Abstract
Purpose
Prostate circulating tumor cells escape into the peripheral blood and enter the bone marrow as disseminated tumor cells, representing an early step before conventionally detectable metastasis. It is unclear how frequently this occurs in localized disease, and existing detection methods rely on epithelial markers with low specificity and sensitivity. Here we employed multiple methodologies of disseminated tumor cell detection in bone marrow harvested at radical prostatectomy.
Materials and Methods
Bone marrow was harvested from 208 clinically localized patients, 16 controls, and 5 metastatic patients, with peripheral blood from 37 metastatic patients. Samples were evaluated at 4 separate centers with 4 distinct platforms that utilized antibody enrichment (AdnaTest, VERSA) or whole sample interrogation (RareCyte, HD-SCA), using traditional epithelial markers and prostate-specific markers. We investigated the sensitivity and specificity of these markers by evaluating their expression levels in both control and metastatic patients.
Results
EpCAM, NKX3.1, and AR were non-specifically expressed in the controls and a majority of all samples with the AdnaTest, with no relation to perioperative variables. Only 1 patient with localized disease was positive for the prostate-specific marker PSA. With the VERSA platform, no localized patient had disseminated tumor cells. With both the RareCyte and HD-SCA platforms, only a single patient had 1 disseminated tumor cell.
Conclusions
Evaluation across multiple platforms revealed that epithelial markers are non-specific in the bone marrow and thus not suitable for disseminated tumor cell detection. Using prostate-specific markers, disseminated tumor cells were typically not detected in localized prostate cancer patients.
Keywords: Prostate cancer, circulating tumor cells, disseminated tumor cells, prostate-specific markers
Introduction
Disseminated tumor cells (DTCs) are cancer cells that escape the primary cancer and enter a target organ, representing a first step towards detectable metastasis [1]. A common landing zone for prostate cancer (PCa) cells is the bone marrow (BM). These cells provide an opportunity for the early detection of cancer spread before the development of clinical metastases that are large enough to be captured on conventional imaging [2]. Previously, Morgan et al reported that DTCs were present in 72% of men with localized PCa prior to radical prostatectomy (RP) [3]. Unexpectedly, the presence of DTCs was not associated with pathological stage, Gleason grade, or prostate-specific antigen (PSA). Notably, this work relied upon epithelial markers for detection of cancer cells in the BM. There has been a recent appreciation of the phenotypic diversity of DTCs and the cellular complexity of the BM, which has called into question the reliability of the traditional epithelial markers such as pan-cytokeratin (CK) and epithelial cell adhesion molecule (EpCAM) [4–7, 8].
The limited sensitivity of epithelial markers was demonstrated recently by McDaniel et al with their detection of CK-negative PCa circulating tumor cells (CTCs), confirmed with fluorescent in situ hybridization for PTEN and ERG, as well as immunofluorescent staining for androgen receptor (AR) expression [8]. Specificity with epithelial markers is also an issue given that EpCAM is known to be ubiquitously expressed on normal resident cells of the BM, including erythroid progenitor cells and human epidermal Langerhans cells [4, 9–11]. AR is also not an ideal prostate marker in the BM, as it facilitates the regulation of hematopoiesis with ubiquitous expression in normal male and female BM [12]. Although NKX3.1 is traditionally considered a prostate-specific marker, Uhlén et al have demonstrated that there is detectable mRNA expression in normal female and male BM tissues [13].
Given the absence of a proven prostate-specific marker that is reliable in the BM niche, little is known regarding when or how often PCa cells disseminate to the BM as DTCs. Previous studies have demonstrated that 35% of localized PCa patients who undergo RP will experience biochemical recurrence within 10 years [14]. Thus, it is imperative to investigate DTCs as an early step in the presently incurable process of metastasis, as nearly all men with metastatic PCa will eventually develop castrate resistance and disease progression [15]. In the present study, we employed a variety of immunofluorescence- and mRNA expression-based rare cell detection platforms to evaluate for the presence of DTCs in localized PCa patients using both traditional epithelial markers and prostate-specific markers. Additionally, we investigated the sensitivity and specificity of these markers by evaluating their expression levels in both control and metastatic PCa patients.
