Abstract
Background
Predictions from conduction velocity data for primate retinal ganglion cell axons indicate that the conduction time to the lateral geniculate nucleus for stimulation of peripheral retina should be no longer than for stimulation of central retina. On this basis, the latency of saccadic eye movements should not increase for more peripherally located targets. However, previous studies have reported relatively very large increases, which has the implication of a very considerable increase in central processing time for the saccade-generating system.
Results
In order to resolve this paradox, we have undertaken an extended series of experiments in which saccadic eye movements were recorded by electro-oculography in response to targets presented in the horizontal meridian in normal young subjects. For stationary or moving targets of either normal beam intensity or reduced red intensity, with the direction of gaze either straight ahead with respect to the head or directed eccentrically, the saccadic latency was shown to remain invariant with respect to a wide range of target angular displacements.
Conclusions
These results indicate that, irrespective of the angular displacement of the target, the direction of gaze or the target intensity, the saccade-generating system operates with a constant generation time.
Background
Specialization of the foveal region of the human retina necessitates a sophisticated ocular motor system to translate an image appearing on the peripheral retina onto the fovea: this is achieved by the generation of saccadic eye movements which effect rapid fixation of the target. The saccadic latency which is the time from the presentation of the target to the commencement of the saccade consists of the transmission times in the afferent and efferent pathways and the central processing time which is likely to be appreciable due to the complexity of the pathway. An important centre in the generation of saccades is the superior colliculus [1]. While a direct projection from the retina via the superior brachium leads to activation of the visual cells of the superior colliculus, the dominant pathway is via the primary visual cortex [2] which projects to the superior colliculus through the thick cytochrome oxidase stripes of area V2 [3]. Saccadic movements are also driven consciously from the frontal eye fields (area 8) which have a projection to the superior colliculus [4]. For a saccade to be generated, there must be activation of the saccade-related burst neurones of the deep layers of the superior colliculus: these drive the excitatory burst neurones of the paramedian pontine reticular formation (PPRF), which in turn drive the extra-ocular motor neurones. There must also be disengagement of the fixation neurones of the foveal representation (rostral zone) of the superior colliculus: these would otherwise drive the omnipause neurones of the PPRF, which in turn inhibit the excitatory burst neurones [5,6]. Saccade generation thus requires release from the inhibitory actions of the fixation neurones; consequently removal of the fixation point prior to presentation of the target causes a marked shortening of the saccadic latency. This has become known as the gap effect and leads to the generation of express saccades of latency of 90–120 ms [7,8]. When the fixation point remains visible (the overlap condition), regular saccades of latencies of 200–220 ms are generated though faster regular saccades of latencies of 135–170 ms have additionally been reported [9].
At first sight it might seem that more peripheral targets might generate saccades with a longer latency due to the more extended conduction pathway across the retina. However, in primate, peripheral ganglion cells possess faster axonal conduction velocities than their centrally located counterparts, so much so that excitation will arrive at the lateral geniculate nucleus 7 ms earlier for stimulation at 40 deg than at 10 deg (calculated from [10]). The expectation is thus that saccadic latencies should at least remain invariant with respect to the retinal location of the target and so provide a standard time reference against which the saccade is generated. However, several studies report, using a non-gap stimulus paradigm, that saccadic latencies increased with increasing target eccentricity, sometimes by considerable amounts: for example, increases as large as 60 ms have been reported [11]. Given that the conduction time in the more peripheral part of the afferent pathway is not increasing and the time delay from the peak of the saccade-related burst response in the superior colliculus to the start of the saccade is only 20 ms [1,5,6], such an increase in saccadic latency implies a considerable increase in central processing time. This inference, however, is at variance with respect to the major function of the peripheral retina in underlying the rapid detection of visual objects, which is mediated through the magnocellular visual pathway. Generally, those studies which reported a consistent increase in saccadic latencies [11-16] employed stationary red light emitting diodes or red neons as targets, which contrasts with those studies reporting no change which used a moving spot and a stationary white target [17,18]. In addition to the target characteristics, the direction of gaze has been reported to have an effect in that adoption of an eccentric direction of gaze reduced the saccadic latency markedly [11,16]. This factor has been invoked as a confounding factor to account for the reported constancy of saccadic latency [11].
Our aim was, therefore, to resolve the issue of how saccadic latency is related to target displacement. To this end, we have by employed a comprehensive range of stimulus conditions in order to determine the effects of targets which were stationary or moving, of normal intensity or of reduced red intensity, and whether the targets were viewed with the eyes directed straight ahead or at an eccentric direction of gaze. A preliminary report of part of this work has previously been published [19].
