Abstract
Rationale
Adolescence is a period of considerable development of brain and behavior and is the time during which most drug use is initiated.
Objective
Age-dependent differences in motivated behaviors may be one of the factors that contribute to heightened vulnerability to developing substance use disorders, so we sought to compare age differences in methamphetamine (METH) and saccharin seeking.
Methods
Beginning during adolescence or adulthood, male and female Sprague-Dawley rats were trained to self-administer 0.1% saccharin (via liquid dipper cup) or intravenous METH at one of three doses (0.02, 0.05, 0.08 mg/kg/inf) under increasing fixed ratio schedules of reinforcement. Subsequently, responding for METH (0.02, 0.05, 0.08 or 0.1 mg/kg/inf) under progressive ratio response requirements was assessed in rats that acquired METH self-administration at the highest dose (0.08 mg/kg/inf).
Results
We found that adult-onset rats acquired METH self-administration more readily and exhibited higher motivation compared to adolescent-onset rats, although there were no differences in METH intake during acquisition. Adult rats also acquired saccharin self-administration more readily, but in contrast to METH, there were age and sex differences in saccharin intake driven by high levels of responding in adult females.
Conclusions
These findings challenge the prevailing notion that adolescents are hypersensitive to reward and instead raise questions about the potential role of methodological factors on which rodent studies often differ.
Keywords: Adolescence, Sex differences, Methamphetamine, Saccharin, Self-administration
1. Introduction
Adolescence is the transitional period between childhood and adulthood, which in humans is estimated to range from about 12 to 25 years old (Dahl 2004), and it is characterized by normal yet substantial changes in neurobiology and behavior (Spear 2000; Sisk and Foster 2004; Somerville et al. 2010). Some adolescents begin using drugs during this time and it has been reported that adolescent-onset users transition to dependence more readily than adults (Chen et al. 2009) and have poorer treatment outcomes (Gonzales et al. 2008; Chen et al. 2009). Reward hypersensitivity is one mechanism that may confer heightened vulnerability for drug use disorders in adolescents. Specifically, it has been hypothesized that adolescents are hypersensitive to rewards and that this characteristic of motivated behavior is one of the factors that contributes to heightened vulnerability to developing substance use disorders (Casey et al. 2008;Doremus-Fitzwater and Spear 2016; Spear 2000; Steinberg, 2010).
Several studies in the human and laboratory animal literature support the idea of reward hypersensitivity in adolescents (Friemel et al. 2010; Marshall et al. 2017; van Duijvenvoorde et al. 2016). For example, self-reported reward-seeking in humans peaks between the ages of 12 to 15, forming an inverted-U relative to older and younger ages (Steinberg et al. 2009). Furthermore, brain regions implicated in reward processing are more active during task demands in adolescents compared to adults (Galvan et al. 2006), even when age differences in behavior are not seen (Strang and Pollak 2014). During tests of reward appraisal or emotion regulation, adolescents are reported to have greater nucleus accumbens (Galvan et al. 2006) and amygdala activation relative to adults (Hare et al. 2008). In addition, adolescent humans and rodents exhibit higher preferences for palatable foods than adults (Desor and Beauchamp 1987; Wilmouth and Spear 2009; Friemel et al. 2010), although this age effect depends on sex (Marshall et al. 2017). In humans, sex differences have been found that support the“telescoping” phenomenon in women, whereby women tend to progress more rapidly than men from initiation of drug use to addiction (Piazza et al. 1989). Generally rodent studies corroborate the human literature as female rats exhibit greater drug-seeking than males (Becker and Koob 2016; Reichel et al. 2012; Roth and Carroll 2004). In rodent studies, there are mixed findings for age-dependent differences in drug-seeking that may depend on drug class (Doherty et al. 2013; Doherty and Frantz 2012; Mayer-Blackwell et al. 2014) or other methodological factors. However, it is most frequently the case that drug seeking in adolescents is found to be similar or heightened relative to adults (Shahbazi et al. 2008; Anker et al. 2012; Wong et al. 2013; Wong and Marinelli 2015).
Neurobiological systems implicated in reward undergo considerable changes during adolescence, which may play a significant role in age differences in reward sensitivity. In the human prefrontal cortex gray matter, synapses, and gene expression of some subtypes of serotonin receptors undergo dynamic changes during adolescence (Huttenlocher and Dabholkar 1997; Giedd et al. 1999; Sowell et al. 1999; Glantz et al. 2007; Lambe et al. 2011). Similar developmentally regulated changes have been observed in rodents during their adolescent period, which has been argued to range from as early as postnatal day (P) 28 to at least P60 based on social, cognitive, hormonal, and neurophysiological changes that parallel markers of human adolescence (Tirelli et al. 2003; Spear 2011). For example, during rodent adolescence there are significant increases in connectivity between the basolateral amygdala and medial prefrontal cortex (Cunningham et al. 2002, 2008), changes in the density of monoamine transporters (Moll et al. 2000; Bradshaw et al. 2016), increases in prefrontal cortex dopamine innervation (Kalsbeek et al. 1988), decreases in medial prefrontal cortex neuron number (Markham et al. 2007; Willing and Juraska 2015), and reorganization of the amygdala (Rubinow and Juraska 2009; Koss et al. 2014).
