Abstract
Fuchs endothelial corneal dystrophy (FECD) is a degenerative disease characterized by corneal endothelial decompensation. FECD causes corneal stromal and epithelial edema and progressively develops into bullous keratopathy, which can eventually lead to blindness. However, the exact pathogenesis is unknown. In this study, we performed an in-depth bioinformatic analysis of the dataset GSE74123 to determine the differentially expressed genes (DEGs) of symptomatic late-onset FECD compared with a normal control. Gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways analysis were used to analyze the pathological molecular mechanism of FECD. We found that cell senescence, reactive oxygen species (ROS), the extracellular matrix (ECM), epithelial-mesenchymal transition (EMT) and immune response-related genes play an important role in the pathological development of symptomatic late-onset FECD. In addition, we revealed that down-regulated IL-6, enhanced NF-κB activity and a suite of orchestrated chemokine responses induce fibrocyte differentiation from monocyte to dendritic cell maturation. PI3K plays a key role in the molecular mechanism of symptomatic late-onset FECD. This study enhances our understanding of the molecular mechanism of FECD pathogenesis and will improve the diagnostics and therapy of FECD patients in the future.
Introduction
Fuchs endothelial corneal dystrophy (FECD), also known as cornea guttata, was originally reported and described by Fuchs in 1910[1]. FECD is a degenerative disease characterized by corneal endothelial decompensation. The characteristic features of FECD are the formation of corneal guttae, local thickening of the corneal Descemet's membrane (DM), a decline in corneal endothelial cell density and ion transport function[2]. The number of corneal endothelial cells (CECs) decreases, causing corneal endothelial dysfunction. Corneal stromal and epithelial edema progressively develop into bullous keratopathy, which can eventually lead to blindness[3]. Corneal transplantation is currently the only way to save a patient's vision[4].
According to the age of onset, FECD can be divided into two subtypes: early-onset (approximately 30 years old) and late-onset (approximately 50 years old). The incidence of late-onset FECD is higher. In America, the prevalence is approximately 4% of the population over the age of 40[5]. There are a variety of criteria for the clinical stage of FECD; the most commonly used system describes the disease progression in four stages[6]. Stage 1 is the corneal guttae period. Corneal guttae protruding from the DM are visible under corneal biomicroscopy and are typical signs of FECD. In Stage 2, as the disease progresses, the number of corneal guttae gradually increase. They gather, fuse with each other and extend to the periphery. Corneal guttae grow along the DM, and the density of corneal endothelial cell decreases. The cells’ hexagonal structure is damaged. The function of the corneal endothelial cell biological pump is impaired and corneal stromal edema increases. In Stage 3, stromal edema is exacerbated, and then epithelial and subepithelial bullae appear[6]. Bullae rupture can cause pain, photophobia, tearing and other symptoms[7, 8], and places a patient at a higher risk of infection. Stage 4, long-term chronic edema leads to the appearance of subepithelial connective tissue with reduced corneal transparency[6]. Corneal neovascularization and corneal scarring may occur with long-term corneal edema, resulting in a further decrease in visual acuity. FECD etiology factors include corneal endothelial cell apoptosis[9], sex hormones[10], inflammation[11], and aqueous humor flow[12] and composition[13], but the exact pathogenesis is unknown.
CECs are in cell cycle arrest and hardly proliferate in vivo. Therefore, corneal endothelial damage and losses in FECD are permanent and irreversible. CEC morphology changes are mainly reflected in changes in cell shape and size. Corneal confocal microscopy showed that CECs were pleomorphic, and that cell volume increased. The dark black area caused by corneal guttae was observed in the abnormal CECs[14]. CECs in symptomatic late-onset FECD are reduced in number and appear attenuated, causing progressive stromal edema.
Genetic analysis shows that FECD is associated with some gene mutations. Mutations in the COL8A2 gene occur in some cases of early-onset FECD[15]. COL8A2 is an extracellular matrix protein that is the main component of DM[16]. The more common delayed-onset FECD usually occurs after age 40 and may be familial. Mutations in the ZEB1, SLC4A11, TCF4, LOXHD1 and AGBL1 genes result in late-onset disease in a minority of familial and/or unrelated cases[17–21].
De Roo et al. reported that circulating fibrocytes and their dendritic derivatives are a new aspect of FECD. They used CEC monolayers with DM collected from patients with symptomatic late-onset FECD (clinical stages II to IV) during endothelial keratoplasty (CEC transplantation). They performed RNA microarray expression analysis (MEA) and Ingenuity Pathway Analysis (IPA), listed differentially expressed genes and plotted gene network comprising MHC class II molecules. The data suggest that both processes epithelial-mesenchymal transition (EMT) and circulating fibrocytes play a role in FECD. In this study, we used a bioinformatic approach which was different from original research, performed an in-depth bioinformatic analysis of the dataset GSE74123 to determine the differentially expressed genes (DEGs) of FECD compared with the normal control. First, the pathological molecular mechanism of FECD is obtained from Gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Then we try to explore the molecular mechanisms for the EMT and fibrocyte differentiation (monocyte/macrophage-dendritic cell maturation) in the corneal endothelial layer of symptomatic late-onset FECD. This study enhances our understanding of the molecular mechanism of FECD pathogenesis and will improve diagnostics and therapy for FECD patients in the future.
