Abstract
Background
Hemangiomas are unique endothelial cell tumors that spontaneously involute, which makes it difficult to interpret response to therapies. The objective of this work was to identify a potential biomarker in the urine of children with infantile hemangiomas that would facilitate testing new therapies.
Methods
A prospective longitudinal study in children with hemangiomas and age matched healthy controls was performed to determine whether microRNA (miR)-126, which is highly abundant in fetal endothelial cells, was more abundant in the urine of affected children. Prospective ultrasound measurements of hemangioma size and blood flow velocity were obtained as secondary endpoints to document longitudinal changes in untreated hemangiomas.
Results
Urinary miR-126 levels were significantly elevated in children with proliferating hemangiomas and relative levels of urinary miR abundance correlated with hemangioma size. Hemangiomas had elevated levels of miR abundance compared to healthy controls. Ultrasound data revealed that hemangioma proliferation typically stopped between 6 to 9 months of age. When hemangioma proliferation stopped urinary miR-126 levels in children with hemangiomas dropped to levels observed in healthy age matched controls.
Conclusions
These are the first reported results to identify a potential miR biomarker in the urine of children with hemangiomas. Measurement of urinary levels of miR-126 could potentially be used to monitor hemangioma response to therapies.
Introduction
Hemangiomas are a unique tumor because 90% spontaneously involute, 10% threaten normal development, 1% threaten the life of the affected child and 50% of affected children have residual deformity(1–3). Judah Folkman described hemangiomas as pure angiogenesis (4–5) and the spontaneous involution of these tumors indicates that there is an “angiogenic switch” that stops tumor growth resulting in involution. The mechanisms underlying this “angiogenic switch” have not been identified in hemangiomas and the fact that most lesions spontaneously involute make it difficult to do rigorous clinical trials that can determine the extent to which observed effects are due to treatment or spontaneous involution. The FDA trial to evaluate oral propranolol, the most rigorous clinical trial ever performed on hemangioma patients (6), represents the current state of the art for therapeutic testing. That trial relied on photographic evidence and caliper measurements to document treatment effects (7). Identification of a biomarker would help predict hemangioma behavior and complications would advance our ability to test new treatment interventions by enabling investigators to determine whether observed involution was spontaneous or treatment induced.
The primary objective of this work was to identify a biomarker candidate expressed during hemangioma growth, but not during involution. Specifically, to observe expression of this biomarker candidate in untreated hemangioma patients over time to establish baseline patterns of expression. The ideal biomarker would be obtained non-invasively in urine. Based on our previous work, we had 2 potential candidates for a urine biomarker including monocyte chemoattractant protein-1 and 8-hydroxy-2′-deoxyguanosine (8-OHdG) (8–9). However, the concentration of these molecules in the urine could be affected by conditions other than hemangioma proliferation. We sought to identify a microRNA biomarker candidate. MicroRNA (miR) are small non-coding RNA molecules generally 21–25 nucleotides in length that regulate biologic responses by targeting multiple genes in a specific biologic pathway. Thus, miR are an upstream “hub” capable of targeting multiple downstream sites for amplified and potentially synergistic effects. In this case, we were looking for a miR that regulated angiogenesis and miR-126 is identified in the literature as a master ‘angiomiR’ (10–13). Furthermore, miR are excreted from cells in exosomes and have been identified in serum and urine as potential biomarkers for multiple pathologic conditions (14–16) making recovery from urine a viable option.
Doppler ultrasound is the screening test of choice to diagnose hemangioma on the basis of flow velocity patterns. Arteriovenous malformations and hemangioma have high flow velocities with hemangioma distinguished from arteriovenous malformations by the presence of tumor stroma (17). The secondary objectives of this work were to examine quantitative changes in hemangioma size and flow velocities as surrogate markers for hemangioma growth and involution and to establish the baseline patterns of changes of these values in children with untreated hemangiomas. These objectives were addressed through the longitudinal collection of ultrasound measurements in children with hemangiomas. The biomarker data was compared to ultrasound measurements to determine how changes in biomarker abundancy correlate with changes in ultrasound measurements.
