Abstract
Background
Tuberculosis (TB) is the leading cause of death in Human immunodeficiency virus (HIV) infected children globally. The aims of this study were to determine the mortality rate and to identify the predictors of mortality among TB/HIV co-infected children at University of Gondar Comprehensive Specialized Hospital.
Method
A retrospective follow-up study was conducted among TB/HIV co-infected children from February 2005 to March 2017. A Kaplan–Meier curve was used to estimate the median survival time. Bivariate and multivariable Cox proportional hazards models were fitted to identify the predictors of mortality.
Results
A total of 271 TB/HIV co-infected children were included in the analysis. Of these, 38(14.02%) children were died during the follow-up period. This gives a total of 1167.67 child-years of observations. The overall mortality rate was 3.27(95%CI: 2.3–4.5) per 100 child-years. The independent predictors of time to death were age 1–5 years (as compared to age <1 year) (AHR = 0.3; 95%CI:0.09–0.98)), being anemic (AHR = 2.6; 95%CI:1.24–5.3), cotrimoxazole preventive therapy(CPT) non-users (AHR = 4.1; 95%CI:1.4–16.75), isoniazid preventive therapy(IPT) non-users (AHR = 2.95; 95%CI:1.16–7.5), having extra pulmonary tuberculosis(EPTB) (AHR = 2.43; 95%CI:1.1–5.3)) and fair or poor adherence to Anti-Retroviral Therapy (ART)(AHR = 3.5; 95%CI:1.7–7.5).
Conclusion
Mortality rate among TB/HIV co-infected children was high at University of Gondar Comprehensive Specialized Hospital. Age, extra-pulmonary tuberculosis, anemia, adherence, CPT and IPT were the independent predictors of mortality.
Background
Tuberculosis (TB) is the leading cause of death among HIV-infected children [1]. It is a major public health problem especially in low and middle-income countries [2–4]. According to the World Health Organization (WHO), in 2016, there were an estimated 10.4 million new cases of TB globally (equivalent to an incidence rate of 142 cases per 100,000 population) and 1.4 million deaths due to TB in 2015 [5]. Approximately, 1.2 million cases of TB occurred in HIV-positive people and 1.0 million cases occurred in children. The burden of TB/HIV co-infection is particularly high in sub-Saharan Africa including in Ethiopia [6].
Ethiopia has been classified as one of the 30 high TB and TB/HIV burden countries [5, 7]. The country is striving to reduce the magnitude of TB and HIV disease in line with the objectives of the sustainable development goal (SDG) [8]. However, the problem still remains high, particularly in children. TB is one of the top ten causes of death [9] and the most commonly reported opportunistic infection in children infected with HIV [10–13]. Even though there were advances in the implementation of prevention of mother to child transmission (PMTCT), and provision of isoniazid preventive therapy (IPT) in Ethiopia, TB is a major cause of hospital admission and death in HIV infected children [14]. The management of TB/HIV co-infection in children is very challenging especially in resource-limited settings such as Ethiopia because of the unavailability of appropriate formulations of drugs, a drug to drug interactions, pill burdens, drug side effects and poor adherence [15–17]. This resulted in high mortality rate among TB/HIV co-infected children.
As of 2015, a WHO report indicated that there were nearly 41,000 children were died due to TB/HIV co-infection. Of these, 34,000 were occurred in Africa [5]. The mortality rate of TB/HIV co-infection was varied in different settings and ranged from 11% to 36.5% [18, 19]. The cause of death among TB/HIV co-infected children are multi-factorial [20]. These include age, nutritional status, immunity status, hemoglobin level, use of CPT and IPT [21]. Thus, the aim of this study was to estimate the mortality rate and to identify the predictors of mortality among TB/HIV co-infected children at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia.
Method
Study setting
The study was conducted at University of Gondar Comprehensive Specialized Hospital HIV care clinic. The hospital is found in Northwest Ethiopia and serves for more than 5 million people in North Gondar and neighboring zones. The HIV care service was established in January 2005. A total of 8581 adults and 1138 children were enrolled in HIV care until March 2017.
Study design and participants
An institutional-based retrospective cohort study was conducted from February 2005 to March 2017. All TB/HIV co-infected children age less than 15 years who were enrolled in Pediatric HIV care Clinic at University of Gondar Comprehensive Specialized Hospital were eligible for this study.
Data collection tools and procedures
Data were collected from medical records using data extraction checklist adapted from National HIV intake and follow-up forms. The checklist comprised socio-demographic, clinical and follow-up variables. The data were collected by four BSc. Nurses working at Pediatric HIV care clinic who had comprehensive HIV care training. The pre-test was conducted among 15 medical records to check the consistency of the data extraction checklist.
