Abstract
Many studies suggest sex differences in memory and hippocampal size, and that hormone therapy (HT) may positively affect these measures in women; however, the parameters of HT use that most likely confer benefits are debated. We evaluated the impact of sex and post-menopausal HT use on verbal learning and memory and hippocampal size in 94 cognitively intact women and 49 men. Using ANCOVAs that controlled for age and education, Women had better total word learning and delayed verbal memory performance than men. HT analyses showed that non-HT users performed similarly to men while HT users performed better than men in delayed memory regardless of whether use was current or in the past. Women had larger hippocampal volumes than men regardless of whether they were HT users. Using univariate linear models we assessed group differences in the predictive value of hippocampal volumes for verbal learning and memory. Hippocampal size significantly predicted memory performance for men and non-HT users, but not for HT users. This lack of relationship between hippocampal size and verbal learning and memory performance in HT users suggests HT use may impact memory through extra-hippocampal neural systems.
Keywords: Aging, Sex, Hormone Therapy, Hippocampal Volume, Cognition
Introduction
Ways to maintain maximum memory functioning as one ages is of great importance to the increasingly aging populations of developed countries. Many factors contribute to the effects of normal aging on memory function, two of which are gender/sex and hormone therapy (HT) use. Women tend to perform better than men on verbal learning and memory paradigms across the lifespan (Caselli et al., 2015; Herlitz, Nilsson, & Backman, 1997; Ystad et al., 2009). In healthy post-menopausal women, HT can prevent (Phillips & Sherwin, 1992; Sherwin, 1998) or diminish (Campbell & Whitehead, 1977; Duka, Tasker, & McGowan, 2000; Joffe et al., 2006; Linzmayer et al., 2001; Maki, Zonderman, & Resnick, 2001; Wolf et al., 1999) verbal memory decline. The hippocampus is critical for the consolidation of episodic memory (Burgess (Burgess, Maguire, & O'Keefe, 2002; Garcia-Lazaro, Ramirez-Carmona, Lara-Romero, & Roldan-Valadez, 2012), and hippocampal size can predict normal age-related memory changes (Chen, Chuah, Sim, & Chee, 2010; Golomb et al., 1993; Hackert et al., 2002; Ystad et al., 2009). There is evidence that hippocampal size in aging populations differs by sex and is affected by HT use, although findings are inconsistent. Some studies have found age-related sex differences in hippocampal size favoring women (Ystad et al., 2009; Zhang et al., 2010), while others have not (Lemaitre et al., 2005; Thomann et al., 2013). Further, cross-sectional studies have found a sharper age-related decline in hippocampal size in men (Li et al., 2014; Pruessner, Collins, Pruessner, & Evans, 2001; Raz, Gunning-Dixon, et al., 2004). Sex differences in memory and regional brain change vulnerability (e.g. hippocampal shrinkage) may be important factors to be taken into account as men and women aim to maximize memory function throughout the lifespan.
Elucidating true sex differences in aging on hippocampal size is complicated by the use of post-menopausal HT. Eberling and colleagues (2003) reported no sex differences in hippocampal volume when HT use in women was not accounted for, but larger hippocampi in women taking HT as compared to men or women not taking HT. These findings were expanded on by Lord et al. (2008) who found larger hippocampi in women currently taking HT compared to men, past HT users, or non-HT users. However, other reports found no difference in hippocampal size between HT users or non-users and men (Greenberg et al., 2006; Ryan et al., 2014). Further, few studies assessing sex and HT effects on hippocampal size have included cognitive measures, leaving the functional impact of effects on memory uncertain. Thus, the first aim of the present retrospective study was to determine if HT use impacts sex differences in both hippocampal size and verbal learning and memory performance in older adults. We predicted only women with a history of HT use would have larger hippocampal volumes and superior verbal learning and memory performance than men. Additionally, we aimed to determine if HT use or sex effects impact the predictive value of hippocampal volumes for verbal learning and memory.
A second aim of the study was to determine if timing of HT use impacts whether or not benefits are observed for verbal learning and memory and hippocampal size. Multiple theories have emerged to reconcile the incongruent HT findings between positive effects in observational studies and negative effects in clinical trials; most notably the unexpected detrimental findings of the large, placebo-controlled, randomized Women’s Health Initiative Memory Study (WHIMS). Briefly, WHIMS participants were 65 years and older and HT assignment was associated with increased risk of dementia (Shumaker et al., 2003), decrements in verbal memory (Resnick et al., 2006), and smaller hippocampi, as compared to the placebo group (Resnick, Espeland, Jaramillo, et al., 2009). Regarding timing of use, HT treatment in the WHIMS began 15+ years post-menopause which may be related to the observed cognitive and brain detriments not found in previous observational studies. The “critical window” hypothesis suggests that HT initiation at or near the time of menopause is related to cognitive benefits, while delayed initiation is not beneficial and can even be detrimental (Acosta (Acosta, Hiroi, Camp, Talboom, & Bimonte-Nelson, 2013; Khoo et al., 2009; Maki, 2006; Sherwin, 2005). The “healthy cell bias of estrogen action” hypothesis suggests HT is beneficial if neurons are healthy, but detrimental if neurological health is compromised (Brinton (Brinton, 2005; Greendale, Derby, & Maki, 2011; Tierney et al., 2009). A method for evaluating timing in retrospective studies is to group users into “past” or “current” HT users, which follows from the notion that past HT use is more likely to only occur near the time of menopause, when neurons are younger and healthier, and can have prolonged positive effects on memory and brain structures. There is evidence that current versus past HT use differentially affects hippocampal size, but results have been mixed. While Boccardi and colleagues (2006), found past users to have the largest hippocampal volumes, Lord et al. (2008) found larger hippocampi in current users, and yet other groups have found no relationship between hippocampal size and current or past HT use (Low et al., 2006; Ryan et al., 2014). Thus, to date “past HT users” versus “current HT users” studies on hippocampal size are contradictory and rarely include cognitive evaluations or longitudinal follow-up. Replication of previous findings, delineation of the relationship between hippocampal size and cognition, and tracking longitudinal trajectories of cognitive and brain aging are all needed to better understand the impact of HT. With a sample of 94 postmenopausal women and 49 men, the current study evaluated the impact of current, past, and never use of HT on verbal learning and memory and hippocampal volumes, and compared findings to men. In a subset of participants, we examined longitudinal age-related hippocampal volume loss, as well as interactions with sex and HT use. Lastly, we investigated the predictive value of hippocampal size on verbal learning and memory performance and interactions across HT groups. We predicted past HT use would convey the largest benefit to post-menopausal women in terms of hippocampal volume and verbal learning and memory, as compared to women who had never used HT and men.
