ABSTRACT
The Wnt/β-catenin pathway controls a variety of cellular behaviors, aberrant activation of which are associated with tumor progression in several types of cancer. The same cellular behaviors are also affected by the mechanical properties of the extracellular matrix (ECM) substratum, which induces signaling through integrins and integrin-linked kinase (ILK). Here, we examined the role of substratum stiffness in the regulation of cell proliferation downstream of Wnt3a. We found that treatment with Wnt3a increased proliferation of cells cultured on stiff substrata, with compliances characteristic of breast tumors, but not of cells on soft substrata, with compliances comparable to that of normal mammary tissue. Depleting ILK rendered cells unresponsive to Wnt3a on both substrata. Ectopic expression of ILK permitted Wnt3a to induce proliferation of cells on both microenvironments, although proliferation on soft substrata remained lower than that on stiff substrata. We further showed that ILK regulates expression of the Wnt receptor frizzled-1 (Fzd1), suggesting the presence of a positive feedback loop between Wnt3a, ILK and Fzd1. These findings suggest that tissue mechanics regulates the cellular response to Wnt under physiological and pathological microenvironmental conditions.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Mechanosensing, Morphogenesis, Morphodynamics, Mechanotransduction, Compliance
Highlighted Article: Stiff extracellular matrix signals through integrin-linked kinase to enhance the expression of frizzled-1, thus promoting signaling and proliferation downstream of Wnt3a in mammary epithelial cells.
INTRODUCTION
The Wnt/β-catenin signaling pathway controls a variety of cellular behaviors including adhesion, migration, differentiation and proliferation (Clevers, 2006; Masckauchán et al., 2005). Upon activation of the frizzled (Fzd) receptors, β-catenin is released from the cytosolic destruction complex and translocates to the nucleus, where it acts as a transcriptional co-activator (Taelman et al., 2010). Aberrant activation of Wnt signaling is closely associated with the progression of various types of cancer (He et al., 2015). In particular, Wnt3a is expressed at higher levels by breast cancer cell lines than by normal mammary epithelial cells (Benhaj et al., 2006).
Cells in epithelial tissues adhere to an underlying extracellular matrix (ECM) and respond to changes in its physical properties by altering gene expression and behavior. The mechanical properties of the ECM are therefore central to developmental, physiological and pathological processes (Discher et al., 2005; Lu et al., 2012). Similar to signaling downstream of Wnt, ECM stiffness alters proliferation, differentiation, adhesion and migration (Engler et al., 2006; Klein et al., 2009; Raab et al., 2012). ECM stiffness also regulates epithelial plasticity by promoting epithelial–mesenchymal transition (EMT) (Lee et al., 2012). Furthermore, stiff substrata with compliances similar to those of mammary tumors have been shown to promote neoplastic progression, cancer stem cell properties and a malignant phenotype (Levental et al., 2009; Pang et al., 2016; Paszek et al., 2005).
These physiological and pathological processes depend on the interactions between mechanical and chemical signals in the cellular microenvironment. Nonetheless, it remains unclear how mechanical signals, such as ECM stiffness, are integrated with biochemical signals, such as Wnt, to control these behaviors. One possible signaling nexus is through the Hippo pathway and its transcriptional coactivators, YAP (Yes-associated protein; also known as YAP1) and TAZ (transcriptional coactivator with PDZ-binding motif) (hereafter YAP/TAZ). The central components of the Hippo pathway include the Mst1/2 and Lats1/2 kinase cascade, which inhibits YAP/TAZ through direct phosphorylation, resulting in cytoplasmic retention and degradation (Piccolo et al., 2014). In addition to the canonical Hippo pathway, recent studies have uncovered a role for Wnt/β-catenin signaling in regulating YAP/TAZ. Stimulation with Wnt diverts YAP/TAZ away from the β-catenin destruction complex, causing stabilization of β-catenin and nuclear accumulation of YAP/TAZ (Azzolin et al., 2014). YAP/TAZ have also been found to mediate cellular responses to mechanical cues, including mechanical stretch (Benham-Pyle et al., 2015) and ECM stiffness (Dupont et al., 2011). Cells cultured on stiff substrata show high YAP/TAZ nuclear localization, whereas YAP/TAZ translocate to the cytoplasm and are thus inactive in cells cultured on soft substrata (Halder et al., 2012; Low et al., 2014). YAP/TAZ can therefore act as mechanosensors to integrate physical and biochemical cues from the microenvironment.
In addition to YAP/TAZ, responses to matrix stiffness are also mediated in part by signaling downstream of integrins through integrin-linked kinase (ILK), with stiffer substrata promoting integrin activation and focal adhesion formation (Levental et al., 2009; Pang et al., 2016; Sakai et al., 2003). These focal adhesion sites are major signaling complexes and ILK functions as a hub around which signaling pathways initiated by both diffusible signals, like Wnt, and cell–matrix interactions are centered. Elevated levels of ILK have been found to promote anchorage-dependent cell cycle progression, cancer stem cell-like properties and tumor progression (Graff et al., 2001; Hsu et al., 2015; Pang et al., 2016). Pharmacologically inhibiting ILK activity blocks protein kinase B/Akt phosphorylation and nuclear translocation of β-catenin in tumorigenic cells but not in their non-tumorigenic counterparts (Oloumi et al., 2006; Troussard et al., 2006). This suggests that ILK may regulate Wnt-induced cellular processes in a cell type- and cellular context-dependent manner.
In this study, we examined the role of the mechanical properties of the cellular microenvironment in the regulation of cell proliferation downstream of Wnt3a. We used engineered synthetic substrata to recapitulate the mechanical stiffness of the normal mammary gland as well as that of breast tumors. We found that mammary epithelial cells responded differently to Wnt3a treatment depending on the stiffness of their underlying substratum. Exposure to Wnt3a increased the percentage of proliferating cells on stiff substrata, but not on soft substrata. Surprisingly, blocking nuclear translocation of YAP/TAZ did not disrupt the ability of Wnt3a to induce proliferation of cells cultured on stiff substrata. Instead, we found that ECM stiffness modulates Wnt3a-induced proliferation by signaling through ILK, which negatively regulates the Wnt receptor frizzled-1 (Fzd1). Taken together, these results highlight the role of tissue mechanics in tuning the proliferative and potential tumorigenic responses of mammary epithelial cells to Wnt3a, providing insight into the molecular mechanisms underlying the effects of a pathological microenvironment on neoplastic progression.
