Abstract
Background
Little is known about the impact of prenatal maternal stress (PNMS) on the developmental trajectory of temperament and few studies have been able to incorporate a natural disaster as a quasi-experimental stressor. The current study investigated PNMS related to Superstorm Sandy (‘Sandy’), a hurricane that struck the New York metropolitan area in October 2012, in terms of objective exposure during pregnancy, subjective stress reaction as assessed by maternal symptoms of post-traumatic stress, and their impact on the developmental changes in temperament during early childhood.
Method
A subsample of 318 mother-child dyads was drawn from the Stress in Pregnancy Study. Temperament was measured at 6, 12, 18, and 24 months of age.
Results
Objective exposure was associated with greater High-Intensity Pleasure, Approach, Perceptual Sensitivity and Fearfulness, but lower Cuddliness and Duration of Orientation at 6 months. Objective exposure and its interaction with subjective stress reaction predicted developmental changes in temperament. In particular, objective exposure was linked to greater increases in Activity Level but decreases in High-Intensity Pleasure, Approach, and Fearfulness. The combination of objective exposure and subjective stress reaction was also associated with greater increases in Activity Level.
Limitations
Temperament was measured solely via maternal report. Trimester-specific effects of Sandy on temperament were not examined.
Conclusion
This is the first study to examine the effects of prenatal maternal exposure to a natural disaster on trajectories of early childhood temperament. Findings suggest that both objective stress exposure and subjective stress reaction in-utero predict developmental trajectories of temperament in early childhood.
Keywords: Prenatal maternal stress, Natural disaster, Objective stress exposure, Subjective stress reaction, Temperament, Longitudinal data
1. Introduction
The origins of many behaviors and health outcomes in life can be traced back to the period in-utero, when rapid alterations in the structure and function of the brain and body of the fetus take place (Barker, 2002; Glover, 2011; Gluckman et al., 2005). Accumulating evidence shows that critical periods exist during pregnancy when the fetus is particularly vulnerable to prenatal maternal stress (PNMS; DiPietro et al., 1996; Wadhwa et al., 2001a). These stressors induce changes in the fetal immune and inflammatory responses, and are believed to be a result, in part, of the effect of physiological mechanisms involving the hypothalamic-pituitary-adrenal (HPA)-axis and the central nervous system (CNS) (Wadhwa et al., 2001a, 2001b). Both animal (Charil et al., 2010; Langley-Evans et al., 1996) and human (Babenko et al., 2015; Buss et al., 2010; Glover, 2011) studies have shown that PNMS alters the structure and function of the offspring’s brain. Prenatal alterations of HPA-axis functioning may lead to an increased susceptibility to long-term neurodevelopmental, health, and behavioral problems (Buitelaar et al., 2003; Talge et al., 2007; Van den Bergh et al., 2008; Van Den Bergh et al., 2005; Ward, 1991).
Fetal programming (conditions during crucial, sensitive periods, such as in-utero, can permanently affect the phenotype of the fetus and the long-lasting risk of disease in adult life) exerts influence on the temperament and behavior of young children born to mothers exposed to PNMS (Barker, 2002). Various forms of PNMS (e.g., depression, anxiety) have consistently been associated with variations in infant temperament characteristics, such as heightened irritability, crying, fearfulness, distractibility, and poor adaptation to stressful situation (Bergman et al., 2007; Brand et al., 2006; Davis et al., 2007, 2004; Milgrom et al., 1995; Nolvi et al., 2016; van der Wal et al., 2007). The potential evolutionary adaptiveness of certain temperament characteristics in infancy may explain why such temperament alterations occur in response to PNMS. Increased impulsivity may contribute to enhanced sensitivity to dangerous signals and readiness to explore new environments; while increased fearfulness may help raise vigilance and alertness to danger (Glover, 2011). Similarly, more distracted attention and scanning behaviors may improve the individual’s ability to monitor and react to threats more effectively (Bradshaw and Sheppard, 2000; Jensen et al., 1997; Shelley-Tremblay and Rosén, 1996). Therefore, biological changes that occur as a result of PNMS exposure during fetal development can be evolutionarily adaptive in harsher environment (Glover, 2011; Gluckman et al., 2005; Pluess et al., 2011). However, these early evolutionary advantages can be precursors to a variety of neurobehavioral problems during later development (e.g., ADHD, conduct problems, and depression) (De Pauw and Mervielde, 2010). This evolutionary duality can be seen through the lens of the fetal programming (Barker, 2002) and mismatch hypotheses (Glover, 2011) which suggests that individuals are more likely to develop behavioral problems if there is discord between early programming and later environment. Increased impulsivity, fearfulness or distracted attention primed by PNMS exposure in-utero may reflect a mismatch with a postnatal environment lacking the acute stressors the fetus was exposed to in-utero. A longitudinal examination of the extent to which PNMS influences early temperament outcomes, and plotting the developmental trajectories of these temperament characteristics in early childhood, will provide additional insight into how temperament could be influenced by in-utero stress in early childhood.
To date, there are several studies of stability and change in temperament during early childhood for typical development (Aksan et al., 1999; Auerbach et al., 2008; Komsi et al., 2006; Putnam et al., 2008; Rothbart et al., 2000a, 2000b). While traditional models conceptualize temperament as stable (Strelau, 1998), recent studies show a lack of stability in temperament profiles from infancy to childhood (Carranza Carnicero et al., 2000; Garstein and Rothbart, 2003; Partridge and Lerner, 2007; Wood, 2011). For instance, researchers have found that “difficult” temperament, including low rhythmicity, withdrawal responses, low adaptability, negative mood and high-intensity of reactions, follows a nonlinear developmental trajectory, increasing around ages 2–3, and decreasing linearly from toddlerhood to preschool (Partridge and Lerner, 2007). Data also suggests that negative affect, such as fearfulness and anger, increases with age from 4 to 16 months (Braungart-Rieker et al., 2010). Some studies show that effortful control (e.g., attention and orienting), which modulates negative emotionality and reactivity, begins to develop by the end of the first year of life (Posner and Rothbart, 2000). Research has also indicated that positive affect, such as smiling and laughter, and approach, appears in the first months of life and develops throughout infancy and toddlerhood (Bridgett et al., 2013; Dinehart et al., 2005; Sallquist et al., 2010).
Furthermore, it has been found that while there are meaningful changes in positive affect during infancy, this temperament characteristic remains relatively consistent throughout early childhood (Bridgett et al., 2013; Putnam et al., 2008; Zentner et al., 2008). Robust prenatal and early childhood longitudinal data would clarify the stability of temperament profiles, as well as the impact of PNMS on these traits during different developmental stages. Furthermore, PMNS has been largely addressed as normative stress in everyday life (Rubonis and Bickman, 1991) that can be increased by other factors such as maternal psychopathology and socioeconomic status. Fewer prospective human studies have examined the effects of large-scale disastrous events (e.g., Quebec Ice Storm, Hurricane Katrina, and World Trade Center terrorism) on developmental programming (Brand et al., 2006; Huizink et al., 2007; King and Laplante, 2005; Kuvacic et al., 1996; Laplante et al., 2016, 2008; Meijer, 1985; Tees et al., 2010; Yehuda et al., 2005), and the findings are inconsistent regarding the impact of maternal stress during pregnancy and temperament. When Laplante et al. (2016) studied the impact of the Quebec Ice Storm, they found that elevated levels of mother’s subjective stress reaction to the ice storm during pregnancy were associated with difficult temperament characteristics (i.e., fussiness, dullness, and attention seeking) among 6-month old offspring. In contrast, Tees et al. (2010) studied just the objective impact of Hurricane Katrina and found no relation to difficult temperament at 2 months and 12 months. Existing studies have not systematically examined the impact of objective exposure to a catastrophic disaster and subjective stress reaction to the disaster during pregnancy while taking into account previous exposure to other traumatic events on longitudinal trajectories of early child temperament. Given that some research has found objective exposure to stress to be more damaging to child outcomes while other studies have found subjective stress reaction more predictive of child outcomes (King and Laplante, 2005), it begs the question of whether it is mere exposure (objective PNMS) or the variation in symptoms as a result of the exposure (subjective PNMS) that is associated with adverse impacts on the fetus. Thus it is important to examine objective and subjective stress, as well as the interaction of the two in determining longitudinal changes in child temperament.