Methods
RNA Extraction and Quantitative Real-Time PCR
Detection of miRNA was performed as previously described (18–19). Briefly, total RNA from cells was extracted using miRVana miRNA isolation kit according to the manufacturer’s protocol (Ambion/Life Technologies). Levels of miR expression in each sample were determined by miR TaqMan assays and TaqMan microRNA reverse transcription kit, followed by quantitative real time PCR (qPCR) using Universal PCR Master Mix (Applied Biosystems/Life Technologies). The probe sequences used for miR qPCR were: (See Table 1).
Table 1.
The probe sequences used for miR qPCR.
| miR ID | Sequence |
|---|---|
| RNU44 | CCTGGATGATGATAGCAAATGCTGACTGAACATGAAGGTCTTAATTAGCTCTA ACTGACT |
| U6snRNA | TGCTCGCTTCGGCAGCACATATACTAAAATTGGAACGATACAGAGAAGATTAG CATGGCCCCTGCGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTT |
| snoRNA202 | GCTGTACTGACTTGATGAAAGTACTTTTGAACCCTTTTCCATCTGATG |
| miR30a | UGUAAACAUCCUCGACUGGAAG |
| miR-126 | UCGUACCGUGAGUAAUAAUGCG |
| miR126* | CAUUAUUACUUUUGGUACGCG |
Results of qPCR were reported as fold change in the target gene relative to the endogenous control miR and calculated using the 2−Δ(ΔCt) method as previously described (18, 20–22):
Human Subjects Protocol
A prospective longitudinal study was done in children with hemangioma (n=34) and age matched healthy volunteers (n=34) (ClinicalTrials.gov identifier: NCT01598116). Subjects were enrolled through the Hemangioma and Vascular Malformation Clinic and the Primary Care Clinic at Nationwide Children’s Hospital. Inclusion criteria were the presence of a hemangioma (HE) on the skin ≥1 cm diameter with diagnosis confirmed by Doppler ultrasound, and age ≤ 5 months to ensure that data was captured while hemangioma were still in the proliferation stage. Exclusion criteria were treatment of hemangioma prior to or during the study, taking steroids or propranolol for other co-morbidities, hemoglobinopathies, PHACES syndrome, rapidly involuting or non-involuting congenital hemangiomas, and parents/guardian unable to provide informed consent. Doppler ultrasound was used to confirm the diagnosis of hemangioma. Data collection was done at 2, 4, 6, 9, 12, 18 and 24 months of age +/− 2 weeks. Bag urine samples and Doppler ultrasound were obtained on the same visit for hemangioma subjects and urine alone for healthy controls. Subjects that missed study visits were allowed to continue in the study.
Urine RNA Isolation
Detection of microRNA in the urine was performed using the mercury™ RNA Isolation Kit- Biofluids (Exiqon) kit according to the manufacturer’s protocol. A minimum sample volume of 3ml urine was needed for testing and samples were centrifuged to remove pelleted debris. The quality and quantity of total RNA was measured using NanoDrop 2000 Spectrophotometer as described earlier (23). RNA quality was detected by uv absorbance at 260nm/280nm. Samples with OD260nm/280nm ratios ≥ 1.6 and ≤ 2.0 were used for qPCR measurements. 100ng of total RNA was used to make cDNA for each miRNA.
Ultrasound Evaluation
Gray scale evaluation was used to assess the size and borders of the hemangioma. Color Doppler images were obtained to evaluate degree of internal vascularity using the same sensitivity setting to monitor changes over time. Spectral Doppler recordings were performed to assess the relative amount of diastolic flow within the lesion, to reflect the degree of arteriovenous shunting. When possible, angle corrected velocities were recorded to allow monitoring of proliferation or involution of the hemangioma. Systolic wave form velocity measurements and resistive indices were calculated in triplicate (if possible) to evaluate changes in the vascular flow over time during hemangioma growth and involution phases. Since precise orientation of feeding or internal vessels was not always possible to determine due to vessel tortuosity, the Doppler frequency shift was also recorded. Studies were performed using a Philips iU22 ultrasound system with high frequency linear array transducers operating between 8 and 17 MHz. Calculation of tumor volume was done as described by Feldman et al (24).
