Abstract
The dentate gyrus is essential for remembering the fine details of experiences that comprise episodic memory. Dentate gyrus granule cells receive highly-processed sensory information and are hypothesized to perform a pattern separation function, whereby similar sensory inputs are transformed into orthogonal neural representations. Behaviorally, this is believed to enable distinct memory for highly interfering stimuli. Since the dentate gyrus is comprised of a large number of adult-born neurons, which have unique synaptic wiring and neurophysiological firing patterns, it has been proposed that neurogenesis may contribute to this process in unique ways. Some behavioral evidence exists to support this role, whereby neurogenesis-deficient rodents are impaired at discriminating the fine visuospatial details of experiences. However, the extent to which newborn neurons contribute to dentate gyrus-dependent learning tasks is unclear. Furthermore, since most studies of dentate gyrus function are conducted in male rats, little is known about how females perform in similar situations, and whether there might be sex differences in the function of adult neurogenesis. To address these issues, we examined spatial discrimination memory in transgenic male and female rats that lacked adult neurogenesis. The first task probed memory for the position of local objects in an open field, assessed by behavioral responses to novel object locations. The second task examined memory for distal environmental cues. All rats were able to successfully discriminate local and distal cue changes. Males and females also performed comparably, although females displayed higher levels of rearing and locomotion. Collectively, our results indicate that rats are capable of learning about local and distal cues in the absence of adult neurogenesis.
Introduction
The dentate gyrus (DG) is the primary interface between the neocortex and the hippocampus proper. It receives highly-processed information about the external environment via the entorhinal cortex and is believed to perform a pattern separation function whereby similar inputs are transformed into distinct neural codes [1,2]. Behaviorally, this has been proposed to enable discrimination of similar sensory stimuli that are prone to interference.
While physiological assays have confirmed a pattern separation function for the DG, these experiments are technically challenging [3,4]. Thus, many have turned to behavioral tests of putative pattern separation-dependent, discrimination functions for the DG. In mice, deletion of DG NMDA receptors leads to impaired context discrimination in a fear conditioning paradigm [5]. DG-specific lesions and/or molecular manipulations also lead to impaired discrimination memory for object location and spatial geometry when stimuli are highly interfering [6–8]. Such a discrimination function is not limited to animals, as imaging studies indicate that the DG-CA3 region is specifically recruited when subjects are asked to distinguish visual stimuli that are distinct, but bear a high resemblance, to previously-studied items [9]. Notably, in conditions such as aging and depression, which are associated with impaired hippocampal and DG function, there is poorer performance on human behavioral pattern separation tasks [10–12].
One feature that sets the DG apart from many regions of the mammalian brain is its ability to produce new neurons throughout adult life. These newborn neurons have enhanced synaptic plasticity in vitro, enhanced morphological plasticity in response to learning, and distinct wiring in hippocampal and entorhinal circuits [13]. While the exact function for newborn neurons remains a topic of intense investigation, a number of animal studies suggest they may be involved in DG discrimination functions. Mice that have reduced or increased levels of neurogenesis show impaired or enhanced context discrimination abilities in fear conditioning paradigms, respectively [14–16] (but see [17,18]). Neurogenesis-deficient rodents are also impaired on spatial discrimination in radial maze and touchscreen paradigms [19], and they are less able to learn lists of interfering odors [20]. Finally, DG-specific manipulation of BDNF and Wnt signalling suggests that new neurons are required for remembering closely-related object locations [21].
While it remains unclear whether and/or how adult-born neurons might contribute to computational pattern separation [22,23], these theoretical perspectives have played a significant role in guiding research on behavioral discrimination functions. The conditions under which new neurons contribute to behavioral discrimination remain unclear however. For example, many behavioral tasks vary in the degree of stress used for motivation, which could differentially recruit adult-born neurons [24]. Differences in behavioral requirements could also arise as a function of the testing paradigm and the sex of the animals. Indeed, in a context fear discrimination task, neurogenesis-deficient female mice discriminate normally but neurogenesis-deficient males show better discrimination learning than intact controls [17]. Also, in a radial maze, male rats that employed spatial strategies were better at discriminating similar locations and had greater neurogenesis compared to females [25]. Here, we therefore tested male and female transgenic neurogenesis-deficient rats in a DG-dependent, object-location discrimination paradigm that measures memory purely based upon rodents’ natural tendency to explore novelty. In a second test, we examined rats’ ability to detect distal cue novelty. While our transgenic manipulation eliminated adult neurogenesis, we found that both male and female rats performed similarly, suggesting that adult neurogenesis is not critical for detecting at least some types of changes to the local and distal cue environment.
Materials and methods
Subjects
To examine behavioral functions of adult-born neurons we used GFAP-TK transgenic rats, in which neurogenesis can be selectively inhibited in adulthood via antiviral drug treatment [26]. Male and female GFAP-TK rats and their wild type littermates were bred in-house on a Long-Evans background, housed with their parents until they were weaned at 21 days of age, and kept on a 12-h light/dark cycle (lights on at 7:00 am). Experimental rats were pair housed in transparent polyurethane bins (48 × 27 × 20 cm) with a single polycarbonate tube, aspen chip bedding and ad libitum rat chow and tap water. The estrous cycle of female subjects was not monitored. To inhibit adult neurogenesis, at 6 weeks of age rats were orally administered 4 mg of valganciclovir (Hoffman La-Roche; delivered in 0.5 g peanut butter+chow pellets) twice per week. All experiments were approved by the Animal Care Committee at the University of British Columbia and conducted in accordance with the Canadian Council on Animal Care.
