Abstract
Background
Diabetes mellitus is associated with left ventricular hypertrophy and dysfunction. Parallel studies have also reported associations between diabetes mellitus and right ventricle dysfunction and reduced survival in patients with pulmonary arterial hypertension. However, the impact of diabetes mellitus on the pulmonary vasculature has not been well-characterized. We hypothesized that diabetes mellitus and hyperglycemia could specifically influence right ventricular afterload and remodeling in patients with Group I pulmonary arterial hypertension, providing a link to their known susceptibility to right ventricular dysfunction.
Methods
Using an adjusted model for age, gender, pulmonary vascular resistance, and medication use, associations of fasting blood glucose, glycated hemoglobin, and the presence of diabetes mellitus were evaluated with markers of disease severity in 162 patients with pulmonary arterial hypertension.
Results
A surrogate measure of increased pulmonary artery stiffness, elevated pulmonary arterial elastance (P=0.012), along with reduced log(pulmonary artery capacitance) (P=0.006) were significantly associated with the presence of diabetes mellitus in patients with pulmonary arterial hypertension in a fully adjusted model. Similar associations between pulmonary arterial elastance and capacitance were noted with both fasting blood glucose and glycated hemoglobin. Furthermore, right ventricular wall thickness on echocardiography was greater in pulmonary arterial hypertension patients with diabetes, supporting the link between right ventricular remodeling and diabetes.
Conclusion
Cumulatively, these data demonstrate that an increase in right ventricular afterload, beyond pulmonary vascular resistance alone, may influence right ventricular remodeling and provide a mechanistic link between the susceptibility to right ventricular dysfunction in patients with both diabetes mellitus and pulmonary arterial hypertension.
Keywords: diabetes mellitus, pulmonary arterial hypertension, pulmonary arterial elastance, pulmonary arterial capacitance
INTRODUCTION
Pulmonary hypertension is defined by an increase in the pressure within the pulmonary circulation. Many conditions are associated with the development of pulmonary hypertension, and there are currently five subgroups that attempt to classify these etiologies.1, 2 Group 1 pulmonary arterial hypertension is characterized by progressive pathological remodeling of the pulmonary vasculature3–5 resulting in both an elevated mean pulmonary artery pressure (≥25 mmHg) and pulmonary vascular resistance (>3 Woods units), in the context of normal left heart filling pressures. Despite recent advances in treatment, there are currently no cures for pulmonary arterial hypertension.6
The morbidity and mortality associated with pulmonary arterial hypertension is largely due to right heart failure. Initial increases in pulmonary vascular resistance and right ventricular afterload due to pulmonary arterial hypertension result in right ventricular hypertrophy as a compensatory adaptation.7, 8 However, similar to long-standing systemic arterial hypertension that results in left ventricular hypertrophy and eventual left heart failure,9 sustained elevations in pulmonary vascular resistance and total right ventricular afterload can eventually lead to right ventricular dilation and failure.
Diabetes mellitus is well-known to contribute to both left ventricular dysfunction and systemic vascular remodeling. Specifically, diabetes mellitus has been shown to be associated with increased left ventricular hypertrophy and systemic vascular resistance.10 Proposed mechanisms behind these associations include endothelial dysfunction leading to unopposed vasoconstriction, increased arterial tone, and increased risk of atherosclerosis.11 Diabetes mellitus has also been reported to negatively affect the left ventricular muscle directly, resulting in increased stiffness and impaired relaxation.12 Increasing evidence indicates that glucose intolerance, insulin resistance, and metabolic syndrome also influence the pathogenesis and prognosis of pulmonary arterial hypertension and the development of right ventricular failure.13–21 Unrecognized glucose intolerance was shown to be common in pulmonary arterial hypertension, though its temporality in the development of pulmonary arterial hypertension is still uncertain.15, 19 Additionally, increasing evidence indicates that comorbid diabetes mellitus may also reduce right ventricular function in patients with pulmonary arterial hypertension and negatively impact their survival.22 However, it is not clear how the presence of diabetes mellitus influences right ventricular structure and function or afterload.