Statistics
The objective of this longitudinal observational study is to collect preliminary data of the changes of selected biomarkers from ultrasound measures (volume and blood flow velocity) and urine samples (MCP-1 and miR) in children with hemangioma, and compare the difference with age matched control at different time point. A sample size of 26 patients and 26 normal age matched control subjects was proposed to detect an effect size of 0.8 standard deviation difference based on a 2 sided 2-sample t-test with 80% power at α=0.05. All data analysis was conducted using SAS 9.4 (SAS Inst. Inc.). Linear mixed models for repeated measures with autoregressive (AR) variance-covariance structure to estimate the changes of tumor volume, miR-126 and blood flow velocity over time. For each measure, the means and the standard errors at each time point were estimated using the LSMEANS statement in the GLIMMIX procedure. The covariance structure was chosen based on AIC/BICC criteria. Sensitivity analyses were conducted use different models and covariance structure to ensure that the results are consistent with the selection of the models. The correlations between the miR-126 levels and the tumor volumes at each time point (4, 6 and 9 month) were summarized using the Spearman correlation due to the non-normal distribution of the variables. The miR-126 differences between the HE patients and the healthy controls were compared using the 2 sample-t-test. Sensitivity analysis was also conducted using 2-sample rank test. A p value of <0.05 was considered statistically significant.
Results
Identification of a housekeeping miR for qPCR standardization was done by measuring commonly reported urinary miR in samples from healthy age matched controls (n=6) and children with hemangiomas (n=6). Results are summarized as mean ± SD and differences between control and HE groups analyzed using Student’s t-test. Normality test of the residual from the mixed models and the sensitivity analysis using 2-sample rank test method were also conducted to ensure the robustness of our conclusions from the t-test. Data were normalized to values observed for the healthy age matched controls and reported as fold-change differences for children with hemangiomas. miR-30a is known to be highly abundant in urine from children and had equivalent abundance in both subject groups(Table 2), so this was selected as the housekeeping miR(25).
Table 2.
Identification of normalization miR in urinary samples from 6 month old subjects (n= 6/group)
| miR | control | HE |
|---|---|---|
| snoRNA202 | 1.0 ± 0.44 | 0.56 ± 0.44* |
| U6 snRNA | 1.0 ± 0.32 | 2.1 ± 0.84 |
| RNU44 | 1.0 ± 0.41 | 0.35 ± 0.21* |
| miR-30a | 1.0 ± 0.22 | 1.1 ± 0.18 |
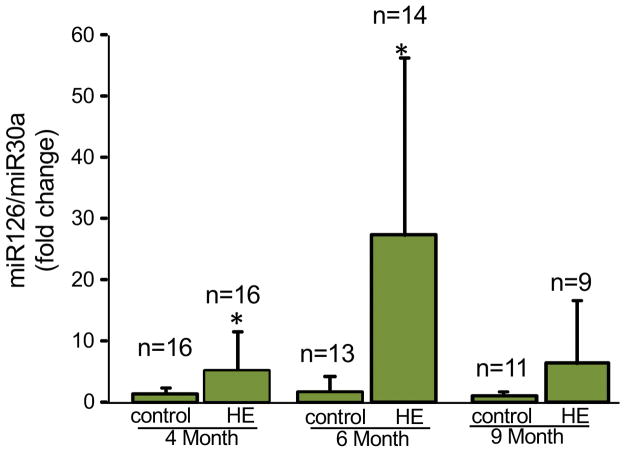
All urine samples that had enough volume to yield sufficient amounts of adequate quality miR of were analyzed by qPCR. miR-126 abundance was significantly elevated in the HE group at age 4 months and 6 months of age, but was not significantly different from healthy controls at age 9 months (Fig. 1). The slight increase in miR-126 abundance at 9 months is attributed to 2 HE children with elevated miR-126 levels at that time point as the other 7 subjects had levels of miR-126 abundance similar to healthy controls. A temporal pattern for miR- 126 levels was identified with elevation occurring at 4 and 6 months of age in the HE group that normalized in most affected children by age 9 months.
Figure 1. miR 126 is more abundant in urine of children with hemangiomas during proliferative phase only.