Results
Saccadic recordings were obtained from 11 subjects, ages 19–23 yr, who had an unaided Snellen acuity of 6/4, normal binocular single vision, and heterophoria determined with the Maddox rod test of no more than 5Δ. The form of the saccade was fundamentally the same in all subjects in that, after a latent period of ca 180–250 ms, there arose the rapid saccadic eye movement which resulted in fixation of the target. In those experiments employing a moving target, the saccadic movement was followed by a smooth pursuit movement. Examples of saccadic movements in response to a stationary target are shown in Fig. 1A &1B. We saw no evidence of express saccades of very short latency. As shown in Fig. 1A &1B, fixation of the target was always attained smoothly without the generation of secondary saccades.
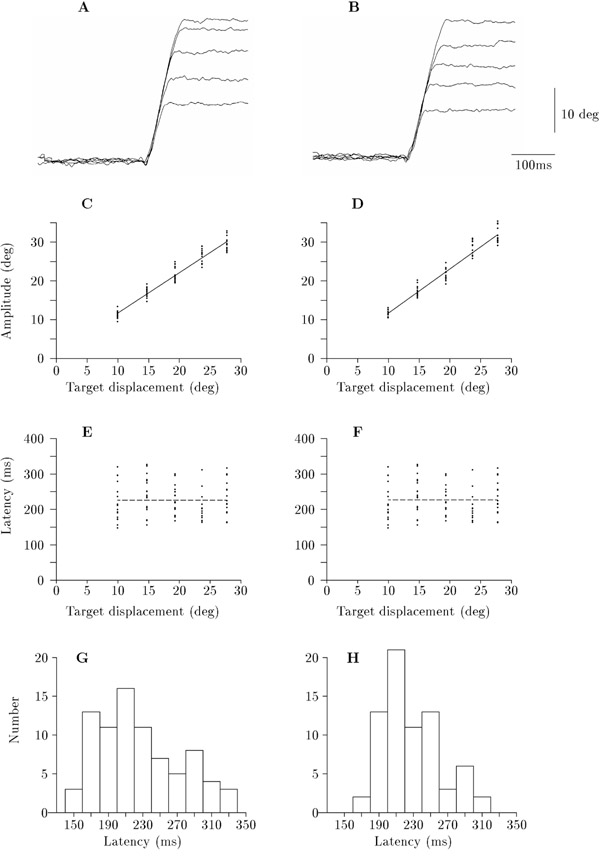
Figure 1.
Comparison of saccades in response to a stationary target of normal intensity (left hand column) and of a reduced intensity red target (right hand column) for direct viewing in the same subject. A & B. Specimen saccadic recordings aligned to the same saccadic latency to show the progressive increase in saccadic amplitude for target displacements, from lower to upper traces, of 10; 15; 20; 24 & 28 deg. C & D. Saccadic amplitude against target displacement showing best fitting regression line (C: y = 1.31 + 1.04x; R2 = 94.8%, Pslope < 0.001; F = 1454; d.f. = 79), D: y = 0.29 + 1.14x; R2 = 94.7%, Pslope < 0.001; F = 1282; d.f. = 72). E & F. Saccadic latency against target displacement with dashed line showing mean value (E: R2 = 0.0%, Pslope = 0.78, F: R2 = 0.0%, Pslope = 0.67). G & H. Histograms of saccadic latencies from E & F, respectively. Mean ± S.E.M. are: (G) 226.4 ± 5.4 ms (n = 81), (H) 227.3 ± 4.1 ms (n = 71).
Saccadic amplitude
In all experiments, the accuracy of the saccadic movements in fixating the target was demonstrated by the highly significant relationship between saccadic amplitude and target displacement. This is illustrated by the montages of increasing saccadic amplitudes generated in response to a stationary target presented at increasing angular displacements from the fixation point (Fig. 1A &1B). Aggregation of the results into plots of saccadic amplitude against target displacement (Fig. 1C &1D) resulted in R2 values of typically 94% (Pslope < 0.001, F > 1200; d.f. > 772). Similar strong, direct relationships between saccadic amplitude and target displacement for moving targets were also obtained as shown by the results in Fig. 2B, D, F &2H.
