Abstract
Background
Empyema affects up to 65,000 patients annually in the United States. Recent consensus guidelines demonstrate ambiguity about optimal treatment. We examined current treatment practices and outcomes for inpatient treatment of empyema using a comprehensive, longitudinal dataset that encompasses an entire state cohort of hospitalized patients.
Methods
We queried the Healthcare Cost and Utilization Project (HCUP) New York State Inpatient Database (2009–2014) for patients with primary empyema and subsequent readmissions. Patients were categorized into three groups by definitive treatment during their initial hospitalization: chest tube drainage, VATS decortication/drainage, or open decortication/drainage. Treatment outcomes including success rates, readmission, reintervention, and mortality were compared between groups.
Results
The cohort included 4,095 patients undergoing intervention for primary empyema discharged during this period with chest tube, VATS, or open drainage and decortication. The majority of patients received definitive operative management (chest tube: 38.2%, VATS: 32.1%, open: 29.8%, p<0.001). Patients had a high mortality rate during their initial hospitalization (chest tube: 15.4%, VATS: 4.7%, open: 6.0%, p<0.001), and a substantial 30-day readmission rate for empyema (chest tube: 7.3%, VATS: 3.8%, open: 4.1%, p<0.001), with reintervention at readmission significantly higher for chest tube (6.1%) vs surgical patients (VATS: 1.9%, open 2.1%, p<0.001).
Conclusions
This study characterizes recent treatment practices of patients with empyema. Higher readmission and reintervention rates were observed in patients managed with chest tubes, suggesting some of these patients may benefit from earlier definitive surgical intervention.
Empyema is a collection of purulent fluid in the pleural space that can occur with pneumonia or secondary to thoracic surgery or chest injury, affecting up to 65,000 patients annually at an estimated cost of 500 million dollars [1], with a mortality of approximately 15% [2]. Empyema evolves through exudative, fibrinopurulent, and organized phases over the course of 3–6 weeks. Treatment includes both antibiotics and complete drainage of the infected fluid, which can be accomplished with a range of interventions.
Initial operative versus non-operative management is controversial. Recent consensus guidelines state further study is needed to examine the use of chest tubes as first line treatment [2]. Small retrospective studies have shown failure rates between 38–47% [3,4], with radiographic loculations among the greatest predictors of failure, and failure of first line treatment associated with increased mortality. Fibrinolytics are sometimes utilized, however a meta-analysis showed mixed data regarding the subsequent need for an operation [5]. A review on the usage of VATS in empyema found higher success rates than chest tube drainage, and improved patient-centered metrics such as length of stay, pain, and complications compared to open drainage, though increasing rates of conversion to an open operation were noted as the empyema became more organized [6]. No consensus currently exists about when each drainage option is the optimal initial treatment.
We studied current treatment and outcomes of primary empyema in hospitalized patients managed medically and surgically. We sought to understand the current practice patterns for empyema management, characterize the types of patients that receive varied treatment modalities, and examine what happens to these patients during their initial hospitalization and over time. We hypothesized that patients managed with more conservative measures would be those who were more acutely ill and would require reintervention more frequently.
Patients and Methods
Patient Cohort
Data Sources
The cohort of patients was created by querying the Healthcare Cost and Utilization Project (HCUP) New York State Inpatient Database (NY SID) [7] for hospitalizations for empyema. This dataset was selected because it includes all inpatient visits to acute care community hospitals statewide, allowing for a comprehensive epidemiologic analysis of patients undergoing bedside or medical procedures and surgical operations in the full range of hospital sizes and settings. A unique identifier is provided to each patient within this dataset for linkage to subsequent admissions, allowing longitudinal follow up of outcomes throughout the years of the study. This study utilized a deidentified administrative database, exempting it from institutional IRB approval.
Inclusion Criteria
The study population included adults ≥ age 18 undergoing any invasive treatment for primary empyema. Empyema was defined using ICD-9 codes 510.9 and 511.1, capturing ‘empyema without mention of a fistula’ and ‘pleurisy with effusion, with mention of a bacterial cause’. These codes were chosen to capture patients with definite documented infection in the pleural space. Invasive treatment was defined using ICD-9 codes 34.04 for chest tube drainage, 34.06 and 34.52 for video assisted thoracoscopic surgery (VATS) drainage and decortication, and 34.09 and 34.51 for open drainage or decortication. Fibrinolytic usage was not analyzed since this was not consistently captured in ICD-9 codes. Index discharges from 2009 through the first two quarters of 2014 were utilized because there was demonstrated code stability, and therefore reliable distinction of open versus minimally invasive treatment, after the introduction of the VATS drainage and decortication codes. Only patients who were residents of New York were included, since residence elsewhere would compromise the ability to track subsequent admissions within this statewide dataset.
