Abstract
Background
Despite increasing reliance on Prescription Drug Monitoring Programs (PDMPs) as a response to the opioid epidemic, the relationship between aberrant drug-related behaviors captured by the PDMP and opioid use disorder is incompletely understood. How PDMP data should guide Emergency Department (ED) assessment has not been studied.
Study Objective
To evaluate a relationship between PDMP opioid prescription records and self-reported non-medical opioid use of prescription opioids in a cohort of opioid dependent ED patients enrolled in a treatment trial.
Methods
PDMP opioid prescription records during one year prior to study enrollment on 329 adults meeting Diagnostic and Statistical Manual IV criteria for opioid dependence entering a randomized clinical trial (RCT) in a large, urban ED were cross tabulated with data on 30-day non-medical prescription opioid use self-report. The association among these two types of data was assessed by the Goodman and Kruskal’s Gamma; a logistic regression was used to explore characteristics of participants who had PDMP record of opioid prescriptions.
Results
During one year prior to study enrollment,118/329 (36%) patients had ≥ 1 opioid prescriptions (range 1–51) in our states’ PDMP. Patients who reported ≥15 out of 30 days of non-medical prescription opioid use were more likely to have ≥4 PDMP opioid prescriptions (20/38; 53%) than patients reporting 1–14 days (14/38, 37%) or zero days of non-medical prescription opioid use (4/38,11%); p=0.002. Female gender and having health insurance were significantly more represented in the PDMP (p<0.05 for both).
Conclusion
PDMPs may be helpful in identifying patients with certain aberrant drug-related behavior, but are unable to detect many patients with OUD. The majority of ED patients with OUD were not captured by the PDMP, highlighting the importance of using additional methods such as screening and clinical history to identify OUDs in ED patients and the limitations of PDMPs to detect OUDs.
Keywords: prescription opioids, prescription drug monitoring program, opioid prescribing, opioid use disorder
INTRODUCTION
The rates of medical and non-medical prescription opioid use have increased markedly in recent years, with a quadrupling of prescription opioid sales and overdose deaths between 1999 and 2012 and more than 33,000 fatal opioid overdoses in 2015.1,2 Local, state and federal agencies have implemented numerous programs and policies to combat this epidemic, including drug take-back programs, educational initiatives, naloxone access legislation, increased access to treatment for substance use disorders and state-based prescription drug monitoring programs (PDMPs).3–6 Emergency Department (ED)-based initiatives to address the opioid use and overdose epidemics include prescriber and patient education, take-home naloxone distribution and linkage of ED-patients to treatment for opioid use disorders.7,8 In 2016, the Centers for Disease Control and Prevention (CDC) released the “CDC Guideline for Prescribing Opioids for Chronic Pain,” which includes specific recommendations for initiating, continuing, and discontinuing opioid medications, PDMP query before initiating chronic opioid therapy and periodically thereafter, as well as assessing the risks and harms of opioid use.9
PDMPs are state-wide databases that collect patient-level information regarding the dispensation of controlled substances by pharmacies and providers and are often used to identify prescription patterns that may indicate aberrant drug-related behavior by the patient (or “doctor shopping”) or aberrant prescribing practices by prescribers.1,10,11 Though state-to-state variation exists, PDMPs data are typically available to registered prescribers and pharmacists, as well as registered law enforcement and state health officials under certain conditions.11,12 “Robust PDMPs,” or those with daily or more frequent data reporting, collection of a wider variety of medications (schedule II-V vs. schedule II), and PDMP query mandates have been associated with decreased prescription opioid fatalities in one analysis.13 Considerable variation exists between states with regard to virtually every aspect of PDMPs, including types or “schedules” of medication information collected, frequency of data uploading to PDMP, the time delay between query and when results are available, who has access to PDMP data and who in each state is responsible for the implementation and operation of the PDMP.13,14 As of 2017, all 50 states and the District of Columbia have operational PDMPs.11 While potential benefits of PDMPs include identifying prescription forgery, aberrant drug-related behavior15,16 and deviant opioid prescribing practices17, it is unclear if PDMPs are effective in identifying patients with specific substance use patterns or substance use disorders, and how PDMP data should guide prescribing practices.6
PDMPs have the potential to provide useful data and expedite care of challenging patients, and their wide-spread use is bolstered by CDC recommendations, state and federal policy maker interest and recent state-based legislation requiring prescriber PDMP registration and PDMP query prior to opioid prescribing.18–20 For example, one of the priorities of the published draft of the Interim Report of the White House Commission on Combating Addiction and the Opioid Crisis was to request federal support for the data-sharing of PDMPs nationwide by July 1, 2018.20 Multiple definitions for prescription pattern concerning for non-medical prescription opioid use or aberrant drug-related behavior15 have been evaluated: ranging from 4 opioid prescriptions from 4 or more providers within the past year, to the past year up to 8 or more prescriptions from 8 or more providers, with some incorporating multiple pharmacies.19,21–23 The importance of understanding how PDMP data should inform opioid prescribing is paramount, as prior studies show that PDMP data can either increase or decrease opioid prescribing practice, yet clear guidelines for how PDMP data should be interpreted for individual patients do not exist.6,21,24 The importance of evidence-based guidelines on how PDMP data should be integrated into clinical practice and decision making are particularly important as PDMP utilization mandates become increasingly common. As of December 2016, 35 states have mandatory PDMP use under certain circumstances,12 and no evidence based guidelines exist to guide how PDMP data should be applied on an individual level.