Sandy was one of the worst natural disasters in the USA (Blake et al., 2013). It caused significant personal and financial harm in the affected area and led to 117 deaths (53 deaths in New York) (Centers for Disease Control and Prevention (CDC), 2013). The northeastern region suffered from power outages, suspension of public transportation service, and gasoline shortage. It was the second costliest cyclone to hit the U.S. since 1900 (Blake et al., 2013). While several studies have examined the mental health impact of Sandy among exposed adults (Boscarino et al., 2014; Caramanica et al., 2015; Neria and Shultz, 2012), no studies have documented the impact of Sandy on pregnant mothers and their offspring over time.
Previous studies suggest a link between PNMS caused by natural disaster and temperament among 6 months-old offspring (Laplante et al., 2016), and between PNMS brought about by other factors and infant temperament (e.g., Brand et al., 2006; Milgrom et al., 1995; van der Wal et al., 2007). However, no study has systematically and precisely investigated the contributions of PNMS to the changes in the fine-grained dimensions of temperament over the early years of life using Rothbart’s conceptual model of infant and child temperament (Rothbart et al., 2000a, 2000b). Analyses of the fine-grained aspects of temperament traits also revealed three higher-order factors of Surgency/Positive Affectivity, Negative Affect and Regulatory Capacity/Effortful Control (Putnam et al., 2008; Rothbart et al., 1994). Surgency/Positive Affectivity refers to sociability, sensation seeking and activity (Rothbart and Bates, 1998) which underpins the behavioral approach system and leads to both reactive approach in reward situations and frustration or irritability in non-reward situations (Gray, 1991). Negative Affect refers to discomfort, fear, anger, sadness, and low soothability (Rothbart and Bates, 1998) which underlies the behavioral inhibition system and leads to heightened vigilance, emotional and physiological arousal, and restricted behavior (Gray, 1991). Regulatory Capacity/Effortful Control refers to processes such as allocating attention which modulates the expression of negative emotionality and reactivity (Putnam et al., 2008; Rothbart et al., 1994). Based on earlier studies and guided by the evolutionary perspective of fetal programming, we proposed to test three hypotheses related to maternal objective exposure to Sandy, their subjective reaction and the interaction of the two. We first examined the effects of PNMS on infant temperament assessed at 6 months. Further, we conducted exploratory analyses to explain the longitudinal connections between these variables; due to the relatively little theory or data available, these speculations were more exploratory. First, we hypothesized that Sandy status, i.e. children who were prenatally exposed to Sandy (i.e., Sandy) versus those who were not (i.e., control), would predict higher levels of Surgency (as indicated by the subcomponents such as Activity Level, High-Intensity Pleasure, and Approach), Negative Affect (as indicated by the subcomponents such as Fearfulness, Distress to Limitations, and Sadness), and lower levels of Regulatory Capacity (as indicated by the subcomponents such as Duration of Orientation/Attention and Cuddliness), which are the features that contribute to a profile of “difficult” temperament; further, we predicted that an increase in these temperament difficulties would be expected to over the next two years (i.e., increased Surgency and Negative Affect with slower growth of Regulatory Capacity). Second, we hypothesized that children of mothers who experienced greater subjective stress reaction to the storm would have a similarly difficult temperament pattern at 6 months and over the next two years. Finally, we hypothesized that children of mothers who had objective exposure to Sandy and experienced more severe subjective stress reaction in-utero would have substantially more temperament difficulties than children of mothers who had less severe subjective stress reactions. Given the exploratory nature of studying the individual factors, we may expect variable trajectories even within the same broader category. In the current study, our main predictors are Sandy status (objective PNMS), subjective stress reaction following Sandy (subjective PNMS), and the interaction of the two. We then carried out a longitudinal examination of the relationship between objective and subjective stress following Sandy and the influence of these factors on temperament trajectories in offspring during their first two years of life.
2. Methods
2.1. Participants
We used a subsample of 318 mother-child dyads (51.3% boys) from a longitudinal study investigating the effect of stress during pregnancy on child development. Pregnant women were recruited from the prenatal obstetrics and gynecological (OB/GYN) clinics at Mount Sinai Medical Center and New York-Presbyterian/Queens in New York City during their second trimester. These women were followed throughout their pregnancy, and subsequently completed questionnaires about their child’s development via mail or in-person at 6, 12, 18, and 24 months of age. Exclusion criteria for participation included HIV infection, maternal psychosis, maternal age < 15 years, life-threatening maternal medical complications, and congenital or chromosomal abnormalities in the fetus. A detailed description of the study can be found elsewhere (Finik and Nomura, 2017). Demographic information, including maternal education, marital status, race, and age was reported by participants during the second trimester (Table 1). All participants gave written consent according to the protocol approved by the Institutional Review Boards at the City University of New York, New York-Presbyterian/Queens, and the Icahn School of Medicine at Mount Sinai.
Table 1.
Characteristics of sample at baseline.
| Child Gender | Boys, N (%) | 163 | 51.3 |
| Girls, N (%) | 155 | 48.7 | |
| Birthweight (g) | Mean (SD) | 3234.91 | 561.76 |
| Gestational Age (weeks) | Mean (SD) | 38.95 | 1.90 |
| Maternal Age | Mean (SD) | 27.18 | 5.94 |
| Mother’s Education | Primary School/Some High School, N (%) | 52 | 16.4 |
| High School/GED, N (%) | 67 | 21.1 | |
| Some College, N (%) | 87 | 27.4 | |
| Associate Degree, N (%) | 32 | 10.1 | |
| Bachelor Degree, N (%) | 45 | 14.2 | |
| Graduate Degree, N (%) | 35 | 11.0 | |
| Marital Status | Married, N (%) | 117 | 36.8 |
| Common Law, N (%) | 17 | 5.3 | |
| Single, N (%) | 179 | 56.3 | |
| Widowed/Divorced/Separated, N (%) | 5 | 1.5 | |
| Mother’s Race | White, N (%) | 44 | 13.8 |
| Black, N (%) | 78 | 24.5 | |
| Hispanic, N (%) | 156 | 49.1 | |
| Asian, N (%) | 34 | 10.7 | |
| Other, N (%) | 6 | 1.9 | |
| Other Trauma Exposure | 0, N (%) | 147 | 46.23 |
| 1–2, N (%) | 107 | 33.65 | |
| 3+, N (%) | 56 | 17.61 | |
| Missing, N (%) | 8 | 2.51 |
Of the 318 mothers contacted for follow-up, temperament data was collected for 235 children at 6 months (wave 1), 156 at 12 months (wave 2), 160 at 18 months (wave 3), and 134 at 24 months (wave 4). There were no significant differences in sex of the child, race, or maternal education between those who provided 1, 2, 3 or 4 waves of data. Additionally, there were no significant demographic differences between this study’s sample (N = 318) and the active participants of the parent study (N = 138) across child sex (p = .082), race (p = .826), and maternal education (p = .053). The control group was composed of mainly participants who resided in Manhattan and received obstetric care at Mount Sinai Hospital, while the Sandy group was composed of women who resided in devastated regions, Queens and Long Island. Except that mothers in the Sandy group had higher education levels than the control group (p < .001), there were no major demographic differences, including sex of child (p = .514), gestational age (p = .371), birthweight (p = .101), maternal age (p = .374) or mother’s race (p = .152) between these two groups.
2.2. Measures
2.2.1. Temperament
The Short Form of the Infant Behavior Questionnaire-Revised (IBQ-R; Garstein and Rothbart, 2003; Putnam et al., 2014; Rothbart, 1981) and Early Childhood Behavior Questionnaire (ECBQ; Putnam et al., 2006) are parent-reported questionnaires of early child temperament for use with infants (IBQ-R) and toddlers (ECBQ) (Putnam et al., 2006). Parents rate the frequency of a wide array of child behaviors on a scale from 1 (never) to 7 (always). The IBQ-R is comprised of 14 subscales, while the ECBQ consists of 18 subscales. Seven scales appear on both questionnaires: Activity Level (the amount of gross motor activity level), Cuddliness (the desire for closeness with others), Sadness (lowered mood), High-Intensity Pleasure (pleasure related to high-intensity stimulus), Low-Intensity Pleasure (pleasure related to low-intensity stimulus), Fearfulness (negative affect related to startle or distress), and Perceptual Sensitivity (the ability to detect low-intensity stimuli) and four scales equivalent on both measures (listed as follows with ECBQ equivalent in brackets): Approach [Positive Anticipation] (excitement in the anticipation of pleasurable activities), Falling Reactivity [Soothability] (the rate of recovery from distress), Duration of Orientation [Attention Focusing] (the ability to sustain attention), and Distress to Limitations [Frustration] (negative affect related to confinement) (Putnam et al., 2008). The remaining scales appear on only one of the instruments and reflect traits specific to that developmental phase (Putnam et al., 2008) and thus were not included in this study. Mothers completed the IBQ-R at 6 and 12 months and the ECBQ at 18, and 24 months postpartum. Both, the IBQ-R (Garstein and Rothbart, 2003; Putnam et al., 2014) and the ECBQ (Putnam et al., 2006) are widely used and have high internal consistency and good interrater reliability among relatives of the child. In the present sample, internal consistency of the temperament measures at different times (6, 12, 18 and 24 months) was adequate with Cronbach’s alphas ranging from .65 to .84.
