brain (Figure 7). The platform interfaces with the skull-anchored connector, which interfaces with external devices. The design of the platform and connector (jointly referred to as the device’s packaging) has significant ramifications on the long-term viability of the device. Extracorporeal connectors are at risk for traumatic damage and material failure due to environmental exposure.[249, 388] Metals, ceramics, and polymers have all been used for the packaging of subcutaneous implants, but they vary in their benefits: ceramics are known for biocompatibility and corrosion resistance yet lack proper mechanical strength and reliability, metals have high ductility and durability but are usually bulky and difficult to scale down in size, and polymers allow for tunable mechanical properties but suffer from absorption of fluids and swelling.[389] The current gold standard is to use a titanium casing with insulated wiring to hermetically seal electrical components from exposure to biological fluids,[390] and help protect device circuitry from electromagnetic interference.[391] Packaging choices for size and form-factor of the platform and connector can also enable on-board electronics such as pre-amplifiers and filters or incorporate wireless chips as modules.[392–393] This is especially true of fully subcutaneous deep brain stimulating (DBS) systems, which house their power source and electronics in an Implanted Pulse Generator (IPG). The IPG is typically secured in a distant tissue compartment, necessitating durable material choices to withstand movements. One of the more common issues for patients using DBS[394] and pacemaker[395] devices is lead breakage and infection. The mechanical mismatch between the hermetically sealed casing and the insulated wires make this joint a preferential failure point (see section 2.11). Efforts to minimize potential failure modes involve wireless devices that power IPGs through radiofrequency (RF) telemetry. Still, this strategy requires significantly more power to operate, bulky device components, and is complicated by tissue that impedes wireless coupling between subcutaneous implants and external devices.[396] Transparent ceramics can avoid this issue as well as enable optical surveying of neural activity and optogenetics (Section 2.15).[397]
Figure 7. Schematic of different neural probe packaging strategies.
Most commercially available technologies are anchored to the skull by cement or bone screws in order to achieve mechanical stability (Top). The platforms and shanks of a device can either be directly attached to the connector (Rigidly anchored) or attached to a wire bundle intermediary (Tethered). Next generation devices could be Unanchored (Bottom). These devices would use telemetry to send neural data to external neural processors. Inset: meningeal fibrosis of the wire bundle of a Utah array that was implanted in a non-human primate for ~4 years (Inset, top). Second-harmonic generation imaging of fibrous tissue around wire bundle shows thick collagenous tissue (~500 µm thick) surrounding wire bundle (Inset, bottom). Non-human primate data is courtesy of the Batista Lab, University of Pittsburgh
Packaging choices also affect the biological response to implants. Devices with larger platforms such as 10×10 or 4×4 shank Utah arrays are prone to fibrous encapsulation by the surrounding meninges. While the burden of penetrating shafts likely plays a role in this outcome, a similar encapsulation process occurs for platforms without penetrating shafts, such as silicone electrocorticography (ECoG) implants, which sit sub- or epi-durally on the surface of the brain.[261, 398–399] The platform can become enveloped in a fibrous capsule as early as 7 days post-implant, with encapsulation persisting for the lifetime of the device.[260–261, 398] For these implants, fibrosis can be mitigated by leaving fenestrations throughout the substrate or using a resorbable substrate that leaves only electrode sites and traces on the surface of the brain.[260] Additionally, one study[400] examining non-fenestrated ECoG grids, found that hematomas formed in 14% of cases after subdural implantation of electrocorticography grids in human children. Subdural hematomas located beneath an ECoG array can interfere with recording. These grids can also form depressions on the surface of the brain, indicating that the weight of an ECoG array is an important consideration. However, mesh-based micro-ECoG devices with a more open architecture suggest that they allow tissue vasculature to wrap up and around electrode sites, reducing the formation of collagen scar tissue between electrodes and the brain in at least one study.[260]
In devices with low-profile or 2D platforms that can be fully embedded in dental acrylic, there are no reports of fibrous encapsulation, however, there can be possible fibroblast and circulating white blood cell influx into the parenchyma from the meningeal space.[260, 400–402] Cell influx can be mitigated by implanting devices fully in the cortex, eliminating any device-meninges interface.[401, 403] While the significance of this cell population is unknown, it presents an important consideration for designing the meninges-device interface in future probes.
Platforms are also important fixation points. Platforms that are not fixed can migrate during the brain’s recovery from implantation surgery. Anchoring can either be accomplished by directly attaching the platform to the connector (rigidly anchored) or held in place by a wire bundle that attaches to the connector (tethered) (Figure 7). In the latter scenario, the connector is cemented and/or screwed into nearby skull bone. The method of tethering and packaging of the wire bundle cables, however, can affect the long-term viability of an implant. For one, due to their proximity and thin insulation layer, wires in the bundle are the primary source of parasitic capacitance (Section 2.2, Eq. 6). Additionally, several studies in rodents have shown that tethering the device leads to increased reactive microglial and astrocytic gliosis and neuronal cell death compared to untethered devices in both planar, Michigan style arrays[401, 403] and microwire style arrays.[296] Untethered microwire arrays also outperform tethered microwire arrays in long-term electrophysiology SNRs.[296] This is likely due to slack in the wire bundle, which can allow for more gross movement of the implanted device during micromotion and impacts. Analysis of the implant tracks for tethered devices corroborate this, which appear to be more elongated than tracks around untethered devices.[404] Movement of the wire bundle can also be a source of motion artifact and lead fracture.[405–409] This could be somewhat reduced by placing the bundle in a trifilar coil, which can allow expansion and contraction of the bundle. It is also possible that the wire bundle generates its own tissue response, which can further affect the relative motion of the brain and the implant. This is seen in the wire bundles of Utah arrays in non-human primates, which can be become fully encapsulated in fibrous tissue after 4 years of implantation (Figure 7). The potential for additional fibrous tissue buildup at the interface of the wire bundle and platform may explain why Utah arrays in rats are frequently slanted and partially explanted.[408] The packaging material and design are critically important components that impact overall functional performance and are often underappreciated.
2.13 Wireless Transducers
While untethered devices have better outcomes than tethered devices, they still must be anchored to the skull, which causes incongruous motion of the brain and implanted device (Figure 7). It is no surprise that devices that are not anchored to the skull or to a gel sealant have reduced astrocytic and microglial gliosis than their anchored counterparts.[401, 403] The percutaneous nature of the connector also presents a risk of infection that is much lower for fully sub-cutaneous deep brain stimulators.[403, 410–411] These issues press the need for fully implantable, wireless recording systems that are not anchored to the skull. While completely wireless, closed-loop systems for motor cortex recording and spinal stimulation has been achieved and able to treat gait dysfunction, the brain-computer interface (BCI) is still bulky and transcranial.[412–413] Smaller, fully implantable wireless modules have been fabricated that can directly interface with commercially available neural probes.[414–417] These devices still require in vivo validation, but are a promising strategy to adapt mature technology to the next generation. Less invasive options include fully injectable millimeter scale electrodes which can interface with neural signal processors via radio-frequency (RF) or ultrasound.[25, 418] Similar devices could also be incorporated on an intravascular stent-electrode device to minimize invasiveness even further.[419] It is unclear, however, if these devices can record single neuronal unit activity.
There are also challenges involved with wireless stimulation of neurons. Clinically available deep brain stimulators require subcutaneous stimulation generators and batteries, but these are bulky and batteries must be periodically replaced. One possible solution for this is to use an external energy source to stimulate neurons. Toward this end, RF or ultrasound stimulation can be targeted to electrode devices to stimulate neurons, though in vivo validation is required.[420–422] Infrared light can be used to thermally stimulate neurons, but it must be delivered through an optically transparent “optrode” device to achieve localized delivery and avoid light scattering.[421, 423–424] Visible light can also be used to stimulate neurons by using a photovoltaic substrate, which can convert light into electric current, though they often require toxic materials and have poor stimulation efficiency.[405, 425–428] A somewhat less invasive strategy is to deliver nanomaterials such as gold or magnetic iron oxide nanoparticles to a neuronal population and stimulate through IR and near-IR light, ultrasound, or magnetic fields.[429–432] This also offers advantages over conventional stimulation through infrared or transcranial magnetic stimulation since nanoparticles can be targeted to specific populations of neurons through localized injection, magnetic targeting, or modified surface chemistry.[424, 431, 433–434] Many nanomaterials that can be incorporated into wireless stimulation can also be used as stimulated drug-release carriers to further increase the therapeutic flexibility of wireless stimulation.[431, 434–438] However, nanoparticles can undergo photothermal reshaping at relatively low energies (0.6–1.5 eV) driven by curvature-induced surface diffusion at temperatures well below the melting point threshold.[439] This is particularly important for nanoparticles that have specific shape and aspect ratio that allow tuning to specific ranges of wavelengths or frequencies.[440] For chronic applications, the long-term stability of nanorods and nanoparticles on stimulation performance and biocompatibility need to be considered at temperatures well below the melting point of these particles. More recently, using carbon fibers as wireless antennas, carbon fiber photoconverters have been stimulated using photovoltaic and photothermal principles and with better spatially localized neural activation patterns than achievable by optogenetics or electrical stimulation.[174] Emerging materials and new wireless transduction technology together promise novel modalities for interfacing with the nervous system and mitigating the risks of percutaneous devices. However, these new systems must be thoroughly characterized to identify unique challenges and limitations.
2.14 Molecular & Biochemical (coatings) Engineering
The chemical and mechanical properties of the probe’s surface play a large role in determining how the brain and device will interact with one another. Researchers exploit surface chemistry to molecular and cellular fouling of the probe, modulate the neurodegenerative and corrosive behavior of encapsulating cells, and deliver therapeutics. As protein adsorption and cellular attachment to the neural probe’s surface are the first steps in forming scar tissue, many groups are pursuing surface chemistry approaches to prevent molecular and cellular adhesion to the probe.[174, 352, 441–443] The most basic approach to this is to modulate the hydrophobicity of the probe’s surface.[444–446] While hydrophobic surfaces prevent hydrophilic domains of proteins from binding to the surface of the probe, hydrophobic domains can still attach.[447] Hydrophilic and zwitter-ionic coatings can prevent both hydrophobic and hydrophilic proteins from attaching by using hydrogen bonds and electrostatic interactions, respectively, to hold a water layer at the interface of the probe and brain. This displaces proteins and cell binding domains, preventing attachment.[443, 448] Various PEG, poly(OEGMA), and hyaluronic acid hydrophilic coatings have been incorporated onto neural probes to prevent fouling and gliosis in vitro and in vivo.[267, 449–450] As an added benefit, hydrophilic or zwitterionic polymer chains can also sterically prevent adsorption and adhesion.[267, 451–452] Unsurprisingly, coatings are more effective in preventing gliosis in vivo if they are applied to the whole penetrating shank as opposed to just the electrode sites, however electrode site coatings can suppress increases in impedance after initial implantation.[450, 452]
Despite promising results, many current anti-fouling coatings fail over time as coating materials oxidize and molecules and cells find vulnerabilities within the coating.[443, 447, 452] Alternative coating strategies seek to modulate the phenotype of contacting cells instead of preventing attachment. These strategies seek to improve neuronal attachment and regeneration while preventing microglial and astrocyte adhesion and activation. By reducing inflammatory cell activation, reactive oxidative species (ROS) output can also be reduced, which has been shown to cause material degradation of most commercially available probe types.[250, 453–455] Cells can be modulated by modifying the surface topography of the probe, immobilizing bioactive molecules to the surface, and/or applying drug-releasing coatings to the probe.
Coating stiff devices with a low density or soft material with a Young’s modulus closer to that of brain tissue can reduce gliosis and improve neuronal survivability.[310, 456–457] Because hydrogel materials lack the electrical, strength, and durability properties necessary for functional devices, they are commonly used as surface coatings with and without bioactive molecules which have been shown to improve the tissue response.[310, 312, 458–460] One disadvantage of hydrogel coatings is that they can push neurons away increasing the distance to the nearest neuron, especially when it swells, which decreases recorded amplitude. Furthermore, depending on the hydrogel crosslink density, it can provide a scaffold for infiltrating macrophages and neutrophils. Hydrophobic, silicone based gels can avoid the issue of swelling and still yield lower glial attachment.[461] Macroscopic gel coatings that cover electrode sites may additionally increase device impedance, though the effect is minimal for swollen hydrogels. Furthermore, conductive polymers can be electropolymerized within the hydrogel to improve the electrical properties.[462] Like soft coatings, patterned surfaces can also have an impact on adherent cell behavior. Etched silicon substrates with 3D grid patterns or pore features reduced microglial attachment while improving neuronal attachment in vitro and in vivo, respectively.[463–465] Improved and directed neuronal cell attachment also been demonstrated in vitro for synthetic polymer, gold, and extracellular-matrix based micro- and nano-patterned surfaces.[466–467] Conductive-polymer and carbon nanotube or graphene oxide composite coatings have also been shown to improve cell attachment and neural stem cell differentiation, though it is unclear if this is due to the topographical features of the coating, or if there are other chemical or mechanical pathways in play.[467–468]
Attaching bioactive molecules to the surfaces of neural probes can also modulate cell behavior. Molecules can either be attached directly to the probe’s surface, or can be chemically tethered[469] or mechanically interlocked[231] to polymer coatings. Since immobilized molecules cannot directly act on intracellular targets, most coating strategies use molecules that target extracellular targets. Toward this, attaching extracellular matrix molecules such as laminin, peptide sequences from laminin, or the proteoglycan hyaluronic acid to the surface can “camouflage” probes such that host cells do not recognize the devices as foreign. These coatings have been shown to reduce gliosis and prevent neuronal cell death.[169, 231, 469–470] Immobilized cell adhesion proteins like L1, which brokers neuron-neuron binding, have been shown to prevent gliosis and neuron death as well as to encourage neurite regeneration in vivo (Figure 8).[449, 471–474] Other immobilization strategies seek to actively suppress inflammation. Attaching antagonists of pro-inflammatory surface receptors or ROS scavengers to remove extracellular inflammatory species can reduce gliosis and spare neurons in vivo.[475–476] Small bioactive molecules, such as superoxide dismutase mimic ROS scavengers, has the added benefit of lower immunogenicity and better in vivo stability compared with fragile peptides and proteins.[476] On the other hand, ligands that mimic the physiological signals for transcytotic transport can be attached to nanoparticles to enable transport across the blood-brain barrier.[477]
Figure 8. Microglial encapsulation of neural probes is attenuated by L1 cell adhesion molecule coating.
Both ultrasmall carbon fiber microthread electrodes (left, blue outline) and silicon Michigan microelectrodes (middle, blue outline) are encapsulated by microglial processes (green) after 6 h of implantation in mice. Electrode devices can be “camouflaged” by covalently attaching bioactive molecules to their surfaces, as shown by an L1 cell adhesion molecule coated Michigan electrode (left, blue outline), which is relatively unburdened by microglial encapsulation. Adapted with permission from[8] Copyright 2015, American Chemical Society and from[472] Copyright 2017, Elsevier.
Drug eluting coatings have the advantage of modulating cell behavior at greater distances and acting on intracellular targets, whereas surface-bound bioactive molecules can only directly act upon membrane targets of adherent cells. Perhaps the most explored drug release target is the anti-inflammatory drug dexamethasone. Dexamethasone, which has been effective in reducing gliosis, sparing gliosis, and reducing electrode impedance, can be administered by release from polymer coatings on devices, electrically stimulated release from conductive polymer coatings or porous metal electrode sites, systemic injection, and microdialysis infusion.[477–486] It is unclear, however, if the effect extends beyond the release period, or if the standard host-tissue response is simply delayed by drug release. Similar dexamethasone-eluting pacemaker leads can have long-periods of drug release (>10 years) with reduction in fibrosis and lower pacing thresholds.[487–488] Other small molecules are being explored as well. Anti-inflammatory cucurmin and resveratrol delivered through systemic injection or resorbable polymeric coatings on devices are effective in preserving neurons and repelling glial scarring, but only for as long as drug is being released.[488–490] Protein release from resorbable polymers, conductive polymesr, and hydrogel coatings have enabled the release of alpha-melanocyte stimulating hormone to reduce inflammation as well as neural growth factor, neurotropin-3, and brain-derived growth factor to stimulate neurite extension toward devices.[491–495] This was demonstrated in glass cone electrodes loaded with growth factor have shown stable neural recordings for at least 5 years, though the glass is brittle and susceptible to shattering and it remains unclear if the outcome is due to the growth factors or other design features.[496–497] Therefore, glass devices should be pursued with extreme caution due to their fragility. In addition to drug delivery, stem cell delivery has also been achieved by seeing stem cells onto laminin or alginate coated probes, though once in the brain most cells differentiate along a non-neuronal fate.[496, 498]
Lastly, conductive polymers have long been used as a method to attach bioactive molecules to the electrode site and for electrically stimulated controlled drug release. The electrical advantages of CPs[498–499] can be further enhanced by co-depositing conductive macromolecules into the coating such as graphene oxide or carbon nano-tubes.[499–500] In vivo, these loaded conductive polymer coatings have proven to be safe, chemically and mechanically durable, and beneficial for long-term neural recordings, with larger spike amplitudes, lower noise floors,[238, 267, 501–503] and are stable over long periods of stimulation.[238, 267] They have also been implicated in encouraging neuronal attachment and neural stem cell differentiation.[468, 504–505] Combined, these features make conductive polymers a versatile tool that can be incorporated into any electrode device, including those with soft substrates.[506] There are molecular and biochemical coating strategies available for devices of all materials, conformations, and dimensions. Each device should consider how to leverage surface chemistry to improve the host-tissue response while maintaining electrical properties.
2.15 Optogenetics and optical engineering
The development of protein based light-sensitive ion channels (opsins) and fluorescent sensors (voltage sensors, calcium sensors, sensing fluorescent reporters) have become a critical tool for achieving cell-specific interrogation. Light delivery and virally targeted opsins have gained increasing scientific and commercial interest as a potential technology for neuromodulation. Optogenetic tools have encountered material challenges related to achieving high-density and efficient light control (see[392] for review), but these technologies are only reliable for months and intended for animal use. This section discusses the material challenges of long-term optical stimulation that includes the packaging of light sources. It is important to note that optogenetics also faces important molecular biology challenges[14, 507–508] including transduction reliability and regulatory concerns.[509]
The central component of an optical stimulation device is typically a light emitting diode (LED) or laser diode, and depending on the application, the use of materials such as quantum dots or photoluminescence elements might be desirable. One key advantage of LEDs (including μLEDs and OLEDs) is that this element can directly illuminate the target tissue and thereby simplify the optical system. LEDs provide a wide illumination profile (typically a cosine function, i.e., Lambertian) and thus are an efficient and effective way to illuminate reasonable volumes of tissue close to the component surface. However, if the presence of the LED(s) interferes with nearby sensors, generates too much heat, or if the required LED lifetime is long (e.g. ≥ 1 yr), then the use of waveguides to deliver the light from an external light source such as a laser diode might be a better long-term strategy. Coupling light into a waveguide can be very efficient when using a laser diode since those have pinpoint directed emission, while the coupling efficiency of an LED is exceptionally poor by comparison unless the waveguide is larger than the surface area of the LED.[510]
A common challenge for all light sources is their energy requirement to generate sufficient irradiance, while minimizing heat generation due to inefficiencies in the current driver, quantum efficiency, and light outcoupling. It has been estimated that optogenetic stimulation requires as much as 1000-fold more power than electrical stimulation to excite matching tissue volumes.[509] Therefore, the electrical insulation must have high dielectric strength to compensate for the additional electrical power necessary to drive LEDs and be robust against insulation failure. This is especially true in locations outside of the brain that are prone to mechanical forces or movement (e.g. muscle). However, power requirements also depend on the distance to the target, opsin sensitivity, and the irradiance required to cause a therapeutic effect. As the efficiency of LEDs and opsins improve, currently available low power μLEDs and OLEDs become practical for in vivo implantation (Figure 9). One inorganic LED system having a pixel size of 10 × 15 µm has been demonstrated to control neural activity with as little as 60 nW (optical power), so even though the power efficiency is only ~1% the heat generation does not exceed safety limits.[511] Organic LEDs having a pixel size of 6 × 9 µm have also been demonstrated to drive cellular activity.[512–513] However, many challenges still remain. Solid state light emission has yet to achieve longevity for at least a year when being used in vivo. Devices also need to be tested for biocompatibility given the potentially reactive materials such as Ag, Al, ITO, GaN, AlGaNP and many other possible light emitting diode layer materials.
