Abstract
Background
Obesity is a heterogeneous condition and specific patterns of body fat distribution are differentially associated with cardiometabolic risk factors. It is not currently known which fat depots contribute most to individual cardiometabolic risk factors. We examined the associations among eight different fat depots accumulated in various anatomical regions and the relationship between these fat depots and multiple cardiometabolic risk factors.
Methods
Participants were from the Framingham Heart Study Offspring (seventh examination) and Third Generation (first examination) who also participated in the multi-detector computed tomography sub-study between 2002 and 2005. Exposures were multi-detector computed tomography-derived fat depots, including abdominal subcutaneous adipose tissue, abdominal visceral adipose tissue, intramuscular fat, intrathoracic fat, pericardial fat, thoracic periaortic fat, intrahepatic fat, and renal sinus fat. Multivariable-adjusted regression analyses with a forward selection procedure were performed to identify the most predictive fat depots.
Results
Of 2,529 participants, 51.9% were women (mean age 51.1 years). Visceral adipose tissue had the strongest correlations with each of the other fat measures (ranging from 0.26 to 0.77), as well as with various cardiometabolic risk factors (ranging from -0.34 to 0.39). As determined by the selection models, visceral adipose tissue was the only fat depot that was associated with all cardiometabolic risk factors evaluated in this study (all p<0.05). Selection models also showed that subcutaneous adipose tissue and intrahepatic fat were associated with cardiometabolic risk factors related to the traits of dysglycemia, dyslipidemia, and hypertension (all p<0.05). However, only associations with visceral adipose tissue and intrahepatic fat persisted after further adjustment for BMI and waist circumference.
Conclusions
Visceral adipose tissue and intrahepatic fat were consistent correlates of cardiometabolic risk factors, above and beyond standard anthropometric indices. Our data provide important insights for understanding the associations between variations in fat distribution and cardiometabolic abnormalities.
Keywords: Ectopic fat, cardiometabolic risk factors, epidemiology, computed tomography
Introduction
Obesity affects more than one-third1 of U.S. adults and is a primary risk factor for cardiovascular morbidity2 and mortality.3, 4 The body mass index (BMI) is the most commonly used measure to assess the risk involved with adiposity-related metabolic derangements. However, individuals with similar BMIs have different cardiometabolic risk, suggesting that obesity is a heterogeneous condition.5, 6 Accordingly, specific patterns of body fat distribution may be differentially associated with cardiometabolic abnormalities.6, 7
Weight gain induces expansion of fat in ectopic sites, such as the heart, liver, kidney, muscles, vasculature, and viscera, which systemically or locally contributes to manifestations of cardiometabolic disease.6 These fat depots can be quantified on advanced imaging techniques, such as multi-detector computed tomography (MDCT) and magnetic resonance imaging (MRI).6, 7 A large set of participants in the Framingham Heart Study were assessed by MDCT to characterize various adipose tissue depots. Using that data, we have previously shown that higher region-specific fat measures, including abdominal subcutaneous, abdominal visceral adipose tissue,8-10 intramuscular fat,11 intrathoracic fat,12, 13 pericardial fat,12, 13 thoracic periaortic fat,14, 15 intrahepatic fat,16 and renal sinus fat,17 are individually associated with more adverse cardiometabolic risk factors.8, 9, 11-16 Importantly, the magnitude of the association between each fat measure and cardiometabolic related traits varied by fat depot type, suggesting that the potential adverse contribution of fat measure on cardiometabolic health are not the same. Yet, current literature lacks studies that systemically explored the associations between various fat depots accumulated in different anatomical regions and a broad array of cardiometabolic risk factors simultaneously. Accordingly, it is not currently known which fat depots contribute most to individual cardiometabolic risk factors. Thus, we examined the associations of eight different fat depots and the associations between these fat depots and a comprehensive list of cardiometabolic risk factors by leveraging our rich data sources. This study is the first to compare the relative importance of eight distinct MDCT-derived fat depots in relation to multiple cardiometabolic risk factors among participants in a large and well-characterized community-dwelling epidemiological study.
Methods
Methods are available in the Supplemental Materials.
Results
Study sample characteristics
Descriptive statistics given in Table 1 describe the demographic, physical, clinical, and lifestyle characteristics of the study participants. The mean age was 51.1 years and 51.9% were women. Hypertriglyceridemia showed the highest prevalence rate (33%), followed by metabolic syndrome (29.8%), hypertension (28.1%), and low HDL cholesterol (26.9%). Diabetes mellitus was least prevalent (5.8%), followed by high LDL cholesterol (9.4%).
Table 1.
Characteristics of the study sample.