To date, few published studies have examined age differences in drug or non-drug reward seeking in rodent models and the limited number that have focus on males and a single reinforcer type (Counotte et al. 2014; Myal et al. 2015). Here, we sought to address this deficiency by testing the hypothesis that adolescents are hypersensitive to rewards by training male and female Sprague-Dawley rats to self-administer 0.1% saccharin (delivered via a liquid dipper cup) or one of three different doses of METH (0.02, 0.05 or 0.08 mg/kg/infusion delivered i.v. via jugular vein catheters). We chose the non-caloric sweetener saccharin as a comparison non-drug reinforcer in order to minimize the potential impact of the differential caloric needs that exist in rapidly growing adolescent compared to adult rats (Spear 2000; Friemel et al. 2010). In a follow-up experiment, we further assessed motivation for METH by allowing rats that had acquired self-administration at 0.08 mg/kg/infusion to respond for one of four different doses of METH (0.02-0.1 mg/kg/infusion) under a progressive ratio (PR) schedule of reinforcement. Consistent with the hypothesis that adolescents are hypersensitive to rewards (Casey et al. 2008; Doremus-Fitzwater and Spear 2016; Spear 2000; Steinberg 2010), we predicted that rats beginning self-administration training during adolescence would more readily acquire the behavior and earn more infusions (i.e., complete higher response ratios) under a PR schedule compared to adults.
2. Methods
2.1 Subjects
Subjects were 80 male and 103 female Sprague-Dawley rats born from vendor-obtained breeders (Harlan; Indianapolis, IN, USA) that were maintained in our facility. They were weaned on PND 22 and housed 2-3 per cage with same-sex littermates and/or same-sex non-littermates with the same date of birth. Group assignments were counterbalanced across litters according to age-of-onset (adolescent or adult) and reinforcer (METH or saccharin). All rats were weighed daily beginning on P30 and food and water were available ad libitum throughout the study, except during the limited periods (≤ 4 h) noted below. Rats were kept on a reversed 12-hour light/dark cycle (lights off at 0900) with experiments performed between 0900 and 1830. Puberty onset was estimated by daily checks for preputial separation in males (Korenbrot et al. 1977) and vaginal opening in females (Hashizume and Ohashi 1984). These checks started on P30 and revealed that age of mean puberty onset in females was P35 (range P31-44) and in males was P43 (range P36-47). Female estrous cycle was not monitored in the current study because drug exposure is known to disrupt cycling (King et al. 1990, 1993; Raap et al. 2000). Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana–Champaign, and were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2 Surgery
Rats were implanted with indwelling jugular vein catheters on or about (± 2 days) P32 (adolescent onset) or P82 (adult onset) using procedures described by Wong et al. (2013). Briefly, rats were anesthetized with isoflurane (2-4%; Midwest Veterinary Supply, Lakeville MN, USA), the right jugular vein was isolated, and a catheter was inserted in the vein and extended to the dorsal torso where an access port was externalized in the mid scapular region. To facilitate catheter patency, rats were flushed daily with 0.1 ml of heparinized saline (50 U/ml) and given an antibiotic. For approximately half the rats in the study, the daily flush solution contained 10 mg/ml cefazolin (Midwest Veterinary Supply), whereas the remainder were given 1.1% Trimethoprim Sulfa (Midwest Veterinary Supply) in their drinking water. This change was made in consultation with campus veterinarians as an approach to provide more appropriate prophylactic treatment. Although catheter patency was not critical for rats in the saccharin self-administration groups, daily flushes were also performed if catheters remained patent. For rats in the i.v. self-administration groups, catheter patency was tested 6-8 days following surgery, 1 day following the final self-administration session, and as needed if patency loss was suspected by infusion with 0.05-0.1 ml of a solution containing 15% ketamine (100 mg/ml) and 15% midazolam (5 mg/ml). Patency was confirmed by immediate loss of muscle tone.
2.3 Apparatus
Self-administration took place in standard operant conditioning chambers (Coulbourn Instruments, Whitehall, PA) that were enclosed in sound-attenuating cubicles. The cubicles were equipped with fans that provided ventilation and masked extraneous noise. One wall of each operant conditioning chamber was equipped with a centrally located food trough outfitted on either side with two recessed nosepoke ports that were equidistant (87 mm) from the trough. White cue lights were located above each lever and a 2.9 kHz Sonalert speaker was located directly above the food trough. A white houselight was located near the chamber ceiling on the wall opposite the nosepoke ports. Entries into the trough and nosepoke ports were monitored using infrared detectors. Graphic State (v3.1; Coulbourn Instruments) was used for automated chamber control and data collection.
2.4 Self-administration
Following 6-8 days of recovery from surgery, rats were allowed 90 min to explore the operant conditioning chamber with access to the nosepoke ports blocked (Wong et al. 2013). The following day, rats were trained in 2-h sessions to perform nosepoke responses for 0.1% saccharin or (+)-METH HCl (Sigma-Aldrich, St. Louis, MO), with the latter available at one of three unit doses: 0.02, 0.05, or 0.08 mg/kg/inf (calculated as weight of the salt and dissolved in 0.9% sterile saline). Doses of METH were based on previous studies reporting the ability to detect age differences (0.05 mg/kg/inf; Anker et al., 2012), different rates of escalation of METH intake (0.05 and 0.1 mg/kg/inf; Kitamura et al., 2006), and sex differences in acquisition and motivation (0.02 and 0.08 mg/kg/inf; Roth & Carroll, 2004). Saccharin concentration was based on a study (Wong, 1985) and our own pilot experiments showing a 0.1% saccharin solution was the most preferred.
For all rats, responses into the right nosepoke port (“reinforced response”) were reinforced with either an infusion of METH (40-80 μl delivered over 2-4 sec) or delivery of saccharin via a dipper cup (0.08 ml/delivery). Responses in the left port (“non-reinforced response”) had no programmed consequence. Reinforcer delivery coincided with presentation of a 4-sec compound stimulus (a 2.9 kHz, 80 dB tone and illumination of the cue light above the right nosepoke port). To encourage exploration of the chamber for the first three days of training, food was removed 2 h prior to the session and returned 30 min to 2 h after the session.