Exclusion Criteria
Individuals with empyema secondary to a surgical intervention were excluded. Patients were excluded for: thoracic surgery within the previous year; cardiac or upper abdominal surgery within the previous month; a diagnosis of empyema with fistula (ICD-9 code 510.0 or procedure code 34.73 indicating closure of a fistula) in the previous year; or codes indicating that the empyema was a complication of another procedure (see full list of code definitions in Supplemental Table 1). Patients were also excluded if their first intervention for the empyema was performed greater than 10 days after admission to the hospital, suggesting that empyema was not the primary problem at admission.
Variable Definitions
Patient comorbidities were defined using the Elixhauser classification including the index admission and hospitalizations during the prior year [8, 9]. Septicemia and shock during the index hospitalization were also captured. Abstracted demographic variables included: age, gender, race, year of treatment. Hospital factors abstracted from the American Hospital Association Annual Survey (Health Forum, LLC, Chicago, IL) included: size, teaching status, and rural-urban location.
Treatment Groups
Initial management was defined as the first therapeutic procedure performed of: chest tube, VATS, or open drainage. Definitive management during the index hospitalization was defined as the most invasive intervention performed within the three groups. Procedures that were started as VATS and converted intraoperatively to open thoracotomies are captured as open cases within the HCUP database.
Outcomes
Treatment success was defined as management with a single procedure during the index hospitalization, no reintervention within 30 days, and survival through 30 days of follow up. Management that occurred during hospital transfers (defined when a subsequent admission date was the same as the calculated day of discharge) was included for analysis as part of the initial management of the empyema. Follow up at 30 and 90 days was evaluated after discharge from either the index hospitalization discharge or discharge from the hospital the patient was transferred to, if applicable. Reintervention was defined as receipt of thoracentesis (ICD-9 code 34.91), chest tube placement, VATS, or open drainage during a subsequent hospitalization as any of these procedures implied incomplete resolution of the parapneumonic fluid and therefore unsuccessful initial therapy. Readmission for empyema was defined as presence of an empyema code during a subsequent hospitalization.
Statistical Analysis
Statistical analyses were performed using SAS statistical software version 9.3 (SAS Institute, Inc., Cary, NC). Continuous variables were compared between groups using the Kruskal-Wallis test. Categorical variables were compared with chi-square tests and the Jonckheere-Terpstra Test for trend which allowed comparison of three groups. Statistical significance was defined by p-values <0.05.
Results
Patient Eligibility
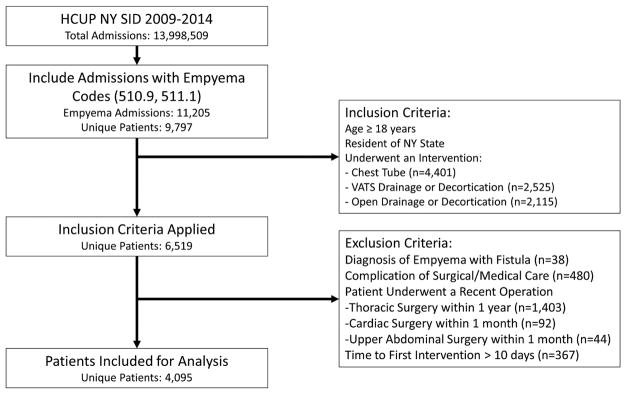
Between 2009 and 2014, the HCUP NY SID captured 11,205 discharges coded for empyema, representing 9,797 unique patients. Application of inclusion criteria to select adult New York residents undergoing an intervention for empyema resulted in a total of 6,519 patients. An additional 2,057 patients for whom the empyema was a complication of surgery and 367 patients with first intervention greater than 10 days following admission were excluded, resulting in 4,095 patients for analysis. Details are illustrated in Figure 1.
Figure 1.
Patient Flow Diagram. This shows the application of inclusion and exclusion criteria, and the resulting patient records used for analysis. Code and treatment frequencies shown here correspond to the percentage of patient records that carried these diagnoses or procedures in any of their codes; many patients had more than one relevant diagnosis or procedure.