Figure 9. Sample light delivery systems for optogenetic stimulation.
a) Optroelectrode design using gallium nitrate (GaN) micro-light emitting diodes (μLEDs) and electrode recording sites stacked together using heterogenous packaging. Reproduced with permission from[318] Copyright 2013, American Association for the Advancement of Science. b) Cross-section along a monolithically fabricated μLED and microelectrode (dash-line on top inset, bottom inset shows the layers along the cross-section). The electrode typically consists of low impedance materials like iridium deposited on layers of SiO2 (green). Gallium nitride (GaN) multi-quantum wells (MQW) are connected to an anode and cathode to form the LED and silicone shank is the base layer. Reproduced with permission from[511] Copyright 2015, Elsevier. c) Organic LED (OLED) layers have also been recently demonstrated in vitro using these dimensions. Potential failure of LED-based illuminating systems is the possibility of failure of the deposited materials and water ingress to leak the voltage to power the LED into the surrounding tissue. This will electrically stimulate the tissue instead of optically. Adapted with permission form[513], Copyright 2016. The American Association for the Advancement of Science. (d–e) Another prototype of optrode where a waveguide is used to deliver light. e) Waveguide cross section showing the transmission, cladding, and silicon substrate layers. Reproduced with permission form[527] Copyright 2015, Nature Publishing Group.
Incoherent μLEDs and coherent vertical cavity surface emitting lasers (VCSELs) are rigid inorganic devices but still have the potential to achieve a flexible form factor suitable for the surface of the brain or conformal wrapping around a nerve.[514–515] To do so, one must thin the light source and heterogeneously package each component onto a substrate to provide flexibility and insulation.[318, 516] The challenge here is to electrically bond the light source to a substrate, eliminate any voids at the interface, and apply a pin-hole-free conformal coating.[517] In practice, this approach usually fails because of defects at sharp edges and voids at interfaces.[518] The usual failure modes of all implantable devices apply including water and ion ingress, hydrolysis, and delamination. Since these are power consuming elements, the failure is accelerated from the electric fields generated with every pulse of current used to generate the light (Figure 9).[107] Long-term use of this technology suitable for human use has yet to be discovered.
OLEDs have the potential to be highly flexible, conformal, and multi-color. However, they are also the most challenging since they are additionally prone to fail in the presence water vapor or oxygen, which have high transmission rates in polymers.[519] Still, consumer demand for flexible displays and wearable electronics has spurred large R&D investments in this technology. The most common approach to reduce the permeability of water and oxygen without compromising flexibility is the deposition of a hybrid stack of alternating organic/inorganic layers (Figure 9C).[519] Atomic layer deposition of Al2O3 and ZnO with low water absorption polymers (e.g. Barix) is one example of the hybrid approach.[513, 520]
If the light source and electronics must be implanted and designed for long-term use, then hermetic packaging is currently the most feasible solution to prevent in vivo failure.[518, 521] A hermetic feedthrough for light is also required, which is another potential material challenge and, unlike electrical feedthroughs, is not a mature medical technology. Usually hermetic enclosures are large and must be positioned many millimeters or centimeters from the therapeutic target, and a waveguide must be engineered with high transmission and biostability.
The core and cladding of a waveguide are equally important material choices and both have optical and lifetime requirements. The index of refraction is the most critical optical parameter and should ideally maximize the transmission efficiency (and reduce Fresnel losses if covering either end of the waveguide). Some common waveguide core and cladding combinations that may be lithographically patterned include SU8/PMMA,[522] SU8/tissue[523] polysiloxane/polysiloxane,[524] and SiON/SiO2 (Figure 9DE).[525–526] A simpler manufacturing technique is fiber pulling using polymer/polymer and many forms of fused silica. Unlike the microfabricated waveguides, these can be combined as bundles and that may increase flexibility. Conversely, the microfabricated systems are particularly useful for multimodal device design like optoelectrodes and drug delivery.[527] One group has also developed a technique for co-polymer-metal extrusion so that the tip becomes a moderate-density optoelectrode tool.[265] Many questions remain about all of these optical systems. A few studies have tested SU8 and EpoCore and found optical losses become stable after 3–6 months of soaking[322, 523] but longer testing is still required and for each optical system. Polymer fibers (extruded cylinders) have the most potential as a flexible waveguide core, which would enable a similar form factor as used in conventional neuromodulation leads. When combined with a hermetically packaged laser diode and accompanying electronics, it is similar to an IPG and flexible lead, e.g. SCS and DBS stimulators. A stylet could provide the stiffness to reach the spinal cord or deep brain structure, then removed to allow the waveguide to move more naturally with the tissue. An optical waveguide, especially one designed for the peripheral nervous system, should be flexible in all directions so as to minimize tissue reactivity. Polymer fibers are typically made of polycarbonate, PMMA, polystyrene,[528] and Cytop (fluorinated polymer) but only Cytop has good transmission between 400–550 nm.[529]
Another constraint of optical stimulation is heat generation. Light requirements usually depend on the tissue volume and distance from the light source. A Monte Carlo simulation tool available online provides an excellent resource for predicting heat generation in tissue due to light absorption and irradiance profiles as a function of the waveguide diameter, optical power, and wavelength.[530] Fortunately, the duty cycle and pulse width for ChR2 stimulation is typically very low (e.g., 2–5 ms duration, 5% –15% duty cycle) and can become even lower at shorter pulse durations. Recent work demonstrated that even when using pulse-width modulation within this small time window the electrophysiological effect is equivalent and therefore power consumption can be significantly reduced.[531] When optical pulses are in the range of one second however, then the rate of heat dissipation is no longer high enough to prevent local tissue heating. Additionally, heat from the light source and any nearby electronics inside the implanted electronics enclosure will have to be added to the radiation heat. Although the heat deposition at these levels might not adversely impact tissue, it is sufficient to elicit changes in tissue perfusion to scavenge increases in heat.[532–533] One recent report stated a maximum energy of 0.5mJ to avoid these physiological responses.[532]
Lastly, there is increased desire for electrode materials that are transparent in tissue for optical stimulation through an electrode, read-out of fluorescent neural activity sensors, and compatibility with optical imaging modalities. In order to image through a probe, the material needs to be both transparent, and have the same refractive index as tissue. This is particularly true with multi-photon microscopy and holography, where non-uniform refraction of light can alter the convergence of the two-photon light path. This can be addressed with uniform ‘sheet’ or ‘slab’ arrays such as an ECoG array, though these devices are prone to dense fibrous encapsulation quickly after implantation (Section 2.12). Transparent electrode site and electrical trace materials have also become desirable. Thin-film indium tin oxide (ITO) is a mostly (~80%) transparent conductor that has been gaining attention for optogenetic applications.[534–535] More recently, graphene conductors with ~90% transparency have also become a popular electrode material for optogenetic applications due to improved strength and lower resistivity.[78–79] However, even with one to four layer thick graphene, these transparent materials generate photoelectric artifacts[78] (see[174] review). Even when light source placement is carefully considered, tissue is a very turbid medium that readily scatters photons. The resulting photoelectrical activation can impact the detected signal[174] and can even cause photoelectrochemical faradaic redox reactions if proper material selections are not made.[536] A long-term challenge for ITO with in vivo optogenetic application is that ITO oxidizes overtime, which results in darkening of the film and a dramatic rise in impedance.[537] Development of new materials for optogenic stimulation and detection need to consider these additional challenges beyond the electrode consideration discussed in 2.1–2.14.
Although numerous issues involving optogenetics are highlighted, its future is bright especially for basic-science researchers mapping neural circuits. Solutions for long-term use of optogenetic neuromodulation will require new advances in materials to provide robust electrical insulation, chemically stable, flexible, and safe light delivery via LEDs or waveguides, and for some applications, near-transparent materials. The opsin transduction challenges will also need to be resolved along with the regulatory challenges, and the therapy will have to be highly efficacious relative to electrical stimulation or pharmaceutical interventions for its long-term translational viability.
3.0 Challenges and Opportunities for Neural-Interfaces Located Outside the Brain
Although implants in the brain often capture the academic and public imagination, neural interfaces for use outside the brain are by far the largest current therapeutic market. The cochlear prosthetic for deafness is one of the earliest neural interfaces FDA approved in the United States. Vagal nerve stimulators for epilepsy, spinal cord stimulators for pain, and Finetech-Brindley stimulator bladder control stimulators were all FDA market approved well before DBS for Parkinson’s disease. Currently, the single largest implantable neural interface market is the epidural spinal cord stimulators for pain. However, recent FDA approvals include: the Argus II Retinal Prosthetic from Second Sight,[538] the CVRx® Rheos Barostim system for hypertension,[539] the Inspire hypoglossal nerve stim system for sleep apnea,[540] the Enteromedics abdominal vagus block for obesity,[541] the Medtronic InterStim sacral nerve stimulator for urinary/fecal incontinence,[542] and the StimWave percutaneously deliverable and wirelessly powered spinal cord stimulation system.[543]
In general, many of the same design constraints and material trade-offs for neural interfaces in the brain apply to neural interfaces placed outside the brain (Figure 10), but the risks/benefits of each trade-off changes for the specific therapeutic application and implant location. More severe side effects such as intracranial hemorrhage leading to paralysis, stroke, and seizures that are risks in brain implants are dramatically less prevalent in the periphery, and replaced with other therapy limiting side-effects such as nerve damage leading to motor dysfunction, unintended muscle activation, bradycardia/tachycardia, dyspnea, pain and paresthesia. Whereas devices such as the CVRx baroreflex activation system or the Finetech-Brindley bladder stimulator work very well as a single minimally invasive stimulating electrode or biopolar/tripolar epineural cuff, systems intended to generate multi-faceted motor signal decoding and tactile/proprioceptive feedback for control of an artificial arm may require dozens if not hundreds of precisely located stimulating and recording electrodes. Similarly, an indwelling longitudinal intrafascicular electrode (LIFE) that violates the epineurium, or the flat interface electrode (FINE) which redefines a nerve’s geometry by flattening the nerve to allow for closer contact with central nerves may be completely suitable for use in the axon fibers.[544–545] However, penetrating electrodes increase the risk of disrupting the blood-nerve barrier (BNB), which is essential in circulating oxygen and nutrients to individual nerve fascicles.[546–548] Likewise, FINE or book-cuff electrodes have been shown to increase BNB permeability,[549] which allows for the leakage of inflammatory plasma proteins into the extracellular space.[542] Additional design considerations are necessary to minimize device related trauma. More recently, regenerative peripheral nerve interfaces (RPNIs) are currently being developed to provide seamless integration of transected peripheral nerve with electronic interfaces by minimizing fibrotic encapsulation, promoting revascularization, and improving signal transduction for neuroprosthetic control.[550–551] Similar to targeted reinnervation, when the nerve terminates on a muscle graft then the action potential is transduced into a large amplitude coordinated muscle response that is read out as an electromyogram. However, unlike targeted reinnervation, RPNIs treat even small diameter nerves in situ using small muscle grafts and can potentially achieve a large number of afferent and efferent interfaces.
Figure 10. Device considerations and challenges encountered during electrode design in the central nervous system and peripheral nervous system.
Axes rank design components from lowest to highest priority for both PNS and CNS devices. Note that device challenges between the two systems are not mutually exclusive. For example, foreign body response is not as disruptive in the PNS (moderate priority) than the CNS (high priority).
Stresses on the neural interface and leads connecting to the implantable pulse generator, as well as relative movement of the targeted tissue with respect to the implanted electrode, also vary greatly based on location of the implant. Epidurally placed spinal cord stimulation electrodes for pain are known to move relative to the underlying spinal cord during changes in postures, motivating strategies to anchor and stimulate on the dorsal root ganglia to provide more consistent therapy. Spinal cord stimulation systems, cochlear implants, and occipital nerve stimulators, suffer most frequently from electrode displacement, extrusion, or breakage.[552–556] Relative movement of nearby tissue inducing migration is less of an issue for vagal nerve stimulators or carotid sinus nerve stimulators, but insulation to direct current preferentially to the underlying nerve of epineural cuffs is more important. Spiral cuff electrodes, such as those used to stimulate the vagus nerve in epilepsy patients, allow for multiple contact points with a flexible geometry that wraps around the nerve and tethers it in place.[557–560] Efforts to reduce migration of PNIs involve device redesign such as suture pads along the lead or mesh electrodes which provide additional points of suturing to the fascia, connective tissue that encloses nerve and muscle tissue.[561] Modifications to surgical techniques have also seen success, such as the use of a silicone elastomer adhesive to cement the electrode to the nerve cable.[562] Electrodes placed on or in peripheral nerves in the arm to record/stimulate for prosthetic control may have leads that are tunneled back over the shoulder joint back to an implantable pulse generator (IPG) or wireless base station for control, and relative movement across that joint is particularly problematic for wear and tear on tissue and lead insulation. Moreover, electrodes placed outside the brain and tunneled back to an IPG tend to be much more consciously perceptible to patients, leading to a host of ‘irritation’ related patient manipulation of the electrode, tunneled lead or pulse generator. For example, there is a common condition known as ‘IPG Twiddler’s Syndrome’ where the patient attempts to spin the IPG on a 2-suture axis within the subcutaneous pocket, which has later led to 3 and 4 suture fixation of the IPG.[563–565] Similarly, one of most common failure points in the cardiac pacemaker or DBS system is at the ISO-1 connector which connects the electrode lead to the IPG. The mechanical mismatch between the hermetically sealed titanium housing of the IPG placed subcutaneously or submuscular in the chest, the bore in the RF-transparent header section, and the lead insulation wears the insulation over time, slowly exposing the underlying trifilar coil leading to extraneous stimulation of the muscles/nerves in the chest.
Although more clinically available, PNS devices face challenges similar to those faced by CNS systems. Flexible materials are required to bend with macromotion and retain fit to nearby anatomy. Greater stresses on implants also demand more mechanically robust materials, both conductors that will not fail due to fracture, insulation that will maintain closure, and traces and connectors that can withstand stresses induced by mechanical mismatch. More creativity is required in defining device geometry, in order to complement the specific strengths of the materials involved. All of the considerations regarding chemical stability of exposed materials also apply as modified for a peripheral environment. Ultimately, materials for PNS devices must work synergistically to maintain functionality within some of the most active regions of the body.
4.0 Regulatory, Reimbursement and Other Practical Considerations
Despite numerous advances in electrode fabrication processes, electrode and insulation materials, and bioactive coatings within the academic realm, existing clinical deep brain stimulators, spinal cord stimulators, and vagal nerve stimulators still draw heavily from the designs, materials, and underlying concepts from the cardiac pacemaker/defibrillator. There are two primary drivers for the slow speed of adoption of new fabrication processes, passive materials, and biologically active materials. The first is the large jump from low volume animal studies across a few academic labs, to widespread clinical use in hundreds of thousands of patients at facilities across the world. Even the cardiac pacemaker/defibrillator, which is implanted in 900,000 patients a year worldwide and relies on a simple surgical procedure and well understood materials that have been in practice for 50 years, has a significant rate of device failure. The comprehensive FOLLOWPACE study, which followed over 1500 pacemaker patients over an average of 5.8 years, found that 12.4 percent of patients developed pacemaker complications within the first two months after surgery, while 9.2 percent of patients experienced complications after two months.[566] Many of the novel materials discussed in this review such as conductive polymers and carbon fibers are manufactured using processes where the intermediate stages of the material may be toxic, or where potentially toxic substances are used to treat the material to achieve the final result. In an industry supply chain designed to manufacture a product at scale, many of the manufacturing steps or constituent materials are provided by an outside supplier. When manufacturing at the scale of 10,000 implants per year, it can be difficulty for the end-system manufacturer to guarantee that the supplier has not changed their process in some small way that might accidentally introduce a leachable toxic substance into material in contact with tissue. Moreover, simple unknowns like shipping the electrode coated with IrOx overseas, or understanding the potential consequences if a surgeon drops the sterile package with the electrode and leads before implantation have to be carefully understood for any change in design. Finally, the introduction of biologically active components can greatly complicate the regulatory pathway. The ‘combined’ devices often have to go through the FDA Office of Combination Products and review from both the FDA Center for Devices and Radiological Health and the FDA Center for Biologics Evaluation and Research, dramatically increasing the regulatory burden and bar for safety. Given the biological component, there is understandably a concern about variability in manufacturer process/supply chain/handling leading to unforeseen consequences in the patient.
The second driver of the slow speed of adoption of new materials/concepts/process into clinical use is the high cost in both time and money to obtain market approval from the Food and Drug Administration (FDA), and the realities of insurance reimbursement. A pivotal trial in the United States for an implantable neuromodulation/neuroprosthetic device to obtain FDA Premarket Approval (PMA) often takes more than five years to complete and can cost well in excess of 100 million dollars. Moreover, obtaining FDA market approval does not guarantee that insurance payers will reimburse for the therapy, or reimburse at a price necessary to sustain a commercial manufacturer. As new materials/processes introduce significant unknowns into the regulatory and reimbursement pathways, the prospective reward for adopting these new concepts must be both highly likely and offset the large costs and risks inherent in regulatory approval given the price for reimbursement set by insurance payers.
Despite these drivers slowing the adoption of new materials/concepts/processes, in 2015 RetroSense Therapeutics LLC received an investigational new drug exemption from the FDA to transduce a very limited number of human subjects in order to restore light sensitive channels in the retina of patients blinded by retinitis pigmentosis.[567] Although this is a Phase 0–1 study to determine safety, and only ambient light is used for illumination, this is a critical step in moving towards optogenetic therapies.[568] The jump from animal models is quite large, however, and demonstrating transduction, specificity of transduction, and maintenance of transduction utilizing an adeno-associated virus gene vector to enable optogenetic therapies in humans has yet to be demonstrated. To date optogenetics has primarily been used in genetically homogenous murine models, with no studies as of yet demonstrating consistent specificity and maintenance of transduction over a long period of time across a large cohort in a large animal model. A study using adeno-associated virus ocular subretinal injection to address proto-oncogene tyrosine-protein kinase MER (MERTK) mutations in six human patients blinded by retinitis pigmentosis, for example, failed to demonstrate any transduction in three of the patients, and only one patient maintained expression out to two years.[568]
Although the retina is attractive for initial demonstrations of optogenetics (as photoreceptors can be illuminated without an implantable device), more diverse therapeutic applications will require implantation of yet to be developed human-ready fiber optics or μLEDs for illumination. This will require a host of developments to enable a safe, reliable, and cost-efficient system at scale, as outlined in the excellent review “From optogenetic technologies to neuromodulation therapies.”[509] Even given a safe and reliable implantable optogenetic transduction and illumination system, clinical adoption will be key for a successful optogenetics business case. At present only 5–20% of the patients indicated for standard DBS for Parkinson’s elect to receive an implant; optogenetics will have to demonstrate a large improvement over electrode technologies to overcome hesitance to undergo both manipulation of the genome in addition surgical implantation of a fiber optic or μLED system.