Behavioral testing
Rats were treated with valganciclovir for 7–9 weeks (to inhibit DG neurogenesis) and began behavioral testing at 13 (males) or 15 (females) weeks of age (age difference because the sexes were tested separately; n = 6-8/group; one male TK rat was excluded from the object location task because the power went out during testing). Valganciclovir treatment stopped once behavioral testing started, but neurogenesis remained reduced until the end of the study (Fig 1). The week before testing started, animals were handled 5 min per day for 5 days. Rats were first tested in a proximal object-location discrimination test and then 7 days later a distal cue discrimination test (Fig 1D and 1E). All handling and behavior testing was conducted by female experimenters. On test days, animals were placed in the hallway in front of the experimental room 45 min before testing started. Both tests were performed in a square open field with transparent acrylic walls (70 cm wide × 70 cm long × 50 cm high), with aspen wood chip bedding covering the floor and 2 objects (sand-filled glass soda bottles) secured to the floor. The open field was placed in a dimly-lit small room (~2 x 2 m) with a door and posters on the walls to provide distal spatial cues. Both tasks consisted of 6 x 5 min trials. For the training trials (1–5) all environmental cues remained constant and rats were placed in an empty cage outside of the room for 3 min between each trial. There was a 10 min intertrial interval prior to trial 6, the testing trial, when cues were manipulated. The object-location discrimination task was modelled after Hunsaker et al. (2008) [7] and Goodrich-Hunsaker et al. (2008) [27], where the intervals prior to the final testing trial were 3 min and 10 min, respectively. Objects were placed in the center of the open field and spaced 45 cm apart for trials 1–5. On trial 6 the objects were shifted to 10 cm apart. In the distal cue novelty task the environment was identical to the object-location training trials (including bottle locations). However, on trial 6 dark curtains were hung on 2 distal room walls. In between trials objects were cleaned with 70% EtOH, feces were removed, and bedding was stirred to prevent odors from accumulating. Between groups of male and females the open field was thoroughly cleaned and bedding was replaced.
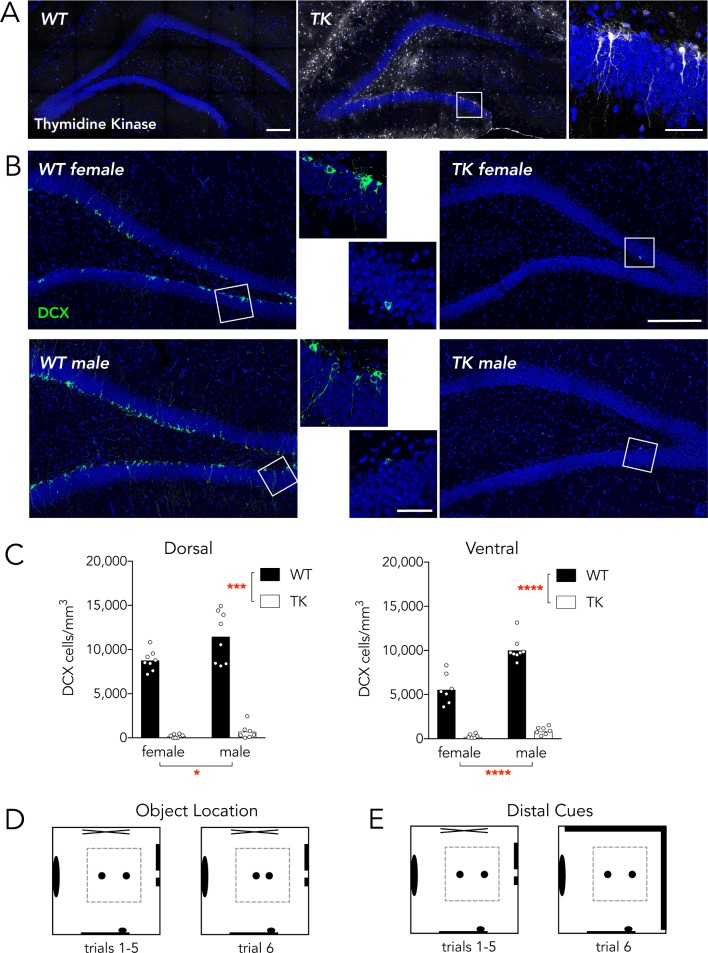
Fig 1. Neurogenesis knockdown and study design.
A) Wild type and TK rats were immunostained for thymidine kinase after testing to confirm genotypes. Thymidine kinase-positive cells can be observed throughout the DG of TK rats. Inset shows thymidine kinase-positive radial glial cells in the subgranular zone. Scale bars: 200 μm for low magnification images, 50 μm for high magnification image. B) Male and female TK rats had dramatic reductions in neurogenesis, as visualized by immunostaining for the immature neuronal marker, DCX. Scale bars as in A. C) Mean DCX+ cell densities were reduced in TK animals and were greater in males than in females (Dorsal DG: effect of genotype F1,27 = 267, P<0.001; effect of sex F1,27 = 7.3, P<0.05; interaction F1,27 = 3.5, P = 0.08; Ventral DG: effect of genotype F1,27 = 280, P<0.0001; effect of sex F1,27 = 34, P<0.0001; interaction F1,27 = 20, P = 0.0002). In the ventral DG, all groups were different from each other (all P<0.0001) except TK males vs TK females (P = 0.9) *P<0.05, ***P<0.001, ****P<0.0001. D) The object location discrimination test took place in a transparent-walled open field. During training, 2 bottles in the open field were spaced 45 cm apart during habituation trials 1–5 and 10 cm apart during test trial 6. E) The distal cue discrimination test used the same habituation environment but for the test trial a dark curtain covered 2 of the room walls.