Acquisition of METH and saccharin self-administration was assessed over 15 sessions of fixed ratio (FR) responding: FR1 for the first 7 sessions, FR3 for 4 sessions, and FR5 for the final 4 sessions. Rats trained to acquire METH self-administration at the 0.08 mg/kg/inf dose were subsequently tested for their motivation to respond for four doses of METH (0.02, 0.05, 0.08, 0.1 mg/kg/inf) during separate sessions performed on a progressive ratio (PR) schedule of reinforcement (Fig. 1). For PR sessions, which were 5 h in duration, the training dose (0.08 mg/kg/inf) was always tested first and the testing order for remaining doses was counterbalanced across rats. Between each PR session, rats were given a 2-h maintenance session with 0.08 mg/kg/inf available on an FR5 schedule of reinforcement. PR breakpoint, which is often analyzed as a measure of motivation for the reinforcer, is typically defined as the final ratio achieved before the rat ceases responding. However, accurate measurement of breakpoint for drugs like METH that have a long half-life requires extended duration sessions (∼24 h) followed by drug-free recovery days (Richardson and Roberts 1996). Because we were interested in age differences during the relatively short developmental period of adolescence, this approach was not practical here, and we therefore chose to use the 5-h timed PR session to complete testing within the fewest number of days possible. Previous studies used timed PR sessions and measures of infusions earned to investigate changes in motivation to respond for METH (Clemens et al. 2006; Cox et al. 2013).
Figure 1.
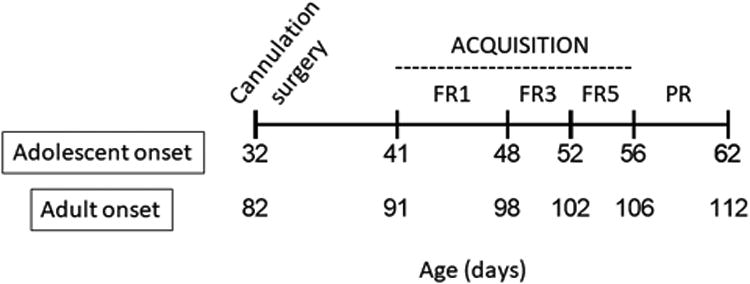
Experimental timeline showing the approximate age in days (± 2) of rats in the adolescent- and adult-onset groups for cannulation surgery and self-administration procedures. Acquisition of METH self-administration was assessed in groups of rats trained at 0.02, 0.05, or 0.08 mg/kg/inf unit doses. Acquisition of 0.1% oral saccharin self-administration was assessed in a fourth group. Progressive ratio (PR) responding, which was assessed only in rats that met acquisition criterion at 0.08 mg/kg/inf, was tested at four doses (0.02, 0.05, 0.08, 0.1 mg/kg/inf) in separate sessions that each were separated by a maintenance session of 0.08 mg/kg/inf available on an FR5 schedule.
2.7 Data Analysis
Dependent measures included number of reinforced responses, number of non-reinforced responses, reinforcers earned, sessions to reach acquisition criterion, and infusions earned during PR sessions. Rats were considered to have acquired METH self-administration if two criteria were met: (1) they infused an average of at least 0.95 mg/kg METH across four consecutive sessions and (2) they maintained this level of consumption through FR5 (sessions 12-15). This second standard was used to insure “acquired” rats continued responding as the ratio requirement increased. For METH, a dose of at least 0.95 mg/kg was achieved when rats earned 48, 19 or 12 infusions at 0.02, 0.05 or 0.08 m/kg/inf, respectively. For saccharin self-administration, the acquisition criterion was set at ≥19 reinforcers to equate required responding with the 0.05 mg/kg/inf dose of METH. For analysis of the rate of acquisition, the final session of the first four-session block was used. Rats that did not meet acquisition criterion were assigned a value of 16 for the sessions to criterion analyses. Sessions to criterion was analyzed separately for saccharin and each dose of METH with a two-way (age × sex) ANOVA. The number of rats per group meeting acquisition criterion by the end of the acquisition period (session 15) was analyzed separately for saccharin and each dose of METH with Chi-square log-rank tests (Mantel-Cox); the specific group comparisons made were determined based on significant differences in the sessions to criterion ANOVAs.
To determine if the acquisition criterion split rats into groups that were behaviorally distinct, reinforced responses were analyzed separately for saccharin and each dose of METH in two-way mixed factor ANOVAs with acquisition (yes or no) and session as factors. Reinforced nosepokes and reinforcers earned from rats that met acquisition criterion were then analyzed for saccharin and each dose of METH in separate three-way (age × sex × session) mixed factor ANOVAs. For PR tests, infusions were analyzed using three-way (age × sex × dose) mixed-factor ANOVAs. Significant main effects and interactions were followed up with Tukey's post-hoc tests.
