Abstract
Background
The aim of this study was to assess the efficacy and safety of acupuncture therapy for patients with hypertension.
Material/Methods
We searched PubMed, Embase, the Cochrane Library, the Chinese Biomedical Literature Database, the Chinese National Knowledge Infrastructure, and the Wan-fang Data Database from inception through 29 April 2017. Randomized controlled trials investigating acupuncture therapy for hypertension were included. Review Manager 5.3 software was used for the data analysis.
Results
A total of 30 RCTs involving 2107 patients were included. The overall methodological quality of the included studies was low. Pooled results demonstrate that acupuncture plus anti-hypertensive drugs is better than anti-hypertensive drugs alone at reducing systolic and diastolic blood pressure (SBP and DBP). The same result was observed for pooled data from experiments that compared acupuncture plus medication to sham acupuncture plus medication at reducing SBP and DBP. However, studies reveal that using acupuncture alone or anti-hypertensive drugs alone do not differ in the effect on lowering blood pressure. Similarly, acupuncture alone also did not differ from sham acupuncture alone, and electroacupuncture versus anti-hypertensive drugs was not significantly different at reducing SBP and DBP.
Conclusions
Our systematic review indicates there is inadequate high quality evidence that acupuncture therapy is useful in treating hypertension, as the exact effect and safety of acupuncture therapy for hypertension is still unclear. Therefore, research with larger sample sizes and higher-quality RCTs is still needed.
MeSH Keywords: Acupuncture, Essential Hypertension, Meta-Analysis, Randomized Controlled Trial
Background
Essential hypertension is a major risk factor for cardiovascular disease and stroke [1]. The number of adults with hypertension worldwide is predicted to reach 1.56 billion individuals by 2025 [2]. In China, hypertension affects more than one-fourth of the population. Moreover, the prevalence of hypertension has increased during recent decades and it has become a major health problem because treatment awareness and hypertension control rates are extremely low [3].
The basic treatment for hypertension is non-pharmacological therapy, and includes weight loss, restricted sodium intake, physical activity, and cessation of smoking and alcohol consumption. However, long-term compliance with non-pharmacological treatment is difficult for most patients. Therefore, anti-hypertensive drugs are the preferred option for treating hypertension [4]. However, they are associated with adverse effects such as drug resistance. Therefore, more effective and safe treatment options are urgently required for hypertension patients.
Acupuncture treatment is an ancient Chinese therapy that has played an important role for over 2500 years in the Chinese healthcare system and has now been adopted worldwide. Several systematic reviews have evaluated the efficacy of acupuncture for hypertension [5–11]. Recently, some new trials have been published, leading us to conduct a systematic review and meta-analysis of all available randomized controlled trials (RCTs), to evaluate the efficacy and safety of acupuncture therapy for treating essential hypertension.
Material and Methods
Systematic review details
This systematic review was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions, and was reported in compliance with the PRISMA statement (see Supplementary Table 1 for PRISMA checklist) [12]. This systematic review has been registered (Reg. No. CRD 42017068032) in PROSPERO (https://www.crd.york.ac.uk/prospero/) [13].
Study search strategy
We systematically searched the PUBMED, EMBASE, the Cochrane Library, the Chinese Biomedical Literature Database (CBM), the Chinese National Knowledge Infrastructure (CNKI), and the Wan-fang databases for inclusion on 29 April 2017 with MeSH terms and key words, and without language restrictions. Search strategy terms were (acupuncture OR electroacupuncture OR acupoint) AND (high blood pressure OR hypertension OR blood pressure) AND (randomized controlled trial OR controlled clinical trial OR randomized OR clinical trials). We also checked the reference lists of relevant reviews and the included trials to identify further studies that met the inclusion criteria for this meta-analysis.
Inclusion criteria
Types of trials
We included randomized controlled trials (RCTs) and quasi-randomized controlled trials (quasi-RCTs) that were published in formal English or Chinese journals.
Types of participants
Based on the International Society of Hypertension Guidelines for the Management of Hypertension (1999 World Health Organization) [14], essential hypertensive patients were those with a systolic blood pressure (SBP) ≥140 mmHg and/or a diastolic blood pressure (DBP) of ≥90 mmHg. All patients with secondary hypertension caused by an identifiable underlying primary cause were all excluded.
Types of interventions
Acupuncture therapy included acupuncture or electroacupuncture with or without lifestyle modifications and/or anti-hypertensive drugs. Control groups received sham acupuncture without any treatment or lifestyle modifications or anti-hypertensive drugs.
Types of outcome measures
Primary outcomes included SBP and DBP changes [pre-treatment BP – post-treatment BP]. Secondary outcomes included the efficacy rates and the adverse events. Efficacy rates were reported as the percentage of the total number of participants that showed reduction of DBP by ≥10 mmHg, or normal DBP (≤90 mmHg), or reduction of SBP by ≥30 mmHg.
Data extraction
Two reviewers (HC and FES) screened all the literature and extracted data independently using a standardized form. The form was pre-designed for collecting information on trial characteristics, including first author, language, number of patients, mean age of the patients, diagnostic criteria, grades of hypertension, acupuncture treatment, control types, sessions of treatment, treatment course, and outcome measures. We defined the change values of blood pressure as pre-treatment BP minus post-treatment BP and extracted the change means and standard deviations (SD) for continuous outcome. For dichotomous outcome measures, we used rates (the number of events out of total number in the study). If change means and standard deviations were missing, we calculated them according to the formula offered by the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.10) (see Supplementary Figure 1 for formula). Disagreements were resolved in consultation with the third reviewer (YHG).
Assessment of the reporting quality of the included studies
Overall reporting quality score was evaluated for 30 parameters (items 1–4, 6–19) of the Consolidated Standards of Reporting Trials (CONSORT) [15]. The discussion section (items 20–22) was excluded because the items under this section could not be rated. We also excluded the section on other information (items 23–25) because they were not relevant for the methodology of the included studies. The Standards for Reporting Interventions in Controlled Trials of Acupuncture (STRICTA) includes 17 items that are substituted for item 5, ‘interventions’ in the CONSORT checklist [16]. Two reviewers (XDT and WBJ) assessed each item for the included studies independently. Each reported item received 1 point, and any item not clearly presented received 0 points. Disagreements were resolved in consultation with the third reviewer (HC).
Risk of bias assessment
Two reviewers (XDT and WBJ) assessed the risk of bias of the included RCTs using the Cochrane Collaboration’s tool for assessing risk of bias [17]. Each trial was scored as high, low, or unclear risk for the following 7 domains: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias); and (7) any other bias. Disagreements were resolved in consultation with the third reviewer (HC).
Statistical analysis
The overall reporting quality of the included studies and the potential differences between the studies from the Chinese journals and English journals were investigated in compliance with the CONSORT and STRICTA statements. The overall scores of the CONSORT and the STRICTA are presented as medians and ranges, and data from each individual item are presented as frequencies. The difference between overall scores of different journals was assessed by the Mann-Whitney U test. Pearson’s chi-square test was used when the sample size was more than 40 and Fisher’s exact test was used when sample size was less than 40 for assessing the reporting difference of each individual item between the different journals. Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) V.19.0 (SPSS Inc, Chicago, Illinois, USA).
Meta-analyses for acupuncture and electroacupuncture were done separately. Continuous data are presented as mean differences (MDs) with 95% confidence interval (CI) and data from studies were pooled using the inverse variance method. Dichotomous data are presented as relative risk (RR) with 95% CI and pooled using Mantel-Haenszel method. We also calculated the required information size based on the standard method [18,19]. Statistical heterogeneity across trials was assessed by the Cochran Q test (P<0.1 for statistical significance) and quantified by the I2 statistic. Following the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.10), we defined I2 >50% as indicating significant heterogeneity. Heterogeneous data were pooled using the random-effects model. We performed subgroup analysis based on the classes of anti-hypertensive drugs such as calcium channel blockers (CCB), β-receptor antagonists, angiotensin-converting enzyme inhibitors (ACEI), and angiotensin receptor blockers (ARB). Moreover, in order to establish robust primary outcomes, we also performed sensitivity analysis for the primary outcomes. Publication bias was evaluated by visually inspecting a funnel plot. Meta-analysis was performed using RevMan 5.3 software.
Results
Study selection
Figure 1 provides a flow chart summarizing the study selection process based on PRISMA guidelines. The initial search yielded 1146 records. After removing duplicate records, screening the titles and abstracts, and doing full text reviews, 30 trials were included in the meta-analysis [20–49].
Figure 1.
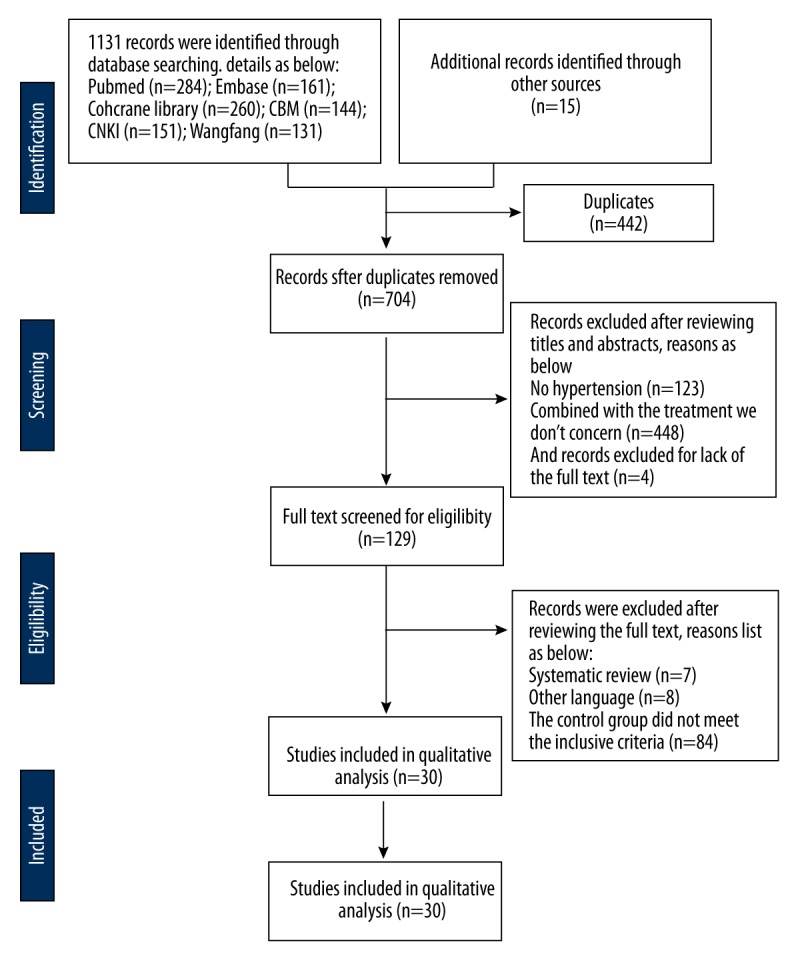
Flow chart of randomized controlled trial selection (based on PRISMA).