Materials and Methods
DTC and CTC Detection Assays
Two immunofluorescence-based assays were employed at two separate centers, the High-Definition Single Cell Assay (HD-SCA) at the University of Southern California (USC), and the Accucyte® System at the Fred Hutchinson Cancer Research Center (FHCRC). Additionally, two mRNA expression-based assays were performed at two separate centers, the AdnaTest at the Johns Hopkins Hospital (JHH) and the Versatile Exclusion-based Rare Sample Analysis (VERSA) platform at University of Wisconsin (UW) (Table 1). All methods were verified with spike-in controls. Of note, the expression patterns of individual cells cannot be queried with mRNA expression applied to a cell population (see Figure 1).
Table 1.
Patients, Markers, and DTC Detection Methods Used at each Site
| JHH | USC | UW | FHCRC | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOCALIZED BM PATIENTS | 106 Localized BM | 43 Localized BM | 18 Localized BM | 41 Localized BM | |||||||||||||||||||||||||||||
| LOCALIZED COHORT OVERLAP | 16 patients overlap between JHH and USC | 2 patients overlap between UW and FHCRC | |||||||||||||||||||||||||||||||
| ADDITIONAL PATIENT SAMPLES | 16 Control BM 5 Metastatic BM 37 Metastatic PB |
5 Localized PB | None | None | |||||||||||||||||||||||||||||
| DTC DETECTION METHOD | AdnaTest | HD-SCA | VERSA | RareCyte | |||||||||||||||||||||||||||||
| TEST CATEGORY | mRNA | IF | mRNA | IF | |||||||||||||||||||||||||||||
|
MARKERS WBC |
|
CD45 |
|
CD45 |
|||||||||||||||||||||||||||||
| LOCALIZED PATIENTS WITH DTCs | 1 patient PSA+ | Non-specific assay 1 patient with AR+ cells | 1 patient PSMA+ | Non-specific assay 1 patient with DTCs = (4P+/AR+/CK+/EpCAM+/CD45−) |
|||||||||||||||||||||||||||||
Figure 1.
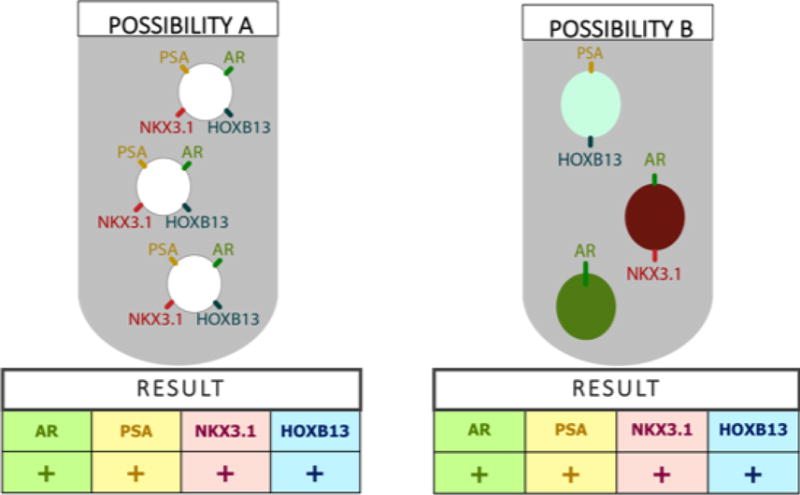
RT-PCR Applied to a Cell Population Cannot Describe Expression Patterns of Individual Cells
Sample Collection and Distribution
All samples were collected consecutively under IRB-approved protocols with no reported complications. Samples from JHH were distributed to all centers, following pre-existing standards for processing time-window and shipping (Table 1). Patients were tested on a maximum of two platforms given the harvest volume limitation for BM aspiration. RP BM samples were taken under anesthesia prior to incision. 79% of BM samples were collected from the pubic bone (P), while 21% were collected from the anterior iliac crest (IC) (JHH: 67/106, 63% P and 39/106, 37% IC; USC: 43/43, 100% P; UW: 17/18, 94% P and 1/18, 6% IC; FHCRC: 37/41, 90% P and 4/41, 10% IC). 18 BM samples from clinically localized patients prior to RP were sent to UW for analysis. 41 localized BM samples were sent to FHCRC; of these 2 patients (5%) were also evaluated at UW. 43 localized BM samples with 5 matched PB samples were sent to USC for analysis with the HD-SCA assay; of these 16 patients (37%) were also evaluated with the JHH AdnaTest. At JHH, BM samples were collected from 106 clinically localized PCa patients prior to RP and 16 control patients specifically defined as individuals without a diagnosis of PCa (either female, or male confirmed to not have prostate cancer). These controls included 1 female patient with renal cell carcinoma undergoing radical nephrectomy, 1 female patient with bladder cancer undergoing transurethral resection of bladder tumor, 1 male patient undergoing a transurethral resection of prostate for benign prostatic hyperplasia, and 13 male patients with bladder cancer undergoing radical cystoprostatectomy (in these patients, the prostate was surgically removed and examined by dedicated urologic pathologists who concluded that no prostate adenocarcinoma was present). 37 PB and 5 BM samples were also collected from metastatic PCa patients. To facilitate collection of control BM from metastatic PCa patients, a cohort that does not commonly undergo anesthesia, a collaboration was formed with the neurosurgery and orthopedic departments to identify patients requiring urgent stabilization of the spine or weight bearing bones due to PCa bony metastasis to supplement clinic patients of the medical oncology investigators.