Importantly, the Diagnostic and Statistical Manual of Mental Disorders (DSM) outlines specific criteria for the diagnosis of opioid dependence (DSM-IV) and opioid use disorders (DSM-V), which excludes the ability to diagnose either condition solely based on PDMP data.25,26 Aberrant drug-related behavior is only one of several indicators that may suggest the presence of an opioid use disorder and is neither required nor sufficient to make a diagnosis of opioid use disorder.26 Although multiple prescriptions from multiple providers over a certain time period could be indicative of an opioid use disorder, it could also be a characteristic of an individual with recurrent injuries or poorly controlled pain without access to reliable primary care. Excellent correspondence between the DSM-4 diagnosis of opioid dependence and the DSM-5 diagnosis of moderate or severe opioid use disorder have been reported, supporting the application of treatments and interventions studied for DSM-IV opioid dependence to patients with moderate or severe opioid use disorder.27,28
The aim of this study was to evaluate the ability of our state’s PDMP to aid ED practitioners in identification of patients with potential opioid dependence by examining a degree of agreement between self-reported non-medical prescription opioid use and the number of prescriptions recorded in the PDMP in a population of ED patients meeting DSM-IV diagnostic criteria for opioid dependence.
METHODS
Study Design
This study consists of an exploratory analysis of data from a cohort of 329 patients meeting DSM-IV criteria for opioid-dependence previously enrolled in an ED-based randomized clinical trial (RCT) of interventions for opioid dependence conducted in a large urban, teaching hospital in New Haven, Connecticut.8
Our states’ PDMP, the Connecticut Prescription Monitoring and Reporting System (CPRMS), has same-day collection of schedule II-V medication data from pharmacies and prescribers, as well as legislative mandates for prescriber registration and PDMP query prior to a new prescription for >72-hour supply.10,11 While our PDMP collects information about routine schedule II-V prescribing including the use of methadone to treat chronic pain, methadone prescribed for treatment of opioid use disorder is not reported. PDMP data on opioid prescriptions for this cohort of DSM-IV opioid dependent patients were obtained for the year prior to their RCT enrollment and cross-referenced with self-report of non-medical prescription opioid use of collected at RCT enrollment. One year is the default time frame for PDMP query in our state’s PDMP. This data analytical study was reviewed and approved by the Yale University Human Investigation Committee (HIC). A data use agreement with Connecticut’s Department of Consumer Protection, the state agency at which our PDMP resides, was obtained.
