Abstract
We have conducted a bibliometric review of drug repurposing by scanning >25 million papers in PubMed and using text-mining methods to gather, count and analyze chemical–disease therapeutic relationships. We find that >60% of the ~35 000 drugs or drug candidates identified in our study have been tried in more than one disease, including 189 drugs that have been tried in >300 diseases each. Whereas in the majority of cases these drugs were applied in therapeutic areas close to their original use, there have been striking, and perhaps instructive, successful attempts of drug repurposing for unexpected, novel therapeutic areas.
Keywords: Drug reprofiling, drug repurposing, bibliometrics, text-mining
Introduction
Drug repurposing (also known as repositioning, reprofiling, redirecting or rediscovering [1]) is defined as developing new uses for a drug beyond its original use or initial approved indication. Drug repurposing has attracted increasing attention in recent years as drug companies seek potentially inexpensive alternatives to compensate for the high costs and disappointing success rate associated with the drug discovery pipeline [2]. Repurposing can help identify new therapies for diseases at lower cost and in a shorter time, particularly in those cases where preclinical safety studies have already been completed. During recent years, several authors have reviewed drug repurposing [2–10]. These reviews for the most part analyze and describe the methodologies, often illustrated with examples of successful repurposing. The compelling case of the repurposing of sildenafil (Viagra®) for erectile dysfunction is common knowledge but there are other stories of repurposing that have gone on to be profitable: bupropion, originally used for depression, was repurposed for smoking cessation; and thalidomide, once a treatment for morning sickness, is now used for multiple myeloma.
Herein, we report on a bibliometric analysis of drug repurposing conducted with the aim of measuring and understanding the scope of the practice over the history of modern drug discovery. We define repurposing as a PubMed report of the use or testing of a drug for a disease different from the originally reported one. Although inexact, this methodology gives unique insight into the scope of the practice. By examining a few drugs in-depth we see striking examples of reasoning and intuition applied to repurposing.
Literature analysis
Our analysis was based on PubMed’s MEDLINE data (http://www.ncbi.nlm.nih.gov/pubmed). At >25 million entries, PubMed is the largest and most comprehensive source of biomedical research citations. To assemble a dataset for this bibliometric analysis, we built on earlier text-mining work [11] and identified articles in PubMed where a chemical entity was described in terms of its therapeutic association with a disease. We determined this relationship by examining the MeSH annotations in a stepwise manner (described in the supplementary material online). These chemical entities represent drugs or drug candidates. For simplicity, these entities will be referred to here as drugs.
All drug–disease combinations were extracted, along with the year the article was published, into a separate dataset. This set included citations with no abstract and those in languages other than English, as long as they were annotated and the annotations met the criteria. The publication-type field was examined for each article and clinical trial publications were flagged. This collection included PubMed articles annotated through June 2016 and will be referred to as the ReprofileSet.
The drug–disease annotations in the ReprofileSet were drawn from various types of articles and include in vitro testing, animal studies and human clinical trials and case studies. By being this inclusive, we could look at repurposing at all stages, from identifying candidate drugs to therapeutic use in humans. Because our metrics are based on the scientific literature and not regulatory or marketing sources, our metrics focus on the investigation part of the lifecycle of a drug. For that reason, there are several aspects of repurposing that our bibliometric data do not reveal: whether the substance was submitted to the FDA (or another regulatory agency), approved, withdrawn, marketed, profitable, safe or effective. These aspects remained outside the scope of this review.
The knowledge that a drug went through a clinical trial is a key piece of information indicating that the drug has crossed some important hurdles. For this reason, we have separately counted articles discussing clinical trials to be able to measure the repurposing attempts that made it to this important stage. In addition, we can make limited inferences about the success of a drug based on the number of articles in the ReprofileSet that associate the drug and the disease. When many articles link a drug and a disease it is likely that the drug successfully treats the disease, whereas low publication counts indicate that using the drug to treat the disease was not actively pursued. Without reading the articles or deeper investigations for each drug, one does not know the reason why the drug was not pursued. It could have failed clinically or might have been a success in the clinic but discontinued later for reasons unrelated to safety and efficacy: scientific, economic or organizational.
Limitations in PubMed, and in the literature itself, affected the composition of the ReprofileSet. Some articles, especially older ones, were annotated with an older vocabulary, so they were not detected by our algorithms and did not make it into the ReprofileSet. Our algorithms looked for therapeutic relationships between a drug and a disease. Errors were likely when an article described more-complex relationships; for example, when a drug treats symptoms of the disease, treats the complications of another treatment (drugs that treat nausea caused by anticancer drugs), measures a symptom of a disease or is used in combination therapy.
Evidence for repurposing might not be part of the literature record; for example, the case where a drug was developed with the aim to treat a specific disease but was repositioned early in the development process before any results were published. This was indeed the case with sildenafil. Indeed, the first article appearing for sildenafil already discussed its use in erectile dysfunction (i.e., repurposing from angina to erectile dysfunction occurred before any publications) [12].
Bibliometric observations
The ReprofileSet contains chemical-disease-article relationships for 35 580 distinct chemicals and 4333 diseases and conditions. Over 60% of the chemicals are associated with more than one disease, suggesting they probably have been reprofiled. The remainder of the list (~13 000 chemicals) is associated therapeutically with only one disease. The histogram in Table 1 shows the distribution of chemicals and disease counts. The last line of the table shows that 189 chemicals have been mentioned in the literature in connection with >300 diseases each. Table 2 shows some of the most frequently repurposed chemicals. The top four chemicals have been studied in >1000 diseases. These chemicals are corticosteroids – drugs that treat inflammation – a testament to the ubiquitous nature of inflammation in disease [13,14]. Prednisolone heads the list and is associated with 1340 diseases. A partial list of the actual diseases that prednisolone has been used to treat or investigated for is shown in Table 3. This table shows that the first disease with a clear connection to prednisolone was pemphigus, described in an article published in 1955 [15]. Since then, prednisolone was tested against many diseases and conditions and research continues to the present day. In 2015, the drug was tested against 16 new diseases, including such ailments as hearing loss [16].
Table 1.
