1. Introduction
Neuronal energy demands are met by a tightly coupled and adaptive vascular network that supplies nutrients and oxygen. The retina is one of the highest energy-consuming organs, exceeding the metabolic rate of the brain; blood vessels grow and regress in reaction to changes in these high demands (Ames et al., 1992b; Anderson and Saltzman, 1964; Yu and Cringle, 2001). Reduced nutrients and reduced oxygen availability instigate compensatory albeit misguided pathological neovascularization in proliferative retinopathies (Chen and Smith, 2007; Sapieha et al., 2010). Conversely, impaired retinal ganglion cell (RGC) and photoreceptor survival are correlated with abrogated vascular development (Pennesi et al., 2008) and as neurons degenerate, the retinal vasculature atrophies to match the reduced metabolic requirements (Wang et al., 2000). In mice, genetic ablation of retinal ganglion cell neurons suppresses the inner retinal vascular development (Sapieha et al., 2008). Ablation of amacrine interneurons also prevents the development of the intermediate vascular plexus (Usui et al., 2015), while photoreceptor degeneration is associated with thinning of the choroid and inner retinal blood vessels (Ayton et al., 2013; Dhoot et al., 2013; Toto et al., 2016). Thinning of the choriocapillaris, in turn, may exacerbate retinal degeneration (Bird, 2010; Whitmore et al., 2015). However, the specific mediators that link neuronal metabolism with retinal angiogenesis in the developing eye and retinal disease remain largely unknown. Conditions such as diabetic retinopathy, vaso-proliferative retinopathy of prematurity and neovascular age-related macular degeneration (AMD) have been characterized as diseases of the vasculature. However, it is becoming more evident that the metabolic needs of the neural retina profoundly influence blood vessel supply in development and in disease.
Retinal oxygen sources and the vaso-proliferative response to low oxygen levels have been well characterized. However, understanding the specific fuels used in the retina to generate ATP and supply building blocks for biosynthesis, as well as understanding the vaso-proliferative response to the lack of fuel are also key to neurovascular development. The metabolic and energy needs of the retina have been assumed to be met by glucose, as the retina is part of the CNS, and the brain relies almost exclusively on glucose (Mergenthaler et al., 2013). There are two primary pathways that cells can use to generate ATP from glucose, glycolysis and oxidative phosphorylation. However, Cohen and Noell concluded in 1960 that a substantial portion of the energy produced through oxidation by the retina (around 65%) was not derived from glucose (Cohen and Noell, 1960). We recently showed that the retina (photoreceptors) can also oxidize lipid through fatty acid β-oxidation to produce ATP, accounting for the energy gap noted by Cohen (Joyal et al., 2016). Little is known about lipid versus glucose fuel substrate preference and its importance during retinal development and pathology. Here we review the neuronal energy demands of the retina, describing both glucose and lipid metabolism as forces that shape the vascular supply of the eye in development and in vaso-proliferative eye diseases.
2. Evolving architecture of the retinal vasculature during development
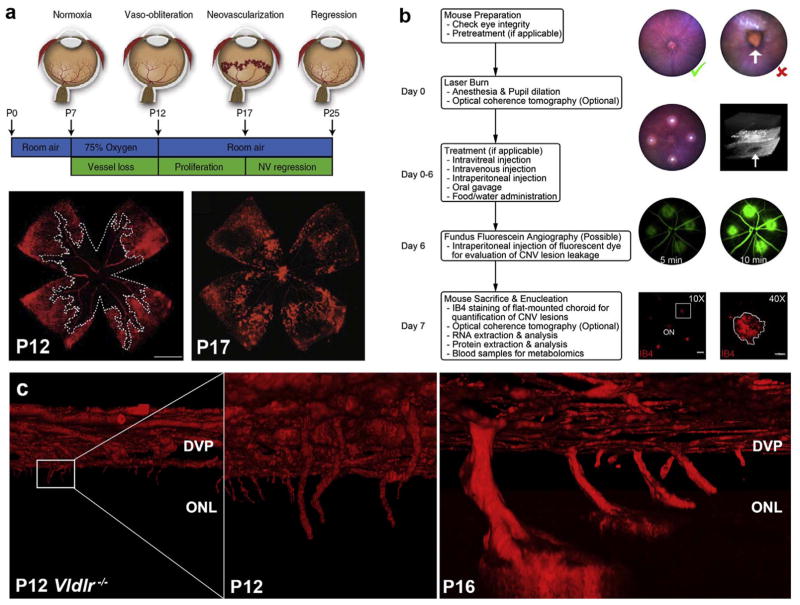
Two vascular networks supply the mature retina, the inner retinal vasculature and the choroid. The inner retinal vasculature provides nutrients and oxygen to the inner two-thirds of the retina and forms three distinct vascular layers originating from branches of the central retinal artery. Murine inner retinal vascular development begins after birth, making it useful for preclinical studies of preterm retinal vascular development. The superficial vasculature forms first and emerges from the optic nerve, migrating radially to reach the periphery of the retina within 7–10 post-natal days (P10; Fig. 1a–e). As photoreceptors increase their metabolic demand, vessels then penetrate the inner retina to form the deep vascular layer, delineated by the outer plexiform layer (OPL), immediately adjacent to photoreceptors (P8-12) (Fig. 1d). The intermediate vascular layer forms last with further maturation of the inner neural retina (P14-20; Fig. 1d). Vascular development is completed shortly before full-term birth in humans and at about P21 in mice (reviewed by (Fruttiger, 2007; Ruhrberg and Bautch, 2013; Ståhlberg and Bengtsson, 2010)). The inner retinal circulation is characterized by low blood flow and a high oxygen extraction; arteriovenous pO2 differences are ~40% (Wangsa-Wirawan and Linsenmeier, 2003). Retinal vascular endothelial cells are not fenestrated and form tight junctions that create a blood-retinal barrier; this barrier is reminiscent of the blood-brain barrier and ensures selective exchanges between the circulation and the neural retina.
Fig. 1. Retinal vascular development follows neuronal maturation.
(a) Similar radial distribution of neurons (RGCs marked with leptin; blue), glia (astrocytes labeled with GFAP; green) and blood vessels (Dextran; red). Arrow points to the optic nerve. (b) Migratory tip cell, which does not proliferate (star; nucleus) with adjacent proliferating stalk cell in cellular division (phospho-histone-H3, green with arrow). (c) Astrocytes lay down a path for growing vessels, starting before birth (E20, left) and completed at birth (P0); vessels are red (right). (d) Inner retinal vascular development begins with the formation of the superficial vascular plexus (1 and e). Formation of the deep vascular plexus (2) corresponds temporally to the maturation of photoreceptors (f). The intermediate vascular plexus forms last (3), likely from vessels of the superficial vascular plexus. (f) Timeline of photoreceptor development from the terminal mitosis of rod precursors to the onset of rhodopsin expression. Mice open their eyes between P12-15, and the outer plexiform layer has developed by P14. Although some rod activity is detectable at P12, photoreceptor outer segments continue to mature until P19-25. Figure modified, with permission, from (Dorrell and Friedlander, 2006; Gariano and Gardner, 2005; Gerhardt et al., 2003; Morrow et al., 1998).
The retinal vascular development follows a highly stereotyped trajectory that suggests a carefully regulated and guided process (Dorrell and Friedlander, 2006). Neurons guide and attract blood vessels, which explains the anatomical coupling and functional crosstalk between these two major networks. Hence, superficial retinal vessels do not physiologically invade the adjacent vitreous body where they would obstruct incoming light to photoreceptors and create a shearing force on the retina. If shared guidance receptors are lost in neurons, such as VEGFR2 (Okabe et al., 2014), or the secretion of Vegf by neurons is aberrant (Mukouyama et al., 2002), the coupling is lost causing misdirected angiogenesis (Witmer et al., 2003). The deep and intermediate layers of inner retinal vessels are also strictly limited to a well-defined neuronal plane, which is violated only in disease states. Under conditions of normal vascular development, growth factors from the organizing neural cells guide vascular positions (Dorrell and Friedlander, 2006; Fantin et al., 2009; Klagsbrun and Eichmann, 2005). The formation of the outer vascular plexus in humans (25–26 weeks of gestation) (Hughes et al., 2000) coincides with the first appearance of neuronal activity (visual evoked potentials), with a corresponding and significant increase in oxygen and fuel consumption by retinal neurons (Cringle et al., 2006; Medrano and Fox, 1995; Yu and Cringle, 2001).
The choroidal vascular plexus arises from posterior ciliary arteries and branches of Sinn’s circle, located around the optic disc, which breaks up into fan-shaped lobules of capillaries (Hardy et al., 2000; Kiel and van Heuven, 1995). The choriocapillaris is a high-flow capillary network that provides nutrients and oxygen to the retinal pigment epithelium (RPE) and the metabolically demanding photoreceptors of the outer retina. Since the choriocapillaris is lined by fenestrated endothelial cells, the RPE instead comprises the entire outer blood-retinal barrier between the choroid and the neurosensory retina. Photoreceptors also interact with the inner retinal vasculature to some extent because mice with photoreceptor loss from retinal degeneration do not develop vascular proliferation in the inner retina in models of proliferative retinopathy (de Gooyer et al., 2006a; de Gooyer et al., 2006b).
2.1. Metabolic needs of maturing neurons sculpt the developing retinal vessels
Neuronal development drives the formation of the mature vasculature. In murine models, differentiation and maturation of the seven retinal neural cell types occur between embryonic day 9 (E9) and postnatal day 7 (P7). The cell types develop in an evolutionarily conserved order, although multiple cell types are generated simultaneously at any given developmental stage. Cones are created early in development, and rods are formed later (Altshuler, 1991; Morrow et al., 1998) (Fig. 1f). In both man and rodents, the vascular network that supplies the eye reorganizes dramatically as this neural development occurs, as an initial fetal network is replaced by the mature vasculature.
During the earliest phase of eye development, the embryonic optic cup is first vascularized through the fetal fissure by the hyaloid artery. With further differentiation and increasing metabolic maturity of the developing cells, the hyaloid artery extends from the optic nerve through the vitreous to form a branching network around the lens (the tunica vasculosa lentis) and extends branches to the retina to supply the inner eye. The choroidal vessels are formed in the second and third months of gestation in man, before the development of retinal vessels (Lutty et al., 2010). The hyaloid system regresses as the retinal vasculature develops (Saint-Geniez and D’Amore, 2004; Zhu et al., 2000), a process that coincides with increasing differentiation and metabolic demands of the maturing neural retina. The metabolic shifts from aerobic to anaerobic metabolism in the differentiating lens fibers correlate with the loss of organelles including mitochondria, making the lens transparent (Ash and Overbeek, 2000; Gogat et al., 2004; Mitchell et al., 1998; Shui et al., 2003). Regression of the hyaloid vessels is controlled by retinal neurons, astrocytes, and macrophages via oxygensensing and Wnt pathways (Kurihara et al., 2010; Lobov et al., 2005; Rao et al., 2013). If hyaloid vessels do not entirely regress, the persistence of the hyaloid vasculature is associated with poor retinal development and poor vision in neonates (JONES, 1963; Liu and Nathans, 2008; Saint-Geniez and D’Amore, 2004). The regression of the hyaloid vessels normally occurs in humans around mid-gestation and mice after birth.
2.2. Migratory tip cells and proliferating endothelial stalk cells guide vascular growth
The primary navigating cell of the vascular front is the endothelial tip cell, which probes and senses chemo-attractive and repulsive environmental cues (Gerhardt et al., 2003 (Gerhardt et al., 2003). Stalk cells follow just behind tip cells and proliferate to elongate the vessels (Gerhardt et al., 2003), then form a vascular lumen (Iruela-Arispe and Davis, 2009) (Fig. 1b). Tight junctions expressed on stalk cells maintain the integrity of the nascent vessel (Dejana et al., 2009). The final maturation of vessels involves pruning of excess vasculature (Ishida et al., 2003) as metabolic and oxygen needs are met and by recruitment of mural cells (Das and McGuire, 2003).
3. Neuronal growth factors and guidance cues that shape the vascular network
3.1. Vascular Endothelial Cell Growth Factor (VEGF)
Neurons with changing energy requirements signal for corresponding adaptations in vascular supply by shared neural and vascular guidance cues, of which a prime example is vascular endothelial growth factor (VEGF) (Robinson et al., 2001). The radial development of the superficial retinal vasculature with an expanding circular wave of hypoxia (and likely energy fuel deficits) corresponds to a wave of VEGF production in front of the developing vasculature and maturing neurons (Chan-Ling et al., 1995; Stone et al., 1995). Endothelial tip cells respond to VEGF by forming motile filopodia enriched in VEGFR2 and Nrp1 (Fig. 1b), and also other guidance receptors such as Unc5b and Eph (Adams et al., 1999; Klagsbrun and Eichmann, 2005; Wilson et al., 2006), which respond to directional cues (Gerhardt et al., 2003). Proliferating stalk endothelial cells responding to VEGF, follow behind tip cells to elongate the vessels (Gerhardt et al., 2003). Posterior to the front of vascularization, the increased oxygen and nutrient supply from newly formed vessels suppresses VEGF expression, while anterior to the front, hypoxic maturing neurons, and glia without a vascular supply cause an increase in VEGF, proceeding in an expanding wave until vascularization is complete. In the maturing retina, neurons are an important source of VEGF to control the development of the superficial and deep vascular layers (Sapieha et al., 2008), more than astrocytes and Müller glial cells as was previously believed (Scott et al., 2010; Stone et al., 1995; Weidemann et al., 2010).
The oxygen-dependent regenerative vascular program is mediated in large part by the hypoxia-inducible factor 1-alpha (HIF1A). HIF1a is a transcription factor for a number of growth factor, including VEGF, that is stabilized by hypoxia and readily degraded by prolyl hydroxylases in the presence of oxygen (Boulahbel et al., 2009; Dor et al., 2001; Fraisl et al., 2009; Pugh and Ratcliffe, 2003; Schofield and Ratcliffe, 2004; Semenza, 2007; Stolze et al., 2006). Regression of the rudimentary hyaloid vessels is also mediated by the suppression of HIF1A and VEGF in the presence of higher retinal oxygen (Kurihara et al., 2011). After the vascular development of the retina is complete, autocrine VEGF expression in endothelial cells (Lee et al., 2007) and neurons (Ruiz de Almodovar et al., 2009) is required to maintain tissue homeostasis. In the outer retina, production of VEGF by RPE is also essential to sustain the viability of the choriocapillaris (Kurihara et al., 2012).
VEGF also plays a critical role in pathologic angiogenesis. While hypoxia stimulates angiogenesis, excess oxygen suppresses VEGF and vascular growth. In preterm infants, supplemental oxygen exposure is a significant risk factor for retinopathy of prematurity, a disease associated with suppression of retinal vascular development (Chan-Ling et al., 2017; Hansen et al., 2017). As the now avascular neural retina matures, increasing its metabolic demands, VEGF is secreted by the hypoxic neuroglia giving rise to pathological neovascularization (Joyal et al., 2012, 2015; Pierce et al., 1995; Pierce et al., 1996; Scott et al., 2010; Sitaras et al., 2015; Stone et al., 1995; Weidemann et al., 2010). Microglial cells also contribute to VEGF secretion and pathological angiogenesis, which is reviewed elsewhere (Binet and Sapieha, 2015; Guillonneau et al., 2017). Similarly in the outer retina, excessive expression of VEGF by photoreceptors and RPE contributes to the subretinal neovascularization that characterizes wet AMD, discussed in more detail later (Campochiaro, 2015; Grisanti and Tatar, 2008; Joyal et al., 2016; Ohno-Matsui et al., 2002; Witmer et al., 2003).
3.2. Other growth factors and guidance cues
Other growth factors also govern vascular patterning. Astrocytes form an early superficial template that guides the initial vascularization of the inner retina. Neurons generate platelet-derived growth factor (PDGF) organizing the astrocytic network apposition, which expresses its corresponding receptor, platelet-derived growth factor receptor-alpha (PDGFRA). Tight junctions with the astrocyte bed (Fruttiger et al., 1996) (via R-Cadherin) and the graded production of VEGF by neuroglia (Stone et al., 1995) and RGCs (Joyal et al., 2014; Sapieha et al., 2008) on the retinal surface ensure directed growth of vessels (Okabe et al., 2014). Although Müller glia and astrocytes were previously believed to be the main source of VEGF, genetic ablation of VEGF in Müller glia and astrocytes does not impact retinal vascular development (Scott et al., 2010; Stone et al., 1995; Weidemann et al., 2010). Moreover, transgenic mice expressing a diffusible VEGF120 isoform present a disorganized astrocytic bed inferring a controlling role of VEGF, dominating that of PDGF, in orchestrating the neuro-glial relationship (Stalmans et al., 2002). Neurons are the most avid oxygen consuming cells in the retina. The recent demonstration of neurons’ ability to liberate angiogenic factors (including VEGF) in response to metabolic needs suggests they may drive vascularization, while the astroglia likely play a more supportive role with amplification of their metabolic signals. Thus, localized regions of retinal hypoxemia or high energy need prompt VEGF, as well as erythropoietin (Epo) secretion (Alon et al., 1995; Chen et al., 2008, 2009; Chen and Smith, 2008), initiating the growth of neo-vessels. O2-independent factors, such as angiopoietins/Tie2 (Ramsauer and D’Amore, 2002) and insulin-like growth factor-1 (IGF-1) (Smith et al., 1999), also participate in the physiological vascularization of the retina. More discussion concerning the role of IGF-1 can be found in reviews (Hellström et al., 2013, 2016; Hellstrom et al., 2001; Liegl et al., 2016a; Liegl et al., 2016b; Lofqvist et al., 2009; Wu et al., 2010).
Both neurons and vessels appear to be guided by a combination of analogous attractant and repellent molecular cues. Of these conserved guidance proteins, the semaphorins, ephrins, netrins and slits and their cognate receptors the neuropilins (Nrp), Eph receptors, roundabout (Robo), and uncoordinated-5 (UNC5) respectively, play central roles in both neuronal patterning and vascular growth. Semaphorin 3A (sema3A) in particular (Fantin et al., 2009; Joyal et al., 2011), ephrinB2 (Ehlken et al., 2011), and to variable extents Netrin-1a and Slit-2 (Wang et al., 2003; Wilson et al., 2006), generally repulse endothelial cells. Netrins and Slits may present opposing actions possibly due to stimulation of different receptor subtypes, cleavage of the original protein, or the modulation of their effects by pro-angiogenic factors (Klagsbrun and Eichmann, 2005). More in-depth discussion of the role of neuronal guidance cues in vascular development can be found in other reviews (Bussolino et al., 2006; Carmeliet and Tessier-Lavigne, 2005; Dorrell and Friedlander, 2006; Klagsbrun and Eichmann, 2005; Sapieha, 2012; Serini and Bussolino, 2004). Neurovascular crosstalk through shared guidance cues may, therefore, allow neuronal energy demands to signal for corresponding adaptations in vascular supply.
4. Neuronal energy requirements to sustain retinal function
The metabolic demands of neurons that determine the vascular network that supplies oxygen and nutrients are intrinsically linked to neuronal structure and function. The primary energetic demands include light sensing via phototransduction and maintenance of electrical gradients, production of the molecules and structures (such as renewable outer segments) that allow vision and managing the oxidative stress arising from these processes.
4.1. Energy cost of phototransduction and the maintenance of electrical gradients
The primary site of phototransduction, which is energy intensive, is the specialized photoreceptors (Fig. 2). Unlike most neurons, photoreceptors signal in absence of stimuli and their neurotransmitter release is higher in the dark. In the absence of light, open channels allow a steady flow of ions in and out of the cell resulting in a cellular depolarization known as the ‘dark current (Stryer, 1991). Upon light stimulation, the ion channels are closed, neurotransmitters release is suppressed causing photoreceptor hyperpolarization, which leads to phototransduction. More than half of photoreceptor energy (ATP) consumption is due to Na+/K+ ATPase ion pumps, which maintain ion levels in the cell (Hagins et al., 1970; Okawa et al., 2008). Concordantly, Na+/K+ ATPase distribution corresponds to areas rich in mitochondria with expression levels proportional to neuronal activity (Fig. 2) (Ames et al., 1992b). Thus photoreceptors (seemingly paradoxically) consume more energy in darkness.
Fig. 2. Photoreceptors and mitochondrial distribution in primate retina.
(a,b) Distribution of cones (c) and rods (r) in the human retina, relative to the optic nerve. Cone density increases in the macula and peaks in the fovea. (c) Macaque retina stained for cytochrome c oxidase (COX) or Na + K + ATPase, markers of mitochondria or areas of higher energy consumption in the retina. Inner segments (IS of photoreceptors strongly express both markers. NFL: Nerve fiber layer, GCL: ganglion cell layer, IPL: Inner plexiform layer, INL: Inner nuclear layer, OPL: Outer plexiform layer, ONL: Outer nuclear layer, IS: inner segment, OS: outer segment. (d) Cone inner segments (CIS) contain much larger and more densely packed mitochondria than rod photoreceptor, by electron microscopy. Figure modified, with permission, from (Curcio et al., 1990; Kageyama and Wong-Riley, 1984; Wong-Riley et al., 1998).
4.2. Energy cost of managing oxidative stress
Dealing with the high oxidative stress experienced by the retina and particularly the photoreceptor is energetically costly. The light that stimulates phototransduction also leads to light-induced damage of lipids, proteins and nucleic acids which must be detoxified and replaced (Kagan et al., 1973). Furthermore, the high levels of oxygen needed for energy production (i.e. oxidative phosphorylation) increases oxidative stress, first by exposure of the retina to free oxygen from high blood flow and second from the reactive oxygen species generated in the mitochondria during oxidative phosphorylation. The retina spends additional energy to prevent, neutralize and repair the effects of oxidative damage.
4.3. Energy cost of continuous replacement of outer segments
To avoid accumulation of light-induced oxidative damage to the non-replicating cell body of the neuron, photoreceptors have evolved a segregated and renewable outer segment, which is a lipid- and protein-rich structure housing light-sensing rhodopsin and cone opsins. Unlike the non-replicating inner segment, which contains the nucleus, the mitochondria, and biosynthetic machinery, the outer segment structure is continuously built, shed, and then regenerated. Approximately 10% of the outer segment is discarded daily from the photoreceptor and phagocytized by the neighboring retinal pigment epithelium (RPE) (LaVail, 1976). The photoreceptors must continually synthesize or scavenge lipids and proteins to maintain the outer segment structure and function. While the anatomical partition of photoreceptors into non-replicating inner segments and replicating outer segments minimizes the risk of cumulative oxidative damage to the photoreceptor nucleus, there is still a high energy cost to ensure the continuous regeneration of outer segments.
5. Pathways to energy production in the retina
How the retina meets its energy demands is not fully understood. There are two primary pathways that cells can use to generate ATP, glycolysis and oxidative phosphorylation (OXPHOS). Work over the past century has highlighted the need of the retina to have both a very high glycolytic and oxidative capacity. The following sections will review the relative contributions of these energy-producing pathways in the retina, as well as discuss the substrate utilization and ability of the retina to use lipids to fuel OXPHOS.
6. Oxygen consumption and oxidative phosphorylation
Oxygen consumption reflects the activity of the electron transport chain and the production of ATP by the mitochondria. The retina is one of the most oxidative tissues in the body, consuming more oxygen than the brain (Ames Iii, 1992) and has the equivalent expression of oxygen-carrying proteins as skeletal muscle (Schmidt et al., 2003). The outer retina, which consists mainly of photoreceptors with some Müller glial feet, is estimated to account for more than 60% of the oxygen consumption of the retina (Du et al., 2016; Medrano and Fox, 1995). This is a consequence of the very high density of mitochondria and oxidative enzymes in the photoreceptors. Approximately 60% of the total mitochondria of the retina are localized specifically to the photoreceptor inner segments (Lowry et al., 1956).
In vivo measurements of oxygen tension in the retina of various animal models have shown that oxygen levels are highest near the choroid and rapidly decrease moving towards to the photoreceptors (Linsenmeier, 1986; Yu and Cringle, 2005) (recently reviewed (Linsenmeier and Zhang, 2017)). Indeed, the oxygen tension is lowest at the ellipsoid zone in the photoreceptors, correlating with the location of the mitochondria. This suggests that the mitochondria in the outer retina are actively consuming the vast majority of the available oxygen as it diffuses from the choroid. In vascularized retinas (those with a blood supply to the inner retina), the oxygen tension increases again after passing the photoreceptor mitochondrial layer towards the inner retina, indicating that the inner retina is supplied by these vessels. In the cat, the choroid provides 90–100% of fuel and oxygen to photoreceptors both in the dark and light and the inner retinal circulation supplies the inner retina with 100% of its supply in dark or light (Linsenmeier and Braun, 1992). Photoreceptors may derive oxygen from the inner retinal vasculature to some extent because photoreceptor loss from retinal degeneration alters the inner vasculature (de Gooyer et al., 2006a; de Gooyer et al., 2006b). This may be indirect though, since the activity of the second and third order neurons are dependent on the presence of the photoreceptors.
