Abstract
Although the hypothalamus functions as a master homeostat for many behaviors, little is known about the transcriptional networks that control its development. To investigate this question, we analyzed mice deficient for the Forkhead domain transcription factor Foxd1. Foxd1 is selectively expressed in neuroepithelial cells of the prethalamus and hypothalamus prior to the onset of neurogenesis, and is later restricted to neural progenitors of the prethalamus and anterior hypothalamus. During early stages of neurogenesis, we observed that Foxd1-deficient mice showed reduced expression of Six3 and Vax1 in anterior hypothalamus, but overall patterning of the prethalamus and hypothalamus is unaffected. After neurogenesis is complete, however, a progressive reduction and eventual loss of expression of molecular markers of the suprachiasmatic, paraventricular and periventricular hypothalamic is observed. These findings demonstrate that Foxd1 acts in hypothalamic progenitors to allow sustained expression of a subset of genes selectively expressed in mature neurons of the anterior hypothalamus.
Keywords: Hypothalamus, prethalamus, development, patterning, cell fate, suprachiasmatic, neuroendocrine, paraventricular
1. Introduction
The hypothalamus is a master regulator of a broad range of homeostatic processes and innate behaviors, including circadian rhythms, sleep, blood pressure, thirst, feeding and body weight, control of the neuroendocrine system, and a range of sexual, emotional and affiliative behaviors (Bedont et al., 2014; Burbridge et al., 2016; Caron and Richard, 2017; Herrera et al., 2017; Lechan and Toni, 2000; Lee et al., 2012; Liu et al., 2017; Morrison, 2016). Despite its behavioral importance, the development of the hypothalamus is poorly understood, largely because of its considerable anatomic and cellular complexity (Braak & Braak, 1992; Flament-Durand,1980; Lechan & Toni, 2000). Although recently some progress has been made in identifying extrinsic and intrinsic factors that control hypothalamic patterning and neurogenesis, much remains unknown (Bedont et al., 2015; Burbridge et al., 2016; Xie and Dorsky, 2017).
Large-scale analysis of gene expression in the developing mouse hypothalamus has provided a starting point for examining hypothalamic development, and has identified candidate genes that are expressed in discrete spatial domains of early hypothalamic neuroepithelium (Shimogori et al., 2010). Among these genes is the Forkhead domain transcription factor Foxd1. Previously identified as a regulator of kidney and retinal ganglion cell development (Carreres et al., 2011; Hatini et al., 1996), Foxd1 was observed to be broadly expressed in neural progenitor cells of the prethalamus and anterior and tuberal hypothalamus during early stages of neurogenesis by E11.5 (Figure 1A,B). By E14.5, however, Foxd1 expression was no longer detected in anterior or tuberal hypothalamus, and became restricted to prethalamic neuroepithelium (Figure 1C,D). Although no hypothalamic defects in Foxd1 mutants have been previously reported (Carreres et al., 2011; Gumbel et al., 2012; Hatini et al., 1996; Levinson et al., 2005), the spatially and temporally dynamic expression pattern of this gene suggests a potential role in patterning the hypothalamus and/or prethalamus.
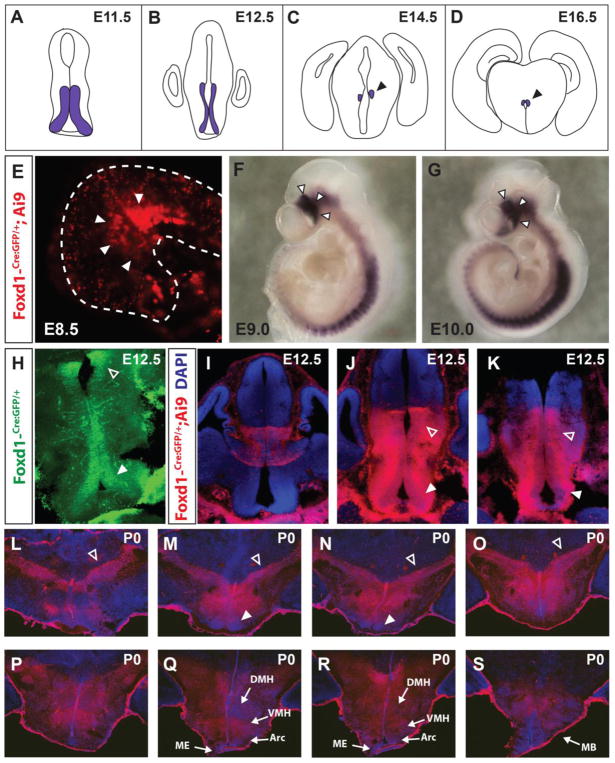
Figure 1. Foxd1 is broadly expressed in hypothalamic and prethalamic neuroepithelial cells.
A–D: Schematic showing Foxd1 mRNA expression in the anterior hypothalamus and prethalamus over the course of development, adapted from Shimogori et al., 2010. E: GFP staining from a Foxd1-Cre/+; Ai9 E8.5 embryo. White arrowheads indicate the presumptive hypothalamus in the ventral neural tube. F, G: Whole mount in situ hybridization of Foxd1 mRNA at E9.0 (F) and E10.0 (G). The hypothalamus is designated by white arrowheads. H: Immunohistochemistry staining against GFP in a Foxd1-Cre/+ E12.5 brain detects expression of Cre-GFP fusion protein expressed from the Foxd1 locus. By this age, Foxd1 is expressed only in the prethalamus (open arrowhead) and the anterior hypothalamus (white arrowhead) along the ventricle. I–K. Despite the limited Foxd1 expression at E12.5, lineage tracing in E12.5 Foxd1-Cre/+; Ai9 brains reveals that every cell in the hypothalamus and prethalamus originates from a Foxd1-positive lineage. Open arrowheads=prethalamus, white arrowheads=hypothalamus. L–S: DsRed immunohistochemistry against in P0.5 Foxd1-Cre/+; Ai9 brain reveals a history of Cre activity throughout the prethalamus and hypothalamus. Blue=DAPI. Open arrowheads=prethalamus, white arrowheads=SCN. Sections are arranged in anterior-posterior sequence from top left to bottom right.
In this study, we have investigated the developmental phenotype of Foxd1 mutants using the region-specific markers identified in our previous work (Shimogori et al., 2010). Despite broad early expression in developing hypothalamus and prethalamus, Foxd1-deficient mice show substantially delayed developmental defects that are essentially confined to the anterior hypothalamus. At E12.5, Foxd1-deficient mice exhibit only modest developmental defects. Early markers of anterior hypothalamic structures such as the suprachiasmatic nucleus (SCN) are detected at E16.5, but by birth are either lost entirely or substantially downregulated. Moreover, markers of terminal differentiation of the SCN, as well as other anterior hypothalamic structures such as the periventricular nucleus (PeVN) and paraventricular nucleus (PvN) are also lost by birth. The contrast between the selective expression of Foxd1 in early-stage hypothalamic progenitors, and the delayed appearance of a developmental phenotype, implies that Foxd1 may control anterior hypothalamic development indirectly by regulating expression of additional transcription factors such as Six3 and Vax1. Alternatively, the delayed developmental phenotype in Foxd1 mutants may result from altered chromatin accessibility at cis-regulatory sequences required for sustained expression of anterior hypothalamic-enriched genes in terminally differentiated neurons. These findings provide new insight into the transcriptional regulatory networks that specify cell identity in the hypothalamus..