RESULTS
Stiff substrata induce nuclear localization of YAP/TAZ and expression of target genes
To investigate how substratum stiffness affects YAP/TAZ signaling in mammary epithelial cells, we used fibronectin-coated synthetic substrata of different stiffness to mimic the average compliance of the microenvironment in the normal mammary gland (elastic modulus, E=130 Pa) and in mammary tumors (E=4020 Pa) (Lee et al., 2012). NMuMG mouse mammary epithelial cells cultured on these substrata exhibited differences in morphology depending on substratum compliance (Fig. 1A). Cells cultured on the soft substrata appeared rounded and formed multicellular clusters. In contrast, cells cultured on stiff substrata were well spread and spindle-shaped in morphology. Cells formed adhesions with their neighbors when cultured on both microenvironments (Fig. S1).
Fig. 1.
Substratum stiffness and Wnt3a synergistically regulate YAP/TAZ nuclear localization and activity. (A) Phase-contrast images of NMuMG cells cultured on soft (E≈130 Pa) or stiff (E≈4020 Pa) fibronectin-coated polyacrylamide substrata. Scale bars: 50 μm. (B) Fluorescence images of cells stained for YAP/TAZ (red) and nuclei (blue). Scale bars: 10 μm. (C) Quantification of the nuclear-to-cytoplasmic ratio of YAP/TAZ (n=3; >60 cells per experiment). The box represents the 25–75th percentiles, and the median is indicated. The whiskers show the 5–95th percentiles. (D–G) Relative transcript levels of (D) Ctgf, (E) Birc5, (F) Ankrd1 and (G) Cyr61. Error bars represent s.e.m. *P<0.05; **P<0.01; ***P<0.001; ns, not significant.
Immunofluorescence analysis revealed that YAP/TAZ were primarily localized to the nucleus in cells cultured on stiff substrata, but were mainly cytoplasmic in those cultured on soft substrata (Fig. 1B,C). These results were confirmed via cellular fractionation followed by immunoblotting analysis of YAP/TAZ (Fig. S2A). These findings are consistent with previous reports of YAP/TAZ localization in MCF10A human mammary epithelial cells (Aragona et al., 2013), human mesenchymal stem cells (Dupont et al., 2011) and IMR90 human lung fibroblasts (Liu et al., 2015), although those studies used different ranges of substratum stiffness (cells were cultured on soft substrata of 400–700 Pa or stiff substrata of >40,000 Pa).
YAP/TAZ also respond to biochemical signaling through the Wnt pathway. When NMuMG cells were treated with Wnt3a, β-catenin translocated to the nucleus within 2 h and remained nuclear for at least 8 h (Fig. S2B). We next examined how Wnt signaling and matrix stiffness are integrated by culturing cells on substrata of different compliance in the presence or absence of Wnt3a. Immunofluorescence analysis revealed that on both soft and stiff substrata, treatment with Wnt3a led to enhanced nuclear translocation of YAP/TAZ (Fig. 1B,C). Quantitative real-time RT-PCR (qRT-PCR) analysis showed that culture on stiff substrata increased the expression of Ctgf, Birc5, Ankrd1 and Cyr61, known targets of YAP/TAZ. However treatment with Wnt3a only led to an additional increase in expression of Birc5 on both substrata (Fig. 1D–G). These data suggest that while Wnt3a enhances nuclear localization of YAP/TAZ regardless of substratum stiffness, this is not sufficient to activate the expression of all YAP/TAZ target genes.
Substratum stiffness modulates Wnt3a-induced proliferation independently of YAP/TAZ
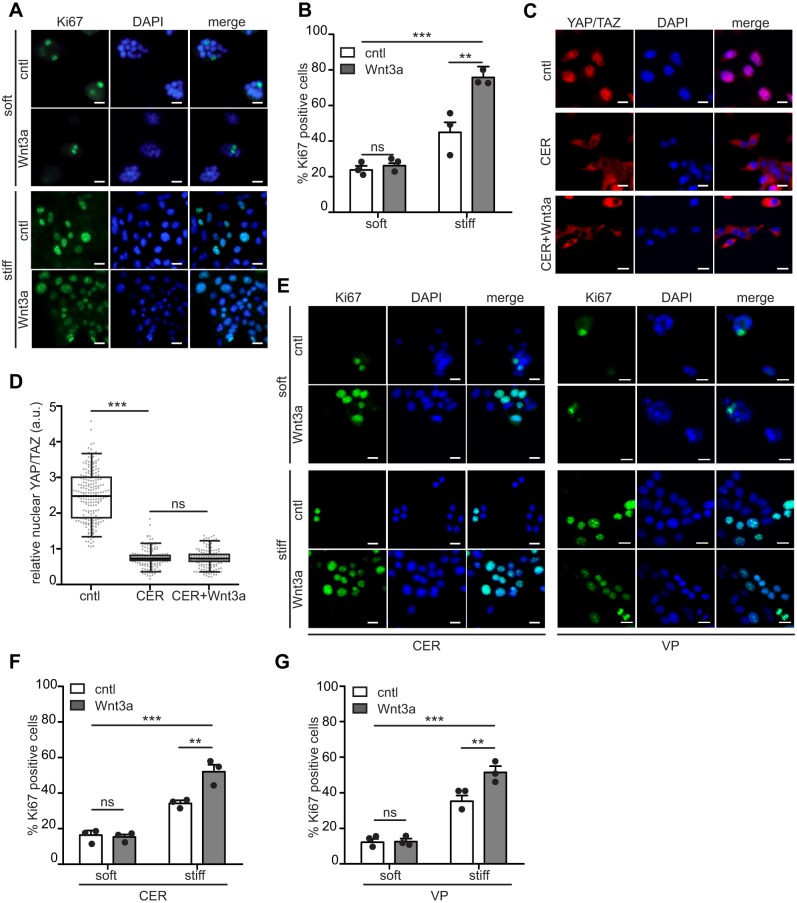
Birc5 (also known as baculoviral IAP repeat containing 5 or survivin) has been found to both promote cell proliferation and prevent apoptosis (Garg et al., 2016; Ito et al., 2000). Consistent with this, recent Gene Ontology analysis has revealed that a large fraction of direct targets of YAP/TAZ are linked to processes related to cell proliferation (Zanconato et al., 2015). We thus sought to determine whether the induction of YAP/TAZ nuclear translocation downstream of Wnt3a and stiffness affects cell proliferation. Immunofluorescence analysis of the proliferation marker Ki67 (also known as MKI67) revealed that cells were more proliferative on stiff substrata (Fig. 2A,B). Treatment with Wnt3a increased the percentage of Ki67-positive cells on stiff substrata, but not on soft substrata (Fig. 2A,B). Exposure to Wnt3a did not affect apoptosis on either soft or stiff substrata (Fig. S3). A microenvironment with physiological compliance thus appears to disrupt the ability of Wnt3a to induce cell proliferation.