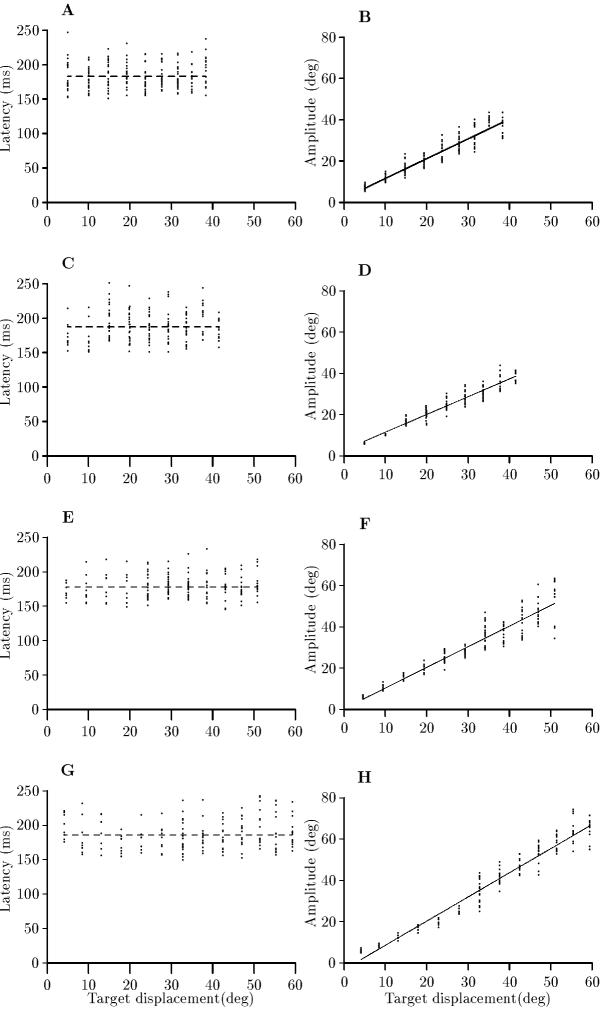
Figure 2.
Saccadic latencies and saccadic amplitudes for increasing target displacements at 0; 10; 20 and 28 deg of steady eccentric gaze shown in descending order for a moving target in the same subject. The graphs of saccadic latency against target displacement show the mean value as a broken line (A: R2 = 0.2%, Pslope = 0.57; y = 182.8 ± 1.33 ms (S.E.M.), C: R2 = 0.3%, Pslope = 0.22; y = 187.3 ± 1.74 ms (S.E.M.), E: R2 = 0.5%, Pslope = 0.17; y = 177.8 ± 1.34 ms (S.E.M.), G: R2 = 0.5%, Pslope = 0.18; y = 185.9 ± 1.78 ms (S.E.M.)) while the graphs of saccadic amplitude against target displacement show the best fitting regression line (B: y = 2.00 + 0.96x; R2 = 89.1%, Pslope < 0.001; F = 1572; d.f. = 193, D: y = 2.86 + 0.86x; R2 = 91.9%, Pslope < 0.001; F = 1858; d.f. = 193, F: y = 0.39 + 1.00x; R2 = 87.7%, Pslope < 0.001; F = 1181; d.f. = 167, H: y = 3.2 + 1.17x; R2 = 95.0%, Pslope < 0.001; F = 3163; d.f. = 167).
Saccadic latency for direct viewing
(a) Stationary target of normal intensity
Initially, a stationary target, which in this case was of normal beam intensity, was tested in 6 subjects with direct viewing of the fixation point. Saccadic latency remained invariant with respect to increasing target displacement in 4 subjects (R2 ≤ 3.4%, Pslope ≥ 0.11) (Fig. 1E). The distribution of the latencies for the subject in Fig. 1E is shown in Fig. 1G in which the data are grouped about a mean value of 226.4 ± 5.4 ms (mean ± S.E.M.). The fifth subject showed a significant but small decrease in the slope of the regression (R2 = 6.8%, Pslope = 0.045; d.f. = 46), while the sixth subject showed a small significant increase in the slope (R2 = 17.1%, Pslope = 0.01; d.f. = 35). However, in both cases, the F values of 4.3 and 8.2, respectively, were both well below the threshold value of 25.0 (see Methods), indicating that these R2 values were without importance. From these results, it was concluded that saccadic latency remained invariant with respect to increasing angular displacement of a stationary target of normal intensity.
(b) Stationary red target
Since those investigators who had reported an increase in saccadic latency with increasing target displacement had employed a target in the form of a red light emitting diode or red neon, we repeated the experiments of the previous section using a red target. These experiments were carried out in 3 subjects, an example of which is shown in Fig. 1F, and showed without exception that saccadic latency remained invariant with respect to target displacement (R2 ≤ 6.1%, Pslope ≥ 0.20). The saccadic latencies were grouped about the mean value which, in the example shown, was 227.3 ± 4.1 ms (mean ± S.E.M.)(Fig. 1H). This result thus marks a major difference from previous studies.