Statistical Analysis
For the JHH localized cohort, the associations of preoperative PSA (continuous variable), RP Gleason Score (GS) (GS=6 vs. GS>6), and pathologic stage (pStage=pT2 vs. pStage>pT2) with AR and NXK3.1 expression were evaluated with the Mann-Whitney U Test (PSA) and Fisher’s exact test (GS, stage).
Results
Localized Cohorts
All localized patients were clinically presumed to have organ confined disease prior to prostatectomy, and had not received any systemic therapy for prostate cancer prior to RP. The localized cohort characteristics are summarized in Table 2.
Table 2.
Clinical Characteristics of the Localized Prostate Cancer Patient Cohorts
| CHARACTERISTIC | JHH N = 106 |
USE N = 43 |
UW N = 18 |
FHCRC N = 41 |
|---|---|---|---|---|
|
| ||||
| Age median (range) | 62 (43-74) | 63 (41-77) | 60 (50-68) | 63 (43-77) |
|
| ||||
| Gleason Score N (%) | ||||
| 6 | 14 (13%) | 6 (14%) | 2 (11%) | 2 (5%) |
| 3+4 | 51 (48%) | 21 (49%) | 10 (57%) | 23 (56%) |
| 4+3 | 20 (19%) | 5 (11%) | 2 (11%) | 7 (17%) |
| 8-10 | 21 (20%) | 11 (26%) | 4(22%) | 9 (22%) |
|
| ||||
| PSA N (%) | ||||
| <10 | 83 (78%) | 33 (77%) | 15(83%) | 33 (80%) |
| 10-20 | 15 (14%) | 8 (19%) | 3 (17%) | 4 (10%) |
| >20 | 8 (8%) | 2 (4%) | 0 (0%) | 4 (10%) |
|
| ||||
| Pathologic T Stage N (%) | ||||
| pT2 | 64 (60%) | 22 (51%) | 8 (44%) | 26 (64%) |
| pT3a | 30 (29%) | 16 (37%) | 9 (50%) | 10 (24%) |
| pT3b | 12 (11%) | 5 (12%) | 0 (0%) | 5 (12%) |
|
| ||||
| Pathologic N Stage N (%) | ||||
| pNx | 15 (14%) | 7 (16%) | 16(89%) | 34 (83%) |
| pN0 | 86 (81%) | 31 (72%) | 2 (11%) | 5 (12%) |
| pN1 | 5 (5%) | 5 (12%) | 0 (0%) | 2 (5%) |
UW Gene Expression Patterns with the VERSA Platforms
With VERSA, EpCAM and/or KRT8 was detected in a majority (72%) of patients tested. The prostate genes AR, ACPP/PAP and NKX3.1 were detected in 50%, 89% and 44% of the patient samples, respectively. However, TMPRSS2, KLK3/PSA and HOXB13 were not detected in any of the BM derived samples. We detected expression of PSMA in cells captured from one patient (pT2Nx) (Figure 2).
Figure 2. Epithelial and Prostate-Specific Gene Expression from EpCAM Capture Cells from Bone Marrow from Localized Prostate Cancer Patients.