Fig. 2.
Wnt3a enhances proliferation on stiff substrata independently of YAP/TAZ nuclear localization. (A) Fluorescence images of NMuMG cells stained for Ki67 (green) and nuclei (blue). (B) Percentage of Ki67-positive cells (n=3; >500 cells per experiment). (C) Fluorescence images of NMuMG cells stained for YAP/TAZ (red) and nuclei (blue) treated with DMSO (cntl) or 1 μM cerivastatin (CER) with or without Wnt3a 24 h before fixation. (D) Quantification of the nuclear-to-cytoplasmic ratio of YAP/TAZ (n=3; >60 cells per experiment) in NMuMG cells treated with DMSO (cntl) or 1 μM CER with or without Wnt3a. The box represents the 25–75th percentiles, and the median is indicated. The whiskers show the 5–95th percentiles. (E) Fluorescence images of NMuMG cells stained for Ki67 (green) and nuclei (blue) treated with 1 µM CER or 5 µM verteporfin (VP). (F) Percentage of Ki67-postive cells after treatment with 1 µM CER. (G) Percentage of Ki67-positive cells after treatment with 5 µM VP. Scale bars: 10 μm. Error bars represent s.e.m. **P<0.01; ***P<0.001; ns, not significant.
To determine whether YAP/TAZ signaling was required for Wnt-induced proliferation, we treated cells with cerivastatin, which inhibits the cholesterol biosynthesis pathway and causes YAP/TAZ to accumulate in the cytoplasm (Sorrentino et al., 2014) (Fig. 2C,D). Surprisingly, blocking YAP/TAZ nuclear localization with cerivastatin did not disrupt the ability of Wnt3a to elevate the proliferation of cells cultured on stiff substrata (Fig. 2E,F). To confirm these findings, we treated cells with verteporfin, which blocks the interaction between YAP and TEAD transcription factors, and thus suppresses downstream signaling (Liu-Chittenden et al., 2012). Exposure to Wnt3a still enhanced proliferation of cells on stiff substrata in the presence of verteporfin (Fig. 2G). Substratum stiffness thus appears to regulate the ability of Wnt3a to induce proliferation independently of YAP/TAZ.
ILK is required for Wnt-induced proliferation
Since soft substrata appeared to prevent Wnt3a from inducing proliferation, we hypothesized that other mechanotransduction pathways activated by stiff ECM might be involved. Mechanical cues from the ECM are transmitted from β1-integrin through ILK (Hannigan et al., 2005). Previously, we found that stiff microenvironments increase the expression of β1-integrin and ILK in breast cancer cells, and that signaling through ILK is essential for cancer stem cell behaviors (Pang et al., 2016). Consistent with this, here, we found that β1-integrin and ILK were upregulated in NMuMG cells cultured on stiff substrata (Fig. 3A–C). Treatment with Wnt3a led to increased ILK expression in cells cultured on stiff substrata but not on soft substrata (Fig. 3B,C). The expression of β1-integrin was not affected by Wnt3a treatment (Fig. 3A).
Fig. 3.
Wnt3a enhances the expression of ILK, which is required for Wnt3a-induced cell proliferation. Relative transcript levels of (A) β1-integrin (ITGB1) and (B) ILK in NMuMG cells cultured on soft or stiff substrata in the presence or absence of Wnt3a. (C) Immunoblotting analysis for ILK in cells cultured on soft or stiff substrata in the presence or absence of Wnt3a. (D) qRT-PCR and immunoblotting analysis for ILK in NMuMG cells stably expressing shRNA against ILK (shILK) or scrambled sequence control (shcntl). (E) Phase-contrast images of NMuMG-shcntl and NMuMG-shILK cells cultured on soft or stiff substrata. Scale bars: 50 μm. (F) Fluorescence images of NMuMG-shILK cells cultured on soft or stiff substrata stained for Ki67 (green) and nuclei (blue). Scale bars: 10 μm. (G) Percentage of Ki67-positive NMuMG-shILK cells (n=3; >500 cells per experiment). (H) Fluorescence images of NMuMG-shILK cells cultured on soft or stiff substrata stained for YAP/TAZ (red) and nuclei (blue). Scale bars: 10 μm. (I) Quantification of the nuclear-to-cytoplasmic ratio of YAP/TAZ (n=3; >50 cells per experiment). Error bars represent s.e.m. Whiskers represent 5th and 95th percentiles. *P<0.05; **P<0.01; ***P<0.001; ns, not significant.
To determine whether ILK plays a role in Wnt3a-induced proliferation, we used short hairpin RNA (shRNA) to stably deplete ILK in mammary epithelial cells (denoted shILK), which led to a ∼50% knockdown of ILK compared to scrambled controls (Fig. 3D). Depleting ILK led to a striking change in the morphology of cells cultured on stiff substrata – cells exhibited a rounded morphology, similar to those on soft substrata (Fig. 3E). Immunofluorescence analysis for Ki67 in shILK-expressing cells revealed that cell proliferation remained elevated on stiff substrata compared to that seen on soft substrata (Fig. 3F,G). However, depleting ILK rendered cells unresponsive to Wnt3a on both microenvironments (Fig. 3F,G). We found similar effects when we examined DNA synthesis using an EdU incorporation assay (Fig. S4). Moreover, immunofluorescence analysis revealed that YAP/TAZ remained primarily in the nucleus in shILK-expressing cells cultured on stiff substrata, similar to what was seen with the scrambled controls (Fig. 3H,I). These data confirm that nuclear localization of YAP/TAZ is not necessary for Wnt3a to induce proliferation of mammary epithelial cells cultured on stiff substrata.