2. Materials and methods
2.1 Mouse breeding and embryo collection
Heterozygous Foxd1-Cre: GFP knock-in mice (Foxd1-Cre/+)(Humphreys et al., 2008) were mated to each other to generate homozygous animals (Foxd1-Cre/Foxd1-Cre) that phenocopy null mutations in Foxd1, and are referred to in this manuscript as Foxd1 mutants. Heterozygous Foxd1-Cre/+ animals are used as controls for all experiments. Mice were maintained and euthanized according to protocols approved by Johns Hopkins Institutional Animal Care and Use Committee.
2.2 In situ hybridization
Embryos were post-fixed in 4% paraformaldehyde in 1xPBS, then embedded in gelatin before sectioning. In situ hybridization was performed as previously described (Shimogori et al., 2010).
2.3 EdU staining and cell counting
Pregnant dams were injected with 50 mg/kg EdU (20 mg/mL in saline) on embryonic day E12.5 and then sacrificed via cervical dislocation 2 hours later. Embryos were post-fixed in 4% PFA, cryopreserved in 30% sucrose, embedded in OCT then cryostat sectioned. EdU staining was performed using the Click-IT EdU imaging kit from Invitrogen, and then the slides were coverslipped using Vectashield. Stained slides were imaged at 40x on a Keyence microscope. Cell counting was conducted using ImageJ on five to six sections per animal. All counting was done blinded and manually.
2.4 TUNEL staining
Post-fixed and cryopreserved E12.5 embryos were sectioned and dry mounted. TUNEL staining was performed according to the protocol for cryopreserved tissue in the In Situ Cell Death Detection Kit-TMR red (Roche). A positive control was used (3000 U/mL DNAse I). Slides were DAPI stained then coverslipped using Vectashield.
2.5. Immunohistochemistry
Embryos were harvested and post-fixed in 4% paraformaldehyde in 1xPBS for a minimum of 16 hours at 4°C. Slides were washed 3x5 min in 1xPBS, then cryopreserved in 30% sucrose in 1xPBS for a minimum of 24 hours at 4°C. Embryos were then embedded in OCT blocks on dry ice, and sectioned on a cryostat at 25μm thickness. Sections were immediately dry mounted onto Superfrost Plus slides and allowed to dry before storing at −20°C. Before beginning the procedure, slides were allowed to dry at room temperature, and then marked with a pap pen to isolate the sections. Slides were incubated in blocking buffer (10% HIHS in 0.1% TX-100 in 1xPBS) for at least two hours at room temperature, before incubating in primary antibody in blocking buffer overnight at 4°C.
Antibodies used are as follows: rabbit anti-GFP 1:1000 (Life Technologies A6455 polyclonal) and rat anti-dsRed 1:1000 (Chromotek 5F8 monoclonal). Slides were washed 3x5 min in 1xPBS-T then incubated with secondary antibody in blocking buffer for two hours at room temperature. Secondary antibodies used were goat anti-rabbit-488 and donkey anti-rat-555. The slides were then washed 5 min in 1xPBS-T before DAPI staining in 1:5000 DAPII in 1xPBS-T for 5 min. Slides were then rinsed 3x5 min in 1x-PBS-T before coverslipping in Vectashield. Stained slides were imaged at 10x on a Keyence microscope.
2.6 Whole-mount in situ hybridization
All incubations were at room temperature unless otherwise noted, and performed in 24-well mesh plates. E9.0 and E10.0 wild-type embryos were harvested in cold 1xPBS-DEPC. E10 embryos were cut down the midline to allow for probe access. Embryos were fixed in 4% PFA in 1xPBS-DEPC for approximately one week. After fixation, the embryos were washed 2x15 min in PTw (1% Tween-20 in 1xPBS-DEPC) then dehydrated with 25%, 50%, then 75% methanol in PTw for 15 min each, then 100% methanol in PTw 3x15 min. Embryos were stored overnight at −20°C, before rehydration in 75%, 50%, and then 25% methanol in PTw for 15 min each, followed by PTw 2x15 min.
Embryos were then incubated in 6% hydrogen peroxide in PTw for 60 min, followed by PTw wash 2x15 min. Embryos were then incubated 3x30 min in detergent mix (1% Igepal CA-630, 1% SDS, 0.5% Na Deoxycholate, 50mM Tris-HCl pH 8.0, 1mM EDTA, 150 mM NaCl) followed by a 10 min PTw wash. Embryos were digested in 10μg/mL Proteinase K in PTw for 20 min, post-fixed in 0.2% glutaraldehyde, 4% PFA in PTw for 20 min, then washed in PTw 2x10 min. A prehybridization step followed, with room temperature hybridization buffer added to the embryos before incubating at 70°C for one hour. Probe was added directly to the vials (25μl per 5mL hybridization buffer) and hybridized overnight at 70°C. Following hybridization, embryos were incubated in Solution X 4x45 min. At this point, the protocol is identical to the high-quality in situ hybridization protocol up to and including adding the antibody. Following antibody incubation, the embryos were washed in 1xTBST 3x10 min, 5x60 min, then overnight. Embryos were washed in NTMT 3x10 min then incubated in an NBT/BCIP color reaction solution at either room temperature or 4°C. Embryos were then washed in 1xTBST to remove the background signal, and the color reaction was stopped in TE.
2.7 RNA-Seq analysis
Hypothalamic tissue was dissected at E12.5 and E17.5 as previously described (Shimogori, et al. 2010) and stored −80°C prior to use. A total of 8 Foxd1-null and 10 control (either wild-type or Foxd1-Cre+/−) embryos were harvested at E17.5, which were pooled into two samples for each genotype, for an n=2. In the litter dissected at E12.5 there were 5 embryos of each genotype, of which 3 were E12.5 and 2 were E12.0, which were also pooled into two samples for an n=2. After genotyping, the RNA was extracted using the RNeasy kit (Qiagen). RNA from individuals of each genotype were then pooled and sent for quality control analysis to determine concentration and purity via Bioanalyzer. The E12.5 RNA had significant gDNA contamination, so DNAse digestion using the Qiagen kit was performed prior to Bioanalzyer analysis of isolated RNA. A cDNA library was generated and barcoded for each pooled RNA sample, then sequenced to a paired-end read length of 100bp using Illumina HiSeq 2500.. The raw RNA-Seq results were mapped onto mm9 genome (NCBI37) using the default parameters in Tophat and Cufflinks (Trapnell et al., 2012) to generate gene expression FPKM (fragments per kilobase of exon per million). Cuffdiff was used to determine differentially expressed genes between the control and mutant samples. Differentially expressed genes with a q-value of <0.05 were considered significant.
2.8 Statistical methods used
For the EdU cell counting, the number of EdU-positive cells was taken as a fraction of the total area of the hypothalamus measured in μm2. The values of the sections were averaged for each individual, and then compared using a two-tailed t-test. Three animals were used for each genotype.