In addition to supplying ATP via oxidative phosphorylation, the mitochondrial TCA cycle is a hub for anabolic reactions that supply many of the building blocks needed for biosynthesis. It is fitting therefore that photoreceptors have a high oxidative capacity and high mitochondrial content to help meet their high ATP requirements as well as to meet the high biosynthetic demands of regenerating their outer segments.
7. Carbohydrate metabolism in the retina
7.1. Role of aerobic glycolysis
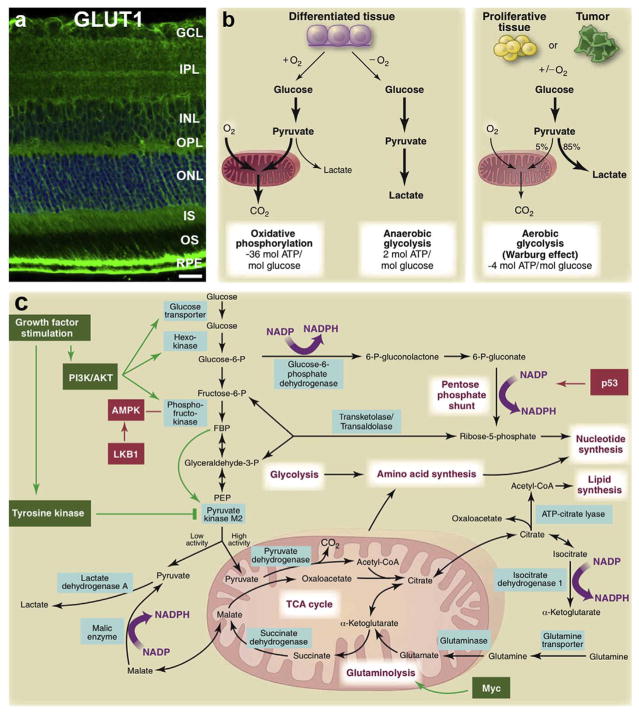
During glycolysis, glucose is oxidized to pyruvate, which can either be converted to lactate or transported into the mitochondria and fully oxidized, yielding substantially more ATP (Fig. 3a and b). When glucose is converted to lactate, approximately 15 times less ATP is generated than when glucose is used for OXPHOS. Nearly a century ago, researchers, including Otto Warburg, noted that a defining feature of retinal glucose metabolism was the rapid production of lactate (Cohen and Noell, 1960; Ng et al., 2015; Warburg et al., 1924). Canonically, high lactate production occurs when oxygen is limiting. However, in the retina, high lactate levels are produced even in the presence of oxygen and elevated mitochondrial respiration (as discussed; Fig. 3a and b). Lactate production in the presence of oxygen is referred to as aerobic glycolysis or the Warburg effect (Fig. 3b and c).
Fig. 3. Glucose metabolism and the Warburg effect in the retina.
(a) Distribution of GLUT1, the main glucose transporter of the retina. (b) Schematic representation of oxidative phosphorylation, anaerobic glycolysis, and aerobic glycolysis (Warburg effect). In the presence of oxygen, differentiated tissues metabolize glucose to pyruvate via glycolysis. Pyruvate is then metabolized in mitochondria by oxidative phosphorylation. The electron transport chain requires oxygen to completely oxidize glucose. When oxygen is scarce, cells redirect pyruvate away from mitochondria to produce lactate (anaerobic glycolysis). Anaerobic glycolysis allows glycolysis to continue by cycling NADH back to NAD+but limits ATP production compared to oxidative phosphorylation. Warburg observed that cancer cells convert most glucose to lactate, irrespective of the presence of oxygen (aerobic glycolysis). The ‘Warburg effect’ is also observed in non cancerous tissues, such as the retina, which, though non proliferative have continuous replacement of outer segments and behave metabolically as “proliferative” cells. Aerobic glycolysis which is less energy efficient may provide building blocks required for growth. In proliferating cells, glucose is in part diverted to biosynthetic pathways upstream of pyruvate production. (c) This schematic summarizes glucose metabolism including glycolysis, oxidative phosphorylation, the pentose phosphate pathway, and glutamine metabolism in “proliferating” cells. Growth factor signaling, such as VEGF, leads to both tyrosine kinase and AKT/PI3K activation. In doing so, growth factors promote glucose uptake and flux through the early part of glycolysis, while inhibiting the late steps; they force glycolytic intermediates towards biosynthetic pathways of macromolecules essential for cell “proliferation” and NADPH production. Metabolic pathways are labeled in purple, and the enzymes controlling critical metabolic steps are shown in blue boxes. Figure modified, with permission, from (Gospe et al., 2010; Vander Heiden et al., 2009).
While there is substantial evidence that the retina primarily metabolizes glucose through glycolysis (discussed below), the benefit of relying on aerobic glycolysis over oxidative phosphorylation of glucose is not fully understood and remains controversial (Liberti and Locasale, 2016; Vander Heiden et al., 2009). Potential benefits may include faster ATP synthesis kinetics and increased production of carbon compounds for biosynthetic pathways that feed off glycolysis. These branching pathways include glycan synthesis, serine biosynthesis and the pentose phosphate pathway (PPP). Many glycans are synthesized from the glycolysis intermediate, fructose-6 phosphate. Glycans are a diverse class of metabolites essential for numerous cellular functions including the glycosylation of many proteins including rhodopsin (Murray et al., 2009). The PPP and serine synthesis are both important for the reduction of NADPH, which is needed for fatty acid synthesis, maintaining redox homeostasis and for reducing all-trans retinal produced from photo-bleaching (Punzo et al., 2012). Both pathways can also contribute to increased nucleotide synthesis, which is necessary for highly proliferative cells, although differentiated photoreceptor nuclei do not replicate.
It is also thought that the high glycolytic demand of the retina may be due to the localization of the mitochondria to the inner segment, which forces the outer segment to rely on aerobic glycolysis (Ng et al., 2015). This idea is supported by the localization of lactate dehydrogenase (LDH) involved in glycolysis to the outer segment whereas mitochondrial enzymes are found in the inner segments (Lowry et al., 1956). However, this problem could potentially be overcome by the shuttling of energy to the outer segment, which is seen at synapses via phosphocreatine (Linton et al., 2010).
The flux through the PPP has been studied in ex vivo rat and rabbit retinas using glucose with radiolabeled carbons at specific sites and measuring the rate of labeled CO2 production (Noell, 1952; Winkler et al., 1997). The PPP was calculated to account for between 1.5 and 10% of glucose oxidation under normoxic conditions (Noell, 1952; Winkler et al., 1997). However, when mitochondrial activity is blocked, the flow of glucose carbons through PPP increases (Noell, 1952; Winkler et al., 1997). Studies in rat retinal cell cultures have shown that under conditions where mitochondrial activity is blocked, blocking glycolysis or PPP leads to increased cell death, suggesting that the PPP is essential for retinal metabolism (Han et al., 2013).
The role of serine synthesis and serine metabolism in the retina is largely unknown. Retinal metabolism is often compared to cancer metabolism given that both have high biosynthetic demands (cancer cells need to supply building blocks for rapid proliferation, and the retina needs to continuously synthesize outer segments) (Ng et al., 2015). Furthermore, like the retina, cancer cells also rely heavily on a large glycolytic flux despite high OXPHOS capacity. More recent work in cancer indicates the dependency of many cell lines on serine metabolism (Yang and Vousden, 2016). Serine is a building block for neurotransmitters, sphingolipids, and ceramide and can be converted to glycine in a reaction that provides carbon for folate-mediated one-carbon metabolism. It is therefore possible that the energetics of the retina might also be reliant on serine metabolism. Supporting this idea, it has recently been shown that the rare macular degenerative and neovascular disease, macular telangiectasia type II, (Mactel), has genetic associations linked to serine and glycine metabolism, as well as decreased serine and glycine levels in patient serum (Scerri et al., 2017).
7.2. Balance of oxygen consumption (OXPHOS) and glycolysis in the retina
Both oxygen consumption (OXPHOS) and aerobic glycolysis have been shown to be essential for retinal function and vision. In humans, if the retinal circulation and therefore oxygen delivery is blocked, vision is lost within ~5 s. The visual function can be prolonged if oxygen is provided, indicating that O2 is a limiting factor (Ames et al., 1992a; Carlisle et al., 1964). Work by Noell in the 1950s showed that injecting iodoacetate (IAA), (which blocks glycolysis by inhibiting GAPDH), very rapidly and profoundly impairs vision while having no other observable effects on the animals, suggesting that the retina is also particularly sensitive to impairment of glycolysis (Noell, 1952).
The relative contributions of aerobic glycolysis versus oxidative phosphorylation to retinal energetics have been assessed using several ex vivo models. The rates of lactate production and oxygen consumption are monitored as a readout of glycolytic flux and oxidative phosphorylation respectively. The electrical activity of the retinal explant can also be monitored in response to light stimuli to indicate phototransduction maintenance. From these types of experiments, we have learned that for optimal electrical activity the retina requires both glucose and O2 (Ames et al., 1992b; Winkler, 1981). Reducing O2 leads to a reduction in energy production and visual function is compromised (Ames et al., 1992b). Similarly, when glucose is removed or glycolysis is chemically blocked, phototransduction, though measurable, is severely reduced (Winkler, 1981). These experiments, as well as in vivo studies, have shown that the vast amount of glucose is metabolized through aerobic glycolysis with ~80% of glucose being converted to lactate rather than oxidized through oxidative phosphorylation (Cohen and Noell, 1960; Wang and Bill, 1997; Wang et al., 1997a, 1997b). However, this reaction accounts for less than 20% of the ATP production in the retina, indicating that mitochondrial OXPHOS generates the vast majority of ATP using only a small portion of the glucose taken up. Therefore, alternative carbon substrates such as lipids can contribute to OXPHOS and ATP production (Joyal et al., 2016).
7.3. Metabolic shift in response to light
As discussed, maintaining the dark current is very energetically expensive. Sustaining the electrical gradient through Na/K ATPase activity accounts for more than 50% of the energy (ATP) demand in the retina (Ames et al., 1992b). This implies that retinal energetics are different in the light versus dark. Studies in ex vivo retinas found that light adapted retinas have a reduced oxygen consumption rate (OCR) (Medrano and Fox, 1995). Ames et al. also found that while O2 consumption is reduced 10–30% in response to light, lactate production does not change, indicating that OXPHOS is responsible for supplying the energy needed for the dark current. They also found that light flashes that increase neurotransmission lead to increased lactate production, suggesting that glycolysis supplies the energy needed for neurotransmission (Ames et al., 1992a). However, in an in vivo porcine model, where lactate and O2 levels were measured in the arterial blood and venous blood supplying the outer retina, constant light causes a marked decrease in lactate production that exceeds the reduction of OXPHOS (Wang et al., 1997b). So, while it seems clear that OXPHOS is increased in the dark and likely fuels the dark current, changes in glycolysis in response to light appear to vary depending on the method, light exposure, and model.
7.4. The metabolic shift in the developing retina
The metabolic demands of the retina change during development. Work in both rabbit and frog retinas indicate that young retinas use very little oxidative phosphorylation and rely more on aerobic glycolysis. As development proceeds and the retina differentiates, there is a shift to higher oxygen consumption. Compared to the young retina, the adult retina increases lactate production by 25% but nearly triples oxygen consumption (Cohen and Noell, 1960). While this may reflect an increase in glucose consumption, it also suggests that as the retina differentiates alternative substrates are used to fuel OXPHOS. Agathocleous et al. have shown that the switch to oxidative phosphorylation only occurs with terminal differentiation and that retinal metabolism is intrinsically linked to development, with the acceleration of differentiation stimulating an earlier shift to oxidative phosphorylation. The young retina also appears to have a greater endogenous energy store and blocking glycogen use also causes the retina to increase oxidative capacity (Agathocleous et al., 2012).
8. Lipid metabolism in the retina
Although the prevailing dogma has been that glucose is the only fuel substrate of the neural retina, as noted above, pioneering work by Cohen and Noell in the 1960s, implied that this was not the case. They reported that almost 65% of the CO2 produced from the TCA cycle by retinas is not derived from glucose (Cohen and Noell, 1960). These results imply that the oxidation of non-carbohydrate carbons is used to meet the retinal ATP demand. In the retina, one might rationalize that the use of both lipids and glucose as fuel would be beneficial in periods of high fuel need or nutrient deprivation.
In this section, we focus on fatty acids’ newly discovered function as fuel in photoreceptors. Lipids diverse roles as signaling molecules have been previously reviewed (Bazan, 2003; Giusto et al., 2010; Marrache et al., 2005; Shimizu, 2009) and will not be explored here. It is not yet known which lipids can be used as fuel so we will also discuss the general lipid composition of the retina as well retinal lipid metabolism, including fatty acids biosynthesis in the endoplasmic reticulum and lipid degradation and oxidation in peroxisomes and mitochondria.
8.1. Use of lipids as fuel: FA β-oxidation in the retina
In the eye, FA β-oxidation disorders are associated with retinopathy (Fletcher et al., 2012b; Roomets et al., 2008; Tyni et al., 2004). Conversely, glucose-uptake deficient patients with GLUT1 deletions, the main retinal glucose transporter, develop intractable seizures but have normal vision (De Vivo et al., 2002; Klepper, 2008; Klepper et al., 2001). Hence, there is strong evidence that the oxidation of FAs is a major contributor to retinal function.
Fatty acids, like glucose, can be oxidized in mitochondria to acetyl-CoA and enter the Krebs cycle to produce energy (Houten and Wanders, 2010). Lipid through FA β-oxidation is an alternative energy source to glucose in organs with high metabolic rates, such as the heart and skeletal muscle (Lopaschuk et al., 2010). These organs are rich in very low-density lipoprotein receptor (VLDLR), which facilitates fatty acid (FA) uptake. Photoreceptors and RPE express high levels of VLDLR, but lipid metabolism has been mostly explored in the retina from the standpoint of membrane biosynthesis, because of the high turnover of photoreceptors outer segments. Functional FA β-oxidation enzymes have been identified in Müller glia, RPE and photoreceptors (Atsuzawa et al., 2010; Oey et al., 2005; Tyni et al., 2002, 2004). Moreover, VLDLR mutations and mutations resulting in mitochondrial deficiency and changes in trifunctional proteins (TFP) that metabolize long-chain FA, result in progressive retinopathies (Fletcher et al., 2012a; Lawlor and Kalina, 1997; Roomets et al., 2008).
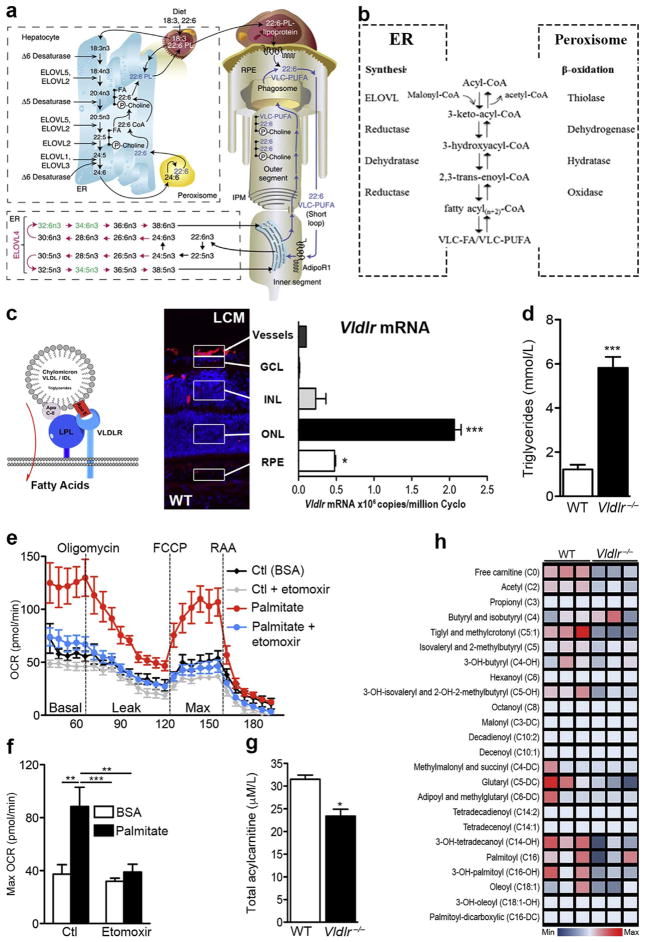
We showed that mouse retina (and specifically photoreceptors) can use FAs to produce energy Fig. 4e–h. VLDLR deficient mice have reduced uptake of fatty acids and decreased fatty acyl intermediates of β-oxidation (Fig. 4d,g,h) resulting in early vascular changes, secondary to energy deficits (Joyal et al., 2016). VLDLR deficient retinas exposed to palmitate increase their oxygen consumption, which is prevented by blocking fatty acid beta oxidation (by inhibiting CPT1 with etomoxir; Fig. 4e and f). Our findings were confirmed in a photoreceptor cell line (661W cone photoreceptors). FA β-oxidation is, therefore, an important metabolic energy pathway in the retina that is only beginning to be explored.
Fig. 4. Very long-chain fatty acids and lipid metabolism in the retina.
(a) Schematic of the formation of VLC-PUFAs. The elongation steps catalyzed by ELOVL4 are unique to photoreceptor inner segments (in red). Shed photoreceptor apical disks are phagocytosed by RPE. DHA (22:6) and VLD-PUFAs are recycled back to photoreceptor inner segments. C, carbons; ELOVL, elongase of the very long-chain fatty acids; ER, endoplasmic reticulum; IPM, interphotoreceptor matrix; PL, phospholipid; RPE, retinal pigment epithelium. (b) Fatty acid elongation and β-oxidation pathways. Each round of elongation involves four successive steps in the endoplasmic reticulum. Elongated fatty acyl-CoA product may undergo further rounds of elongation, be released for use in the cell, or, β-oxidized in the peroxisome. (c) VLDL receptors bind triglyceride-rich chylomicrons and VLDLs that express Apo-E, allowing lipoprotein lipase (LPL) to release long-chain fatty acids. VLDLR is highly expressed in photoreceptors (outer nuclear layer; ONL) by laser capture microdissection (LCM and qRT-PCR). GCL: ganglion cell layer, INL: inner nuclear layer, RPE: retinal pigment epithelium. n = 3 retinas, scale: 50 μm. (d) Circulating plasma triglyceride levels in WT and Vldlr−/− mice. P < 0.0001. (e) Oxygen consumption rate (OCR) and (f) maximal OCR of wild-type (WT) retinas provided with long-chain fatty acid (FA) palmitate or control (Ctl: bovine serum albumin or BSA) in the presence or absence of FA oxidation inhibitor, etomoxir (40 μM). n=6–8 retinas. (g) Total acylcarnitine levels (P = 0.0108) and (h) metabolite array of FA β-oxidation, measured by LC/MS/MS. n = 3 animal retinas. Figure modified, with permission, from (Joyal et al., 2016; Rice et al., 2015).
8.2. Peroxisomes and mitochondria in lipid oxidation
Peroxisomes play complementary roles to mitochondria in lipid metabolism. As discussed later, mitochondrial diseases can present with retinal dysfunction. Similarly, peroxisomal disorders such as Zellweger syndrome, adrenoleukodystrophy and Refsum’s disease cause severe retinal degeneration (Braverman et al., 2016), suggesting that both organelles are vital for retinal function.
Peroxisomes were initially described by Christian De Duve as ‘microbodies’ with oxidase and catalase activity able to metabolize hydrogen peroxide. Several lines of evidence point to their unique contribution of metabolizing long-chain fatty acids to shorter chains that can be further oxidized in the mitochondria (Wanders et al., 2010). Anatomically, peroxisomes are in close juxtaposition to lipid droplets seen in most living cells which are an intracellular source of energy for growth or use during starvation (Novikoff and Novikoff, 1982). Lipid droplets also prevent cellular exposure to high levels of free fatty acids that can be readily oxidized. As seen in yeast, peroxisomes extend pexopodia, or little foot processes, which are rich in peroxisomal β-oxidation enzymes, into the core of lipid droplets (Binns et al., 2006). When energy is required, VLC-FA stored as triglycerides can readily be oxidized by peroxisomes. Conversely, peroxisomes can synthesize and store neutral lipids in droplets creating a bidirectional energy stream for intracellular lipid storage and utilization adapting fuel supply to the cellular metabolic demands.
Fatty acid oxidation occurs in both mitochondria and peroxisomes, but peroxisomal fatty acid oxidation does not result directly in ATP synthesis. Insight into the complementary role of peroxisomes emerged from peroxisomal disorders where increased levels of branched and very long chain fatty acids accumulate in serum (Brown et al., 1982; Poulos et al., 1986). Branched-chain fatty acids, such as phytanic acid, must first undergo oxidative decarboxylation (α-fatty acid oxidation) in peroxisomes to become adequate substrates for further metabolism by β-oxidation in either peroxisome or mitochondria. Interestingly, FA β-oxidation of very long chain FA (≥26 carbons) occurs exclusively in peroxisomes, whereas shorter FA can be oxidized by either organelle (Wanders et al., 2010). FA oxidation is, however, less energetically favorable in peroxisomes (with no ATP production) compared to mitochondria, and the process is not carried to completion. Hence, peroxisomes shorten VLC-FA by oxidation, which can then be fully metabolized in mitochondria or utilized for membrane biosynthesis.
Fatty acid β-oxidation, in both peroxisomes and mitochondria, can be summarized by four consecutive enzymatic steps: dehydrogenation, hydration, second dehydrogenation and thiolytic cleavage. The first dehydrogenation step is catalyzed by peroxisomal straight-chain acyl-CoA oxygenase, followed by hydration and dehydrogenation, both of which are catalyzed by the bifunctional enzyme D-bifunctional protein. Lastly, 3-ketoacyl-CoA thiolase carries out the thiolytic cleavage reaction, forming a new acyl CoA molecule that is shorter by two carbons. Carnitine acyltransferase expressed in peroxisomes converts acyl CoAs to acylcarnitines so that they can be transferred to mitochondria for further oxidation (Wanders, 2013). Shorter chain fatty acyl CoA uptake across the double mitochondrial membranes requires a carnitine exchange shuttle, consisting of carnitine palmitoyl transferases (CPT) and the transporter protein carnitine-acylcarnitine translocase (CACT). Located on the outer mitochondrial membrane, CPT1 exchanges CoA for carnitine, and CACT transports the FA carnitine inside the mitochondrial matrix, where it is converted back to fatty acyl CoA by CPT2. FA oxidation is regulated transcriptionally in both organelles by PPARα (Aoyama et al., 1998), but mitochondrial oxidation is also tightly regulated by malonyl-CoA and CPT1 levels (McGarry et al., 1977, 1978). Fatty acid oxidation, therefore, requires complementary contributions from both peroxisomes and mitochondria. Interestingly, peroxisomes have unique genetic forms of enzymes common to mitochondria (Lodhi and Semenkovich, 2014). However, specifically in the retina, very little is known about the role of the peroxisome and much work remains to determine why peroxisomal disorders result in retinal degeneration.
9. Lipids versus glucose as fuel: the Randle cycle and fatty acid receptors
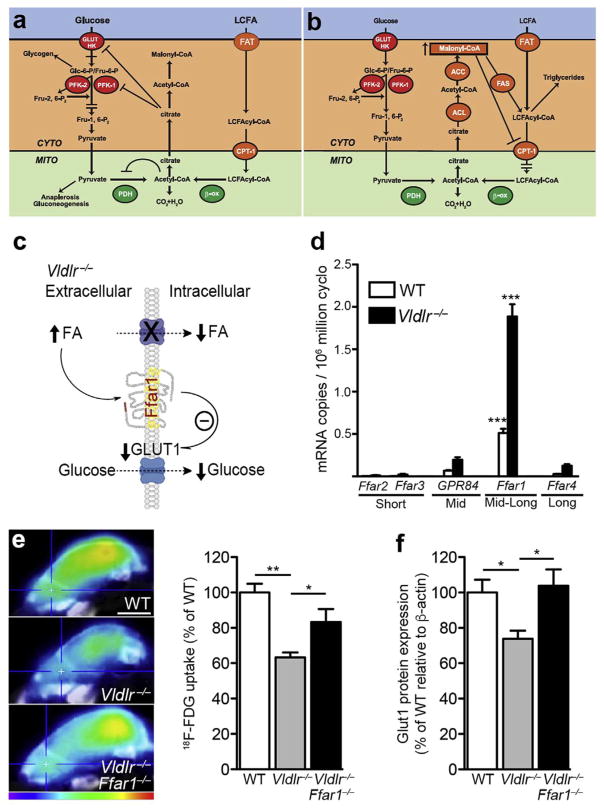
Adapting fuel utilization to match nutrient availability might improve metabolic efficiency in the retina as in other tissues. Hormones, such as insulin and glucagon, help to control the relative abundance of fuel substrate in circulation but different mechanisms are needed at the cellular level to determine which substrates are used. Randle and colleagues first proposed a mechanism for fuel selection by tissue, independent of hormonal control (Fig. 5a and b). Tissues that use lipids to produce energy, curb glucose uptake during starvation (Cahill, 1970; Ferrannini et al., 1983; Owen et al., 1979). The glucose-fatty acid cycle, or Randle cycle, describes a graded inhibition of various enzymes of glycolysis (mostly pyruvate dehydrogenase) mediated by the accumulation of acetyl-CoA and NADH resulting from fatty acid oxidation (Fig. 5a). Preferential FA oxidation, therefore, reroutes glucose towards glucose-dependent tissues, such as the brain, as well as glycogen synthesis or gluconeogenesis.