Characteristics of the included studies
Characteristics of the included trials are summarized in Table 1. The 28 RCTs and 2 qusi-RCTs evaluated a total of 2107 patients (range: 28 to 160 patients per trial) and were published between 2000 and 2016. Six of the included studies were published in English [25–27,29,30,49] and the remaining 24 studies were published in Chinese [20–24,28,31–42,47,48]. Four studies reported adverse effects [22,26,40,41], 5 studies reported no adverse effects [25,27,29,30,38], and the remaining 21 studies did not report any information on adverse effects. Twenty-eight of the 30 studies defined the criteria for hypertension as a systolic blood pressure (SBP) ≥140mmHg or diastolic blood pressure (DBP) ≥90 mmHg [22–29,31–48]. Liu et al. [30] included hypertension patients with SBP ranging from 120–159 mmHg or DBP ranging from 80–99 mmHg. Yin et al. [49] included patients with SBP ranging from 120 to 180 mmHg or DBP 80 to 100 mmHg.
Table 1.
Characteristics of included studies.
| Study ID | Language | Mean age (T/C) | Gender (Male/Female) | *Included criteria | Hypertension grades | Intervention | No. of patients evaluated | Course | #Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | ||||||||
| Chen BG et al., 2006 | Chinese | 54.75±7.12/51.7210.38 | 41/19 | 1 | 1, 2 | AC (30 mins a day) | Metroprolol (100 mg per day) | 30 | 30 | 4 weeks | 1, 2, 3 |
| Chen J et al., 2010 | Chinese | 48.2±7.2/50.5± 8.4 | 31/29 | 1 | 1 | AC (30 mins a day) plus felodipine (5 mg per day) | Felodipine (5 mg per day) | 30 | 30 | 15 days | 3 |
| Chen NY et al., 2010 | Chinese | 61.3±8.0/62.0± 7.1 | 41/39 | 1 | Not reported | AC (30 mins a day) | Diovan (80 mg per day) | 40 | 40 | 30 days | 1, 3, 4 |
| Chen Q et al., 2011 | Chinese | 59±8/59± 8 | 29/31 | 1 | 1, 2 | AC (30 mins a day) | Metroprolol (100 mg per day) | 30 | 30 | 30 days | 3 |
| Chen YF et al., 2000 | Chinese | 63.57±8.08/65.20± 8.86 | 38/32 | 1 | 2 | AC (30 mins a day) | Nifedipine (10–20 mg tid) | 35 | 35 | 2 weeks | 1, 3 |
| Choi WJ et al., 2015 | English | 48.04±6.13/46.20±9.26 | Not reported | 1 | Not reported | AC (20 mins every treatment, 4 times totally) | SA | 25 | 25 | 2 weeks | 2, 4 |
| Cui JK et al., 2013 | English | 56.7±8.9/54.7±8.1 | 55/37 | 1 | Not reported | AC (once a day except Sunday) plus irbesartan | Irbesartan (150 mg per day) | 46 | 46 | 4 weeks | 3, 4 |
| Flachskampf FA et al., 2007 | English | 58.8±8.2/58.0±7.9 | 66/74 | 1 | 1, 2 | AC (30 mins; 22 sessions) | SA | 83 | 77 | 6 weeks | 1, 2, 4 |
| Huang F et al., 2007 | Chinese | 56.51±6.28/58.12±6.15 | 27/33 | 1 | 1, 2 | AC (30 mins a day) plus captopril (25 mg tid) | Captopril (25 mg tid) | 30 | 30 | 4 weeks | 1, 3 |
| Kim HM et al., 2012 | English | 52.08±8.69/52.38±10.3 | 16/12 | 1 | 1 | AC (20 mins, twice a week) | SA | 12 | 16 | 8 weeks | 1, 2 |
| Liu Y et al., 2015 | English | 49.4±8.4/53.4±8.2 | 7/24 | 2 | 1,2 | AC (30 mins, twice a week) | No treatment | 15 | 15 | 8 weeks | 2 |
| Luo H et al., 2015 | Chinese | 45–75 (range) | 66/34 | 1 | 2 | AC (30 mins a day) plus felodipine (5mg) | Felodipine (5 mg) | 44 | 46 | 20 days | 1, 3 |
| Ma ZY et al., 2011 | Chinese | 66.39±5.47/64.58±7.13 | 47/33 | 1 | 1,2 | EA (10 mins a day) | Nicardipine (20 mg tid) | 40 | 40 | 15 days | 1, 3 |
| Shen ZK et al., 2007 | Chinese | 32±8.24/21±7.31 | 31/19 | 1 | Not reported | AC (30 mins a day) plus nifedipine (20 mg bid) | Nifedipine (20mg bid) | 25 | 25 | 20 days | 1, 2, 3 |
| Sun J et al., 2009 | Chinese | 47.23±5.66/48.42±6.13 | 48/39 | 1 | 1 | AC (30 mins a day) | lifestyle | 44 | 43 | Not reported | 2 |
| Tian L et al., 2008 | Chinese | 59.17±3.16/59±3.01 | 33/27 | 1 | 1,2 | AC (30 mins a day) | Levamlodipine (2.5 mg a day) | 30 | 30 | 30 days | 1, 2, 3 |
| Yin C et al., 2007 | English | 52/54 | 9/21 | 3 | 1,2 | AC plus antihypertensive | SA plus antihypertensive | 15 | 15 | 8 weeks | 1 |
| Wan WJ et al., 2009 | Chinese | 63.72±8.23/65.24±6.41 | 36/24 | 1 | 1,2 | AC (10mins a day) | nicardipine (20 mg tid) | 30 | 30 | 15 days | 1,3 |
| Wang C et al., 2006 | Chinese | 25–60(range) | 34/25 | 1 | Not reported | EA (30 mins a day) | Lotensin (10 mg a day) | 30 | 29 | 8 weeks | 1,3 |
| Wu XM et al., 2015 | Chinese | 49. 10±8. 7/48. 08±8. 8 | 52/47 | 1 | 1,2 | AC (10 mins a day) | lifestyle | 50 | 49 | 4 weeks | 1, 2, 3 |
| Wu YR et al., 2011 | Chinese | 54.75±7.10/51.72±10.3 | 70/50 | 1 | 1,2 | AC (30 mins a day) | Metroprolol (100 mg a day) | 60 | 60 | 20 days | 1, 3 |
| Xie B et al., 2014 | Chinese | 56±11/53±10 | 30/30 | 1 | Not reported | AC (30 mins a day) | Captopril (25 mg tid) | 30 | 30 | 3 weeks | 1, 3 |
| Xing H et al., 2016 | Chinese | 61.83±9.10/57.14±9.33 | 35/28 | 1 | 1,2 | AC (30 mins a day) | Captopril (25 mg tid) | 31 | 32 | 4 weeks | 1, 3 |
| Yang DH et al., 2010 | Chinese | 40.4±5.2/41.7±4.2 | 37/23 | 1 | 1,2 | EA (30 mins a day) | Captopril (25 mg tid) | 30 | 30 | 2 weeks | 1, 2, 3 |
| Zhao DJ et al., 2003 | Chinese | 40.3±11.4/46.1±14.2 | 37/23 | 1 | 1,2 | AC plus lifesytle | Lifestyle | 30 | 30 | 40 days | 1 |
| Zhang Y et al., 2012 | Chinese | 42–46 (range) | Not reported | 1 | Not reported | AC (30 mins a day) | Captopril (25 mg tid) | 14 | 14 | 8 weeks | 1 |
| Zhang YB et al., 2011 | Chinese | 53.62±9.83/52.16±10.04 | 53/27 | 1 | Not reported | AC (20 mins a day) | Amlodipine (2.5 mg a day) | 45 | 35 | 4 weeks | 1, 3 |
| Zhang YL et al., 2005 | Chinese | 63.60±8.20/65.20±8.00 | 47/28 | 1 | Not reported | AC (30 mins a day) plus nifedipine (10mg tid) | Nifedipine (10 mg tid) | 45 | 30 | 20 days | 1, 3 |
| Zhang ZH et al., 2004 | Chinese | 56.5/55.5 | 42/18 | 1 | 1,2 | AC (30 mins a day) | Compounds of Reserpine and Hydrochlorothiazidec | 30 | 30 | 15 days | 3 |
| Zheng Y et al., 2016 | English | 56.53±7.52/56.73±4.91 | 8/22 | 1 | 1,2 | AC (30 mins a day except weekends) | SA | 15 | 15 | 2 weeks | 1 |
T – treatment; C – control; mins – minutes; AC – acupuncture; SA – sham acupuncture; EA – electro acupuncture; SBP – systolic blood pressure; DBP – diastolic blood pressure.
Included criteria: (1) SBP: ≥140 mmHg or DBP: ≥90 mmHg; (2) SBP: 120–159 mmHg or DBP: 80–99 mmHg; (3) SBP: 120–180 mmHg and DBP: 80–100 mmHg
Outcomes: (1) Blood pressure after intervention; (2) Changes in magnitude of blood pressure after intervention; (3) Efficacy rate; (4) Adverse effects.
We analyzed the therapeutic outcomes of acupuncture or electroacupuncture with or without lifestyle modifications or anti-hypertensive drugs. The 5 most frequently used acu-points were LI11 (quchi; 18 studies), LR3 (taichong; 15 studies), GB20 (fengchi; 12 studies), ST36 (zusanli; 9 studies), and DU20 (baihui; 7 studies). Control groups included untreated patients and patients undergoing treatment by lifestyle modifications or anti-hypertensive drugs. The median course of the included studies was 28 days (range: 14–56).