3. Results
3.1 Foxd1 is broadly expressed in neuroepithelial cells of the prethalamus and hypothalamus
We first sought to expand upon our previous study of Foxd1 expression (Shimogori et al., 2010) by more fully exploring the spatial and temporal expression pattern of Foxd1 in the developing hypothalamus and prethalamus. For these studies, we used a mouse line in which a Cre-GFP fusion had been inserted in frame with the start codon of the endogenous Foxd1 locus (Hatini et al., 1996; Humphreys et al., 2008), which we then crossed to the Cre-dependent tdTomato reporter line Ai9 (Madisen et al., 2010). Using this approach, we observed Cre activity as early as E8.5 in the region of the presumptive hypothalamus (Figure 1E). Using whole-mount in situ hybridization, Foxd1 mRNA was found to be strongly expressed in prethalamic and hypothalamic neuroepithelium at E9.0 (Figure 1F) and E10.0 (Figure 1G), as well as in paraxial mesoderm, as previously described (Hatini et al., 1994). Using immunohistochemistry against GFP in heterozygous Foxd1-Cre-GFP knock-in animals, we found that Foxd1 expression was restricted to hypothalamic and prethalamic neural progenitors at E12.5 (Figure 1H), consistent with our previous in situ hybridization analysis (Shimogori et al., 2010). Analysis of Foxd1-Cre-GFP; Ai9 mice at E12.5, however, revealed that virtually the entirety of both hypothalamic and prethalamic regions expressed tdTomato, demonstrating that these cells arise from Foxd1-expressing progenitors (Figure 1I–K). This was the also seen in Foxd1-Cre-GFP; Ai9 mice at P0.5 (Figure 1L–S), consistent with previous analysis of adult Foxd1-Cre-GFP; Ai9 mice (Salvatierra et al., 2014).
Further support comes from analysis of Foxd1-Cre; Sun1-GFP hypothalamus at P30. In these mice, GFP labels nuclei of cells that have expressed Foxd1-Cre at some during development (Mo et al., 2015). We stained for GFP and the neuronal marker NeuN and calculated the percentage of co-labeled cells within the hypothalamus, and found that at least 80% of GFP-positive cells co-labeled for NeuN in all hypothalamic areas investigated (Figure S1)
3.2 Foxd1-deficient mice show modest reductions in Six3 and Vax1 expression at E12.5, a modest decrease in cell proliferation and no change in cell death
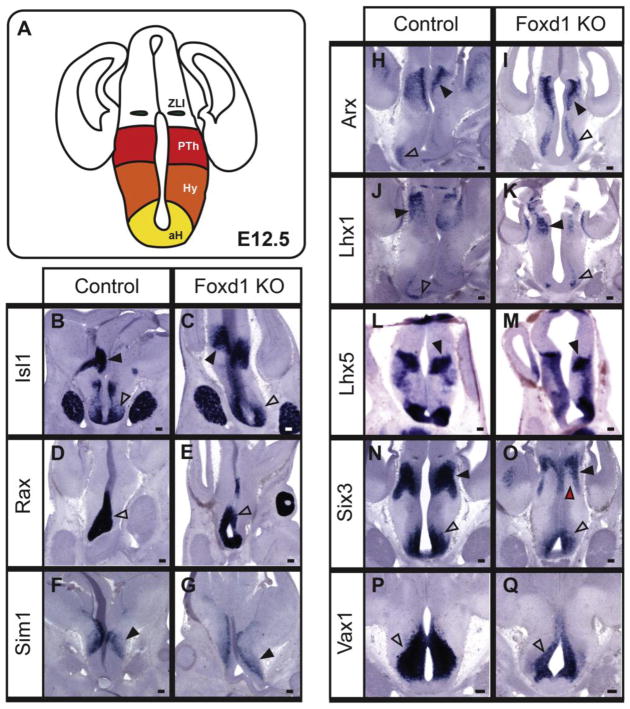
We generated Foxd1-deficient mice by creating homozygous Foxd1-Cre-GFP/Foxd1-Cre-GFP mice, which, as previously reported, show no detectable expression of native protein and die neonatal (Hatini et al., 1996). We first analyzed these mice at E12.5, conducting section in situ hybridization using a panel of markers that label discrete regions within prethalamus and/or hypothalamus (Figure 2A) (Shimogori et al., 2010). Most probes tested, including Isl1, Rax, Sim1, Arx, Lhx1 and Lhx5 (Figure 2B–M), showed no detectable difference in either expression pattern or signal intensity.
Figure 2. At E12.5, most hypothalamic markers are unaffected by loss of Foxd1.
A: Coronal schematic demonstrating the organization of the hypothalamus and prethalamus shown in B–Q. B–Q: In situ hybridization on E12.5 heterozygote control and Foxd1 mutant coronal sections. Most selective markers of the ventral AH (open arrowheads, B–E, H–K), and prethalamus (black arrowheads, B, C, H–K) were unaffected in Foxd1 mutant brains. However, Vax1 and Six3 expression was reduced in the ventral AH (open arrowheads, F, G, N, O) and Six3 expression in the prethalamus was also reduced (black arrowheads indicate the prethalamus, red arrowhead shows missing expression domain, F, G). Sim1 expression in the presumptive PVN was unaffected (black arrowheads, L, M). (Number of animals analyzed: Lhx1=3; Arx=3; Isl1=3; Sim1=3; Rax=2; Lhx5=1; Vax1=1; Six3=1)
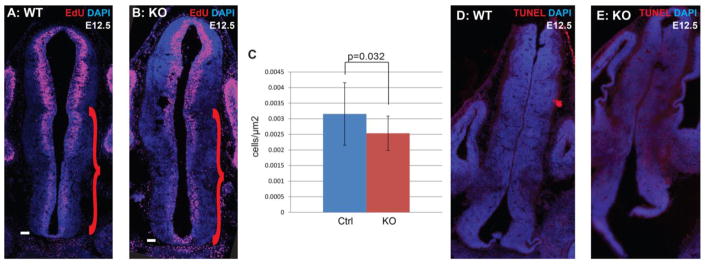
In contrast, expression levels of the transcription factors Six3 (Figure 2N,O) and Vax1 (Figure 2P,Q) showed reduced expression in the ventral hypothalamus of Foxd1-deficient embryos. Six3 also showed reduced expression in its prethalamic expression domain, with a more dramatic reduction observed in the ventricular zone (Figure 2O; red arrowhead) than in the mantle zone (Figure 2O; black arrowhead). In addition, although no changes in cell death were observed at this age in Foxd1-deficient embryos (Figure 3D,E), there was a modest but significant decrease in proliferation (Figure 3A–C).
Figure 3. Progenitor proliferation, but not apoptosis, is affected by loss of Foxd1.
A–B: Immunohistochemistry staining against EdU (red) and DAPI (blue) on E12.5 heterozygote control and Foxd1 mutant coronal sections after a 2hr EdU pulse. Red scroll bars indicate the counted portion of the section containing the prethalamus and hypothalamus. C: Quantification of EdU-positive cells relative to area (μm2) in control versus Foxd1 mutant (n=3). D–E: There was no obvious change in the number of TUNEL-positive cells in Foxd1 mutants.