(c) Moving target of normal intensity
These experiments were undertaken on the basis that a moving target additionally requires a prediction of the target location in order to achieve fixation. For a total of 7 subjects with direct viewing, the general result was that saccadic latency remained constant with respect to target displacement. In 6 out of the 7 subjects, saccadic latency was not significantly related to target displacement (R2 ≤ 3.1%, Pslope ≥ 0.13). In the seventh subject, saccadic latency increased significantly with target displacement (R2 = 19.7%, Pslope= 0.001; d.f. = 44), though this was without meaning since the F value of the regression (F = 11.8) was well below that value of 25.0 required for the relationship to be accorded any importance. Typical results are shown in Fig. 2A in which case the latency was most appropriately described by a constant value of 182.8 ± 1.3 ms (mean ± S.E.M.).
Saccadic latency for eccentric directions of gaze
The relationship between saccadic latency and target displacement at different directions of gaze in a leftwards direction with generation of saccades in a rightwards direction was investigated in response to both moving and stationary targets.
(a) Moving target
A typical result is shown in Fig. 2A,C,E,G in which the experiment was carried out at eccentric gazes of 10, 20 and 28 deg, as well as in the straight ahead direction: saccadic latency remained invariant with respect to increasing target displacement at each eccentricity (R2 ≤ 1.1%, Pslope ≥ 0.17). This result was reproduced in 3 more subjects, one of whom provided saccadic latencies at the same eccentric gazes while two subjects undertook the experiment at an eccentric gaze of 20 deg as well as in the straight ahead direction. In all 4 subjects, there was no significant effect of the angle of eye eccentricity on saccadic latency (P > 0.10, ANOVA).
(b) Stationary target
In 2 subjects who viewed at 10, 20 & 40 deg eccentricity and in one subject who viewed at 20 deg eccentricity, as well as in the straight ahead direction, saccadic latency was invariant with respect to eccentricity (P > 0.1, ANOVA). In a fourth subject, the saccadic latency showed a small increase of 15 ms for viewing at 20 deg eccentricity (P = 0.001, ANOVA). Hence, for both sets of experiments, there was no evidence that the saccadic latency was reduced on adoption of an eccentric direction of gaze.
Additional experiments
Saccadic recordings obtained in response to a leftwards as well as a rightwards directed moving target in one subject showed no change in saccadic latency (P = 0.40, ANOVA). Likewise, saccadic latencies were very similar for left and right eye monocular recordings (P = 0.57, ANOVA). In both cases, saccadic latency did not change significantly with increasing target displacement (R2 = 0.0%, Pslope ≥ 0.40). Saccadic recordings in response to angular displacements of 10–38 deg were made in response to a moving target with direct viewing on two further occasions in two subjects. In each of these new sets of data, saccadic latency was again not significantly related to target displacement (R2 ≤ 1.7%, Pslope ≥ 0.07). For the 3 sets of data, the mean values ± S.E.M. for one subject were 182.8 ± 1.33 ms (shown in Fig. 2A), 181.2 ± 0.22 ms and 188.6 ± 1.45 ms, and for the second subject were 195.1 ± 1.58 ms, 199.5 ± 1.46 ms and 192.2 ± 3.52 ms. Hence, the reproducibility of the data on different recording days was very high.
Discussion
We have undertaken an investigation of the effects of target eccentricity on the latency of saccade generation with target presentation during steady fixation (overlap condition). The outcome of this study was that saccadic latency remained invariant with respect to the magnitude of target displacement, irrespective of whether the target was moving or stationary, whether fixation was straight ahead or was directed eccentrically, or whether the target was of normal intensity or consisted of a dim red target. From an examination of the outcomes of regression analysis of all the types of experiment undertaken, out of 32 experiments in 11 different subjects, Pslope did not attain statistical significance in 27 cases. In 5 cases, in which Pslope < 0.05, the relationships were discounted as unimportant [20]. We also confirmed the validity of the results for saccadic movements in both leftwards and rightwards directions and for monocular recordings. The distribution of our saccadic latencies (Fig. 1G &1H) which was similar to those reported previously [18,21] fell predominantly into the slow regular group though the faster saccades would qualify as fast regular saccades [9]. Express saccades were never observed which is consistent with the overlap mode of target presentation. Since our data were collected in triplets, there was plainly some prior knowledge of the target location though is without effect on saccadic latencies [22].
Thus, with respect to saccadic latency, the present study confirms previous results for a normal intensity moving target [17] for a normal intensity stationary target [18] and for a yellow-green target in excess of 3 logarithmic units above foveal threshold presented in the nasal hemifield outside of the foveal area [15], but has contradicts other studies [11-14,16]. The previous report that the saccadic latency was reduced by as much as 60 ms on adoption of an eccentric direction of gaze [11] was also not confirmed: our results indicated unequivocally that there was no such reduction in latency, which is consistent with evidence of an absence an effect of proprioceptive feedback on position sense of the eye in man [23,24].
