Abstract
Background
Postoperative Atrial Fibrillation (POAF) following cardiac surgery results in a significant increase in morbidity, mortality, and healthcare cost. Prophylactic amiodarone has been shown to reduce the incidence of POAF, however the cost-effectiveness of a protocol driven approach remains unknown.
Methods
All patients with a Society of Thoracic Surgeons (STS) risk score enrolled in a prophylactic amiodarone protocol (n=153) were propensity score matched 1:3 with patients prior to protocol implementation (n=3574). Using the matched cohort, multivariate logistic and linear regressions assessed the relative risks (POAF reduction and adverse medication effects) of amiodarone therapy and costs respectively. TreeAge cost-effectiveness software modeled effects of prophylactic amiodarone costs, complication rates and quality of life.
Results
Of patients eligible for the prophylactic amiodarone protocol, 94.3% (281/298) were enrolled. Prophylactic amiodarone significantly reduced the rate of POAF (25.7 vs 16.8%, p<0.0001). A total of 600 matched patients demonstrate no baseline differences in demographics, comorbidities, disease state, or operative factors with a significant reduction in POAF without an increase in other associated complications. Using these adjusted estimates, the prophylactic amiodarone protocol demonstrated a cost savings of $458 per patient. Sensitivity analysis confirmed the protocol is cost-effective for all protocol related POAF risk reductions below OR 0.726.
Conclusions
Implementation of a prophylactic amiodarone protocol significantly reduced risk-adjusted rates of POAF with a cost savings of $458 per patient. This analysis demonstrates how rigorous quantitative analysis can evaluate the benefits of quality improvement projects.
Keywords: Amiodarone, Postoperative Atrial Fibrillation, POAF, cost-effectiveness
Atrial fibrillation is the most common postoperative complication following cardiac surgery affecting 15–65% of patients.(1–3) Postoperative Atrial Fibrillation (POAF) following cardiac surgery results in a significant increase in morbidity and mortality.(2, 4, 5) This has in turn led to increased hospital costs, resource utilization, and hospital length of stay (LOS).(3, 6) Previous studies have shown an additional $7,000 to $14,000 cost increase in association with POAF.(3, 6, 7) Given the high incidence and substantial cost of POAF this has been a frequent target of quality improvement projects to provide superior outcomes at a lower cost resulting in better value.(8, 9)
Several pharmacologic interventions aimed at preventing POAF have been studied.(10) The successful use of amiodarone for preventing POAF has been well described in the literature.(10–15) In one randomized controlled trial, one dose of IV amiodarone was given in the immediate post-operative period followed by 5 days of oral amiodarone prophylaxis. The effect was 32% of the patients in the placebo group experienced POAF compared to only 14% in the treatment group.(14) Prophylactic amiodarone has been shown to reduce the incidence of POAF, however the cost-effectiveness of a protocol driven approach remains unknown. A 2008 randomized controlled study of 250 patients in Europe demonstrated that implementation of an amiodarone protocol for POAF lead to a 2% savings per patient.(12)
The purpose of this study was to evaluate the cost-effectiveness of a prophylactic amiodarone protocol implemented at our institution to reduce the incidence of POAF. We hypothesized a prophylactic amiodarone protocol would significantly reduce the incidence of POAF in a cost-effective manner.
Patients and Methods
Patient and Cost Data
All patients undergoing cardiac surgery with a Society of Thoracic Surgery (STS) risk score from January 2013 to April 2017 were identified using an institutional STS database. Patients were stratified by enrollment in a prophylactic amiodarone protocol. Hospital finance records obtained from the Clinical Data Repository (CDR) were matched to each patient record. Every hospital charge code has an associated cost valuation that is derived from direct and indirect costs, which were then totaled for the entire admission. Hospital costs were adjusted to 2016 equivalent dollars using the Center for Medicare and Medicaid Services (CMS) Inpatient Prospective Payment System (IPPS) adjustment for medical related inflation. Cost data was presented as mean ± SD to more fully represent all cost variation and outliers. The University of Virginia Institutional Review Board approved this study with a waiver of patient consent due to its retrospective nature and minimal risk to participants (IRB Protocol # 19762).