Data analysis
Data were entered into EPI-info version 7 and then exported to STATA version 12 for analysis. WHO Anthro-Plus software was used to classify indices variables and to assess the nutritional status of the children. Descriptive statistics were carried out and summarized using tables and graphs. Mortality rate was calculated by dividing the number of children died during the follow-up period by the Child-Years of follow-up. Kaplan–Meier curve was used to estimate the median survival time. The Log-rank test was used to compare survival curves between the categories of the explanatory variables. A life table was used to estimate the probability of survival at a different time interval in the follow-up time.
Both bivariate and multivariable Cox proportional hazard model were used to identify the predictors of time to death of TB/HIV co-infected children. A bivariate Cox proportional hazard model was first fitted, and the variables significant at P-value <0.2 in the bivariate analysis were selected for the final multivariable Cox proportional hazard model. Then the final Cox proportional hazard model was fitted using backward stepwise selection. Variables having p-value less than 0.05 at 95% CI in the final multivariable Cox proportional hazards model were considered as significantly associated with the dependent variable. The necessary assumption of Cox proportional hazard model was checked by using Schoenfield residuals test.
Ethical considerations
Ethical clearance was obtained from the Institutional Review Board of the University of Gondar. Permission letter was also obtained from University of Gondar Comprehensive Specialized Hospital’s management and HIV care clinics focal person to use the secondary data for the purpose of this study. Since we used secondary data, we did not get informed consents from each study participants.
Results
Socio-demographic characteristics
A total of 301 TB/HIV co-infected children’s medical records were reviewed. Of these, 30(9%) were excluded from the analysis due to missing of data. The remaining, 271 TB/HIV co-infected children were included in the analysis. The mean age of the study participants was 6.6(±3.5 SD) years. Nearly one-third 88 (32.47%) of the children were under 5 years and half of them 137(50.55%) were males (Table 1). The majorities 219 (80.81%) of the respondent were living in urban and 220 (81.18%) children were lives with their parents. Half 135 (49.82) of children’s caregiver were between the age group of 25 and 34 years with a median age of 30 (IQR (27–38)) years. Approximately, two third 176 (64.94%) of the children’s caregivers were HIV positive.
Table 1. Socio-demographic characteristics of TB/HIV co-infected children at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from February 2005 to March 2017 (n = 271).
| Characteristics | Total N (%) | Death N (%) | Censored N (%) | |
|---|---|---|---|---|
| N = 271 | N = 38 | N = 233 | ||
| Age (Years) | <1 | 11(4.06) | 5(1.85) | 6(2.21) |
| 1–5 | 77(28.41) | 8(2.95) | 69(25.46) | |
| 6–10 | 125(46.13) | 19(7.01) | 106(39.11) | |
| 11–14 | 58(21.40) | 6(2.21) | 52(19.19) | |
| Sex | Male | 137(50.55) | 19(7.01) | 118(43.54) |
| Female | 134(49.45) | 19(7.01) | 115(42.44) | |
| Age of caregiver(Years) | 15–24 | 32(11.81) | 5(1.85) | 27((9.96) |
| 25–34 | 135(49.82) | 21(7.75) | 114(42.07) | |
| 35–44 | 68(25.09) | 7(2.58) | 61(22.51) | |
| >44 | 36(13.28) | 5(1.85) | 31(11.44) | |
| Residence | Urban | 219(80.81) | 28(10.33) | 191(70.48) |
| Rural | 52(19.19) | 10(3.69) | 42(15.50) | |
| Family size | < = 2 | 44(16.24) | 10(3.69) | 34(12.55) |
| 2–4 | 142(52.40) | 18(6.64) | 124(45.76) | |
| > = 5 | 85(31.37) | 10(3.69) | 75(27.68) | |
| Caregiver of the child | Parents | 220(81.18) | 31(11.4) | 189(69.74) |
| Siblings | 19(7.01) | 1(0.37) | 18(6.64) | |
| Grand-parents | 23(8.49) | 3(1.11) | 20(7.38) | |
| Others | 9(3.32) | 3(1.11) | 6(2.21) | |
| Child lives with | Parents | 247(91.14) | 34(12.55) | 213(78.60) |
| Orphaned | 11(4.06) | 2(0.74) | 9(3.32) | |
| Others | 13(4.80) | 2(0.74) | 11(4.06) | |
Clinical characteristics
A total of 237(87.45%) children had an advanced baseline WHO clinical stage (i.e. 3 and 4) (Table 2). More than one-third 95 (35.06%) of children had experienced with an initial regiment change during the follow-up period. Of these, 33(34.74%) were due to TB infections. Twenty-eight (10.33%) children had experienced with ART treatment failure. Of these 8(28.6%) children were initiated second-line ART.
