Abstract
Background
Chemotherapy can cause adverse effects such as chemotherapy-related cognitive impairment (CRCI). In this prospective study, the efficacy of traditional Chinese medicine acupuncture therapy in relieving CRCI and its impact on serum brain-derived neurotrophic factor (BDNF) are evaluated.
Material/Methods
Eighty patients were randomly divided into a treatment group and a control group with 40 patients in each group. The treatment group was treated at the following acupuncture points: Baihui (DU20), Sishencong (EX-HN1), Shenting (DU24), Zusanli (ST36), Taixi (K13), Dazhong (K14), and Juegu (GB39). Cognitive function was assessed using the functional assessment of cancer treatment cognition test (FACT-COG, version 3), the auditory-verbal learning test (AVLT), the verbal fluency test (VFT), the symbol digit modality test (SDMT), the clock-drawing test (CDT), and the trail-making test part B (TMT-B). In addition, blood serum levels of BDNF were measured before and after treatment. Correlations between change in BDNF levels and cognitive function were also analyzed.
Results
CRCI was ameliorated in the acupuncture treatment group, with scores on FACT-COG, AVLT-recognition and CDT assessments all significantly increased (P<0.05 in all cases). In addition, serum BDNF levels after acupuncture treatment were significantly higher than before treatment (t=3.242, P<0.01). Moreover, the level of BDNF was positively correlated with the total score of FACT-COG, AVLT-recognition, and CDT (r=0.694, 0.628, and 0.532, respectively; all P<0.05). The control group showed no statistically significant difference in any measures over the same period.
Conclusions
Acupuncture therapy is effective in the treatment of CRCI in breast cancer patients through a mechanism that may be related to an increase of BDNF.
MeSH Keywords: Acupuncture - Methods, Breast, Brain-Derived Neurotrophic Factor, Chemical Synthesis, Mild Cognitive Impairment
Background
Breast cancer (BC) is a common malignant disease in women. When treated with chemotherapy, patients often complain of memory loss; this symptom is referred to as chemotherapy-related cognitive impairment (CRCI) or “chemotherapy brain” [1]. It is widely recognized as one of the adverse effects of chemotherapy used to treat malignant tumors. CRCI is defined as a cognitive decline in memory, learning, attention, reasoning, visual-spatial functioning, and information processing during and after discontinuation of chemotherapy in cancer patients [2,3]. A recent nationwide, multicenter, prospective, longitudinal study noted that chemotherapy can cause CRCI and affect quality of life [4]; however, CRCI treatment methods are limited. Therefore, an effective rehabilitation approach to CRCI is desirable. A randomized controlled trial has established that traditional Chinese medicine acupuncture is safe, well-tolerated, and effective in treating mild cognitive impairment in people with Alzheimer’s disease [5]. Acupuncture has been widely used to treat other diseases and shown to be safe and effective, with little risk and no complication, in many studies [6–8]. Acupuncture has a history of over 2000 years and is important in traditional Chinese medicine [9]. The philosophy behind acupuncture is the balance between yin and yang energies, similar to the need for sympathetic and parasympathetic activity to be balanced [10]. In the clinic, hair-thin needles are inserted deep into the skin at specific sites known as acupuncture points by skilled practitioners, followed by stimulation to those points through different methods, including manual stimulation (acupressure), heat (moxibustion), electrical pulses (electro-acupuncture), or laser light (laser acupuncture) [9]. Acupuncture is widely used in cancer patients for its ability to relieve pain, fatigue, xerostomia, and other symptoms [11,12]. This study evaluated the effect of acupuncture on CRCI in breast cancer patients who received chemotherapy and assessed changes in BDNF levels in these patients.
Material and Methods
Participants
Breast cancer patients treated with chemotherapy were recruited from the local hospital from May 2017 to October 2017. Patients were diagnosed with early breast cancer (stage 0–II) and received chemotherapy. Inclusion criteria were: (1) age 21–55 years (i.e., premenopausal); (2) newly diagnosed with breast cancer after surgery and treated with common standard-dose chemotherapy regimens; (3) a chief complaint of memory impairment that was confirmed by a family member; (4) a level of education sufficient to understand the information content of the Mini-Mental State Examination (MMSE); (5) expected to survive >1 year. Exclusion criteria were: metastatic breast cancer, prior cancer, substance abuse, brain injury, a history of neurological or psychiatric disorders, or currently taking psychoactive medications that might affect brain structure and function.
Patients were recruited according to the above criteria and were treated with acupuncture therapy (n=40) or not treated with acupuncture (control, n=40). All subjects were right-handed. No demographic differences were found between groups (P>0.05).
