Abstract
Background
Cognitive reserve (CR) is one factor that helps to maintain cognitive function in patients with Alzheimer’s disease (AD). Whether the effects of CR depend on the semantic/executive components of the task remains unknown.
Methods
470 patients (138 with AD, 332 with mild cognitive impairment (MCI)) were selected from the Alzheimer’s Disease Neuroimaging Initiative database. Linear regression models were used to determine the effects of CR (years of education) on cognitive performance after controlling for demographic factors and regional cortical atrophy. First, we assessed memory tasks with low (Auditory Verbal Learning Test (AVLT) discriminability), moderate (AVLT delayed recall) and high (Logical Memory Test (LMT) delayed recall) executive/semantic components. Next, we assessed tasks with lower (digit span forward, Trails A) or higher (digit span backwards, Trails B) executive demands, and lower (figure copying) or higher (naming, semantic fluency) semantic demands.
Results
High CR was significantly associated with performance on the LMT delayed recall, approached significance in the AVLT delayed recall and was not significantly associated with performance on AVLT discriminability. High CR was significantly associated with performance on the Trails B and digit span backwards, mildly associated with Trails A performance and was not associated with performance on digit span forwards. High CR was associated with performance on semantic but not visuospatial tasks. High CR was associated with semantic tasks in patients with both MCI and AD, but was only associated with executive functions in patients with MCI.
Conclusion
CR may relate to executive functioning and semantic knowledge, leading to preserved cognitive performance in patients with AD pathology.
INTRODUCTION
Cognitive reserve (CR) is the retained ability to perform cognitive tasks despite loss of neuronal function from neurological disease.1 Many factors have been found to influence CR, including education, occupational status and participation in social and leisure activities.2,3 In persons with Alzheimer’s disease (AD) pathology, CR is thought to be one factor that explains why patients with similar amounts of disease burden can have markedly different performance on clinical and neuropsychological assessments.4
The biological mechanisms through which CR exerts its influence remain unknown, although potential explanations include brain reserve, brain maintenance, neural reserve and neural compensation.1,5 Brain reserve refers to structural changes at baseline, such as increased numbers of neurons or synapses, which allow the brain to tolerate a greater degree of pathology. Brain maintenance proposes that CR results in neuroprotective effects, such that neuronal tissue is less susceptible or resistant to neuropathology. Neural reserve is the retained ability to perform a cognitive task due to increased efficiency or capacity within the neural network typically involved in performing that task, while neural compensation maintains functionality through the use of general semantic knowledge, problem-solving and executive functions. Determining the mechanism of CR may provide insight into targeted, novel interventions for patients with mild cognitive impairment (MCI) and AD, with the aim to potentially delay the onset of clinically significant impairment from AD pathology.
Neural compensation can be due to increased general knowledge, sometimes referred to as semantic knowledge,5 or due to executive functions that allow for flexible problem-solving.1 Therefore, tasks that allow a subject to use semantic and/or executive skills to improve performance should show particularly strong effects of CR if neural compensation is contributing. In contrast, CR would be expected to improve performance on all tasks within a cognitive domain equally if neural reserve is responsible. Here, we assess for neural compensation by determining whether the beneficial effects of CR are due to the semantic and/or executive components of the task. First, we test the effects of CR on memory tests with low, moderate and high semantic and/or executive components. We hypothesise that CR will exert a greater influence on tasks with high versus low executive and/or semantic components. Next, we directly assess tasks with lower versus higher executive functions, and lower versus higher semantic components, to determine if CR also exerts a greater effect on high component tasks.
METHODS
Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by the principal investigator Michael W Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, positron emission tomography (PET), other biological markers and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Subjects in the study include those with MCI and AD as well as normal controls between the ages of 55 and 90. For up-to-date information regarding these specific protocols, please see www.adni-info.org. Our study included subjects with a diagnosis of MCI or AD who had baseline MRI data and neuropsychological testing available, which included 138 subjects with AD and 332 subjects with MCI for a total of 470 subjects.
Informed consent
Each subject gave written informed consent for imaging and neuropsychological testing in accordance with the Human Subjects Research Committee Guidelines. Please see www.adni-info.org for further details.
Diagnosis
Patients were diagnosed as amnestic MCI or mild AD according to the inclusion/exclusion criteria for the ADNI study. A diagnosis of MCI required a subjective memory complaint; clinical dementia rating (CDR) of 0.5, with a score of at least 0.5 on the memory box; impaired performance on the logical memory task part II delayed recall below the education-adjusted cut-off; Mini Mental Status Exam (MMSE) between 24 and 30 (inclusive); and general preservation of function and cognition such that a diagnosis of dementia cannot be made. A diagnosis of mild AD required a subjective memory complaint, CDR of 0.5–1, impaired performance on the logical memory task part II delayed recall below the education-adjusted cut-off, MMSE between 20 and 26 (inclusive) and National Institute of Neurological and Communicative Disorders and Stroke (NINCDS)/Alzheimer’s Disease and Related Disorders Association (ADRDA) criteria for probable AD.
Cognitive reserve
Years of education, starting with first grade, were used as a surrogate marker for CR: for the purposes of this study, completion of a general education degree (GED) or high school diploma=12 years, associate degree=14 years, completion of a bachelor’s degree=16 years, completion of an MS/MA=18 years, completion of a law degree=19 years, and completion of an MD, PhD or both=20 years. We chose this measure over the American National Adult Reading Test (ANART) test, which is strongly correlated with CR and may have less of a sexual bias than education,6 because ANART was shown to be positively correlated with performance on some cognitive tasks in age-matched control subjects,7 and has been used as an independent measure of semantic knowledge or crystallised intelligence,5,8 which could potentially bias our results.