Fig. 5. Choosing between lipids and glucose as fuel: the Randle cycle and fatty acid receptors.
(a) Randle cycle: inhibition of glucose utilization by fatty acid oxidation. Accumulation of acetyl-CoA and NADH from FA oxidation inhibits pyruvate dehydrogenase (PDH), whereas cytosolic citrate regulates 6-phosphofructo-1-kinase (PFK) activity. Glucose uptake regulation is not fully explained by the Randle cycle. (b) Randle cycle: inhibition of fatty acid oxidation by glucose. Malonyl-CoA, which is produced by ACC when glucose is abundant, governs the expression of CPT1, hence regulating the entry of long-chain FA into the mitochondria. This effect re-routes fatty acids toward esterification and storage. CYTO: cytosol; MITO: mitochondria; GLUT: glucose transporter; HK: hexokinase; Glc-6-P: glucose 6-phosphate; Fru-6-P: fructose 6-phosphate; CPTI: carnitine palmitoyltransferase I; β-ox: β-oxidation, ACC: Acetyl-CoA carboxylase, ACL: ATP-citrate lyase; FAS, fatty acid synthase. (c) Elevated circulating fatty acid levels, as seen in Vldlr−/− retina, activate fatty acid receptors (such as Ffar1) that suppress GLUT1 expression and glucose uptake when lipids are abundant. (d) FA sensing receptors are expressed in WT and Vldlr−/−retinas (qRT-PCR). ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer. n = 3 animal retinas. (g) Glucose uptake (18F-FDG, scale: 4 mm) and Glut1 protein expression of WT and Vldlr−/− mice compared to littermate Vldlr−/−/Ffar1−/− mice (P16). Figure modified, with permission, from (Hue and Taegtmeyer, 2009; Joyal et al., 2016).
Subsequently, a general molecular explanation was also offered for the converse inhibition of FA oxidation by glucose (Collier et al., 1993; Sidossis and Wolfe, 1996) (Fig. 5b). Glucose through glycolysis, which also contributes to the production of acetyl-CoA in mitochondria, is oxidized by the citric acid cycle to citrate. Increased citrate levels promote transportation of citrate back to the cytosol where it regenerates acetyl-CoA, and ultimately forms malonyl-CoA. Malonyl-CoA is a molecular switch that inhibits CPT1, preventing entry of long-chain FA in mitochondria (McGarry et al., 1977, 1978). Hence, excess FA are redirected towards storage in lipid droplets locally or adipocytes systemically. If the glucose-fatty acid cycle helps elucidate preferential substrate metabolism inside the cell based on relative abundance, it fails to adequately explain how nutrient uptake is regulated at the cell surface to redirect unwanted fuel to distant storage locations, such as liver or adipocytes. The control mechanisms for substrate selection in the retina have not yet been fully determined although we found that high circulating lipids signal through Ffar1 to suppress glut 1 and glucose uptake in the retina (Joyal et al., 2016).
9.1. Lipid-sensing G-protein coupled receptor control of glucose uptake
Fatty acids are key endocrine regulators of lipid and carbohydrate metabolism, in part through the activation of lipid-sensing G-protein coupled receptors (GPCRs) or free fatty acid receptors (FFAR). GPCRs are plasma membrane environmental sensors, and several are specialized in the detection of nutrients, including amino acids, glucose, and lipids (Ichimura et al., 2009; Kraakman et al., 1999; Wauson et al., 2013). Free FA receptors Ffar2 and Ffar3 are activated by short-chain fatty acids (Brown et al., 2003), Ffar1 is activated by medium- and long-chain fatty acids (Briscoe et al., 2003; Costanzi et al., 2008; Oh et al., 2010; Poitout, 2003), while Ffar4 is primarily activated by long-chain FA, such as ω-3 FA (DHA and EPA) (Oh et al., 2010).
Ffar1 is abundantly expressed in the CNS (Boneva et al., 2011; Briscoe et al., 2003; Ma et al., 2007, 2010; Nakamoto et al., 2012; Yamashima, 2008) and is expressed in the retina, where its function has only recently been investigated (Joyal et al., 2016). First discovered in the pancreas, Ffar1 regulates insulin secretion (Itoh et al., 2003). In β-islet cells, circulating FA and Ffar1 agonists (GW9508 and TAK-875) (Briscoe et al., 2006; Burant et al., 2012; Leifke et al., 2012; Naik et al., 2012; Tsujihata et al., 2011; Yashiro et al., 2012) release insulin in the presence of glucose; it was explored as a target to treat type II diabetes. Interestingly, Ffar1 over-expression that mimics long-term high FA exposure eventually decreases insulin secretion leading to overt diabetes. Chronic Ffar1 signaling inhibits GLUT2 expression, a constitutive glucose transporter in the pancreas (Steneberg et al., 2005). As a result, lower intracellular glucose reduces insulin secretion (Itoh et al., 2003; Salehi et al., 2005; Schnell et al., 2007; Steneberg et al., 2005). Ffar1 signaling in the presence of high FA uptake also activates PPARα, an enhancer of FA β-oxidation; this metabolic switch may also inhibit glycolysis via the Randle cycle (Steneberg et al., 2005). Therefore, by sensing circulating FA nutrients, Ffar1 in the pancreas determines whether glucose or FA will be used as fuel, in part by regulating glucose uptake. Similarly, in the retina, we found that Ffar1 is expressed in photoreceptors where it regulates glucose uptake (Fig. 5c and d). High serum palmitate levels or pharmacological agonists of Ffar1 suppress GLUT1 and retinal glucose uptake, which is corrected in Ffar1 deficient mice (Joyal et al., 2016). Lipid sensing plasma membrane GPCRs may, therefore, govern glucose uptake. We speculate that long-term suppression of glucose entry by Ffar1 in photoreceptors (perhaps secondary to that by increased levels of circulating lipids) might contribute to age-related mitochondrial dysfunction in AMD or MacTel. The retinal effects of Ffar1 agonists, which are currently being considered for the treatment of type 2 diabetes, should be carefully monitored, particularly in older individuals who are at increased risk for AMD. Lipid metabolism in the eye is, therefore, an essential area of research, both because of the unique biosynthetic composition of the retina and because of its energy requirements, which may become dysregulated with aging and under pathological conditions.
10. Retinal lipid composition
As we do not yet know what lipids are used as fuel in the retina we will review lipid composition and lipid metabolism in the retina.
10.1. Long-chain polyunsaturated fatty acids (LC-PUFA)
The retina is rich in lipids derived from essential ω3 and ω6 long chain polyunsaturated fatty acids (LC-PUFA), which are critical for many retinal functions. Since humans lack key enzymes to synthesize α-linolenic acid (ω3) and linoleic acid (ω6), they must be obtained through dietary sources (SanGiovanni and Chew, 2005). Longer chain FA are then synthesized from these essential FA by iterative steps of desaturation (by insertion of double bonds) and elongation (by adding 2 carbons) in the endoplasmic reticulum of liver and to a lesser extent, in situ in retina (Bazan, 1989a,b; Li et al., 2001). Fatty acid structural nomenclature describes the number of carbons, double bonds and the position of the first double bond relative to the methyl terminal (ω) of the acyl chain. α-LLNA (or C18:3ω–3) therefore has 18 carbons, 3 double bonds, and the first unsaturated double bond is inserted at carbon 3. α-Linolenic acid (C18:3ω–3) is the dietary precursor to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), whereas linoleic acid (C18:2ω–6) is the dietary precursor to arachidonic acid (AA). Pro-inflammatory prostaglandins and leukotrienes are derived from the ω-6 AA. Conversely, the anti-inflammatory neuroprotectins and D-series resolvins originate from the ω-3 DHA and the E-series resolvins from EPA. The molecular basis for the health benefits of ω-3-PUFAs is thought to occur primarily through the direct integration of EPA and DHA in membrane phospholipids, replacing ω-6-LC-PUFAs such as linoleic acid (18:2ω-6) and AA. The biological impact of altering lipid intake is thought to be primarily through modification of membrane microdomain composition and specific receptors. Dietary ω3 to ω6 fatty acids may, therefore, impact retinal function and retinal neovascularization (Connor et al., 2007; Fu et al., 2015; Gong et al., 2015, 2016a, 2016b, 2017; Hård et al., 2013; Rezende et al., 2014; Sapieha et al., 2011, 2012; Shao et al., 2014; Stahl et al., 2010). There is no evidence these essential fatty acids are used for fuel, and without adequate synthetic machinery, it would be surprising if they were not used primarily in other capacities.
10.2. Phospholipids in membranes
LC-PUFA are incorporated predominantly into phospholipids in the neural retina (Bazan et al., 1997). Four types of phospholipids constitute structural elements of retinal membranes; phosphatidylcholine (PC; 40–50%) forms the outer leaflet, while phosphatidylethanolamine (PEA; 30–35%) and phosphatidylserine (PS; 5–10%) localize mostly on the cytoplasmic leaflet of membranes. Phosphatidylinositol (PI; 3–6%) is concentrated in membrane signaling domains (Bazan et al., 1997). Disc membranes of photoreceptor outer segments have the highest concentrations of DHA in the body (30% of total retinal fatty acids) (Neuringer, 1993). DHA comprises 20% of outer segment PC and 30% for both PEA and PS. Half of all PC and PEA fatty acids are saturated (30% and 10% palmitic acid; 20% and 36% stearic acid, respectively), compared to a third of PS (28% stearic acid (Anderson, 1970; Fliesler and Anderson, 1983). While on average the phospholipids throughout the retina contain a PUFA in one of the two acyl positions, the phospholipids in the outer segments predominately have PUFAs in both acyl positions. (Aveldano and Sprecher, 1987; Choe and Anderson, 1990; Choe et al., 1990; Wiegand et al., 1991). The high concentration of PUFAs in the outer segments improve rhodopsin activation and phototransduction in model membrane systems (Fliesler and Anderson, 1983; Litman and Mitchell, 1996). Fliesler and Anderson (1983) provide a detailed review of the chemistry and metabolism of lipids in the vertebrate retina. In short, biophysical and biochemical properties of DHA and longer chain fatty acids govern membrane function in photoreceptors and impact vision.
10.3. Lipid uptake of LC-PUFA and processing to very low-density lipoproteins (VLDL)
Retinal uptake of preformed long-chain (LC)-PUFA is much more efficient than in situ retinal biosynthesis (Su et al., 1999; Wetzel et al., 1991). The liver is a key site for LC-PUFA biosynthesis (Bazan, 1989a,b; Li et al., 2001) (Fig. 4a). Dietary lipids absorbed by the gut form chylomicrons and very low-density lipoprotein (VLDL), which are either readily metabolized to produce energy or stored in the liver or adipose tissues (Scott and Bazan, 1989). They are secreted into the lymphatic system and reach the blood circulation via the thoracic duct. Levels of fuel reserve in adipocytes are gauged by secretion of adipokines, such as leptin and adiponectin, which signal to the brain and peripheral tissues and regulate energy homeostasis. Adipose tissue through cycles of lipolysis and re-esterification ensures fatty acid availability for oxidative tissues. During periods of starvation, adipocytes liberate FA to fuel tissues. The liver is an essential homeostat for transient energy fluctuation, storing excessive FA from circulation following postprandial elevated triglyceride levels, as benign less reactive triacylglycerol (TAG), and secreting VLDL following the peak lipid load (Bordin et al., 1998; Nenseter et al., 1992; Nestel, 2000; Vasandani et al., 2002). Oxidative tissues capable of using lipid as fuel, such as heart, skeletal muscle, and retina, express VLDL receptors, which increase FA uptake. Indeed, deletion of VLDLR or LPL prevents efficient lipid uptake and β-oxidation in the heart (Augustus et al., 2006; Niu and Evans, 2011; Perman et al., 2011).
Once in the liver, essential FA, such as α-linolenic, are released in hepatocytes and form a complex with fatty acid synthase in the presence of malonyl coenzyme A (CoA). FA are elongated and desaturated in the endoplasmic reticulum (ER) to form DHA-CoA, which is then esterified to phospholipids (Fig. 4b). Apoproteins and phospholipids are transported in vesicles to the Golgi, where very low-density lipoproteins (VLDL) are assembled before being secreted (Bazan, 1990). DHA of hepatic origin is transported with dietary DHA as VLDL and chylomicrons to the choriocapillaris. Uptake of radiolabeled DHA in photoreceptors is highly efficient, beginning 1 h post-ingestion and peaking at 24 h (Li et al., 2001). Lipoprotein lipase (LPL) expressed in retinal choriocapillaris hydrolyzes chylomicron remnants and VLDL, liberating free fatty acids. Free fatty acids may subsequently form non-covalent bonds with albumin in blood plasma for tissue delivery.
Passage of fatty acids from blood to retina may proceed by facilitated transport or diffusion (Kawamura et al., 2003; Kelley et al., 1987; Matsugi et al., 1997; Wu et al., 2003). Transport of fatty acids from vessels to the retinal pigment epithelium (RPE), and to outer and inner segments of photoreceptors appears to be mediated by a high-affinity receptor (Bazan et al., 1997). Fatty acids are hydrophobic and therefore require specialized cytoplasmic transport systems, binding proteins, and receptors to reach photoreceptors. Since photoreceptors are not in direct contact with the vascular supply, adjacent cell types (RPE, astrocytes, and Müller cells) intercede in the process (Bazan et al., 1997). Fatty acid transport proteins (FATP)-1, FATP-4 and CD36 are the predominant transporters expressed on blood-brain barrier endothelial cells (Mitchell et al., 2011). Very low-density lipoprotein receptor (VLDLR) (Donati et al., 2006), FATP-1 and 4 (Chekroud et al., 2012) are also expressed in RPE and photoreceptors. Thus, fatty acid transport to the retina is inferred although more evidence is still needed. Organs with high metabolic rates that are capable of using lipids as an energy substrate, such as the heart and skeletal muscles (Lopaschuk et al., 2010) are rich in VLDLR to help FA uptake (Fig. 4c and d). VLDLR anchors ApoE of triglyceride (TG)-rich chylomicrons and VLDL, enabling the cleavage of long-chain FA from TG by lipoprotein lipase (LPL) (Fig. 4c). VLDLR also participate in the transcytosis of active LPL across endothelial cells (Obunike et al., 2001). VLDLR may, therefore, facilitate the delivery of TG-derived free FA across capillary beds to fuel tissues (Beisiegel and Heeren, 1997; Takahashi et al., 1995).
10.4. Very long-chain fatty acid biosynthesis and function in retina
In situ biosynthesis of very long chain (VLC)-PUFAs is a distinctive feature of the neural retina (Fig. 4a and b). ELOVL4, a condensing enzyme, is currently the only known elongase responsible for the synthesis of VLC fatty acids (≥22 carbons). ELOVL4 is highly expressed in photoreceptors inner segments (Kuny et al., 2014), and to a lesser extent in brain, testis, and skin (Agbaga et al., 2008), but not in liver (Zadravec et al., 2011). ELOVL4 elongase sequentially attaches two-carbon units to the acyl backbone of shorter long chain (LC)-PUFAs (McMahon and Kedzierski, 2009). Interestingly, n-3 VLC-PUFAs are predominantly synthesized from EPA, and less from DHA despite their retinal abundance (Suh and Clandinin, 2005). Major retinal VLC-PUFA end products are 32:5n-3 and 34:5n-3 (Agbaga et al., 2008), but their specific function remains essentially unknown.
The recent discovery of EVOLV4 mutations in Stargardt macular dystrophy type 3 (STG3) highlights the integral role of VLC-PUFAs for vision. STG3 is an autosomal dominant genetic disorder with deficient VLC-PUFAs associated with macular dystrophy and loss of central vision, suggesting a regional importance of these lipids in the macula. VLC-PUFAs are presumed to improve membrane fluidity and packing density, potentially stabilizing the unique folding pattern of outer segment discs, which may allow adequate shedding of rods and cones photoreceptor outer segments (Agbaga et al., 2010; Suh et al., 2000, 2009). Aging eyes are substantially depleted in VLC-PUFA, and their levels are further reduced in age-related macular degeneration (AMD) (Liu et al., 2010). The retinal lipid composition and in situ biosynthesis of VLC-PUFA might, therefore, contribute to the onset of AMD, and may offer a therapeutic target to address age-related retinal diseases.
11. Retinal cell-specific metabolism
In vivo or ex vivo measurements of retinal metabolism is the sum of the activity of multiple cell types. These cells have distinct metabolic activities and potentially compartmentalized and opposing metabolic reactions. In this review, we will focus on the metabolism of cells of the outer retina, namely photoreceptors, Müller glia, and RPE.
11.1. Photoreceptors
The majority of both retinal OXPHOS and glycolysis occurs in photoreceptors. As previously noted, in accordance with their high energy demand, photoreceptors have over 60% of retinal mitochondria (located in the photoreceptor inner segments) (Cohen, 1961; Hoang et al., 2002), as well as the highest electron transport chain enzyme Cytochrome C oxidase activity (Giulian et al., 1989; Kageyama and Wong-Riley, 1984). Based on ex vivo analysis with selective inhibitors, the outer retina (photoreceptors) is estimated to have 2–3 times greater oxygen consumption compared to the inner retina (Medrano and Fox, 1995). In vivo analysis finds that the inner retina has comparable O2 consumption in the light (when less ATP is needed for the Na+/K+ ATPase ion pumps to maintain gradients) but in the dark the outer retinal (photoreceptor) O2 consumption increases where the inner retina is unchanged, supporting that OXPHOS fuels the dark current in photoreceptors (Wang et al., 1997a, 1997b). Concerning lactate production during aerobic glycolysis, the outer retina (photoreceptors) accounts for the vast majority with very little detected in the inner retina (Wang et al., 1997a, 1997b). Wild-type rat retinas have>50% more lactate production and O2 consumption than dystrophic retina lacking photoreceptors (Graymore, 1959; Graymore and Tansley, 1959). Combined, these data suggest that the photoreceptors are the primary consumers of energy in the retina and likely the site of the majority of OXPHOS and aerobic glycolysis as discussed below.
Photoreceptors (both rods and cones) have a unique mitochondrial localization and morphology (Winkler et al., 1997) with a high concentration of mitochondria in the ellipsoid region of the inner segment, near the ciliary junction to the outer segments (Goldberg et al., 2016). As noted, maintaining the sodium gradient in the photoreceptors is a crucial physiological feature that requires substantial energy derived from ATP hydrolysis (Hagins et al., 1970). The sodium pumps are most dense in the ellipsoid region of the photoreceptor inner segment and as noted earlier (when comparing light and dark metabolism) are likely fuelled via oxidative phosphorylation rather than through glycolysis (Ames et al., 1992b).
11.2. Photoreceptors are the primary site for glycolysis
Enzymes for aerobic glycolysis localize to photoreceptors, indicating that they are the primary site of aerobic glycolysis in the retina. Photoreceptors express high levels of enzyme isoforms that favor the conversion of pyruvate into lactate, including hexokinase II (HKII), the pyruvate kinase M2 isoform (PKM2) and lactate dehydrogenase subunit A, LDHA (Aït-Ali et al., 2015; Chinchore et al., 2017; Casson et al., 2016 Lindsay et al., 2014; Rajala et al., 2016; Reidel et al., 2011). Hexokinase catalyzes the first reaction of glycolysis via the phosphorylation of glucose. The HKII isoform localizes to the mitochondrial membrane and specifically to the ATP transporter (VDAC) providing the enzyme an ample supply of the ATP needed (Mathupala et al., 2006; Rueda et al., 2016). The preferential expression of HKII, compared to the more widely expressed HKI isoform, is associated with aerobic glycolysis (and increased growth in cancer cells) (Wolf et al., 2011).
Pyruvate kinase mediates the final reaction in glycolysis converting PEP to pyruvate. The M2 isoform, as opposed to the M1 isoform, is allosterically regulated between a tetramer with high affinity for PEP and a less active dimeric form. The less active dimer leads to reduced pyruvate formation and a build-up of glycolytic intermediates and potentially leads to increased anabolic reactions from these intermediates (reviewed in (Ng et al., 2015)). This enzyme is also preferentially expressed in rapidly dividing cells including cancer cells. While some controversy exists regarding the expression of PKM1 in photoreceptors, with some reporting the absence of PKM1 and others observing it (Chinchore et al., 2017; Casson et al., 2016; Lindsay et al., 2014; Rajala et al., 2016; Rueda et al., 2016), PKM2 is indisputably the dominant isoform in photoreceptors.
Lactate dehydrogenase (LDH) exists as a tetramer comprised of A and B subunits. A tetramer consisting solely of A subunit favors the conversion of pyruvate to lactate and is highly expressed in cancer cells, whereas the tetramer of B subunit favors the reverse reaction (reviewed in (Vander Heiden et al., 2009)). Graymore first noted that photoreceptors might be the site of LDHA expression in the retina (Graymore, 1964). Since then many groups have noted the high expression of LDHA in the retina and specifically in photoreceptors, in a pattern matching the expression of PKM2 (Chinchore et al., 2017; Casson et al., 2016; Rueda et al., 2016).
11.3. Glycolysis is required for photoreceptor outer segment synthesis
There is increasing evidence connecting light stimulation in photoreceptors to the regulation of aerobic glycolysis. Rajala et al. observed PI3K-mediate phosphorylation of PKM2 with light stimulation, leading to enzyme inhibition, suggesting a reduced production of pyruvate with light and potentially a build-up of glycolytic intermediates leading to anabolic reactions (Rajala et al., 2016). Chinchore et al. have recently shown that impairing glycolysis specifically in rods with a variety of methods (interfering with the activity of LDHA, PFK, and PK) leads to shorter rod outer segments (OS), which is thought to be the result of reduced OS synthesis (Chinchore et al., 2017). Their data suggest that high glycolytic flux and the production of lactate is necessary for the biosynthesis of OS. Interestingly, while reducing PKM2 led to shorter OS, overexpression of PKM1 had a similar effect. The observation that OS are shorter with both reduced glycolytic flux and increased flux suggests that the ability of the photoreceptor to precisely regulate the glycolytic rate is crucial to the OS length (Chinchore et al., 2017). Supporting the hypothesis that glycolytic intermediates are needed for biosynthesis during light exposure, keeping animals with impaired glycolytic function in the dark rescued OS length (Chinchore et al., 2017). However, it remains to be shown directly if there is an altered flux through anabolic reactions derived from glycolytic intermediates (such as PPP or serine biosynthesis) in response to light and if these pathways are necessary for OS biosynthesis.
11.4. Metabolic differences between cones and rods
The function and morphology of rods and cones are different, leading to differences in metabolic needs and photosensitivities. While rods are highly sensitive to light and able to respond to single photons, cones are less sensitive but respond and recover more quickly. The OS of a rod also differs from that of a cone, consisting of enclosed membrane discs in the outer segment as opposed to a continuous membrane in the cone. Cones and rods also differ in the number of mitochondria per cell. In mice, cones have twice the number of mitochondria, whereas in primates it is estimated to be ~10 times more than rods (Perkins et al., 2003). Furthermore, cones contain more ATP than rods (Scarpelli and Craig, 1963) and potentially cones have glycogen stores while rods do not (Nihira et al., 1995). It is possible that increased OXPHOS (mitochondrial) energetic capacity in cones is merely a response to an increased energetic demand compared to rods. Cones may also have increased energy capacity to allow increase resistance to metabolic insult and apoptosis. Rods and cones also differ with respect to their reliance on aerobic glycolysis. Noell reported that monkey cones survive an injection of iodoacetate, which blocks glycolysis, while rods rapidly die, suggesting that rods rely more on glycolysis than cones (Noell, 1952). Rod function is impacted by subtle increases or decreases in glucose levels, whereas cones maintain normal function with similar glucose changes glucose and only show defects when glucose levels are severely reduced (Macaluso et al., 1992). In accord, in diabetes, rod function appears to be affected early as diabetic patients have difficulty seeing in the dark (Bailey and Sparrow, 2001).
There is metabolic cross-talk between rods and cones. Rods help support the metabolism and survival of cones through the release of RdCVF (rod-derived cone viability factor) (Aït-Ali et al., 2015). RdCVF is released from rods and increases glucose uptake in cones (Aït-Ali et al., 2015). When rods die and no longer secrete RdCVF cones are secondarily impacted.
