Abstract
Epstein-Barr virus associated gastric cancer (EBVaGC) comprises approximately 10% of gastric carcinomas. Multiple factors contribute to tumorigenesis, including EBV driven hypermethylation of tumor suppressor genes, inflammatory changes in gastric mucosa, host immune evasion by EBV and changes in cell cycle pathways. The unique molecular characteristics of EBVaGC, such as programmed death ligand 1 (PD-L1) overexpression, highlight the potential for using EBV as a biomarker for response to immunotherapy. Few studies have reported benefit from immunotherapy in EBV positive cancers, and clinical trials investigating the impact of checkpoint inhibitors in EBVaGC are currently underway. This review provides the most recent updates on molecular pathophysiology, epidemiology, clinical features and treatment advances pertaining to EBVaGC.
Keywords: EBV, Immunotherapy, PDL-1, Gastric Cancer, Viruses, Biomarker
Introduction
Until the late 1930s, gastric cancer was the leading cause of cancer death in the United States [1]. Today, it is the third most common cause of cancer-related mortality and the fifth most common cancer worldwide [2]. In the United States, it is the 14th most common cause of cancer, with approximately 10,960 deaths per year [1]. Gastric cancer has a significant socioeconomic, ethnic and geographic disparity, with highest rates in Eastern Asia, followed by Central and Eastern Europe, and lowest in North America and Western Africa [3]. Although the worldwide incidence of gastric cancer has declined over the last few years, the incidence of proximal gastric cancer has increased globally [4].
The overall 5-year survival rate in most parts of the world is dismal at 20% with median survival less than 12 months [5]. Gastric cancer’s aggressive nature and its heterogeneity warrant the identification of new sensitive and specific biomarkers. To facilitate biomarker discovery and personalized treatment development, global efforts have been undertaken to molecularly classify gastric cancer. In 2014, The Cancer Genome Atlas (TCGA) network used six genomic and molecular platforms to comprehensively characterize 295 tumors into four molecular subtypes: Epstein-Barr virus (EBV)-positive tumors, microsatellite instable (MSI) tumors, genomically stable (GS) tumors, and tumors with chromosomal instability (CIN) [6]. In 2015, the Asian Cancer Research Group (ACRG) conducted gene expression data on 300 gastric cancers, leading to four different subtypes with prognostic data: MSI, microsatellite stable/epithelial-to-mesenchymal transition (MSS/EMT), MSS/TP53+ and MSS/Tp53− [7]. The ACRG found that EBV infection occurred in 6.5% of overall patients, and more frequently in the MSS/TP53+ subgroup, which had the second-best overall survival [7].
EBV-positive tumors comprised 9% of TCGA gastric cancer samples [6] and 6.5% of the ACRG samples [7]. EBV-positive tumors also exhibited a higher prevalence of DNA hypermethylation and elevated levels of programmed death ligands 1 and 2 (PD-L1/2) in TCGA samples. Although the ACRG analysis did not identify hypermutation among EBV-positive gastric cancers, it did find EBV to be more frequent in the MSS/TP53+ subtype, with significant enrichment of PIK3CA and ARID1A mutations, and increased immune infiltrates [7].
These classification results suggest that EBV associated gastric cancers (EBVaGC) have a distinct tumorigenic profile, and present the opportunity for using EBV as a novel biomarker in gastric cancer for targeted treatment development. Limited progress has been made by adding targeted therapy to gastric cancer treatments. The addition of trastuzumab for gastric cancers with overexpression of human epidermal growth factor receptor 2 (Her-2) (3+/2+ on immunohistochemistry or FISH positive) has had a modest improvement in survival, with a median survival increase of 2.5 months based on the ToGA trial [8]. Recent meta-analyses have shown that gastric cancers with EBV positivity and microsatellite instability are most likely to overexpress PDL-1 [9]. Microsatellite instability (MSI-high) already serves as a biomarker in predicting utility of immune checkpoint inhibitors [10], where the PD-1 antibody, pembrolizumab, is FDA approved for use in MSI-high gastric cancers that have progressed on standard treatment. Most recently, results from the global phase II KEYNOTE-059 trial [11] showed an improved overall response rate to pembrolizumab in gastric cancer patients with overexpression of PDL-1. This led to the FDA approval of pembrolizumab for gastric cancer patients with overexpression of PDL-1 who have failed two or more lines of systemic chemotherapy. Nivolumab is another PD-1 antibody, which for the first time, has shown an overall survival benefit in gastric cancer patients in the ATTRACTION-2 phase 3 trial conducted in Japan, South Korea and Taiwan [12].
As the role of immunotherapy in gastric cancer gains momentum, the need for identifying biomarkers of response becomes crucial. Patients with EBVaGC could be the next subgroup most likely to benefit from immunotherapy. This review provides an overview of EBVaGC, the current clinical trials including EBVaGC and its implications for advancing personalized medicine in the care of gastric cancer patients.
EBV and cancer
EBV, also known as human herpesvirus 4 (HHV4), is a double-stranded DNA virus infecting over 90% of the adult population [13]. It was first discovered in 1964 by Tony Epstein and Yvonne Barr, when they used electron microscopy to identify herpesvirus-type particles in a subpopulation of Burkitt’s lymphoma (BL) cell lines from African patients [13]. Since then, EBV has been recognized as the first virus to be directly associated with human cancer. It is currently categorized as a group-1 carcinogen due to its association with the development of a wide spectrum of cancers, including BL, post-transplant lymphoproliferative disorder (PTLD), Hodgkin and non-Hodgkin lymphomas, nasopharyngeal carcinoma (NPC) and more recently, gastric carcinoma [14].
Defined by the presence of EBV in gastric cancer cells, EBVaGC has an annual incidence of 75,000–90,000 cases per year, representing the largest subpopulation among EBV-related tumors [15]. EBVaGC was first reported by Burke et al in 1990 using polymerase chain reaction (PCR) in gastric carcinoma cells resembling lymphoepithelioma [15]. In 1992, in situ hybridization technique allowed Shibata et al to identify EBV in 16% of gastric adenocarcinoma samples by localizing EBV-encoded small RNA 1 (EBER1) [16]. Its etiological involvement in gastric tumorigenesis is strongly suggested by the detection of monoclonal episomes and uniform presence of EBER in tumor cells but not in the adjacent normal mucosa [16].
Pathophysiology and tumorigenesis
EBV is considered a direct transforming pathogen [17] by expressing its own regulatory genes affecting host cell cycle pathways [18]. It enters epithelial cells from the oropharynx and subsequently spreads to the lymphoid tissues where it infects B lymphocytes [19].
After primary infection via the oral route, EBV establishes a lifelong virus carrier state, called latent infection, where it persists as an episome within the nucleus [20]. During its latency cycle, it constitutively expresses a limited set of latent gene products known as: EBV nuclear antigens (EBNAs 1, 2, 3A, 3B, 3C and EBNA-LP) [21], EBV-encoded small RNAs (EBERs) 1 and 2, latent membrane protein (LMP 1, 2A and 2B) and 40 microRNAs from BamHI-A rightward transcripts, known as BARTs [22]. Depending on the various combinations of these gene products, four latency patterns have been classified: latency Ia, Ib, II, and III [23]. EBVaGC belongs to latency I, which is limited to EBERs, BARTs and EBNA1 [23]. The absence or presence of LMP2A distinguishes latency type Ia or Ib, respectively. In over 50% of EBVaGC cases, LMP2A is expressed [21].