5.0 Conclusion
The complexity of neural interface design, coupled with regulatory and reimbursement hurdles, forecasts a challenging road forward for medical device innovators. Understanding how individual material properties contribute to the success or failure of a neural interface has allowed researchers to experiment with the optimization of specific design variables. Although this has resulted in advancements in materials science and the current understanding of ideal design (Table 1), major obstacles remain for translation to functional implantable devices. This is because single parameter optimization alone is insufficient in producing long-term functional neural interfaces, due to unavoidable tradeoffs that result from the interdependence of design variables. For example, hydrogels can be engineered to have a similar elastic modulus as the brain tissue, however they lack many other important characteristics, including electrical and insulating properties, chemical stability, or material strength and durability. Conductive polymers provide benefits of corrosion resistance, high ESA, and favorable electrical properties, but they are lacking in mechanical stability, biostability, and softness. And subcellular devices have favorable softness and flexibility, and induce minimal strain on the surrounding tissue, yet they suffer from high resistance and low mechanical strength. It is clear that there is a complex network of interplay between intrinsic and extrinsic material and design properties, and the resulting biological, mechanical and electrical responses (Figure 11). Therefore, the ultimate challenge in developing functional devices is understanding interdependence of these variables, and then carefully balancing and titrating these variables with existing or emerging biomaterials. Alternatively, new modes of technology (e.g. wireless transducers) could eliminate the need for some of these variables. These innovations, however, require careful perspective and consideration to critically analyze what new limiting variables are introduced with these new signal transduction modes. Only through honest evaluation of limitations and failure modes, as well as outliers, are we able to engineer transformative next generation technologies.
Table 1.
Constraints on electrode device design and ideal solutions for recording and stimulating applications. Constraint columns represent opposing extremes for different engineering parameters.
| Constraint | Ideal | Constraint | |
|---|---|---|---|
|
|
|||
| Conductor | Low conductivity High electrical resistance Ohmic Heating | Infinite conductivity Zero cross-sectional area Electrochemically stable materials Flexibility Mechanical strength | High cross-sectional area Increased stiffness Low stretchability Increased device footprint |
| Dielectric Thickness | Ultra-Thin Low dielectric strength Dielectric constant of vacuum (1) High shunt capacitance Greater impact from water absorption Electron tunneling Parasitic capacitance | No capacitance No water absorption Low dielectric constant Self-healing | Thick Insulation Large device size & footprint Increased susceptibility for delamination Increased stiffness |
| Site Size | Very small High impedance Charge density Safety Limit (stimulation) | Zero surface area electrode site Zero impedance | Very large Signal is distributed and attenuated Large activation area (Stimulation) |
| Site Coating | Resistive materials Corrosion or Material dissolution | High ESA:GSA ratio High charge injection capability Non-faradaic charge transfer Good biocompatiblity | Capacitive materials Low charge injection capabilities |
| Size & Geometry | Small cross-sectional area Very flexible Poor Strength | Low device volume High device strength | Large cross-sectional area Increased stiffness Increased tissue strain, increased glial encapsulation |
| Volumetric Density | Low shank density Discontinuous sampling volume | Spatially continuous recording/stimulating radius Large spacing between electrode shanks Low tissue strain | High shank density Increased tissue strain BBB disruption Meningeal gliosis |
| Flexibility | Very stiff Mechanical mismatch with soft tissue | Stiff enough to insert/handle Flexible enough to not activate inflammatory mechanosensesitive receptors Rigid enough to minimize motion-related electromagnetic artifact and prevent migration | Very flexible Difficult to penetrate pia mater Greater motion artifact Device migration |
| Insertion | Slow insertion BBB disruption Tissue Strain and Dimpling | No vascular disruption No tissue strain Controlled insertion speed and angle | Fast insertion Ballistic Trauma Increased gliosis |
| Elastic Modulus (Softness) | Very Rigid Mechanical mismatch with soft tissue Activates inflammatory mechanosensitive receptors | Elastic Modulus of Tissue Good conductivity at low cross-sectional area Good dielectric at thin insulation layer High yield strength | Very Soft Low Conductivity Low Dielectric Constant Difficult to Insert |
| Packaging | Rigidly anchored device Increased tissue strain Impact Trauma Infection Route | Unanchored to the skull Wireless device | Flexibly Tethered device Increased fibrotic encapsulation Implant migration Infection route Motion artifacts |
| Molecular & Cellular | Pharmaceutical Drug release side effects Toxic polymer degradation Hyrdogels swell | No biofouling No inflammation or scarring No neurodegeneration Neurotrophic, Neuroregeneration, Neural Growth Factors Neurocamouflaging/Neural Adhering Surface | Biological Hyrdogels swell Stem cell rejection by immune system Proteins degrade quickly |
Figure 11. Complexity of Functional Neural Interface Engineering.
There is a complex network of interdependence between design properties and material constraints, which must be carefully considered when engineering functional neural interface devices. Optimizing a single material property can often negatively impact another material characteristics directly or indirectly through downstream relationships. This can push the overall design outside functional performance constraint windows, ultimately resulting in a non-functional device. Therefore, neural interface engineering requires precise balancing between intrinsic and extrinsic material properties, and their impact on overall performance. Bolded words represent tunable design parameters, and italics denote influencing factors or constraints. Functional performance characteristics are listed in the center, where the biological, electrical and mechanical domains overlap. Blue and red arrows represent positive or negative correlations between terms. Abbreviations include BBB (blood brain barrier), PC (pseudocapacitive), µ/n (micro/nano surface), ESA:GSA (electrochemical to geometric surface area ratio), VD (volumetric density of tissue recording). See Table 1 for simplified form.
Acknowledgments
TDYK, SMW, ALV, and NJM were supported by NIH NINDS 1R01NS094396. KAL was supported by The Grainger Foundation. JPS was supported by NIH (U01-NS090526-01) and the NSF (1545858). ALV was supported by NIH (R01-NS094404)
The authors will like to thank Dr. Aaron Batista Lab for providing the histology samples used in Figure 7.
Biographies

Kip Ludwig is the Associate Director of the Mayo Clinic Neural Engineering Laboratories in Rochester, Minnesota. He received his Ph.D. in Biomedical Engineering from the University of Michigan. Prior to Mayo he led translational device programs at the National Institutes of Health under the SPARC, BRAIN and CREATE Devices Initiatives. He also developed a minimally-invasive neuromodulation therapy that is for sale in 7 countries and in a U.S. Pivotal Trial.

John Seymour’s research is in the area of advanced neurotechnology with a focus on circuit mapping tools. His research has addressed reduced tissue reactivity and improved optical, electrical, and mechanical performance. He earned his B.S. with Honors in Engineering Physics from Ohio State University and his M.S. and Ph.D in Biomedical Engineering from the University of Michigan. His industry experience includes working at NeuroNexus as a Principal Scientist. He now holds a faculty research position of Assistant Research Scientist in the Department of Electrical Engineering at the University of Michigan developing novel neural interface systems.
Alberto Vazquez is currently a Research Assistant Professor in the Departments of Radiology and Bionengineering at the University of Pittsburgh. He currently works on neuronal, metabolic and vascular brain imaging approaches to assess brain function and dysfunction. He obtained his BS in Biomedical Engineering from Rensselear Polytechnic Institute in Troy, NY, his M.S. in Bioengineering from the University of Pittsburgh and his Ph.D. in Biomedical Engineering from the University of Michigan.

Takashi D. Y. Kozai is an Assistant Professor in the Bioenigneering Department at the University of Pittsburgh. He received his Ph.D. in Biomedical Engineering from the University of Michigan, and his BAs from the University of Colorado at Boulder in Molecular, Cellular, & Developmental Biology and Biochemistry. His primary research interest is in seamlessly interfacing neurobiology and neurotechnology through multimodal analysis including in vivo multiphoton imaging, functional in vivo electrophysiology, and biomaterials engineering. He is also interested in elucidating molecular and cellular pathways of brain injuries and diseases as well as developing intervention strategies.
Contributor Information
Steven M. Wellman, Department of Bioengineering, Center for the Basis of Neural Cognition, McGowan Institute of Regenerative Medicine, NeuroTech Center, University of Pittsburgh Brain Institute, Center for Neuroscience at the University of Pittsburgh, University of Pittsburgh, 208 Center for Biotechnology, 300 Technology Dr., Pittsburgh, PA 15219, United States
James R. Eles, Department of Bioengineering, Center for the Basis of Neural Cognition, McGowan Institute of Regenerative Medicine, NeuroTech Center, University of Pittsburgh Brain Institute, Center for Neuroscience at the University of Pittsburgh, University of Pittsburgh, 208 Center for Biotechnology, 300 Technology Dr., Pittsburgh, PA 15219, United States
Dr Kip A. Ludwig, Department of Neurologic Surgery, 200 First St. SW, Rochester, MN 55905.
Dr John P. Seymour, Electrical & Computer Engineering, 1301 Beal Ave., 2227 EECS, Ann Arbor, MI 48109.
Nicholas J. Michelson, Department of Bioengineering, Center for the Basis of Neural Cognition, McGowan Institute of Regenerative Medicine, NeuroTech Center, University of Pittsburgh Brain Institute, Center for Neuroscience at the University of Pittsburgh, University of Pittsburgh, 208 Center for Biotechnology, 300 Technology Dr., Pittsburgh, PA 15219, United States
William E. McFadden, Department of Bioengineering, Center for the Basis of Neural Cognition, McGowan Institute of Regenerative Medicine, NeuroTech Center, University of Pittsburgh Brain Institute, Center for Neuroscience at the University of Pittsburgh, University of Pittsburgh, 208 Center for Biotechnology, 300 Technology Dr., Pittsburgh, PA 15219, United States
Prof Alberto L. Vazquez, Department of Bioengineering, Center for the Basis of Neural Cognition, McGowan Institute of Regenerative Medicine, NeuroTech Center, University of Pittsburgh Brain Institute, Center for Neuroscience at the University of Pittsburgh, University of Pittsburgh, 208 Center for Biotechnology, 300 Technology Dr., Pittsburgh, PA 15219, United States.
Prof Takashi D.Y. Kozai, Department of Bioengineering, Center for the Basis of Neural Cognition, McGowan Institute of Regenerative Medicine, NeuroTech Center, University of Pittsburgh Brain Institute, Center for Neuroscience at the University of Pittsburgh, University of Pittsburgh, 208 Center for Biotechnology, 300 Technology Dr., Pittsburgh, PA 15219, United States.
References
- 1.Huber D, Gutnisky DA, Peron S, O'Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Nature. 2012;484:473. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collinger JL, Kryger MA, Barbara R, Betler T, Bowsher K, Brown EH, Clanton ST, Degenhart AD, Foldes ST, Gaunt RA, Gyulai FE, Harchick EA, Harrington D, Helder JB, Hemmes T, Johannes MS, Katyal KD, Ling GS, McMorland AJ, Palko K, Para MP, Scheuermann J, Schwartz AB, Skidmore ER, Solzbacher F, Srikameswaran AV, Swanson DP, Swetz S, Tyler-Kabara EC, Velliste M, Wang W, Weber DJ, Wodlinger B, Boninger ML. Clin Transl Sci. 2014;7:52. doi: 10.1111/cts.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Nature. 2006;442:164. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 4.Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJ, Velliste M, Boninger ML, Schwartz AB. Lancet. 2013;381:557. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Journal of Neural Engineering. 2013;10:066014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chestek CA, Gilja V, Nuyujukian P, Foster JD, Fan JM, Kaufman MT, Churchland MM, Rivera-Alvidrez Z, Cunningham JP, Ryu SI, Shenoy KV. Journal of Neural Engineering. 2011;8:045005. doi: 10.1088/1741-2560/8/4/045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozai TDY, Du Z, Gugel ZV, Smith MA, Chase SM, Bodily LM, Caparosa EM, Friedlander RM, Cui XT. Journal of Neuroscience Methods. 2015;242:15. doi: 10.1016/j.jneumeth.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozai TDY, Jaquins-Gerstl A, Vazquez AL, Michael AC, Cui XT. ACS Chemical Neuroscience. 2015;6:48. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SY, Kim I, Marsh MP, Jang DP, Hwang SC, Van Gompel JJ, Goerss SJ, Kimble CJ, Bennet KE, Garris PA, Blaha CD, Lee KH. Mayo Clin Proc. 2012;87:760. doi: 10.1016/j.mayocp.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishida KT, Sandberg SG, Lohrenz T, Comair YG, Saez I, Phillips PE, Montague PR. PLoS One. 2011;6:e23291. doi: 10.1371/journal.pone.0023291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang S-Y, Kimble CJ, Kim I, Paek SB, Kressin KR, Boesche JB, Whitlock SV, Eaker DR, Kasasbeh A, Horne AE. Journal of neurosurgery. 2013;119:1556. doi: 10.3171/2013.8.JNS122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevathan JK, Yousefi A, Park HO, Bartoletta JJ, Ludwig KA, Lee KH, Lujan JL. ACS Chemical Neuroscience. 2017;8:394. doi: 10.1021/acschemneuro.6b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang XW, Gong S. Curr Protoc Neurosci. 2005;(Unit 5):20. doi: 10.1002/0471142301.ns0520s31. Chapter 5. [DOI] [PubMed] [Google Scholar]
- 14.Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O'Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V. Nature methods. 2012;9:159. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rousche PJ, Normann RA. J Neurosci Methods. 1998;82:1. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 16.Roitbak T, Sykova E. Glia. 1999;28:40. doi: 10.1002/(sici)1098-1136(199910)28:1<40::aid-glia5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.McConnell GC, Rees HD, Levey AI, Gutekunst CA, Gross RE, Bellamkonda RV. Journal of Neural Engineering. 2009;6:56003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- 18.Biran R, Martin DC, Tresco PA. Experimental Neurology. 2005;195:115. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Potter KA, Buck AC, Self WK, Capadona JR. Journal of Neural Engineering. 2012;9:046020. doi: 10.1088/1741-2560/9/4/046020. [DOI] [PubMed] [Google Scholar]
- 20.Kozai TD, Gugel Z, Li X, Gilgunn PJ, Khilwani R, Ozdoganlar OB, Fedder GK, Weber DJ, Cui XT. Biomaterials. 2014;35:9255. doi: 10.1016/j.biomaterials.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Jorfi M, Skousen JL, Weder C, Capadona JR. Journal of Neural Engineering. 2015;12:011001. doi: 10.1088/1741-2560/12/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacour SP, Courtine G, Guck J. Nature Reviews Materials. 2016;1:16063. [Google Scholar]
- 23.Thompson CH, Zoratti MJ, Langhals NB, Purcell EK. Tissue engineering. Part B, Reviews. 2016;22:125. doi: 10.1089/ten.TEB.2015.0279. [DOI] [PubMed] [Google Scholar]
- 24.Patil AC, Thakor NV. Medical & Biological Engineering & Computing. 2016;54:23. doi: 10.1007/s11517-015-1430-4. [DOI] [PubMed] [Google Scholar]
- 25.Im C, Seo J-M. Biomedical Engineering Letters. 2016;6:104. [Google Scholar]
- 26.Jeong J-W, Shin G, Park Sung I, Yu Ki J, Xu L, Rogers John A. Neuron. 86:175. doi: 10.1016/j.neuron.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Kim H, Kim JH, Lee S-H. Lab on a Chip. 2016;16:959. doi: 10.1039/c5lc00842e. [DOI] [PubMed] [Google Scholar]
- 28.Kozai T, Alba N, Zhang H, Kotov N, Gaunt R, Cui X. In: Nanotechnology and Neuroscience: Nano-electronic, Photonic and Mechanical Neuronal Interfacing. Vittorio MD, Martiradonna L, Assad J, editors. Springer New York; New York, NY: 2014. p. 71. Ch. Chapter 4: Nanostructured Coatings for Improved Charge Delivery to Neurons. [DOI] [Google Scholar]