RCT Setting and Population
The initial RCT was set in a large urban, teaching hospital in New Haven, Connecticut between April 7, 2009 and June 25, 2013. All patients met criteria for opioid dependence on the MINI-International Neuropsychiatric Interview (MINI) using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,25 and had urine toxicology screens positive for opiates or oxycodone. Patients were excluded from the RCT if they were currently enrolled in a drug treatment program, required hospitalization, were non-English speaking, critically ill, unable to communicate due to dementia or psychosis, suicidal, in police custody, had been engaged in formal addiction treatment within the past 30 days, had a psychiatric or medical condition requiring hospitalization, or required opioid medication for a pain condition.8
Protocol of the current data-analytical study
Two sources of de-identified data were created, linked, cross-referenced, and analyzed: a) data collected at RCT enrollment including demographics, self-reported past 30-day non-medical prescription opioid use using timeline follow back method, and healthcare utilization characteristics such as insurance status and having a primary care doctor;29 b) PDMP data on opioid prescriptions during the year prior to the RCT enrollment. RCT data were collected by patient self-report and were coded in the following manner: age (continuous variable), days of self-reported prescription opioid use out of past 30 (continuous variable), having a primary care doctor (binary), and past 30-day heroin use (binary). The PDMP based data set was created by accessing and reviewing one by one individual patient records in the State of Connecticut PDMP for the 329 patients enrolled in the RCT. PDMP data for one year prior to study enrollment was collected based on prior studies evaluating PDMP data over the past 12 months.19,21 Data on prescriptions of opioids, including oxycodone, hydrocodone, codeine, hydromorphone, oxymorphone, fentanyl, morphine, tramadol, and methadone, consisting of the prescription date, medication name, number of prescribers, and the number of prescriptions during the year prior to the RCT enrollment were transcribed into a secure electronic data set using numerical ids corresponding to the RCT enrollment record without patient identifiers.
While buprenorphine prescription data is collected by our PDMP, during the study period we assumed that buprenorphine was primarily prescribed for its’ FDA approved indication of the treatment of opioid use disorder and not for other indications. Therefore, including patients receiving prescriptions for buprenorphine in the current study would confound the aim of the analytical approach. We assumed that buprenorphine prescriptions were not indicative of potentially aberrant behaviors, but rather indicative of ongoing opioid use disorder treatment compliance and participation. Of the 21 patients with buprenorphine prescriptions in the PDMP, only a single patient received overlapping buprenorphine prescriptions from more than one prescriber. This overlap appeared to occur during transition from a hospital to a community provider. Therefore, potential illicit buprenorphine use and diversion, which has been reported, did not significantly contribute to aberrant drug-related behavior captured in this population during the study period.30
Outcome Measures and Statistical analyses
Descriptive statistics were used to characterize the study sample on past year overall numbers and distributional characteristics of opioid prescriptions and prescribers in PDMP and on self-reported days of non-medical prescription opioid use during 30 days prior to RCT enrollment. Using data extracted form PDMP, the participants were categorized into 3 non-overlapping groups: I) zero opioid prescriptions (nI=211), II) 1 to 3 opioid prescriptions (nII=80), III) 4 or more opioid prescriptions (nIII=38) in the year prior to RCT enrollment. This categorization scheme represents an ordinal gradient of potential risk of non-medical prescription opioid use based on the least restrictive definition of prescription pattern identified in the literature: from zero opioid prescriptions indicative of low or no risk, to 4 or more opioid prescriptions indicative of potentially substantial risk.21 Using self-reported data on the number of days with non-medical prescription opioid use in the prior 30 days, collected at RCT entry and approximating other published classifications indicating potential patterns concerning severity of non-medical prescription opioid use,21 participants were also categorized into 3 non-overlapping groups based on ordinal gradient of severity of non-medical prescription opioid use: A) zero days (nA=131), B) 1 to 14 days (nB=91), and C) 15 or more days (nC=107). This categorization resulted in dividing the entire sample into approximately teritles. Subsequently, measures of association or agreement between these two ordinal categorization methods were evaluated using the cross tabulation method and the chi-square test and the Goodman and Kruskal’s Gamma measure of association between ordinal variables.31
Additionally, characteristics including gender, age, race, insurance status, having a primary care provider, and history of heroin use were analyzed using direct multivariate logistic regression to evaluate for differences between patients who did and did not have past-year opioid prescriptions in the PDMP. These covariates were chosen from previously collected data based face validity and prior studies.32–34 Alpha was set at 0.05. Analyses were conducted with STATA 13.1 (College Station, TX)
RESULTS
Baseline demographic and clinical characteristics of patients have been previously reported and are briefly summarized in Table 1.8 Of note, patients were primarily male, insured (31.6% commercial, 43.2% Medicaid), and without a primary care physician (58.1%).
Table 1.