| Characteristics | Women (n=1313) | Men (n=1216) | Overall Participants (n=2529) |
|---|---|---|---|
| Demographics | |||
| Age,a years | 51.9 (9.8) | 50.2 (10.8) | 51.1 (10.3) |
| Adiposity Measures | |||
| Body Mass Index, kg/m2 | 26.8 (5.8) | 28.0 (4.5) | 27.4 (5.2) |
| Waist Circumference, cm | 92.5 (15.6) | 99.7 (11.6) | 96.0 (14.3) |
| Subcutaneous Adipose Tissue, cm3 | 3082 (1503) | 2503 (1152) | 2804 (1377) |
| Visceral Adipose Tissue, cm3 | 1325 (820) | 2172 (1042) | 1733 (1025) |
| Intramuscular Fat, HU | 54.4 (6.4) | 57.6 (6.5) | 56.0 (6.6) |
| Intrathoracic Fat, cm3 | 65.6 (40.2) | 123.4 (63.0) | 93.4 (59.8) |
| Pericardial Fat, cm3 | 99.7 (38.1) | 122.6 (45.4) | 110.7 (43.3) |
| Thoracic Periaortic Fat, cm3 | 9.7 (5.3) | 16.8 (8.6) | 13.1 (7.9) |
| Intrahepatic Fat, liver phantom ratio | 0.36 (0.05) | 0.36 (0.06) | 0.36 (0.05) |
| Renal Sinus Fat,b cm2 | 0.19 (0.05-0.43) | 0.43 (0.19-0.89) | 0.28 (0.10-0.63) |
| Cardiometabolic Risk Factors | |||
| Systolic Blood Pressure, mmHg | 119.8 (17.6) | 123.1 (14.8) | 121.4 (16.4) |
| Diastolic Blood Pressure, mmHg | 73.4 (9.2) | 77.4 (9.1) | 75.3 (9.3) |
| Fasting Plasma Glucose, mg/dL | 95.5 (17.9) | 101.7 (23.8) | 98.5 (21.1) |
| Total Cholesterol, mg/dL | 197.7 (35.7) | 194.4 (33.8) | 196.1 (34.8) |
| LDL Cholesterol, mg/dL | 113.9 (32.7) | 121.8 (30.4) | 117.7 (31.9) |
| HDL Cholesterol, mg/dL | 61.5 (16.4) | 47.1 (12.4) | 54.6 (16.3) |
| Triglycerides,b mg/dL | 94.0 (66-139) | 108.5 (73-162) | 101.0 (70-150) |
| Overweight,c % | 55.0 (722) | 74.3 (903) | 64.3 (1625) |
| Obesity,c % | 23.8 (313) | 26.2 (319) | 25.0 (632) |
| Hypertension, % | 26.2 (344) | 30.1 (366) | 28.1 (710) |
| Diabetes Mellitus, % | 5.2 (68) | 6.5 (79) | 5.8 (147) |
| Hypercholesterolemia, % | 19.8 (260) | 25.6 (311) | 22.6 (571) |
| High LDL Cholesterol, % | 8.2 (108) | 10.6 (129) | 9.4 (237) |
| Low HDL Cholesterol, % | 24.8 (326) | 29.2 (355) | 26.9 (681) |
| Hypertriglyceridemia, % | 26.1 (343) | 40.4 (491) | 33.0 (834) |
| Metabolic Syndrome, % | 26.4 (346) | 33.6 (408) | 29.8 (754) |
| Lifestyle Factors | |||
| Current Smoking, % | 12.0 (158) | 13.4 (163) | 12.7 (321) |
| Moderate to Heavy Alcohol Use, % | 15.0 (197) | 16.7 (203) | 15.8 (400) |
| Diabetes Treatment, % | 2.8 (37) | 3.2 (39) | 3.0 (76) |
| Antihypertensive Treatment, % | 18.8 (247) | 18.2 (221) | 18.5 (468) |
| Lipid-Lowering Treatment, % | 9.4 (124) | 17.2 (209) | 13.2 (333) |
| Postmenopausal Status, % | 50.9 (668) | - | - |
| Hormone Replacement Therapy, % | 19.9 (261) | - | - |
Unless otherwise indicated, data are shown as means (standard deviations) for continuous variables or percentages (counts) for dichotomous variables.
Median (25th-75th percentile) age of our participants was 50.0 (44.0-58.0) years for women; 49.0 (42.0-57.0) for men; and 49.0 (43.0-58.0) for overall participants.
Values were shown as median (25th-75th percentile) due to their skewed distribution.
Overweight was defined as BMI 25 kg/m2 or higher and less than 30 kg/m2; obesity was defined as BMI 30 kg/m2 or higher.
Percentages (counts) for hypercholesterolemia defined based on the cutoff point of >200 mg/dL was 49.5% (650) for women; 53.9% (655) for men; and 51.6% (1305) for overall participants. Percentages (counts) for high LDL defined based on the cutoff point of >100 mg/dL was 64.1% (841) for women; 75.2% (914) for men; and 69.4% (1755) for overall participants.
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; HU, Hounsfield unit; LDL, low-density lipoprotein.
Associations among the fat measures
A heat map depicting the age- and sex- adjusted Pearson correlation coefficients between fat measures is given in Figure 1a. All fat measures, including MDCT-acquired fat depots, BMI, and waist circumference were correlated with each other (all p<0.05). However, the strength of these associations varied greatly. In particular, visceral adipose tissue had the strongest correlations (highest magnitudes) with each of the MDCT-derived fat depot. Both subcutaneous and visceral adipose tissue were highly correlated with BMI and waist circumference (r-values ranging from 0.72 to 0.88). Intramuscular fat, intrahepatic fat, and renal sinus fat were only weakly to moderately correlated with all the other fat measures evaluated. Similar patterns of the associations were observed in both women and men (Supplemental Figure 1a).
Figure 1.