- 29.Rousche PJ, Pellinen DS, Pivin DP, Jr, Williams JC, Vetter RJ, Kipke DR. IEEE Trans Biomed Eng. 2001;48:361. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- 30.Williams JC, Hippensteel JA, Dilgen J, Shain W, Kipke DR. Journal of Neural Engineering. 2007;4:410. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- 31.Williams JC, Rennaker RL, Kipke DR. Brain Res Brain Res Protoc. 1999;4:303. doi: 10.1016/s1385-299x(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 32.Kozai TD, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT. Biomaterials. 2016;87:157. doi: 10.1016/j.biomaterials.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozai TD, Marzullo TC, Hooi F, Langhals NB, Majewska AK, Brown EB, Kipke DR. Journal of Neural Engineering. 2010;7:046011. doi: 10.1088/1741-2560/7/4/046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozai TDY, Li X, Bodily LM, Caparosa EM, Zenonos GA, Carlisle DL, Friedlander RM, Cui XT. Biomaterials. 2014;35:9620. doi: 10.1016/j.biomaterials.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozai TDY, Catt K, Li X, Gugel ZV, Olafsson VT, Vazquez AL, Cui XT. Biomaterials. 2015;37:25. doi: 10.1016/j.biomaterials.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilgunn PJ, Xiao Chuan O, Flesher SN, Schwartz AB, Gaunt RA. presented at Neural Engineering (NER), 2013 6th International IEEE/EMBS Conference on, San Diego, California, 6–8 November, 2013. [Google Scholar]
- 37.McCreery D, Cogan S, Kane S, Pikov V. Journal of Neural Engineering. 2016;13:036012. doi: 10.1088/1741-2560/13/3/036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadol JB, Jr, O'Malley JT, Burgess BJ, Galler D. Hear Res. 2014;318:11. doi: 10.1016/j.heares.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiers K, Cardamone T, Furness JB, Clark JC, Patrick JF, Clark GM. Cochlear Implants Int. 2016;17:129. doi: 10.1080/14670100.2016.1157943. [DOI] [PubMed] [Google Scholar]
- 40.Clark GM, Clark J, Cardamone T, Clarke M, Nielsen P, Jones R, Arhatari B, Birbilis N, Curtain R, Xu J. Cochlear implants international. 2014;15:S1. doi: 10.1179/1754762814Y.0000000087. [DOI] [PubMed] [Google Scholar]
- 41.Clark GM, Clark JC, Furness JB. Jama. 2013;310:1225. doi: 10.1001/jama.2013.278142. [DOI] [PubMed] [Google Scholar]
- 42.Gunasekera B, Saxena T, Bellamkonda R, Karumbaiah L. ACS Chem Neurosci. 2015;6:68. doi: 10.1021/cn5002864. [DOI] [PubMed] [Google Scholar]
- 43.Woldring S, Dirken MN. Acta Physiol Pharmacol Neerl. 1950;1:369. [PubMed] [Google Scholar]
- 44.Renshaw B, Forbes A, Morison BR. Journal of Neurophysiology. 1940;3:74. [Google Scholar]
- 45.Mott NF, Jones H. The theory of the properties of metals and alloys. Courier Corporation; Toronto, Ontario: 1958. [Google Scholar]
- 46.Oliveros A, Guiseppi-Elie A, Saddow SE. Biomedical Microdevices. 2013;15:353. doi: 10.1007/s10544-013-9742-3. [DOI] [PubMed] [Google Scholar]
- 47.Tschöpe A. Solid State Ionics. 2001;139:267. [Google Scholar]
- 48.Badwal SPS. Journal of Materials Science. 1984;19:1767. [Google Scholar]
- 49.Seto JYW. Journal of Applied Physics. 1975;46:5247. [Google Scholar]
- 50.Saha R, Muthuswamy J. Biomedical Microdevices. 2007;9:345. doi: 10.1007/s10544-006-9039-x. [DOI] [PubMed] [Google Scholar]
- 51.Muthuswamy J, Okandan M, Jain T, Gilletti A. IEEE transactions on biomedical engineering. 2005;52:1748. doi: 10.1109/TBME.2005.855712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berggren M, Richter-Dahlfors A. Advanced Materials. 2007;19:3201. [Google Scholar]
- 53.Svennersten K, Larsson KC, Berggren M, Richter-Dahlfors A. Biochimica et Biophysica Acta (BBA)-General Subjects. 2011;1810:276. doi: 10.1016/j.bbagen.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Simon DT, Gabrielsson EO, Tybrandt K, Berggren M. Chemical Reviews. 2016;116:13009. doi: 10.1021/acs.chemrev.6b00146. [DOI] [PubMed] [Google Scholar]
- 55.Green R, Abidian MR. Advanced Materials. 2015;27:7620. doi: 10.1002/adma.201501810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin DC. MRS Communications. 2015;5:131. [Google Scholar]
- 57.Rivnay J, Owens RM, Malliaras GG. Chemistry of Materials. 2013;26:679. [Google Scholar]
- 58.Aregueta-Robles UA, Woolley AJ, Poole-Warren LA, Lovell NH, Green RA. Frontiers in Neuroengineering. 2015;7:57. doi: 10.3389/fneng.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui X, Wiler J, Dzaman M, Altschuler RA, Martin DC. Biomaterials. 2003;24:777. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- 60.Bidez PR, Li S, MacDiarmid AG, Venancio EC, Wei Y, Lelkes PI. Journal of Biomaterials Science, Polymer Edition. 2006;17:199. doi: 10.1163/156856206774879180. [DOI] [PubMed] [Google Scholar]
- 61.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Biomaterials. 2008;29:3393. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 62.Svirskis D, Travas-Sejdic J, Rodgers A, Garg S. Journal of Controlled Release. 2010;146:6. doi: 10.1016/j.jconrel.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Kim J, Jung J, Lee D, Joo J. Synthetic Metals. 2002;126:311. [Google Scholar]
- 64.Kolarcik CL, Luebben SD, Sapp SA, Hanner J, Snyder N, Kozai TDY, Chang E, Nabity JA, Nabity ST, Lagenaur CF, Cui XT. Soft Matter. 2015;11:4847. doi: 10.1039/c5sm00174a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esrafilzadeh D, Razal JM, Moulton SE, Stewart EM, Wallace GG. Journal of Controlled Release. 2013;169:313. doi: 10.1016/j.jconrel.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 66.Zhanhong JD, Kolarcik CL, Kozai TDY, Luebben SD, Sapp SA, Sally Zheng X, Nabity JA, Tracy Cui X. Acta Biomaterialia. 2017;53:46. doi: 10.1016/j.actbio.2017.02.010. DOI: http://dx.doi.org/10.1016/j.actbio.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armstrong-James M, Millar J. Journal of Neuroscience Methods. 1979;1:279. doi: 10.1016/0165-0270(79)90039-6. [DOI] [PubMed] [Google Scholar]
- 68.Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR. Nat Mater. 2012;11:1065. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guitchounts G, Markowitz JE, Liberti WA, Gardner TJ. Journal of Neural Engineering. 2013;10:046016. doi: 10.1088/1741-2560/10/4/046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel PR, Zhang H, Robbins MT, Nofar JB, Marshall SP, Kobylarek MJ, Kozai TDY, Kotov NA, Chestek CA. 2016;13:6. doi: 10.1088/1741-2560/13/6/066002. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel PR, Na K, Zhang H, Kozai TDY, Kotov NA, Yoon E, Chestek CA. Journal of Neural Engineering. 2015;12:046009. doi: 10.1088/1741-2560/12/4/046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Science. 2004;306:666. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 73.Chen C-H, Lin C-T, Hsu W-L, Chang Y-C, Yeh S-R, Li L-J, Yao D-J. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9:600. doi: 10.1016/j.nano.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Li B, Cao X, Ong HG, Cheah JW, Zhou X, Yin Z, Li H, Wang J, Boey F, Huang W, Zhang H. Advanced Materials. 2010;22:3058. doi: 10.1002/adma.201000736. [DOI] [PubMed] [Google Scholar]
- 75.Luo X, Weaver CL, Tan S, Cui XT. Journal of Materials Chemistry B. 2013;1:1340. doi: 10.1039/C3TB00006K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian H-C, Liu J-Q, Wei D-X, Kang X-Y, Zhang C, Du J-C, Yang B, Chen X, Zhu H-Y, NuLi Y-N, Yang C-S. Biomaterials. 2014;35:2120. doi: 10.1016/j.biomaterials.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 77.Rakhilin N, Barth B, Choi J, Munoz NL, Kulkarni S, Jones JS, Small DM, Cheng YT, Cao Y, LaVinka C, Kan E, Dong X, Spencer M, Pasricha P, Nishimura N, Shen X. Nat Commun. 2016;7:11800. doi: 10.1038/ncomms11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park DW, Schendel AA, Mikael S, Brodnick SK, Richner TJ, Ness JP, Hayat MR, Atry F, Frye ST, Pashaie R, Thongpang S, Ma Z, Williams JC. Nat Commun. 2014;5:5258. doi: 10.1038/ncomms6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuzum D, Takano H, Shim E, Reed JC, Juul H, Richardson AG, de Vries J, Bink H, Dichter MA, Lucas TH, Coulter DA, Cubukcu E, Litt B. Nat Commun. 2014;5:5259. doi: 10.1038/ncomms6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L, Zhao Y, Xu W, Shi E, Wei W, Li X, Cao A, Cao Y, Fang Y. Nano Lett. 2017;17:71. doi: 10.1021/acs.nanolett.6b03356. [DOI] [PubMed] [Google Scholar]
- 81.Fabbro A, Prato M, Ballerini L. Adv Drug Deliver Rev. 2013;65:2034. doi: 10.1016/j.addr.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 82.Jan E, Hendricks JL, Husaini V, Richardson-Burns SM, Sereno A, Martin DC, Kotov NA. Nano Lett. 2009;9:4012. doi: 10.1021/nl902187z. [DOI] [PubMed] [Google Scholar]
- 83.Keefer EW, Botterman BR, Romero MI, Rossi AF, Gross GW. Nat Nano. 2008;3:434. doi: 10.1038/nnano.2008.174. [DOI] [PubMed] [Google Scholar]
- 84.Odom TW, Huang J-L, Kim P, Lieber CM. Nature. 1998;391:62. [Google Scholar]
- 85.Saito R, Fujita M, Dresselhaus G, Dresselhaus MS. Applied Physics Letters. 1992;60:2204. [Google Scholar]
- 86.Jan E, Kotov NA. Nano Lett. 2007;7:1123. doi: 10.1021/nl0620132. [DOI] [PubMed] [Google Scholar]
- 87.Bai YX, Ho SS, Kotov NA. Nanoscale. 2012;4:4393. doi: 10.1039/c2nr30197k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H, Patel PR, Xie Z, Swanson SD, Wang X, Kotov NA. ACS Nano. 2013;7:7619. doi: 10.1021/nn402074y. [DOI] [PubMed] [Google Scholar]
- 89.Vitale F, Summerson SR, Aazhang B, Kemere C, Pasquali M. ACS Nano. 2015;9:4465. doi: 10.1021/acsnano.5b01060. [DOI] [PubMed] [Google Scholar]
- 90.Sekitani T, Noguchi Y, Hata K, Fukushima T, Aida T, Someya T. Science. 2008;321:1468. doi: 10.1126/science.1160309. [DOI] [PubMed] [Google Scholar]
- 91.Rolston JD, Desai SA, Laxpati NG, Gross RE. Neurosurg Clin N Am. 2011;22:425. doi: 10.1016/j.nec.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robinson DA. Proceedings of the IEEE. 1968;56:1065. [Google Scholar]
- 93.Obien MEJ, Deligkaris K, Bullmann T, Bakkum DJ, Frey U. Frontiers in Neuroscience. 2014;8:423. doi: 10.3389/fnins.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nelson MJ, Pouget P, Nilsen EA, Patten CD, Schall JD. Journal of Neuroscience Methods. 2008;169:141. doi: 10.1016/j.jneumeth.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilke R, Moghadam GK, Lovell N, Suaning G, Dokos S. Journal of Neural Engineering. 2011;8:046016. doi: 10.1088/1741-2560/8/4/046016. [DOI] [PubMed] [Google Scholar]
- 96.Najafi K, Ji J, Wise K. IEEE Transactions on Biomedical Engineering. 1990;37:1. doi: 10.1109/10.43605. [DOI] [PubMed] [Google Scholar]
- 97.Burmeister JJ, Moxon K, Gerhardt GA. Analytical chemistry. 2000;72:187. doi: 10.1021/ac9907991. [DOI] [PubMed] [Google Scholar]
- 98.Wilke R, Khalili Moghadam G, Lovell N, Suaning G, Dokos S. Journal of Neural Engineering. 2011;8:6016. doi: 10.1088/1741-2560/8/4/046016. [DOI] [PubMed] [Google Scholar]
- 99.Loeb GE, Bak MJ, Salcman M, Schmidt EM. IEEE Transactions on Biomedical Engineering. 1977;BME-24:121. doi: 10.1109/TBME.1977.326115. [DOI] [PubMed] [Google Scholar]
- 100.Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA. IEEE Transactions on Biomedical Engineering. 1991;38:758. doi: 10.1109/10.83588. [DOI] [PubMed] [Google Scholar]
- 101.Lee K-K, He J, Singh A, Massia S, Ehteshami G, Kim B, Raupp G. Journal of Micromechanics and Microengineering. 2003;14:32. [Google Scholar]
- 102.Lee DS, Kim SJ, Sohn JH, Kim IG, Kim SW, Sohn DW, Kim JH, Choi B. Chinese J Polym Sci. 2012;30:242. [Google Scholar]
- 103.Naka K-I, Kido R. Brain Research. 1967;5:422. [Google Scholar]
- 104.Sankar V, Patrick E, Dieme R, Sanchez JC, Prasad A, Nishida T. Front Neuroeng. 2014;7:13. doi: 10.3389/fneng.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McPherson J, Kim JY, Shanware A, Mogul H. Applied Physics Letters. 2003;82:2121. [Google Scholar]
- 106.Dissado LA, Fothergill JC. Electrical degradation and breakdown in polymers. IET; London, United Kingdom: 1992. [Google Scholar]
- 107.Xie X, Rieth L, Caldwell R, Negi S, Bhandari R, Sharma R, Tathireddy P, Solzbacher F. Biomedical microdevices. 2015;17:1. doi: 10.1007/s10544-014-9904-y. [DOI] [PubMed] [Google Scholar]
- 108.Kozai TD, Vazquez AL. J Mater Chem B Mater Biol Med. 2015;3:4965. doi: 10.1039/C5TB00108K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marg E. Nature. 1964;202:601. doi: 10.1038/202601a0. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt E, McIntosh J, Bak M. Medical and Biological Engineering and Computing. 1988;26:96. doi: 10.1007/BF02441836. [DOI] [PubMed] [Google Scholar]
- 111.Bard AJ, Mirkin MV. Scanning Electrochemical Microscopy. CRC Press; Boca Raton, Florida: 2012. [Google Scholar]
- 112.Morton KC, Morris CA, Derylo MA, Thakar R, Baker LA. Anal Chem. 2011;83:5447. doi: 10.1021/ac200885w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boyd IW. Laser processing of thin films and microstructures: oxidation, deposition and etching of insulators. Springer Science & Business Media; Berlin, Heidelberg: 2013. [Google Scholar]
- 114.Prasad A, Xue QS, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Journal of Neural Engineering. 2012;9:056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- 115.Omura M, Hirata M, Tanaka A, Zhao M, Makita Y, Inoue N, Gotoh K, Ishinishi N. Toxicology Letters. 1996;89:123. doi: 10.1016/s0378-4274(96)03796-4. [DOI] [PubMed] [Google Scholar]
- 116.Cherevko S, Topalov AA, Zeradjanin AR, Katsounaros I, Mayrhofer KJJ. Rsc Adv. 2013;3:16516. [Google Scholar]
- 117.Seymour JP, Langhals NB, Anderson DJ, Kipke DR. Biomed Microdevices. 2011;13:441. doi: 10.1007/s10544-011-9512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Batey J, Tierney E. Journal of Applied Physics. 1986;60:3136. [Google Scholar]
- 119.Yang TC, Saraswat KC. IEEE T Electron Dev. 2000;47:746. [Google Scholar]
- 120.Liu Y, Lin IK, Zhang X. Mat Sci Eng a-Struct. 2008;489:294. [Google Scholar]
- 121.Hetke JF, Lund JL, Najafi K, Wise KD, Anderson DJ. IEEE Trans Biomed Eng. 1994;41:314. doi: 10.1109/10.284959. [DOI] [PubMed] [Google Scholar]
- 122.Ogurtani TO. The Journal of Chemical Physics. 2006;124:144706. doi: 10.1063/1.2185625. [DOI] [PubMed] [Google Scholar]
- 123.Schenk O, Urai JL, Piazolo S. Geofluids. 2006;6:93. [Google Scholar]
- 124.Schenk O, Urai J. Contrib Mineral Petrol. 2004;146:671. [Google Scholar]
- 125.Kim BJ, Washabaugh EP, Meng E. presented at Micro Electro Mechanical Systems (MEMS), 2014 IEEE 27th International Conference on, San Francisco, California, 26–30 January, 2014. [Google Scholar]
- 126.Kim BJ, Gutierrez CA, Meng E. J Microelectromech S. 2015;24:1534. [Google Scholar]
- 127.Yoshida M, Lahann J. ACS Nano. 2008;2:1101. doi: 10.1021/nn800332g. [DOI] [PubMed] [Google Scholar]
- 128.Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR. Nat Mater. 2012;11:1065. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nandivada H, Chen HY, Bondarenko L, Lahann J. Angew Chem Int Ed Engl. 2006;45:3360. doi: 10.1002/anie.200600357. [DOI] [PubMed] [Google Scholar]
- 130.Lahann J. Polymer International. 2006;55:1361. [Google Scholar]
- 131.Elkasabi Y, Chen HY, Lahann J. Advanced Materials. 2006;18:1521. [Google Scholar]
- 132.Lahann J, Chen HY. Abstr Pap Am Chem S. 2005;230:U4292. [Google Scholar]
- 133.Lahann J. Abstr Pap Am Chem S. 2005;229:U1106. [Google Scholar]
- 134.Lahann J, Balcells M, Lu H, Rodon T, Jensen KF, Langer R. Anal Chem. 2003;75:2117. doi: 10.1021/ac020557s. [DOI] [PubMed] [Google Scholar]
- 135.Lahann J, Mitragotri S, Tran TN, Kaido H, Sundaram J, Choi IS, Hoffer S, Somorjai GA, Langer R. Science. 2003;299:371. doi: 10.1126/science.1078933. [DOI] [PubMed] [Google Scholar]
- 136.Lahann J, Hocker H, Langer R. Angew Chem Int Ed Engl. 2001;40:2947. doi: 10.1002/1521-3773(20010817)40:16<2947::AID-ANIE22222947>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 137.Lahann J, Klee D, Pluester W, Hoecker H. Biomaterials. 2001;22:817. doi: 10.1016/s0142-9612(00)00244-1. [DOI] [PubMed] [Google Scholar]
- 138.Lahann J, Choi IS, Lee J, Jenson KF, Langer R. Angew Chem Int Edit. 2001;40:3166. doi: 10.1002/1521-3773(20010903)40:17<3166::AID-ANIE3166>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 139.Bally F, Cheng K, Nandivada H, Deng X, Ross AM, Panades A, Lahann J. ACS Appl Mater Interfaces. 2013;5:9262. doi: 10.1021/am401875x. [DOI] [PubMed] [Google Scholar]
- 140.Deng X, Eyster TW, Elkasabi Y, Lahann J. Macromol Rapid Commun. 2012;33:640. doi: 10.1002/marc.201100819. [DOI] [PubMed] [Google Scholar]
- 141.Seymour JP, Elkasabi YM, Chen HY, Lahann J, Kipke DR. Biomaterials. 2009;30:6158. doi: 10.1016/j.biomaterials.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prasad A, Xue QS, Dieme R, Sankar V, Mayrand RC, Nishida T, Streit WJ, Sanchez JC. Front Neuroeng. 2014;7:2. doi: 10.3389/fneng.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mechler F, Victor JD. Journal of Computational Neuroscience. 2012;32:73. doi: 10.1007/s10827-011-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du J, Roukes ML, Masmanidis SC. Journal of Micromechanics and Microengineering. 2009;19:075008. [Google Scholar]
- 145.Moffitt MA, McIntyre CC. Clin Neurophysiol. 2005;116:2240. doi: 10.1016/j.clinph.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 146.Swindale NV, Spacek MA. Frontiers in systems neuroscience. 2014;8:6. doi: 10.3389/fnsys.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Blanche TJ, Spacek MA, Hetke JF, Swindale NV. Journal of neurophysiology. 2005;93:2987. doi: 10.1152/jn.01023.2004. [DOI] [PubMed] [Google Scholar]