Methods
Participants
Participants were recruited from several institutions of the Arizona Alzheimer’s Consortium: Barrow Neurological Institute, Banner Sun Health Research Institute, and Mayo Clinic-Arizona. All participants provided written consent approved by the individual institutions’ Institutional Review Boards. We recruited cognitively normal participants from the longitudinal Alzheimer’s disease studies at the home institutions. Participants consisted of 49 men and 94 post-menopausal women. General eligibility requirements for study participants were: 1) Cognitively intact, with no clinical diagnosis of mild cognitive impairment or dementia from the study neurologist and a score of ≥25 on the Mini Mental State Exam (Folstein, Folstein, & McHugh, 1975); 2) No evidence of depression as measured by either the Geriatric Depression Scale (GDS; Yesavage et al., 1982) or Beck Depression Inventory-II (BDI; Beck, Steer, Ball, & Ranieri, 1996); and 3) For women, post-menopausal, as defined as one year or more since the final menstrual period. Detailed histories of self-reported post-menopausal HT exposure were gathered from female participants by the attending clinician of each institution. When possible, women were asked to bring in their prescriptions to confirm accuracy and reliability of self-report. Data concerning specific information about type of HT formulation (Current, n = 31; Past, n = 29), age at HT initiation, HT initiation delay with regard to menopause, and hysterectomy status was not recorded unless participants were confident in the accuracy, preferably with documentation when possible (Current, n = 22; Past, n = 25; Never, n = 12; Table 1). Women were divided into three groups based on HT exposure: Current (n = 32), Past (n = 41), and Never (n = 21) users. Based on effect sizes (approx. d=1.1) in Lord et al. (2008) we had sufficient power (β=0.80) to detect to detect a significant (α=0.05) HT-related benefit for hippocampal volume with respect to Never Users and Men. Our hypothesis that Past HT Users would have larger hippocampal volumes than Current Users and Never Users was based on findings from Boccardi et al. (2006). Effect size for the hippocampus could not be computed from this report, as the author took a whole-brain statistical approach; however, our sample size for each group was larger, giving us confidence in our power to detect a significant effect. Finally, based on effect size (η2=0.12) in Carlson & Sherwin (1999), we had sufficient power (β=0.80) to detect significant (α=0.05) HT-related benefits for verbal memory as compared to Never Users and Men. Thirty-one participants (7 Current, 8 Past, 5 Never, and 11 Men) returned for longitudinal analysis an average of three years after their initial study visit. Genetic determination of Apolipoprotein E (APOE) allelic status, which is highly related to risk of late-onset Alzheimer’s disease (Burke & Roses, 1991) and memory decline, was performed in a subset of participants with the use of polymerase-chain-reaction assays (Hixson & Vernier, 1990).
Table 1.
Participant Demographic and Cognitive Scores
| Variable | Total (n = 143) Mean (S.D.) Range |
Current (n = 32) Mean (S.D.) Range |
Past (n = 41) Mean (S.D.) Range |
Never (n = 21) Mean (S.D.) Range |
Men (n = 49) Mean (S.D.) Range |
|---|---|---|---|---|---|
| Age | 71.4 (9.4) | 71.7 (10.5) | 70.0 (7.7) | 72.4 (11.7) | 70.0 (11.6) |
| 73–91 | 38–86 | 56–87 | 49–91 | 37–91 | |
| Median: 72 | Median: 72.5 | Median: 70 | Median: 73 | Median: 71 | |
|
| |||||
| Education | 14.9 (2.6) | 15.0 (2.3) | 14.9 (2.4) | 14.2 (2.5) | 15.3 (2.8) |
| 8–20 | 10–20 | 11–20 | 9–18 | 8–20 | |
|
| |||||
| E/E+P1 (# of users) | 38/22 | 20/11 | 18/11 | ||
|
| |||||
| Hysterectomy | 67% | 82% | 68% | 43% | |
|
| |||||
| Years since menopause | 28.2 (9.4) | 29.7 (9.8) | 30.0 (8.4) | 25.8 (11.1) | |
| 3–46 | 8–46 | 11–44 | 3–42 | ||
|
| |||||
| Age at HT initiation | 48.6 (8.8) | 48.9 (8.6) | 48.2 (9.1) | ||
| 30–75 | 32–75 | 30–69 | |||
|
| |||||
| HT initiation delay (years) | 3.5 (6.2) | 2.6 (6.0) | 4.3 (6.3) | ||
| 0–25 | 0–23 | 0–25 | |||
|
| |||||
| APOE E4+ (carrier/non-carrier) | 29/55 | 8/15 | 9/17 | 5/8 | 7/15 |
|
| |||||
| Total Words (z-score) | 0.48 (1.2) | 0.47 (0.98) | 0.28 (1.1) | −0.44 (1.2) | |
|
| |||||
| Delayed Memory (z-score) | 0.56 (1.2) | 0.58 (0.81) | 0.27 (1.3) | −0.14 (1.2) | |
estrogens/estrogens+progestogen
Cognitive Testing
Participants were administered either the California Verbal Learning Test (CVLT-II; Delis, Freeland, Kramer, & Kaplan, 1988) or the Rey Auditory Verbal Learning Test (RAVLT; Rey, 1964), which are both auditory learning and memory tasks consisting of a supra-span word list that is repeated 5 times for a total word recall learning (“Total Words”) measure as well as a free recall trial 20–30 minutes later to assess delayed memory (CVLT-II/RAVLT by group: Current: 19/13; Past: 13/28; Never: 12/9; Men: 15/36). The only difference between the two tests is the CVLT-II word list contains 16 words, while the AVLT contains 15 words. Memory performance for Total Words and Delayed Memory are presented as age-adjusted z-scores (Ivnik, 1990; Paolo, Troster, & Ryan, 1997) in order to pool data across these very similar tests.
Magnetic Resonance Image Acquisition
All participants underwent imaging on a 1.5- or 3- tesla GE scanner at Barrow Neurological Institute (1.5T/3T by group: Current: 13/19; Past: 7/34; Never: 9/12; Men: 11/40). Scanners had identical gradient sets (cardiac resonance modules with identical coefficients) in order to minimize image distortion and facilitate pooling data across magnet strength. Images of the whole brain were collected using standard axial SPGR (spoiled gradient) T1-weighted, 3-dimensional acquisition with in plane resolution = 0.9375 square mm voxels.