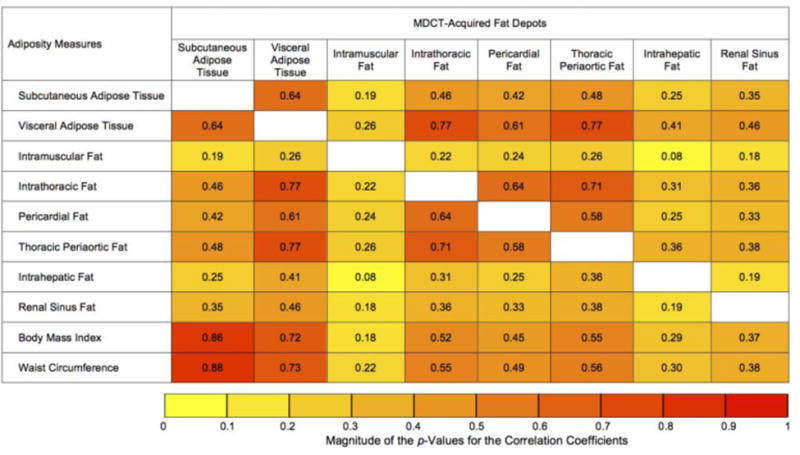
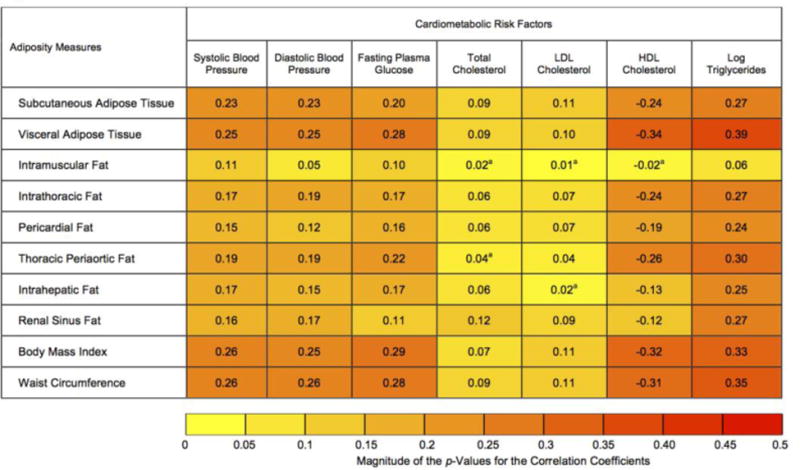
Heat map of the association a) among fat measures; b) between fat measures and cardiometabolic risk factors based on age and sex-adjusted partial Pearson correlation coefficients (r). The color depth of each cell represents the magnitude of the absolute values of the correlation coefficients from low (|r|=0 for Figure 1a and 1b; yellow) to high (|r|=1 for Figure 1a and |r|=0.5 for Figure 1b; red). Unless otherwise specified, all results were significant at p<0.05. ap>0.05. For Figure 1b, correlation coefficients associated with higher abdominal subcutaneous adipose tissue and visceral adipose tissue, intrathoracic fat, pericardial fat, thoracic periaortic fat, and renal sinus fat; and lower intramuscular fat and intrahepatic fat are presented. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDCT, multi-detector computed tomography.
Associations between the fat measures and cardiometabolic risk factors
The associations between fat measures and continuous cardiometabolic risk factors are visually presented in a heat map (Figure 1b). Higher fat measures were associated with higher systolic blood pressure, diastolic blood pressure, fasting plasma glucose, total cholesterol, LDL cholesterol, triglycerides and lower HDL cholesterol. Among the MDCT-derived fat depots, visceral adipose tissue showed the strongest correlations with each of the cardiometabolic risk factors. Among the cardiometabolic risk factors, the strongest correlations were observed with triglycerides. Total cholesterol and LDL cholesterol were only weakly associated with the fat measures (strongest correlation, r=0.12).
In the sex-stratified analyses, most of the fat measures were significantly associated with total and LDL cholesterol in women; whereas, in men, a majority of the associations between fat measures with total and LDL cholesterol were statistically non-significant (Supplemental Figure 1b). In the BMI-stratified analyses, a majority of the associations between MDCT-derived fat depots and cardiometabolic risk factors shown by correlation coefficients were stronger among participants with BMI 25 kg/m2 or higher (Supplemental Figure 2b), as compared to the participants with BMI less than 25kg/m2. (Supplemental Figure 2a).
Fat measures as risk factor correlates of cardiometabolic risk profile
Stepwise forward selection regression analyses identified the subset of MDCT-acquired fat depots that were associated with continuous (Table 2) and categorical (Table 3) cardiometabolic risk factors. Visceral adipose tissue was identified via the selection procedure in each of the cardiometabolic risk factor models. After further adjustment for BMI, visceral adipose tissue remained associated with all cardiometabolic risk factors, with the exception of diabetes mellitus. Similar results were obtained for models that were additionally adjusted for waist circumference instead of BMI (data not shown).
Table 2.
Associations between continuous cardiometabolic risk factors and MDCT-acquired fat depots (as determined by stepwise forward selection).