- 148.Lempka SF, Johnson MD, Moffitt MA, Otto KJ, Kipke DR, McIntyre CC. Journal of Neural Engineering. 2011;8:045006. doi: 10.1088/1741-2560/8/4/045006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dryg ID, Ward MP, Qing KY, Mei H, Schaffer JE, Irazoqui PP. IEEE Trans Neural Syst Rehabil Eng. 2015;23:562. doi: 10.1109/TNSRE.2015.2399856. [DOI] [PubMed] [Google Scholar]
- 150.Dymond AM. IEEE Transactions on Biomedical Engineering. 1976;BME-23:274. [Google Scholar]
- 151.Cogan SF, Garrett DJ, Green RA. Neurobionics: The Biomedical Engineering of Neural Prostheses. John Wiley & Sons, Inc.; Hoboken, NJ: 2016. p. 55. [DOI] [Google Scholar]
- 152.Macpherson JV. Phys Chem Chem Phys. 2015;17:2935. doi: 10.1039/c4cp04022h. [DOI] [PubMed] [Google Scholar]
- 153.Martin HB, Argoitia A, Landau U, Anderson AB, Angus JC. J Electrochem Soc. 1996;143:L133. [Google Scholar]
- 154.Mortimer JT, Kaufman D, Roessman U. Ann Biomed Eng. 1980;8:235. doi: 10.1007/BF02364479. [DOI] [PubMed] [Google Scholar]
- 155.White RL, Gross TJ. IEEE Transactions on Biomedical Engineering. 1974;BME-21:487. [Google Scholar]
- 156.Johnson PF, Hench LL. Brain, Behavior and Evolution. 1977;14:23. doi: 10.1159/000124612. [DOI] [PubMed] [Google Scholar]
- 157.Cogan SF, Troyk PR, Ehrlich J, Plante TD. IEEE Trans Biomed Eng. 2005;52:1612. doi: 10.1109/TBME.2005.851503. [DOI] [PubMed] [Google Scholar]
- 158.Rose TL, Kelliher EM, Robblee LS. J Neurosci Methods. 1985;12:181. doi: 10.1016/0165-0270(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 159.Rose T, Robblee L. IEEE Transactions on Biomedical Engineering. 1990;37:1118. doi: 10.1109/10.61038. [DOI] [PubMed] [Google Scholar]
- 160.Robblee LS, McHardy J, Agnew WF, Bullara LA. Journal of Neuroscience Methods. 1983;9:301. doi: 10.1016/0165-0270(83)90062-6. [DOI] [PubMed] [Google Scholar]
- 161.Beebe X, Rose TL. IEEE Transactions on Biomedical Engineering. 1988;35:494. doi: 10.1109/10.2122. [DOI] [PubMed] [Google Scholar]
- 162.Cogan SF, Troyk PR, Ehrlich J, Plante TD, Detlefsen DE. IEEE Transactions on Biomedical Engineering. 2006;53:327. doi: 10.1109/TBME.2005.862572. [DOI] [PubMed] [Google Scholar]
- 163.Cogan SF, Plante TD, Ehrlich J. presented at Engineering in Medicine and Biology Society, 2004. IEMBS '04. 26th Annual International Conference of the IEEE, San Francisco, California, 1–5 September, 2004. [Google Scholar]
- 164.Meyer RD, Cogan SF, Nguyen TH, Rauh RD. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2001;9:2. doi: 10.1109/7333.918271. [DOI] [PubMed] [Google Scholar]
- 165.Negi S, Bhandari R, Solzbacher F. Journal of Sensor Technology. 2015;17:1. [Google Scholar]
- 166.Jayalakshmi M, Balasubramanian K. Int J Electrochem Sc. 2008;3:1196. [Google Scholar]
- 167.Weiland JD, Anderson DJ, Humayun MS. IEEE Transactions on Biomedical Engineering. 2002;49:1574. doi: 10.1109/TBME.2002.805487. [DOI] [PubMed] [Google Scholar]
- 168.Egert U, Heck D, Aertsen A. Experimental Brain Research. 2002;142:268. doi: 10.1007/s00221-001-0932-5. [DOI] [PubMed] [Google Scholar]
- 169.Franks W, Schenker I, Schmutz P, Hierlemann A. IEEE Trans Biomed Eng. 2005;52:1295. doi: 10.1109/TBME.2005.847523. [DOI] [PubMed] [Google Scholar]
- 170.Jiang LQ, Gao L. J Am Ceram Soc. 2006;89:156. [Google Scholar]
- 171.Weiland JD, Anderson DJ, Humayun MS. IEEE Trans Biomed Eng. 2002;49:1574. doi: 10.1109/TBME.2002.805487. [DOI] [PubMed] [Google Scholar]
- 172.Janders M, Egert U, Stelzle M, Nisch W. presented at Engineering in Medicine and Biology Society, 1996. Bridging Disciplines for Biomedicine. Proceedings of the 18th Annual International Conference of the IEEE, Amsterdam, Netherlands, 31 October-3 November, 1996. [Google Scholar]
- 173.Gaylor JM, Raman G, Chung M, Lee J, Rao M, Lau J, Poe DS. Jama Otolaryngol. 2013;139:265. doi: 10.1001/jamaoto.2013.1744. [DOI] [PubMed] [Google Scholar]
- 174.Kozai TDY, Vazquez AL. Journal of Materials Chemistry B. 2015;3:4965. doi: 10.1039/C5TB00108K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Histed MH, Bonin V, Reid RC. Neuron. 2009;63:508. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rattay F, Wenger C. Neuroscience. 2010;170:399. doi: 10.1016/j.neuroscience.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cogan SF, Ludwig KA, Welle CG, Takmakov P. Journal of Neural Engineering. 2016;13:021001. doi: 10.1088/1741-2560/13/2/021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Merrill DR, Bikson M, Jefferys JGR. Journal of Neuroscience Methods. 2005;141:171. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 179.Shannon RV. IEEE Trans Biomed Eng. 1992;39:424. doi: 10.1109/10.126616. [DOI] [PubMed] [Google Scholar]
- 180.Negi S, Bhandari R, Solzbacher F. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:5142. doi: 10.1109/EMBC.2012.6347151. [DOI] [PubMed] [Google Scholar]
- 181.Norlin A, Pan J, Leygraf C. J Electrochem Soc. 2005;152:J85. [Google Scholar]
- 182.Song HK, Jung YH, Lee KH, Dao LH. Electrochim Acta. 1999;44:3513. [Google Scholar]
- 183.Wei XF, Grill WM. Frontiers in neuroengineering. 2009;2:15. doi: 10.3389/neuro.16.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Han J, Han J, Lee BS, Lim J, Kim SM, Kim H, Kang S. Journal of Micromechanics and Microengineering. 2012;22:6. [Google Scholar]
- 185.Wang B, Petrossians A, Weiland JD. IEEE Transactions on Biomedical Engineering. 2014;61:2254. doi: 10.1109/TBME.2014.2300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ludwig KA, Langhals NB, Joseph MD, Richardson-Burns SM, Hendricks JL, Kipke DR. Journal of Neural Engineering. 2011;8:014001. doi: 10.1088/1741-2560/8/1/014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cui XY, Wiler J, Dzaman M, Altschuler RA, Martin DC. Biomaterials. 2003;24:777. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- 188.Green RA, Hassarati RT, Bouchinet L, Lee CS, Cheong GLM, Yu JF, Dodds CW, Suaning GJ, Poole-Warren LA, Lovell NH. Biomaterials. 2012;33:5875. doi: 10.1016/j.biomaterials.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 189.Cui XT, Zhou DD. IEEE Trans Neural Syst Rehabil Eng. 2007;15:502. doi: 10.1109/TNSRE.2007.909811. [DOI] [PubMed] [Google Scholar]
- 190.Cogan SF. Annu Rev Biomed Eng. 2008;10:275. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 191.Baek S, Green RA, Poole-Warren LA. Acta Biomaterialia. 2014;10:3048. doi: 10.1016/j.actbio.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 192.Martin DC, Feldman KE. 8,586,702. Patent U.S. Patent No. 2013
- 193.Kawagoe KT, Zimmerman JB, Wightman RM. J Neurosci Methods. 1993;48:225. doi: 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- 194.Swamy BK, Venton BJ. Analytical chemistry. 2007;79:744. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- 195.Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS, Wightman RM. Analytical chemistry. 2010;82:2020. doi: 10.1021/ac902753x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zachek MK, Hermans A, Wightman RM, McCarty GS. Journal of Electroanalytical Chemistry. 2008;614:113. doi: 10.1016/j.jelechem.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jackowska K, Krysinski P. Anal Bioanal Chem. 2013;405:3753. doi: 10.1007/s00216-012-6578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Roberts JG, Hamilton KL, Sombers LA. Analyst. 2011;136:3550. doi: 10.1039/c1an15337d. [DOI] [PubMed] [Google Scholar]
- 199.Johnson MD, Kao OE, Kipke DR. J Neurosci Methods. 2007;160:276. doi: 10.1016/j.jneumeth.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 200.Pihel K, Walker QD, Wightman RM. Analytical chemistry. 1996;68:2084. doi: 10.1021/ac960153y. [DOI] [PubMed] [Google Scholar]
- 201.Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, Porreca F, Heien ML. Analytical chemistry. 2015;87:2600. doi: 10.1021/ac502165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sarada B, Rao TN, Tryk D, Fujishima A. Analytical chemistry. 2000;72:1632. doi: 10.1021/ac9908748. [DOI] [PubMed] [Google Scholar]
- 203.Zhu M, Zeng C, Ye J. Electroanalysis. 2011;23:907. [Google Scholar]
- 204.Zachek MK, Takmakov P, Moody B, Wightman RM, McCarty GS. Analytical Chemistry. 2009;81:6258. doi: 10.1021/ac900790m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Halpern JM, Cullins MJ, Chiel HJ, Martin HB. Diamond and Related Materials. 2010;19:178. [Google Scholar]
- 206.Xiao X, Wang J, Liu C, Carlisle JA, Mech B, Greenberg R, Guven D, Freda R, Humayun MS, Weiland J, Auciello O. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;77B:273. doi: 10.1002/jbm.b.30448. [DOI] [PubMed] [Google Scholar]
- 207.Cogan SF, Guzelian AA, Agnew WF, Yuen TGH, McCreery DB. Journal of Neuroscience Methods. 2004;137:141. doi: 10.1016/j.jneumeth.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 208.Robblee LS, McHardy J, Agnew WF, Bullara LA. J Neurosci Methods. 1983;9:301. doi: 10.1016/0165-0270(83)90062-6. [DOI] [PubMed] [Google Scholar]
- 209.Patrick E, Orazem ME, Sanchez JC, Nishida T. Journal of Neuroscience Methods. 2011;198:158. doi: 10.1016/j.jneumeth.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Prasad A, Xue Q-S, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Journal of Neural Engineering. 2012;9:056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- 211.Patrick E, Orazem ME, Sanchez JC, Nishida T. J Neurosci Methods. 2011;198:158. doi: 10.1016/j.jneumeth.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pudenz RH. Surgery. 1942;12:791. [Google Scholar]
- 213.Dymond Anthony M, Kaechele Lloyd E, Jurist John M, Crandall Paul H. Journal of Neurosurgery. 1970;33:574. doi: 10.3171/jns.1970.33.5.0574. [DOI] [PubMed] [Google Scholar]
- 214.Robinson FR, Johnson MT. DTIC Document. 1961 [Google Scholar]
- 215.McFadden Joseph T. Journal of Neurosurgery. 1969;31:373. doi: 10.3171/jns.1969.31.4.0373. [DOI] [PubMed] [Google Scholar]
- 216.Piersma BJ, Greatbarch W. J Electrochem Soc. 1987;134:2458. [Google Scholar]
- 217.Robblee LS, Rose TL. Neural prostheses: Fundamental studies. Prentice-Hall; Englewood Cliffs: 1990. p. 25. [Google Scholar]
- 218.Brummer SB, Robblee LS, Hambrecht FT. Annals of the New York Academy of Sciences. 1983;405:159. doi: 10.1111/j.1749-6632.1983.tb31628.x. [DOI] [PubMed] [Google Scholar]
- 219.Petrossians A, Whalen JJ, Weiland JD, Mansfeld F. J Electrochem Soc. 2011;158:D269. [Google Scholar]
- 220.Marrese CA. Analytical Chemistry. 1987;59:217. doi: 10.1021/ac00129a007. [DOI] [PubMed] [Google Scholar]
- 221.Weiland JD, Anderson DJ. IEEE Trans Biomed Eng. 2000;47:911. doi: 10.1109/10.846685. [DOI] [PubMed] [Google Scholar]
- 222.Moxon KA, Kalkhoran NM, Markert M, Sambito MA, McKenzie JL, Webster JT. IEEE Transactions on Biomedical Engineering. 2004;51:881. doi: 10.1109/TBME.2004.827465. [DOI] [PubMed] [Google Scholar]
- 223.Desai SA, Rolston JD, Guo L, Potter SM. Front Neuroeng. 2010;3:5. doi: 10.3389/fneng.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Heim M, Yvert B, Kuhn A. J Physiol Paris. 2012;106:137. doi: 10.1016/j.jphysparis.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 225.Ocón P, Cristobal AB, Herrasti P, Fatas E. Corrosion Science. 2005;47:649. [Google Scholar]
- 226.Boretius T, Schuettler M, Stieglitz T. Artificial Organs. 2011;35:245. doi: 10.1111/j.1525-1594.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 227.Green RA, Lovell NH, Poole-Warren LA. Acta Biomaterialia. 2010;6:63. doi: 10.1016/j.actbio.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 228.Cui X, Martin DC. Sensors and Actuators B: Chemical. 2003;89:92. [Google Scholar]
- 229.Nishizawa M, Nozaki H, Kaji H, Kitazume T, Kobayashi N, Ishibashi T, Abe T. Biomaterials. 2007;28:1480. doi: 10.1016/j.biomaterials.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 230.Green RA, Hassarati RT, Bouchinet L, Lee CS, Cheong GL, Yu JF, Dodds CW, Suaning GJ, Poole-Warren LA, Lovell NH. Biomaterials. 2012;33:5875. doi: 10.1016/j.biomaterials.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 231.Green RA, Hassarati RT, Bouchinet L, Lee CS, Cheong GL, Jin FY, Dodds CW, Suaning GJ, Poole-Warren LA, Lovell NH. Biomaterials. 2012;33:5875. doi: 10.1016/j.biomaterials.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 232.Kozai TDY, Alba NA, Zhang H, Kotov NA, Gaunt RA, Cui XT. In: Nanotechnology and Neuroscience: Nano-electronic, Photonic and Mechanical Neuronal Interfacing. De Vittorio M, Martiradonna L, Assad J, editors. Springer New York; New York, NY: 2014. p. 71. [DOI] [Google Scholar]
- 233.Burke M, Clarke B, Rochev Y, Gorelov A, Carroll W. J Mater Sci Mater Med. 2008;19:1971. doi: 10.1007/s10856-007-3274-4. [DOI] [PubMed] [Google Scholar]
- 234.Tallman DE, Vang C, Wallace GG, Bierwagen GP. J Electrochem Soc. 2002;149:C173. [Google Scholar]
- 235.Mortimer JT, Kaufman D, Roessmann U. Annals of Biomedical Engineering. 1980;8:235. doi: 10.1007/BF02364479. [DOI] [PubMed] [Google Scholar]
- 236.Luo X, Weaver CL, Zhou DD, Greenberg R, Cui XT. Biomaterials. 2011;32:5551. doi: 10.1016/j.biomaterials.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cui XT, Zhou DD. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2007;15:502. doi: 10.1109/TNSRE.2007.909811. [DOI] [PubMed] [Google Scholar]
- 238.Kozai TDY, Catt K, Du Z, Na K, Srivannavit O, Haque R-UM, Seymour J, Wise KD, Yoon E, Cui XT. IEEE Transactions on Bio-medical Engineering. 2016;63:111. doi: 10.1109/TBME.2015.2445713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhou H, Cheng X, Rao L, Li T, Duan YY. Acta Biomaterialia. 2013;9:6439. doi: 10.1016/j.actbio.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 240.Wilks S, Richardson-Burns SM, Hendricks JL, Martin DC, Otto KJ. Frontiers in Neuroengineering. 2009;2:7. doi: 10.3389/neuro.16.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kolarcik CL, Catt K, Rost E, Albrecht IN, Bourbeau D, Du Z, Kozai TD, Luo X, Weber DJ, Tracy Cui X. Journal of Neural Engineering. 2015;12:016008. doi: 10.1088/1741-2560/12/1/016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chen GZ, Shaffer MSP, Coleby D, Dixon G, Zhou W, Fray DJ, Windle AH. Advanced Materials. 2000;12:522. [Google Scholar]
- 243.Wang K, Fishman HA, Dai H, Harris JS. Nano Lett. 2006;6:2043. doi: 10.1021/nl061241t. [DOI] [PubMed] [Google Scholar]
- 244.Kozai TDY, Catt K, Du Z, Kyounghwan N, Srivannavit O, Haque RM, Seymour JP, Yoon E, Cui XT. IEEE Trans Biomed Eng. 2015;63:11. doi: 10.1109/TBME.2015.2445713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Webster TJ, Waid MC, McKenzie JL, Price RL, Ejiofor JU. Nanotechnology. 2003;15:48. doi: 10.1088/0957-4484/15/1/009. [DOI] [PubMed] [Google Scholar]
- 246.Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M. Chemical Society Reviews. 2012;41:2323. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Song H, Jung Y, Lee K, Dao L. Electrochim Acta. 1999;44:3513. [Google Scholar]
- 248.Santini M, De Seta F, T. I. M. S. G. o. L. O. Stimulation PACE. 1993;16:722. doi: 10.1111/j.1540-8159.1993.tb01651.x. [DOI] [PubMed] [Google Scholar]
- 249.James CB, Juan A, John PD. Journal of Neural Engineering. 2016;13:026003. doi: 10.1088/1741-2560/13/2/026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Takmakov P, Ruda K, Scott Phillips K, Isayeva IS, Krauthamer V, Welle CG. Journal of Neural Engineering. 2015;12:026003. doi: 10.1088/1741-2560/12/2/026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kozai TD, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR. Nat Mater. 2012;11:1065. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Meijs S, Alcaide M, Sorensen C, McDonald M, Sorensen S, Rechendorff K, Gerhardt A, Nesladek M, Rijkhoff NJ, Pennisi CP. Journal of Neural Engineering. 2016;13:056011. doi: 10.1088/1741-2560/13/5/056011. [DOI] [PubMed] [Google Scholar]
- 253.Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain Res. 2003;983:23. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- 254.Bernatchez SF, Parks PJ, Gibbons DF. Biomaterials. 1996;17:2077. doi: 10.1016/0142-9612(96)00014-2. [DOI] [PubMed] [Google Scholar]
- 255.Sanders JE, Stiles CE, Hayes CL. J Biomed Mater Res. 2000;52:231. doi: 10.1002/1097-4636(200010)52:1<231::aid-jbm29>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 256.Seymour JP, Kipke DR. Biomaterials. 2007;28:3594. doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 257.Polikov VS, Tresco PA, Reichert WM. J Neurosci Methods. 2005;148:1. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 258.Ersen A, Elkabes S, Freedman DS, Sahin M. Journal of Neural Engineering. 2015;12:016019. doi: 10.1088/1741-2560/12/1/016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Skousen JL, Bridge MJ, Tresco PA. Biomaterials. 2015;36:33. doi: 10.1016/j.biomaterials.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 260.Schendel Aa, Nonte MW, Vokoun C, Richner TJ, Brodnick SK, Atry F, Frye S, Bostrom P, Pashaie R, Thongpang S, Eliceiri KW, Williams JC. Journal of Neural Engineering. 2014;11:046011. doi: 10.1088/1741-2560/11/4/046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Schendel AA, Thongpang S, Brodnick SK, Richner TJ, Lindevig BDB, Krugner-Higby L, Williams JC. Journal of Neuroscience Methods. 2013;218:121. doi: 10.1016/j.jneumeth.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wang W, Tan W, Luo D, Lin J, Yu Y, Wang Q, Zhao W, Wu B, Chen J, He J. BMC Neuroscience. 2012;13:147. doi: 10.1186/1471-2202-13-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Nature. 2014;505:223. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fujishiro A, Kaneko H, Kawashima T, Ishida M, Kawano T. Scientific Reports. 2014;4:4868. doi: 10.1038/srep04868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P. Nat Biotech. 2015;33:277. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- 266.Gunning DE, Beggs JM, Dabrowski W, Hottowy P, Kenney CJ, Sher A, Litke AM, Mathieson K. Journal of Neural Engineering. 2013;10:016007. doi: 10.1088/1741-2560/10/1/016007. [DOI] [PubMed] [Google Scholar]