11.5. RPE metabolism
The retinal pigment epithelium (RPE) is a pigmented monolayer of cells that comprises the entire blood outer blood-retinal barrier between the choroidal vascular plexus and the neurosensory retina. Photoreceptors depend heavily on the RPE to maintain homeostasis; there is a constant molecular exchange between these two cell types and their metabolism is tightly linked. Indeed, loss of RPE function in mice leads to photoreceptor degeneration and loss or dysfunction of the RPE in humans occurs in many diseases, which comprise leading causes of vision loss, including age-related macular degeneration, retinitis pigmentosa, and diabetic retinopathy.
In many ways, the metabolic demands of photoreceptors translate to metabolic requirements for the supportive RPE. The RPE phagocytize the shed outer segments, detoxifying and degrading the light-damaged components and recycling the usable fatty acids, particularly DHA and the restored retinal photosensitive pigments back to the photoreceptors (Strauss, 2005). The RPE cells are responsible for vectorially transporting H2O, ions, energy substrates and other nutrients between the photoreceptors and choroid (Strauss, 2005). Oxidative stress further increases the metabolic burden on the RPE. The RPE is exposed to high photo-oxidative stress from direct light exposure. Additional sources of oxidative stress include photoreceptor light-damaged outer segment lipid, protein and photopigment components before detoxification (Winkler et al., 2008) as well as direct exposure to oxygen from the high rate of blood flow of the choroid (Bill et al., 1983). To deal with the excessive oxidative stress, RPE invests energy into increasing the anti-oxidative capacity (Strauss, 2005). The RPE cells have an unusually high rate of reductive carboxylation, whereby glutamine enters the TCA cycle and is carboxylated to yield citrate. Increased flux through this pathway appears to enhance the redox potential of RPE and feed lipid synthesis (Du et al., 2016). Despite the susbtantial investment into antioxidant capacity, age correlates with a decrease in mitochondrial number, reduced ATP levels and increased apoptosis in RPE cells. This phenomenon is exacerbated in patients with AMD, suggesting long-term stress leads to RPE dysfunction and ultimately degeneration of photoreceptors (Bhutto and Lutty, 2012; Feher et al., 2006; Zhao et al., 2011a).
RPE have high mitochondrial activity and also appear to have high aerobic glycolysis in cell culture models (Adijanto and Philp, 2014; Kurihara et al., 2016). While cell culture models suggest high glycolytic rates in RPE, it is thought that RPE preferentially passes glucose to the photoreceptors (Strauss, 2005). Supporting this notion, the RPE express low levels of hexokinase, which would allow glucose to pass through as opposed to entering glycolysis to be consumed in the RPE (Lowry et al., 1961; Wang et al., 2016). Kurihara et al. have shown that increasing glycolytic rates in RPE may lead to photoreceptor degeneration. Inducing hypoxia or hypoxia-signaling in RPE eventually results in photoreceptor cell death (Kurihara et al., 2016). In response to hypoxia, oxidative phosphorylation is reduced, and glycolysis is increased in RPE cells, and glucose transport is reduced which might be mostly responsible for the photoreceptor degeneration (Kurihara et al., 2016).
In addition to transporting glucose, the RPE might also produce additional energy substrates for the photoreceptors. Adijanto et al. have found that RPE oxidizes lipids to yield and secrete ketone bodies. They propose a cycle in which lipids are taken up by the RPE during outer segment recycling and are oxidized to produce ketones, which are then returned to the photoreceptors and enter the TCA cycle to produce ATP and amino acids (Adijanto et al., 2014).
11.6. Müller glia metabolism
The Müller glia span the retina. The processes are highly branched and have arbors that extend into all of the retinal layers. The function and the metabolism of these cells are still enigmatic (Reviewed in (Hurley et al., 2015)).
Similar to a lactate shuttle proposed in the brain, it has been proposed that Müller glia constitute a significant site of aerobic glycolysis in the retina. This hypothesis suggests that the Müller glia take up glucose to produce and secrete lactate that is then used by the photoreceptors for oxidative phosphorylation. Supporting this idea, Poitry-Yamate et al. showed that freshly isolated Müller glia provide high levels of lactate and when Müller glia were isolated in combination with photoreceptors the lactate release was reduced, suggesting that the photoreceptors were consuming the lactate (Poitry-Yamate et al., 1995). Winkler et al. supported this idea by showing that in cultured Müller glia, essentially all glucose is used to produce lactate and there is little oxidative phosphorylation of glucose (Winkler et al., 2000). It is important to note that isolation of Müller glia intact and without other cell parts attached is very difficult.
Conversely, it has also been proposed that Müller glia lack the ability to produce lactate and instead use lactate and amino acids produced by the photoreceptors to generate energy and metabolites via the TCA cycle (Lindsay et al., 2014). As described earlier, there is substantial evidence supporting the idea that photoreceptors have high levels of aerobic glycolysis and secrete lactate. The lack of expression of various glycolytic enzymes in Müller glia further suggests that these cells do not have high glycolytic rates in vivo. Several groups have noted the lack of pyruvate kinase (PKM1 or PKM2) in Müller glia in the rodent retina (Casson et al., 2016; Lindsay et al., 2014; Rajala et al., 2016). It has also been noted that Müller glia do not express LDHA (Casson et al., 2016) but may express LDHB (Chinchore et al., 2017). Lindsay et al. suggest that Müller glia can utilize lactate and aspartate generated by the photoreceptors as energetic and anaplerotic substrates (Lindsay et al., 2014).
Most evidence for Müller glia metabolism comes from cultured cells, which are imperfectly isolated from the retina, and are unlikely to reflect Müller glia metabolism in vivo in the absence of physiological cell/cell interaction. Given the cellular morphology of Müller glia (spanning the retina with multiple cellular interactions), it is likely that the metabolic pathways vary in various parts of the cells depending on their location and their cellular neighbors. Further work determining the in vivo metabolic properties of Müller glia and their relationship to photoreceptors is needed.
12. Neuroglial mitochondria: nutrient and oxygen sensors that drive angiogenesis
Elaborate mechanisms preserve the critical homeostasis between the vascular supply of nutrients and oxygen, and the neuronal energy demands fuelled by mitochondria, the cell’s powerhouse. Major mitochondrial energy pathways including the TCA cycle, fatty acid β-oxidation, and oxidative phosphorylation require nutrients and oxygen to produce energy. Glucose, amino acids and fatty acids fuel the Krebs cycle by generating acetyl-CoA. Nutrient deficiency may, therefore, decrease acetyl-CoA synthesis and downstream metabolites of the Krebs cycle, such as α-ketoglutarate.
The incomplete reduction of oxygen in mitochondria by complex III of oxidative phosphorylation leads to the generation of reactive oxygen species (ROS) (Guzy and Schumacker, 2006). Many heme-containing enzymes, such as prolyl hydroxylases (PHDs) are tightly regulated by oxygen and ROS. The important Krebs cycle metabolite α-ketoglutarate is also a co-factor of prolyl hydroxylase domain-containing protein (PHD), required for the hydroxylation and degradation of hypoxia-inducible factor 1 (HIF1). Hence, a nutrient shortage that decreases Krebs cycle metabolites, hypoxia, or ROS will inhibit PHD and stabilize HIF1a, by preventing its immediate degradation (Pouysségur and Mechta-Grigoriou, 2006). HIF1a is a critical transcription factor for more than 60 genes, including VEGF, which triggers cytoprotective adaptation and compensatory angiogenesis during ischemia (and during fuel shortages) (Joyal et al., 2016).
Oxygen is also directly coupled to mitochondrial oxidative phosphorylation (Johnson and Hansford, 1975). Complex IV of the electron transport chain (ETC) reduces oxygen to water, enabling the flow of electron and the reduction of flavin (FAD) and nicotinamide (NAD) nucleotide, which is then used by enzymes of the Krebs cycle (King et al., 2006). Though the Krebs cycle does not directly use oxygen, it can only take place when oxygen is present, since it relies on by-products of the ETC. Hypoxia, therefore, inhibits both the ETC and the Krebs cycle leading to the accumulation of key metabolites, particularly succinate which is used by both metabolic pathways (Folbergrová et al., 1974; Hoyer and Krier, 1986). Moreover, HIF and succinate dehydrogenase activity are intertwined, such that during hypoxia, HIF is stabilized and succinate dehydrogenase levels are decreased (Strumilo, 2005); the latter increases HIF stabilization through reactive species generation (Pouysségur and Mechta-Grigoriou, 2006; Tretter and Adam-Vizi, 2005), HIF, in turn, can directly suppress levels of succinate dehydrogenase ‘B’ (Dahia and Consortium, 2006), and together amplify succinate accumulation. Physiological functions for the Krebs cycle intermediates beyond their traditional roles as metabolites of this pathway were only recently described (Bénit et al., 2014). However, several indicators suggest their potential roles in ischemic adaptations, such as vasomotor regulation and angiogenesis. Succinate administration after an ischemic event improves brain recovery (Cannella et al., 1989; Gurvitch et al., 1997) and dysfunctional mutations in succinate dehydrogenase (which converts succinate to fumarate) lead to succinate accumulation, which is associated with tumorigenesis (Gottlieb and Tomlinson, 2005; King et al., 2006).
Although succinate has been studied for over 60 years, the discovery of the G-protein coupled receptor GPR91 (previously designated as an orphan receptor) that specifically binds succinate implied biological functions beyond energy production. GPR91 is predominantly expressed in highly vascularized tissues such as the kidney, placenta, liver and the retina (He et al., 2004; Wittenberger et al., 2001) suggesting a possible sensitivity to hypoxia. GPR91 is a purinergic-like receptor with ~53% homology to P2 purinoceptors. As in the case of Krebs cycle intermediates, metabolic products of purines (ATP, ADP, AMP, and adenosine) accumulate during hypoxia and activate purinergic receptors that may participate in neovascularization (Linden, 2005). But whether they act directly or through the release of proangiogenic factors is controversial (Grant et al., 1999; Wakai et al., 2001).
In the retina, GRP91 is almost exclusively expressed in retinal ganglion cells (RGCs) where it senses hypoxic stress during retinal vascular development and disease. These ischemic neurons through succinate production and GPR91 signaling secrete angiogenic factors, particularly VEGF, known for its chemotactic properties (Sapieha et al., 2008). RGCs shape their microvascular environment to re-instate metabolic equilibrium.
13. Pathological angiogenesis in disease as a marker of retinal energy failure
Vessels supplying oxygen and nutrients to neurons, continually adapt to neuronal energy requirements. Vascular remodeling is, therefore, an early sign of changes in retinal neuron metabolism, possibly driven by energy needs. Hence, diseases involving mitochondria may present a unique vascular signature.
Mitochondrial ocular diseases are categorized as either primary or secondary. Primary retinal disorders result from direct impairment of mitochondrial functions by mutations in either mitochondrial DNA (mtDNA) or nuclear genes coding for mitochondrial proteins (which contribute the largest number involved in mitochondrial function). Mitochondrial diseases, therefore, reflect the contributions of two genomes, mitochondrial and nuclear, in addition to environmental influences. Many mitochondrial gene mutations may be lethal, but of those mutations that survive, the heterogeneous, slowly progressive and often late presentations of primary mitochondropathies highlight the complexity of the compensatory mechanisms in place to palliate energy failure in the retina. Acquired (or secondary) mitochondrial dysfunction is believed to contribute to the development of diabetic retinopathy (from adaptation to hyperglycemia and dyslipidemia) and age-related macular degeneration (AMD) (from mitochondrial aging leading to a dysfunction of photoreceptors and support cells). These are the most common causes of blindness in adults. We will describe the presentations of classical mitochondrial retinal diseases to illustrate the importance of angiogenesis as a marker of energy failure.
14. Primary mitochondropathies and ocular diseases
Neuro-ophthalmic mitochondrial disorders can be broadly divided into four groups: (1) bilateral optic neuropathies; (2) pigmentary retinopathies that affect the inner and outer retina respectively; (3) ophthalmoplegia with ptosis; and (4) retrochiasmal visual loss (Fig. 6a). The extra-retinal ocular manifestations and are reviewed elsewhere (Biousse and Newman, 2003; Newman, 2012).
Fig. 6. Mitochondrial disorders and vascular adaptation.
(a) Cellular homeostasis is under the dual control of nuclear (blue) and mitochondrial genomes. Mitochondrial diseases (bold text), many of which impact vision, arise from mutations in genes (italic text) from either genome. Mitochondrial DNA encodes 13 structural subunits of the electron transport chain (complexes I, III, IV, and V) and the RNA needed for gene translation. All other mitochondrial proteins are encoded by the nuclear genome. Abbreviations: CMT2A, Charcot-Marie-Tooth disease, type 2A; DCMA, dilated cardiomyopathy with ataxia; DDON, deafness, dystonia and optic neuropathy; DOA, dominant optic atrophy; DOA+, dominant optic atrophy-plus syndrome; FRDA, Friedreich ataxia; HSP7, hereditary spastic paraplegia, type 7; LHON, Leber hereditary optic neuropathy; OXPHOS, oxidative phosphorylation; ROS detox, reactive oxygen species detoxification; rRNA, ribosomal RNA; tRNA, transfer RNA; 3MGA, 3-methylglutaconic aciduria, type III. (b) Fluorescein angiography of fundus during the acute stage of LHON, showing tortuous vessels and telangiectasias. Energy metabolism has a direct impact on the retinal vascular phenotype. Figure modified, with permission, from (Newman, 2012).
14.1. Leber’s hereditary optic neuropathy (LHON): paradigm of the inner retina
Leber’s hereditary optic neuropathy is the most common mitochondrial ocular disease. The condition was initially described by Von Graefe in 1858 (von Graefe, 1858a; Von Graefe, 1858b), but was only coined as a formal clinical entity by Leber in 1871 (Leber, 1871a, b). LHON was the first maternally inherited (Erickson, 1972) mitochondrial disorder of the eye (Wallace, 1999) to be discovered. Three different point mutations in mitochondrial DNA account for more than 90% of cases (Wallace et al., 1988). However, if the presence of a mitochondrial mutation is necessary for the pathogenic expression, it is not sufficient. The co-existence of both mutant and normal mitochondria (heteroplasmy) in a given tissue is often offered as an explanation for the variation in penetrance of mitochondrial disease. However, many carriers of mitochondrial point DNA mutations without significant heteroplasmy will never suffer visual loss. Other factors, genetic and environmental, therefore modify the expression of mitochondrial disorders (Carelli et al., 2015; Giordano et al., 2013).
LHON presents as an acute or subacute loss of central vision that generally progresses within a few months, and affects mostly young males (Hwang et al., 2017). Almost 50% of affected men will suffer significant vision loss compared to only 10% of females (Hwang et al., 2017). The reason for this gender bias is suggested to be related to estrogen effects on mitochondrial DNA (Pisano et al., 2015). In affected patients, color and visual acuity fades progressively in one eye, rapidly followed by a similar involvement of the contralateral eyes days to months later (rarely years). Visual field defects mainly involve central vision, creating a large central absolute scotoma, while preserving peripheral vision. Preferential loss of central high acuity color vision is also a generalizable characteristic of many mitochondrial disorders, possibly correlating geographically with an area of higher retinal energy demands of the cone-rich central retina. Interestingly, earlier onset of visual loss (younger than 15 years) and a subacute time course, have a better visual prognosis (Nakamura and Yamamoto, 2000). Rarely patients, mostly carrying the 14484 mtDNA mutation, will experience significant vision recovery, which suggests the presence of yet unknown rescue mechanisms for energy-depleted neurons. Finally, LHON patients usually do not have symptoms until early adult life, when some ill-defined trigger, endogenous or environmental, is believed to initiate acute vision loss. A large case-control study of LHON patients did not show, however, an association between usual environmental culprits of mitochondrial stress, such as tobacco or alcohol consumption, and the onset of visual loss although other studies suggest an association (Kerrison et al., 2000). LHON illustrates that environmental triggers result in tissue-specific energy failure and that compensatory mechanisms may alleviate the energy crisis (Carelli et al., 2004).
The pathogenesis of the selective damage to the optic nerve (retinal ganglion cells) in LHON remains uncertain. Unmyelinated prelaminar retinal ganglion cell axons are mitochondria-rich and have shown a high degree of mitochondrial respiration (Pan et al., 2012). This is, therefore, an area of high metabolic energy requirements for the retina. Moreover, the acute-angle turn made by axons as they enter the optic nerve may represent a ‘choke-point’ for axoplasmic mitochondrial transport, associated with an inherent metabolic vulnerability. Mutations of the electron transport chain complexes, as observed in LHON, may result in abnormal oxidative phosphorylation (respiration), decreased ATP production, and more ROS production, together resulting in damage to ganglion neurons and their axons. Mitochondrial biogenesis appears to be more active in carriers of mitochondrial mutations that do not develop the disease, suggesting that more mitochondria, albeit defective, may collectively better maintain the energy requirement of ganglion cells above the optic nerve degeneration threshold (Giordano et al., 2013). Similarly, estrogen receptors were recently shown to trigger mitochondrial biogenesis providing a compelling explanation for the gender bias observed in LHON (Pisano et al., 2015). A mouse model of complex I deficiency presents histopathological features of LHON (Lin et al., 2012), highlighting the inherent vulnerability of retinal axons and their dependence on mitochondrial respiration. Further exploration of mitochondrial diseases in mouse models will be key to explore the pathogenesis of human mitochondrial disorders.
Vascular remodeling in LHON is an early sign of the underlying mitochondrial disorder (Fig. 6b). Microangiopathy may be seen for years before the onset of the optic neuropathy and is also seen in asymptomatic disease carriers (Wallace et al., 1988). During the active phase of the disease, however, retinal artery branches become acutely dilated and tortuous, forming arteriovenous shunts and telangiectasia. Hyperemia of the optic disc is associated with occasional peripapillary hemorrhages (Nikoskelainen, 1984; Nikoskelainen et al., 1983, 1984). Eventually, as neurons atrophy and their energy demands fall, the microangiopathy completely disappears. Optic disc pallor observed in the late phase of the disease correlates with loss of ganglion cells and a paucity of vessels in the affected central retina. Closer inspection of the endothelium and smooth muscle of microangiopathy lesions suggest an accumulation of morphologically aberrant mitochondria (Wallace et al., 1988). Whether angiogenesis directly contributes to the disease etiology or is a consequence of neuronal energy demands is unknown. Similar proliferative vascular changes are noted surrounding necrotizing lesions in Leigh and MELAS syndrome, mitochondrial disorders affecting the central nervous system more severely (Grönlund et al., 2010; Isashiki et al., 1998; Uziel et al., 1997). It is therefore plausible that increases in neuronal energy demands compel an increase in angiogenesis to reinstate energy homeostasis. Conversely, as neurons degenerate and their energy demand fall, so may the drive to increase vascular supply leading to neurovascular atrophy.
14.2. Outer retina (photoreceptor) bioenergetics and pigmentary retinopathies
Retinal pigment epithelium (RPE) and photoreceptors constitute a bioenergetic unit fuelled primarily by the choroidal vasculature. Disorders that impair energy production often present with pigmentary changes of the retina, sometimes referred to as salt-and-pepper retinopathy, secondary to aberrant migration of RPE. Unaffected carrier relatives of patients with a more severe mitochondrial disease often have isolated pigmentary retinal changes. Degeneration of RPE, and sometimes degeneration of rod and cone photoreceptors, contributes to (usually mild) vision loss, affecting roughly 50% of patients with known mitochondrial mutations (Bhatti, 2006; de Crecchio et al., 2006; Durlu et al., 1997; Lowes, 1975; McDonald, 2003).
Pigmentary retinopathy is a diagnostic criterion for Kearns-Sayre Syndrome (KSS) (Kearns and Sayre, 1958). KSS is the result of duplication or deletions in mitochondrial DNA and is often associated with chronic progressive external ophthalmoplegia (CPEO). Mitochondrial encephalopathy, lactic acidosis and stroke-like episode (MELAS), is also a disease of mitochondrial genes, which often presents with pigmentary retinal changes (Isashiki et al., 1998). A specific mutation associated with MELAS (3243) may also give rise to more severe macular dystrophy (Isashiki et al., 1998). Mitochondrial mutations in the ATPase-6 gene, which account for approximately a third of patients with Leigh’s syndrome is often associated with a pigmentary retinopathy and sometimes the loss of photoreceptors. Leigh syndrome, also called subacute necrotizing encephalomyelopathy, is a disorder of aerobic energy production characterized by early childhood encephalopathy, lactic acidosis, and central hypoventilation (Uziel et al., 1997). Progressive pigmentary retinopathy is also noted in some cases of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHAD: EC1.1.1.211), a defect in mitochondrial fatty acid β-oxidation (Tyni et al., 2002). In short, mitochondrial disorders that impair energy production often present with pigmentary retinopathies, with some degree of photoreceptor atrophy, reflecting the energy dependency of the outer retina.
15. Secondary mitochondropathies and ocular diseases
Acquired (or secondary) mitochondrial dysfunction is likely involved in the development of diabetic retinopathy, and age-related macular degeneration (AMD). AMD is primarily caused by aging changes, which include aging mitochondria, while fuel shifts induced by hyperglycemia and dyslipidemia contribute to DR. Both diseases are associated with secondary energetic dysfunction of photoreceptors and vessels. Retinal degenerative disease, such as retinitis pigmentosa, often also present signs of secondary mitochondrial dysfunction that likely exacerbate disease progression.
15.1. Retinitis pigmentosa: cone degeneration after rod loss
Genetically determined photoreceptor degeneration in retinitis pigmentosa (RP) is associated with changes in cellular energetics and vascular attenuation (Ayton et al., 2013; Campochiaro and Mir, 2017; Dhoot et al., 2013; Toto et al., 2016). RP is used to describe any inherited monogenic disorder associated with photoreceptors and frequently, pigmentary degeneration. RP is characterized initially by progressive loss of night vision due to many different mutations affecting primarily rod function (Hartong et al., 2006). Peripheral visual fields are lost first as the peripheral retina primarily consists of rods, leaving only a small island of central vision due to surviving macular cones. Secondary cone loss follows in most RP subtypes (Punzo et al., 2009); Punzo and Cepko found that cone loss may be a result of cone starvation (as well as oxidative stress). They assessed four mouse models of photoreceptor degeneration secondary to mutations in rod-specific genes. Alterations in the insulin/mammalian target of rapamycin pathway (mTOR) aligned with the activation of autophagy during cone death. Suppression of endogenous insulin caused an increase in cone loss and exogenous insulin increased cone survival. These data suggest that cone death in retinitis pigmentosa could, at least in part, be a result of the starvation of cones, a bystander effect of rod loss.
There is other evidence suggesting the involvement of photoreceptor energetics in RP. The function of many genes implicated in photoreceptor degeneration, highlight two converging themes: bioenergetic dysfunction and the accumulation of reactive oxygen and nitrogen species (RONS). Photoreceptors have a narrow cilium connecting the inner segment mitochondria and biosynthetic machinery to the continuously regenerating membranes of the light-sensing outer segments. Cilium dysfunction compromises cargo delivery from the inner segment to the outer segment of photoreceptors, and impairs lipid membrane regeneration, and energy utilization, which increases the vulnerability to oxidative damage. Mutations involved in ciliary transport accounts for 25% of genetically determined photoreceptor degenerations, such as Usher syndrome. Mutations affecting lipid metabolism, phototransduction and the visual cycle, all associated with cell metabolism, together account for another 25% of photoreceptor degenerations (Wright et al., 2010).
The RP visual cycle mutations affect energy metabolism, lipid metabolism, and regeneration of outer segments. Photo-excitation of rhodopsin initiates the visual cycle, converting 11-cis retinal to all-trans retinal, liberating singlet oxygen in the process. All-trans retinal released from rhodopsin is then conjugated to lipid phosphatidylethanolamine and transported to the cytoplasm by ATP-binding cassette, subfamily A, member 4 (ABCA4). More than 180 mutations in the ABCA4 gene have been implicated in photoreceptor degeneration, including autosomal recessive Stargardt macular dystrophy, fundus flavimaculatus, autosomal recessive retinitis pigmentosa, cone-rod dystrophy and somewhat controversially for age-related macular degeneration (Michaelides et al., 2006; Molday et al., 2009; Tsybovsky et al., 2010; Zhong and Molday, 2010).
Lipid oxidation of the very long-chain polyunsaturated fatty acid (PUFA) abundant in the outer segment can cause a build-up of oxidized lipids that must be cleared by RPE phagocytosis, which processes 10% of photoreceptors outer segment each day. Disorders of PUFA synthesis can also result in photoreceptor degeneration, as seen with ELOVL4 autosomal dominant mutations, a subtype of Stargardt disease (STG3) (Agbaga et al., 2008).
Altered bioenergetic functions may also directly result in photoreceptor demise. Mutation in NAD-dependent mitochondrial isocitrate dehydrogenase 3 (IDH3), a critical enzyme of the Krebs cycle, occurs in a familial form of RP through metabolic dysfunction (Hartong et al., 2008). Finally, as photoreceptors degenerate and retinal ATP demands fall, more oxygen may be delivered to the retina then can be reduced to H2O by mitochondrial oxidative phosphorylation. Excess tissue oxygen is partially reduced by the mitochondrial electron transport chain (ETC), generating more RONS and potentially increasing retinal toxicity. Despite the genetic heterogeneity of disease mechanisms, mitochondrial dysfunction and oxidative damage are common denominators of photoreceptor degeneration. Similar to inner retina mitochondrial diseases, the choroid plexus atrophies as the energy demand of degenerating photoreceptors declines, confirming the intimate coupling between vascular supply and retinal energy demands.