We have also shown constancy of saccadic latency with increasing target angular displacement, whether the target was of normal intensity or was of dim red intensity, which was all the more remarkable in that the effective intensity of the red target relative to the photopic threshold was very low. This result differs from the reported increase in saccadic latency in response to a red target of luminance up to 3 logarithmic units above foveal threshold [15]. However, the latter result is unexpected since, in the same study, a yellow-green target which was photopically equivalent to the red target resulted in essentially constant saccadic latencies [15]. The difference may have arisen on account of the very deep red target (660 nm) which was used: this may also explain the difference from the present results showing constancy of latency. When the intensity of a blue/green target was reduced by 2 logarithmic units, the saccadic latency in response to a small target displacement of 6 deg was increased by 40 ms [25], though this result reflects operation in the scotopic range since the subjects were dark adapted for 40 min prior to the experiments.
The explanation proposed to account for the large increase in saccadic latency with target angular displacement was that it allowed time for the generation of a compensatory head movement [11]; however, should the head not move, the saccadic latency would still remain greatly extended. The difference between our results and those of the previous studies are not readily reconcilable except previously reported results are not as clear cut as they may seem. Many studies had very small sample sizes. Hallet and Lightstone [13] reported an increase in saccadic latency in one subject and no change in the other while the results of Zambarbieri et al. [16] contradict those of an earlier study in which saccadic latency remained constant [26]. In the data of Fuller [11] despite, in many cases, an apparent monotonic relationship between saccadic latency and target angular displacement, none of the correlation coefficients for iso-orbital viewing were statistically significant.
Our results showing constancy of saccadic latency are, however, fully consistent with the inferences which may be drawn from primate conduction velocity data [10] in that there should be no increase in conduction time for stimulation of more peripheral parts of the retina. Furthermore, we have shown that the specific nature of the stimulus is not critical: it may be stationary, moving or of reduced intensity but the saccadic latency remains invariant. This accords with studies in primates trained to undertake fixation of novel objects appearing in the peripheral visual field: saccadic latencies were similar in response to the presentation of novel objects which differed in hue, luminance and size from the surrounding objects [27].
Our result has relevance to attempts to model the control system for saccadic eye movements. Recent models show an emphasis on explaining the gap effect. In neuronal terms, this can be viewed as the prior disenabling of the fixation neurones by extinction of the fixation point thus removing this stage in the saccade generation process [6] though more recently it has been shown express saccade generation is better predicted by the activity of buildup neurones of the superior colliculus [28]. Thus, for a gap between fixation extinction and target presentation of a fixed value (usually 200 ms), this may be regarded as a visual cue to the subject that the target is about to be presented [29,30]: under these circumstances, it is thus not surprising that express saccades should be generated. However, in keeping with previous studies which investigated the effects of target eccentricities, we have used an overlap paradigm which requires both the disengagement of fixation and generation of the saccade.
Winner-take-all models are based on the operation of reciprocal inhibition between two competing centres such that, should one centre dominant, it becomes subject to positive feedback resulting in saccade generation [31,32]. The values of saccadic latency generated by Clark's model are, however, unrealistically high for both the gap and overlap conditions and show a strong dependence on target intensity [31]. Saccadic latency showed a reduction with increasing target eccentricities over a limited range, though latency became asymptotic for targets in excess of 5 deg [31]. By contrast, in Findlay & Walker's model, it is proposed that the generation of an unequivocal salience peak in response to the target presentation through a non-linear triggering process leads to a "fixed and stereotyped burst of activity"[32]. The defining characteristic is the location of the burst in the salience map rather than on other characteristics like intensity. This is consistent with our results.
In the three loop model [33], the delays between the proposed three central modules are proposed to arise from stochastic variations in inputs without invoking the need for inhibitory interactions. This model does not examine the dependence of saccadic latency on target eccentricity but it does generate latency values which correspond to the present results for an overlap paradigm. However, the chosen response latency for visual cortical neurones of 30 ms is unrealistic since the conduction time from the retinal ganglion cells to the lateral geniculate nucleus is of this order of magnitude [10], irrespective of the retinal delay and conduction from lateral geniculate nucleus to visual cortex. Since the brain stem mechanisms for saccade generation have very short time delays between neurones which operate in a pulsatile manner, the overall time delay in the motor pathway amounts to only 20 ms [1,5,6]. Hence, the major component of delay is thus afferent and central. This is reflected in the Robinson model which incorporates a 0.2 s delay for processing of the target position in space [5]. There was no explicit provision for an increased delay as the target displacement increases. On the basis of the present results, this is indeed unnecessary. The reason for invariant saccadic latencies probably lies at the level of the retina: the sensitivity to the appearance of a peripherally located target is likely to be high since luminance detection is mediated by both M retinal ganglion cells (parasol ganglion cells) [34] and P retinal ganglion cells (midget ganglion cells) [35]. Furthermore, luminance detection would be accentuated by the steepness of the response-luminance contrast relationship of M retinal ganglion cells [36]. Thereafter, in the visual pathway, increasing target eccentricities do not lead to longer conduction times [10]. Hence, there is a strong neural basis as to why saccadic latencies should not increase with increasing target eccentricity or reduced target intensity. This is what is implied by the present results in which saccadic latency remains remarkably constant.
