Abstract
Unpredictable aversive experiences, or stressors, lead to changes in depression- and anxiety-related behavior and to changes in hippocampal structure including decreases in adult neurogenesis, granule cell and pyramidal cell dendritic morphology, and volume. Here we review the relationship between these behavioral and structural changes and discuss the possibility that these changes may be largely adaptive. Specifically, we suggest that new neurons in the dentate gyrus enhance behavioral adaptability to changes in the environment, biasing behavior in novel situations based on previous experience with stress. Conversely, atrophy-like changes in the hippocampus and decreased adult neurogenesis following chronic stress may serve to limit stress responses and stabilize behavior during chronic stress.
Keywords: adult neurogenesis, dentate gyrus, hippocampus, volume, dendrites, morphology, stress, behavioral adaptation
INTRODUCTION
Behavioral and physiological stress responses are critical for survival under adverse conditions. Rapid responses to acute stress help organisms avoid actual harm and to cope with injuries if the threat cannot be avoided. However, these rapid responses are themselves potentially harmful if they must be repeated frequently over long periods of time in response to chronic exposure to stressors in dangerous environments. Management of chronic stress, therefore, likely requires different strategies. For example, fear learning can enhance prediction and planning, increasing the likelihood of avoiding future stressors. Prior expectation of danger can also enable anticipatory physical changes to better cope with injury. However, avoidance behaviors and repeated physiological preparation can have adverse physical consequences if continued or repeated over long periods. The ability to flexibly adapt stress responses to changing environments is critical for the capacity to recover from challenges or stressors, often referred to as resilience (McEwen et al., 2015a). Such flexibility depends on previous experience, both during development and in adulthood: “The individual traits that allow the more flexible outcomes undoubtedly depend upon a foundational capacity of that individual that is built upon experiences in the life course…” (McEwen et al., 2015a). The tremendous amount of neuroplasticity in the developing brain may provide the substrate that allows developmental environments to set the stage for stress response tendencies throughout life (Gluckman et al., 2005; Oitzl et al., 2010). Similarly, adult neurogenesis, a dramatic form of neuroplasticity in adulthood that adds new neurons to the hippocampus throughout life, may provide ongoing flexibility that allows stress response and stress perception to continue to adapt to environmental challenges throughout life.
Fear generalization is important for response to chronic stress
Rapid learning is important in aversive environments, as it enables an organism to avoid or appropriately respond to previously encountered threats (Do-Monte et al., 2015; Ghosh and Chattarji, 2015). Generalization of fear learning is similarly adaptive, as it enables avoidance of threats not specifically encountered before. However, excessive generalization is maladaptive if it leads to fear responses that are too strong or that occur in inappropriate situations, interfering with more rewarding actions. For example, fear of novel environments could be protective. But it could also enhance freezing and decrease exploration in rodents to such a degree that it interferes with successful foraging for food, and it could decrease participation in pleasurable activities or events in humans. This decrease in rewarding activity due to inappropriate fear is the cardinal feature of anxiety disorders (Dunsmoor and Paz, 2015; Lissek, 2012; Luyten et al., 2011). There is a balance, then, or optimal level of fear generalization for a given situation, which depends on the likelihood of encountering adversity and reward within the environment (Figure 1). Elucidating the neural circuits and mechanisms that determine the level of fear generalization and mediate optimization of the stress response is critical for understanding and treating stress-related disorders such as generalized anxiety disorder, post-traumatic stress disorder, and depressive illness.
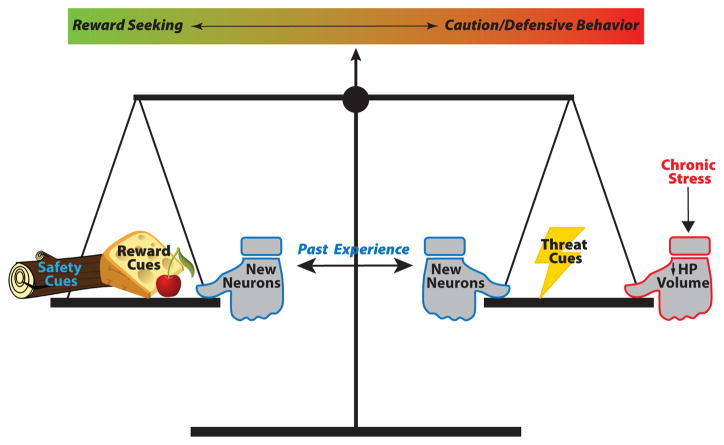
Figure 1.
Reward seeking and cautious or anxiety-like behavior must be balanced to appropriately respond in new or unpredictable situations. Safety and reward cues drive behavior toward reward seeking, while cues signaling actual or potential threats tip the balance toward defensive or cautious behaviors such as freezing or approach-avoidance investigation. New neurons generated in the adult dentate gyrus can bias this balance in either direction based on the predictability of prior threats. Stress and the resulting structural changes in the hippocampus bias the system toward caution.
Extreme threats such as very strong negative stimuli (e.g., strong shocks), which may model the type of traumatic experience that can lead to PTSD, lead to high levels of generalization, i.e., freezing to cues never associated with the shock (Ghosh and Chattarji, 2015). This effect is non-associative, generalizing to stimuli that are very different from those present during the aversive experience and can be seen even after a few moderate footshocks (Kamprath and Wotjak, 2004; Seo et al., 2015). Importantly, this non-associative generalization can affect results in learning and memory tasks that depend on freezing as a behavioral outcome (Kamprath and Wotjak, 2004; Seo et al., 2015). Another key feature affecting the impact of threat is predictability. Even mild threats can lead to prolonged HPA axis responses and fear generalization if they are unpredictable (Glover et al., 2017; Koolhaas et al., 2011; Onat and Büchel, 2015). Evidence suggests that plasticity in the amygdala is critical for the long-lasting behavioral changes seen in response to strong threat (Ghosh and Chattarji, 2015; Perusini et al., 2016). In contrast, the hippocampus may be more critical for long-lasting effects of milder but unpredictable stressors (Glover et al., 2017). The hippocampus is a key brain region for stress response (McEwen et al., 2015b), and evidence also suggests that it plays a role in prediction, particularly in situations of conflicting goals or uncertainty (Bannerman et al., 2014; Buckner, 2010; Cameron and Glover, 2015; Chudasama et al., 2009; Gray and McNaughton, 2000; Tsetsenis et al., 2007). Within the hippocampus, new granule neurons are added throughout adulthood, and this neurogenesis has been implicated in both stress response and anxiodepressive-like behavior (David et al., 2009; Groves et al., 2013; Santarelli et al., 2003; Snyder et al., 2011). The links between stress, unpredictability, and the hippocampus suggest that new neurons may function to provide plasticity needed to adapt behavioral strategies in response to changing environments (Glasper et al., 2012).