Neuropsychological testing
Measures of delayed recall and discriminability memory were derived from the Auditory Verbal Learning Test (AVLT),9 which consists of five learning trials where subjects are read a list of 15 words and asked to immediately recall as many items as possible. After a sixth trial of 15 distractor words, subjects are then asked to remember as many objects on the list at 30 min spontaneously (delayed recall) and to select previously learnt words on a list consisting of 15 learnt words and 15 distractor words (delayed recognition). In order to correct for word registration, which is a function of verbal working memory, we measured the delayed recall score as the proportion of words registered on trial 5 that were spontaneously recalled after a 30 min delay. Discriminability was determined using recognition scores at 30 min. To account for false alarms to non-studied items, we derived a measure of discriminability, d-prime (d′), which was calculated in a standard fashion based on classic signal detection theory.10 Additionally, because d′ is undefined when either proportion is 0 or 1, we used standard formulas to convert these values: Hits=(#Hits+0.5)/(#studied items+1) and FA=(#FA+0.5)/(#unstudied items+1).11
The Logical Memory Test (LMT) is a modification of the episodic memory measure from the Wechsler Memory Scale-Revised and involves the immediate and delayed (30 min) free recall of a short story that is read aloud to the patient and contains 25 elements of information (perfect score is 25). We used the 30 min delayed recall score in order to compare directly with the 30 min delayed recall from the AVLT.
Because AVLT discriminability involves minimal demands on memory retrieval, there is a lower opportunity to use semantic and/or executive skills to improve performance. In contrast, the AVLT delayed recall provides some opportunity for semantic/executive skills to be incorporated, although this capacity is limited because none of the words are designed to be phonetically or semantically related.9 Finally, the LMT involves remembering semantically related elements of a story, allowing for the greatest potential to use semantic and executive skills to improve task performance. This categorisation is consistent with prior models of memory function that have differentiated verbal memory tasks according to whether the information is arbitrary or semantically associated.12
Executive processing was measured using the Trail Making Test Parts A and B,13 digit span forwards and digit span backwards. Semantic tasks included the number of animals generated in 1 min (fluency animals) and the Boston Naming Test (BNT). On the BNT we additionally added in correct responses to phonetic cues, as previously described,14 in order to better control for lexical-retrieval biases.
MRI imaging and analysis
MRI scans were collected on a 1.5T scanner using a standardised magnetization-prepared rapid gradient-echo (MPRAGE) protocol: sagittal plane, repitition time (TR) of 2400 ms, echo time (TE) of 3 ms, inversion time (TI) 1000 ms, flip angle 8°, 24 cm field of view (FOV), 192×192 in-plane matrix and 1.2 mm slice thickness.15 Fully preprocessed scans were downloaded for analysis.
T1 image volumes were examined quantitatively by a cortical surface-based reconstruction and analysis of cortical thickness, in nine regions of interest (ROIs) corresponding to the ‘cortical signature’ of AD, as described previously.16–20 Each ROI from the left and right hemispheres was evaluated, for a total of 18 regions per subject.
Statistical analysis
For each test, a three-block hierarchical linear regression model was used to determine the effects of CR on cognitive task performance (figure 1). Age and gender were entered in the first block to control for potential confounding demographic factors. Because we expected performance on neuropsychological tests within specific domains to be correlated to regional, as opposed to global, atrophy, in block 2, cortical thickness measurements from each of the 18 individual ROIs were added in a stepwise manner with a threshold of p<0.05 for inclusion in the model and p>0.1 for exclusion from the model. Finally, in block 3, years of education were added to the model to determine if CR modulated performance after controlling for demographic factors and regional atrophy. Significance findings were confirmed using Bonferroni corrections for multiple comparisons by multiplying the p value by the total number of neuropsychological tasks evaluated (n=10). Statistical analyses were performed using IBM SPSS V.22.0.
Figure 1.
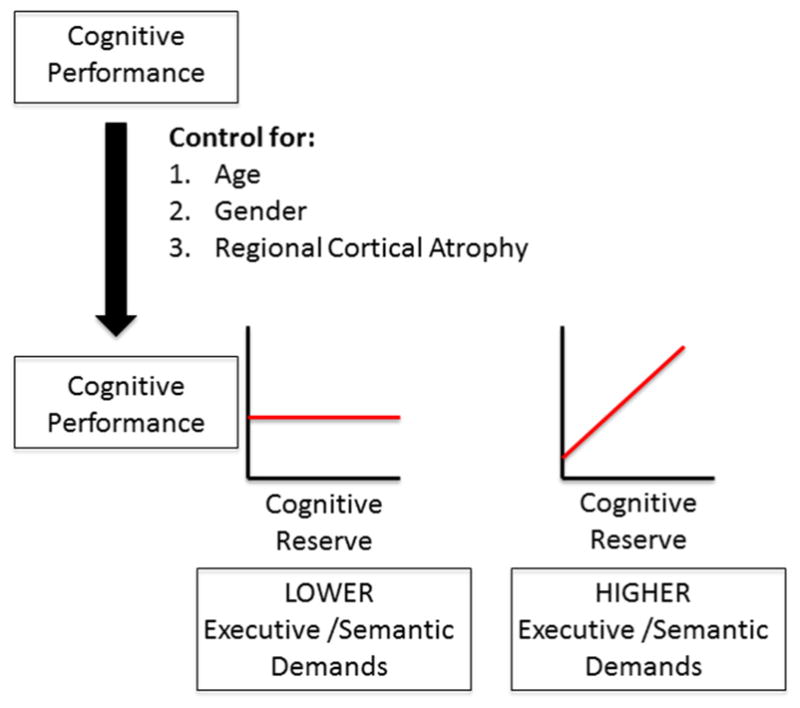
Hierarchical linear regression model and predictions. Cognitive task performance is first controlled for age, gender and regional cortical atrophy. Next, the effects of cognitive reserve (CR) on task performance are plotted on the graph (y-axis) in relation to CR (x-axis). We predict that CR will not modulate cognitive performance on tasks with lower executive/semantic demands, but will improve cognitive performance on tasks with higher executive/semantic demands.