Based on these established observations of left ventricular and systemic vascular complications, we hypothesized that the presence of hyperglycemia and diabetes mellitus may also influence pulmonary artery stiffness and right ventricular remodeling in patients with Group 1 pulmonary arterial hypertension, providing a link to their known susceptibility to right ventricular dysfunction. To test this hypothesis, the associations of fasting blood glucose, glycated hemoglobin, and the presence of diabetes mellitus were evaluated with markers of pulmonary arterial hypertension severity in a cohort of Group I pulmonary arterial hypertension patients.
MATERIALS AND METHODS
Study Design and Patient Population
A chart review was performed on a cohort of patients seen at The University of Arizona Pulmonary Hypertension Clinic with Group 1 pulmonary arterial hypertension (confirmed by right heart catheterization) between 12/2011 and 1/2016. This study was approved by The University of Arizona institutional human subjects review board (#1502660424). Written informed consent was obtained from all patients. Diabetic status was determined by either documented clinical diagnosis or treatment with anti-diabetic medications and all diabetic patients included in the study were diagnosed with type 2 diabetes mellitus. Demographic data and results from clinical testing for pulmonary arterial hypertension, including echocardiography, right heart catheterization, and six-minute walk distance, were acquired from medical records and were selected based on testing completed closest to the date of right heart catheterization. All right heart catheterizations were performed by a single primary pulmonary hypertension provider and operator, and cardiac output was measured by thermodilution. Pulmonary arterial capacitance was defined from right heart catheterization as stroke volume (mL)/[pulmonary artery systolic pressure - pulmonary artery diastolic pressure (mm Hg)] and effective pulmonary arterial elastance was estimated by pulmonary artery systolic pressure (mm Hg)/stroke volume (mL).23 Echocardiography parameters were assessed using American Society of Echocardiography criteria24 including right ventricular wall thickness (right ventricular lateral wall) measured by a board-certified cardiologist. Other data, including the use of pulmonary arterial hypertension-specific medications (at time of right heart catheterization) and laboratory testing (such as fasting blood glucose, high-density lipoprotein, low-density lipoprotein, and glycated hemoglobin levels, closest to right heart catheterization date), were also acquired from the electronic medical records when available.
Statistical Analysis
Strength and direction of the linear relationships between variables were performed using Pearson’s correlation. Univariate as well as multivariate analyses were performed. Adjustment was performed to control for gender, age, indexed pulmonary vascular resistance, pulmonary arterial hypertension-specific medication use, and pulmonary artery occlusion pressure (for analyses on pulmonary arterial capacitance and pulmonary arterial elastance). For right ventricular wall thickness analysis, the data were additionally adjusted for left ventricular posterior wall end diastolic diameter and interventricular septal end diastolic diameter. Log transformations were performed for any dataset that did not follow normal distribution. A p-value of less than 0.05 was considered significant. All analyses were performed using R25 statistical software. Additional R packages used in the analyses were ggplot2,26 gdata,27 reshape2,28 gtable,29 and stargazer.30
RESULTS
Cohort Characteristics
The study cohort included 162 patients with Group 1 pulmonary arterial hypertension, 27 of whom were diagnosed with type 2 diabetes mellitus (16.7%). Demographic data for the cohort is shown in Table 1. While there were more males in the diabetes mellitus subgroup, the non-diabetes mellitus group was composed of predominantly female subjects (P<0.0001) and reflected the typical gender predisposition in pulmonary arterial hypertension.15 As would be expected, body mass index (P=0.005), glycated hemoglobin (P<0.001), and fasting blood glucose (P<0.001) were also significantly higher in diabetic compared to non-diabetic patients. While there were no significant differences in mean pulmonary artery pressure or pulmonary vascular resistance, pulmonary arterial compliance was reduced and pulmonary arterial elastance was increased significantly in patients with diabetes mellitus. Both increased right ventricular wall thickness and reduced six-minute walk distance demonstrated trends toward differences between those with and without diabetes mellitus. These select parameters were then further assessed for differences in a multivariate model.
Table 1.