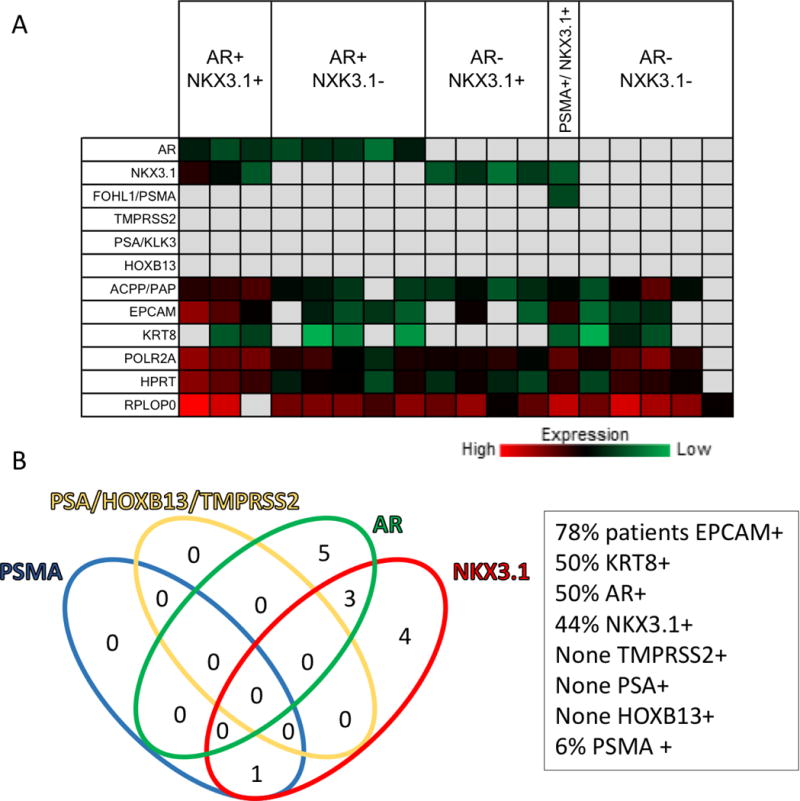
A. Results from quantitative RT-PCR are presented as CT values represented as a heat map.
B. Venn diagram illustrating the number of patients positive for the indicated markers.
USC DTC and CTC detection with the HD-SCA Platform
Using the high definition single cell assay (HD-SCA) workflow, 43 BM samples and 5 PB samples from treatment-naïve organ-confined PCa patients were assayed. As seen in Figure S1, the incidence of DTCs in BM is zero for 42 patients and one for a single patient (pT2N0, below threshold of 3 cells) and no CTCs were detected in PB.
JHH Gene Expression Patterns with the AdnaTest
EpCAM, NKX3.1, and AR were expressed in the control patient BM and a majority of all BM samples and thus displayed a lack of specificity for prostate DTC detection (Figure 3). Explicitly, 98% (n=125/127) of all BM samples contained cells that expressed EpCAM. Neither PSA nor HOXB13 were found in any control patient BM, yet they were expressed in the majority of metastatic patient blood (n=27/37, 73% PSA+ n=26/37, 70% HOXB13+) and BM samples (n=3/5, 60% PSA+; n=3/5, 60% HOXB13+) (Figure 3B–D). Only 1 patient (1%, pT3bN0) from the localized PCa patient cohort was positive for PSA in the BM and none expressed HOXB13, implying that DTCs are a rare event in localized PCa (Figure 3A).
Figure 3.