Table 2. Clinical characteristics of TB/HIV co-infected children at University of Gondar Comprehensive Specialized Hospital Northwest Ethiopia, from February 2005 to March 20017 (n = 271).
| Characteristics | Total N (%) N = 271 |
Death N (%) N = 38 |
Censored N (%) N = 233 |
|
|---|---|---|---|---|
| Baseline WHO stage | I & II | 34(12.55) | 3(1.11) | 31(11.40) |
| III & IV | 237(87.45) | 35(12.90) | 202(74.54) | |
| ART Eligibility criteria | CD4+ cell | 37(13.65) | 4(1.48) | 33(12.80) |
| WHO stage | 104(38.38) | 11(4.06) | 93(34.32) | |
| Both | 125(46.13) | 23(8.49) | 102(37.32) | |
| Not recorded | 5(1.85) | 0(0) | 5(1.85) | |
| Initial ART regiment based on NRTIs | ABC-based | 10(3.69) | 5(1.85) | 5(1.85) |
| AZT-based | 185(68.27) | 20(7.38) | 165(60.89) | |
| D4T-based | 67(24.72) | 12(4.43) | 55(20.30) | |
| TDF-based | 9(3.32) | 1(0.37) | 8(2.95) | |
| Initial ART regiments based on NNRTs | EFV-based | 102(37.64) | 12(4.43) | 90(33.21) |
| NVP, PI and other based | 169(62.36) | 26(9.59) | 143(52.77) | |
| Initial regiment change | Yes | 95(35.06) | 13(4.80) | 82(30.26) |
| No | 176(64.94) | 25(9.23) | 151(55.72) | |
| Reason for regiment change | Side effect/toxicities | 23(24.21) | 6(6.32) | 17(17.89) |
| Treatment failure | 2(2.11) | 0(0) | 2(2.11) | |
| TB | 33(34.74) | 5(5.26) | 28(29.47) | |
| Stock out | 37(38.95) | 2(2.11) | 35(36.84) | |
| Treatment failure | Yes | 28(10.33) | 5(1.85) | 23(8.49) |
| No | 243(89.67) | 33(12.18) | 210(77.49) | |
| Immunologic failure | Yes | 20(7.38) | 4(1.48) | 16(5.90) |
| No | 251(92.62) | 34(12.55) | 217(80.07) | |
| Virologic failure | Yes | 17(6.27) | 3(1.11) | 14(5.17) |
| No | 254(93.73) | 35(12.92) | 219(80.81) | |
| Clinical failure | Yes | 5(1.85) | 3(1.11) | 2(0.74) |
| No | 266(98.15) | 35(12.92) | 233(85.24) | |
| Baseline HIV associated Immunosuppression status | Non-significant/Mild | 87(32.10) | 8(2.95) | 79(29.15) |
| Advanced | 73(26.95) | 8(2.95) | 65(23.99) | |
| Sever | 111(40.96) | 22(8.12) | 89(32.84) | |
| Isoniazid | Yes | 97(35.79) | 6(2.21) | 91(33.58) |
| No | 174(64.21) | 32(11.81) | 142(52.40) | |
| Hemoglobing/dl | <10 | 48(17.70) | 14(5.17) | 34(12.50) |
| > = 10 | 223(82.29) | 24(8.86) | 199(73.43) | |
| Co-trimoxazole preventive therapy | Yes | 236(87.08) | 28(10.33) | 208(76.75) |
| No | 35(12.92) | 10(3.69) | 25(9.23) | |
| Weight for age | Normal | 77(28.41) | 12(4.43) | 65(23.99) |
| Underweight | 194(71.59) | 26(9.59) | 168(61.99) | |
| Height for age | Normal | 110(40.59) | 14(5.17) | 96(35.42) |
| Stunting | 161(59.41) | 24(8.86) | 137(50.55) | |
| Adherence | Good | 231(85.24) | 23(8.49) | 208(76.75) |
| Fair | 27(9.96) | 12(4.43) | 15(5.53) | |
| Poor | 13(4.80) | 3(1.11) | 10(3.69) | |
| Site of TB | PTB | 186(68.63) | 13(4.80) | 173(63.84) |
| EPTB | 85(31.37) | 25(9.23) | 60(22.14) | |
| Time at which TB is developed | PRE ART | 206(76.01) | 25(9.23) | 181(66.79) |
| ART | 65(23.99) | 13(4.80) | 52(19.19) | |
Forty-eight (17.7%) children were anemic at baseline with a median Hgb level of 12 (IQR; 10.6–13). Regarding prophylaxis use, 236(87.45%) of the respondents were on co-trimoxazole Preventive Therapy (CPT) and ninety-seven (35.79%) were on isoniazid preventive therapy (IPT). At TB diagnosis, stunting and underweight were 161(59.41%) and 194(71.59%) respectively.