Materials and methods
Microarray data of FECD and normal control
The data set GSE74123 provided by De Roo et al. was downloaded from the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74123). The preparation of samples was described in a previous study[22]. Briefly, fresh CE monolayers with DM were prospectively collected from patients with symptomatic late-onset FECD (clinical stages II to IV) during endothelial keratoplasty (CE transplantation). The RNA of samples was extracted. Eight RNA samples were selected for microarray expression analysis: four FECD (age 75.5 ± 4.0; female: male ratio = 3:1) and four normal control samples (age 52.8 ± 18.8; female: male ratio = 0:4). An Affymetrix Human Gene 1.0 ST Array was used as the platform.
Microarray data processing and DEGs screening
Data normalization was performed using the “Shengxin.ren” tool, a free software for data analysis (http://gap.shengxin.ren). All samples and genes were clustered by MATLAB. Principal component analysis (PCA) was performed using ImageGP (http://www.ehbio.com/ImageGP/index.php/Home/Index/index.html). Pearson’s Correlation Coefficient was calculated to evaluate the degree of linear correlation between the two samples. The expression of the FECD group was compared to the control group. Genes with | log2 (fold change) | > 1 and P value < 0.05 were selected as the DEGs.
Functional enrichment analysis of DEGs
All DEGs were submitted to the Database for Annotation, Visualization and Integrated Discovery (DAVID, version 6.8; https://david.ncifcrf.gov/). Gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were screened with P value <0.05.
Protein-protein interaction (PPI) network
Protein-protein interaction (PPI) analysis is necessary to illustrate the molecular mechanisms. In this study, the Search Tool for the Retrieval of Interacting Genes (STRING; http://string-db.org/) database was used to construct a PPI network. We picked the genes that appeared more than 10 times in significantly different KEGG pathways. These genes were submitted to STRING. An interaction score of 0.4 was defined as the screened threshold. The important, significant pathways were labeled on the nodes.
Results
Preliminary analysis of dataset GSE74123
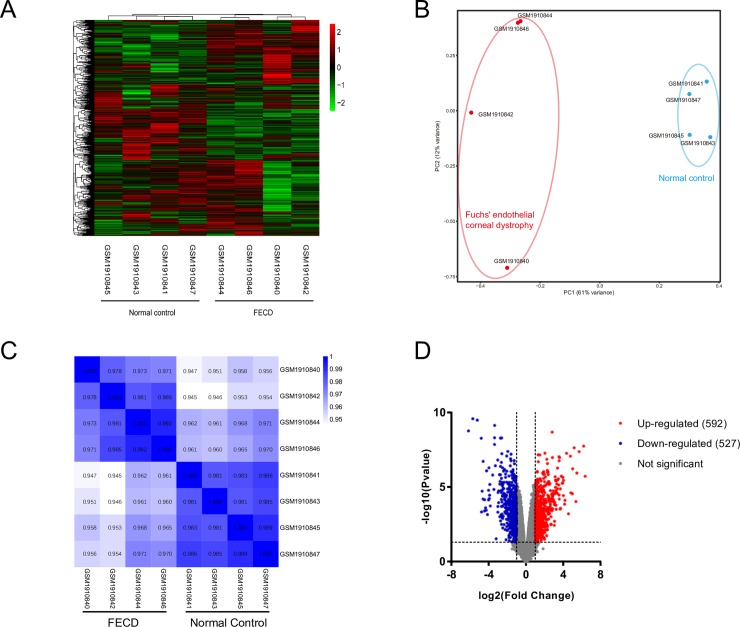
GSE74123 was downloaded from the GEO database. After normalization, cluster analysis of the samples and genes was performed. Fig 1A shows significant differences between the FECD group and normal control group. PCA is a statistical method that can analyze the major influencing factors from multiple sources. We performed a PCA analysis of the normalized relative gene expression to show the grouping information for both groups. Similarly, significant differences between the two groups of samples were also found in PCA analysis. Fig 1B shows that the FECD group and control group were clustered into two separate regions, indicating that they had good biological reproducibility. A Pearson’s heatmap (Fig 1C) shows the correlation between the two groups of samples. There was a high correlation within each group of samples, but the control group was higher. A good biological replicate proved that the project's biological experiments can be repeated with little variation.
Fig 1. The summary of the dataset GSE74123.
(A) The cluster heatmap of GSE74123. (B) The PCA of GSE74123 showed that significant differences between the two groups of samples. (C) Pearson heatmap showed the correlation between the two groups of samples. There was a high correlation within each group of samples. (D) The volcano plot showed the distribution of all genes by fold change and P value. The expression of FECD group was compared with control group. Genes with |log2 (fold change) | > 1 and P value < 0.05 were selected as the DEGs.
The expression of the FECD group was compared with the control group. Genes with | log2 (fold change) | > 1 and P value < 0.05 were selected as the DEGs. There were 592 DEGs up-regulated and 527 DEGs that were down-regulated. The volcano plot shows the distribution of all genes in terms of fold change and P value. Red dots represent up-regulated genes, and blue dots represent down-regulated genes (Fig 1D).
These results demonstrated that the FECD group and control group had good biological repeatability. The gene expression of samples in the two groups were significantly different, and thus in-depth bioinformatic analysis could be performed.