3. Results
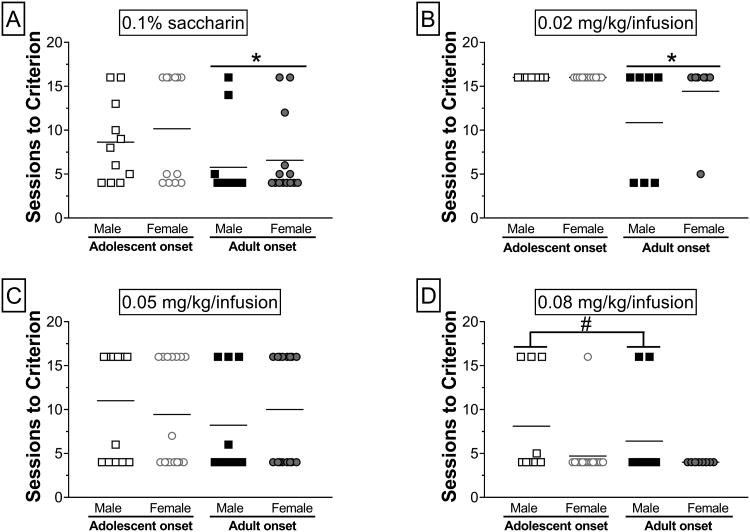
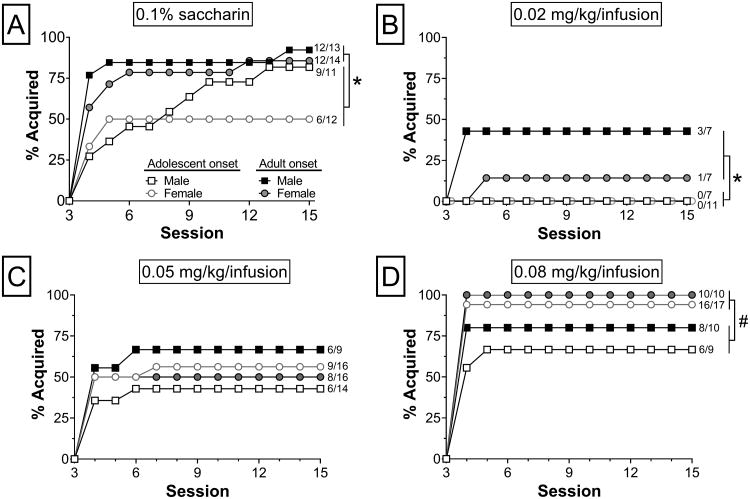
Acquistion of intravenous METH (0.02, 0.05 and 0.08 mg/kg/inf) and oral saccharin (0.1%) self-administration was assessed across 15 daily sessions with increasing FR schedules of reinforcement. Separate two-way ANOVAs of sessions to acquisition criterion revealed a main effect of age for saccharin (F1,46 = 5.44, p< 0.05), a significant main effect of age for 0.02 mg/kg/inf (F1,28 = 6.93, p< 0.05), and a main effect of sex for 0.08 mg/kg/inf (F1,42 = 5.92, p< 0.05). Tukey's post-hoc analyses demonstrated that for saccharin (Fig. 2A) and 0.02 mg/kg/inf METH (Fig. 2B), adolescent-onset rats required significantly more sessions to reach criterion than adult-onset rats. At the highest dose of METH (0.08 mg/kg/inf), males required more sessions to reach acquisition criterion than females (Fig. 2D). Increases in the percent of rats meeting acquisition criterion differed by reinforcer and unit dose of METH, and these effects varied by age of onset and sex. Consistent with the sessions to criterion data for saccharin (χ2 = 4.06, p< 0.05; Fig. 3A) and 0.02 mg/kg/inf (χ2 = 5.88, p< 0.05; Fig. 3B), significantly more adult-onset rats reached acquisition criterion by the final session than adolescent-onset rats regardless of sex. At 0.08 mg/kg/inf, more females met acquisition criterion by the 15th session than males and this was driven by a significant difference between adolescent-onset females and adolescent-onset males (χ2 = 5.03, p< 0.05; Fig. 3D). There were no significant group differences in acquisition at 0.05 mg/kg/inf (Fig. 2C, 3C).
Figure 2.
Sessions to reach acquisition criterion for saccharin and METH self-administration. Individual plots of number of sessions to acquisition criterion for (A) 0.1% saccharin (n = 11-14/group), (B) 0.02 mg/kg/inf METH (n = 7-11/group), (C) 0.05 mg/kg/inf METH (n = 9-16/group), and (D) 0.08 mg/kg/inf METH (n = 9-17/group). The horizontal line within each group indicates the group mean. #p< 0.05 males vs. females; * p< 0.05 adolescent-onset vs. adult-onset.
Figure 3.
Acquisition of 0.1% saccharin and METH (0.02, 0.05, or 0.08 mg/kg/inf) self-administration across 15 daily sessions of training. Shown are the percent of rats meeting acquisition criterion for (A) 0.1% saccharin (n = 11-14/group), (B) 0.02 mg/kg/inf METH (n = 7-11/group), (C) 0.05 mg/kg/inf METH (n = 9-16/group), and (D) 0.08 mg/kg/inf METH (n = 9-17/group). The ratio of rats that acquired to the total number that completed training for each group is indicated next to the corresponding acquisition curve. *p< 0.05 adolescent- vs. adultonset;#p< 0.05 female vs. male.