All assessments were completed twice: before acupuncture therapy (time 1) and after completion of acupuncture therapy (time 2). The control group completed assessments at the same time points. Data collectors were blind to the study participants’ treatment group.
This study was approved by the Ethics Committee of the Affiliated Hospital of Jiangnan University. All participants provided informed written consent.
Chemotherapy
Based on the current guidelines for the treatment of breast cancer, we chose the 3 most commonly used chemotherapy schemes. Specific regimens were as follows: (1) TC chemotherapy: 5 mg/m2 docetaxel + 600 mg/m2 cyclophosphamide by intravenous (IV) on day1; cycled every 21 days for 4 cycles; (2) TCb chemotherapy: 75 mg/m2 docetaxel + AUC 6 dose of carboplatin by IV on day 1; cycled every 21 days for 4 cycles; and, 3) AC followed by docetaxel chemotherapy: 60 mg/m2 doxorubicin + 600 mg/m2 cyclophosphamide by IV on day 1; cycled every 21 days for 4 cycles and followed by 100 mg/m2 docetaxel by IV on day 1; cycled every 21 days for 4 cycles. The total duration of chemotherapy lasted 3–6 months. Adjuvant drugs included antacids (lansoprazole and omeprazole), antiemetics (ondansetron and palonosetron) and anti-allergy medicine (dexamethasone). Granulocyte colony-stimulating factor was used when complications such as anemia and bone marrow suppression occurred.
Neuropsychological assessment
Neuropsychological assessment, including self-report measures and neuropsychological tests, were performed within 14 days of the final chemotherapy treatment. Self-report measures were: (1) health information and medical history and (2) functional assessment of cancer treatment cognition (FACT-COG, version 3). FACT-COG is the cancer patient’s own cognitive function assessment; it includes 4 aspects: perceived cognitive impairments (PCI), impact on quality of life (QOL), comments from others (OTH), and perceived cognitive abilities (PCA). Neuropsychological tests performed were: (1) the auditory-verbal learning test (AVLT); (2) the verbal fluency test (VFT); (3) the symbol digit modality test (SDMT); (4) the clock-drawing test (CDT); and, (5) the trail-making test part B (TMT-B). AVLT is to assess memory, including short-term memory (AVLT1), delayed recall (AVLT2), and recognition (AVLT3). The verbal fluency test (semantic categories “animals/minute”) aims to verify language, semantic memory, and executive functions by evaluating word retrieval ability established in long-term memory. The symbol digit modality test (SDMT) is a measure of attention (perception and coding), cognitive processing speed, and visual working memory. The clock-drawing test (CDT) is a visual (non-verbal) screening instrument for measuring mild-to-moderate cognitive impairment. The trail-making test part B (TMT-B) is used to evaluate driving abilities and includes testing for executive functions. All participants were also required to complete the MMSE, State Anxiety Inventory (S-AI) and Beck Depression Inventory (BDI). S-AI was used to exclude patients with anxiety disorder and was measured as baseline values. BDI was used to exclude depressive disorder and was measured for inclusion as covariates in analyses.
Serum BDNF detection
On the mornings before and after acupuncture treatment, 5 ml of venous blood was drawn from each subject on the same day as the neuropsychological assessment. Blood samples were centrifuged at 3000 rpm for 10 min after standing for 15 min, and then kept at −80°C after separation. An enzyme-linked immunosorbent assay (ELISA) kit was used to measure expression levels of BDNF in the serum twice, and the mean value was used in analysis.
Acupuncture therapy
Acupuncture therapy was performed by 2 skilled acupuncturists with >5 years’ experience in neurological rehabilitation with acupuncture. Sterile, disposable needles 40 mm long and 0.25 mm in diameter (Huatuo, Suzhou Medical Instruments Factory, China) were used by acupuncturists. Basic acupuncture formulas Baihui (DU20), Sishencong (EX-HN1), and Taixi (KI3) were used. Based on symptoms and tongue manifestation, other acu-points could also be stimulated as follows. The angle insertion of Baihui, Shenting (DU24), and Sishencong are approximately 10–20° (between needle and scalp), with 10–15 mm as the best insertion depth. Taixi, Dazhong (KI4), and Juegu (GB39) were inserted 15–20 mm deep with a 0.25×25 mm acupuncture needle. Zusanli (ST36) was inserted 25–35 mm deep with a 0.25×40 mm acupuncture needle. Effective needling was accompanied by needling feelings of numbness, tingling, swelling, or muscle weakness, known as “de qi” in acupuncture; the needle was kept in situ for 30 min after stimulation. Patients received two 4-week courses of acupuncture with a 3-day rest between the 2 courses. Every week, patients were treated once a day for 5 days, followed by 2 days of rest.