Demographics and clinical characteristics of the University of Arizona cohort.
| Diabetic (n=27) |
Non-diabetic (n=135) |
P value | |
|---|---|---|---|
| Age on enrollment (years) | 62.2 ± 11.3 | 58.7 ± 13.1 | 0.165 |
| Gender (F/M) | 10/17 | 114/21 | <0.0001 |
| Hispanic Ethnicity (n) | 4 | 21 | 1 |
| Body mass index (kg/m2) | 33.8 ± 8.4 | 28.6 ± 7.5 | 0.005 |
| Glycated hemoglobin (%) | 6.5 ± 0.9 (n=19) | 5.5 ± 0.4 (n=30) | <0.001 |
| Fasting blood glucose (mg/dL, n=157) | 132.5 ± 42.8 | 91.8 ± 8.8 | <0.001 |
| Systolic blood pressure (mmHg) | 128.8 ± 16.1 | 120.4 ± 17.2 | 0.019 |
| Diastolic blood pressure (mmHg) | 74.2 ± 13.4 | 71.6 ± 12.2 | 0.375 |
| High-density lipoprotein (mg/dL) | 42.4 ± 17.0 | 46.2 ± 18.3 | 0.562 |
| Low-density lipoprotein (mg/dL) | 96.4 ± 31.1 | 90.5 ± 34.3 | 0.613 |
|
Echocardiography Variables | |||
| Tricuspid annular plane systolic excursion (cm) | 3.22 ± 4.39 | 2.06 ± 0.51 | 0.2 |
| Right ventricular wall thickness (cm) | 0.91 ± 0.21 | 0.77 ± 0.21 | 0.06 |
| Left ventricular ejection fraction (%) | 61.8 ± 9.7 | 63.2 ± 6.5 | 0.513 |
| Left ventricular posterior wall diameter (cm) | 1.10 ± 0.19 | 1.03 ± 0.23 | 0.119 |
|
Right Heart Catheterization Parameters | |||
| Heart Rate (1/min) | 79.4 ± 14.9 | 83.7 ± 14.2 | 0.091 |
| Mean right atrial pressure (mmHg) | 8.8 ± 3.8 | 7.8 ± 5.6 | 0.285 |
| Mean pulmonary artery pressure (mmHg) | 42.4 ± 14.9 | 36.8 ± 16.0 | 0.067 |
| Pulmonary artery systolic pressure (mmHg) | 70.7 ± 25.0 | 60.9 ± 25.0 | 0.110 |
| Pulmonary artery diastolic pressure (mmHg) | 26.5 ± 10.9 | 24.2 ± 11.7 | 0.330 |
| Pulmonary capillary wedge pressure (mmHg) | 10.5 ± 3.6 | 10.0 ± 4.2 | 0.487 |
| Pulmonary artery saturation (%) | 67.8 ± 6.7 | 68.7 ± 7.2 | 0.572 |
| Pulmonary vascular resistance (Woods unit) | 5.68 ± 2.37 | 5.54 ± 3.91 | 0.804 |
| Indexed pulmonary vascular resistance (Woods unit.m2) | 2.8 ± 1.3 | 3.1 ± 2.3 | 0.533 |
| Cardiac output (L/min) | 5.88 ± 1.31 | 5.75 ± 1.64 | 0.650 |
| Cardiac index (L/min/m2) | 2.97 ± 0.80 | 3.16 ± 0.95 | 0.300 |
| Stroke Volume (mL) | 72.4 ± 16.8 | 78.4 ± 24.3 | 0.15 |
| Pulmonary artery capacitance (mL/mmHg) | 1.82 ± 0.84 | 2.79 ± 2.00 | <0.001 |
| Pulmonary artery elastance (mmHg/mL) | 1.04 ± 0.39 | 0.86 ± 0.52 | 0.05 |
|
Functional Outcomes | |||
| Six-minute walk distance (m) | 289.8 ± 117.8 | 339.4 ± 136.1 | 0.095 |
Diabetes Mellitus and Pulmonary Arterial Hypertension Severity
Indices of pulmonary arterial hypertension severity as measured by right heart catheterization and echocardiography in patients with and without diabetes mellitus are displayed in Table 1. There were no significant differences across the variables for measures of severity of pulmonary arterial hypertension except for derived measures of log(pulmonary artery capacitance) (P=0.001) and pulmonary arterial elastance (P=0.05) on right heart catheterization. There was a trend towards significance of univariate right ventricular wall thickness (P=0.06) in patients with diabetes mellitus versus without diabetes mellitus.