Our main analysis tested the relationship between CR and task performance across all patients with a diagnosis of MCI or AD. To determine if the relationship between CR and task performance is different in patients with MCI versus AD, we performed separate, post-hoc analyses within each diagnostic group separately. Finally, as our use of education as a marker for the LMT task was potentially prone to bias, we repeated this analysis to determine the relationship between ANART as a marker of CR and performance on the LMT.
RESULTS
Demographics
Four hundred and seventy patients patients (332 with MCI, 138 with AD, 61% male) were used in this study (table 1). The mean education in years was 15.4 (range: 4–20) and the mean age was 75 (range: 54–91).
Table 1.
Demographics and neuropsychological test scores
| MCI (n=332) | AD (n=138) | Total (n=470) | ||||
|---|---|---|---|---|---|---|
| Mean | (95%CI) | Mean | (95%CI) | Mean | (95%CI) | |
| Age (years) | 74.8 | (74.0 to 75.6) | 75.5 | (74.3 to 76.8) | 75.0 | (74.3 to 75.7) |
| Gender (% male) | 65.0 | 53.0 | 60.0 | |||
| Education (years) | 15.7 | (15.4 to 16.0) | 14.6 | (14.0 to 15.1) | 15.4 | (15.1 to 15.7) |
| 0–12 years | 19% | 31% | 23% | |||
| 12–16 years | 42% | 47% | 43% | |||
| 17–20 years | 39% | 22% | 34% | |||
| MMSE | 27.1 | (26.9 to 27.3) | 23.4 | (23.1 to 23.8) | 26.0 | (25.8 to 26.2) |
| AVLT delayed recall | 3.0 | (2.7 to 3.4) | 0.5 | (0.3 to 0.7) | 2.3 | (2.0 to 2.6) |
| AVLT delayed recognition | 9.8 | (9.4 to 10.2) | 6.9 | (6.3 to 7.5) | 9.0 | (8.6 to 9.3) |
| LMT delayed recall | 3.9 | (3.6 to 4.2) | 1.2 | (0.9 to 1.5) | 3.1 | (2.8 to 3.3) |
| Trails A (s) | 44.1 | (41.8 to 46.4) | 65.6 | (59.9 to 71.3) | 50.5 | (48.0 to 53.0) |
| Trails B (s) | 130.2 | (122.4 to 137.9) | 198.5 | (184.0 to 213.0) | 150.4 | (142.9 to 157.8) |
| Digit span forwards | 6.5 | (6.4 to 6.7) | 6.3 | (6.1 to 6.5) | 6.5 | (6.4 to 6.6) |
| Digit span backwards | 4.6 | (4.5 to 4.7) | 4.0 | (3.8 to 4.2) | 4.4 | (4.3 to 4.5) |
| Boston Naming Test | 28.4 | (28.0 to 28.7) | 25.7 | (24.7 to 26.7) | 27.6 | (27.2 to 28.0) |
| Fluency (animals) | 16.2 | (15.7 to 16.7) | 12.4 | (11.6 to 13.2) | 15.1 | (14.6 to 15.6) |
| Copy | 4.7 | (4.6 to 4.7) | 4.4 | (4.2 to 4.6) | 4.6 | (4.5 to 4.7) |
Baseline demographic and neuropsychological test scores for patients with MCI and AD. The mean values and 95% CI displayed unless otherwise noted. Items in bold showed significant correlation (p<0.05) with education at baseline.
AD, Alzheimer’s disease; AVLT, Auditory-Verbal Learning Task; LMT, Logical Memory Test; MCI, mild cognitive impairment; MMSE, Mini Mental Status Exam.
Regional cortical atrophy associated with cognitive performance
Significant regions of cortical atrophy for each cognitive task and domain are presented in table 2. Notably, both delayed recall memory tasks were significantly associated with left mesial temporal lobe cortical thickness, all executive function tasks except for digit span forwards were associated with left angular gyrus thickness, and naming was associated with left temporal pole thickness.
Table 2.