Amiodarone Prophylaxis Protocol
Starting in November 2016 all patients undergoing cardiac surgery were enrolled in the protocol unless they met exclusion criteria preoperatively (Supplemental Figure 1). The protocol included a loading dose of amiodarone four hours after admission to the Intensive Care Unit (ICU) postoperatively. Loading dose was amiodarone 900mg in 500 ml of 5% dextrose water given at 1 mg /minute over 6 hours, followed by 0.5 mg/minute over 18 hours. On postoperative day one, the patient transitioned to amiodarone 200 mg TID. This is continued to 42 doses (14 days) or until the patient is discharged, whichever occurs first. If patients were unable to tolerate the intravenous amiodarone load on postoperative day zero, they were started on amiodarone 200mg oral three times daily on postoperative day one. If patient could not tolerate oral medication on postoperative day one they were given amiodarone 150 mg intravenous every 12 hours.
Statistical Analysis
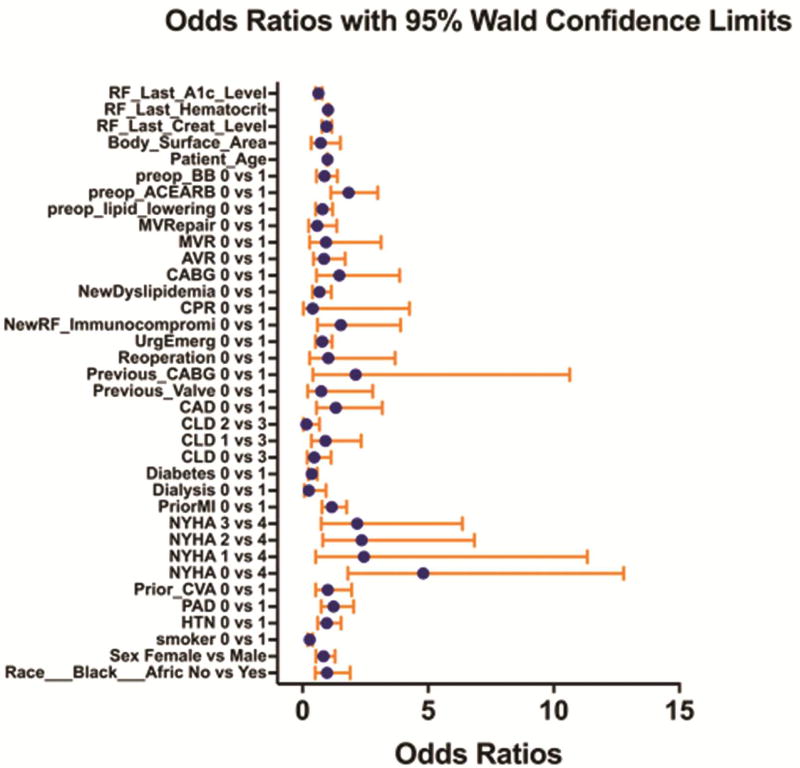
Continuous variables are presented as median (IQR) and categorical variables as number (%). To control for selection bias in our control group propensity score matching was utilized. All patients with a STS risk score enrolled in a prophylactic amiodarone protocol (n=153) were propensity score matched 1:3 with all patients meeting enrollment criteria with STS risk scores prior to initiation of the protocol (n=3574). The propensity score matching was performed using only preoperative variables including demographics, medications, and comorbid disease in a logistic regression model (Figure 1). Matching was completed using a greedy algorithm without replacement. Balance was assessed by standardized mean differences, with all results <20%.
Figure 1.
Propensity Score Matching. Demonstrates odds ratios with 95% confidence intervals contributed by each variable in the propensity score matching algorithm.
Patient demographics, operative characteristics and short-term outcomes were compared using Chi-Square test, Mann Whitney U-test for categorical and continuous variables as appropriate. After matching, groups were compared by paired univariate analysis. Categorical variables were compared using McNemar’s Test and continuous variables compared by Signed Rank Test. Multivariate logistic regression was used to identify the risk-adjusted impact of the prophylactic amiodarone protocol on the occurrence of POAF adjusting for year of surgery in the matched cohort. Similarly, adverse events attributed to amiodarone were identified as the incidence of any in hospital postoperative event occurring in the amiodarone protocol cohort compared to the event rate in the control group, excluding atrial fibrillation. Linear regression identified the risk-adjusted costs associated development of POAF as well as the cost of adverse events attributed to prophylactic amiodarone. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC).
