Abstract
Metal ions are essential for many reactions, however, metal excess can be toxic. In bacteria, metal limitation activates pathways for import and mobilization of metals, whereas metal excess induces efflux and storage. In this Review, we highlight recent insights into metal homeostasis, including protein- and RNA-based sensors that interact directly with metals or metal-containing cofactors. The resulting transcriptional response to metal stress is deployed in a stepwise manner, and is reinforced by post-transcriptional regulatory systems. Metal limitation and intoxication by the host is an evolutionarily ancient strategy to limit bacterial growth. The details of the resulting growth restriction are beginning to be understood, and appear to be organism-specific.
Metal ions are essential for life, and are indispensable for nearly all aspects of metabolism. Indeed, many central bioenergetic and biogeochemical processes on Earth, including respiration, photosynthesis, and nitrogen fixation, are absolutely dependent on metal ion co-factors1. Here, we focus on those processes that allow bacteria to maintain metal homeostasis, in particular, of three most important metals for metabolism — zinc, iron and manganese. Building on recent advances, primarily from studies of Bacillus subtilis and other well-studied model organisms, we highlight new insights into the varied and nuanced ways in which bacteria sense and respond to changes in metal ion status. Studies in various organisms are also revealing insights into how metal limitation restricts bacterial growth2, and how metal intoxication can kill bacteria3, which are relevant to host-pathogen interactions.
Bacteria respond to metal ion limitation and excess by activating the expression of specific sets of genes in regulons that are typically controlled by a metal-sensing regulatory transcription factor, that is, a metalloregulatory protein. Metalloregulatory proteins are multimeric, DNA-binding proteins that undergo an allosteric transition when binding metals4,5. Here, we will focus on the prototypic proteins that regulate Zn(II), Fe(II), and Mn(II) homeostasis. Generally, metalloregulators bind directly to their effector metals, but cellular iron status may instead be monitored by sensing iron-sulfur clusters6 or heme7 as a proxy. Finally, as documented for Mg(II)8 and Mn(II)9,10, some metals may also be sensed by binding to riboswitches in the nascent RNA transcript to control RNA synthesis or mRNA translation (Figure 1).
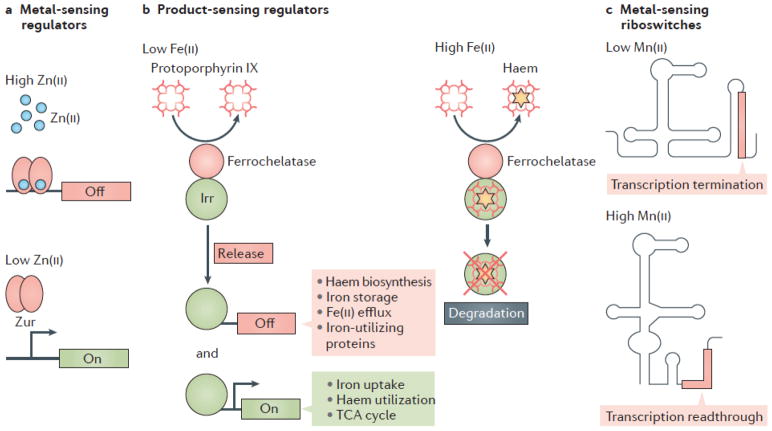
Figure 1. Types of metalloregulatory systems.
Metal sensing regulators can be divided into three classes: proteins that bind metal directly, proteins that bind a metal dependent cofactor, and riboswitches that bind metal directly. (A) Direct metal sensors are those which modulate transcription in response to direct metal binding (for example, Zn(II) binding to Zur)34. (B) Product sensing metalloregulators use the levels of a metal dependent metabolite as a proxy for intracellular metal levels. In the case of B. japonicum Irr, heme serves as a proxy for Fe(II) levels. Irr binds directly to ferrochelatase, which catalyzes formation of heme through the insertion of iron into protoporphyrin. Under conditions of Fe(II) sufficiency, heme is produced by ferrochelatase. Heme can then bind Irr, leading to its degradation. However, under conditions of Fe(II) limitation, apo-Irr is released and active for transcription regulation7. (C) Metal sensing riboswitches, such as the yybP-ykoY Mn(II) sensing riboswitch, can act at the level of transcription and translation9,10. In B. subtilis, binding of Mn(II) favors a RNA conformation that prevents the formation of an intrinsic transcription termination hairpin.
Metals cannot be synthesized or degraded, and therefore homeostasis primarily relies on modulating transport into and out of the cell. However, adaptation to metal limitation and excess is complex. In addition to increased import, metal limitation may lead to the mobilization of stored metal, the activation of alternative pathways independent of the limiting metal, and down-regulation of some metal-dependent enzymes and processes (a metal-sparing response)1. Conversely, metal excess typically leads to the expression of efflux systems11. However, the importance of abundant metal binding metabolites and metal storage and sequestration mechanisms in buffering metal fluctuations is becoming increasingly appreciated12,13. Despite the activation of these various adaptive responses, bacteria will cease growth and ultimately die under conditions of severe metal limitation (starvation) or excess (intoxication). The cellular processes that fail under these conditions are not well understood, but insights are emerging in several organisms. For example, the expression of specific zinc-independent14,15 or iron-independent16 enzymes when zinc and iron, respectively, are limited implies that the corresponding metal-dependent enzymes may fail to be activated. Conversely, metal ion intoxication leads to growth arrest that can often be traced to mismetallation of a metalloprotein with a non-preferred metal17. Efflux pumps for Mn(II)18–20, Zn(II)21 and Cu(I)22 have been well defined, and recent work in B. subtilis has revealed the role of P-type ATPases in Fe(II) efflux23. These types of efflux systems function as virulence factors in several bacterial pathogens3,20,24–26, which implies that metal intoxication is a selective pressure during bacterial growth in hosts. Metal intoxication is likely an antibacterial strategy that complements the better known process of metal ion limitation (nutritional immunity27), which is reviewed in detail elsewhere2,28. In this Review, we will discuss different metalloregulatory systems that are used by bacteria and how they respond to metal limitation and intoxication, as well as how these systems influence host-pathogen interactions.
Metalloregulatory systems
Complex mechanisms are required to maintain essential metals at sufficient levels to meet cellular demands, yet low enough to avoid toxicity. When considering the metal needs of the cell, two key parameters can be defined. First, the overall content of metal is defined as the quota (for example, in atoms per cell). Second, the labile pool refers to that subset of the quota that is kinetically accessible (rapidly exchanging) and available for incorporation into nascent proteins and for sensing by regulators. The absolute size of the labile pool can be difficult to measure, but it functions as a buffered pool of metal ions in equilibrium with thermodynamically free (hydrated) ions (BOX 1).
BOX 1. Bacterial metal ion quotas and the labile pool.
Understanding bacterial metal homeostasis relies on three key concepts: the metal quota, the labile metal pool, and the thermodynamically ‘free’ concentration of metal in the cell at equilibrium. The total metal content of the bacterial cell (atoms/cell) is defined as the quota and reflects the overall demand for metals to support growth. For zinc, iron and manganese the majority of cellular metal is bound to proteins, often in the form of a metal cofactor. Examples include Zn(II), which is bound during protein folding and iron, which is incorporated into FeS cluster enzymes, hemoproteins, and mononuclear Fe(II)-dependent enzymes.
The labile pool is difficult to measure, but can be operationally defined. For example, the labile iron pool can be defined as that portion of the quota that is accessible to a chelator in vivo. In E. coli, the labile iron concentration has been measured in the range of ~10 μM147. Nascent proteins acquire their metal cofactors during synthesis from this labile pool of exchangeable metal ions, and metalloregulatory proteins monitor the status of this same pool. The properties of the labile pool are determined, in part by the overall avidity with which metals bind to ligands, as described in the Irving-Williams series [Cu(I) > Zn(II) > Ni(II) > Co(II) > Fe(II) > Mn(II) > Mg(II) > Ca(II)148], and by the variety and affinity of potential metal ligands in the cytosol149.
The labile metal pool is in equilibrium with, and serves to buffer, metal ions at a thermodynamically defined concentration of free metal ions. Metalloregulator ion affinity provides insight into the buffered metal pools in the cell: when free metals exceed the dissociation constant (Kd) of the metalloregulator, the cell responds appropriately by repression of uptake or derepression of efflux and storage functions. The equilibrium concentration of free metals in the cell varies over many orders of magnitude. Cytosolic copper levels are typically low (there are few cytosolic enzymes that require copper) and the free Cu(I) level is buffered, due to the high avidity with which Cu(I) binds to ligands, in the attomolar (10−18 M) range 150. Cytosolic Cu(I) is mobilized by protein chaperones, which transport it to appropriate recipient enzymes and prevent toxicity due to adventitious mismetallation151,152. Cytosolic zinc levels are overall much higher (an ~1 mM quota), but the high affinity of Zn(II) for its ligands results in a labile pool that is buffered to the sub-picomolar range ( ~10−12–10−14 M), which indicates that virtually no ‘free’ (fully hydrated) Zn(II) ions exist in the cell95. Conversely, cytosolic magnesium (Mg(II)) is maintained at high physiological levels (~10−3 M) and is well hydrated and highly mobile 153. Iron and manganese are a particular challenge for the cell because both metals are relatively abundant, for example, in B. subtilis, the pools of free Fe(II) and Mn(II) are estimated to be in the range of 10−6 to 10−5 M 154. The potential consequence of this is that cytosolic levels of free (hydrated and mobile) metal may be very similar, resulting in competition for metallation of the same enzymes and regulators154.
In B. subtilis, a Gram positive model bacterium, the zinc, iron and manganese quotas correspond to ~0.1 to 0.5 mM averaged over the cell volume1. Whereas cellular zinc quotas are relatively constant across species, the demand for iron and manganese is more variable. For example, Lactobacillus acidophilus29 and the human Lyme disease pathogen Borrelia burgdorferii30 have no demonstrable requirement for iron. Conversely, Escherichia coli requires little if any manganese when iron is sufficient31. By contrast, both iron and manganese32 are absolutely required for growth of B. subtilis. Metalloregulatory systems are responsible for ensuring that cellular metal needs are met, and they do this by monitoring the kinetically accessible (labile) pool of metal ions in the cell, and appropriately modulating gene expression5 (Figure 1).
Direct metal-sensing regulators
Metalloregulatory proteins ensure that metal acquisition systems are expressed when metals are limiting for growth, and that storage and efflux systems are expressed under conditions of excess. For example, when the intracellular metal concentration is sufficient, a metal-bound holorepressor may repress genes that function in metal uptake (Figure 1), whereas metallation of sensors of excess may lead to the expression of genes that are required for metal storage or efflux4.