In multivariate regression analysis (Table 2), right ventricular wall thickness remained significantly increased in patients with diabetes mellitus as compared to those without diabetes mellitus (coefficient 0.18 cm, P=0.034) in a fully adjusted model. Diabetic patients also exhibited significantly reduced log(pulmonary artery capacitance) (0.262 mL/mmHg, P=0.006) and increased pulmonary arterial elastance (0.117 mmHg/mL, P=0.012). While six-minute walk distance was reduced in patients with diabetes mellitus, it was not significant in univariate analysis (−49.6 m, P=0.122) or after multivariate regression analysis (−28.3 m, P=0.379).
Table 2.
Comparison of pulmonary arterial hypertension severity measures stratified by diabetes mellitus status.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Coefficient | P value | n | Coefficient | P value | |
| Right ventricular wall thickness | 72 | 0.14 | 0.05 | 53 | 0.18 | 0.034 |
| Log(pulmonary artery capacitance) | 137 | −0.371 | 0.007 | 137 | −0.262 | 0.006 |
| Pulmonary artery elastance | 142 | 0.199 | 0.063 | 142 | 0.117 | 0.012 |
| Pulmonary artery saturation | 151 | −0.9 | 0.538 | 147 | 0.0 | 0.999 |
| Six-minute walk distance | 129 | −49.6 | 0.122 | 123 | −28.3 | 0.379 |
Table 3 reveals both correlation coefficient values and their significance of association between pulmonary arterial hypertension severity measures and indices of hyperglycemia (glycated hemoglobin and fasting blood glucose levels). Figure 1 illustrates moderate and significant linear relationships between pulmonary arterial capacitance, pulmonary arterial elastance, and pulmonary artery saturation and glycated hemoglobin levels in the cohort. Similarly, significant but weaker associations were evident between fasting blood glucose levels and pulmonary artery saturation and six-minute walk distance including trends toward associations with right ventricular wall thickness.
Table 3.
Correlation between hyperglycemic indices and pulmonary arterial hypertension severity measures in the University of Arizona cohort.
| n | r | P value | |
|---|---|---|---|
| Glycated Hemoglobin | |||
|
| |||
| Indexed pulmonary vascular resistance | 47 | 0.202 | 0.174 |
| Right ventricular wall thickness | 23 | 0.275 | 0.204 |
| Pulmonary artery capacitance | 42 | −0.384 | 0.020 |
| Pulmonary artery elastance | 42 | 0.358 | 0.012 |
| Pulmonary artery saturation | 44 | −0.401 | 0.007 |
| Six-minute walk distance | 36 | −0.177 | 0.302 |
|
Fasting Blood Glucose | |||
| Indexed pulmonary vascular resistance | 152 | 0.098 | 0.231 |
| Right ventricular wall thickness | 72 | 0.225 | 0.058 |
| Pulmonary artery capacitance | 137 | −0.143 | 0.095 |
| Pulmonary artery elastance | 142 | 0.121 | 0.152 |
| Pulmonary artery saturation | 148 | −0.171 | 0.038 |
| Six-minute walk distance | 125 | −0.177 | 0.048 |
Figure 1. Correlation plots between indices of hyperglycemia and measures of pulmonary arterial hypertension severity.
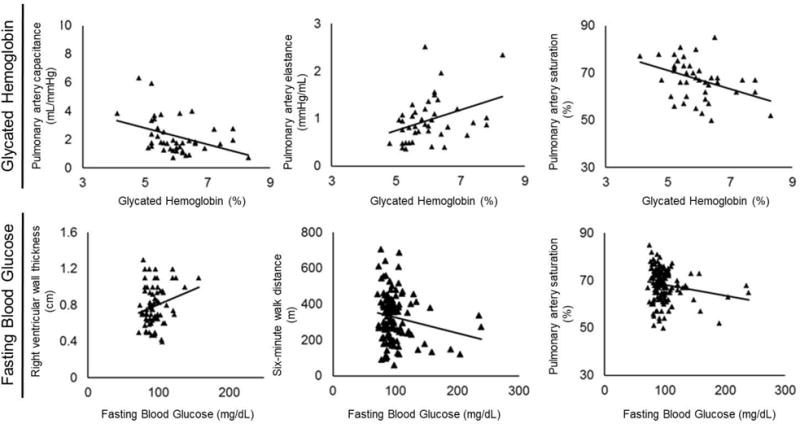
The figures below depict the significant associations between glycated hemoglobin levels and pulmonary arterial capacitance, pulmonary arterial elastance, as well as pulmonary artery saturation in the pulmonary arterial hypertension cohort. Similarly, significant but weaker associations are evident between fasting blood glucose levels and pulmonary artery saturation and six-minute walk distance including trends toward associations with right ventricular wall thickness.