Baseline characteristics of study participants on enrollment
| Baseline Characteristics | No.(%) of total patients (n=329) | |
|---|---|---|
| Age, mean (SD) y | 31.4 (10.6) | |
| Men, N (%) | 251 (76.3) | |
| Has a Primary Care Doctor (%) | 138 (41.9) | |
| Past 30-day Heroin Use (%) | 247 (75.1) | |
| Race/Ethnicity, N (%) | White | 248 (75.4) |
| Black | 23 (7.0) | |
| Hispanic | 54 (16.4) | |
| Other | 4 (10.6) | |
| Education, N (%) | High School/GED/No College | 136 (41.3) |
| Some College | 113 (34.4) | |
| ≥College degree | 20 (6.1) | |
| Health Insurance, N (%) | Private/commercial | 104 (31.6) |
| Medicare | 6 (1.8) | |
| Medicaid | 142 (43.2) | |
| None | 71 (21.6) | |
| Usual Source of Care, N (%) | Private Physicians Office | 92 (27.9) |
| Clinic | 88 (26.7) | |
| Emergency Department | 149 (45.3) | |
One hundred eighteen of the 329 (36%) enrolled participants had at least one opioid prescription listed in the PDMP in the year prior to study enrollment. Of the 118 participants with any past year PDMP opioid prescription, the median number of opioid prescriptions was 2 and the maximum was 51 prescriptions [Interquartile Range: 1, 6]. Overall, 38 of the 329 (12%) patients had 4 or more opioid prescriptions in the past 12 months, with 23 (7%) having 4 or more opioid prescriptions from 4 or more unique providers. Of the 211 patients with no past year opioid prescriptions, 96 reported no past 30-day prescription opioid use (45%), 55 reported between 1 and 14 days (26%) and 60 reported ≥ 15 days of prescription opioid use (28%). Of the 198/329 who reported any non-medical prescription opioid use in the prior 30 days, 91 reported 1–14 days, and 107 reported 15–30 days of non-medical prescription opioid use.
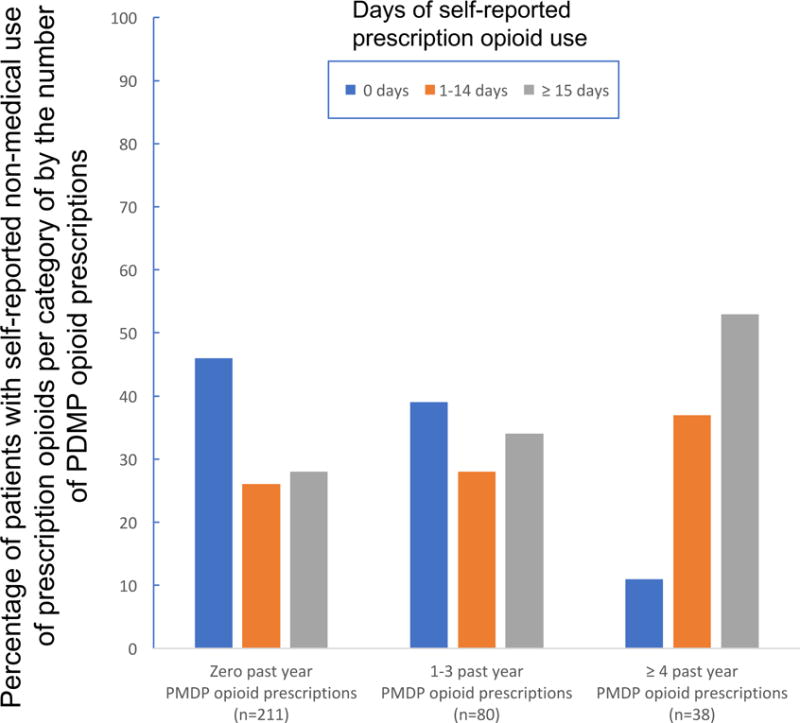
The cross-tabulation of patient categories based on PDMP and self-reported non-medical prescription opioid use data showed significantly different distribution pattern for patients with 4 or more opioid prescriptions in PDMP. In this PDMP category, there were significantly more patients who reported 15 to 30 days of non-medical prescription opioid use (20/38, 53%) as compared to patients reporting 1–14 days (14/38, 37%) or zero days (4/38, 11%). In contrast, in the two other PDMP categories (0 prescriptions and 1–3 prescriptions), the distributions of patients who reported no use or 1–14 days of non-medical prescription opioid use were comparable (chi-square=17.12, p=0.002). The measure of ordinal association gamma=0.3 was also statistically significant for this cross-tabulation. Figure 1 illustrates the distributions of past 30-day self-reported prescription opioid use groups by each PDMP category.
Figure 1.