Reporting quality of the included studies
We evaluated the reporting quality of the included studies according to CONSORT and STRICTA guidelines. The CONSORT median quality score was 10 (range: 4–29, Table 2), Based on the CONSORT, Chinese journals (median score: 10; range: 6–17) and English journals (median score: 9; range: 4–29) had similar reporting quality (P=0.875). However, consideration of individual items shows that the quality of English journals is better than Chinese journals for reporting items 1a, 2b, 8b, 9, 11a, 13b, 15, and 19 (all P<0.05; Table 3).
Table 2.
Overall score of the CONSORT and STRICTA reporting quality of the included studies (N=30; 24 studies from Chinese journals; 6 studies from English journals).
| Journals | CONSORT Median (range) |
STRICTA Median (range) |
|---|---|---|
| Chinese journals (N=24) | 10 (6–17) | 11 (7–12) |
| English journals (N=6) | 9 (4–29) | 12 (11–17)* |
| All journals (N=30) | 10 (4–29) | 11 (7–17) |
Indicate a significant difference with the studies published in Chinese journals.
Table 3.
CONSORT assessments of the reporting characteristics of the included studies (N=30; 24 studies from Chinese journals; 6 studies from English journals).
| Section/ topic | Item | Description | Positive studies | Positive Chinese journals | Positive English journals |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Title and Abstract | 1a | Identifying randomized trial in the title | 4 (13%) | 0 (0) | 4 (67%)* |
| 1b | Structured summary of trial design, methods, results, and conclusions; for specific guidance see CONSORT for Abstracts | 28 (93) | 22 (92) | 6 (100) | |
| Background and objectives | 2a | Scientific background and explanation of rationale | 23 (77) | 17 (71) | 6 (100) |
| 2b | Specific objectives or hypotheses | 12 (40) | 6 (25) | 6 (100)* | |
| Trial design | 3a | Description of trial design (e.g., parallel, factorial) including allocation ratio | 29 (97) | 23 (96) | 6 (100) |
| 3b | Important changes to methods after trial commencement (e.g. eligibility criteria), with reasons | 0 | 0 (0) | 0 (0) | |
| Participants | 4a | Eligibility criteria for participants | 29 (97%) | 23 (96) | 6 (100) |
| 4b | Settings and locations where the data were collected | 24 (80%) | 18 (75) | 6 (100) | |
| Outcomes | 6a | Definition of pre-specified primary and secondary outcome measures, including how and when they were assessed | 28 (93) | 22 (92) | 6 (100) |
| 6b | Reasoning of any changes to trial outcomes after the trial commenced | 0 (0) | 0 (0) | 0 (0) | |
| Sample size | 7a | Protocol of determining sample size | 1 (3) | 0 (0) | 1 (17) |
| 7b | Explanation of any interim analyses and stopping guidelines, whenever applicable | 0 (0) | 0 (0) | 0 (0) | |
| Sequence generation | 8a | Method used to generate the random allocation sequence | 21 (70) | 18 (75) | 3 (50) |
| 8b | Type of randomization and details of any restrictions (e.g., blocking and block size) | 3 (10) | 0 (0) | 3 (50)* | |
| Allocation concealment | 9 | Mechanism used to implement the random allocation sequence (e.g., sequentially numbered containers) and description of any steps taken to conceal the sequence until interventions were assigned | 4 (13) | 0 (0) | 4 (67)* |
| Implementation | 10 | Individuals that generated the random allocation sequence, enrolled participants, and assigned participants to interventions | 1 (3) | 0 (0) | 1 (17) |
| Blinding | 11a | The group that was blinded after assignment to interventions (e.g. participants, care providers, those assessing outcomes) and the protocol of blinding, if performed | 6 (20) | 0 (0) | 6 (100)* |
| 11b | If relevant, description of the similarity of interventions | 0 (0) | 0 (0) | 0 (0) | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 23 (77) | 17 (71) | 6 (100) |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 3 (10) | 1 (4) | 2 (33) | |
| Participant flow (A diagram is strongly recommended) | 13a | The number of participants that were randomly assigned, received intended treatment, and were analyzed for the primary outcome are shown for each group | 3 (10) | 1 (4) | 2 (33) |
| 13b | The number of participants that were lost or excluded after randomization and the reasons | 2 (13) | 0 (0) | 2 (33)* | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 19 (63) | 15 (63) | 4 (67) |
| 14b | Reasons for ending or stopping the trial | 0 (0) | 0 (0) | 0 (0) | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 14 (47) | 8 (33) | 6 (100)* |
| Numbers analyzed | 16 | For each group, the number of participants (denominator) included in each analysis and if the analysis was performed as originally assigned | 3 (10) | 1 (4%) | 2 (33) |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (e.g., 95% confidence interval) | 29 (97) | 23 (96) | 6 (100) |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | 30 (100) | 24 (100) | 6 (100) | |
| Ancillary analyses | 18 | Results of any other analyses performed, such as subgroup and adjusted analyses; distinguishing pre-specified from exploratory analyses | 0 (0) | 0 (0) | 0 (0) |
| Harms | 19 | All important harms or unintended adverse effects in each group; for specific guidance see CONSORT for Harms# | 8 (27) | 3 (13) | 5 (83)* |
Indicate a significant difference with the studies published in Chinese journals;
CONSORT for harms can be seen on http://www.consort-statement.org/checklists/view/32--consort-2010/116-harms.
The STRICTA median score was 11 (range: 7–11, Table 2), and using STRICTA, English journals (median score: 12; range: 11–17) have a better reporting quality (P=0.03) than Chinese journals (median score: 11; range: 7–12). Similarly, the STRICTA report shows that the quality of English journals is better than Chinese journals for items 1b, 1c, 4a, and 6a (all P<0.05; Table 4).
Table 4.
STRICTA assessment of the reporting characteristics of included studies (N=30; 24 studies from Chinese journals; 6 studies from English journals).
| Section/ topic | Item | Description | Positive studies | Positive Chinese journals | Positive English journals |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Acupuncture rationale | 1a | Style of acupuncture (e.g. Traditional Chinese Medicine, Japanese, Korean, Western medical, Five Element, ear acupuncture, etc) | 30 (100) | 24 (100%) | 6 (100%) |
| 1b | Reasoning for treatment provided, historical context, literature sources, and/or consensus methods, with references provided wherever necessary | 9 (30) | 5 (21) | 4 (67)* | |
| 1c | Extent to which treatment varied | 2 (7) | 0 (0) | 2 (33)* | |
| Details of needling | 2a | Number of needle insertions per subject per session (mean and range wherever relevant) | 23 (77) | 20 (83) | 3 (50) |
| 2b | Names (or location if no standard name) of points used (uni/bilateral) | 30 (100) | 24 (24) | 6 (100) | |
| 2c | Depth of insertion, based on a specified unit of measurement or a particular tissue level | 20 (67) | 15 (63) | 5 (83) | |
| 2d | Response sought (e.g. de qi or muscle twitch response) | 23 (77) | 17 (71) | 6 (100) | |
| 2e | Needle stimulation (e.g. manual, electrical) | 29 (97) | 23 (96) | 6 (100) | |
| 2f | Needle retention time | 29 (97) | 23 (96) | 6 (100) | |
| 2g | Needle type (diameter, length, and manufacturer or material) | 22 (73) | 16 (67) | 6 (100) | |
| Treatment regimen | 3a | Number of treatment sessions | 30 (100) | 24 (100) | 6 (100) |
| 3b | Frequency and duration of treatment sessions | 30 (100) | 24 (100) | 6 (100) | |
| Other components of treatment | 4a | Details of other interventions administered to the acupuncture group (e.g. moxibustion, cupping, herbs, exercises, lifestyle modification advice) | 10 (33) | 5 (21) | 5 (83)* |
| 4b | Setting and context of treatment, including instructions to practitioners, and information to patients | 1 (3) | 0 (0) | 1 (17) | |
| Practitioner background | 5 | Description of participating acupuncturists (qualification or professional affiliation, years in acupuncture practice and other relevant experience) | 1 (3) | 0 (0) | 1 (17) |
| Control or comparator interventions | 6a | Rationale for the control or comparator in the context of the research question and sources justifying the choice | 2 (7) | 0 (0) | 2 (33)* |
| 6b | Precise description of the control or comparator. If sham acupuncture or any other type of acupuncture-like control is used, provide details as for Items 1 to 3 above | 28 (93) | 22 (92) | 6 (100) |
Indicates a significant difference with the studies published in Chinese journals.
Risk of bias of the included studies
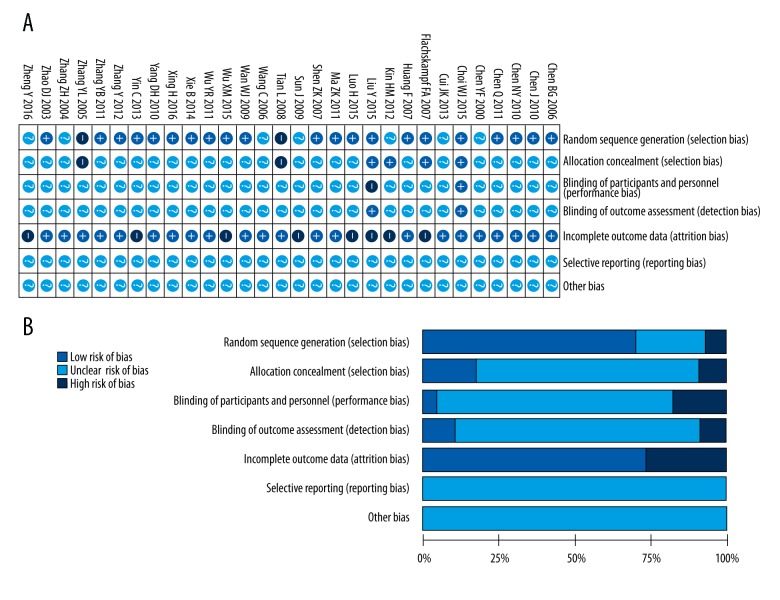
Most included studies had poor methodological quality because they lacked sufficient information to assess special items by the Cochrane risk of bias tool (Figure 2). Two trials were quasi-RCTs [35,45], in which the patients were randomized according to even and odd numbers. Twenty-one trials generated a randomized sequence for patients by using a random number table or software [20–23,25,27,28,30–33,36,38–40,42,44,47–49]. The remaining 7 studies did not indicate how random assignments were made. Appropriate allocation concealment was reported in only 5 trials, all published in English [25,27,29,30,49]. Adequate blinding of patients and the doctors was not reported by any of the 30 trials. Blinding of the outcome assessment was mentioned in only 3 trials [25,30,44]. Only 1 study used intention-to-treat analysis [27]. Eight trials reported dropouts [27,29–31,34,38,41,48]. Selective reporting is unknown for any of the included studies, since we have no access to the study protocol.