Glycated Hemoglobin and Pulmonary Arterial Hypertension Severity
Measures of pulmonary arterial hypertension severity were similarly assessed to determine their association with glycated hemoglobin values (Table 4). Measures of right ventricular afterload were also increased, with higher pulmonary arterial elastance (0.152 mmHg/mL, P=0.0005) in a fully adjusted model and lower log(pulmonary artery capacitance), significant in univariate analysis (−0.236 mL/mmHg, P=0.009). Pulmonary artery saturation was also significantly associated with glycated hemoglobin levels in univariate analysis (−3.9%, P=0.006) but was no longer significant in multivariate analysis (−2.2%, P=0.155).
Table 4.
Association of glycated hemoglobin with measures of pulmonary arterial hypertension severity.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Coefficient | P value | n | Coefficient | P value | |
| Right ventricular wall thickness | 23 | 0.08 | 0.204 | 20 | 0.16 | 0.094 |
| Log(pulmonary artery capacitance) | 41 | −0.236 | 0.009 | 41 | −0.114 | 0.151 |
| Pulmonary artery elastance | 41 | 0.224 | 0.006 | 41 | 0.152 | 0.0005 |
| Pulmonary artery saturation | 43 | −3.9 | 0.006 | 41 | −2.2 | 0.155 |
| Six-minute walk distance | 36 | −23.7 | 0.303 | 33 | −4.5 | 0.873 |
Fasting Blood Glucose and Pulmonary Arterial Hypertension Severity
Finally, measures of pulmonary arterial hypertension severity were similarly assessed to determine their association with fasting blood glucose levels (Table 5). Right ventricular wall thickness, which revealed a significant association with diabetes mellitus and a trend with glycated hemoglobin in multivariate analyses, again demonstrated a significant difference (0.006 cm, P=0.005) in a fully adjusted model. Similar to the observations noted for diabetes mellitus and glycated hemoglobin, right ventricular afterload was also higher. This was demonstrated by an increase in pulmonary arterial elastance (0.001 mmHg/mL, P=0.04). A significant reduction in log(pulmonary artery capacitance) was also observed during univariate analysis (−0.004 mL/mmHg, P=0.039), which remained trending in a fully adjusted model (−0.002 mL/mmHg, P=0.124). As was shown with glycated hemoglobin, pulmonary artery saturation was also significantly associated with fasting blood glucose in univariate analysis (−0.053%, P=0.024) and a trend was nearly significant in multivariate analysis (−0.045%, P=0.054). Six-minute walk distance showed a difference by univariate analysis (−0.88 m, P=0.049) but the significance was lost in multivariate analysis (−0.52 m, P=0.245).
Table 5.
Association of fasting blood glucose with measures of pulmonary arterial hypertension severity.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Coefficient | P value | n | Coefficient | P value | |
| Right ventricular wall thickness | 72 | 0.003 | 0.058 | 53 | 0.006 | 0.005 |
| Log(pulmonary artery capacitance) | 136 | −0.004 | 0.039 | 136 | −0.002 | 0.124 |
| Pulmonary artery elastance | 141 | 0.003 | 0.095 | 141 | 0.001 | 0.04 |
| Pulmonary artery saturation | 147 | −0.05 | 0.024 | 144 | −0.05 | 0.054 |
| Six-minute walk distance | 125 | −0.89 | 0.049 | 119 | −0.52 | 0.245 |
DISCUSSION
In the current study, the presence of diabetes mellitus was associated with measures of increased pulmonary artery stiffness, including reduced pulmonary arterial capacitance and increased pulmonary arterial elastance along with right ventricular remodeling (hypertrophy), in patients with Group I pulmonary arterial hypertension. These findings were further supported by observing parallel changes in elastance and right ventricular hypertrophy with two additional diabetic parameters including levels of fasting blood glucose and glycated hemoglobin. Finally, trends toward reduced pulmonary artery saturation and six-minute walk distance associations with diabetes mellitus and the degree of hyperglycemia suggest susceptibility to manifestations of right heart failure and poor functional outcomes in pulmonary arterial hypertension patients.