All trials were recorded by an overhead camera for offline analyses. The distance covered during each trial was measured by Ethovision software (Noldus). A human experimenter manually quantified the frequency of object exploration and rearing events. Exploration events were defined as continuous bouts of bottle sniffing and investigation (regardless of duration) where the rat’s nose was ≤ 2 cm from the bottle and the rat was directly exploring the bottle. Nondirected body/tail contact with the bottle, or contact when rearing (relatively rare) was not counted as bottle exploration. Rearing events, i.e. standing upright on hind legs, were interpreted as information-gathering behaviors directed at the distal environment [28]. The absolute time spent investigating and rearing was also quantified but these data were more variable. Since the patterns and trends were closely correlated between the 2 measures we therefore focused our analyses on the frequency data (all data are available in S1 File). To further probe novelty responses on trial 6 compared to trial 5 we also calculated discrimination indices for each measure (trial 6/(trial 5+6)), which provided a single quantification of memory performance for each animal where values above 0.5 indicate greater-than-chance responses to novelty.
Histology and microscopy
Following testing rat’s brains were extracted and immersed in 4% paraformaldehyde for 48 hours. Brains were then transferred to a 10% glycerol solution for 1 day and a 20% glycerol solution for at least 2 days before being sectioned at 40 μm on a freezing sliding microtome. Dorsal hippocampal sections (~4 mm posterior to Bregma) were stained for thymidine kinase to confirm genotypes and dorsal and ventral (~6 mm posterior to Bregma) sections were stained for the immature neuronal marker doublecortin to confirm inhibition of neurogenesis (Fig 1). Immunohistochemistry was performed with goat anti-HSV-1 thymidine kinase (sc-28038; Santa Cruz Biotechnology, USA) and goat anti-doublecortin (sc-8066; Santa Cruz Biotechnology, USA) primary antibodies, diluted in PBS with 0.05% triton-x and 3% normal horse serum, on free-floating sections. Secondary detection was performed with Alexa488-conjugated secondary antibodies (Life Technologies, USA) diluted in PBS. Sections were counterstained with DAPI, mounted onto slides, coverslipped with PVA-DABCO, and imaged with a Leica SP8 confocal microscope. DCX+ cell density measurements were calculated by dividing the number of DCX+ cells by the granule cell layer volume in one dorsal and one ventral section per animal.
Statistical analyses
Behavior across trials 1–6 was analyzed by 3-way repeated measures ANOVA. Mauchly’s test of sphericity was used to test for equal within-subject variance across trials and, when sphericity was violated, the Greenhouse-Geisser correction was applied. Where significant main effects or interactions were observed, post hoc comparisons were performed using Sidak’s test. Discrimination indices were analyzed by sex x genotype ANOVAs and individual groups were compared to chance using a one sample t-test. In all cases significance was set at p = 0.05.
Results
Neurogenesis reduction
Immunohistochemical staining for doublecortin (DCX) in the dentate gyrus following behavioral testing revealed extensive neurogenesis in valganciclovir-treated WT rats, as indicated by immature neurons scattered throughout the DG. In contrast, valganciclovir nearly completely inhibited adult neurogenesis in all male and female TK rats, in both the dorsal and ventral DG (Fig 1B and 1C). The density of DCX+ cells was greater in males than in females, in both the dorsal and ventral DG.
The reduction of DCX+ cells in TK rats is likely to reflect reductions in neurogenesis, and unlikely to be confounded by compensatory neurogenesis, for several reasons. First, GFAP+ stem cells are likely at the top of the neurogenic hierarchy, and so by killing them we eliminate all possible neurogenic cells. Second, we have shown that DCX labels ~90% of immature BrdU+ cells, which is comparable to the proportion of neurons that later express pan-neuronal markers such as NeuN [29]. Thus, even if additional neurons were generated from a distinct type of precursor (ie non-GFAP+), we expect that they would still be labelled by DCX during their immature stages (as far as we know there are no studies showing that newborn DG neurons do not express DCX). Finally, other studies that have reported reductions in DCX+ cells in GFAP-TK rats have confirmed the efficacy of the model using other markers such as BrdU/NeuN [26] and NeuroD [30].
Object location discrimination task
For 5 consecutive trials, rats explored the open field with 2 objects spaced 45 cm apart; on the 6th trial the distance between the objects was reduced to 10 cm. The first measure of performance in the object-location task was the frequency of object exploration events. There was a main effect of trial on object exploration but no effect of sex or genotype (see statistical analyses in figure legend). Rats explored the bottle less across the 5 habituation trials (Fig 2A). Critically, exploration time doubled on trial 6 relative to trial 5, indicating that rats detected the new bottle locations. This pattern was also apparent in the discrimination indices, all of which were greater than chance, indicating successful discrimination of object location (Fig 2B).
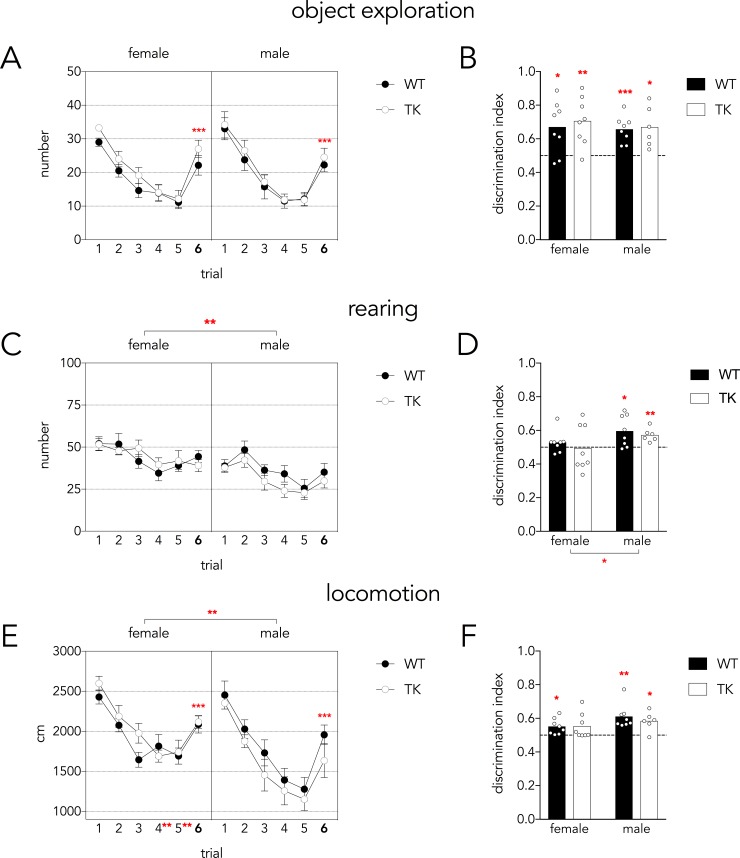
Fig 2. Object location discrimination task.