Key to this process is the ability of the metalloregulator to bind and respond to its cognate effector, while ignoring non-cognate (competing) metals. If metalloregulators are mismetallated by a non-cognate metal, metal homeostasis systems may be inappropriately regulated, which has potentially disastrous consequences. This is generally avoided by a combination of four distinct mechanisms5,33: carefully tuning the affinity of the regulator to the level of free metal that is buffered by the labile pool, restricting access of non-cognate metals to the regulator, restricting allostery to the cognate metal, and modulating the abundance of the regulator in the cell (BOX 2). Detailed biochemical and genetic studies of many metalloregulators, supported by structural studies of both metallated and unbound (apo-) forms, document these various mechanisms and have been reviewed in detail elsewhere4.
BOX 2. Biochemical basis of metal-specific sensing.
Metalloregulatory systems have evolved to detect and respond to specific metal ions in the complex milieu of the cell, which often contains similar ions at higher concentrations. To respond appropriately, metalloregulators use four principles: affinity, access, allostery, and abundance33.
Affinity refers to the biochemical affinity for cognate and non-cognate metal ions (equilibrium binding constant, often expressed as a dissociation constant, Kd). The affinity is determined by chemical principles, and is generally tuned to detect changes in the labile pool that represent an increase or decrease relative to the equilibrium value. Affinity is determined by the nature of the binding site, which includes the identity and coordination geometry of the metal ligands4,5. For example, Fe(II) prefers an octahedral geometry whereas both Mn(II) and Zn(II) may have tetrahedral (or higher in the case of Mn) coordination, with less preference for specific bond angles. Metals also differ in their preference for hard vs. soft (polarizable) ligands, with cysteine-rich coordination sites favoring Zn(II) or Fe(II) rather than Mn(II), for example155.
Access refers to the availability of a particular ion for binding and reflects the fact that metals that could compete for a specific metal sensor do not have access to the regulator due to limited uptake into the cell, effective sequestration or buffering at concentrations below those that would lead to mismetallation. For example, metalloregulators that bind strongly in vitro to non-cognate metals higher in the Irving-Williams series (Box 1) may be maintained in cells at low levels due to buffering and repression of uptake by their cognate metalloregulator156. In some cases, the ambient levels of free metal in the cytosol can vary substantially between bacteria. This has led to the common observation that the responsiveness of metalloregulators may be different when expressed in a heterologous host that maintains labile metal pools at different levels157,158.
Allostery refers to the ability of metals that bind to metalloregulatory proteins to trigger the allosteric conformational changes necessary to affect gene expression. For example, the B. subtilis CzrA protein dissociates from DNA in the presence of Zn(II), which leads to the expression of Zn(II) efflux genes56. However, mismetallation of CzrA with Cu(I) in vitro fails to trigger this allosteric transition, and competitively inhibits the ability of Zn(II) to do so159. Conversely, the Cu(I) sensor CsoR can also bind various divalent metals (including Zn), but these fail to trigger the allosteric change needed for derepression of Cu(I) efflux functions160.
Abundance refers to the intracellular level of a metalloregulator and can also affect selectivity. One notable example comes from studies of Fe(II)-sensing by B. subtilis Fur. Under most conditions Fur responds to and senses Fe(II) with high selectivity. However, a mutation that affects expression of the fur gene and leads to a two-fold increase in Fur protein levels results in mismetallation of Fur with Mn(II), inappropriate repression of Fe(II) uptake and homeostasis, and ultimately in a strong growth defect44.
In this section, we briefly summarize the key features of metal sensing as exemplified by those proteins that control zinc, iron and manganese homeostasis in B. subtilis. These regulators are representative of widespread classes of regulators and we can therefore draw parallels with homologous and analogous regulators in other bacteria. In B. subtilis, Zn(II) is sensed by Zur34 (zinc-specific metallo-regulatory protein; a sensor of sufficiency) and CzrA35 (a sensor of excess), Fe(II) by Fur (ferric uptake regulation protein)36, and Mn(II) by MntR37 and by Mn(II)-sensing riboswitches9 (Figure 2). For many sensors, including Zur, Fur, and MntR, the binding of a metal ion is required to bind DNA with high affinity. By contrast, CzrA is representative of metalloregulators that bind DNA in their unmetallated state (apo-repressor) and dissociate upon metal-binding, leading to derepression of efflux.
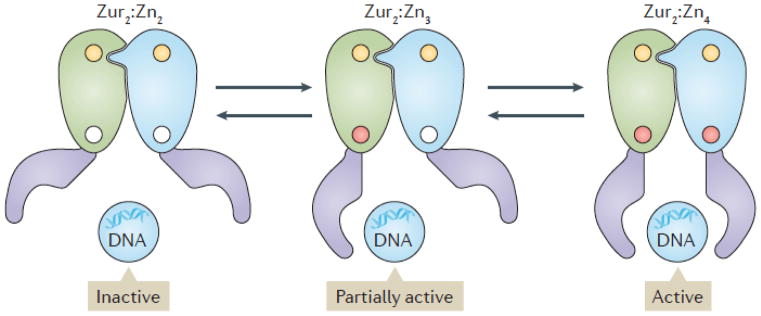
Figure 2. Mechanisms of stepwise regulation of the Zur regulon.
Under conditions of Zn(II) sufficiency (right), the dimeric Zur repressor is present in its fully metallated (Zur2:Zn4) state and the full Zur regulon is repressed. As Zn(II) levels fall, the first sets of genes (see Fig. 3) are derepressed as Zur transitions to the intermediate metallated form (Zur2:Zn3; middle) that binds DNA with lower affinity. As Zn(II) levels fall further, the remaining, more tightly bound, Zn(II) ion dissociates leading to formation of Zur with only the structural Zn(II) site is occupied ( Zur2:Zn2; left). This leads to derepression of additional adaptive responses, including the expression of the alternate folate synthesis enzyme FolEB and an alternate S14 ribosomal protein.
Fe(II)-sensing by Fur
In B. subtilis, iron sufficiency is sensed by Fur, a classic ferric uptake regulator36. Fur was originally described in E. coli over 30 years ago38 and is a prototypic metalloregulator: a dimeric DNA-binding protein that requires binding of divalent cations to bind DNA with high affinity. Despite several decades of study, and numerous crystal structures of Fur and Fur-like regulators in various states of metallation39–43, the precise site of metal sensing has been controversial.
Biochemical studies of Fur in B. subtilis have provided insights into the sites of metal sensing44. Similar to several representative Fur family members, this Fur protein has a tightly associated structural Zn(II), which is bound at site 1 and which is required for protein folding and dimerization45. DNA-binding is activated by binding of divalent metal ions at two additional sites (sites 2 and 3). Site 3 binds metal (presumably iron) tighter than site 2, and it is ultimately the binding of iron to site 2 that leads to full activation of DNA-binding. The fully metallated (active) form of Fur likely is a dimer with two Zn(II) atoms and up to four Fe(II) atoms (Fur2:Zn2Fe4)44.
Whereas Fur in B. subtilis normally responds specifically to Fe(II) in vivo, mismetallation with Mn(II) can also occur44. Indeed, in several proteobacteria it has long been known that under conditions of Mn(II) intoxication mutants arise that have reduced or eliminated Fur activity, which suggests that Fur metallated by Mn(II) inappropriately represses Fe(II) uptake46–48. By contrast, Fur in B. subtilis is selectively activated by Fe(II), with a dissociation constant (Kd) of ~1 μM, but generally non-responsive to Mn(II), which binds with a Kd of ~24 μM44. The affinity of Fur for Mn(II) is only slightly lower than the affinity of MntR for Mn(II), which has a Kd of ~6 μM, a concentration presumed to reflect the in vivo level of free Mn(II) at equilibrium44. As a result, the selectivity of Fur for Fe(II) over Mn(II) is delicately balanced, and regulation of Fur abundance is crucial: an increase in Fur protein levels of even two-fold can lead to a dysregulation of Fe(II) homeostasis in which the ambient cellular Mn(II) levels activate Fur and repress iron import, which leads to iron starvation44.
Mn(II)-sensing by MntR
Another metalloregulator, MntR, is the central regulator of Mn(II) homeostasis37. MntR in B. subtilis is representative of a Mn(II)-sensing subset of the DtxR (diphtheria toxin repressor) family of Fe(II) sensors that are found in several Gram positive bacteria49. The MntR dimer binds four atoms of Mn(II) (MntR2:Mn4) at the A and C sites within each protomer, which activates DNA binding50. Metal selectivity in this system results, in part, from these two sequential binding events: Mn(II) binding at the A site helps organize the C site for binding of the second Mn(II) atom51. Fe(II) binds to the A site with a similar affinity as Mn(II), but with a different coordination geometry51. As a result, the C site is distorted and binding to the C site is inhibited. Thus, Fe(II) fails to trigger the allosteric transition that is required for DNA-binding and may thereby act as an antagonist of MntR function. MntR homologs are widely conserved in bacterial species, including E. coli52, and are frequently involved in sensing Mn(II), and sometimes also Fe(II)53.
Zn(II)-sensing by Zur and CzrA
Regulation of Zn(II) homeostasis involves, Zur, a paralog of Fur that is activated and binds DNA in response to Zn(II)34,54. Zur is also a dimeric protein with both a structural Zn(II) and a second, Zn(II)-sensing site (site 2)55. Activation of DNA-binding of Zur occurs in two steps — binding of Zn(II) to one protomer (Kd ~56 pM) in the dimer and then, with ~20-fold lower affinity (Kd ~1.2 nM), binding to the second protomer. Due to this negative cooperativity in binding, the inactive Zur dimer (Zur2:Zn2) is sequentially metallated to first form Zur2:Zn3 and then Zur2:Zn4 (Figure 3). The implications of this negative cooperativity for gene regulation are considered below. Zur homologs are widely distributed in bacteria, and where absent, functionally analogous regulators often control sets of genes that are similar to those controlled by Zur.