Statistical analysis
SPSS v. 19.0 (SPSS Inc., IL, USA) was used for statistical analysis. Paired t tests were used to assess changes in neuropsychological test performance between time 1 (t1) and time 2 (t2) within each treatment group. Differences in cognitive tests and subjective measures were assessed using an ANCOVA with age, radiotherapy, tamoxifen, S-AI, and BDI scores initially included as covariates (and removed from the model if p>0.05). Relationships between change in BDNF and change in neuropsychological measures (scores that changed from t1 to t2) were explored within each group separately using a two-tailed Pearson or Spearman correlation, as appropriate.
Results
Demographic characteristics and clinical data
A total of 80 subjects were initially enrolled in the study. Figure 1 show the flow of patients through the study. One treatment subject and 3 control subjects were excluded from analysis because they were unable to finish the neuropsychological assessment or acupuncture therapy. One control subject was diagnosed with brain metastases in the second assessment and was also dropped from the analysis. Therefore, n=39 for the treatment group and n=36 for the control group.
Figure 1.
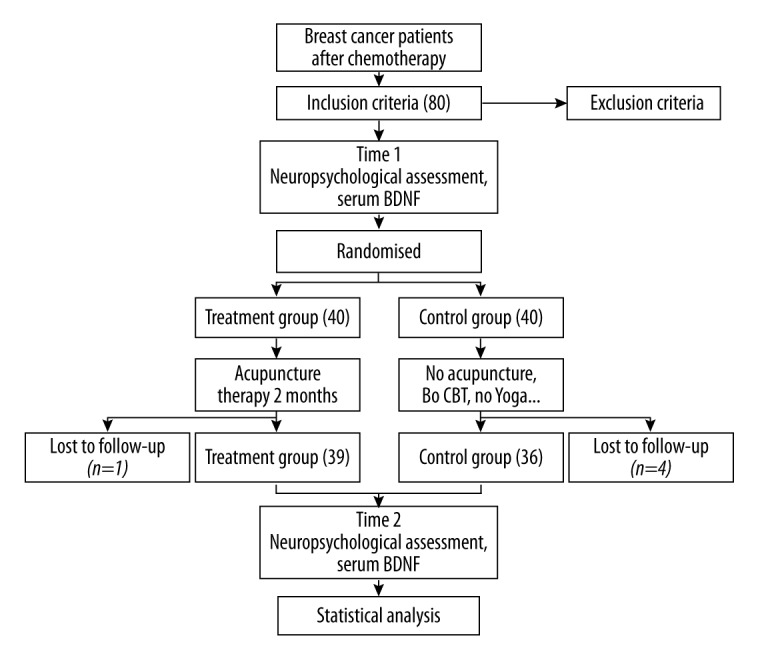
Flow chart of the study. BT: cognitive-behavior therapy.
Patients in the treatment and control groups did not differ in terms of age, education, MMSE, or depression or anxiety symptoms at time 1 (Table 1). S-AI and BDI scores were initially included as covariates, but none had significant effects and they were thus removed from the final model. The second neuropsychological assessment was conducted, on average, 2 months after time 1; inter-scan intervals did not differ significantly between the 2 groups.
Table 1.
Demographic and clinical characteristics.
| Treatment group (n=39) | Control group (n=36) | Paired t test | P | |
|---|---|---|---|---|
| Age, mean (SD), years | 43.11±4.23 | 42.26±4.42 | 0.70 | 0.50 |
| TNM stage, No (%) | ||||
| 0 | 0 (0.0) | 0 (0.0) | – | – |
| I | 24 (61.5) | 22 (61.1) | – | – |
| II | 15 (38.5) | 14 (38.9) | – | – |
| Surgery method, No (%) | ||||
| Conservative surgery | 8 (20.5) | 7 (19.4) | – | – |
| Mastectomy + SLNB | 20 (51.3) | 18 (50.0) | – | |
| Modify mastectomy | 11 (28.2) | 11 (30.6) | – | – |
| Subtype, No (%) | ||||
| Luminal A (HR/HER2−) | 0 (0.0) | 0 (0.0) | – | – |
| Luminal B (HR/HER2) | 20 (51.3) | 19 (52.8) | – | – |
| Erb-B2 (HR−/HER2) | 9 (23.1) | 8 (22.2) | – | – |
| Basal-like (HR−/HER2−) | 10 (25.6) | 9 (25.0) | – | – |
| Radiotherapy, No (%) | 12 (30.8) | 11 (30.6) | – | – |
| Hormonal therapy, No (%) | 21 (53.8) | 20 (55.6) | – | – |
| Chemotherapy regimen, No (%) | ||||
| TC | 19 (48.7) | 18 (50.0) | – | – |
| TP | 9 (23.1) | 7 (19.4) | – | – |
| ECx4→Tx4 | 11 (28.2) | 11 (30.6) | – | – |
| T1 to T2, mean (SD), days | 58.94±5.73 | 55.85±4.86 | 1.76 | 0.09 |
| Depression BDI, mean (SD) | 7.81±4.35 | 7.33±4.47 | 0.56 | 0.58 |
| Anxiety S-AI, mean (SD) | 34.91±7.20 | 33.95±6.27 | 2.01 | 0.06 |
| Education, mean (SD), years | 14.26±2.06 | 13.87±2.58 | 0.28 | 0.78 |
| MMSE, mean (SD) | 24.81±1.48 | 25.19±1.72 | 0.84 | 0.41 |
N/A – not applicable; SD – standard deviation; SLNB – sentinel lymph node biopsy; TC – Docetaxel + cyclophosphamide; TP – Paclitaxel + Carboplatin; ECx4→Tx4 – (Epirubicin + Cyclophosphamide)×4+Docetaxel×4; BDI – Beck Depression Inventory; S-AI – State Anxiety Inventory; MMSE – Mini-Mental State Examination. P-values are the result of t tests for continuous variables, or Fisher’s Exact test for categorical variables * Statistically significant (p<.05).