Therapeutic chemical–disease pairs
| Number of diseases | Number of chemicals | % Chemicals |
|---|---|---|
| 1 | 13 972 | 39.27 |
| 2 | 6657 | 18.71 |
| 5 | 6605 | 18.56 |
| 10 | 3119 | 8.77 |
| 20 | 1980 | 5.56 |
| 30 | 804 | 2.26 |
| 40 | 513 | 1.44 |
| 50 | 300 | 0.84 |
| 60 | 245 | 0.69 |
| 70 | 196 | 0.55 |
| 80 | 151 | 0.42 |
| 90 | 111 | 0.31 |
| 100 | 104 | 0.29 |
| 110 | 93 | 0.26 |
| 120 | 58 | 0.16 |
| 130 | 54 | 0.15 |
| 140 | 58 | 0.16 |
| 150 | 44 | 0.12 |
| 160 | 44 | 0.12 |
| 170 | 33 | 0.09 |
| 180 | 29 | 0.08 |
| 190 | 24 | 0.07 |
| 200 | 26 | 0.07 |
| 210 | 30 | 0.08 |
| 220 | 27 | 0.08 |
| 230 | 32 | 0.09 |
| 240 | 16 | 0.04 |
| 250 | 15 | 0.04 |
| 300 | 51 | 0.14 |
| >300 | 189 | 0.53 |
| Total | 35 580 | 100.00 |
Over 13 000 chemicals have been tested on only one disease whereas 189 chemicals(the most highly repurposed chemicals according to the definition used here) have been tested for >300 diseases each.
Table 2.
Drugs that have been used or tested in the largest number of diseases
| Chemical name | Disease counta | Article countb | Clinical article countc |
|---|---|---|---|
| Prednisolone | 1340 | 8472 | 1541 |
| Dexamethasone | 1317 | 8386 | 1990 |
| Prednisone | 1162 | 8388 | 1567 |
| Methylprednisolone | 1135 | 6664 | 1396 |
| Interferon-α | 879 | 17 323 | 4924 |
| Ascorbic acid | 840 | 3579 | 746 |
| Cyclosporine | 838 | 6369 | 1288 |
| Cyclophosphamide | 817 | 11 086 | 2044 |
| Hydrocortisone | 809 | 3472 | 640 |
| Methotrexate | 798 | 12 584 | 2166 |
| Aspirin | 789 | 9072 | 2534 |
| Vitamin E | 787 | 3621 | 819 |
| Heparin | 779 | 8726 | 1416 |
| Immunoglobulin G | 692 | 4844 | 918 |
| Indomethacin | 688 | 4520 | 1113 |
| Ethanol | 688 | 4284 | 466 |
| Lidocaine | 646 | 3800 | 1185 |
| Doxycycline | 633 | 3058 | 977 |
| Acetylcysteine | 627 | 2324 | 534 |
| Adrenocorticotropic hormone | 627 | 4149 | 266 |
| Propranolol | 622 | 7079 | 1895 |
Count of diseases occurring with the drug in ReprofileSet drug–disease pairs.
Count of articles total in which the drug occurs with any disease in ReprofileSet.
Count of articles describing clinical trials.
Table 3.
Diseases treated by prednisolone: selection of first diseases co-occurring with the drug and a selection of the more recent ones
| Disease | First publication yeara |
Article countb |
Clinical article countc |
|---|---|---|---|
| Pemphigus | 1955 | 51 | 11 |
| Adrenogenital syndrome | 1956 | 3 | 0 |
| Anemia, hemolytic | 1956 | 13 | 0 |
| Arthritis | 1956 | 33 | 0 |
| Arthritis, rheumatoid | 1956 | 262 | 63 |
| Asthma | 1956 | 343 | 120 |
| Celiac disease | 1956 | 10 | 1 |
| Eye diseases | 1956 | 37 | 4 |
| Hematologic diseases | 1956 | 12 | 1 |
| Hepatitis | 1956 | 51 | 10 |
| Hodgkin disease | 1956 | 26 | 4 |
| Hypersensitivity | 1956 | 30 | 1 |
| Inflammation | 1956 | 42 | 11 |
| Jaundice | 1956 | 5 | 0 |
| Leukemia | 1956 | 50 | 4 |
| Liver cirrhosis | 1956 | 32 | 2 |
| Multiple sclerosis | 1956 | 34 | 6 |
| Periarthritis | 1956 | 5 | 0 |
| Rheumatic diseases | 1956 | 52 | 6 |
| Rheumatic heart disease | 1956 | 20 | 0 |
| Rheumatoid nodule | 1956 | 1 | 0 |
| Skin diseases | 1956 | 71 | 4 |
| Tuberculosis | 1956 | 12 | 0 |
| Tuberculosis, pulmonary | 1956 | 60 | 8 |
| >1000 diseases | |||
| Anemia, iron deficiency | 2015 | 1 | 0 |
| Blepharoptosis | 2015 | 2 | 0 |
| Bulbar palsy, progressive | 2015 | 1 | 0 |
| Eosinophilic esophagitis | 2015 | 1 | 0 |
| Hearing loss, central | 2015 | 1 | 0 |
| Hypocalcemia | 2015 | 1 | 0 |
| Lymphatic abnormalities | 2015 | 1 | 0 |
| Multiple pulmonary nodules | 2015 | 1 | 0 |
| Neoplasms, muscle tissue | 2015 | 1 | 0 |
| Palatal neoplasms | 2015 | 1 | 0 |
| Pharyngeal neoplasms | 2015 | 1 | 0 |
| Plaque, atherosclerotic | 2015 | 1 | 1 |
| Precursor T cell lymphoblastic leukemia–lymphoma | 2015 | 1 | 0 |
| Psoas abscess | 2015 | 1 | 0 |
| Pulmonary alveolar proteinosis | 2015 | 1 | 0 |
| Vascular malformations | 2015 | 1 | 0 |
Year of first occurrence in ReprofileSet for this disease with prednisolone.
Count of articles in which the drug occurs with this disease in ReprofileSet.
Count of articles describing clinical trials.
Most of the diseases treated by prednisolone have an inflammation component that is the targeted physiological endpoint of the drug. The repurposing in these cases is straightforward and does not redirect the drug to a new therapeutic area. We see similar patterns with cancer drugs: when successful in treating one type of cancer the drugs are used for other cancer types. There are, however, less obvious repurposing examples with prednisolone. For example, the drug was tested successfully against restless legs syndrome in 2010 [17]. These data suggest that redirecting a drug for a disease in the same therapeutic area is a common phenomenon. This is understandable, because taking a drug that works on one type of cancer or inflammation and trying it on another type is an obvious step. Repositioning a drug to a completely new therapeutic area happens less often and is ultimately more interesting, because the motivation is less obvious. This kind of repurposing could ultimately be more valuable because it could extend the drug to a new market and, from a scientific point of view, it could offer further understanding of the disease physiology and the mechanism of action. It is also likely to be the riskiest repurposing approach. To look for examples for repurposing over a large therapeutic distance we will examine in-depth two drugs with a long history of use: the antipsychotic chlorpromazine and the antimalarial chloroquine.