A role for adult neurogenesis in adapting to ambiguous threat
Work with rodent models in which adult neurogenesis is specifically inhibited have provided evidence for a role of new neurons in adaptive stress responses. We have found that mice that are fear conditioned with an ambiguously cued shock behave differently than their counterparts conditioned with a reliable shock, even though the shocks experienced by all animals were mild and identical in number, strength, and duration (Glover et al., 2017). Although mice in both groups respond identically to the cue, showing high levels of freezing, mice trained with the ambiguous cue show high levels of anxiety-like behavior in a novel situation days later, while mice trained with the reliably cued shock show low anxiety as though they were never shocked. These results suggest that unpredictable shock, or negative experience more generally, causes mice to be more cautious in future novel situations, which is likely to be adaptive in environments containing many unpredictable threats. In contrast, animals that experience the same aversive stimulus, but in a reliably predictable manner, do not show enhanced caution or anxiety-like behavior. This is also likely adaptive, as excessive caution limits reward opportunities, thereby incurring costs that outweigh benefits in environments in which threats are few or are predictable. Thus, normal animals show adaptive behavioral changes determined by the unpredictability or ambiguity of threats (Hawley et al., 2012; Koolhaas et al., 2011; Reser, 2016). However, mice lacking adult neurogenesis fail to show this behavioral flexibility (Glover et al., 2017). Instead, these animals show an intermediate increase in anxiety-like behavior after experiencing the shocks, regardless of whether they are reliably or ambiguously cued.
Unpredictable threats meet the strict definition of stressors (Koolhaas et al., 2011), suggesting that the behavioral adaptations seen in response to ambiguously-predicted threats may reflect changes in behavior induced by stress more broadly. Rats do consistently show an anxiety-like phenotype following chronic stress, which typically manifests as a lack of exploration into novel, and therefore potentially threatening, environments (Bondi et al., 2008; Eiland and McEwen, 2012). They also exhibit enhanced aversive conditioning (Conrad et al., 1999; Servatius and Shors, 1994; Shors et al., 1992), suggesting that stress enhances the salience of threatening stimuli and cautious behavior, both of which are advantageous for survival when exploring a new environment with a high likelihood of danger. After chronic restraint stress, rats show greater hesitation than non-stressed controls when eating in an unknown arena but show increased intake of caloric sucrose in the safety of their home cage (Schoenfeld et al., 2017). Together, these behavioral changes suggest adaptations that favor limiting exposure to potentially unsafe environments in the presence of persistent stress. Inhibition of adult neurogenesis does not alter these consumption behaviors in stressed rats (Schoenfeld et al., 2017), possibly because of the long duration of relatively strong stress.
Failure to rapidly adjust levels of caution could be adaptive in some environments
In the wild, failure to adjust behavior based on threat predictability would likely result in excessive anxiety in predictable environments and too little caution in unpredictable environments. But is this maladaptive or does it reflect another layer of adaptation? Adult neurogenesis is strongly and rapidly inhibited by stress hormones (Cameron and Gould, 1996; 1994), suggesting that decreased neurogenesis may be an adaptive stress response. Acute stress briefly decreases adult neurogenesis, while chronic stress has variable effects that may depend on the specific paradigm (Gould et al., 1997; Mirescu and Gould, 2006; Schoenfeld and Gould, 2013). Several weeks of 6-hour/day restraint stress has a relatively small effect on adult neurogenesis, decreasing immature neuron number in rats by only 30%, and only in the ventral part of the hippocampus (Schoenfeld et al., 2017). However, a truly chronic, non-remitting stress produced by neuropathic pain can decrease adult neurogenesis 70% throughout the entire hippocampus (Dimitrov et al., 2014). In contrast, rewarding experiences increase adult neurogenesis and buffer stress effects (Schoenfeld and Gould, 2013), suggesting that the overall nature of the environment, or the sum total of daily rewards and stresses may set the level of neurogenesis.
A decrease in neurogenesis due to chronic stress should limit stress response flexibility within a few weeks, when the missing new neurons would be mature enough to participate in hippocampal function. Evidence for such a change has been observed in a social defeat stress paradigm, where mice that were irradiated to reduce adult neurogenesis four weeks earlier showed less social avoidance than neurogenesis-normal mice (Lagace et al., 2010). Decreased neurogenesis, therefore, may have the effect of blunting response to ongoing stressors and possibly of relieving the organism of the cognitive burden associated with predicting patterns to threats. Increasing the number of new neurons, on the other hand, may enhance sensitivity to the environment. If cognitive and environmental features that triggered a depressive episode have resolved, then enhancing neurogenesis could hasten recovery. However, if neurogenesis were increased while stresses remain, the additional new neurons could potentially worsen anxiety and other stress-related mental and behavioral changes. It seems clear from work in animals that new neurons affect mood-related behavior but do so in a complex way rather than simply enhancing mood. It is critical to understand specifically how changes in adult neurogenesis influence mood – whether by increasing the influence of the environment or through some another role – in order to understand whether and how such manipulations may be effective therapeutically.
Identifying threat cues – pattern separation and task difficulty
Distinguishing cues that predict threat from those that are non-predictive is key to optimizing stress responses and avoiding excessive anxiety and depressive behavior. Some threats are clearly and reliably predictable based on cues that precede them, while others are impossible to predict based on environmental stimuli. Many threats, however, may be predicted but only by complex patterns of cues or by cues that differ only subtly from safety cues. Situations in which outcomes are difficult or impossible to predict are likely to engage the hippocampus (Redish, 2016).