EBV infection induces extensive CpG island methylation [23] within approximately 18 weeks of infection [24], and is significantly correlated with CpG island methylator phenotype (CIMP)-high status [25]. This characterizes an important pathogenic mechanism, known as EBV-specific methylation epigenotype [24]. It is currently under investigation whether the host cell initiates genome-wide methylation as a defense mechanism, or whether EBV directly begins the process. LMP2A is reported to induce host DNA methyltransferase 1 (DNMT1) [24] or DNMT3b [26] overexpression and initiate genome-wide methylation. Overall, 886 genes are known to be methylated [26], with downregulation of approximately 216 genes [23]. Table 1 lists important host genes that are affected by EBV infection, and their role in tumorigenesis.
Table 1.
Important host genes and their role in EBVaGC tumorigenesis
| Gene | Mechanism of tumorigenesis | Reference |
|---|---|---|
| Upregulated Genes | ||
| ZEB1 | Inhibits latent to lytic switch of EBV, enabling a longer latency duration | [26] |
| PIK3CA | Increases cell proliferation and survival by activating downstream PI3K/Akt pathway | [63] |
| PeBOW | A protein complex that enhances cell survival and ribosome biogenesis | [36] |
| PD-1/2 | Suppresses immune surveillance and facilitate tumor development | [63] |
| JAK2 | Stimulates cell proliferation, survival and differentiation | [63] |
| Bcl-2 | Anti-apoptotic protein | [48] |
| Cyclin D1 | Allows cell cycle progression through G1 phase | [48] |
| IHH | increases metastatic potential through angiogenesis, Snail protein expression, as well as a decrease in e-cadherin and tight junctions | [23] |
| Downregulated Genes | ||
| SSTR1 | Expression is decreased by eight-fold in EBVaGC, allowing cell proliferation, loss of apoptosis and invasion | [26] |
| PTEN | Loss of this tumor suppressor leads to PI3K/Akt pathway activation, and increased cell growth, angiogenesis, migration, loss of cell adhesion, and cell cycle regulation | [63] |
| ARID1A | Loss leads to enhanced tumor migration and lymphovascular invasion through downregulation of e-cadherin | [63] |
| P16 | Loss leads to uncontrolled cell growth, and may induce expression of thymidine phosphorylase, which facilitates tumor angiogenic activity | [23] |
A comprehensive analysis of promoter methylation status of 51 gastric carcinoma cases was conducted by Shinozaki et al [27], who subsequently classified gastric carcinoma into three epigenotypes characterized by different sets of methylation genes: EBV+/extensively high-methylation, EBV−/high-methylation and EBV−/low-methylation. Methylated genes specific for the EBV+ subtype included CXXC4, TIMP2 and PLXND1. COL9A2, EYA1 and ZNF365 were highly methylated in EBV+ and EB−/high-methylation subtypes, whereas AMPH, SORC33 and AJAP1 were frequently methylated in all epigenotypes. They discovered that EBVaGC had approximately 270 genes which were uniquely methylated. Interestingly, MLH1 was frequently methylated (46%) in the EBV−/high-methylation type, whereas none of the EBVaGC cases showed MLH1 methylation. Different methylation patterns might explain why EBVaGC is frequently found in MSS subtypes of gastric cancer and displays a different molecular biology than the MSI-high subtype.
Whether there are particular EBV oncogenes contributing to gastric carcinogenesis is currently under investigation. LMP1 is an EBV-encoded oncoprotein that has been linked with EBV-associated lymphomas and nasopharyngeal carcinomas, however, LMP1 expression is not observed in gastric cancer [28]. Different cell types may respond differently to EBV oncogenes. For instance, EBV BamHI-A rightward frame 1 (BARF-1) gene encodes a secretory protein with cell transforming and mitogenic properties, which is consistently expressed during latency cycle in EBVaGC [29]. In rodent cell lines, BARF-1 induces apoptosis, a process not observed in human gastric epithelial cell models [30]. BARF-1 has been shown to enhance cell proliferation through upregulation of NF-κB and cyclin-D1 in EBV infected gastric carcinoma cells [29]. BARF-1 also reduces expression of the cell cycle inhibitor p21 [29]. Recently, preclinical studies conducted by Turrini et al [31] highlight the role of a BARF-1 monoclonal antibody, which exerts direct anti-tumor effects on EBV positive cells by binding to BARF-1 and inducing antibody dependent cellular cytotoxicity (ADCC), while also limiting the mitogenic activity of BARF-1 molecules in the tumor microenvironment. Therefore, understanding the oncogenic mechanisms of the EBV genes is crucial for developing new targets. Table 2 lists EBV oncogenes with their pathogenic mechanisms in EBVaGC.
Table 2.
EBV proteins and their role in tumorigenesis
| Gene | Mechanism of tumorigenesis | Reference |
|---|---|---|
| LMP2A | Upregulates cellular oncogenes. Increases survivin production and cellular growth. Downregulates WnT pathway, increasing cell migration and invasion. | [21, 60, 83] |
| BARF-1 | Stimulate cell proliferation, growth and survival via NF-κB/cyclinD1 pathway. Plays an anti-apoptotic role. | [20, 83, 84] |
| EBER | Induce insulin-like growth factor 1 (IGF1) which serves as an autocrine growth factor | [83] |
| EBNA | Target the nucleus and affect gene expression. Upregulate oncogenes and inhibit tumor suppressors, such as gastrokine 1 and 2, which increases cell growth. | [24, 85] |
Chronic inflammation is a risk factor for EBVaGC as it sets the optimal conditions necessary for tumorigenesis, and therefore, certain injuries may condition the gastric mucosa to develop EBV associated carcinomas, a process known as field cancerization [32]. According to Kaneda et al, atrophic gastritis serves as a lesion which enables cell to cell contact between infiltrated EBV-carrying lymphocytes and gastric epithelial cells [24]. EBER is greatly expressed in Crohn’s disease and UC, and not expressed in adjacent normal mucosa [33]. Repetitive injuries, such as those from bile reflux can also predispose to EBVaGC [32].
Tumor microenvironment can play a critical role in tumorigenesis, metastasis and angiogenesis [34]. Tumor infiltrating lymphocytes (TILs) create an immune-active tumor microenvironment and their presence improves patient survival [34]. TILs boost anti-tumor immunity by eradicating EBV-positive malignant cells, and their presence is shown to be increased in EBVaGC. A low density of TILs could predict regional lymph node metastasis and poor prognosis in gastric cancer [35]. EBVaGC is accompanied by CD8 CTLs and mature dendritic cells more often than non-EBVaGC. However, there are also particular mechanisms which allow EBV to evade the host immune response and allow infection to persist, as shown in Table 3.
Table 3.