3.3 Foxd1-deficient mice show reduced expression of a subset of anterior hypothalamic markers by E16.5
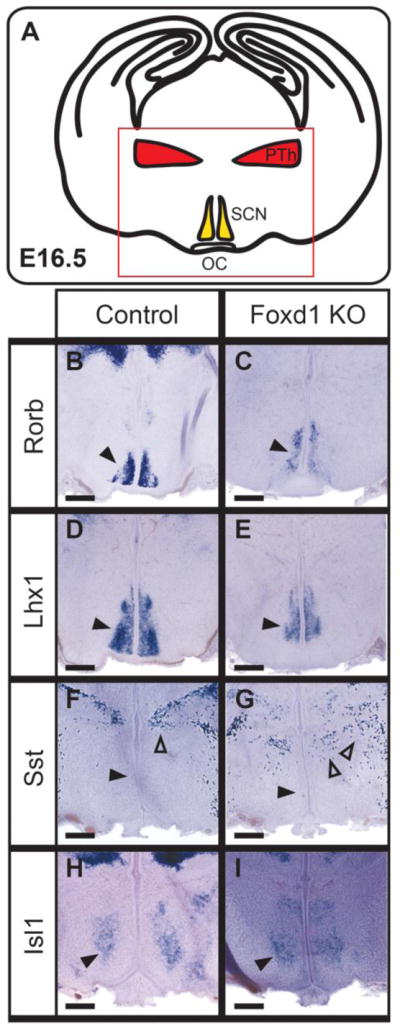
At E16.5, we observed selective defects in the expression of molecular markers for individual neuronal populations in the SCN and prethalamus in Foxd1-deficient embryos (Figure 4A). Although the broadly expressed gene Isl1 did not show altered expression (Figure 4B,C), the expression domain was significantly more medial in the Foxd1-null mutant, and located close to the third ventricle. We also observed a reduction in the expression levels of the SCN markers Rorb (Figure 4D,E) and Lhx1 (Figure 4F,G). Expression of somatostatin (Sst) was also disrupted in the thalamic reticular nucleus – a prethalamic structure (Inamura et al., 2011) – but was not detectably altered in lateral hypothalamus (Figure 4H,I).
Figure 4. A subset of anterior hypothalamic markers show reduced expression at E16.5.
I: A schematic demonstrating the organization of the hypothalamus and prethalamus at the coronal plane shown in A–I. A–I: In situ hybridization on E16.5 heterozygote controls and Foxd1 mutant brains. B–E: A subset of SCN-specific markers show modestly reduced expression at E16.5 (black arrowheads in B–E indicate the SCN). F, G: At E16.5, Sst expression is not yet detectable in the PeVN (black arrowheads), but shows altered organization in the prethalamus (open arrowheads). H, I: Isl1, which is broadly expressed in anterior hypothalamus, was unaffected at E16.5 (black arrowheads). (Number of animals analyzed: Lhx1=3; Isl1=1; Sim1=1; Rorb=2; Sst=1)
3.4 Foxd1-deficient mice show SCN agenesis and severe reduction of a subset of anterior hypothalamic markers by P0.5
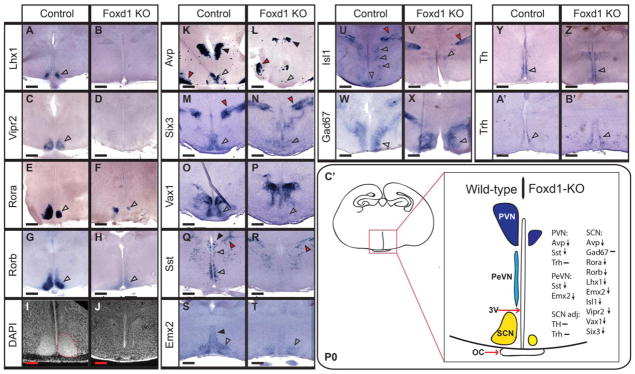
By P0.5, severe defects in SCN, PeVN and PVN differentiation were observed in Foxd1-deficient mice. Expression of Lhx1, a master regulator of terminal SCN differentiation (Bedont et al., 2014; Bedont and Blackshaw, 2015; Bedont, et al. 2017), was completely absent (Figure 5A, B), as was expression of Vipr2, an essential mediator of circadian entrainment in the SCN (Figure 5C,D) (Harmar et al., 2002). Rora and Rorb, two selective markers of the SCN and key transcriptional regulators of the core circadian clock, showed substantially reduced expression (Figure 5E–H). The histology of the SCN was also severely disrupted, with DAPI staining showing no remaining detectable nuclear organization (Figure 5I, J).
Figure 5. Loss of Foxd1 results in a broad and selective loss of anterior hypothalamic markers at P0.
A–H; K–B’: In situ hybridization of coronal sections from P0 heterozygote controls and Foxd1 mutant brain. Expression of selective markers of the SCN was either absent or greatly reduced in Foxd1 mutants relative to controls (open arrowheads, A–H, K–P, S, T). Gad67 expression, however, was unaffected (open arrowheads, W, X), as were Th and Trh (open arrowheads, Y–B’), which are expressed in regions of the AH adjacent to the SCN. In sharp contrast to wild-type animals (red dashed outline, I) the majority of Foxd1 mutants showed no evidence of an organized SCN (J). Markers of the PVN were also negatively affected by the loss of Foxd1 (black arrowheads, K, L, Q, R), as was Sst in the PeVN (open arrowheads, Q, R). The broadly expressed hypothalamic marker Isl1 was strongly reduced in anterior hypothalamus, but not in prethalamus (open arrowheads, U, V). Other prethalamic markers were unaffected (red arrowheads, M, N, Q, R). C’: Schematic summarizing developmental defects in the anterior hypothalamus. Expression of anterior hypothalamic markers was either reduced (down arrow) or unaffected (−). (Number of animals analyzed: Lhx1=3; Isl1=4; Sst=10; Gad67=7; AVP=6; Vipr2=3; Rorb=5; Trh=4; Rora=1; Emx2=1; Th=2; Six3=1; Vax1=1)
Genes that are expressed in SCN, as well as other anterior hypothalamic regions, also showed altered expression. Avp expression in SCN was virtually absent, and was substantially reduced in PVN, although its expression in SON was unaffected (Figure 5K, L). Expression of Six3, an essential transcriptional regulator of SCN development (VanDunk et al., 2011), showed dramatically reduced expression in the SCN region, as did the transcription factor Vax1 (Figure 4M–P). Sst expression was absent from the PeVN and PVN (Figure 5Q,R), and expression of the transcription factor Emx2 was likewise absent from the PeVN, but continued to show expression in the subparaventricular zone (vSPZ) (Figure 5S,T). Isl1, which is broadly expressed in the prethalamic-derived reticular nucleus, as well as the anterior and tuberal hypothalamus at this age, also showed substantially reduced expression throughout the hypothalamus, although expression in reticular nucleus was unaffected (Figure 5U,V; Figure 6A,B).
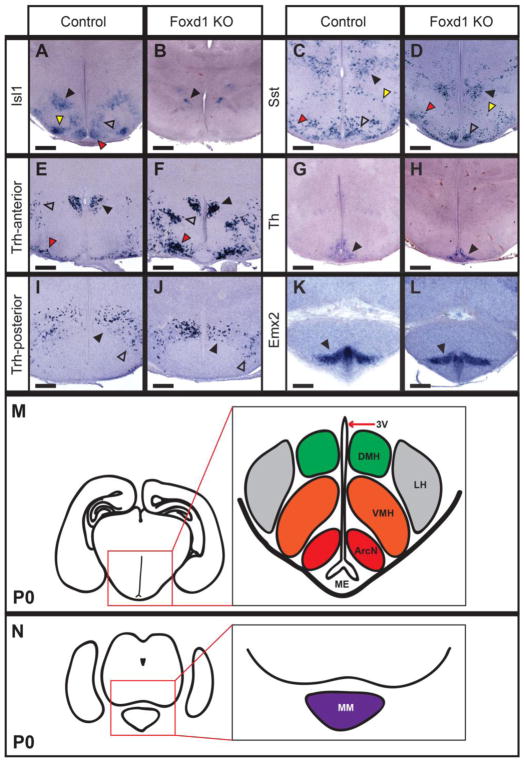
Figure 6. Posterior and lateral hypothalamic structures are largely unaffected in Foxd1-deficient animals at P0.