Several studies have demonstrated that loss of dentate gyrus granule neurons affects performance of difficult stimulus discrimination tasks (Gazzara and Altman, 1981; Hvoslef-Eide and Oomen, 2016). In a typical study of this type, animals are trained to associate cues (discrete stimuli, spatial locations, or contexts) with a shock but are also exposed to similar cues that are not associated with the shock. Animals are then expected to behave differently in the presence of the reward/shock-associated cue and the similar cue; failure to do so is interpreted as an inability to discriminate between the cues or, alternatively, to recall the cues with sufficient specificity to know which one is associated with the shock. This impairment, in turn, is believed to reflect a failure of hippocampal circuitry to generate sufficiently distinct patterns of activity (“pattern separation”) for each exposure to the cues, leading to interference between activity patterns that results in memory interference. These behavior tests have been called pattern separation tasks, based on an assumption that they reflect the proposed dentate gyrus function in separating patterns of network activity. However, because there is little evidence actually linking behavior in these tasks with circuitry level changes in pattern separation, these tests are better described as difficult discrimination tasks (Santoro, 2013). These tasks clearly involve a discrimination, but the specific impairment leading to the change in behavior is unclear. The deficit could be in discriminating the cues, i.e., a sensory impairment, but it could also reflect a memory impairment, or a change in task performance, e.g., behaviors related to threat avoidance. If the impairment is in sensory discrimination or memory for the cues, then animals should react similarly to both cues regardless of the specific behavior examined. Most pattern separation studies have used shock as the unconditioned stimulus and have measured freezing behavior to test for discrimination. However, as discussed above, freezing behavior is highly susceptible to generalization (Ghosh and Chattarji, 2015; Glover et al., 2017; Kamprath and Wotjak, 2004; Seo et al., 2015), which could mask an ability to recall and distinguish the two contexts. Such masking has been demonstrated by differences in cue- or context-specific place preference or startle behaviors even in the absence of differential freezing (Antoniadis and McDonald, 1999; McNish et al., 1997). Performance effects that masquerade as spatial learning and memory deficits have also been found in water maze tasks (Devan et al., 2003). Interestingly, these effects were observed only under inherently ambiguous partial reinforcement conditions and occurred in a stressful water-based task but not in a non-threatening dry-land version of the same task (Gonzalez et al., 2000). Taken together, these studies suggest that in difficult or ambiguous learning tasks, stress responses can affect task performance in ways that mask or mimic learning effects.
Like stress, adult neurogenesis may impact difficult discrimination tasks because of their difficulty rather than because of the discrimination itself. Task difficulty has been suggested as a defining feature of neurogenesis-dependent tasks (Beylin et al., 2001), and conversely, survival of new neurons is affected by the difficulty of tasks on which animals are trained, even when tasks are not hippocampus-dependent (Curlik and Shors, 2011; Epp et al., 2010; but see Swan et al., 2014). An early study found that rats with severe granule cell loss, due to irradiation in the first postnatal week, found that these animals were impaired in discrimination tasks with highly similar, but not distinct, stimuli (Gazzara and Altman, 1981). The authors concluded that the impairment reflected task difficulty rather than a sensory deficit, in part based on an earlier study that found that hippocampal lesion-induced impairments disappear when animals learn simple discriminations before moving on to more difficult ones (Truax and Thompson, 1969). A similar effect has been seen with adult neurogenesis-deficient rats in difficult eyeblink conditioning tasks (Beylin et al., 2001; Curlik et al., 2013; Curlik and Shors, 2011), consistent with the possibility that these animals can discriminate cues and learn and recall associations normally but have difficulty focusing on the correct predictive stimuli. A role for the hippocampus in difficult tasks also appears to be supported by proposed functions in decision making under conditions of closely matched but conflicting goals (Gray and McNaughton, 2000) and, more recently, in the use of hippocampus-dependent vicarious trial and error to aid decision making (Redish, 2016).
There are several different roles new neurons might play in difficult tasks. As discussed above, it seems unlikely that their role is directly related to sensory discrimination, per se, because evidence of discrimination can be found using alternative behavioral measures and because similar impairments are observed using visual (Clelland et al., 2009), auditory (Beylin et al., 2001), and tactile (Gazzara and Altman, 1981) stimuli. The ambiguous cue study described above (Glover et al., 2017) might be thought of as a difficult discrimination (i.e., pattern separation) task but with cues that are impossible, rather than difficult, to discriminate. Because the tone associated with a shock and the tone associated with no shock are identical rather than highly similar, none of the animals can discriminate the cues and predict which trials would include a shock. Yet despite having the same inability to discriminate the identical cues, the behavior of neurogenesis-deficient mice and control mice in the task differs. The ambiguity that is an inherent aspect of the design in this task is also likely to be present in difficult discrimination tasks during the relatively long phase of training when the cue associations are not yet understood.
Stress, neurogenesis, and depression
Although stress-induced changes in behavior may be adaptive by helping to match behavior to the environment, stress is also strongly associated with depression. It is currently unknown how stress alters hippocampal network activity and how these circuit changes translate into depression-related mood and behavior changes. However, stress causes several changes to hippocampal structure that likely affect connectivity and function. As described above, loss of adult neurogenesis decreases neuronal activity throughout the hippocampus in response to ambiguous stress (Glover et al., 2017). Decreased adult neurogenesis has been proposed as a mechanism through which stress leads to depression and, conversely, through which treatments halt depression (Duman et al., 1999; Madsen et al., 2000). This idea is based on 1) findings that all classes of antidepressant medications enhance proliferation of granule cell precursors; 2) the delayed time course of antidepressant action, which could potentially be explained by the similar time course of new granule cell maturation; and 3) correlations between the neurogenesis and depressive-like behavior in animals (Miller and Hen, 2015). Chronic stress, including psychogenic stress without physical harm, reduces proliferation, differentiation, maturation and survival of new granule cells (Czéh et al., 2007; Dagyte et al., 2009; Pham et al., 2003; Schoenfeld et al., 2017; Snyder et al., 2009b). Some stress procedures can produce regional differences in the effects of stress on neurogenesis (Alves et al., 2017; Schoenfeld et al., 2017; Tanti et al., 2013), however it is unclear how consistent these regional effects are and whether any in particular are more clearly associated with development of depressive-like behavior.