According to Rothbart’s conceptual model, there are three broader dimensions of infant and child temperament, comprised by the 11 common dimensions of temperament between the IBQ-R and ECBQ instruments. Surgency/Positive Affectivity is comprised by the following subscales: Activity Level, High-Intensity Pleasure, Approach, and Perceptual Sensitivity (IBQ-R); Negative Affect is comprised by: Falling Reactivity, Fearfulness, Distress to Limitations, Sadness, and Perceptual Sensitivity (ECBQ); and Regulatory Capacity/Effortful Control is comprised by: Duration of Orientation, Low-Intensity Pleasure, and Cuddliness (Posner and Rothbart, 2007; Putnam et al., 2008). The present study uses the component subscales rather than the broader dimensions of temperament to enable more specific analysis (Table 2).
Table 2.
Child temperament between age 6 and 24 months.
| Temperament Outcome | 6 Month | 12 Month | 18 Month | 24 Month | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Activity Levela | 4.10 | 1.16 | 4.26 | 1.02 | 4.69 | 1.12 | 4.80 | 1.21 |
| Approacha | 5.32 | 1.25 | 5.89 | 0.92 | 5.12 | 1.21 | 5.33 | 1.07 |
| Cuddlinessc | 5.68 | 0.95 | 5.20 | 1.06 | 5.20 | 0.98 | 5.31 | 0.94 |
| Distress to Limitationsb | 3.88 | 1.23 | 4.38 | 1.12 | 3.63 | 1.29 | 3.79 | 1.32 |
| Duration of Orientationc | 4.65 | 1.40 | 4.71 | 1.33 | 4.64 | 0.94 | 4.63 | 0.89 |
| Falling Reactivityb | 4.98 | 1.08 | 4.88 | 0.97 | 4.73 | 1.05 | 4.86 | 1.04 |
| Fearfulnessb | 3.26 | 1.41 | 3.91 | 1.31 | 2.78 | 1.18 | 2.69 | 1.12 |
| Sadnessb | 3.48 | 1.22 | 3.62 | 1.16 | 3.03 | 1.11 | 2.84 | 1.08 |
| High-Intensity Pleasurea | 6.14 | 1.02 | 6.45 | 0.73 | 4.90 | 1.34 | 5.07 | 1.27 |
| Low-Intensity Pleasurec | 5.65 | 0.92 | 5.53 | 1.01 | 5.09 | 1.10 | 5.23 | 1.20 |
| Perceptual Sensitivitya,b | 4.49 | 1.60 | 4.97 | 1.40 | 4.50 | 1.28 | 4.64 | 1.32 |
Subscales loaded on the Surgency/Positive Affectivity.
Subscales loaded on the Negative Affect.
Subscales loaded on the Regulatory Capacity/Effortful Control.
2.2.2. Maternal traumatic stress
Sandy status was defined as whether mothers were pregnant during Sandy (1), or either before or after Sandy (0). As fetal programming is of interest to this investigation, those not pregnant during Sandy comprised a single group to isolate the impact of Sandy in-utero. A total of 135 mothers (42.5%) were pregnant, and 183 mothers were not pregnant during Sandy (164 delivered children prior to and 19 became pregnant after Sandy). Participants were assigned to a Sandy group or a comparison group based on this measure.
The Post-traumatic Stress Diagnostic Scale (PDS; Foa, 1996) is a reliable self-report questionnaire that measures symptom severity of Post-Traumatic Stress Disorder (PTSD). It was adapted to assess symptoms specifically related to Sandy and served as a measure of mother’s subjective stress reaction. This variable is referred to henceforth as ‘Subjective stress reaction’. Mothers rated how much they were bothered by each of the PTSD symptoms as outlined in DSM-IV on a four-point Likert scale from 0 (Not at all or only one time) to 3 (5 or more times a week/almost always). Internal consistency was .90.
2.2.3. Covariates
2.2.3.1. Other trauma
Mothers reported a history of other lifetime trauma besides Sandy (e.g., serious accident, violence, severe illness, etc.) on the PDS (Foa, 1996). The number of other traumatic events derived from this measure constitutes our “other trauma” variable.
2.2.3.2. Prenatal depression
Prenatal depression has been associated with increased temperament problems and negative emotionality during early child development (Davis et al., 2007; Field, 2011; Zuckerman et al., 1990). The Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987), a validated 10 item self-report measure, was used to measure mother’s depressive symptomatology during pregnancy. Mothers reported their feelings in the past 7 days on a 4 point Likert scale from 0 to 3. The internal consistency was .81.
2.2.3.3. Gestational diabetes mellitus (GDM) and preeclampsia
A combination index score (0, “No”, and 1, “Yes”) indicated absence or presence of preeclampsia and/or GDM. Preeclampsia and GDM status was determined by participant medical record review throughout pregnancy. Two conditions were required for diagnosis of preeclampsia at the clinic from which the participants were drawn: 1) elevated blood pressure at 140 systolic or 90 diastolic on at least two separate occasions and 2) presence of proteinuria in at least 300 mg/24 h or 2+ on urine dip. GDM status was defined as glucose intolerance with first onset during pregnancy as determined by a glucose tolerance test.
2.2.3.4. Mother’s drug abuse
A combination index score of (0, “No”, and 1, “Yes”), indicated absence or presence of smoking, drinking, marijuana, cocaine, heroin, methadone, opiate, glue, prescription, and/or other drugs according to maternal self-report during pregnancy at the baseline.
2.2.3.5. Demographic variables
A priori confounder selection included child gender and maternal education. Maternal education was considered the best indicator of socioeconomic status (SES) among pregnant women, as other indicators (i.e. employment and income) may introduce systematic bias as mothers may choose to stay at home during pregnancy. Findings regarding gender differences in temperament are varied and inconsistent. Females exhibit greater distress to novelty and sensitivity to stimuli in infancy as compared to their male counterparts (Martin et al., 1997). However, a longitudinal analysis of infant reactive temperament found no sex differences among infants 3–9 months old (Rothbart, 1986). Though we did not conduct separate analyses for males and females, to remain conservative in our analysis, we have adjusted for gender to account for this potential confound.
2.3. Statistical analyses
Hierarchical linear modeling (HLM) was used to assess both within-person longitudinal models and between-person models (Raudenbush and Bryk, 2002). The within-person model mapped the developmental trajectory of temperament at four data points from age 6–24 months. The between-person model estimated how traumatic stress due to Sandy influenced changes in child temperament. All models in the analysis were corrected for non-normal distributions of level 2 residuals by applying the full maximum likelihood estimation with robust standard errors (Maas and Hox, 2004).
2.3.1. Model 1: Change in temperament over time
Model 1 was designed to characterize the trajectories of temperament across 4 time points ranging from 6 to 24 months of age. For each outcome, we first tested a model of linear change (a). As temperament may not display a linear change, especially in early development (Partridge and Lerner, 2007; Sukigara et al., 2015), we also tested for curvilinearity in the linear trajectory for each outcome by adding a quadratic term for age to the model (b). If no significant quadratic effect was observed, the term was removed from the model. In addition, tests of relative model fit were computed by comparing the deviance statistics of both the quadratic model and linear model. The quadratic model was retained if it yielded a significant reduction in deviances according to the Chi-square difference test (p < .05). Random effects were included on the intercept and change coefficients (all linear terms and the quadratic where retained). Age was centered at 6 months, meaning that the intercept represented the average temperament level when children were 6 months old.