Neuropsychological assessment
Treatment and control groups did not differ in self-report measures or neuropsychological tests at baseline (Table 2). The treatment group had significantly higher scores after acupuncture therapy on FACT-COG, AVLT3, and CDT compared with baseline (paired t test, P<0.05). In contrast, the control group showed no significant differences in performance at time 2 compared to baseline. Moreover, a repeated-measures ANOVA revealed significant interactions between groups and performance change over time for self-report measures (FACT-COG), CDT, and AVLT3.
Table 2.
Summary of neuropsychologic assessment.
| Metabolites | Treatment (n=39) | Control (n=36) | Repeated measures ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T value | T1 | T2 | T value | F | P | |
| Mean ±SD | Mean ±SD | Mean ±SD | Mean ±SD | |||||
| FACT-COG | 98.75±12.94 | 102.38±13.78 | 4.840** | 99.60±11.05 | 99.80±10.77 | 1.489 | 5.77 | 0.001 |
| PCI | 55.42±10.95 | 56.29±11.49 | 3.494** | 57.55±8.43 | 57.35±8.99 | 0.721 | 3.21 | 0.027 |
| QOL | 11.33±3.42 | 11.75±3.38 | 2.632* | 11.70±2.41 | 11.55±2.24 | 0.326 | 1.30 | 0.279 |
| OTH | 11.63±2.89 | 12.54±3.31 | 2.991** | 11.10±2.65 | 11.30±1.92 | 1.189 | 0.48 | 0.697 |
| PCA | 20.38±4.19 | 21.79±4.40 | 2.298* | 19.25±3.31 | 19.60±3.33 | 1.285 | 3.75 | 0.014 |
| AVLT1 | 9.13±1.48 | 9.17±1.55 | 0.440 | 9.25±1.55 | 9.50±1.82 | 1.561 | 0.23 | 0.873 |
| AVLT2 | 9.42±1.61 | 9.63±1.50 | 2.005 | 9.45±1.36 | 9.65±1.50 | 1.453 | 0.14 | 0.936 |
| AVLT3 | 10.92±1.44 | 11.42±1.18 | 2.202* | 10.75±1.59 | 10.70±1.49 | 0.357 | 5.21 | 0.002 |
| VFT | 17.88±3.33 | 18.21±3.74 | 1.163 | 18.50±3.38 | 19.15±2.83 | 1.412 | 0.56 | 0.642 |
| SDMT | 34.75±5.15 | 35.71±5.54 | 1.558 | 36.70±5.50 | 38.05±6.62 | 2.077 | 1.33 | 0.269 |
| CDT | 8.08±1.50 | 8.54±1.14 | 2.696* | 8.10±1.21 | 8.05±1.36 | 0.438 | 5.50 | 0.002 |
| TMT-B | 95.58±26.67 | 95.46±26.80 | 0.901 | 92.35±27.06 | 90.40±26.19 | 1.698 | 0.19 | 0.901 |
ANOVA – analysis of variance; FACT-COG – Functional Assessment of Cancer Therapy-Cognitive Function; PCI – perceived cognitive impairments; QOL – impact of perceived impairments on quality of life; OTH – comments from others; PCA – perceived cognitive abilities; AVLT1 – Auditory-Verbal Learning Test – Immediately recall; AVLT2 – Auditory-Verbal Learning Test – Delayed Recall; AVLT3 – Auditory-Verbal Learning Test – recognition; VFT – Verbal Fluency Test; SDMT – Symbol digit modality test; CDT – Clock-Drawing Test; TMT-B – Trail-Making Test part B. Statistically significant * (p<.05, ** p<.01).