A) The number of object exploration events decreased over the 5 habituation trials but doubled on trial 6 when object locations were changed (Mauchly’s test P = 0.3; effect of trial F5,130 = 54, P<0.001, partial η2 = 0.68; trial 6 vs trials 3,4,5 all P<0.001). There were no differences between sexes, genotypes and no interactions (all P>0.16). B) All groups showed above-chance discrimination indices, indicating increased object exploration on trial 6 relative to trial 5. Variance was not different across groups (Levene’s test F3,26 = 1.9, P = 0.16). C) The number of rearing events decreased over the 5 habituation trials (Mauchly’s test P = 0.6; effect of trial F5,130 = 11, P<0.001, partial η2 = 0.30; trial 4&5 vs 1&2 all P<0.001) but did not change between trials 5 and 6 (P = 0.8). There were no genotype differences or interactions (all P>0.1) but females reared significantly more than males (F1,26 = 14, P<0.01, partial η2 = 0.35). D) The rearing discrimination index was above chance only in males and was greater in males than females (effect of sex F1,26 = 4.5, P = 0.04, partial η2 = 0.15). Due to unequal variance (Levene’s test P = 0.002) and a lack of sex x genotype interaction, genotypes were pooled and the sex difference was reanalyzed, confirming a greater rearing discrimination index in males and homogeneity of variance across groups (T28 = 2.2, P = 0.03; Levene’s P = 0.25) E) Locomotion decreased over the 5 habituation trials in both sexes (Mauchly’s test P = 0.4; effect of trial, F5,130 = 56, P<0.001, partial η2 = 0.68) and increased from trial 5 to trial 6 following object displacement (P<0.001). Locomotion was greater in females than males, specifically on trials 4 and 5 (effect of sex, F1,26 = 10, P<0.01, partial η2 = 0.27; trial x sex interaction F5,130 = 2.3, P<0.05, partial η2 = 0.82; trials 4&5 different between males and females P<0.01). F) The locomotor discrimination index did not differ between genotypes (P = 0.6, partial η2 = 0.01) or sexes (P = 0.07, partial η2 = 0.12). Discrimination indices revealed above-chance levels of discrimination, except for female TK rats. Variance was not different across groups (Levene’s test F3,26 = 0.7, P = 0.6). For all graphs: *P<0.05, **P<0.01, ***P<0.001. Graphs in panels A,C,E indicate mean ± standard error.
We next examined the frequency of rearing events. Again, we found a main effect of trial, indicating that rats habituated to the distal testing environment over the course of testing (Fig 2C). Females reared 32% more than males, but the number of rearing events did not change between trials 5 and 6, and the rearing discrimination indices were at chance. In males, there was no significant increase in the absolute number of rearing events from trial 5 to 6. However, the discrimination indices were greater in males than in females (Fig 2D). Moreover, only male discrimination indices were greater than chance, indicating sex differences in the behavioral response to local cue manipulations.
We then examined the distance travelled over the course of the 6 trials of the object-location task. There was a main effect of trial, with a significant increase in locomotion between trials 5–6 (22% in females, 48% in males), indicating that rats habituated to the environment and successfully detected the novel object location. While males and females initially covered similar distances on the early trials, males showed greater habituation over time such that locomotion was significantly reduced on trials 4 and 5 relative to females (Fig 2E). Discrimination indices were not different between males and females or WT and TK rats, and most groups were significantly above chance performance (Fig 2F).
Distal cue novelty task
To test rats’ ability to detect changes in distal cues they were exposed to the open field for 5 consecutive training trials. On the 6th trial a black curtain covered 2 adjacent walls (Fig 1E). Over the 6 trials, rats explored the objects progressively less. They did not show any signs of increased bottle exploration following the distal cue manipulation, in terms of trial 5–6 differences or in the discrimination indices (Fig 3A and 3B).
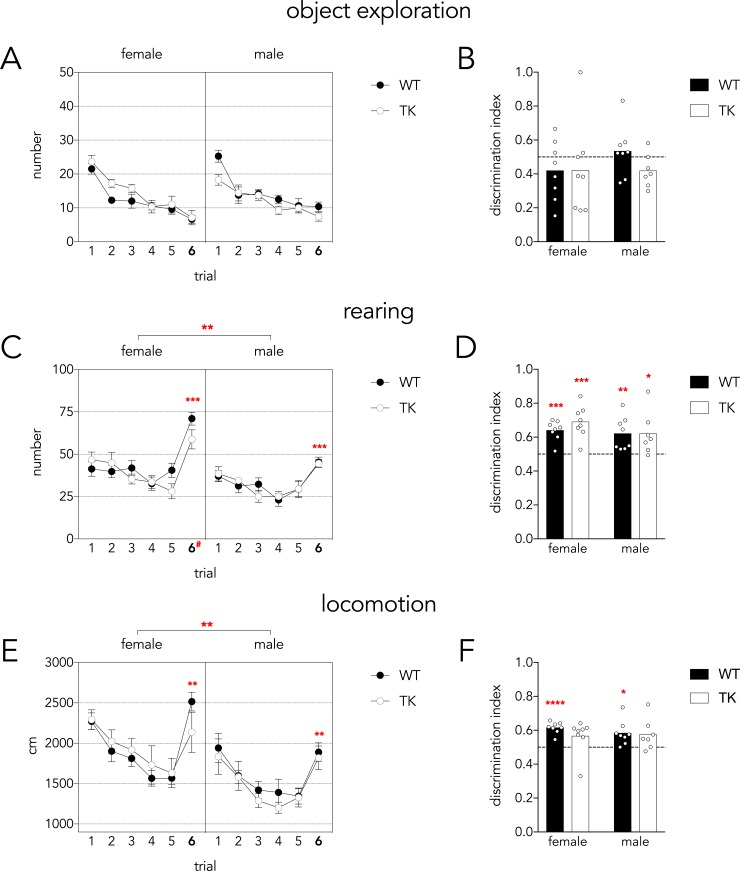
Fig 3. Distal cue novelty task.