Initial Treatment Success during Index Hospitalization by Category of Procedure
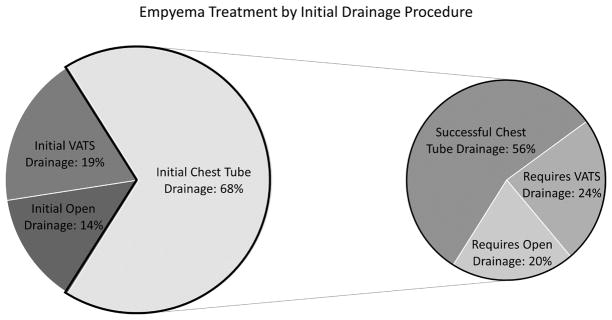
Initial chest tube placement was performed in 67.8% (2,780) of patients, whereas upfront VATS was performed in 18.5% (758) and upfront open operation in 13.6% (557). For patients with initial chest tube drainage, nonsurgical treatment during the index hospitalization was achieved in 56% (1,563), 24% (665) subsequently required VATS, and 20% (552) subsequently required an open operation (Figure 2). Of the patients for whom initial VATS treatment was attempted, 85% (648) were treated successfully and 15% (110) ultimately required an open procedure. While these numbers represent the treatment rates with each category of intervention performed, these patients frequently required more than one procedure of that type during the initial hospitalization.
Figure 2.
Initial Management Breakdown and Success of Chest Tube Drainage during the Index Hospitalization.
Single-Procedure Treatment Success
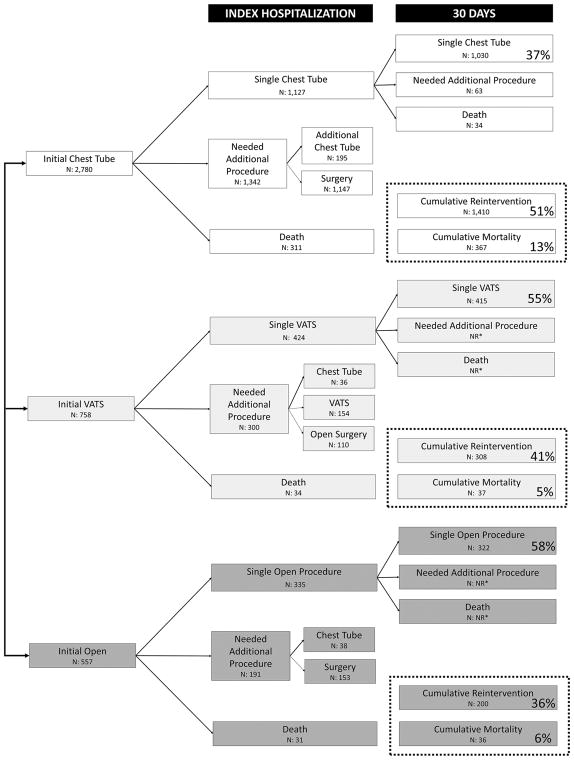
Treatment success with a single procedure during the index hospitalization, no reintervention through 30 days, and survival at 30 days was achieved in 37% of patients managed with a chest tube, 55% of patients managed with VATS, and 58% of patients managed with an open procedure (p<0.001). A flow diagram of the management of these patients including reintervention and mortality rates by initial treatment group through 30 days can be seen in Figure 3. It was common for patients to require multiple procedures across all treatment modalities. Length of stay was significantly shorter for patients with single-procedure treatment success with a median of 12 days (IQR 9–19 days) versus 15 days when multiple procedures were required (IQR 10–22 days, p<0.001).
Figure 3.
Success Rates with a Single Procedure by Initial Intervention. (NR*=not reported. HCUP prohibits reporting numbers of records <11.) Percentages correspond to the proportion of each initial treatment group (chest tube, VATS, or open) with the outcome of interest: single procedure success, reintervention, and mortality.
Definitive Treatment Trends over Time
During the five and a half year study period, rates of definitive treatment of empyema during the index hospitalization remained relatively stable (Figure 4). Overall, the majority received definitive surgical management (p<0.001): 1,563 patients (38%) were managed with chest tube drainage, 1,313 (32%) managed with VATS, and 1,219 (30%) managed with an open procedure. There was a small increase in VATS procedures (29% to 33%) and decrease in open procedures (33% to 27%) from 2009 to 2014 that was not statistically significant (p=0.07).