Conclusions
In conclusion, irrespective of the nature of the stimulus- a moving or stationary target, of normal or reduced intensity, viewed either directly or eccentrically- saccadic latency remains remarkably invariant with respect to target angular displacement. The corollary of this result is that there is therefore no increase in the central processing time under these conditions.
Methods
Recording apparatus
Saccadic eye movements were recorded by electro-oculography in which the active electrode was placed on the right temple, the indifferent on the left temple and the earth on the forehead. The electrodes were connected to a differential preamplifier of gain X100 and bandwidth DC-100 Hz and standing potentials were annulled with a variable DC offset control. The preamplifier output was passed through a further amplification stage of X10, which incorporated a 50 Hz notch filter. The output was recorded and analyzed with a computer-based data acquisition system. At each recording session, calibration of the electro-oculogram was undertaken by recording the steady DC voltage change in response to deflection of the eyes both leftwards and rightwards across a metre rule viewed from 57 cm in 10 cm steps up to 40 cm. The recorded voltage showed a very strong linear relationship against angular displacement (typically, R2 = 99%, Pslope < 0.001). From these relationships, the recorded saccadic amplitudes were converted into angular subtenses.
Stimulus display
The target took the form of the triggered beam of one of the electron beams of a Tektronix 502 oscilloscope (P2 phosphor, peak emission ca. 540 nm) from which the graticule had been removed. The beam which had an angular subtense 0.6 min arc was set to the maximum intensity without the presence of a halo. The experiments were carried out in normal room lighting. For direct viewing, the effective intensity, determined by attenuation of the beam with neutral density filters until the beam was just visible, was measured to be 4.6 logarithmic units above the photopic threshold. The respective intensities at eccentricities of 10, 20 and 28 deg were 4.2, 3.9 and 3.6 logarithmic units above the photopic threshold. To generate a stationary red target, a red Kodak Wratten 29F filter was affixed to the screen of the 502 oscilloscope over the location of the appearance of the oscilloscope beam. This was measured to have an effective intensity of 2.1 logarithmic units above foveal threshold for direct viewing and, at eccentricities of 10, 20 and 28 deg, the effective intensities were 1.6, 0.9 and 0.6 logarithmic units, respectively.
The subject, with the head stabilized in a rest, viewed from 57 cm, the oscilloscope screen which had mounted coplanar to, and to the left of, either a white tangent screen or a white arc shaped screen on which a black fixation point of diameter 1.8 min arc was mounted at the required angular displacement from the appearance of the oscilloscope beam. For direct viewing i.e. with the eyes in the straight ahead position in the orbit with respect to the head (denoted as 0 deg eccentricity), the display was positioned so that the fixation point was straight ahead and the target beam appeared to the right. For an eccentric direction of gaze, the straight ahead position was first determined and then the display was moved leftwards by the required amount. This was followed by translation of the eyes leftwards to fixate the fixation point whilst keeping the head immobile and the target again appeared to the right. The oscilloscope beam which was either stationary or moved with a velocity of 10 deg s-1 was triggered by a Digitimer which also triggered the sweep of the computer-based data acquisition system. These events were confirmed to be synchronized by comparison of the recorded stimulus marker with the appearance of the oscilloscope beam detected with a photodiode-amplifier.
The instruction to the subjects were to hold their gaze steady towards the black fixation point and fixate the target appearing appearing in the peripheral visual field. For a stationary target fixation was to be maintained as long as the target was visible (1.00 s) and for the moving target it was to be tracked to the edge of the oscilloscope screen. Fixation of the black fixation point was then to be resumed. The cycle period of the target presentation was varied continually within the range of 4–8 s. An auditory warning cue was not employed. At each target displacement, 3 saccadic recordings were obtained and the target displacement changed until normally a total of 12–24 recordings, which produced a stable saccadic latency distribution, was obtained at each target displacement. The range of target displacements generally ranged from 10 deg to 40 deg.