In multivariate logistic regression analysis, patients that were female (OR=1.73, 95%CI=1.02–2.97) or had insurance (OR=1.96, 95%CI=1.07–3.58) were more likely to have at least one opioid prescription in the PDMP in the year prior to enrollment, and no difference was detected based on primary care provider, age, or prior heroin use. (Table 2). The Hosmer-Lemeshow test was used to evaluate the model fit. The value of the test was small, and the p=0.1 indicates a good fit to the data. The overall significance of the model was p=0.004.
Table 2.
Logistic regression for having ≥ 1 PDMP opioid prescription in PDMP in year prior to study enrollment using the following co-variates: gender, having insurance, having a doctor, age, heroin use in past 30 days (n=329).
| Adjusted Odds Ratio | 95% CI | |
|---|---|---|
| Female Gender | 1.73* | 1.02–2.97 |
| Has Insurance | 1.96* | 1.07–3.58 |
| Has Primary Care Doctor | 1.4‡ | 0.86–2.26 |
| Age (years) | 1.01‡ | 0.99–1.04 |
| Heroin Use | 1.01‡ | 0.40–1.19 |
p=<0.05
p=>0.10
Discussion
Cross-referencing PDMP opioid prescription data with self-report on non-medical prescription opioid use, we found a significant association between these two independently obtained indices: 53% of patients identified with 4 or more past year PDMP opioid prescriptions endorsed non-medical prescription opioid use in more than half of the 30 days prior to study enrollment. The overall measure of association between the ordinal categories of risk for non-medical prescription opioid use was also statistically significant and indicative of a weak positive relationship (gamma=0.3). Of note, a total of 211 (64%) of DSM-IV opioid dependent patients in our study had no prescriptions recorded in PDMP in the year prior to enrollment. Additionally, only 23 (7%) of DSM-IV opioid dependent patients enrolled in the study had 4 or more opioid prescriptions from 4 or more unique providers, a criterion often used as indicative of aberrant drug-related behavior.21 Collectively, these findings highlight the importance of using screening, good history taking, clinician impression, electronic medical records, in addition to utilizing the PDMP to identify patients with opioid use disorder. Also, the presence of buprenorphine prescriptions in the PDMP should alert a provider to the potential diagnosis of opioid use disorder.
The relative absence of past-year PDMP opioid prescriptions in an opioid-dependent ED patient population has not previously been demonstrated, although it is consistent with previous reports of prescription opioid sources for non-medical use. Analysis of National Survey on Drug Use and Health (NSDUH) data determined that approximately 1 out of 5 of respondents reporting non-medical prescription opioid use within the past 30 days reported one or more medical provider as their most recent opioid source.35,36 Of those with frequent non-medical prescription opioid use (≥ 200 days in the past year), 27.3% most recently obtained opioids from a physician, 26.4% obtained them from a friend or relative for free, 23% purchased them from a friend or relative, and 15% purchased from a dealer or stranger.35 Our findings that opioid-dependent women enrolled in our study were more likely than opioid-dependent men to have any prescriptions in the PDMP has also not previously been demonstrated, and a NSDUH analysis found that women were less likely to have a physician source for non-medical prescription opioid use than men.36
Despite the promise of PDMPs and their potential to provide useful and practice-changing information to prescribers, their ability to detect opioid use disorder has not been characterized and the sensitivity of PDMPs to identify patients with opioid use disorder or aberrant-drug related behavior is unknown.6 We do identify a potential harm of PDMP utilization if providers are “falsely reassured” about patients’ opioid use by the absence of patient data in the PDMP concerning for aberrant or non-medical prescription opioid use, because providers sometimes increase planned opioid prescribing after certain PDMP query results.21,24,37 Two ED-based studies found that accessing PDMP data changes opioid prescribing behavior, not infrequently resulting in increased opioid prescribing, presumably based on a “reassuring” PDMP report of few or no prior opioid prescriptions.21,24 Another study of prescribers across medical specialties found increased quantity of opioids prescribed by attending providers after checking the PDMP in 37% of cases.37 Importantly, these attending providers reported a high suspicion for drug diversion (47%) and prescription drug abuse (89%), suggesting that the PDMP data may have outweighed clinician impression, patient history or other clinical indicators.37
Our results also shed light on a recent ED-based study that found only fair concordance (kappa=0.3) between a PDMP definition and clinician impression of drug-seeking behavior.21 In addition to identifying some clinical factors such as “requesting opioid medication by name”, “multiple visits for same complaint”, and “suspicious history”, this study found that ED providers had a relatively low positive predictive value (41.2%) of identifying prescription pattern concerning for non-medical prescription opioid use based on clinician impression in comparison to the PDMP definition of 4 or more opioid prescriptions from 4 or more providers within the past year. 21 The authors hypothesized that the low positive predictive value was due to a large disconnect in the ability of ED clinicians or the PDMP to detect drug seeking behavior or prescription patterns concerning for non-medical prescriptions opioid use.21 Our results suggest that the deficit may lie in the ability of the PDMP to assist in the detection of aberrant-drug seeking behavior in certain populations (such as those who procure prescription opioids through non-physician sources), and suggests a more prominent role for clinician impression and clinical history in the development of safe prescribing practices.