Volumetric Analyses
Hippocampal volumes were manually traced by three individuals who were blinded to sex, HT status, age, and other variables. Inter-rater reliability was assessed using a two-way mixed, average-measures, intra-class correlation (ICC; McGraw, 1996), resulting in the excellent range (Cicchetti, 1994) for both consistency (ICC = 0.99) and absolute agreement (ICC = 0.99). Hippocampal outlines were traced from the three-dimensional SPGR images using ANALYZE (version 7.5; Rochester, MN). Structures were first visualized in all three planes, landmarked in the sagittal plane, and drawn in the coronal plane. We employed the guidelines of Machulda et al. (2001) to define the hippocampal boundaries, including the hippocampal head and body. The anterior boundary was defined by observing the white matter band and/or the cerebrospinal fluid space between the amygdala and hippocampus in the sagittal plane (Machulda et al., 2001). The posterior aspect was initially landmarked in the sagittal plane by locating the posterior edge of the hippocampus and then confirming in the coronal plane that the fornices were completely visualized. Since total brain size can influence hippocampal size, we used a corrected value calculated as the percentage of total brain volume (HVC). This within-subject correction provided further confidence that magnet strength would not confound results. Total intracranial volume (TIV) was generated using the Voxel-Based Morphometry Toolbox in Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/).
Statistical Analyses
ANOVA was performed to assess sex and HT group differences on demographic variables (age, education, years since menopause, age at HT initiation, and HT initiation delay). Although groups were well-matched for age and education, because these factors can affect verbal learning and memory and hippocampal size, they were added as covariates in the ANCOVA assessing sex and HT effects on cognitive assessment (Total Words, Delayed Memory) and volumetric data (left hippocampus, right hippocampus, and TIV). In the presence of a significant omnibus ANCOVA (p<0.05), post-hoc analyses with were conducted. Significant group differences were interpreted as p<0.05 after Bonferroni correction for multiple comparisons. We tested for possible sex and HT group by instrument interactions using a two-way ANOVA and confirmed group effects in each instrument separately using ANCOVA. Repeated measures ANCOVA with age, education, and time between visits as covariates, was used to asses longitudinal hippocampal volume loss. Univariate linear models were used to assess the interactions between our categorical (group) and continuous (hippocampal volumes) predictors for continuous outcomes (Total Words and Delayed Memory). Univariate tests were followed with post-hoc contrasts and Pearson’s r correlations, in order to assess direction of relationships.
Results
Demographics
The age range of participants was 49–91 years. There were no sex differences in age, t(141)=0.13, MSE=88.74, p=0.89, or years of education, t(141)=1.11, MSE=6.57, p=0.27, nor did HT groups differ on these variables, age: F(3, 139)=0.64, MSE=88.80, p=0.59; years of education: F(3, 139)=0.79, MSE=6.61, p=0.50. Participants were mostly Caucasian (80%) or Latino (10%). Type of HT formulation used was available for 82% of participants (Table 1). The most common medications were Premarin (conjugated equine estrogens; CEE; HT Groups: Current: 48%; Past: 41%) and Prempro (CEE+medroxyprogesterone acetate (MPA); HT Groups: Current: 10%; Past: 10%). Hysterectomy status, age at HT initiation, and HT initiation delay with regard to menopause information was available for 65% of women. Of this subsample, 67% (n=41) reported that they had undergone a hysterectomy (Current: 82% (n=18); Past: 68% (n=17); Never: 43% (n=6); Table 1). The high rates of hysterectomy are consistent with the high rates of estrogen-only HT use. HT groups did not differ on the number of years since menopause, F(2, 56)=0.65, MSE=90.31, p=0.53, age at menopause, F(2, 56)=1.03, MSE=54.5, p=0.36, age at HT initiation, t(45)=0.22, MSE=78.63, p=0.82, or delay of HT initiation with regard to menopause, t(45)=0.91, MSE=38.24, p=0.37 (Table 1). APOE genotype was available for a subsample of the participants (57%). Presence of an ε4 allele, the allele related to increased risk of late-onset Alzheimer’s disease, was distributed equally among the groups, suggesting that the dependent measures were not influenced by uneven distribution of APOE genotype (Table 1).
Cognitive Measures
Sex Differences
We observed the expected sex difference for verbal learning and memory, with Women performing better than Men on Total Words, F(3, 139)=22.1, MSE=1.23, p<0.001, and Delayed Memory, F(3, 139)=12.1, MSE=1.23, p=0.001.
HT Use
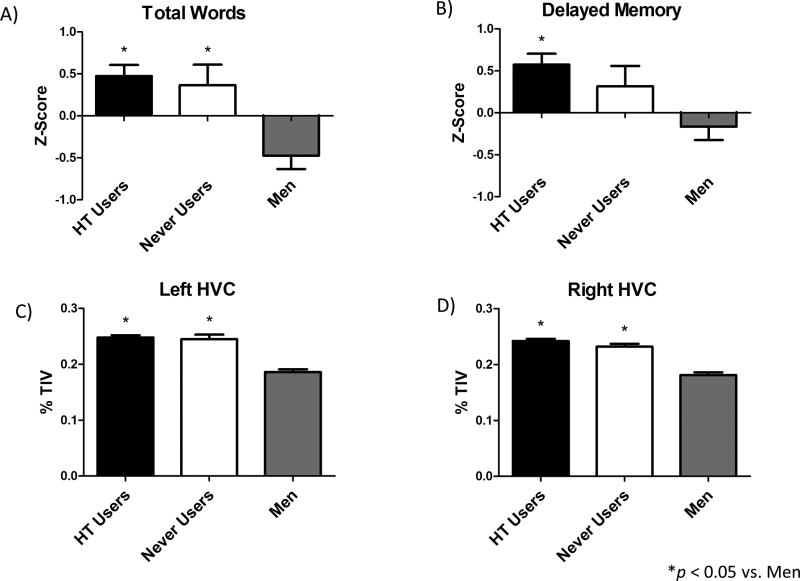
To address the question of whether use of post-menopausal HT at any time impacted these sex differences, Women were grouped as HT Users (Current and Past) or Never Users. There was a significant omnibus ANCOVA for Total Words, F(4, 138)=11.04, MSE=1.24, p < 0.001, and Delayed Memory, F(4, 138)=6.47, MSE=1.24, p=0.002. Post-hoc analyses revealed that while Users performed better than Men on Total Words, p<0.001 (Fig. 1A), and Delayed Memory, p<0.001 (Fig. 1B), Never Users only performed better than Men on Total Words, p=0.005 (Fig. 1A), but not Delayed Memory, p=0.10, (Fig. 1B). Users and Never Users did not differ on either measure (Fig. 1A&B).
Figure 1.
Age and education adjusted mean z-scores (+SE) for (A) total words and (B) delayed memory. Age and education adjusted mean %TIV for (C) left and (D) right hippocampal volumes. HT Users (n = 73), Never Users (n = 21), Men (n=49). *p < 0.05 vs. Men
HT Timing
To address the question of whether timing of post-menopausal HT impacted sex differences, we further categorized Women based on Current or Past Use. The omnibus ANCOVAs remained significant when including Men and Never users for both Total Words, F(5, 137)=7.31, MSE=1.25, p<0.001, and Delayed Memory, F(5, 137)=4.29, MSE=1.25, p=0.006. However, timing of HT use did not seem to impact the verbal learning or memory sex difference, as both Current and Past Users performed better than Men on Total Words and Delayed Memory, all p<0.006. Current, Past, and Never Users did not differ on either measure.