Mortality rate
Two hundred seventy-one children were followed for different periods (1 month to 12 years) that gives a total of 1167.67 Child-Years of observation. The median follow-up period was 4(IQR; 1.9–6.5) years. From a total of 271 children who were included in the analysis, 38(14.02%) new deaths were observed, 186 (68.6%) were alive at the end of the follow-up, 22 (8.1%) were transfer out to other treatment centres, and 25 (9.2%) were lost to follow-up. Thus, the overall mortality rate was 3.27 (95%CI: 2.43–4.52) per 100 Child-Years. Among children who died during the follow-up period, half (50%) of them were males and 23 (60.5%) died within the first year of follow-up (Table 3). Twenty-five children had extra-pulmonary or/and disseminated tuberculosis.
Table 3. Mortality rate stratified by socio-demographic and clinical characteristics of TB/HIV co-infected children at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from February 2005 to March 2017.
| Characteristics | Total N (%) | PY | Death N (%) | IDR | |
|---|---|---|---|---|---|
| Age (years) | <1 | 11(4.06) | 56.10 | 5(1.85) | 8.92 |
| > = 1 | 260(95.94) | 1105.20 | 33(12.18) | 2.99 | |
| Residence | Urban | 219(80.81) | 1015.25 | 28(10.33) | 2.76 |
| Rural | 52(19.19) | 152.40 | 10(3.69) | 6.56 | |
| Child lives with | Parents | 247(91.14) | 1094.58 | 34(12.55) | 3.10 |
| Orphaned | 11(4.06) | 36.00 | 2(0.74) | 5.56 | |
| Others | 13(4.80) | 37.10 | 2(0.74) | 5.40 | |
| Baseline WHO stage | I & II | 34(12.55) | 114.25 | 3(1.11) | 2.63 |
| III & IV | 237(87.45) | 1047.00 | 35(12.9) | 3.34 | |
| Baseline immunity status | Non-significant/Mild | 87(32.10) | 337.83 | 8(2.95) | 2.37 |
| Advanced/ severe | 184(67.90) | 823.42 | 30(11.07) | 3.64 | |
| Initial ART regiment based on NRTIs | ABC-based | 10(3.69) | 20.17 | 5(1.85) | 24.81 |
| AZT-based | 185(68.27) | 828.33 | 20(7.38) | 2.40 | |
| D4T-based | 67(24.72) | 298.17 | 12(4.43) | 4.00 | |
| TDF-based | 9(3.32) | 20.99 | 1(0.37) | 4.81 | |
| Treatment failure | Yes | 28(10.33) | 149.58 | 5(1.85) | 3.34 |
| No | 243(89.67) | 1018.10 | 33(12.18) | 3.24 | |
| Clinical failure | Yes | 5(1.85) | 12.67 | 3(1.11) | 23.70 |
| No | 266(98.15) | 1155.00 | 35(12.92) | 3.00 | |
| Isoniazid | Yes | 97(35.79) | 542.00 | 6(2.21) | 1.11 |
| No | 174(64.21) | 625.66 | 32(11.81) | 5.11 | |
| Hemoglobin g/dl | <10 | 48(17.71) | 167.58 | 14(5.17) | 8.41 |
| > = 10 | 223(82.29) | 1000.10 | 24(8.86) | 2.40 | |
| CPT | Yes | 236(87.08) | 1079.58 | 28(10.33) | 2.60 |
| No | 35(12.92) | 88.10 | 10(3.69) | 11.40 | |
| Adherence | Good | 231(85.24) | 1037.92 | 23(8.49) | 2.22 |
| Fair/poor | 40(14.76) | 123.33 | 15(5.54) | 12.20 | |
| Site of TB | PTB | 186(68.63) | 801.67 | 13(4.80) | 1.62 |
| EPTB | 85(31.37) | 365.999 | 25(9.23) | 6.83 | |
| Follow-up years | <1 | 44(16.24) | 15.33 | 23(8.49) | 150.00 |
| 1–5 | 116(42.8) | 345.92 | 9(3.32) | 2.60 | |
| >5 | 111(40.96) | 800.00 | 6(2.21) | 0.75 | |
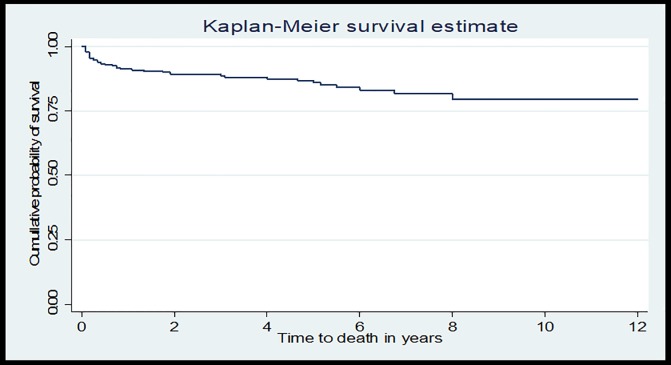
The cumulative probability of survival at the end of 1 year was 91.2%, at the end of 3 years was 88.6%, at the end of 5 years was 85.8%, and at the end of 12 years was 79.4% respectively (Fig 1).