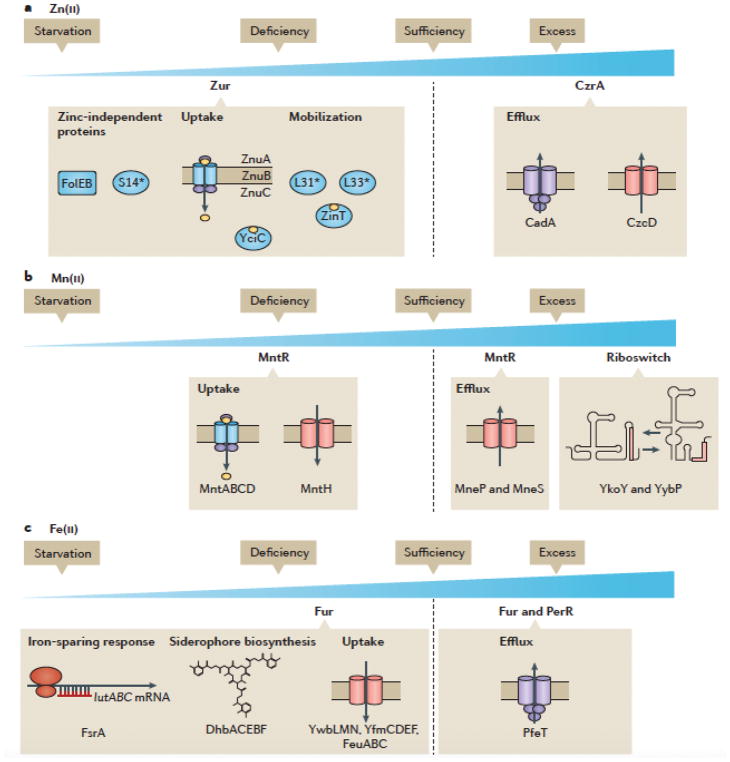
Figure 3. Metalloregulation in B. subtilis as a model system.
As cells transition from Zn(II) sufficiency to Zn(II) deficiency, the Zur regulon is derepressed in three stages90. First, Zn(II) independent L31 and L33 ribosomal protein paralogs are expressed to liberate Zn(II) from the ribosome. Then, the ZnuABC Zn(II) uptake system is expressed to import Zn(II) from the environment. Finally, the Zn(II)-independent S14 ribosomal protein paralog is expressed to ensure continued synthesis of ribosomes, and the Zn(II)-independent. FolEB GTP cyclohydrolase is produced to support folate synthesis. Under conditions of Zn(II) excess, expression of Zn(II) efflux pumps is derepressed when Zn(II) binds to CzrA, which impairs its ability to bind to its operator sites. Whereas conditions of Zn(II) limitation and excess are sensed by two metalloregulators, Mn(II) and Fe(II) homeostasis are controlled by single metalloregulatory proteins (Mn(II) by MntR and Fe(II) by Fur). Under conditions of Mn(II) limitation, the MntR regulon is derepressed leading to expression of two Mn(II) uptake systems37. When cells are exposed to Mn(II) excess, MntR directly activates the expression of Mn(II) efflux pumps18. As cells become severely Mn(II) overloaded, genes regulated by the Mn(II) sensing yybP-ykoY riboswitch are induced9,10. Fe(II) limitation leads to derepression of the Fur regulon, including genes required for Fe(II) uptake (siderophore biosynthesis and uptake, elemental iron import, and an iron-citrate importer)75 and the Fe(II)-sparing response65. Under conditions of Fe(II) intoxication, Fur directly induces expression of Fe(II) efflux mediated by PfeT 23.
In addition to sensing metal sufficiency, cells also have mechanisms to sense metal excess. In B. subtilis, Zn(II) excess is sensed by a separate regulatory protein, CzrA56. CzrA binds DNA as a repressor in the absence of regulatory metal ions and, upon metallation with Zn(II), CzrA dissociates from DNA, which results in induction of two efflux systems, the CadA P-type ATPase and the CzcD cation diffusion facilitator type transporter56,57. In general, efflux is the most expedient mechanism for bacteria to deal with metal ion excess. Recent results have identified analogous proteins that mediate efflux of both Fe(II)23 and Mn(II)18 in B. subtilis. However, in these cases the same regulatory protein that represses uptake also activates the expression of the efflux genes. Thus, Fur19,23 and MntR18 are both bifunctional regulators that can repress and activate gene expression in response to metal status (Figure 2).
Product sensing as an alternative to direct metal sensing
Fe(II) can also be selectively sensed by monitoring the main products of iron metabolism, rather than the level of the ion itself7. In the cells, iron is mostly used for the assembly of heme-containing proteins and FeS-containing enzymes58. Therefore, bacteria have evolved metalloregulatory systems that sense these iron-dependent products to indirectly monitor intracellular iron levels. For example, the iron response regulator Irr, which was first described in Bradyrhizobium japonicum, is a Fur family protein that regulates iron homeostasis by monitoring heme through a direct interaction with ferrochelatase59,60 (Figure 1b). Unlike other Fur family members that function during iron sufficiency, Irr binds DNA under conditions of iron limitation. Irr represses transcription of genes involved in heme biosynthesis, iron storage, Fe(II) efflux and iron-utilizing proteins, and directly activates genes involved in iron uptake, heme utilization and the TCA cycle61. Ferrochelatase catalyzes the final step in heme biosynthesis, the insertion of Fe(II) into a protoporphyrin ring62. When Fe(II) is sufficient, the heme synthesized by ferrochelatase binds directly to Irr, which triggers Irr protein degradation60. When Fe(II) is limiting, protoporphyrin binds to Irr, which leads to release of Irr from ferrochelatase and subsequent regulation of target genes61. This prevents the toxic overaccumulation of protoporphyrin.
Bacteria also commonly monitor the cellular capacity for the biogenesis of FeS clusters through a regulatory protein, IscR, that requires insertion of an FeS center for function6. IscR is a member of the AraC family of transcription factors and contains three cysteine residues located in between the two helix-turn-helix binding motifs63. Interestingly, both the apo-form and the holo- form of IscR function as transcription factors and recognize two classes of DNA-binding motifs (type 1 and 2)64. Upon binding of an [2Fe-2S] cluster to an atypical Cys3His1 binding site in IscR the IscR-DNA interface is remodeled, which allows recognition of both type 1 and 2 motifs and regulation of the full IscR regulon65.
Recognition of metal ions by riboswitches
Selectively recognizing and responding to metal ions within the complex milieu of the cell presents a challenge for proteins, even for those proteins that can arrange chemically diverse ligands (typically oxygen, nitrogen, and sulfur) in a defined geometry33. Despite their more limited choice of ligands, RNA molecules have also evolved as metal-selective sensors. The first metal-sensing riboswitch was identified upstream of the S. enterica mgtA gene, which encodes a Mg(II) uptake protein8. The structural and mechanistic basis for Mg(II) sensing was revealed in a later study characterizing the B. subtilis mgtE riboswitch66. Transcription read-through is regulated by the Mg(II) sensing ‘magnesium-box (M-box)’ riboswitch8,66. Upon Mg(II) binding, the riboswitch adopts a compact conformation, which sequesters an antiterminator element and favors transcription termination66. Interestingly, the MgtE channel itself is also allosterically regulated by intracellular Mg(II) and is completely inhibited by ~5–10 mM Mg(II)67 (BOX 3), which is near the in vitro affinity of the M-box riboswitch (half maximal effective concentration (EC50) ~3 mM)66. This suggests that MgtE activity and mgtE expression are shut off concurrently. This presumably allows for the efficient inactivation of Mg(II) uptake during rapid fluctuations in Mg(II) availability to avoid intoxication.
BOX 3. Allosteric regulation of metal import.
Adapting to changing availability of metal ions by altering gene expression to effect changes in import and export is a relatively slow process. Therefore, it is advantageous for bacteria to have mechanisms to regulate the activity of existing uptake and efflux systems. Two major mechanisms prevent inappropriate activity of metal transporters: allosteric regulation and an appropriately tuned affinity for substrate.
Allosteric regulation is best understood in the case of the Mg(II) importer MgtE161. MgtE family transporters function as homodimers and possess an N-terminal cytosolic domain that senses intracellular Mg(II) and a C-terminal transmembrane conduction pore162. When intracellular Mg(II) increases, Mg(II) binding to the cytosolic domain stabilizes the closed, inactive conformation of the pore. This feedback inhibition can provide a rapid shutoff of uptake when Mg(II) levels are sufficient. However, high concentrations of Mn(II) may inappropriately lock MgtE in the closed conformation, which potentially leads to intracellular Mg(II) deficiency124. To date, there are surprisingly few examples of allosterically regulated transport systems for other metals in bacteria, which may reflect the difficulty of evolving allosteric sites of sufficient specificity to prevent dysregulation by non-cognate metals. As an alternative, it may be possible to regulate transport using a conditionally expressed protein. For example, Mn(II) efflux in E. coli is carried out by the MntP efflux pump129. As cells transition to Mn(II) limitation the MntR-regulated MntS protein is synthesized and is proposed to inhibit MntP activity, which leads to rapid shutoff of efflux and thus prevents Mn(II) starvation.
Alternatively, it may not be necessary to inhibit efflux pump activity if the affinity of the transporter is appropriately tuned to be highly active only under conditions of metal excess. Support for this mechanism comes from studies in Saccharomyces cerevisiae. In this organism, Zn(II) limitation leads to induction of both the Zrt1 Zn(II) uptake transporter163 and Zrc1, a Zn(II) transporter that is involved in vacuolar Zn(II) storage164. The synthesis of Zrc1 has been hypothesized to prevent Zn(II) overload due to Zrt1 activity. Importantly, the affinity of the Zrc1 storage transporter is tuned such that it is largely inactive under Zn(II) limitation, which prevents depletion of cytosolic Zn(II). A similar strategy may exist in B. subtilis iron efflux by PfeT, an Fe(II) efflux P1B-type ATPase23. PfeT has a surprisingly low affinity for Fe(II) (K1/2 ~0.5 mM), perhaps to ensure that efflux only active when cytosolic Fe(II) is in excess. In contrast with PfeT, the Listeria monocytogenes ortholog FrvA has a relatively high affinity for Fe(II) (K1/2 ~0.1 mM)24. Consequently, expression of FrvA in B. subtilis depletes the cytosol of Fe(II) and induces Fe(II) limitation, as evidenced by derepression of the Fur and PerR regulons. Why L. monocytogenes has a comparatively high affinity Fe(II) efflux pump is an open question.
Given the high (millimolar) concentration of Mg(II) in the bacterial cell, and the role of Mg(II) in RNA folding and structure, the ability of riboswitches to respond to Mg(II) is perhaps not surprising. However, recent results suggest that riboswitches can also sense Mn(II). Specifically, the widely distributed yybP-ykoY family of riboswitches (named after the associated genes in B. subtilis) responds selectively to Mn(II)9,10 (Figure 1c), despite the fact that ambient levels of free Mn(II) in the cell are thought to be near 10 μM, several orders of magnitude below those of Mg(II)68. Structural studies of the Mn-sensing riboswitch in Lactococcus lactis demonstrates that Mn(II)-sensing requires both oxygen ligands and a nitrogen ligand from a specifically oriented adenine residue in the riboswitch9. Riboswitches with selectivity for nickel and cobalt (and perhaps other metal ions) have also been reported, which further highlights the versatility of RNA-based systems for metal sensing69.