Results of linear regression models
| Test | 3 | SE | p Value | Partial correlation |
|---|---|---|---|---|
| AVLT discriminability | ||||
| Constant | 11.562 | 2.752 | 0 | n/a |
| Age | −0.004 | 0.021 | 0.85 | −0.009 |
| Gender | −0.442 | 0.321 | 0.169 | −0.063 |
| Education | −0.044 | 0.052 | 0.399 | −0.039 |
| AVLT delayed recall | ||||
| Constant | −0.985 | 0.212 | 0 | n/a |
| Age | 0.004 | 0.002 | 0.021 | 0.1 |
| Gender | 0.032 | 0.028 | 0.257 | 0.049 |
| Left MTL | 0.156 | 0.038 | 0.00004 | 0.189 |
| Right MTL | 0.099 | 0.03 | 0.001 | 0.152 |
| Education | 0.008 | 0.004 | 0.069 | 0.084 |
| LMT delayed recall | ||||
| Constant | −11.407 | 1.956 | 0 | n/a |
| Age | 0.033 | 0.016 | 0.035 | 0.085 |
| Gender | −0.343 | 0.234 | 0.145 | −0.059 |
| Left MTL | 1.08 | 0.316 | 0.001 | 0.157 |
| Right MTL | 0.771 | 0.25 | 0.002 | 0.142 |
| Left SPL | 1.121 | 0.421 | 0.008 | 0.123 |
| Education | 0.303 | 0.037 | 3.58E-15 | 0.354 |
| Trails A | ||||
| Constant | 181.249 | 19.455 | 0 | n/a |
| Age | −0.236 | 0.157 | 0.133 | −0.064 |
| Gender | 1.687 | 2.393 | 0.481 | 0.03 |
| Left AG | −26.973 | 4.882 | 5.51E-08 | −0.236 |
| Education | −0.987 | 0.389 | 0.012 | −0.108 |
| Trails B | ||||
| (Constant) | 613.766 | 62.943 | 0 | n/a |
| Age | −0.19 | 0.476 | 0.689 | −0.017 |
| Gender | −0.619 | 7.08 | 0.93 | −0.004 |
| Left AG | −84.21 | 14.543 | 1.30E-08 | −0.239 |
| Right temporal pole | −25.62 | 11.823 | 0.031 | −0.09 |
| Left precuneus | −33.699 | 15.108 | 0.026 | −0.092 |
| Education | −6.761 | 1.16 | 1.04E-08 | −0.241 |
| Digit span forwards | ||||
| Constant | 5.708 | 0.614 | 0 | n/a |
| Age | 0.006 | 0.007 | 0.381 | 0.04 |
| Gender | −0.068 | 0.103 | 0.51 | −0.03 |
| Education | 0.025 | 0.017 | 0.13 | 0.07 |
| Digit span backwards | ||||
| Constant | −1.009 | 0.938 | 0.283 | n/a |
| Age | 0.016 | 0.007 | 0.023 | 0.099 |
| Gender | 0.316 | 0.102 | 0.002 | 0.135 |
| Left precuneus | 0.545 | 0.222 | 0.014 | 0.107 |
| Left AG | 0.777 | 0.231 | 0.001 | 0.146 |
| Left SFG | −0.817 | 0.223 | 0.00028 | −0.159 |
| Left IFG | 0.66 | 0.285 | 0.021 | 0.101 |
| Education | 0.084 | 0.017 | 5.00E-07 | 0.222 |
| Boston Naming Test | ||||
| (Constant) | 7.266 | 3.672 | 0.048 | n/a |
| Age | −0.04 | 0.028 | 0.157 | −0.066 |
| Gender | 0.377 | 0.424 | 0.375 | 0.041 |
| Left temporal pole | 2.525 | 0.76 | 0.001 | 0.153 |
| Left MTL | 1.093 | 0.51 | 0.033 | 0.099 |
| Left ITG | 2.194 | 0.805 | 0.007 | 0.126 |
| Education | 0.422 | 0.068 | 1.04E-09 | 0.278 |
| Fluency: animals | ||||
| (Constant) | −0.228 | 3.71 | 0.951 | n/a |
| Age | −0.058 | 0.03 | 0.052 | −0.09 |
| Gender | −1.059 | 0.463 | 0.023 | −0.106 |
| Left SMG | 3.558 | 0.888 | 0.000071 | 0.183 |
| Left ITG | 2.765 | 0.824 | 0.001 | 0.154 |
| Education | 0.368 | 0.074 | 9.59E-07 | 0.225 |
| Copy | ||||
| (Constant) | 2.179 | 0.554 | 0 | n/a |
| Age | 0.003 | 0.004 | 0.504 | 0.031 |
| Gender | −0.017 | 0.067 | 0.797 | −0.012 |
| Left AG | 0.496 | 0.123 | 0.000067 | 0.184 |
| Right ITG | 0.277 | 0.098 | 0.005 | 0.129 |
| Education | 0.02 | 0.011 | 0.075 | 0.083 |
β values, standard errors and uncorrected significance values for each of the variables included in the final regression model for each neuropsychological task. Variables with an uncorrected p<0.05 are in bold.
AG, angular gyrus; AVLT, Auditory-Verbal Learning Task; IFG, inferior frontal gyrus; ITG, inferior temporal lobe; LMT, Logical Memory Test; MTL, mesial temporal lobe; n/a, not applicable; SFG, superior frontal gyrus; SMG, supramarginal gyrus; SPL, superior parietal lobule.
Effects of CR on cognitive performance
The results for each variable in the final regression model for each task are presented in table 2.
Memory
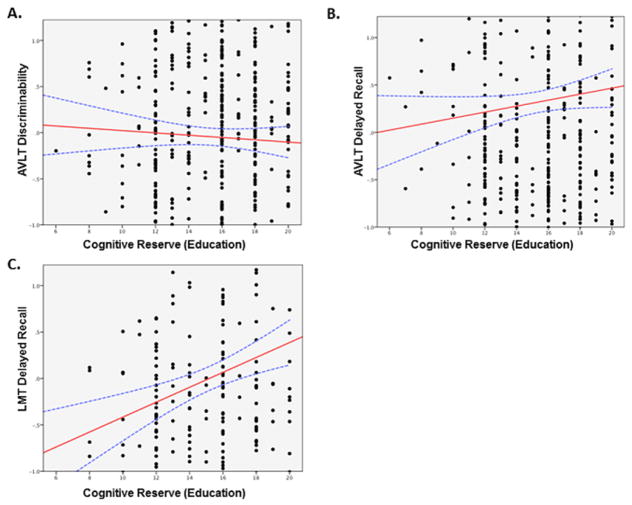
There was no significant relationship between CR and performance on AVLT discriminability (p=0.4, not significant (ns); figure 2A), a task with minimal semantic/executive components. There was a trend towards a significant relationship between CR and performance on the ALVT delayed recall, a task with moderate semantic/executive components (p=0.07, ns; figure 2B). Higher CR was associated with significantly better performance on the LMT delayed recall task, a memory task with relatively higher semantic/executive components (β=0.3; SE=0.04; p<0.00001; figure 2C).