Direct evidence of a role for new neurons in depression can be tested using rodent models in which adult neurogenesis has been specifically inhibited using irradiation or pharmacogenetic methods. However, these investigations are subject to the limitations of rodent depressive-like behavior to mimic depression (Dzirasa and Covington, 2012; Nestler and Hyman, 2010). Most studies do not find any effect of neurogenesis ablation on depressive behavior when testing otherwise naïve rats (Airan et al., 2007; Meshi et al., 2006; Santarelli et al., 2003; Surget et al., 2011). However, exposure to threats, either acute or repeated, differentially enhances anxiety-like and depressive-like behavior in rodents without neurogenesis (Glover et al., 2017; Seo et al., 2015; Snyder et al., 2011), strengthening the link between new neurons and stress response. Under both stress and non-stress conditions, however, new neurons are necessary for a variety of antidepressant medications to decrease depressive-like behavior in rodents (Airan et al., 2007; Santarelli et al., 2003; Surget et al., 2011). Interestingly, although social defeat stress increases social avoidance in control mice, this stress effect is actually diminished in mice lacking adult neurogenesis (Lagace et al., 2010). Behavioral changes that accompany decreased neurogenesis in the presence of stress may serve to limit exposure to the outside environment and enhance social connectedness in order to avoid future threats and thereby increases survival.
Stress and depression decrease hippocampal volume
Stress and depression have been linked not only with changes in adult neurogenesis but with other structural changes in the hippocampus as well. In humans, stressful life episodes are associated with lower hippocampal volumes (Bremner et al., 1995; Papagni et al., 2011; Winter and Irle, 2004) and are predicators of developing depression (Caspi et al., 2003; Kendler et al., 1999). Patients with major depressive disorder consistently have smaller hippocampi, with volume showing inverse correlations with symptom severity, number of depressive episodes, and likelihood of relapse (Brown et al., 2014a; Kronmüller et al., 2008; Videbech and Ravnkilde, 2004). These volume changes suggest that hippocampal volume may one day be a useful biomarker of depression and/or antidepressant efficacy, though high intrinsic variability currently precludes this. In addition, although the aforementioned effects were observed in mixed sex subject groups, and differences have been observed in at least one study using only female subjects (Tae et al., 2011), several reports suggest that decreased hippocampal volume due to stress and depression is more apparent in males than in females (Carlson et al., 2017; Colle et al., 2017; Frodl et al., 2002; Spalletta et al., 2014). The association of low hippocampal volume with depression suggests that stress and hippocampal changes may be important drivers of depression (Lupien et al., 2007; Maller et al., 2017). Association between endogenous glucocorticoid levels and hippocampal volume, and effects of exogenous glucocorticoid treatment on hippocampal volume in non-depressed populations (Brown et al., 2014b; Lupien et al., 1998; Sindi et al., 2014), suggest that stress hormones, and not depression-related behaviors, are the primary cause of hippocampal volume decrease. Effects of glucocorticoid treatment on hippocampal volume can be detected within 3 days (Brown et al., 2014b), and volume can be increased by antidepressants (Abbott et al., 2014; Maller et al., 2017), together suggesting that changes in hippocampal volume may reflect potentially adaptive neuroplasticity rather than atrophy.
Studies using both chronic restraint or chronic unpredictable mild stress in rodents demonstrate that stress produces a decrease in overall hippocampal volume that can be detected by MRI (Lee et al., 2009; Luo et al., 2014; Schoenfeld et al., 2017). In recently published work from our lab, high-resolution MRI and 3-dimensional reconstruction of traced sections were used to measure volume shrinkage in subregions of the hippocampus (Schoenfeld et al., 2017). Four weeks of unpredictable restraint stress decreased the volume of the dentate gyrus, CA3, and CA1 subregions, across both dorsal and ventral portions of the hippocampus. Earlier work suggests that milder stress paradigms might produce uneven volume reductions affecting dorsal but not ventral hippocampal segments (Pinto et al., 2014). All of these studies have been carried out in male rats and mice, so further study will be needed to determine whether the hippocampus of female rodents is more resistant to volume changes as appears to be the case in humans (see above).
Other structural changes that may contribute to volume decrease
The parallels between stress effects on neurogenesis and hippocampal volume have led researchers to suggest that new neurons may underlie stress-induced changes in volume (Boku et al., 2018; Czéh and Lucassen, 2007; Eker et al., 2011; Joshi et al., 2016). However, volume reductions could alternatively reflect a number of other known cellular changes. A tremendous body of work from Bruce McEwen’s lab over the last three decades has demonstrated that chronic stress causes atrophy of CA3 pyramidal neurons, shrinking the apical dendritic trees in male rats (Conrad et al., 1999; Eiland and McEwen, 2012; Galea et al., 1997; Magariños et al., 1999; 2011; McCall et al., 2013; Watanabe et al., 1992a; 1992b; Wood et al., 2004). The timing, reversibility, sexual dimorphism, and functional consequences of this atrophy have been reviewed elsewhere (Conrad, 2006). We have recently observed similar effects on dendritic atrophy in CA3 pyramidal neurons in the dorsal hippocampus, the portion of the hippocampus that was the focus of previous studies. However, we found that CA3 pyramidal cells in the ventral hippocampus showed no decrease in dendritic length following the same four-week stress period but instead reorganized their trees, becoming less complex proximal to the soma and more complex more distally (Schoenfeld et al., 2017). This reorganization could shift the weight of various dendritic inputs relative to the normal unstressed condition without altering the total number of synaptic inputs to these neurons. Glial and vascular changes within the hippocampus could also contribute significantly to volume loss (reviewed in Murthy, 2017).
Stress and inhibition of adult neurogenesis decrease volume differently
To directly address the impact of adult neurogenesis on hippocampal volume, the effect of complete ablation of adult neurogenesis on hippocampal volume in male rats has been compared to that of chronic stress (Schoenfeld et al., 2017). Inhibition of adult neurogenesis produces shrinkage first in the dentate gyrus and later in area CA3 as well, resulting in detectable volume loss in the hippocampus as a whole. However, although the loss of new neurons and chronic stress both decrease hippocampal volume, the shrinkage due to ablation is more restricted and occurs more slowly than that due to chronic stress (Schoenfeld et al., 2017). Moreover, the effect of chronic daily restraint on neurogenesis is small, resulting in a decrease of only ~33%, and restricted to the ventral segment, while stress decreases volume throughout all hippocampal subregions (Schoenfeld et al., 2017). Therefore, although adult neurogenesis is vital to maintaining normal hippocampal structure, and may contribute to stress-induced hippocampal shrinkage in the dentate gyrus, it is unlikely to be responsible for the majority of the stress- or depression-associated hippocampal volume loss, particularly in humans, where the ablation-resistant CA1 region makes up a larger proportion of the hippocampus than it does in rodents (Joelving et al., 2006).