Similar to metalloregulatory proteins, riboswitches may also use a product sensing strategy by monitoring the major products of metabolism. This is the case for cobalt, which is primarily used in bacteria as part of the cobalamin (vitamin B12) cofactor required for the activity of many enzymes, including methionine synthase in E. coli 70.71. Although some metalloregulators respond to excess Co(II) directly (although often without high selectivity) to activate broad specificity efflux pumps, uptake of Co(II) does not appear to be regulated by direct Co(II) sensing72. Instead, bacteria regulate their need for Co(II) by monitoring cobalamin availability, often through a riboswitch-mediated mechanism71.
Metal ion limitation and its consequences
Bacteria have a complex and diverse set of mechanisms to respond to the limitation of essential metals1. Insights into the physiological stresses imposed by metal limitation have emerged, in part, from the detailed characterization of the regulons controlled by those metalloregulators that distinguish metal limitation from sufficiency.
As might be expected, a dominant theme in the physiological responses to metal limitation is the derepression of high-affinity uptake systems to help counter the deficiency. A second theme is the substitution of an enzyme or protein that depends on a limiting metal with one that can function independent of that metal (and perhaps with a distinct metal cofactor). Third, cells mobilize limiting metals from reservoirs and remodel their proteomes by translational repression of less important enzymes to enable the limiting metal ion to be used for synthesis of those enzymes that are most essential for viability and growth (metal-sparing). Thus, cellular responses to metal limitation typically extend well beyond simply regulating metal import, and their analysis provides insights into the types of processes that typically fail and thereby limit growth.
Iron limitation
These three intertwined themes are well represented in bacterial iron homeostasis. First, bacteria generally use a set of iron uptake pathways (often involving siderophores), as reviewed in detail elsewhere58. Second, bacteria will often replace some iron-dependent functions with alternate proteins that do not require iron. Indeed, this type of substitution is exceptionally widespread in biology1. Third, bacteria often activate an iron-sparing response73,74.
In B. subtilis, iron limitation leads to induction of both siderophore biosynthesis and the expression of various iron import pathways75. Fur also regulates a flavodoxin that can functionally replace the abundant iron-containing electron transfer protein ferredoxin under iron limitation76. Indeed, this particular adaptive response is so widespread that induction of flavodoxin is a frequently used biomarker for environmental iron limitation77. Fur in B. subtilis also regulates a small, noncoding RNA (sRNA)65, FsrA, that appears to function in concert with two small, basic proteins, FbpA and FbpB, to mediate the translational repression of iron enzymes73,78. This is representative of a widespread adaptive response to iron limitation, which is often referred to as an iron-sparing response 79.
The corresponding adaptive responses to iron limitation are especially well understood in E. coli. In this organism, there are several examples of iron-independent enzymes that replace their counterparts under conditions of iron limitation. E. coli encodes two homologous superoxide dismutases (SODs), one of which is an Fe-containing enzyme80 (FeSOD), whereas the other utilizes Mn81 (MnSOD). The FeSOD is active under most growth conditions, whereas MnSOD expression is induced during periods of iron starvation82. Similarly, E. coli encodes two ribonucleotide reductases (RNR) that catalyze the conversion of ribonucleotides to deoxyribonucleotides83. The major RNR is Fe-dependent (encoded by nrdAB) and essential for aerobic growth. The second (encoded by nrdEF) is regulated by regulated and is Mn-dependent16. This alternative RNR is crucial for survival under conditions of H2O2 stress and during iron starvation when NrdAB is inactive16. In collaboration with these metalloenzyme substitution processes, the RyhB sRNA down-regulates the translation of numerous iron-containing enzymes (an iron-sparing response) to help prioritize iron usage74,84.
Despite these various mechanisms to acquire and mobilize iron, growth ultimately ceases when iron is unavailable. It is not clear why growth ceases, and this presumably varies between organisms and depends on the growth medium and conditions. The processes that are most susceptible to iron limitation might be inferred from the induction of pathways that help bypass limitation and forestall growth arrest. However, further investigation will be required to understand which processes are the most dependent on iron. In many bacteria, iron has several distinct roles — it serves as a cofactor for iron-dependent enzymes, for the assembly of FeS complexes, and as an essential component of heme. One active area of research is to define the mechanisms of iron allocation to satisfy these needs, which likely involves accessory proteins such as frataxin.85
Zinc limitation
Insights into how bacteria adapt to zinc limitation have emerged from the characterization of the Zn(II) deficiency response that is regulated by Zur or its functional equivalent. For example, genes regulated by Zur often have functions that can replace Zn(II)-dependent enzymes14,15. In each case, this implies that the Zn(II)-dependent process would otherwise fail when Zn(II) is depleted. Here, we consider the specific example of the regulon controlled by Zur in B. subtilis (Figures 2 and 3).
Under conditions of Zn(II) sufficiency, Zur represses genes that encode a high-affinity Zn(II) uptake system, an alternative folate biosynthesis enzyme, putative Zn(II) chaperones (ZinT and YciC), and Zn(II)-independent ribosomal protein paralogs34,86. The induction of a Zn(II)-independent folate biosynthesis enzyme (FolE2) suggests that the dominant, Zn(II)-cofactored enzyme fails when Zn(II) levels fall, which causes folate auxotrophy14. This presumably imposed a selective pressure on an ancestral strain that led to the acquisition of an alternative, Zn(II)-independent enzyme that is now under Zur control. Similarly, induction of an alternative, Zn-independent form of the ribosomal protein S14 (designated S14*), which assembles early during ribosome biogenesis, likely enables continued ribosome synthesis even when intracellular Zn(II) levels are insufficient to metallate the constitutively expressed S1415. Two other ribosomal protein paralogs, L31* and L33*, can also replace their Zn(II)-containing counterparts87,88. In these cases, these large subunit ribosomal protein paralogs displace their Zn(II)-containing counterparts (L31, L33) from the surface of assembled ribosomes87. This Zn(II) mobilization mechanism allows redistribution of Zn(II) from abundant ribosomal proteins, which thereby serve as a storage reservoir, to support the synthesis of essential, Zn(II)-requiring enzymes87,89.
As metalloregulators monitor intracellular metal ion concentration, the onset of metal limitation is generally expected to be gradual as cells deplete their available intracellular pools during growth. Consequently, regulation of gene expression is often not an all or none event, but rather is finely tuned across operons with metalloregulatory binding sites of varying affinity and responsiveness90,91. Analysis of the resulting graded response has provided insights into how the cell prioritizes its responses to the gradual decline in available Zn(II) and may provide insights into which processes fail first. In the case of B. subtilis, derepression of the Zur regulon occurs in three distinct stages upon Zn(II) starvation, and these stages are correlated with the sequential loading of Zn(II) into the sensing sites of the dimeric repressor (Figure 2)90. In the first stage, Zn(II) that is already present in the cell and associated with the L31 and L33 ribosomal proteins is mobilized by expression of the Zn(II)-independent paralogs L31* and L33*90. As Zn(II) levels decline further the ZnuABC uptake system and the YciC and ZinT metallochaperones enable high-affinity uptake and presumably facilitate Zn trafficking90. As Zn(II) levels decline still further, the substitution functions are derepressed to enable continued de novo synthesis of ribosomes (S14*) and folate production90. Although it is perhaps surprising that mobilization of intracellular Zn(II) stores precedes the expression of high-affinity uptake systems, this makes sense because high-affinity uptake is energetically expensive. Moreover, high-affinity uptake systems have the disadvantage that they can potentially lead to metal overload if there is a sudden increase in metal availability (BOX 3).
Graded responses are likely a general feature of many bacterial stress responses, and a variety of mechanisms support the sequential expression of discrete sets of genes as ion stores deplete. In the specific case of Zur in B. subtilis, one mechanism that contributes to the graded response is negative cooperativity in Zn(II) binding (Figure 2)55,90. Under Zn(II) sufficiency, the regulon is repressed by fully metallated Zur (Zur2:Zn4). As Zn(II) levels fall, the weakly bound Zn(II) ion dissociates to generate the Zur2:Zn3 form of the repressor, which leads to the derepression of the first two classes of stress response genes; however, the mechanism mediating their stepwise derepression is not yet known90. Finally, as Zn(II) levels decline further the tightly bound Zn(II) ion dissociates leading to the Zur2:Zn2 form and derepression of the S14* and FolE2 proteins (Figure 3). The molecular mechanisms that support graded responses in other systems include multiple metal-binding sites that enable sequential loading of metal, as noted for Streptomyces coelicolor Zur91 and possibly B. subtilis Fur44, and cooperative binding of multiple regulators to a single regulatory region, as noted for the E. coli Zur92 and Fur93 regulons.
Metal ion intoxication and its consequences
Fluctuating environmental conditions pose a challenge for maintaining homeostasis. For example, if a metal-starved bacterium encounters a metal-rich environment, the action of high-affinity uptake systems can rapidly lead to metal intoxication, a problem that is exacerbated by the lack of allosteric feedback inhibition for most metal uptake systems (BOX 3). In addition, eukaryotes use metal intoxication as an antibacterial strategy (BOX 4), as reviewed in detail for Cu(I) and Zn(II)3. Recent findings suggest that this is an ancient strategy, which likely evolved in response to predation of bacteria by protozoa94. Here, we consider some mechanisms that serve to prevent metal overload.
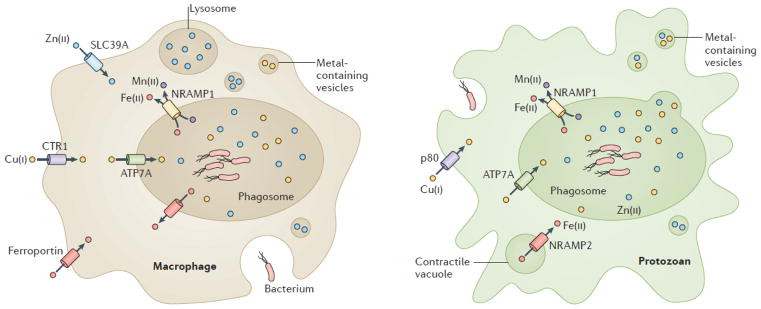
BOX 4. Metal homeostasis during bacteria-host interactions and the evolutionary origins of innate immunity.
Obtaining sufficient metal ions for growth presents a major challenge for bacteria in the host environment2. In mammals, much of the iron is sequestered by binding to proteins such as transferrin and hemoglobin. Similarly, zinc and manganese are sequestered by calprotectin, which is released by neutrophils at sites of infection. In addition, phagocytic vacuoles of macrophages that have engulfed bacteria are depleted of iron and manganese by the action of the natural resistance associated macrophage protein 1 (NRAMP1)136. Furthermore, NRAMP1, together with ferroportin, lowers Fe(II) levels165.