Mechanisms of Immune Evasion in EBVaGC
| Mechanism of Immune Evasion | Description |
|---|---|
| Overexpression of IL-1β | |
| Overexpression of IFN-γ | |
| PDL-1 amplification | |
| Expression of early lytic gene-BNLF2α |
|
| LMP2A mutations |
|
| EBNA1 repeats and polymorphisms |
|
EBV can also activate several important cellular pathways which promote gastric cancer development. Wang et al [36] characterized EBVaGC-specific cellular pathways, and found alterations in macromolecular biosynthetic processes such as carbohydrate, lipid and protein biosynthesis. Deregulation of cholesterol transport and lipoprotein clearance pathways is also evident in EBVaGC [37]. Zhao et al showed 13 pathways to be deregulated in EBVaGC, including: neuroactive ligand-receptor interaction, pathways in cancer, mitogen-activated protein kinase (MAPK) signaling pathway, cytokine-cytokine receptor interaction, axon guidance, regulation of actin cytoskeleton, insulin signaling pathway, cell adhesion molecules, endocytosis, calcium signaling pathway, Wnt signaling pathway, glutamatergic synapse, and focal adhesion [26]. These mechanisms are altered to facilitate rapid tumor growth [36]. A gene set enrichment analysis of EBV-specific gene expression signatures using ingenuity pathway analysis by Sohn et al [38] revealed that EBVaGC had significant genetic alterations in pathways involving energy production and metabolism, with a downregulation of metabolism genes.
Small microRNAs (miRNAs) and long noncoding RNAs (IncRNAs) play important post-transcriptional gene regulatory roles in EBVaGC [39, 40]. EBV was the first virus in which miRNAs were discovered [40]. It contains approximately 25 miRNA precursors and 44 mature miRNAs, which are divided into two major clusters: (a) BART and (b) BHRF-1 [41]. The genes targeted by EBV miRNAs are associated with oncogenesis, cell adhesion, signal transduction and apoptosis, all of which contribute to carcinogenesis [41]. For instance, BART reduces the expression of Bid, which is an apoptotic molecule [40].
The host cellular miRNAs can also be downregulated by EBV latency genes, encouraging epithelial-to-mesenchymal transition. Two host miRNAs, has-miR-200a and has-miR-200b, are downregulated in EBVaGC, which reduces e-cadherin expression and triggers tumorigenesis [41].
IncRNAs are associated with larger tumors, greater invasion and reduced survival rates in gastric cancer [39]. Huang et al showed increased expression of an IncRNA, known as SNHG8 in EBVaGC [39]. SNHG8 interacts with EBV proteins, and amplifies carcinogenic pathways such as DNA repair, epithelial-mesenchymal transition and ribosomal function [39]. A summary of the pathophysiology of EBV tumorigenesis is shown in Figure 1.
Figure 1. Pathogenesis of EBVaGC.
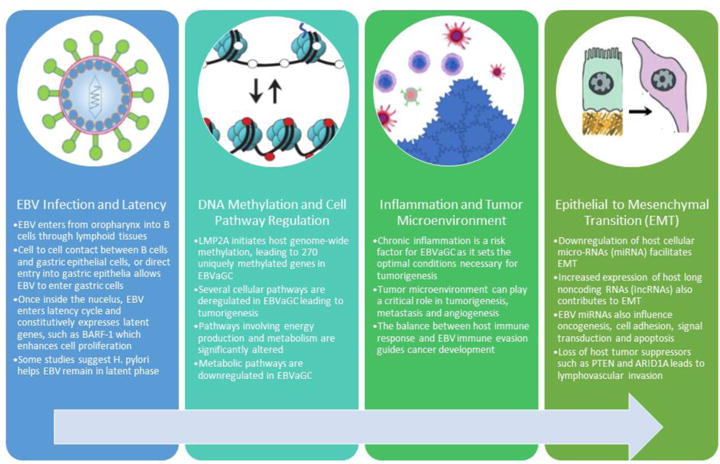
Although the pathogenesis of EBVaGC is not clearly established, several factors allow EBV to stimulate oncogenesis in the gastric epithelium. Once EBV infects the gastric epithelial cell, it enters its latency phase and leads to genome-wide methylation and cell pathway regulation. Aberrant genetic expression and the interaction of EBV infected gastric epithelial cell with the tumor microenvironment dictates cell fate, ultimately leading to cancer development and epithelial to mesenchymal transition.
Epidemiology of EBVaGC
Geographical Variance
The frequency of EBVaGC ranges from 1.3%–30.9% in different regions, with an average of 10% worldwide [33]. The pooled estimate of EBVaGC frequencies in North and South America, Asia and Europe is 9.9%, 8.3% and 9.2% respectively [15]. Table 4 provides a list of different countries with their EBVaGC frequencies reported to date.
Table 4.
EBVaGC frequencies around the world listed in ascending order
| Country | EBVaGC Frequency (%) |
Reference |
|---|---|---|
| Papa New Guinea | 1.3 | [89] |
| Pakistan | 1.9 | [90] |
| Peru | 4 | [83] |
| China-Guangzhou | 6.7 | [88] |
| Japan | 6.9 | [87] |
| Brazil | 7 | [19] |
| China-Beijing | 7.3 | [91] |
| Denmark | 8.5 | [53] |
| Malaysia | 10 | [92] |
| China-Tangshan | 10.6 | [48] |
| Iran | 11.1 | [93] |
| Colombia | 16 | [83] |
| United States | 16 | [94] |
| Chile | 17 | [87] |
| Zambia | 23 | [47] |
| Brunei | 30.9 | [92] |
Genetic polymorphisms have given rise to different EBV strains thought to contribute to the geographical variance observed in EBVaGC incidence (Table 5). Liu et al identified nine newly sequenced EBV genomes from EBVaGC, named EBVaGC1 to 9 [42]. Different studies have shown that both healthy controls and EBVaGC patients carry the same strain, suggesting these polymorphisms are restricted to geography, not disease-type [43].
Table 5.
Geographic distribution of EBVaGC polymorphisms
| Genotype | Properties | Regional Prevalence | Reference |
|---|---|---|---|
| Genotype A | Strong capacity to transform B cells into a state of continuous immortalization. Common in gastric remnant cancer. Increases risk of EBVaGC | Western and Asian countries | [83, 88] |
| Genotype B | Lower B cell transforming efficiency, poorer initial outgrowth, more common in immunosuppressed patients | Equatorial Africa | [17, 83] |
| Type D | Presence of restriction site at the BamHI W1/I1 region, common in recurrent gastric cancer | Western countries, Tunisia | [42] |
| Type C | Absence of restriction site at the BamHI W1/I1 region, common in gastric remnant cancer | Japan and China | [42] |
| Type f | Presence of restriction site at the BamHI F region | [42] | |
| Type F | Absence of restriction site at the BamHI F region, common in gastric remnant cancer | Southern China, Southern Japan, Latin America | [42] |
| P-ala | Signature changes at amino acid residue 487 of EBNA1. Common in LELC | [43] | |
| P-thr | Signature changes at amino acid residue 487 of EBNA1 | America | [43] |
| V-leu | Signature changes at amino acid residue 487 of EBNA1. Common in gastric remnant cancer and LELC | [17, 43] | |
| V-val | Signature changes at amino acid residue 487 of EBNA1 | China, Japan | [43] |
| V-pro | Signature changes at amino acid residue 487 of EBNA1 | [43] | |
| Wt-LMP1 | Intact LMP1 gene exon 3 | [43] | |
| Del-LMP1 | 30-bp sequence deletion in LMP1 gene exon 3 | [43] | |
| XhoI− | lack of XhoI restriction site at exon 1 of the LMP1 gene | Asia | [95] |
| XhoI+ | Retention of XhoI region on exon 1 of LMP1 gene. Increases risk of EBVaGC | Western countries | [83, 95] |
| EB-6m | EBER gene polymorphism | [42] | |
| EB-8m | EBER gene polymorphism | [42] | |
| EB-10m | EBER gene polymorphism | [42] |
Age and Sex Distribution
A large Dutch prospective randomized control trial, called D1D2, was conducted between 1989–1993 to prospectively study pathological and surgical features among 566 gastric cancer patients [44]. Beek et al conducted a subgroup analysis of D1D2 patients, and found EBVaGC to be significantly male predominant and associated with patients under age 60 [44]. Most meta-analyses have shown a strong association between EBVaGC and male gender, where reported male to female ratios are 2:1 or 3:1 [45].