- 267.Yoshida Kozai TD, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR. Nature Materials. 2012;11:1065. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ojovan SM, Rabieh N, Shmoel N, Erez H, Maydan E, Cohen A, Spira ME. Scientific Reports. 2015;5:14100. doi: 10.1038/srep14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Suyatin DB, Wallman L, Thelin J, Prinz CN, Jörntell H, Samuelson L, Montelius L, Schouenborg J. PLoS ONE. 2013;8:e56673. doi: 10.1371/journal.pone.0056673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xie C, Lin Z, Hanson L, Cui Y, Cui B. Nat Nano. 2012;7:185. doi: 10.1038/nnano.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wnęk M, Górzny MŁ, Ward MB, Wälti C, Davies AG, Brydson R, Evans SD, Stockley PG. Nanotechnology. 2013;24:025605. doi: 10.1088/0957-4484/24/2/025605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shenton W, Douglas T, Young M, Stubbs G, Mann S. Advanced Materials. 1999;11:253. [Google Scholar]
- 273.Dujardin E, Peet C, Stubbs G, Culver JN, Mann S. Nano Letters. 2003;3:413. [Google Scholar]
- 274.Lim JS, Kim SM, Lee SY, Stach EA, Culver JN, Harris MT. Journal of colloid and interface science. 2010;342:455. doi: 10.1016/j.jcis.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 275.Knez M, Bittner AM, Boes F, Wege C, Jeske H, Maiß E, Kern K. Nano Letters. 2003;3:1079. [Google Scholar]
- 276.Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V. Nature methods. 2010;7:126. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Singh YS, Sawarynski LE, Dabiri PD, Choi WR, Andrews AM. Analytical chemistry. 2011;83:6658. doi: 10.1021/ac2011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bennet KE, Tomshine JR, Min H-K, Manciu FS, Marsh MP, Paek SB, Settell ML, Nicolai EN, Blaha CD, Kouzani AZ. Frontiers in human neuroscience. 2016;10:102. doi: 10.3389/fnhum.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Arumugam PU, Zeng H, Siddiqui S, Covey DP, Carlisle JA, Garris PA. Applied physics letters. 2013;102:253107. doi: 10.1063/1.4811785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Borland LM, Shi G, Yang H, Michael AC. Journal of Neuroscience Methods. 2005;146:149. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 281.Khan AS, Michael AC. TrAC Trends in Analytical Chemistry. 2003;22:503. [Google Scholar]
- 282.Jaquins-Gerstl A, Michael AC. Journal of Neuroscience Methods. 2009;183:127. doi: 10.1016/j.jneumeth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Frédéric M, Arno A, Tobias H, Patrick R, Gustaaf B, Bruce M, Fabian K. Journal of Neural Engineering. 2016;13:046018. [Google Scholar]
- 284.Gray CM, Maldonado PE, Wilson M, McNaughton B. Journal of Neuroscience Methods. 1995;63:43. doi: 10.1016/0165-0270(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 285.Kozai TDY, Marzullo TC, Hooi F, Langhals NB, Majewska AK, Brown EB, Kipke DR. Journal of Neural Engineering. 2010;7:046011. doi: 10.1088/1741-2560/7/4/046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fernández E, Greger B, House PA, Aranda I, Botella C, Albisua J, Soto-Sánchez C, Alfaro A, Normann RA. Frontiers in Neuroengineering. 2014;7:24. doi: 10.3389/fneng.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.House PA, MacDonald JD, Tresco PA, Normann RA. Neurosurgical focus. 2006;20:E4. doi: 10.3171/foc.2006.20.5.5. [DOI] [PubMed] [Google Scholar]
- 288.Nolta NF, Christensen MB, Crane PD, Skousen JL, Tresco PA. Biomaterials. 2015;53:753. doi: 10.1016/j.biomaterials.2015.02.081. [DOI] [PubMed] [Google Scholar]
- 289.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. Nature Neuroscience. 2012;16:55. doi: 10.1038/nn.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lind G, Gällentoft L, Danielsen N, Schouenborg J, Pettersson LME. PLoS ONE. 2012;7:e47509. doi: 10.1371/journal.pone.0047509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.McConnell GC, Rees HD, Levey AI, Gutekunst C-A, Gross RE, Bellamkonda RV. Journal of Neural Engineering. 2009;6:056003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- 292.Liu X, McCreery DB, Bullara LA, Agnew WF. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2006;14:91. doi: 10.1109/TNSRE.2006.870495. [DOI] [PubMed] [Google Scholar]
- 293.Baumbach GL, Siems JE, Faraci FM, Heistad DD. The American journal of physiology. 1989;256:H493. doi: 10.1152/ajpheart.1989.256.2.H493. [DOI] [PubMed] [Google Scholar]
- 294.Ward MP, Rajdev P, Ellison C, Irazoqui PP. Brain Research. 2009;1282:183. doi: 10.1016/j.brainres.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 295.Saxena T, Karumbaiah L, Gaupp EA, Patkar R, Patil K, Betancur M, Stanley GB, Bellamkonda RV. Biomaterials. 2013;34:4703. doi: 10.1016/j.biomaterials.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 296.Karumbaiah L, Saxena T, Carlson D, Patil K, Patkar R, Gaupp EA, Betancur M, Stanley GB, Carin L, Bellamkonda RV. Biomaterials. 2013;34:8061. doi: 10.1016/j.biomaterials.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 297.Gilletti A, Muthuswamy J. Journal of Neural Engineering. 2006;3:189. doi: 10.1088/1741-2560/3/3/001. [DOI] [PubMed] [Google Scholar]
- 298.Kozai TDY, Catt K, Li X, Gugel ZV, Olafsson VT, Vazquez AL, Cui XT. Biomaterials. 2015;37:25. doi: 10.1016/j.biomaterials.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Paukert M, Bergles DE. J Physiol-London. 2012;590:2955. doi: 10.1113/jphysiol.2012.228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Feinberg DA, Mark AS. Radiology. 1987;163:793. doi: 10.1148/radiology.163.3.3575734. [DOI] [PubMed] [Google Scholar]
- 301.Gilletti A, Muthuswamy J. Journal of Neural Engineering. 2006;3:189. doi: 10.1088/1741-2560/3/3/001. [DOI] [PubMed] [Google Scholar]
- 302.Subbaroyan J, Martin DC, Kipke DR. Journal of Neural Engineering. 2005;2:103. doi: 10.1088/1741-2560/2/4/006. [DOI] [PubMed] [Google Scholar]
- 303.Lee H, Bellamkonda RV, Sun W, Levenston ME. J Neural Eng. 2005;2:81. doi: 10.1088/1741-2560/2/4/003. [DOI] [PubMed] [Google Scholar]
- 304.Moshayedi P, Ng G, Kwok JC, Yeo GS, Bryant CE, Fawcett JW, Franze K, Guck J. Biomaterials. 2014;35:3919. doi: 10.1016/j.biomaterials.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 305.Someya T, Bao Z, Malliaras GG. Nature. 2016;540:379. doi: 10.1038/nature21004. [DOI] [PubMed] [Google Scholar]
- 306.Liao C, Zhang M, Yao MY, Hua T, Li L, Yan F. Advanced Materials. 2015;27:7493. doi: 10.1002/adma.201402625. [DOI] [PubMed] [Google Scholar]
- 307.Aregueta-Robles UA, Woolley AJ, Poole-Warren LA, Lovell NH, Green RA. Front Neuroeng. 2014;7:15. doi: 10.3389/fneng.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Green RA, Baek S, Poole-Warren LA, Martens PJ. Sci Technol Adv Mater. 2010;11:014107. doi: 10.1088/1468-6996/11/1/014107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhong Y, Bellamkonda RV. J R Soc Interface. 2008;5:957. doi: 10.1098/rsif.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Bollmann L, Koser DE, Shahapure R, Gautier HOB, Holzapfel GA, Scarcelli G, Gather MC, Ulbricht E, Franze K. Frontiers in cellular neuroscience. 2015;9:363. doi: 10.3389/fncel.2015.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gutowski SM, Templeman KL, South AB, Gaulding JC, Shoemaker JT, LaPlaca MC, Bellamkonda RV, Lyon LA, García AJ. Journal of Biomedical Materials Research Part A. 2014;102:1486. doi: 10.1002/jbm.a.34799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rao L, Zhou H, Li T, Li C, Duan YY. Acta Biomaterialia. 2012;8:2233. doi: 10.1016/j.actbio.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 313.Gilgunn PJ, Khilwani R, Kozai TDY, Weber DJ, Cui XT, Erdos G, Ozdoganlar OB, Fedder GK. presented at Micro Electro Mechanical Systems (MEMS), 2012 IEEE 25th International Conference on, Paris, France, January 29-February 2, 2012. [Google Scholar]
- 314.Khilwani R, Gilgunn PJ, Kozai TDY, Ong XC, Korkmaz E, Gunalan PK, Cui XT, Fedder GK, Ozdoganlar OB. Biomedical Microdevices. 2016;18:97. doi: 10.1007/s10544-016-0125-4. [DOI] [PubMed] [Google Scholar]
- 315.Sohal HS, Jackson A, Jackson R, Clowry GJ, Vassilevski K, O’Neill A, Baker SN. Frontiers in Neuroengineering. 2015 doi: 10.3389/fneng.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Subbaroyan J. Investigations of tethering induced injury response in brain tissue by intracortical implants through modeling and in vivo experiments. University of Michigan; Ann Arbor, Michigan: 2007. [Google Scholar]
- 317.Shin U-H, Jeong D-W, Park S-M, Kim S-H, Lee HW, Kim J-M. Carbon. 2014;80:396. [Google Scholar]
- 318.Kim T-i, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim R-H. Science. 2013;340:211. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Norton JJ, Lee DS, Lee JW, Lee W, Kwon O, Won P, Jung S-Y, Cheng H, Jeong J-W, Akce A. Proceedings of the National Academy of Sciences. 2015;112:3920. doi: 10.1073/pnas.1424875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chun K-Y, Oh Y, Rho J, Ahn J-H, Kim Y-J, Choi HR, Baik S. Nature nanotechnology. 2010;5:853. doi: 10.1038/nnano.2010.232. [DOI] [PubMed] [Google Scholar]
- 321.Kim D-H, Lu N, Ma R, Kim Y-S, Kim R-H, Wang S, Wu J, Won SM, Tao H, Islam A. Science. 2011;333:838. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- 322.Fiedler E, Haas N, Stieglitz T. presented at Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE, Chicago, Illinois August 2014; [DOI] [PubMed] [Google Scholar]
- 323.Loffler S, Xie Y, Klimach P, Richter A, Detemple P, Stieglitz T, Moser A, Hofmann UG. Biomed Tech (Berl) 2012;57:104. doi: 10.1515/bmt-2012-4434. /j/bmte.2012.57.issue-s1-S/bmt-2012-4434/bmt-2012-4434.xml [pii] [DOI] [Google Scholar]
- 324.Hassler C, Boretius T, Stieglitz T. Journal of Polymer Science Part B: Polymer Physics. 2011;49:18. [Google Scholar]
- 325.Hassler C, von Metzen RP, Ruther P, Stieglitz T. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2010;93B:266. doi: 10.1002/jbm.b.31584. [DOI] [PubMed] [Google Scholar]
- 326.Hassler C, Metzen R, Stieglitz T. In: 4th European Conference of the International Federation for Medical and Biological Engineering. Sloten J, Verdonck P, Nyssen M, Haueisen J, editors. Vol. 22. Springer; Berlin, Heidelberg: 2009. p. 2439. Ch. 585. [Google Scholar]
- 327.Kisban S, Herwik S, Seidl K, Rubehn B, Jezzini A, Umilta MA, Fogassi L, Stieglitz T, Paul O, Ruther P. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:175. doi: 10.1109/IEMBS.2007.4352251. [DOI] [PubMed] [Google Scholar]
- 328.Rodriguez FJ, Ceballos D, Schuttler M, Valero A, Valderrama E, Stieglitz T, Navarro X. J Neurosci Methods. 2000;98:105. doi: 10.1016/s0165-0270(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 329.Stieglitz T, Beutel Hr, Schuettler M, Meyer J-U. Biomedical Microdevices. 2000;2:283. [Google Scholar]
- 330.Stieglitz T, Beutel H, Meyer JU. Sensor Actuat a-Phys. 1997;60:240. [Google Scholar]
- 331.Kuo JT, Kim BJ, Hara SA, Lee CD, Gutierrez CA, Hoang TQ, Meng E. Lab on a Chip. 2013;13:554. doi: 10.1039/c2lc40935f. [DOI] [PubMed] [Google Scholar]
- 332.Kim BJ, Kuo JT, Hara SA, Lee CD, Yu L, Gutierrez CA, Hoang TQ, Pikov V, Meng E. Journal of Neural Engineering. 2013;10:045002. doi: 10.1088/1741-2560/10/4/045002. [DOI] [PubMed] [Google Scholar]
- 333.Fu TM, Hong G, Zhou T, Schuhmann TG, Viveros RD, Lieber CM. Nat Methods. 2016;13:875. doi: 10.1038/nmeth.3969. [DOI] [PubMed] [Google Scholar]
- 334.Dai X, Zhou W, Gao T, Liu J, Lieber CM. Nat Nanotechnol. 2016;11:776. doi: 10.1038/nnano.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Xie C, Liu J, Fu TM, Dai X, Zhou W, Lieber CM. Nat Mater. 2015;14:1286. doi: 10.1038/nmat4427. [DOI] [PubMed] [Google Scholar]
- 336.Luan L, Wei X, Zhao Z, Siegel JJ, Potnis O, Tuppen CA, Lin S, Kazmi S, Fowler RA, Holloway S. Science Advances. 2017;3:e1601966. doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kozai TDY, Kipke DR. J Neurosci Methods. 2009;184:199. doi: 10.1016/j.jneumeth.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rennaker RL, Street S, Ruyle AM, Sloan AM. J Neurosci Methods. 2005;142:169. doi: 10.1016/j.jneumeth.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 339.Harris JP, Hess AE, Rowan SJ, Weder C, Zorman CA, Tyler DJ, Capadona JR. Journal of Neural Engineering. 2011;8:046010. doi: 10.1088/1741-2560/8/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Singh S, Lo M-C, Damodaran V, Kaplan H, Kohn J, Zahn J, Shreiber D. Sensors. 2016;16:330. doi: 10.3390/s16030330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Karumbaiah L, Norman SE, Rajan NB, Anand S, Saxena T, Betancur M, Patkar R, Bellamkonda RV. Biomaterials. 2012;33:5983. doi: 10.1016/j.biomaterials.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 342.Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, De S, Roysam B, Shain W, Kim SJ. Journal of Neural Engineering. 2006;3:196. doi: 10.1088/1741-2560/3/3/002. [DOI] [PubMed] [Google Scholar]
- 343.Morrison B, Cater HL, Wang CC-B, Thomas FC, Hung CT, Ateshian GA, Sundstrom LE. Stapp car crash journal. 2003;47:93. doi: 10.4271/2003-22-0006. [DOI] [PubMed] [Google Scholar]
- 344.Morrison B, Cater HL, Benham CD, Sundstrom LE. Journal of Neuroscience Methods. 2006;150:192. doi: 10.1016/j.jneumeth.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 345.Bar-Kochba E, Scimone MT, Estrada JB, Franck C. Scientific Reports. 2016;6:30550. doi: 10.1038/srep30550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, De S, Roysam B, Shain W, Kim SJ. J Neural Eng. 2006;3:196. doi: 10.1088/1741-2560/3/3/002. [DOI] [PubMed] [Google Scholar]
- 347.Edell DJ, Toi VV, McNeil VM, Clark LD. IEEE Trans Biomed Eng. 1992;39:635. doi: 10.1109/10.141202. [DOI] [PubMed] [Google Scholar]
- 348.Andrei A, Welkenhuysen M, Nuttin B, Eberle W. Journal of Neural Engineering. 2012;9:016005. doi: 10.1088/1741-2560/9/1/016005. [DOI] [PubMed] [Google Scholar]
- 349.Haj Hosseini N, Hoffmann R, Kisban S, Stieglitz T, Paul O, Ruther P. 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; Lyon, France: 2007. p. 4711. [DOI] [PubMed] [Google Scholar]
- 350.Jensen W, Yoshida K, Hofmann UG. IEEE Transactions on Biomedical Engineering. 2006;53:934. doi: 10.1109/TBME.2006.872824. [DOI] [PubMed] [Google Scholar]
- 351.Sharp AA, Ortega AM, Restrepo D, Curran-Everett D, Gall K. IEEE Transactions on Biomedical Engineering. 2009;56:45. doi: 10.1109/TBME.2008.2003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain research. 2003;983:23. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- 353.Elkin BS, Morrison B. Journal of Biomechanical Engineering. 2013;135:114507. doi: 10.1115/1.4025386. [DOI] [PubMed] [Google Scholar]
- 354.Lee H, Bellamkonda RV, Sun W, Levenston ME. Journal of Neural Engineering. 2005;2:81. doi: 10.1088/1741-2560/2/4/003. [DOI] [PubMed] [Google Scholar]
- 355.MacManus DB, Pierrat B, Murphy JG, Gilchrist MD. Scientific Reports. 2016;6:21569. doi: 10.1038/srep21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Rousche PJ, Normann RA. Annals of Biomedical Engineering. 1992;20:413. doi: 10.1007/BF02368133. [DOI] [PubMed] [Google Scholar]
- 357.Subbaroyan J, Martin DC, Kipke DR. Journal of Neural Engineering. 2005;2:103. doi: 10.1088/1741-2560/2/4/006. [DOI] [PubMed] [Google Scholar]
- 358.Casanova F, Carney PR, Sarntinoranont M. Journal of Neuroscience Methods. 2014;237:79. doi: 10.1016/j.jneumeth.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Sharp AA, Panchawagh HV, Ortega A, Artale R, Richardson-Burns S, Finch DS, Gall K, Mahajan RL, Restrepo D. Journal of Neural Engineering. 2006;3:L23. doi: 10.1088/1741-2560/3/4/L02. [DOI] [PubMed] [Google Scholar]
- 360.Thelin J, Jorntell H, Psouni E, Garwicz M, Schouenborg J, Danielsen N, Linsmeier CE. PLoS One. 2011;6:e16267. doi: 10.1371/journal.pone.0016267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Harris JP, Hess AE, Rowan SJ, Weder C, Zorman CA, Tyler DJ, Capadona JR. Journal of Neural Engineering. 2011;8:046010. doi: 10.1088/1741-2560/8/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kozai TDY, Kipke DR. Journal of Neuroscience Methods. 2009;184:199. doi: 10.1016/j.jneumeth.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Hanson TL, Maharbiz MM, SABES PN. WO2016126340. Patent. 2016:A3.