Measurements of pulmonary arterial capacitance and elastance in this study were estimated by right heart catheterization. While total right ventricular afterload is defined by pulmonary input impedance, the Windkessel model has been shown to estimate right ventricular afterload and is composed of pulmonary artery: resistance, characteristic impedance, and compliance.31 While pulmonary vascular resistance is routinely used to describe total right ventricular afterload, it accounts for only about 75% of right ventricular afterload32 and does not incorporate the pulsatile component. Input impedance of the proximal pulmonary arteries is also a relatively small component of right ventricular afterload. Therefore, the role of pulmonary artery compliance is significant as it reflects the pulsatile afterload and accounts for approximately one-fourth of the total right ventricular afterload.32 Both increased pulmonary arterial elastance and reduced pulmonary arterial capacitance have been shown to be associated with pulmonary arterial hypertension including right ventricular dysfunction and associated poor outcomes.33–35 In the current study, these measures of pulmonary artery stiffness were significantly associated with both diabetes mellitus and granular measures of hyperglycemia. These data provide for the first time an association between hyperglycemia and increased right ventricular afterload which is independent of pulmonary vascular resistance. These data parallel known effects of diabetes mellitus and hyperglycemia on the stiffness of systemic vasculature and the development of hypertension.36, 37 These data therefore highlight the need to further study whether diabetes mellitus causally impacts both vascular cell proliferation/remodeling as well as vascular stiffness independently, including consideration of changes in metabolism and endothelial cell dysfunction.
Several studies have attempted to address the roles of aberrant glucose metabolism in the right ventricle and within the pulmonary vasculature in the pathogenesis of pulmonary arterial hypertension.13–21 Specifically, it is hypothesized that early in the development of pulmonary arterial hypertension, a mitochondrial metabolic shift occurs including aerobic glycolysis and reduced glucose oxidation within the pulmonary circulation and the right ventricle, resulting in inefficient glucose utilization and resulting in the development of maladaptive right ventricular hypertrophy.13, 20, 21 However, it is unknown to what extent the presence of diabetes mellitus or hyperglycemia contributes to this metabolic derangement and importantly to the pulmonary vascular or right ventricular remodeling. This study shows for the first time that the presence of diabetes may play a role beyond the development of hyperproliferative vascular remodeling resulting in rising pulmonary vascular resistance alone and may also contribute to increases in vascular stiffness. While the molecular mechanisms of this association are unclear, a recent study performed in diabetic mice demonstrated further attenuated endothelium-dependent relaxation in pulmonary arteries of chronically hypoxic diabetic mice18 compared to control mice. The study further proposed a novel molecular mechanism involving the role of mitochondria in generating excessive amounts of reactive oxygen species in pulmonary endothelial cells isolated from diabetic mice, resulting in abnormal endothelium-dependent pulmonary artery relaxation and worsening pulmonary hypertension in diabetes.18 Whether roles of mitochondrial reactive oxygen species and the endothelium may also extend in modifying pulmonary artery stiffness in pulmonary arterial hypertension with diabetes mellitus represents a source for further investigation.
Current observations also highlight the association of diabetes mellitus and hyperglycemia on right ventricular remodeling in Group I pulmonary arterial hypertension. Specifically, the effects of diabetes mellitus and hyperglycemia on vascular stiffness may add another layer of contribution to right ventricular afterload leading to the observation of increased right ventricular wall thickness. Alternatively, diabetes mellitus and hyperglycemia may have effects on right ventricular muscle itself independent of the pulmonary vascular afterload. In fact, changes to right ventricular remodeling remained significant in the study despite adjusting for markers of left ventricular wall thickness. These data then begin to provide possible mechanisms for the previously reported decrease in right ventricular function (demonstrated by decreased right ventricular stroke work index) and increase in mortality among patients with pulmonary arterial hypertension and comorbid diabetes mellitus.22 Consistent with this claim, the current work demonstrates decreased trends in pulmonary artery saturation and six-minute walk distance with hyperglycemia, indicative of worsening right ventricular function.