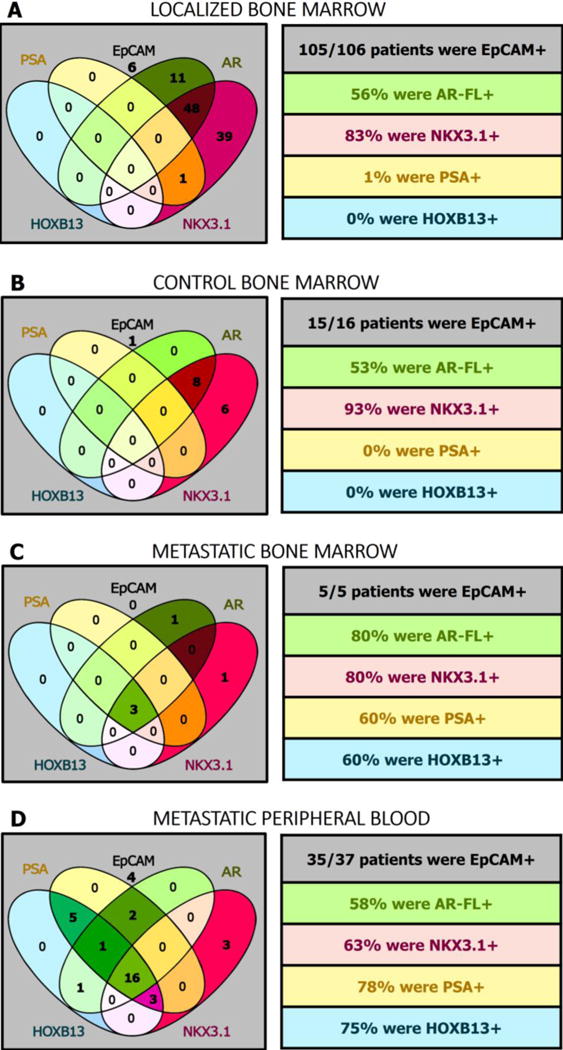
Epithelial and Prostate-Specific Gene Expression Patterns in the Localized, Control, and Metastatic Settings
JHH Cohort Statistical Analysis
Given that only 1 patient was EpCAM negative in the localized cohort, we did not examine the significance of EpCAM expression as it relates to clinicopathologic features of the cohort patients. For NKX3.1 and AR n=88/106, 83% and n=59/106, 56% of the localized cohort were positive for expression, thus we compared these patients with their marker negative counterparts to see if there was any significant association with RP GS, pathologic stage, or PSA (Table S1). None of these comparisons achieved statistical significance (Table S1).
FHCRC Gene Expression Patterns with the RareCyte Platform
Two slides from each of the 41 patients with localized PCa were stained and analyzed. Figure 4E shows a single DTC identified in the BM from one patient (pT2N0). DTCs were not detected in the remaining 40 patients. Three clusters of cells with larger nuclei (compared to the surrounding leukocytes), strong CK, variable EpCAM, and no CD45 from the same JHH patient were also detected, but were not classified as DTCs as they lacked AR and 4P staining (Figure S2).
Figure 4.
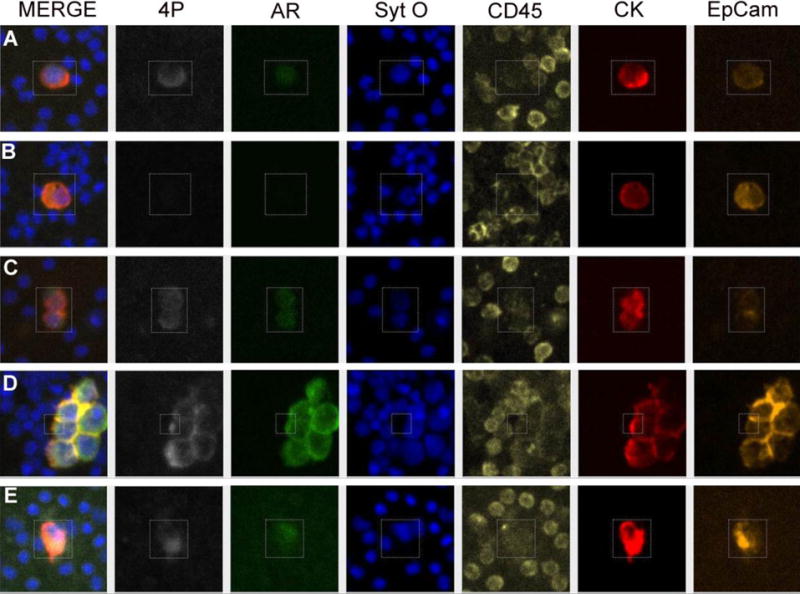
Examples of various PCa cells. 6-Channel images show 4P (PSAP, PSCA, PSMA, and PSA cocktail) (white), AR (green), nuclear stain (Sytox Orange) (Blue), CD45 (yellow), CK (red), and EpCam (Orange) staining of cells isolated from (A) LnCap PB spike-in (B) PC3 PB spike-in, (C) 22RV1 PB spike-in, (D) PCa cell isolated from BMA from a patient with bone lesions, and (E) DTC isolated from BMA from a JHU patient.