EBVaGC is predominantly found in the proximal stomach [46]. The incidence of proximal gastric cancer has been on the rise among younger patients, as reported in United States [4] and Zambia [47]. However, data is conflicted about the association between EBVaGC and younger age. Meta-analyses from 2009 [45] and 2010 [48] failed to show the significance of age in EBVaGC. Conversely, in a 2011 meta-analysis by Camargo et al of 5081 gastric cancer patients, EBVaGC was more common among younger patients [49]. Most recently, Anderson et al conducted a large-scale epidemiological study using the North American Association of Central Cancer Registries (NAACR) database, and included patients diagnosed with gastric cancer from 1995 till 2013 [50]. They reported that the incidence of non-cardia gastric cancer (including the gastric fundus, body, antrum and pylorus) among non-Hispanic whites has been increasing among younger patients (<50 years) and decreasing among older patients (>50 years) across the US. This trend was of moderate significance among Hispanics, and not observed in Blacks or patients of other ethnicities. Hence, the distribution of EBVaGC is influenced by ethnicity and lifestyle factors; therefore, studies including diverse populations need to be conducted to better understand this relationship.
Risk Factors
Environmental risk factors could predispose one to developing EBVaGC. According to a meta-analysis by Bae et al [51], the risk of acquiring EBVaGC shows regional variation, with the highest risk observed in Far East Asia, which also has the highest incidence of gastric cancer.
Certain co-infections could also increase one’s risk, such as HIV and H pylori [1]. Kayamba et al reported EBVaGC among 60% of HIV patients in Zambia, suggesting HIV increases risk for EBV related gastric cancers [47].
Lifestyle factors, such as cigarette smoke, have been shown to reactivate EBV in gastric cell lines [52]. Camargo et al showed that EBVaGC is 2.4 times more frequent in current smokers and 2 times more frequent in former smokers [52]. Smoking also increases the risk of EBV-positive, but not EBV-negative, Hodgkin lymphoma [52].
In a study by Kim et al [37], a history of gastric ulcer increased the risk of EBVaGC, when compared with non-EBVaGC, suggesting that chemical injury through peptic ulcers could contribute to tumorigenesis. EBV is more frequently detected (approximately 35%) in postsurgical gastric remnants, where the injury and inflammation is believed to facilitate EBV entry [37]. Atrophic gastritis and pernicious anemia could also increase the risk of developing EBVaGC. Boysen et al conducted a nationwide Danish study showing patients with pernicious anemia were twice as likely to have EBVaGC then those without [53].
Clinical Features and Histopathology
EBV shows an anatomic preference during gastric tumorigenesis. EBVaGC is predominantly present in the proximal stomach, with reported rates being 11.6% in the cardia and 9.5% in the body of the stomach [48]. A Korean study by Park et al showed that 84.4% of EBVaGCs were located in the upper or middle third of the stomach [46].
Several studies have shown EBVaGC to be associated with a lower T stage (depth of tumor invasion), and N stage (nodal status) [54]. The D1D2 trial reported a lower frequency of lymph node metastases in EBVaGC [44]. A correlation with earlier clinical stage of gastric cancer was also found by Song et al, where 37.4% of EBVaGC patients presented in stage I, compared to only 4.9% of non-EBVaGC (p<0.0001) control [55].
EBVaGC and HER2
HER2 is a known proto-oncogene overexpressed in approximately 10–30% of GCs [56]. Whether HER2 positivity predicts a poor or better prognosis is currently controversial, and data regarding its relationship with EBVaGC is scarce. Zhang et al explored HER2 expression in 78 EBVaGC and 216 EBV-negative GCs and clinicopathologically matched cases using immunohistochemistry [21]. They found that HER2 expression is significantly reduced in EBVaGC compared with non-EBVaGC. They concluded that LMP2A down-regulates HER2 expression. Zhang et al showed that patients with LMP2A+/HER2low EBVaGC show better overall survival compared with LMP2A−/HER2low cases. This relationship needs to be explored further through translational studies.
Histopathology
EBVaGC frequently grows in ulcerated or saucer-like tumors featured by well-delineated and pushing borders [15]. In areas where mild to moderate gastritis is found, EBV is frequently found near the atrophic border [57]. It also displays moderate to poor degree of differentiation [15, 44, 58]. In the D1D2 trial, 76.2% of EBVaGC were poorly differentiated [44]. Inconsistency exists regarding Laurén classification, where some studies report a predominantly intestinal-type histology, whereas others report diffuse-type. Meta-analysis by Li et al [48] and a retrospective analysis by Kayamba et al [47] showed no association between Laurén histological type and EBVaGC. However, the D1D2 trial showed that 63.4% of EBVaGC cases exhibited intestinal histology, compared to 4.9% of diffuse-type [44]. According to the World Health Organization (WHO) histology classification, EBVaGC falls under the tubular type histology [44].
EBVaGC can be divided into three histologic subtypes based on the microscopic characterization of host cellular immune response [33]: 1) Lymphoepithelioma-like carcinoma (LELC), 2) Carcinoma with Crohn’s disease-like lymphoid reaction (CLR), which is defined by three or more lymphoid follicles with active germinal centers at the advancing edge of the tumor [41], and 3) Conventional-type adenocarcinoma (CA), which is classified by cases showing infiltration of scattered lymphocytes with prominent desmoplasia in the absence of lymphoid follicles, or with only one or two lymphoid aggregates per tissue section [59].
Prognosis and Survival
Whether EBVaGC is associated with improved survival or not is controversial. Current studies have largely included localized disease, and do not all adjust for TNM staging. Camargo et al reported median survival times for EBVaGC to be 8.5 years, compared to 5.3 years for EBV-negative tumors [54]. Song et al reported 5 year OS and DFS rates in EBVaGC of 71.4% and 67.5%, compared to 56.1% and 55.2% in control group, respectively [55, 59]. The survival advantage from EBV also seems to be regionally restricted. Liu et al showed a better OS among Asian patients, but not in European and American patients [60].
Recently, gene expression data from the TCGA cohort was used to identify subgroups with the highest survival advantage from adjuvant chemotherapy, and EBVaGC was associated with the best prognosis for both recurrence-free survival (p=0.006) and OS (p=0.004) [38]. Patients with GS subtype had the worst prognosis whereas those with MSI and CIN had a moderate prognosis. Prospective trials with outcome data are needed to better understand the prognostic value of EBV in gastric cancer.
Recurrence rates of EBVaGC are reported to be 0% in stage I, 21.1% in stage II, 33.3% in stage III, and 83.3% in stage IV [55]. According to Huang et al, tumor size > 5cm is an independent parameter of poor prognosis in EBVaGC stages I–III, instead of tumor depth of invasion [15].