Linear model (a)
In the linear model, temperament is a function of an intercept plus a linear effect for age. The linear model equation is as follows:
- Level 1
- Level 2
Quadratic model (b)
- Level 1
- Level 2
2.3.2. Model 2: Predictors of intercepts and slopes
After choosing the linear (a) or quadratic (b) model where appropriate, we explored whether Sandy status, subjective stress reaction, and their interaction (Sandy status x Subjective stress reaction) explained significant variance in mean intercept or slope of child temperament. If any temperament dimension displayed neither linear nor quadratic change overtime, predictors were added to calculate the intercept only. Subjective stress reaction was grand mean centered. If there was a significant interaction, sequential analyses were performed to compare the effect of Subjective stress reaction on temperament by Sandy status. High and low Subjective stress reaction groups were created based on upper versus lower quartile to display the effect of Subjective stress reaction on temperament. Exposure to other trauma, child gender, maternal education, prenatal depression, GDM and preeclampsia, and drug abuse were included as covariates in modeling the predictors of change in temperament.
Linear Model (c)
- Level 1
- Level 2
Quadratic Model (d)
- Level 1
- Level 2
2.3.3. Missing data
Missing data represents serious methodological problems in many longitudinal empirical studies. HLM yields parameter estimates for missing time points for dependent variable data (temperament) at level 1 (i.e., within subject variability) but not for predictor variables at level 2 (i.e., between subject variability) (Raudenbush and Bryk, 2002). Rather than removing a sizable portion of the sample by using a repeated-measure analysis, we leveraged this central methodological strength of HLM, the generation of estimates for missing data at certain time points. Given that the majority of the children in the current sample were missing temperament data for at least one-time point (42.1%), HLM provided the most robust method of dealing with the missing data. At level 2, missing values for predictor variables were imputed with the default multiple imputation procedure of SPSS (5 imputations), in which missing data is replaced with estimated values. Multiple imputation is superior to traditional pairwise and listwise deletion (Allison, 2002), as including cases with only complete data may not be representative of the whole population, which would introduce systematic bias. In the current sample, the percentage of missing data for the predictor variables and covariates ranged from .3% for subjective stress reaction to 4% for prenatal depression at level 2. Following Schafer and Graham (2002), HLM computes average estimates that reflect the uncertainty of missing level 2 data.
3. Results
3.1. Change in temperament over time
We modeled temperament as a function of the intercept plus the linear/quadratic effect of age (Table 3). Fig. 1 depicts the developmental trajectories of each temperament domain. Activity Level increased linearly with age. Sadness, High-Intensity Pleasure, and Low-Intensity Pleasure decreased linearly with age. Approach, Distress to Limitations, Fearfulness and Perceptual Sensitivity increased or remained stagnant in the earlier age range (6–12 months) but then decreased. Cuddliness decreased in the earlier age range (6–18 months) but then increased. Neither the linear nor the quadratic slope for Duration of Orientation and Falling Reactivity were significant.
Table 3.
Linear and quadratic change in child temperament over time.
| Temperament Outcome | Intercepts | Linear | Quadratic | Model Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| γ00: M | SE | t-ratio | γ10: M | SE | t-ratio | γ20: M | SE | t-ratio | ΔX2 (4) | p | |
| Activity Levela | 4.08 | .07 | 59.47** | .52 | .08 | 6.43*** | – | – | – | – | – |
| Sadnessb | 3.60 | .07 | 48.50*** | −.48 | .08 | −6.24*** | – | – | – | – | – |
| High-Intensity Pleasurea | 6.30 | .06 | 101.64*** | −.95 | .09 | −11.04*** | – | – | – | – | – |
| Low-Intensity Pleasurec | 5.66 | .06 | 100.01*** | −.39 | .07 | −5.88*** | – | – | – | – | – |
| Approacha | 5.43 | .08 | 66.79*** | .53 | .22 | 2.36* | −.47 | .13 | −3.59*** | 23.10 | < .001 |
| Cuddlinessc | 5.68 | .06 | 94.57*** | −1.10 | .18 | −6.11*** | .57 | .12 | 4.92*** | 28.41 | < .001 |
| Distress to Limitationsb | 3.96 | .08 | 50.93*** | .42 | .19 | 2.19* | −.43 | .12 | −3.50*** | 18.83 | .001 |
| Fearfulnessb | 3.43 | .09 | 37.81*** | .36 | .23 | 1.57+ | −.65 | .15 | −4.44*** | 16.99 | .002 |
| Perceptual Sensitivitya,b | 4.58 | .10 | 44.68*** | .40 | .24 | 1.66+ | −.30 | .14 | −2.11* | 9.99 | .040 |
| Duration of Orientationc | 4.68 | .08 | 55.33*** | −.03 | .08 | −.32 | – | – | – | – | – |
| Falling Reactivityb | 4.94 | .06 | 76.78*** | −.10 | .07 | −1.46 | – | – | – | – | – |
Note: γ’s (M) represent the average, or fixed effects. SE = standard error. Chi-square difference test (ΔX2) yields model comparison between the linear and quadratic models. Model comparison statistics are presented when the quadratic term is significant and significant ΔX2. Negative quadratic effects reflect concave trajectories; positive quadratic effects reflect convex trajectories. Higher values reflect more/greater/longer Activity Level, Cuddliness, Sadness, High-Intensity Pleasure, Low-Intensity Pleasure, Fearfulness, Approach, Falling Reactivity, Distress to Limitations, Duration of Orientation, and Perceptual Sensitivity.
p < .05.
p < .01.
p < .001.
p < .10.
Subscales loaded on the Surgency/Positive Affectivity.
Subscales loaded on the Negative Affect.
Subscales loaded on the Regulatory Capacity/Effortful Control.
Fig. 1.
Developmental trajectories of temperament for the overall sample. Linear models are selected for Activity (a), Sadness (b), High-Intensity Pleasure (c), and Low-Intensity Pleasure (d). Quadratic models are selected for Approach (e), Cuddliness (f), Distress to Limitations (g), Fearfulness (h), and Perceptual Sensitivity (i). Neither linear nor quadratic models are selected for Duration of Orientation (j) and Falling Reactivity (k).
3.2. Predictors of Intercepts and Slopes
After retaining the best fitting model (i.e., linear or quadratic), we explored whether Sandy status, subjective stress reaction, or their combination predicted mean intercepts (i.e., temperament at 6 months) or slopes (i.e., rate/direction of change over time). In the interest of brevity, only significant results related to PNMS are reported here. All analyses included exposure to other trauma, prenatal depression, maternal substance use and GDM/preeclampsia, child gender, and maternal education as covariates.
3.3. Sandy status
3.3.1. Intercept
There was a significant main effect of Sandy status in predicting intercepts for High-Intensity Pleasure (t-ratio = 2.26, p = .025), Approach (t-ratio = 4.35, p < .001), Cuddliness (t-ratio = −2.64, p = .009), Fearfulness (t-ratio=2.31, p = .022), Perceptual Sensitivity (t-ratio = 2.17, p = .031) and Duration of Orientation (t-ratio = −1.95, p = .050). At 6 months, children exposed to Sandy in-utero were rated higher on High-Intensity Pleasure, Approach, Fearfulness, and Perceptual Sensitivity, but lower on Cuddliness and Duration of Orientation as compared to those unexposed (Fig. 2).
Fig. 2.
Comparisons of temperament at 6 months between control and Sandy groups. There were significant differences in High-Intensity Pleasures, Approach, Cuddliness, Fearfulness, Perceptual Sensitivity, and Duration of Orientation. The differences were not significant in Activity Level, Sadness, Low-Intensity Pleasure, Distress to Limitations, and Falling Reactivity.
3.3.2. Slopes
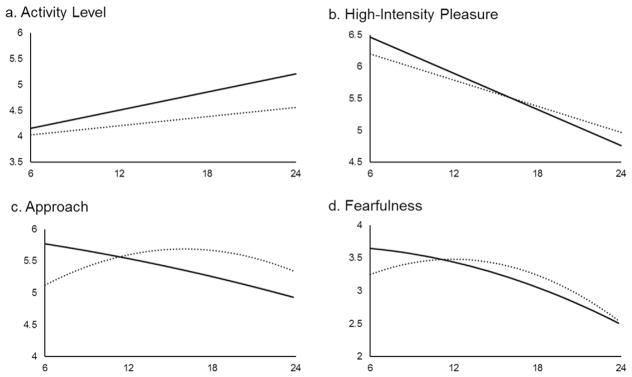
For Activity Level, there was a significant main effect of Sandy status in predicting the linear slope (t-ratio = 2.24, p = .026). At 6–24 months, children exposed to Sandy in-utero had a greater increase on reported Activity Level than those unexposed.
For High-Intensity Pleasure, there was a significant main effect of Sandy status in predicting the linear slope (t-ratio = −2.00, p = .046). At 6–24 months, children exposed to Sandy in-utero showed a greater decrease on High-Intensity Pleasure than those unexposed.