Figure 2.
Cognitive reserve (CR) improves memory performance on tasks with high executive/semantic components. Relationship between standardised Z-scores of test performance (controlled for age, gender and regional cortical atrophy) and CR (years of education) for memory tasks with low (A), moderate (B) or high (C) executive/semantic demands. AVLT, Auditory Verbal Learning Test; LMT, Logical Memory Test.
Executive function
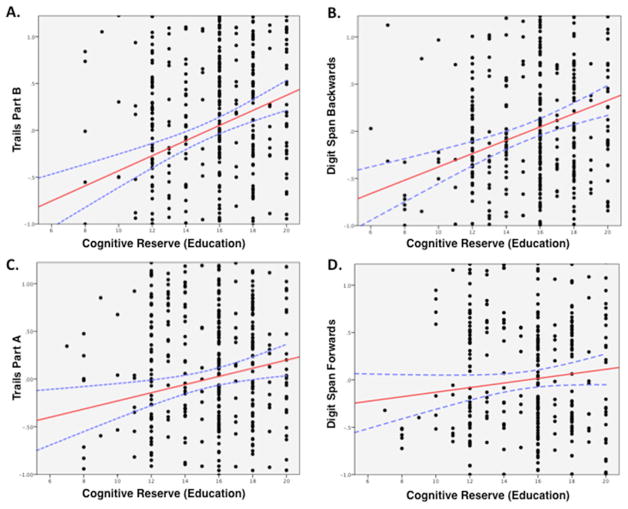
Higher CR was significantly associated with performance on the Trails Part B test (β = −6.8; SE=1.2; p<0.00001; figure 3A) and digit span backwards (β=0.08; SE=0.02; p<0.00001; figure 3B), tasks with high executive demands. There was a smaller but significant effect of CR on Trails Part A performance (β = −0.987; SE=0.389; p=0.01; figure 3C); however, this was no longer significant after correcting for multiple comparisons (pc=0.12, ns). Performance on digit span forwards was not significantly associated with CR (p=0.13, ns; figure 3D).
Figure 3.
Cognitive reserve (CR) improves performance on tasks with high executive component. Relationship between standardised Z-scores of test performance (controlled for age, gender and regional cortical atrophy) and CR (years of education) for tasks with high (A,B) versus low (C,D) executive component.
Semantic knowledge
Higher CR was significantly associated with performance on semantic fluency (β=0.37; SE=0.07; p<0.00001; figure 4A) and naming (β=0.4; SE=0.07; p<0.00001; figure 4B), tasks with high semantic demands, but not figure copying (p=0.08, ns; figure 4C), a task with low semantic demands.
Figure 4.
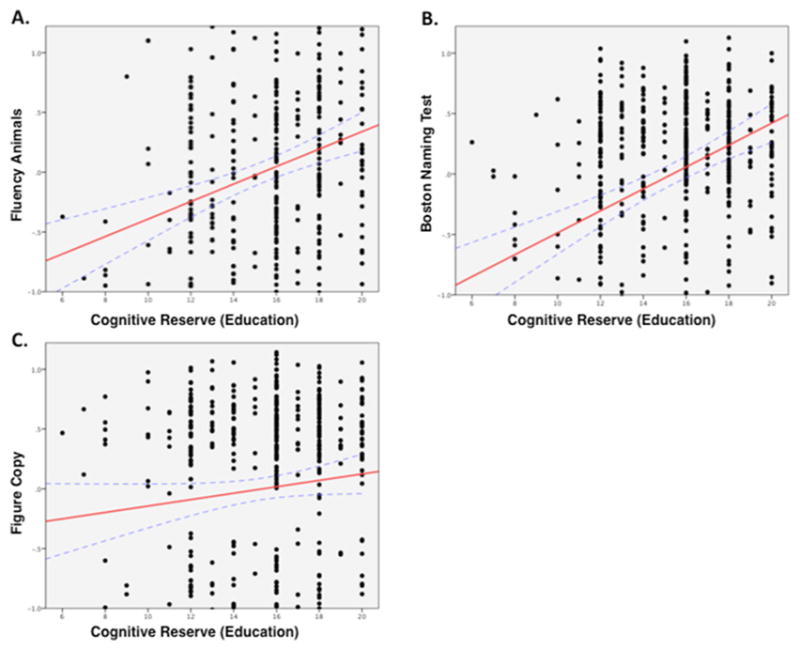
Cognitive reserve (CR) improves performance on tasks with high semantic component. Relationship between standardised Z-scores of test performance (controlled for age, gender and regional cortical atrophy) and CR (years of education) for tasks with high (A,B) versus low (C) semantic component.
Differences in the effects of CR in patients with MCI versus AD
The MCI group showed a similar relationship between CR and task performance as in our combined MCI/AD group (table 3). In the AD group, CR was also significantly associated with performance on the LMT and the BNT. However, unlike the MCI group, there was no significant relationship between CR and performance on digit span backwards, Trail Making Test Part B or semantic fluency (table 3).
Table 3.
Partial correlations of CR with neuropsychological tasks
| Task | Partial correlations (r) | |
|---|---|---|
| MCI (n=332) | AD (n=138) | |
| AVLT discriminability | −0.07 | 0.01 |
| AVLT delayed recall | 0.08 | 0.03 |
| LMT delayed recall | 0.36*** | 0.22* |
| Trails Part A | −0.18* | 0.05 |
| Trails Part B | −0.26*** | −0.15 |
| Digit span forward | 0.09 | 0.002 |
| Digit span backwards | 0.19** | 0.21 |
| Boston Naming Test | 0.31*** | 0.26** |
| Fluency animals | 0.24*** | 0.09 |
| Copy | 0.11 | −0.01 |
Partial correlations between years of education (CR) and neuropsychological tasks in the final regression model when patients with MCI (n=332) and AD (n=138) were evaluated separately.
p<0.05,
p<0.001,
p<0.0001.