Cost-Effectiveness Model
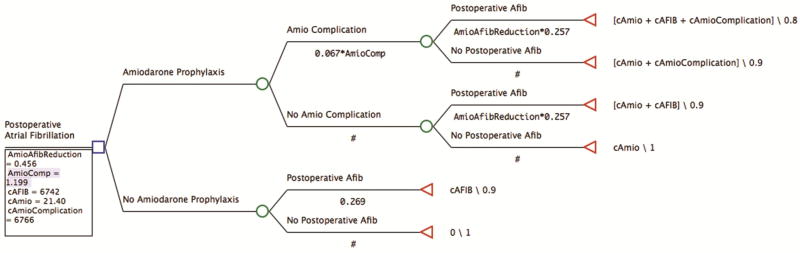
A model was developed to analyze the financial impact of the prophylactic amiodarone protocol at our institution (Figure 1). All cost-effectiveness modeling was performed using TreeAge Pro Version 2017 (TreeAge Software, Inc., Williamstown, MA). Institutional rates of POAF were applied to both arms of the analysis. Patients enrolled in the prophylactic amiodarone protocol had POAF rates multiplied by the risk reduction associated with the amiodarone protocol risk reduction (the odds ratio identified by logistic regression). Also for the amiodarone protocol group, the rate of adverse events was included based as the odds ratio from logistic regression.
The institutional risk-adjusted costs for POAF and adverse events attributable to amiodarone identified by linear regression were also included. The cost of the medication was applied to all patients in the treatment arm. Quality of life was included as utilities set to 1.0 for patients without POAF or amiodarone associated adverse events. No estimates were available for the utility of POAF, but based on the relatively modest impacts on additional complications and mortality, a 10% reduction in utility was assigned for POAF as well as adverse events attributed to amiodarone with a combined 20% reduction for the occurrence of both.
One-way sensitivity analyses were performed for all variables included in the model by allowing them to range between the 95% confidence intervals of the parameter estimates. Two-way sensitivity analysis was performed for those variables identified on one-way sensitivity analysis to influence cost-effectiveness.
Results
Baseline Characteristics
Of patients eligible for the prophylactic amiodarone protocol since implementation, 94.3% (281/298) were enrolled. Prophylactic amiodarone significantly reduced the institutional rate of POAF (25.7 vs 16.8%, p<0.0001) in the first 6 months of implementation. Propensity score matching was utilized since significant baseline differences existed between the pre and post protocol populations (Supplemental Table 1). A total of 600 matched patients demonstrate no baseline differences in demographics, comorbidities, disease state, or operative factors (Table 1). Within these matched groups, the prophylactic amiodarone protocol patients demonstrate a significant reduction in POAF without a significant increase in other associated complications (Table 1).
Table 1.
Matched Cohort Eligible For Amiodarone Protocol with STS Risk Score
| Baseline Characteristics | Amiodarone (n = 150) |
No Amiodarone (n = 450) |
p value |
|---|---|---|---|
| Age (Years) | 65.5 (15) | 65 (17) | 0.74 |
| Male | 111 (74.0%) | 336 (74.7%) | 0.87 |
| PROM (%) | 1.2 (1.6) | 1.1 (1.5) | 0.65 |
| PROMM (%) | 12.5 (10.4) | 11.1 (8.3) | 0.37 |
| Operative Characteristics | |||
|
| |||
| Reoperative Surgery | 9 (6.0%) | 21 (4.7%) | 0.51 |
| Elective | 81 (54.0%) | 251 (55.8%) | 0.70 |
| Coronary Artery Bypass Graft | 106 (69.3%) | 2553 (70.1%) | 0.83 |
| CPB Time (Minutes) | 100.5 (45) | 95 (38) | 0.17 |
| Outcomes | |||
|
| |||
| Postoperative Atrial Fibrillation | 28 (18.7%) | 121 (26.9%) | 0.04 |
| Operative Mortality | 1 (0.7%) | 3 (0.7%) | 1.00 |
| Major Morbidity | 13 (8.7%) | 41 (9.1%) | 0.87 |
| ICU stay (Hours) | 44.8 (49) | 44.8 (51) | 0.21 |
| LOS (Days) | 5 (3) | 5 (2) | 0.48 |
| Operative Mortality O:E | 0.58 | 0.64 | |
| Morbidity/Mortality O:E | 0.70 | 0.82 | |
| IPPS Adjusted Cost ($) | 59,154±38,069 | 45,576±41,031 | <0.0001 |
Continuous variables presented as Median (IQR)
PROM = Society of Thoracic Surgeons predicted risk of mortality
PROMM = Society of Thoracic Surgeons predicted risk of morbidity or mortality
CPB = cardiopulmonary bypass
Cost and Risk Modeling of POAF
Multivariate logistic regression for the incidence of POAF demonstrated the prophylactic amiodarone protocol independently reduced the risk of POAF (OR 0.46, p=0.003) in the matched cohort even after accounting for time bias (Table 2). Additionally, modeling for adverse events demonstrated amiodarone contributed a non-statistically significant increase in adverse events (OR 1.20, p=0.44, Table 2). Multivariate linear regression for total hospital costs in the matched cohort provided an estimate of $6743±3639 attributable to POAF when adjusting for STS major morbidities and year of surgery (Table 2). Finally, the cost of adverse events attributable to amiodarone were estimated as $6766±5046 adjusting for the occurrence of POAF and year of surgery using the matched cohort (Table 2). All models performed adequately with moderate predictive power, c-statistic range 0.78–0.87.