These results suggest that on stiff substrata, ILK plays a critical role in regulating cell morphology and proliferative responses to Wnt. Specifically, ILK is required for Wnt3a to induce proliferation of cells on stiff microenvironments.
Ectopic expression of ILK permits Wnt3a to induce proliferation of cells on soft substrata
To determine whether ILK expression is sufficient for Wnt3a to induce proliferation in the absence of a stiff microenvironment, we ectopically expressed ILK using a recombinant bicistronic adenovirus encoding for ILK and GFP (adILK). As a control, we used adenovirus encoding for GFP alone (adGFP). Transduction with adILK resulted in a doubling of ILK expression, as determined by qRT-PCR and immunoblotting analysis (Fig. 4A). adILK-transduced cells exhibited round or spread morphology on soft or stiff substrata, respectively (Fig. 4B), which was indistinguishable from untransduced cells (Fig. 1A). Immunofluorescence analysis for Ki67 revealed that in adILK-transduced cells, Wnt3a treatment increased proliferation on both soft and stiff substrata (Fig. 4C,D). Overall, cell proliferation remained significantly higher on stiff substrata than on soft substrata, indicating that ILK is sufficient for Wnt-induced proliferation regardless of substratum stiffness, but not sufficient to induce proliferation on soft substrata in the absence of exogenous Wnt3a.
Fig. 4.

Ectopic expression of ILK permits Wnt3a to induce proliferation of cells on soft substrata. (A) qRT-PCR and immunoblotting analysis for ILK in NMuMG cells transduced with adGFP or adILK. (B) Phase-contrast images of NMuMG cells transduced with adGFP or adILK cultured on soft or stiff substrata. Scale bars: 50 μm. (C) Fluorescence images of NMuMG cells transduced with adILK (IRES-GFP, green) and cultured on soft or stiff substrata stained for Ki67 (red) and nuclei (blue). Scale bars: 10 μm. (D) Percentage of Ki67-positive adGFP- or adILK-transduced NMuMG cells (n=3; >500 cells per experiment). Error bars represent s.e.m. *P<0.05; **P<0.01; ***P<0.001.
ILK signals through Fzd1 to regulate Wnt-induced proliferation
Our data suggest that culture on soft substratum blocks signaling downstream of Wnt3a. Consistent with that, we found that depleting ILK abrogated the ability of Wnt3a to induce nuclear translocation of β-catenin (Fig. 5A,B). Conversely, treatment with Wnt3a led to enhanced nuclear translocation of β-catenin in adILK-transduced cells compared to what was seen in control cells (Fig. 5C,D). Since depleting ILK rendered cells incapable of proliferating in response to Wnt3a, we hypothesized that knockdown of ILK might negatively regulate frizzled receptor(s), thus rendering the cells insensitive to the ligand. We focused on Fzd1, Fzd4 and Fzd6, which are known to be expressed by NMuMG mouse mammary epithelial cells (Cheung et al., 2015). We found that the levels of Fzd4 and Fzd6 were unaffected by substratum stiffness (Fig. 5E,F); however, stiff substrata increased the expression of Fzd1 at both the transcript (Fig. 5G) and protein levels (Fig. 5H), as determined by qRT-PCR and immunoblotting analysis, respectively. We further found that depleting ILK resulted in a decrease in transcript (Fig. 5I) and protein (Fig. 5J,K) levels of Fzd1. Conversely, ectopic expression of ILK led to an increase in Fzd1 transcript (Fig. 5L) and protein (Fig. 5M,N) compared to control cells. These results suggest that ILK regulates signaling downstream of Wnt3a by altering the expression of Fzd1.
Fig. 5.
ILK regulates Wnt signaling by altering the expression of Fzd1. (A) Fluorescence images of NMuMG cells stably expressing scrambled sequence control (shcntl) or shRNA against ILK (shILK) stained for β-catenin (red) and nuclei (blue) in the presence or absence of Wnt3a. (B) Quantification of relative nuclear translocation of β-catenin (n=3; >200 cells per experiment). (C) Fluorescence images of NMuMG cells transduced with adGFP or adILK stained for β-catenin (red) and nuclei (blue) in the presence or absence of Wnt3a. (D) Quantification of relative nuclear translocation of β-catenin (n=3; >50 cells per experiment). In B and D, the box represents the 25–75th percentiles, and the median is indicated. The whiskers show the 5–95th percentiles. (E–G) Relative transcript level of (E) Fzd4, (F) Fzd6 and (G) Fzd1 in NMuMG cells cultured on soft or stiff substrata. (H) Immunoblotting analysis for Fzd1 in NMuMG cells cultured on soft or stiff substrata. (I) qRT-PCR, (J) immunoblotting and (K) immunofluorescence analysis for Fzd1 in shILK-expressing NMuMG cells or control cells. (L) qRT-PCR analysis for Fzd1 in NMuMG cells transduced with adGFP or adILK. (M) Immunofluorescence analysis for Fzd1 (red), GFP (green) and nuclei (blue) in NMuMG cells transduced with adGFP or adILK. (N) Immunoblotting analysis for Fzd1 or ILK in NMuMG cells transduced with adGFP or adILK. Scale bars: 10 μm. Error bars represent s.e.m. *P<0.05; **P<0.01; ***P<0.001; ns, not significant.