Figure 2.
Risk of bias graph of the included trials. (A) Summary of the risk of bias in 7 domains in the 30 RCTs. (B) Graphical representation of the overall risk of bias in the 7 domains. Green, yellow and red represent low, unclear and high risk of bias. Length of the rectangles (green, yellow or red) show the percentage of studies with low, unclear, or high risk of bias for the 7 domains analyzed.
Analyzing the effects of different interventions
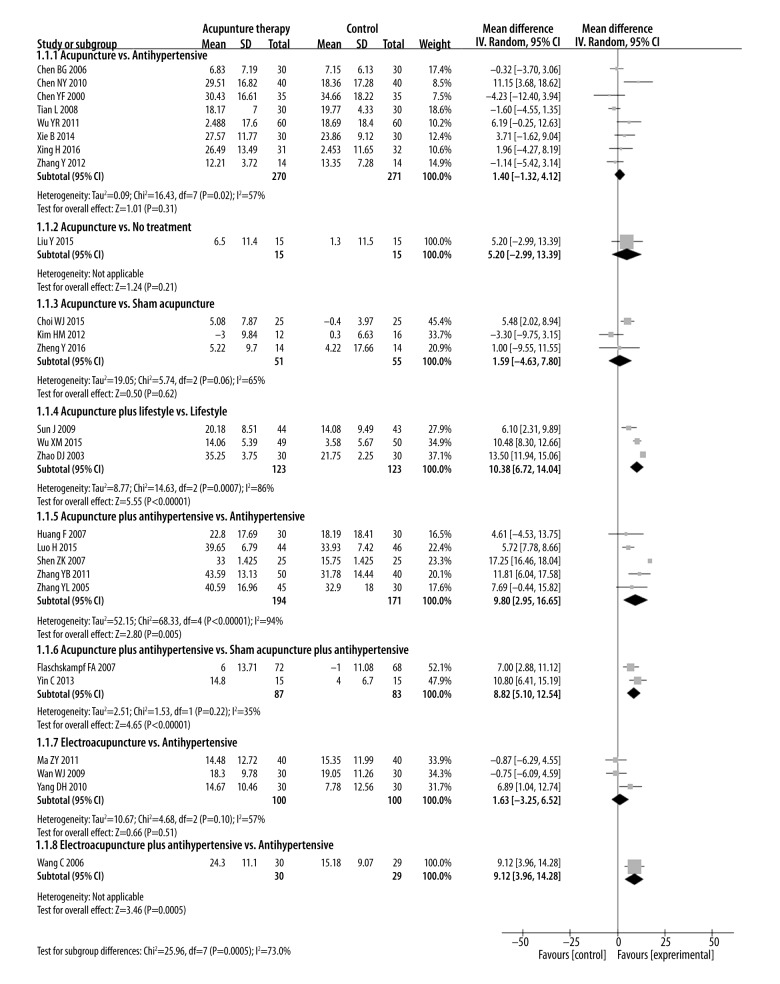
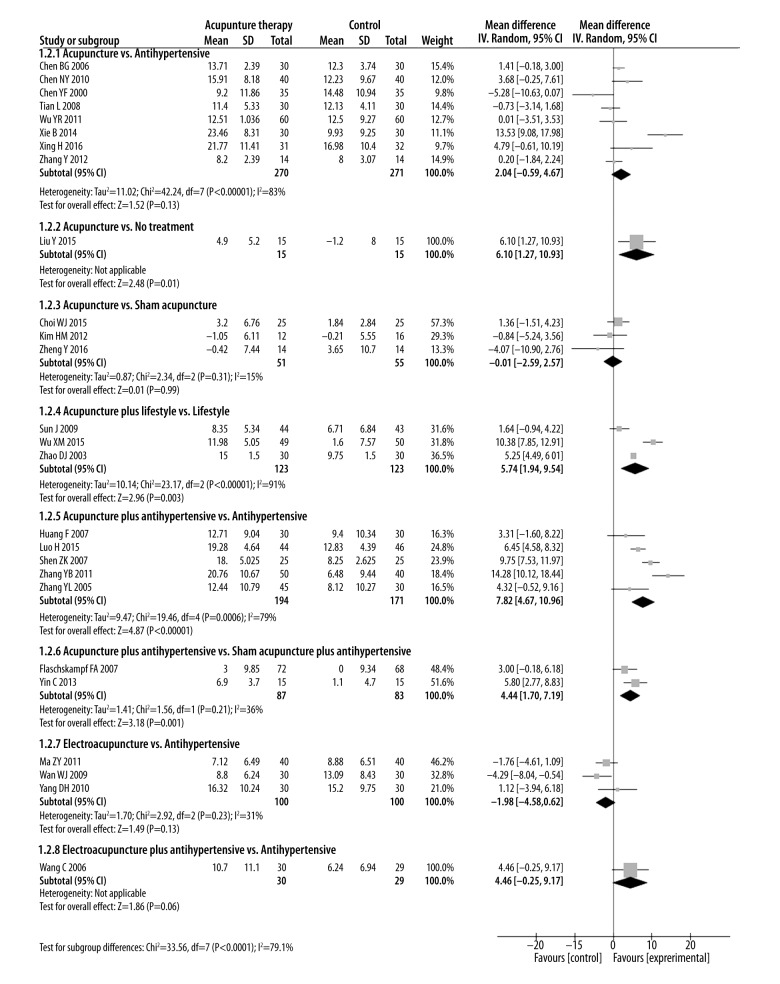
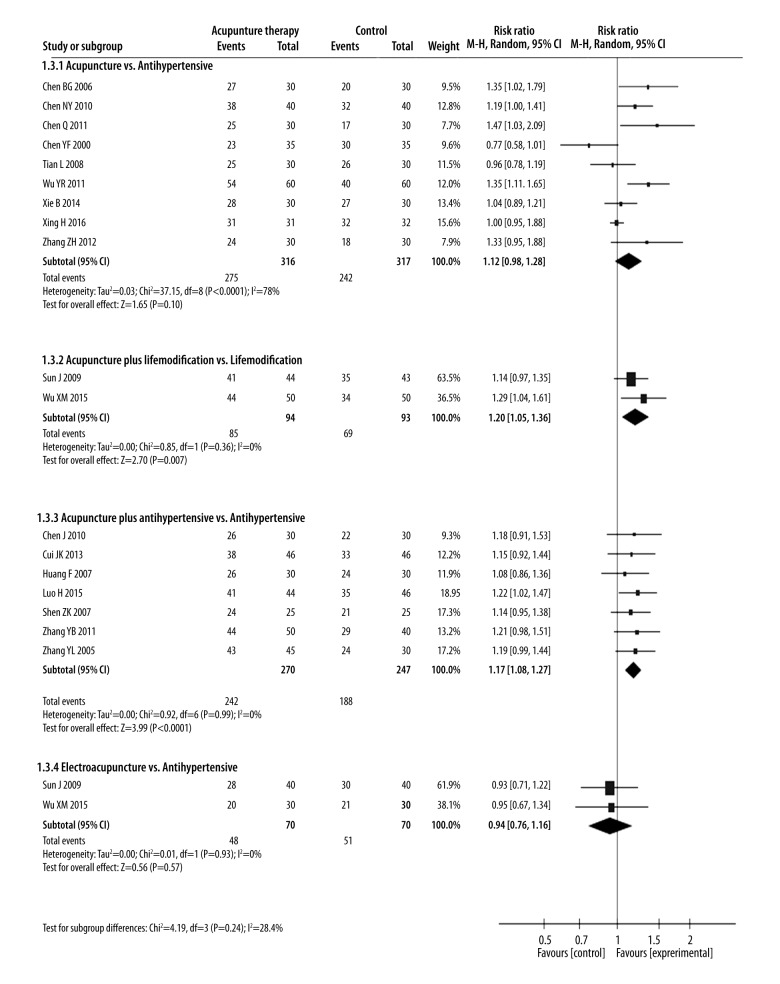
We performed the following 8 comparisons based on different types of interventions evaluated in the included studies: (1) acupuncture vs. anti-hypertensive drugs; (2) acupuncture vs. no treatment; (3) acupuncture vs. sham acupuncture; (4) acupuncture plus lifestyle modifications vs. lifestyle modifications; (5) acupuncture plus anti-hypertensive drugs vs. anti-hypertensive drugs; (6) acupuncture plus anti-hypertensive drugs vs. sham acupuncture plus anti-hypertensive drugs; (7) electroacupuncture vs. anti-hypertensive drugs; and (8) electroacupuncture plus anti-hypertensive drugs vs. anti-hypertensive drugs. The pooled effect estimates for all these comparisons are shown in Figures 3–5.
Figure 3.
Forest plot of SBP magnitude changes in all 30 trials.
Figure 4.
Forest plot of DBP magnitude changes in all 30 trials.
Figure 5.
Forest plot of the efficacy rate of acupuncture therapy in all trials.
1. Acupuncture vs. anti-hypertensive drugs
Eight studies with 541 patients reported SBP and DBP compared acupuncture and anti-hypertensive drug treatments [20,22,24,35,39–41,43]. SBP and DBP changes were similar for acupuncture and anti-hypertensive drug treatments [SBP: MD=1.4 mmHg (95% CI: −1.32 to 4.12), I2=57%; DBP: MD=2.04 mmHg (95% CI: −0.59 to 4.67), I2=83%].