A–L: In situ hybridization on P0 heterozygote control and Foxd1 mutant coronal sections. Expression of Sst (open arrowheads, C, D), and Th (black arrowheads, G, H) in the ArcN were unchanged in Foxd1 mutants, but Isl1 expression (red arrowheads, A, B) was lost. DMH markers were unaffected (black arrowheads, C, D, I, J), with the exception of Isl1 (open arrowheads, A, B), which was also reduced in the ventrolateral VMH (yellow arrowheads, A, B) and LH (black arrowheads, A, B). The lateral hypothalamus had normal Trh expression (open arrowheads, E, F, I, J). Markers of the tuberomammillary terminal (red arrowheads, C, D), and the supraoptic nucleus (red arrowheads, E, F), and mammillary nucleus (black arrowheads, K, L) were also unaffected. M, N: Schematics showing the overall organization of posterior hypothalamic nuclei on two different coronal planes. M corresponds to the coronal plane featured in A–D, G–J and N corresponds to sections K, L.
Not all anterior hypothalamic markers showed reduced expression in Foxd1-deficient mice at P0.5, however. Expression of Th in A14 dopaminergic cells of the PeVN was unaffected (Figure 5Y, Z). Trh expression in the PeVN was likewise not reduced, although ectopic clusters of Trh-positive cells were observed (Figure 5A’,B’) and the PVN (Figure 6E,F). Gad67, which is expressed in GABAergic neurons throughout the anterior hypothalamus (O’Hara et al., 1995), likewise was not affected in Foxd1-deficient mice (Figure 5W, X). Despite the fact that reduced expression of Six3 was observed in prethalamus at E12.5 (Figure 2N, O), its expression in thalamic reticular nucleus was unaffected at P0.5 (Figure 5N; red arrowhead). Likewise, no obvious changes in Sst and Isl1 expression were seen in the prethalamus (Figure 5Q,R,U,V; red arrowhead), despite the fact that hypothalamic expression of both genes was severely disrupted, and the fact that prethalamic Sst expression was disrupted at E16.5 (Figure 4G). Finally, aside from decreased Isl1 expression, no changes in expression of markers of the tuberal and posterior hypothalamus were observed (Figure 6C–N).
3.5 RNA-Seq analysis identified additional genes showing altered expression in Foxd1-deficient mice at E12.5 and E17.5
To further characterize changes in gene expression resulting from loss of function of Foxd1, we conducted RNA-Seq analysis of E12.5 and E17.5 hypothalamus and prethalamus derived from heterozygote controls and Foxd1-deficient mice. At both ages we observed a dramatic downregulation of Foxd1 mRNA levels, as expected, but otherwise saw essentially no other overlap in genes that showed significantly altered expression at E12.5 and E17.5 (q<0.05). We observed 2 genes that were upregulated and 49 genes that were downregulated at E12.5, and 16 genes that were upregulated and 10 that were downregulated at E17.5. Several of the genes upregulated at E17.5 are selectively expressed in telencephalic structures, such as Foxg1, Neurod2 and Tbr1 (Table 2). However, analysis of Foxg1 at P0 revealed no ectopic expression within the hypothalamus (Figure S2), which suggests that these upregulated telencephalic markers might reflect contamination of the RNA-Seq samples by telencephalic structures.
Table 2. RNA-Seq analysis of control and Foxd1-deficient E17.5 hypothalamus.
RNA-Seq analysis of E17.5 hypothalamus from heterozygote control and Foxd1 mutants. Genes are listed that show significant differential expression and/or were analyzed by in situ hybridization. Differentially expressed genes were determined by q-value<0.05. Transcription factors and coregulators are highlighted in italic.
| Gene | Fold Change (log2) | q-value | Expression | In situ validated? |
|---|---|---|---|---|
| Avp | −3.38 | 0.04 | PeVN, SCN, PVN | yes |
| Foxd1 | −3.22 | 0.04 | TARGET | no |
| Oxt | −2.49 | 0.04 | PeVN | no |
| Slc6a3 | −1.65 | 0.04 | ZI/AH | no |
| 4930555G | ||||
| 01Rik | −1.49 | 0.04 | High in AH | no |
| Vipr2 | −1.32 | 0.49 | SCN | yes |
| Crh | −1.29 | 0.04 | PeVN | no |
| Vax1 | −1.21 | 0.04 | AH | yes |
| Cdh23 | −1.04 | 0.04 | High in AH | no |
| Rora | −1.01 | 1 | SCN | yes |
| Rorb | −0.20 | 1 | SCN | yes |
| Th | −0.17 | 1 | SCN adjacent | yes |
| Six3 | −0.16 | 1 | AH | yes |
| Isl1 | −0.10 | 1 | AH | yes |
| Lhx1 | −0.10 | 1 | SCN | yes |
| Gad1 | 0.01 | 1 | AH | yes |
| Sst | 0.11 | 1 | PeVN | yes |
| Trh | 0.23 | 1 | AH | yes |
| Emx2 | 0.31 | 1 | SCN and PeVN | yes |
| Zbtb18 | 0.66 | 0.04 | vAH | no |
| Tbr1 | 0.77 | 0.04 | Telencephalon | no |
| Satb2 | 0.89 | 0.04 | Telencephalon | no |
| Eomes | 1.06 | 0.04 | EmThal | no |
| Foxg1 | 1.06 | 0.04 | Telencephalon | yes |
| Kdm5d | 1.17 | 0.04 | ND | no |
| Neurod2 | 1.18 | 0.04 | Telencephalon | no |
At E12.5, we also observed substantially reduced expression of Zfhx2 and Zfhx3, two transcription factors selectively expressed in anterior hypothalamus, as well as Six3 (Table 1). Other transcriptional regulators that show both reduced expression in Foxd1-deficient mice and selective expression in embryonic anterior hypothalamus include the transcription factor Tox2, the histone demethylase Kdm6b, and the transcriptional coregulator Rere (Eichele and Diez-Roux, 2011; Lein et al., 2007).
Table 1.