Certain genetic changes in EBVaGC have prognostic significance as well. For instance, EBVaGC is associated with PD-L1 overexpression, and PDL1 overexpression in EBVaGC is correlated with poor OS and disease-specific survival [61]. Loss of tumor suppressors such as PTEN and ARID1A contributes to lymphovascular invasion and poor 5-year mortality among EBVaGC [62]. Mutations in EBVaGC leading to amplification of PIK3CA also lead to poor survival [63]. Ongoing trials testing the efficacy of targeted treatments against PD-L1 and PIK3CA mutations in EBVaGC are further discussed in potential treatments below.
EBV and Helicobacter pylori
Approximately 50% of the world’s population is infected with Helicobacter pylori, which induces gastric inflammation and may set the conditions necessary for EBV related epigenetic changes and tumorigenesis [1]. Hypermethylation and upregulation of inflammatory markers (TNFα, IL-1β) associated with EBVaGC has been linked to H. pylori [1]. Whether H. pylori and EBV work synergistically to develop EBVaGC is still being investigated. Cardenas-Mondragon et al published a study showing severe gastric lesions in pediatric patients who were co-infected with EBV and H. pylori [64]. They also showed that without EBV, H. pylori did not trigger severe gastritis, and concluded that both pathogens synergistically contribute to inflammation and damage to the gastric mucosa. Camargo et al also suggested that H. pylori catalase could dampen host oxidative stress response, and allow EBV to remain in latent phase for a longer duration, thereby enabling EBVaGC development [65]. EBV positive tumors show higher seroreactivity to catalase [65]. Interestingly, presence of EBV has been shown to increase the oncogenic potential of the H. pylori protein, CagA [1]. Conversely, a study by Shukla et al suggested that H. pylori might prevent EBVaGC by attenuating TGF-β expression, which is required for EBV reactivation [66].
Several studies do not show any relationship between the two pathogens. For instance, a study conducted by deSouza et al did not show any association between EBV infection and H pylori among 226 patients with gastritis and GC [19]. A meta-analysis done by Lee et al found no association between EBV and H. pylori status among 9738 patients in 48 articles [67]. Hence, conflicting results from studies make it difficult to draw conclusions about the relationship between H. pylori and EBV, and whether they synergistically contribute to GC remains unknown. Further studies are essential for understanding their relationship.
Potential Treatments
Although very limited data in patients is known, pre-clinical data has shown EBVaGC to be resistant to current chemotherapy options including docetaxel [68] and 5-FU [69]. However, the potential for testing clinical response to anti-PD1 inhibitors in EBVaGC is currently underway. Based on the KEYNOTE-059 phase II trial [11], the US Food and Drug Administration approved pembrolizumab for patients with PD-L1+ advanced GCs who have progressed on more than two chemotherapy regimens. This trial showed an improved Overall Response Rate (ORR) among PD-L1+ tumors, when compared with PD-L1− tumors (15.5% vs. 5.5%). Pembrolizumab has proven to be highly effective in treating EBV positive T-cell lymphomas refractory to two lines of chemotherapy [70].
Furthermore, ATTRACTION-2 is the first phase III trial to report OS benefit of an anti-PD-1 inhibitor, nivolumab, in GC patients [12]. This was a randomized, double-blind, placebo-controlled phase III trial including 493 Asian patients with advanced or recurrent gastric or gastroesophageal junction cancer refractory to at least two previous chemotherapy regimens, who were randomized to receive either nivolumab or placebo. Nivolumab showed a better median OS (5.3 vs 4.1 months), with doubling of the OS rate at 1 year (26.2% vs. 10.9%). Findings from the phase I/II CheckMate 032 [71] study show anti-tumor activity of nivolumab in GC patients who are of non-Asian ethnicity, suggesting the efficacy of immune checkpoint inhibitors is similar across different ethnicities.
Avelumab is a human IgG PD-L1 antibody currently being tested in the JAVELIN Gastric 100 trial (NCT02625610) [72], which is a global, randomized, open-label, phase 3 trial comparing maintenance therapy with avelumab versus continuation of first-line chemotherapy in patients with metastatic gastric cancer who have not progressed after 12 weeks of first-line oxaliplatin/fluoropyrimidine chemotherapy. Selected patients have no prior chemotherapy for advanced disease, and are not preselected based on PD-L1 expression. JAVELIN Gastric 300 is another phase 3 trial (NCT02625623) [73] comparing avelumab and best supportive care (BSC) with BSC with/without chemotherapy as third-line treatment for patients with recurrent or metastatic gastric or gastroesophageal cancer, and its most recent results have not shown OS benefits from single-agent avelumab when compared with chemotherapy. Recently, avelumab was shown to be beneficial [74] for a metastatic gastric cancer patient enrolled in the phase 1 JAVELIN Solid Tumor trial (NCT01772004) assessing safety and pharmacokinetics of avelumab [75]. This was a 57 year old female patient with EBV-positive metastatic gastric cancer refractory to three lines of chemotherapy, who received over 24 cycles of avelumab and showed significant resolution of metastases [74]. Hence, the potential of EBV as a biomarker of response to avelumab should be explored in future clinical trials.
Currently, there are actively recruiting phase II trials stratifying GC patients based on EBV status, and assessing response to anti-PD-1 inhibitors. NCT03257163 is a phase II trial assessing the efficacy of pembrolizumab in EBVaGC and MSI-high GCs in combination with capecitabine and radiation [76]. Another phase II trial (NCT02589496) is using whole exome and RNA sequencing to identify the association of GC subtypes (including EBV positivity) with treatment response to pembrolizumab in advanced GC patients who have failed first-line treatment [77]. Another open label, multi-arm, phase II trial (NCT02951091) is testing the efficacy of nivolumab in EBVaGC as second-line treatment [78]. A phase I trial (NCT03044743) is evaluating the safety of CRISPR-Cas9 mediated PD-1 knockout EBV-CTL cells (cytotoxic-T cells) in stage IV GCs [79].
EBVaGC also shows an enrichment of PIK3CA mutations, which is part of the PI3K-AKT-mTOR pathway leading to increased cell proliferation [80]. Various PI3K inhibitors have been studied in GC without definitive clinical results, and would be useful to study in EBVaGC. A phase I trial of an oral PI3K inhibitor, buparlisib (BKM120), showed a manageable safety profile in Japanese patients with solid tumors [81]. Another inhibitor of this pathway, everolimus, was evaluated in the randomized, double-blind phase III GRANITE-1 study in advanced gastric cancer patients who had progressed after one or two lines of systemic chemotherapy. Everolimus was given in combination with best supportive care. However, it did not show an OS benefit, with some improvement in median PFS (1.7 vs. 1.4 months) [82].
As one of the pathogenic mechanisms of EBVaGC is hypermethylation, demethylating agents such as 5-Aza cytidine have been investigated as potential treatments. In preclinical studies, 5-Aza has been shown to restore expression of all methylated genes, activate lytic infection and lead to cell lysis [32]. It is currently approved in blood malignancies and tested in vitro in colorectal cancers, but its role in EBVaGC needs to be further investigated [26]. As some EBV miRNAs inhibit apoptosis, specific targeting of such miRNAs and their precursors may be therapeutically effective [27].
Summary and Conclusions
Worldwide, most individuals are infected with EBV during childhood, which gives rise to EBVaGC in 75,000–90,000 people every year. We discussed unique environmental, genetic and virus-specific factors which enable tumorigenesis. Better understanding of the epidemiology of EBV related gastric cancers and other environmental co-variates will facilitate preventative efforts. Personalized medicine holds great promises in advancing cancer treatment and improving patient outcomes, especially the recent advancements in PD-1 inhibitors in gastric cancer. Identification of new biomarkers for targeted treatment development is essential, and the unique molecular and clinical features of EBVaGC must be utilized to improve outlooks for patients with this disease. EBV can serve as a biomarker for immunotherapy and therefore, better understanding of its epidemiology will enable dedicated trials for this unique patient population.