A) Rats decreased exploration of the objects over the 6 trials (Mauchly’s test P = 0.2; trial effect, F5,135 = 44, P<0.001, partial η2 = 0.62) and did not increase exploration upon changes to the distal context on trial 6 (trial 6 vs trials 1–4 all P<0.01, trial 6 vs trial 5 P = 0.3). There were no genotype or sex differences (all P>0.5). B) Discrimination indices were not different between groups (genotype and sex effects P>0.4) and were not different from chance (all P>0.2), indicating that object exploratory behavior was unaffected by changes to distal cues. Variance was not different across groups (Levene’s test F3,27 = 1.5, P = 0.25). C) Rearing events declined modestly over the 5 habituation trials and increased upon changes to the distal context on trial 6 (Mauchly’s test P = 0.2; effect of trial, F5,135 = 32, P<0.001, partial η2 = 0.54; trial 6 vs all other trials P<0.001). Females reared more than males, particularly on trial 6 (sex effect F1,27 = 14, P<0.01, partial η2 = 0.33; trial x sex interaction F5,135 = 2.6, P = 0.03, partial η2 = 0.087; females vs males on trial 6 #P<0.0001). WT and TK rats did not differ (F1,27 = 2, P = 0.15, partial η2 = 0.015). D) Rearing discrimination indices did not differ between sexes or genotypes (both effects P>0.2) and all indices showed greater than chance levels of discrimination. Variance was not different across groups (Levene’s test F3,27 = 1.1, P = 0.4). E) Distance travelled declined over trials until trial 6 when the distal cues were changed (Mauchly’s test P = 0.001; Greenhouse-Geisser corrected trial effect F2.9,135 = 22, P<0.001, partial η2 = 0.45; trial 6 significantly greater than trials 3–5, P<0.01). Females covered a greater distance than males (sex effect F1,27 = 17, P<0.001, partial η2 = 0.39) and WT and TK rats did not differ (P = 0.7). F) The locomotion discrimination index did not differ between genotypes or sexes (main effects P>0.3). Locomotion discrimination indices tended to be above chance levels of performance but only female TK and male WT indices were significantly above chance. Variance was not different across groups (Levene’s test F3,27 = 0.9, P = 0.4). For all graphs: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). Graphs in panels A,C,E indicate mean ± standard error.
The most robust response to the contextual change was observed in rearing behavior. Rearing decreased slightly over the first 5 trials but increased significantly on trial 6, indicating rats detected the changes to the distal context (Fig 3C). Females reared significantly more than males (30% more overall), especially after the context change on trial 6. Rearing discrimination indices were above chance for all groups and did not differ between sexes or genotypes.
Locomotor behavior also changed in response to the distal cue changes. There was a decline in distance travelled over trials 1–5 but distance increased significantly on trial 6 (42% increase; Fig 3E). Females covered 25% greater distance than males. Locomotor discrimination indices were consistent with distal cue novelty detection, though not all groups’ indices were statistically greater than chance (Fig 3F).
Discussion
Summary of main findings
Here we investigated the requirement of adult-born hippocampal neurons in an object location memory task that is designed to tap into DG functions in fine grained spatial processing. We also examined whether adult-born neurons are required to detect changes to the distal cue environment. Our principal finding is that adult neurogenesis was not required for learning either of these tasks. For the most part, males and females learned the tasks similarly. This is notable since no studies have systematically examined behavioral requirements for adult neurogenesis in male and females, and the vast majority of open field, novelty-based memory studies have exclusively used males. Consistent with previous studies, females were more active in the open field. Also, only males showed increased rearing behavior in response to local object location changes, suggesting sex differences in the behavioral response to local cue reconfigurations.
Dentate gyrus and neurogenesis functions in memory
The DG is a major site of convergence of different forms of sensory information and, given the ability of DG neurons to undergo changes in synaptic strength, is well-positioned to mediate rapid encoding of sensory experience. Specifically, DG neurons receive inputs from the medial and lateral entorhinal cortices; the medial entorhinal cortex conveys spatial signals and information about self-motion and position in space [31–33]. In contrast, the lateral entorhinal cortex conveys information about the identity and spatial location of objects in the environment [34,35]. The convergence of these inputs in the hippocampus produces high level representations that are the result of unique configurations of spatial, self and object-related cues [36–38]. The specificity of DG place fields is consistent with this function and supports the computational role of the DG in pattern separation, whereby input patterns are orthogonalized to reduce memory interference [3]. A critical behavioral role for the DG in spatial processing extends back to studies demonstrating that DG dysfunction leads to deficits in spatial water maze learning and context-specific expression of fear [5,39,40]. A specific role for adult-generated DG neurons is supported by several studies that have found that they promote context-specific fear discrimination [14,15,41], context-specific ensemble codes in CA3 [42], memory for highly-interfering spatial locations [19] (but see [43]) and lists of interfering odor pairs [20]. Furthermore, emerging evidence suggests that mature neurons are more critical for behaviors that may rely on pattern completion functions whereas immature neurons necessary for behaviors that may rely more on pattern separation functions [16,44].