Type of Verbal Learning and Memory Test
There were no group by type of verbal learning test (AVLT vs. CVLT) interactions for any analysis, all p>0.45. Furthermore, when sex/HT effects were investigated in each verbal learning test separately, findings were consistent with pooled data. Women performed better than Men on both tests for Total Words, AVLT: F(3, 80)=12.16, MSE=1.16, p=0.001; CVLT: F(3, 55)=11.22, MSE=1.28, p=0.001, and Delayed Memory, AVLT: F(3, 80)=5.79, MSE=0.99, p=0.018; CVLT: F(3, 55)=7.88, MSE=1.45, p=0.007. HT Users and Never Users performed better than Men for Total Words, AVLT: HT Users vs. Men, p=0.002; Never Users vs. Men, p=0.033; CVLT: HT Users vs. Men, p=0.001; Never Users vs. Men, p=0.03, but only HT Users performed better than Men for Delayed Memory, AVLT: HT Users vs. Men, p=0.027; Never Users vs. Men, p=0.14; CVLT: HT Users vs. Men, p=0.003; Never Users vs. Men, p=0.17. HT Users and Never Users did not differ on either measure for either test. These results collectively suggest type of verbal learning test administered did not affect the group outcomes observed, however, Bonferroni adjustments were not carried out for separate verbal learning tests as these were confirmatory analyses.
Hippocampal volume
Sex differences
There was a sex difference for hippocampal volume-corrected (HVC), with Women having larger HVCs bilaterally, left: F(3, 139)=94.4, MSE=0.001, p<0.001; right: F(3, 139)=108.9, MSE=0.001, p<0.001.
HT use
When Women were grouped as HT Users or Never Users, there was a significant omnibus ANCOVA for each HVC when including Men and Never Users, left: F(4, 138)=47.0, MSE=0.001, p<0.001; right: F(4, 138)=55.36, MSE=0.001, p<0 .001. However, post hoc analyses revealed that HT Use did not impact HVC, as both Users and Never Users had larger bilateral HVC than Men, all p<0.001 (Fig. 1C&D), and did not differ from each other.
Timing of HT
The omnibus ANCOVAs for timing (Current or Past) remained significant for each analysis when including Men and Never Users, left: F(5, 137)=31.3, MSE=0.001, p<0.001; right: F(5, 137)=36.7, MSE=0.001, p<0 .001. However, timing of HT use did not impact HVC, as both Current and Past Users were larger than Men, all p<0.001, and Current, Past, and Never Users did not differ.
TIV and raw hippocampal volumes
Group differences in HVC were observed in the presence of a significant sex difference for TIV, a measure of global brain size, favoring Men, F(3, 139)=78.0, MSE=11929.4, p<0.001. Sex differences in HVC were, however, not merely an effect of larger TIV in the Men, as Women had larger raw left and right HVs, left: F(3, 139)=27.5, MSE=254114.0, p<0.001; right: F(3, 139)=31.6, MSE=200165.9, p<0.001, even when further categorized by HT use or timing, all p<0.05. Interestingly, Past Users had larger raw right HVs than Never Users, p=0.04, however, Bonferroni adjustments were not carried out for raw hippocampal volumes as these were confirmatory analyses.
Strength of MRI Magnet
There were no group by MRI scanner (1.5T vs. 3T) interactions for any volumetric measure, all p>0.18. Furthermore, when sex/HT effects were investigated in each MRI scanner separately, findings were consistent with pooled data. Women had larger HVC than Men on both MRI scanners, left: 1.5T: F(3, 36)=46.07, MSE=0.001, p<0.001; 3T: F(3, 99)=66.17, MSE=0.001, p<0.001; right: 1.5T: F(3, 36)=32.58, MSE=0.001, p<0.001; 3T: F(3, 99)=81.44, MSE=0.001, p<0.001. HT Users and Never Users separately also had larger HVC than Men for both scanners, left: 1.5T: HT Users vs. Men, p<0.001; Never Users vs. Men, p<0.001; 3T: HT Users vs. Men, p<0.001; Never Users vs. Men, p<0.001; right: 1.5T: HT Users vs. Men, p<0.001; Never Users vs. Men, p=0.001; 3T: HT Users vs. Men, p<0.001; Never Users vs. Men, p<0.001. HT Users and Never Users did not differ on HVC for either MRI scanner. All results survived Bonferroni adjustments and collectively suggest that strength of the magnet did not affect group outcomes observed.
Longitudinal and Cross-Sectional Age-related Hippocampal Volume Differences
A subset of 31 participants (7 Current, 8 Past, 5 Never, and 11 Men) returned for longitudinal analysis an average of 3 years after their initial study visit. For the Women, all participants maintained their HT status during this time. HVC was assessed across time with the time between visits, age, and education as covariates. Hippocampi were significantly smaller at the follow-up visit, left: p<0.001; right: p<0.001, but there were no significant interactions with sex, left: F(1, 26)=0.80, MSE=0.00, p=0.38 (Supp. Fig. 1A); right: F(1, 26)=3.25, MSE=0.00, p=0.08 (Supp. Fig. 1A & B), HT Use, left: F(1, 25)=1.15, MSE=18928.4, p=0.33; right: F(1, 25)=1.56, MSE=75095.5, p=0.23, or timing, left: F(1, 24)=0.85, MSE=19470.6, p=0.48; right: F(1, 24)=2.43, MSE=0.00, p=0.09.
In cross-sectional analyses including all participants, age accounted for a significant amount of the variance in left, F(1, 140)=11.0, MSE=0.001, p=0.001, and right, F(1, 140)=24.35, MSE=0.001, p<0.001, HVCs but did not interact with sex, left: F(1, 140)=0.3, MSE=0.001, p=0.58; right: F(1, 140)=0.08, MSE= 0.001, p=0.78, HT Use, left: F(1, 138)=1.71, MSE=0.001, p=0.19; right: F(1, 138)=1.37, MSE= 0.001, p=0.26, or timing, left: F(1, 136)=1.32, MSE=0.001, p=0.27; right: F(1, 136)=1.06, MSE= 0.001, p=0.37.