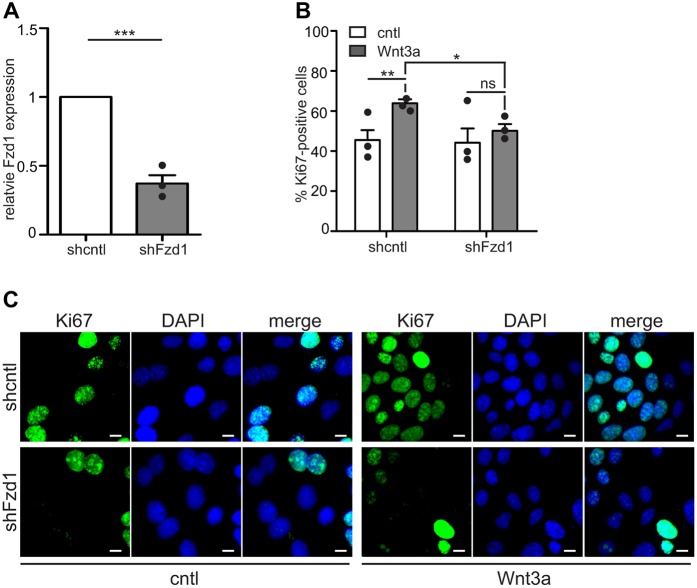
To determine whether Fzd1 is required for Wnt3a-induced proliferation, we transiently decreased its expression in mammary epithelial cells using shRNA (shFzd1, Fig. 6A). Immunofluorescence analysis for Ki67 showed that Wnt3a was unable to induce proliferation in shFzd1-expressing cells (Fig. 6B,C). Taken together, these results suggest that ILK transduces mechanical signals from the cellular microenvironment to regulate the expression of Fzd1, and thereby modulate proliferative signaling downstream of Wnt3a.
Fig. 6.
Fzd1 is required for proliferation downstream of Wnt3a. (A) qRT-PCR analysis for Fzd1 in NMuMG cells transiently transfected with shRNA against Fzd1 (shFzd1) or scrambled sequence control (shcntl). (B) Percentage of Ki67-positive shcntl or shFzd1-expressing NMuMG cells (n=3; >300 cells per experiment). (C) Fluorescence images of shFzd1-expressing NMuMG cells stained for Ki67 (green) and nuclei (blue). Scale bars: 10 μm. Error bars represent s.e.m. *P<0.05; **P<0.01; ***P<0.001; ns, not significant.
DISCUSSION
Canonical Wnt signaling regulates a diverse array of cellular behaviors. Here, we found that the canonical Wnt pathway is in turn controlled by the mechanical properties of the microenvironment. In particular, Wnt3a induces proliferation of mammary epithelial cells cultured on stiff but not soft substrata. Our data support a model in which a mechanically stiff microenvironment elevates the expression of ILK to prime mammary epithelial cells to be more sensitive to Wnt3a by enhancing the expression of the Wnt receptor Fzd1. Conversely, a soft microenvironment characteristic of the normal mammary gland protects mammary epithelial cells from excessive growth in the presence of elevated levels of Wnt3a. This may be particularly relevant for breast cancer. Wnt signaling has been found to be aberrantly activated in breast tumor tissue (Roelink et al., 1990; Zhang et al., 2010), and ILK is increasingly recognized for its role in promoting tumor progression, angiogenesis and metastasis (Graff et al., 2001; Hannigan et al., 2005; Ito et al., 2003). Our data suggest the presence of a feedback loop linking Wnt, ECM and ILK: Wnt signaling increases the expression of ILK in stiff microenvironments, which alters the levels of Fzd1 to enhance mitogenic signals downstream of Wnt, resulting in elevated proliferation; this would further increase mechanical stresses within a spatially restricted tumor microenvironment. This model suggests that therapeutic strategies aiming at pharmacologically reducing ILK activity or softening the tumor microenvironment back to a normal state may ameliorate malignant effects, such as uncontrolled proliferation, that are initiated by abnormally high levels of Wnt signaling.
There is extensive evidence for crosstalk between the Wnt and Hippo signaling pathways (Bernascone and Martin-Belmonte, 2013). In several colon cancer cell lines, YAP is required for β-catenin-dependent proliferation (Rosenbluh et al., 2012). Surprisingly, we found that the effects of substratum stiffness on proliferation downstream of Wnt3a were independent of YAP in NMuMG mouse mammary epithelial cells. Other systems, however, use YAP as a relay between Wnt and proliferative signaling. In confluent monolayers of Madin–Darby canine kidney (MDCK) epithelial cells, mechanical stretch of the underlying substratum induces proliferation by activating YAP in an E-cadherin-dependent manner (Benham-Pyle et al., 2015). Conversely, in the developing mouse heart, Hippo signaling through YAP negatively regulates the expression of Wnt target genes and thereby decreases the proliferation of cardiomyocytes (Heallen et al., 2011). It is likely that the specific physiological (and mechanical) context defines the role of YAP in Wnt-mediated induction of tissue growth and that this regulation depends on the strength or stability of cell–cell adhesions and differs between epithelial and mesenchymal cells (Du et al., 2016). Regardless, our study is the first to show a connection between canonical Wnt signaling and mechanosensing by ILK.
Fzd1 is the latest example of a protein whose expression is modulated by ILK. In pancreatic cancer cells, ILK enhances the expression of the KRAS oncogene by altering the levels of hnRNP1, which binds to the KRAS promoter (Chu et al., 2016). ILK also stabilizes Mucin-1 protein by decreasing its phosphorylation via protein kinase-C, thus altering Mucin-1 levels post-translationally (Huang et al., 2017). The ILK protein itself appears to contain a functional nuclear localization sequence and can translocate to the nucleus, and chromatin immunoprecipitation assays have revealed that ILK can interact directly with regulatory motifs within DNA (Acconcia et al., 2007). Our data suggest that ILK regulates the transcription of Fzd1, but it remains unclear whether it does so by directly binding to the Fzd1 promoter or enhancer regions, or by indirectly altering signaling through another pathway.
Cell shape has long been coupled with proliferation in various cell types. Cell spreading and integrin-mediated adhesion have been considered to be essential regulators of cell proliferation (Ben-Ze'ev et al., 1980; Chen et al., 1997; Mammoto et al., 2004; Singhvi et al., 1994). Our results show that despite having rounded morphology on both soft and stiff substrata, ILK-depleted cells are still more proliferative on stiff substrata than on soft substrata. Our data suggest an interesting disconnect between the mechanical regulation of cell shape and the regulation of proliferation by the microenvironment. Based on the striking morphological change observed in shILK-expressing cells, we expect ILK to act as a crucial regulator of cell morphology. We further suspect that additional mechanosensing mediators collaborate with ILK to translate changes in the mechanical properties of the ECM to downstream signaling, and may control other cellular behaviors in addition to proliferation.