BDNF change
The expression of BDNF significantly increased after acupuncture treatment (BDNF Mean ±SD, before treatment (t1): 19.17±4.63 ng/ml; after treatment (t2): 22.52±4.99 ng/ml; t=3.242, P<0.01). No significant difference was observed in the control group (BDNF Mean ±SD, t1: 18.97±4.52 ng/ml; t2: 18.67±4.61 ng/ml) (Figure 2).
Figure 2.
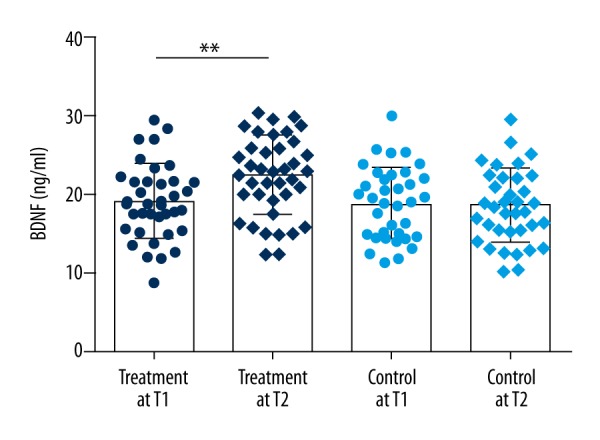
Levels of BDNF in serum. ** p<.01.
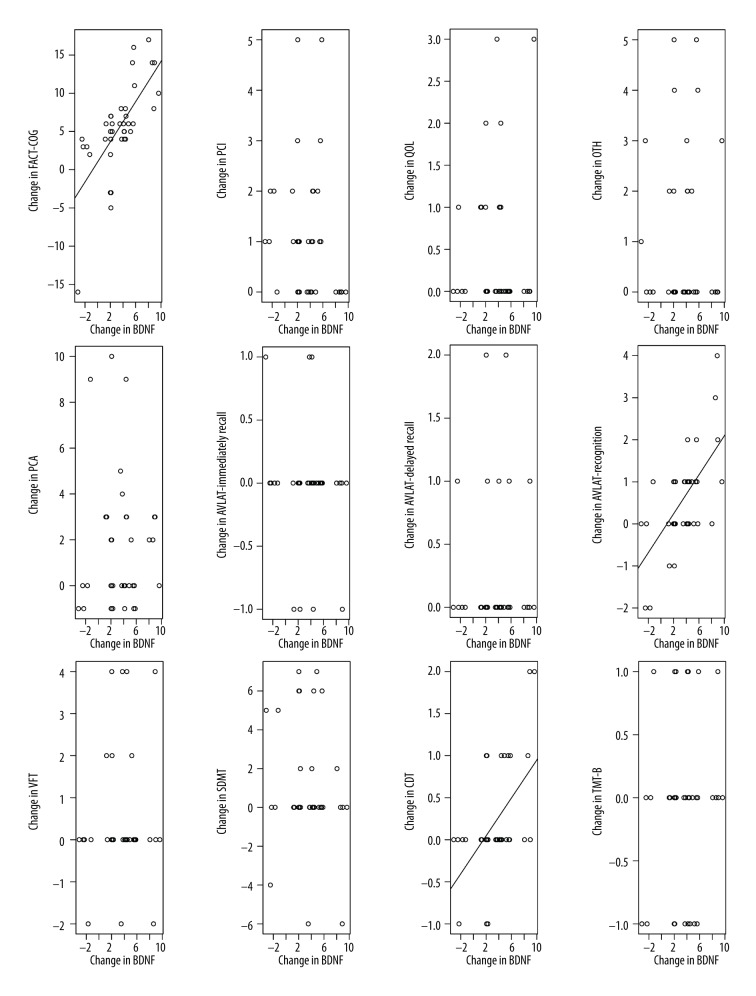
Correlation analysis
BDNF levels were positively correlated with cognitive scores (Figure 3). Change in FACT-COG, AVLT3, and CDT scores were positively correlated with BDNF levels in the treatment group (r=0.694, 0.628, and 0.532, respectively, all P<0.05). No other cognitive score changes were correlated with BDNF change and no significant correlations were observed in the control group.
Figure 3.
Correlation in BDNF changes and cognitive changes (treatment group).
Discussion
This study on the efficacy of acupuncture treatment to CRCI showed, using neurocognitive assessments, that acupuncture can improve the cognitive ability of breast cancer patients who receive chemotherapy. Both subjective and objective cognitive function tests were performed, and the results of the objective AVLT3 and CDT tests were consistent with the subjective FACT-COG test, showing that the CRCI is ameliorated after acupuncture treatment.