Vessels in RP regress as photoreceptors degenerate (Rezaei et al., 2017); 13 patients with RP at various stages were imaged by optical coherence tomography angiography. The resulting optical microangiograms provide detailed visualization of retinal and choroidal vascular networks within the retina and choroid. All patients with moderate to severe RP showed abnormal microvasculature in both deep retinal and choroidal layers. Images of patients with only peripheral abnormalities demonstrated relatively normal vasculature networks. Microvascular changes in the retinal and choroidal vasculature correlate with structural changes in IS/OS junction to RPE layer.
15.2. Secondary mitochondropathies and common ocular diseases: DR and AMD
Mitochondrial dysfunction may be accelerated in certain diseases, such as diabetic retinopathy and age-related macular degeneration (Feher et al., 2006; Mueller et al., 2012). Mitochondria-rich retinal neurons exposed to light (and oxygen) generate ROS. Antioxidant enzyme defenses, such as superoxide dismutase (SOD), glutathione (GSH), and catalase (found only in peroxisomes) help protect the retina against excess ROS in a highly oxidizing microenvironment (Dorrell et al., 2009). The process of mitochondrial DNA repair previously believed non-existent, is in fact highly regulated and can be impaired by oxidative stress (Berneburg et al., 2006; Liu and Demple, 2010). Hence, mitochondrial mutations may accumulate over time, leading to age-acquired mitochondrial dysfunction (Ceriello, 2012; de Zeeuw et al., 2015; Giacco and Brownlee, 2010; Ihnat et al., 2007).
15.2.1. Diabetic retinopathy
Diabetic retinopathy is the leading cause of vision loss in working-age adults and is the most prevalent microvascular complication of diabetes. Diabetic retinopathy primarily affects vessels in the inner retina. Within 15 years of diagnosis, almost 50% of patients with type I diabetes and 10% of patients with type II diabetes develop microvascular complications, classified into two phases (Antonetti et al., 2012). Non-proliferative diabetic retinopathy (NPDR) is associated with capillary drop out, leading to retinal ischemia, loss of nutrients and loss of waste removal. This initial retinal insult eventually triggers compensatory but ultimately pathological neovascular proliferation. This second phase of the disease, proliferative diabetic retinopathy (PDR), is associated with more severe vision loss. Macular edema can develop in both phases from increased vascular permeability (Antonetti et al., 2012; Stitt et al., 2016).
Hyperglycemia-induced metabolic abnormalities coincide with and likely cause mitochondrial dysfunction and oxidative damage. Advanced glycation end products (AGEs), increased activity of the polyol pathway and protein kinase C signaling are involved in the progressive metabolic dysfunction seen in diabetic retinopathy, together mediating oxidative stress (Geraldes et al., 2009). Free radicals are generated by mitochondrial respiration and are necessary for normal cellular function (Chouchani et al., 2016; Divakaruni and Brand, 2011; Spiegelman, 2007). Excessive suppression of free radicals impairs mitochondrial function (Chouchani et al., 2016) as does the excess production of free radicals (Gonzalez-Lima et al., 2014; Scatena, 2012; Shi and Gibson, 2007). Oxidative stress results from the accumulation of RONS secondary to the inability to sufficiently scavenge excess free radicals, either because of excessive production or impaired removal of RONS (Barot et al., 2011; Bell and Guarente, 2011; Qiu et al., 2010).
Mitochondria are the most abundant source of endogenous superoxides, peroxynitrites and hydroxyl radicals (Balaban et al., 2005). Antioxidant scavengers that detoxify excess levels of these naturally occurring free radicals may be suppressed in diabetic retina and high-glucose cultured retinal mitochondria (Jarrett et al., 2008). Indeed, elevated levels of lipid peroxides, superoxide, and hydrogen peroxide, together with suppression of SOD and glutathione reductase are reported in murine diabetic retinopathy models (Giacco and Brownlee, 2010; Lamoke et al., 2015). Increased oxidative stress is therefore widely regarded as pathogenic in diabetic retinopathy. Changes in mitochondrial permeability from lipid membrane oxidative damages may explain in part the mitochondrial swelling observed in diabetic mice (Hammes, 2005). Ultimately, severe mitochondrial damage releases cytochrome c from mitochondria to the cytosol, initiating apoptosis in diabetic retinal capillaries (Caldwell and Slapnick, 1989). However, the exact mechanism by which high-glucose mediates changes in mitochondria redox state and the proliferative vascular signal likely governed by metabolism remains ill-defined. Our limited mechanistic understanding of proliferative DR is in part due to the lack of proper animal models, since diabetic mice present with capillary dropout (NPDR) (Lutty et al., 1997), but these models fail to develop the proliferative neovascular disease (PDR). We can however infer from primary mitochondropathies that surviving neurons faced with reduced vascular supply from capillary dropout will signal a need for energy, driving vaso-proliferative DR.
15.2.2. Age-related macular degeneration
Age-related macular degeneration (AMD) is a progressive outer retinal neurodegenerative disease of the central retina and macula and the leading cause of vision loss in aging adults. More than 20% of our aging population is expected to develop AMD (Lim et al., 2012). Drusen deposition and areas of hyper- or de-pigmentation are early signs dry AMD, which may progress to photoreceptors and retinal pigment epithelium atrophy, sometimes referred to as geographic atrophy. Neovascular or wet AMD is associated with the invasion of neovessels into the photoreceptor layer, which is often associated with vision loss. Pathological neovessels in AMD may originate from the choroid (85–90%), or inner retinal vessels (10–15%). This latter sub-type of AMD is also called retinal angiomatous proliferation (RAP) (Bottoni et al., 2005; Donati et al., 2006), and resembles to some extent, macular telangiectasia (MacTel), a rare multifactorial inherited disease of the macula (Shukla et al., 2012; Toy et al., 2012; Yannuzzi et al., 2012).
Mitochondrial dysfunction is correlated with AMD. Mitochondrial DNA polymorphism (Mueller et al., 2012; Park et al., 2012; Udar et al., 2009) and variants of the age-related maculopathy susceptibility 2 protein (ARMS2) are powerful AMD predictors. ARMS2 by some reports is found in mitochondria (Fritsche et al., 2008; Kanda et al., 2007), although its localization is debated (Kortvely et al., 2010). Human induced pluripotent stem cells (hiPSCs) from AMD patients transformed into RPE, secreted more complement and inflammatory factors, which was exaggerated in cells from ARMS2/HTRA1 homozygous patients. Nicotinamide improved the AMD phenotype of hiPSCs-derived RPE cells (Saini et al., 2017). Retinal pigment abnormalities and RPE atrophy characteristic of early dry AMD are noted in 75% of individuals with mitochondrial mutation A3243G associated with MELAS syndrome (Primary mitochondropathies and ocular diseases) (Isashiki et al., 1998). Mitochondrial haplotypes have also been correlated with changes in the prevalence of AMD. RONS primarily produced by complex III of the mitochondrial electron transport chain (ETC), accumulate in the aging macula and correlate both geographically and temporally with AMD progression (Ting et al., 2009). Despite evidence converging on the role of energy deregulation, surprisingly little is known about the energy signals that may govern AMD. Few suitable animal models replicate all aspects of AMD, but several transgenic mice present some characteristic signs of the disease (Table 1) (Ambati et al., 2003; Baba et al., 2010; Cao et al., 2010; Cashman et al., 2011; Chen et al., 2007; Combadière et al., 2007; Hahn et al., 2004; Heckenlively et al., 2003; Imamura et al., 2006; Jo et al., 2011; Luhmann et al., 2009; Lyzogubov et al., 2011; Malek et al., 2005; Markovets et al., 2011; Shen et al., 2006; Takada et al., 1994; Tuo et al., 2007; Zeiss, 2010; Zhao et al., 2011b).
Table 1. Animal models of neovascular age-related macular degeneration.
Table adapted, with permission, from (Pennesi et al., 2012).
| CNV | RAP-Like | Reference | Comment | |
|---|---|---|---|---|
| Complement Factor Pathway | ||||
| Transgenic C3 Overexpression mice | X | Cashman et al., 2011 | ||
| Chemokines | ||||
| Ccl2 −/− and Ccr2 −/− mice | X | Ambati et al., 2003 | ||
| Ccl2 −/− mice1 | – | Luhmann et al., 2009 | 1 - Results differ from Ambati et al., (2003). | |
| Cx3cr1 −/− mice | X2 | Combadière et al., 2007 | 2 - Increased laser-induced CNV. | |
| Ccl2/Cx3cr1 −/− mice | X | Tuo et al., 2007 | ||
| Oxidative Damage models | ||||
| Ceruloplasmin/hephaestin −/− mice | X | Hahn et al., 2004 | ||
| SOD1 knockout mice | X | Imamura et al., 2006 | ||
| NRF2 −/− mice | X | Zhao et al., 2011 | ||
| OXYS Rat | X | Markovets et al., 2011 | ||
| Glucose/Lipid Metabolism | ||||
| APOEe2/e4 transgenic mice ± high fat | X3 | Malek et al., 2005 | 3 - Only on ApoE4 mice when on high fat. | |
| Vldlr −/− mice | X | X | Heckenlively et al., 2003, Chen et al., 2007 | |
| Other | ||||
| Senescence accelerated mouse | X4 | Takada et al., 1994 | 4 - Intrachoroidal NV | |
| Induced CNV | ||||
| Matrigel induced CNV | X | Shen et al., 2006; Cao et al., 2010 | ||
| PEG-8 injection | X | Lyzogubov et al., 2011 | ||
| PEC-injected MCP mice | X | Jo et al., 2011 | ||
| Rat Subretinal lipid hydroperoxide injection | X | Baba et al., 2010 | 5 - intrachoroidal NV (VMD2/VEGF mice) | |
| VEGF Transgenic mice | X5 | X6 | Multiple - see text | 5 - CNV if subretinal injection 6 - rho/VEGF mice |
Dyslipidemia and other common cardiovascular risk factors are associated with AMD (Haines et al., 2006; Lim et al., 2012). Interestingly, human VLDLR deletions result in photoreceptor degeneration and a maculopathy (Boycott et al., 2009; Sarac et al., 2012). Vldlr−/− mice display some salient pathologic features that may inform us about causes of neovascular AMD, including dyslipidemia and macular telangiectasia reminiscent of retinal angiomatous proliferation (RAP) as well as choroidal neovascularization. Poor lipid uptake in Vldlr−/− mice results in high circulating triglycerides and FA levels (Goudriaan et al., 2004). Finally, RONS accumulate in Vldlr−/− photoreceptors (Dorrell et al., 2009; Zhou et al., 2011); also an essential aspect of AMD (Barot et al., 2011; Schrier and Falk, 2011). Therefore Vldlr−/− mice may be a relevant model of RAP in which to explore how retinal lipid energy metabolism impacts aberrant vessel growth. Very low-density lipoprotein receptor (Vldlr), which is present in photoreceptors (Dorrell et al., 2009; Joyal et al., 2016) and is expressed in other tissues with a high metabolic rate, facilitates the uptake of triglyceride-derived fatty acids (Goudriaan et al., 2004; Lopaschuk et al., 2010). In the retinas of Vldlr−/− mice with low fatty acid uptake (Goudriaan et al., 2004) but high circulating lipid levels, we found that Ffar1 suppresses the expression of the glucose transporter Glut1 (Fig. 5c–f). Impaired glucose entry into photoreceptors results in a dual (lipid and glucose) fuel shortage and a reduction in the levels of the TCA cycle intermediate α-ketoglutarate (α-KG; Fig. 7a). Low α-KG levels promote the stabilization of Hif1a and the secretion of Vegfa by starved Vldlr−/− photoreceptors, leading to neovascularization (Fig. 7b–g). We also confirmed the presence of high vitreous VEGFA levels in humans with AMD and RAP (Fig. 7h). Dysregulated lipid and glucose photoreceptor energy metabolism might be a driving force in neovascular AMD and other retinal diseases.
Fig. 7. Energy-deficient photoreceptors drive angiogenesis.
(a) Dual shortage of glucose (b, metabolized to pyruvate) and FA uptake reduces acetyl-CoA (c, estimated by measuring acetylcarnitine) and (d) TCA (Krebs) cycle intermediate α-KG in Vldlr−/− retina (LC/MS/MS). Together with oxygen (O2), α-KG is an essential co-activator of propyl-hydroxylase dehydrogenase (PHD) that tags HIF-1α for degradation by proline hydroxylation (hydroxyproline). (e) Levels of hydroxyproline residues in WT and Vldlr−/− retinas measured by LC/MS/MS (n = 15 WT, 12 Vldlr−/− animal retinas, P = 0.0004). (f) Hif1a retinal expression in Vldlr−/− photoreceptor layer (P12 retinal flat mounts, Scale: 100 μm; left: extended focus; middle and right panels: 3D confocal IHC, n = 3) where (g) Vegfa was then also secreted and localized (P16 retinal flat mounts, Scale: 100 μm; left: extended focus; middle and right panels: 3D confocal IHC, n = 3 retinas). (h) Human subjects with AMD, either retinal angiomatous proliferation (RAP, n =3) or choroidal neovascularization (CNV, n = 7) had higher VEGFA vitreous levels by ELISA compared to control subjects without pathologic neovessels (macular hole; n = 8). Results are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Figure modified, with permission, from (Joyal et al., 2016).
Mouse models used to investigate secondary proliferative retinopathies and AMD are described in Fig. 8.
Fig. 8. Experimental models of pathological angiogenesis.
(a) Oxygen-induced retinopathy: the mouse model of proliferative retinopathy. Neonatal mice are exposed to 75% oxygen from P7 to P12, which induces loss of immature retinal vessels, leading to a central zone of vaso-obliteration (VO). Mice are returned to room air at P12, and the central avascular retina becomes hypoxic, triggering both vascular regrowth and the formation of pathologic neovascular (NV) tufts culminating at P17. (b) Laser-induced choroidal neovascularization (CNV) model. Detailed flowchart of the procedure to obtain consistent laser-induced CNV lesions. Flat-mounted choroid vessels are stained with IB4 at day 7 after laser photocoagulation. 10× Scale bar: 200 μm, 40× Scale bar: 50 μm. ON, optic nerve. (c) Mice model of retinal angiomatous proliferation (RAP): Vldlr−/− mice. Mice deficient for Vldlr spontaneously develop pathological RAP-like vascular lesions invading the photoreceptor layer at P12 and reaching the RPE at P16. Figure modified, with permission, from (Connor et al., 2009; Gong et al., 2015; Joyal et al., 2016).
16. Kinetics of photoreceptor degeneration and mitochondrial adaptation
Our mechanistic understanding of photoreceptor and neuronal decay due to bioenergetic failure and excess RONS would predict progressive damage accumulation, and accelerating cell death with aging (Clarke et al., 2000) (Fig. 9a). This conflicts with all investigated examples of inherited photoreceptor loss, where cell death kinetics is almost constant, and declines slightly in later stages of the disease (stretched exponential kinetics; Fig. 9b) (Clarke et al., 2000). Exponential decay of photoreceptors would, therefore, be proportional to the number of surviving photoreceptors, with a rate constant that differs according to disease severity. However, a decline in cell death rate as diseases progress may suggest a compensatory process. More importantly, the consistency of this paradoxical decay kinetics across many neurodegenerative disorders may suggest a universal mechanism of neuronal death and compensatory mechanisms. To reconcile the apparent discordance between mechanistic and kinetic data, it is argued that diverse mutations in photoreceptor functions have in common mitochondrial signaling, which would ultimately control neuronal survival. Hence, numerous cellular stressors would lead to a steady-state level of apoptotic and survival mitochondrial signals, reflecting the equilibrium between the cost of damage repair required for a neuron to survive versus the wasteful expenditure on a futile repair when survival is unlikely. Evolution of the biochemical functions of mitochondria in neurons is consistent with this theory of integrated damages and repairs leading to neuronal death or survival (Wright et al., 2010).
Fig. 9. Constant risk of photoreceptor death and survival.
(a) Irrespective of the underlying cause, the risk of neuronal death appears constant, manifesting as an exponential decline in cell number. If the risk of neuronal death were increasing, as presumed with mitochondrial diseases and the accumulation of free radicals over time, one would observe a sigmoidal decline in cell number, which is rarely reported. Compensatory pathways must, therefore, exist to palliate for neuronal death. (b) Examples of the exponential decline of photoreceptor degeneration. Wild-type and Rom1−/− mice, and photoreceptor dysplasia (pd/pd) in miniature schnauzers (left). Retinal degeneration of heterozygous (Rds+/−) and homozygous mice (Rds−/−) carrying a null mutation in the gene encoding peripherin/rds (middle). Experimental retinal detachment in the cat (right). Figure modified, with permission, from (Clarke et al., 2000).
17. Conclusion and perspectives
Vascular remodeling is intimately coupled to retinal energy metabolism. Surges in neuronal energy demands are met with vascular proliferation. Conversely, neuronal atrophy and lower retinal metabolic requirements result in vascular pruning. Vascular remodeling is, therefore, an early sign of neuronal metabolic changes. Classic evidence describes the importance of glucose as a primary retinal fuel, sustaining energy production and the biosynthesis of building blocks for growth through aerobic glycolysis. The prevailing assumption has been that glucose is the only fuel used by photoreceptors. However, a substantial proportion of retinal fuel for oxidation (OXPHOS) was shown not to originate from glucose, in work conducted half a century ago. We recently showed that lipids, are used by photoreceptors as fuel for OXPHOS through fatty acid β-oxidation, and are an important energy source for the retina.
Nutrient availability and downstream Krebs cycle metabolites help govern the retinal vascular supply, ensuring adequate neuronal energy homeostasis. Mitochondrial disorders, inherited or acquired, tilt this precisely regulated energy balance often resulting in retinal vascular changes and neuronal loss. Photoreceptors and retinal ganglion cells are uniquely susceptible to bioenergetic dysfunction because of their considerable metabolic requirements. Many important questions arise from the variable penetrance of mitochondrial disorders in the eye, such as their regional susceptibility, their delayed onset, and the gender-specific and environmental triggers that initiate disease. More importantly, the complex compensatory mitochondrial adaptation that almost universally delays neuronal demise with disease progression is ill understood. Therapeutic strategies that harness these inherent protective mechanisms may help prevent neuronal death and pathological angiogenesis, delaying vision loss.
Acknowledgments
This work was supported by: JSJ: Burroughs Wellcome Fund Career Award for Medical Scientists, Fondation Fighting Blindness, Natural Sciences and Engineering Research Council of Canada (NSERC) 06743, Fonds de Recherche du Québec – Santé (FRQS), Canadian Child Health Clinician Scientist Program, and CIHR New Investigator Award. LEHS: NIH EY024864, EY017017, P01 HD18655, BCH IDDRC, 1U54HD090255, Lowy Medical Research Institute, European Commission FP7 project 305485 PREVENT-ROP.