Why do mammary epithelial cells respond differently to Wnt depending on the physical properties of the microenvironment? Our proposed mechanism may provide clues for the role of Wnt signaling in the embryonic and pubertal development of the mammary gland. Canonical Wnt activity has been shown to localize to developing mammary placodes and buds (Boras-Granic et al., 2006; Chu et al., 2004), and is essential for mammary gland morphogenesis (Chu et al., 2004; Lindvall et al., 2006). It was suggested that the localized activation of Wnt signaling might be achieved by restricted expression of a specific Wnt pathway gene; however, no obvious candidate was identified for this role (Chu et al., 2004). Our investigation provides an alternative mechanism for Wnt-induced mammary epithelial cell proliferation during mammary gland development. Instead of localized Wnt gene expression and activity, it is possible that localized differences in the mechanical stiffness (perhaps of the underlying mesenchyme) at placodes and buds modulate the levels of frizzled receptors and thereby control epithelial cell proliferation downstream of a non-localized Wnt signal.
Future investigations of how the physical properties of the cellular microenvironment modulate other Wnt-induced processes, such as adhesion and migration, will broaden our current understanding of tissue mechanics as it relates to both physiological processes like mammary gland morphogenesis and pathological processes like tumor progression.
MATERIALS AND METHODS
Cell culture and reagents
NMuMG mouse mammary epithelial cells (passed at a 1:10 ratio, used before passage 20, and authenticated by the supplier) were obtained from the American Type Culture Collection and cultured in high glucose DMEM (HyClone) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals) and 50 μg/ml gentamicin (Gibco). NMuMG cells were seeded at a density of 100,000 cells/cm2 and cultured at 37°C and 5% CO2 on fibronectin-coated polyacrylamide substrata of soft (E≈130 Pa) or stiff (E≈4020 Pa) compliances for 48 h as described previously (Lee et al., 2012); the initial seeding density resulted in ∼20% confluence on soft and ∼50% confluence on stiff substrata within a few hours because of differences in cell spreading. A saturating dose of purified Wnt3a (80 ng/ml; Peprotech) was added to the medium for the final 8 h of the experiment (Azzolin et al., 2014). Cerivastatin (1 μM; Sigma) or verteporfin (5 µM; Sigma) were added for the last 24 h of the experiment to inhibit nuclear localization of YAP/TAZ or interaction between YAP and TEAD transcription factors, respectively.
Transfections and viral transductions
Expression plasmids for shRNA against Fzd1 were obtained from Sigma (TRCN0000341044). The control scrambled shRNA plasmid, pLKO.1-scramble-shRNA (shcntl), was obtained from Addgene (plasmid 1864, deposited by D. Sabatini; Sarbassov et al., 2005). Cells were transiently transfected with shRNA expression constructs by using FuGENE HD transfection reagent (Promega).
To knock down the expression of ILK, NMuMG cells were transduced with lentiviral particles carrying short hairpin RNA (shRNA) against ILK (sc-35667-V and sc-35666-V; Santa Cruz Biotechnology) or control lentivirus expressing a scrambled shRNA sequence. Stable shRNA-expressing clones were produced according to the manufacturer's instructions and selected using puromycin.
Recombinant adenoviruses encoding ILK (bicistronic with GFP) or GFP alone were obtained from Vector Biolabs (Pang et al., 2016). Adenovirus was added directly to culture medium at a multiplicity of infection (MOI) of 100, and the medium was replaced 24 h later.
Immunofluorescence analysis
Samples were fixed with 4% paraformaldehyde (PFA) for 15 min and washed with PBS. After blocking for 1 h with 5% goat serum (Sigma) and 0.2% bovine serum albumin (BSA), the samples were incubated with primary antibody against YAP/TAZ (D24E4, 1:500; Cell Signaling), β-catenin (D10A8, 1:100; Cell Signaling), Ki67 (Ab15580, 1:500; Abcam), E-cadherin (3195, 1:300; Cell Signaling), cleaved caspase-3 (9961, 1:200; Cell Signaling) or Fzd1 (sc-398082, 1:50; Santa Cruz Biotechnology). Samples were then washed with PBS and incubated with Alexa Fluor-conjugated secondary antibodies (1:400; Invitrogen). Nuclei were counterstained with Hoechst 33342 (Invitrogen). After additional washes with PBS, samples were visualized using a 20×/0.45 NA air objective on a Nikon Eclipse Ti-U inverted fluorescence microscope (Nikon) equipped with a Hamamatsu ORCA charge-coupled device camera. Image analysis was performed on at least 50 cells for each experimental group over three independent experiments using ImageJ.
EdU incorporation assay
Samples were incubated with 10 μM EdU for 1 h before fixation, permeabilization and EdU staining, using the Click-iT EdU Alexa Fluor 594 Imaging kit (Thermo Scientific) according to the manufacturer's instructions.
Quantitative real-time reverse-transcription PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). Isolated RNA was used to synthesize cDNA using a Verso cDNA Synthesis Kit (Thermo Scientific). Transcript levels were measured by qRT-PCR using a Bio-Rad Mini-Opticon instrument and iTaq Universal SYBR Green Supermix (Bio-Rad). Primers for Ctgf, Birc5, Ankrd1, Cyr61, Itgb1, ILK, Fzd1, Fzd4, Fzd6 and 18S rRNA (Table S1) were determined to be specific by using BLAST and dissociation curve analysis. All transcript levels were normalized to that of 18S rRNA in the same sample.
Immunoblotting analysis
Samples were lysed in RIPA lysis buffer (Thermo Scientific) supplemented with protease inhibitors (Roche) and protein concentrations were measured using the Pierce bicinchoninic acid (BCA) Protein Assay Kit (Thermo Scientific). Samples were then mixed with Laemmli sample buffer, boiled at 95°C for 5 min, resolved by SDS-PAGE, and transferred onto nitrocellulose membranes. Membranes were then blocked in 5% milk and incubated overnight at 4°C in blocking buffer containing antibodies specific for YAP/TAZ (1:1000; Cell Signaling), Fzd1 (1:100; Santa Cruz Biotechnology), E-cadherin (1:800; Cell Signaling), ILK (1:1000; Cell Signaling) or GAPDH (1:1000; Cell Signaling). For nuclear extracts, samples were prepared with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific).