| Cardio- metabolic Risk Factors |
Model Typea |
Effect Size |
MDCT-Acquired Fat Depots | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subcutaneous Adipose Tissue |
Visceral Adipose Tissue |
Intramuscular Fat |
Intrathoracic Fat |
Pericardial Fat |
Thoracic Periaortic Fat |
Intrahepatic Fat |
Renal Sinus Fat |
|||
| BMI | MV | β (95% CI) | 3.60 (3.48,3.73) | 1.75 (1.61,1.90) | 0.14 (0.03,0.26) | |||||
| P Value | <.001 | <.001 | 0.02 | |||||||
| Systolic Blood Pressureb | MV | β (95% CI) | 1.75 (0.99,2.51) | 1.95 (1.02,2.87) | 0.99 (0.37,1.61) | |||||
| P Value | <.001 | <.001 | 0.002 | |||||||
| MV+ BMI | β (95% CI) | -0.07 (-1.21,1.07) | 1.12 (0.12,2.12) | 0.95 (0.34,1.57) | ||||||
| P Value | 0.91 | 0.03 | 0.002 | |||||||
| Diastolic Blood Pressureb | MV | β (95% CI) | 1.03 (0.56,1.49) | 1.71 (1.05,2.36) | -0.57 (-1.05,-0.09) | 0.49 (0.11,0.86) | 0.53 (0.08,0.97) | |||
| P Value | <.001 | <.001 | 0.02 | 0.01 | 0.02 | |||||
| MV+ BMI | β (95% CI) | 0.27 (-0.43,0.96) | 1.35 (0.66,2.05) | -0.56 (-1.03,- | 0.47 (0.10,0.84) | 0.52 (0.08,0.97) | ||||
| P Value | 0.45 | <.001 | 0.02 | 0.01 | 0.02 | |||||
| Fasting Plasma Glucosec | MV | β (95% CI) | 6.45 (5.15,7.75) | -2.26 (-3.57,-0.95) | 0.95 (0.20,1.69) | |||||
| P Value | <.001 | <.001 | 0.01 | |||||||
| MV+ BMI | β (95% CI) | 4.18 (2.62,5.74) | -2.07 (-3.37,-0.77) | 0.92 (0.18,1.66) | ||||||
| P Value | <.001 | .002 | 0.01 | |||||||
| Total Cholesterold | MV | β (95% CI) | 5.05 (2.52,7.58) | -2.91 (-5.49,-0.32) | 2.98 (1.29,4.68) | |||||
| P Value | <.001 | 0.03 | <.001 | |||||||
| MV+ BMI | β (95% CI) | 4.86 (1.92,7.80) | -2.91 (-5.50,- | 2.97 (1.27,4.67) | ||||||
| P Value | 0.001 | 0.03 | <.001 | |||||||
| LDL Cholesterold | MV | β (95% CI) | 1.70 (0.12,3.29) | 5.55 (3.05,8.04) | -2.91 (-5.26,-0.56) | |||||
| P Value | 0.04 | <.001 | 0.02 | |||||||
| MV+ BMI | β (95% CI) | 1.53 (-0.86,3.92) | 5.47 (2.85,8.09) | -2.92 (-5.27,- | ||||||
| P Value | 0.21 | <.001 | 0.02 | |||||||
| HDL Cholesterold | MV | β (95% CI) | -5.99 (-6.63,-5.36) | -1.08 (-1.74,-0.42) | ||||||
| P Value | <.001 | 0.001 | ||||||||
| MV+ BMI | β (95% CI) | -4.41 (-5.30,- | -1.08 (-1.73,-0.43) | |||||||
| P Value | <.001 | 0.001 | ||||||||
| Log Triglyceridesd | MV | β (95% CI) | 0.22 (0.18,0.25) | 0.03 (0.01,0.06) | -0.04 (-0.08,-0.01) | 0.06 (0.04,0.08) | 0.06 (0.04,0.08) | |||
| P Value | <.001 | 0.004 | 0.01 | <.001 | <.001 | |||||
| MV+ BM | β (95% CI) | 0.18 (0.14,0.23) | 0.03 (0.01,0.06) | -0.04 (-0.08,-0.01) | 0.06 (0.04,0.08) | 0.06 (0.03,0.08) | ||||
| P Value | <.001 | 0.004 | 0.02 | <.001 | <.001 | |||||
Data are shown as β coefficients (95% CIs) and p-values. The β coefficients describe the association with continuous cardiometabolic risk factor per 1 standard deviation decrement in intramuscular fat and intrahepatic fat; and per 1 standard deviation increment in other MDCT-acquired fat depots. Shaded cells indicate that the fat measures were not selected via the stepwise forward selection regression procedure.
MV model included following covariates: age, sex, current smoking, alcohol use, physical activity index, postmenopausal status (women only), and hormone replacement therapy (women only). BMI was additionally adjusted for in the models labelled “MV+BMI model.”
Antihypertensive treatment was included as a covariate for systolic and diastolic blood pressure models.
Diabetes treatment was included as a covariate for fasting plasma glucose model.
Lipid-lowering treatment was included as a covariate for total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides models. Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDCT, multi-detector computed tomography; MV, multivariable model.
Table 3.
Associations between dichotomous cardiometabolic risk factors and MDCT-acquired fat depots (as determined by stepwise forward selection).