Table 2.
Risk and Cost Estimates From Multivariate Regression
| Amiodarone Effects | Adjusted Odds Ratio | 95% CI | p-value | |
|
| ||||
| POAF Reduction | 0.456 | 0.27 | 0.769 | 0.0033 |
| Amiodarone Complications | 1.199 | 0.953 | 1.445 | 0.4353 |
| Cost Estimates | Adjusted Estimate | 95% CI | p-value | |
|
| ||||
| POAF | $6743 | 3104 | 10382 | 0.0644 |
| Amiodarone Complications | $6766 | 1720 | 11812 | 0.1805 |
Cost-Effectiveness Modeling
Cost-effectiveness of the prophylactic amiodarone protocol was modeled using the estimates calculated above. A standard willingness-to-pay threshold of $50,000 per utility was used for the calculation. The model demonstrates the prophylactic amiodarone protocol is cost effective with an average savings of $458 for every patient treated (Figure 2). The protocol provided an incremental cost-effectiveness ratio with savings of $64,156 per utility adjusted unit gained.
Figure 2.
TreeAge Cost-Effectiveness Model. Decision tree with estimated costs, POAF rates, adverse event rates and outcome utilities.
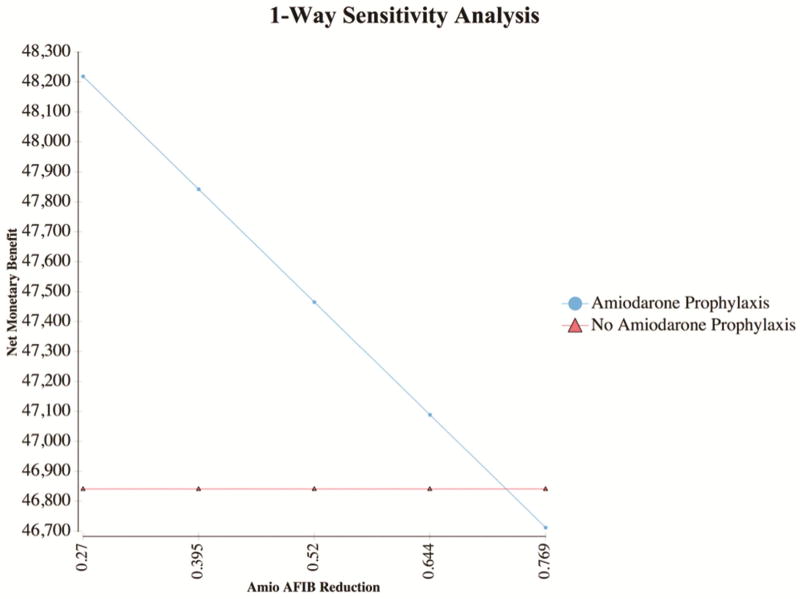
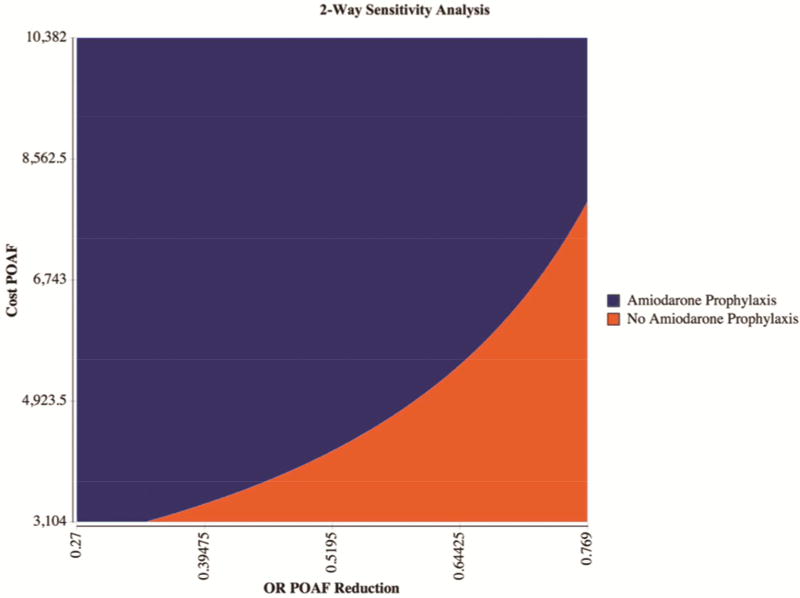
Within the 95% confidence intervals of the estimates from above, the model is only sensitive to amount of reduction in POAF associated with the prophylactic amiodarone protocol, where any odds ratio below 0.726 is cost-effective (Figure 3). Furthermore, two-way sensitivity analysis varying both the cost of POAF and the odds ratio of POAF reduction with amiodarone demonstrates a prophylactic amiodarone protocol is cost effective at most estimates (Figure 4).
Figure 3.
One-Way Sensitivity Analysis. One-way sensitivity analysis demonstrates the model is only sensitive to variation in the odds ratio of POAF reduction provided by a prophylactic amiodarone protocol above 0.726
Figure 4.
Two-Way Sensitivity Analysis. Two-way sensitivity analysis varying both cost of POAF and odds ratio of POAF reduction with amiodarone demonstrates a prophylactic amiodarone protocol is cost effective at most estimates. Blue indicates amiodarone Prophylaxis is more cost effective while Orange indicates when it is not more cost effective. The 95% CI were used to vary the axes.
Comment
This study demonstrates implementation of a prophylactic amiodarone protocol provides a cost-effective reduction in POAF. Our institution demonstrated successful application of this protocol driven care with almost 95% enrollment. Furthermore, these data demonstrate a significant reduction in rates of POAF without an increase in risk adjusted adverse events. Not only did the protocol improve outcomes, but also reduced cost with an estimated average savings of $458 per patient. Finally, these results are robust and not influenced by the cost of atrial fibrillation or the rates of adverse events due to amiodarone. The model was only sensitive to the risk-reduction seen with amiodarone, and the odds ratio cutoff (0.726) is higher than any estimates in the published literature.