Fig 1. Kaplan-Meier curve of survival proportion for TB/HIV co-infected children at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from February 2005 to March 2017.
Predictors of mortality
In the bivariate Cox Proportional Hazard model age, Hemoglobin level, co-trimoxazole preventive therapy (CPT), isoniazid prophylaxis (IPT), site of tuberculosis (TB) infections, severe immunosuppression and adherence to ART were statistically significant (Table 4). However, in multivariable Cox-Proportional Hazard model age, CPT, IPT, site of TB infection, adherence to ARV drugs and hemoglobin level remained statistically significant.
Table 4. Predictors of time to death of TB/HIV co-infected children at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from February 2005 to March 2017 (n = 271).
| Characteristics | Death | Censored | CHR(95%CI) | AHR(95%CI) | |
|---|---|---|---|---|---|
| Age | <1year | 5 | 6 | 1.00 | 1.00 |
| 1–5 years | 8 | 69 | 0.25 (0.08–0.77)* | 0.3(0.09–0.98)** | |
| 5–10 | 19 | 106 | 0.37(0.14–1.00) | 0.7(0.22–2.3) | |
| 10–14 | 6 | 52 | 0.29(0.09–0.96) | 0.33(0.084–1.33) | |
| Mother HIV status | Positive | 22 | 160 | 1.00 | 1.00 |
| Negative/Unknown | 16 | 73 | 1.77(0.93–3.37) | 1.14(0.26–5) | |
| Address | Urban | 28 | 191 | 1.00 | 1.00 |
| Rural | 10 | 42 | 1.99(0.96–4.11) | 1.58(0.696–3.6) | |
| HIV status of caregiver | Positive | 22 | 154 | 1.00 | 1.00 |
| Negative /Unknown | 16 | 79 | 1.57(0.82–2.997) | 1.69(0.38–7.5) | |
| Immunity status at TB diagnosis | N.S & mild | 8 | 86 | 1.00 | 1.00 |
| Advanced IS | 7 | 59 | 1.17(0.42–3.23)* | 1.3(0.44–3.94) | |
| Sever IS | 23 | 88 | 2.32(1.04–5.199)* | 2.45(0.97–6.2)** | |
| Anemia status at TB diagnosis | Anemic | 14 | 34 | 3.1(1.6–6)* | 2.6(1.24–5.3)** |
| Non-anemic | 24 | 199 | 1.00 | 1.00 | |
| CPT | Yes | 28 | 208 | 1.00 | 1.00 |
| No | 10 | 25 | 3.51(1.68–7.31)* | 4.1(1.76–9.7)** | |
| IPT | Yes | 6 | 91 | 1.00 | 1.00 |
| No | 32 | 142 | 3.87(1.6–9.28)* | 2.95(1.16–7.45)** | |
| Site of TB | PTB | 13 | 173 | ||
| EPTB | 25 | 60 | 4.43(2.26–8.67)* | 2.43(1.1–5.3)** | |
| Adherence | Good | 23 | 208 | 1.00 | 1.00 |
| Fair/poor | 15 | 25 | 4.86(2.5–9.3)* | 3.57(1.7–7.5)** | |
| Time of TB occurrence | Pre ART | 25 | 181 | 1.00 | 1.00 |
| ART | 13 | 52 | 1.68(0.86–3.29) | 1.46(0.68–3.15) | |
N.S = non-significant, I.S = immunosuppression.
* = variables significant in the baviriable at p-value less than 0.05 at 95%CI.