Our finding that the number of self-reported days of non-medical prescription opioid use correlates with number of PDMP opioid prescriptions in an opioid-dependent population has not been previously demonstrated. This correlation, in combination with other studies suggesting improved opioid-related outcomes after PDMP implementation, supports a role of PDMP utilization in patient care.13,14,38 An interrupted time series analysis evaluating the effect of PDMP implementation across individual states found that a state’s implementation of a PDMP program was associated with an average reduction of 1.12 opioid-related overdose deaths per 100,000 people in the year after implementation, although a prior study found no such reduction in opioid-associated mortality.14,39 A reduction in prescription opioid-associated mortality was found to be associated with PDMPs that are more robust, such as those that require more frequent reporting or that include schedule III, IV and V medications in addition to schedule II medications, and those with laws mandating PDMP use.13 Though the PDMP can accurately reflect the history of prescription opioids of a patient, the ability to accurately differentiate between the phenotype of aberrant opioid use or opioid use disorder and that of that of un or under treated pain based on PDMP information alone, particularly amongst patients who are uninsured or do not have a primary care provider, remains a challenge. Despite a small body of work suggesting a positive benefit of PDMPs, multiple challenges limit the ability of PDMPs to exert their full potential including robustness, ease of navigation and integration electronic medical records, further research into how data associated with PDMPs should influence clinical care and changing physician attitudes regarding PDMP utilization.6,40–42
Collectively, our results should be interpreted as a caution for over-reliance on the PDMP to screen for aberrant drug-related behavior, and emphasizes the importance and role of good history taking and ED-based screening. Our results should not be interpreted as a reason for prescribers not to use PDMPs, but rather, be a reminder to consider the multitude of scenarios resulting in a low number of PDMP opioid prescriptions, before increasing opioid prescribing based on a paucity of PDMP prescriptions.
Limitations
Several limitations to this analysis and its interpretation in a broader medical context exist. These findings are based on ED patients who agreed to enroll in an opioid treatment based study and may not be generalizable to patient without opioid use disorder, or patients unwilling to enter a treatment trial for DSM-IV opioid dependence. Individual opioid use patterns of prescription opioids and heroin may vary significantly between the past 30 days and past year, and variations in individual patterns of prescription opioid use to non-medical prescription opioid use to heroin use may not be adequately captured by this analysis. This cohort reported high rates of heroin use (75%), which may limit the generalizability of our findings to other patient populations, although they did not exclusively use heroin as >60% of the cohort reported non-medical prescription opioid use within 30 days prior to study enrollment. PDMPs are limited to interstate data-sharing agreements and may not reflect all prescriptions obtained. It is also possible that data upload errors by pharmacies and provider uploading prescription information to the PDMP may result in inaccurate PDMP data. Additionally, it is possible that patients may have received prescriptions under an alias, which would not be associated with the patients’ name and date of birth when searching the PDMP. Finally, the results of the multivariate logistic regression model may be effected by the presence of unmeasured covariates and or cofounders.
Conclusion
The PDMP may be helpful in identifying patients with opioid use disorder with certain aberrant drug related behavior, but is unable to detect many patients with opioid use disorders. In our analysis, only 36% of DSM-IV opioid dependent ED patients enrolled in a treatment trial had one or more PDMP opioid prescriptions in the year prior to enrollment, with only 7% meeting our criteria for prescription pattern concerning for non-medical prescription opioid use. Although association or agreement between self-reported past 30-day prescription opioid use and PDMP opioid prescriptions was detected, a paucity of PDMP opioid prescriptions should be interpreted cautiously. This work highlights the importance of using the PDMP in conjunction with other screening tools, including patient history, biochemical analysis (when indicated), and other clinical indicators to identify patients with non-medical prescription opioid use.