For Approach, there was a significant main effect of Sandy status in predicting the linear and quadratic slopes (linear: t-ratio = −4.13, p < .001; quadratic: t-ratio = 2.69, p = .008). Children exposed to Sandy in-utero showed a continuous decrease on levels of Approach from 6 to 24 months, whereas those in the control group showed an increase from 6 to 12 months followed by a decrease from 18 to 24 months, with no significant difference between 12 and 18 months.
For Fearfulness, there was a significant main effect of Sandy status in predicting the linear and quadratic slopes (linear: t-ratio = −2.61, p = .009; quadratic: t-ratio = 2.12, p = .035). Children exposed to Sandy in-utero showed a continuous decrease on Fearfulness, whereas those in the control group showed an increase from 6 to 12 months followed by a decrease from 12 to 24 months (Fig. 3).
Fig. 3.
Developmental trajectories of temperament by Sandy status (solid line=Sandy; dashed line=control). Linear/quadratic slopes were significantly predicted by Sandy status for Activity Level (a), High-Intensity Pleasure (b) Approach (c), and Fearfulness (d).
3.4. Subjective stress reaction
There was no significant main effect of subjective stress reaction in predicting either intercept or slopes.
3.5. Sandy status × Subjective stress reaction interaction
3.5.1. Slopes
For Activity Level, there was a significant interaction effect in predicting the linear slope (t-ratio = 2.33, p = .021), further analyses revealed that subjective stress reaction marginally predicted the linear slope in temperament growth for the Sandy group (t-ratio = 1.74, p = .084) but not in the control group (t-ratio = −.15, p = .881). Among children exposed to Sandy in-utero, greater subjective stress reaction was associated with a greater increase of Activity Level from 6 m to 24 months, whereas lower subjective stress reaction was associated with a smaller increase (Fig. 4).
Fig. 4.

Developmental trajectories of temperament by maternal subjective stress reaction to Sandy (solid line=high stress reaction; dashed line=low stress reaction) in children exposed to Sandy in-utero.
4. Discussion
The current study investigated the effects of PNMS due to Superstorm Sandy on the developmental trajectory of temperament in early childhood using an evolutionary developmental perspective. Prior research on disaster-related PNMS and child development has had mixed results, with King et al. (2012) reporting a clear link between both objective and subjective PNMS due to the Quebec Ice Storm and child development and Tees et al. (2010) reporting no significant relation between objective hardship related to Hurricane Katrina and infant temperament. Our findings demonstrated that exposure to Sandy in-utero alone, without accounting for the degree of objective exposure, had a considerable impact on both infant temperament and on the developmental trajectory of temperament from infancy to childhood. One reason why our results deviate from the nonsignificant findings in Tees et al.’s (2010) study might be the relatively small proportion of pregnant women during Hurricane Katrina were included in the cohort of pregnant women that were examined in their study. Additionally, our study included repeated measures of child temperament, as opposed to a one-time measurement, to examine differences in the pattern of change in temperament.
Our results suggest that objective exposure to PNMS would be a robust predictor of emotionally reactive (positive and negative) and dysregulated affective traits in 6-month old infants. These results are consistent with the findings of various prior studies in this area (Austin et al., 2005; Davis et al., 2007, 2004; McGrath et al., 2008; Nolvi et al., 2016; Pesonen et al., 2005). In line with our hypotheses, children exposed to Sandy in-utero exhibited elevated positive emotionality, more specifically characterized by higher pleasure seeking (High-Intensity Pleasure), approach tendency (Approach), and higher reactivity to environmental cues (Perceptual Sensitivity). In terms of negative emotionality and self-regulation, they displayed higher levels of vigilance (Fearfulness) and more problems with affiliation (Cuddliness) and attention (Duration of Orientation). This profile of temperament characteristics may improve the survival of young infants according to the evolutionary framework underlying fetal programming via PNMS. Fearfulness may protect the infants by priming them to avoid danger in potentially harmful environments (Bergman et al., 2007; Braungart-Rieker et al., 2010). Greater sensitivity, and reactivity might enable the child to more efficiently sense potential threats and elicit parental attention. However, these children also may be more likely to display maladaptive behaviors in a subsequently nonthreatening postnatal environment owing to a mismatch between the environment the child was prepared for and the environment the child was born into.
Although there was no notable difference in the scale of Activity Level at 6 months, a greater increase in Activity Level from 6 to 24 months was found for children exposed to Sandy in-utero. Additionally, among these children, a marginally greater increase in activity was found for those whose mother experienced greater subjective stress reaction to Sandy. Activity level has been associated with peer rejection (Walker et al., 2001), aggression, and externalizing behaviors (e.g., attention-deficit/hyperactivity disorder, ADHD) (Auerbach et al., 2008, 2005). The consistent findings on high activity are notable, as this scale is an important early behavioral risk marker for possible ADHD and behavior after age 2 (Buss et al., 1980; Campbell et al., 1994), as well as early onset and persistence of ADHD by age 4 (Sonuga-Barke et al., 2005). As our participants grow, we may be able to observe if children who were exposed to greater subjective PNMS in-utero exhibit a higher or accelerated rate of behavioral problems compared to their peers.
At 6 months, children exposed to Sandy in-utero, relative to their unexposed peers, showed greater values of High-Intensity Pleasure and Approach, exhibited lower ratings in these measures over time, and had lower values at 24 months. The high-intensity pleasure exhibited has been related to sensation seeking (Zuckerman, 1990). Additionally, the high approach tendency may lead to frustration when an individual is being blocked from reaching goals (Rothbart et al., 2000a, 2000b). It is possible that infants with initially higher pleasure-seeking tendencies and approach are more likely to be discouraged by frustrations, thereby reducing their approach tendencies, even if they usually exhibit positive affect. Lower sensation seeking and lower approach motivation characteristics appear to be a risk factor for increased vulnerability to depression in childhood and adolescence (Schneider, 2014). On the other hand, it is possible that highly reactive infants attract greater attention from their caregivers than low-reactive infants, thereby having learned emotion regulation and more adaptive behavior over time.
Similarly, children exposed to Sandy in-utero showed greater Fearfulness at 6 months, then a greater decrease in Fearfulness over time, though there was no difference observed at 24 months. Our findings are consistent with some prior studies. For example, Barker et al. (2011) revealed that PNMS was associated with fearless temperament at age 2 years, which was further associated with greater conduct problems and callous-unemotional traits at age 13, independent of parenting and prenatal risk factors. Relatedly, researchers have also studied cortisol, a stress hormone that indexes stress activity in the HPA-axis. Lower cortisol levels indicate blunted stress reactivity and has been associated with fearlessness and callous-unemotional traits (Loney et al., 2006; Stadler et al., 2011). A study by Yehuda et al. (2005) on the effects of exposure to 9/11 showed that offspring of women who developed PTSD predicted lower cortisol levels at 9 months of age compared to offspring of women who did not develop PTSD. Fearless temperament has been associated with greater risk for externalizing behaviors (Eisenberg et al., 2001).
The process of expression and regulation of emotion exhibits dramatic shifts over infancy and early childhood (Camras, 1994; Posner and Rothbart, 2007; Ruff and Rothbart, 2001). Prior temperament literature illustrates the inter-relationship between temperament traits, via heterotypic continuity (Bridgett et al., 2011; Carranza et al., 2013; De Pauw and Mervielde, 2010; Putnam et al., 2008), which posits the extent to which specific temperament traits that are phenotypically different share common underlying attributes during different stages of development. Effortful control is one of the biggest heterotypic change that reflects neuro-maturation after infancy (Auerbach et al., 2008). Our data shows that negative characteristics, e.g., Distress to Limitations and Fearfulness, increased or remained stagnant between 6 and 12 months followed by decreasing levels around 12–18 months, which may be attributed to the development of the effortful control. Similarly, the regulatory temperament characteristics, i.e., Cuddliness, decreased from 6 to 18 months and then increased. While gaining a further understanding of these issues (the heterotypic continuity of different temperament domains) is important, especially in the context of the complex nature of the PNMS and temperament association, it is beyond the scope of this study.