Urine specimens collected from children with HE and healthy age matched controls at 4, 6 and 9 months of age show significantly increased abundance of miR126 during HE proliferation (4 & 6 months) which subsequently reverts to baseline level when proliferation stops (9 months). Results are expressed as mean ± SD; *p <0.05 Students’ t-test, n= number of subjects analyzed.
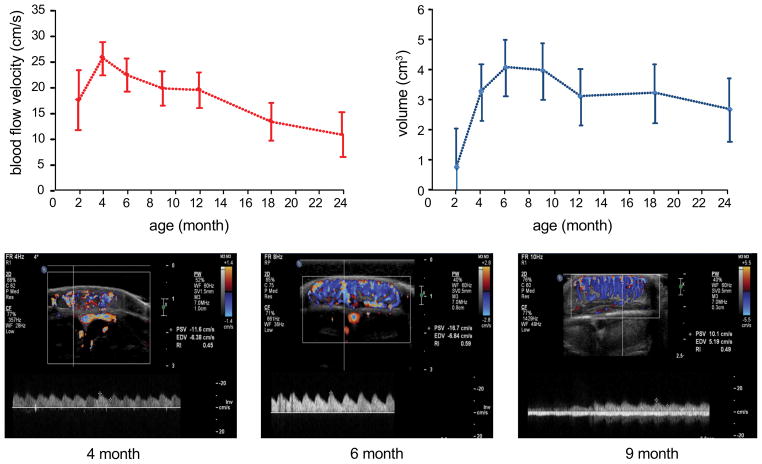
Ultrasound measurements of hemangioma size and blood flow velocity were obtained to understand how these values change as hemangiomas progress from the proliferative to the involuting phase and to serve as an objective comparator for the miR-126 biomarker data. The numbers of subjects with both valid miR-126 and ultrasound measures were 14, 13, and 9 at time point of 4, 6 and 9 month respectively. The mean and standard errors of the blood flow velocities and volumes were estimated using the LSMEANS statement based on the linear mixed model for repeated measures. Mean blood flow velocities peaked at 4 months of age and had progressive decline to age 24 months with flow velocities that were roughly half the peak flow velocities observed at age 4 months (Fig. 2a). Blood flow velocity may peak at 4 months because this is when the rate of tumor growth is greatest based on the change in tumor volume from 2–4 months. Hemangioma size did not increase past 6 months of age and began to decrease in size between 9–12 months of age (Fig. 2b). Representative ultrasound images for measuring flow velocity in a single subject are shown in Fig. 2c–e. Temporal changes in volume are consistent with clinical observations where hemangiomas reach a plateau in size during and gradually involute. In this cohort there was no additional growth after 6 months of age and that plateau in hemangioma growth occurred between 6–9 months of age.
Figure 2. Ultrasound measurements track longitudinal changes in hemangioma blood flow and size.
a, Changes in mean blood flow velocity (+/− SD) for all subjects at each time point in the study indicate increasing flow velocities that peak at 4 months of age then progressively decline. b, HE size (volume) peaks at 6 months marking the end of HE proliferation, remains stable in size between 6–9 months and then begins to decrease in size. Representative color Doppler ultrasound images from single subject at (c) 4 months, (d) 6 months and (e) 9 months of age. The calculated flow velocity measurements (cm/s) are displayed on the right hand side of each image where PSV= peak systolic velocity and EDV= end diastolic velocity.