Chlorpromazine
Chlorpromazine is a relatively old drug in the modern pharmacopeia. It was originally synthesized by scientists at Rhone–Poulenc as one of a group of phenothiazine derivatives with the hopes that it would be an effective antimalarial [18]. In 1950, the Rhone–Poulenc scientist Paul Charpentier gave a sample of chlorpromazine he had synthesized to a surgeon-anesthesiologist, Henri Laborit, who administered the drug to patients before surgery. Laborit found that his patients went into surgery less anxious and more relaxed. Recognizing these sedative effects from Laborit’s trials, another colleague successfully tried the compound as an adjunct therapy to barbiturates on an individual diagnosed with acute mania; and, shortly afterwards, the drug was used to treat mania and similar conditions. The results were remarkable and the publications describing the clinical effects of chlorpromazine sparked enormous interest [18,19].
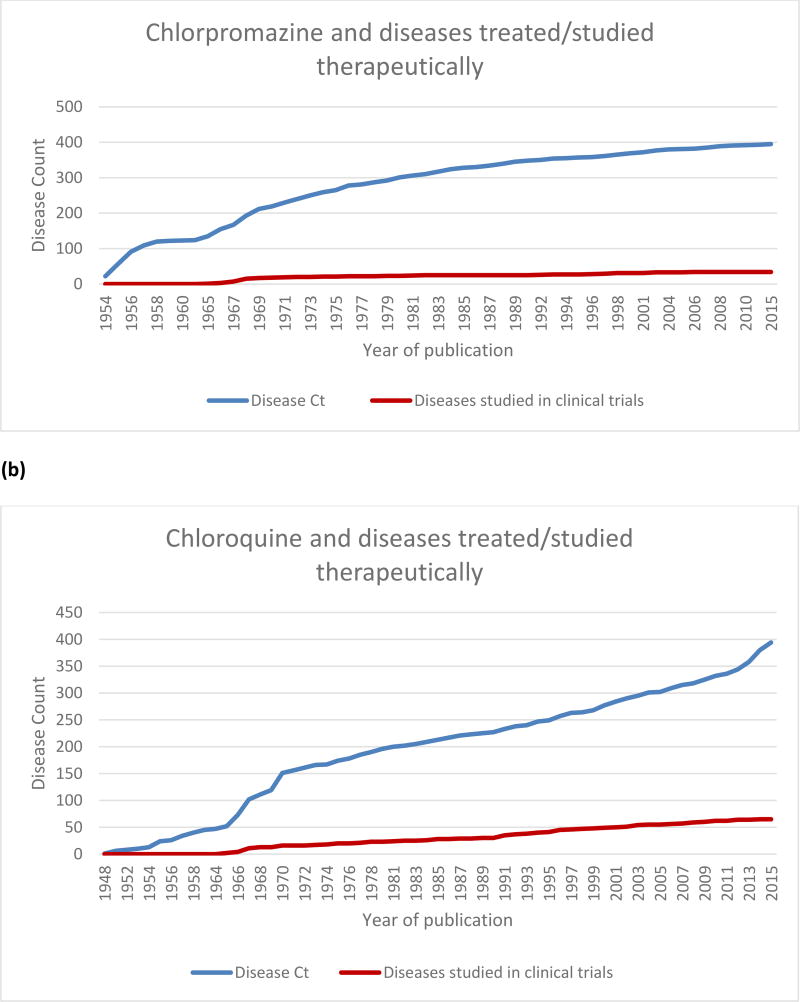
The first publications included in PubMed date from 1952 but the first articles that fit the criteria for inclusion in ReprofileSet appeared in 1954. By that time the drug had attracted widespread attention evidenced by the 60 articles published in that year alone (data not shown), and there had already been efforts to reprofile the drug. In 1954, chlorpromazine was studied as a treatment for 22 diseases. Table 4 shows a partial list of those diseases, starting with the earliest reports and ending with the most recent ones. Figure 1a shows the growth of cases for chlorpromazine repurposing starting with the first publication in ReprofileSet in 1954.
Table 4.
Diseases treated by chlorpromazine: of the total list with 394 diseases, a selection of the earliest and the most recent is included here
| Disease | First publication yeara |
Article countb | Clinical article countc |
|---|---|---|---|
| Schizophrenia | 1954 | 581 | 192 |
| Mental disorders | 1954 | 271 | 18 |
| Psychotic disorders | 1954 | 181 | 23 |
| Bipolar disorder | 1954 | 63 | 25 |
| Hypertension | 1954 | 43 | 3 |
| Depression | 1954 | 40 | 18 |
| Neurotic disorders | 1954 | 34 | 7 |
| Vomiting | 1954 | 26 | 8 |
| Neoplasms | 1954 | 13 | 3 |
| Hallucinations | 1954 | 11 | 3 |
| Nausea | 1954 | 11 | 4 |
| Movement disorders | 1954 | 11 | 1 |
| Tuberculosis, pulmonary | 1954 | 8 | 0 |
| Radiation injuries | 1954 | 5 | 0 |
| Parkinson’s disease | 1954 | 5 | 0 |
| Chorea | 1954 | 4 | 0 |
| Angina pectoris | 1954 | 4 | 0 |
| Infant nutrition disorders | 1954 | 3 | 0 |
| Peripheral vascular diseases | 1954 | 2 | 0 |
| Neuralgia | 1954 | 2 | 0 |
| Alopecia | 1954 | 1 | 0 |
| Rabies | 1954 | 1 | 0 |
| >500 diseases | |||
| Neonatal abstinence syndrome | 2008 | 1 | 0 |
| Otitis media | 2008 | 1 | 0 |
| Jaundice, obstructive | 2008 | 1 | 0 |
| Central nervous system protozoal infections | 2008 | 1 | 0 |
| Staphylococcal infections | 2009 | 1 | 0 |
| Breast neoplasms | 2009 | 1 | 0 |
| Cadmium poisoning | 2010 | 1 | 0 |
| Infarction, middle cerebral artery | 2014 | 1 | 0 |
| Stroke | 2015 | 1 | 0 |
Year of first occurrence in ReprofileSet for this disease with chlorpromazine.
Count of articles in which the drug occurs with this disease in ReprofileSet.
Count of articles describing clinical trials.
Figure 1.
Growth of repurposing over time for (a) chlorpromazine and (b) chloroquine. Although both drugs have been tried in nearly 400 diseases, the rate of growth and the number of diseases for which studies progressed to clinical trials differ.