GO enrichment analysis
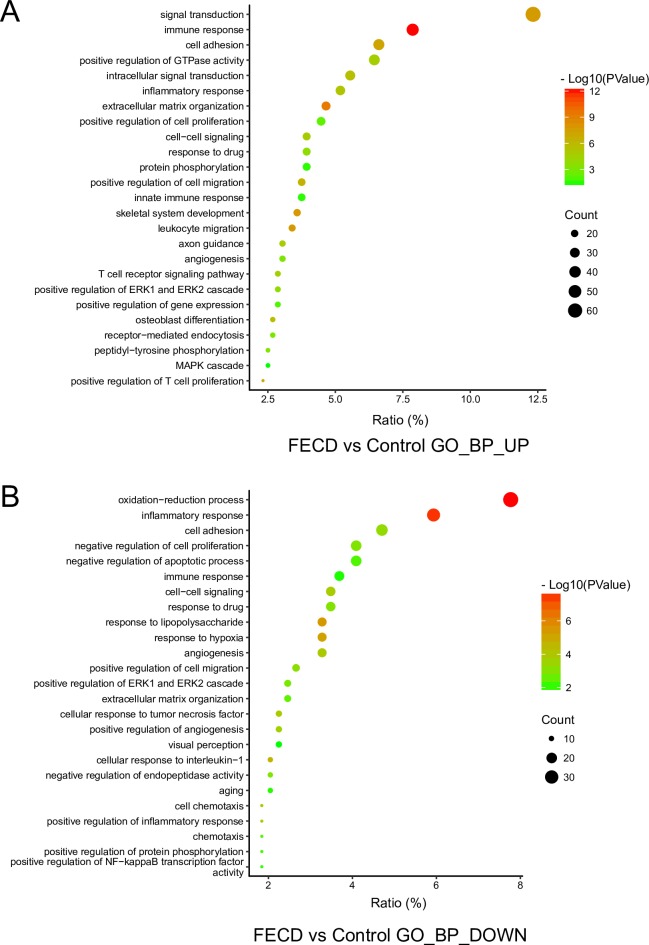
GO terms consist of Biological Process (BP), Cellular Component (CC) and Molecular Function (MF). The up-regulated DEGs and down-regulated DEGs were submitted to DAVID to analyze GO enrichment. The entire list is shown in S1 Table. We picked the top 25 significantly up-regulated BP GO terms (Fig 2A), including immune response, extracellular matrix organization, positive regulation of cell proliferation, and leukocyte migration. The Top 25 significantly down-regulated BP GO terms were also selected (Fig 2B), including oxidation-reduction process, negative regulation of cell proliferation, negative regulation of apoptotic processes, and visual perception. Notably, the second top GO term of up-regulated BP is immune response, and the top GO term of down-regulated BP is oxidation-reduction process. The Top 25 significantly CC and MF GO terms are shown in S1 Fig. The top GO terms of up-regulated CC and MF are plasma membrane and calcium ion binding, respectively. The top GO terms of down-regulated CC and MF are plasma membrane and receptor binding respectively.
Fig 2. The biological process (BP) in GO enrichment of DEGs.
(A) The top 25 significantly up-regulated BP GO terms in FECD group. (B) The top 25 significantly down-regulated BP GO terms in FECD group.
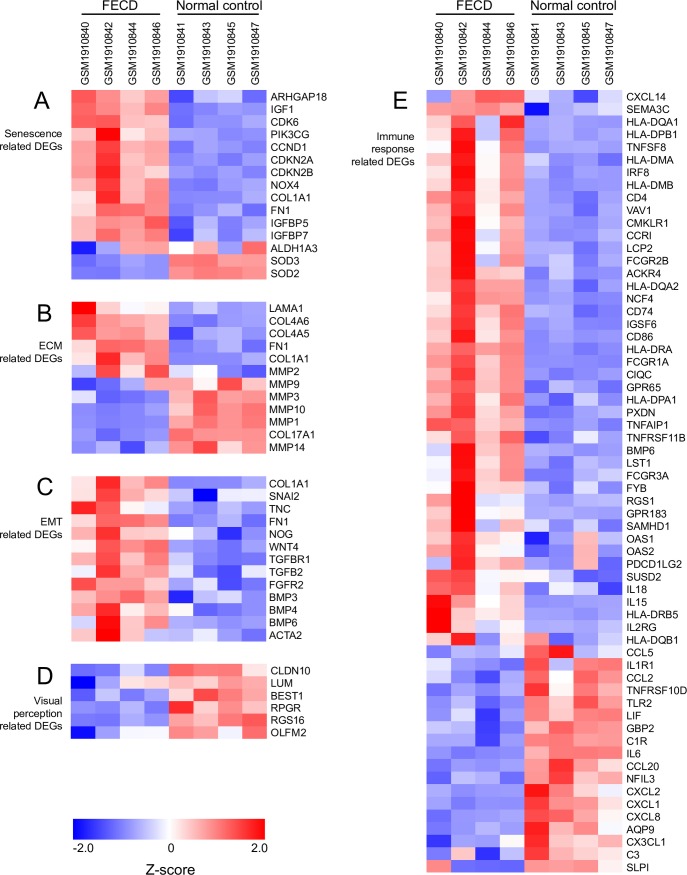
Expression changes of cell senescence, ECM, EMT and immune response related genes
According to the results of DEGs and GO enrichment, five major types of genes were selected for analysis. First, 15 senescence-related DEGs were selected, based on the study of Matthaei et al.[23]. Compared to the control, genes in FECD related to cell senescence, such as CDKN2A and CDKN2B, were up-regulated. Oxidase NOX4 and proliferation genes CCND1, CDK6 were also up-regulated. Antioxidase SOD2, SOD3 were down-regulated (Fig 3A).