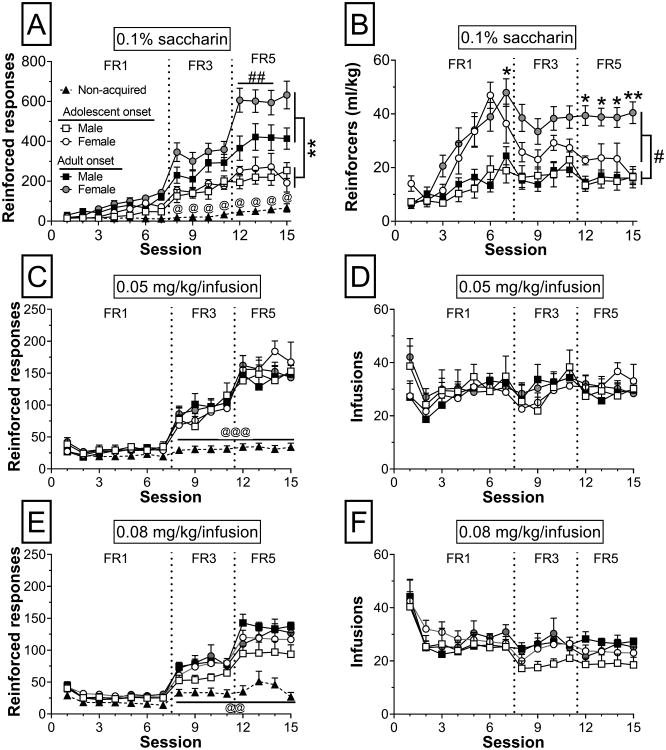
Reinforced responses for rats that acquired METH or saccharin self-administration, as well as those that did not (collapsed across group), are shown in Fig. 4. For saccharin, we found a significant session by acquisition interaction (F14,672 = 4.26, p< 0.0001). We also found a significant session by acquisition interaction for each dose of METH (0.02 mg/kg/inf: F14,417 = 11.57, p< 0.0001; 0.05 mg/kg/inf: F14,742 = 16.03, p< 0.0001; 0.08 mg/kg/inf: F14,616 = 4.38, p< 0.0001). The pattern of differences between rats that acquired and those that did not was consistent for saccharin and all doses of METH. Generally, there were no differences between acquired and not acquired rats for sessions 1 through 7 (exception: sessions 3, 4, and 7 at 0.02 mg/kg/inf and session 7 for saccharin). Differences emerged when the response requirement was increased to FR3 on session 8 and were maintained through the end of acquisition (Fig. 4A,C,E). Specifically, acquired rats made significantly more responses than rats that did not acquire on sessions 8 through 15. We performed this same analysis on non-reinforced nosepoke responses and found a significant acquired by session interaction at the 0.02 mg/kg/inf dose of METH (F14,417 = 2.32, p < 0.01; data not shown). Post-hoc tests indicated that on session 10, the small number of rats that met acquisition criteria at this dose (n = 4 adult-onset rats) had more non-reinforced responses compared to non-acquired rats (p < 0.01; data not shown). Subsequent analyses were not performed at the 0.02 mg/kg/inf dose due to the low number of rats that met acquisition criterion at this dose.
Figure 4.
Number of reinforced nosepoke responses and reinforcers earned across 15 days of acquisition for (A,B) 0.1% saccharin, (C,D) 0.05 mg/kg/inf METH, and (E,F) 0.08 mg/kg/inf METH. @@p< 0.01, @@@p< 0.001 acquired vs. not acquired for sessions 8-15; ** p< 0.01 adolescent- vs. adult-onset for sessions 8-15; ##p< 0.01 females vs. males for sessions 12-14; * p< 0.05, ** p< 0.01, adolescent- vs. adult-onset;#p< 0.05 females vs. males 3-15.
In the subset of rats that acquired saccharin (n = 39) and METH (0.05 mg/kg/inf: n = 29; 0.08 mg/kg/inf: n = 40) self-administration, reinforced nosepoke responses and reinforcers earned were analyzed in three-way ANOVAs. For saccharin, there was a main effect of session for reinforced nosepokes (F14,490 = 20.44, p< 0.0001). For both doses of METH, we also found a main effect of session for reinforced nosepokes (0.05 mg/kg/inf: F14,350 = 27.68, p< 0.0001; 0.08 mg/kg/inf: F14,504 = 39.22, p< 0.0001). Three contrasts were constructed to compare reinforced nosepokes averaged across days 1-7 (FR1), 8-11 (FR3), and 12-15 (FR5). For saccharin and both doses of METH, average nosepokes at each FR schedule differed significantly from the other two (p< 0.001 for all comparisons), demonstrating that reinforced responses increased with each escalating response requirement (FR1 < FR3 < FR5). For saccharin, there was also an age by session interaction (F14,490 = 4.25, p< 0.0001), and a sex by session interaction (F14,490 = 2.38, p< 0.01). Females made more nosepokes than males when the response requirement was increased to FR5 on session 12 and were maintained through session 14 (Fig. 4A). This effect seemed to be driven by adult-onset rats who made more nosepokes than adolescent-onset rats from session 8 through 15. Analysis of reinforcers earned (ml/kg) for saccharin rats revealed significant age by session (F14,490 = 3.84, p< 0.0001) and sex by session (F14,490 = 3.18, p< 0.0001) interactions. Females earned significantly more reinforcers per body weight than males on sessions 3 through 15, which appears largely driven by adult females once the fixed ratio requirement was increased on session 8. Adult-onset rats earned more reinforcers per body weight than adolescent-onset rats on sessions 7, and 12 through 15 (Fig. 4B). Analysis of reinforcers earned revealed only significant main effects of session for both doses of METH (0.05 mg/kg/inf: F14,350 = 4.32, p< 0.0001; 0.08 mg/kg/inf: F14,504 = 15.66, p< 0.0001). Post-hoc analysis of reinforcers at the 0.05 mg/kg/inf dose revealed that rats earned significantly less reinforcers on session 2 than sessions 1 and 11 (Fig.4D, significance not shown). At the 0.08 mg/kg/inf dose, rats earned significantly more reinforcers on session 1 than all other sessions (Fig.4F, significance not shown).
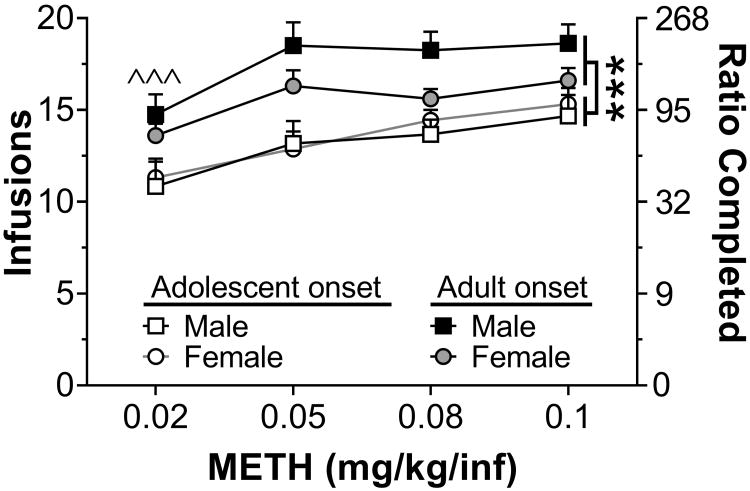
PR responding for 0.02-1.0 mg/kg/inf METH was then assessed in the subset of rats that met acquisition criterion at the 0.08 mg/kg/inf dose (Fig. 5). Analysis of infusions revealed significant main effects of dose (F3,111 = 13.6, p< 0.001) and age (F1,37 = 8.11, p< 0.01), but no interaction. Compared to all other doses tested, rats earned significantly fewer infusions at the 0.02 mg/kg/inf dose, consistent with data from acquisition (Fig. 5B). The main effect of age revealed that adult-onset rats, regardless of sex, earned significantly more infusions than adolescent-onset rats.