AD, Alzheimer’s disease; AVLT, Auditory Verbal Learning Test; CR, cognitive reserve; MCI, mild cognitive impairment.
Addressing potential confounds
Because education-normed LMT scores were used as inclusion criteria for the ADNI study, using education as a marker of CR may have introduced bias into our results for the LMT test specifically. To address this concern, we repeated our analysis of the association between CR and LMT using the ANART as a surrogate marker of CR, not education. We obtained similar results; CR was significantly associated with LMT performance in the combined MCI/AD cohort (partial correlation r=0.19, p<0.0001), as well as the separated MCI (partial correlation r=0.19, p<0.005) and AD (partial correlation r=0.20, p<0.05) cohorts.
DISCUSSION
The main results of our study demonstrate that the beneficial effects of CR in patients with MCI and AD depend on the executive and semantic components of the cognitive task. Patients with higher CR had superior performance on memory tasks with higher executive and semantic components, while there was no effect of CR on memory tests with lower executive and semantic components. This hypothesis was confirmed by comparing other tasks with low versus high executive and semantic components. More specifically, CR was only associated with performance on tasks with higher executive components in patients with MCI, but not AD, while both diagnostic groups showed an association between CR and performance on tasks with higher semantic components. Taken together, our results suggest that neural compensation, through the use of executive functions and semantic knowledge, may be one mechanism by which CR is associated with improved cognitive performance.
Performance in specific cognitive domains is related to regional cortical atrophy
Impaired performance on delayed recall in patients with MCI and AD has been shown to correlate with hippocampal atrophy,11,21–23 while deficits in other aspects of memory function have been associated with atrophy in other mesial temporal lobe cortical structures11,22 as well as frontoparietal regions.11 Regional atrophy is also associated with impaired performance in patients with AD in executive tasks19 and naming.14 Our study found that delay recall performance was correlated with cortical thickness in the left mesial temporal lobe, that executive function tasks correlated with cortical thickness in the left angular gyrus, and that naming was associated with left temporal pole atrophy. Our results therefore complement prior studies, providing evidence that regionally specific cortical atrophy correlates with domain-specific cognitive performance.
CR is associated with performance within specific cognitive domains
Prior studies looking at the effects of CR on domain-specific tests were unable to control for the amount of disease severity. A prior study found that patients with higher education had superior performance on tests of delayed recall memory and cognitive control as measured by the Stroop task, and approached significance in performance on the Trail Making Test Part B.24 Their study used MMSE scores to control for disease severity; however, the MMSE is affected by many factors other than the pathological changes of AD, including by CR itself. Our results therefore add further evidence that CR modulates specific cognitive domains, as well as show that the effects of CR persist after controlling for regional cortical atrophy.
Mechanism of CR
Our study provides evidence for the effects of CR after controlling for regional cortical atrophy, suggesting that brain reserve may not fully explain the modulatory effects of CR. This is in line with several previous studies demonstrating higher levels of pathology in those with high CR compared with those with low CR at similar estimations of disease severity and neuropsychological performance.4,7,25–33 However, it is also possible that microanatomical differences, such as synaptic count and differences in dendritic branching, could contribute to brain reserve and would not be reflected in measures of cortical atrophy or pathological disease burden.34
Brain maintenance can be tested by looking at the rates of disease progression in patients with high and low CR. While some studies have found slower rates of amyloid-beta deposition in normal controls with high CR and slower progression of hypometabolism on fluorodeoxyglucose-PET longitudinally in patients with AD with higher CR,35 others have shown no difference in cortical atrophy over time in low versus high CR patients with MCI and AD.32 Additionally, while prospective studies in non-demented older individuals have shown that patients with higher CR determined by education, occupational status and leisure activities have lower risk of progressing to dementia,2,3,36 those with high CR who are diagnosed with AD show faster rates of decline in memory function37,38 as well as processing speed and global cognitive function.39,40 These data suggest that CR modulates performance up to a certain level of disease burden, but that continued disease progression overwhelms compensatory mechanisms involved in CR, leading to more rapid deterioration in cognition late in the disease course.1 Our study was not designed to assess the longitudinal progression of disease severity over time; however, we did find that CR exerted broader effects on task performance in patients with milder (MCI) versus more significant (AD) impairments in a cross-sectional sample.
Our study more specifically attempted to differentiate between neural reserve, in which CR is associated with task performance for all tasks within a cognitive domain equally, and neural compensation, in which CR specifically modulates performance dependent on the semantic and/or executive components of the task. When analysing each patient group separately, the association of CR with task performance in patients with MCI was related to both executive and semantic components, while CR only influenced performance on tasks with semantic components in patients with AD. One possible explanation is that executive functions contribute to CR in patients with MCI, while in patients with AD this capacity is lost due to disease progression. This would be consistent with prior studies showing an association between executive functions and functional preservation in patients with MCI.41 Our finding that CR is associated with performance on tasks with higher semantic components fits with prior studies showing patients with MCI have hyperactivation in brain regions involved in semantic memory, compared with age-matched controls.42 This is also consistent with models differentiating arbitrary versus semantic components of verbal memory.12 While learning arbitrary verbal associations are hypothesised to localise to mesial temporal lobe structures, semantically associated verbal memory is thought to involve a more widely distributed network of brain regions, allowing for neural compensation.12
As the cognitive effects of education result from years of intervention, it remains unclear whether similar effects can be seen after relatively shorter durations of cognitive rehabilitation interventions. However, prior systematic reviews have found that multifaceted cognitive and social interventions improved cognitive performance in patients with MCI and AD, while interventions targeting specific cognitive domains may be less effective.43–46 Interventions targeting general, college-level educational classes, for instance, led to improved cognitive performance in a group of elderly individuals.47
Limitations
Our study has several limitations. First, our population was taken from the ADNI database, which may not accurately reflect the characteristics of the general population. Specifically, the average educational level in this cohort is much higher compared with the general population or populations used to study CR in the past. Nevertheless, finding significant effects of CR even within this highly educated population (eg, finding significant effects of CR between college and graduate levels of education) suggests a relatively high ceiling effect for CR.