Nine studies with 517 patients reported efficacy rates of acupuncture and anti-hypertensive drug treatments [20,22–24,35,39–41,46]. The efficacy rates of both these treatments were similar [RR=1.12 (95% CI: 0.98 to 1.28), I2=78%].
2. Acupuncture vs. no treatment
Only 1 study with 30 patients reported SBP and DBP in patients treated with acupuncture compared to an untreated control group [30]. No differences were observed between treatments in SBP change [SBP: MD=5.2 mmHg (95% CI: −2.99 to 13.39)]. However, DBP changes were greater in patients treated with acupuncture than in untreated patients [DBP: MD=6.1mmHg (95% CI: 1.27 to 10.93)].
3. Acupuncture vs. sham acupuncture
Three studies with 106 patients reported SBP and DBP for acupuncture versus sham acupuncture treatments [25,48,49]. The SBP and DBP changes were similar for both acupuncture and sham acupuncture treatments [SBP: MD=1.59 mmHg (95% CI: −4.63 to 7.8 mmHg), I2=65%; DBP: MD=−0.01 mmHg (95% CI: −2.59 to 2.57), I2=15%].
4. Acupuncture plus lifestyle modifications vs. lifestyle modifications
Three studies with 246 patients compared acupuncture plus lifestyle modifications and lifestyle modifications alone [34,38,49]. Two of the three studies (n=187) also reported the efficacy rate of acupuncture plus lifestyle medications and lifestyle modifications alone [34,38]. SBP and DBP changes, as well as efficacy rate, were greater in the combined therapy than in lifestyle modifications alone [SBP: MD=10.38 mmHg (95% CI: 6.72 to 14.04), I2=86%; DBP: MD=5.74 mmHg (95% CI: 1.94 to 9.54), I2=91%; RR: 1.2 (95% CI: 1.05 to 1.36), I2=0%]. This suggests a synergy occurs between acupuncture and lifestyle modification.
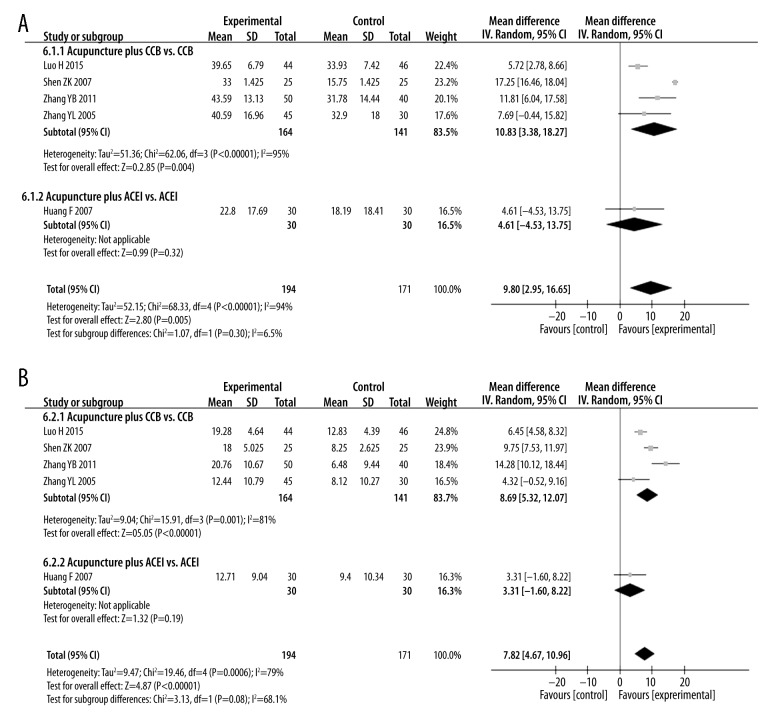
5. Acupuncture plus anti-hypertensive drugs vs. anti-hypertensive drugs
Five studies with 365 patients compared treatments of acupuncture plus anti-hypertensive drugs vs. anti-hypertensive drugs alone for blood pressure effects [28,31,33,44,45], and 7 studies with 517 patients reported efficacy rates for these 2 treatments [21,26,28,31,33,44,45]. Both SBP and DBP changes, as well as efficacy rates, were higher in acupuncture plus anti-hypertensive drug treatment than in treatment with anti-hypertensive drugs alone [SBP: MD=9.8 mmHg (95% CI: 2.95 to 16.65), I2=94%; DBP: MD=7.82 mmHg (95% CI: 4.67 to 10.96), I2=79%; RR=1.17 (95% CI: 1.08 to 1.27), I2=0%].
6. Acupuncture plus anti-hypertensive drugs vs. sham acupuncture plus anti-hypertensive drugs
Two studies with 170 patients compared the treatments of acupuncture plus anti-hypertensive drugs and sham acupuncture plus anti-hypertensive drugs treatments on blood pressure [27,49]. Changes in SBP and DBP differed between these treatment groups (SBP: MD=8.82 mmHg) (95% CI; 5.1 to 12.54), I2=35%; DBP: MD=4.44 mmHg (95% CI: 1.7 to 7.19) I2=36%)
7. Electro-acupuncture vs. anti-hypertensive drugs
Three studies with 200 patients reported SBP and DBP changes with electroacupuncture vs. anti-hypertensive drugs [32,36,42] and two (n=99) reported on efficacy rate [32,36]. Electroacupuncture and anti-hypertensive drugs treatments showed similar SBP and DBP magnitude changes and efficacy rates [SBP: MD=1.63 mmHg (95% CI: −3.25 to 6.52), I2=57%; DBP: MD=−1.98 mmHg (95% CI: −4.85 to 0.62), I2=31%; RR=0.94 (95% CI: 0.76 to 1.16), I2=0%].
8. Electro-acupuncture plus anti-hypertensive drugs vs. anti-hypertensive drugs
Only 1 study with 59 patients compared treatment effects of electro-acupuncture plus Lotensin vs. Lotensin alone on blood pressure [37]. SBP changes were greater in the combined therapy than with lotensin alone [SBP: MD=9.12 mmHg (95% CI: 3.96 to 14.28)]. However, DBP changes were similar in treatment groups [DBP: MD=4.46 mmHg (95% CI: −0.25 to 9.17)].
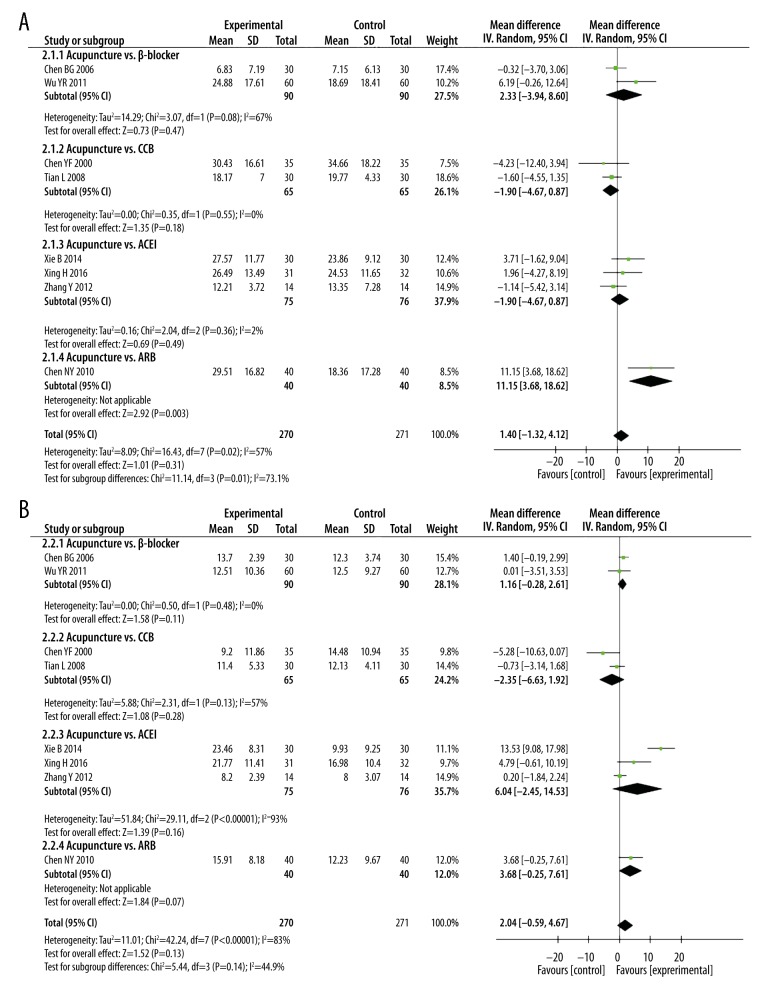
Subgroup analysis
Clinical heterogeneity is attributed in part to the use of different classes of anti-hypertensive drugs; drugs used included CCB, β-receptor antagonists, ACEI, and ARB. To control for this heterogeneity, we performed subgroup analyses of blood pressure for different classes of anti-hypertensive drugs. Pooled data indicated that DBP changes are similar in acupuncture and anti-hypertensive drug treatments (Figures 6, 7). One study [22] showed that acupuncture lowered SBP better than ARB [SBP: MD=11.15 mmHg (95% CI: 3.68 to 18.62]. Pooled result from 4 studies [31,33,44,45] showed that SBP and DBP changes were also higher in the acupuncture plus CCB treatment than with CCB treatment alone [SBP: MD=10.83 mmHg (95% CI: 3.38 to 18.27), I2=95%; DBP: MD=8.69 mmHg (95% CI: 5.32 to 12.07), I2=81%].
Figure 6.
Subgroup analyses of (A) SBP and (B) DBP magnitude changes in patients that underwent acupuncture or anti-hypertensive drug therapies.
Figure 7.
Subgroup analyses of (A) SBP and (B) DBP magnitude changes in patients that underwent therapy with acupuncture plus anti-hypertensive drugs or anti-hypertensive drugs alone.
Sensitivity analysis for the primary outcomes
We did a sensitivity analysis for SBP and DBP and efficacy rate by omitting studies one at a time. Meta-analysis results for reduced data were similar to the original results (Tables 5–7), suggesting that the pooled data results are robust.
Table 5.