Several pharmacologic therapies have been used to reduce the risk of POAF after cardiac surgery.(16–18) Prophylactic amiodarone has been demonstrated to successfully reduce the risk of POAF.(19–21) However, there some concerns exists about medication related adverse events including bradycardia, heart block, and hypotension that may prove more costly then justified by the reduction in POAF.(22, 23) After controlling for these effects we demonstrate implementation of a prophylactic amiodarone protocol at our institution still provides a cost effective reduction in POAF. These results were not sensitive to variation in rates of medication related adverse events or associated costs. The cost savings are secondary to reduced resource utilization associated with the development of POAF.
Studies in the literature provide different estimates for POAF risk reduction with use of prophylactic amiodarone.(19, 21, 24) A recent meta-analysis looking at six prospective randomized trials demonstrated an OR of 0.50 with a 95% confidence interval of 0.42–0.60.(25) Our cost-effectiveness model demonstrated prophylactic amiodarone was cost effective at all OR below 0.726, which was at the top of the confidence interval for our population. Therefore, based on meta-analysis data, the cost-effectiveness of prophylactic amiodarone would not be sensitive to variation in any of the parameters out to the 95% confidence intervals.
The amiodarone protocol utilized; including dosing, route and timing can have significant effects on rates of medication related adverse events, efficacy of therapy, and success of implementation. Our protocol utilized intravenous dosing postoperatively which is associated with a higher rate of medication related adverse events in the literature.(18) However, we demonstrated the cost-effectiveness of our amiodarone protocol is not sensitive to these effects up to the 95% confidence interval. Furthermore, since patients only require dosing postoperatively, the implementation of our protocol was highly successful with over 95% of patients eligible being enrolled. Other centers should consider this as a guide for implementation of their own protocols to provide the highest compliance with a prophylactic amiodarone protocol.