Fig 3. Heatmaps of four major types of genes.
(A) Senescence, (B) ECM, (C) EMT, (D) visual perception and (E) immune response related DEGs were picked and normalized by Z-score. Red represents up-regulated expression. Blue represents up-regulated expression.
Furthermore, the extracellular matrix- (ECM) related genes were up-regulated, and matrix metalloproteinases (MMPs) related genes were down-regulated (Fig 3B). Additionally, epithelial-mesenchymal transition- (EMT) related genes were all up-regulated, especially the key EMT markers COL1A1, SNAI2, TNC, FN1 and ACTA2 (Fig 3C). The visual perception-related genes, such as CLDN10 and LUM, were down-regulated (Fig 3D). In addition, in the immune response-related genes, human leukocyte antigen (HLA) genes were up-regulated. CD4, CD74 and CD86 were up-regulated. Interleukin IL15 and IL18 were up-regulated, while IL6 was down-regulated. Inflammatory chemokines (CCL2, CCL20, CXCL1, CXCL2, CXCL8) were down-regulated (Fig 3E).
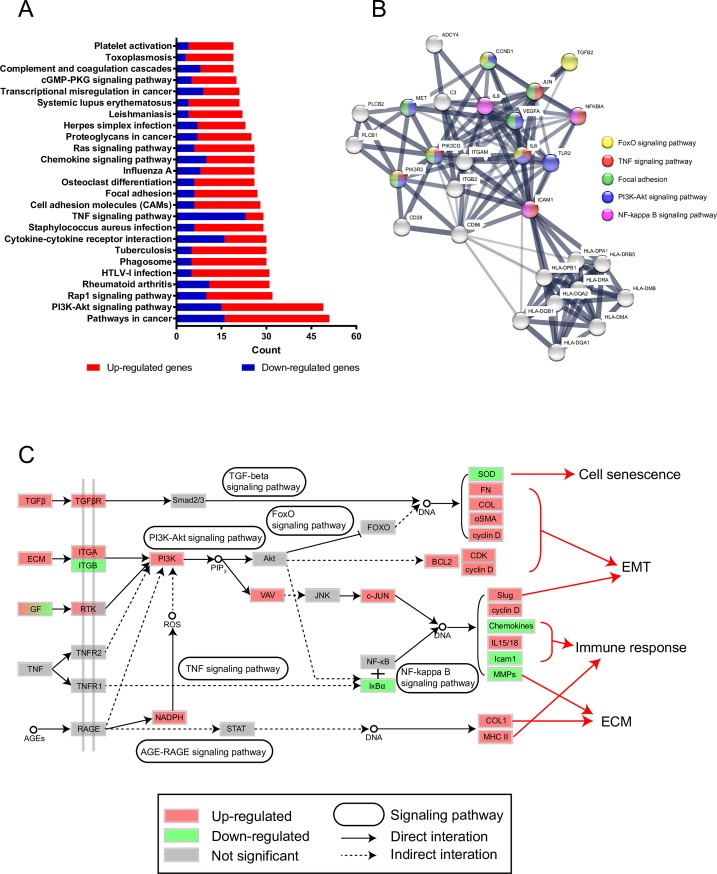
KEGG signaling pathway analysis
Various genes coordinate with each other to exercise their biological functions. The most important biochemical metabolic pathways and signal transduction pathways involved in differentially expressed genes can be identified using KEGG significant enrichment. In this study, we submitted all DEGs to DAVID to analyze the KEGG signaling pathways. The entire list is shown in S2 Table. The top 25 significantly different pathways were selected (Fig 4A), including pathways in cancer, the PI3K-Akt signaling pathway, Rap1 signaling pathway, TNF signaling pathway, focal adhesion, and some disease pathways such as rheumatoid arthritis, tuberculosis, Influenza A, and Toxoplasmosis. Then, we listed the genes of all significantly different pathways and counted genes that appeared more than ten times. We plotted PPI with these genes and labeled some important pathways (Fig 4B). It is obvious that PI3K play a key role in the molecular mechanism.
Fig 4. Signaling pathway analysis in symptomatic late-onset FECD.
(A) Top 25 significantly different pathways were selected according to DEGs in FECD. (B) PPI of genes which appeared more than 10 times in significantly different pathways. Some important pathways in this study were labeled on it. (C) Signaling pathway network was plotted according to KEGG database.
According to the above results, we plotted the possible signaling pathway network (Fig 4C). Up-regulated TGF-β activated its receptor, resulting in increased signal in the TGF-β signaling pathway. EMT-related proteins such as fibronectin, collagen and α-SMA were up-regulated, while cyclin D was also up-regulated. The increase in integrin, growth factor (GF) receptor, TNF receptor and reactive oxygen species (ROS) directly or indirectly activated PI3K protein, which increased the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), activated the PI3K-Akt pathway and indirectly affected the FOXO signaling pathway, resulting in the down-regulation of SOD2 and SOD3. Up-regulation of PIP3 activated Vav and indirectly activated the transcription factor c-Jun. Through the TNF signaling pathway, the expression of IκBα was down-regulated so that the transcription factor NF-κB can enter the nucleus. c-Jun and NF-κB caused up-regulation of Slug, cyclin D, IL15, IL18, and down-regulation of chemokines, ICAM1, and MMPs. The increase in advanced glycation end products (AGEs) led to activation of the AGE-RAGE signaling pathway and up-regulation of collagen I and the major histocompatibility complex (MHC) II.