Here we exploited rats’ innate exploratory response to novelty [45]. This approach has been used on numerous occasions to reveal functions for medial temporal lobe structures in memory for objects, contexts, time, and their combinations. An advantage of novelty-induced exploration is that it does not rely on aversive stimuli or food deprivation for motivation. This methodological difference is significant because adult-born neurons (and the hippocampus more broadly) regulate behavioral and endocrine responses to stressors [24,46–48], and their functions in these more cognitive, less stressful, learning paradigms may therefore be distinct.
Novelty-induced exploration paradigms have identified roles for the DG and adult-born neurons in object-related memory. Rats with DG lesions, and mice with disrupted juvenile DG neurogenesis, are specifically impaired at detecting changes in local object positions when there is a high degree of spatial similarity between training and testing [8,27,49]. Learning highly-interfering spatial object configurations upregulates BDNF specifically in the DG and both BDNF and new neurons have been implicated in 24 hour object location memory [6,21]. Neurogenesis-deficient mice are impaired on (flexible) spatial discrimination of object-like cues presented on a touchscreen [19,43]. Notably, in addition to their role in remembering object location, adult-born neurons are also involved in object novelty detection [50–52]. Given the role of the lateral entorhinal cortex in these various types of object-related processing [34,35], and its preferential connectivity with adult-born neurons [53], there is broad support for a role for neurogenesis in object-related memory.
In our study, ablating adult neurogenesis did not impact rats’ ability to discriminate changes in the spatial positioning of objects. While this would seem to be at odds with previous studies, a number of methodological factors might have contributed to the differing reports. For example, deficits in the object-location discrimination task have been reported in mice that have had reduced neurogenesis since the juvenile period [54]. Behavioral effects may have been more apparent due to a greater net reduction in neurogenesis. Our task may have been easier than the one employed by Hunsaker et al. (2008) since we used 5 training trials instead of 3 (we found that rats did not learn reliably with only 3 training trials). Smaller spatial changes might also be more likely to reveal deficits, though ours were broadly comparable to those used previously and we did not even observe a trend for impaired performance in the TK rats. Our findings would seem to be at odds with the observation of Bekinschtein et al. (2014) that rats with reduced Wnt signalling and DG neurogenesis had impaired memory for object position. This could be due to differences in the spatial environment (they used a larger, circular open field with more objects and smaller changes to object location) or the testing schedule (theirs had only a single training trial and a longer, 24 hr, training-test interval) that could have made their task more difficult. Additionally, different methods to reduce neurogenesis could be at play (e.g. Wnt signalling contributes to synaptic plasticity [55]).
In contrast to the local cue manipulations of the first task, in the second task we manipulated the distal cue environment. In hippocampal-dependent tasks such as the water maze, distal cues are the primary source of visuospatial information. Manipulation of the distal environment is also known to regulate hippocampal place fields [56]. Since, neurogenesis deficient animals have been found to be impaired in spatial tasks that rely on accurate memory for distal cues [19,57,58], we reasoned that TK rats might show differences in responding to changes in the distal cue environment. However, as in the object discrimination task, neurogenesis-deficient rats performed normally. Given the role of the hippocampus in high-order, conjunctive encoding, future studies should expand the investigation to include novelty-based exploration tasks that require differential association of stimuli with spatial locations, contexts and temporal intervals [59].
Behavioral patterns following local and distal cue changes
There were specific and complementary patterns of behavior depending on task and sex. Over the 5 learning trials in both tasks, all measures tended to decrease as rats habituated to the environment. Males and females tended to show similar habituation curves except for locomotion, where males habituated to a greater extent on the later trials (particularly in the object location task, where the open field was more novel). Females also displayed greater amounts of rearing and locomotion in both tasks. This is consistent with a large literature indicating that female rodents are more active than males. In open field environments, and when given access to running wheels, females consistently rear more and cover greater distance [60–62]. As studies incorporating both males and females become more common, it will be important to account for activity differences when using novelty responses to assess memory (e.g. to ensure that high baseline activity does not obscure novelty responses).
The raw scores and discrimination indices revealed distinct behavioral patterns in the two tasks: object exploration increased only when object positions changed, rearing increased only when the distal cues changed, and locomotion increased following changes to both proximal objects and distal cues. While the discrimination indices may seem small it is unlikely that they are at floor, and unable to detect differences between groups. They are well within the range of what is commonly observed in tasks that use innate exploration preferences as a measure of memory. Furthermore, for the primary measures (object exploration in the object task and rearing in the distal cue task) the indices are consistently 0.6 to 0.7, where 0.66 corresponds to a doubling in absolute measures across trials 5–6. It is also worth noting that “chance” performance (0.5) indicates equivalent behavior on trials 5 and 6 but, in previous studies, impaired DG function actually results in discrimination indices that are below chance, presumably because the animals do not notice the environmental change on the test trial and continue to habituate [27]. Finally, floor effects for the discrimination indices are unlikely to be an issue because, in the case of the rearing discrimination index in the object task, it was able to reveal a male-specific rearing response even though the rearing indices were less robust than the object discrimination indices. Since rearing is typically interpreted as a behavior that promotes investigation of the distal environment, this suggests that males may be more likely to re-explore the distal environment when there are changes to local cues. Considered another way, this finding may be consistent with evidence that women tend to have better object-related memory than men and, while rodent data are equivocal, it might also fit with reports of sex differences in object-location memory (reviewed in [63]).