Data analysis
Measurements were made as follows. (1) Latency was the time from the appearance of the target to the start of the saccadic eye movement. 50% subjects showed the generation of a small notch indicative of synchronized depolarization of the extra-ocular muscles ([37]) which provided a sharply defined point for the latency measurement (Fig. 1A &1B). For the other 50% subjects, the latency was measured to when the saccade exceeded the baseline noise. (2) The amplitude was measured from the initial notch prior to the saccade or from the baseline to the highest point of the saccade prior to the steady fixation achieved with stationary targets or the start of the smooth pursuit movement generated in response to a moving target.
Latency and amplitude were tested against angular displacement by linear regression analysis using the Minitab 11 statistical package [38]. Statistical significance for regression was taken when Pslope < 0.05. Sometimes, statistical significance was indicated for R2 values as low as 2.0%, which implied that, while the slope of the regression line was non-zero, its importance was very doubtful. We therefore adopted the standard procedure for assessing the usefulness or importance of the regression equation [20]. This involves application of the γm criterion from which the multiple by which the F ratio for the significance of regression must be exceeded before the slope of regression can be accorded any importance. For 30 degrees of freedom, the least conservative multiple is 6: accordingly, the minimum F value required for the slope of the regression to be important is 25.0. In those cases where the regression was either without statistical significance or without importance due to a F value of less than 25.0, the mean value was taken as the most likely value of the predicted variable for each displacement [20]. Comparisons between sets of data were made by analysis of variance (ANOVA). Statistical significance was taken when P < 0.05.
Acknowledgments
Acknowledgements
We express our appreciation to our subjects for their participation in the experiments. We are indebted to Dr J Shaw Dunn for making available the prosections for measurement of the visual pathway and to Dr KA Lindsay for his contribution to the preparation of the manuscript.
Contributor Information
Jennifer H Darrien, Email: jdm1u@udcf.gla.ac.uk.
Katrina Herd, Email: jdm1u@udcf.gla.ac.uk.
Lisa-Jo Starling, Email: jdm1u@udcf.gla.ac.uk.
Jay R Rosenberg, Email: GPAA07@udcf.gla.ac.uk.
James D Morrison, Email: jdm1u@udcf.gla.ac.uk.
References
- Mays LE, Sparks DL. Dissociation of visual and saccade related responses in superior colliculus neurones. J Neurophysiol. 1980;43:207–232. doi: 10.1152/jn.1980.43.1.207. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Stryker M, Cynader M, Berman N. Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortex. J Neurophysiol. 1974;37:181–194. doi: 10.1152/jn.1974.37.1.181. [DOI] [PubMed] [Google Scholar]
- Abel PL, O'Brien BJ, Lia B, Olavarria JF. Distribution of neurons projecting to the superior colliculus correlates with thick cytochrome oxidase stripes in macaque visual area V2. J Comp Neurol. 1997;377:313–323. doi: 10.1002/(SICI)1096-9861(19970120)377:3<313::AID-CNE1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Kaneko CRS, Scudder CA. Brainstem control of saccadic eye movements. Ann Rev Neurosci. 1985;8:307–317. doi: 10.1146/annurev.ne.08.030185.001515. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Vision for the control of movement. Invest Ophthal Vis Sci. 1996;17:2131–2145. [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movements. J Opt Soc Am. 1967;57:1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: exremely short reaction times of goal directed movements. Exp Brain Res. 1984;57:191–195. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Gezeck S, Fischer B, Timmer J. Saccadic reaction times: a statistical analysis of multimodal distributions. Vision Res. 1997;37:2119–2131. doi: 10.1016/S0042-6989(97)00022-9. [DOI] [PubMed] [Google Scholar]
- Ogden TE, Miller RF. Studies of the optic nerve of the rhesus monkey: nerve fibre spectrum and physiological properties. Vision Res. 1966;6:485–506. [PubMed] [Google Scholar]
- Fuller JH. Eye position and target amplitude effects on human visual saccadic latencies. Exp Brain Res. 1996;109:457–466. doi: 10.1007/BF00229630. [DOI] [PubMed] [Google Scholar]
- White CT, Eason RG, Bartlett NR. Latency and duration of eye movements in the horizontal plane. J Opt Soc Am. 1962;52:210–213. doi: 10.1364/josa.52.000210. [DOI] [PubMed] [Google Scholar]