Figure 4.
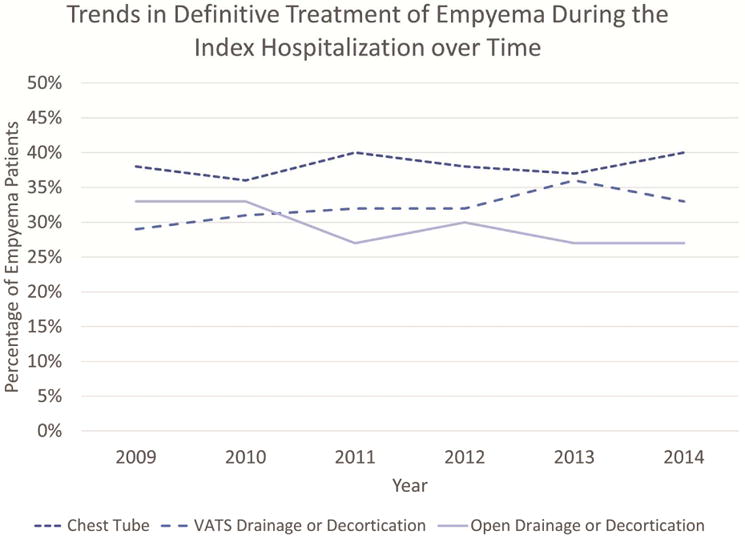
Definitive treatment options have remained stable over time.
Characteristics by Definitive Treatment Provided
Patient Characteristics
Patients managed operatively were younger (median age VATS: 56 years, open: 57 years, chest tube: 64 years, p<0.001) and less acutely and chronically ill than patients managed definitively with a chest tube. Nonsurgically managed patients had significantly higher rates of major medical comorbidities: congestive heart failure, valvular disease, peripheral vascular disease, pulmonary hypertension, chronic pulmonary disease, diabetes with complications, renal disease, metastatic cancer, coagulopathy, and anemia of chronic disease (all p≤0.02). The diagnosis of shock (chest tube: 15% vs VATS: 8% and open: 10%, p<0.001) and septicemia (chest tube: 41% vs VATS: 34% and open: 33%) were much higher in nonsurgical versus surgical patients There was no significant difference between the groups with regards to race or clinically meaningful difference in median income. (Table 1)
Table 1.
Descriptive Characteristics by Definitive Treatment Strategy during the Initial Hospitalization
| Median (IQR) or n (%) | Median (IQR) or n (%) | Median (IQR) or n (%) | ||
|---|---|---|---|---|
| Variable | Chest Tube | VATS | Open | p |
| n=4,095 | 1,563 (38.2%) | 1,313 (32.1%) | 1,219 (29.8%) | |
| Patient Factors | ||||
| Age | 64 years (52–79) | 56 years (45–69) | 57 years (47–69) | <0.001 |
| Race | 0.331 | |||
| White | 1,102 (70.5%) | 896 (68.2%) | 814 (66.8%) | |
| Black | 147 (9.4%) | 134 (10.2%) | 135 (11.1%) | |
| Hispanic | 145 (9.3%) | 115 (8.8%) | 122 (10.0%) | |
| Other | 169 (10.8%) | 168 (12.8%) | 148 (12.1%) | |
| Median Income by Zipcode | 0.020 | |||
| Quartile 1 | 293 (18.6%) | 210 (16.0%) | 213 (17.5%) | |
| Quartile 2 | 335 (21.4%) | 286 (21.8%) | 282 (23.1%) | |
| Quartile 3 | 377 (24.1%) | 306 (23.3%) | 241 (19.8%) | |
| Quartile 4 | 486 (31.1%) | 465 (35.4%) | 423 (34.7%) | |
| Missing | 72 (4.6%) | 46 (3.5%) | 60 (4.9%) | |
| Comorbidities | ||||
| Congestive Heart Failure | 366 (23.4%) | 144 (11.0%) | 155 (12.7%) | <0.001 |
| Valve Disease | 142 (9.1%) | 61 (4.7%) | 67 (5.5%) | <0.001 |
| Pulmonary Circulation Disease | 145 (9.3%) | 66 (5.0%) | 85 (7.0%) | <0.001 |
| Peripheral Vascular Disease | 118 (7.6%) | 41 (3.1%) | 53 (4.4%) | <0.001 |
| Chronic Pulmonary Disease | 518 (33.1%) | 338 (25.7%) | 344 (28.2%) | <0.001 |
| Diabetes without Complications | 345 (22.1%) | 258 (19.7%) | 275 (22.6%) | 0.151 |
| Diabetes with Complications | 101 (6.5%) | 57 (4.3%) | 56 (4.6%) | 0.020 |