** = variables significant in multivariable at p-value less than 0.05 at 95%CI. Anemic: <10 mg/dl; non-anemic: ≥10 mg/dl.
According to the analysis, children whose age group 1–5 years were less likely to die as compared to children with age less than one year (AHR = 0.3; 95%CI: 0.09–0.98). Anemic Children were 2.6 times at higher risk of death as compared to non-anemic children (AHR = 2.6; 95%CI: 1.24–5.3). Similarly, children with extra-pulmonary or/and disseminated tuberculosis were 2.43 times at higher risk of death as compared children with pulmonary tuberculosis (AHR = 2.43; 95%CI: 1.1–5.3). Children who did not use CPT were 4.1 times at higher risk of death than children who used CPT (AHR = 4.1; 95%CI = 1.76–9.7). Similarly, IPT non-users were 2.95 times at higher risk of death as compared with IPT users (AHR = 2.95; 95%CI = 1.16–7.45).
A child with fair or poor adherence to ART drugs was 3.57 times at higher risk of death than a child with good adherence to ART drugs.
Discussion
This is the first published study that has presented the mortality rate and predictors of mortality from a cohort of TB and HIV co-infected children in Northwest Ethiopia. The overall mortality rate was 3.27 (95%CI: 2.4–4.5) per 100Child-Year of follow-up. This result is consistent with mortality rate reported in systematic review and meta-analysis [22], and in other studies conducted in high TB and TB/HIV burden countries such as South Africa [23, 19, 24], and Indian [25]. However, our finding is higher than a study conducted in Nigeria (1.4 per 100 Child-Year follow-ups) [26].
The highest mortality rate (8.92/100CY) was observed in the first year of follow-up. The peak mortality rate in the first year might be associated with the progression of the sub-clinical disease, which remains undetected during enrollment and progresses rapidly. Late arrival at health care means late diagnosis which is one predictor of death among TB/HIV co-infected children supported by a study conducted in South Africa [27]. The other fact could be Immune reconstitution inflammatory syndrome (IRIS) which is common within 6 months of ART initiation. The result also showed that a high number of children were started ART within the first year of follow-up which increases the probability of IRIS occurrence [28].The other possible reason for increased TB/HIV co-infected children survival with duration of follow-up could be the result of the progressive increase in CD4 cell counts which builds the immune system and this may again decrease the viral load across time, finally, increase the survival rate.
The cumulative survival rate in this study was 79.4% (95%CI: 71% -85.6%) which is in line with a retrospective cohort study conducted in Nigeria 73% [29]. The survival rate in our study at 1, 2 and 3 years were 91.2%, 89.1%, and 88.6% respectively, which were similar with a cohort study in Thailand 96.1%, 94% and 87.7% at 1, 2 and 3years respectively among ART users, and much higher than 44.4%, 19.2%, and 9.3% among non-ART user group [20]. The discrepancy in those of pre-ART may be due to the effect of ART drugs. Anti-retroviral drugs are responsible for viral suppression, which increase the CD4 cell, finally, increase the survival of children and decrease the risk of death which is supported, by a study conducted in Malawi [30].
In our study, similar with other studies conducted in South Africa [23] and Nigeria [31], TB/HIV co-infected children with age less than one year were at higher risk of death than children with age 1–5 years. This is due to the fact that, children with age less than one year had an immature immune system, especially in TB/HIV co-infected children who have the tendency to develop the more severe disease, that leads to death. Anemic children were at higher risk of death than non-anemic children, which were similar to studies conducted in Tanzania [32], and Malawi [30]. This might be due to the effect of anemia on the oxygen intake capacity which had a synergistic effect with tuberculosis and HIV co-infections that increase the prognosis of the disease process which may end-up with death.
Co-trimoxazole preventive therapy non-users were four times at higher risk of death than CPT users. This may be due to the fact that CPT can prevent most of the opportunistic infections in HIV and TB co-infected children and, finally CPT use may reduce the mortality rate. Isoniazid Preventive Therapy (IPT) non-users were also at higher risk of death than IPT users. Similar findings were reported in South Africa [33] and Nigeria [31]. IPT prevents the reoccurrence of TB infections, severity and dissemination of TB. Our result showed that a child infected with extra-pulmonary or/and disseminated TB was at higher risk of death than a child infected with PTB.
We have also found that children who had fair or poor adherence to ART drugs had a higher risk of death than children who had good adherence to ART drugs. Similar findings were reported in other studies conducted in Indian [18] and Addis Ababa, Ethiopia [34]. Adhere to ART drugs will suppress viral replications and increase CD4 cells counts. This increases the survival of children and reduces mortality. On the other hand, children who had poor adherence to ART drugs will face several problems such as treatment failure, drug-resistant, the occurrence of OIs that could lead to death and poor outcomes. Thus, it would be important to provide treatment adherence counseling to the parents and caregivers of the child at the start of treatment and during the follow-up periods.