In the current environment of quality improvement and focus on protocol driven care it is critical to evaluate these interventions using the value framework.(9) There are many pitfalls in designing and implementing and evaluating the success of quality improvement projects as described in a recent paper by Ebinger et al.(26) However, these authors do not mention the significant investment of personnel hours to develop and successfully deploy a major service line wide initiative. While these estimates are difficult to identify and quantify, this prophylactic amiodarone protocol required over 100 hours of work from surgeons, nurses, and quality officers with an estimated cost of $3,530. While these expenses were quickly recovered through protocol cost savings ($458 per patient), it must be considered when planning a major quality improvement initiative.
This analysis is limited by its retrospective nature with potential for selection bias using a time-based analysis. Propensity score matching was utilized to control for these confounders. Additionally, multivariate logistic and linear regression was utilized to estimate the effects of the protocol on POAF occurrence, adverse events, and cost; however, these models are susceptible to bias and cannot control for unidentified variables. Finally, this analysis is based on the actual practice patterns at our academic cardiac surgery program and therefore may not be generalizable to other centers.
In conclusion, implementation of a prophylactic amiodarone protocol significantly reduced risk-adjusted rates of POAF with a cost savings of $458 per patient. This effect was independent of cost variation and was durable at all prior estimates of POAF risk reduction using prophylactic amiodarone. This analysis demonstrates how rigorous quantitative analysis can evaluate the benefits of quality improvement projects. In an era of value driven care, these critical evaluations are necessary to ensure we are delivering the best care.
Supplementary Material
Supplemental Figure 1. Preoperative Exclusion Criteria
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Annals of internal medicine. 2001;135(12):1061–1073. doi: 10.7326/0003-4819-135-12-200112180-00010. [DOI] [PubMed] [Google Scholar]
- 2.Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: A major morbid event? Annals of surgery. 1997;226(4):501–511. doi: 10.1097/00000658-199710000-00011. discussion 511-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hravnak M, Hoffman LA, Saul MI, Zullo TG, Whitman GR. Resource utilization related to atrial fibrillation after coronary artery bypass grafting. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2002;11(3):228–238. [PMC free article] [PubMed] [Google Scholar]
- 4.Almassi GH, Pecsi SA, Collins JF, Shroyer AL, Zenati MA, Grover FL. Predictors and impact of postoperative atrial fibrillation on patients' outcomes: A report from the randomized on versus off bypass trial. The Journal of thoracic and cardiovascular surgery. 2012;143(1):93–102. doi: 10.1016/j.jtcvs.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Bramer S, van Straten AH, Soliman Hamad MA, Berreklouw E, Martens EJ, Maessen JG. The impact of new-onset postoperative atrial fibrillation on mortality after coronary artery bypass grafting. The Annals of thoracic surgery. 2010;90(2):443–449. doi: 10.1016/j.athoracsur.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 6.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94(3):390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 7.Almassi GH, Wagner TH, Carr B, et al. Postoperative atrial fibrillation impacts on costs and one-year clinical outcomes: The veterans affairs randomized on/off bypass trial. The Annals of thoracic surgery. 2015;99(1):109–114. doi: 10.1016/j.athoracsur.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Speir AM, Kasirajan V, Barnett SD, Fonner E., Jr Additive costs of postoperative complications for isolated coronary artery bypass grafting patients in virginia. Ann Thorac Surg. 2009;88(1):40–45. doi: 10.1016/j.athoracsur.2009.03.076. discussion 45–46. [DOI] [PubMed] [Google Scholar]
- 9.Rich JB, Speir AM, Fonner E, Jr Virginia Cardiac Surgery Quality I. Making a business case for quality by regional information sharing involving cardiothoracic surgery. Am Heart Hosp J. 2006;4(2):142–147. doi: 10.1111/j.1541-9215.2006.04577.x. [DOI] [PubMed] [Google Scholar]