Although the role of metal sequestration has long been appreciated2, evidence of a role for metal intoxication (particularly Cu(I) and Zn(II)) as an antimicrobial strategy of the immune response has emerged only recently3. Upon engulfment, Cu(I) accumulates in the phagocytic vacuole due to transport into the cell by CTR1 and subsequently into the vacuole by the ATP7A Cu(I) transporters137. Zn(II) levels may rise due to the action of SLC39A family Zn(II) transporters166. Furthermore, Zn(II) accumulates in metal-containing vesicles, which may fuse with the phagocytic vacuole within the macrophage138. The strongest evidence for a direct role for metal intoxication in the innate immune response comes from the decreased virulence of bacteria in which metal efflux genes have been mutated3. Mn(II) and Fe(II) intoxication may also have an antimicrobial role during infection, although this area of research is still in its early stages20,24,167.
Although host metal sequestration and intoxication, and the corresponding bacterial defenses, are intensively studied in human pathogens166, this is an ‘arms-race’ with a long evolutionary history168. A comparison of phagocytosis between protozoa and macrophages reveals remarkable similarities (see Figure 4). Similar to macrophages, some predatory protozoa use NRAMP-type transporters to deplete the phagosome of Fe(II) and Mn(II). For example, Nramp1 localizes to the phagosome169 and Nramp2 to the contractile vacuole170 in the social amoeba Dictoyostelium discoideum. Additionally, an ortholog of the Cu(I) transporter ATP7A in D. discoideum localized to both the cytoplasmic membrane and vacuolar structures, which indicates a possible use in flooding the phagocytic vacuole with Cu(I)171. Zn(II) and Cu(II) containing vesicles have also been observed before fusion with the protozoal phagosome138,172. Thus, selection for survival in protozoa may have primed bacterial cells to survive engulfment by mammalian phagocytes. Indeed, some human pathogens may have gained important virulence properties from their association with environmental protozoa. For example, the Dot/Icm type IV secretion system of Legionella pneumophila, the causative agent of Legionnaire’s disease173, is required both for survival in protozoa and in macrophages174.
[H3] Preventing metal overload: buffering, efflux, and storage
Bacteria limit the damage caused by a sudden influx of metals through cytosolic ‘buffering’ (as first defined for Zn(II) homeostasis12), sequestration in storage proteins, and induction of efflux pumps. Under steady state conditions, cytosolic Zn(II)-binding proteins and molecules can buffer the relatively large Zn(II) content of most cells to a cytosolic concentration of free Zn(II) ions in the picomolar range95. When there is an influx of Zn(II) ions buffering reactions dampen the resulting rise in the cytosolic free Zn(II) ion concentration, which gives the Zn(II) uptake systems time to be repressed and Zn(II) efflux or sequestration systems to be activated.
The precise components of the cellular metal buffering systems are beginning to be elucidated. In general, they likely consist of small molecules such as amino acids, glutathione, organic acids, inorganic ligands, and weak ligands on the surface of macromolecules, specific buffering proteins, and a subset of delivery proteins. In B. subtilis, bacillithiol (BSH) comprises a major component of the Zn(II) buffering system96. BSH functionally replaces glutathione and is the major low-molecular-weight thiol in the bacterial phylum Firmicutes97. The experimentally determined BSH:Zn affinity would allow the cellular BSH pool of 1–5 mM98 to effectively buffer Zn in the physiologically relevant pM–nM range96. Similarly, glutathione may also serve as a Zn(II) buffer99. Alternatively, some bacteria and many eukaryotes express a small cysteine-rich metal-binding protein called metallothionein that provides a buffer for Zn(II)100. Histidine has also been identified as a possible buffer of labile Zn(II) in Acinetobacter baumanii101.
Insights into proteins and molecules that buffer and transport metal ions in the cytosol are also emerging for other nutrient metals. Recently, methionine was identified as a possible intracellular Cu(I) chelator in E. coli102. However, glutathione also likely has a role99 and BSH has recently been linked to Cu(I) homeostasis in B. subtilis103. The importance of intracellular Cu(I) and Zn(II) buffering by low molecular mass thiols in the protection against metal toxicity was also demonstrated for Streptococcus pneumoniae, in which mutants that lack the ability to import GSH are hypersensitive to Cu(I) and Zn(II)104
The labile Mn(II) pool has been investigated in organisms from lactic acid bacteria to yeast, and seems to be largely bound to abundant phosphoryl-containing ligands like phosphosugars and pyrophosphate105,106. Electron paramagnetic resonance studies of the Mn(II) accumulating bacterium Deinococcus radiodurans suggest that a large fraction of intracellular Mn(II) forms low molecular weight complexes with nitrogenous compounds107. In vitro studies support this model, as Mn(II) can associate with short peptides, some nucleotides, free amino acids, and inorganic phosphate108. Additionally, a major pool of Mn(II) in stationary phase D. radiodurans108 and B. anthracis109 is associated with the enzyme superoxide dismutase, which is involved in alleviating oxidative stress.
Metal ion buffering provides a window of opportunity for cells to express other proteins to facilitate adaptation to metal excess. Prominent among these proteins are the specific metal efflux systems, as well as storage and sequestration proteins. Bacteria, archaea, and eukaryotes store Fe(III) in ferritin and ferritin-related proteins for use under conditions of iron limitation110. Ferritins are essential for the survival of several pathogenic organisms in infected hosts111. Ferritin subunits form a spherical shell, in which up to 4,500 atoms of iron can be sequestered112,113. The uptake of iron by ferritins involves an initial oxidation of Fe(II) by molecular oxygen at the ferroxidase center, and iron is stored as Fe(III)114. Release of iron from ferritin requires reduction, but the precise molecular mechanisms are poorly understood. In the specific case of Corynebacterium glutamicum, post-translational modification of ferritin by the pupylation machinery triggers the disassembly of the ferritin-iron complex in a process that does not require active protein degradation115.
Consequences of metal intoxication
Intoxication results in some cases from poisoning of surface exposed enzymes or proteins, which are not protected by the homeostasis mechanisms that maintain intracellular metal concentrations. However, when the influx of metal ions overwhelms the buffering capacity of the cell, and efflux and storage systems are unable to respond rapidly enough, cells experience cytosolic metal intoxication. Here, we summarize some of the better understood mechanisms of metal intoxication. Of note, metal intoxication is increasingly appreciated for its role in host-pathogen interactions (BOX 4).
Intoxication from without: inhibition of respiration and metal uptake
Metal ions may inhibit key biological processes even in the absence of their transport into the cell. Zn(II), Cd(II), and Co(II) can all inhibit the activity of the electron transport chain in bacteria and mitochondria116–118. The precise site(s) of Zn(II) inactivation of the electron transport chain is still unclear. Genetic evidence from E. coli116, S. coelicolor117, and B. subtilis119 suggests specific inhibition of the major aerobic cytochrome oxidase as bacteria upregulate the relatively Zn(II)-insensitive cytochrome bd quinol oxidase under conditions of Zn(II) intoxication conditions. This may be of particular importance for host-pathogen interactions because induction of the cytochrome bd system also confers tolerance to nitric oxide120,121 and hydrogen sulfide122, which can be abundant in the host environment.
Zn(II) also has an antimicrobial effect by antagonizing metal uptake. In S. pneumoniae, excess Zn(II) prevents the uptake of Mn(II) by binding irreversibly to the extracellular Mn(II) binding protein PsaA123. Thus, upon Zn(II) intoxication, S. pneumoniae becomes Mn(II) starved and therefore hypersensitive to oxidative stress due to a decrease in activity of MnSOD123. Inhibition of Mn(II) import by competing metal ions may explain why many bacteria have redundant import systems, often including both an ABC transporter and an MntH protein. Metal ions might also antagonize metal uptake by inappropriately signaling sufficiency to transporters that are regulated allosterically. One possible example is the Mg(II) importer MgtE, which is potentially inhibited by binding of Mn(II) to its allosteric regulatory site124 (BOX 3).
Intoxication from within: enzyme and regulator mismetallation
Enzyme mismetallation has emerged as an important consequence of both metal intoxication and peroxide stress. Under conditions of peroxide stress several mononuclear Fe-dependent enzymes lose function in E. coli when bound Fe(II) is oxidized to Fe(III) and dissociates, which can lead to enzyme inactivation when Zn(II) binds instead125. Recent studies revealed that a family of bacterial Fe–S dehydratases is particularly vulnerable to inactivation by toxic metals126–128. Additionally, excess Mn inhibits ferrochelatase, which leads to inhibition of heme-dependent enzymes such as catalase and cytochrome oxidases129.
Despite the many mechanisms metalloregulators use to ensure specificity (BOX 2), mismetallation of metalloregulators has been observed. Fur, which senses Fe(II), can be mismetallated by Mn(II) under conditions of Mn(II) intoxication or, as described above, when Fur is expressed at high levels in B. subtilis68. Recently, Cd(II) was shown to dysregulate Zn(II) homeostasis in S. pneumoniae due to inappropriate repression of Zn(II) uptake systems and activation of gene expression of Zn(II) efflux systems130. However, direct interaction of Cd(II) with the Zn(II) sensing transcription factors AdcR and SczA has not yet been shown. Furthermore, in a B. subtilis mutant with defective Zn(II) efflux, zinc intoxication results from mismetallation of PerR and dysregulation of the PerR regulon119. PerR is a member of the Fur family in B. subtilis and functions as both a metal sensor and as an Fe(II)-dependent H2O2 sensor131. Normally, PerR functions with either Fe(II) or Mn(II) as a corepressor and coordinately regulates genes that are involved in oxidative stress132. However, when mismetallated by Zn(II), PerR fails to repress the hemA heme biosynthesis operon but maintains repression of katA, which encodes the highly abundant heme-containing catalase119. As a result, cells accumulate toxic levels of heme. As shown in Staphylococcus aureus, heme toxicity results from redox cycling between excess heme, which associates with the cell membrane, and menaquinone133.
Outlook
Metal ion homeostasis is a delicate balance in which cells must maintain sufficient levels of metals to ensure proper functioning of essential enzymes, while preventing metal intoxication. Although bacterial metal homeostasis has been studied for decades, we have only recently begun to understand the molecular details of how metal buffering, protein chaperones and proteome remodeling ensure efficient metallation of key enzymes during metal limitation. The intracellular sites for storage and sequestration of excess metal are also only now being discovered. Key questions still remain unanswered regarding the nature of the labile metal pool, the precise cellular metal quotas, and the effects of environmental factors on metal requirements.