Conclusions
In sum, our results indicate that neurogenesis is not essential for short-term memory for local and distal cues, in males and females. These findings are in contrast with previous studies showing that the DG and neurogenesis contributes to various types of spatial discrimination memory. Functions of adult neurogenesis may be specific for certain types of conjunctive associations, and they could also be critically dependent on task parameters. Another possibility, is that new neurons may become involved in learning when there is a stressful component involved [24]. Given the fact that sex differences in hippocampal function emerge often in response to stress [64,65], it could be fruitful to investigate whether new neuron functions in (spatial discrimination) memory emerge in stressful situations.
Supporting information
Behavioral data for all of the animals, including some data that was analyzed but not included in the published manuscript (e.g. behavioral event durations).
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (JSS), the German Research Foundation (DRS), the Brain and Behavior Research Foundation (DRS), the Michael Smith Foundation for Health Research (to JSS), and the Canadian Institutes of Health Research (to JSS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34: 515–525. doi: 10.1016/j.tins.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79: 1–48. doi: 10.1016/j.pneurobio.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 3.Neunuebel JP, Knierim JJ. CA3 Retrieves Coherent Representations from Degraded Input: Direct Evidence for CA3 Pattern Completion and Dentate Gyrus Pattern Separation. Neuron. Elsevier Inc; 2014;81: 416–427. doi: 10.1016/j.neuron.2013.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leutgeb JK, Leutgeb S, Moser M-B, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315: 961–966. doi: 10.1126/science.1135801 [DOI] [PubMed] [Google Scholar]
- 5.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317: 94–99. doi: 10.1126/science.1140263 [DOI] [PubMed] [Google Scholar]
- 6.Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, et al. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 2013;5: 759–768. doi: 10.1016/j.celrep.2013.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18: 955–964. doi: 10.1002/hipo.20455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11: 626–636. doi: 10.1002/hipo.1077 [DOI] [PubMed] [Google Scholar]
- 9.Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319: 1640–1642. doi: 10.1126/science.1152882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21: 968–979. doi: 10.1002/hipo.20808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Déry N, Pilgrim M, Gibala M, Gillen J, Wojtowicz JM, Macqueen G, et al. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front Neurosci. 2013;7: 66 doi: 10.3389/fnins.2013.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shelton DJ, Kirwan CB. A possible negative influence of depression on the ability to overcome memory interference. Behav Brain Res. 2013;256: 20–26. doi: 10.1016/j.bbr.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 13.Vivar C, van Praag H. Functional circuits of new neurons in the dentate gyrus. Front Neural Circuits. 2013;7: 15 doi: 10.3389/fncir.2013.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tronel S, Belnoue L, Grosjean N, Revest J-M, Piazza P-V, Koehl M, et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22: 292–298. doi: 10.1002/hipo.20895 [DOI] [PubMed] [Google Scholar]
- 15.Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472: 466–470. doi: 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149: 188–201. doi: 10.1016/j.cell.2012.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cushman JD, Maldonado J, Kwon EE, Garcia AD, Fan G, Imura T, et al. Juvenile neurogenesis makes essential contributions to adult brain structure and plays a sex-dependent role in fear memories. Front Behav Neurosci. 2012;6: 3 doi: 10.3389/fnbeh.2012.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder JS, Cameron HA. Reduced adult neurogenesis alters behavioural and endocrine discriminative fear conditioning. Figshare. 2014; doi: 10.6084/m9.figshare.884597.v4 [Google Scholar]
- 19.Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. 2009;325: 210–213. doi: 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luu P, Sill OC, Gao L, Becker S, Wojtowicz JM, Smith DM. The role of adult hippocampal neurogenesis in reducing interference. Behav Neurosci. 2012;126: 381–391. doi: 10.1037/a0028252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, et al. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus. 2014;24: 905–911. doi: 10.1002/hipo.22304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70: 589–596. doi: 10.1016/j.neuron.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker S. Neurogenesis and pattern separation: time for a divorce. WIREs Cogn Sci. John Wiley & Sons, Inc; 2017;8: e1427 doi: 10.1002/wcs.1427 [DOI] [PubMed] [Google Scholar]
- 24.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476: 458–461. doi: 10.1038/nature10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow C. Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus. 2016;26: 87–101. doi: 10.1002/hipo.22493 [DOI] [PubMed] [Google Scholar]
- 26.Snyder JS, Grigereit L, Russo A, Seib DR, Brewer M, Pickel J, et al. A Transgenic Rat for Specifically Inhibiting Adult Neurogenesis. eNeuro. 2016;3 doi: 10.1523/ENEURO.0064-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122: 16–26. doi: 10.1037/0735-7044.122.1.16 [DOI] [PubMed] [Google Scholar]
- 28.Lever C, Burton S, O'Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Reviews in the neurosciences. 2006;17: 111–133. [DOI] [PubMed] [Google Scholar]
- 29.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29: 14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galinato MH, Takashima Y, Fannon MJ, Quach LW, Morales Silva RJ, Mysore KK, et al. Neurogenesis during abstinence is necessary for context-driven methamphetamine-related memory. J Neurosci. Society for Neuroscience; 2018;38: 2011–17–14. doi: 10.1523/JNEUROSCI.2011-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B. Spatial representation in the entorhinal cortex. Science. 2004;305: 1258–1264. doi: 10.1126/science.1099901 [DOI] [PubMed] [Google Scholar]
- 32.Hafting T, Fyhn M, Molden S, Moser M-B, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nat Cell Biol. Nature Publishing Group; 2005;436: 801–806. doi: 10.1038/nature03721 [DOI] [PubMed] [Google Scholar]