References
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adijanto J, Philp NJ. Cultured primary human fetal retinal pigment epithelium (hfRPE) as a model for evaluating RPE metabolism. Exp Eye Res. 2014;126C:77–84. doi: 10.1016/j.exer.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adijanto J, Du J, Moffat C, Seifert EL, Hurley JB, Philp NJ. The retinal pigment epithelium utilizes fatty acids for ketogenesis: IMPLICATIONS for metabolic coupling with the outer retina. J Biol Chem. 2014;289:20570–20582. doi: 10.1074/jbc.M114.565457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agathocleous M, Love NK, Randlett O, Harris JJ, Liu J, Murray AJ, Harris WA. Metabolic differentiation in the embryonic retina. Nat Cell Biol. 2012;14:859–864. doi: 10.1038/ncb2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbaga MP, Brush RS, Mandal MNA, Henry K, Elliott MH, Anderson RE. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc Natl Acad Sci U S A. 2008;105:12843–12848. doi: 10.1073/pnas.0802607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbaga MP, Mandal MNA, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res. 2010;51:1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aït-Ali N, Fridlich R, Millet-Puel G, Clérin E, Delalande F, Jaillard C, Blond F, Perrocheau L, Reichman S, Byrne LC, Olivier-Bandini A, Bellalou J, Moyse E, Bouillaud F, Nicol X, Dalkara D, van Dorsselaer A, Sahel JA, Léveillard T. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161:817–832. doi: 10.1016/j.cell.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Altshuler D. Specification of Cell Type in the Vertebrate Retina. Development of the Visual System 1991 [Google Scholar]
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- Ames A, 3rd, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci Offic J Soc Neurosci. 1992a;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci. 1992b;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., III Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can J Physiol Pharmacol. 1992;70:S158–S164. doi: 10.1139/y92-257. [DOI] [PubMed] [Google Scholar]
- Anderson RE. Lipids of ocular tissues: IV. A comparison of the phospholipids from the retina of six mammalian species. Exp eye Res. 1970;10:339–344. doi: 10.1016/s0014-4835(70)80046-x. [DOI] [PubMed] [Google Scholar]
- Anderson B, Saltzman H. Retinal oxygen utilization mesured by hyperbaric blackout. Arch Ophthalmol. 1964;72:792–795. doi: 10.1001/archopht.1964.00970020794009. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Thomas W, Gardner MD. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Ash JD, Overbeek PA. Lens-specific VEGF-A expression induces angioblast migration and proliferation and stimulates angiogenic remodeling. Dev Biol. 2000;223:383–398. doi: 10.1006/dbio.2000.9755. [DOI] [PubMed] [Google Scholar]
- Atsuzawa K, Nakazawa A, Mizutani K, Fukasawa M, Yamamoto N, Hashimoto T, Usuda N. Immunohistochemical localization of mitochondrial fatty acid β-oxidation enzymes in Müller cells of the retina. Histochem Cell Biol. 2010;134:565–579. doi: 10.1007/s00418-010-0752-4. [DOI] [PubMed] [Google Scholar]
- Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D’armiento J, Abel ED, Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–8723. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- Aveldano M, Sprecher H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J Biol Chem. 1987;262:1180–1186. [PubMed] [Google Scholar]
- Ayton LN, Guymer RH, Luu CD. Choroidal thickness profiles in retinitis pigmentosa. Clin Exp Ophthalmol. 2013;41:396–403. doi: 10.1111/j.1442-9071.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- Baba T, Bhutto IA, Merges C, Grebe R, Emmert D, McLeod DS, Armstrong D, Lutty GA. A rat model for choroidal neovascularization using subretinal lipid hydroperoxide injection. Am J Pathol. 2010;176:3085–3097. doi: 10.2353/ajpath.2010.090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CC, Sparrow JM. Visual symptomatology in patients with sight-threatening diabetic retinopathy. Diabet Med. 2001;18:883–888. doi: 10.1046/j.1464-5491.2001.00589.x. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barot M, Gokulgandhi MR, Mitra AK. Mitochondrial dysfunction in retinal diseases. Curr Eye Res. 2011;36:1069–1077. doi: 10.3109/02713683.2011.607536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Metabolism of arachidonic acid in the retina and retinal pigment epithelium: biological effects of oxygenated metabolites of arachidonic acid. Prog Clin Biol Res. 1989a;312:15–37. [PubMed] [Google Scholar]
- Bazan NG. The metabolism of omega-3 polyunsaturated fatty acids in the eye: the possible role of docosahexaenoic acid and docosanoids in retinal physiology and ocular pathology. Prog Clin Biol Res. 1989b;312:95–112. [PubMed] [Google Scholar]
- Bazan N. Supply of n-3 polyunsaturated fatty acids and their significance in the central nervous system. Nutr Brain. 1990;8:1–24. [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Bazan N, Gordon W, Marcheselli V, Lukiw W, Duhault J, Koenig-Berard E, Linn D, DeCoster M, Mukherjee P. Experimental models and their use in studies of diabetic retinal microangiopathy. Therapie. 1997;52:447–451. [PubMed] [Google Scholar]
- Beisiegel U, Heeren J. Lipoprotein lipase (EC 3.1.1.34) targeting of lipoproteins to receptors. Proc Nutr Soc. 1997;56:731–737. doi: 10.1079/pns19970073. [DOI] [PubMed] [Google Scholar]
- Bell EL, Guarente L. The SirT3 divining rod points to oxidative stress. Mol Cell. 2011;42:561–568. doi: 10.1016/j.molcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénit P, Letouzé E, Rak M, Aubry L, Burnichon N, Favier J, Gimenez-Roqueplo AP, Rustin P. Unsuspected task for an old team: succinate, fumarate and other Krebs cycle acids in metabolic remodeling. Biochim Biophys Acta. 2014;1837:1330–1337. doi: 10.1016/j.bbabio.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Berneburg M, Kamenisch Y, Krutmann J, Röcken M. ‘To repair or not to repair - no longer a question’: repair of mitochondrial DNA shielding against age and cancer. Exp Dermatol. 2006;15:1005–1015. doi: 10.1111/j.1600-0625.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- Bhatti MT. Retinitis pigmentosa, pigmentary retinopathies, and neurologic diseases. Curr Neurol Neurosci Rep. 2006;6:403–413. doi: 10.1007/s11910-996-0021-z. [DOI] [PubMed] [Google Scholar]
- Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Asp Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill A, Sperber G, Ujiie K. Physiology of the choroidal vascular bed. Int Ophthalmol. 1983;6:101–107. doi: 10.1007/BF00127638. [DOI] [PubMed] [Google Scholar]
- Binet F, Sapieha P. ER stress and angiogenesis. Cell Metab. 2015;22:560–575. doi: 10.1016/j.cmet.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Binns D, Januszewski T, Chen Y, Hill J, Markin VS, Zhao Y, Gilpin C, Chapman KD, Anderson RG, Goodman JM. An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol. 2006;173:719–731. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biousse V, Newman NJ. Neuro-ophthalmology of mitochondrial diseases. Curr Opin Neurol. 2003;16:35–43. doi: 10.1097/01.wco.0000053592.70044.57. [DOI] [PubMed] [Google Scholar]
- Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010;120:3033–3041. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boneva NB, Kaplamadzhiev DB, Sahara S, Kikuchi H, Pyko IV, Kikuchi M, Tonchev AB, Yamashima T. Expression of fatty acid-binding proteins in adult hippocampal neurogenic niche of postischemic monkeys. Hippocampus. 2011;21:162–171. doi: 10.1002/hipo.20732. [DOI] [PubMed] [Google Scholar]
- Bordin P, Bodamer O, Venkatesan S, Gray R, Bannister P, Halliday D. Effects of fish oil supplementation on apolipoprotein B100 production and lipoprotein metabolism in normolipidaemic males. Eur J Clin Nutr. 1998;52:104–109. doi: 10.1038/sj.ejcn.1600522. [DOI] [PubMed] [Google Scholar]
- Bottoni F, Massacesi A, Cigada M, Viola F, Musicco I, Staurenghi G. Treatment of retinal angiomatous proliferation in age-related macular degeneration: a series of 104 cases of retinal angiomatous proliferation. Arch Ophthalmol. 2005;123:1644–1650. doi: 10.1001/archopht.123.12.1644. [DOI] [PubMed] [Google Scholar]
- Boulahbel H, Durán RV, Gottlieb E. Prolyl hydroxylases as regulators of cell metabolism. Biochem Soc Trans. 2009;37:291–294. doi: 10.1042/BST0370291. [DOI] [PubMed] [Google Scholar]
- Boycott KM, Bonnemann C, Herz J, Neuert S, Beaulieu C, Scott JN, Venkatasubramanian A, Parboosingh JS. Mutations in VLDLR as a cause for autosomal recessive cerebellar ataxia with mental retardation (dysequilibrium syndrome) J Child Neurol. 2009;24:1310–1315. doi: 10.1177/0883073809332696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman NE, Raymond GV, Rizzo WB, Moser AB, Wilkinson ME, Stone EM, Steinberg SJ, Wangler MF, Rush ET, Hacia JG. Peroxisome biogenesis disorders in the Zellweger spectrum: an overview of current diagnosis, clinical manifestations, and treatment guidelines. Mol Genet Metabol. 2016;117:313–321. doi: 10.1016/j.ymgme.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148:619–628. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F, McAdams A, Cummins J, Konkol R, Singh I, Moser A, Moser H. Cerebro-hepato-renal (Zellweger) syndrome and neonatal adrenoleukodystrophy: similarities in phenotype and accumulation of very long chain fatty acids. Johns Hopkins Med J. 1982;151:344–351. [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, Leifke E. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1403–1411. doi: 10.1016/S0140-6736(11)61879-5. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Valdembri D, Caccavari F, Serini G. Semaphoring vascular morphogenesis. Endothelium. 2006:81–91. doi: 10.1080/10623320600698003. [DOI] [PubMed] [Google Scholar]
- Cahill GF. Starvation in man. N Engl J Med. 1970;282:668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Caldwell RB, Slapnick SM. Increased cytochrome oxidase activity in the diabetic rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1989;30:591–599. [PubMed] [Google Scholar]
- Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. doi: 10.1016/j.preteyeres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA, Mir TA. The mechanism of cone cell death in retinitis pigmentosa. Prog Retin Eye Res. 2017 Sep 27; doi: 10.1016/j.preteyeres.2017.08.004. http://dx.doi.org/10.1016/j.preteyeres.2017.08.004. pii: S1350–9462(17)30071-X, [Epub ahead of print] [DOI] [PubMed]
- Cannella DM, Kapp JP, Munschauer FE, Markov AK, Shucard DW. Cerebral resuscitation with succinate and fructose-1, 6-diphosphate. Surg Neurol. 1989;31:177–182. doi: 10.1016/0090-3019(89)90113-4. [DOI] [PubMed] [Google Scholar]
- Cao J, Zhao L, Li Y, Liu Y, Xiao W, Song Y, Luo L, Huang D, Yancopoulos GD, Wiegand SJ, Wen R. A subretinal matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Invest Ophthalmol Vis Sci. 2010;51:6009–6017. doi: 10.1167/iovs.09-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23:53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Carelli V, d’Adamo P, Valentino ML, La Morgia C, Ross-Cisneros FN, Caporali L, Maresca A, Loguercio Polosa P, Barboni P, De Negri A. Parsing the differences in affected with LHON: genetic versus environmental triggers of disease conversion. Brain. 2015:139e17–e17. doi: 10.1093/brain/awv339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle R, Lanphier EH, Rahn H. Hyperbaric oxygen and persistence of vision in retinal ischemia. J Appl Physiol. 1964;19:914–918. doi: 10.1152/jappl.1964.19.5.914. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Desai A, Ramo K, Kumar-Singh R. Expression of complement component 3 (C3) from an adenovirus leads to pathology in the murine retina. Invest Ophthalmol Vis Sci. 2011;52:3436–3445. doi: 10.1167/iovs.10-6002. [DOI] [PubMed] [Google Scholar]
- Casson RJ, Wood JPM, Han G, Kittipassorn T, Peet DJ, Chidlow G. MType pyruvate kinase isoforms and lactate dehydrogenase a in the mammalian retina: metabolic ImplicationsPKM1 and LDH-a in the mammalian retina. Invest Ophthalmol Vis Sci. 2016;57:66–80. doi: 10.1167/iovs.15-17962. [DOI] [PubMed] [Google Scholar]
- Ceriello A. The emerging challenge in diabetes: the “metabolic memory”. Vasc Pharmacol. 2012;57:133–138. doi: 10.1016/j.vph.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- Chan-Ling T, Gole GA, Quinn GE, Adamson SJ, Darlow BA. Pathophysiology, screening and treatment of ROP: a multi-disciplinary perspective. Prog Retin Eye Res. 2017 Sep 27; doi: 10.1016/j.preteyeres.2017.09.002. http://dx.doi.org/10.1016/j.preteyeres.2017.09.002. pii: S1350–9462(16)30077-5, [Epub ahead of print] [DOI] [PubMed]
- Chekroud K, Guillou L, Grégoire S, Ducharme G, Brun E, Cazevieille C, Bretillon L, Hamel CP, Brabet P, Pequignot MO. Fatp1 deficiency affects retinal light response and dark adaptation, and induces age-related alterations. PLoS One. 2012;7:e50231. doi: 10.1371/journal.pone.0050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Smith LEH. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Smith LEH. A double-edged sword: erythropoietin eyed in retinopathy of prematurity. J AAPOS Offic Publ Am Assoc Pediatr Ophthalmol Strabismus/Am Assoc Pediatr Ophthalmol Strabismus. 2008;12:221–222. doi: 10.1016/j.jaapos.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem. 2007;282:34420–34428. doi: 10.1074/jbc.M611289200. [DOI] [PubMed] [Google Scholar]
- Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LEH. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchore Y, Begaj T, Wu D, Drokhlyansky E, Cepko CL. Glycolytic reliance promotes anabolism in photoreceptors. Elife. 2017:6. doi: 10.7554/eLife.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe HG, Anderson RE. Unique molecular species composition of glycerolipids of frog rod outer segments. Exp eye Res. 1990;51:159–165. doi: 10.1016/0014-4835(90)90068-6. [DOI] [PubMed] [Google Scholar]
- Choe HG, Ghalayini AJ, Anderson RE. Phosphoinositide metabolism in frog rod outer segments. Exp eye Res. 1990;51:167–176. doi: 10.1016/0014-4835(90)90069-7. [DOI] [PubMed] [Google Scholar]
- Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, Pierce KA, Laznik-Bogoslavski D, Vetrivelan R, Clish CB, Robinson AJ, Gygi SP, Spiegelman BM. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532(7597):112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Collins RA, Leavitt BR, Andrews DF, Hayden MR, Lumsden CJ, McInnes RR. A one-hit model of cell death in inherited neuronal degenerations. Nature. 2000;406:195–199. doi: 10.1038/35018098. [DOI] [PubMed] [Google Scholar]
- Cohen AI. The fine structure of the extrafoveal receptors of the Rhesus monkey. Exp eye Res. 1961;1:128–136. doi: 10.1016/s0014-4835(61)80018-3. [DOI] [PubMed] [Google Scholar]
- Cohen LH, Noell WK. Glucose catabolism of rabbit retina before and after development of visual function. J Neurochem. 1960;5:253–276. doi: 10.1111/j.1471-4159.1960.tb13363.x. [DOI] [PubMed] [Google Scholar]
- Collier G, Traianedes K, Macaulay S, O’Dea K. Effect of fatty acid oxidation inhibition on glucose metabolism in diabetic rats. Hormone Metabol Res. 1993;25:9–12. doi: 10.1055/s-2007-1002035. [DOI] [PubMed] [Google Scholar]
- Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A, Lavalette S, Houssier M, Jonet L, Picard E, Debré P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Serhan CN, Smith LEH. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LEH. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzi S, Neumann S, Gershengorn MC. Seven transmembrane-spanning receptors for free fatty acids as therapeutic targets for diabetes mellitus: pharmacological, phylogenetic, and drug discovery aspects. J Biol Chem. 2008;283:16269–16273. doi: 10.1074/jbc.R800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cringle SJ, Yu PK, Su EN, Yu DY. Oxygen distribution and consumption in the developing rat retina. Invest Ophthalmol Vis Sci. 2006;47:4072–4076. doi: 10.1167/iovs.05-1638. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Dahia PLM, Consortium FP. Transcription association of VHL and SDH mutations link hypoxia and oxidoreductase signals in pheochromocytomas. Ann N Y Acad Sci. 2006;1073:208–220. doi: 10.1196/annals.1353.023. [DOI] [PubMed] [Google Scholar]
- Das A, McGuire PG. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin eye Res. 2003;22:721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- de Crecchio G, Alfieri MC, Cennamo G, D’Esposito F, Forte R. Pericentral pigmentary retinopathy: long-term follow-up. Eye (Lond) 2006;20:1408–1410. doi: 10.1038/sj.eye.6702263. [DOI] [PubMed] [Google Scholar]
- de Gooyer TE, Stevenson KA, Humphries P, Simpson DAC, Curtis TM, Gardiner TA, Stitt AW. Rod photoreceptor loss in Rho−/− mice reduces retinal hypoxia and hypoxia-regulated gene expression. Invest Ophthalmol Vis Sci. 2006a;47:5553–5560. doi: 10.1167/iovs.06-0646. [DOI] [PubMed] [Google Scholar]
- de Gooyer TE, Stevenson KA, Humphries P, Simpson DAC, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci. 2006b;47:5561–5568. doi: 10.1167/iovs.06-0647. [DOI] [PubMed] [Google Scholar]
- De Vivo DC, Leary L, Wang D. Glucose transporter 1 deficiency syndrome and other glycolytic defects. J Child Neurol. 2002;17(Suppl 3):3S15–23. discussion 13S24-15. [PubMed] [Google Scholar]
- de Zeeuw P, Wong BW, Carmeliet P. Metabolic adaptations in diabetic endothelial cells. Circ J. 2015;79:934–941. doi: 10.1253/circj.CJ-15-0230. [DOI] [PubMed] [Google Scholar]
- Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Dhoot DS, Huo S, Yuan A, Xu D, Srivistava S, Ehlers JP, Traboulsi E, Kaiser PK. Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol. 2013;97:66–69. doi: 10.1136/bjophthalmol-2012-301917. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Brand MD. The regulation and physiology of mitochondrial proton leak. Physiol (Bethesda) 2011;26:192–205. doi: 10.1152/physiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- Donati MC, Carifi G, Virgili G, Menchini U. Retinal angiomatous proliferation: association with clinical and angiographic features. Ophthalmologica. 2006;220:31–36. doi: 10.1159/000089272. [DOI] [PubMed] [Google Scholar]
- Dor Y, Porat R, Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol. 2001;280:C1367–C1374. doi: 10.1152/ajpcell.2001.280.6.C1367. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin eye Res. 2006;25:277–295. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Jacobson R, Yanes O, Gariano R, Heckenlively J, Banin E, Ramirez GA, Gasmi M, Bird A, Siuzdak G, Friedlander M. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J Clin Invest. 2009;119:611–623. doi: 10.1172/JCI35977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Rountree A, Cleghorn WM, Contreras L, Lindsay KJ, Sadilek M, Gu H, Djukovic D, Raftery D, Satrustegui J, Kanow M, Chan L, Tsang SH, Sweet IR, Hurley JB. Phototransduction influences metabolic flux and nucleotide metabolism in mouse retina. J Biol Chem. 2016;291:4698–4710. doi: 10.1074/jbc.M115.698985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlu YK, Burumcek E, Devranoglu K, Mudun AB, Karacorlu S, Arslan MO. Associated ocular findings in pericentral pigmentary retinopathy. Acta Ophthalmol Scand. 1997;75:101–103. doi: 10.1111/j.1600-0420.1997.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Ehlken C, Martin G, Lange C, Gogaki EG, Fiedler U, Schaffner F, Hansen LL, Augustin HG, Agostini HT. Therapeutic interference with EphrinB2 signalling inhibits oxygen-induced angioproliferative retinopathy. Acta Ophthalmol. 2011;89:82–90. doi: 10.1111/j.1755-3768.2009.01609.x. [DOI] [PubMed] [Google Scholar]
- Erickson RP. Leber’s optic atrophy, a possible example of maternal inheritance. Am J Hum Genet. 1972;24:348. [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Maden CH, Ruhrberg C. Neuropilin ligands in vascular and neuronal patterning. Biochem Soc Trans. 2009;37:1228–1232. doi: 10.1042/BST0371228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27:983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AL, Pennesi ME, Harding CO, Weleber RG, Gillingham MB. Observations regarding retinopathy in mitochondrial trifunctional protein deficiencies. Mol Genet Metabol. 2012a;106:18–24. doi: 10.1016/j.ymgme.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AL, Pennesi ME, Harding CO, Weleber RG, Gillingham MB. Observations regarding retinopathy in mitochondrial trifunctional protein deficiencies. Mol Genet Metab. 2012b;106:18–24. doi: 10.1016/j.ymgme.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler AJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Folbergrová J, Ljunggren B, Norberg K, Siesjö BK. Influence of complete ischemia on glycolytic metabolites, citric acid cycle intermediates, and associated amino acids in the rat cerebral cortex. Brain Res. 1974;80:265–279. doi: 10.1016/0006-8993(74)90690-8. [DOI] [PubMed] [Google Scholar]
- Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Loenhardt T, Janssen A, Fisher SA, Rivera A, Keilhauer CN, Weber BHF. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40:892–896. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Calver AR, Krüger WH, Mudhar HS, Michalovich D, Takakura N, Nishikawa S, Richardson WD. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron. 1996;17:1117–1131. doi: 10.1016/s0896-6273(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Fu Z, Lofqvist CA, Shao Z, Sun Y, Joyal JS, Hurst CG, Cui RZ, Evans LP, Tian K, SanGiovanni JP, Chen J, Ley D, Hansen Pupp I, Hellstrom A, Smith LE. Dietary ω-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am J Clin Nutr. 2015;101:879–888. doi: 10.3945/ajcn.114.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano C, Iommarini L, Giordano L, Maresca A, Pisano A, Valentino ML, Caporali L, Liguori R, Deceglie S, Roberti M, Fanelli F, Fracasso F, Ross-Cisneros FN, D’Adamo P, Hudson G, Pyle A, Yu-Wai-Man P, Chinnery PF, Zeviani M, Salomao SR, Berezovsky A, Belfort R, Ventura DF, Moraes M, Moraes Filho M, Barboni P, Sadun F, De Negri A, Sadun AA, Tancredi A, Mancini M, d’Amati G, Loguercio Polosa P, Cantatore P, Carelli V. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain. 2013;137:335–353. doi: 10.1093/brain/awt343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Chen J, Ingeman JE, George JK, Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci Offic J Soc Neurosci. 1989;9:4416–4429. doi: 10.1523/JNEUROSCI.09-12-04416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusto NM, Pasquaré SJ, Salvador GA, Ilincheta de Boschero MG. Lipid second messengers and related enzymes in vertebrate rod outer segments. J Lipid Res. 2010;51:685–700. doi: 10.1194/jlr.R001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogat K, Le Gat L, Van Den Berghe L, Marchant D, Kobetz A, Gadin S, Gasser B, Quéré I, Abitbol M, Menasche M. VEGF and KDR gene expression during human embryonic and fetal eye development. Invest Ophthalmol Vis Sci. 2004;45:7–14. doi: 10.1167/iovs.02-1096. [DOI] [PubMed] [Google Scholar]
- Goldberg AFX, Moritz OL, Williams DS. Molecular basis for photoreceptor outer segment architecture. Prog Retin Eye Res. 2016;55:52–81. doi: 10.1016/j.preteyeres.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Li J, Sun Y, Fu Z, Liu CH, Evans L, Tian K, Saba N, Fredrick T, Morss P, Chen J, Smith LEH. Optimization of an image-guided laser-induced choroidal neovascularization model in mice. PLoS One. 2015;10:e0132643. doi: 10.1371/journal.pone.0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Fu Z, Edin ML, Liu CH, Wang Z, Shao Z, Fredrick TW, Saba NJ, Morss PC, Burnim SB, Meng SS, Lih FB, Lee KSS, Moran EP, SanGiovanni JP, Hellström A, Hammock BD, Zeldin DC, Smith LEH. Cytochrome P450 oxidase 2C inhibition adds to ω-3 long-chain polyunsaturated fatty acids protection against retinal and choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2016a;36:1919–1927. doi: 10.1161/ATVBAHA.116.307558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Shao Z, Fu Z, Edin ML, Sun Y, Liegl RG, Wang Z, Liu CH, Burnim SB, Meng SS, Lih FB, SanGiovanni JP, Zeldin DC, Hellström A, Smith LEH. Fenofibrate inhibits cytochrome P450 epoxygenase 2C activity to suppress pathological ocular angiogenesis. EBioMedicine. 2016b;13:201–211. doi: 10.1016/j.ebiom.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Fu Z, Liegl R, Chen J, Hellström A, Smith LE. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am J Clin Nutr. 2017;106:16–26. doi: 10.3945/ajcn.117.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Barksdale BR, Rojas JC. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol. 2014;88:584–593. doi: 10.1016/j.bcp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Gospe SM, Baker SA, Arshavsky VY. Facilitative glucose transporter Glut1 is actively excluded from rod outer segments. J Cell Sci. 2010;123:3639–3644. doi: 10.1242/jcs.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E, Tomlinson IPM. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- Goudriaan JR, Espirito Santo SMS, Voshol PJ, Teusink B, van Dijk KW, van Vlijmen BJM, Romijn JA, Havekes LM, Rensen PCN. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res. 2004;45:1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- Grant MB, Tarnuzzer RW, Caballero S, Ozeck MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC, Belardinelli L. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res. 1999;85:699–706. doi: 10.1161/01.res.85.8.699. [DOI] [PubMed] [Google Scholar]
- Graymore C. Metabolism of the developing retina. I Aerobic and anaerobic glycolysis in the developing rat retina. Br J Ophthalmol. 1959;43:34–39. doi: 10.1136/bjo.43.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graymore C. Possible significance of the isoenzymes of lactic dehydrogenase in the retina of the rat. Nature. 1964;201:615–616. doi: 10.1038/201615b0. [DOI] [PubMed] [Google Scholar]