Most of the early efforts to find new uses for chlorpromazine did not stray far from its original uses: pre-operative relaxation and control of mental disorders. On the premise that chlorpromazine controlled autonomic responses, the drug was tried for treatment of coughs, particularly whooping cough, and over the following decades chlorpromazine was tried in the treatment of many unwanted movements of the body such as chorea, nausea, vomiting, labor, epilepsy, pre-eclampsia, the muscle spasms associated with tetanus and intractable hiccups; for example, see [20–25]. In cancer treatment chlorpromazine was used to treat the symptoms of radiation treatment such as nausea, vomiting and loss of appetite [26].
In a letter published in 1972, a physician noted the well-established observation that cancer mortality was lower in mentally ill patients than in the general population and, therefore, because most of these patients were on drugs like chlorpromazine, these drugs could have antineoplasic effects. Numerous studies were also cited regarding the in vitro and in vivo effects of chlorpromazine on cancer and these called for further controlled clinical trials [27]. Interest continued and chlorpromazine has subsequently been studied in >30 types of cancer. Many researchers have since studied chlorpromazine and cancer at the molecular and cellular level, trying to determine whether the drug had an effect on tumorigenesis [28–30]. In 2009, chlorpromazine was found to enhance the cytotoxic effect of tamoxifen on cancer cells [31]. The mechanism was thought to occur through modifying the uptake properties of membranes.
As stated above, chlorpromazine was originally synthesized as a potentially effective treatment for malaria [19]. The drug was not found to be effective against malaria but did find a use early on to treat the psychoses associated with the high fever accompanying malaria. In more recent years, the relationship of chlorpromazine to malaria has become more complex. It was found to have some antimalarial activity but its potency was too low to be clinically effective [32]. However, in more recent studies, chlorpromazine has been found to enhance the potency of the antimalarial drug chloroquine against chloroquine-resistant strains of the parasite [33]. In a turn of fortune, chlorpromazine could have a place in the antimalarial arsenal after all. A very early observation of the effects of chlorpromazine showed it lowered body temperature [34] while inhibiting shivering. Recently, this old observation was put to new use in a rat model for stroke where the drug was administered as a combination therapy with induced hypothermia [35]. Thus, although repurposing of chlorpromazine has slowed it certainly still continues.
Chloroquine
Chloroquine was developed to treat malaria but, unlike chlorpromazine, chloroquine has had many years of successful use as an antimalarial. Chloroquine was originally synthesized as Resochin® in 1934 by Hans Andersag, a scientist at IG Farben. Thought to be too toxic, chloroquine was shelved by IG Farben and eventually licensed to Winthrop Chemical Company in the USA, where it was eventually resurrected, in part by the war effort; and from 1946 the drug was widely available for use [36,37]. The first article in PubMed about chloroquine is dated April 1946 and describes the drug as a treatment for malaria [38].
The fact that chloroquine was successful in treating the disease it was targeted for has not kept the drug from being redirected at many other diseases. Table 5 contains a sampling of the diseases that chloroquine has been studied in and the growth trajectory is plotted in Figure 1b. The first records that made it into the ReprofileSet were published in 1948. Since then, there have been nearly 400 diseases linked to chloroquine in the literature. Because of the effectiveness of chloroquine in counteracting the malarial parasite, the drug was redirected early on to other parasitic diseases. As Table 5 shows, chloroquine was tried as a treatment against a variety of parasitic diseases before 1960, including amebic dysentery, giardiasis, clonorchiasis, hookworm and trichomonas.
Table 5.
Diseases treated by chloroquine: of the total list of 392 diseases, a selection of the earliest and the most recent is included here
| Disease | First publication yeara |
Article countb |
Clinical article countc |
|---|---|---|---|
| Malaria | 1948 | 902 | 70 |
| Typhoid fever | 1951 | 1 | 0 |
| Dysentery, amebic | 1951 | 18 | 0 |
| Amebiasis | 1951 | 28 | 0 |
| Cestode infections | 1951 | 2 | 0 |
| Hepatitis | 1951 | 5 | 0 |
| Giardiasis | 1952 | 12 | 2 |
| Trichomonas infections | 1952 | 2 | 0 |
| Liver abscess, amebic | 1953 | 31 | 2 |
| Lupus erythematosus, systemic | 1953 | 104 | 8 |
| Rheumatic fever | 1954 | 8 | 1 |
| Arthritis | 1954 | 13 | 2 |
| Pemphigus | 1954 | 2 | 0 |
| Arthritis, rheumatoid | 1955 | 152 | 19 |
| Erythema | 1955 | 9 | 0 |
| Lichen planus | 1955 | 6 | 0 |
| Vitiligo | 1955 | 2 | 0 |
| Dermatitis | 1955 | 1 | 0 |
| Clonorchiasis | 1955 | 6 | 0 |
| Rosacea | 1955 | 2 | 0 |
| Hookworm infections | 1955 | 2 | 0 |
| Acrodermatitis | 1955 | 1 | 0 |
| Miliaria | 1955 | 1 | 0 |
| Liver diseases, parasitic | 1955 | 5 | 0 |
| Neurosyphilis | 1956 | 1 | 0 |
| Atrial fibrillation | 1956 | 5 | 0 |
| >500 diseases | |||
| Carcinoma, non-small-cell lung cancer | 2013 | 5 | 0 |
| Liver neoplasms | 2013 | 2 | 0 |
| Reperfusion injury | 2013 | 2 | 0 |
| Spinal cord diseases | 2013 | 1 | 0 |
| Bone neoplasms | 2013 | 1 | 0 |
| Skin neoplasms | 2013 | 4 | 0 |
| Acute lung injury | 2013 | 1 | 0 |
| Hypertension, pulmonary | 2013 | 1 | 0 |
| Multiple sclerosis | 2013 | 2 | 0 |
| Osteosarcoma | 2013 | 1 | 0 |
| Familial primary pulmonary hypertension | 2013 | 1 | 0 |
| Encephalomyelitis, autoimmune, experimental | 2013 | 3 | 0 |
| Feline infectious peritonitis | 2013 | 1 | 0 |
| Huntington disease | 2014 | 1 | 0 |
| Melanoma, experimental | 2014 | 2 | 0 |
| Bone resorption | 2014 | 1 | 0 |
| Bipolar disorder | 2014 | 1 | 0 |
| Hemochromatosis | 2014 | 1 | 0 |
| Osteoporosis | 2014 | 1 | 0 |
| Colorectal neoplasms | 2014 | 1 | 0 |
| Pancreatic neoplasms | 2014 | 2 | 0 |
| Hyperglycemia | 2014 | 1 | 0 |
| Simian acquired immunodeficiency syndrome | 2014 | 1 | 0 |
| Lupus vasculitis, central nervous system | 2014 | 1 | 0 |
| Liver cirrhosis, experimental | 2014 | 1 | 0 |
| Neovascularization, pathologic | 2014 | 2 | 0 |
| Prostatic neoplasms, castration-resistant | 2014 | 1 | 0 |
| Pelizaeus–Merzbacher disease | 2014 | 1 | 0 |
| Triple-negative breast neoplasms | 2014 | 1 | 0 |
| Carcinoma, pancreatic ductal | 2014 | 1 | 0 |
| Scleroderma, diffuse | 2014 | 1 | 0 |
| Inflammatory bowel diseases | 2014 | 1 | 0 |
| Esophageal neoplasms | 2014 | 1 | 0 |
| Acute kidney injury | 2014 | 1 | 0 |
| Musculoskeletal pain | 2014 | 1 | 1 |
| Reoviridae infections | 2015 | 1 | 0 |
| Kidney neoplasms | 2015 | 1 | 0 |
| Hypopharyngeal neoplasms | 2015 | 1 | 0 |
| Stomach neoplasms | 2015 | 1 | 0 |
| Brain edema | 2015 | 1 | 0 |
| Aortic aneurysm, abdominal | 2015 | 1 | 0 |
| Endometrial neoplasms | 2015 | 1 | 0 |
| Alzheimer’s disease | 2015 | 1 | 0 |
| Carcinoma, hepatocellular | 2015 | 1 | 0 |
| Cholangiocarcinoma | 2015 | 1 | 0 |
| Brain injuries | 2015 | 1 | 0 |
| Carcinoma, renal cell | 2015 | 1 | 0 |
Year of first occurrence in ReprofileSet for this disease with chloroquine.