Sensitivity analysis – systolic pressure.
| Study omitted | Estimate | 95% Confidence interval (CI) | |
|---|---|---|---|
| Lower CI limit | Upper CI limit | ||
| Acupuncture vs. medication | |||
| Chen BG 2006 | 1.92 | −1.41 | 5.25 |
| Chen NY 2010 | 0.27 | −1.79 | 2.33 |
| Chen YF 2000 | 1.90 | −0.93 | 4.72 |
| Tian L 2008 | 2.15 | −0.97 | 5.27 |
| Wu YR 2011 | 0.84 | −1.88 | 3.55 |
| Xie B 2014 | 1.13 | −1.83 | 4.10 |
| Xing H 2016 | 1.43 | −1.59 | 4.45 |
| Zhang Y 2012 | 1.94 | −1.23 | 5.10 |
| Acupuncture vs. sham acupuncture | |||
| Choi WJ 2015 | −2.13 | −7.63 | 3.36 |
| Kim HM 2012 | 5.05 | 1.76 | 8.33 |
| Zheng Y 2016 | 1.53 | −7.03 | 10.09 |
| Acupuncture plus lifestyle vs. lifestyle | |||
| Sun J 2009 | 12.09 | 9.14 | 15.04 |
| WU XM 2015 | 10.01 | 2.77 | 17.25 |
| Zhao DJ 2003 | 8.58 | 4.32 | 12.83 |
| Acupuncture plus antihypertensive vs. antihypertensive | |||
| Huang F 2007 | 10.83 | 3.38 | 18.27 |
| Luo H 2015 | 11.36 | 5.20 | 17.53 |
| Shen ZK 2007 | 7.20 | 4.19 | 10.22 |
| Zhang YB 2011 | 9.23 | 0.99 | 17.47 |
| Zhang YL 2005 | 10.24 | 2.56 | 17.91 |
| Acupuncture plus antihypertensive vs. sham acupuncture plus antihypertensive | |||
| Flachskampf FA 2007 | 10.80 | 6.41 | 15.19 |
| Yin C 2013 | 7.00 | 2.88 | 11.12 |
| Electroacupuncture vs. antihypertensive | |||
| Ma ZY 2011 | 2.97 | −4.51 | 10.46 |
| Wan WJ 2009 | 2.92 | −4.67 | 10.53 |
| Yang DH 2010 | −0.81 | −4.61 | 2.99 |
Table 6.
Sensitivity analysis – diastolic pressure.
| Study omitted | Estimate | 95% Confidence interval (CI) | |
|---|---|---|---|
| Lower CI limit | Upper CI limit | ||
| Acupuncture vs. medication | |||
| Chen BG 2006 | 2.21 | −1.25 | 5.67 |
| Chen NY 2010 | 1.83 | −1.07 | 4.72 |
| Chen YF 2000 | 2.80 | 0.14 | 5.46 |
| Tian L 2008 | 2.53 | −0.53 | 5.59 |
| Wu YR 2011 | 2.36 | −0.61 | 5.32 |
| Xie B 2014 | 0.61 | −0.95 | 2.16 |
| Xing H 2016 | 1.75 | −1.05 | 4.55 |
| Zhang Y 2012 | 2.40 | −0.85 | 5.65 |
| Acupuncture vs. sham acupuncture | |||
| Choi WJ 2015 | −1.79 | −5.48 | 1.91 |
| Kim HM 2012 | −0.44 | −5.44 | 4.57 |
| Zheng Y 2016 | 0.70 | −1.70 | 3.10 |
| Acupuncture plus lifestyle vs. lifestyle | |||
| Sun J 2009 | 7.67 | 2.65 | 12.69 |
| WU XM 2015 | 3.66 | 0.15 | 7.18 |
| Zhao DJ 2003 | 6.01 | −2.55 | 14.58 |
| Acupuncture plus antihypertensive vs. antihypertensive | |||
| Huang F 2007 | 8.69 | 5.32 | 12.07 |
| Luo H 2015 | 8.16 | 3.78 | 12.54 |
| Shen ZK 2007 | 7.18 | 2.93 | 11.44 |
| Zhang YB 2011 | 6.57 | 3.87 | 9.28 |
| Zhang YL 2005 | 8.51 | 4.99 | 12.03 |
| Acupuncture plus antihypertensive vs. sham acupuncture plus antihypertensive | |||
| Flachskampf FA 2007 | 5.80 | 2.77 | 8.83 |
| Yin C 2013 | 3.00 | −0.18 | 6.18 |
| Electroacupuncture vs. antihypertensive | |||
| Ma ZY 2011 | −1.86 | −7.14 | 3.41 |
| Wan WJ 2009 | −1.07 | −3.55 | 1.42 |
| Yang DH 2010 | −2.72 | −5.13 | −0.31 |
Table 7.
Sensitivity analysis – efficacy rate.
| Study omitted | Estimate | 95% Confidence interval (CI) | |
|---|---|---|---|
| Lower CI limit | Upper CI limit | ||
| Acupuncture vs. antihypertensive | |||
| Chen BG 2006 | 1.12 | 0.97 | 1.30 |
| Chen NY 2010 | 1.14 | 0.96 | 1.34 |
| Chen Q 2011 | 1.12 | 0.97 | 1.28 |
| Chen YF 2000 | 1.19 | 1.06 | 1.35 |
| Tian L 2008 | 1.17 | 1.01 | 1.36 |
| Wu YR 2011 | 1.11 | 0.96 | 1.28 |
| Xie B 2014 | 1.17 | 0.99 | 1.37 |
| Xing H 2016 | 1.14 | 1.00 | 1.31 |
| Zhang ZH 2004 | 1.13 | 0.97 | 1.30 |
| Acupuncture plus lifemodification vs. lifemodification | |||
| Su J 2009 | 1.29 | 1.04 | 1.61 |
| Wu XM 2015 | 1.14 | 0.97 | 1.35 |
| Acupuncture plus antihypertensive vs. antihypertensive | |||
| Chen J 2010 | 1.17 | 1.08 | 1.27 |
| Cui JK 2013 | 1.18 | 1.08 | 1.28 |
| Huang F 2007 | 1.19 | 1.09 | 1.29 |
| Luo H 2015 | 1.16 | 1.06 | 1.27 |
| Shen ZK 2007 | 1.18 | 1.08 | 1.29 |
| Zhang YB 2011 | 1.17 | 1.07 | 1.27 |
| Zhang YL 2005 | 1.17 | 1.07 | 1.27 |
| Electroacupuncture vs. antihypertensive | |||
| Ma ZY 2011 | 0.95 | 0.67 | 1.34 |
| Wan WJ 2009 | 0.93 | 0.71 | 1.22 |
Safety evaluation
Nine of the included studies reported adverse events during the trial [22,25–27,29,30,38,40,41]. No study reported subject dropouts due to adverse events. In 4 studies [22,26,40,41], adverse events such as headache, syncope, dizziness, pain, cough, and bleeding were reported in the treatment group. The adverse effects of headache, dizziness, cough, and hypotension were reported in the control group [22,26,40,41]. The incidence of the adverse events was similar for both groups of patients [RR=0.48 (95% CI: 0.14 to 1.61), I2=52%].
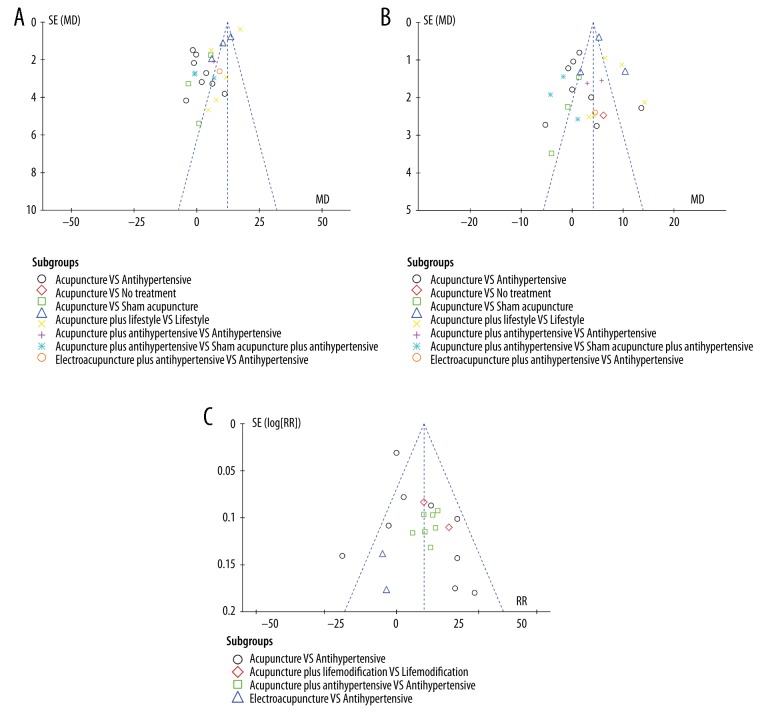
Publication bias
A funnel plot analysis revealed strong asymmetry (Figure 8), suggesting potential publication bias, probably due to the small sample sizes of the included studies.
Figure 8.
Funnel plot of (A) SBP, (B) DBP, and (C) efficacy rate in all trials.
Discussion
This systematic review of 28 RCTs and 2 quasi-RCTs showed that acupuncture plus anti-hypertensive drug treatment was better than anti-hypertensive drugs alone or sham acupuncture plus anti-hypertensive drugs, based on change in SBP and DBP. These results suggest that acupuncture enhances the beneficial effects of anti-hypertensive drugs.
DBP changes were greater in patients treated with acupuncture than in untreated patients. Moreover, SBP changes were greater in patients treated with electro-acupuncture plus Lotensin than in Lotensin alone. However, since only 1 study was available to assess both of these comparisons [30], these findings are preliminary and need further evidence.
Our findings also show that lowering of blood pressure is similar in treatments with acupuncture alone and with anti-hypertensive drugs alone. Blood pressure changes are similar for sham acupuncture and acupuncture treatments. Moreover, pooled data from 3 studies showed that blood pressure changes are similar for treatments with electro-acupuncture and anti-hypertensive drugs alone [32,36,42]. These results showed that acupuncture therapy alone was not sufficient for treating hypertension. However, there is significant heterogeneity among the studies; therefore, the quality of the results is low.