Although acupuncture has a long history of, and is widely accepted in, clinical use, the mechanism by which it works remains elusive. Recent studies on acupuncture have provided some possible insights into its mechanism. For example, through neuroimaging, researchers studying Alzheimer disease have shown that acupuncture increases hippocampal connectivity, activates certain cognitive-related areas, and adjusts default network activity patterns [13]. In a cognitively-impaired rat model, stimulating Shenmen (HT7) with laser acupuncture inhibits the expression of acetylcholinesterase in the hippocampus [14].
In the expresses mutated amyloid precursor protein (APP) and presenilin-1 mouse model, electro-acupuncturing Baihui can reduce the abnormally high expression of β-amyloid-42, inhibit the apoptosis of nerve cells, enhance BDNF level, and relieve cognitive impairment [15]. Moreover, experiments have shown that acupuncture on aging mice can: induce cell proliferation and improve learning and memory in different brain regions [16]; upregulate the activity of phospho-isomerase in the hippocampus [17]; and regulate the function of cytoskeletal-related synapses, induce neurotransmitter secretion, and promote the recovery of neuroplasticity [18,19].
In the present study, sham acupuncture was not used in the control group because the acupuncture control method is not yet mature and should not be considered as a standard model of acupuncture research [20,21]. The so-called “sham acupuncture control trial” may simply be a comparative study of different acupuncture methods [22]. Scholars hold differing views on whether sham acupuncture has a placebo effect or a therapeutic effect [23]. The commonly used sham acupuncture methods include adjacent sham acupuncture control, non-condition-related acupoint control, shallow needling acupoint control, minimal stimuli acupuncture control, and soothing acupuncture control. All of these have obvious defects and may cause infection if not done properly. Researchers have no standard method to follow when conducting clinical trials [21] and can only minimize the therapeutic effect of the placebo through a rational and rigorous design to better verify the therapeutic effect of acupuncture. The 2 groups were not allowed to receive cognitive therapy, yoga, or other physical therapy throughout the present study. Many factors can influence CRCI, making it difficult to isolate any one aspect; we controlled other factors to the best of our ability by assigning patients to treatment groups at random and taking baseline measures for all patients to control for individual differences.
The Chinese version of FACT-COG is clinically convenient and makes it easy to diagnose patients with cognitive function difficulties [24]. In the objective test, the Auditory-Verbal Learning Test (AVLT) was used for cognitive dysfunction among cancer survivors with chemotherapy. In fact, the AVLT, the California Verbal Learning Test (CVLT), and the Hopkins Verbal Learning Test – Revised (HVLT-R) are equivalent to the memory tests and are similar in structure [25], but the HVLT-R is shorter. There is no standard procedure for selection of a scale in cognitive function research; HVLT and AVLT are both commonly used in published literature. The AVLT is easier to use and a revised version for Chinese has been created. The results of AVLT3 and CDT after acupuncture were significantly higher than those before acupuncture treatment. Given that AVLT reflects word memory function and CDT reflects executive function and visual space structure ability, we speculate that acupuncture can significantly improve memory and executive function.
In further analysis, the type of surgical technique did not affect the overall quality of life and sexual satisfaction [26]. Problems with memory and attention are not directly correlated with surgical adverse effects [26], but breast reconstruction has a negative effect on the cognitive function of breast cancer survivors [27]. In this study, we selected premenopausal patients to exclude the effect of hormonal changes at menopause, with an average age of 43 years. A mastectomy was done in 50% of patients. However, according to the current Chinese national data, breast reconstruction occurs in less than 1% of the all breast cancers patients. Thus, regrettably, no analysis on this can occur because of the lack of data.
BDNF in peripheral serum can reflect the level of central BDNF [28]. BDNF is related to cognition [29, 30]. After acupuncture treatment, BDNF significantly changes and correlates positively with improved cognitive function. We speculate that acupuncture relieves “chemotherapy brain” through a mechanism mediated by BDNF. Acupuncture treatment may promote the physiological formation of BDNF, increasing levels of BDNF, which, in turn, accelerates recovery of the central nervous system from chemotherapy damage [19] affecting attention, memory and executive function, and other cognitive tasks. We found acupuncture effective in reducing CRCI without any report of adverse effects. Acupuncture treatment may also provide other benefits, such as improved mood [31], reduced pain [32], and improved quality of life; thus, it warrants further study as part of cancer patient care.