Discussion
FECD usually seriously affects binocular vision. It may cause blurred vision or even blindness[24, 25]. Previous studies have found that changes in cell function, such as ECM deposition[26], oxidative stress[27], apoptosis[9] and EMT[26, 28], play a key role in the formation of FECD. However, FECD pathogenesis and the molecular mechanism are incomplete[24–26, 29, 30]. De Roo et al. [22] performed MEA and IPA, listed differentially expressed genes and plotted gene network comprising MHC class II molecules. In this study, we used bioinformatic analysis to select DEGs between FECD and the normal control in GSE74123. The pathological molecular mechanism of FECD is obtained from GO terms and KEGG pathway analysis. We try to explore the molecular mechanisms for the EMT and fibrocyte differentiation in the corneal endothelial layer of symptomatic late-onset FECD.
The main function of CECs is to maintain the transparency of the cornea through the selective barrier and active pump functions. In this study, for symptomatic late-onset FECD, the expression of CLDN10 (claudin10) was significantly down-regulated in FECD (Fig 3D). This indicates that both the CEC barrier and pump functions are damaged in symptomatic late-onset FECD. Members of the Claudin family play a key role in maintaining tight junctions where located in various types of epithelial cells and endothelial cells[31–33]. Tight junctions form biological barriers with ion selective permeability. CECs positively express claudin-10 and negatively express claudin-14. This protein expression combination can be used to identify CECs[34]. Under normal circumstances, sodium ions enter anterior chamber through tight junctions to enable corneal endothelium pumps to work properly. Claudin-10 plays a key role in sodium ion transport of CEC tight junctions. The down-regulated expression of claudin-10 might prevent sodium ion transport through tight junctions[34].
Furthermore, in the present study, the expression of immune response-related genes was up-regulated. HLA genes were up-regulated. CD4, CD74 and CD86 were up-regulated. Interleukin IL15 and IL18 were up-regulated, while IL6, CXCL1, CXCL2, CXCL8, CX3CL1, LIF, C3, CCL2, C1R, TLR2, GBP2 and SLPI were down-regulated (Fig 3E). CD74 is involved in the formation and transportation of MHC class II (HLA) molecules[35]. CD86 is a protein expressed on antigen-presenting cells that provides costimulatory signals necessary for T cell activation and survival[36]. IL15 regulates the activity and proliferation of T cells and NK cells[37]. After stimulation with IL-18, NK cells and certain T cells release another important cytokine—interferon-γ (IFN-γ) or type II interferon—that plays an important role in activating macrophages or other cells[38]. The up-regulation of the above gene expression shows a marked inflammatory and immune response in corneal endothelium and DM in symptomatic late-onset FECD. Meaningfully, here we showed the down-regulated genes of IL6, TLR, CXCL1, CXCL2, CXCL8 in symptomatic late-onset FECD. Previous reports showed that IL-6 skewed the differentiation of dendritic cells into macrophages[39, 40]. IL-6 secretion by dendritic cells followed TLR activation. IL-6 has been shown to inhibit NF-κB activity in dendritic cells[41], suggesting that IL-6 may influence their maturation or trafficking. Normally, IκBα blocks the ability of NF-κB transcription factors to bind to DNA[42]. In this study, IκBα was expressed less in FECD, leading to increased NF-κB activity, further causing changes in the expression of EMT, MMPs and immune-related genes. Therefore, we hypothesize that down-regulated IL-6, enhanced NF-κB activity and a suite of orchestrated chemokine responses induce fibrocyte differentiation from monocytes to dendritic cell maturation in the corneal endothelial layer of symptomatic late-onset FECD.
The production of reactive oxygen species (ROS) may aggravate many inflammatory diseases. There are lots of ROS produced by polymorphonuclear neutrophils (PMNs) in inflammation. While ROS cause vascular endothelial dysfunction and tissue damage [43]. In many kinds of cells, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase leads to the production of large amounts of ROS, especially in phagocytes and vascular endothelial cells[44]. Superoxide dismutase (SOD) not only eliminates the effects of ROS, but it is also an important mechanism for cell anti-apoptosis[45]. In the present study, NOX4 (NADPH oxidase 4) was significantly up-regulated, while SOD2 and SOD3 were significantly down-regulated (Fig 3A). This demonstrated a significant increase in ROS activity in the corneal endothelium of symptomatic late-onset FECD. ROS can induce cell senescence and death. In some cell lines, sublethal amounts of ROS lead to cell cycle arrest and senescence-related changes[46]. In this study, the senescence-related markers CDKN2A and CDKN2B were significantly up-regulated (Fig 3A), demonstrating the senescence of CECs in symptomatic late-onset FECD. Due to the senescence and death of some CECs, other CECs migrate or alter their shape to maintain the integrity of the corneal endothelial barrier. Corneal endothelial pump function will show a compensatory increase to maintain the corneal deturgescent state[24, 25]. However, as time goes on, the senescence and death of CECs increases, and corneal endothelial barrier function is gradually lost.
Inflammation and ROS can further cause EMT[47]. Befitting EMT is beneficial for wound healing, but excessive EMT causes corneal endothelial fibrosis and the loss of barrier and pump function. Increased EMT leads cells to accumulate more ECM[48]. In this study, for symptomatic late-onset FECD, COL1A1, SNAI2, FN1 and ACTA2 were up-regulated. The accumulation of ECM is the main reason for the thickening of DM and is also a factor in the formation of guttae[29]. The number of guttae is inversely proportional to the corneal endothelial cell density, as the coalescence of guttae is accompanied by a continual loss of CECs[24]. It also results in the loss of corneal endothelial barrier function and further exacerbates corneal edema, forming a vicious cycle.