- Hallet PE, Lightstone AD. Saccadic eye movements towards stimuli triggered by prior saccades. Vision Res. 1976;16:99–106. doi: 10.1016/0042-6989(76)90083-3. [DOI] [PubMed] [Google Scholar]
- Biguer B, Prablanc C, Jeannerod M. The contribution of coordinated eye and head movements in hand pointing accuracy. Exp Brain Res. 1984;55:462–469. doi: 10.1007/BF00235277. [DOI] [PubMed] [Google Scholar]
- Kalesnykas RP, Hallet PE. Retinal eccentricity and latency of eye saccades. Vision Res. 1994;34:517–531. doi: 10.1016/0042-6989(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Zambarbieri D, Beltrami G, Versino M. Saccadic latency towards auditory targets depends on the relative position of the sound source with respect to the eyes. Vision Res. 1995;35:3305–3312. doi: 10.1016/0042-6989(95)00065-M. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Honrubia V. Reaction time and accuracy of the saccadic eye movements of normal subjects in a moving-target task. Aviation Space Environ Med. 1976;47:1165–1167. [Google Scholar]
- Frost D, Pöppel E. Different programming modes of human saccadic eye movements as a function of stimulus eccentricity: indications of functional subdivision of the visual field. Biol Cybern. 1976;23:39–48. doi: 10.1007/BF00344150. [DOI] [PubMed] [Google Scholar]
- Al-Janabi Z, Brown S, Grimsley S, Jeffrey VJ, Joshi A, Love SM, Sellwood WB, Thampey N, Tindell VJ, Wong LY, Morrison JD, Rosenberg JR. Relationship between saccadic latency and target displacement at different directions of gaze. J Physiol. 1999;521:49–50. [Google Scholar]
- Draper N, Smith H. Applied Regression Analysis 2nd Ed. New York, John Wiley & Sons. 1981.
- Tanaka M, Yoshida T, Fukushima K. Latency of saccades during smooth-pursuit eye movement in man. Exp Brain Res. 1996;121:92–98. doi: 10.1007/s002210050440. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Latency for saccadic eye movement. J Opt Soc Am. 1967;57:1030–1033. doi: 10.1364/josa.57.001030. [DOI] [PubMed] [Google Scholar]
- Irvine SR, Ludvigh EJ. Is ocular proprioceptive sense concerned in vision? Arch Ophthal. 1936;15:1037–1049. [Google Scholar]
- Brindley GS, Merton PA. The absence of position sense in the human eye. J Physiol. 1960;153:127–130. doi: 10.1113/jphysiol.1960.sp006523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeless LL, Cohen GH, Boynton RM. Luminance as a parameter of the eye-movement control system. J Opt Soc Am. 1967;57:394–400. [Google Scholar]
- Zambarbieri D, Schimd R, Magenes G, Prablanc C. Saccadic responses evoked by presentation of visual and auditory targets. Exp Brain Res. 1982;47:417–427. doi: 10.1007/BF00239359. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Lee K. The role of primate extrastriate area V4 in vision. Science. 1991;251:1251–1253. doi: 10.1126/science.2006413. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Pare M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci. 1997;17:8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LE, Ross SM. Saccadic latency and warning signals: stimulus onset, offset and change as warning events. Percept Psych. 1980;27:251–257. doi: 10.3758/bf03204262. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Oonk HM, Barnes LL, Hughes HC. Effects of warning signals and fixation point offsets on the latencies of pro- versus antisaccades: implications of the interpretation of the gap effect. Exp Brain Res. 1995;103:287–293. doi: 10.1007/BF00231715. [DOI] [PubMed] [Google Scholar]
- Clark JJ. Spatial attention and latencies of saccadic eye movements. Vision Res. 1999;39:585–602. doi: 10.1016/S0042-6989(98)00190-4. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Walker R. A model of saccade processing based on parallel processing and competitive inhibition. Behav Brain Sci. 1999;22:661–721. doi: 10.1017/S0140525X99002150. [DOI] [PubMed] [Google Scholar]
- Fischer B, Gezeck S, Huber W. The three-loop model: a neural network for the generation of saccades. Biol Cybern. 1995;72:185–196. doi: 10.1007/s004220050123. [DOI] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol. 1989;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Katz LM, Maunsell JHR. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. J Neurosci. 1991;11:994–1001. doi: 10.1523/JNEUROSCI.11-04-00994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R, Perry VH. Cat and monkey retinal ganglion cells and their visual functional roles. TINS. 1986;9:229–235. doi: 10.1016/0166-2236(86)90064-0. [DOI] [Google Scholar]
- Thickbroom GW, Mastaglia L. Presaccadic 'spike' potential: investigation of topography and source. Brain Res. 1985;339:271–280. doi: 10.1016/0006-8993(85)90092-7. [DOI] [PubMed] [Google Scholar]
- Ryan BE, Joiner BL. Minitab Handbook 3rd Ed. Belmont, California, Duxbury Press. 1994.