The role of metal ions during host interactions is also an active area of research, with recent advances being made in understanding the role of both metal sequestration in limiting bacterial growth (through the action of host sequestration proteins and efflux of Fe and Mn from the phagocytic vacuole)27,134–136 and the role of metal ion intoxication 25,137,138. The importance of metal intoxication in host-pathogen interactions was first noted due to the virulence defects of bacteria that are defective in Zn(II) and Cu(I) efflux 25,139–141. However, this general model can now be extended to Fe(II) and Mn(II) intoxication20,24,142,143. Key remaining questions include the locations and mechanisms of metal intoxication during infection. These recent insights have suggested new vaccine strategies that target metal transport systems. For example, the outer membrane Zn(II) uptake transporter ZnuD in Neisseria meningitidis144 and the Mn(II) uptake systems MntC in S. aureus145 and PsaA in S. pneumoniae146 were both identified as potential vaccine targets. To develop new and effective antimicrobial therapies, it will be crucial to better understand how bacteria manage metal homeostasis and to identify the mechanisms and timing of metal sequestration and intoxication by the host.
Figure 4.
Metal homeostasis during bacteria-host interactions and the evolutionary origins of innate immunity. (see Box 4 text).
Acknowledgments
This work was funded by a grant from the National Institutes of Health awarded to JDH (GM059323). C.R. is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grants XDB15020402 and XDB15020302) and the 100 Talent Program of Fujian Province China.
References
- 1.Merchant SS, Helmann JD. Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. Advances in microbial physiology. 2012;60:91–210. doi: 10.1016/b978-0-12-398264-3.00002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nature reviews Microbiology. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djoko KY, Ong CL, Walker MJ, McEwan AG. The Role of Copper and Zinc Toxicity in Innate Immune Defense against Bacterial Pathogens. The Journal of biological chemistry. 2015;290:18954–18961. doi: 10.1074/jbc.R115.647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chemical reviews. 2009;109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 6.Mettert EL, Kiley PJ. Fe-S proteins that regulate gene expression. Biochimica et biophysica acta. 2015;1853:1284–1293. doi: 10.1016/j.bbamcr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brian MR. Perception and Homeostatic Control of Iron in the Rhizobia and Related Bacteria. Annual review of microbiology. 2015;69:229–245. doi: 10.1146/annurev-micro-091014-104432. [DOI] [PubMed] [Google Scholar]
- 8.Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg(2+) Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Price IR, Gaballa A, Ding F, Helmann JD, Ke A. Mn(2+)-sensing mechanisms of yybP-ykoY orphan riboswitches. Molecular cell. 2015;57:1110–1123. doi: 10.1016/j.molcel.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dambach M, et al. The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Molecular cell. 2015;57:1099–1109. doi: 10.1016/j.molcel.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS microbiology reviews. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 12.Colvin RA, Holmes WR, Fontaine CP, Maret W. Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics : integrated biometal science. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 13.Capdevila DA, Wang J, Giedroc DP. Bacterial Strategies to Maintain Zinc Metallostasis at the Host-Pathogen Interface. The Journal of biological chemistry. 2016;291:20858–20868. doi: 10.1074/jbc.R116.742023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sankaran B, et al. Zinc-independent folate biosynthesis: genetic, biochemical, and structural investigations reveal new metal dependence for GTP cyclohydrolase IB. Journal of bacteriology. 2009;191:6936–6949. doi: 10.1128/jb.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natori Y, et al. A fail-safe system for the ribosome under zinc-limiting conditions in Bacillus subtilis. Molecular microbiology. 2007;63:294–307. doi: 10.1111/j.1365-2958.2006.05513.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin JE, Imlay JA. The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Molecular microbiology. 2011;80:319–334. doi: 10.1111/j.1365-2958.2011.07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imlay JA. The mismetallation of enzymes during oxidative stress. The Journal of biological chemistry. 2014;289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Shin JH, Pinochet-Barros A, Su TT, Helmann JD. Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Molecular microbiology. 2016 doi: 10.1111/mmi.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters LS, Sandoval M, Storz G. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. Journal of bacteriology. 2011;193:5887–5897. doi: 10.1128/jb.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Molecular microbiology. 2009;72:12–25. doi: 10.1111/j.1365-2958.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hantke K. Bacterial zinc transporters and regulators. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2001;14:239–249. doi: 10.1023/a:1012984713391. [DOI] [PubMed] [Google Scholar]
- 22.Rensing C, Grass G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS microbiology reviews. 2003;27:197–213. doi: 10.1016/S0168-6445(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 23.Guan G, et al. PfeT, a P1B4 -type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Molecular microbiology. 2015;98:787–803. doi: 10.1111/mmi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pi H, Patel SJ, Arguello JM, Helmann JD. The Listeria monocytogenes Fur-regulated virulence protein FrvA is an Fe(II) efflux P1B4 -type ATPase. Molecular microbiology. 2016;100:1066–1079. doi: 10.1111/mmi.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botella H, et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell host & microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SJ, et al. Fine-tuning of Substrate Affinity Leads to Alternative Roles of Mycobacterium tuberculosis Fe2+-ATPases. The Journal of biological chemistry. 2016;291:11529–11539. doi: 10.1074/jbc.M116.718239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg ED. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. Jama. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Skaar EP, Raffatellu M. Metals in infectious diseases and nutritional immunity. Metallomics : integrated biometal science. 2015;7:926–928. doi: 10.1039/c5mt90021b. [DOI] [PubMed] [Google Scholar]
- 29.Sabine DB, Vaselekos J. Trace element requirements of Lactobacillus acidophilus. Nature. 1967;214:520. doi: 10.1038/214520a0. [DOI] [PubMed] [Google Scholar]
- 30.Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science (New York, NY) 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 31.Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Molecular microbiology. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasantha N, Freese E. The role of manganese in growth and sporulation of Bacillus subtilis. Journal of general microbiology. 1979;112:329–336. doi: 10.1099/00221287-112-2-329. [DOI] [PubMed] [Google Scholar]
- 33.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nature reviews. Microbiology. 2009;7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 34.Gaballa A, Helmann JD. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. Journal of bacteriology. 1998;180:5815–5821. doi: 10.1128/jb.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore CM, Helmann JD. Metal ion homeostasis in Bacillus subtilis. Current opinion in microbiology. 2005;8:188–195. doi: 10.1016/j.mib.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann JD. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Molecular microbiology. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 37.Que Q, Helmann JD. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Molecular microbiology. 2000;35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 38.Hantke K. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Molecular & general genetics : MGG. 1984;197:337–341. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- 39.Deng Z, et al. Mechanistic insights into metal ion activation and operator recognition by the ferric uptake regulator. Nature communications. 2015;6:7642. doi: 10.1038/ncomms8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dian C, et al. The structure of the Helicobacter pylori ferric uptake regulator Fur reveals three functional metal binding sites. Molecular microbiology. 2011;79:1260–1275. doi: 10.1111/j.1365-2958.2010.07517.x. [DOI] [PubMed] [Google Scholar]
- 41.Butcher J, Sarvan S, Brunzelle JS, Couture JF, Stintzi A. Structure and regulon of Campylobacter jejuni ferric uptake regulator Fur define apo-Fur regulation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10047–10052. doi: 10.1073/pnas.1118321109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheikh MA, Taylor GL. Crystal structure of the Vibrio cholerae ferric uptake regulator (Fur) reveals insights into metal co-ordination. Molecular microbiology. 2009;72:1208–1220. doi: 10.1111/j.1365-2958.2009.06718.x. [DOI] [PubMed] [Google Scholar]
- 43.Pohl E, et al. Architecture of a protein central to iron homeostasis: crystal structure and spectroscopic analysis of the ferric uptake regulator. Molecular microbiology. 2003;47:903–915. doi: 10.1046/j.1365-2958.2003.03337.x. [DOI] [PubMed] [Google Scholar]
- 44.Gaballa A, MacLellan S, Helmann JD. Transcription activation by the siderophore sensor Btr is mediated by ligand-dependent stimulation of promoter clearance. Nucleic acids research. 2012;40:3585–3595. doi: 10.1093/nar/gkr1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JW, Helmann JD. Functional specialization within the Fur family of metalloregulators. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2007;20:485–499. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 46.Funahashi T, et al. Characterization of Vibrio parahaemolyticus manganese-resistant mutants in reference to the function of the ferric uptake regulatory protein. Microbiology and immunology. 2000;44:963–970. doi: 10.1111/j.1348-0421.2000.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 47.Benson HP, LeVier K, Guerinot ML. A dominant-negative fur mutation in Bradyrhizobium japonicum. Journal of bacteriology. 2004;186:1409–1414. doi: 10.1128/JB.186.5.1409-1414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Molecular & general genetics : MGG. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 49.Merchant AT, Spatafora GA. A role for the DtxR family of metalloregulators in gram-positive pathogenesis. Molecular oral microbiology. 2014;29:1–10. doi: 10.1111/omi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kliegman JI, Griner SL, Helmann JD, Brennan RG, Glasfeld A. Structural basis for the metal-selective activation of the manganese transport regulator of Bacillus subtilis. Biochemistry. 2006;45:3493–3505. doi: 10.1021/bi0524215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGuire AM, et al. Roles of the A and C sites in the manganese-specific activation of MntR. Biochemistry. 2013;52:701–713. doi: 10.1021/bi301550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patzer SI, Hantke K. Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli. Journal of bacteriology. 2001;183:4806–4813. doi: 10.1128/jb.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikeda JS, Janakiraman A, Kehres DG, Maguire ME, Slauch JM. Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. Journal of bacteriology. 2005;187:912–922. doi: 10.1128/jb.187.3.912-922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patzer SI, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Molecular microbiology. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 55.Ma Z, Lee JW, Helmann JD. Identification of altered function alleles that affect Bacillus subtilis PerR metal ion selectivity. Nucleic acids research. 2011;39:5036–5044. doi: 10.1093/nar/gkr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore CM, Gaballa A, Hui M, Ye RW, Helmann JD. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Molecular microbiology. 2005;57:27–40. doi: 10.1111/j.1365-2958.2005.04642.x. [DOI] [PubMed] [Google Scholar]