This study has several limitations. First, child temperament measures were based solely on maternal self-report. Adding observational measures or multiple informant measures (e.g., paternal or teacher measures) in future studies could strengthen our findings. Second, although we illustrated the longitudinal effects of PNMS on temperament, developmental trajectories could be more appropriately studied if more time-points were included. Relatedly, measures of behavior and the emergence of symptomatology in later childhood would bolster the validity of using temperament as a marker for underlying neurobehavioral alterations related to PNMS. Third, there were no significant differences in major demographic characteristics between the exposed and control groups except that mothers in the Sandy group had higher educational level (which was controlled for in the analysis). However, given that the groups were recruited from different residential areas in NY, it is possible that they may differ in unmeasured ways. Forth, this study did not include the timing of stress exposure, which some prior research has shown to be an important predictor of later development, though this finding is not unanimous (Laplante et al., 2016; Tees et al., 2010). Further, despite evidence of both homotypic and heterotypic continuity in Rothbart’s measure of temperament between infancy and childhood (Komsi et al., 2006), the findings need to be interpreted with caution given that item descriptions are not identical on the IBQ-R and ECBQ. Moreover, the study lacks a biological measure of HPA-axis functioning. Higher prenatal stress has been linked to altered levels of maternal cortisol, the end product of the HPA-axis (Chrousos, 1992). In this regard, research targeting this psychobiological substrate could further our knowledge of prenatal stress and temperament. As stress is an intangible concept, much of the interpretation is subjective and it is difficult to synthesize and integrate results among studies measuring different types of stress, such as maternal depression and anxiety (Van den Bergh and Marcoen, 2004; Glover, 2015), stressful life events (Li et al., 2014), daily hassles (Buitelaar et al., 2003; Huizink et al., 2003) and traumatic experiences. Finally, the underlying mechanism of the effect of maternal traumatic stress on child temperament was not investigated in this study. Although we attempted to control for potentially confounding prenatal variables (i.e., lifetime trauma other than Sandy, prenatal depression, GDM & preeclampsia, substance abuse) to investigate the stress brought on by Sandy in a longitudinal framework, we acknowledge the need for further research to identify the differential contribution of prenatal stress types on offspring temperament.
Despite these limitations, our research suggests that disaster-related prenatal stress exposure was associated with more surgent, negative and dysregulated temperament traits among exposed offspring at 6 months of age, with a progressive increase in activity level and a progressive decrease in risk-taking, positive anticipation, and fear in the early development. These findings support the fetal programming and evolutionary perspective. In particular, temperament characteristics observed in the 6-month-old children who were affected by Sandy may have better coping skills for postnatal environmental risks especially during early development stage. While adaptive in early life, difficult temperament during this period in childhood may be a precursor for a range of problematic outcomes (De Pauw and Mervielde, 2010; Hellemans et al., 2010).
The clinical significance of our study is that maternal exposure to disaster-related stress may produce alterations in infant temperament that may have implications for future behavioral problems. If replicated and extended to behavioral difficulties later in development, these findings underscore the benefits of mental health intervention during prenatal care to reduce downstream developmental health burdens. Our findings also add support to calls for additional investment in pediatric practices to increase their capacity for involvement in national disaster preparedness. In particular, our results highlight that pregnant mothers are an especially vulnerable group and there is an urgent need for vigorous post-disaster outreach to this population. Natural disasters are not preventable, but armed with the knowledge that in-utero exposure to traumatic events adversely impact child temperament, we can encourage early intervention to ameliorate their adverse effects on child outcomes.
Acknowledgments
Funding
This research work was supported by the Grants K01MH080062, K01MH080062S and R01MH102729 from the National Institutes of Mental Health (NIMH), and PSC-CUNY, Queens College Research Enhancement Grant (to Nomura).
We would like to thank the children and parents who consented to participate in this study. We thank current and former research staff and assistants at Queens College, City University of New York for their contributions to this study.
None of the authors have any conflict of interest.
References
- Aksan N, Goldsmith HH, Smider NA, Essex MJ, Clark R, Hyde JS, Klein MH, Vandell DL. Derivation and prediction of temperamental types among pre-schoolers. Dev Psychol. 1999;35:958–971. doi: 10.1037//0012-1649.35.4.958. http://dx.doi.org/10.1037/0012-1649.35.4.958. [DOI] [PubMed] [Google Scholar]
- Allison P. Missing Data. Sage Publication; Thousand Oaks, CA: 2002. [Google Scholar]
- Auerbach JG, Landau R, Berger A, Arbelle S, Faroy M, Karplus M. Neonatal behavior of infants at familial risk for ADHD. Infant Behav Dev. 2005;28:220–224. http://dx.doi.org/10.1016/j.infbeh.2004.12.002. [Google Scholar]
- Auerbach JG, Berger A, Atzaba-Poria N, Arbelle S, Cypin N, Friedman A, Landau R. Temperament at 7, 12, and 25 months in children at familial risk for ADHD. Infant Child Dev. 2008;17:321–338. http://dx.doi.org/10.1002/icd.579. [Google Scholar]
- Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Hum Dev. 2005;81:183–190. doi: 10.1016/j.earlhumdev.2004.07.001. http://dx.doi.org/10.1016/j.earlhumdev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. http://dx.doi.org/10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. http://dx.doi.org/10.1016/S1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Barker ED, Oliver BR, Viding E, Salekin RT, Maughan B. The impact of prenatal maternal risk, fearless temperament and early parenting on adolescent callous-unemotional traits: a 14-year longitudinal investigation. J Child Psychol Psychiatry. 2011;52:878–888. doi: 10.1111/j.1469-7610.2011.02397.x. http://dx.doi.org/10.1111/j.1469-7610.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’connor TG, Modi N, Glover V, Connor TGO. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. http://dx.doi.org/10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Blake ES, Kimberlain TB, Berg RJ, Cangialosi JP, Beven JL., II . Tropical Cyclone Report Hurricane Sandy Rep. National Hurricane Center; 2013. AL182012. [Google Scholar]
- Boscarino JA, Hoffman SN, Adams RE, Figley CR, Solhkhah R. Mental health outcomes among vulnerable residents after Hurricane Sandy: implications for disaster research and planning. Am J Disaster Med. 2014;9:107–120. doi: 10.5055/ajdm.2014.0147. http://dx.doi.org/10.5055/ajdm.2014.0147. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Sheppard DM. The neurodevelopmental frontostriatal disorders: evolutionary adaptiveness and anomalous lateralization. Brain Lang. 2000;73:297–320. doi: 10.1006/brln.2000.2308. http://dx.doi.org/10.1006/brln.2000.2308. [DOI] [PubMed] [Google Scholar]
- Brand SR, Engel SM, Canfield RL, Yehuda R. The effect of maternal PTSD following in utero trauma exposure on behavior and temperament in the 9-month-old infant. Ann NY Acad Sci. 2006;1071:454–458. doi: 10.1196/annals.1364.041. http://dx.doi.org/10.1196/annals.1364.041. [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker JM, Hill-Soderlund AL, Karrass J. Fear and anger reactivity trajectories from 4 to 16 months: the roles of temperament, regulation, and maternal sensitivity. Dev Psychol. 2010;46:791–804. doi: 10.1037/a0019673. http://dx.doi.org/10.1037/a0019673. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Gartstein MA, Putnam SP, Lance KO, Iddins E, Waits R, Vanvleet J, Lee L. Emerging effortful control in toddlerhood: the role of infant orienting/regulation, maternal effortful control, and maternal time spent in caregiving activities. Infant Behav Dev. 2011;34:189–199. doi: 10.1016/j.infbeh.2010.12.008. http://dx.doi.org/10.1016/j.infbeh.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Laake LM, Gartstein MA, Dorn D. Development of infant positive emotionality: the contribution of maternal characteristics and effects on subsequent parenting. Infant Child Dev. 2013;22:362–382. http://dx.doi.org/10.1002/icd.1795. [Google Scholar]