In this study, we also found that visual perception-related gene expression levels were significantly down-regulated in FECD (Fig 3D). LUM (lumican) is a major keratan sulfate proteoglycan of the cornea. It can be combined with collagen fibers, regulating their spatial arrangement[49]. The down regulation of lumican may be related to the disordered arrangement of DM collagen layers in FECD. Interestingly, some retina-related genes (BEST1, RPGR, RGS16, OLFM2) were remarkably down-regulated in the present study. Mutations in the BEST1 (bestrophin 1) gene are causally associated with as many as five clinically distinct retinal degenerative diseases[50]. The Retinitis Pigmentosa GTPase Regulator (RPGR) is located in the photoreceptor connecting cilia. It interacts with a wide variety of ciliary proteins[51]. Snow et al. found that RGS16 (Regulator of G-protein signaling 16) was abundantly expressed in retina, with lower levels of expression in most of the other tissues examined[52]. Sultana et al revealed that olfactomedin 2 (OLFM2) may play an important role in the course of retinal and eye development[53]. However, the function of these genes in FECD is currently unknown.
In summary, we picked the DEGs of FECD in GSE74123 using bioinformatics analysis. The FECD phenotype and related gene expression levels were related using GO enrichment and KEGG pathway results. We not only confirmed MHC class II type antigen presentation, inflammation, EMT, and ECM production found in previous study, but also drew some new conclusions such as cell senescence, downregulation of CLDN10 and visual perception related genes. In addition, GO terms, KEGG pathways and PPI network were performed. It was inferred that FECD was related to the PI3K-Akt, TGF-β and NFκB signaling pathway. Down-regulated IL-6, enhanced NF-κB activity and a suite of orchestrated chemokine responses induce fibrocyte differentiation from monocyte to dendritic cell maturation. PI3K plays a key role in the molecular mechanism. The original authors' subsequent research also confirmed the role of NFκB signaling pathway in FECD[11]. The possible pathological and molecular mechanisms of symptomatic late-onset FECD were preliminarily analyzed and diagrammatized (Fig 5). Therefore, for FECD nonsurgical therapy, we might consider therapeutic strategies on anti-inflammatory, anti-ROS and anti-EMT activities, as well as inhibition of NF-κB activity and PI3K activity. However, these conclusions are merely hypotheses, suggested by the results of gene expression analysis, which need further biologically verification in the future.
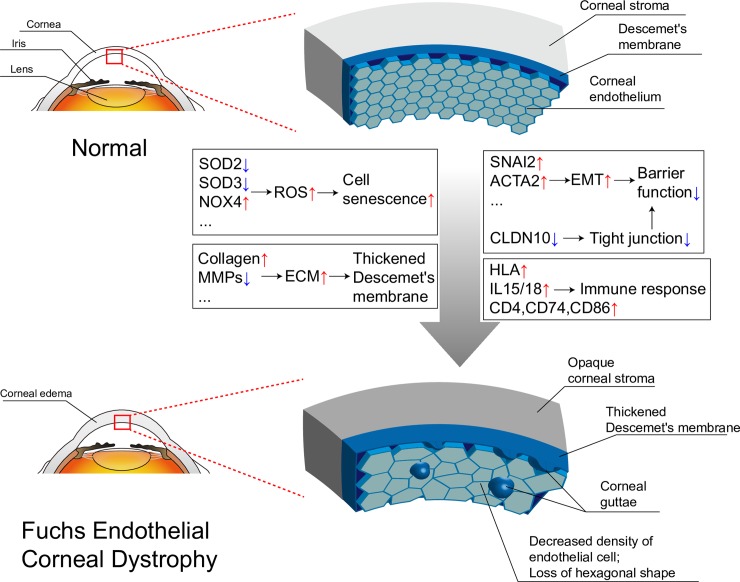
Fig 5. The schematic diagram of possible pathological and molecular mechanisms of symptomatic late-onset FECD.
In the symptomatic late-onset of FECD, corneal stroma is cloudy. DM becomes thick. Corneal guttae appear. The density of CECs is decreased. CECs lose their hexagonal shape. The reason for these phenomena is that the expression of cell senescence, EMT, ECM and immune response related genes changes.
Supporting information
The top 25 significantly up-regulated CC (A) and MF (B) GO terms in FECD group. The top 25 significantly down-regulated CC (C) and MF (D) GO terms in FECD group.
(PDF)
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Natural Science Foundation of China (no. 81371689) and Special Funds for Major Science and Technology Projects of Guangdong Province (2015B010125007).