Hippocampal Volumes as Cognitive Predictors: Sex and HT Interactions
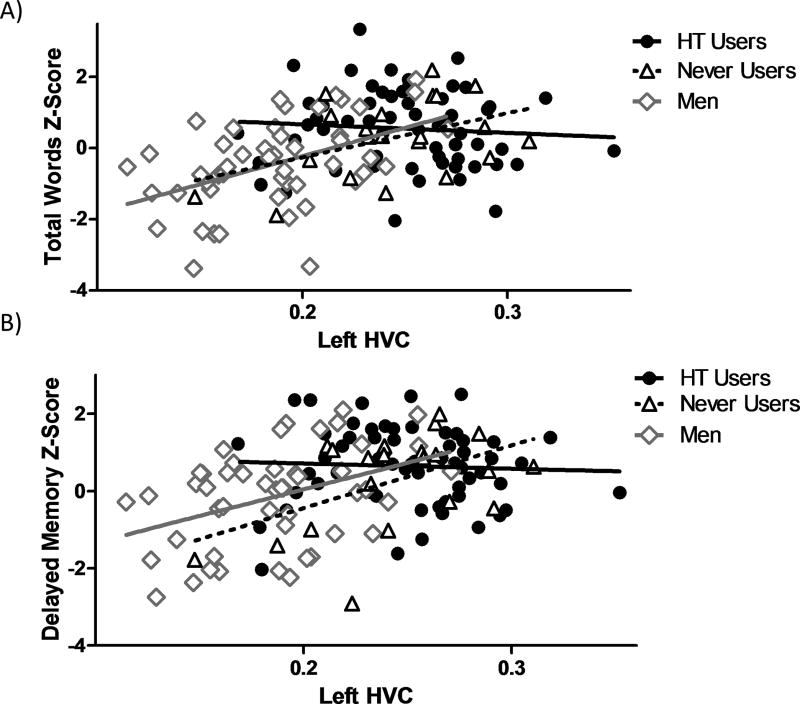
As expected, univariate linear models revealed a significant influence of hippocampal size on verbal memory performance for both Total Words, left: F(1, 139)=6.87, MSE=1.21, p=0.01; right: F(1, 139)=15.11, MSE=1.18, p<0.001, and Delayed Memory, left: F(1, 140)=8.54, MSE=1.17, p=0.004; right: F(1, 139)=14.15, MSE=1.15, p<0.001, where larger hippocampal size was related to better verbal learning and memory. The model indicated that for the left HVC, this relationship differed by sex, Total Words: F(1, 139)=6.86, MSE=1.21, p=0.01, and between Men and HT groups, Total Words: F(2, 137)=5.4, MSE=1.19, p=0.006; Delayed Memory: F(2, 137)=4.41, MSE=1.19, p=0.014. Post-hoc contrasts revealed that for both measures the relationship with left HVC differed between Users and Men, Total Words: F(1, 137)=9.78, MSE=1.19, p=0.002; Delayed Memory: F(1, 137)=6.33, MSE=1.14, p=0.013. The relationship between Delayed Memory and left HVC also differed between Users and Never Users, F(1, 137)=5.21, MSE=1.14, p=0.024. Indeed, Pearson’s r correlations demonstrated that for Men and Never Users, there was a significant positive relationship between left HVC and Total Words, Men: r(47)=0.46; p<0.001; Never Users: r(19)=0.43; p=0.049 (Fig. 2A), and left HVC and Delayed Memory, Men: r(47)=0.40; p=0.004; Never Users: r(19)=0.49; p=0.023 (Fig. 2B). For HT Users, however, there was not a significant correlation between left HVC and Total Words, r(74)=0.05; p=0.62, or left HVC and Delayed Memory, r(74)=0.06; p=0.68 (Fig. 2A&B). Timing of HT use did not affect this relationship as neither Current nor Past Users had left HVCs that significantly correlated with verbal learning or memory performance, all p>0.53.
Figure 2.
Intergroup correlations between (A) left HVC and total words z-score, and (B) left HVC and delayed memory z-score, for Men, HT Users, and Never Users.
Discussion
In a community-based sample of 143 older adults, controlling for age and education, the present study replicated previous findings that women perform better on verbal learning and memory tasks and have larger bilateral hippocampi than men (Caselli et al., 2015; Herlitz et al., 1997; Ystad et al., 2009; Zhang et al., 2010). We did not find support for beneficial effects of post-menopausal HT use regardless of timing (current or past) on verbal learning and memory, with respect to women who had never used HT, as seen in other reports (Campbell & Whitehead, 1977; Carlson & Sherwin, 1999; Duka et al., 2000; Joffe et al., 2006; Linzmayer et al., 2001; Maki et al., 2001; Phillips & Sherwin, 1992; Wolf et al., 1999). Recently, the Cognitive and Affective Study of the Kronos Early Estrogen Prevention Study (KEEPS-Cog) also aimed to address the "critical window" and “healthy cell bias of estrogen action” hypotheses by initiating HT in younger, more recently post-menopausal women (Gleason et al., 2015). In line with our findings, KEEPS-Cog found no impact of HT on cognitive change, as compared to placebo (Gleason et al., 2015). HT use in our participants did, however, impact the female advantage for delayed verbal memory. Only women who had used HT (current or past) performed better than men on delayed memory, while women who never used HT performed similarly to men. Few investigations have compared verbal memory in women on and off post-menopausal HT to men, and the present findings suggest that while HT may not elevate verbal memory abilities above women who did not take HT, it plays a role in maintaining the female verbal memory advantage compared to men. This may be important to women as they aim to maximize memory function throughout the lifespan.
The female advantage for hippocampal size was, however, independent of HT use and timing; even women who never used HT had larger hippocampi than men, and HT users did not differ from non-users. Notably, the previous investigations which found benefits of HT use on hippocampal size were studies with the smallest sample sizes (Boccardi et al., 2006; Eberling et al., 2003; Lord et al., 2008), while the largest studies to date also found a lack of support for HT benefits (Low et al., 2006; Ryan et al., 2014). Organizational effects of ovarian hormones on the female brain may explain why women have larger hippocampi than men, independent of, or interacting with, post-menopausal HT use. Satterthwaite and colleagues (2014) found females had larger hippocampi post- but not pre- puberty, even when controlling for age. Thus, pubertal ovarian hormones may organize the brain to enlarge female hippocampi, relative to the size of male hippocampi, such that differences in size are evident between the sexes across the lifespan (see Koebele & Bimonte-Nelson, 2015 for further discussion).