Acknowledgments
The authors would like to acknowledge Rodrick Marriott, PharmD, Director of Drug Control in the Connecticut Department of Consumer Protection and Xaviel Soto, Program Manager of the state of Connecticut PDMP for their collaboration, insight, and review of the manuscript, and thank Andrew Taylor and Seth Luty for technical assistance.
Funding Sources/Disclosures: NIDA Grant# K12DA033312, 5R01DA025991
Footnotes
Prior Presentations: Association for Medical Education and Research in Substance Abuse Annual Conference, 2015.
References
- 1.Prescription Drug Overdose | Prescription Drug Overdose | CDC Injury Center [Internet]. [cited 2016 Jan 14];Available from: http://www.cdc.gov/drugoverdose/
- 2.Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–52. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 3.Kolodny A, Courtwright DT, Hwang CS, et al. The Prescription Opioid and Heroin Crisis: A Public Health Approach to an Epidemic of Addiction. Annu Rev Public Health. 2015;36(1):559–74. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 4.Hawk KF, Vaca FE, D’Onofrio G. Reducing Fatal Opioid Overdose: Prevention, Treatment and Harm Reduction Strategies. Yale J Biol Med. 2015;88(3):235–45. [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies–tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–6. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 6.Gugelmann H, Perrone J, Nelson L. Windmills and pill mills: can PDMPs tilt the prescription drug epidemic? J Med Toxicol. 2012;8(4):378–86. doi: 10.1007/s13181-012-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuels EA, Dwyer K, Mello MJ, Baird J, Kellogg A, Bernstein E. Emergency Department-Based Opioid Harm Reduction: moving physicians from willing to doing. Acad Emerg Med. 2016;23(4):455–65. doi: 10.1111/acem.12910. [DOI] [PubMed] [Google Scholar]
- 8.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–44. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Reports. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 10.DCP: Prescription Monitoring Program [Internet]. [cited 2017 Feb 21];Available from: http://www.ct.gov/dcp/cwp/view.asp?a=1620&q=411378&dcpNav_GID=1881
- 11.The PDMP Training and Technical Assistance Center [Internet]. [cited 2017 Feb 22];Available from: http://www.pdmpassist.org/
- 12.The National Alliance for Model State Drug Laws (NAMSDL) [Internet]. [cited 2017 Apr 21];Available from: http://www.namsdl.org/index.cfm
- 13.Pardo B. Do More Robust Prescription Drug Monitoring Programs Reduce Prescription Opioid Overdose? Addiction. 2016;112(10):1773–83. doi: 10.1111/add.13741. [DOI] [PubMed] [Google Scholar]
- 14.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation Of Prescription Drug Monitoring Programs Associated With Reductions In Opioid-Related Death Rates. Health Aff (Millwood) 2016;35(7):1324–32. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passik SD, Kirsh KL. The Need to Identify Predictors of Aberrant Drug-Related Behavior and Addiction in Patients Being Treated with Opioids for Pain. Pain Med. 2003;4(2):186–9. doi: 10.1046/j.1526-4637.2003.03018.x. [DOI] [PubMed] [Google Scholar]
- 16.Todd KH. Chronic Pain and Aberrant Drug-Related Behavior in the Emergency Department. J Law, Med Ethics. 2005;33(4):761–9. doi: 10.1111/j.1748-720x.2005.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 17.Kreiner PW, Strickler GK, Undurraga EA, Torres ME, Nikitin RV, Rogers A. Validation of prescriber risk indicators obtained from prescription drug monitoring program data. Drug Alcohol Depend. 2017;173:S31–8. doi: 10.1016/j.drugalcdep.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA. 2015;313(9):891–2. doi: 10.1001/jama.2014.18514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner SG, Griggs CA, Langlois BK, et al. Characteristics of emergency department “doctor shoppers”. J Emerg Med. 2015;48(4):424–31.e1. doi: 10.1016/j.jemermed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 20.White House Commission on Combating Drug Addiction and the Opioid Crisis. Interim Report: White House Commission on Combating Drug Addiction and the Opioid Crisis. 2017 [Google Scholar]