References
- 1.Fuchs E. Dystrophia epithelialis corneae. Graefe's Archive for Clinical and Experimental Ophthalmology. 1910;76(3):478–508. [Google Scholar]
- 2.Iwamoto T, DEVOE AG. Electron microscopic studies on Fuchs' combined dystrophy I. Posterior portion of the cornea. Investigative Ophthalmology & Visual Science. 1971;10(1):9–28. [PubMed] [Google Scholar]
- 3.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7(1):2–18. [PubMed] [Google Scholar]
- 4.Thompson RW, Price MO, Bowers PJ, Price FW. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110(7):1396–402. doi: 10.1016/S0161-6420(03)00463-9 [DOI] [PubMed] [Google Scholar]
- 5.Krachmer JH, Purcell JJ, Young CW, Bucher KD. Corneal endothelial dystrophy: a study of 64 families. Archives of ophthalmology. 1978;96(11):2036–9. [DOI] [PubMed] [Google Scholar]
- 6.Adamis AP, Filatov V, Tripathi BJ. Fuchs' endothelial dystrophy of the cornea. Survey of ophthalmology. 1993;38(2):149–68. [DOI] [PubMed] [Google Scholar]
- 7.Yanoff M. BS Fine Ocular pathology Mosby-Wolfe, Barcelona: 1996:276–8. [Google Scholar]
- 8.Roy F. Current ocular therapy WB. Saunders Company Philadelphia, Pa; 1995. [Google Scholar]
- 9.Li QJ, Ashraf MF, Shen D, Green WR, Stark WJ, Chan C-C, et al. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Archives of ophthalmology. 2001;119(11):1597–604. [DOI] [PubMed] [Google Scholar]
- 10.Kim JJ, Yu HG, Ku SY. Sex Steroid Hormone and Ophthalmic Disease. Korean Journal of Reproductive Medicine. 2010;37(2):89–98. [Google Scholar]
- 11.De Roo A-K, Janssens T, Foets B, van den Oord JJ. Immunohistochemical Profiling of Corneas With Fuchs Endothelial Corneal Dystrophy. Cornea. 2017;36(7):866–74. doi: 10.1097/ICO.0000000000001212 [DOI] [PubMed] [Google Scholar]
- 12.Wilson SE, Bourne WM, O’Brien PC, Brubaker RF. Endothelial function and aqueous humor flow rate in patients with Fuchs’ dystrophy. American journal of ophthalmology. 1988;106(3):270–8. [DOI] [PubMed] [Google Scholar]
- 13.Richardson MR, Segu ZM, Price MO, Lai X, Witzmann FA, Mechref Y, et al. Alterations in the aqueous humor proteome in patients with Fuchs endothelial corneal dystrophy. Molecular vision. 2010;16:2376 [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman SC, Beuerman RW, Kaufman HE. Diagnosis of advanced Fuchs' endothelial dystrophy with the confocal microscope. American journal of ophthalmology. 1993;116(5):652–3. [DOI] [PubMed] [Google Scholar]
- 15.Mok J, Kim H, Joo C. Q455V mutation in COL8A2 is associated with Fuchs' corneal dystrophy in Korean patients. Eye. 2009;23(4):895–903. doi: 10.1038/eye.2008.116 [DOI] [PubMed] [Google Scholar]
- 16.Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, et al. Compositional differences between infant and adult human corneal basement membranes. Investigative ophthalmology & visual science. 2007;48(11):4989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riazuddin SA, Zaghloul NA, Al-Saif A, Davey L, Diplas BH, Meadows DN, et al. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. The American Journal of Human Genetics. 2010;86(1):45–53. doi: 10.1016/j.ajhg.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vithana EN, Morgan PE, Ramprasad V, Tan DT, Yong VH, Venkataraman D, et al. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Human molecular genetics. 2007;17(5):656–66. doi: 10.1093/hmg/ddm337 [DOI] [PubMed] [Google Scholar]
- 19.Mehta JS, Vithana EN, Tan DT, Yong VH, Yam GH, Law RW, et al. Analysis of the posterior polymorphous corneal dystrophy 3 gene, TCF8, in late-onset Fuchs endothelial corneal dystrophy. Investigative ophthalmology & visual science. 2008;49(1):184–8. [DOI] [PubMed] [Google Scholar]
- 20.Riazuddin SA, Parker DS, McGlumphy EJ, Oh EC, Iliff BW, Schmedt T, et al. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. The American Journal of Human Genetics. 2012;90(3):533–9. doi: 10.1016/j.ajhg.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riazuddin SA, Vasanth S, Katsanis N, Gottsch JD. Mutations in AGBL1 cause dominant late-onset Fuchs corneal dystrophy and alter protein-protein interaction with TCF4. The American Journal of Human Genetics. 2013;93(4):758–64. doi: 10.1016/j.ajhg.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Roo A-K, Wouters J, Govaere O, Foets B, van den Oord JJ. Identification of Circulating Fibrocytes and Dendritic Derivatives in Corneal Endothelium of Patients With Fuchs' DystrophyFibrocytes in Fuchs' Endothelial Corneal Dystrophy. Investigative Ophthalmology & Visual Science. 2017;58(1):670–81. [DOI] [PubMed] [Google Scholar]
- 23.Matthaei M, Zhu AY, Kallay L, Eberhart CG, Cursiefen C, Jun AS. Transcript profile of cellular senescence-related genes in Fuchs endothelial corneal dystrophy. Experimental eye research. 2014;129:13–7. doi: 10.1016/j.exer.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elhalis H, Azizi B, Jurkunas UV. Fuchs endothelial corneal dystrophy. The ocular surface. 2010;8(4):173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Patel DV. The pathophysiology of Fuchs' endothelial dystrophy–a review of molecular and cellular insights. Experimental eye research. 2015;130:97–105. doi: 10.1016/j.exer.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 26.Okumura N, Minamiyama R, Ho LT, Kay EP, Kawasaki S, Tourtas T, et al. Involvement of ZEB1 and Snail1 in excessive production of extracellular matrix in Fuchs endothelial corneal dystrophy. Laboratory Investigation. 2015;95(11):1291–304. doi: 10.1038/labinvest.2015.111 [DOI] [PubMed] [Google Scholar]