There are some limitations to our research. First, the small sample size is not sufficient to provide strong support to the conclusion and further studies are warranted in multicenter, large-sample trials. Second, patients willing to participate in studies like this tend to be positive, and thus participants may represent a biased sample. Third, subjective and memory-learning effects occur when using cognitive function scales, and more convincing results can be achieved if combined with neuroimaging technology. In addition, there was no follow-up observation. The effect of chemotherapy-related cognitive dysfunction is obvious in the short term [33,34], especially within 6 months. Subjective complaints of cognitive impairment are most often reported 1 month after chemotherapy; some are reported perennially, while others partially recover. Many factors affect the rehabilitation of cognitive impairment after chemotherapy [35]. This study focused on short-term chemotherapy-related cognition impairment; if an appropriate experimental control design method can be found, long-term cognitive effects warrant further study.
Conclusions
Acupuncture therapy is effective in the treatment of chemotherapy-related cognitive impairment in breast cancer patients through a mechanism that may be related to the observed concomitant increase of BDNF.
Footnotes
Source of support: Departmental sources
References
- 1.Pullens MJ, De Vries J, Van Warmerdam LJ, et al. Chemotherapy and cognitive complaints in women with breast cancer. Psychooncology. 2013;22:1783–89. doi: 10.1002/pon.3214. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence JA, Griffin L, Balcueva EP, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv. 2016;10:176–84. doi: 10.1007/s11764-015-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JH, Bae SH, Jung YS, Jung YM. Prevalence and characteristics of chemotherapy-related cognitive impairment in patients with breast cancer. J Korean Acad Nurs. 2015;45:118–28. doi: 10.4040/jkan.2015.45.1.118. [DOI] [PubMed] [Google Scholar]
- 4.Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol. 2016;35(5):506–14. doi: 10.1200/JCO.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia Y, Zhang X, Yu J, et al. Acupuncture for patients with mild to moderate Alzheimer’s disease: A randomized controlled trial. BMC Complement Altern Med. 2017;17(1):556. doi: 10.1186/s12906-017-2064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, Wan Y, Huang J, Xu F. Clinical analysis of electroacupuncture and multiple acupoint stimulation in relieving cancer pain in patients with advanced hepatocellular carcinoma. J Cancer Res Ther. 2018;14(1):99–102. doi: 10.4103/jcrt.JCRT_736_17. [DOI] [PubMed] [Google Scholar]
- 7.Pan JX, Chen YP, Wei NN. Improvement of upper limb and hand functions of stroke patients by balancing acupuncture combined with motor relearning training. Zhen Ci Yan Jiu. 2018;43(2):123–26. doi: 10.13702/j.1000-0607.170484. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SK, Gao WB, Liu Y, He H. [Therapeutic effect of cervical Jiaji electroacupuncture on postoperative intractable hiccup of liver neoplasms]. Zhonghua Zhong Liu Za Zhi. 2018;40(2):138–40. doi: 10.3760/cma.j.issn.0253-3766.2018.02.011. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 9.Deng G, Cassileth BR. Complementary therapies in pain management. In: Cherny N, Fallon M, Kaasa S, et al., editors. Oxford Textbook of Palliative Medicine. 5th ed. Oxford: Oxford University Press; 2015. pp. 628–31. [Google Scholar]
- 10.Cassileth BR, editor. Acupuncture. Singapore: World Scientific Publishing Co., Ptv., Ltd; 2011. The complete guide to complementary therapies in cancer care: Essential information for patients, survivors and health professionals; pp. 5–12. [Google Scholar]
- 11.Chien TJ, Liu CY, Hsu CH. Integrating acupuncture into cancer care. J Tradit Complement Med. 2013;3:234–39. doi: 10.4103/2225-4110.119733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Regan D, Filshie J. Acupuncture and cancer. Auton Neurosci. 2010;157:96–100. doi: 10.1016/j.autneu.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Liang P, Zhao Z, et al. Acupuncture modulates resting state hippocampal functional connectivity in Alzheimer disease. PLoS One. 2014;9(3):e91160. doi: 10.1371/journal.pone.0091160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutalangka C, Wattanathorn J, Muchimapura S, et al. Laser acupuncture improves memory impairment in an animal model of Alzheimer’s disease. J Acupunct Meridian Stud. 2013;6(5):247–51. doi: 10.1016/j.jams.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Lin R, Chen J, Li X, et al. Electroacupuncture at the Baihui acupoint alleviates cognitive impairment and exerts neuroprotective effects by modulating the expression and processing of brain-derived neurotrophic factor in APP/PS1 transgenic mice. Mol Med Rep. 2016;13(2):1611–17. doi: 10.3892/mmr.2015.