Subgroup analysis for different classes of anti-hypertensive drugs reveals that SBP changes are greater for acupuncture treatment than treatment with ARB and β-receptor antagonists. No significant differences are present in DBP changes between acupuncture and the different classes of anti-hypertensive drugs. However, the subgroup analysis reveals that acupuncture combined with CCB was more effective than CCB alone. These results are inconsistent and the data are insufficient to draw any conclusions.
We found that the reporting quality of the included studies was very low, especially for the Chinese journals. STRICTA statement analysis shows that the reporting quality of English journals is better than in Chinese journals. CONSORT statement analysis found no difference between the 2 groups overall, but the English journals had better reporting of the methodological section of the CONSORT statement (sequence generation, allocation, blinding, baseline data, and harms/adverse effects) than Chinese journals. Failure to report details of design methodology is a potential source of increased heterogeneity in the included studies. Therefore, these issues affected the analyses of acupuncture therapy for hypertension.
We also collected the published systematic reviews on the topic (Tables 8, 9). Compared with these systematic reviews, the current systematic review updates the latest evidence, and provides subgroup analysis based on the different classes of anti-hypertensive drugs, which generates most of the clinical heterogeneity. Nonetheless, several limitations to our meta-analysis exist. First, substantial heterogeneity exists among the included studies. In clinics, the methods of acupuncture and selection of the acu-points may vary because the treatment is based on the syndrome differentiation of Traditional Chinese Medicine, which leads to heterogeneity. Moreover, reporting quality of the included studies is low, especially in the methodology section of the study design, which can also be a source for heterogeneity. Second, a lack of translators meant we could only include Chinese and English studies, which leads to a selection bias. Third, sample sizes of the included studies, especially in Chinese trials, are small and the wide confidence intervals indicate high variability.
Table 8.
List and details of reviews (including this review) analyzing acupuncture therapy for hypertension.
| Author/year | Language | Clinical characteristics | No. of trials | Search date | Primary outcomes | Subgroup analysis |
|---|---|---|---|---|---|---|
| Zhang et al., 2013 | Chinese | AC vs. medication; AC vs. SA; AC plus medication vs. medication | 11 | October 2012 | SBP and DBP change magnitude/adverse effect | Mainly based on different interventions |
| Guo W et al., 2013 | Chinese | AC plus medication vs. medication | 10 | May 31, 2012 | SBP and DBP after intervention/efficacy rate/adverse effect | Not performed |
| Lee H et al., 2009 | English | AC plus medication vs. SA plus medication; AC vs. SA; AC plus medication vs. medication; AC vs. medication | 11 (3 in meta-analysis) | June, 2007 | SBP and DBP change magnitude/adverse effect | Not performed |
| Li DZ et al., 2014 | English | AC vs. SA; AC plus medication vs. SA plus medication | 4 | November, 2012 | SBP and DBP change magnitude/adverse effect | Mainly based on different interventions |
| Wang J et al., 2013 | English | AC vs. SA; AC plus medication vs. medication; AC vs. medication; AC plus medication vs. SA plus medication; AC plus lifestyle modification vs. lifestyle modification | 35 (24 in meta-analysis) | January, 2013 | SBP and DBP change magnitude/adverse effect | Mainly based on different interventions |
| Zhang YJ et al., 2013 | Chinese | AC vs. medication; AC plus medication vs. medication | 13 | July, 2013 | SBP and DBP after intervention/efficacy rate/adverse effect | Not performed |
| Zhao XF et al., 2015 | English | AC plus medication vs. medication; AC vs. medication; AC plus medication vs. SA plus medication; AC plus lifestyle modification vs. lifestyle modification | 23 | April 13, 2014 | SBP and DBP change magnitude/SBP and DBP after intervention/efficacy rate/adverse effect/ | Mainly based on different interventions |
| The current review | English | AC vs. medication; AC vs. SA; AC vs. no-treatment; AC plus medication vs. medication; AC plus medication vs. SA plus medication | 31 | April 30, 2017 | SBP and DBP change magnitude/efficacy rate/adverse effect | Mainly based on different classes of antihypertensive drugs |
AC – acupuncture; SA – sham acupuncture.
Table 9.
Main findings of previous reviews that analyzed acupuncture therapy outcomes in hypertension patients.
| Author/year | Comparison | No. of trials | Outcomes*,** | ||
|---|---|---|---|---|---|
| Blood pressure after intervention | Blood pressure change magnitude | Efficacy rate | |||
| Guo et al., 2013 | AC plus medication vs. medication | 4 | SBP: −8.35 (−10.89, −5.81) DBP: −5.25 (−11.19, −.69) |
Not reported | OR: 5.23 (3.24, 8.44) |
| Lee H et al., 2009 | AC vs. SA | 3 | Not reported | SBP: −5 (−12, 1) DBP: −3 (−6, 0) |
Not reported |
| Lee H et al., 2009 | AC plus medication vs. SA plus medication | 2 | Not reported | SBP: −8 (−10, −5) DBP: −4 (−6, −2) |
Not reported |
| Li DZ et al., 2014 | AC vs. SA | 2 | Not reported | SBP: 1.33 (−0.25, 5.16) DBP: −0.18 (−3.98, 3.62) |
Not reported |
| Li DZ et al., 2014 | AC plus medication vs. SA plus medication | 2 | Not reported | SBP: −8.58 (−10.13, −7.13) DBP: −2.82 (−5.22, −0.43) |
Not reported |
| Wang J et al., 2013 | AC vs. medication | 11 | Not reported | SBP: −0.77 (−3.89, 2.35) DBP: 0. 1 (−1.6, 1.79) |
Not reported |
| Wang J et al. 2013 | AC plus medication vs. medication | 7 | Not reported | SBP: −10.2 (−14, −6.4) DBP: −4.34 (−6.79, −1.9) |
Not reported |
| Wang J et al. 2013 | AC vs. SA | 3 | Not reported | SBP: 0.26 (−2.4, 2.91) DBP: −1.04 (−2.56, 0.47) |
Not reported |
| Wang J et al., 2013 | AC plus medication vs. SA plus medication | 2 | Not reported | SBP: −7.74 (−10.43, −4.51) DBP: −4.22 (−6.26, −2.18) |
Not reported |
| Wang J et al., 2013 | AC plus lifestyle modification vs. lifestyle modification | 1 | Not reported | SBP: −13.5 (−15.06, −11.94) DBP: −5.25 (−6.01, −4.49) |
Not reported |
| Zhang YJ et al., 2013 | AC vs. medication | 7 | SBP: −3.26 (−7.98, 1.46) DBP: −2.17 (−5.02, 0.68) |
Not reported | OR: 0.95 (0.45,2) |
| Zhang YJ et al., 2013 | AC plus medication vs. medication | 4 | SBP: −9.5 (−13.66, −5.34) DBP: −0.16 (−2.52, 2.19) |
Not reported | OR: 5.13 (2.6,10.11) |
| Zhao XF et al., 2015 | AC vs. medication | 7 | SBP: −0.56 (−3.02, 1.89) DBP: −1.01 (−2.26, 0.24) |
Not reported | OR: 1.14 (0.7, 1.85) |
| Zhao XF et al., 2015 | AC plus medication vs. medication | 3 | SBP: −9.04 (−20.11,2.02) DBP: −2.87 (−8.45, 2.72) |
Not reported | OR: 4.19 (1.65, 10.67) |
| Zhao XF et al., 2015 | AC plus lifestyle modification vs. lifestyle modification | 1 | SBP: −10.53 (−27.52, 6.46) DBP: −7.52 (−15.06, 0.02) |
Not reported | Not reported |
| Zhao XF et al., 2015 | AC vs. SA | 2 | Not reported | SBP: 0.3 (−0.27, 0.88) DBP: −1.4 (−2.37, −0.44) |
Not reported |
| Zhao XF et al., 2015 | AC plus medication vs. SA plus medication | 2 | Not reported | SBP: −7.47 (−10.43, −4.51) DBP: −4.22 (−6.26, −2.18) |
Not reported |
AC – acupuncture; SA – sham acupuncture; No. – number.
Effect size was presented with mean difference (MD, 95% confidence interval [lower limit, upper limit]) in continuous variables or risk ratio or odds ratio (RR or OR, 95% confidence interval [lower limit, upper limit]) in dichotomous variables;
Lower is better for continuous variables.
Therefore, the precise effects of acupuncture therapy for treating hypertension remain uncertain given the high overall risk of bias in our included studies. Thus, well-designed and large-sized RCTs are needed.
Conclusions
In conclusion, this review provides evidence that acupuncture enhances the therapeutic effects of anti-hypertensive drugs. However, the benefits and the safety of acupuncture therapy for treating hypertension are still unclear because of methodology flaws and low reporting quality of published studies. High-quality RCTs with larger sample sizes are required to better assess the outcomes of acupuncture therapy as treatment for hypertension.
Supplementary Files
Supplementary Table 1.
PRISMA statement.
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS | 4 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | 3 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 4 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3–4 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 3–4 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 5 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 5 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 5 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 5 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 6 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 6 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 5–6 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | 6 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 6–7 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | 7 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 8 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | 8–9 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | 8–9 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15 | 8 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | 10 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 11–12 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 12–13 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 13 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 1 |
The formula for calculating the missing change-from-baseline standard deviation.
Footnotes
Conflicts of interest
None.