A lack of statistical significance of these particular findings (functional outcomes and right ventricular function) in an adjusted model highlights the limitations of the current study including the small sample size and limited timeframe evaluated. Nonetheless, these observations warrant further investigations in an independent and larger cohort of Group I pulmonary arterial hypertension patients. Other limitations of the study were primarily related to the inherent retrospective nature of the data collection. Not every patient had all data available, and patient follow up was inconsistent. Additionally, pulmonary arterial capacitance and pulmonary arterial elastance can be derived by multiple measures; however, only one method was used in this study. This method overestimates compliance, as it does not account for blood flow from the pulmonary circulation into the capillary bed during systole. Nevertheless, it highly correlates with pulmonary artery compliance measured on the basis of the lumped two-element and three-element Windkessel models.31 A further limitation of this study was the lack of available values for resistance-compliance time, well-established for evaluating pulsatile load.
CONCLUSIONS
The current work provides multiple layers of evidence to support the influence of diabetes mellitus and hyperglycemia on reduced pulmonary artery compliance, increased pulmonary artery elastance, and right ventricular remodeling in pulmonary arterial hypertension including associations with a broader diagnosis of diabetes mellitus (vs. no diabetes mellitus) and also with granular phenotypes using glycated hemoglobin and fasting blood glucose levels. This may represent a contributing mechanism to previously reported poor outcomes in pulmonary arterial hypertension with diabetes mellitus. While there is no cure for pulmonary arterial hypertension, identifying modifiable risk factors may lead to an improved quality of life and delay or modify progression of the disease by allowing for targeted treatment of contributing comorbid conditions.16 These data, therefore, present the possibility that further targeted measures could be taken to improve prognosis in this subset of patients, including consideration of increased surveillance for diabetes mellitus in patients with pulmonary arterial hypertension, more aggressive pulmonary arterial hypertension and diabetes mellitus treatment in patients with comorbid diabetes mellitus, increased patient awareness regarding the risks of comorbid diabetes mellitus and pulmonary arterial hypertension, and increased pulmonary hypertension testing of diabetics17 in the community who show symptoms of pulmonary arterial hypertension.
Highlights.
Diabetes mellitus is associated with increased right ventricular afterload in pulmonary arterial hypertension.
Diabetes mellitus is associated with increased right ventricular hypertrophy in pulmonary arterial hypertension.
The study findings may link diabetes to susceptibility in right ventricular dysfunction in pulmonary arterial hypertension.
Acknowledgments
We are grateful to Anand Kadakia, MBBS for his contributions to the clinical data collection.
Funding Information: This study received support from the National Institutes of Health - National Heart, Lung, and Blood Institute (NHLBI) U01 RFA-HL-14-027 Award (PVDOMICS), NHLBI HL60190, and NHLBI R01 [HL141281 (AAD)].
Financial/Non-Financial Disclosures: Dr. Anna Hemnes receives personal fees (on Ad board) from Actelion, GSK, Bayer, and United Therapeutics and a grant from the NIH. Dr. Eric Austin receives personal fees (on Ad board) from Acceleron Pharma, Inc., and a grant from the NIH. Dr. Franz Rischard receives grants from United Therapeutics, Gilead, Actelion, and Bayer.
All authors had access to the data and a role in writing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
GUARANTOR STATEMENT
Ankit A. Desai MD is the guarantor of this manuscript and takes responsibility for the content, data, and analysis.