- 57.Pennella MA, Arunkumar AI, Giedroc DP. Individual metal ligands play distinct functional roles in the zinc sensor Staphylococcus aureus CzrA. Journal of molecular biology. 2006;356:1124–1136. doi: 10.1016/j.jmb.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS microbiology reviews. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 59.Hamza I, Qi Z, King ND, O’Brian MR. Fur-independent regulation of iron metabolism by Irr in Bradyrhizobium japonicum. Microbiology (Reading, England) 2000;146( Pt 3):669–676. doi: 10.1099/00221287-146-3-669. [DOI] [PubMed] [Google Scholar]
- 60.Qi Z, O’Brian MR. Interaction between the bacterial iron response regulator and ferrochelatase mediates genetic control of heme biosynthesis. Molecular cell. 2002;9:155–162. doi: 10.1016/s1097-2765(01)00431-2. [DOI] [PubMed] [Google Scholar]
- 61.Rudolph G, et al. The Iron control element, acting in positive and negative control of iron-regulated Bradyrhizobium japonicum genes, is a target for the Irr protein. Journal of bacteriology. 2006;188:733–744. doi: 10.1128/jb.188.2.733-744.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferreira GC. Heme biosynthesis: biochemistry, molecular biology, and relationship to disease. Journal of bioenergetics and biomembranes. 1995;27:147–150. doi: 10.1007/BF02110029. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz CJ, et al. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14895–14900. doi: 10.1073/pnas.251550898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giel JL, Rodionov D, Liu M, Blattner FR, Kiley PJ. IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Molecular microbiology. 2006;60:1058–1075. doi: 10.1111/j.1365-2958.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- 65.Rajagopalan S, et al. Studies of IscR reveal a unique mechanism for metal-dependent regulation of DNA binding specificity. Nature structural & molecular biology. 2013;20:740–747. doi: 10.1038/nsmb.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dann CE, 3rd, et al. Structure and mechanism of a metal-sensing regulatory RNA. Cell. 2007;130:878–892. doi: 10.1016/j.cell.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 67.Hattori M, et al. Mg(2+)-dependent gating of bacterial MgtE channel underlies Mg(2+) homeostasis. The EMBO journal. 2009;28:3602–3612. doi: 10.1038/emboj.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma Z, Faulkner MJ, Helmann JD. Origins of specificity and cross-talk in metal ion sensing by Bacillus subtilis Fur. Molecular microbiology. 2012;86:1144–1155. doi: 10.1111/mmi.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furukawa K, et al. Bacterial riboswitches cooperatively bind Ni(2+) or Co(2+) ions and control expression of heavy metal transporters. Molecular cell. 2015;57:1088–1098. doi: 10.1016/j.molcel.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banerjee RV, Johnston NL, Sobeski JK, Datta P, Matthews RG. Cloning and sequence analysis of the Escherichia coli metH gene encoding cobalamin-dependent methionine synthase and isolation of a tryptic fragment containing the cobalamin-binding domain. The Journal of biological chemistry. 1989;264:13888–13895. [PubMed] [Google Scholar]
- 71.Nahvi A, et al. Genetic control by a metabolite binding mRNA. Chemistry & biology. 2002;9:1043. doi: 10.1016/s1074-5521(02)00224-7. [DOI] [PubMed] [Google Scholar]
- 72.Rodionov DA, Hebbeln P, Gelfand MS, Eitinger T. Comparative and functional genomic analysis of prokaryotic nickel and cobalt uptake transporters: evidence for a novel group of ATP-binding cassette transporters. Journal of bacteriology. 2006;188:317–327. doi: 10.1128/jb.188.1.317-327.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaballa A, et al. The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11927–11932. doi: 10.1073/pnas.0711752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baichoo N, Wang T, Ye R, Helmann JD. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Molecular microbiology. 2002;45:1613–1629. doi: 10.1046/j.1365-2958.2002.03113.x. [DOI] [PubMed] [Google Scholar]
- 76.Yoch DC, Valentine RC. Ferredoxins and flavodoxins of bacteria. Annual review of microbiology. 1972;26:139–162. doi: 10.1146/annurev.mi.26.100172.001035. [DOI] [PubMed] [Google Scholar]
- 77.Erdner DL, Anderson DM. Ferredoxin and flavodoxin as biochemical indicators of iron limitation during open-ocean iron enrichment. Limnology and Oceanography. 1999;44:1609–1615. doi: 10.4319/lo.1999.44.7.1609. [DOI] [Google Scholar]
- 78.Smaldone GT, et al. A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism. Journal of bacteriology. 2012;194:2594–2605. doi: 10.1128/jb.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masse E, Arguin M. Ironing out the problem: new mechanisms of iron homeostasis. Trends Biochem Sci. 2005;30:462–468. doi: 10.1016/j.tibs.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 80.Yost FJ, Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. The Journal of biological chemistry. 1973;248:4905–4908. [PubMed] [Google Scholar]
- 81.Keele BB, Jr, McCord JM, Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. The Journal of biological chemistry. 1970;245:6176–6181. [PubMed] [Google Scholar]
- 82.Fee JA. Regulation of sod genes in Escherichia coli: relevance to superoxide dismutase function. Molecular microbiology. 1991;5:2599–2610. doi: 10.1111/j.1365-2958.1991.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 83.Andrews SC. Making DNA without iron - induction of a manganese-dependent ribonucleotide reductase in response to iron starvation. Molecular microbiology. 2011;80:286–289. doi: 10.1111/j.1365-2958.2011.07594.x. [DOI] [PubMed] [Google Scholar]
- 84.Masse E, Vanderpool CK, Gottesman S. Effect of RyhB small RNA on global iron use in Escherichia coli. Journal of bacteriology. 2005;187:6962–6971. doi: 10.1128/jb.187.20.6962-6971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mielcarek A, Blauenburg B, Miethke M, Marahiel MA. Molecular insights into frataxin-mediated iron supply for heme biosynthesis in Bacillus subtilis. PloS one. 2015;10:e0122538. doi: 10.1371/journal.pone.0122538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaballa A, Wang T, Ye RW, Helmann JD. Functional analysis of the Bacillus subtilis Zur regulon. Journal of bacteriology. 2002;184:6508–6514. doi: 10.1128/JB.184.23.6508-6514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akanuma G, Nanamiya H, Natori Y, Nomura N, Kawamura F. Liberation of zinc-containing L31 (RpmE) from ribosomes by its paralogous gene product, YtiA, in Bacillus subtilis. Journal of bacteriology. 2006;188:2715–2720. doi: 10.1128/jb.188.7.2715-2720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nanamiya H, et al. Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Molecular microbiology. 2004;52:273–283. doi: 10.1111/j.1365-2958.2003.03972.x. [DOI] [PubMed] [Google Scholar]
- 89.Gabriel SE, Helmann JD. Contributions of Zur-controlled ribosomal proteins to growth under zinc starvation conditions. Journal of bacteriology. 2009;191:6116–6122. doi: 10.1128/jb.00802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin JH, Helmann JD. Molecular logic of the Zur-regulated zinc deprivation response in Bacillus subtilis. Nature communications. 2016;7:12612. doi: 10.1038/ncomms12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin JH, et al. Graded expression of zinc-responsive genes through two regulatory zinc-binding sites in Zur. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5045–5050. doi: 10.1073/pnas.1017744108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilston BA, et al. Structural and mechanistic basis of zinc regulation across the E. coli Zur regulon. PLoS biology. 2014;12:e1001987. doi: 10.1371/journal.pbio.1001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beauchene NA, et al. Impact of Anaerobiosis on Expression of the Iron-Responsive Fur and RyhB Regulons. mBio. 2015;6:e01947–01915. doi: 10.1128/mBio.01947-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.German N, Doyscher D, Rensing C. Bacterial killing in macrophages and amoeba: do they all use a brass dagger? Future microbiology. 2013;8:1257–1264. doi: 10.2217/fmb.13.100. [DOI] [PubMed] [Google Scholar]
- 95.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science (New York, NY) 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 96.Ma Z, et al. Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Molecular microbiology. 2014;94:756–770. doi: 10.1111/mmi.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Newton GL, et al. Bacillithiol is an antioxidant thiol produced in Bacilli. Nature chemical biology. 2009;5:625–627. doi: 10.1038/nchembio.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma SV, et al. Chemical and Chemoenzymatic syntheses of bacillithiol: a unique low-molecular-weight thiol amongst low G + C Gram-positive bacteria. Angewandte Chemie (International ed in English) 2011;50:7101–7104. doi: 10.1002/anie.201100196. [DOI] [PubMed] [Google Scholar]
- 99.Helbig K, Bleuel C, Krauss GJ, Nies DH. Glutathione and transition-metal homeostasis in Escherichia coli. Journal of bacteriology. 2008;190:5431–5438. doi: 10.1128/jb.00271-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nairn BL, et al. The Response of Acinetobacter baumannii to Zinc Starvation. Cell host & microbe. 2016;19:826–836. doi: 10.1016/j.chom.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fung DK, Lau WY, Chan WT, Yan A. Copper efflux is induced during anaerobic amino acid limitation in Escherichia coli to protect iron-sulfur cluster enzymes and biogenesis. Journal of bacteriology. 2013;195:4556–4568. doi: 10.1128/jb.00543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kay KL, Hamilton CJ, Le Brun NE. Mass spectrometry of B. subtilis CopZ: Cu(i)-binding and interactions with bacillithiol. Metallomics : integrated biometal science. 2016;8:709–719. doi: 10.1039/c6mt00036c. [DOI] [PubMed] [Google Scholar]
- 104.Potter AJ, Trappetti C, Paton JC. Streptococcus pneumoniae uses glutathione to defend against oxidative stress and metal ion toxicity. Journal of bacteriology. 2012;194:6248–6254. doi: 10.1128/jb.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Culotta VC, Daly MJ. Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxidants & redox signaling. 2013;19:933–944. doi: 10.1089/ars.2012.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bruch EM, Thomine S, Tabares LC, Un S. Variations in Mn(II) speciation among organisms: what makes D. radiodurans different. Metallomics : integrated biometal science. 2015;7:136–144. doi: 10.1039/c4mt00265b. [DOI] [PubMed] [Google Scholar]
- 107.Sharma A, et al. Responses of Mn2+ speciation in Deinococcus radiodurans and Escherichia coli to gamma-radiation by advanced paramagnetic resonance methods. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5945–5950. doi: 10.1073/pnas.1303376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tabares LC, Un S. In situ determination of manganese(II) speciation in Deinococcus radiodurans by high magnetic field EPR: detection of high levels of Mn(II) bound to proteins. The Journal of biological chemistry. 2013;288:5050–5055. doi: 10.1074/jbc.C112.444992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tu WY, et al. Cellular iron distribution in Bacillus anthracis. Journal of bacteriology. 2012;194:932–940. doi: 10.1128/jb.06195-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andrews SC. Iron storage in bacteria. Advances in microbial physiology. 1998;40:281–351. doi: 10.1016/s0065-2911(08)60134-4. [DOI] [PubMed] [Google Scholar]
- 111.Waidner B, et al. Essential role of ferritin Pfr in Helicobacter pylori iron metabolism and gastric colonization. Infection and immunity. 2002;70:3923–3929. doi: 10.1128/IAI.70.7.3923-3929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Macedo S, et al. The nature of the di-iron site in the bacterioferritin from Desulfovibrio desulfuricans. Nature structural biology. 2003;10:285–290. doi: 10.1038/nsb909. [DOI] [PubMed] [Google Scholar]