Figure 5.
Responding for 0.02-0.1 mg/kg/inf METH under a PR schedule of reinforcement. Shown are infusions (left y-axis) and ratio completed (right y-axis) during the 5-h PR sessions. ***p< 0.001 adolescent-onset vs. adult-onset; ˆˆˆp< 0.001 lowest dose vs. all other doses, collapsed across groups.
4. Discussion
Adolescence is a time of behavioral, cognitive, and neurobiological change that coincides with the period of greatest drug use initiation. Reward hypersensitivity has been postulated as a potential mechanism contributing to adolescent-onset drug use (Casey et al. 2008; Doremus-Fitzwater and Spear 2016; Spear, 2000; Steinberg 2010). In this study, we tested this hypothesis by examining age and sex differences in METH and saccharin self-administration. We found that adult-onset rats acquired saccharin and the lowest dose of METH (0.02 mg/kg/inf) self-administration more readily than adolescent-onset rats and that adolescent-onset males consistently had the lowest levels of METH acquisition across all training doses. Females also acquired self-administration of the highest does of METH (0.08 mg/kg/inf) more readily than males. In spite of similarities in acquisition of saccharin self-administration, adult-onset female rats self-administered signficantly more saccharin than their male counterparts. However, for rats that met acquisition criteria, there were no group differences in responding or intake of METH across the 15 sessions of acquisition. When motivation for METH was assessed in the subset of rats that met acquisition criterion at the highest training dose (0.08 mg/kg/inf) using a PR schedule of reinforcement, adult-onset rats earned the most infusions across all doses tested. Together, these data demonstrate that under the conditions used in the current experiments, adult-onset rats engage in more reward seeking behavior than adolescent-onset rats and that females are hypersensitive to nondrug rewards. Thus, the notion that adolescents are hypersensitive to rewards was not supported here.
Studies examining age differences in nondrug reward seeking have found that adult males engage in more reward seeking than adolescent males, but consume similar amounts by body weight (Counotte et al. 2014; Myal et al. 2015). Our results are consistent with this and further demonstrate that both male and female adults exhibit higher operant responding for saccharin relative to their adolescent counterparts, but that females consume more saccharin per body weight than males. This latter finding contrasts with a study demonstrating adult males and females did not differ in sucrose pellet intake by body weight (Zhou et al. 2015). However, an important methodological difference was that in the current study we used a non-caloric reward to mitigate the influence of age and sex differences in caloric need.
In addition to sex differences in nondrug reward seeking, we found evidence for sex differences in self-administration of the highest dose of METH (0.08 mg/kg/inf), with females acquiring more readily than males. This rapid initiation of METH use in our females is consistent with previous studies (Perry et al. 2013, 2015; Roth and Carroll 2004). In contrast, females did not display characteristics that are linked with a transition to addiction, i.e. greater intake of, or motivation for, METH. Thus, our METH self-administration data only partially support the notion that females exhibit the “telescoping” phenomenon (Piazza et al. 1989).
Our METH self-administration findings add to a growing literature that reveals nuanced effects of age and sex on drug seeking. Evidence for heightened self-administration in adolescents and females may depend on several methodological factors, such as duration of access, dose, and pubertal status. Typically, when cocaine or amphetamines are available for short periods of time (≤ 2 h), adolescents (Shahbazi et al. 2008; Harvey et al. 2009; Anker et al. 2012; Wong and Marinelli 2015) and females (Reichel et al. 2012) self-administer similar amounts of drug as their counterparts. Consistent with findings from the current study, these age and sex differences are dependent on dose (Kantak et al. 2007; Shahbazi et al. 2008; Wong et al. 2013; Schassburger et al. 2016). However, under long access conditions (∼4-6 h), adolescents (Shahbazi et al. 2008; Anker and Carroll 2010; Wong et al. 2013) and females (Roth and Carroll 2004; Reichel et al. 2012) have been reported to self-administer psychostimulants more readily than adults and males, respectively. Puberty appears to play a role as well given that males who were post-pubertal at the onset of self-administration infuse cocaine more readily than adults (Wong et al. 2013). Males that were pre-pubertal at the onset of self-administration, but were post-pubertal by the end of testing, earned the same number of infusions as adult males (Wong et al. 2013). In our adolescent-onset groups, most of the males had not yet reached puberty at the onset of METH self-administration, which could account for the low levels of self-administration in our adolescent-onset males. On the other hand, our adolescent-onset females were mostly post-pubertal at the start of self-administration. Studies assessing motivation reveal similar nuanced effects, such that adolescents and females sometimes exhibit higher motivation than their counterparts, but this depends on dose and housing conditions (Roth and Carroll 2004; Shahbazi et al. 2008; Westenbroek et al. 2013; Wong et al. 2013). Taken together, the findings suggest that propensity to self-administer drugs may not be conferred in an absolute sense and instead depends on a number of factors (Kosten et al. 2000; Walker et al. 2008; Lewis et al. 2013, 2016; Westenbroek et al. 2013; Baarendse et al. 2014). In the current study, the methodological features of note are that the rats we used were born in our facility and thus did not experience shipment stress at different ages, were socially housed, tested during their dark cycle, and underwent sessions with increasing FR response requirements across the acquisition period.