| Renal Disease | 274 (17.5%) | 127 (9.7%) | 131(10.8%) | <0.001 |
| Liver Disease | 111 (7.1%) | 76 (5.8%) | 60 (4.9%) | 0.051 |
| Metastatic Cancer | 149 (9.5%) | 37 (2.8%) | 52 (4.3%) | <0.001 |
| Localized Tumor | 126 (8.1%) | 31 (2.4%) | 49 (4.0%) | <0.001 |
| Rheumatic Disease | 54 (3.5%) | 41 (3.1%) | 41 (3.4%) | 0.880 |
| Coagulopathy | 220 (14.1%) | 115 (8.8%) | 110 (9.0%) | <0.001 |
| Obesity | 134 (8.6%) | 146 (11.1%) | 139 (11.4%) | 0.022 |
| Acute Blood Loss Anemia | 38 (2.4%) | 20 (1.5%) | 30 (2.5%) | 0.165 |
| Anemia of Chronic Disease | 562 (36.0%) | 405 (30.9%) | 363 (29.8%) | <0.001 |
| Alcohol Abuse | 124 (7.9%) | 108 (8.2%) | 103 (8.5%) | 0.883 |
| Drug Use | 84 (5.4%) | 94 (7.2%) | 96 (7.9%) | 0.023 |
| Psychiatric Disease | 100 (6.4%) | 86 (6.6%) | 67 (5.5%) | 0.491 |
| Septicemia | 646 (41.3%) | 442 (33.7%) | 401 (32.9%) | <0.001 |
| Shock | 237 (15.2%) | 103 (7.8%) | 123 (10.1%) | <0.001 |
| Hospital/Treatment Factors | ||||
| Time to Intervention | 2 days (1–5) | 3 days (1–5) | 3 days (1–5) | <0.001 |
| Hospital Size | <0.001 | |||
| 0–99 beds | 57 (3.7%) | 33 (2.5%) | 33 (2.7%) | |
| 100–299 beds | 380 (24.3%) | 324 (24.7%) | 352 (28.9%) | |
| 300–499 beds | 485 (31.0%) | 346 (26.4%) | 287 (23.5%) | |
| 500+ beds | 641 (41.0%) | 610 (46.5%) | 547 (44.9%) | |
| Teaching Hospital | 1,124 (71.9%) | 935 (71.2%) | 821 (67.4%) | 0.079 |
| Urban Hospital Location | 1,469 (94.0%) | 1,267 (96.5%) | 1,165 (95.6%) | 0.009 |
| Diagnosis Codes | ||||
| 510.9 | 1,487 (95.1%) | 1,298 (98.9%) | 1,197 (98.2%) | <0.001 |
| 511.1 | 135 (8.6%) | 60 (4.6%) | 68 (5.6%) | <0.001 |
Hospital Characteristics
There was a trend towards less invasive surgical management in larger hospitals: patients managed in hospitals with at least 300 beds were more likely to undergo a VATS procedure than open drainage, whereas the opposite was true in smaller hospitals (p<0.001). There was no significant difference in management strategy between academic and nonacademic centers, and the overwhelming majority of these patients were treated in urban hospitals (≥94% for all treatment modalities). (Table 1)
Outcomes by Definitive Treatment Modality
In-hospital stay was longest for patients undergoing open drainage or decortication (median: 15 days vs chest tube: 14 days and VATS: 12 days, p<0.001). Overall, these patients had a high mortality rate during the index admission (chest tube: 15.4%, VATS: 4.7%, open: 6.0%, p<0.001), that continued to increase at 30 and 90 days. Mortality was lowest at all time points for VATS patients (5.4% and 6.3%, vs chest tube: 18.3% and 20.6%, open: 6.8% and 7.5% at 30 and 90 days, respectively, p<0.001).