- 113.Carrondo MA. Ferritins, iron uptake and storage from the bacterioferritin viewpoint. The EMBO journal. 2003;22:1959–1968. doi: 10.1093/emboj/cdg215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang X, Le Brun NE, Thomson AJ, Moore GR, Chasteen ND. The iron oxidation and hydrolysis chemistry of Escherichia coli bacterioferritin. Biochemistry. 2000;39:4915–4923. doi: 10.1021/bi992631f. [DOI] [PubMed] [Google Scholar]
- 115.Kuberl A, Polen T, Bott M. The pupylation machinery is involved in iron homeostasis by targeting the iron storage protein ferritin. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4806–4811. doi: 10.1073/pnas.1514529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beard SJ, Hughes MN, Poole RK. Inhibition of the cytochrome bd-terminated NADH oxidase system in Escherichia coli K-12 by divalent metal cations. FEMS microbiology letters. 1995;131:205–210. doi: 10.1111/j.1574-6968.1995.tb07778.x. [DOI] [PubMed] [Google Scholar]
- 117.Brekasis D, Paget MS. A novel sensor of NADH/NAD+ redox poise in Streptomyces coelicolor A3(2) The EMBO journal. 2003;22:4856–4865. doi: 10.1093/emboj/cdg453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alhasawi A, Auger C, Appanna VP, Chahma M, Appanna VD. Zinc toxicity and ATP production in Pseudomonas fluorescens. Journal of applied microbiology. 2014;117:65–73. doi: 10.1111/jam.12497. [DOI] [PubMed] [Google Scholar]
- 119.Chandrangsu P, Helmann JD. Intracellular Zn(II) Intoxication Leads to Dysregulation of the PerR Regulon Resulting in Heme Toxicity in Bacillus subtilis. PLoS genetics. 2016;12:e1006515. doi: 10.1371/journal.pgen.1006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shepherd M, et al. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Scientific reports. 2016;6:35285. doi: 10.1038/srep35285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mason MG, et al. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nature chemical biology. 2009;5:94–96. doi: 10.1038/nchembio.135. [DOI] [PubMed] [Google Scholar]
- 122.Korshunov S, Imlay KR, Imlay JA. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Molecular microbiology. 2016;101:62–77. doi: 10.1111/mmi.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McDevitt CA, et al. A molecular mechanism for bacterial susceptibility to zinc. PLoS pathogens. 2011;7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takeda H, et al. Structural basis for ion selectivity revealed by high-resolution crystal structure of Mg2+ channel MgtE. Nature communications. 2014;5:5374. doi: 10.1038/ncomms6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anjem A, Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. The Journal of biological chemistry. 2012;287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu FF, Imlay JA. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Applied and environmental microbiology. 2012;78:3614–3621. doi: 10.1128/aem.07368-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chillappagari S, et al. Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis. Journal of bacteriology. 2010;192:2512–2524. doi: 10.1128/jb.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin JE, Waters LS, Storz G, Imlay JA. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS genetics. 2015;11:e1004977. doi: 10.1371/journal.pgen.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Begg SL, et al. Dysregulation of transition metal ion homeostasis is the molecular basis for cadmium toxicity in Streptococcus pneumoniae. Nature communications. 2015;6:6418. doi: 10.1038/ncomms7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 132.Faulkner MJ, Ma Z, Fuangthong M, Helmann JD. Derepression of the Bacillus subtilis PerR peroxide stress response leads to iron deficiency. Journal of bacteriology. 2012;194:1226–1235. doi: 10.1128/jb.06566-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wakeman CA, et al. Menaquinone biosynthesis potentiates haem toxicity in Staphylococcus aureus. Molecular microbiology. 2012;86:1376–1392. doi: 10.1111/mmi.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu JZ, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell host & microbe. 2012;11:227–239. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Damo SM, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gunshin H, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 137.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. The Journal of biological chemistry. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kapetanovic R, et al. Salmonella employs multiple mechanisms to subvert the TLR-inducible zinc-mediated antimicrobial response of human macrophages. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:1901–1912. doi: 10.1096/fj.201500061. [DOI] [PubMed] [Google Scholar]
- 139.Ong CL, Walker MJ, McEwan AG. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Scientific reports. 2015;5:10799. doi: 10.1038/srep10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Francis MS, Thomas CJ. Mutants in the CtpA copper transporting P-type ATPase reduce virulence of Listeria monocytogenes. Microbial pathogenesis. 1997;22:67–78. doi: 10.1006/mpat.1996.0092. [DOI] [PubMed] [Google Scholar]
- 141.Achard ME, et al. The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infection and immunity. 2010;78:2312–2319. doi: 10.1128/iai.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Turner AG, et al. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio. 2015;6 doi: 10.1128/mBio.00278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McLaughlin HP, et al. A putative P-type ATPase required for virulence and resistance to haem toxicity in Listeria monocytogenes. PloS one. 2012;7:e30928. doi: 10.1371/journal.pone.0030928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hubert K, et al. ZnuD, a potential candidate for a simple and universal Neisseria meningitidis vaccine. Infection and immunity. 2013;81:1915–1927. doi: 10.1128/iai.01312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Anderson AS, et al. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. The Journal of infectious diseases. 2012;205:1688–1696. doi: 10.1093/infdis/jis272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miyaji EN, et al. PsaA (pneumococcal surface adhesin A) and PspA (pneumococcal surface protein A) DNA vaccines induce humoral and cellular immune responses against Streptococcus pneumoniae. Vaccine. 2001;20:805–812. doi: 10.1016/s0264-410x(01)00395-4. [DOI] [PubMed] [Google Scholar]
- 147.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Irving H, Williams RJP. 637. The stability of transition-metal complexes. Journal of the Chemical Society (Resumed) 1953:3192–3210. doi: 10.1039/JR9530003192. [DOI] [Google Scholar]
- 149.Foster AW, Osman D, Robinson NJ. Metal preferences and metallation. The Journal of biological chemistry. 2014;289:28095–28103. doi: 10.1074/jbc.R114.588145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Braymer JJ, Giedroc DP. Recent developments in copper and zinc homeostasis in bacterial pathogens. Curr Opin Chem Biol. 2014;19:59–66. doi: 10.1016/j.cbpa.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Outten FW, Huffman DL, Hale JA, O’Halloran TV. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. The Journal of biological chemistry. 2001;276:30670–30677. doi: 10.1074/jbc.M104122200. [DOI] [PubMed] [Google Scholar]
- 152.Rensing C, McDevitt SF. The copper metallome in prokaryotic cells. Metal ions in life sciences. 2013;12:417–450. doi: 10.1007/978-94-007-5561-1_12. [DOI] [PubMed] [Google Scholar]
- 153.Maguire ME, Cowan JA. Magnesium chemistry and biochemistry. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2002;15:203–210. doi: 10.1023/a:1016058229972. [DOI] [PubMed] [Google Scholar]
- 154.Helmann JD. Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. The Journal of biological chemistry. 2014;289:28112–28120. doi: 10.1074/jbc.R114.587071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nature reviews. Microbiology. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 156.Tottey S, et al. Cyanobacterial metallochaperone inhibits deleterious side reactions of copper. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:95–100. doi: 10.1073/pnas.1117515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guedon E, Helmann JD. Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators. Molecular microbiology. 2003;48:495–506. doi: 10.1046/j.1365-2958.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- 158.Cavet JS, et al. A nickel-cobalt-sensing ArsR-SmtB family repressor. Contributions of cytosol and effector binding sites to metal selectivity. The Journal of biological chemistry. 2002;277:38441–38448. doi: 10.1074/jbc.M207677200. [DOI] [PubMed] [Google Scholar]
- 159.Harvie DR, et al. Predicting metals sensed by ArsR-SmtB repressors: allosteric interference by a non-effector metal. Molecular microbiology. 2006;59:1341–1356. doi: 10.1111/j.1365-2958.2006.05029.x. [DOI] [PubMed] [Google Scholar]
- 160.Ma Z, Cowart DM, Scott RA, Giedroc DP. Molecular insights into the metal selectivity of the copper(I)-sensing repressor CsoR from Bacillus subtilis. Biochemistry. 2009;48:3325–3334. doi: 10.1021/bi900115w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dalmas O, Sompornpisut P, Bezanilla F, Perozo E. Molecular mechanism of Mg2+-dependent gating in CorA. Nature communications. 2014;5:3590. doi: 10.1038/ncomms4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. Crystal structure of the MgtE Mg2+ transporter. Nature. 2007;448:1072–1075. doi: 10.1038/nature06093. [DOI] [PubMed] [Google Scholar]
- 163.Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.MacDiarmid CW, Milanick MA, Eide DJ. Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. The Journal of biological chemistry. 2003;278:15065–15072. doi: 10.1074/jbc.M300568200. [DOI] [PubMed] [Google Scholar]
- 165.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stafford SL, et al. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Bioscience reports. 2013;33 doi: 10.1042/bsr20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Raimunda D, Long JE, Sassetti CM, Arguello JM. Role in metal homeostasis of CtpD, a Co(2)(+) transporting P(1B4)-ATPase of Mycobacterium smegmatis. Molecular microbiology. 2012;84:1139–1149. doi: 10.1111/j.1365-2958.2012.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hao X, et al. Survival in amoeba--a major selection pressure on the presence of bacterial copper and zinc resistance determinants? Identification of a “copper pathogenicity island”. Applied microbiology and biotechnology. 2015;99:5817–5824. doi: 10.1007/s00253-015-6749-0. [DOI] [PubMed] [Google Scholar]
- 169.Buracco S, et al. Dictyostelium Nramp1, which is structurally and functionally similar to mammalian DMT1 transporter, mediates phagosomal iron efflux. Journal of cell science. 2015;128:3304–3316. doi: 10.1242/jcs.173153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Peracino B, Buracco S, Bozzaro S. The Nramp (Slc11) proteins regulate development, resistance to pathogenic bacteria and iron homeostasis in Dictyostelium discoideum. Journal of cell science. 2013;126:301–311. doi: 10.1242/jcs.116210. [DOI] [PubMed] [Google Scholar]
- 171.Burlando B, et al. Occurrence of Cu-ATPase in Dictyostelium: possible role in resistance to copper. Biochemical and biophysical research communications. 2002;291:476–483. doi: 10.1006/bbrc.2002.6463. [DOI] [PubMed] [Google Scholar]
- 172.Hao X, et al. A role for copper in protozoan grazing - two billion years selecting for bacterial copper resistance. Molecular microbiology. 2016 doi: 10.1111/mmi.13483. [DOI] [PubMed] [Google Scholar]
- 173.Fields BS. The molecular ecology of legionellae. Trends in microbiology. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 174.Hilbi H, Segal G, Shuman HA. Icm/dot-dependent upregulation of phagocytosis by Legionella pneumophila. Molecular microbiology. 2001;42:603–617. doi: 10.1046/j.1365-2958.2001.02645.x. [DOI] [PubMed] [Google Scholar]