RNA-Seq analysis of control and Foxd1-deficient E12 hypothalamus. RNA-Seq analysis of E12 hypothalamus from heterozygote control and Foxd1 mutants. Genes are listed that show significant differential expression and/or were analyzed by in situ hybridization. Two replicates from E12 and E12.5 were combined for this analysis. Differentially expressed genes were determined by q-value<0.05. Transcription factors and coregulators are highlighted in italic.
| Gene | Fold-change (log2) | q-value | Expression | In situ validated? |
|---|---|---|---|---|
| Foxd1 | −3.32 | 0.015 | TARGET | no |
| Atn1 | −2.38 | 0.015 | ND | no |
| Nova2 | −2.16 | 0.015 | AH | no |
| Kdm6b | −2.10 | 0.015 | AH | no |
| Zfhx2 | −1.82 | 0.015 | AH | no |
| Six3 | −1.72 | 0.015 | AH | yes |
| Rere | −1.29 | 0.015 | AH | no |
| Zfhx3 | −1.25 | 0.015 | AH | no |
| Fign | −1.18 | 0.015 | AH | no |
| Atp1b2 | −1.87 | 0.029 | AH | no |
| Tox2 | −1.61 | 0.039 | AH | no |
| Isl1 | −0.29 | 1 | AH | yes |
| Rax | −0.33 | 1 | AH | yes |
| Sim1 | 0.14 | 1 | PVN | yes |
| Arx | −0.16 | 1 | ID | yes |
| Lhx1 | −0.99 | 0.15 | AH | yes |
| Lhx5 | −0.22 | 1 | ID and MM | yes |
| Vax1 | 0.09 | 1 | AH | yes |
With the exception of Pou4f1 and Irx1, which likely represent dissection contaminants from habenula, all of the genes that are significantly downregulated in E17.5 Foxd1-deficient mice are selectively expressed in anterior hypothalamus. These include the neurohormones Avp, Oxt and Crh; the transcription factor Vax1, and the cell adhesion molecule Cdh23 (Table 2).
4. Discussion
In this study, we have identified Foxd1 as an essential regulator of anterior hypothalamic development. Despite showing strong and selective expression in early hypothalamic and prethalamic neuroepithelial and neural progenitor cells, the effects of loss of function of Foxd1 are initially quite modest at E12.5, and are primarily confined to downregulation of a handful of genes that are selectively expressed in developing anterior hypothalamus, including the transcription factors Six3, Vax1 and Zfhx3. Most anterior hypothalamic markers, and all tuberal and posterior hypothalamic markers tested were unaffected at this age, and only Six3 showed reduced expression in neural progenitors of the prethalamus. At E16.5, when neurogenesis is essentially complete in hypothalamus and prethalamus (Byerly and Blackshaw, 2009; Ratié et al., 2013; Shimada and Nakamura, 1973) and Foxd1 expression is barely detectable, we observed reduced expression of early markers of the developing SCN, including Lhx1 and Rorb, along with reduced Sst expression in thalamic reticular nucleus (which is derived from prethalamus (Inamura et al., 2011)). In addition, there was a medial shift in the Isl1-expression domain, along with the emergence of clusters of Trh-positive neurons in the more lateral regions of the anterior hypothalamus. This may result from a of loss of cells of the presumptive PeVN, which normally occupies this domain, but this is difficult to test directly given that many markers of the PeVN are not expressed at this time point (e.g. Sst; Figure 4F,G; black arrowhead indicating the PeVN). By P0.5, expression of SCN-specific markers is either absent or greatly reduced, and nucleogenesis of the SCN does not occur. In addition, there is a reduction or absence of expression of a subset of PeVN and PVN-specific markers, including Avp and Sst. Although there appears to be disrupted expression of a limited number of prethalamic markers at earlier timepoints, this is no longer observed at P0.5.
These findings lead us to draw several conclusions about the mechanism by which Foxd1 regulates the development of the prethalamus and hypothalamus. First, despite its initially broad expression throughout both structures, and selective expression in anterior hypothalamus following the onset of neurogenesis, Foxd1 is not required for spatial patterning or neurogenesis in either region; no obvious transformations in the identity of individual cell types are observed following Foxd1 loss of function.
Second, the effects of Foxd1 loss of function in hypothalamus primarily reflect a failure to either maintain expression of early-onset cell specific genes or to undergo terminal differentiation. An example of the former is Lhx1, the earliest detectable selective marker of the developing SCN, which initiates expression normally, but which shows reduced and then absent expression at later stages of development. An example of the latter is Sst expression in the PeVN and PVN, which is never initiated. It is, however, likely that failure of terminal differentiation results from disrupted maintenance of expression of master transcriptional regulators, such as Lhx1 itself in the case of the SCN. It is also important to note that in no hypothalamic region examined was there a complete failure of differentiation. Although the SCN was the most severely affected area, cells in this region continued to express the GABAergic marker Gad67, and also showed reduced but still detectable expression of Rora and Rorb. In the PeVN, Sst and Emx2 expression were lost but Th and Trh expression were preserved. Likewise, Avp and Sst expression in the PVN were sharply downregulated but Trh expression was unaffected. Taken together, these data imply that Foxd1 does not regulate cell identity per se, but is instead necessary to maintain expression of a subset of cell type-specific genes in anterior hypothalamus.
Third, despite strong and persistent expression of Foxd1 in prethalamic neuroepithelium, only modest and transient changes in prethalamic markers were observed. Although reduced Six3 and Sst expression were observed in embryonic prethalamus, at P0.5 there were no changes in either the pattern or intensity of prethalamic markers detected. This implies that while Foxd1 may regulate the kinetics of differentiation of certain prethalamic cell types, it is dispensable for their terminal differentiation.
Finally, the discrepancy between the neural progenitor-specific expression pattern of Foxd1 and the late onset of the developmental phenotypes observed in Foxd1 loss-of-function mutants implies that Foxd1 acts indirectly to control expression of genes associated with terminal differentiation. The most obvious mechanism by which this could occur is by regulation of expression of other transcription factors, which in turn go on to guide terminal differentiation of specific neuronal subtypes.
Several potential candidate factors were identified in our analysis, including Six3, Zfhx3 and Vax1. Six3 is expressed broadly in the developing anterior hypothalamus, and is one of the most strongly downregulated genes in Foxd1 mutants, as measured by both RNA-Seq and in situ hybridization. Previous work has shown that Six3 is essential for terminal differentiation of the SCN (VanDunk et al., 2011). The same study also suggested a possible role in regulating Avp expression in the PVN (VanDunk et al., 2011). Another gene known to regulate SCN-specific gene expression is the zinc homeodomain factor Zfhx3, which is significantly downregulated in RNA-Seq analysis of E12.5 Foxd1 mutant hypothalamus. Hypomorphic mutations of Zfhx3 have been shown to lead to reduced expression of SCN-specific genes and altered circadian function (Balzani et al., 2016; Parsons et al., 2015; Wilcox et al., 2017). A final candidate gene is Vax1, also shown to be strongly downregulated as measured by both RNA-Seq and in situ hybridization, which is also expressed in ventral hypothalamus, and has been reported to be required for expression of SCN-specific genes and maintenance of normal circadian activity rhythms (Hoffmann, et al. 2016).
Foxd1-dependent regulation of Six3, Zfhx3 and Vax1 may thus account for the severe defects in SCN development observed in Foxd1 mutants. It is important to note that expression of these genes is reduced but not eliminated in Foxd1 mutants, implying that additional, as yet unidentified, transcription factors are required to maintain expression of these genes in anterior hypothalamus. Further and more detailed analysis of mice mutant for these genes will be needed to determine whether disruption of these genes also accounts for observed defects in PeVN and PVN.