Second, we used only one marker of CR, education, although occupation2 and leisure activities3 have also been found to be markers of CR. Additionally, several recent studies6,7 have used the ANART as a marker of CR, which may more accurately reflect CR compared with education and show less gender bias. However, while the ANART as a marker of premorbid IQ is felt to be relatively resistant to the deleterious effects of ageing and neurodegenerative disease, this is not universally accepted.48 While ANART is relatively preserved through prodromal AD, there is deterioration in the ANART score in patients with AD as their disease progresses.49 Furthermore, the effects of education and ANART on neuropsychological task performance are similar.6 As the ANART was shown to independently correlate with performance on the BNT in normal controls,7 and the ANART has been used as a surrogate test of semantic knowledge and verbal intelligence,5 using the ANART would have introduced a potential confounding factor in our analysis. For these reasons, education was thought to be a more appropriate measure of CR for the main analyses in this study.
Finally, our study was limited by specific neuropsychological tasks available from the ADNI study. A more direct, prospective evaluation of performance on neuropsychological tasks specifically designed to assess the effects of CR on tasks varying in semantic and executive components is therefore necessary. Additionally, our study was a cross-sectional analysis of the relationship between cognitive performance and CR. Longitudinal and/or interventional studies are needed in order to infer causality between CR and mechanisms of task performance. Finally, impaired performance on the LMT was used as part of the inclusion criteria for patients enrolled in the ADNI study. Because these measures are normed according to education, this introduces bias into our results. However, finding similar effects of CR on the LMT performance using the ANART instead of education, despite the limitations in using the ANART mentioned above, supports the validity of our results.
CONCLUSIONS
Our study demonstrates that the beneficial effects of CR in patients with MCI and AD are dependent on the executive and semantic components of the cognitive task. This provides evidence supporting the hypothesis that CR improves cognitive performance, at least in part, through neural compensation. Further studies using cognitive tasks specifically designed to vary in the degree of executive and semantic demands are needed to verify these findings. If replicated, these results could help to guide cognitive rehabilitation efforts in the future.
Acknowledgments
Funding Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica, Biogen, Bristol-Myers Squibb Company, CereSpir, Cogstate, Eisai, Elan Pharmaceuticals, Eli Lilly and Company, EuroImmun, F Hoffmann-La Roche and its affiliated company Genentech, Fujirebio, GE Healthcare, IXICO, Janssen Alzheimer Immunotherapy Research & Development, Johnson & Johnson Pharmaceutical Research & Development, Lumosity, Lundbeck, Merck & Co, Meso Scale Diagnostics, NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer, Piramal Imaging, Servier, Takeda Pharmaceutical Company, and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organisation is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Correction notice This paper has been amended since it was published Online First. Owing to a scripting error, some of the publisher names in the references were replaced with ‘BMJ Publishing Group’. This only affected the full text version, not the PDF. We have since corrected these errors and the correct publishers have been inserted into the references.
Contributors The corresponding author (RRD) performed all statistical analyses. RRD: Conception of study, acquisition of data, analysis of data, writing of manuscript. MB: Acquisition of data, analysis of data. DAW: Critical revisions to the manuscript. BCD: Conception of study, critical revisions to manuscript.
Disclaimer Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed. © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
References
- 1.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–10. http://jama.jamanetwork.com/article.aspx?articleid=368856. [PubMed] [Google Scholar]
- 3.Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–42. doi: 10.1212/wnl.57.12.2236. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3025284&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–15. doi: 10.1212/01.wnl.0000069923.64550.9f. http://www.ncbi.nlm.nih.gov/pubmed/12821732. [DOI] [PubMed] [Google Scholar]
- 5.Barulli D, Stern Y, Efficiency SY. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17:502–9. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–64. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vemuri P, Weigand SD, Przybelski SA, et al. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain. 2011;134:1479–92. doi: 10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de OMO, Nitrini R, Yassuda MS, et al. Vocabulary is an Appropriate measure of Premorbid Intelligence in a sample with Heterogeneous Educational Level in Brazil. Behav Neurol. 2014;2014:1–6. doi: 10.1155/2014/875960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rey A. L’examen clinique en psychologie. Paris: Presses universitaires de France; 1964. [Google Scholar]
- 10.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. http://www.ncbi.nlm.nih.gov/pubmed/2966230. [DOI] [PubMed] [Google Scholar]
- 11.Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage. 2011;54:1530–9. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain. 2009;132:570–82. doi: 10.1093/brain/awp012. [DOI] [PubMed] [Google Scholar]
- 13.Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 14.Domoto-Reilly K, Sapolsky D, Brickhouse M, et al. Naming impairment in Alzheimer’s disease is associated with left anterior temporal lobe atrophy. Neuroimage. 2012;63:348–55. doi: 10.1016/j.neuroimage.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack CR, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–55. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickerson BC, Stoub TR, Shah RC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolk DA, Dickerson BC Alzheimer’s Disease Neuroimaging Initiative. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentionalexecutive network function in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107:10256–61. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickerson BC, Wolk DA Alzheimer’s Disease Neuroimaging Initiative. Biomarker-based prediction of progression in MCI: comparison of AD signature and hippocampal volume with spinal fluid amyloid-β and tau. Front Aging Neurosci. 2013;5:55. doi: 10.3389/fnagi.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köhler S, Black SE, Sinden M, et al. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: an MR volumetry study in Alzheimer’s disease. Neuropsychologia. 1998;36:901–14. doi: 10.1016/s0028-3932(98)00017-7. http://www.ncbi.nlm.nih.gov/pubmed/9740363. [DOI] [PubMed] [Google Scholar]