Statistical analysis
Data represent the mean±s.e.m. of at least three independent experiments unless otherwise specified. Statistical analysis was performed using GraphPad Prism 5.0. Differences between means were tested for significance using either a Student's t-test (normally distributed data) or a Mann–Whitney test (non-normally distributed data). When two different categorical independent variables were present, differences between means were tested for significance using a two-way ANOVA followed by Bonferroni post-test. P<0.05 was considered to represent a significant difference between experimental groups.
Supplementary Material
Acknowledgements
The authors thank members of the Tissue Morphodynamics Group for helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.H., C.M.N.; Investigation: S.H., M.-F.P.; Resources: M.-F.P.; Writing - original draft: S.H.; Writing - review & editing: C.M.N.; Supervision: C.M.N.; Project administration: C.M.N.; Funding acquisition: C.M.N.
Funding
This work was supported in part by grants from the National Institutes of Health (HL110335, HL118532, HL120142, CA187692, CA214292), the David & Lucile Packard Foundation, the Alfred P. Sloan Foundation, the Camille & Henry Dreyfus Foundation, the Burroughs Wellcome Fund, and a Faculty Scholars Award from the Howard Hughes Medical Institute. M.-F.P. was supported in part by a postdoctoral fellowship from the New Jersey Commission on Cancer Research. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.210476.supplemental
References
- Acconcia F., Barnes C. J., Singh R. R., Talukder A. H. and Kumar R. (2007). Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. 104, 6782–6787. 10.1073/pnas.0701999104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S. and Piccolo S. (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. 154, 1047–1059. 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V. et al. (2014). YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. 158, 157–170. 10.1016/j.cell.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R. and Penman S. (1980). Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. 21, 365–372. 10.1016/0092-8674(80)90473-0 [DOI] [PubMed] [Google Scholar]
- Benhaj K., Akcali K. C. and Ozturk M. (2006). Redundant expression of canonical Wnt ligands in human breast cancer cell lines. 15, 701–707. 10.3892/or.15.3.701 [DOI] [PubMed] [Google Scholar]
- Benham-Pyle B. W., Pruitt B. L. and Nelson W. J. (2015). Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. 348, 1024–1027. 10.1126/science.aaa4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernascone I. and Martin-Belmonte F. (2013). Crossroads of Wnt and Hippo in epithelial tissues. 23, 380–389. 10.1016/j.tcb.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Boras-Granic K., Chang H., Grosschedl R. and Hamel P. A. (2006). Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. 295, 219–231. 10.1016/j.ydbio.2006.03.030 [DOI] [PubMed] [Google Scholar]
- Chen C. S., Mrksich M., Huang S., Whitesides G. M. and Ingber D. E. (1997). Geometric control of cell life and death. 276, 1425–1428. 10.1126/science.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- Cheung C. T., Bendris N., Paul C., Hamieh A., Anouar Y., Hahne M., Blanchard J.-M. and Lemmers B. (2015). Cyclin A2 modulates EMT via beta-catenin and phospholipase C pathways. 36, 914–924. 10.1093/carcin/bgv069 [DOI] [PubMed] [Google Scholar]
- Chu E. Y., Hens J., Andl T., Kairo A., Yamaguchi T. P., Brisken C., Glick A., Wysolmerski J. J. and Millar S. E. (2004). Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. 131, 4819–4829. 10.1242/dev.01347 [DOI] [PubMed] [Google Scholar]
- Chu P.-C., Yang M.-C., Kulp S. K., Salunke S. B., Himmel L. E., Fang C.-S., Jadhav A. M., Shan Y.-S., Lee C.-T., Lai M.-D. et al. (2016). Regulation of oncogenic KRAS signaling via a novel KRAS-integrin-linked kinase-hnRNPA1 regulatory loop in human pancreatic cancer cells. 35, 3897–3908. 10.1038/onc.2015.458 [DOI] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. 127, 469–480. 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Discher D. E., Janmey P. and Wang Y. L. (2005). Tissue cells feel and respond to the stiffness of their substrate. 310, 1139–1143. 10.1126/science.1116995 [DOI] [PubMed] [Google Scholar]
- Du J., Zu Y., Li J., Du S., Xu Y., Zhang L., Jiang L., Wang Z., Chien S. and Yang C. (2016). Extracellular matrix stiffness dictates Wnt expression through integrin pathway. 6, 20395 10.1038/srep20395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S. et al. (2011). Role of YAP/TAZ in mechanotransduction. 474, 179–183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L. and Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. 126, 677–689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Garg H., Suri P., Gupta J. C., Talwar G. P. and Dubey S. (2016). Survivin: a unique target for tumor therapy. 16, 49 10.1186/s12935-016-0326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J. R., Deddens J. A., Konicek B. W., Colligan B. M., Hurst B. M., Carter H. W. and Carter J. H. (2001). Integrin-linked kinase expression increases with prostate tumor grade. 7, 1987–1991. [PubMed] [Google Scholar]
- Halder G., Dupont S. and Piccolo S. (2012). Transduction of mechanical and cytoskeletal cues by YAP and TAZ. 13, 591–600. 10.1038/nrm3416 [DOI] [PubMed] [Google Scholar]
- Hannigan G., Troussard A. A. and Dedhar S. (2005). Integrin-linked kinase: a cancer therapeutic target unique among its ILK. 5, 51–63. 10.1038/nrc1524 [DOI] [PubMed] [Google Scholar]
- He S., Lu Y., Liu X., Huang X., Keller E. T., Qian C. N. and Zhang J. (2015). Wnt3a: functions and implications in cancer. 34, 554–562. 10.1186/s40880-015-0052-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L. and Martin J. F. (2011). Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. 332, 458–461. 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E.-C., Kulp S. K., Huang H.-L., Tu H.-J., Salunke S. B., Sullivan N. J., Sun D., Wicha M. S., Shapiro C. L. and Chen C.-S. (2015). Function of integrin-linked kinase in modulating the stemness of IL-6-abundant breast cancer cells by regulating gamma-secretase-mediated notch1 activation in caveolae. 17, 497–508. 10.1016/j.neo.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-L., Wu H.-Y., Chu P.-C., Lai I.-L., Huang P.-H., Kulp S. K., Pan S.-L., Teng C.-M. and Chen C.-S. (2017). Role of integrin-linked kinase in regulating the protein stability of the MUC1-C oncoprotein in pancreatic cancer cells. 6, e359 10.1038/oncsis.2017.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Shiraki K., Sugimoto K., Yamanaka T., Fujikawa K., Ito M., Takase K., Moriyama M., Kawano H., Hayashida M. et al. (2000). Survivin promotes cell proliferation in human hepatocellular carcinoma. 31, 1080–1085. 10.1053/he.2000.6496 [DOI] [PubMed] [Google Scholar]