This study has some limitations. First, since this study was based on secondary data, some important variables were not available in the registers and therefore were not included in our study.
Second, those study subjects whose chart was not available in the ART clinic were not included in the study which may undermine the result if it is related to the study outcome. Finally, since we were unable to record the baseline socio-demographic and clinical characteristics for the incomplete and excluded records, we could not compare the study outcome between the excluded and included study subjects.
Conclusion
Mortality rate was high among TB/HIV co-infected children at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Age less than one year, having extra-pulmonary tuberculosis, being anemic, having fair or poor adherence, co-trimoxazole preventive therapy non-user, and isoniazid preventive therapy non-user were significant predictors of mortality among TB/HIV co-infected children.
Supporting information
(XLS)
Acknowledgments
The authors would like to thank the University of Gondar for financial support that made this study possible. They would also like to thank the data collectors for their tolerance and collaboration during the work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funding organization was University of Gondar.
References
- 1.Venturini E, Turkova A, Chiappini E, Galli L, de Martino M, Thorne C. Tuberculosis and HIV co-infection in children. BMC Infectious Diseases. 2014;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulson T. Epidemiology: a mortal foe. Nature. 2013;502(7470):S2–S3. doi: 10.1038/502S2a [DOI] [PubMed] [Google Scholar]
- 3.Liestøl K, Tretli S, Tverdal A, Mæhlen J. Tuberculin status, socioeconomic differences and differences in all-cause mortality: experience from Norwegian cohorts born 1910–49. International journal of epidemiology. 2009;38(2):427–34. doi: 10.1093/ije/dyn347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Social Science & Medicine. 2009;68(12):2240–6. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Global tuberculosis report 2016. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 6.Organization WH. Global tuberculosis report 2015: World Health Organization; 2015. [Google Scholar]
- 7.WHO. Use of high burden country lists for TB by WHO in the post-2015 era. Geneva, Switzerland; 2015.
- 8.WHO. The End-TB strategies IMPLEMENTING THE END-TB STRATEGY: THE ESSENTIALS. Geneva, Switzerland: WHO Press; 2015. [Google Scholar]
- 9.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50 Suppl 3:S184–94. [DOI] [PubMed] [Google Scholar]
- 10.Nwokeukwu HI, Okafor PN, Okorie O, Ukpabi IK. Paediatric Multidrug-Resistant Tuberculosis with HIV Coinfection: A Case Report. Case reports in medicine. 2013;2013:756152 doi: 10.1155/2013/756152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manosuthi W, Wongsawat J. Treatment challenges in co-infected HIV and TB children. Indian pediatrics. 2011;48(12):937–8. [DOI] [PubMed] [Google Scholar]
- 12.Yumo HA, Kuaban C, Neuhann F. WHO recommended collaborative TB/HIV activities: evaluation of implementation and performance in a rural district hospital in Cameroon. The Pan African medical journal. 2011;10:30 [PMC free article] [PubMed] [Google Scholar]
- 13.Bakeera-Kitaka S, Conesa-Botella A, Dhabangi A, Maganda A, Kekitiinwa A, Colebunders R, et al. Tuberculosis in human immunodeficiency virus-infected Ugandan children starting on antiretroviral therapy. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2011;15(8):1082–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos JM, Reyes F, Tesfamariam A. Childhood and adult tuberculosis in a rural hospital in Southeast Ethiopia: a ten-year retrospective study. BMC public health. 2010;10:215 doi: 10.1186/1471-2458-10-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlowski A JM, Sköld M, Rottenberg ME, Källenius G Tuberculosis and HIV Co-Infection. doi:101371/journalppat1002464. 2012; PLoS Pathog 8(2): e1002464 doi: 10.1371/journal.ppat.1002464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marais B, Graham S, Cotton M, Beyers N. Diagnostic and management challenges for childhood tuberculosis in the era of HIV. Journal of Infectious Diseases. 2007;196(Supplement 1):S76–S85. [DOI] [PubMed] [Google Scholar]
- 17.Marais B, Rabie H, Cotton M. TB and HIV in children–advances in prevention and management. Pediatric respiratory reviews. 2011;12(1):39–45. [DOI] [PubMed] [Google Scholar]