| Cardiometabolic Risk Factors | Model Typea | Effect Size | MDCT-Acquired Fat Depots | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Subcutaneous Adipose Tissue | Visceral Adipose Tissue | Intramuscular Fat | Intrahepatic Fat | Renal Sinus Fat | |||||
| Obesity | MV | OR (95% CI) | 14.20 (10.72, 18.79) | 4.69 (3.71, 5.92) | |||||
| p | <.001 | <.001 | |||||||
| Hypertension | MV | OR (95% CI) | 1.15 (1.01, 1.31) | 1.55 (1.32, 1.82) | 1.27 (1.15, 1.40) | 1.22 (1.05, 1.41) | |||
| p | 0.03 | <.001 | <.001 | 0.008 | |||||
| MV+BMI | OR (95% CI) | 0.82 (0.68, 1.00) | 1.32 (1.11, 1.57) | 1.26 (1.15, 1.40) | 1.21 (1.05, 1.40) | ||||
| p | 0.05 | 0.002 | <.001 | 0.009 | |||||
| Diabetes Mellitus | MV | OR (95% CI) | 1.36 (1.10, 1.68) | 1.51 (1.21, 1.89) | 1.28 (1.11, 1.48) | ||||
| p | 0.005 | <.001 | <.001 | ||||||
| MV+BMI | OR (95% CI) | 0.69 (0.50, 0.95) | 1.13 (0.89, 1.45) | 1.27 (1.10, 1.48) | |||||
| p | 0.02 | 0.32 | 0.001 | ||||||
| Hyper-cholesterolemia | MV | OR (95% CI) | 1.50 (1.34, 1.67) | ||||||
| p | <.001 | ||||||||
| MV+BMI | OR (95% CI) | 1.46 (1.25, 1.71) | |||||||
| p | <.001 | ||||||||
| High LDL Cholesterol | MV | OR (95% CI) | 1.25 (1.08, 1.45) | ||||||
| p | 0.003 | ||||||||
| MV+BMI | OR (95% CI) | 1.31 (1.06, 1.62) | |||||||
| p | 0.01 | ||||||||
| Low HDL Cholesterol | MV | OR (95% CI) | 2.02 (1.78, 2.29) | 0.81 (0.71, 0.92) | 1.17 (1.06, 1.29) | ||||
| p | <.001 | 0.001 | 0.001 | ||||||
| MV+BMI | OR (95% CI) | 1.72 (1.46, 2.04) | 0.81 (0.71, 0.92) | 1.17 (1.06, 1.29) | |||||
| p | <.001 | 0.001 | 0.002 | ||||||
| Hyper-triglyceridemia | MV | OR (95% CI) | 1.79 (1.57, 2.04) | 1.22 (1.10, 1.34) | 1.23 (1.08, 1.40) | ||||
| p | <.001 | <.001 | 0.003 | ||||||
| MV+BMI | OR (95% CI) | 1.69 (1.43, 1.99) | 1.21 (1.10, 1.34) | 1.22 (1.07, 1.40) | |||||
| p | <.001 | <.001 | 0.003 | ||||||
| Metabolic Syndrome | MV | OR (95% CI) | 1.46 (1.27, 1.68) | 3.35 (2.79, 4.01) | 1.33 (1.19, 1.49) | ||||
| p | <.001 | <.001 | <.001 | ||||||
| MV+BMI | OR (95% CI) | 0.85 (0.69, 1.05) | 2.62 (2.15, 3.18) | 1.33 (1.19, 1.48) | |||||
| p | 0.13 | <.001 | <.001 | ||||||
Data are shown as ORs (95% CIs) and p-values. Intrathoracic fat, pericardial fat, and thoracic periaortic fat are not included in the table as those fat depots were not selected for any of the outcomes (cardiometabolic risk factors) via the stepwise forward selection regression procedure. The ORs describe the association with dichotomous cardiometabolic risk factors per 1 standard deviation decrement in intramuscular fat and intrahepatic fat; and per 1 standard deviation increment in other MDCT-acquired fat depots. Shaded cells indicate that the fat measures were not selected via the stepwise forward selection regression procedure.
MV model was adjusted for following covariates: age, sex, current smoking, alcohol use, physical activity index, postmenopausal status (women only), and hormone replacement therapy (women only). BMI was additionally adjusted for in the models labeled “MV+BMI model.”
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDCT, multi-detector computed tomography; MV, multivariable model; OR, odds ratio.
The stepwise forward selection regression analysis identified subcutaneous adipose tissue in half of the risk factor models including those predicting: BMI, systolic blood pressure, diastolic blood pressure, LDL cholesterol, obesity, hypertension, diabetes mellitus, and metabolic syndrome. However, additional adjustment for BMI or waist circumference substantially attenuated these associations (all p>0.05). Additional adjustment for BMI changed the direction of some of the associations between subcutaneous adipose tissue and cardiometabolic risk factors (i.e., subcutaneous adipose tissue with systolic blood pressure, hypertension, diabetes mellitus, and metabolic syndrome in Tables 2 and 3), however none of these associations were statistically significant (all p>0.05).
Similarly, intrahepatic fat was selected in more than half of the cardiometabolic risk factor models, including those predicting systolic blood pressure, diastolic blood pressure, fasting plasma glucose, log triglycerides, hypertension, diabetes, low HDL, hypertriglyceridemia, and metabolic syndrome. Intrahepatic fat remained associated with all of these cardiometabolic risk factors even after further adjustment for BMI or waist circumference (separately).
When we used lower cutoff points for defining hypercholesterolemia (>200 mg/dL) and high LDL cholesterol (>100 mg/dL), visceral adipose tissue and renal sinus fat were selected as significant predictors of hypercholesterolemia; however, for high LDL cholesterol, only renal sinus fat was selected as a predictor (Supplemental Table 1).
Sex-stratified analysis
The trends of the associations identified in the sex-pooled analysis were generally consistent with the results of the sex-stratified analysis (Supplemental Tables 2 and 3). In particular, the direction of the associations was generally consistent between women and men. In women, visceral adipose tissue was consistently associated with all the cardiometabolic risk factors evaluated regardless of the additional adjustment for BMI or waist circumference. In contrast, several exceptions were noted in men.
Discussion
Principal findings
Our investigation explored associations among a broad list of region-specific fat measures, including radiographic measures of fat depots and traditional anthropometric measures of adiposity. Our principal findings are three-fold. First, visceral adipose tissue generally showed the highest correlations with all of the other fat measures. While subcutaneous and visceral adipose tissue were both strongly correlated with BMI and waist circumference, intrahepatic fat was not, which may indicate that BMI and waist circumference are poor surrogates for intrahepatic fat. Second, visceral adipose tissue was the only fat depot that was consistently associated with each cardiometabolic risk factor evaluated, and these associations were attenuated, but generally remained, after additional adjustment for BMI or waist circumference. While subcutaneous adipose tissue was included in the selection models for many cardiometabolic risk factors, these associations were attenuated and no longer statistically significant after BMI or waist circumference was added to the multivariable-adjusted model indicating that subcutaneous adipose tissue does not contribute to cardiometabolic risk factors beyond a measure of generalized or central adiposity. Intrahepatic fat was also associated with multiple cardiometabolic risk factors related to glucose and lipid metabolism, as well as measures of hypertension, and these associations remained even after adjustment for the standard anthropometric indices. Finally, in addition to visceral adipose tissue, subcutaneous adipose tissue and intrahepatic fat, we identified differential associations between various fat depots (i.e., intramuscular fat, intrathoracic fat, pericardial fat, thoracic periaortic fat, and renal sinus) and cardiometabolic risk factors.