Highlights.
Approximately 10% of all gastric cancers are related to EBV infection
EBV associated gastric cancers have a distinct molecular and clinical profile
These tumors have higher PDL-1 expression, PIK3CA mutations and hypermethylation
The role of immunotherapy in EBV associated gastric cancer is currently unknown
EBV is a promising biomarker in gastric cancer
Acknowledgments
This work was partly supported by the National Cancer Institute [grant number P30CA014089[; The Gloria Borges WunderGlo Foundation-The Wunder Project; Dhont Family Foundation; San Pedro Peninsula Cancer Guild; Daniel Butler Research Fund; and Call to Cure Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
manuscript title: eBSTEIN-BARR VIRUS ASSOCIATED GASTRIC CANCER: A COMPREHENSIVE REVIEW
- Madiha Naseem
- Afsaneh Barzi
- Christine Brezden-Masley
- Alberto Puccini
- Martin D Berger
- Ryuma Tokunaga
- Francesca Battaglin
- Shivani Soni
- Michelle McSkane
- Wu Zhang
- Heinz-Josef Lenz
References
- 1.Singh S, Jha HC. Status of Epstein-Barr Virus Coinfection with Helicobacter pylori in Gastric Cancer. J Oncol. 2017;2017:3456264. doi: 10.1155/2017/3456264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan BA, et al. Improving Outcomes in Resectable Gastric Cancer: A Review of Current and Future Strategies. Oncology (Williston Park) 2016;30(7):635–45. [PubMed] [Google Scholar]
- 3.Kalnina Z, et al. Emerging blood-based biomarkers for detection of gastric cancer. World J Gastroenterol. 2015;21(41):11636–53. doi: 10.3748/wjg.v21.i41.11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Medical Journal. 2014;55(12):621–628. doi: 10.11622/smedj.2014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimi P, et al. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiology Biomarkers & Prevention. 2014;23(5):700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W. TCGA divides gastric cancer into four molecular subtypes: implications for individualized therapeutics. Chinese Journal of Cancer. 2014;33(10):469–470. doi: 10.5732/cjc.014.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristescu R, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nature Medicine. 2015;21(5):449–U217. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 8.Gunturu KS, et al. Gastric cancer and trastuzumab: first biologic therapy in gastric cancer. Therapeutic Advances in Medical Oncology. 2013;5(2):143–151. doi: 10.1177/1758834012469429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu LH, et al. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. Plos One. 2017;12(8) doi: 10.1371/journal.pone.0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee V, Le DT. Efficacy of PD-1 blockade in tumors with MMR deficiency. Immunotherapy. 2016;8(1):1–3. doi: 10.2217/imt.15.97. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs CS, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. Journal of Clinical Oncology. 2017;35 [Google Scholar]
- 12.Kang YK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 13.Silver B, Krell J, Frampton AE. Do miRNAs hold the key to controlling EBV-driven tumorigenesis? Future Virology. 2012;7(11):1045–1049. [Google Scholar]
- 14.Ning S. Innate immune modulation in EBV infection. Herpesviridae. 2011;2(1):1. doi: 10.1186/2042-4280-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SC, et al. Prognostic factors in Epstein-Barr virus-associated stage I–III gastric carcinoma: Implications for a unique type of carcinogenesis. Oncology Reports. 2014;32(2):530–538. doi: 10.3892/or.2014.3234. [DOI] [PubMed] [Google Scholar]
- 16.Truong CD, et al. Characteristics of Epstein-Barr virus-associated gastric cancer: A study of 235 cases at a comprehensive cancer center in USA. Journal of Experimental & Clinical Cancer Research. 2009;28 doi: 10.1186/1756-9966-28-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JN, et al. Epstein-Barr virus-associated gastric carcinoma: a newly defined entity. J Clin Gastroenterol. 2012;46(4):262–71. doi: 10.1097/MCG.0b013e318249c4b8. [DOI] [PubMed] [Google Scholar]
- 18.Ryan JL, et al. Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa. Dig Dis Sci. 2012;57(7):1887–98. doi: 10.1007/s10620-012-2116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Souza CRT, et al. Occurrence of Helicobacter pylori and Epstein-Barr virus infection in endoscopic and gastric cancer patients from Northern Brazil. Bmc Gastroenterology. 2014;14 doi: 10.1186/1471-230X-14-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, et al. Xenophagy in Helicobacter pylori- and Epstein-Barr virus-induced gastric cancer. J Pathol. 2014;233(2):103–12. doi: 10.1002/path.4351. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YW, et al. Epstein-Barr virus latent membrane protein 2A suppresses the expression of HER2 via a pathway involving TWIST and YB-1 in Epstein-Barr virus-associated gastric carcinomas. Oncotarget. 2015;6(1):207–220. doi: 10.18632/oncotarget.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He B, et al. Epstein-Barr virus-encoded miR-BART6-3p inhibits cancer cell metastasis and invasion by targeting long non-coding RNA LOC553103. Cell Death Dis. 2016;7(9):e2353. doi: 10.1038/cddis.2016.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yau TO, Tang CM, Yu J. Epigenetic dysregulation in Epstein-Barr virus-associated gastric carcinoma: disease and treatments. World J Gastroenterol. 2014;20(21):6448–56. doi: 10.3748/wjg.v20.i21.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneda A, et al. Epstein-Barr virus infection as an epigenetic driver of tumorigenesis. Cancer Res. 2012;72(14):3445–50. doi: 10.1158/0008-5472.CAN-11-3919. [DOI] [PubMed] [Google Scholar]
- 25.Chang MS, et al. CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr virus. Clinical Cancer Research. 2006;12(10):2995–3002. doi: 10.1158/1078-0432.CCR-05-1601. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, et al. Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells. Cancer. 2013;119(2):304–12. doi: 10.1002/cncr.27724. [DOI] [PubMed] [Google Scholar]
- 27.Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer. International Journal of Oncology. 2015;46(4):1421–1434. doi: 10.3892/ijo.2015.2856. [DOI] [PubMed] [Google Scholar]
- 28.Morris MA, et al. The Epstein-Barr virus encoded LMP1 oncoprotein modulates cell adhesion via regulation of activin A/TGF beta and beta 1 integrin signalling. Scientific Reports. 2016;6 doi: 10.1038/srep19533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang MS, et al. Epstein-Barr Virus-Encoded BARF1 Promotes Proliferation of Gastric Carcinoma Cells through Regulation of NF-kappa B. Journal of Virology. 2013;87(19):10515–10523. doi: 10.1128/JVI.00955-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng W, et al. N-terminal domain of BARF1 gene encoded by Epstein-Barr virus is essential for malignant transformation of rodent fibroblasts and activation of BCL-2. Oncogene. 2001;20(10):1176–1185. doi: 10.1038/sj.onc.1204217. [DOI] [PubMed] [Google Scholar]