Count of articles in which the drug occurs with this disease in ReprofileSet.
Count of articles describing clinical trials.
Observing the effects of other antimalarials spurred the earliest repurposing of chloroquine [39]. The antimalarial drug quinine was known to reduce fever, thereby restoring pallor, and probably for that reason in 1894 a physician used quinine to treat a patient with lupus skin rash and noted improvements in the patient’s condition [40]. When synthetic antimalarials became available, this study was followed with more-successful studies on lupus erythematosus [39,40]. During WWII observations of large groups of soldiers taking quinacrine for prevention or treatment of malaria showed that the drug ameliorated symptoms of lupus erythematosus as well as inflammatory arthritis. These observations were followed by clinical studies with quinacrine in 1951 [41], and later chloroquine in both diseases [42,43].
It is generally understood that chloroquine is mainly active in lysosomes [39,44]. Chloroquine, a weak base, diffuses easily into lysosomes, where it becomes protonated and loses its ability to diffuse out of the vesicle. In the context of malaria, chloroquine invades the lysosomes of the parasite and prevents the digestion of heme, effectively killing the organism. In human cells, the accumulation of chloroquine in the lysosomes results in inhibition of certain enzymes such as phospholipase A2, thereby hampering the breakdown of proteins and cell signaling pathways. Chloroquine buildup in the vesicles changes the intracellular pH, increasing it slightly, and, as a consequence, cellular processes requiring a specific pH are thwarted. Chloroquine also has activities unrelated to its lysosomotropic mechanisms of action. These include DNA intercalation or the tendency of the drug to occupy the minor groove of DNA and disrupt transcription and translation, and inhibition of tumor necrosis factor (TNF)-α – an activity thought to be central to the anti-inflammatory effects of the drug in lupus erythematosus and rheumatoid arthritis [39,45]. The full picture of the downstream effects of the drug is still being elucidated [46].
With so many potentially useful mechanisms, chloroquine has been investigated for use in cancer, viral and bacterial infections. The use of chloroquine in cancer, for instance, is a growing area of repositioning [8,47], as seen in the uptick of the line in Figure 1b. The rationale behind using the drug in cancer is based on a variety of known (and suspected) mechanisms. Chloroquine and glioblastoma provide an interesting example. In a study published in 2000, researchers were testing a form of the diphtheria toxin called Tf-CRM107 to treat brain tumors in mice [48]. They knew the toxic form of the diphtheria toxin was produced by breakdown of the original substance in the cell lysosomes and that chloroquine accumulated in lysosomes and prevented many of the normal breakdown processes. They showed that adding chloroquine to the regimen allowed them to give the mice higher doses of Tf-CRM107 without an increase in toxicity.
More recently, chloroquine has been described as an autophagy inhibitor. Autophagy is a cell process that involves shipping damaged organelles to lysosomes for degradation and recycling the energy from the process. It is thought to be a way that cancer cells survive when put under stress. Inhibiting autophagy, therefore, is a strategy to fight cancer [49]. Because of the known effects of chloroquine on lysosomes, it has been considered that the drug inhibits autophagy in cancer cells. There is still uncertainty about the exact relationship between autophagy, cancer and chloroquine [50], and studies continue.
The same reasoning that led to positive results in cancer motivated the attempts to find synergy between chloroquine and HIV treatments. Viruses evidently use autophagy to recover energy for survival and HIV is known to survive by hiding out latently in parts of the cell (reservoirs) where antiviral drugs cannot find it [51]. This new area of study is not yet providing success stories, but it continues [52,53]. Each attempt to repurpose chloroquine results in learning more about the drug and, often, more about the disease it is used against. The pleiotropic activity of chloroquine probably means it will continue to be directed at other diseases beyond malaria.
Disease-specific repurposing
We also examined repurposing from the disease perspective to see whether trends and patterns could be observed. To obtain an overview of the relationships between diseases and drugs, we looked at the number of drugs tried in the treatment of each of the 4333 diseases in the ReprofileSet. Table 6 contains the diseases with the most associated drugs.
Table 6.
Diseases associated therapeutically with the most drugs
| Disease | Count of drugsa | Count of drugs studied in clinical trialsb |
|---|---|---|
| Neoplasms | 4709 | 1897 |
| Breast neoplasms | 3373 | 1080 |
| Lung neoplasms | 3052 | 994 |
| Inflammation | 2985 | 727 |
| Pain | 2392 | 1290 |
| Neoplasms, experimental | 2285 | 127 |
| Colonic neoplasms | 2206 | 320 |
| Adenocarcinoma | 2200 | 809 |
| Prostatic neoplasms | 2120 | 678 |
| Hypertension | 1921 | 1100 |
| Edema | 1799 | 342 |
| Liver neoplasms | 1797 | 589 |
| Diabetes mellitus, experimental | 1723 | 26 |
| Melanoma | 1695 | 632 |
| Asthma | 1669 | 985 |
| Ovarian neoplasms | 1586 | 573 |
| Myocardial infarction | 1575 | 740 |
| Brain ischemia | 1485 | 331 |
Count of drugs occurring in drug–disease pairs with the disease in ReprofileSet.