- 364.Chen CH, Chuang SC, Su HC, Hsu WL, Yew TR, Chang YC, Yeh SR, Yao DJ. Lab Chip. 2011;11:1647. doi: 10.1039/c0lc00718h. [DOI] [PubMed] [Google Scholar]
- 365.Lewitus D, Smith KL, Shain W, Kohn J. Acta Biomaterialia. 2011;7:2483. doi: 10.1016/j.actbio.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 366.Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. J Control Release. 2006;111:252. doi: 10.1016/j.jconrel.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 367.Kozai TDY, Gugel Z, Li X, Gilgunn PJ, Khilwani R, Ozdoganlar OB, Fedder GK, Weber DJ, Cui XT. Biomaterials. 2014;35:9255. doi: 10.1016/j.biomaterials.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 368.McClain MA, Clements IP, Shafer RH, Bellamkonda RV, LaPlaca MC, Allen MG. Biomed Microdevices. 2011;13:361. doi: 10.1007/s10544-010-9505-3. [DOI] [PubMed] [Google Scholar]
- 369.Patel PR, Na K, Zhang H, Kozai TDY, Kotov NA, Yoon E, Chestek CA. Journal of Neural Engineering. 2015;12:4. doi: 10.1088/1741-2560/12/4/046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lo M-c, Wang S, Singh S, Damodaran VB, Kaplan HM, Kohn J, Shreiber DI, Zahn JD. Biomedical Microdevices. 2015;17:34. doi: 10.1007/s10544-015-9927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Kozai TDY, Gugel Z, Li X, Gilgunn PJ, Khilwani R, Ozdoganlar OB, Fedder GK, Weber DJ, Cui XT. Biomaterials. 2014;35:9255. doi: 10.1016/j.biomaterials.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 372.Wolbarsht ML, MacNichol EF, Wagner HG. Science. 1960;132:1309. doi: 10.1126/science.132.3436.1309. [DOI] [PubMed] [Google Scholar]
- 373.Dowben RM, Rose JE. Science. 1953;118:22. doi: 10.1126/science.118.3053.22. [DOI] [PubMed] [Google Scholar]
- 374.McCarthy PT, Otto KJ, Rao MP. Biomedical Microdevices. 2011;13:503. doi: 10.1007/s10544-011-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Maruyama Y, Oishi Y, Kakimoto M, Imai Y. Macromolecules. 1988;21:2305. [Google Scholar]
- 376.Srinivasan G, Reneker DH. Polymer international. 1995;36:195. [Google Scholar]
- 377.Guitchounts G, Markowitz JE, Liberti WA, Gardner TJ. J Neural Eng. 2013;10:046016. doi: 10.1088/1741-2560/10/4/046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR. Nature materials. 2012;11:1065. doi: 10.1038/nmat3468. Supplementary Information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Shim BS, Zhu J, Jan E, Critchley K, Kotov NA. ACS Nano. 2010;4:3725. doi: 10.1021/nn100026n. [DOI] [PubMed] [Google Scholar]
- 380.Shim BS, Zhu J, Jan E, Critchley K, Ho S, Podsiadlo P, Sun K, Kotov NA. ACS Nano. 2009;3:1711. doi: 10.1021/nn9002743. [DOI] [PubMed] [Google Scholar]
- 381.Shim BS, Chen W, Doty C, Xu C, Kotov NA. Nano Lett. 2008;8:4151. doi: 10.1021/nl801495p. [DOI] [PubMed] [Google Scholar]
- 382.Skorb EV, Andreeva DV. Polymer Chemistry. 2013;4:4834. [Google Scholar]
- 383.Lacour SP, Chan D, Wagner S, Li T, Suo Z. Applied Physics Letters. 2006;88:204103. [Google Scholar]
- 384.Saddow SE, Agarwal A. Advances in silicon carbide processing and applications. Artech House; Norwood, Massachusetts: 2004. [Google Scholar]
- 385.Reddy JD, Volinsky A, Frewin C, Locke C, Saddow S. presented at Mater. Res. Soc. Symp. Proc. Strasburg, PA: Jan, 2008. [Google Scholar]
- 386.Rzany A, Harder C, Schaldach M. Silicon carbide as an anti-thrombogenic stent coating: an example of a science-based development strategy. Progress in Biomedical Research. 5:168. [Google Scholar]
- 387.Mavinakuli P, Wei S, Wang Q, Karki AB, Dhage S, Wang Z, Young DP, Guo Z. The Journal of Physical Chemistry C. 2010;114:3874. [Google Scholar]
- 388.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Journal of Neural Engineering. 2013;10:066014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Teo AJ, Mishra A, Park I, Kim Y-J, Park W-T, Yoon Y-J. ACS Biomaterials Science & Engineering. 2016;2:454. doi: 10.1021/acsbiomaterials.5b00429. [DOI] [PubMed] [Google Scholar]
- 390.Amanat N, James NL, McKenzie DR. Medical engineering & physics. 2010;32:690. doi: 10.1016/j.medengphy.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 391.Erdogan O. Indian Pacing and Electrophysiology Journal. 2002;2:74. [PMC free article] [PubMed] [Google Scholar]
- 392.Seymour JP, Wu F, Wise KD, Yoon E. Microsystems & Nanoengineering. 2017;3:16066. doi: 10.1038/micronano.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Nurmikko A, Borton D, Yin M. In: Neurobionics: The Biomedical Engineering of Neural Prostheses. Shepherd RK, editor. John Wiley & Sons, Inc.; Hoboken, New Jersey: 2016. p. 123. Ch. 5. [DOI] [Google Scholar]
- 394.Benabid AL, Chabardes S, Mitrofanis J, Pollak P. The Lancet Neurology. 2009;8:67. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- 395.Hauser RG, Katsiyiannis WT, Gornick CC, Almquist AK, Kallinen LM. Europace. 2010;12:395. doi: 10.1093/europace/eup375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ferguson JE, Redish AD. Expert Review of Medical Devices. 2011;8:427. doi: 10.1586/erd.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Damestani Y, Reynolds CL, Szu J, Hsu MS, Kodera Y, Binder DK, Park BH, Garay JE, Rao MP, Aguilar G. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9:1135. doi: 10.1016/j.nano.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 398.Degenhart AD, Eles J, Dum R, Mischel JL, Smalianchuk I, Endler B, Ashmore RC, Tyler-Kabara EC, Hatsopoulos NG, Wang W, Batista AP, Cui XT. Journal of Neural Engineering. 2016;13:046019. doi: 10.1088/1741-2560/13/4/046019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Ryapolova-Webb E, Afshar P, Stanslaski S, Denison T, de Hemptinne C, Bankiewicz K, Starr PA. Journal of Neural Engineering. 2014;11:016009. doi: 10.1088/1741-2560/11/1/016009. [DOI] [PubMed] [Google Scholar]
- 400.Onal C, Otsubo H, Araki T, Chitoku S, Ochi A, Weiss S, Elliott I, Snead OC, 3rd, Rutka JT, Logan W. J Neurosurg. 2003;98:1017. doi: 10.3171/jns.2003.98.5.1017. [DOI] [PubMed] [Google Scholar]
- 401.Markwardt NT, Stokol J, Rennaker RL., II Journal of Neuroscience Methods. 2013;214:119. doi: 10.1016/j.jneumeth.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ravikumar M, Sunil S, Black J, Barkauskas DS, Haung AY, Miller RH, Selkirk SM, Capadona JR. Biomaterials. 2014;35:8049. doi: 10.1016/j.biomaterials.2014.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Kim Y-T, Hitchcock RW, Bridge MJ, Tresco PA. Biomaterials. 2004;25:2229. doi: 10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 404.Biran R, Martin DC, Tresco PA. Journal of Biomedical Materials Research Part A. 2007;82A:169. doi: 10.1002/jbm.a.31138. [DOI] [PubMed] [Google Scholar]
- 405.Abdo A, Jayasinha V, Spuhler PS, Unlu M, Sahin M. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:626. doi: 10.1109/IEMBS.2009.5334073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Musial PG, Baker SN, Gerstein GL, King EA, Keating JG. Journal of Neuroscience Methods. 2002;115:29. doi: 10.1016/s0165-0270(01)00516-7. [DOI] [PubMed] [Google Scholar]
- 407.Paralikar KJ, Rao CR, Clement RS. J Neurosci Methods. 2009;181:27. doi: 10.1016/j.jneumeth.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Garg A, Mohan AL, Garell PC. Parkinson’s Disease. 2010;2010:189371. doi: 10.4061/2010/189371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Fernández FS, Alvarez Vega MA, Antuña Ramos A, Fernández González F, Lozano Aragoneses B. Parkinson’s Disease. 2009;2010:409356. doi: 10.4061/2010/409356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Bjerknes S, Skogseid IM, Sæhle T, Dietrichs E, Toft M. PLOS ONE. 2014;9:e105288. doi: 10.1371/journal.pone.0105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. IEEE Transactions on Neural Systems and Rehabilitation Engineering: A Publication of the IEEE Engineering in Medicine and Biology Society. 2005;13:524. doi: 10.1109/TNSRE.2005.857687. [DOI] [PubMed] [Google Scholar]
- 412.Borton DA, Yin M, Aceros J, Nurmikko A. Journal of Neural Engineering. 2013;10:026010. doi: 10.1088/1741-2560/10/2/026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot J-B, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WKD, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, Courtine G. Nature. 2016;539:284. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Harrison RR, Kier RJ, Kim S, Rieth L, Warren DJ, Ledbetter NM, Clark GA, Solzbacher F, Chestek CA, Gilja V, Nuyujukian P, Ryu SI, Shenoy KV. 2008 IEEE Biomedical Circuits and Systems Conference. IEEE; Baltimore, Maryland: 2008. p. 125. [DOI] [Google Scholar]
- 415.Kim S, Bhandari R, Klein M, Negi S, Rieth L, Tathireddy P, Toepper M, Oppermann H, Solzbacher F. Biomedical Microdevices. 2009;11:453. doi: 10.1007/s10544-008-9251-y. [DOI] [PubMed] [Google Scholar]
- 416.Rafflenbeul L, Werthschützky R, Gail A. Procedia Engineering. 2012;47:1097. [Google Scholar]
- 417.Kiourti A, Lee CWL, Chae J, Volakis JL. IEEE Transactions on Biomedical Engineering. 2016;63:131. doi: 10.1109/TBME.2015.2458583. [DOI] [PubMed] [Google Scholar]
- 418.Schwerdt HN, Xu W, Shekhar S, Abbaspour-Tamijani A, Towe BC, Miranda FA, Chae J. Journal of microelectromechanical systems : a joint IEEE and ASME publication on microstructures, microactuators, microsensors, and microsystems. 2011;20:1119. doi: 10.1109/JMEMS.2011.2162487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Oxley TJ, Opie NL, John SE, Rind GS, Ronayne SM, Wheeler TL, Judy JW, McDonald AJ, Dornom A, Lovell TJH, Steward C, Garrett DJ, Moffat BA, Lui EH, Yassi N, Campbell BCV, Wong YT, Fox KE, Nurse ES, Bennett IE, Bauquier SH, Liyanage KA, van der Nagel NR, Perucca P, Ahnood A, Gill KP, Yan B, Churilov L, French CR, Desmond PM, Horne MK, Kiers L, Prawer S, Davis SM, Burkitt AN, Mitchell PJ, Grayden DB, May CN, O'Brien TJ. Nat Biotech. 2016;34:320. [Google Scholar]
- 420.Ciofani G, Danti S, D'Alessandro D, Ricotti L, Moscato S, Bertoni G, Falqui A, Berrettini S, Petrini M, Mattoli V, Menciassi A. ACS Nano. 2010;4:6267. doi: 10.1021/nn101985a. [DOI] [PubMed] [Google Scholar]
- 421.Larson PJ, Towe BC. presented at Neural Engineering (NER), 2011 5th International IEEE/EMBS Conference on, Cancun, Mexico, April 27-May 1, 2011. [Google Scholar]
- 422.Harrison RR, Watkins PT, Kier RJ, Lovejoy RO, Black DJ, Greger B, Solzbacher F. Solid-State Circuits, IEEE Journal of. 2007;42:123. [Google Scholar]
- 423.Abaya TVF, Diwekar M, Blair S, Tathireddy P, Rieth L, Clark GA, Solzbacher F. Biomedical optics express. 2012;3:2200. doi: 10.1364/BOE.3.002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Shapiro MG, Homma K, Villarreal S, Richter C-P, Bezanilla F. Nature communications. 2012;3:736. doi: 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Abdo A, Sahin M. IEEE transactions on biomedical circuits and systems. 2011;2011:1. doi: 10.1109/TBCAS.2011.2114882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Abdo A, Sahin M, Freedman DS, Cevik E, Spuhler PS, Unlu MS. Journal of Neural Engineering. 2011;8:056012. doi: 10.1088/1741-2560/8/5/056012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Sahin M, Ur-Rahman SS. IEEE Trans Neural Syst Rehabil Eng. 2007;15:227. doi: 10.1109/TNSRE.2007.897027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Seymour EÇ, Freedman DS, Gökkavas M, Özbay E, Sahin M, Ünlü MS. Frontiers in Neuroengineering. 2014;7:00005. doi: 10.3389/fneng.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Romero G, Christiansen MG, Stocche Barbosa L, Garcia F, Anikeeva P. Advanced Functional Materials. 2016;26:6471. [Google Scholar]
- 430.Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Science. 2015;347:1477. doi: 10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- 431.Wang Y, Guo L. Frontiers in Neuroscience. 2016;10:69. doi: 10.3389/fnins.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Lavoie-Cardinal F, Salesse C, Bergeron É, Meunier M, De Koninck P. Scientific Reports. 2016;6:20619. doi: 10.1038/srep20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Frontiers in Human Neuroscience. 2015;9:303. doi: 10.3389/fnhum.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Hallett M. Nature. 2000;406:147. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 435.Luo X, Cui XT. Electrochemistry Communications. 2009;11:1956. doi: 10.1016/j.elecom.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Jiang S, Sun Y, Cui X, Huang X, He Y, Ji S, Shi W, Ge D. Synthetic Metals. 2013;163:19. [Google Scholar]
- 437.Freedman MS, Cui XT. Physical Chemistry Communications. 2014;1:15. [Google Scholar]
- 438.Cho Y, Shi R, Ivanisevic A, Ben Borgens R. Nanotechnology. 2009;20:275102. doi: 10.1088/0957-4484/20/27/275102. [DOI] [PubMed] [Google Scholar]
- 439.Taylor AB, Siddiquee AM, Chon JW. ACS Nano. 2014;8:12071. doi: 10.1021/nn5055283. [DOI] [PubMed] [Google Scholar]
- 440.Yong J, Needham K, Brown WG, Nayagam BA, McArthur SL, Yu A, Stoddart PR. Advanced Healthcare Materials. 2014;3:1862. doi: 10.1002/adhm.201400027. [DOI] [PubMed] [Google Scholar]
- 441.Kozai TDY, Vazquez AL, Weaver CL. Journal of Neural Engineering. 2012;9:6. doi: 10.1088/1741-2560/9/6/066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Purcell EK, Seymour JP, Yandamuri S, Kipke DR. Journal of Neural Engineering. 2009;6:026005. doi: 10.1088/1741-2560/6/2/026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Bridges AW, García AJ. Journal of Diabetes Science and Technology. 2008;2:984. doi: 10.1177/193229680800200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Biran R, Noble MD, Tresco PA. Journal of Biomedical Materials Research. 1999;46:150. doi: 10.1002/(sici)1097-4636(199908)46:2<150::aid-jbm3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 445.Leung BK, Biran R, Underwood CJ, Tresco PA. Biomaterials. 2008;29:3289. doi: 10.1016/j.biomaterials.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 446.Manwaring ME, Biran R, Tresco PA. Biomaterials. 2001;22:3155. doi: 10.1016/s0142-9612(01)00068-0. [DOI] [PubMed] [Google Scholar]
- 447.Zhang H, Chiao M. Journal of Medical and Biological Engineering. 2015;35:143. doi: 10.1007/s40846-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Harding JL, Reynolds MM. Trends in Biotechnology. 2014;32:140. doi: 10.1016/j.tibtech.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 449.Azemi E, Stauffer WR, Gostock MS, Lagenaur CF, Cui XT. Acta Biomaterialia. 2008;4:1208. doi: 10.1016/j.actbio.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 450.Lee JY, Khaing ZZ, Siegel JJ, Schmidt CE. RSC Adv. 2015;5:39228. doi: 10.1039/c5ra03294f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Zhao H, Zhu B, Luo S-C, Lin H-A, Nakao A, Yamashita Y, Yu H-h. ACS Applied Materials & Interfaces. 2013;5:4536. doi: 10.1021/am400135c. [DOI] [PubMed] [Google Scholar]
- 452.Hu Y, Yang G, Liang B, Fang L, Ma G, Zhu Q, Chen S, Ye X. Acta Biomaterialia. 2015;13:142. doi: 10.1016/j.actbio.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 453.Patrick E, Orazem ME, Sanchez JC, Nishida T. Journal of Neuroscience Methods. 2011;198:158. doi: 10.1016/j.jneumeth.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Prasad A, Xue Q-S, Dieme R, Sankar V, Mayrand RC, Nishida T, Streit WJ, Sanchez JC. Frontiers in neuroengineering. 2014;7:2. doi: 10.3389/fneng.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Sanchez JC, Alba N, Nishida T, Batich C, Carney PR. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2006;14:217. doi: 10.1109/TNSRE.2006.875581. [DOI] [PubMed] [Google Scholar]
- 456.Sridharan A, Nguyen JK, Capadona JR, Muthuswamy J. Journal of Neural Engineering. 2015;12:036002. doi: 10.1088/1741-2560/12/3/036002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Lind G, Linsmeier CE, Schouenborg J. Scientific Reports. 2013;3:11830. doi: 10.1038/srep02942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Bridges AW, Whitmire RE, Singh N, Templeman KL, Babensee JE, Lyon LA, García AJ. Journal of Biomedical Materials Research Part A. 2010;94A:252. doi: 10.1002/jbm.a.32669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Lu Y, Wang D, Li T, Zhao X, Cao Y, Yang H, Duan YY. Biomaterials. 2009;30:4143. doi: 10.1016/j.biomaterials.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 460.Li M, Zhou H-H, Li T, Li C-Y, Xia Z-Y, Duan YY. Neural Regeneration Research. 2015;10:2048. doi: 10.4103/1673-5374.172325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Achyuta AKH, Polikov VS, White AJ, Lewis HGP, Murthy SK. Macromolecular Bioscience. 2010;10:872. doi: 10.1002/mabi.200900451. [DOI] [PubMed] [Google Scholar]
- 462.Kim D-H, Abidian M, Martin DC. Journal of Biomedical Materials Research. 2004;71A:577. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 463.Fan YW, Cui FZ, Hou SP, Xu QY, Chen LN, Lee I-S. Journal of Neuroscience Methods. 2002;120:17. doi: 10.1016/s0165-0270(02)00181-4. [DOI] [PubMed] [Google Scholar]
- 464.Moxon KA, Kalkhoran NM, Markert M, Sambito MA, McKenzie JL, Webster JT. IEEE Transactions on Biomedical Engineering. 2004;51:881. doi: 10.1109/TBME.2004.827465. [DOI] [PubMed] [Google Scholar]