- 21.Weiner SG, Griggs CA, Mitchell PM, et al. Clinician impression versus prescription drug monitoring program criteria in the assessment of drug-seeking behavior in the emergency department. Ann Emerg Med. 2013;62(4):281–9. doi: 10.1016/j.annemergmed.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–20. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 23.Lev R, Lee O, Petro S, et al. Who is prescribing controlled medications to patients who die of prescription drug abuse? Am J Emerg Med. 2016;34(1):30–5. doi: 10.1016/j.ajem.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Baehren DF, Marco CA, Droz DE, Sinha S, Callan EM, Akpunonu P. A statewide prescription monitoring program affects emergency department prescribing behaviors. Ann Emerg Med. 2010;56(1):19–23-3. doi: 10.1016/j.annemergmed.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-4. Washington, D.C.: American Psychiatric Association; 2010. [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. American Psychiatric Association; 2013. American Psychiatric Association. DSM-5 Task Force. [Google Scholar]
- 27.Boscarino JA, Rukstalis MR, Hoffman SN, et al. Prevalence of Prescription Opioid-Use Disorder Among Chronic Pain Patients: Comparison of the DSM-5 vs. DSM-4 Diagnostic Criteria J Addict Dis. 2011;30(3):185–94. doi: 10.1080/10550887.2011.581961. [DOI] [PubMed] [Google Scholar]
- 28.Compton WM, Dawson DA, Goldstein RB, Grant BF. Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug Alcohol Depend. 2013;132(1–2):387–90. doi: 10.1016/j.drugalcdep.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobell L, Sobell M. sobell TLFB.pdf. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 30.Lavonas EJ, Severtson SG, Martinez EM, et al. Abuse and diversion of buprenorphine sublingual tablets and film. J Subst Abuse Treat. 2014;47(1):27–34. doi: 10.1016/j.jsat.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Goodman LA, Kruskal WH. Measures of Association for Cross Classifications, IV: Simplification of Asymptotic Variances. J Am Stat Assoc. 1972;67(338):415–21. [Google Scholar]
- 32.Green TC, Grimes Serrano JM, Licari A, Budman SH, Butler SF. Women who abuse prescription opioids: Findings from the Addiction Severity Index-Multimedia Version® Connect prescription opioid database. Drug Alcohol Depend. 2009;103(1–2):65–73. doi: 10.1016/j.drugalcdep.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han H, Kass PH, Wilsey BL, Li C-S. Individual and County-Level Factors Associated with Use of Multiple Prescribers and Multiple Pharmacies to Obtain Opioid Prescriptions in California. PLoS One. 2012;7(9):e46246. doi: 10.1371/journal.pone.0046246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McHugh RK, DeVito EE, Dodd D, et al. Gender differences in a clinical trial for prescription opioid dependence. J Subst Abuse Treat. 2013;45(1):38–43. doi: 10.1016/j.jsat.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use United States, 2008–2011. JAMA Intern Med. 2014;174(5):802–3. doi: 10.1001/jamainternmed.2013.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saloner B, Bachhuber M, Barry CL. Physicians as a Source of Medications for Nonmedical Use: Comparison of Opioid Analgesic, Stimulant, and Sedative Use in a National Sample. Psychiatr Serv. 2017;68(1):56–62. doi: 10.1176/appi.ps.201500245. [DOI] [PubMed] [Google Scholar]
- 37.Feldman L, Skeel Williams K, Knox M, Coates J. Influencing controlled substance prescribing: attending and resident physician use of a state prescription monitoring program. Pain Med. 2012;13(7):908–14. doi: 10.1111/j.1526-4637.2012.01412.x. [DOI] [PubMed] [Google Scholar]
- 38.Rasubala L, Pernapati L, Velasquez X, Burk J, Ren Y-F. Impact of a Mandatory Prescription Drug Monitoring Program on Prescription of Opioid Analgesics by Dentists. PLoS One. 2015;10(8):e0135957. doi: 10.1371/journal.pone.0135957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(5):747–54. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 40.Hildebran C, Cohen DJ, Irvine JM, et al. How clinicians use prescription drug monitoring programs: a qualitative inquiry. Pain Med. 2014;15(7):1179–86. doi: 10.1111/pme.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrone J, DeRoos FJ, Nelson LS. Prescribing practices, knowledge, and use of prescription drug monitoring programs (PDMP) by a national sample of medical toxicologists, 2012. J Med Toxicol. 2012;8(4):341–52. doi: 10.1007/s13181-012-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poon SJ, Greenwood-Ericksen MB, Gish RE, et al. Usability of the Massachusetts Prescription Drug Monitoring Program in the Emergency Department: A Mixed Methods Study. Acad Emerg Med. 2016;23(4):406–14. doi: 10.1111/acem.12905. [DOI] [PubMed] [Google Scholar]