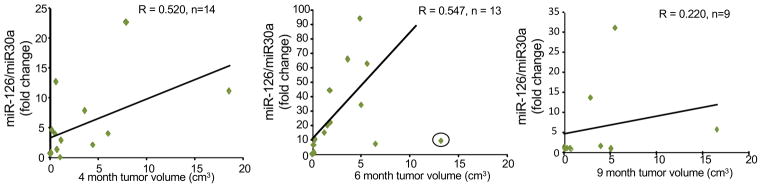
Scatter plots were used to visually evaluate whether urinary miR-126 abundance correlated with hemangioma size at ages 4, 6, and 9 months. All subjects that had miR-126 qPCR values and ultrasound volume measurements were included in the analysis. At 4 months of age there was a moderately strong correlation between HE size and urinary miR abundance (Fig. 3a). Increased urinary miR-126 abundance was detectable for all HE. At 6 months there was a moderately strong correlation between HE size and miR-126 abundance. All HE had increased miR-126 abundance compared to controls. This was most pronounced at age 6 months (Fig. 3b). In the 6 month group there was 1 subject that was treated as an outlier and not included in the calculation of the Spearman correlation co-efficient. In this subject the relative abundance of miR-126 was higher at 4 months (11.18 fold change) compared to 6 months (9.93 fold change) with a corresponding decrease in tumor volume from 18.6 cm3 to 13.2 cm3 indicating that this HE was beginning to involute prior to age 6 months. The clinical course for this subject indicates that this was likely a rapidly involuting congenital hemangioma, which should have been excluded. By 9 months of age miR-126 abundance for all HE subjects decreased, so that there was no statistically significant difference in values between HE and age matched controls. For 2 subjects relative miR-126 abundance was high, but it was decreasing. The decrease from 6 months to 9 months in 1 subject was 62.58 to 31.21 fold and 15.17 to 13.6 fold for the other subject. All the other subjects had miR-126 abundance levels that essentially dropped to levels observed with healthy controls even though the HE were still present (Fig. 3c). This indicates that the expression of miR-126 is not needed to maintain HE tumor volume and that the biology of the HE has changed.
Figure 3. Urinary miR126 abundance correlates with tumor size.
The Spearman correlation coefficient (R) indicates a moderately strong positive correlation between urinary miR126 abundance and tumor volume that is observed during HE proliferation at (a) 4 months and (b) 6 months of age, but not at 9 months of age (c). The circled value in panel B was excluded as a rapidly involuting congenital hemangioma, n= number of subjects analyzed.
Discussion
In 1998 the National Institutes of Health Biomarkers Definition Working Group defined biomarkers as, “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.” (26). There have been previous attempts to identify urine biomarkers for hemangioma. In a study reported by Marler et al, elevation of urinary MMP-2, MMP-9, bFGF or VEGF was not observed in children with infantile hemangioma, but they did identify 2 high molecular weight matrix metalloproteases in 60% of children with hemangiomas (27). Kleber et al monitored urinary MMP-2 and MMP-9 levels in response to propranolol treatment of hemangiomas and found increased MMP-2 levels, but no changes in MMP-9 levels (28). However, MMP-2 and MMP-9 are found in the urine of healthy children in response to normal tissue remodeling, so the levels of these proteins are not necessarily specific for hemangioma growth (29). At this time there is no urine biomarker that has been adopted on a widespread clinical basis to monitor growth of infantile hemangioma.
There is evidence that points to miR-126 as a potential biomarker for hemangiomas as it is the most abundant miR in fetal endothelial cells (30–31). Endothelial cell specific expression of miR-126 regulates endothelial cell proliferation, development of fetal vasculature and vascular integrity (31–32). Expression of miR-126 is substantially elevated during fetal development and drops significantly post-partum (31, 33–35), which is consistent with the theory of hemangiomas as fetal rests (10, 12). These results provide first evidence that elevated urinary miR-126 levels can be measured and used as an indicator of a hemangioma growth in affected children. The elevated levels and correlation between HE size and miR-126 abundance at 4 and 6 months, the drop in miR-126 expression once proliferation stops, along with essentially undetectable levels of miR 126 in controls indicates that HE are the source of the miR-126. Thus, miR-126 meets the biomarker criteria as, “a characteristic that is objectively measured and evaluated as an indicator of … pathogenic processes.”
This is the first reported longitudinal ultrasound data for changes in blood flow velocity and tumor volume in children with untreated hemangiomas. As with the miR biomarker, it is important to establish the changes in ultrasound measurements over time to determine whether this method of monitoring HE has merit for future studies. In addition, the ultrasound data was collected to provide quantitative data that documents changes in hemangioma size and to correlate these findings with miR126 abundance levels. Ultrasound data is challenging to use because HE size and blood flow will change if the child is crying and we could not control for this variable. Nonetheless, the changes in blood flow velocity and tumor volume observed in this study are consistent with a report by Chang, et al. that performed serial measurements (using tape measures) of hemangioma growth on 367 children. They found that 80% of HE growth was complete by age 5 months and 97% had completed proliferation by age 9 months (36). Both ultrasound and miR-126 data reported in this study are consistent with this clinical picture. Thus, ultrasound monitoring of flow velocity and size may be a reasonable surrogate for monitoring changes in hemangioma growth and proliferation, but collection of additional data is need to validate this method of monitoring.