- 33.Solstad T, Boccara CN, Kropff E, Moser M-B, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. American Association for the Advancement of Science; 2008;322: 1865–1868. doi: 10.1126/science.1166466 [DOI] [PubMed] [Google Scholar]
- 34.Tsao A, Moser M-B, Moser EI. Traces of Experiencein the Lateral Entorhinal Cortex. Curr Biol. Elsevier Ltd; 2013;23: 399–405. doi: 10.1016/j.cub.2013.01.036 [DOI] [PubMed] [Google Scholar]
- 35.Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5: 69 doi: 10.3389/fnbeh.2011.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369: 20130369 doi: 10.1098/rstb.2013.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neuroscience and biobehavioral reviews. 2012;36: 1597–1608. doi: 10.1016/j.neubiorev.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunsaker MR, Chen V, Tran GT, Kesner RP. The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: a test of the binding of items and context model. Hippocampus. 2013;23: 380–391. doi: 10.1002/hipo.22097 [DOI] [PubMed] [Google Scholar]
- 39.Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7: 133–153. [DOI] [PubMed] [Google Scholar]
- 40.Xavier GF, Oliveira-Filho FJ, Santos AM. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in “place strategy” because of a lack of flexibility in the use of environmental cues? Hippocampus. John Wiley & Sons, Inc; 1999;9: 668–681. doi: 10.1002/(SICI)1098-1063(1999)9:6<668::AID-HIPO8>3.0.CO;2-9 [DOI] [PubMed] [Google Scholar]
- 41.Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012;32: 8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niibori Y, Yu T-S, Epp JR, Akers KG, Josselyn SA, Frankland PW. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Comms. 2012;3: 1253 doi: 10.1038/ncomms2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swan AA, Clutton JE, Chary PK, Cook SG, Liu GG, Drew MR. Characterization of the role of adult neurogenesis in touch-screen discrimination learning. Hippocampus. 2014;24: 1581–1591. doi: 10.1002/hipo.22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, et al. Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron. 2016;90: 101–112. doi: 10.1016/j.neuron.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31: 47–59. [DOI] [PubMed] [Google Scholar]
- 46.Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16: 1177–1188. doi: 10.1038/mp.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. Glucocorticoids Orchestrate Divergent Effects on Mood through Adult Neurogenesis. J Neurosci. 2013;33: 2961–2972. doi: 10.1523/JNEUROSCI.3878-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Culig L, Surget A, Bourdey M, Khemissi W, Le Guisquet A-M, Vogel E, et al. Increasing adult hippocampal neurogenesis in mice after exposure to unpredictable chronic mild stress may counteract some of the effects of stress. Neuropharmacology. 2017;126: 179–189. doi: 10.1016/j.neuropharm.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 49.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18: 1064–1073. doi: 10.1002/hipo.20464 [DOI] [PubMed] [Google Scholar]
- 50.Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16: 147–154. doi: 10.1101/lm.1172609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22: 1188–1201. doi: 10.1002/hipo.20964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolz L, Heigele S, Bischofberger J. Running Improves Pattern Separation during Novel Object Recognition. van Praag H, Christie B, editors. BPL. IOS Press; 2015;1: 129–141. doi: 10.3233/BPL-150010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vivar C, Potter MC, Choi J, Lee J-Y, Stringer TP, Callaway EM, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Comms. 2012;3: 1107 doi: 10.1038/ncomms2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kesner RP, Hui X, Sommer T, Wright C, Barrera VR, Fanselow MS. The role of postnatal neurogenesis in supporting remote memory and spatial metric processing. Hippocampus. 2014. doi: 10.1002/hipo.22346 [DOI] [PubMed] [Google Scholar]
- 55.Ivanova OY, Dobryakova YV, Salozhin SV, Aniol VA, Onufriev MV, Gulyaeva NV, et al. Lentiviral Modulation of Wnt/β-Catenin Signaling Affects In Vivo LTP. Cell Mol Neurobiol. 5 ed. Springer US; 2017;37: 1227–1241. doi: 10.1007/s10571-016-0455-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser M-B. Independent Codes for Spatial and Episodic Memory in Hippocampal Neuronal Ensembles. Science. American Association for the Advancement of Science; 2005;309: 619–623. doi: 10.1126/science.1114037 [DOI] [PubMed] [Google Scholar]
- 57.Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4: e5464 doi: 10.1371/journal.pone.0005464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130: 843–852. doi: 10.1016/j.neuroscience.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 59.Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. Wiley Subscription Services, Inc., A Wiley Company; 2010;20: 1139–1153. doi: 10.1002/hipo.20714 [DOI] [PubMed] [Google Scholar]
- 60.Archer J. Rodent sex differences in emotional and related behavior. Behav Biol. 1975;14: 451–479. [DOI] [PubMed] [Google Scholar]
- 61.Bartling B, Al-Robaiy S, Lehnich H, Binder L, Hiebl B, Simm A. Sex-related differences in the wheel-running activity of mice decline with increasing age. Exp Gerontol. 2017;87: 139–147. doi: 10.1016/j.exger.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 62.Basso JC, Morrell JI. Using wheel availability to shape running behavior of the rat towards improved behavioral and neurobiological outcomes. J Neurosci Methods. 2017;290: 13–23. doi: 10.1016/j.jneumeth.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 63.Koss WA, Frick KM. Sex differences in hippocampal function. Cahill L, editor. J Neurosci Res. 2017;95: 539–562. doi: 10.1002/jnr.23864 [DOI] [PubMed] [Google Scholar]
- 64.Luine V. Sex differences in chronic stress effects on memory in rats. Stress (Amsterdam, Netherlands). 2002;5: 205–216. doi: 10.1080/1025389021000010549 [DOI] [PubMed] [Google Scholar]
- 65.Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci. 2007;10: 1401–1403. doi: 10.1038/nn1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Behavioral data for all of the animals, including some data that was analyzed but not included in the published manuscript (e.g. behavioral event durations).
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.