Source of support: This study was support both by the National Natural Science Foundation of China (Grant No. 81674063) and the Traditional Chinese Medicine Science and Technology Project funded by the Bureau of Traditional Chinese Medicine of Jiangsu province (Grant No. YB2015006)
References
- 1.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15(9):913–24. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Yang L, Wang L, et al. Burden of hypertension in China: A nationally representative survey of 174,621 adults. Int J Cardiol. 2017;227:516–23. doi: 10.1016/j.ijcard.2016.10.110. [DOI] [PubMed] [Google Scholar]
- 4.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 5.Guo W, Pei QW, Dong WL, et al. [Acupuncture combined with medication for hypertension: a systematic and meta-analysis]. Journal of Shandong Unversity of TCM. 2013;37(2):99–103. [in Chinese] [Google Scholar]
- 6.Lee H, Kim SY, Park J, et al. Acupuncture for lowering blood pressure: Systematic review and meta-analysis. Am J Hypertens. 2009;22(1):122–28. doi: 10.1038/ajh.2008.311. [DOI] [PubMed] [Google Scholar]
- 7.Li DZ, Zhou Y, Yang YN, et al. Acupuncture for essential hypertension: A meta-analysis of randomized sham-controlled clinical trials. Evid Based Complement Alternat Med. 2014;2014:279478. doi: 10.1155/2014/279478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Xiong X, Liu W. Acupuncture for essential hypertension. Int J Cardiol. 2013;169(5):317–26. doi: 10.1016/j.ijcard.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhao XF, Hu HT, Li JS, et al. Is Acupuncture Effective for Hypertension? A Systematic Review and Meta-Analysis. PLoS One. 2015;10(7):e0127019. doi: 10.1371/journal.pone.0127019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang LL, KH, Yang C, Ban HP, et al. [Effect of acupuncture for hypertension and frequency of acupoints]. Liaoning Journal of Traditional Chinese Medicine. 2013;40(10):2115–19. [in Chinese] [Google Scholar]
- 11.Zhang YJ, Li ZJ, Gao Y, Du YZ. [Meta-analysis on efficacy of acupuncture and acupuncture combined with medicine in treatment for mild to moderate essential hypertension]. Liaoning Journal of Traditional Chinese Medicine. 2014;41(9):1802–6. [in Chinese] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int j Surgery (London, England) 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst Rev. 2012;1:2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens. 1999;17(2):151–83. [PubMed] [Google Scholar]
- 15.Schulz KF, Altman DG, Moher DL CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT statement. PLoS Med. 2010;7(6):e1000261. doi: 10.1371/journal.pmed.1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 18.Casgrande JT, Pike MC, Smith P. An improved approximate formula for calculating sample sizes for comparing two binomial distributions. Biometrics. 1978;34(3):4. [PubMed] [Google Scholar]
- 19.Chow SC, Shao J, Wang H. Sample size calculation in clinical research. New York: Marcel Dekker; 2003. [Google Scholar]
- 20.Chen BG, Qian CY, Zhang JN, Mao HR. [Clinical observations on the antihypotensive effect of point Fengchi]. Shanghai Journal of Acupuncture and Moxibustion. 2006;25(3):15–16. [Google Scholar]
- 21.Chen J, Li J, Wang ZR. [Therapeutic effect on essential hypertension treated with combined therapy of acupuncture and medication]. Zhongguo Zhen Jiu. 2010;30(11):896–98. [PubMed] [Google Scholar]
- 22.Chen NY, Zhou Y, Dong Q, Zhou CX. [Observation on therapeutic effect of acupuncture in the treatment of German hypertension patients]. Zhen Ci Yan Jiu. 2010;35(6):462–66. [PubMed] [Google Scholar]
- 23.Chen Q, Chen BG. [Clinical observation on acupuncture at Quchi (LI 11) and Fengchi (GB 20) for Hypertension]. Shanghai Journal of Acupuncture and Moxibustion. 2011;30(10):659–60. [in Chinese] [Google Scholar]
- 24.Chen YF, Qian H, Li L, et al. [Effects of acupuncture on contents of plasma endothelin and angiotensin II in the patient of hypertensiton]. Chinese Journal of Acupuncture and Moxibustion. 2000;(11):691–93. [in Chinese] [Google Scholar]
- 25.Choi WJ, Cho YY, Sun SH. The effects of Sa-am acupuncture Simpo-jeongkyeok treatment on the blood pressure, pulse rate, and body temperature. J Pharmacopuncture. 2015;18(2):33–41. doi: 10.3831/KPI.2015.18.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui JK, Ge XY. [The clinical study of acupuncture combined with irbesartan for treating hypertension]. Acta Chinese Medicine and Pharmacology. 2013;41(6):111–12. [in Chinese] [Google Scholar]
- 27.Flachskampf FA, Gallasch J, Gefeller O, et al. Randomized trial of acupuncture to lower blood pressure. Circulation. 2007;115(24):3121–29. doi: 10.1161/CIRCULATIONAHA.106.661140. [DOI] [PubMed] [Google Scholar]
- 28.Huang F, Yao GX, Huang XL, Liu YN. [Clinical observation on acupuncture for treatment of hypertension of phlegm-stasis blocking collateral type]. Zhongguo Zhen Jiu. 2007;27(6):403–6. [PubMed] [Google Scholar]
- 29.Kim HM, Cho SY, Park SU, et al. Can acupuncture affect the circadian rhythm of blood pressure? A randomized, double-blind, controlled trial. J Altern Complement Med. 2012;18(10):918–23. doi: 10.1089/acm.2011.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Park JE, Shin KM, et al. Acupuncture lowers blood pressure in mild hypertension patients: A randomized, controlled, assessor-blinded pilot trial. Complement Ther Med. 2015;23(5):658–65. doi: 10.1016/j.ctim.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Luo H, Li K. [The efficacy of midnight-midday ebb flow acupuncture for treating 44 hypertensive patients]. Hunan Journal of Traditional Chinese Medicine. 2015;31(10):80–82. [Google Scholar]
- 32.Ma ZY, Wang YF, Wan WJ, et al. [The effect of electroacupuncture in Quchi (LI 11) for patients with hypertension on concentration of plasma NPY and NT]. Journal of new Chinese Medicine. 2011;43(4):89–91. [in Chinese] [Google Scholar]
- 33.Shen ZK, Shao CH, Jiang PY, et al. Clinical observation on acupuncture Zusanli and western medicine for treatment of 25 patients with resistant hypertension. Shanxi Journal of Traditional Chinese Medicine. 2007;28(10):1377–79. [Google Scholar]
- 34.Sun J, Duan Q, Wang Q, Zhu XP. [The clinical study of acupuncture for mild hypertension(Liver Yang syndrone)]. Journal of Mew Chinese Medicine. 2009;41(8):94–95. [Google Scholar]
- 35.Tian L. [The clinical summary of acupuncture for treating 30 patients with hypertension of hyperactivity of liver-yang pattern]. Hunan Journal of Traditional Chinese Medicine. 2008;24(2):14–15. [Google Scholar]
- 36.Wan WJ, Ma CY, Xiong XA, et al. [Clinical observation on therapeutic effect of electroacupuncture at Quchi (LI 11) for treatment of essential hypertension]. Zhongguo Zhen Jiu. 2009;29(5):349–52. [PubMed] [Google Scholar]
- 37.Wang C, Cheng ZQ. Clinical Effective evaluation and its machenical analysis of acupuncture on obese hypertensive patients. Liaoning Journal of Traditional Chinese Medicine. 2006 [Google Scholar]
- 38.Wu XM, Li B, Wu B, et al. [Effect of puncturing from Baihui(GV20) to Qianding(GV21) with penetrating method on ambulatory blood pressure in grade 1 and 2 primary hypertension]. Beijing Journal of Traditional Chinese Medicine. 2015;34(12):931–35. [Google Scholar]
- 39.Wu YR, Li HX. The clinical observation on acupuncture for treating hypertension. China Medical Herald. 2011;8(19):102–3. [Google Scholar]
- 40.Xie B, Lin YP. [Efficacy observation on acupuncture for essential hypertension of yin deficiency due to yang hyperactivity pattern]]. Zhongguo Zhen Jiu. 2014;34(6):547–50. [PubMed] [Google Scholar]
- 41.Xing H, Zhang Y, Liu XD, et al. [Clinical study of acupuncture in treating essential hypertension of accumulation of phlegm and dampness]. Shandong Journal of Traditional Chinese Medicine. 2016;35(9):802–6. [in Chinese] [Google Scholar]
- 42.Yang DH. Effect of electroacupuncture on Quchi (LI 11) and Taichong (LR 3) on blood pressure variability in young patients with hypertension. Zhongguo zhen jiu = Chinese acupuncture & moxibustion. 2010;30(7):547–50. doi: 10.13703/j.0255-2930.2010.07.008. Epub 2010/09/25. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Liu XG, Dong XY, et al. Clinical reserach of acupuncture adding psychotherapy in treating essential hypertension. Journal of Chengdu University of Traditional Chinese Medicine. 2012;35(4):16–18. [Google Scholar]
- 44.Zhang YB. [Clinical research of acupuncture and medicine in the treatment of liver yang hyperactivity type hypertension]. China Journal of Chinese Medicine. 2011;26(162):1397–98. [Google Scholar]
- 45.Zhang YL, Li CP, Peng M, Yang HS. Effect of acupuncture combined with medicine on neuropeptide Y in the patient of hypertension. Zhongguo Zhen Jiu. 2005;25(3):155–57. [PubMed] [Google Scholar]
- 46.Zhang ZH, Zhou J, Wang Q, Ma X. [Acupuncture for treatment of primary hypertension and effect on functions of vascular endothelium]. Chinese Acupuncture & Moxibustion. 2004;24(8):539–40. [in Chinese] [Google Scholar]
- 47.Zhao DJ, Fan QL. [Effect of acupuncture on insulin resistance in the patient of hypertension]. Chinese Acupuncture & Moxibustion. 2003;23(3):165–67. [in Chinese] [Google Scholar]
- 48.Zheng Y, Zhang J, Wang Y, et al. Acupuncture decreases blood pressure related to hypothalamus functional connectivity with frontal lobe, cerebellum, and insula: A study of instantaneous and short-term acupuncture treatment in essential hypertension. Evid Based Complement Alternat Med. 2016;2016:6908710. doi: 10.1155/2016/6908710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin C, Seo B, Park HJ, et al. Acupuncture, a promising adjunctive therapy for essential hypertension: A double-blind, randomized, controlled trial. Neurol Res. 2007;29(Suppl 1):S98–103. doi: 10.1179/016164107X172220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
PRISMA statement.
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS | 4 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | 3 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 4 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3–4 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 3–4 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 5 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 5 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 5 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 5 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 6 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 6 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 5–6 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | 6 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 6–7 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | 7 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 8 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | 8–9 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | 8–9 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15 | 8 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | 10 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 11–12 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 12–13 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 13 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 1 |
The formula for calculating the missing change-from-baseline standard deviation.