- 10.Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. The Cochrane database of systematic reviews. 2013;(1):Cd003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkerson KA, Gillespie EL, White CM, et al. Impact of prophylactic amiodarone on length of hospital stay, stroke, and atrial fibrillation after cardiothoracic surgery. Pharmacotherapy. 2005;25(3):320–324. doi: 10.1592/phco.25.3.320.61595. [DOI] [PubMed] [Google Scholar]
- 12.Zebis LR, Christensen TD, Kristiansen IS, Hjortdal VE. Amiodarone cost effectiveness in preventing atrial fibrillation after coronary artery bypass graft surgery. Ann Thorac Surg. 2008;85(1):28–32. doi: 10.1016/j.athoracsur.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Chang CM, Lu MJ, et al. Intravenous amiodarone for prevention of atrial fibrillation after coronary artery bypass grafting. The Annals of thoracic surgery. 2000;70(1):157– 161. doi: 10.1016/s0003-4975(00)01308-4. [DOI] [PubMed] [Google Scholar]
- 14.Zebis LR, Christensen TD, Thomsen HF, et al. Practical regimen for amiodarone use in preventing postoperative atrial fibrillation. The Annals of thoracic surgery. 2007;83(4):1326–1331. doi: 10.1016/j.athoracsur.2006.09.096. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell LB, Exner DV, Wyse DG, et al. Prophylactic oral amiodarone for the prevention of arrhythmias that begin early after revascularization, valve replacement, or repair: Papabear: A randomized controlled trial. JAMA : the journal of the American Medical Association. 2005;294(24):3093–3100. doi: 10.1001/jama.294.24.3093. [DOI] [PubMed] [Google Scholar]
- 16.Davis EM, Packard KA, Hilleman DE. Pharmacologic prophylaxis of postoperative atrial fibrillation in patients undergoing cardiac surgery: Beyond beta-blockers. Pharmacotherapy. 2010;30(7):749, 274e–318e. doi: 10.1592/phco.30.7.749. [DOI] [PubMed] [Google Scholar]
- 17.Echahidi N, Pibarot P, O'Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51(8):793–801. doi: 10.1016/j.jacc.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Turagam MK, Downey FX, Kress DC, Sra J, Tajik AJ, Jahangir A. Pharmacological strategies for prevention of postoperative atrial fibrillation. Expert Rev Clin Pharmacol. 2015;8(2):233–250. doi: 10.1586/17512433.2015.1018182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann MC. Evaluation of an evidence-based practice implementation: Prophylactic amiodarone following coronary artery revascularization. Dimens Crit Care Nurs. 2012;31(3):193–201. doi: 10.1097/DCC.0b013e31824e0150. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Wang C, Gao D, et al. Meta-analysis of amiodarone versus beta-blocker as a prophylactic therapy against atrial fibrillation following cardiac surgery. Intern Med J. 2012;42(10):1078–1087. doi: 10.1111/j.1445-5994.2012.02844.x. [DOI] [PubMed] [Google Scholar]
- 21.Butler J, Harriss DR, Sinclair M, Westaby S. Amiodarone prophylaxis for tachycardias after coronary artery surgery: A randomised, double blind, placebo controlled trial. Br Heart J. 1993;70(1):56–60. doi: 10.1136/hrt.70.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rostagno C, La Meir M, Gelsomino S, et al. Atrial fibrillation after cardiac surgery: Incidence, risk factors, and economic burden. J Cardiothorac Vasc Anesth. 2010;24(6):952–958. doi: 10.1053/j.jvca.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Barnes BJ, Kirkland EA, Howard PA, et al. Risk-stratified evaluation of amiodarone to prevent atrial fibrillation after cardiac surgery. Ann Thorac Surg. 2006;82(4):1332–1337. doi: 10.1016/j.athoracsur.2006.04.081. [DOI] [PubMed] [Google Scholar]
- 24.Rostagno C. Recent developments in pharmacologic prophylaxis of atrial fibrillation in patients undergoing surgical revascularization. Cardiovasc Hematol Agents Med Chem. 2009;7(2):137–146. doi: 10.2174/187152509787847074. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee S, Sardar P, Mukherjee D, Lichstein E, Aikat S. Timing and route of amiodarone for prevention of postoperative atrial fibrillation after cardiac surgery: A network regression meta-analysis. Pacing Clin Electrophysiol. 2013;36(8):1017–1023. doi: 10.1111/pace.12140. [DOI] [PubMed] [Google Scholar]
- 26.Ebinger JE, Porten BR, Strauss CE, et al. Design, challenges, and implications of quality improvement projects using the electronic medical record: Case study: A protocol to reduce the burden of postoperative atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2016;9(5):593–599. doi: 10.1161/CIRCOUTCOMES.116.003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Preoperative Exclusion Criteria