Count of drugs occurring in drug–disease pairs with the disease in ReprofileSet where the article described a clinical trial.
The general term ‘neoplasms’ tops the list with 4709 chemicals. Seven of the top ten diseases are forms of cancer. Inflammation and pain also occur in the top ten. At the other end of the spectrum (not shown), 176 diseases are linked therapeutically to only one drug. To examine repurposing trends more closely on the disease level we will focus on migraine.
Migraine
To determine which drugs were repurposed for migraine we searched ReprofileSet for the year the drug was first associated therapeutically with any disease. If that year was earlier than the drug was associated therapeutically with migraine then we considered the drug to be repurposed. Using these measures (which should be taken as a rough estimate only) we found 109 drugs for which migraine was the first disease linked therapeutically, with sumatriptan and the follow-on triptan drugs at the top of the list. Nearly 500 drugs, by contrast, were tried in another disease before migraine. These drugs are considered repurposed based on our definition. A selection of these drugs is listed in Table 7.
Table 7.
Drugs repurposed for migraine (counts are estimates)
| Chemical | Migraine article counta |
First publication year of treatment for any diseaseb |
Publication year of first migraine articlec |
|---|---|---|---|
| Ergotamine | 202 | 1964 | 1965 |
| Dihydroergotamine | 129 | 1973 | 1976 |
| Topiramate | 109 | 1987 | 2002 |
| Propranolol | 101 | 1965 | 1968 |
| Aspirin | 99 | 1951 | 1974 |
| Metoclopramide | 86 | 1970 | 1974 |
| Valproic acid | 77 | 1973 | 1993 |
| Acetaminophen | 72 | 1948 | 1972 |
| Naproxen | 63 | 1974 | 1985 |
| Indomethacin | 54 | 1964 | 1968 |
| Amitriptyline | 33 | 1963 | 1969 |
| Prochlorperazine | 29 | 1964 | 1973 |
| Cinnarizine | 28 | 1973 | 1977 |
| Diclofenac | 28 | 1975 | 1979 |
Number of articles in which drug and migraine appear as drug–disease pair.
Year of publication in which drug appears in drug–disease pair with any disease.
Year of publication in which drug appears in drug–disease pair with migraine.
The reprofiled drug with the most articles is ergotamine. Ergotamine is a very old, naturally occurring chemical. PubMed does not accurately capture its archaic history because its therapeutic use predates its appearance in PubMed by well over 100 years and its overall therapeutic use perhaps by several centuries. Ergotamine is an alkaloid produced by a fungus that, over recorded history, has periodically infected cereal crops [54]. At some point, people observed that eating infected rye initiated labor [55]. This observation led to using an extract from the infected grain to trigger labor deliberately through initiating some kind of contraction mechanism. However, difficulties in getting the dosage correct led to complications and use of ergotamine was limited to stopping postpartum bleeding, another area where contraction (presumably of blood vessels) had been observed. The first written record of ergotamine use in migraine therapy was published in Italy in 1862 [56]. In England, successful use of ergotamine against migraine and other neuralgia was published by Woakes in 1868. The combination of knowledge and reasoning that led Woakes to try ergotamine on migraine patients started with his observations of pain. In shingles patients Woakes had noticed exudation of fluid, apparently from tissues into the nerves, and assumed the fluid was released by vasodilation. He reasoned that a substance that counteracted vasodilation (i.e., a vasoconstrictor) would ameliorate the pain and he chose ergotamine – a known vasoconstrictor. The chemical was thought to accelerate childbirth and stem postpartum bleeding through the constriction of uterine arteries. With these two lines of evidence he reasoned that, if ergotamine could constrict vessels in the head, pain would be relieved. Woakes’ record of successful treatment of migraine did not attract a lot of attention, possibly because of the difficulty getting reliable dosing of the naturally occurring chemical. Once ergotamine could be produced reliably, the drug received more attention, and for many years thereafter it was the most effective therapy for acute treatment of migraine [56].
Several classes of drugs have been routinely reprofiled for migraine prevention. Antihypertensives such as propranolol and metoprolol and several antiepileptic drugs (e.g., acetazolamide, valproic acid, topiramate) are all prescribed to prevent attacks. Despite the success of the long-standing ergotamine and newer triptan drugs, these treatments do not bring relief to all patients, and so the search for new therapies continues. Reasoning from the knowledge that migraine has also been treated by mechanical techniques, including cooling (i.e., ice packs) and compression, researchers conducted a clinical trial on a cryotherapy agent perflexane administered intranasally [57]. Perhaps reasoning in a similar vein, the capsaicin or vanilloid receptors are thought to play a part in the migraine pathway. In a recent study [58], capsaicin was used to induce migraine in a mouse model and vanilloid receptor antagonists originally thought to be useful in irritable bowel syndrome [59] were tested to see whether they could ameliorate the effects on the putative migraine pathway. The connection from migraine to the vanilloid receptor introduces an emerging line of reasoning that could bring a new set of future therapies for migraine.
Concluding remarks
Here, we have provided the first bibliometric overview of drug repurposing. Our results show that the number of drugs that have been repurposed for new indications is surprisingly high. Data show that nearly two-thirds of all drugs annotated in MEDLINE have been tried on at least one disease beyond the original use and several hundred drugs have been used in scores of diseases. Whereas many repurposing efforts can be regarded as obvious (using the drug to treat a disease in a similar therapeutic area), there are striking cases where a drug has been redirected to diseases that would be considered therapeutically distant and far from obvious.
Through the close examination of specific interesting examples of repurposing, such as the history of ergotamine, we see consistent evidence that humans observe the effects of chemicals and reason from those effects toward new applications. Reasoning from observations in a modern setting is part of a long lineage stretching back through eons of practitioners of the healing arts: observant mothers, herbalists, shamans, community healers and, of course, doctors.
Today’s physician has much more evidence to reason with. Observing the effects of chemicals on patients is no longer limited to the bedside but can be performed on a larger scale through analyzing patient databases, clinical trial data, chemical genomics and systems chemical biology data, literature reviews, patient online forums and even social media including tweets and blogs. In addition, practitioners and researchers today are not limited to a patient’s temperature and pulse; they can view human biology at the molecular level. These observations of in vitro activity and cellular mechanisms have become jumping off points to new lines of reasoning. The mechanistic understanding that chloroquine disrupts lysosome homeostasis, for instance, provided the link to autophagy and the disruption of cancer cell survival. Such lines of reasoning can be enhanced with computers that increase the scope and the speed of the observations and offer new analytical methods that outstrip the capabilities of the brain.