A second, and not mutually exclusive, potential mechanism of action of Foxd1 might be to prime the activation of cis-regulatory elements that control expression of cell type-specific genes during late stages of differentiation in hypothalamic neural progenitor cells. Other members of the Forkhead family such as Foxa1 are known to possess pioneering activity, or the ability to directly bind to closed chromatin and induce a shift to an open conformation (Lupien et al., 2008; Taube et al., 2010). It is thus possible that Foxd1 selectively targets enhancer elements that are necessary for transcription of critical transcriptional regulators such as Lhx1 in terminally differentiated neurons, altering their chromatin conformation and rendering them accessible for binding by other cell type-specific transcription factors. Global analysis of chromatin conformation using techniques such as ATAC-Seq (Buenrostro et al., 2013) in Foxd1-deficient hypothalamic progenitors can potentially address this question.
Third, loss of Foxd1 has a modest but significant negative effect on cell proliferation at E12.5, which may contribute to the developmental defect observed in the SCN and other anterior structures by P0. Loss of Foxd1 does not appear to lead to an increase in cell death at E12.5, suggesting that this is not a mechanism by which loss of Foxd1 leads to aberrant development of the anterior hypothalamus. However, although we cannot discount the possibility that increased cell death may occur at a later developmental time point, this is unlikely given the relatively normal appearance of the hypothalamus at E12.5 and the fact that Foxd1 expression is largely absent from the anterior hypothalamus after this age.
Supplementary Material
Immunohistochemistry analysis of GFP and NeuN in Foxd1-Cre; Sun-GFP mice at P30 in different hypothalamic regions. A–D: GFP in green channel. E–H: NeuN in far red channel. I–L: merged. M: PVN. N: DMH. O: VMH. P: LH. Q: PH. Scale bars represent 200μm.
A–J: In situ hybridization on P0 heterozygote control and Foxd1 mutant coronal sections. Limited expression of the telencephalic marker Foxg1 is observed in the preoptic area and anterior hypothalamus of the control hypothalamus, which was unchanged in the mutant hypothalamus (black arrowheads). Sections are arranged in anterior-posterior sequence from left to right.
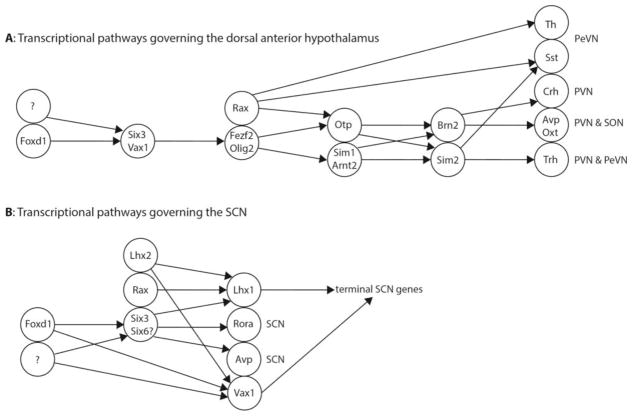
Figure 7. Schematic demonstrating model of the role of Foxd1 in anterior hypothalamic development.
A: The known transcriptional regulatory pathways governing development of the dorsal anterior hypothalamus. We hypothesize that Foxd1 regulates Six3 and Vax1, which in turn may regulate Fezf2 and Olig2. B: The transcription factors known to be involved in development of the SCN. We hypothesize that Foxd1 is controlling SCN development through regulation of Six3 and Vax1. In both the dorsal anterior hypothalamus and the SCN, we hypothesize that Foxd1 is working in conjunction with other, unknown transcription factors, due to the incomplete loss of Six3 and Vax1 in the Foxd1-null animals.
Highlights.
Foxd1 is broadly expressed in neuroepithelial cells of the hypothalamus and prethalamus.
Foxd1 mutants show severe defects in anterior hypothalamic development, although prethalamic development is only modestly affected.
Loss of Foxd1 results does not affect initial patterning of the hypothalamus, but leads to a progressive loss of expression of markers specific to neurons of the suprachiasmatic, paraventricular and periventricular nuclei.
Foxd1 regulates expression of multiple transcription factors expressed in developing anterior hypothalamus.
Acknowledgments
We thank W. Yap for comments on the manuscript. This work was supported by NIH R01DK108230, and a Hopkins Synergy Award (to S.B.)
Abbreviations
- AH
anterior hypothalamus
- DMH
dorsomedial hypothalamus
- ID
intrahypothalamic diagonal
- PeVN
periventricular nucleus
- PvN
paraventricular nucleus
- PrThal
prethalamus
- SCN
suprachiasmatic nucleus
- VMH
ventromedial hypothalamus
Footnotes
Author contributions
EAN conducted experiments and analyzed all data. JW and JQ analyzed RNA-Seq data. EAN and SB interpreted data, and wrote the manuscript, with input from all authors.
Author information
RNA-Seq data is available in GEO as accession number GSE108400. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balzani E, Lassi G, Maggi S, Sethi S, Parsons MJ, Simon M, Nolan PM, Tucci V. The Zfhx3-Mediated Axis Regulates Sleep and Interval Timing in Mice. Cell Rep. 2016;16:615–21. doi: 10.1016/j.celrep.2016.06.017. https://doi.org/10.1016/j.celrep.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedont JL, Blackshaw S. Constructing the suprachiasmatic nucleus: a watchmaker’s perspective on the central clockworks. Front Syst Neurosci. 2015;9:74. doi: 10.3389/fnsys.2015.00074. https://doi.org/10.3389/fnsys.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedont JL, LeGates TA, Slat EA, Byerly MS, Wang H, Hu J, Rupp AC, Qian J, Wong GW, Herzog ED, Hattar S, Blackshaw S. Lhx1 controls terminal differentiation and circadian function of the suprachiasmatic nucleus. Cell Rep. 2014;7:609–22. doi: 10.1016/j.celrep.2014.03.060. https://doi.org/10.1016/j.celrep.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedont JL, Newman EA, Blackshaw S. Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip Rev Dev Biol. 2015;4:445–68. doi: 10.1002/wdev.187. https://doi.org/10.1002/wdev.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Anatomy of the human hypothalamus (chiasmatic and tuberal region) Prog Brain Res. 1992;93:3–14–6. doi: 10.1016/s0079-6123(08)64559-8. [DOI] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–8. doi: 10.1038/nmeth.2688. https://doi.org/10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbridge S, Stewart I, Placzek M. Development of the Neuroendocrine Hypothalamus. Compr Physiol. 2016;6:623–43. doi: 10.1002/cphy.c150023. https://doi.org/10.1002/cphy.c150023. [DOI] [PubMed] [Google Scholar]
- Byerly MS, Blackshaw S. Vertebrate retina and hypothalamus development. Wiley Interdiscip Rev Syst Biol Med. n.d;1:380–389. doi: 10.1002/wsbm.22. https://doi.org/10.1002/wsbm.22. [DOI] [PubMed] [Google Scholar]
- Caron A, Richard D. Neuronal systems and circuits involved in the control of food intake and adaptive thermogenesis. Ann N Y Acad Sci. 2017;1391:35–53. doi: 10.1111/nyas.13263. https://doi.org/10.1111/nyas.13263. [DOI] [PubMed] [Google Scholar]
- Carreres MI, Escalante A, Murillo B, Chauvin G, Gaspar P, Vegar C, Herrera E. Transcription factor Foxd1 is required for the specification of the temporal retina in mammals. J Neurosci. 2011;31:5673–81. doi: 10.1523/JNEUROSCI.0394-11.2011. https://doi.org/10.1523/JNEUROSCI.0394-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele G, Diez-Roux G. High-throughput analysis of gene expression on tissue sections by in situ hybridization. Methods. 2011;53:417–23. doi: 10.1016/j.ymeth.2010.12.020. https://doi.org/10.1016/j.ymeth.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Flament-Durand J. The hypothalamus: anatomy and functions. Acta Psychiatr Belg. n.d;80:364–75. [PubMed] [Google Scholar]