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng H, Yu J, Jiang Z, et al. Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neurosci Lett. 2008;432(2):111–16. doi: 10.1016/j.neulet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Jia Y, Yan D, et al. Aging-related changes of triose phosphate isomerase in hippocampus of senescence accelerated mouse and the intervention of acupuncture. Neurosci Lett. 2013;542:59–64. doi: 10.1016/j.neulet.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Nie K, Zhang XZ, Zhao L, et al. [Effect of acupuncture on transmembrane signal pathway in AD mice: an analysis based on lipid-raft proteomics]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34(8):991–96. [in Chinese] [PubMed] [Google Scholar]
- 19.Wang X, Ju S, Chen S, et al. Effect of electro-acupuncture on neuroplasticity of spinal cord-transected rats. Med Sci Monit. 2017;23:4241–51. doi: 10.12659/MSM.903056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macpherson H. Pragmatic clinical trials. Complement Ther Med. 2004;12(2–3):136–40. doi: 10.1016/j.ctim.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 21.Yong-Zhou W. [Placebo effect of acupuncture: A critique and reflection on methodology of clinical research]. Zhongguo Zhen Jiu. 2012;32(8):731–35. [in Chinese] [PubMed] [Google Scholar]
- 22.Xiangjun L, Rui L. Challenges of “acupuncture and moxibustion is only a placebo” – based on the comparative study of domestic and foreign acupuncture literatures. Tianjin Traditional Chinese Medicine. 2013;30(10):632–36. [Google Scholar]
- 23.He W, Tong Y, Zhao Y, et al. Review of controlled clinical trials on acupuncture versus sham acupuncture in Germany. J Traditi Chin Med. 2013;33(3):403–7. doi: 10.1016/s0254-6272(13)60187-9. [DOI] [PubMed] [Google Scholar]
- 24.Cheung YT, Lim SR, Shwe M, et al. Psychometric properties and measurement equivalence of the English and Chinese versions of the functional assessment of cancer therapy-cognitive in Asian patients with breast cancer. Value Health. 2013;16(6):1001–13. doi: 10.1016/j.jval.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Guo QH. Neuropsychologicalassessment. 2nd ed. Shanghai science and Technology Press; 2016. p. 104. [Google Scholar]
- 26.Słowik AJ, Jabłoński MJ, Michałowska-Kaczmarczyk AM, Jach R. Evaluation of quality of life in women with breast cancer, with particular emphasis on sexual satisfaction, future perspectives and body image, depending on the method of surgery. Psychiatr Pol. 2017;51(5):871–88. doi: 10.12740/PP/OnlineFirst/63787. [DOI] [PubMed] [Google Scholar]
- 27.Penha TR, Botter B, Heuts EM, et al. Quality of life in patients with breast cancer-related lymphedema and reconstructive breast surgery. J Reconstr Microsurg. 2016;32(6):484–90. doi: 10.1055/s-0036-1572538. [DOI] [PubMed] [Google Scholar]
- 28.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–64. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- 29.Tu F, Pang Q, Huang T, et al. Apigenin ameliorates post-stroke cognitive deficits in rats through histone acetylation-mediated neurochemical alterations. Med Sci Monit. 2017;23:4004–13. doi: 10.12659/MSM.902770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azimi M, Gharakhanlou R, Naghdi N, et al. Moderate treadmill exercise ameliorates amyloid-β-induced learning and memory impairment, possibly via increasing AMPK activity and up-regulation of the PGC-1α/FNDC5/BDNF pathway. Peptides. 2018;102:78–88. doi: 10.1016/j.peptides.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Luo D, Ma R, Wu Y, et al. Mechanism underlying acupuncture-ameliorated depressive behaviors by enhancing glial glutamate transporter in chronic unpredictable mild stress (CUMS) Rats. Med Sci Monit. 2017;23:3080–87. doi: 10.12659/MSM.902549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y, Liu Y, May BH, et al. Effectiveness of acupuncture for cancer pain: Protocol for an umbrella review and meta-analyses of controlled trials. BMJ Open. 2017;7(12):e018494. doi: 10.1136/bmjopen-2017-018494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pendergrass JC, Targum SD, Harrison JE. Cognitive impairment associated with cancer: a brief review. Innov Clin Neurosci. 2018;15(1–2):36–44. [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstein LJ, McCreath GA, Komeylian Z, Rich JB. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: A multilevel meta-analysis. Neurosci Biobehav Rev. 2017;83:417–28. doi: 10.1016/j.neubiorev.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Vitali M, Ripamonti CI, Roila F, et al. Cognitive impairment and chemotherapy: A brief overview. Crit Rev Oncol Hematol. 2017;118:7–14. doi: 10.1016/j.critrevonc.2017.08.001. [DOI] [PubMed] [Google Scholar]