Performing a longitudinal study on infants and small children with minimal compensation or incentive for participation, i.e. no treatment, was a significant challenge. Many subjects either dropped out, missed study visits or the urine samples were not adequate for testing. As a result the number of valid samples at each time point was much smaller than expected. The team is greatly indebted to the parents who did bring their children for 2 hour study visits as their only real incentive was to help future children born with hemangiomas. Nonetheless, the results provide critical first evidence that justifies performing additional studies to validate miR-126 as a biomarker. These are the first reported results of a miR biomarker for hemangiomas and the only reported urine biomarker for hemangiomas with elevated expression only during the proliferative phase. This work provides a new paradigm for identifying the transition from hemangioma growth to involution. These results provide an important baseline for future studies to test whether urinary miR-126 levels decrease in response to therapeutic intervention. The use of quantitative values derived from biologic events that can be used as surrogate endpoints to monitor hemangioma proliferation and involution will enable investigators to test new treatments for hemangioma, so that all children with hemangiomas can be safely treated.
Acknowledgments
This work was supported by NIH/NIGMS R01 GM095657 (to GMG) and an NIH Clinical Translational Science Award to The Ohio State University (UL1TR001070). This work was also made possible by the Primary Care Yellow team at Nationwide Children’s Hospital, under the direction of Dr. Melissa Meyer, who enrolled the healthy age matched control subjects. The authors also wish to posthumously acknowledge the contributions to this work by Dr. Bill Shiels, the former Chairman of Radiology at Nationwide Children’s Hospital
Footnotes
ClinicalTrials.gov identifier: NCT01598116
References
- 1.Paller AS. Responses to anti-angiogenic therapies. J Investig Dermatol Symp Proc. 2000;5:83–86. doi: 10.1046/j.1087-0024.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 2.Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218–222. [PubMed] [Google Scholar]
- 4.Ezekowitz RA, Mulliken JB, Folkman J. Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326:1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Leaute-Labreze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735–746. doi: 10.1056/NEJMoa1404710. [DOI] [PubMed] [Google Scholar]
- 7.Leaute-Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 8.Gordillo GM, Onat D, Stockinger M, et al. A key angiogenic role of monocyte chemoattractant protein-1 in hemangioendothelioma proliferation. Am J Physiol Cell Physiol. 2004;287:C866–873. doi: 10.1152/ajpcell.00238.2003. [DOI] [PubMed] [Google Scholar]
- 9.Gordillo G, Fang H, Park H, Roy S. Nox-4-dependent nuclear H2O2 drives DNA oxidation resulting in 8-OHdG as urinary biomarker and hemangioendothelioma formation. Antioxid Redox Signal. 2010;12:933–943. doi: 10.1089/ars.2009.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Olson EN. AngiomiRs--key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fish JE, Srivastava D. MicroRNAs: opening a new vein in angiogenesis research. Sci Signal. 2009;2:pe1. doi: 10.1126/scisignal.252pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goerke SM, Kiefer LS, Stark GB, Simunovic F, Finkenzeller G. miR-126 modulates angiogenic growth parameters of peripheral blood endothelial progenitor cells. Biol Chem. 2015;396:245–252. doi: 10.1515/hsz-2014-0259. [DOI] [PubMed] [Google Scholar]