Reintervention and readmission rates were also higher for primary chest tube management, with 21% readmitted at 30 days, and 6–7% readmitted with empyema and requiring further intervention. Readmission rates at 30 days were lower for patients receiving VATS and open treatment: 11.5% and 13.4%, respectively (p<0.001), as was the reintervention rate (VATS: 1.9% and open 2.1%). This trend persisted at 90 days (Table 2).
Table 2.
In-hospital, Mortality, Readmission, and Reintervention Outcomes by Definitive Treatment
| Chest Tube | VATS Drainage or Decortication | Open Drainage or Decortication | ||
|---|---|---|---|---|
| n=4,095 | 1,563 (38.2%) | 1,313 (32.1%) | 1,219 (29.8%) | |
| Outcome Measure | n (%) | n (%) | n (%) | p |
| Length of Stay (Median and IQR) | 14 days (9–22) | 12 days (9–19) | 15 days (10–21) | <0.001 |
| Mortality (Index Hospitalization) | 241 (15.4%) | 62 (4.7%) | 73 (6.0%) | <0.001 |
| 30 Day Mortality | 286 (18.3%) | 71 (5.4%) | 83 (6.8%) | <0.001 |
| 90 Day Mortality | 322 (20.6%) | 83 (6.3%) | 91 (7.5%) | <0.001 |
| 30 Day Readmission for Any Reason | 276 (20.9%) | 144 (11.5%) | 154 (13.4%) | <0.001 |
| 30 Day Readmission for Empyema | 96 (7.3%) | 48 (3.8%) | 47 (4.1%) | <0.001 |
| 90 Day Readmission for Any Reason | 391 (29.6%) | 236 (18.9%) | 236 (20.6%) | <0.001 |
| 90 Day Readmission for Empyema | 113 (8.9%) | 55 (4.4%) | 63 (5.6%) | <0.001 |
| 30 Day Reintervention Rate | 80 (6.1%) | 24 (1.9%) | 24 (2.1%) | <0.001 |
| 90 Day Reintervention Rate | 113 (8.8%) | 37 (3.0%) | 45 (4.0%) | <0.001 |
Comment
We evaluated the current state of empyema management using a comprehensive statewide database to capture patients treated both in and out of the operating room. Notably, we were able to evaluate patients treated with chest tubes, which are not included in purely surgical databases. The inclusion of all hospitalized patients in New York state provides a complete picture of current treatment practices and outcomes for empyema across inpatient practice settings and types. The longitudinal nature of this dataset allowed for assessment of mortality and morbidity outcomes of these three treatment paradigms. These features of the HCUP NY SID allowed for a unique analysis of empyema management.
Our data shows persistent high rates of initial chest tube utilization, although prior studies demonstrated high failure rates of this treatment modality [3, 4] and the majority of patients in our study ultimately required definitive operative intervention. Over half of the patients initially treated with a chest tube required multiple procedures during the index hospitalization and 9% required reintervention within 90 days. Chest tube clogging or dislodgement are known to be common problems [10], and even with appropriate tube function, this intervention alone may not provide complete reexpansion of the lung with drainage in the setting of loculations and organization of the empyema. In these settings, a trial of chest tube drainage may delay definitive therapy.
We found that when the first treatment was successful, hospital stay was significantly shorter by 3 days. Current guidelines provide a general protocol for treatment of empyema: patients with early empyema should be treated with drainage; patients in the fibrinopurulent stage can be treated with VATS or possibly fibrinolytics; and patients in the organized stage need a decortication to remove the rind and fully reexpand the lung [2]. In patients who are appropriate for operative intervention and a surgeon can identify that one will be needed, the intervention should occur as early as possible as these are not elective cases. While it may seem obvious that immediate provision of definitive treatment improves patient outcomes, our data show that this is not typical: only 53% of patients overall were treated with a single procedure. Furthermore, these patients have high rates of readmission and reintervention within thirty and ninety days, especially with nonsurgical management. When categorizing patients by definitive treatment strategy, both readmission rates specifically for empyema (7.3% vs 3.8–4.1%) and reintervention for patients managed with chest tube drainage (6.1% vs 1.9–2.1% at 30 days) were substantially higher than the rates for patients managed surgically. Timely provision of definitive treatment may improve outcomes.