Age-Related Hippocampal Volume Differences
Despite strong sex differences in verbal learning and memory performance and hippocampal size, we found no sex differences on longitudinal age-related hippocampal volume loss or cross-sectional age-related hippocampal volume differences. Longitudinal findings should be interpreted with caution as high drop-out rate led to reduced power. Our cross-sectional findings, however, are in line with others who failed to find sex differences in cross-sectional age-related volumetric differences when participants were middle-age and older (Lemaitre et al., 2005; Thomann et al., 2013). Other studies including young-adults, report greater age-related volumetric differences in men (Li et al., 2014; Pruessner et al., 2001; Raz, Gunning-Dixon, et al., 2004). Taken together, sex differences in age-related slopes of hippocampal volumes may be restricted to young-adulthood and no longer evident in middle age and elderly adults. The mechanisms by which the sex differences in age-related slopes change as people grow older warrants further investigation and may involve menopause. Further, we found no evidence that post-menopausal HT use impacts longitudinal age-related hippocampal volume loss or age-related volumetric differences in cross-sectional analysis. This finding is in alignment with the WHIMS and other longitudinal reports of hippocampal volume changes which found no differences in rates of decline between groups previously assigned to HT or placebo conditions (Coker et al., 2014; Raz, Rodrigue, Kennedy, & Acker, 2004).
Hippocampal Volumes as Cognitive Predictors
The predictive value of left hippocampal size on verbal learning and memory performance differed by sex and HT use. Hippocampal size was a valuable predictor for performance in women who never used HT and men. However, regardless of timing, HT use obfuscated the relationship between hippocampal volume and verbal learning and memory. This lack of correlation is not merely lack of power, as significant correlations were detected in never users which represented the smallest group. These findings suggest HT affects extra-hippocampal regions, such as the prefrontal cortex, that have been shown to be involved in memory performance (Simons & Spiers, 2003). Functional neuroimaging research provides some support for this notion. In multiple reports, estrogen administration, with or without a progestin, induced changes in fMRI brain activation patterns in the frontal lobe and other brain regions during the performance of memory tasks (Dumas (Dumas, Kutz, Naylor, Johnson, & Newhouse, 2010; Joffe et al., 2006; Persad et al., 2009; Shaywitz et al., 1999; Y. R. Smith et al., 2006). Using PET imaging, the Baltimore Aging Study found that women using HT showed increased regional cerebral blood flow over time in regions important for memory processing, such as the frontal cortex, entorhinal cortex, and hippocampus (Resnick & Maki, 2001). Our data add to the notion that HT actions on the brain which are related to learning and memory likely involve regions other than or in addition to the hippocampus. Future research is warranted, as delineating HT-induced regional and mechanistic brain effects is necessary to optimize memory function in women through the lifespan.
Other HT and Menopause Considerations
Type of HT formulation
Type of HT formulation (combination estrogens+progestogen vs. estrogen-only) has also emerged as a consideration to reconcile disparate findings of HT effects on cognition and brain. Women who have a uterus must include a progestogen in their HT regimen to protect against endometrial hyperplasia (Smith, Prentice, Thompson, & Herrmann, 1975). Collectively, the WHIMS combination HT trial reported statistically significant detrimental effects of HT while the estrogen-only trial reported marginally significant detriments (Resnick, Espeland, An, et al., 2009; Resnick, Espeland, Jaramillo, et al., 2009; Resnick et al., 2006; Shumaker et al., 2004; Shumaker et al., 2003). Further, in a more recent review of randomized clinical trials, Maki & Sundermann (2009) conclude beneficial effects of HT are more commonly associated with estrogen-only HT and detrimental effects are more commonly seen after combination HT, specifically the combination HT formulation used in the WHIMS. Type of HT formulation, based on self-report, was available in 82% of participants in the present study (estrogen-only HT n=38; combination HT n=22). When verbal learning and memory and hippocampal volumes were compared between women based on type of HT formulation, we found no differences between estrogen-only HT, combination HT, and no HT. Type of HT formulation did, however, impact the female advantage for delayed verbal memory. Only women who used estrogen-only HT performed better than men on delayed memory (p<0.001) while women who used combination HT performed similarly to and men (p=0.10). Type of HT did not affect hippocampal size. Our finding that estrogen-only HT was the most favorable for verbal memory is in alignment with the collective results of other studies (Maki & Sundermann, 2009), however, we did not find an advantage over women who did not use HT, but rather an advantage over men. This exploratory result should be interpreted with caution, as our sample size was reduced for women whom type of HT formulation was known, questioning the power to detect true effects. Nevertheless, we did not find HT-related cognitive detriments as seen in the WHIMS. The difference may be due to sample size, but may also be due to the fact that many participants in our study used combination formulations which contained progestogens other than MPA (the progestogen component of the WHIMS). Clinical and animal research suggests MPA is less favorable for cognition and brain health than other progestogens (Braden et al., 2010; Maki & Sundermann, 2009; Nilsen & Brinton, 2002). Our exploratory results add to the growing body of literature suggesting type of formulation may be a critical parameter to be considered, with regards to the cognitive effects of HT.
Type of menopause
When possible (65% of sample), we recorded type of menopause; surgical (hysterectomy) vs. natural. Of this subsample, women who had undergone hysterectomy were significantly younger at menopause. HT users were also more likely to have undergone surgical menopause than non-users (74% of users vs. 43% of non-users), presenting somewhat of a confound in interpreting the HT-related verbal memory benefit as compared to men. A possible confound lies in whether surgical menopause itself is beneficial to memory. In our participants, we did not find this to be the case, as type of menopause did not impact memory performance in women who never used HT. The literature would also suggest that if anything surgical menopause is more detrimental to memory than natural menopause (Nappi et al., 1999). Understanding the relation between type of menopause and HT effects on memory and brain is inherently confounded by type of HT formulation, as women who have a uterus must include a progestogen in their HT regimen (combination formulations). Animal studies, however, can help address this question; Acosta et al. (2010) found that CEE improved memory in ovariectomized rats but impaired memory in rats that underwent a transitional model of menopause. The impairment may in part be due to high levels of androstenedione released from the follicle deplete ovary (Acosta et al., 2009; Camp et al., 2012).This becomes an important consideration for retrospective self-report when women may be unclear about whether or not their hysterectomy included an oophorectomy. Understanding the role follicle deplete ovaries play in the effects of HT on cognition and brain is important for future prospective studies.
Limitations
Although there are many practical reasons why retrospective studies may be the most reasonable method to investigate long-term effects of HT use, there are some limitations that arise. First, HT information relied on self-report. This concern is somewhat mitigated as our primary questions surrounded the effects of HT use, past or current, as opposed to factors that rely on precise recollection (e.g. HT formulation, duration, timing with respect to menopause etc.). To this point, we were limited in other health related factors to be included in our analyses. Recent data suggests systemic health conditions such as diabetes can interact with HT effects on brain health (Espeland et al., 2015), further adding to the list of factors to be considered when investigating HT use. Second, our sample size was modest, especially for never users. Based on the literature, however, we had adequate power to detect HT-related verbal memory and hippocampal volume effects. In order to achieve adequate power, data were also somewhat heterogeneous for methodology (type of memory and scanning instruments) and type of menopause. In an effort to address heterogeneity of methodology we demonstrated similar effects of group when analyses were separated by memory and scanning instruments, albeit Bonferroni alpha adjustment was not made for these confirmatory analyses. Further, there were no statistically significant interactions between groups and instruments, making it unlikely that the heterogeneity of instruments played a role in the pooled findings. Third, sample size was further reduced for longitudinal hippocampal volume change due to high drop-out and for genetic determination of APOE allelic status. We may have been underpowered to detect longitudinal HT effects on hippocampal volumes were they present, although it is difficult to speculate about necessary power, as HT-related effects for longitudinal change have not been demonstrated (Coker et al., 2014; Raz, Rodrigue, et al., 2004).Within the subset of participants for which APOE allelic status was available (57%), presence of an ε4 allele, the allele related to increased risk of late-onset Alzheimer’s disease, was distributed equally among the groups. However, APOE cannot be ruled out with certainty as playing a role in observed memory and brain effects.