References
- 1.Desai AA, Machado RF. Diagnostic and therapeutic algorithm for pulmonary arterial hypertension. Pulm Circ. 2011;1:122–4. doi: 10.4103/2045-8932.78096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oudiz RJ. Classification of Pulmonary Hypertension. Cardiol Clin. 2016;34:359–61. doi: 10.1016/j.ccl.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Maron BA, Loscalzo J. Pulmonary hypertension: pathophysiology and signaling pathways. Handbook of experimental pharmacology. 2013;218:31–58. doi: 10.1007/978-3-642-38664-0_2. [DOI] [PubMed] [Google Scholar]
- 4.Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2017;367:643–649. doi: 10.1007/s00441-016-2539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuder RM, Abman SH, Braun T, et al. Development and pathology of pulmonary hypertension. Journal of the American College of Cardiology. 2009;54:S3–9. doi: 10.1016/j.jacc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Fritz JS, Smith KA. The Pulmonary Hypertension Consult: Clinical and Coding Considerations. Chest. 2016;150:705–13. doi: 10.1016/j.chest.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. Journal of the American College of Cardiology. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Mehari A, Valle O, Gillum RF. Trends in pulmonary hypertension mortality and morbidity. Pulm Med. 2014;2014:105864. doi: 10.1155/2014/105864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazzeroni D, Rimoldi O, Camici PG. From Left Ventricular Hypertrophy to Dysfunction and Failure. Circ J. 2016;80:555–64. doi: 10.1253/circj.CJ-16-0062. [DOI] [PubMed] [Google Scholar]
- 10.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–9. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 11.Tabit CE, Chung WB, Hamburg NM, et al. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldi JC, Wilson GA, Wilson LC, et al. The Type 2 Diabetic Heart: Its Role in Exercise Intolerance and the Challenge to Find Effective Exercise Interventions. Sports Med. 2016;46:1605–1617. doi: 10.1007/s40279-016-0542-9. [DOI] [PubMed] [Google Scholar]
- 13.Archer SL. Mitochondrial fission and fusion in human diseases. N Engl J Med. 2014;370:1074. doi: 10.1056/NEJMc1316254. [DOI] [PubMed] [Google Scholar]
- 14.Gopal DM, Santhanakrishnan R, Wang YC, et al. Impaired right ventricular hemodynamics indicate preclinical pulmonary hypertension in patients with metabolic syndrome. J Am Heart Assoc. 2015;4:e001597. doi: 10.1161/JAHA.114.001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grinnan D, Farr G, Fox A, et al. The Role of Hyperglycemia and Insulin Resistance in the Development and Progression of Pulmonary Arterial Hypertension. J Diabetes Res. 2016;2016:2481659. doi: 10.1155/2016/2481659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai YC, Tabima DM, Dube JJ, et al. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation. 2016;133:717–31. doi: 10.1161/CIRCULATIONAHA.115.018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsboom G, Wietholt C, Haney CR, et al. Lung (1)(8)F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:670–9. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan M, Han Y, Si R, et al. Hypoxia-induced pulmonary hypertension in type 2 diabetic mice. Pulm Circ. 2017;7:175–185. doi: 10.1086/690206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pugh ME, Robbins IM, Rice TW, et al. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30:904–11. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation. 2015;131:1691–702. doi: 10.1161/CIRCULATIONAHA.114.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talati M, Hemnes A. Fatty acid metabolism in pulmonary arterial hypertension: role in right ventricular dysfunction and hypertrophy. Pulm Circ. 2015;5:269–78. doi: 10.1086/681227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benson L, Brittain EL, Pugh ME, et al. Impact of diabetes on survival and right ventricular compensation in pulmonary arterial hypertension. Pulm Circ. 2014;4:311–8. doi: 10.1086/675994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6:953–63. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 25.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 26.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. 2009 [Google Scholar]
- 27.Warnes GR, Bolker B, Gorjanc G, et al. Gdata: Various R Programming Tools for Data Manipulation. 2015 [Google Scholar]
- 28.Wickham H. Reshaping Data with the reshape Package. Journal of Statistical Software. 2007;21:1–20. [Google Scholar]
- 29.Wickham H. Gtable: Arrange ’Grobs’ in Tables. 2016 [Google Scholar]
- 30.Hlavac M. Stargazer: Well-Formatted Regression and Summary Statistics Tables. 2015 [Google Scholar]
- 31.Saouti N, Westerhof N, Postmus PE, et al. The arterial load in pulmonary hypertension. Eur Respir Rev. 2010;19:197–203. doi: 10.1183/09059180.00002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thenappan T, Prins KW, Pritzker MR, et al. The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Ann Am Thorac Soc. 2016;13:276–84. doi: 10.1513/AnnalsATS.201509-599FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahapatra S, Nishimura RA, Sorajja P, et al. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. Journal of the American College of Cardiology. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 34.Stevens GR, Garcia-Alvarez A, Sahni S, et al. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012;5:378–87. doi: 10.1016/j.jcmg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Swift AJ, Capener D, Johns C, et al. Magnetic Resonance Imaging in the Prognostic Evaluation of Patients with Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2017;196:228–239. doi: 10.1164/rccm.201611-2365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab. 2016;20:546–51. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44:721–6. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]