The age-dependent differences in METH self-administration we observed suggest that adolescent-onset rats are less sensitive to the reinforcing effects of METH compared to their adult-onset counterparts. Although there is evidence for adolescent reduced sensitivity to the reinforcing properties of opiate drugs (Doherty et al. 2013; Doherty and Frantz 2012; Mayer-Blackwell et al. 2014; Niikura et al. 2013), the existing research on age-dependent differences in the reinforcing and locomotor-activating effects of psychostimulants is equivocal. Studies have shown that adolescents are more sensitive (Zakharova et al. 2009; Mathews et al. 2011), less sensitive (Adriani and Laviola 2003; Mathews and McCormick 2007; Zakharova et al. 2009), or no different (Mathews and McCormick, 2007) than their adult counterparts. One possibility is that age differences in pharmacokinetics could account for reduced sensitivity to METH in adolescents, but findings are again mixed. Studies examining plasma and brain levels of stimulants have found that adolescents have lower (Spear and Brake 1983; Kokoshka et al. 2000; McCarthy et al. 2004; Craig et al. 2014), higher (Caster et al., 2005), or similar levels (McCarthy et al., 2004; Caster et al., 2005) compared to adults. Based on the extensive changes occurring in the brain during adolescence (Andersen et al. 1997, 2000; Dagher et al. 2001; Koss et al. 2014; Juraska and Willing 2017), another possibility is that differences in pharmacodynamics (e.g. dopamine transporter function; Volz et al., 2009) contribute to our findings. Further studies will be required to determine the impact of pharmacokinetics and pharmacodynamics on age- and sex-differences in drug seeking.
In summary, our investigation of differences in saccharin and METH seeking revealed that age differences in reward seeking depend on sex and reward type. Adult male and female rats were more sensitive to the reinforcing effects of METH and acquired saccharin self-administration more readily than adolescent rats. However, age and sex differences emerged when a nondrug reward was assessed as females, regardless of age, consumed more saccharin per body weight than males. Overall, adult females exhibited heightened sensitivity to rewards relative to all other groups. These findings challenge the prevailing notion that adolescents are hypersensitive to reward. Within the context of the existing literature, these results highlight the importance of factors including sex, reward type, and experimental conditions (e.g., group housing) on group differences in reward seeking. To reconcile the conflicting reports on this subject, further study is required to elucidate specific factors that determine the direction and magnitude of age and sex differences in drug seeking.
Acknowledgments
The authors thank Adam Gold, Courtney Hong, Laura Cortes, Kristen Hughes, Ashley Wehrheim, Sarah Rahman, Shawn Kurian, and Emily Kroeger for excellent technical assistance.
This work was supported by the National Institutes of Health [F31 DA036330 and T32 DA016176 to E.R.H and R01 DA029815 to J.M.G.]
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: Two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, et al. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, et al. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Baron TR, Zlebnik NE, Carroll ME. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend. 2012;124:149–53. doi: 10.1016/j.drugalcdep.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208:211–22. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJJ, Limpens JHW, Vanderschuren LJMJ. Disrupted social development enhances the motivation for cocaine in rats. Psychopharmacology (Berl) 2014;231:1695–1704. doi: 10.1007/s00213-013-3362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacological Reviews. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw SE, Agster KL, Waterhouse BD, Mcgaughy JA. Age-related changes in prefrontal norepinephrine transporter density: The basis for improved cognitive flexibility after low doses of atomoxetine in adolescent rats. Brain Res. 2016;1641:245–257. doi: 10.1016/j.brainres.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–25. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addict Behav. 2009;34:319–322. doi: 10.1016/j.addbeh.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, McGregor IS. Intravenous methamphetamine self-administration in rats: effects of intravenous or intraperitoneal MDMA co-administration. Pharmacology, Biochemistry, and Behavior. 2006;85(2):454–63. doi: 10.1016/j.pbb.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Council NR. Guide for the Care and Use of Laboratory Animals: Eighth Edition 2010 [Google Scholar]
- Counotte DS, Schiefer C, Shaham Y, O'Donnell P. Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology (Berl) 2014;231:1675–1684. doi: 10.1007/s00213-013-3294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology. 2013;38(10):2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EL, Zhao B, Cui JZ, et al. Nicotine Pharmacokinetics in Rats is altered as a function of Age, impacting the Interpretation of Animal Model Data. Drug Metab Dispos. 2014;42:1447–1455. doi: 10.1124/dmd.114.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2008;18:1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston Ja, et al. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42:48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Desor JA, Beauchamp GK. Longitudinal changes in sweet preferences in humans. Physiol Behav. 1987;39:639–641. doi: 10.1016/0031-9384(87)90166-1. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Frantz KJ. Heroin self-administration and reinstatement of heroin-seeking in adolescent vs. adult male rats. Psychopharmacology. 2012;219:763–773. doi: 10.1007/s00213-011-2398-x. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Cooke BM, Frantz KJ. A Role For The Prefrontal Cortex In Heroin-Seeking After Forced Abstinence By Adult Male Rats But Not Adolescents. Neuropsychopharmacology. 2013;38(3):446–454. doi: 10.1038/npp.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: An adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev. 2016;54:1970–1977. doi: 10.1016/j.neubiorev.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friemel CM, Spanagel R, Schneider M. Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Behav Neurosci. 2010;4:1–10. doi: 10.3389/fnbeh.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, et al. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Ang A, McCann MJ, Rawson RA. An Emerging Problem: Methamphetamine Abuse Among Treatment Seeking Youth. Subst Abus. 2008;29:71–80. doi: 10.1080/08897070802093312. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, et al. Biological Substrates of Emotional Reactivity and Regulation in Adolescence During an Emotional Go-Nogo Task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Dembro Ka, Rajagopalan K, et al. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology (Berl) 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume K, Ohashi K. Timing of sexual receptivity and the release of gonadotrophins during puberty in female rats. J Reprod Fert. 1984;72:87–91. doi: 10.1530/jrf.0.0720087. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Willing J. Pubertal onset as a critical transition for neural development and cognition. Brain Res. 2017;1654:87–94. doi: 10.1016/j.brainres.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, et al. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Goodrich CM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2007;15:37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- King TS, Canez MS, Gaskill S, et al. Chronic cocaine disruption of estrous cyclicity in the rat: dose-dependent effects. J Pharmacol Exp Ther. 1993;264:29–34. [PubMed] [Google Scholar]