This overview of repurposing is timely. The US National Institutes of Health (NIH) National Center for Advancing Translational Sciences has recently issued a series of requests for proposals designed to encourage repurposing [60]. The NIH initiative could result in even more inventive, intuitive repurposing. It might enable the translation of early-stage repurposing hypotheses into actual treatments or make repurposing a routine part of the drug discovery process. Although not addressing the approaches and tools for drug repurposing, our study highlighting the wide scope of the practice should serve to further encourage researchers and physicians to concentrate their efforts on finding new uses for existing drugs.
Supplementary Material
Highlights.
A bibliometric review of drug repurposing provides novel insights into the practice
Some drugs have been tried in hundreds of diseases
Even an old drug like chloroquine is actively being tested in new therapeutic applications
Acknowledgments
S.E. kindly acknowledges funding from 1R21TR001718-01 and 1UH2TR002084-01 from NIH NCATS. Grant support: 1U01CA207160 from the National Cancer Institute and the Russian Government Program of Competitive Growth of Kazan Federal University are also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Teaser: A bibliometric review of biomedical literature shows that a surprisingly high number of drugs have been repurposed.
Conflicts of interest
S.E. is CEO of Collaborations Pharmaceuticals and Phoenix Nest.
References
- 1.Simsek M, et al. Finding hidden treasures in old drugs: the challenges and importance of licensing generics. Drug Discov. Today. 2017 doi: 10.1016/j.drudis.2017.08.008. doi.org/10.1016/j.drudis.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 3.Andronis C, et al. Literature mining, ontologies and information visualization for drug repurposing. Brief. Bioinform. 2011;12:357–368. doi: 10.1093/bib/bbr005. [DOI] [PubMed] [Google Scholar]
- 4.Dudley JT, et al. Exploiting drug–disease relationships for computational drug repositioning. Brief. Bioinform. 2011;12:303–311. doi: 10.1093/bib/bbr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekins S, et al. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov. Today. 2011;16:298–310. doi: 10.1016/j.drudis.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Sardana D, et al. Drug repositioning for orphan diseases. Brief. Bioinform. 2011;12:346–356. doi: 10.1093/bib/bbr021. [DOI] [PubMed] [Google Scholar]
- 7.Pantziarka P, et al. The Repurposing Drugs in Oncology (ReDO) Project. Ecancermedicalscience. 2014;8:442. doi: 10.3332/ecancer.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlahopoulos S, et al. New use for old drugs? Prospective targets of chloroquines in cancer therapy. Curr. Drug Targets. 2014;15:843–851. doi: 10.2174/1389450115666140714121514. [DOI] [PubMed] [Google Scholar]
- 9.Pantziarka P, et al. Repurposing drugs in your medicine cabinet: untapped opportunities for cancer therapy? Future Oncol. 2015;11:181–184. doi: 10.2217/fon.14.244. [DOI] [PubMed] [Google Scholar]
- 10.Klug DM, et al. Repurposing strategies for tropical disease drug discovery. Bioorg. Med. Chem. Lett. 2016;26:2569–2576. doi: 10.1016/j.bmcl.2016.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker NC, Hemminger BM. Mining connections between chemicals, proteins, and diseases extracted from Medline annotations. J. Biomed. Inform. 2010;43:510–519. doi: 10.1016/j.jbi.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghofrani HA, et al. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat. Rev. Drug Discov. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.[No authors listed] Inflammation: a unifying theory of disease? Research is showing that chronic inflammation may be the common factor in many diseases. Harv. Health Lett. 2006;31:4–5. [PubMed] [Google Scholar]
- 14.Hunter P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 2012;13:968–970. doi: 10.1038/embor.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruni L, Serri F. Urinary steroids in pemphigus patients treated with cortisone, prednisone and prednisolone. Soc. Ital. Dermatol. Sifilogr. Sezioni. Interprov. 1955;6:593–594. (in Italian) [PubMed] [Google Scholar]
- 16.Maeda Y, et al. Steroid-dependent sensorineural hearing loss in a patient with Charcot-Marie-Tooth disease showing auditory neuropathy. Auris Nasus Larynx. 2015;42:249–253. doi: 10.1016/j.anl.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Oscroft NS, Smith IE. Oral glucocorticosteroids: effective in a case of restless legs syndrome resistant to other therapies. Sleep Med. 2010;11:596. doi: 10.1016/j.sleep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Munoz F, et al. History of the discovery and clinical introduction of chlorpromazine. Ann. Clin. Psychiatry. 2005;17:113–135. doi: 10.1080/10401230591002002. [DOI] [PubMed] [Google Scholar]
- 19.Frankenburg FR, Baldessarini RJ. Neurosyphilis, malaria, and the discovery of antipsychotic agents. Harv. Rev. Psychiatry. 2008;16:299–307. doi: 10.1080/10673220802432350. [DOI] [PubMed] [Google Scholar]
- 20.Cole AC, Robertson DH. Chlorpromazine in the management of tetanus. Lancet. 1955;269:1063–1064. doi: 10.1016/s0140-6736(55)92850-6. [DOI] [PubMed] [Google Scholar]
- 21.Friedgood CE, Ripstein CB. Chlorpromazine (thorazine) in the treatment of intractable hiccups. J. Am. Med. Assoc. 1955;157:309–310. doi: 10.1001/jama.1955.02950210005002. [DOI] [PubMed] [Google Scholar]
- 22.Head RG. The use of chlorpromazine as an adjunct in the treatment of psychomotor epilepsy: a preliminary report. Bull. Tulane Univ. Med. Fac. 1955;15:23–25. [PubMed] [Google Scholar]
- 23.Fernandes M, et al. Thorazine-Phenergan in the treatment of preeclampsia. An. Bras. Ginecol. 1956;41:15–22. (in Portuguese) [PubMed] [Google Scholar]
- 24.Harer WB. Chlorpromazine in normal labor. Obstet. Gynecol. 1956;8:1–9. [PubMed] [Google Scholar]
- 25.Fortes EB. Treatment of minor chorea with chlorpromazine. Port. Med. 1957;41:482–489. (in Italian) [PubMed] [Google Scholar]
- 26.Campbell M, Gordon RA. The use of chlorpromazine in intractable pain associated with terminal carcinoma. Can. Med. Assoc. J. 1956;75:420–421. [PMC free article] [PubMed] [Google Scholar]