Potential mechanisms
The most compelling finding of this investigation was that among the eight MDCT-acquired fat depots, only visceral adipose tissue was consistently associated with each of the cardiometabolic risk factors. Although subcutaneous adipose tissue was also associated with multiple cardiometabolic risk factors, the association did not persist after adjustment of BMI or waist circumference. More specifically, additional adjustment of BMI attenuated the associations or changed the direction of the associations (all p<0.05) that were observed between subcutaneous adipose tissue and cardiometabolic risk factors based on the multivariable-adjusted models. These findings are mainly due to the high correlation between subcutaneous adipose tissue and BMI (r=0.86, p<0.05), and suggesting that subcutaneous adipose tissue does not contribute to cardiometabolic risk factors beyond a measure of generalized or central adiposity.
Multiple lines of evidence support that abdominal subcutaneous and visceral adipose tissue are associated with different cardiometabolic consequences; in particular, a more adverse effect may be attributed to visceral adipose tissue.18 In the state of positive energy balance, expansion of visceral adipose tissue reflects an inability to expand fat depot subcutaneously due to the decreased capacity of subcutaneous adipose tissue hypertrophy, hyperplasia, and angiogenesis.18 As an active endocrine organ, the expansion of visceral adipose tissue is associated with overproduction of metabolically active substances, such as pro-inflammatory,18, 19 hemostasis, and fibrinolysis biomarkers20 that mediate the relations with cardiometabolic risk factors. As compared to subcutaneous adipose tissue, hypertrophy of visceral adipocytes was related to measures of insulin resistance21 where adipocyte size is an important determinant of cellular functionality.22 Taken together, visceral and subcutaneous adipose tissue may differentially contribute to cardiometabolic risk factors owing to their distinctive functions.
In this study, intrahepatic fat was associated with a number of cardiometabolic risk factors related to dysglycemia and dyslipidemia traits, as well as measures of blood pressure. This may be explained by the joint role of visceral adipose tissue and intrahepatic fat in glucose and lipid metabolism.23 Expansion of visceral adipose tissue volume with concomitant enlargement of the adipocyte size has been associated with increased visceral adipose tissue lipolytic activity, substantially increasing the free fatty acids released from visceral adipose tissue to the liver via the portal vein by approximately 20%.24 In response to the elevated hepatic fatty acids presented to the liver, the liver must store the non-esterified free acids in the form of triglycerides.25 In addition, increased hepatic fatty acids may be responsible for hepatic insulin resistance and an increase in the production and release of very-low-density lipoprotein cholesterol.25 Our findings support that both visceral and intrahepatic fat may be contributing fat depots for lipid regulation and metabolism. Yet, the precise mechanisms are still unclear.
In addition to our primary findings, we found no correlation or only weak correlations with total and LDL cholesterol and the MDCT-derived fat depots. Of note, metabolic syndrome is determined by the combination of physiological, biochemical, clinical, and metabolic factors.26 Among the measures of lipid metabolism, only HDL cholesterol and triglycerides levels are used to define metabolic syndrome. In particular, total cholesterol and LDL cholesterol are risk factors of atherosclerotic cardiovascular disease, which does not directly fit into the category of metabolic disease traits. Collectively, we have shown that ectopic fat depots are more strongly associated metabolic risk factors, rather than clustering risk factors for atherosclerotic heart disease.
It is well-established that risk of cardiometabolic disease increases with both increasing age and fat accumulation, while accumulation of fat also increases with advanced age.10, 27-30 In a similar context, demographic and lifestyle factors, such as sex, smoking status, alcohol use, physical activity, postmenopausal status, and hormone replacement therapy, are also considered as traditional risk factors for cardiometabolic disease, that are also related to accumulation of fat.31-37 Accordingly, the relationships between fat measures and cardiometabolic risk factors are not uniform across individuals with various demographic and lifestyle factors. This suggests that there are potential effects of measuring fat distributions at different ages and depending on various factors (e.g., alcohol intake, smoking status) in an individual's lifetime. And whether the effects of fat deposits are constant over time or only correlated with specific cardiometabolic risk factors will vary. Although adjusting for traditional risk factors (e.g., age and sex) statistically ruled out their contributions in a pooled analysis, the important issue of how adiposity affects disease risk in younger vs. older adults and in different factors would be obscured. The lack of association between fat measures and LDL cholesterol may be a measurement issue. Further studies that include more specific fat profiles, such as LDL particles, LDL size, apolipoprotein B would be useful to confirm the associations between fat depots and lipid profiles.
Implications
This study adds an important facet to the obesity research spectrum in which, within the heterogeneity of fat distribution, visceral adipose tissue and, to a lesser degree, intrahepatic fat, are the fat depots most closely associated with multiple cardiometabolic risk factors, above and beyond generalized and central adiposity. BMI is the most cost effective method to determine overall obesity status that can predict cardiometabolic disease risk. Accordingly, BMI is ideal for preliminary screening in clinical and field settings. However, the sensitivity involved with screening populations by this method is relatively crude because the index does not provide information on body fat distribution, such as visceral and subcutaneous fat. Our findings add to evidence that imaging fat volume provides important clues to cardiometabolic risk that BMI may not explain. In regards to the preventive implications of the present study, our findings support that making recommendations solely by BMI may result in misclassifying individuals at high risk for those who are “metabolically healthy obese”. And those who are “metabolically unhealthy non-obese” and “metabolically unhealthy normal weight” will be misclassified as being at lower risk.38, 39 To date, CT and MRI are the gold standard for noninvasively measuring abdominal visceral fat. More cost-effective methods for assessing abdominal visceral fat are needed before screening at risk populations can be recommended.