- 18.Isaakidis P, Paryani R, Khan S, Mansoor H, Manglani M, Valiyakath A, et al. Poor outcomes in a cohort of HIV-infected adolescents undergoing treatment for multidrug-resistant tuberculosis in Mumbai, India. PLoS ONE. 2013;8(7):e68869 doi: 10.1371/journal.pone.0068869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks RM, Padayatchi N, Shah NS, Wolf A, Werner L, Sunkari VB, et al. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2014;18(9):1074–83. [DOI] [PubMed] [Google Scholar]
- 20.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;43(1):42–6. doi: 10.1097/01.qai.0000230521.86964.86 [DOI] [PubMed] [Google Scholar]
- 21.Salvadori N, Ngo-Giang-Huong N, Duclercq C, Kanjanavanit S, Ngampiyaskul C, Techakunakorn P, et al. Incidence of Tuberculosis and Associated Mortality in a Cohort of Human Immunodeficiency Virus-Infected Children Initiating Antiretroviral Therapy. Journal of the Pediatric Infectious Diseases Society. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaakidis P, Casas E, Das M, Tseretopoulou X, Ntzani E, Ford N. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. The International Journal of Tuberculosis and Lung Disease. 2015;19(8):969–78. doi: 10.5588/ijtld.15.0123 [DOI] [PubMed] [Google Scholar]
- 23.Jeena P, Pillay P, Pillay T, Coovadia H. Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture-proven pulmonary tuberculosis in Durban, South Africa. The International Journal of Tuberculosis and Lung Disease. 2002;6(8):672–8. [PubMed] [Google Scholar]
- 24.Adejumo OA, Daniel OJ, Adebayo BI, Adejumo EN, Jaiyesimi EO, Akang G, et al. Treatment Outcomes of Childhood TB in Lagos, Nigeria. Journal of tropical pediatrics. 206;62(2):131–8. doi: 10.1093/tropej/fmv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesseling AC, Westra A, Werschkull H, Donald P, Beyers N, Hussey G, et al. Outcome of HIV-infected children with culture-confirmed tuberculosis. Archives of disease in childhood. 2005;90(11):1171–4. doi: 10.1136/adc.2004.070466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebonyi AO, Oguche S, Agbaji OO, Sagay AS, Okonkwo PI, Idoko JA, et al. Mortality among pulmonary tuberculosis and HIV-1 co-infected Nigerian children being treated for pulmonary tuberculosis and on antiretroviral therapy: a retrospective cohort study. Germs. 2016;6(4):139–50. doi: 10.11599/germs.2016.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madhi SA. Impact of HIV-1 co-infection on tuberculosis and value of CD4+ lymphocyte counts and concurrent antigen testing in interpretation of tuberculin reactions in hospitalized children with tuberculosis in South Africa 2014. [Google Scholar]
- 28.Stephen D, Wilkinson R, Lipman M, Wood R. Immune Reconstitution and ‘‘Unmasking” of Tuberculosis during Antiretroviral Therapy. AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE. 2008;177:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaakidis P, Cox HS, Varghese B, Montaldo C, Da Silva E, Mansoor H, et al. Ambulatory multi-drug resistant tuberculosis treatment outcomes in a cohort of HIV-infected patients in a slum setting in Mumbai, India. PLoS ONE. 2011;6(12):e28066 doi: 10.1371/journal.pone.0028066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buck WC, Olson D, Kabue M, Ahmed S, Nchama L, Munthali A, et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. The International Journal of Tuberculosis and Lung Disease. 2013;17(11):1389–95. doi: 10.5588/ijtld.13.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamla DD, Asadu C, Davies A, de Wagt A, Ilesanmi O, Adeyinka D, et al. Patching the gaps towards the 90-90-90 targets: outcomes of Nigerian children receiving antiretroviral treatment who are co-infected with tuberculosis. Journal of the International AIDS Society. 2015;18(Suppl 6):20251 doi: 10.7448/IAS.18.7.20251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwiru RS, Spiegelman D, Duggan C, Seage GR, Semu H, Chalamilla G, et al. Nutritional status and other baseline predictors of mortality among HIV-infected children initiating antiretroviral therapy in Tanzania. Journal of the International Association of Providers of AIDS Care (JIAPAC). 2013:2325957413500852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zar HJ, Cotton MF, Strauss S, Karpakis J, Hussey G, Schaaf HS, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomized controlled trial. The BMJ. 2007;334(7585):136 doi: 10.1136/bmj.39000.486400.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palme IB, Gudetta B, Bruchfeld J, Muhe L, Giesecke J. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. The Pediatric infectious disease journal. 2002;21(11):1053–61. doi: 10.1097/01.inf.0000036090.75121.f3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.