- 13.Kuhnert F, Mancuso MR, Hampton J, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 14.Lv LL, Cao YH, Ni HF, et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305:F1220–1227. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 15.Omran A, Elimam D, Webster KA, Shehadeh LA, Yin F. MicroRNAs: a new piece in the paediatric cardiovascular disease puzzle. Cardiol Young. 2013;23:642–655. doi: 10.1017/S1047951113000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omran A, Elimam D, He F, Peng J, Yin F. Potential role of blood microRNAs as non-invasive biomarkers for early detection of asymptomatic coronary atherosclerosis in obese children with metabolic syndrome. Med Hypotheses. 2012;79:889–893. doi: 10.1016/j.mehy.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Paltiel HJ, Burrows PE, Kozakewich HP, Zurakowski D, Mulliken JB. Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology. 2000;214:747–754. doi: 10.1148/radiology.214.3.r00mr21747. [DOI] [PubMed] [Google Scholar]
- 18.Gordillo GM, Biswas A, Khanna S, et al. Dicer knockdown inhibits endothelial cell tumor growth via microRNA 21a-3p targeting of Nox-4. J Biol Chem. 2014;289:9027–9038. doi: 10.1074/jbc.M113.519264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas A, Khanna S, Roy S, Pan X, Sen CK, Gordillo GM. Endothelial cell tumor growth is Ape/ref-1 dependent. Am J Physiol Cell Physiol. 2015;309:C296–307. doi: 10.1152/ajpcell.00022.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Zhu S, Cai C, et al. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature. 2014;509:487–491. doi: 10.1038/nature13166. [DOI] [PubMed] [Google Scholar]
- 21.Biswas S, Roy S, Banerjee J, et al. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci U S A. 2010;107:6976–6981. doi: 10.1073/pnas.1001653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordillo GM, Biswas A, Khanna S, Spieldenner JM, Pan X, Sen CK. Multidrug Resistance-associated Protein-1 (MRP-1)-dependent Glutathione Disulfide (GSSG) Efflux as a Critical Survival Factor for Oxidant-enriched Tumorigenic Endothelial Cells. J Biol Chem. 2016;291:10089–10103. doi: 10.1074/jbc.M115.688879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S, Patel D, Khanna S, et al. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci U S A. 2007;104:14472–14477. doi: 10.1073/pnas.0706793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pahr DH, Zysset PK. A comparison of enhanced continuum FE with micro FE models of human vertebral bodies. J Biomech. 2009;42:455–462. doi: 10.1016/j.jbiomech.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 25.Luo Y, Wang C, Chen X, et al. Increased serum and urinary microRNAs in children with idiopathic nephrotic syndrome. Clin Chem. 2013;59:658–666. doi: 10.1373/clinchem.2012.195297. [DOI] [PubMed] [Google Scholar]
- 26.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 27.Marler JJ, Fishman SJ, Kilroy SM, et al. Increased expression of urinary matrix metalloproteinases parallels the extent and activity of vascular anomalies. Pediatrics. 2005;116:38–45. doi: 10.1542/peds.2004-1518. [DOI] [PubMed] [Google Scholar]
- 28.Kleber CJ, Spiess A, Kleber JB, Hinz U, Holland-Cunz S, Weiss J. Urinary matrix metalloproteinases-2/9 in healthy infants and haemangioma patients prior to and during propranolol therapy. Eur J Pediatr. 2012;171:941–946. doi: 10.1007/s00431-011-1660-x. [DOI] [PubMed] [Google Scholar]
- 29.Thrailkill KM, Kumar S, Rosenberg CK, Auten KJ, Fowlkes JL. Characterization of matrix metalloproteinases in human urine: alterations during adolescence. Pediatr Nephrol. 1999;13:223–229. doi: 10.1007/s004670050597. [DOI] [PubMed] [Google Scholar]
- 30.Chistiakov DA, Orekhov AN, Bobryshev YV. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J Mol Cell Cardiol. 2016;97:47–55. doi: 10.1016/j.yjmcc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishizaki T, Tamiya T, Taniguchi K, et al. miR126 positively regulates mast cell proliferation and cytokine production through suppressing Spred1. Genes Cells. 2011;16:803–814. doi: 10.1111/j.1365-2443.2011.01529.x. [DOI] [PubMed] [Google Scholar]
- 34.Poissonnier L, Villain G, Soncin F, Mattot V. miR126–5p repression of ALCAM and SetD5 in endothelial cells regulates leucocyte adhesion and transmigration. Cardiovasc Res. 2014;102:436–447. doi: 10.1093/cvr/cvu040. [DOI] [PubMed] [Google Scholar]
- 35.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360–367. doi: 10.1542/peds.2007-2767. [DOI] [PubMed] [Google Scholar]