Our work also demonstrated associations between intramuscular fat, intrathoracic fat, pericardial fat, thoracic periaortic fat, and renal sinus fat with several different cardiometabolic risk factors, supporting that these fat depots should not be overlooked as pathologic fat depots contributing to cardiometabolic disease. Future studies are warranted to explore the biology of ectopic adipose tissue storage to precisely decipher the pathogenicity of excess adiposity.
In the context of the current literature, strengths, and limitations
Additional discussions on our findings in the context of current literature, strengths and limitations are described in Supplemental Materials.
Conclusions
On the basis of a community-based epidemiologic study, visceral adipose tissue was consistently associated with all cardiometabolic risk factors evaluated in this study. subcutaneous adipose tissue and intrahepatic fat were associated with multiple cardiometabolic traits related to glucose and lipid metabolism, as well as measures of blood pressure. However, these associations only remained significant for visceral and intrahepatic fat above and beyond the contribution of generalized obesity and central adiposity. Our findings highlight the importance of the role of ectopic fat distribution for a better understanding of the metabolic complications of obesity.
Supplementary Material
Supplemental Figure 1. Heat map of the association a) among fat measures; b) between fat measures and cardiometabolic risk factors based on age-adjusted sex-specific partial Pearson correlation coefficients (r). The color depth of each cell represents the magnitude of the absolute values of the correlation coefficients from low (|r|=0 for Supplemental Figure 1a and 1b; yellow) to high (|r|=1 for Supplemental Figure 1a and |r|=0.5 for Supplemental Figure 1b; red). Unless otherwise specified, all results were significant at p<0.05. ap>0.05. For Supplemental Figure 1b, correlation coefficients associated with higher abdominal subcutaneous adipose tissue and visceral adipose tissue, intrathoracic fat, pericardial fat, thoracic periaortic fat, and renal sinus fat; and lower intramuscular fat and intrahepatic fat are presented. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDCT, multi-detector computed tomography.
Supplemental Figure 2. Heat map of the association between fat measures and cardiometabolic risk factors based on age- and sex-adjusted partial Pearson correlation coefficients for pooled models; and age-adjusted partial Pearson correlation coefficients for sex-stratified models according to the BMI status (BMI < 25 kg/m2 for Supplemental Figure 2a and BMI ≥ 25 kg/m2 for Supplemental Figure 2b). The color depth of each cell represents the magnitude of the absolute values of the correlation coefficients from low (|r|=0; yellow) to high (|r|=0.5; red). Correlation coefficients associated with higher abdominal subcutaneous adipose tissue and visceral adipose tissue, intrathoracic fat, pericardial fat, thoracic periaortic fat, and renal sinus fat; and lower intramuscular fat and intrahepatic fat are presented. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDCT, multi-detector computed tomography.
Supplemental Table 1. Associations between dichotomous hypercholesterolemia and high low-density lipoprotein (LDL) cholesterol (defined by using different cutoff points of >200 mg/dL for hypercholesterolemia and >100 mg/dL for LDL cholesterol) with MDCT-acquired fat depots (as determined by stepwise forward selection).
Supplemental Table 2. Associations between continuous cardiometabolic risk factors and fat depots stratified by sex (as determined by multivariable-adjusted linear regression analysis).
Supplemental Table 3. Associations between dichotomous cardiometabolic risk factors and fat depots stratified by sex (as determined by multivariable-adjusted linear regression).
Clinical Significance.
Relation of eight fat measurements and cardiometabolic risk factors were assessed.
Eight different fat depots accumulated in various anatomical regions were evaluated.
Visceral adipose tissue was consistently associated with cardiometabolic risk factors.
Intrahepatic fat was also associated with multiple cardiometabolic risk factors.
These associations were independent of standard anthropometric indices.
Acknowledgments
Funding Source: This work was supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (contract N01-HC-25195).
Footnotes
Authors' Statement: All authors had access to the data and a role in writing the manuscript.
Conflict of Interest Disclosures: Alison Pedley is an employee of Merck & Company, Inc. There is nothing to disclose for any author other than Alison Pedley.