Discussion
Though localized PCa patients can be cured with surgery in many cases, a significant fraction will experience recurrence of their cancer, often within the first 5 years after surgery [16, 17]. DTCs have the potential to serve as an early indicator of disease progression, yet little is understood regarding their natural history [18]. This is largely due to the difficulty of identifying rare cells in the BM. Conventional methods of rare cell detection have relied on epithelial markers, which are now understood to be non-specific in the BM [19, 20, 21, 22]. In the present report, we investigated the presence of DTCs across multiple methodologies for rare cell analysis.
With the AdnaTest for mRNA-expression, EpCAM, NKX3.1, and AR were non-specifically expressed in the control patient BM and a majority of all BM samples, with no relation to perioperative variables. Explicitly, 98% (n=125/127) of all BM samples contained cells that expressed EpCAM. HOXB13 and PSA were not found in any control, yet were expressed in the majority of metastatic patient samples. Only 1 patient from the localized PCa patient cohort was positive for PSA. Similarly, with the VERSA platform, the epithelial markers EpCAM and/or KRT8 were detected in a majority (72%) of patients tested. On both platforms, NKX3.1 and AR were detected in many samples. On the VERSA platform, it was noted that prostate acid phosphatase (PAP) was detected in 89% of the patient samples. Importantly, both PAP and NKX3.1 are generally assumed to be prostate-specific markers, but these results are consistent with external mRNA expression data showing low expression levels of both genes in normal male and female BM (PAP - 11.1 transcripts per kilobase million (TPM), NKX3.1 – 1.3 TPM) [13]. With VERSA, TMPRSS2, PSA and HOXB13 were not detected in any of the BM derived samples, and only one patient was positive for PSMA. Taken together, these results imply that DTCs are a rare event in localized PCa.
The immunofluorescence-based platforms offered the ability to evaluate co-localization of markers at the single cell level. The HD-SCA platform detects AR+/CK+/CD45- nucleated cells, and only 1 localized patient was found to have this expression signature. Whereas AR is known to be expressed in normal BM, and was detected in many samples by the VERSA and AdnaTest mRNA based platform, these results imply that AR is not normally co-localized with the epithelial marker CK in normal tissues [8]. Similarly, with the FHCRC RareCyte platform, only 1 patient had a prostate DTC defined using the criteria of 4P+/AR+/CK+/EpCAM+/CD45-. Unfortunately, none of these markers are definitively prostate-specific in the BM – 4P refers to the combination of PSA (which is believed to be prostate-specific) with PAP, PSCA, and PSMA all in one immunofluorescent channel. Given that the VERSA platform found PAP expression in the majority of samples, more work is necessary to show that this co-localized expression signature is sufficiently prostate-specific. The requirement for AR expression in addition to a prostate cell marker such as PSA provides additional specificity for DTC classification, but at the potential cost of sensitivity.
Small case series of 2 to 13 PCa patient BM samples have previously been studied for epithelial and prostate-specific marker expression [4, 6, 7]. One such group used an epithelial marker, human-epithelial antigen (HEA), to isolate cells from 11 BM samples, and then pooled them and evaluated individual cells for the expression of NKX3.1, HOXB13, and AR. They found that only 44 of the 85 total cells they analyzed expressed a prostatic signature [4]. In a larger study of 105 M0 PCa patients, Guzvic et al isolated EpCAM+ cells from the BM and analyzed gene expression of one prostate-specific marker, PSA. They found positive PSA expression in just 1 localized PCa patient. Yet, given their hypothesis that all EpCAM+ cells represented DTCs, they reasonably only evaluated a small subset of the EpCAM+ cells, as opposed to querying the entire cell population. This group was unable to reliably differentiate the gene expression signature of PCa patients from controls [5].
In contrast to what was previously accepted, the present study suggests that DTCs are uncommon in patients with localized PCa utilizing current detection methods. This could be because they are very rare and present at a rate below the limit of detection. Another limitation is that with some methods such as the AdnaTest and VERSA platform, only EpCAM+ cells were captured and evaluated. We now know that epithelial marker negative PCa CTCs have been found in the blood, and if this is extrapolated to the BM there may be EpCAM- DTCs that were not identified by our assay [8]. This study was also limited by the small number of control and metastatic BM samples, and heterogeneity of BM aspirate sites. Future studies will also validate the specificity and sensitivity of PSA and HOXB13 in a larger number of metastatic BM samples and would confirm the nonspecific expression of NKX3.1 and AR in a larger number of control BM samples. It is possible that this study is not powered to detect a significant difference in clinicopathologic variables in NKX3.1+ and AR+ cells, however the analysis here is presented to show at least that no difference was detected in the current small cohort. The main evidence for the conclusion that EpCAM, NKX3.1 and AR are unsuitable markers for prostate DTCs is that they were detected in the BM of control patients who were known not to have prostate cancer.