Interestingly, surgically managed patients also frequently required multiple procedures during the index hospitalization. This highlights the complexity of empyema management: effective surgical treatment mandates drainage of all purulent fluid collections as well as removal of granulation tissue to reexpand the lung and obliterate the infected space. The quality of care delivered may vary widely between surgeons and hospitals. These differences are difficult to measure and no quality standards currently exist.
Finally, mortality rates remained surprisingly high ranging from 5.4–18.3% within 30 days, depending on the treatment modality. We noted substantially higher mortality rates in patients treated with chest tubes, likely due in part to the higher rates of underlying serious systemic comorbidities and higher rates of septicemia and shock on presentation. Some of this increased risk may also be due to inadequate source control and failure to provide timely definitive therapy.
Our study has several limitations inherent to use of an administrative database. First, data regarding whether the empyema was in the exudative, fibrinopurulent, or organizing stage was unavailable because radiographic and temporal historical details are not included in HCUP. This clinical information is often paramount to the physician when deciding on treatment. Additionally, fibrinolytic usage could not be accurately assessed using this dataset, and the group of patients receiving treatment with a chest tube is likely a composite of patients who had a tube placed for drainage only and patients who received fibrinolytic therapy. Similarly, there are no data available on the intent of initial chest tube placement. The chest tube cohort was very likely a heterogenous group of patients, including individuals who were too acutely or chronically ill for surgical intervention, those needing source control as a bridge to a definitive operation, and patients with early empyema who might have been spared an operation through a trial of chest tube drainage with or without fibrinolytics. Therefore, it is unclear whether the patients who progressed to operative management after tube placement truly failed adequate drainage, or if the tube was intended to be temporary source control until a definitive procedure could be performed due to either patient or hospital factors. Finally, there is some degree of selection bias present in determining treatment modalities for the patients in this administrative database, as observed by the fact that the cohort treated more conservatively with chest tube drainage tended to be older and sicker than the operative groups. In these cases, multimodality treatment may indicate that conservative therapy was appropriately attempted in order to spare a number of older, sicker, or frailer patients a morbid operation. These differences in patient characteristics, both observed within the database and in uncaptured factors, contribute to the variation in outcomes seen between treatment modalities.
Although these limitations prevent creation of a predictive model for treatment success with this dataset, the observed real-world results in this study provide a useful basis for understanding outcomes in empyema treatment in the current era. With continued high rates of mortality, readmission, and reintervention, there is room for improvement in management of this disease. Provision of care by qualified surgeons and early attention to selecting appropriate definitive care for these patients may improve outcomes.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor MD, Kozower BD. Surgical Spectrum in the Management of Empyemas. Thorac Surg Clin. 2012;22:431–40. doi: 10.1016/j.thorsurg.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Shen KR, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg. 2017;153:e129–46. doi: 10.1016/j.jtcvs.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Huang HC, et al. Predicting factors for outcome of tube thoracostomy in complicated parapneumonic effusion for empyema. Chest. 1999;115:751–6. doi: 10.1378/chest.115.3.751. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak CJ, et al. Choice of first intervention is related to outcomes in the management of empyema. Ann Thorac Surg. 2009;87:1525–30-1. doi: 10.1016/j.athoracsur.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Janda S, Swiston J. Intrapleural Fibrinolytic Therapy for Treatment of Adult Parapneumonic Effusions and Empyemas. Chest. 2012;142:401–11. doi: 10.1378/chest.11-3071. [DOI] [PubMed] [Google Scholar]
- 6.Chambers A, et al. Is video-assisted thoracoscopic surgical decortication superior to open surgery in the management of adults with primary empyema? Interact Cardiovasc Thorac Surg. 2010;11:171–7. doi: 10.1510/icvts.2010.240408. [DOI] [PubMed] [Google Scholar]
- 7.HCUP State Inpatient Databases (SID) Healthcare Cost and Utilization Project (HCUP). 2009–2014. Agency for Healthcare Research and Quality; Rockville, MD: n.d. [accessed October 19, 2017]. https://hcup-us.ahrq.gov/sidoverview.jsp. [Google Scholar]
- 8.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Research and Quality, Rockville M. HCUP Comorbidity Software. Healthcare Cost and Utilization Project (HCUP); 2008. [accessed March 21, 2017]. www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. [Google Scholar]
- 10.Davies HE, et al. A study of the complications of small bore “Seldinger” intercostal chest drains. Respirology. 2008;13:603–7. doi: 10.1111/j.1440-1843.2008.01296.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.