NHLBI Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the U.S. Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obesity Research. 1995;3:73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 3.McTigue KM, Chang YF, Eaton C, et al. Severe obesity, heart disease, and death among white, African American, and Hispanic postmenopausal women. Obesity. 2014;22:801–810. doi: 10.1002/oby.20224. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics. Mortality multiple cause micro-data files, 2013 public-use data file and documentation: NHLBI tabulations. [Accessed May 19, 2015]. [Google Scholar]
- 5.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167:642–648. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 6.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 7.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–176. doi: 10.1016/j.ijcard.2013.08.077. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 9.Pou KM, Massaro JM, Hoffmann U, et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–485. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS. Association of changes in abdominal fat quantity and quality with incident cardiovascular disease risk factors. J Am Coll Cardiol. 2016;68:1509–1521. doi: 10.1016/j.jacc.2016.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Therkelsen KE, Pedley A, Speliotes EK, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33:863–870. doi: 10.1161/ATVBAHA.112.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 13.Thanassoulis G, Massaro JM, Hoffmann U, et al. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham heart study. Circ Cardiovasc Imaging. 2010;3:559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britton KA, Pedley A, Massaro JM, et al. Prevalence, distribution, and risk factor correlates of high thoracic periaortic fat in the Framingham Heart Study. J Am Heart Assocn. 2012;1:e004200. doi: 10.1161/JAHA.112.004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman SJ, Massaro JM, Schlett CL, et al. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster MC, Hwang SJ, Porter SA, et al. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 19.Lee JJ, Pedley A, Hoffmann U, et al. Cross-Sectional Associations of Computed Tomography (CT)- Derived Adipose Tissue Density and Adipokines: The Framingham Heart Study. J Am Heart Assoc. 2016;5:e002545. doi: 10.1161/JAHA.115.002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertens I, Van Gaal LF. Visceral fat as a determinant of fibrinolysis and hemostasis. Semin Vasc Med. 2005;5:48–55. doi: 10.1055/s-2005-871741. [DOI] [PubMed] [Google Scholar]
- 21.Ledoux S, Coupaye M, Essig M, et al. Traditional anthropometric parameters still predict metabolic disorders in women with severe obesity. Obesity. 2010;18:1026–1032. doi: 10.1038/oby.2009.349. [DOI] [PubMed] [Google Scholar]
- 22.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 23.Björntorp P. Abdominal obesity and the metabolic syndrome. Annals of medicine. 1992;24:465–468. doi: 10.3109/07853899209166997. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 27.Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr. 2005;81:1330–1334. doi: 10.1093/ajcn/81.6.1330. [DOI] [PubMed] [Google Scholar]
- 28.Utzschneider KM, Carr DB, Hull RL, et al. Impact of intra-abdominal fat and age on insulin sensitivity and beta-cell function. Diabetes. 2004;53:2867–2872. doi: 10.2337/diabetes.53.11.2867. [DOI] [PubMed] [Google Scholar]
- 29.Demerath EW, Sun SS, Rogers N, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: The Healthy Transitions Study. Obes Res. 2001;9:10–16. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]
- 32.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 33.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 34.Kanaley JA, Sames C, Swisher L, et al. Abdominal fat distribution in pre- and postmenopausal women: The impact of physical activity, age, and menopausal status. Metabolism. 2001;50:976–982. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- 35.Komiya H, Mori Y, Yokose T, Tajima N. Smoking as a risk factor for visceral fat accumulation in Japanese men. Tohoku J Exp Med. 2006;208:123–132. doi: 10.1620/tjem.208.123. [DOI] [PubMed] [Google Scholar]
- 36.Barlow CE, Shuval K, Balasubramanian BA, et al. Association Between Sitting Time and Cardiometabolic Risk Factors After Adjustment for Cardiorespiratory Fitness, Cooper Center Longitudinal Study, 2010-2013. Prev Chronic Dis. 2016;13:E181. doi: 10.5888/pcd13.160263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shuval K, Finley CE, Barlow CE, et al. Sedentary behavior, cardiorespiratory fitness, physical activity, and cardiometabolic risk in men: the cooper center longitudinal study. Mayo Clinic Proceedings. 2014;89:1052–1062. doi: 10.1016/j.mayocp.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Jung CH, Lee WJ, Song KH. Metabolically healthy obesity: a friend or foe? Korean J Intern Med. 2017;32:611. doi: 10.3904/kjim.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese”, normal-weight individual. Am J Clin Nutr. 1981;34:1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Heat map of the association a) among fat measures; b) between fat measures and cardiometabolic risk factors based on age-adjusted sex-specific partial Pearson correlation coefficients (r). The color depth of each cell represents the magnitude of the absolute values of the correlation coefficients from low (|r|=0 for Supplemental Figure 1a and 1b; yellow) to high (|r|=1 for Supplemental Figure 1a and |r|=0.5 for Supplemental Figure 1b; red). Unless otherwise specified, all results were significant at p<0.05. ap>0.05. For Supplemental Figure 1b, correlation coefficients associated with higher abdominal subcutaneous adipose tissue and visceral adipose tissue, intrathoracic fat, pericardial fat, thoracic periaortic fat, and renal sinus fat; and lower intramuscular fat and intrahepatic fat are presented. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDCT, multi-detector computed tomography.
Supplemental Figure 2. Heat map of the association between fat measures and cardiometabolic risk factors based on age- and sex-adjusted partial Pearson correlation coefficients for pooled models; and age-adjusted partial Pearson correlation coefficients for sex-stratified models according to the BMI status (BMI < 25 kg/m2 for Supplemental Figure 2a and BMI ≥ 25 kg/m2 for Supplemental Figure 2b). The color depth of each cell represents the magnitude of the absolute values of the correlation coefficients from low (|r|=0; yellow) to high (|r|=0.5; red). Correlation coefficients associated with higher abdominal subcutaneous adipose tissue and visceral adipose tissue, intrathoracic fat, pericardial fat, thoracic periaortic fat, and renal sinus fat; and lower intramuscular fat and intrahepatic fat are presented. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDCT, multi-detector computed tomography.
Supplemental Table 1. Associations between dichotomous hypercholesterolemia and high low-density lipoprotein (LDL) cholesterol (defined by using different cutoff points of >200 mg/dL for hypercholesterolemia and >100 mg/dL for LDL cholesterol) with MDCT-acquired fat depots (as determined by stepwise forward selection).
Supplemental Table 2. Associations between continuous cardiometabolic risk factors and fat depots stratified by sex (as determined by multivariable-adjusted linear regression analysis).
Supplemental Table 3. Associations between dichotomous cardiometabolic risk factors and fat depots stratified by sex (as determined by multivariable-adjusted linear regression).