Conclusion
In conclusion, evaluation across multiple platforms revealed that DTCs were very rare in localized PCa patients at the time of RP, changing the way we understand the process of metastasis in prostate cancer. Potential explanations for these negative results include: 1). The step of bone metastasis in which CTCs invade into the BM niche as DTCs may have not occurred at the time of prostatectomy in the majority of localized men, suggesting the reservoir for recurrent disease is elsewhere; 2). The cells that reside in the BM are not detectable by current methodology; 3). DTCs are present in the BM at eventual sites of recurrence and not detectable in BM derived from the studied locations. Those rare patients in whom DTCs are detected may be uniquely suited to different management than their DTC-negative counterparts. As such, we are expanding our cohort of men with high-grade cancer to determine if DTCs are more prevalent in this population. Importantly, epithelial markers were found to be non-specific in the BM and thus are not suitable for DTC detection.
Supplementary Material
Acknowledgments
Yezi Zhua, Misop Hana, Alan W. Partina, Misop Hana, Trinity J. Bivalacquaa, Mohamad E. Allafa, Ashley Rossa, Arthur Burnetta, Edward M. Schaeffera, Trinity J. Bivalacquaa, H. Ballentine Cartera, Yezi Zhua, Emma E. van der Tooma, James E. Verdonea, Michael Morikadob, Anders Carlssonb, Anand Kolatkarb, Mariam Rodriguez Leeb, Jim Hicksb, Anupama Singhc, Erika Heningerc, Daniel M. Sciubbadb, Ali Bydondb, Timothy F. Withamdb, Jean-Paul Wolinskydb, Alexandra Corellae, and Juanita Ongkowijoyoe
Funding: This work was supported by the Urology Care Foundation’s Resident Research Award (H.J.C.), the Stutt family (K.J.P.), NCI grant numbers. U54CA143803, CA163124, CA093900 and CA143055 (K.J.P.) CA181648 (J.M.L.), CA097186 (P.S.N.), CDMRP award W81XWH-15-1-0430 (P.S.N.), and the Prostate Cancer Foundation (K.J.P., J.M.L., P.N., P.K.), Burroughs Wellcome Fund (C.R.G.), and the Polak Foundation Fellow (P.M.).
Footnotes
Disclosures: K.J.P. is a consultant and holds equity for Cue Biopharma, is a consultant for Celsee Diagnostics and board member of Curis, Inc. J.M.L. holds equity in Salus Discovery, LLC and has served as a consultant for Sanofi-Genzyme. J.H. is on the Clinical Advisory Boards of Epic Sciences, Inc. La Jolla, CA and CelMatix, Inc. of NY, NY. P.K. is an Advisor, receives royalties and is a shareholder at Epic Sciences, Inc., La Jolla, CA.