- 31.Turrini R, et al. A BARF1-specific mAb as a new immunotherapeutic tool for the management of EBV-related tumors. Oncoimmunology. 2017;6(4) doi: 10.1080/2162402X.2017.1304338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukayama M, Ushiku T. Epstein-Barr virus-associated gastric carcinoma. Pathol Res Pract. 2011;207(9):529–37. doi: 10.1016/j.prp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Akiba S, et al. Epstein-Barr virus associated gastric carcinoma: Epidemiological and clinicopathological features. Cancer Science. 2008;99(2):195–201. doi: 10.1111/j.1349-7006.2007.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho J, Kang MS, Kim KM. Epstein-Barr Virus-Associated Gastric Carcinoma and Specific Features of the Accompanying Immune Response. Journal of Gastric Cancer. 2016;16(1):1–7. doi: 10.5230/jgc.2016.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang BW, et al. Prognostic value of tumor-infiltrating lymphocytes in Epstein-Barr virus-associated gastric cancer. Ann Oncol. 2016;27(3):494–501. doi: 10.1093/annonc/mdv610. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, et al. Interpreting the distinct and shared genetic characteristics between Epstein-Barr virus associated and non-associated gastric carcinoma. Gene. 2016;576(2 Pt 2):798–806. doi: 10.1016/j.gene.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Kim SY, et al. Deregulation of immune response genes in patients with Epstein-Barr virus-associated gastric cancer and outcomes. Gastroenterology. 2015;148(1):137–147 e9. doi: 10.1053/j.gastro.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Sohn BH, et al. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clinical Cancer Research. 2017;23(15):4441–4449. doi: 10.1158/1078-0432.CCR-16-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang T, et al. SNHG8 is identified as a key regulator of epstein-barr virus(EBV) –associated gastric cancer by an integrative analysis of IncRNA and mRNA expression. Oncotarget. 2016;7(49):80990–81002. doi: 10.18632/oncotarget.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinozaki A, et al. Downregulation of MicroRNA-200 in EBV-Associated Gastric Carcinoma. Cancer Research. 2010;70(11):4719–4727. doi: 10.1158/0008-5472.CAN-09-4620. [DOI] [PubMed] [Google Scholar]
- 41.Shinozaki-Ushiku A, et al. Profiling of Virus-Encoded MicroRNAs in Epstein-Barr Virus-Associated Gastric Carcinoma and Their Roles in Gastric Carcinogenesis. Journal of Virology. 2015;89(10):5581–5591. doi: 10.1128/JVI.03639-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu SZ, et al. Epstein-Barr Virus Infection in Gastric Remnant Carcinoma and Recurrent Gastric Carcinoma in Qingdao of Northern China. Plos One. 2016;11(2) doi: 10.1371/journal.pone.0148342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng N, et al. Is gastric lymphoepithelioma-like carcinoma a special subtype of EBV-associated gastric carcinoma? New insight based on clinicopathological features and EBV genome polymorphisms. Gastric Cancer. 2015;18(2):246–55. doi: 10.1007/s10120-014-0376-9. [DOI] [PubMed] [Google Scholar]
- 44.van Beek J, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22(4):664–70. doi: 10.1200/JCO.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 45.Murphy G, et al. Meta-analysis Shows That Prevalence of Epstein-Barr Virus-Positive Gastric Cancer Differs Based on Sex and Anatomic Location. Gastroenterology. 2009;137(3):824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JH, et al. Epstein-Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer. 2016;19(4):1041–1051. doi: 10.1007/s10120-015-0565-1. [DOI] [PubMed] [Google Scholar]
- 47.Kayamba V, et al. Serological response to Epstein-Barr virus early antigen is associated with gastric cancer and human immunodeficiency virus infection in Zambian adults: a case-control study. Pan African Medical Journal. 2016;23 doi: 10.11604/pamj.2016.23.45.8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, et al. Meta-analysis of the relationship between Epstein-Barr virus infection and clinicopathological features of patients with gastric carcinoma. Sci China Life Sci. 2010;53(4):524–30. doi: 10.1007/s11427-010-0082-8. [DOI] [PubMed] [Google Scholar]
- 49.Camargo MC, et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. British Journal of Cancer. 2011;105(1):38–43. doi: 10.1038/bjc.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson WF, et al. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst. 2018 doi: 10.1093/jnci/djx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae JM, Kim EH. Epstein-Barr Virus and Gastric Cancer Risk: A Meta-analysis With Meta-regression of Case-control Studies. J Prev Med Public Health. 2016;49(2):97–107. doi: 10.3961/jpmph.15.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camargo MC, et al. Case-case comparison of smoking and alcohol risk associations with Epstein-Barr virus-positive gastric cancer. International Journal of Cancer. 2014;134(4):948–953. doi: 10.1002/ijc.28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boysen T, et al. Epstein-Barr virus-associated gastric carcinoma among patients with pernicious anemia. Int J Cancer. 2011;129(11):2756–60. doi: 10.1002/ijc.25925. [DOI] [PubMed] [Google Scholar]
- 54.Camargo MC, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63(2):236–43. doi: 10.1136/gutjnl-2013-304531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song HJ, et al. Host Inflammatory Response Predicts Survival of Patients With Epstein-Barr Virus-Associated Gastric Carcinoma. Gastroenterology. 2010;139(1):84–U123. doi: 10.1053/j.gastro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Wang HB, Liao XF, Zhang J. Clinicopathological factors associated with HER2-positive gastric cancer: A meta-analysis. Medicine. 2017;96(44) doi: 10.1097/MD.0000000000008437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iizasa H, et al. Epstein-Barr Virus (EBV)-associated Gastric Carcinoma. Viruses-Basel. 2012;4(12):3420–3439. doi: 10.3390/v4123420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rymbai ML, et al. Frequency of Epstein - Barr virus infection as detected by messenger RNA for EBNA 1 in histologically proven gastric adenocarcinoma in patients presenting to a tertiary care center in South India. Indian Journal of Medical Microbiology. 2015;33(3):369–373. doi: 10.4103/0255-0857.158556. [DOI] [PubMed] [Google Scholar]
- 59.Song HJ, Kim KM. Pathology of Epstein-Barr Virus-Associated Gastric Carcinoma and Its Relationship to Prognosis. Gut and Liver. 2011;5(2):143–148. doi: 10.5009/gnl.2011.5.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X, et al. Epigenetic silencing of WNT5A in Epstein-Barr virus-associated gastric carcinoma. Arch Virol. 2013;158(1):123–32. doi: 10.1007/s00705-012-1481-x. [DOI] [PubMed] [Google Scholar]
- 61.Saito R, et al. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol. 2017;30(3):427–439. doi: 10.1038/modpathol.2016.202. [DOI] [PubMed] [Google Scholar]
- 62.Kang HJ, et al. Biomarkers of EBV-positive Gastric Cancers: Loss of PTEN Expression is Associated with Poor Prognosis and Nodal Metastasis. Annals of Surgical Oncology. 2016;23(11):3684–3692. doi: 10.1245/s10434-016-5284-2. [DOI] [PubMed] [Google Scholar]
- 63.Sunakawa Y, Lenz HJ. Molecular classification of gastric adenocarcinoma: translating new insights from the cancer genome atlas research network. Curr Treat Options Oncol. 2015;16(4):17. doi: 10.1007/s11864-015-0331-y. [DOI] [PubMed] [Google Scholar]