Volume loss during stress on these dendrites likely reflects primarily loss of dendritic mass, along with possible changes in glial cells and/or the extracellular matrix. Inhibition of adult neurogenesis produces atrophy of CA3 pyramidal cells within 4 weeks, but the effect of stress is somewhat more pronounced (Schoenfeld et al., 2017). Stress also decreases dendritic length in preexisting dentate gyrus granule cells, while ablation of new neurons has no effect on these cells (Schoenfeld et al., 2017), consistent with a more limited role for loss of adult neurogenesis in stress-induced volume decrease. The changes in CA3 pyramidal cell morphology resulting from decreased adult neurogenesis make the functional and behavioral effects of decreased neurogenesis and CA3 dendritic atrophy difficult to parse. However, the use of chemogenetic methods to rapidly silence young neurons (Gu et al., 2012) may soon enable direct investigation of dendritic shrinkage effects.
Loss of adult neurogenesis may limit structural reorganization
The effects of stress on structural plasticity and behavior are typically viewed in a negative light due to the role that stress plays in the development of neuropsychiatric conditions (Cattaneo and Riva, 2016; Gold et al., 2015 for review; Romeo, 2017; see Ross et al., 2017; Tafet and Nemeroff, 2016). However, as discussed above, stress produces changes in behavior that are likely to be adaptive during exposure to real-world dangers. Stress-induced changes in hippocampal structure may similarly be adaptive rather than damaging. Decreased CA3 dendritic morphology enhances glucocorticoid response (Conrad, 2006), enabling stronger physical and behavioral responses to stress that may be beneficial in dangerous situations. However, the same structural and behavioral changes that might be protective over a period of days to weeks may also be maladaptive if threatening environments or perceived stresses continue over long periods of time.
Decreases in adult neurogenesis that accompany stress could serve an adaptive function structurally as well as behaviorally, by limiting the morphological and volume changes brought about by stress. Recent findings from our lab hint that even though inhibition of adult neurogenesis decreases hippocampal volume and dendritic morphology on its own this same loss of new neurons does not increase, and may even diminish the shrinkage caused by chronic stress. Specifically, pharmacogenetic inhibition of adult neurogenesis reduces the volume of the dentate gyrus and CA3 in unstressed rats, and the addition of stress produces no further atrophy in those regions (Schoenfeld et al., 2017). Moreover, the effects of stress on atrophy of granule cell and CA3 pyramidal cell dendrites are more pronounced in rats with ongoing neurogenesis than in rats without adult neurogenesis, and stress-induced CA3 dendritic atrophy occurs in the dorsal region, where neurogenesis is unaffected, but not in the ventral region, where neurogenesis is decreased (Schoenfeld et al., 2017).
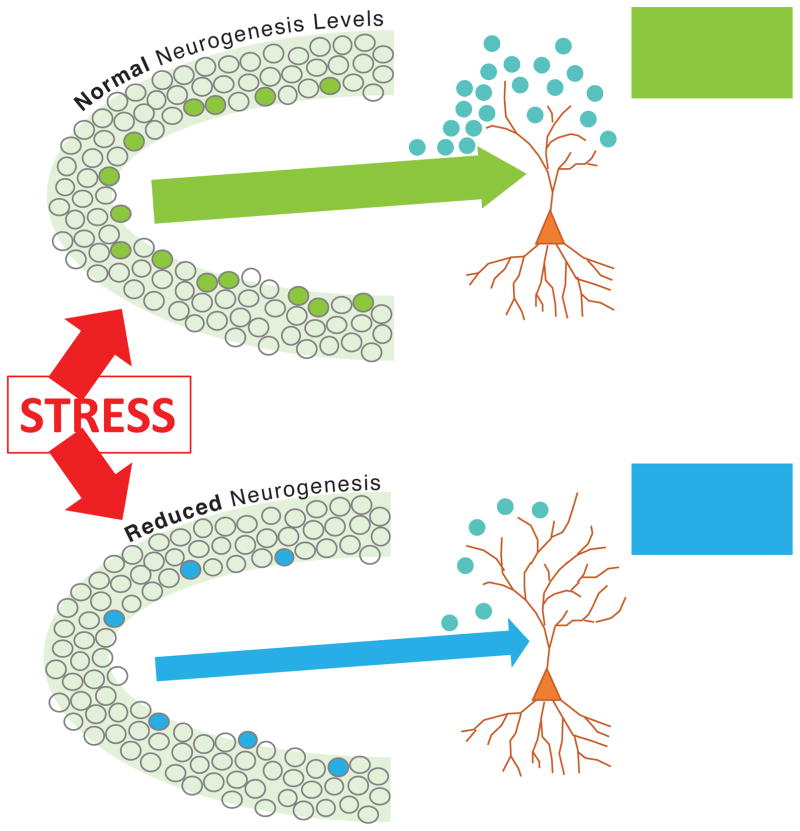
Although it is not known how inhibition of neurogenesis would limit structural change during chronic stress, evidence from several types of studies suggests that the loss of new neurons limits excitability in downstream CA3 pyramidal cells. New neurons release glutamate onto CA3 dendrites through mossy fibers, like other granule cells (Toni et al., 2008), so having fewer granule cells may reduce the total number of glutamatergic synapses onto CA3. In addition, young neurons appear to be more excitable than mature neurons (Mongiat et al., 2009; Schmidt-Hieber et al., 2004), so removal of new neurons might shift the excitability of the dentate gyrus downward. This possibility is supported by work showing that dentate gyrus LTP is reduced following neurogenesis ablation (Saxe et al., 2006; Singer et al., 2011) and that immediate-early gene activation of granule cells and pyramidal cells is lower in mice lacking adult neurogenesis, compared to normal mice, during stressful experience (Glover et al., 2017). Pharmacological studies suggest that reduced CA3 dendritic complexity following stress results from hyperexcitability of the dentate gyrus leading to glutamate excitotoxicity (Watanabe et al., 1992a). A reduction in the number of highly excitable young neurons in the dentate gyrus, then, may serve to buffer the effects of glutamate excitotoxicity on CA3 structure (Fig. 2).
Figure 2.
With adult neurogenesis intact, stress increases activation of the dentate gyrus, leading to increased excitation and glutamate release onto downstream CA3 pyramidal cells, causing excitotoxic dendritic atrophy. When neurogenesis is reduced, due to ongoing stress or other factors, the dentate gyrus may be less activated by stress, causing mossy fiber input to the CA3 pyramidal cells to be buffered, limiting dendritic atrophy.