- Buitelaar JK, Huizink AC, Mulder EJ, de Medina PGR, Visser GHA. Prenatal stress and cognitive development and temperament in infants. Neurobiol Aging. 2003;24:S53–S60. doi: 10.1016/s0197-4580(03)00050-2. http://dx.doi.org/10.1016/S0197-4580(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6-9-year-old children. Psychoneuroendocrinology. 2010;35:141–153. doi: 10.1016/j.psyneuen.2009.07.010. http://dx.doi.org/10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss DM, Block JH, Block J, Development C, Jun N. Preschool activity level: personality correlates and developmental implications. Child Dev. 1980;51:401–408. [Google Scholar]
- Campbell SB, Pierce EW, March CL, Ewing LJ, Szumowski EK. Hard-to-manage preschool boys: symptomatic behavior across contexts and time. Child Dev. 1994;65:836–851. http://dx.doi.org/10.1111/j.1467-8624.1994.tb00787.x. [PubMed] [Google Scholar]
- Camras LA. The Nature of Emotion: Fundamental Questions. Oxford University Press; New York: 1994. Two aspects of emotional development: expression and elicitation; pp. 347–351. [Google Scholar]
- Caramanica K, Brackbill RM, Stellman SD, Farfel MR. Posttraumatic stress disorder after hurricane sandy among persons exposed to the 9/11 disaster. Int J Emerg Ment Health. 2015;17:356–362. doi: 10.4172/1522-4821.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza JA, González-Salinas C, Ato E. A longitudinal study of temperament continuity through IBQ, TBAQ and CBQ. Infant Behav Dev. 2013;36:749–761. doi: 10.1016/j.infbeh.2013.08.002. http://dx.doi.org/10.1016/j.infbeh.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Carranza Carnicero JA, Pérez-López J, Del Carmen GS, Martínez-Fuentes MT. A longitudinal study of temperament in infancy: stability and convergence of measures. Eur J Personal. 2000;37:21–37. http://dx.doi.org/10.1002/(SICI)1099-0984(200001/02)14:1<21::AID-PER367>3.0.CO;2-A. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Deaths associated with Hurricane Sandy - October-November 2012. MMWR Morb Mortal Wkly Rep. 2013;62:393–397. [PMC free article] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. http://dx.doi.org/10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The concepts of stress and stress system disorders. JAMA. 1992;267:1244. http://dx.doi.org/10.1001/jama.1992.03480090092034. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale Detection of Postnatal Depression Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. http://dx.doi.org/10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6:319–331. http://dx.doi.org/10.1207/s15327078in0603_1. [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. http://dx.doi.org/10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- De Pauw SSW, Mervielde I. Temperament, personality and developmental psychopathology: a review based on the conceptual dimensions underlying childhood traits. Child Psychiatry Hum Dev. 2010;41:313–329. doi: 10.1007/s10578-009-0171-8. http://dx.doi.org/10.1007/s10578-009-0171-8. [DOI] [PubMed] [Google Scholar]
- Dinehart LHB, Messinger DS, Acosta SI, Cassel T, Ambadar Z, Cohn J. Adult perceptions of positive and negative infant emotional expressions. Infancy. 2005;8:279–303. http://dx.doi.org/10.1207/s15327078in0803_5. [Google Scholar]
- DiPietro JA, Hodgson DM, Costigan KA, Johnson TRB. Fetal antecedents of infant temperament. Child Dev. 1996;67:2568–2583. http://dx.doi.org/10.1111/j.1467-8624.1996.tb01875.x. [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes Ra, Shepard Sa, Reiser M, Murphy BC, Losoya SH, Guthrie IK. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Dev. 2001;72:1112–1134. doi: 10.1111/1467-8624.00337. http://dx.doi.org/10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34:1–14. doi: 10.1016/j.infbeh.2010.09.008. http://dx.doi.org/10.1016/j.infbeh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Finik J, Nomura Y. Cohort profile: stress in pregnancy (SIP) study. Int J Epidemiol. 2017:dyw264. doi: 10.1093/ije/dyw264. http://dx.doi.org/10.1093/ije/dyw264. [DOI] [PMC free article] [PubMed]
- Foa E. Posttraumatic Diagnostic Scale Manual. National Computer Systems; Minneapolis, MN: 1996. [Google Scholar]
- Garstein M, Rothbart MK. Studying infant temperament via a revision of the Infant behavior Questionnaire. Infant Behav Dev. 2003;26:64–86. [Google Scholar]
- Glover V. Annual research review: prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry Allied Discip. 2011;52:356–367. doi: 10.1111/j.1469-7610.2011.02371.x. http://dx.doi.org/10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Glover V. Advances in Neurobiology. Springer; New York: 2015. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms; pp. 269–283. doi: < http://doi.org/10.1007/978-1-4939-1372-5_13>. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. http://dx.doi.org/10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Gray JA. Explorations in Temperament: International Perspectives on Theory and Measurement. Plenum Press; New York: 1991. The neuropsychology of temperament; pp. 105–128. [Google Scholar]
- Hellemans KGC, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. http://dx.doi.org/10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJH, Visser GHA, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Dick DM, Sihvola E, Pulkkinen L, Rose RJ, Kaprio J. Chernobyl exposure as stressor during pregnancy and behaviour in adolescent off-spring. Acta Psychiatr Scand. 2007;116:438–446. doi: 10.1111/j.1600-0447.2007.01050.x. http://dx.doi.org/10.1111/j.1600-0447.2007.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, Mrazek D, Knapp PK, Steinberg L, Pfeffer C, Schowalter J, Shapiro T. Evolution and revolution in child psychiatry: ADHD as a disorder of adaptation. J Am Acad Child Adolesc Psychiatry. 1997;36:1672–1681. doi: 10.1097/00004583-199712000-00015. http://dx.doi.org/10.1097/00004583-199712000-00015. [DOI] [PubMed] [Google Scholar]
- King S, Laplante DP. The effects of prenatal maternal stress on children’s cognitive development: project ice storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. http://dx.doi.org/10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- King S, Dancause K, Turcotte-Tremblay AM, Veru F, Laplante DP. Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res Part C Embryo Today Rev. 2012;96:273–288. doi: 10.1002/bdrc.21026. http://dx.doi.org/10.1002/bdrc.21026. [DOI] [PubMed] [Google Scholar]
- Komsi N, Räikkönen K, Pesonen AK, Heinonen K, Keskivaara P, Järvenpää AL, Strandberg TE. Continuity of temperament from infancy to middle childhood. Infant Behav Dev. 2006;29:494–508. doi: 10.1016/j.infbeh.2006.05.002. http://dx.doi.org/10.1016/j.infbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Kuvacic I, Skrablin S, Hodzic D, Milkovic G. Possible influence of expatriation on perinatal outcome. Acta Obstet Gynecol Scand. 1996;75:367–371. doi: 10.3109/00016349609033333. http://dx.doi.org/10.3109/00016349609033333. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CRW, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. http://dx.doi.org/10.1016/S0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project ice storm: prenatal maternal stress affects cognitive and linguistic functioning in 5½-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063–1072. doi: 10.1097/CHI.0b013e31817eec80. http://dx.doi.org/10.1097/CHI.0b013e31817eec80. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, King S. The effects of maternal stress and illness during pregnancy on infant temperament: project ice storm. Pediatr Res. 2016;79:107–113. doi: 10.1038/pr.2015.177. http://dx.doi.org/10.1038/pr.2015.177. [DOI] [PubMed] [Google Scholar]
- Li X, Wang Z, Hou Y, Wang Y, Liu J, Wang C. Effects of childhood trauma on personality in a sample of Chinese adolescents. Child Abus Negl. 2014;38:788–796. doi: 10.1016/j.chiabu.2013.09.002. http://dx.doi.org/10.1016/j.chiabu.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Loney BR, Butler MA, Lima EN, Counts CA, Eckel LA. The relation between salivary cortisol, callous-unemotional traits, and conduct problems in an adolescent non-referred sample. J Child Psychol Psychiatry Allied Discip. 2006;47:30–36. doi: 10.1111/j.1469-7610.2005.01444.x. http://dx.doi.org/10.1111/j.1469-7610.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- Maas CJM, Hox JJ. The influence of violations of assumptions on multilevel parameter estimates and their standard errors. Comput Stat Data Anal. 2004;46:427–440. http://dx.doi.org/10.1016/j.csda.2003.08.006. [Google Scholar]
- Martin RP, Wisenbaker J, Baker J, Huttunen MO. Gender differences in temperament at six months and five years. Infant Behav Dev. 1997;20:339–347. http://dx.doi.org/10.1016/S0163-6383(97)90005-9. [Google Scholar]