- King TS, Schenken RS, Kang IS, et al. Cocaine disrupts estrous cyclicity and alters reproductive neuroendocrine axis in the rat. Neuroendocrinology. 1990;51:15–22. doi: 10.1159/000125310. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: A dose-effect function. Psychopharmacology. 2006;186(1):48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kokoshka JM, Fleckenstein a E, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J Neurochem. 2000;75:2095–102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Fillman SG, Webster MJ, Weickert CS. Serotonin receptor expression in human prefrontal cortex: Balancing excitation and inhibition across postnatal development. PLoS One. 2011;6:1–10. doi: 10.1371/journal.pone.0022799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Scheck L, Olive FF. The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Front Psychiatry. 2013;4:1–9. doi: 10.3389/fpsyt.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Tomek SE, et al. Early life stress and chronic variable stress in adulthood interact to influence methamphetamine self-administration in male rats. Behav Pharmacol. 2016;27:182–184. doi: 10.1097/FBP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Marshall AT, Liu AT, Murphy NP, Maidment NT, Ostlund SB. Sex-specific enhancement of palatability-driven feeding in adolescent rats. Plos One. 2017;12(7):e0180907. doi: 10.1371/journal.pone.0180907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews IZ, Kelly H, McCormick CM. Low doses of amphetamine lead to immediate and lasting locomotor sensitization in adolescent, not adult, male rats. Pharmacol Biochem Behav. 2011;97:640–646. doi: 10.1016/j.pbb.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, McCormick CM. Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Behav Pharmacol. 2007;18:641–650. doi: 10.1097/FBP.0b013e3282effbf5. [DOI] [PubMed] [Google Scholar]
- Mayer-Blackwell B, Schlussman SD, Butelman ER, Ho A, Ott J, Kreek MJ, Zhang Y. Self administration of oxycodone by adolescent and adult mice affects striatal neurotransmitter receptor gene expression. Neuroscience. 2014;258:280–291. doi: 10.1016/j.neuroscience.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy LE, Mannelli P, Niculescu M, et al. The distribution of cocaine in mice differs by age and strain. Neurotoxicol Teratol. 2004;26:839–848. doi: 10.1016/j.ntt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, et al. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Myal S, O'Donnell P, Counotte DS. Nucleus accumbens injections of the mGluR2/3 agonist LY379268 increase cue-induced sucrose seeking following adult, but not adolescent sucrose self-administration. Neuroscience. 2015;305:309–15. doi: 10.1016/j.neuroscience.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacology Biochemistry and Behavior. 2013;110:112–116. doi: 10.1016/j.pbb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Jagannathan L, Becker JB. The Roles of Dopamine and α1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology. 2015;40(12):2696–2704. doi: 10.1038/npp.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza NJ, Vrbka JL, Yeager RD. Telescoping of alcoholism in women alcoholics. International Journal of the Addictions. 1989;24(1):19–28. doi: 10.3109/10826088909047272. [DOI] [PubMed] [Google Scholar]
- Raap DK, Morin B, Medici CN, Smith RF. Adolescent cocaine and injection stress effects on the estrous cycle. Physiol Behav. 2000;70:417–424. doi: 10.1016/s0031-9384(00)00287-0. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: A stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schassburger RL, Pitzer EM, Smith TT, et al. Adolescent rats self-administer less nicotine than adults at low doses. Nicotine Tob Res epub ahead of print. 2016 doi: 10.1093/ntr/ntw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, et al. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: Age-Dependent Behavior and Psychopharmacological Responsivity in Rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O'Brien L, et al. Age Differences in Future Orientation and Delay Discounting. Child Dev. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A behavioral scientist looks at the science of adolescent brain development. Brain and Cognition. 2010;72(1):160–4. doi: 10.1016/j.bandc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang NM, Pollak SD. Developmental continuity in reward-related enhancement of cognitive control. Dev Cogn Neurosci. 2014;10:34–43. doi: 10.1016/j.dcn.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Peters S, Braams BR, Crone EA. What motivates adolescents? Neural responses to rewards and their influence on adolescents' risk taking, learning, and cognitive control. Neurosci Biobehav Rev. 2016;70:135–147. doi: 10.1016/j.neubiorev.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Rowley SD, et al. Vesicular Monoamine Transporter-2 Function and Their Implications for Methamphetamine. Pharmacology. 2009;63:147–151. doi: 10.1002/syn.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek C, Perry AN, Becker JB. Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: Taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav. 2009;92:566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. Saccharin preferences of hamsters, gerbils and rats in the “two-food” situation. Behavioural Processes. 1985;10:87–99. doi: 10.1016/0376-6357(85)90120-2. [DOI] [PubMed] [Google Scholar]
- Wong WC, Ford KA, Pagels NE, et al. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33:4913–22. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WC, Marinelli M. Adolescent-onset of cocaine use is associated with heightened stress-induced reinstatement of cocaine seeking. Addict Biol. 2015;21:634–645. doi: 10.1111/adb.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin differentially affects sucrose taking and seeking in male and female rats. Behav Brain Res. 2015;283:184–190. doi: 10.1016/j.bbr.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]