The time course of stress effects on hippocampal structure has not been extensively studied, but some evidence suggests that the changes may begin much earlier than the time points investigated in most chronic stress experiments. Detectable decreases in hippocampal volume have been observed after only 3 days of restraint stress (Rahman et al., 2016), and CA3 pyramidal cell dendritic spines can be lost within a few hours of stress in vivo (Chen et al., 2010) and glucocorticoids in vitro (Chen et al., 2008). Stress- and glucocorticoid-induced changes in proliferation of granule cell precursors also occur within hours (Schoenfeld and Gould, 2013), though the resulting new neurons are unlikely to be functional for 2–4 weeks (Snyder et al., 2009a). Adult-born neurons generated prior to stress, however, can be rapidly activated by stress (Glover et al., 2017; Schoenfeld et al., 2013) and may contribute to the response differently than mature granule cells.
Ideally, when active stress in the environment dissipates, behavior should rapidly rebound to match the new, safer conditions. Consistent with this, CA3 atrophy reverses to baseline levels within 10 days following the cessation of stress (Conrad et al., 1999). The time course of recovery of behavioral impairments in spatial learning and memory following stress corresponds with that of morphological recovery (Hoffman et al., 2011; Sousa et al., 2000). However, the recovery of neurogenesis following cessation of chronic stress is slower than the normalization of dendritic morphology (Heine et al., 2004). Mice and rats with reduced adult neurogenesis also show weaker recovery of depressive-like behavior following chronic stress in several different paradigms (Levone et al., 2015; Llorens-Martín et al., 2011; Mateus-Pinheiro et al., 2013), suggesting that the very slow return of fear-related behavior to baseline following severe stress (Perusini et al., 2016) may also reflect large or persistent changes in adult neurogenesis.
Conclusions
Taken together, the evidence suggests that neurons produced in the adult dentate gyrus enhance behavioral adaptation to the environment, biasing behavior toward cautious, anxiety- or depressive-like behavior in stressful environments containing unpredictable threats while having the opposite effect, enhancing reward seeking and recovery from stress, in safe environments. The reduction of adult neurogenesis during chronic stress may slow recovery when stress is removed but may also limit and stabilize behavior, protecting against reckless behavior when threats are temporarily absent. Structural changes in the hippocampus follow a similar pattern, with stress shrinking dendritic trees and overall volume and loss of new neurons also leading to decreases in morphology and volume but also possibly limiting the changes brought about by stress. Although decreased adult neurogenesis may limit structural damage and maintain moderate levels of cautious behavior for days to weeks in case threats have not been eliminated, stabilization of hippocampal structure at a reduced size may be expected to impede recovery from inappropriate anxiety and/or depressive behavior in safe environments.
HIGHLIGHTS.
Increased fear generalization may be adaptive in stressful environments.
Adult neurogenesis allows flexible matching of behavior to environmental threat levels.
Chronic stress has several atrophy-like structural effects, but they are reversible.
Decreased adult neurogenesis may limit structural and behavioral effects of stress.
Stabilization of structure/behavior helps if stress continues but may slow recovery.
Acknowledgments
Funding information: This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health [project number ZIAMH002784].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, Bustillo J, Calhoun VD. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry. 2014;4:e483. doi: 10.1038/tp.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Alves ND, Correia JS, Patrício P, Mateus-Pinheiro A, Machado-Santos AR, Loureiro-Campos E, Morais M, Bessa JM, Sousa N, Pinto L. Adult hippocampal neuroplasticity triggers susceptibility to recurrent depression. Transl Psychiatry. 2017;7:e1058. doi: 10.1038/tp.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, McDonald RJ. Discriminative fear conditioning to context expressed by multiple measures of fear in the rat. Behav Brain Res. 1999;101:1–13. doi: 10.1016/s0166-4328(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Sprengel R, Sanderson DJ, Mchugh SB, Rawlins JNP, Monyer H, Seeburg PH. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15:181–192. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Boku S, Nakagawa S, Toda H, Hishimoto A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72:3–12. doi: 10.1111/pcn.12604. https://doi.org/10.1111/pcn.12604. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Hughes CW, McColl R, Peshock R, King KS, Rush AJ. Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacology. 2014a;39:770–779. doi: 10.1038/npp.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Jeon-Slaughter H, Lu H, Jamadar R, Issac S, Shad M, Denniston D, Tamminga C, Nakamura A, Thomas BP. Hippocampal Volume in Healthy Controls Given 3-Day Stress Doses of Hydrocortisone. Neuropsychopharmacology. 2014b doi: 10.1038/npp.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annual review of psychology. 2010;61:27–48. C1–8. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annual review of psychology. 2015;66:53–81. doi: 10.1146/annurev-psych-010814-015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Distinct populations of cells in the adult dentate gyrus undergo mitosis or apoptosis in response to adrenalectomy. J Comp Neurol. 1996;369:56–63. doi: 10.1002/(SICI)1096-9861(19960520)369:1<56::AID-CNE4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Kim J, Khan DA, King K, Lucarelli RT, McColl R, Peshock R, Brown ES. Hippocampal volume in patients with asthma: Results from the Dallas Heart Study. J Asthma. 2017;54:9–16. doi: 10.1080/02770903.2016.1186174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Riva MA. Stress-induced mechanisms in mental illness: A role for glucocorticoid signalling. J Steroid Biochem Mol Biol. 2016;160:169–174. doi: 10.1016/j.jsbmb.2015.07.021. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dubé CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci USA. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci. 2009;30:2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle R, Segawa T, Chupin M, Tran Dong MNTK, Hardy P, Falissard B, Colliot O, Ducreux D, Corruble E. Early life adversity is associated with a smaller hippocampus in male but not female depressed in-patients: a case-control study. BMC Psychiatry. 2017;17:71. doi: 10.1186/s12888-017-1233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Ledoux JE, Magariños AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Curlik DM, Maeng LY, Agarwal PR, Shors TJ. Physical skill training increases the number of surviving new cells in the adult hippocampus. PLoS ONE. 2013;8:e55850. doi: 10.1371/journal.pone.0055850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlik DM, Shors TJ. Learning increases the survival of newborn neurons provided that learning is difficult to achieve and successful. J Cogn Neurosci. 2011;23:2159–2170. doi: 10.1162/jocn.2010.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? European archives of psychiatry and clinical neuroscience. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Czéh B, Müller-Keuker JIH, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Dagyte G, Van der Zee EA, Postema F, Luiten PGM, Den Boer JA, Trentani A, Meerlo P. Chronic but not acute foot-shock stress leads to temporary suppression of cell proliferation in rat hippocampus. Neuroscience. 2009;162:904–913. doi: 10.1016/j.neuroscience.2009.05.053. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan BD, Stouffer EM, Petri HL, McDonald RJ, Olds JL. Partial reinforcement across trials impairs escape performance but spares place learning in the water maze. Behav Brain Res. 2003;141:91–104. doi: 10.1016/s0166-4328(02)00294-2. [DOI] [PubMed] [Google Scholar]