Conclusion
In conclusion, our findings shed light on the complexities of how sex and post-menopausal HT use contribute to normal cognitive and brain aging. Overall, results add to the growing body of literature which suggests HT may be safe for verbal learning and memory and hippocampal size. HT use may confer some benefit for the female verbal memory advantage. But, while post-menopausal women have larger hippocampi than age-matched men, size is not impacted by HT use. Finally, a failure to find a relationship between hippocampal size and verbal learning and memory performance in women with a history of HT use suggests HT use may be impacting verbal memory in other ways. While understanding the impact of post-menopausal HT use on brain and cognition has indeed proven to be complicated, the importance of this understanding is not reduced. From basic science to clinical investigations, findings suggest that under optimal parameters, post-menopausal HT use can contribute to maintaining maximum cognitive functioning with age. In an increasingly aging population, understanding these parameters is essential, and future prospective studies aimed to optimize HT use for cognition and brain are warranted.
Supplementary Material
Supplemental Figure 1. Mean %TIV for (A) left and (B) right hippocampal volumes across two longitudinal time points, adjusted for age, education, and time between visits.
Acknowledgments
Funding: This work was supported by the National Institute on Aging under Grant P30-AG19610 and the State of Arizona (the Arizona Alzheimer’s Consortium)
Footnotes
Disclosures: None.
References
- Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. An update on the cognitive impact of clinically-used hormone therapies in the female rat: Models, mazes, and mechanisms. Brain research. 2013 doi: 10.1016/j.brainres.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009 doi: 10.1210/en.2008-1802. doi: en.2008-1802 [pii]10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151(8):3795–3804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Ghidoni R, Govoni S, Testa C, Benussi L, Bonetti M, Frisoni GB. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a Voxel-based morphometry study. Menopause. 2006;13(4):584–591. doi: 10.1097/01.gme.0000196811.88505.10. [DOI] [PubMed] [Google Scholar]
- Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93(3):444–453. doi: 10.1016/j.nlm.2010.01.002. doi: S1074-7427(10)00003-1 [pii]10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Annals of the New York Academy of Sciences. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burke JR, Roses AD. Genetics of Alzheimer's disease. International journal of neurology. 1991;25–26:41–51. [PubMed] [Google Scholar]
- Camp BW, Gerson JE, Tsang CW, Villa SR, Acosta JI, Blair Braden B, Bimonte-Nelson HA. High serum androstenedione levels correlate with impaired memory in the surgically menopausal rat: a replication and new findings. The European journal of neuroscience. 2012;36(8):3086–3095. doi: 10.1111/j.1460-9568.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Whitehead M. Oestrogens for menopausal flushing. Br Med J. 1977;1(6053):104–105. doi: 10.1136/bmj.1.6053.104-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB. Relationships among cortisol (CRT), dehydroepiandrosterone-sulfate (DHEAS), and memory in a longitudinal study of healthy elderly men and women. Neurobiology of Aging. 1999;20(3):315–324. doi: 10.1016/s0197-4580(99)00052-4. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DE, Baxter LC, Woodruff BK, Geda YE. Sex-based memory advantages and cognitive aging: a challenge to the cognitive reserve construct? Journal of the International Neuropsychological Society : JINS. 2015;21(2):95–104. doi: 10.1017/S1355617715000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Chuah LY, Sim SK, Chee MW. Hippocampal region-specific contributions to memory performance in normal elderly. Brain and cognition. 2010;72(3):400–407. doi: 10.1016/j.bandc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychological Assessment. 1994;6(4):284–290. [Google Scholar]
- Coker LH, Espeland MA, Hogan PE, Resnick SM, Bryan RN, Robinson JG, Shumaker SA. Change in brain and lesion volumes after CEE therapies: the WHIMS-MRI studies. Neurology. 2014;82(5):427–434. doi: 10.1212/WNL.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56(1):123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology (Berl) 2000;149(2):129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Hormones and behavior. 2010;58(5):929–935. doi: 10.1016/j.yhbeh.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Wu C, Haan MN, Mungas D, Buonocore M, Jagust WJ. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiol Aging. 2003;24(5):725–732. doi: 10.1016/s0197-4580(02)00056-8. doi: S0197458002000568 [pii] [DOI] [PubMed] [Google Scholar]
- Espeland MA, Brinton RD, Manson JE, Yaffe K, Hugenschmidt C, Vaughan L, Resnick SM. Postmenopausal hormone therapy, type 2 diabetes mellitus, and brain volumes. Neurology. 2015;85(13):1131–1138. doi: 10.1212/WNL.0000000000001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Lazaro HG, Ramirez-Carmona R, Lara-Romero R, Roldan-Valadez E. Neuroanatomy of episodic and semantic memory in humans: a brief review of neuroimaging studies. Neurology India. 2012;60(6):613–617. doi: 10.4103/0028-3886.105196. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Asthana S. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS medicine. 2015;12(6):e1001833. doi: 10.1371/journal.pmed.1001833. discussion e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH. Hippocampal atrophy in normal aging. An association with recent memory impairment. Archives of neurology. 1993;50(9):967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Payne ME, MacFall JR, Provenzale JM, Steffens DC, Krishnan RR. Differences in brain volumes among males and female hormone-therapy users and nonusers. Psychiatry Res. 2006;147(2–3):127–134. doi: 10.1016/j.pscychresns.2006.01.001. doi: S0925-4927(06)00012-6 [pii]10.1016/j.pscychresns.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Derby CA, Maki PM. Perimenopause and cognition. Obstetrics and gynecology clinics of North America. 2011;38(3):519–535. doi: 10.1016/j.ogc.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002;17(3):1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Nilsson LG, Backman L. Gender differences in episodic memory. Memory & cognition. 1997;25(6):801–811. doi: 10.3758/bf03211324. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of lipid research. 1990;31(3):545–548. [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. The Auditory Verbal Learning Test: Norms for ages 55 and older. Psychological Assessment. 1990;2:304–312. [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13(3):411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Khoo SK, O'Neill S, Byrne G, King R, Travers C, Tripcony L. Postmenopausal hormone therapy and cognition: effects of timing and treatment type. Climacteric. 2009 doi: 10.3109/13697130903370316. doi: 10.3109/13697130903370316 [pii]10.3109/13697130903370316. [DOI] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson HA. Trajectories and phenotypes with estrogen exposures across the lifespan: What does Goldilocks have to do with it? Hormones and behavior. 2015 doi: 10.1016/j.yhbeh.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26(3):900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Li W, van Tol MJ, Li M, Miao W, Jiao Y, Heinze HJ, Walter M. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Human brain mapping. 2014;35(1):238–247. doi: 10.1002/hbm.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzmayer L, Semlitsch HV, Saletu B, Bock G, Saletu-Zyhlarz G, Zoghlami A, Grunberger J. Double-blind, placebo-controlled psychometric studies on the effects of a combined estrogen-progestin regimen versus estrogen alone on performance, mood and personality of menopausal syndrome patients. Arzneimittelforschung. 2001;51(3):238–245. doi: 10.1055/s-0031-1300030. [DOI] [PubMed] [Google Scholar]
- Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol Aging. 2008;29(1):95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. doi: S0197-4580(06)00331-9 [pii]10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Low LF, Anstey KJ, Maller J, Kumar R, Wen W, Lux O, Sachdev P. Hormone replacement therapy, brain volumes and white matter in postmenopausal women aged 60–64 years. Neuroreport. 2006;17(1):101–104. doi: 10.1097/01.wnr.0000194385.10622.8e. doi: 00001756-200601230-00021 [pii] [DOI] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Cha R, O'Brien P, Jack CR., Jr Functional inferences vary with the method of analysis in fMRI. Neuroimage. 2001;14(5):1122–1127. doi: 10.1006/nimg.2001.0926. doi: 10.1006/nimg.2001.0926S1053-8119(01)90926-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138(3):1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. doi: S0306-4522(06)00042-X [pii]10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Maki PM, Sundermann E. Hormone therapy and cognitive function. Hum Reprod Update. 2009;15(6):667–681. doi: 10.1093/humupd/dmp022. doi: dmp022 [pii]10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158(2):227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming Inferences About Some Intraclass Correlation Coefficients. Psychological Assessment. 1996;1(1):30–46. [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47(1):29–36. doi: 10.1159/000010058. doi: goi47029 [pii] [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143(1):205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Paolo AM, Troster AI, Ryan JJ. California Verbal Learning Test: normative data for the elderly. Journal of clinical and experimental neuropsychology. 1997;19(2):220–234. doi: 10.1080/01688639708403853. [DOI] [PubMed] [Google Scholar]
- Persad CC, Zubieta JK, Love T, Wang H, Tkaczyk A, Smith YR. Enhanced neuroactivation during verbal memory processing in postmenopausal women receiving short-term hormone therapy. Fertil Steril. 2009;92(1):197–204. doi: 10.1016/j.fertnstert.2008.04.040. doi: S0015-0282(08)00955-2 [pii]10.1016/j.fertnstert.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17(5):485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(1):194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiology of Aging. 2004;25(3):377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Hormone replacement therapy and age-related brain shrinkage: regional effects. Neuroreport. 2004;15(16):2531–2534. doi: 10.1097/00001756-200411150-00020. doi: 00001756-200411150-00020 [pii] [DOI] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, An Y, Maki PM, Coker LH, Jackson R, Rapp SR. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab. 2009;94(11):4152–4161. doi: 10.1210/jc.2009-1340. doi: jc.2009-1340 [pii]10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72(2):135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. doi: 72/2/135 [pii]10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Annals of the New York Academy of Sciences. 2001;949:203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91(5):1802–1810. doi: 10.1210/jc.2005-2097. doi: jc.2005-2097 [pii]10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie. Paris: Presses Universitaries de France; 1964. [Google Scholar]
- Ryan J, Artero S, Carriere I, Scali J, Maller JJ, Meslin C, Ancelin ML. Brain volumes in late life: gender, hormone treatment, and estrogen receptor variants. Neurobiology of Aging. 2014;35(3):645–654. doi: 10.1016/j.neurobiolaging.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, Gur RC. Sex differences in the effect of puberty on hippocampal morphology. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(3):341–350. e341. doi: 10.1016/j.jaac.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Gore JC. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281(13):1197–1202. doi: 10.1001/jama.281.13.1197. doi: jci90007 [pii] [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Proc Soc Exp Biol Med. 1998;217(1):17–22. doi: 10.3181/00379727-217-44200. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 2005;47(3):371–375. doi: 10.1016/j.yhbeh.2004.12.002. doi: S0018-506X(04)00268-5 [pii]10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. doi: 10.1001/jama.291.24.2947291/24/2947 [pii] [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. doi: 10.1001/jama.289.20.2651289/20/2651 [pii] [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature reviews. Neuroscience. 2003;4(8):637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293(23):1164–1167. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. The Journal of clinical endocrinology and metabolism. 2006;91(11):4476–4481. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann PA, Wustenberg T, Nolte HM, Menzel PB, Wolf RC, Essig M, Schroder J. Hippocampal and entorhinal cortex volume decline in cognitively intact elderly. Psychiatry research. 2013;211(1):31–36. doi: 10.1016/j.pscychresns.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Oh P, Moineddin R, Greenblatt EM, Snow WG, Fisher RH, MacLusky NJ. A randomized double-blind trial of the effects of hormone therapy on delayed verbal recall in older women. Psychoneuroendocrinology. 2009;34(7):1065–1074. doi: 10.1016/j.psyneuen.2009.02.009. doi: S0306-4530(09)00060-2 [pii]10.1016/j.psyneuen.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Torber S, McEwen BS, Kirschbaum C. Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology. 1999;24(7):727–741. doi: 10.1016/s0306-4530(99)00025-6. doi: S0306-4530(99)00025-6 [pii] [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Ystad MA, Lundervold AJ, Wehling E, Espeseth T, Rootwelt H, Westlye LT, Lundervold A. Hippocampal volumes are important predictors for memory function in elderly women. BMC medical imaging. 2009;9:17. doi: 10.1186/1471-2342-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qiu C, Lindberg O, Bronge L, Aspelin P, Backman L, Wahlund LO. Acceleration of hippocampal atrophy in a non-demented elderly population: the SNAC-K study. International psychogeriatrics/IPA. 2010;22(1):14–25. doi: 10.1017/S1041610209991396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Mean %TIV for (A) left and (B) right hippocampal volumes across two longitudinal time points, adjusted for age, education, and time between visits.