- Ito R., Oue N., Zhu X. D., Yoshida K., Nakayama H., Yokozaki H. and Yasui W. (2003). Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. 442, 118–123. [DOI] [PubMed] [Google Scholar]
- Klein E. A., Yin L., Kothapalli D., Castagnino P., Byfield F. J., Xu T., Levental I., Hawthorne E., Janmey P. A. and Assoian R. K. (2009). Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. 19, 1511–1518. 10.1016/j.cub.2009.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Chen Q. K., Lui C., Cichon M. A., Radisky D. C. and Nelson C. M. (2012). Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial-mesenchymal transition. 23, 4097–4108. 10.1091/mbc.E12-02-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F. T., Csiszar K., Giaccia A., Weninger W. et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. 139, 891–906. 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall C., Evans N. C., Zylstra C. R., Li Y., Alexander C. M. and Williams B. O. (2006). The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. 281, 35081–35087. 10.1074/jbc.M607571200 [DOI] [PubMed] [Google Scholar]
- Liu F., Lagares D., Choi K. M., Stopfer L., Marinković A., Vrbanac V., Probst C. K., Hiemer S. E., Sisson T. H., Horowitz J. C. et al. (2015). Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. 308, L344–L357. 10.1152/ajplung.00300.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y., Huang B., Shim J. S., Chen Q., Lee S.-J., Anders R. A., Liu J. O. and Pan D. (2012). Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. 26, 1300–1305. 10.1101/gad.192856.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. C., Pan C. Q., Shivashankar G. V., Bershadsky A., Sudol M. and Sheetz M. (2014). YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. 588, 2663–2670. 10.1016/j.febslet.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Lu P., Weaver V. M. and Werb Z. (2012). The extracellular matrix: a dynamic niche in cancer progression. 196, 395–406. 10.1083/jcb.201102147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A., Huang S., Moore K., Oh P. and Ingber D. E. (2004). Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. 279, 26323–26330. 10.1074/jbc.M402725200 [DOI] [PubMed] [Google Scholar]
- Masckauchán T. N. H., Shawber C. J., Funahashi Y., Li C.-M. and Kitajewski J. (2005). Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. 8, 43–51. 10.1007/s10456-005-5612-9 [DOI] [PubMed] [Google Scholar]
- Oloumi A., Syam S. and Dedhar S. (2006). Modulation of Wnt3a-mediated nuclear beta-catenin accumulation and activation by integrin-linked kinase in mammalian cells. 25, 7747–7757. 10.1038/sj.onc.1209752 [DOI] [PubMed] [Google Scholar]
- Pang M.-F., Siedlik M. J., Han S., Stallings-Mann M., Radisky D. C. and Nelson C. M. (2016). Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. 76, 5277–5287. 10.1158/0008-5472.CAN-16-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D. et al. (2005). Tensional homeostasis and the malignant phenotype. 8, 241–254. 10.1016/j.ccr.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Piccolo S., Dupont S. and Cordenonsi M. (2014). The biology of YAP/TAZ: hippo signaling and beyond. 94, 1287–1312. 10.1152/physrev.00005.2014 [DOI] [PubMed] [Google Scholar]
- Raab M., Swift J., Dingal P. C. D., Shah P., Shin J.-W. and Discher D. E. (2012). Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. 199, 669–683. 10.1083/jcb.201205056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H., Wagenaar E., Lopes da Silva S. L. and Nusse R. (1990). Wnt-3, a gene activated by proviral insertion in mouse mammary-tumors, is homologous to Int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. 87, 4519–4523. 10.1073/pnas.87.12.4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh J., Nijhawan D., Cox A. G., Li X., Neal J. T., Schafer E. J., Zack T. I., Wang X., Tsherniak A., Schinzel A. C. et al. (2012). beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. 151, 1457–1473. 10.1016/j.cell.2012.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Li S., Docheva D., Grashoff C., Sakai K., Kostka G., Braun A., Pfeifer A., Yurchenco P. D. and Fassler R. (2003). Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. 17, 926–940. 10.1101/gad.255603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. 307(5712): 1098–1101. doi:10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- Singhvi R., Kumar A., Lopez G. P., Stephanopoulos G. N., Wang D. I., Whitesides G. M. and Ingber D. E. (1994). Engineering cell shape and function. 264, 696–698. 10.1126/science.8171320 [DOI] [PubMed] [Google Scholar]
- Sorrentino G., Ruggeri N., Specchia V., Cordenonsi M., Mano M., Dupont S., Manfrin A., Ingallina E., Sommaggio R., Piazza S. et al. (2014). Metabolic control of YAP and TAZ by the mevalonate pathway. 16, 357–366. 10.1038/ncb2936 [DOI] [PubMed] [Google Scholar]
- Taelman V. F., Dobrowolski R., Plouhinec J.-L., Fuentealba L. C., Vorwald P. P., Gumper I., Sabatini D. D. and De Robertis E. M. (2010). Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. 143, 1136–1148. 10.1016/j.cell.2010.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troussard A. A., McDonald P. C., Wederell E. D., Mawji N. M., Filipenko N. R., Gelmon K. A., Kucab J. E., Dunn S. E., Emerman J. T., Bally M. B. et al. (2006). Preferential dependence of breast cancer cells versus normal cells on integrin-linked kinase for protein kinase B/Akt activation and cell survival. 66, 393–403. 10.1158/0008-5472.CAN-05-2304 [DOI] [PubMed] [Google Scholar]
- Zanconato F., Forcato M., Battilana G., Azzolin L., Quaranta E., Bodega B., Rosato A., Bicciato S., Cordenonsi M. and Piccolo S. (2015). Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. 17, 1218–1227. 10.1038/ncb3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Li Y., Liu Q., Lu W. and Bu G. (2010). Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. 29, 539–549. 10.1038/onc.2009.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.