- Dimitrov EL, Tsuda MC, Cameron HA, Usdin TB. Anxiety- and Depression-Like Behavior and Impaired Neurogenesis Evoked by Peripheral Neuropathy Persist following Resolution of Prolonged Tactile Hypersensitivity. J Neurosci. 2014;34:12304–12312. doi: 10.1523/JNEUROSCI.0312-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Quiñones-Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519:460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Paz R. Fear Generalization and Anxiety: Behavioral and Neural Mechanisms. Biol Psychiatry. 2015;78:336–343. doi: 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Covington HE. Increasing the validity of experimental models for depression. Ann N Y Acad Sci. 2012;1265:36–45. doi: 10.1111/j.1749-6632.2012.06669.x. [DOI] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2012;22:82–91. doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Eker MC, Kitis O, Okur H, Eker OD, Ozan E, Isikli S, Akarsu N, Gonul AS. Smaller hippocampus volume is associated with short variant of 5-HTTLPR polymorphism in medication-free major depressive disorder patients. Neuropsychobiology. 2011;63:22–28. doi: 10.1159/000321834. https://doi.org/10.1159/000321834. [DOI] [PubMed] [Google Scholar]
- Epp JR, Haack AK, Galea LAM. Task difficulty in the Morris water task influences the survival of new neurons in the dentate gyrus. Hippocampus. 2010;20:866–876. doi: 10.1002/hipo.20692. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jäger M, Leinsinger G, Bottlender R, Hahn K, Möller HJ. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gazzara RA, Altman J. Early postnatal x-irradiation of the hippocampus and discrimination learning in adult rats. J Comp Physiol Psychol. 1981;95:484–495. doi: 10.1037/h0077783. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci. 2015;18:112–120. doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- Glasper ER, Schoenfeld TJ, Gould E. Adult neurogenesis: optimizing hippocampal function to suit the environment. Behav Brain Res. 2012;227:380–383. doi: 10.1016/j.bbr.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Glover LR, Schoenfeld TJ, Karlsson RM, Bannerman DM, Cameron HA. Ongoing neurogenesis in the adult dentate gyrus mediates behavioral responses to ambiguous threat cues. PLoS Biol. 2017;15:e2001154. doi: 10.1371/journal.pbio.2001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev. 2005;81:51–59. doi: 10.1016/j.earlhumdev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gold PW, Machado-Vieira R, Pavlatou MG. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast. 2015;2015:581976. doi: 10.1155/2015/581976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CL, Kolb B, Whishaw IQ. A cautionary note regarding drug and brain lesion studies that use swimming pool tasks: partial reinforcement impairs acquisition of place learning in a swimming pool but not on dry land. Behav Brain Res. 2000;112:43–52. doi: 10.1016/s0166-4328(00)00162-5. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the function of the septo-hippocampal system. 2. Oxford University Press; 2000. [Google Scholar]
- Groves JO, Leslie I, Huang G-J, Mchugh SB, Taylor A, Mott R, Munafo M, Bannerman DM, Flint J. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genet. 2013;9:e1003718. doi: 10.1371/journal.pgen.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Janoschka S, Ge S. Neurogenesis and Hippocampal Plasticity in Adult Brain. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2012_217. [DOI] [PubMed] [Google Scholar]
- Hawley DF, Morch K, Christie BR, Leasure JL. Differential Response of Hippocampal Subregions to Stress and Learning. PLoS ONE. 2012;7:e53126. doi: 10.1371/journal.pone.0053126.g004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joëls M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Krigbaum A, Ortiz JB, Mika A, Hutchinson KM, Bimonte-Nelson HA, Conrad CD. Recovery after chronic stress within spatial reference and working memory domains: correspondence with hippocampal morphology. Eur J Neurosci. 2011;34:1023–1030. doi: 10.1111/j.1460-9568.2011.07820.x. [DOI] [PubMed] [Google Scholar]
- Hvoslef-Eide M, Oomen CA. Adult neurogenesis and pattern separation in rodents: A critical evaluation of data, tasks and interpretation. Frontiers in Biology. 2016 doi: 10.1007/s11515-016-1406-2. [DOI] [Google Scholar]
- Joelving FC, Billeskov R, Christensen JR, West M, Pakkenberg B. Hippocampal neuron and glial cell numbers in Parkinson’s disease--a stereological study. Hippocampus. 2006;16:826–833. doi: 10.1002/hipo.20212. [DOI] [PubMed] [Google Scholar]
- Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, Leaver A, Woods RP, Narr KL. Structural Plasticity of the Hippocampus and Amygdala Induced by Electroconvulsive Therapy in Major Depression. Biol Psychiatry. 2016;79:282–292. doi: 10.1016/j.biopsych.2015.02.029. https://doi.org/10.1016/j.biopsych.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, De Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kronmüller KT, Pantel J, Köhler S, Victor D, Giesel F, Magnotta VA, Mundt C, Essig M, Schröder J. Hippocampal volume and 2-year outcome in depression. Br J Psychiatry. 2008;192:472–473. doi: 10.1192/bjp.bp.107.040378. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Jarome T, Li SJ, Kim JJ, Helmstetter FJ. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport. 2009;20:1554–1558. doi: 10.1097/WNR.0b013e328332bb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levone BR, Cryan JF, O’Leary OF. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol Stress. 2015;1:147–155. doi: 10.1016/j.ynstr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depress Anxiety. 2012;29:257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martín M, Tejeda GS, Trejo JL. Antidepressant and proneurogenic influence of environmental enrichment in mice: protective effects vs recovery. Neuropsychopharmacology. 2011;36:2460–2468. doi: 10.1038/npp.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Cao Z, Wang D, Wu L, Li Y, Sun W, Zhu Y. Dynamic study of the hippocampal volume by structural MRI in a rat model of depression. Neurol Sci. 2014;35:1777–1783. doi: 10.1007/s10072-014-1837-y. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. Neuroimage. 2007;34:479–485. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Luyten L, Vansteenwegen D, van Kuyck K, Gabriëls L, Nuttin B. Contextual conditioning in rats as an animal model for generalized anxiety disorder. Cogn Affect Behav Neurosci. 2011;11:228–244. doi: 10.3758/s13415-011-0021-6. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, McEwen BS. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Broadhouse K, Rush AJ, Gordon E, Koslow S, Grieve SM. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.224. [DOI] [PubMed] [Google Scholar]