- Graymore C, Tansley K. Iodoacetate poisoning of the rat retina. II Glycolysis in the poisoned retina. Br J Ophthalmol. 1959;43:486–493. doi: 10.1136/bjo.43.8.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin eye Res. 2008;27:372–390. doi: 10.1016/j.preteyeres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Grönlund MA, Honarvar AKS, Andersson S, Moslemi AR, Oldfors A, Holme E, Tulinius M, Darin N. Ophthalmological findings in children and young adults with genetically verified mitochondrial disease. Br J Ophthalmol. 2010;94:121–127. doi: 10.1136/bjo.2008.154187. [DOI] [PubMed] [Google Scholar]
- Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, Sennlaub F. On phagocytes and macular degeneration. Prog Retin Eye Res. 2017;61:98–128. doi: 10.1016/j.preteyeres.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Gurvitch AM, Mutuskina EA, Zarzhetsky YV, Trubina IE, Avruschenko MS, Pylova SI, Volkov AV, Lazareva NA, Stepanichev MY, Onufriev MV, Gulyaeva NV. Prophylaxis of encephalopathies and risk factors of atherogenesis development in the postresuscitation period in rats by means of succinic acid. Resuscitation. 1997;35:165–170. doi: 10.1016/s0300-9572(97)00045-2. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- Hagins WA, Penn RD, Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970;10:380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc Natl Acad Sci U S A. 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Schnetz-Boutaud N, Schmidt S, Scott WK, Agarwal A, Postel EA, Olson L, Kenealy SJ, Hauser M, Gilbert JR, Pericak-Vance MA. Functional candidate genes in age-related macular degeneration: significant association with VEGF, VLDLR, and LRP6. Invest Ophthalmol Vis Sci. 2006;47:329–335. doi: 10.1167/iovs.05-0116. [DOI] [PubMed] [Google Scholar]
- Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Hormone Metabol Res (Hormon- und Stoffwechselforschung = Hormones et métabolisme) 2005;37(Suppl 1):39–43. doi: 10.1055/s-2005-861361. [DOI] [PubMed] [Google Scholar]
- Han G, Wood JPM, Chidlow G, Mammone T, Casson RJ. Mechanisms of neuroprotection by glucose in rat retinal cell cultures subjected to respiratory inhibition. Invest Ophthalmol Vis Sci. 2013;54:7567–7577. doi: 10.1167/iovs.13-12200. [DOI] [PubMed] [Google Scholar]
- Hansen RM, Moskowitz A, Akula JD, Fulton AB. The neural retina in retinopathy of prematurity. Prog Retin Eye Res. 2017;56:32–57. doi: 10.1016/j.preteyeres.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hård A-L, Smith LE, Hellström A. Nutrition, Insulin-like Growth Factor-1 and Retinopathy of Prematurity, Seminars in Fetal and Neonatal Medicine. Elsevier, pp. 2013:136–142. doi: 10.1016/j.siny.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P, Dumont I, Bhattacharya M, Hou X, Lachapelle P, Varma DR, Chemtob S. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovasc Res. 2000:489–509. doi: 10.1016/s0008-6363(00)00084-5. [DOI] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Hartong DT, DM, McGee TL, Berson EL, Dryja TP, Colman RF. Novel insights into the contributions of isocitrate dehydrogenases to the Krebs cycle from patients with retinitis pigmentosa. Nat Genet. 2008;40:1230. doi: 10.1038/ng.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Miao FJP, Lin DCH, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- Heckenlively JR, Hawes NL, Friedlander M, Nusinowitz S, Hurd R, Davisson M, Chang B. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 2003;23:518–522. doi: 10.1097/00006982-200308000-00012. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, Albertsson-Wikland K, Carlsson B, Niklasson A, Sjodell L, LeRoith D, Senger DR, Smith LE. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001;98:5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström A, Smith LEH, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström A, Ley D, Hansen-Pupp I, Hallberg B, Ramenghi LA, Löfqvist C, Smith LEH, Hård AL. Role of insulinlike growth factor 1 in fetal development and in the early postnatal life of premature infants. Am J Perinatol. 2016;33:1067–1071. doi: 10.1055/s-0036-1586109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang QV, Linsenmeier RA, Chung CK, Curcio CA. Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Vis Neurosci. 2002;19:395–407. doi: 10.1017/s0952523802194028. [DOI] [PubMed] [Google Scholar]
- Houten SM, Wanders RJA. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer S, Krier C. Ischemia and aging brain. Studies on glucose and energy metabolism in rat cerebral cortex. Neurobiol Aging. 1986;7:23–29. doi: 10.1016/0197-4580(86)90022-9. [DOI] [PubMed] [Google Scholar]
- Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41:1217–1228. [PubMed] [Google Scholar]
- Hurley JB, Lindsay KJ, Du J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J Neurosci Res. 2015;93:1079–1092. doi: 10.1002/jnr.23583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TJ, Karanjia R, Moraes-Filho MN, Gale J, Tran JS, Chu ER, Salomao SR, Berezovsky A, Belfort R, Moraes MN. Natural history of conversion of Leber’s Hereditary Optic Neuropathy: a prospective case series. Ophthalmology. 2017;124:843–850. doi: 10.1016/j.ophtha.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostagl other lipid Mediat. 2009;89:82–88. doi: 10.1016/j.prostaglandins.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Ihnat MA, Thorpe JE, Ceriello A. Hypothesis: the ‘metabolic memory’, the new challenge of diabetes. Diabet Med. 2007;24:582–586. doi: 10.1111/j.1464-5491.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isashiki Y, Nakagawa M, Ohba N, Kamimura K, Sakoda Y, Higuchi I, Izumo S, Osame M. Retinal manifestations in mitochondrial diseases associated with mitochondrial DNA mutation. Acta Ophthalmol Scand. 1998;76:6–13. doi: 10.1034/j.1600-0420.1998.760103.x. [DOI] [PubMed] [Google Scholar]
- Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, Honda Y, Oguchi Y, Adamis AP. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9:781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Jarrett SG, Lin H, Godley BF, Boulton ME. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog Retin Eye Res. 2008;27:596–607. doi: 10.1016/j.preteyeres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Jo YJ, Sonoda KH, Oshima Y, Takeda A, Kohno R-i, Yamada J, Hamuro J, Yang Y, Notomi S, Hisatomi T, Ishibashi T. Establishment of a new animal model of focal subretinal fibrosis that resembles disciform lesion in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:6089–6095. doi: 10.1167/iovs.10-5189. [DOI] [PubMed] [Google Scholar]
- Johnson RN, Hansford RG. The control of tricarboxylate-cycle oxidations in blowfly flight muscle. The steady-state concentrations of citrate, isocitrate 2-oxoglutarate and malate in flight muscle and isolated mitochondria. Biochem J. 1975;146:527–535. doi: 10.1042/bj1460527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE. Hyaloid remnants in the eyes of premature babies. Br J Ophthalmol. 1963;47:39–44. doi: 10.1136/bjo.47.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, Djavari M, Kunik D, Honoré JC, Picard E, Zabeida A, Varma DR, Hickson G, Mancini J, Klagsbrun M, Costantino S, Beauséjour C, Lachapelle P, Smith LEH, Chemtob S, Sapieha P. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011;117:6024–6035. doi: 10.1182/blood-2010-10-311589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JS, Omri S, Sitaras N, Rivera JC, Sapieha P, Chemtob S. Neovascularization in retinopathy of prematurity: opposing actions of neuronal factors GPR91 and semaphorins 3. Acta Paediatr. 2012;101:819–826. doi: 10.1111/j.1651-2227.2012.02692.x. [DOI] [PubMed] [Google Scholar]
- Joyal JS, Nim S, Zhu T, Sitaras N, Rivera JC, Shao Z, Sapieha P, Hamel D, Sanchez M, Zaniolo K, St-Louis M, Ouellette J, Montoya-Zavala M, Zabeida A, Picard E, Hardy P, Bhosle V, Varma DR, Gobeil F, Beauséjour C, Boileau C, Klein W, Hollenberg M, Ribeiro-da-Silva A, Andelfinger G, Chemtob S. Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat Med. 2014;20:1165–1173. doi: 10.1038/nm.3669. [DOI] [PubMed] [Google Scholar]
- Joyal JS, Bhosle VK, Chemtob S. Subcellular G-protein coupled receptor signaling hints at greater therapeutic selectivity. Expert Opin Ther Targets. 2015;19:717–721. doi: 10.1517/14728222.2015.1042365. [DOI] [PubMed] [Google Scholar]
- Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N, Fredrick T, Burnim S, Kim JS, Patel G, Juan AM, Hurst CG, Hatton CJ, Cui Z, Pierce KA, Bherer P, Aguilar E, Powner MB, Vevis K, Boisvert M, Fu Z, Levy E, Fruttiger M, Packard A, Rezende FA, Maranda B, Sapieha P, Chen J, Friedlander M, Clish CB, Smith LEH. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med. 2016;22:439–445. doi: 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Shvedova AA, Novikov KN, Kozlov YP. Light-induced free radical oxidation of membrane lipids in photoreceptors of frog retina. Biochim Biophys Acta. 1973;330:76–79. doi: 10.1016/0005-2736(73)90285-x. [DOI] [PubMed] [Google Scholar]
- Kageyama GH, Wong-Riley MT. The histochemical localization of cytochrome oxidase in the retina and lateral geniculate nucleus of the ferret, cat, and monkey, with particular reference to retinal mosaics and ON/OFF-center visual channels. J Neurosci. 1984;4:2445–2459. doi: 10.1523/JNEUROSCI.04-10-02445.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda A, Chen W, Othman M, Branham KEH, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2007;104:16227–16232. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551:787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns TP, Sayre GP. Retinitis pigmentosa, external ophthalmoplegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA archives Ophthalmol. 1958;60:280–289. [PubMed] [Google Scholar]
- Kelley C, D’Amore P, Hechtman HB, Shepro D. Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. J Cell Biol. 1987;104:483–490. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrison JB, Miller NR, Hsu F, Beaty TH, Maumenee IH, Smith KH, Savino PJ, Stone EM, Newman NJ. A case-control study of tobacco and alcohol consumption in Leber hereditary optic neuropathy. Am J Ophthalmol. 2000;130:803–812. doi: 10.1016/s0002-9394(00)00603-6. [DOI] [PubMed] [Google Scholar]
- Kiel JW, van Heuven WA. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1995;36:579–585. [PubMed] [Google Scholar]
- King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine & growth factor Rev. 2005;16:535–548. doi: 10.1016/j.cytogfr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Klepper J. Glucose transporter deficiency syndrome (GLUT1DS) and the ketogenic diet. Epilepsia. 2008;49(Suppl 8):46–49. doi: 10.1111/j.1528-1167.2008.01833.x. [DOI] [PubMed] [Google Scholar]
- Klepper J, Willemsen M, Verrips A, Guertsen E, Herrmann R, Kutzick C, Flörcken A, Voit T. Autosomal dominant transmission of GLUT1 deficiency. Hum Mol Genet. 2001;10:63–68. doi: 10.1093/hmg/10.1.63. [DOI] [PubMed] [Google Scholar]
- Kortvely E, Hauck SM, Duetsch G, Gloeckner CJ, Kremmer E, Alge-Priglinger CS, Deeg CA, Ueffing M. ARMS2 is a constituent of the extracellular matrix providing a link between familial and sporadic age-related macular degenerations. Invest Ophthalmol Vis Sci. 2010;51:79–88. doi: 10.1167/iovs.09-3850. [DOI] [PubMed] [Google Scholar]
- Kraakman L, Lemaire K, Ma P, Teunissen AW, Donaton MC, Van Dijck P, Winderickx J, de Winde JH, Thevelein JM. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol Microbiol. 1999;32:1002–1012. doi: 10.1046/j.1365-2958.1999.01413.x. [DOI] [PubMed] [Google Scholar]
- Kuny S, Filion MA, Suh M, Gaillard F, Sauvé Y. Long-term retinal cone survival and delayed alteration of the cone mosaic in a transgenic mouse model of stargardt-like dystrophy (STGD3) cone degeneration in STGD3 mouse. Invest Ophthalmol Vis Sci. 2014;55:424–439. doi: 10.1167/iovs.13-13457. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Kubota Y, Ozawa Y, Takubo K, Noda K, Simon MC, Johnson RS, Suematsu M, Tsubota K, Ishida S, Goda N, Suda T, Okano H. Von Hippel-Lindau protein regulates transition from the fetal to the adult circulatory system in retina. Development. 2010;137:1563–1571. doi: 10.1242/dev.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Krohne TU, Aguilar E, Johnson RS, Friedlander M. Astrocyte pVHL and HIF-α isoforms are required for embryonic-to-adult vascular transition in the eye. J Cell Biol. 2011;195:689–701. doi: 10.1083/jcb.201107029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012;122:4213–4217. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Gantner ML, Usui Y, Schultz A, Bravo S, Aguilar E, Wittgrove C, Friedlander MS, Paris LP. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife. 2016;5:e14319. doi: 10.7554/eLife.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoke F, Shaw S, Yuan J, Ananth S, Duncan M, Martin P, Bartoli M. Increased oxidative and nitrative stress accelerates aging of the retinal vasculature in the diabetic retina. PLoS One. 2015;10:e0139664. doi: 10.1371/journal.pone.0139664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Lawlor DP, Kalina RE. Pigmentary retinopathy in long chain 3-hydroxyacylcoenzyme A dehydrogenase deficiency. Am J Ophthalmol. 1997;123:846–848. doi: 10.1016/s0002-9394(14)71141-9. [DOI] [PubMed] [Google Scholar]
- Leber T. Über hereditäre und congenital-angelegte Sehnervenleiden. Graefe’s Arch Clin Exp Ophthalmol. 1871a;17:249–291. [Google Scholar]
- Leber T. Ueber anomale Formen der Retinitis pigmentosa. Graefe’s Arch Clin Exp Ophthalmol. 1871b;17:314–341. [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifke E, Naik H, Wu J, Viswanathan P, Demanno D, Kipnes M, Vakilynejad M. A multiple-ascending-dose study to evaluate safety, pharmacokinetics, and pharmacodynamics of a novel GPR40 agonist, TAK-875, in subjects with type 2 diabetes. Clin Pharmacol Ther. 2012;92:29–39. doi: 10.1038/clpt.2012.43. [DOI] [PubMed] [Google Scholar]
- Li F, Chen H, Anderson RE. Biosynthesis of docosahexaenoate-containing glycerolipid molecular species in the retina. J Mol Neurosci. 2001;16:205–214. doi: 10.1385/JMN:16:2-3:205. [DOI] [PubMed] [Google Scholar]
- Liberti MV, Locasale JW. Metabolism: a new layer of glycolysis. Nat Chem Biol. 2016;12:577–578. doi: 10.1038/nchembio.2133. [DOI] [PubMed] [Google Scholar]
- Liegl R, Hellström A, Smith LE. Retinopathy of prematurity: the need for prevention. Eye Brain. 2016a;8:91–102. doi: 10.2147/EB.S99038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegl R, Löfqvist C, Hellström A, Smith LEH. IGF-1 in retinopathy of prematurity, a CNS neurovascular disease. Early Hum Dev. 2016b;102:13–19. doi: 10.1016/j.earlhumdev.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- Lin CS, Sharpley MS, Fan W, Waymire KG, Sadun AA, Carelli V, Ross-Cisneros FN, Baciu P, Sung E, McManus MJ, Pan BX, Gil DW, Macgregor GR, Wallace DC. Mouse mtDNA mutant model of Leber hereditary optic neuropathy. Proc Natl Acad Sci U S A. 2012;109:20065–20070. doi: 10.1073/pnas.1217113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- Lindsay KJ, Du J, Sloat SR, Contreras L, Linton JD, Turner SJ, Sadilek M, Satrustegui J, Hurley JB. Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc Natl Acad Sci U S A. 2014;111:15579–15584. doi: 10.1073/pnas.1412441111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J general Physiol. 1986;88:521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Braun RD. Oxygen distribution and consumption in the cat retina during normoxia and hypoxemia. J general Physiol. 1992;99:177–197. doi: 10.1085/jgp.99.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. Prog Retin eye Res. 2017;58:115–151. doi: 10.1016/j.preteyeres.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton JD, Holzhausen LC, Babai N, Song H, Miyagishima KJ, Stearns GW, Lindsay K, Wei J, Chertov AO, Peters TA, Caffe R, Pluk H, Seeliger MW, Tanimoto N, Fong K, Bolton L, Kuok DLT, Sweet IR, Bartoletti TM, Radu RA, Travis GH, Zagotta WN, Townes-Anderson E, Parker E, Van der Zee CEEM, Sampath AP, Sokolov M, Thoreson WB, Hurley JB. Flow of energy in the outer retina in darkness and in light. Proc Natl Acad Sci U S A. 2010;107:8599–8604. doi: 10.1073/pnas.1002471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman BJ, Mitchell DC. A role for phospholipid polyunsaturation in modulating membrane protein function. Lipids. 1996;31:S193–S197. doi: 10.1007/BF02637075. [DOI] [PubMed] [Google Scholar]
- Liu P, Demple B. DNA repair in mammalian mitochondria: much more than we thought? Environ Mol Mutagen. 2010;51:417–426. doi: 10.1002/em.20576. [DOI] [PubMed] [Google Scholar]
- Liu C, Nathans J. An essential role for frizzled 5 in mammalian ocular development. Development. 2008;135:3567–3576. doi: 10.1242/dev.028076. [DOI] [PubMed] [Google Scholar]
- Liu A, Chang J, Lin Y, Shen Z, Bernstein PS. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J lipid Res. 2010;51:3217–3229. doi: 10.1194/jlr.M007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Semenkovich CF. Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab. 2014;19:380–392. doi: 10.1016/j.cmet.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofqvist C, Willett KL, Aspegren O, Smith ACH, Aderman CM, Connor KM, Chen J, Hellstrom A, Smith LEH. Quantification and localization of the IGF/insulin system expression in retinal blood vessels and neurons during oxygen-induced retinopathy in mice. Invest Ophthalmol Vis Sci. 2009;50:1831–1837. doi: 10.1167/iovs.08-2903. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- Lowes M. Chronic progressive external ophthalmoplegia, pigmentary retinopathy, and heart block (KEARNS-SAYRE syndrome) Acta Ophthalmol. 1975;53:610–619. doi: 10.1111/j.1755-3768.1975.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Roberts NR, Lewis C. The quantitative histochemistry of the retina. J Biol Chem. 1956;220:879–892. [PubMed] [Google Scholar]
- Lowry OH, Roberts NR, Schulz DW, Clow JE, Clark JR. Quantitative histochemistry of retina II. Enzymes of glucose metabolism. J Biol Chem. 1961;236:2813–2820. [PubMed] [Google Scholar]
- Luhmann UFO, Robbie S, Munro PMG, Barker SE, Duran Y, Luong V, Fitzke FW, Bainbridge JWB, Ali RR, MacLaren RE. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009;50:5934–5943. doi: 10.1167/iovs.09-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty GA, Cao J, McLeod DS. Relationship of polymorphonuclear leukocytes to capillary dropout in the human diabetic choroid. Am J Pathol. 1997;151:707–714. [PMC free article] [PubMed] [Google Scholar]
- Lutty GA, Hasegawa T, Baba T, Grebe R, Bhutto I, McLeod DS. Development of the human choriocapillaris. Eye (Lond) 2010;24:408–415. doi: 10.1038/eye.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzogubov VV, Tytarenko RG, Liu J, Bora NS, Bora PS. Polyethylene glycol (PEG)-induced mouse model of choroidal neovascularization. J Biol Chem. 2011;286:16229–16237. doi: 10.1074/jbc.M110.204701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Tao B, Warashina S, Kotani S, Lu L, Kaplamadzhiev DB, Mori Y, Tonchev AB, Yamashima T. Expression of free fatty acid receptor GPR40 in the central nervous system of adult monkeys. Neurosci Res. 2007;58:394–401. doi: 10.1016/j.neures.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Ma D, Zhang M, Larsen CP, Xu F, Hua W, Yamashima T, Mao Y, Zhou L. DHA promotes the neuronal differentiation of rat neural stem cells transfected with GPR40 gene. Brain Res. 2010;1330:1–8. doi: 10.1016/j.brainres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Macaluso C, Onoe S, Niemeyer G. Changes in glucose level affect rod function more than cone function in the isolated, perfused cat eye. Invest Ophthalmol Vis Sci. 1992;33:2798–2808. [PubMed] [Google Scholar]
- Malek G, Johnson LV, Mace BE, Saloupis P, Schmechel DE, Rickman DW, Toth CA, Sullivan PM, Bowes Rickman C. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A. 2005;102:11900–11905. doi: 10.1073/pnas.0503015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovets AM, Saprunova VB, Zhdankina AA, Fursova AZ, Bakeeva LE, Kolosova NG. Alterations of retinal pigment epithelium cause AMD-like retinopathy in senescence-accelerated OXYS rats. Aging (Albany NY) 2011;3:44–54. doi: 10.18632/aging.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrache AM, Gobeil F, Zhu T, Chemtob S. Intracellular signaling of lipid mediators via cognate nuclear G protein-coupled receptors. Endothelium J Endothelial Cell Res. 2005;12:63–72. doi: 10.1080/10623320590933815. [DOI] [PubMed] [Google Scholar]
- Mathupala S, Ko Ya, Pedersen P. Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsugi T, Chen Q, Anderson DR. Suppression of CO2-induced relaxation of bovine retinal pericytes by angiotensin II. Invest Ophthalmol Vis Sci. 1997;38:652–657. [PubMed] [Google Scholar]
- McDonald HR. Diagnostic and therapeutic challenges. Pediatric pigmentary retinopathy Retina. 2003;23:387–391. doi: 10.1097/00006982-200306000-00016. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Mannaerts G, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977;60:265. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Takabayashi Y, Foster DW. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978;253:8294–8300. [PubMed] [Google Scholar]
- McMahon A, Kedzierski W. Polyunsaturated very-long-chain C28–C36 fatty acids and retinal physiology. Br J Ophthalmol. 2009 doi: 10.1136/bjo.2008.149286. bjo 2008 149286. [DOI] [PubMed] [Google Scholar]
- Medrano CJ, Fox DA. Oxygen consumption in the rat outer and inner retina: light- and pharmacologically-induced inhibition. Exp Eye Res. 1995;61:273–284. doi: 10.1016/s0014-4835(05)80122-8. [DOI] [PubMed] [Google Scholar]
- Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Surv Ophthalmol. 2006;51:232–258. doi: 10.1016/j.survophthal.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Mitchell CA, Risau W, Drexler HC. Regression of vessels in the tunica vasculosa lentis is initiated by coordinated endothelial apoptosis: a role for vascular endothelial growth factor as a survival factor for endothelium. Dev Dyn. 1998;213:322–333. doi: 10.1002/(SICI)1097-0177(199811)213:3<322::AID-AJA8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem. 2011;117:735–746. doi: 10.1111/j.1471-4159.2011.07245.x. [DOI] [PubMed] [Google Scholar]
- Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791:573–583. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Cepko CL. Vertebrate photoreceptor cell development and disease. Trends Cell Biol. 1998;8:353–358. doi: 10.1016/s0962-8924(98)01341-5. [DOI] [PubMed] [Google Scholar]
- Mueller EE, Schaier E, Brunner SM, Eder W, Mayr JA, Egger SF, Nischler C, Oberkofler H, Reitsamer HA, Patsch W, Sperl W, Kofler B. Mitochondrial haplogroups and control region polymorphisms in age-related macular degeneration: a case-control study. PLoS One. 2012;7:e30874. doi: 10.1371/journal.pone.0030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama Y-s, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Murray AR, Fliesler SJ, Al-Ubaidi MR. Rhodopsin: the functional significance of asn-linked glycosylation and other post-translational modifications. Ophthalmic Genet. 2009;30:109–120. doi: 10.1080/13816810902962405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik H, Vakilynejad M, Wu J, Viswanathan P, Dote N, Higuchi T, Leifke E. Safety, tolerability, pharmacokinetics, and pharmacodynamic properties of the GPR40 agonist TAK-875: results from a double-blind, placebo-controlled single oral dose rising study in healthy volunteers. J Clin Pharmacol. 2012;52:1007–1016. doi: 10.1177/0091270011409230. [DOI] [PubMed] [Google Scholar]
- Nakamoto K, Nishinaka T, Matsumoto K, Kasuya F, Mankura M, Koyama Y, Tokuyama S. Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system. Brain Res. 2012;1432:74–83. doi: 10.1016/j.brainres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yamamoto M. Variable pattern of visual recovery of Leber’s hereditary optic neuropathy. Br J Ophthalmol. 2000;84:534–535. doi: 10.1136/bjo.84.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenseter MS, Rustan AC, Lund-Katz S, Søyland E, Maelandsmo G, Phillips MC, Drevon CA. Effect of dietary supplementation with n-3 polyunsaturated fatty acids on physical properties and metabolism of low density lipoprotein in humans. Arterioscler, Thromb, Vasc Biol. 1992;12:369–379. doi: 10.1161/01.atv.12.3.369. [DOI] [PubMed] [Google Scholar]
- Nestel PJ. Fish oil and cardiovascular disease: lipids and arterial function. Am J Clin Nutr. 2000;71:228S–231S. doi: 10.1093/ajcn/71.1.228S. [DOI] [PubMed] [Google Scholar]
- Neuringer M. The relationship of fatty acid composition to function in the retina and visual system. Lipids, Learn. Brain Fats Infant Formulas. 1993:134–163. [Google Scholar]
- Newman NJ. Treatment of hereditary optic neuropathies. Nat Rev Neurol. 2012;8:545–556. doi: 10.1038/nrneurol.2012.167. [DOI] [PubMed] [Google Scholar]
- Ng SK, Wood JP, Chidlow G, Han G, Kittipassorn T, Peet DJ, Casson RJ. Cancer-like metabolism of the mammalian retina. Clin Exp Ophthalmol. 2015;43:367–376. doi: 10.1111/ceo.12462. [DOI] [PubMed] [Google Scholar]
- Nihira M, Anderson K, Gorin FA, Burns MS. Primate rod and cone photoreceptors may differ in glucose accessibility. Invest Ophthalmol Vis Sci. 1995;36:1259–1270. [PubMed] [Google Scholar]
- Nikoskelainen E. New aspects of the genetic, etiologic, and clinical puzzle of Leber’s disease. Neurology 34. 1984:1482–1482. doi: 10.1212/wnl.34.11.1482. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen E, Hoyt WF, Nummelin K. Ophthalmoscopic findings in Leber’s hereditary optic neuropathy. II The fundus findings in the affected family members. Arch Ophthalmol. 1983;101:1059–1068. doi: 10.1001/archopht.1983.01040020061011. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen E, Hoyt WF, Nummelin K, Schatz H. Fundus findings in Leber’s hereditary optic neuroretinopathy: III. Fluorescein angiographic studies. Arch Ophthalmol. 1984;102:981–989. doi: 10.1001/archopht.1984.01040030783017. [DOI] [PubMed] [Google Scholar]
- Niu YG, Evans RD. Very-low-density lipoprotein: complex particles in cardiac energy metabolism. J Lipids. 2011;2011:189876. doi: 10.1155/2011/189876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell WK. Electrophysiologic study of the retina during metabolic impairment. Am J Ophthalmol. 1952;35:126–133. doi: 10.1016/0002-9394(52)90265-1. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Novikoff PM. Microperoxisomes and peroxisomes in relation to lipid metabolism. Ann N Y Acad Sci. 1982;386:138–152. doi: 10.1111/j.1749-6632.1982.tb21412.x. [DOI] [PubMed] [Google Scholar]