- 27.Csatary LK. Chlorpromazines and cancer. Lancet. 1972;2:338–339. doi: 10.1016/s0140-6736(72)92955-8. [DOI] [PubMed] [Google Scholar]
- 28.Belkin M, Hardy WG. Effect of reserpine and chlorpromazine on sarcoma 37. Science. 1957;125:233–234. doi: 10.1126/science.125.3241.233. [DOI] [PubMed] [Google Scholar]
- 29.Cranston EM. Effects of some tranquilizers on a mammary adenocarcinoma in mice. Cancer Res. 1958;18:897–899. [PubMed] [Google Scholar]
- 30.Polliack A, Levij IS. Antineoplastic effect of chlorpromazine in chemical carcinogenesis in the hamster cheek pouch. Cancer Res. 1972;32:1912–1915. [PubMed] [Google Scholar]
- 31.Yde CW, et al. The antipsychotic drug chlorpromazine enhances the cytotoxic effect of tamoxifen in tamoxifen-sensitive and tamoxifen-resistant human breast cancer cells. Anticancer Drugs. 2009;20:723–735. doi: 10.1097/CAD.0b013e32832ec041. [DOI] [PubMed] [Google Scholar]
- 32.Kalkanidis M, et al. Novel phenothiazine antimalarials: synthesis, antimalarial activity, and inhibition of the formation of beta-haematin. Biochem. Pharmacol. 2002;63:833–842. doi: 10.1016/s0006-2952(01)00840-1. [DOI] [PubMed] [Google Scholar]
- 33.Basco LK, Le Bras J. In vitro activities of chloroquine in combination with chlorpromazine or prochlorperazine against isolates of Plasmodium falciparum. Antimicrob. Agents Chemother. 1992;36:209–213. doi: 10.1128/aac.36.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dundee JW, et al. Chlorpromazine and the production of hypothermia. Anaesthesia. 1954;9:296–302. doi: 10.1111/j.1365-2044.1954.tb01927.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, et al. Enhanced beneficial effects of mild hypothermia by phenothiazine drugs in stroke therapy. Neurol. Res. 2015;37:454–460. doi: 10.1179/1743132815Y.0000000031. [DOI] [PubMed] [Google Scholar]
- 36.Schlitzer M. Malaria chemotherapeutics part I: history of antimalarial drug development, currently used therapeutics, and drugs in clinical development. ChemMedChem. 2007;2:944–986. doi: 10.1002/cmdc.200600240. [DOI] [PubMed] [Google Scholar]
- 37.Krafts K, et al. From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy. Parasitol. Res. 2012;111:1–6. doi: 10.1007/s00436-012-2886-x. [DOI] [PubMed] [Google Scholar]
- 38.[No authors listed] ACTIVITY of a new antimalarial agent, chloroquine (SN 7618) J. Am. Med. Assoc. 1946;130:1069. doi: 10.1001/jama.1946.02870160015006. [DOI] [PubMed] [Google Scholar]
- 39.Mackenzie AH. An appraisal of chloroquine. Arthritis Rheum. 1970;13:280–291. doi: 10.1002/art.1780130310. [DOI] [PubMed] [Google Scholar]
- 40.Wallace DJ. The history of antimalarials. Lupus. 1996;5(suppl. 1):2–3. [PubMed] [Google Scholar]
- 41.Page F. Treatment of lupus erythematosus with mepacrine. Lancet. 1951;2:755–758. doi: 10.1016/s0140-6736(51)91643-1. [DOI] [PubMed] [Google Scholar]
- 42.Goldman L, et al. Chloroquine diphosphate in treatment of discoid lupus erythematosus. J. Am. Med. Assoc. 1953;152:1428–1429. doi: 10.1001/jama.1953.63690150002009a. [DOI] [PubMed] [Google Scholar]
- 43.Haydu GG. Rheumatoid arthritis therapy: a rationale and the use of chloroquine diphosphate. Am. J. Med. Sci. 1953;225:71–75. [PubMed] [Google Scholar]
- 44.Jensen M, Mehlhorn H. Seventy-five years of Resochin in the fight against malaria. Parasitol. Res. 2009;105:609–627. doi: 10.1007/s00436-009-1524-8. [DOI] [PubMed] [Google Scholar]
- 45.Cooper RG, Magwere T. Chloroquine: novel uses & manifestations. Indian. J. Med. Res. 2008;127:305–316. [PubMed] [Google Scholar]
- 46.Fox R. Anti-malarial drugs: possible mechanisms of action in autoimmune disease and prospects for drug development. Lupus. 1996;5(suppl. 1):4–10. [PubMed] [Google Scholar]
- 47.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 2009;625:220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 48.Hagihara N, et al. Vascular protection by chloroquine during brain tumor therapy with Tf-CRM107. Cancer Res. 2000;60:230–234. [PubMed] [Google Scholar]
- 49.Sui X, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eng CH, et al. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc. Natl. Acad. Sci. U. S. A. 2016;113:182–187. doi: 10.1073/pnas.1515617113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alonso-Villaverde C, et al. Metabolic stress in infected cells may represent a therapeutic target for human immunodeficiency virus infection. Med. Hypotheses. 2013;81:125–130. doi: 10.1016/j.mehy.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Chauhan A. Enigma of HIV-1 latent infection in astrocytes: an in-vitro study using protein kinase C agonist as a latency reversing agent. Microbes Infect. 2015;17:651–659. doi: 10.1016/j.micinf.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savarino A, Shytaj IL. Chloroquine and beyond: exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology. 2015;12:51. doi: 10.1186/s12977-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miedaner T, Geiger HH. Biology, genetics, and management of ergot (Claviceps spp.) in rye, sorghum, and pearl millet. Toxins. 2015;7:659–678. doi: 10.3390/toxins7030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Dongen PW, de Groot AN. History of ergot alkaloids from ergotism to ergometrine. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995;60:109–116. doi: 10.1016/0028-2243(95)02104-z. [DOI] [PubMed] [Google Scholar]
- 56.Eadie MJ. Ergot of rye-the first specific for migraine. J. Clin. Neurosci. 2004;11:4–7. doi: 10.1016/j.jocn.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Vanderpol J, et al. Therapeutic effect of intranasal evaporative cooling in patients with migraine: a pilot study. J. Headache Pain. 2015;16:5. doi: 10.1186/1129-2377-16-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meents JE, et al. Two TRPV1 receptor antagonists are effective in two different experimental models of migraine. J. Headache Pain. 2015;16:57. doi: 10.1186/s10194-015-0539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiskur BJ, et al. A novel TRPV1 receptor antagonist JNJ-17203212 attenuates colonic hypersensitivity in rats. Methods Find. Exp. Clin. Pharmacol. 2010;32:557–564. doi: 10.1358/mf.2010.32.8.1507853. [DOI] [PubMed] [Google Scholar]
- 60.Repurposing Drugs. 2017 Available at: https://ncats.nih.gov/preclinical/repurpose.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.