- Mateus-Pinheiro A, Pinto L, Bessa JM, Morais M, Alves ND, Monteiro S, Patrício P, Almeida OFX, Sousa N. Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Transl Psychiatry. 2013;3:e210. doi: 10.1038/tp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall T, Weil ZM, Nacher J, Bloss EB, El Maarouf A, Rutishauser U, McEwen BS. Depletion of polysialic acid from neural cell adhesion molecule (PSA-NCAM) increases CA3 dendritic arborization and increases vulnerability to excitotoxicity. Experimental Neurology. 2013;241:5–12. doi: 10.1016/j.expneurol.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gray J, Nasca C. Recognizing Resilience: Learning from the Effects of Stress on the Brain. Neurobiol Stress. 2015a;1:1–11. doi: 10.1016/j.ynstr.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala and Prefrontal Cortex. Neuropsychopharmacology. 2015b doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNish KA, Gewirtz JC, Davis M. Evidence of contextual fear after lesions of the hippocampus: a disruption of freezing but not fear-potentiated startle. J Neurosci. 1997;17:9353–9360. doi: 10.1523/JNEUROSCI.17-23-09353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mongiat LA, Espósito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS ONE. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S. Stress-Induced Hippocampal Volume Loss Is Adult Neurogenesis Independent. Biol Psychiatry. 2017;82:e91–e93. doi: 10.1016/j.biopsych.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34:853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Onat S, Büchel C. The neuronal basis of fear generalization in humans. Nat Neurosci. 2015;18:1811–1818. doi: 10.1038/nn.4166. [DOI] [PubMed] [Google Scholar]
- Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress (Amsterdam, Netherlands) 2011;14:227–232. doi: 10.3109/10253890.2010.522279. [DOI] [PubMed] [Google Scholar]
- Perusini JN, Meyer EM, Long VA, Rau V, Nocera N, Avershal J, Maksymetz J, Spigelman I, Fanselow MS. Induction and Expression of Fear Sensitization Caused by Acute Traumatic Stress. Neuropsychopharmacology. 2016;41:45–57. doi: 10.1038/npp.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pinto V, Costa JC, Morgado P, Mota C, Miranda A, Bravo FV, Oliveira TG, Cerqueira JJ, Sousa N. Differential impact of chronic stress along the hippocampal dorsal-ventral axis. Brain structure & function. 2014 doi: 10.1007/s00429-014-0713-0. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O’Mara SM. Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep. 2016;6:29127. doi: 10.1038/srep29127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD. Vicarious trial and error. Nat Rev Neurosci. 2016;17:147–159. doi: 10.1038/nrn.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reser JE. Chronic stress, cortical plasticity and neuroecology. Behav Processes. 2016;129:105–115. doi: 10.1016/j.beproc.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Romeo RD. The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Res. 2017;1654:185–191. doi: 10.1016/j.brainres.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Ross JA, Gliebus G, Van Bockstaele EJ. Stress induced neural reorganization: A conceptual framework linking depression and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2017 doi: 10.1016/j.pnpbp.2017.08.004. [DOI] [PMC free article] [PubMed]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Santoro A. Reassessing pattern separation in the dentate gyrus. Front Behav Neurosci. 2013;7:96. doi: 10.3389/fnbeh.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Differential effects of stress and glucocorticoids on adult neurogenesis. Curr Top Behav Neurosci. 2013;15:139–164. doi: 10.1007/7854_2012_233. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, Cameron HA. Stress and Loss of Adult Neurogenesis Differentially Reduce Hippocampal Volume. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J Neurosci. 2013;33:7770–7777. doi: 10.1523/JNEUROSCI.5352-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR. Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. J Neurosci. 2015;35:11330–11345. doi: 10.1523/JNEUROSCI.0483-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servatius RJ, Shors TJ. Exposure to inescapable stress persistently facilitates associative and nonassociative learning in rats. Behav Neurosci. 1994;108:1101–1106. doi: 10.1037//0735-7044.108.6.1101. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Sindi S, Fiocco AJ, Juster R-P, Lord C, Pruessner J, Lupien SJ. Now you see it, now you don’t: Testing environments modulate the association between hippocampal volume and cortisol levels in young and older adults. Hippocampus. 2014 doi: 10.1002/hipo.22341. [DOI] [PubMed] [Google Scholar]
- Singer BH, Gamelli AE, Fuller CL, Temme SJ, Parent JM, Murphy GG. Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice. Proc Natl Acad Sci USA. 2011;108:5437–5442. doi: 10.1073/pnas.1015425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009a;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009b;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Piras F, Caltagirone C, Fagioli S. Hippocampal multimodal structural changes and subclinical depression in healthy individuals. J Affect Disord. 2014;152–154:105–112. doi: 10.1016/j.jad.2013.05.068. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan AA, Clutton JE, Chary PK, Cook SG, Liu GG, Drew MR. Characterization of the role of adult neurogenesis in touch-screen discrimination learning. Hippocampus. 2014;24:1581–1591. doi: 10.1002/hipo.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam EC, Choi JW, Park JI. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR American journal of neuroradiology. 2011;32:671–676. doi: 10.3174/ajnr.A2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafet GE, Nemeroff CB. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J Neuropsychiatry Clin Neurosci. 2016;28:77–88. doi: 10.1176/appi.neuropsych.15030053. [DOI] [PubMed] [Google Scholar]
- Tanti A, Westphal WP, Girault V, Brizard B, Devers S, Leguisquet AM, Surget A, Belzung C. Region-dependent and stage-specific effects of stress, environmental enrichment, and antidepressant treatment on hippocampal neurogenesis. Hippocampus. 2013;23:797–811. doi: 10.1002/hipo.22134. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truax T, Thompson R. Role of the hippocampus in performance of easy and difficult visual discrimination tasks. J Comp Physiol Psychol. 1969;67:228–234. doi: 10.1037/h0026763. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992b;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am J Psychiatry. 2004;161:2194–2200. doi: 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci USA. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]