- Obunike JC, Lutz EP, Li Z, Paka L, Katopodis T, Strickland DK, Kozarsky KF, Pillarisetti S, Goldberg IJ. Transcytosis of lipoprotein lipase across cultured endothelial cells requires both heparan sulfate proteoglycans and the very low density lipoprotein receptor. J Biol Chem. 2001;276:8934–8941. doi: 10.1074/jbc.M008813200. [DOI] [PubMed] [Google Scholar]
- Oey NA, den Boer MEJ, Wijburg FA, Vekemans M, Augé J, Steiner C, Wanders RJA, Waterham HR, Ruiter JPN, Attié-Bitach T. Long-chain fatty acid oxidation during early human development. Pediatr Res. 2005;57:755–759. doi: 10.1203/01.PDR.0000161413.42874.74. [DOI] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an Omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Matsui K, Hirose A, Yamamoto S, Saikia J, Okamoto N, Gehlbach P, Duh EJ, Hackett S, Chang M, Bok D, Zack DJ, Campochiaro PA. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. 2002;160:711–719. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K, Kobayashi S, Yamada T, Kurihara T, Tai-Nagara I, Miyamoto T, Mukouyama Y-s, Sato TN, Suda T, Ema M, Kubota Y. Neurons limit angiogenesis by titrating VEGF in retina. Cell. 2014;159:584–596. doi: 10.1016/j.cell.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Reichard GA, Patel MS, Boden G. Energy metabolism in feasting and fasting. Adv Exp Med Biol. 1979;111:169–188. doi: 10.1007/978-1-4757-0734-2_8. [DOI] [PubMed] [Google Scholar]
- Pan BX, Ross-Cisneros FN, Carelli V, Rue KS, Salomao SR, Moraes-Filho MN, Moraes MN, Berezovsky A, Belfort R, Sadun AA. Mathematically modeling the involvement of axons in Leber’s hereditary optic NeuropathyInvolvement of axons in hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2012;53:7608–7617. doi: 10.1167/iovs.12-10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee S, Yu HG, Kim JI, Seo JS. Copy number variation of agerelated macular degeneration relevant genes in the Korean population. PLoS One. 2012;7:e31243. doi: 10.1371/journal.pone.0031243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Nishikawa S, Matthes MT, Yasumura D, LaVail MM. The relationship of photoreceptor degeneration to retinal vascular development and loss in mutant rhodopsin transgenic and RCS rats. Exp Eye Res. 2008;87:561–570. doi: 10.1016/j.exer.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol Asp Med. 2012;33:487–509. doi: 10.1016/j.mam.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins GA, Ellisman MH, Fox DA. Three-dimensional analysis of mouse rod and cone mitochondrial cristae architecture: bioenergetic and functional implications. Mol Vis. 2003;9:60–73. [PubMed] [Google Scholar]
- Perman JC, Boström P, Lindbom M, Lidberg U, Ståhlman M, Hägg D, Lindskog H, Scharin Täng M, Omerovic E, Mattsson Hultén L, Jeppsson A, Petursson P, Herlitz J, Olivecrona G, Strickland DK, Ekroos K, Olofsson SO, Borén J. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest. 2011;121:2625–2640. doi: 10.1172/JCI43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith L. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- Pisano A, Preziuso C, Iommarini L, Perli E, Grazioli P, Campese AF, Maresca A, Montopoli M, Masuelli L, Sadun AA, d’Amati G, Carelli V, Ghelli A, Giordano C. Targeting estrogen receptor β as preventive therapeutic strategy for Leber’s hereditary optic neuropathy. Hum Mol Genet. 2015;24:6921–6931. doi: 10.1093/hmg/ddv396. [DOI] [PubMed] [Google Scholar]
- Poitout V. The ins and outs of fatty acids on the pancreatic beta cell. Trends Endocrinol Metab. 2003;14:201–203. doi: 10.1016/s1043-2760(03)00086-9. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci. 1995;15:5179–5191. doi: 10.1523/JNEUROSCI.15-07-05179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos A, Sharp P, Singh H, Johnson D, Fellenberg A, Pollard A. Detection of a homologous series of C26-C38 polyenoic fatty acids in the brain of patients without peroxisomes (Zellweger’s syndrome) Biochem J. 1986;235:607–610. doi: 10.1042/bj2350607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J, Mechta-Grigoriou F. Redox regulation of the hypoxia-inducible factor. Biol Chem. 2006;387:1337–1346. doi: 10.1515/BC.2006.167. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2012;287:1642–1648. doi: 10.1074/jbc.R111.304428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Rajala RV, Rajala A, Kooker C, Wang Y, Anderson RE. The Warburg effect mediator pyruvate kinase M2 expression and regulation in the retina. Sci Rep. 2016;6:37727. doi: 10.1038/srep37727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer M, D’Amore PA. Getting Tie(2)d up in angiogenesis. J Clin Invest. 2002:1615–1617. doi: 10.1172/JCI17326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel B, Thompson JW, Farsiu S, Moseley MA, Skiba NP, Arshavsky VY. Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cells. Mol Cell Proteomics. 2011;10:M110002469. doi: 10.1074/mcp.M110.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei KA, Zhang Q, Chen CL, Chao J, Wang RK. Retinal and choroidal vascular features in patients with retinitis pigmentosa imaged by OCT based microangiography. Graefes Arch Clin Exp Ophthalmol. 2017;255:1287–1295. doi: 10.1007/s00417-017-3633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende FA, Lapalme E, Qian CX, Smith LE, SanGiovanni JP, Sapieha P. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am J Ophthalmol. 2014;158:1071–1078. doi: 10.1016/j.ajo.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DS, Calandria JM, Gordon WC, Jun B, Zhou Y, Gelfman CM, Li S, Jin M, Knott EJ, Chang B, Abuin A, Issa T, Potter D, Platt KA, Bazan NG. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat Commun. 2015;6:6228. doi: 10.1038/ncomms7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GS, Ju M, Shih SC, Xu X, McMahon G, Caldwell RB, Smith LE. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J Offic Publ Fed Am Soc Exp Biol. 2001;15:1215–1217. doi: 10.1096/fj.00-0598fje. [DOI] [PubMed] [Google Scholar]
- Roomets E, Kivelä T, Tyni T. Carnitine palmitoyltransferase I and Acyl-CoA dehydrogenase 9 in retina: insights of retinopathy in mitochondrial trifunctional protein defects. Invest Ophthalmol Vis Sci. 2008;49:1660–1664. doi: 10.1167/iovs.07-1094. [DOI] [PubMed] [Google Scholar]
- Rueda EM, Johnson JE, Giddabasappa A, Swaroop A, Brooks MJ, Sigel I, Chaney SY, Fox DA. The cellular and compartmental profile of mouse retinal glycolysis, tricarboxylic acid cycle, oxidative phosphorylation, and ~P transferring kinases. Mol Vis. 2016;22:847–885. [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Bautch VL. Neurovascular development and links to disease. Cell Mol Life Sci. 2013;70:1675–1684. doi: 10.1007/s00018-013-1277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- Saini JS, Corneo B, Miller JD, Kiehl TR, Wang Q, Boles NC, Blenkinsop TA, Stern JH, Temple S. Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration. Cell Stem Cell. 2017;20:635–647. e637. doi: 10.1016/j.stem.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Salehi A, Flodgren E, Nilsson NE, Jimenez-Feltstrom J, Miyazaki J, Owman C, Olde B. Free fatty acid receptor 1 (FFA(1)R/GPR40) and its involvement in fatty-acid-stimulated insulin secretion. Cell Tissue Res. 2005;322:207–215. doi: 10.1007/s00441-005-0017-z. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Sapieha P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood. 2012;120:2182–2194. doi: 10.1182/blood-2012-04-396846. [DOI] [PubMed] [Google Scholar]
- Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honoré JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O, Lachapelle P, Di Polo A, Beauséjour C, Andelfinger G, Mitchell G, Sennlaub F, Chemtob S. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- Sapieha P, Joyal JS, Rivera JC, Kermorvant-Duchemin E, Sennlaub F, Hardy P, Lachapelle P, Chemtob S. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest. 2010;120:3022–3032. doi: 10.1172/JCI42142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, SanGiovanni JP, Gronert K, Smith LEH. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM, Hatton CJ, Joyal JS, Krah NM, Dennison RJ, Tang J, Kern TS, Akula JD, Smith LEH. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr Diabetes. 2012;2:e36. doi: 10.1038/nutd.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarac O, Gulsuner S, Yildiz-Tasci Y, Ozcelik T, Kansu T. Neuro-ophthalmologic findings in humans with quadrupedal locomotion. Ophthalmic Genet. 2012;33:249–252. doi: 10.3109/13816810.2012.689412. [DOI] [PubMed] [Google Scholar]
- Scarpelli DG, Craig EL. The fine localization of nucleoside triphosphatase activity in the retina of the frog. J Cell Biol. 1963;17:279–288. doi: 10.1083/jcb.17.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatena R. Mitochondria and cancer: a growing role in apoptosis, cancer cell metabolism and dedifferentiation. Adv Exp Med Biol. 2012;942:287–308. doi: 10.1007/978-94-007-2869-1_13. [DOI] [PubMed] [Google Scholar]
- Scerri TS, Quaglieri A, Cai C, Zernant J, Matsunami N, Baird L, Scheppke L, Bonelli R, Yannuzzi LA, Friedlander M, Egan CA, Fruttiger M, Leppert M, Allikmets R, Bahlo M MacTel Project C. Genome-wide analyses identify common variants associated with macular telangiectasia type 2. Nat Genet. 2017;49:559–567. doi: 10.1038/ng.3799. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- Schnell S, Schaefer M, Schöfl C. Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40. Mol Cell Endocrinol. 2007;263:173–180. doi: 10.1016/j.mce.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Schrier SA, Falk MJ. Mitochondrial disorders and the eye. Curr Opin Ophthalmol. 2011;22:325–331. doi: 10.1097/ICU.0b013e328349419d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci. 1989;86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One. 2010;5:e11863. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Serini G, Bussolino F. Common cues in vascular and axon guidance. Physiol (Bethesda, Md) 2004:348–354. doi: 10.1152/physiol.00021.2004. [DOI] [PubMed] [Google Scholar]
- Shao Z, Fu Z, Stahl A, Joyal JS, Hatton C, Juan A, Hurst C, Evans L, Cui Z, Pei D, Gong Y, Xu D, Tian K, Bogardus H, Edin ML, Lih F, Sapieha P, Chen J, Panigrahy D, Hellstrom A, Zeldin DC, Smith LEH. Cytochrome P450 2C8 ω3-long-chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization–brief report. Arterioscler Thromb Vasc Biol. 2014;34:581–586. doi: 10.1161/ATVBAHA.113.302927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Wen R, Tuo J, Bojanowski CM, Chan CC. Exacerbation of retinal degeneration and choroidal neovascularization induced by subretinal injection of Matrigel in CCL2/MCP-1-deficient mice. Ophthalmic Res. 2006;38:71–73. doi: 10.1159/000090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Gibson GE. Oxidative stress and transcriptional regulation in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:276–291. doi: 10.1097/WAD.0b013e31815721c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- Shui YB, Wang X, Hu JS, Wang SP, Garcia CM, Potts JD, Sharma Y, Beebe DC. Vascular endothelial growth factor expression and signaling in the lens. Invest Ophthalmol Vis Sci. 2003;44:3911–3919. doi: 10.1167/iovs.02-1226. [DOI] [PubMed] [Google Scholar]
- Shukla D, Gupta SR, Neelakantan N, Tiwari S, Gupta S, Patwardhan AR, Soubhya TB. Type 2 idiopathic macular telangiectasia. Retina. 2012;32:265–274. doi: 10.1097/IAE.0b013e31822091b0. [DOI] [PubMed] [Google Scholar]
- Sidossis LS, Wolfe RR. Glucose and insulin-induced inhibition of fatty acid oxidation: the glucose-fatty acid cycle reversed. Am J Physiol Endocrinol Metabol. 1996;270:E733–E738. doi: 10.1152/ajpendo.1996.270.4.E733. [DOI] [PubMed] [Google Scholar]
- Sitaras N, Rivera JC, Noueihed B, Bien-Aimé M, Zaniolo K, Omri S, Hamel D, Zhu T, Hardy P, Sapieha P, Joyal JS, Chemtob S. Retinal neurons curb inflammation and enhance revascularization in ischemic retinopathies via proteinaseactivated receptor-2. Am J Pathol. 2015;185:581–595. doi: 10.1016/j.ajpath.2014.10.020. [DOI] [PubMed] [Google Scholar]
- Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM. Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp. 2007;287:60–63. discussion 63–69. [PubMed] [Google Scholar]
- Stahl A, Sapieha P, Connor KM, SanGiovanni JP, Chen J, Aderman CM, Willett KL, Krah NM, Dennison RJ, Seaward MR, Guerin KI, Hua J, Smith LEH. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ Res. 2010;107:495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhlberg A, Bengtsson M. Single-cell gene expression profiling using reverse transcription quantitative real-time PCR. Methods. 2010;50:282–288. doi: 10.1016/j.ymeth.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R, Lois N. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156–186. doi: 10.1016/j.preteyeres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Stolze IP, Mole DR, Ratcliffe PJ. Regulation of HIF: prolyl hydroxylases. Novartis Found Symp. 2006;272:15–25. discussion 25–36. [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci Offic J Soc Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Strumilo S. Short-term regulation of the alpha-ketoglutarate dehydrogenase complex by energy-linked and some other effectors. Biochem Mosc. 2005;70:726–729. doi: 10.1007/s10541-005-0177-1. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991;266:10711–10714. [PubMed] [Google Scholar]
- Su HM, Bernardo L, Mirmiran M, Ma XH, Corso TN, Nathanielsz PW, Brenna JT. Bioequivalence of dietary α-linolenic and docosahexaenoic acids as sources of docosahexaenoate accretion in brain and associated organs of neonatal baboons. Pediatr Res. 1999;45:87–93. doi: 10.1203/00006450-199901000-00015. [DOI] [PubMed] [Google Scholar]
- Suh M, Clandinin MT. 20: 5n-3 but not 22: 6n-3 is a preferred substrate for synthesis of n-3 very-long-chain fatty acids (C24–C36) in retina. Curr eye Res. 2005;30:959–968. doi: 10.1080/02713680500246957. [DOI] [PubMed] [Google Scholar]
- Suh M, Wierzbicki AA, Lien EL, Clandinin MT. Dietary 20: 4n-6 and 22: 6n-3 modulates the profile of long-and very-long-chain fatty acids, rhodopsin content, and kinetics in developing photoreceptor cells. Pediatr Res. 2000;48:524–530. doi: 10.1203/00006450-200010000-00017. [DOI] [PubMed] [Google Scholar]
- Suh M, Sauvé Y, Merrells KJ, Kang JX, Ma DW. Supranormal electroretinogram in fat-1 mice with retinas enriched in docosahexaenoic acid and n-3 very long chain fatty acids (C24–C36) Invest Ophthalmol Vis Sci. 2009;50:4394–4401. doi: 10.1167/iovs.08-2565. [DOI] [PubMed] [Google Scholar]
- Takada Y, Ohkuma H, Ogata N, Matsushima M, Sugasawa K, Uyama M. Proteoglycan in Bruch’s membrane of senescence accelerated mouse: localization and age-related changes. Nippon Ganka Gakkai Zasshi. 1994;98:469–476. [PubMed] [Google Scholar]
- Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, Yamamoto T, Nakai T. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem. 1995;270:15747–15754. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- Ting AYC, Lee TKM, MacDonald IM. Genetics of age-related macular degeneration. Curr Opin Ophthalmol. 2009;20:369–376. doi: 10.1097/ICU.0b013e32832f8016. [DOI] [PubMed] [Google Scholar]
- Toto L, Borrelli E, Mastropasqua R, Senatore A, Di Antonio L, Di Nicola M, Carpineto P, Mastropasqua L. Macular features in retinitis pigmentosa: correlations among ganglion cell complex thickness, capillary density, and macular function. Invest Ophthalmol Vis Sci. 2016;57:6360–6366. doi: 10.1167/iovs.16-20544. [DOI] [PubMed] [Google Scholar]
- Toy BC, Koo E, Cukras C, Meyerle CB, Chew EY, Wong WT. Treatment of nonneovascular idiopathic macular telangiectasia type 2 with intravitreal ranibizumab: results of a phase II clinical trial. Retina. 2012;32:996–1006. doi: 10.1097/IAE.0b013e31824690a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond, B, Biol Sci. 2005;360:2335–2345. doi: 10.1098/rstb.2005.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujihata Y, Ito R, Suzuki M, Harada A, Negoro N, Yasuma T, Momose Y, Takeuchi K. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther. 2011;339:228–237. doi: 10.1124/jpet.111.183772. [DOI] [PubMed] [Google Scholar]
- Tsybovsky Y, Molday RS, Palczewski K. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv Exp Med Biol. 2010;703:105–125. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, Zhang K, Chan CC. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyni T, Johnson M, Eaton S, Pourfarzam M, Andrews R, Turnbull DM. Mitochondrial fatty acid beta-oxidation in the retinal pigment epithelium. Pediatr Res. 2002;52:595–600. doi: 10.1203/00006450-200210000-00021. [DOI] [PubMed] [Google Scholar]
- Tyni T, Paetau A, Strauss AW, Middleton B, Kivelä T. Mitochondrial fatty acid beta-oxidation in the human eye and brain: implications for the retinopathy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Pediatr Res. 2004;56:744–750. doi: 10.1203/01.PDR.0000141967.52759.83. [DOI] [PubMed] [Google Scholar]
- Udar N, Atilano SR, Memarzadeh M, Boyer DS, Chwa M, Lu S, Maguen B, Langberg J, Coskun P, Wallace DC, Nesburn AB, Khatibi N, Hertzog D, Le K, Hwang D, Kenney MC. Mitochondrial DNA haplogroups associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:2966–2974. doi: 10.1167/iovs.08-2646. [DOI] [PubMed] [Google Scholar]
- Usui Y, Westenskow PD, Kurihara T, Aguilar E, Sakimoto S, Paris LP, Wittgrove C, Feitelberg D, Friedlander MSH, Moreno SK, Dorrell MI, Friedlander M. Neurovascular crosstalk between interneurons and capillaries is required for vision. J Clin Invest. 2015;125:2335–2346. doi: 10.1172/JCI80297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel G, Moroni I, Lamantea E, Fratta GM, Ciceri E, Carrara F, Zeviani M. Mitochondrial disease associated with the T8993G mutation of the mitochondrial ATPase 6 gene: a clinical, biochemical, and molecular study in six families. J Neurol Neurosurg Psychiatry. 1997;63:16–22. doi: 10.1136/jnnp.63.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasandani C, Kafrouni AI, Caronna A, Bashmakov Y, Gotthardt M, Horton JD, Spady DK. Upregulation of hepatic LDL transport by n-3 fatty acids in LDL receptor knockout mice. J lipid Res. 2002;43:772–784. [PubMed] [Google Scholar]
- von Graefe A. Ein ungewöhnlicher Fall von hereditäre amaurose. Arch Ophthalmol. 1858a;4:266–268. [Google Scholar]
- Von Graefe A. Exceptionelles verhalten des gesichtsfeldes bei pigmententartung der netzhaut. Arch für Ophthalmol. 1858b;4:250–253. [Google Scholar]
- Wakai A, Wang JH, Winter DC, Street JT, O’Sullivan RG, Redmond HP. Adenosine inhibits neutrophil vascular endothelial growth factor release and transendothelial migration via A2B receptor activation. Shock. 2001;15:297–301. doi: 10.1097/00024382-200115040-00008. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Wanders RJ. Peroxisomes in Human Health and Disease: Metabolic Pathways, Metabolite Transport, Interplay with Other Organelles and Signal Transduction, Peroxisomes and Their Key Role in Cellular Signaling and Metabolism. Springer, pp. 2013:23–44. doi: 10.1007/978-94-007-6889-5_2. [DOI] [PubMed] [Google Scholar]
- Wanders R, Ferdinandusse S, Brites P, Kemp S. Peroxisomes, lipid metabolism and lipotoxicity. Biochim Biophysica Acta (BBA)-Molecular Cell Biol Lipids. 2010;1801:272–280. doi: 10.1016/j.bbalip.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Wang L, Bill A. Effects of constant and flickering light on retinal metabolism in rabbits. Acta Ophthalmol Scand. 1997;75:227–231. doi: 10.1111/j.1600-0420.1997.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Kondo M, Bill A. Glucose metabolism in cat outer retina. Effects of light and hyperoxia. Invest Ophthalmol Vis Sci. 1997a;38:48–55. [PubMed] [Google Scholar]
- Wang L, Tornquist P, Bill A. Glucose metabolism in pig outer retina in light and darkness. Acta Physiol Scand. 1997b;160:75–81. doi: 10.1046/j.1365-201X.1997.00030.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Villegas-Pérez MP, Vidal-Sanz M, Lund RD. Progressive optic axon dystrophy and vacuslar changes in rd mice. Invest Ophthalmol Vis Sci. 2000;41:537–545. [PubMed] [Google Scholar]
- Wang B, Xiao Y, Ding BB, Zhang N, Yuan X, Gui L, Qian KX, Duan S, Chen Z, Rao Y, Geng JG. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Wang W, Lee SJ, Scott PA, Lu X, Emery D, Liu Y, Ezashi T, Roberts MR, Ross JW, Kaplan HJ, Dean DC. Two-step reactivation of dormant cones in retinitis pigmentosa. Cell Rep. 2016;15:372–385. doi: 10.1016/j.celrep.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- Warburg O, Posener K, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Zeitschr. 1924;152:309–344. [Google Scholar]
- Wauson EM, Lorente-Rodríguez A, Cobb MH. Minireview: nutrient sensing by G protein-coupled receptors. Mol Endocrinol. 2013;27:1188–1197. doi: 10.1210/me.2013-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel MG, Li J, Alvarez RA, Anderson RE, O’Brien PJ. Metabolism of linolenic acid and docosahexaenoic acid in rat retinas and rod outer segments. Exp eye Res. 1991;53:437–446. doi: 10.1016/0014-4835(91)90161-7. [DOI] [PubMed] [Google Scholar]
- Whitmore SS, Sohn EH, Chirco KR, Drack AV, Stone EM, Tucker BA, Mullins RF. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1–29. doi: 10.1016/j.preteyeres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand RD, Koutz CA, Stinson AM, Anderson RE. Conservation of docosahexaenoic acid in rod outer segments of rat retina during n-3 and n-6 fatty acid deficiency. J Neurochem. 1991;57:1690–1699. doi: 10.1111/j.1471-4159.1991.tb06369.x. [DOI] [PubMed] [Google Scholar]
- Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, Urness LD, Suh W, Asai J, Kock GAH, Thorne T, Silver M, Thomas KR, Chien CB, Losordo DW, Li DY. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Arnold MJ, Brassell MA, Sliter DR. Glucose dependence of glycolysis, hexose monophosphate shunt activity, energy status, and the polyol pathway in retinas isolated from normal (nondiabetic) rats. Invest Ophthalmol Vis Sci. 1997;38:62–71. [PubMed] [Google Scholar]
- Winkler BS, Arnold MJ, Brassell MA, Puro DG. Energy metabolism in human retinal Müller cells. Invest Ophthalmol Vis Sci. 2000;41:3183–3190. [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Starnes CA, Twardy BS, Brault D, Taylor RC. Nuclear magnetic resonance and biochemical measurements of glucose utilization in the cone-dominant ground squirrel retina. Invest Ophthalmol Vis Sci. 2008;49:4613–4619. doi: 10.1167/iovs.08-2004. [DOI] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GFJM, Van Noorden CJF, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Wittenberger T, Schaller HC, Hellebrand S. An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J Mol Biol. 2001;307:799–813. doi: 10.1006/jmbi.2001.4520. [DOI] [PubMed] [Google Scholar]
- Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Huang Z, Liebl W, Nie F, Xu H, Zhang C. Neurochemical organization of the macaque retina: effect of TTX on levels and gene expression of cytochrome oxidase and nitric oxide synthase and on the immunoreactivity of Na+ K + ATPase and NMDA receptor subunit I. Vis Res. 1998;38:1455–1477. doi: 10.1016/s0042-6989(98)00001-7. [DOI] [PubMed] [Google Scholar]
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- Wu DM, Kawamura H, Sakagami K, Kobayashi M, Puro DG. Cholinergic regulation of pericyte-containing retinal microvessels. Am J Physiol-Heart Circ Physiol. 2003;284:H2083–H2090. doi: 10.1152/ajpheart.01007.2002. [DOI] [PubMed] [Google Scholar]
- Wu C, Vanderveen DK, Hellström A, Löfqvist C, Smith LEH. Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2010;128:443–447. doi: 10.1001/archophthalmol.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashima T. A putative link of PUFA, GPR40 and adult-born hippocampal neurons for memory. Prog Neurobiol. 2008;84:105–115. doi: 10.1016/j.pneurobio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA, Bardal AMC, Freund KB, Chen KJ, Eandi CM, Blodi B. Idiopathic macular telangiectasia. Retina. 2012;32(Suppl 1):450–460. doi: 10.1097/iae.0b013e31823f9a59. [DOI] [PubMed] [Google Scholar]
- Yashiro H, Tsujihata Y, Takeuchi K, Hazama M, Johnson PRV, Rorsman P. The effects of TAK-875, a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. J Pharmacol Exp Ther. 2012;340:483–489. doi: 10.1124/jpet.111.187708. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ. Retinal degeneration and local oxygen metabolism. Exp Eye Res. 2005;80:745–751. doi: 10.1016/j.exer.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Zadravec D, Tvrdik P, Guillou H, Haslam R, Kobayashi T, Napier JA, Capecchi MR, Jacobsson A. ELOVL2 controls the level of n-6 28: 5 and 30: 5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J lipid Res. 2011;52:245–255. doi: 10.1194/jlr.M011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiss CJ. Animals as models of age-related macular degeneration: an imperfect measure of the truth. Vet Pathol. 2010;47:396–413. doi: 10.1177/0300985809359598. [DOI] [PubMed] [Google Scholar]
- Zhao C, Yasumura D, Li X, Matthes M, Lloyd M, Nielsen G, Ahern K, Snyder M, Bok D, Dunaief JL, LaVail MM, Vollrath D. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J Clin Invest. 2011a;121:369–383. doi: 10.1172/JCI44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011b;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Molday RS. Binding of retinoids to ABCA4, the photoreceptor ABC transporter associated with Stargardt macular degeneration. Methods Mol Biol. 2010;652:163–176. doi: 10.1007/978-1-60327-325-1_9. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wong LL, Karakoti AS, Seal S, McGinnis JF. Nanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldlr knockout mouse. PLoS One. 2011;6:e16733. doi: 10.1371/journal.pone.0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WH, Guo X, Villaschi S, Francesco Nicosia R. Regulation of vascular growth and regression by matrix metalloproteinases in the rat aorta model of angiogenesis. Lab Invest. 2000;80:545–555. doi: 10.1038/labinvest.3780060. [DOI] [PubMed] [Google Scholar]