- McGrath JM, Records K, Rice M. Maternal depression and infant temperament characteristics. Infant Behav Dev. 2008;31:71–80. doi: 10.1016/j.infbeh.2007.07.001. http://dx.doi.org/10.1016/j.infbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. Child psychiatric sequelae of maternal war stress. Acta Psychiatr Scand. 1985;72:505–511. doi: 10.1111/j.1600-0447.1985.tb02647.x. http://dx.doi.org/10.1111/j.1600-0447.1985.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Westley DT, Mccloud PI. Do infants of depressed mothers cry more than other infants? J Paediatr Child Health. 1995;31:218–221. doi: 10.1111/j.1440-1754.1995.tb00789.x. http://dx.doi.org/10.1111/j.1440-1754.1995.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Neria Y, Shultz JM. Mental health effects of Hurricane Sandy: characteristics, potential aftermath, and response. JAMA. 2012;308:2571–2572. doi: 10.1001/jama.2012.110700. http://dx.doi.org/10.1001/jama.2012.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolvi S, Karlsson L, Bridgett DJ, Korja R, Huizink AC, Kataja EL, Karlsson H. Maternal prenatal stress and infant emotional reactivity six months post-partum. J Affect Disord. 2016;199:163–170. doi: 10.1016/j.jad.2016.04.020. http://dx.doi.org/10.1016/j.jad.2016.04.020. [DOI] [PubMed] [Google Scholar]
- Partridge T, Lerner JV. A latent growth-curve approach to difficult temperament. Infant Child Dev. 2007;16:255–265. http://dx.doi.org/10.1002/icd.465. [Google Scholar]
- Pesonen AK, Räikkönen K, Strandberg TE, Järvenpää AL. Continuity of maternal stress from the pre- to the postnatal period: associations with infant’s positive, negative and overall temperamental reactivity. Infant Behav Dev. 2005;28:36–47. http://dx.doi.org/10.1016/j.infbeh.2004.09.001. [Google Scholar]
- Pluess M, Velders FP, Belsky J, Van IJzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VWV, Hofman A, Arp PP, Verhulst FC, Tiemeier H. Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biol Psychiatry. 2011;69:520–525. doi: 10.1016/j.biopsych.2010.10.006. http://dx.doi.org/10.1016/j.biopsych.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self regulation. Dev Psychopathol. 2000;12:427–441. doi: 10.1017/s0954579400003096. http://dx.doi.org/10.1017/S0954579400003096. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. http://dx.doi.org/10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: the Early Childhood Behavior Questionnaire. Infant Behav Dev. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. http://dx.doi.org/10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Rothbart MK, Gartstein MA. Homotypic and heterotypic continuity of fine-grained temperament during infancy, toddlerhood, and early childhood. Infant Child Dev. 2008;17:387–405. http://dx.doi.org/10.1002/icd.582. [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E. Development and assessment of short and very short forms of the Infant Behavior Questionnaire–Revised. J Personal Assess. 2014;96:445–458. doi: 10.1080/00223891.2013.841171. http://dx.doi.org/10.1080/00223891.2013.841171. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Sage; Thousand Oaks, CA: 2002. 9780761919049. [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Dev. 1981;52:569–578. http://dx.doi.org/10.1111/j.1467-8624.1981.tb03082.x. [Google Scholar]
- Rothbart MK. Longitudinal observation of infant temperament. Dev Psychol. 1986;22:356–365. http://dx.doi.org/10.1037//0012-1649.22.3.356. [Google Scholar]
- Rothbart MK, Bates JE. Handbook of Child Psychology, editor. Social, Emotional, and Personality Development. Vol. 3. Wiley; New York: 1998. Temperament; pp. 105–176. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL. Temperament and social behavior in childhood. J Dev Psychol. 1994;40:21–39. [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. J Personal Soc Psychol. 2000a;78:122–135. doi: 10.1037//0022-3514.78.1.122. http://dx.doi.org/10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Derryberry D, Hershey K. Stability of temperament in childhood: laboratory infant assessment to parent report at seven years. In: Molfese V, Molfese D, editors. Temperament and Personality Development across the Life Span. Erlbaum; Mahwah, NJ: 2000b. pp. 85–119. [Google Scholar]
- Rubonis AV, Bickman L. Psychological impairment in the wake of disaster: the disaster-psychopathology relationship. Psychol Bull. 1991;109:384–399. doi: 10.1037/0033-2909.109.3.384. http://dx.doi.org/10.1037/0033-2909.109.3.384. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Rothbart MK. Attention in Early Development: Themes and Variations. Oxford University Press; 2001. [Google Scholar]
- Sallquist J, Eisenberg N, Spinrad TL, Gaertner BM, Eggum ND, Zhou N. Mothers’ and children’s positive emotion: relations and trajectories across four years. Soc Dev. 2010;19:799–821. doi: 10.1111/j.1467-9507.2009.00565.x. http://dx.doi.org/10.1111/j.1467-9507.2009.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. http://dx.doi.org/10.1037/1082-989X.7.2.147. [PubMed] [Google Scholar]
- Schneider BH. Child Psychopathology: from Infancy to Adolescence. Cambridge University Press; Cambridge: 2014. [Google Scholar]
- Shelley-Tremblay JF, Rosén LA. Attention deficit hyperactivity disorder: an evolutionary perspective. J Genet Psychol. 1996;157:443–453. doi: 10.1080/00221325.1996.9914877. http://dx.doi.org/10.1080/00221325.1996.9914877. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Auerbach J, Campbell SB, Daley D, Thompson M. Varieties of preschool hyperactivity: multiple pathways from risk to disorder. Dev Sci. 2005 doi: 10.1111/j.1467-7687.2005.00401.x. http://dx.doi.org/10.1111/j.1467-7687.2005.00401.x. [DOI] [PubMed]
- Stadler C, Kroeger A, Weyers P, Grasmann D, Horschinek M, Freitag C, Clement HWW. Cortisol reactivity in boys with attention-deficit/hyperactivity disorder and disruptive behavior problems: the impact of callous unemotional traits. Psychiatry Res. 2011;187:204–209. doi: 10.1016/j.psychres.2010.05.004. http://dx.doi.org/10.1016/j.psychres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Strelau J. Temperament: A Psychological Perspective. Plenum Press; New York, NY: 1998. http://dx.doi.org/10.4135/9780857025753.n199. [Google Scholar]
- Sukigara M, Nakagawa A, Mizuno R. Development of a Japanese version of the Early Childhood Behavior Questionnaire (ECBQ) using cross-sectional and longitudinal data. SAGE Open. 2015:5. http://dx.doi.org/10.1177/2158244015590443.
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry Allied Discip. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. http://dx.doi.org/10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tees MT, Harville EW, Xiong X, Buekens P, Pridjian G, Elkind-Hirsch K. Hurricane Katrina-related maternal stress, maternal mental health, and early infant temperament. Matern Child Health J. 2010;14:511–518. doi: 10.1007/s10995-009-0486-x. http://dx.doi.org/10.1007/s10995-009-0486-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BRH, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-Year-Olds. Child Dev. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. http://dx.doi.org/10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. http://dx.doi.org/10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Van Den Bergh BRHH, Mulder EJHH, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. http://dx.doi.org/10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- van der Wal MF, van Eijsden M, Bonsel GJ. Stress and emotional problems during pregnancy and excessive infant crying. J Dev Behav Pediatr. 2007;28:431–437. doi: 10.1097/DBP.0b013e31811ff8f4. http://dx.doi.org/10.1097/DBP.0b013e31811ff8f4. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001a;5:119–125. doi: 10.1023/a:1011353216619. http://dx.doi.org/10.1023/A:1011353216619. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Garite TJ. Chapter 9 The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Prog Brain Res. 2001b;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. http://dx.doi.org/10.1016/S0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- Walker S, Berthelsen D, Irving K. Temperament and peer acceptance in early childhood: Sex and social status differences. Child Study J. 2001;31:177–193. [Google Scholar]
- Ward AJ. Prenatal stress and childhood psychopathology. Child Psychiatry Hum Dev. 1991;22:97–110. doi: 10.1007/BF00707788. http://dx.doi.org/10.1007/BF00707788. [DOI] [PubMed] [Google Scholar]
- Wood PK. Developmental models for children’s temperament: alternatives to chronometric polynomial curves. Infant Child Dev. 2011;20:194–212. http://dx.doi.org/10.1002/icd.692. [Google Scholar]
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. http://dx.doi.org/10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- Zentner M, Bates JE, Article R. Child temperament: an integrative review of concepts, research programs, and measures. Int J Dev Sci. 2008;2:7–37. http://dx.doi.org/10.3233/DEV-2008-21203. [Google Scholar]
- Zuckerman B, Bauchner H, Parker S, Cabral H. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11:190–194. http://dx.doi.org/10.1097/00004703-199008000-00006. [PubMed] [Google Scholar]
- Zuckerman M. The psychophysiology of sensation seeking. J Personal. 1990;58:313–345. doi: 10.1111/j.1467-6494.1990.tb00918.x. http://dx.doi.org/10.1111/j.1467-6494.1990.tb00918.x. [DOI] [PubMed] [Google Scholar]