- 27.Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. The American journal of pathology. 2010;177(5):2278–89. doi: 10.2353/ajpath.2010.100279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katikireddy KR, White TL, Miyajima T, Vasanth S, Raoof D, Chen Y, et al. NQO1 downregulation potentiates menadione-induced endothelial-mesenchymal transition during rosette formation in Fuchs endothelial corneal dystrophy. Free Radical Biology and Medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmedt T, Silva MM, Ziaei A, Jurkunas U. Molecular bases of corneal endothelial dystrophies. Experimental eye research. 2012;95(1):24–34. doi: 10.1016/j.exer.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulsen ET, Dyrlund TF, Runager K, Scavenius C, Krogager TP, Højrup P, et al. Proteomics of Fuchs’ endothelial corneal dystrophy support that the extracellular matrix of Descemet’s membrane is disordered. Journal of proteome research. 2014;13(11):4659–67. doi: 10.1021/pr500252r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troy T-C, Arabzadeh A, Larivière NM, Enikanolaiye A, Turksen K. Dermatitis and aging-related barrier dysfunction in transgenic mice overexpressing an epidermal-targeted claudin 6 tail deletion mutant. PloS one. 2009;4(11):e7814 doi: 10.1371/journal.pone.0007814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turksen K. Wasted salts and wasted bodies: new insight into the role of claudin 7 in the kidney. Am Physiological Soc; 2010. [DOI] [PubMed] [Google Scholar]
- 33.Arabzadeh A, Troy T-C, Turksen K. Changes in the distribution pattern of Claudin tight junction proteins during the progression of mouse skin tumorigenesis. BMC cancer. 2007;7(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inagaki E, Hatou S, Yoshida S, Miyashita H, Tsubota K, Shimmura S. Expression and Distribution of Claudin Subtypes in Human Corneal EndotheliumClaudin Subtypes in Human Corneal Endothelium. Investigative ophthalmology & visual science. 2013;54(12):7258–65. [DOI] [PubMed] [Google Scholar]
- 35.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annual review of immunology. 1994;12(1):259–91. [DOI] [PubMed] [Google Scholar]
- 36.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1(9):793–801. [DOI] [PubMed] [Google Scholar]
- 37.Carson WE, Giri JG, Lindemann M, Linett ML, Ahdieh M, Paxton R, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. Journal of Experimental Medicine. 1994;180(4):1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novick D, Kim S, Kaplanski G, Dinarello CA, editors. Interleukin-18, more than a Th1 cytokine Seminars in immunology; 2013: Elsevier. [DOI] [PubMed] [Google Scholar]
- 39.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nature immunology. 2000;1(6):510–4. doi: 10.1038/82763 [DOI] [PubMed] [Google Scholar]
- 40.Mitani H, Katayama N, Araki H, Ohishi K, Kobayashi K, Suzuki H, et al. Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. British journal of haematology. 2000;109(2):288–95. [DOI] [PubMed] [Google Scholar]
- 41.Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-κB binding activity and CCR7 expression. The FASEB journal. 2004;18(12):1439–41. doi: 10.1096/fj.03-0969fje [DOI] [PubMed] [Google Scholar]
- 42.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes & development. 1995;9(22):2723–35. [DOI] [PubMed] [Google Scholar]
- 43.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxidants & redox signaling. 2014;20(7):1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pendyala S, Natarajan V. Redox regulation of Nox proteins. Respiratory physiology & neurobiology. 2010;174(3):265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adler V, Yin Z, Tew KD, Ronai Ze. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18(45). [DOI] [PubMed] [Google Scholar]
- 46.Lu T, Finkel T. Free radicals and senescence. Experimental cell research. 2008;314(9):1918–22. doi: 10.1016/j.yexcr.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer research. 2004;64(20):7464–72. doi: 10.1158/0008-5472.CAN-04-1725 [DOI] [PubMed] [Google Scholar]
- 48.Radisky DC. Epithelial-mesenchymal transition. Journal of cell science. 2005;118(19):4325–6. [DOI] [PubMed] [Google Scholar]
- 49.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. The Journal of cell biology. 1998;141(5):1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson AA, Guziewicz KE, Lee CJ, Kalathar RC, Pulido JS, Marmorstein LY, et al. Bestrophin 1 and retinal disease. Progress in Retinal and Eye Research. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Megaw RD, Soares DC, Wright AF. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Experimental eye research. 2015;138:32–41. doi: 10.1016/j.exer.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snow BE, Antonio L, Suggs S, Siderovski DP. Cloning of a retinally abundant regulator of G-protein signaling (RGS-r/RGS16): genomic structure and chromosomal localization of the human gene. Gene. 1998;206(2):247–53. [DOI] [PubMed] [Google Scholar]
- 53.Sultana A, Nakaya N, Senatorov VV, Tomarev SI. Olfactomedin 2: Expression in the Eye and Interaction with Other Olfactomedin Domain–Containing Proteins. Investigative ophthalmology & visual science. 2011;52(5):2584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The top 25 significantly up-regulated CC (A) and MF (B) GO terms in FECD group. The top 25 significantly down-regulated CC (C) and MF (D) GO terms in FECD group.
(PDF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.