- 22.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–96. doi: 10.1212/wnl.58.8.1188. http://www.ncbi.nlm.nih.gov/pubmed/11971085. [DOI] [PubMed] [Google Scholar]
- 23.Kramer JH, Schuff N, Reed BR, et al. Hippocampal volume and retention in Alzheimer’s disease. J Int Neuropsychol Soc. 2004;10:639–43. doi: 10.1017/S1355617704104050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Carret N, Auriacombe S, Letenneur L, et al. Influence of education on the pattern of cognitive deterioration in AD patients: the cognitive reserve hypothesis. Brain Cogn. 2005;57:120–6. doi: 10.1016/j.bandc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: pittsburgh compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63:112–8. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- 26.Roe CM, Mintun MA, D’Angelo G, et al. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh compound B uptake. Arch Neurol. 2008;65:1467–71. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern Y, Alexander GE, Prohovnik I, et al. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32:371–5. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- 28.Dumurgier J, Paquet C, Benisty S, et al. Inverse association between CSF aβ 42 levels and years of education in mild form of Alzheimer’s disease: the cognitive reserve theory. Neurobiol Dis. 2010;40:456–9. doi: 10.1016/j.nbd.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Arenaza-Urquijo EM, Molinuevo JL, Sala-Llonch R, et al. Cognitive reserve proxies relate to gray matter loss in cognitively healthy elderly with abnormal cerebrospinal fluid amyloid-β levels. J Alzheimers Dis. 2013;35:715–26. doi: 10.3233/JAD-121906. [DOI] [PubMed] [Google Scholar]
- 30.Querbes O, Aubry F, Pariente J, et al. Alzheimer’s Disease Neuroimaging Initiative. Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132:2036–47. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo SW, Im K, Lee JM, et al. Effects of demographic factors on cortical thickness in Alzheimer’s disease. Neurobiol Aging. 2011;32:200–9. doi: 10.1016/j.neurobiolaging.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Pillai JA, McEvoy LK, Hagler DJ, et al. Higher education is not associated with greater cortical thickness in brain areas related to literacy or intelligence in normal aging or mild cognitive impairment. J Clin Exp Neuropsychol. 2012;34:925–35. doi: 10.1080/13803395.2012.702733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Julkunen V, Paajanen T, et al. AddNeuroMed Consortium. Education increases reserve against Alzheimer’s disease--evidence from structural MRI analysis. Neuroradiology. 2012;54:929–38. doi: 10.1007/s00234-012-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W, Yu JT, Tan MS, et al. Cognitive reserve and Alzheimer’s disease. Mol Neurobiol. 2015;51:187–208. doi: 10.1007/s12035-014-8720-y. [DOI] [PubMed] [Google Scholar]
- 35.Lo RY, Jagust WJ Alzheimer’s Disease Neuroimaging Initiative. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord. 2013;27:343–50. doi: 10.1097/WAD.0b013e3182900b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441–54. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- 37.Stern Y, Albert S, Tang MX, et al. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942. doi: 10.1212/wnl.53.9.1942. http://www.ncbi.nlm.nih.gov/pubmed/10599762. [DOI] [PubMed] [Google Scholar]
- 38.Hall CB, Derby C, LeValley A, et al. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657–64. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- 39.Scarmeas N, Albert SM, Manly JJ, et al. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:308–16. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruandet A, Richard F, Bombois S, et al. Cognitive decline and survival in Alzheimer’s disease according to education level. Dement Geriatr Cogn Disord. 2008;25:74–80. doi: 10.1159/000111693. [DOI] [PubMed] [Google Scholar]
- 41.Chang YL, Bondi MW, McEvoy LK, et al. Global clinical dementia rating of 0. 5 in MCI masks variability related to level of function. Neurology. 2011;76:652–9. doi: 10.1212/WNL.0b013e31820ce6a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodard JL, Seidenberg M, Nielson KA, et al. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132:2068–78. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huntley JD, Gould RL, Liu K, et al. Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open. 2015;5:e005247. doi: 10.1136/bmjopen-2014-005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurz AF, Leucht S, Lautenschlager NT. The clinical significance of cognition-focused interventions for cognitively impaired older adults: a systematic review of randomized controlled trials. Int Psychogeriatr. 2011;23:1364–75. doi: 10.1017/S1041610211001001. [DOI] [PubMed] [Google Scholar]
- 45.Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. In: Woods B, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2012. p. CD005562. [DOI] [PubMed] [Google Scholar]
- 46.Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. In: Bahar-Fuchs A, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2013. p. CD003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lenehan ME, Summers MJ, Saunders NL, et al. Sending your grandparents to university increases cognitive reserve: the tasmanian healthy brain project. Neuropsychology. 2016:30. doi: 10.1037/neu0000249. [DOI] [PubMed] [Google Scholar]
- 48.Taylor KI, Salmon DP, Rice VA, et al. Longitudinal examination of American National adult reading test (AMNART) performance in dementia of the alzheimer type (DAT): validation and correction based on degree of cognitive decline. J Clin Exp Neuropsychol. 1996;18:883–91. doi: 10.1080/01688639608408309. [DOI] [PubMed] [Google Scholar]
- 49.Grober E, Hall CB, Lipton RB, et al. Memory impairment, executive dysfunction, and intellectual decline in preclinical alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266–78. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]