- Gumbel JH, Patterson EM, Owusu SA, Kabat BE, Jung DO, Simmons J, Hopkins T, Ellsworth BS. The forkhead transcription factor, Foxd1, is necessary for pituitary luteinizing hormone expression in mice. PLoS One. 2012;7:e52156. doi: 10.1371/journal.pone.0052156. https://doi.org/10.1371/journal.pone.0052156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann Hanne Mette, Meadows Jason D, Trang Crystal, Hereford Brittainy, Bharti Kapil, MRG, PLM Deletion of SIX3 or VAX1 in the SCN Impairs Circadian Rhythms and Fertility. Endocrine Society’s 98th Annual Meeting and Expo.2016. [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–78. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- Hatini V, Tao W, Lai E. Expression of winged helix genes, BF-1 and BF-2, define adjacent domains within the developing forebrain and retina. J Neurobiol. 1994;25:1293–309. doi: 10.1002/neu.480251010. https://doi.org/10.1002/neu.480251010. [DOI] [PubMed] [Google Scholar]
- Herrera CG, Ponomarenko A, Korotkova T, Burdakov D, Adamantidis A. Sleep & metabolism: The multitasking ability of lateral hypothalamic inhibitory circuitries. Front Neuroendocrinol. 2017;44:27–34. doi: 10.1016/j.yfrne.2016.11.002. https://doi.org/10.1016/j.yfrne.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–91. doi: 10.1016/j.stem.2008.01.014. https://doi.org/10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Inamura N, Ono K, Takebayashi H, Zalc B, Ikenaka K. Olig2 lineage cells generate GABAergic neurons in the prethalamic nuclei, including the zona incerta, ventral lateral geniculate nucleus and reticular thalamic nucleus. Dev Neurosci. 2011;33:118–29. doi: 10.1159/000328974. https://doi.org/10.1159/000328974. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Toni R. Functional Anatomy of the Hypothalamus and Pituitary. Endotext. 2000 [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–2. doi: 10.1038/nn.3079. https://doi.org/10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. https://doi.org/10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Levinson RS, Batourina E, Choi C, Vorontchikhina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–39. doi: 10.1242/dev.01604. https://doi.org/10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- Liu K, Kim J, Kim DW, Zhang YS, Bao H, Denaxa M, Lim SA, Kim E, Liu C, Wickersham IR, Pachinis V, Hattar S, Song J, Brown SP, Blackshaw S. Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature. 2017;548:582–587. doi: 10.1038/nature23663. https://doi.org/10.1038/nature23663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–70. doi: 10.1016/j.cell.2008.01.018. https://doi.org/10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40. doi: 10.1038/nn.2467. https://doi.org/10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo A, Mukamel EA, Davis FP, Luo C, Henry GL, Picard S, Urich MA, Nery JR, Sejnowski TJ, Lister R, Eddy SR, Ecker JR, Nathans J. Epigenomic Signatures of Neuronal Diversity in the Mammalian Brain. Neuron. 2015;86:1369–84. doi: 10.1016/j.neuron.2015.05.018. https://doi.org/10.1016/j.neuron.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF. Central control of body temperature. F1000Research. 2016:5. doi: 10.12688/f1000research.7958.1. https://doi.org/10.12688/f1000research.7958.1. [DOI] [PMC free article] [PubMed]
- Parsons MJ, Brancaccio M, Sethi S, Maywood ES, Satija R, Edwards JK, Jagannath A, Couch Y, Finelli MJ, Smyllie NJ, Esapa C, Butler R, Barnard AR, Chesham JE, Saito S, Joynson G, Wells S, Foster RG, Oliver PL, Simon MM, Mallon AM, Hastings MH, Nolan PM. The Regulatory Factor ZFHX3 Modifies Circadian Function in SCN via an AT Motif-Driven Axis. Cell. 2015;162:607–21. doi: 10.1016/j.cell.2015.06.060. https://doi.org/10.1016/j.cell.2015.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratié L, Ware M, Barloy-Hubler F, Romé H, Gicquel I, Dubourg C, David V, Dupé V. Novel genes upregulated when NOTCH signalling is disrupted during hypothalamic development. Neural Dev. 2013;8:25. doi: 10.1186/1749-8104-8-25. https://doi.org/10.1186/1749-8104-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatierra J, Lee DA, Zibetti C, Duran-Moreno M, Yoo S, Newman EA, Wang H, Bedont JL, de Melo J, Miranda-Angulo AL, Gil-Perotin S, Garcia-Verdugo JM, Blackshaw S. The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. J Neurosci. 2014;34:16809–20. doi: 10.1523/JNEUROSCI.1711-14.2014. https://doi.org/10.1523/JNEUROSCI.1711-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Nakamura T. Time of neuron origin in mouse hypothalamic nuclei. Exp Neurol. 1973;41:163–73. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J, Blackshaw S. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–75. doi: 10.1038/nn.2545. https://doi.org/10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JH, Allton K, Duncan SA, Shen L, Barton MC. Foxa1 functions as a pioneer transcription factor at transposable elements to activate Afp during differentiation of embryonic stem cells. J Biol Chem. 2010;285:16135–44. doi: 10.1074/jbc.M109.088096. https://doi.org/10.1074/jbc.M109.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–78. doi: 10.1038/nprot.2012.016. https://doi.org/10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDunk C, Hunter LA, Gray PA. Development, maturation, and necessity of transcription factors in the mouse suprachiasmatic nucleus. J Neurosci. 2011;31:6457–67. doi: 10.1523/JNEUROSCI.5385-10.2011. https://doi.org/10.1523/JNEUROSCI.5385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AG, Vizor L, Parsons MJ, Banks G, Nolan PM. Inducible Knockout of Mouse Zfhx3 Emphasizes Its Key Role in Setting the Pace and Amplitude of the Adult Circadian Clock. J Biol Rhythms. 2017;32:433–443. doi: 10.1177/0748730417722631. https://doi.org/10.1177/0748730417722631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Dorsky RI. Development of the hypothalamus: conservation, modification and innovation. Development. 2017;144:1588–1599. doi: 10.1242/dev.139055. https://doi.org/10.1242/dev.139055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemistry analysis of GFP and NeuN in Foxd1-Cre; Sun-GFP mice at P30 in different hypothalamic regions. A–D: GFP in green channel. E–H: NeuN in far red channel. I–L: merged. M: PVN. N: DMH. O: VMH. P: LH. Q: PH. Scale bars represent 200μm.
A–J: In situ hybridization on P0 heterozygote control and Foxd1 mutant coronal sections. Limited expression of the telencephalic marker Foxg1 is observed in the preoptic area and anterior hypothalamus of the control hypothalamus, which was unchanged in the mutant hypothalamus (black arrowheads). Sections are arranged in anterior-posterior sequence from left to right.