- 64.Cardenas-Mondragon MG, et al. Epstein Barr virus and Helicobacter pylori co-infection are positively associated with severe gastritis in pediatric patients. PLoS One. 2013;8(4):e62850. doi: 10.1371/journal.pone.0062850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camargo MC, et al. Anti-Helicobacter pylori Antibody Profiles in Epstein-Barr virus (EBV)-Positive and EBV-Negative Gastric Cancer. Helicobacter. 2016;21(2):153–7. doi: 10.1111/hel.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shukla SK, et al. Transforming growth factor beta 1 (TGF-beta1) modulates Epstein-Barr virus reactivation in absence of Helicobacter pylori infection in patients with gastric cancer. Cytokine. 2016;77:176–9. doi: 10.1016/j.cyto.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 67.Lee JH, et al. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: A meta-analysis. Journal of Gastroenterology and Hepatology. 2009;24(3):354–365. doi: 10.1111/j.1440-1746.2009.05775.x. [DOI] [PubMed] [Google Scholar]
- 68.Shin HJ, Kim DN, Lee SK. Association between Epstein-Barr virus infection and chemoresistance to docetaxel in gastric carcinoma. Mol Cells. 2011;32(2):173–9. doi: 10.1007/s10059-011-0066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin JY, et al. LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein-Barr virus-positive gastric cancer cells. BMC Cancer. 2010;10:425. doi: 10.1186/1471-2407-10-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwong YL, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing L-asparaginase. Blood. 2017;129(17):2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 71.Janjigian YYBJ, Calvo E, Kim JW, Ascierto PA, Sharma P. CheckMate-032: Phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC) Journal of Clinical Oncology. 2016;35(supplement 15):4014. [Google Scholar]
- 72.Moehler MHRM, Lee KW, Coskun HS, WOng R, Ozguroglu M. JAVELIN Gastric 100: Phase 3 trial of avelumab (anti-PD-L1) maintenance therapy versus continuation of first-line chemotherapy in patients with advanced gastric or gastroesophageal junction cancer (GC/GEJC) Journal of Clinical Oncology. 2018;36(supplement 4) [Google Scholar]
- 73.Bang YJ, et al. Avelumab (MSB0010718C; anti-PD-L1) + best supportive care (BSC) vs BSC chemotherapy as third-line treatment for patients with unresectable, recurrent, or metastatic gastric cancer: The phase 3 JAVELIN Gastric 300 trial. Journal of Clinical Oncology. 2016;34(15) [Google Scholar]
- 74.Panda A, et al. Immune Activation and Benefit From Avelumab in EBV-Positive Gastric Cancer. J Natl Cancer Inst. 2018;110(3):316–320. doi: 10.1093/jnci/djx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heery CR, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncology. 2017;18(5):587–598. doi: 10.1016/S1470-2045(17)30239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.S J. Pembrolizumab, Capecitabine, and Radiation Therapy in Treating Patients With Mismatch-Repair Deficient and Epstein-Barr Virus Positive Gastric Cancer. 2017 Available from: https://clinicaltrials.gov/ct2/show/NCT03257163.
- 77.J L. Study of Pembrolizumab in Subjects With Advanced Gastric or Gastroesophageal Junction Adenocarcinoma Who Progressed After First-Line Therapy With Platinum and Fluoropyrimidine: Integration of Molecular Subtypes Through Integrative Genomic Analysis. 2017 Available from: https://clinicaltrials.gov/ct2/show/NCT02589496.
- 78.SY R. Biomarker-Integrated Umbrella, Advanced Gastric Cancer. 2016 Available from: https://clinicaltrials.gov/ct2/show/NCT02951091.
- 79.B L. PD-1 Knockout EBV-CTLs for Advanced Stage Epstein-Barr Virus (EBV) Associated Malignancies. 2017 Available from: https://clinicaltrials.gov/ct2/show/NCT03044743.
- 80.Ang YLE, Yong WP, Tan P. Translating gastric cancer genomics into targeted therapies. Critical Reviews in Oncology Hematology. 2016;100:141–146. doi: 10.1016/j.critrevonc.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Ando Y, et al. Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Science. 2014;105(3):347–353. doi: 10.1111/cas.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohtsu A, et al. Everolimus for Previously Treated Advanced Gastric Cancer: Results of the Randomized, Double-Blind, Phase III GRANITE-1 Study. Journal of Clinical Oncology. 2013;31(31):3935–+. doi: 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ordonez P, et al. Identification of The Distinctive Type i/XhoI plus Strain of Epstein-Barr Virus in Gastric Carcinoma in Peru. Anticancer Research. 2011;31(10):3607–3613. [PubMed] [Google Scholar]
- 84.Wang AL, et al. Differential expression of EBV proteins LMP1 and BHFR1 in EBV-associated gastric and nasopharyngeal cancer tissues. Molecular Medicine Reports. 2016;13(5):4151–4158. doi: 10.3892/mmr.2016.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu F, et al. EBNA1 binding and epigenetic regulation of gastrokine tumor suppressor genes in gastric carcinoma cells. Virology Journal. 2014;11 doi: 10.1186/1743-422X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukayama M, Hino R, Uozaki H. Epstein-Barr virus and gastric carcinoma: virus-host interactions leading to carcinoma. Cancer Sci. 2008;99(9):1726–33. doi: 10.1111/j.1349-7006.2008.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strong MJ, et al. Differences in Gastric Carcinoma Microenvironment Stratify According to EBV Infection Intensity: Implications for Possible Immune Adjuvant Therapy. Plos Pathogens. 2013;9(5) doi: 10.1371/journal.ppat.1003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen JN, et al. Epstein-Barr virus genome polymorphisms of Epstein-Barr virus-associated gastric carcinoma in gastric remnant carcinoma in Guangzhou, southern China, an endemic area of nasopharyngeal carcinoma. Virus Research. 2011;160(1–2):191–199. doi: 10.1016/j.virusres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 89.Morewaya J, et al. Epstein-Barr virus-associated gastric carcinoma in Papua New Guinea. Oncology Reports. 2004;12(5):1093–1098. [PubMed] [Google Scholar]
- 90.Anwar MKC, Naveed IA, Hamid S, Ahmad M, Itoh T, et al. Ebstein-barr virus detection in tumors of upper gastrointestinal tract. An in situ hybridization study in Pakistan. J Exp Clin Cancer Research. 2005;24:379–385. [PubMed] [Google Scholar]
- 91.Liu Y, et al. Genome-wide analysis of Epstein-Barr virus (EBV) isolated from EBV-associated gastric carcinoma (EBVaGC) Oncotarget. 2016;7(4):4903–4914. doi: 10.18632/oncotarget.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yen RL, et al. Profiles of Epstein-Barr virus associated gastric carcinomas in Brunei Darussalam. Asian Pac J Cancer Prev. 2014;15(23):10489–93. doi: 10.7314/apjcp.2014.15.23.10489. [DOI] [PubMed] [Google Scholar]
- 93.Leila Z, et al. Detection of Epstein-Barr Virus and Cytomegalovirus in Gastric Cancers in Kerman, Iran. Asian Pac J Cancer Prev. 2016;17(5):2423–8. [PubMed] [Google Scholar]
- 94.Shibata D, et al. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139(3):469–74. [PMC free article] [PubMed] [Google Scholar]
- 95.Hu LF, et al. Isolation and Sequencing of the Epstein-Barr-Virus Bnlf-1 Gene (Lmp1) from a Chinese Nasopharyngeal Carcinoma. Journal of General Virology. 1991;72:2399–2409. doi: 10.1099/0022-1317-72-10-2399. [DOI] [PubMed] [Google Scholar]