References
- 1.Alix-Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008 Aug 15;14(16):5013–21. doi: 10.1158/1078-0432.CCR-07-5125. Review. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, Schoenborn JR, Morrissey C, et al. High-Resolution Genomic Profiling of Disseminated Tumor Cells in Prostate Cancer. J Mol Diagn. 2016 Jan;18(1):131–43. doi: 10.1016/j.jmoldx.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan TM, Lange PH, Porter MP, et al. Disseminated Tumor Cells in Prostate Cancer Patients after Radical Prostatectomy and without Evidence of Disease Predicts Biochemical Recurrence. Clinical cancer research. 2009;15(2):677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chéry L, Lam HM, Coleman I, Lakely B, et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget. 2014 Oct 30;5(20):9939–51. doi: 10.18632/oncotarget.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gužvić M, Braun B, Ganzer R, et al. Combined genome and transcriptome analysis of single disseminated cancer cells from bone marrow of prostate cancer patients reveals unexpected transcriptomes. Cancer Res. 2014 Dec 15;74(24):7383–94. doi: 10.1158/0008-5472.CAN-14-0934. [DOI] [PubMed] [Google Scholar]
- 6.Welty CJ, Coleman I, Coleman R, et al. Single cell transcriptomic analysis of prostate cancer cells. BMC Mol Biol. 2013 Feb 16;14:6. doi: 10.1186/1471-2199-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cann GM, Gulzar ZG, Cooper S, et al. mRNA-Seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLoS One. 2012;7(11):e49144. doi: 10.1371/journal.pone.0049144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDaniel AS, Ferraldeschi R, Krupa R, et al. Phenotypic diversity of circulating tumour cells in patients with metastatic castration-resistant prostate cancer. BJU Int. 2016 Aug 18; doi: 10.1111/bju.13631. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lammers R, Giesert C, Grünebach F, et al. Monoclonal antibody 9C4 recognizes epithelial cellular adhesion molecule, a cell surface antigen expressed in early steps of erythropoiesis. Exp Hematol. 2002 Jun;30(6):537–45. doi: 10.1016/s0301-472x(02)00798-1. [DOI] [PubMed] [Google Scholar]
- 10.Shetye JD, Liljefors ML, Emdin SO, et al. Spectrum of cytokeratin-positive cells in the bone marrows of colorectal carcinoma patients. Anticancer Res. 2004 Jul-Aug;24(4):2375–83. [PubMed] [Google Scholar]
- 11.Eisenwort G, Jurkin J, Yasmin N, et al. Identification of TROP2 (TACSTD2), an EpCAM-like molecule, as a specific marker for TGF-β1-dependent human epidermal Langerhans cells. J Invest Dermatol. 2011 Oct;131(10):2049–57. doi: 10.1038/jid.2011.164. [DOI] [PubMed] [Google Scholar]
- 12.Mantalaris A, Panoskaltsis N, Sakai Y, et al. Localization of androgen receptor expression in human bone marrow. J Pathol. 2001 Mar;193(3):361–6. doi: 10.1002/1096-9896(0000)9999:9999<::AID-PATH803>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Uhlen M, Fagerberg L, Hallstrom EM, et al. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.11226/science.1260419. [DOI] [PubMed] [Google Scholar]
- 14.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005 Jul 27;294(4):433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 15.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015 May 21;161(5):1215–28. doi: 10.1016/j.cell.2015.05.001. Erratum in: Cell. 2015 Jul 16;162(2):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loeb S, Feng Z, Ross A, et al. Can we stop prostate specific antigen testing 10 years after radical prostatectomy? J Urol. 2011 Aug;186(2):500–5. doi: 10.1016/j.juro.2011.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amling CL, Blute ML, Bergstralh EJ, et al. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000 Jul;164(1):101–5. [PubMed] [Google Scholar]
- 18.Köllermann J, Weikert S, Schostak M, et al. Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J Clin Oncol. 2008 Oct 20;26(30):4928–33. doi: 10.1200/JCO.2007.15.0441. [DOI] [PubMed] [Google Scholar]
- 19.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008 Oct 1;14(19):6302–9. doi: 10.1158/1078-0432.CCR-08-0872. Erratum in: Clin Cancer Res. 2009 Feb 15;15(4):1506. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008 Jul 1;26(19):3213–21. doi: 10.1200/JCO.2007.15.8923. Erratum in: J Clin Oncol. 2009 Apr 10;27(11):1923. [DOI] [PubMed] [Google Scholar]
- 21.Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006 Jul 15;12(14 Pt 1):4218–24. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 22.Murlidhar V, Zeinali M, Grabauskiene S, et al. A radial flow microfluidic device for ultra-high-throughput affinity-based isolation of circulating tumor cells. Small. 2014 Dec 10;10(23):4895–904. doi: 10.1002/smll.201400719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrinucci D, Bethel K, Kolatkar A, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. 2012 Feb;9(1):016003. doi: 10.1088/1478-3975/9/1/016003. Epub 2012 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperger M, Strotman LN, Welsh A, et al. Integrated Analysis of Multiple Biomarkers from Circulating Tumor Cells Enabled by Exclusion-Based Analyte Isolation. Clin Cancer Res. 2016 Jul 11; doi: 10.1158/1078-0432.CCR-16-1021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campton DE, Ramirez AB, Nordberg JJ, et al. High-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence staining. BMC Cancer. 2015 May 6;15:360. doi: 10.1186/s12885-015-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