- 465.Moxon KA, Hallman S, Aslani A, Kalkhoran NM, Lelkes PI. Journal of Biomaterials Science, Polymer Edition. 2007;18:1263. doi: 10.1163/156856207782177882. [DOI] [PubMed] [Google Scholar]
- 466.Chen S, Allen MG. MRS Bulletin. 2012;37:606. [Google Scholar]
- 467.Chapman CAR, Wang L, Chen H, Garrison J, Lein PJ, Seker E. Advanced Functional Materials. 2017;27:1604631. doi: 10.1002/adfm.201604631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Luo X, Weaver CL, Zhou DD, Greenberg R, Cui XT. Biomaterials. 2011;32:5551. doi: 10.1016/j.biomaterials.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Labaye DE, Jérôme C, Geskin V, Louette P, Lazzaroni R, Martinot L, Jérôme R. Langmuir. 2002;18:5222. [Google Scholar]
- 470.Stauffer WR, Cui XT. Biomaterials. 2006;27:2405. doi: 10.1016/j.biomaterials.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 471.Kolarcik CL, Bourbeau D, Azemi E, Rost E, Zhang L, Lagenaur CF, Weber DJ, Cui XT. Acta Biomaterialia. 2012;8:3561. doi: 10.1016/j.actbio.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Eles JR, Vazquez AL, Snyder NR, Lagenaur CF, Murphy MC, Kozai TDY, Cui XT. Biomaterials. 2017;113:279. doi: 10.1016/j.biomaterials.2016.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA. Biomaterials. 2001;22:1017. doi: 10.1016/s0142-9612(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 474.Azemi E, Lagenaur CF, Cui XT. Biomaterials. 2011;32:681. doi: 10.1016/j.biomaterials.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Taub AH, Hogri R, Magal A, Mintz M, Shacham-Diamand Y. Journal of Biomedical Materials Research Part A. 2012;100A:1854. doi: 10.1002/jbm.a.34152. [DOI] [PubMed] [Google Scholar]
- 476.Potter-Baker KA, Nguyen JK, Kovach KM, Gitomer MM, Srail TW, Stewart WG, Skousen JL, Capadona JR. J. Mater. Chem. B. 2014;2:2248. doi: 10.1039/C4TB00125G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Chang J, Jallouli Y, Kroubi M, Yuan XB, Feng W, Kang CS, Pu PY, Betbeder D. International journal of pharmaceutics. 2009;379:285. doi: 10.1016/j.ijpharm.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 478.Kozai TDY, Jaquins-gerstl AS, Vazquez AL, Michael AC, Cui XT. Biomaterials. 2016;87:157. doi: 10.1016/j.biomaterials.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Mercanzini A, Reddy ST, Velluto D, Colin P, Maillard A, Bensadoun J-C, Hubbell JA, Renaud P. Journal of Controlled Release. 2010;145:196. doi: 10.1016/j.jconrel.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 480.Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltzman M, Turner JN. IEEE Transactions on Neural Systems and Rehabilitation Engineering: A Publication of the IEEE Engineering in Medicine and Biology Society. 2003;11:186. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- 481.Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W. Experimental Neurology. 2005;194:289. doi: 10.1016/j.expneurol.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 482.Zhong Y, Bellamkonda RV. Brain Research. 2007;1148:15. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Wadhwa R, Lagenaur CF, Cui XT. Journal of Controlled Release. 2006;110:531. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 484.Kim D-H, Martin DC. Biomaterials. 2006;27:3031. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 485.Weaver CL, LaRosa JM, Luo X, Cui XT. ACS Nano. 2014;8:1834. doi: 10.1021/nn406223e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Mond H, Stokes K, Helland J, Grigg L, Kertes P, Pate B, Hunt D. Pacing Clin Electrophysiol. 1988;11:214. doi: 10.1111/j.1540-8159.1988.tb04543.x. [DOI] [PubMed] [Google Scholar]
- 487.Crossley GH, Brinker JA, Reynolds D, Spencer W, Johnson WB, Hurd H, Tonder L, Zmijewski M. Circulation. 1995;92:2935. doi: 10.1161/01.cir.92.10.2935. [DOI] [PubMed] [Google Scholar]
- 488.Kutyifa V, Zima E, Molnar L, Kuehne C, Theiss S, Herrmann G, Geller L, Merkely B. Cardiology journal. 2013;20:431. doi: 10.5603/CJ.2013.0103. [DOI] [PubMed] [Google Scholar]
- 489.Nguyen JK, Jorfi M, Buchanan KL, Park DJ, Foster EJ, Tyler DJ, Rowan SJ, Weder C, Capadona JR. Acta Biomaterialia. 2016;29:81. doi: 10.1016/j.actbio.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Potter-Baker KA, Stewart WG, Tomaszewski WH, Wong CT, Meador WD, Ziats NP, Capadona JR. Journal of Neural Engineering. 2015;12:046002. doi: 10.1088/1741-2560/12/4/046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Zhong Y, Bellamkonda RV. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2005;106:309. doi: 10.1016/j.jconrel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 492.Han N, Rao SS, Johnson J, Parikh KS, Bradley PA, Lannutti JJ, Winter JO. Frontiers in Neuroengineering. 2011;4:2. doi: 10.3389/fneng.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Jhaveri SJ, Hynd MR, Dowell-Mesfin N, Turner JN, Shain W, Ober CK. Biomacromolecules. 2009;10:174. doi: 10.1021/bm801101e. [DOI] [PubMed] [Google Scholar]
- 494.Evans AJ, Thompson BC, Wallace GG, Millard R, O'Leary SJ, Clark GM, Shepherd RK, Richardson RT. Journal of Biomedical Materials Research Part A. 2009;91A:241. doi: 10.1002/jbm.a.32228. [DOI] [PubMed] [Google Scholar]
- 495.Kennedy PR, Mirra SS, Bakay RA. Neuroscience letters. 1992;142:89. doi: 10.1016/0304-3940(92)90627-j. [DOI] [PubMed] [Google Scholar]
- 496.Kennedy P, Andreasen D, Bartels J, Ehirim P, Mao H, Velliste M, Wichmann T, Wright J. Progress in brain research. 2011;194:1. doi: 10.1016/B978-0-444-53815-4.00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Bartels J, Andreasen D, Ehirim P, Mao H, Seibert S, Wright EJ, Kennedy P. Journal of Neuroscience Methods. 2008;174:168. doi: 10.1016/j.jneumeth.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Azemi E, Gobbel GT, Cui XT. J Neurosurg. 2010;113:673. doi: 10.3171/2010.1.JNS09313. [DOI] [PubMed] [Google Scholar]
- 499.Cui X, Hetke JF, Wiler JA, Anderson DJ, Martin DC. Sensors and Actuators A: Physical. 2001;93:8. [Google Scholar]
- 500.Weaver CL, Li H, Luo X, Cui XT. Journal of Materials Chemistry B. 2014;2:5209. doi: 10.1039/c4tb00789a. [DOI] [PubMed] [Google Scholar]
- 501.Alba NA, Du ZJ, Catt KA, Kozai TDY, Cui XT. Biosensors. 2015;5:618. doi: 10.3390/bios5040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Fattahi P, Yang G, Kim G, Abidian MR. Advanced Materials. 2014;26:1846. doi: 10.1002/adma.201304496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Kolarcik CL, Catt K, Rost E, Albrecht IN, Bourbeau D, Du Z, Kozai TDY, Luo X, Weber DJ, Cui XT. Journal of Neural Engineering. 2015;12:016008. doi: 10.1088/1741-2560/12/1/016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Weaver CL, Cui XT. Advanced Healthcare Materials. 2015;4:1408. doi: 10.1002/adhm.201500056. [DOI] [PubMed] [Google Scholar]
- 505.Matsumoto K, Sato C, Naka Y, Whitby R, Shimizu N. Nanotechnology. 2010;21:115101. doi: 10.1088/0957-4484/21/11/115101. [DOI] [PubMed] [Google Scholar]
- 506.Moser T, Celma C, Lebert A, Charrault E, Brooke R, Murphy PJ, Browne G, Young R, Higgs T, Evans D. ACS Applied Materials & Interfaces. 2016;8:974. doi: 10.1021/acsami.5b10831. [DOI] [PubMed] [Google Scholar]
- 507.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Cell. 2010;141:154. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Chow BY, Boyden ES. Science Translational Medicine. 2013;5:177ps5. doi: 10.1126/scitranslmed.3003101. [DOI] [PubMed] [Google Scholar]
- 509.Williams JC, Denison T. Science Translational Medicine. 2013;5:177ps6. doi: 10.1126/scitranslmed.3003100. [DOI] [PubMed] [Google Scholar]
- 510.Hudson MC, Thiel FL. Applied optics. 1974;13:2540. doi: 10.1364/AO.13.002540. [DOI] [PubMed] [Google Scholar]
- 511.Wu F, Stark E, Ku P-C, Wise KD, Buzsáki G, Yoon E. Neuron. 2015;88:1136. doi: 10.1016/j.neuron.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Steude A, Jahnel M, Thomschke M, Schober M, Gather MC. Advanced Materials. 2015;27:7657. doi: 10.1002/adma.201503253. [DOI] [PubMed] [Google Scholar]
- 513.Steude A, Witts EC, Miles GB, Gather MC. Science Advances. 2016;2:e1600061. doi: 10.1126/sciadv.1600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Kim DH, Viventi J, Amsden JJ, Xiao J, Vigeland L, Kim YS, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang Y, Hwang KC, Zakin MR, Litt B, Rogers JA. Nat Mater. 2010;9:511. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Choi C, Lin L, Liu Y, Choi J, Wang L, Haas D, Magera J, Chen RT. Journal of Lightwave Technology. 2004;22:2168. [Google Scholar]
- 516.Schwaerzle M, Pothof F, Paul O, Ruther P. presented at Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), 2015 Transducers-2015 18th International Conference on, Anchorage, Alaska June 2015. [Google Scholar]
- 517.Wu J, Pike RT, Wong C, Kim NP, Tanielian MH. IEEE Transactions on advanced packaging. 2000;23:721. [Google Scholar]
- 518.Jiang G, Zhou DD. Implantable Neural Prostheses 2. Springer; New York, New York: 2009. p. 27. [Google Scholar]
- 519.Lewis JS, Weaver MS. IEEE Journal of selected topics in quantum electronics. 2004;10:45. [Google Scholar]
- 520.Jeong EG, Han YC, Im H-G, Bae B-S, Choi KC. Organic Electronics. 2016;33:150. [Google Scholar]
- 521.Joung Y-H. International neurourology journal. 2013;17:98. doi: 10.5213/inj.2013.17.3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Borreman A, Musa S, Kok A, Diemeer M, Driessen A. IEEE/LEOS Benelux Chapter 2002 Annual Symposium. 2002;83 [Google Scholar]
- 523.Son Y, Lee HJ, Kim J, Lee CJ, Yoon E-S, Kim TG, Cho I-J. presented at 28th IEEE Int. Conf. on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, January 2015. [Google Scholar]
- 524.Han T, Madden S, Zhang M, Charters R, Luther-Davies B. presented at Optoelectronic and Microelectronic Materials and Devices, 2008. COMMAD 2008. Conference on, Sydney, Australia July 2008. [Google Scholar]
- 525.Im M, Cho I-J, Wu F, Wise KD, Yoon E. presented at Micro Electro Mechanical Systems (MEMS), 2011 IEEE 24th International Conference on, Cancun, Mexico January 2011. [Google Scholar]
- 526.Kampasi K, Stark E, Seymour J, Na K, Winful HG, Buzsáki G, Wise KD, Yoon E. Scientific Reports. 2016;6:30961. doi: 10.1038/srep30961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Son Y, Lee HJ, Kim J, Shin H, Choi N, Lee CJ, Yoon ES, Yoon E, Wise KD, Kim TG, Cho IJ. Scientific Reports. 2015;5:15466. doi: 10.1038/srep15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Ioannides N, Chunga E, Bachmatiuk A, Gonzalez-Martinez I, Trzebicka B, Adebimpe D, Kalymnios D, Rümmeli M. Materials Research Express. 2014;1:032002. [Google Scholar]
- 529.Yang Y, Turnbull G, Samuel I. Applied Physics Letters. 2008;92:150. [Google Scholar]
- 530.Stujenske JM, Spellman T, Gordon JA. Cell reports. 2015;12:525. doi: 10.1016/j.celrep.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Chen F-YB, Budgett DM, Sun Y, Malpas S, McCormick D, Freestone PS. IEEE transactions on biomedical circuits and systems. 2016;11:28. doi: 10.1109/TBCAS.2016.2577042. [DOI] [PubMed] [Google Scholar]
- 532.Rungta RL, Osmanski B-F, Boido D, Tanter M, Charpak S. Nature Communications. 2017;8:14191. doi: 10.1038/ncomms14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Christie IN, Wells JA, Southern P, Marina N, Kasparov S, Gourine AV, Lythgoe MF. Neuroimage. 2013;66:634. doi: 10.1016/j.neuroimage.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 534.Zochowski M, Wachowiak M, Falk CX, Cohen LB, Lam YW, Antic S, Zecevic D. Biol Bull. 2000;198:1. doi: 10.2307/1542798. [DOI] [PubMed] [Google Scholar]
- 535.Gross GW, Rhoades BK, Reust DL, Schwalm FU. Journal of Neuroscience Methods. 1993;50:131. doi: 10.1016/0165-0270(93)90001-8. [DOI] [PubMed] [Google Scholar]
- 536.Khurram A, Seymour JP. presented at Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE, Osaka, Japan July 2013; [DOI] [PubMed] [Google Scholar]
- 537.Fraser DB, Cook HD. J Electrochem Soc. 1972;119:1368. [Google Scholar]
- 538. [accessed: February 28, 2017];US FDA Second Sight Argus II Retinal Prosthesis HDE H110002 System Summary of Safety and Probable Benefit. http://www.accessdata.fda.gov/cdrh_docs/pdf11/H110002b.pdf.
- 539. [accessed: February 28, 2017];US FDA CVRx Barostim neo Legacy System HDE H130007. www.accessdata.fda.gov/cdrh_docs/pdf13/H130007a.pdf.
- 540. [accessed: February 28, 2017];US FDA Inspire Upper Airway Stimulation PMA P130008: FDA Summary of Safety and Effectiveness Data. http://www.accessdata.fda.gov/cdrh_docs/pdf13/P130008b.pdf.
- 541. [accessed: February 28, 2017];US FDA Enteromedics Maestro Rechargeable System PMA P130019. www.accessdata.fda.gov/cdrh_docs/pdf13/P130019a.pdf.
- 542.Liu LY, Zheng H, Xiao HL, She ZJ, Zhao SM, Chen ZL, Zhou GM. Neuroscience letters. 2008;434:155. doi: 10.1016/j.neulet.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 543. [accessed: February 28, 2017];US FDA Stimwave Freedom Spinal Cord Stimulator (SCS) System K162161. http://www.accessdata.fda.gov/cdrh_docs/pdf16/K162161.pdf.
- 544.Yoshida K, Horch K. IEEE Transactions on Biomedical Engineering. 1993;40:492. doi: 10.1109/10.243412. [DOI] [PubMed] [Google Scholar]
- 545.Leventhal DK, Durand DM. Annals of Biomedical Engineering. 2003;31:643. doi: 10.1114/1.1569266. [DOI] [PubMed] [Google Scholar]
- 546.Christensen MB, Pearce SM, Ledbetter NM, Warren DJ, Clark GA, Tresco PA. Acta Biomater. 2014;10:4650. doi: 10.1016/j.actbio.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 547.Branner A, Normann RA. Brain Res Bull. 2000;51:293. doi: 10.1016/s0361-9230(99)00231-2. [DOI] [PubMed] [Google Scholar]
- 548.Bouldin TW, Earnhardt TS, Goines ND. J Neuropathol Exp Neurol. 1991;50:719. doi: 10.1097/00005072-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 549.Tyler DJ, Durand DM. Ann Biomed Eng. 2003;31:633. doi: 10.1114/1.1569263. [DOI] [PubMed] [Google Scholar]
- 550.Kung TA, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG. Plastic and Reconstructive Surgery. 2014;133:1380. doi: 10.1097/PRS.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 551.Urbanchek MG, Kung TA, Frost CM, Martin DC, Larkin LM, Wollstein A, Cederna PS. BioMed Research International. 2016;2016:5726730. doi: 10.1155/2016/5726730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Cameron T. J Neurosurg. 2004;100:254. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- 553.Dietz A, Wennstrom M, Lehtimaki A, Lopponen H, Valtonen H. Eur Arch Otorhinolaryngol. 2016;273:1411. doi: 10.1007/s00405-015-3716-4. [DOI] [PubMed] [Google Scholar]
- 554.Schwedt TJ, Dodick DW, Hentz J, Trentman TL, Zimmerman RS. Cephalalgia. 2007;27:153. doi: 10.1111/j.1468-2982.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 555.Dodick DW, Silberstein SD, Reed KL, Deer TR, Slavin KV, Huh B, Sharan AD, Narouze S, Mogilner AY, Trentman TL, Ordia J, Vaisman J, Goldstein J, Mekhail N. Cephalalgia. 2015;35:344. doi: 10.1177/0333102414543331. [DOI] [PubMed] [Google Scholar]
- 556.Falowski S, Wang D, Sabesan A, Sharan A. Neuromodulation. 2010;13:121. doi: 10.1111/j.1525-1403.2009.00261.x. [DOI] [PubMed] [Google Scholar]
- 557.Ben-Menachem E. Lancet Neurol. 2002;1:477. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 558.Grill WM, Mortimer JT. IEEE Trans Rehabil Eng. 1998;6:364. doi: 10.1109/86.736150. [DOI] [PubMed] [Google Scholar]
- 559.Naples GG, Mortimer JT, Scheiner A, Sweeney JD. IEEE Trans Biomed Eng. 1988;35:905. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- 560.Espinosa J, Aiello MT, Naritoku DK. Surg Neurol. 1999;51:659. doi: 10.1016/s0090-3019(99)00046-4. [DOI] [PubMed] [Google Scholar]
- 561.Mobbs RJ, Blum P, Rossato R. J Clin Neurosci. 2003;10:476. doi: 10.1016/s0967-5868(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 562.Renard VM, North RB. J Neurosurg Spine. 2006;4:300. doi: 10.3171/spi.2006.4.4.300. [DOI] [PubMed] [Google Scholar]
- 563.Geissinger G, Neal JH. Surgical neurology. 2007;68:454. doi: 10.1016/j.surneu.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 564.Burdick AP, Okun MS, Haq IU, Ward HE, Bova F, Jacobson CE, Bowers D, Zeilman P, Foote KD. Stereotactic and functional neurosurgery. 2010;88:353. doi: 10.1159/000319039. [DOI] [PubMed] [Google Scholar]
- 565.Ellis GL. The American journal of emergency medicine. 1990;8:48. doi: 10.1016/0735-6757(90)90296-c. [DOI] [PubMed] [Google Scholar]
- 566.Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, Moons KG. Heart Rhythm. 2012;9:728. doi: 10.1016/j.hrthm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 567. [accessed: March 7, 2017];US FDA RST-001 Phase I/II Trial for Retinitis Pigmentosa. https://clinicaltrials.gov/ct2/show/NCT02556736.
- 568.Ghazi NG, Abboud EB, Nowilaty SR, Alkuraya H, Alhommadi A, Cai H, Hou R, Deng W-T, Boye SL, Almaghamsi A. Human genetics. 2016;135:327. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- 569.Zhang H, Shih J, Zhu J, Kotov NA. Nano Lett. 2012;12:3391. doi: 10.1021/nl3015632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Dias NS, Carmo JP, da Silva AF, Mendes PM, Correia JH. Sensor Actuat a-Phys. 2010;164:28. [Google Scholar]